Abstract
A truncated version of the nef gene of simian immunodeficiency virus SIVmac239 capable of encoding amino acids 98 to 263 was used as bait to screen a cDNA library from activated lymphocytes in a yeast two-hybrid system. The zeta chain of the T-cell receptor (TCRζ) was found to interact specifically not only with truncated SIV nef in yeast cells but also with full-length glutathione S-transferase (GST)-SIVnef fusion protein in vitro. Coimmunoprecipitation of TCRζ with full-length SIV nef was demonstrated in transfected Jurkat cells and in Cos 18 cells which express the cytoplasmic domain of TCRζ fused to the external domain of CD8 via the CD8 transmembrane domain. Using a series of nef deletion mutants, we have mapped the binding site within the central core domain of nef (amino acids 98 to 235). Binding of TCRζ was specific for nef isolated from SIVmac239, SIVsmH4, and human immunodeficiency virus (HIV)-2ST and was not detected with nef from five different HIV-1 isolates. An active tyrosine kinase was coprecipitated with nef-TCRζ complexes from Jurkat cells but not from J.CAM1.6 cells which lack a functional Lck tyrosine kinase. These results demonstrate a specific association of SIV and HIV-2 nef, but not HIV-1 nef, with TCRζ.
A nef gene is found in all subgroups of primate lentiviruses (49, 53). nef is considered a nonessential auxiliary gene because it can be deleted without dramatically affecting the ability of virus to replicate in cell culture (31, 56, 57). Evidence for the importance of nef for the efficiency of viral replication in the intact organism and for the maintenance of high virus loads has been derived both from studies with simian immunodeficiency virus (SIV) in monkeys and from studies with human immunodeficiency virus type 1 (HIV-1) in humans. Monkeys infected with a derivative of a pathogenic molecular clone of SIV from which nef gene sequences were specifically removed maintain low or undetectable virus loads and usually show no signs of disease progression (31). Similarly, one human in central Massachusetts (32) and several in Australia (16) are infected with nef-deleted forms of HIV-1, and they too are long-term nonprogressors who maintain low viral loads. In Australia, a single blood donor clearly transmitted nef-deleted HIV-1 to several recipients via blood donations, and this virus clearly behaved with a markedly attenuated phenotype.
Information is beginning to emerge which suggests that nef may have evolved a number of different, independent functional activities to enhance the replication and survival of virus. These include downregulation of the CD4 receptor from the surface of the cell (1, 21, 43, 50), downregulation of major histocompatibility complex class I molecules (52) which may protect infected cells from killing by cytotoxic T lymphocytes (15), infectivity enhancement (2, 38), and lymphocyte activation (3, 6, 17, 18) or inhibition of lymphocyte activation (23, 26, 39). Each of these functional activities clearly involves interactions with the host cell. Finding the cellular partners that couple with nef to achieve these functional activities is important for defining the biochemical activities and eventually delineating their relative importance. Cellular proteins that have been found to couple with nef include src family kinase (5, 14, 34, 46), a serine/threonine kinase (24, 37, 51), protein kinase C (PKC) theta (55), β-cop (7), a thioesterase (36), and CD4 (45).
In this report, we describe the specific interaction of the zeta chain of the T-cell receptor (TCRζ) with nef of SIVmac, SIVsm, and HIV-2. Specific binding to TCRζ was not observed with five different nef alleles of HIV-1. An active tyrosine kinase was found to coprecipitate with the nef-TCRζ complex, suggesting that the interaction might influence T-cell signaling.
MATERIALS AND METHODS
Cell lines and plasmids.
The Jurkat human T-cell line and the J.CAM1.6 cell line were obtained from the American Type Culture Collection (Rockville, Md.) and grown in RPMI 1640 medium which contained 25 mM HEPES, 10% fetal calf serum (Gibco/BRL, Grand Island, N.Y.), penicillin-streptomycin (50 IU and 50 μg/ml, respectively), and 2 mM l-glutamine (Gibco/BRL). Cos 18 cells were kindly provided by A. Weiss (Howard Hughes Medical Institute, University of California, San Francisco, Calif.) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal calf serum, 0.4 mg of G418 per ml, penicillin-streptomycin (50 IU and 50 μg/ml, respectively), and 2 mM l-glutamine (Gibco/BRL). The 221 cell line was grown in RPMI 1640 medium supplemented with 5% fetal calf serum, 10% interleukin-2 (IL-2), penicillin-streptomycin (50 IU and 50 μg/ml, respectively), and 2 mM l-glutamine (Gibco/BRL).
Plasmid pJSP4-27 containing the HIV-2 ST nef gene and plconsnefSN were obtained from the AIDS Research and Reference Reagent Program (McKesson Bioservices, Rockville, Md.). pSIVsmH4 was obtained from V. Hirsch (National Institute of Allergy and Infectious Diseases, Rockville, Md.). pGEX2T-SF2nef was a gift from D. Baltimore (Massachusetts Institute of Technology, Cambridge, Mass.).
Yeast two-hybrid screen.
Yeast two-hybrid screening was performed according to the protocol suggested by the MATCHMAKER TWO-HYBRID SYSTEM 2 (Clontech, Palo Alto, Calif.). nef hybrid expression plasmid pBD-239Δnef2 was constructed by fusing a truncated SIVmac239 nef gene encoding amino acids (aa) 1 to 15 and aa 98 to 263 (Δnef2; see Fig. 1) to the GAL4-DNA binding domain in the pAS2-1 vector. Δnef2 was used for the yeast two-hybrid screen in order to minimize high, nonspecific backgrounds seen with the full-length nef and because nef sequences missing aa 16 to 97 are still capable of associating with a serine/threonine kinase (data not shown) (51). A cDNA library from phytohemagglutinin (PHA)-activated human lymphocytes fused to the GAL4-DNA activation domain was purchased from Clontech. The yeast strain Saccharomyces cerevisiae Y190 (leu his trp auxotroph) harboring the two reporter genes HIS3 and lacZ was sequentially transformed with the nef hybrid expression plasmid and the PHA-activated human lymphocyte cDNA library by using the lithium acetate transformation method (Clontech). Double transformants were plated onto synthetic medium agar plates lacking leucine, tryptophan, and histidine in the presence of 3-amino-1,2,4-triazole (−L−Y−H+3AT). After 10 days of incubation at 30°C, His+ colonies were rescued and patched onto −L−Y−H+3AT plates. β-Galactosidase activities in these His+ colonies were tested by replica plating on nylon filters which were dipped into liquid nitrogen, soaked in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) buffer, and incubated at room temperature for 3 to 5 h. Colonies of the LacZ+ clones were restreaked onto −L−Y−H+3AT plates to isolate single colony and were retested for β-galactosidase activity. Confirmed positive clones (His+ LacZ+) were grown in leucine-minus synthetic medium in the presence of 10 μg of cycloheximide per ml for 5 days at 30°C to counterselect pBD-239Δnef2. Plasmids from the segregants (Leu+ Trp− Cyhr) containing only the pAD-cDNA were isolated, transformed into Escherichia coli, and sequenced.
FIG. 1.
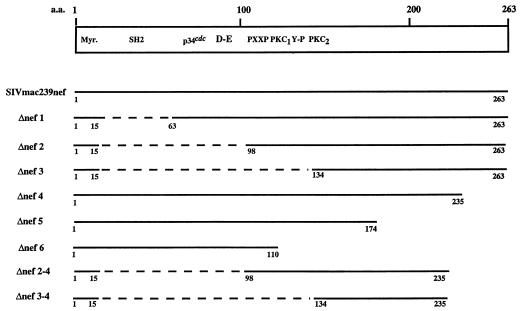
Schematic representation of deletion mutations in nef of SIVmac239. Sequence motifs are described by Shugars et al. (53) and Samuel et al. (49). Myr, myristoylation site; SH2, putative consensus SH2 binding sequence; p34cdc, consensus cyclin-dependent kinase substrate sequence; D-E, acidic stretch; PXXP, putative SH3 binding motif; Y-P, tyrosine kinase recognition sequence; PKC1 and PKC2, PKC recognition sequences. Clones for expression in mammalian cells contained an AU1 epitope tag at the carboxyl termini. Dashed lines represent deleted regions; numbers indicate the positions of the amino acids.
Yeast mating assay.
To verify specific interaction of nef and the protein expressed by the cDNA clones, S. cerevisiae Y190 containing the pAD-cDNA was mated with another strain, Y187, previously transformed with the pAS2-1 fused to the wild type or the truncated SIV nef genes in complete YPD medium for 18 h at 30°C. Diploid yeast cells were plated onto −L−Y−H+3AT plates and incubated for 5 days at 30°C. His and LacZ phenotypes were scored as described above.
Construction of Nef expression plasmids.
The plasmid p239SpE3′ containing the 3′ half of the SIVmac239 open proviral genome (42) was used as the template for the PCR amplification of the truncated nef fragments. Δnef1, Δnef2, and Δnef3 fragments were amplified by PCR by using the 5′ primers AH1 (5′-GGAAGATCTGGGACAGTATATGAATACTCCATG-3′), AH2 (5′-GGAAGATCTGGGGGTATCAGTGAGGCCAAAA-3′), and AH3 (5′-GGAAGATCTGTACAGTGCAAGAAGACATAGA-3′) and the 3′ primer 3′ NefPsIAU1 (5′-ACG CTGCAGTTATATATAGCGATAGGTGTCGCGAGTTTCCTTCTTGTCAGC- 3′), respectively, which introduced the unique BglII and PstI restriction sites (underlined) and a sequence encoding an AU1 epitope tag (in boldface). Δnef4, Δnef5, and Δnef6 fragments were amplified by using the 5′ primer 5′NefEcoRI (5′-GCGGAATTCATGGGTGGAGCTATTTCCATG-3′) and the 3′ primers AH4 (5′-ACGCTGCAGTTATATATAGCGATAGGTGTCGCTTCCAAACTCTTCT GGGTA-3′), AH5 (5′-ACGCTGCAGTTATATATAGCGATAGGTGTCTAGCCAGCCAAATGTCTTTGG-3′), and AH6 (5′-ACGCTGCAGTTATATATAGCGATAGGTGTCGTAACTCATTGTTCTTAGGGG-3′), respectively, which introduced the unique EcoRI and PstI restriction sites (underlined) and an AU1 epitope (in boldface). Δnef2-4 and Δnef3-4 were constructed by PCR amplification of p239SPE3′ with the 5′ primers AH2 and AH3, respectively, with the 3′ primer AH4. The Δnef1, Δnef2, Δnef3, Δnef2-4, and Δnef3-4 fragments digested with BglII and PstI and the Δnef4, Δnef1, and Δnef6 fragments digested with EcoRI and PstI were cloned into pFJ-239nef (18) previously digested with the corresponding enzymes to create pFJΔnef1, pFJΔnef2, pFJΔnef3, pFJΔnef4, pFJΔnef5, pFJΔnef6, pFJΔnef2-4, and pFJΔnef3-4.
To construct pFJSF2nef, the nef open reading frame (ORF) of pGEX2T-SF2nef was amplified by PCR with 5′EcoRISF2 (5′-GTCCAGAATTCGCCGCCATGGGTGGCAAGTGGTCAAAA-3′) and 3′PstIAU1SF2 (5′-ACGCTGCAGTTATATATAGCGATAGGTGTCGCAGTCTTTGTAGTACTCCGG-3′). The PCR DNA fragment was digested with EcoRI and PstI and cloned into pFJ expression vector (30) previously digested with the similar restriction enzymes to construct pFJSF2nef. pGST-SF2nef was constructed by removal of the SF2 nef DNA fragment from the pFJSF2nef at the EcoRI and XhoI sites and subcloned into a vector pGEX-4T (Pharmacia, Piscataway, N.J.). pGST-239nef was derived from pFJ239nef (18) by restricting at the EcoRI and XhoI sites and subcloned into the expression vector pGEX-4T.
The nef ORFs of the plasmids pFJNL4-3nef, pFJSHIVnef-153, and pFJSHIVnef-259 were generated by PCR amplification of pNL4-3 and of proviral clones recovered from two animals infected with a recombinant SHIVnef virus (16a). The primers used for PCR were 5′EcoRISF2 and 3′PstIAU1NL4-3 (5′-ACGC TGCAGTTATATATAGCGATAGGTGTCGCAGTTCTTGAAGTACTCCGG- 3′). The PCR fragments were subsequently cloned into the expression vector pFJ. pFJRulda was constructed in a similar manner by using PCR amplification from the proviral clone recovered from the animal infected with SHIVnefRulda with primers 5′EcoRIRulda (5′-GTCCAGAATTCGCCGCCATGGGGGGCAAGTGGTCAAAA-3′) and 3′PstIAU1Rulda (5′-ACGCTGCAGTTATATATAGCGATAGGTGTCGTTCTTGAAGTACTCCGGATG-3′). pFJSIVsmH4nef and pFJHIV-2ST were constructed by PCR amplification of the plasmids pSIVsmH4 and pJSP4-27, respectively, with primers SmH45′EcoRI (5′-GTCCAGAATTCGCCGCCATGGGTGGCGCTATTTCCAAG-3′) and SmH43′AU1PstI (5′-AC GCTGCAGTTATATATAGCGATAGGTGTCATCTGCCAGCCTCTCCGCAG A-3′) for pFJSIVsmH4nef and primers 5′EcoRIHIV-2 (5′-CCGGAATTCATGGGGGCGAGTGGATCCAAGAAG-3′) and 3′PSTIAU1HIV-2 (5′-ACGCTGCAGTTATATATAGCGATAGGTGTC) for pFJHIV-2ST. The PCR fragments were digested with EcoRI and PstI and cloned into pFJ digested with the similar enzymes.
The nef ORF of the consensus nef was amplified from pJSP4-27 by PCR with primers 5′XEConsef (5′-CTTCAGTCTAGAATTCGCCACCATGGGTGGCAAG-3′) and 3′BamHIConsnef (5′-CGCGGATCCTTATATATAGCGATAGGTGTCGCAGTCTTTGTAGTACTCCGGATG-3′). The PCR DNA fragment was cloned into pFJ previously digested with EcoRI and BglII to form pFJconsnef. Each mutant form of nef was completely sequenced to verify the presence of the mutation and the absence of any other changes.
All hybrid expression plasmids used for the yeast transformation were derived from the corresponding pFJ-nef expression plasmids with digestion at the EcoRI and PstI sites and cloned into the pAS2-1 vector (Clontech).
Expression and purification of recombinant glutathione S-transferase (GST)-fusion protein.
Ten milliliters of overnight cultures of E. coli transformed with pGEX-4T or recombinant plasmids were diluted 1:20 with fresh medium and grown for 2 h at 37°C before inducing with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After a further 6 h of incubation, the cells were pelleted and resuspended in 10 ml of bacteria lysis buffer containing 1% Triton X-100, 0.1% N-lauryl sarconsinate, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1% aprotinin, and 1 μg of leupeptin per ml in phosphate-buffered saline (PBS). Cells were lysed by sonication followed by centrifugation at 10,000 × g for 5 min at 4°C. The cell pellets were sonicated again and centrifuged at 10,000 × g for 15 min at 4°C. The supernatant was collected and incubated with 500 μl of the preswollen glutathione-Sepharose beads (Pharmacia) for 2 h at 4°C. The beads were washed three times with ice-cold PBS and stored at 4°C.
In vitro binding assay.
A total of 107 cells were lysed in 1 ml of cell lysis buffer (0.5% Nonidet P-40, 50 mM HEPES [pH 7.5], and 150 mM NaCl) containing 2 mM NaVO3, 10 mM NaF, 1 mM PMSF, 1 μg of leupeptin per ml, and 1% aprotinin (Sigma Chemical, St. Louis, Mo.). The cell lysates were centrifuged at 13,000 × g for 30 min at 4°C. The supernatant was mixed with 30 μl of the glutathione-Sepharose beads (beads) and 20 μl of the immobilized GST (GST beads; 5 mg/ml) and incubated for 30 min at 4°C. Precleared cell extracts were incubated with approximately 30 μg of the soluble GST and 10 μl of the immobilized GSTnef fusion proteins or GST beads (all loaded beads contained approximately 2 mg of protein per ml) for 3 h at 4°C. The coprecipitated proteins were washed three times with ice-cold lysis buffer, boiled in Laemmli sample treatment buffer (33) for 5 min, separated by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE) and electroblotted onto the Immobilon membrane (Millipore, Bedford, Mass.). Immunodetection was performed with a 1:3,000 dilution of anti-TCRζ mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) and developed by the enhanced chemiluminescence (ECL) system with procedures suggested by the manufacturer (Amersham, Chicago, Ill.).
Association of tyrosine kinase with the nef coprecipitation complex was performed by procedures similar to those described above, except that the coprecipitated complexes were washed three times with the cell lysis buffer and once with the kinase buffer (50 mM Tris-HCl [pH 7.4] and 10 mM MgCl2). In vitro kinase reaction with the coprecipitated proteins was carried out in the presence of the kinase buffer and 1 mM ATP (final volume, 20 μl) for 30 min at room temperature. Tyrosine-phosphorylated proteins were analyzed by SDS-10% PAGE, transferred onto the Immobilon membrane (Millipore), and immunodetected by the antiphosphotyrosine mouse monoclonal antibody 4G10 (immunoglobulin G2b [IgG2b]; 1:10,000 dilution; Upstate Biotechnology Inc., Lake Placid, N.Y.). The immunoblot was developed by the ECL system.
Transfection, immunoprecipitation, and immunoblotting.
Cos 18 cells were transfected with 5 μg of the nef expression plasmids by the standard DEAE-dextran method (47). Cells were harvested 48 h after transfection and lysed with 1 ml of the cell lysis buffer containing a cocktail of protease inhibitors as described above. The cell lysates were cleared by centrifugation at 13,000 × g for 30 min at 4°C and divided into aliquots of 50 and 950 μl, respectively. The aliquot containing 50 μl of the cell lysate was used for the analysis of nef protein expression, while the aliquot containing 950 μl of the cell lysate was used for the immunoprecipitation. Immunoprecipitation was performed by adding 1 μl of anti-AU1 mouse monoclonal antibody (BABCO Biotech, Berkeley, Calif.) and 30 μl of protein A-agarose (Santa Cruz Biotechnology) to the cell lysates. The suspension mixtures were rocked at 4°C for 3 h, and the immune complexes were washed three times with the cell lysis buffer and once with 50 mM Tris-HCl (pH 7.4). Immunoprecipitated proteins were separated by SDS-10% PAGE, transferred onto the Immobilon membrane (Millipore), and reacted with the anti-TCRζ mouse monoclonal antibody (diluted 1:3,000; Santa Cruz Biotechnology), and detected by the ECL system (Amersham).
Transfection of Jurkat cells was carried out by electroporating 107 cells at 210 V and 960 μF with 50 μg of the plasmid DNA. Cells were harvested 20 h posttransfection, and immunoprecipitation of nef complexes was conducted as described above.
RESULTS
SIVmac239 nef binds to a TCR sequence in yeast cells.
We used a deleted form of SIVmac239 nef protein (aa 1 to 15 and 98 to 263; Δnef2 in Fig. 1) fused to the DNA binding domain of the GAL4 transcription factor as bait in a yeast two-hybrid screen. The expression plasmid for this fusion construct was called pBD-239 Δnef2. A cDNA library prepared from PHA-activated human lymphocytes was fused to the transcription activation domain of the GAL4 transcription factor (pAD-cDNA) and introduced into S. cerevisiae Y190 previously transformed with pBD-239Δnef2. When the protein encoded by pBD-239Δnef2 interacts with that encoded by pAD-cDNA, it will reconstitute a functional GAL4 transcription factor and activate transcription of two reporter genes, lacZ and HIS3, respectively, which are present in the S. cerevisiae Y190. Transformants harboring the positive interacting clones are expected to grow in the absence of histidine and to produce β-galactosidase.
Approximately 106 individual cDNA clones were screened. Ninety-one colonies were able to grow in the absence of histidine. When these colonies were assayed for the production of β-galactosidase, 20 of the 91 HIS+ colonies were found to have β-galactosidase activity. To further eliminate false positives, candidate yeast colonies were subjected to cycloheximide counterselection to remove pBD-239Δnef2 from the cells. The resulting cycloheximide-resistant yeast colonies, which carried only the candidate pAD-cDNA, were then used in mating assays to determine the specificity of the interaction. Six independent clones remained positive. Sequence analysis of these six clones revealed that one (clone 69) had 97% nucleotide sequence identity to the human TCRζ. Comparison of the amino acid sequence encoded by clone 69 and the TCRζ cDNA showed that clone 69 encoded a 100-aa polypeptide similar to the cytoplasmic region of the human TCRζ (Fig. 2). There were three amino acid differences between the amino acid sequence deduced from clone 69 and the published sequence of TCRζ (Fig. 2) (61).
FIG. 2.
Comparison of amino acid sequence of clone 69 with that of the human TCRζ. The published sequence of the TCR (61) from the initiating methionine is shown. Dots indicate amino acid identity. Boldface letters indicate different amino acid sequences.
Diploid cells expressing vectors without inserts (pBD and pAD), expressing the truncated nef fusion without clone 69 (pBD-239Δnef2 and pAD), or expressing the clone 69 fusion without the nef fusion (pBD and pAD-cDNA69), did not grow in the selective medium and were negative for the lacZ phenotype (Table 1). In contrast, interaction of the truncated nef (pBD-239Δnef2) and the TCRζ (pAD-cDNA69) conferred on the yeast cells the ability to grow in −L−Y−H+3AT medium and to produce β-galactosidase (Table 1). This interaction was specific, since negative phenotypes were observed in cells expressing clone 69 or the truncated nef fusion protein in combination with the p53 or the simian virus 40 large T antigen (SV40 T-Ag), respectively (Table 1). As positive controls, yeast cells expressing the wild-type GAL4 transcription factor or the combination of p53 and SV40 large T antigen previously shown to interact with each other (35) were positive for the lacZ phenotype (Table 1). Specific interaction with clone 69 was also observed by using the full-length SIVmac239nef and by using a truncated nef containing the central core region containing aa 98 to 235 (239Δnef2-4) (Table 1). Further deletion from 98 to 134 (Δnef3; Fig. 1 and Table 1), however, resulted in the loss of interaction with clone 69, suggesting that aa 98 to 134 in nef are required to bind to the clone 69 sequence.
TABLE 1.
Growth and lacZ phenotypes in diploid S. cerevisiae harboring hybrid expression plasmids
| Strains containing the indicated plasmid
|
Growth on −L−T−H+3AT | lacZ phenotypea | |
|---|---|---|---|
| GAL4-DB (TRP1) | GAL4-AD (LEU2) | ||
| pVA3-1 (p53) | pTD1-1 (SV40 T-Ag) | + | Blue |
| pBD | pAD | − | White |
| pBD-239ΔNef2 | pAD | − | White |
| pBD | pAD-cDNA69 | − | White |
| pBD-239ΔNef2 | pAD-cDNA69 | + | Blue |
| pVA3-1 (p53) | pAD-cDNA69 | − | White |
| pBD-239ΔNef2 | pTD1-1 (SV40 T-Ag) | − | White |
| pBD-w.t.239Nef | pAD-cDNA69 | + | Blue |
| pBD-239ΔNef2-4 | pAD-cDNA69 | + | Blue |
| pBD-239ΔNef3 | pAD-cDNA69 | − | White |
S. cerevisiae Y190 and Y187 containing the two expression plasmids as indicated were mated in the presence of complete YPD medium. An aliquot of the diploid cells was plated onto −L−T−H+3AT plates as well as SD synthetic medium-agar plates lacking leucine and tryptophan (−L−T). In the case of diploid yeast cells which did not grow on −L−T−H+3AT plates, colonies from −L−T plates were used to analyze for lacZ phenotype.
Use of GST fusions to demonstrate specific interaction.
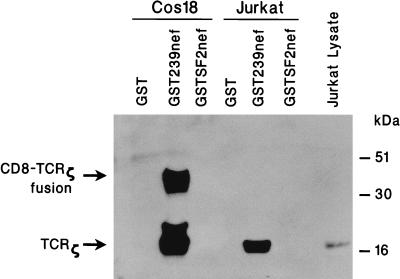
To confirm a possible interaction of nef with TCRζ, nef was expressed as a GST-fusion protein in E. coli, purified, immobilized on glutathione-Sepharose beads, and incubated with lysates of CD4+ Jurkat T cells or with lysates of Cos 18 cells which stably express CD8-TCRζ fusion protein (28). Proteins coprecipitated with nef were separated by electrophoresis in an SDS-12% polyacrylamide gel and electroblotted onto a nylon membrane; the presence of TCRζ was detected with mouse anti-TCRζ monoclonal antibody. A protein band of approximately 40 kDa, which was the size of the CD8-TCRζ fusion protein (22), was specifically detected in the sample containing the extract of Cos 18 cells and GST239nef (Fig. 3). Smaller species of approximately 16 and 22 kDa, which might have resulted from the degradation of the CD8-TCRζ fusion, were also detected. No CD8-TCRζ was detected when GST without nef was used (Fig. 3). Similarly, TCRζ (18 kDa) was also specifically detected when GST-SIVmac239 nef (GST239nef) was incubated with the Jurkat cell lysate (Fig. 3). Again, the nef-TCRζ interaction was specific, since no TCRζ was found to interact with GST in the absence of nef. Further evidence for the specificity of the interaction was obtained by the absence of signal when nef of HIV-1 strain SF2 was fused to GST and used for the assays (Fig. 3). The failure of HIV-1 SF2nef to interact with TCRζ is consistent with other results described in more detail below.
FIG. 3.
Binding of GSTnef with TCRζ in vitro. GST, recombinant GST239nef, and GSTSF2nef proteins were expressed in E. coli, affinity purified, and immobilized by using glutathione-Sepharose beads. The immobilized GST proteins were incubated with extracts of Cos 18 or Jurkat cells previously precleared with immobilized GST and glutathione-Sepharose beads. After extensive washing, proteins coprecipitated with the complexes were analyzed by SDS-12% PAGE, electroblotted onto a nylon membrane, and reacted with anti-TCRζ monoclonal antibody.
Coprecipitation of TCRζ and SIVmac239 nef from CD8-TCRζ-expressing cells.
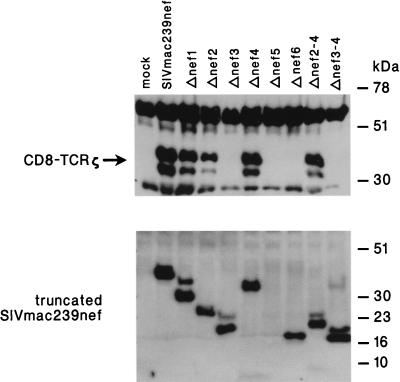
We also analyzed the ability of TCRζ to be coprecipitated with nef from CD8-TCRζ-expressing cells. For this purpose, an AU1 epitope tag was placed at the carboxyl terminus of nef-coding sequence in the pFJ expression plasmid and used for transfection into Cos 18 cells, which expressed the CD8-TCRζ fusion protein. Transfected cell lysates were incubated with anti-AU1 monoclonal antibody, and precipitated proteins were separated by SDS-12% PAGE. The presence of CD8-TCRζ in the nef immunoprecipitates was detected by reactivity of immunoblotted proteins with anti-TCRζ monoclonal antibody. Specific coimmunoprecipitation of CD8-TCRζ was readily detected with nef from SIVmac239 but not from mock-transfected cells (Fig. 4).
FIG. 4.
Coprecipitation of TCRζ with nef from Cos 18 cells. Cos 18 cells expressing CD8-TCRζ fusion protein were mock transfected (mock) or transfected with plasmids encoding full-length (SIVmac239nef) or truncated (Δnef1 to Δnef3-4) SIVmac239 nef. nef immune complexes were precipitated with anti-AU1 monoclonal antibody, washed extensively, resolved by SDS-12% PAGE, and transferred onto a membrane filter. CD8-TCRζ coimmunoprecipitated with nef was detected by the anti-TCRζ monoclonal antibody (upper panel). Expression of the full-length or the truncated SIVmac239 nef proteins in the corresponding transfected Cos 18 cells was detected by separating the whole-cell lysates in an SDS-12% polyacrylamide gel, transferring onto a nylon membrane, and probing with the anti-AU1 monoclonal antibody (lower panel).
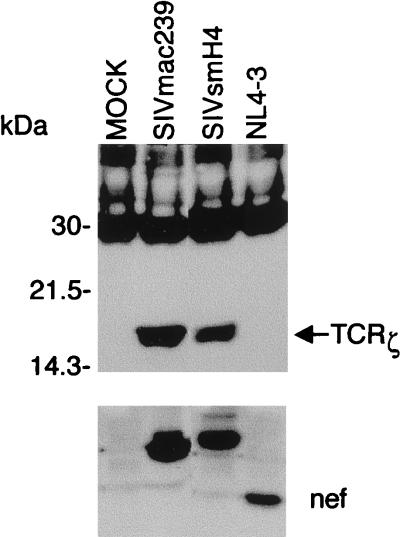
The nef gene of SIVsm strain H4 (25) was also AU1 tagged and cloned into the pFJ expression vector. Coprecipitation of authentic TCRζ with nef was examined following electroporation into Jurkat T cells. The endogenous TCRζ coprecipitated with both SIVmac239 nef and SIVsmH4 nef (Fig. 5). In contrast, AU1-tagged nef from HIV-1 strain NL4-3 did not associate with TCRζ in these assays (Fig. 5).
FIG. 5.
SIV nef interacts with the endogenous TCRζ in Jurkat cells. Jurkat cells were electroporated with expression plasmids encoding SIVmac239 nef, SIVsmH4 nef, or HIV-1 NL4-3 nef (NL4-3) tagged with an AU1 epitope. After 20 h, cells were harvested and nef proteins were immunoprecipitated with anti-AU1 monoclonal antibody. Proteins coimmunoprecipitated with nef were analyzed by SDS-12% PAGE and immunoblotted onto a nylon membrane which was probed with the anti-TCRζ monoclonal antibody (upper panel). Expression of the nef proteins was detected by separating the whole-cell lysates in an SDS-12% polyacrylamide gel and immunoblotting with the anti-AU1 monoclonal antibody (lower panel).
The central region of SIVmac239 nef is required to bind to TCRζ.
In order to delineate the regions of nef responsible for binding to TCRζ, a series of SIVmac239 nef deletion mutants was constructed and tested for binding to TCRζ. All nef mutants contained aa 1 to 15 at the amino terminus for myristoylation. The deletion mutants were constructed with an AU1 tag at the carboxyl terminus and analyzed following transfection of Cos 18 cells. The cells were lysed and incubated with AU1 antibody; precipitated proteins were analyzed by SDS-12% PAGE, and the presence of CD8-TCRζ was detected by reactivity of anti-TCRζ monoclonal antibody with immunoblotted proteins (Fig. 4). As shown in the lower panel of Fig. 4, comparable amounts of the full-length and the truncated nef proteins were detected in the transfected Cos 18 cells. When analyzed without heating prior to the electrophoresis, Δnef5 was detected, and the level of its expression was found to be similar to that of the control (not shown). Deletion of the amino terminus of nef from aa 16 to 97 (Δnef2 in Fig. 1) did not affect the binding to CD8-TCRζ (Fig. 4), suggesting that the amino-terminal region of nef which contains the putative SH2 binding motif and the acidic stretch may not be required for interaction with the TCRζ. However, further deletion up to aa 133, including the putative PXXP motif and the potential PKC phosphorylation site (PKC1) (Δnef3 in Fig. 1) resulted in the loss of binding to TCRζ (Fig. 4). While extensive deletion at the amino-terminal region of nef did not affect its interaction with the TCRζ, only minor deletion at the carboxyl terminus of the protein was tolerated (Δnef4 versus Δnef5 and Δnef6; Fig. 1 and 4). A minimal construct containing only aa 98 to 235 and 1 to 15 (Δnef2-4 in Fig. 1) was shown to bind CD8-TCRζ in these assays (Fig. 4). Thus, we have mapped aa 98 to 235 as a minimal region in nef of SIVmac239 capable of interacting with the TCRζ.
SIV nef and HIV-2 nef, but not HIV-1 nef, associate with TCRζ.
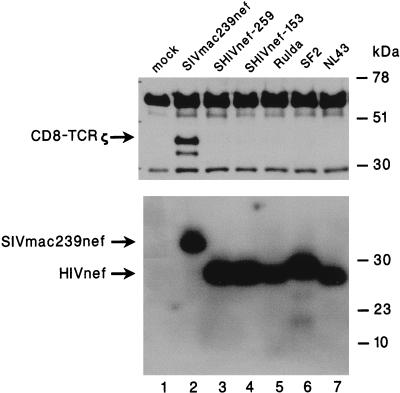
The failure of TCRζ to bind to GSTnef from HIV-1 strain SF2 (Fig. 3) and to coimmunoprecipitate with nef from HIV-1 strain NL4-3 in transfected Jurkat cells (Fig. 5) prompted us to investigate further this apparent restriction in specificity. We expressed AU1-tagged nef from five different HIV-1 isolates and analyzed their association with CD8-TCRζ in transfected Cos 18 cells. NL4-3 and SF2 nef were derived from two laboratory strains of HIV-1, whereas Rulda nef was derived from a primary clinical isolate. nef-153 and nef-259 are derivatives of NL4-3 nef from monkeys with progressive disease following infection by SHIVnef chimeras (16a). Expression plasmids containing these HIV-1 nef genes were transfected into Cos 18 cells, and TCRζ was detected in immunoprecipitates with TCRζ monoclonal antiserum as described for the above experiments. In contrast to nef of SIVmac239, which coimmunoprecipitated the TCRζ, none of the HIV-1 nefs associated with the TCRζ (Fig. 6, upper panel). Failure to coprecipitate the TCRζ was not due to instability or inefficient expression of the HIV-1 nef genes, since comparable amounts of the nef proteins were detected in the HIV-1 and the SIV nef-transfected cells (Fig. 6, lower panel).
FIG. 6.
HIV-1 nef does not associate with TCRζ. Cos 18 cells were transfected with expression plasmids encoding SIVmac239 or HIV-1 nef tagged with an AU1 epitope. nef immune complexes were precipitated with anti-AU1 monoclonal antibody. Immunoprecipitation complexes were washed extensively, separated by SDS-12% PAGE, and transferred onto a nylon membrane which was probed with the anti-TCRζ monoclonal antibody (upper panel). Expression of the nef proteins in the corresponding transfected Cos 18 cells was detected by separating the whole-cell lysates in an SDS-12% polyacrylamide gel, immunoblotting onto a nylon membrane, and probing with the anti-AU1 monoclonal antibody (lower panel). Lanes: 1, mock transfected; 2, wild-type SIVmac239 nef; 3 and 4, HIV nef obtained from two animals (259 and 153, respectively) infected with a recombinant SIV in which the SIV nef gene was replaced by the HIV NL4-3 nef allele (SHIVnef); 5, HIV nef derived from a primary clinical isolate (Rulda); 6, HIV-1 SF2 nef; and 7, HIV-1 NL4-3 nef.
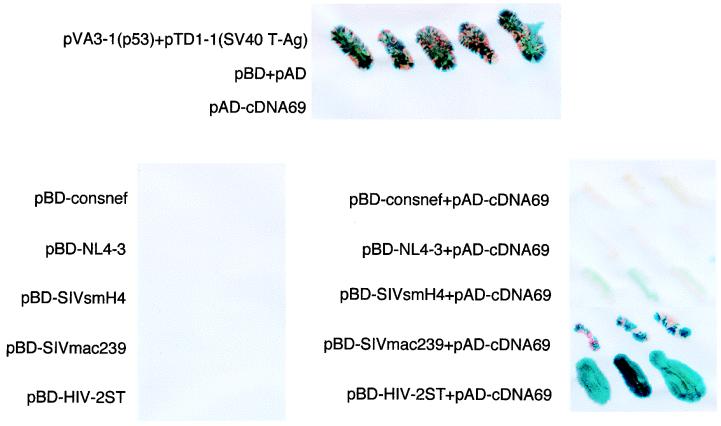
Proteins which have low affinity binding and transient interaction might evade detection by the coimmunoprecipitation technique. Yeast two-hybrid systems have been shown to detect protein-protein interactions which were not detected by other conventional methods (19, 45, 59). We thus cotransformed S. cerevisiae Y190 with pAD-cDNA69 and hybrid expression plasmids containing the GAL4 DNA binding domain fused to the nef genes of various HIV-1, SIV, and HIV-2 isolates. As shown in Fig. 7, specific interactions of TCRζ and nef resulting in the production of β-galactosidase were found in yeast cells cotransformed with pAD-cDNA69 and pBD-Nef derived from SIVsmH4, SIVmac239, and HIV-2ST, whereas neither pBD-NL4-3nef and pAD-cDNA69 nor pBD-consnef and pAD-cDNA69 enabled the yeast cells to grow well in the presence of the selective medium or to produce β-galactosidase, consistent with the results obtained in the coimmunoprecipitation experiment. Similar negative results were obtained when a consensus HIV-1 nef sequence was used (Fig. 7).
FIG. 7.
SIV and HIV-2 nef, but not HIV-1 nef, associates with TCRζ in yeast cells. S. cerevisiae Y190 was cotransformed with hybrid plasmid encoding the fusion protein of the GAL4 DNA binding domain and the HIV-1 consensus nef (pBD-consnef), HIV-1 NL4-3 nef (pBD-NL4-3), SIVsmH4 nef (pBD-SIVsmH4), SIVmac239 nef (pBD-SIVmac239), or HIV-2 ST nef (pBD-HIV-2ST) alone (left panel) or in combination with the pAD-cDNA69 (right panel). Transformed yeast cells were plated onto −L−Y−H+3AT plates, and colonies were assayed for the production of β-galactosidase (blue). The top panel indicates the positive and negative controls with the yeast cells transformed with expression plasmids of pVA3-1 (p53) and pTD1-1 (SV40 T-Ag), vectors with no inserts (pBD+pAD), or pAD-cDNA69 (TCRζ) alone.
Tyrosine kinase activity in nef complexes.
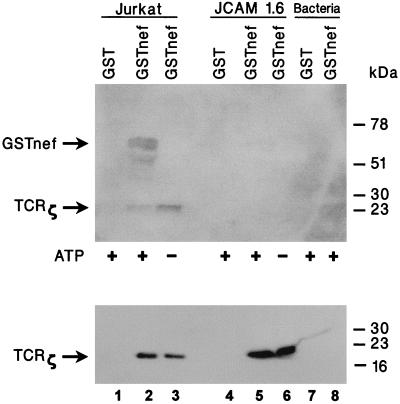
Protein tyrosine phosphorylation is one of the earliest biochemical events elicited by stimulation of B-cell receptor (BCR) and TCR in B and T cells, respectively (10, 29). Neither the BCR nor the TCR has intrinsic tyrosine kinase activity; both appear to interact with cytoplasmic protein tyrosine kinase (PTK). Three cytoplasmic PTKs (lck, fyn, and ZAP70) have been implicated in intracellular TCR signal transduction (11, 12, 48, 58). In the ensuing experiment, we asked if the nef-TCRζ complex coprecipitated with a PTK. Immobilized GSTnef from SIVmac239 was incubated with the Jurkat cell lysate as described previously. After extensive washing, the coprecipitation complexes were incubated under the conditions for assay of kinase activity in the presence or absence of ATP. The reaction products were analyzed by SDS-12% PAGE and immunoblotted onto a nylon membrane. Tyrosine-phosphorylated proteins were detected with an antiphosphotyrosine antibody. In the sample supplied with ATP, two major bands with migration corresponding to the molecular weights of GSTnef and TCRζ were detected by phosphotyrosine-specific antiserum (Fig. 8, upper panel, lane 2). The presence of TCRζ was confirmed when the same blot was reprobed with TCRζ-specific antiserum (Fig. 8, lower panel, lane 2). In contrast, tyrosine phosphorylation of the protein band with a molecular weight similar to that of GSTnef was not detected in the sample without the addition of ATP (Fig. 8, upper panel, lane 3). No tyrosine phosphorylation was observed in the samples without prior incubation with the Jurkat cell lysate (Fig. 8, lanes 7 and 8).
FIG. 8.
TCRζ-nef complex contains tyrosine kinase activity. Affinity-purified and immobilized recombinant GST or GSTnef was incubated in the presence of Jurkat T or J.CAM1.6 cell lysates previously precleared with immobilized GST and glutathione-Sepharose beads. Complexes precipitated with GSTnef were washed extensively and incubated in kinase buffer in the presence or absence of ATP. The reaction products were analyzed by SDS-12% PAGE, transferred onto a nylon membrane, and probed with antiphosphotyrosine monoclonal antibody. As negative controls, GST and recombinant GSTnef were purified from the bacterial lysates and assayed directly for kinase activity without prior incubation with the cell extracts (upper panel). To confirm the presence of TCRζ in the coprecipitation complexes, antibodies were stripped and the filter was reprobed with the anti-TCRζ monoclonal antibody (lower panel).
Next, we examined if the tyrosine kinase activity present in the nef-TCRζ complex might be dependent on lck. In vitro binding assays with GST or GSTnef were performed in the presence of extracts prepared from J.CAM1.6 cells, a Jurkat-derived mutant cell line which lacks a functional lck but has equivalent amounts of ZAP70, TCR, and TCRζ proteins (22, 29). As shown in Fig. 8, lower panel, GSTnef precipitated TCRζ from the lysates of Jurkat cells and J.CAM1.6 cells (lanes 2, 3, 5, and 6). However, tyrosine phosphorylation activity was detected only in complexes prepared from the Jurkat cell lysate but not in the ones prepared from the J.CAM1.6 cell lysate (Fig. 8, upper panel, compare lane 2 with lane 5). This result suggested that binding of nef to TCRζ is independent of any prior tyrosine phosphorylation of TCRζ and that the tyrosine kinase coprecipitated with the nef-TCRζ complex is dependent on the presence of a functional lck.
DISCUSSION
The TCR is a multimolecular complex containing the polymorphic TCR α and β subunits, the invariant CD3 γ, δ, and ɛ chains, and a homodimer of ζ chains or, in a minority of receptors, a heterodimer of ζ and η chains (4, 13, 20). The disulfide-linked TCR α and β heterodimer is responsible for antigen recognition, but the short, 5-aa cytoplasmic domains of the TCR α and β subunits are insufficient to couple to the intracellular signaling molecules. In contrast, the ζ chain has an extracellular region of only 9 aa but an extensive intracellular domain of 113 aa (60). Using a chimeric protein consisting of the extracellular and transmembrane domains of CD8 fused to the cytoplasmic domain of the ζ chain, Irving and Weiss elegantly showed that this fusion protein could elicit transducing signals indistinguishable from those generated by the intact TCR (28). One characteristic feature of the ζ chain is the presence of the immunoreceptor tyrosine-based activation motif (ITAM) (EX2YX2L/IX7YX2L/I), which is crucial for ζ chain coupling to the intracellular tyrosine kinases and adapter proteins and, hence, is absolutely required for all subsequent TCR signaling responses (8, 12, 41). Phosphorylated ITAM sequences function as SH2 binding domains (27, 44). One of the earliest biochemical events in TCR signaling is the activation of the src family tyrosine kinase lck, which in turn recruits various enzymes and signaling molecules leading to an altered pattern of gene expression and cellular activation (9–11, 40).
We used a truncated SIVmac239 nef as bait to screen an activated lymphocyte cDNA library in a yeast two-hybrid system. We identified TCRζ as one of the cellular proteins associating with nef. The clone that was identified in the screen actually differed from the published sequence of TCRζ (61) at three amino acid positions. The reasons for this are not clear. However, specific binding of nef to authentic TCRζ present in Jurkat cells and to authentic TCR sequences present in the CD8-TCRζ fusion in Cos 18 cells was demonstrated. Binding of TCRζ to full-length nef was demonstrated in vitro (Fig. 3) and in cells coexpressing TCRζ and nef (Fig. 4, 5, and 6). The association with TCRζ was specific for SIVmac, SIVsm, and HIV-2 nef but was not observed with HIV-1 nef. In addition, the nef-TCRζ complex coprecipitated active tyrosine kinase, which is present in Jurkat cells but not in J.CAM1.6 cells lacking a functional Lck. Finally, using a series of amino- and carboxyl-terminal deletion mutants of SIVmac239 nef, we mapped the central core region (aa 98 to 235) of nef responsible for binding to the ζ chain.
We can speculate that the binding of SIV nef to TCRζ might be related to reported activities of nef in causing T-lymphocyte activation. An unusual nef allele of SIV, called Y nef, is responsible for causing activation of primary lymphocytes in culture and an unusually acute disease course in monkeys (17, 18). Natural nef alleles of both SIV and HIV allow virus replication in an IL-2-dependent cell line and activate the production of IL-2 from these cells (3). HIV-1 nef was earlier reported to cause activation signals in Jurkat cells (6). HIV-1 nef has a highly conserved SH3 binding element, PXXPXXP, which is principally but not exclusively responsible for binding to src family kinases (34). SIV and HIV-2 nefs have a single PXXP element, and, although these can bind src family kinases, they appear to do so less well than HIV-1 nefs (14, 18, 46). Perhaps HIV-1 nef can influence signaling by direct interaction with src family kinases through the highly conserved PXXPXXP SH3 binding domain, while nef of SIVmac, SIVsm, and HIV-2, as an alternative means to the same end, may interact first with TCRζ. The interaction of nef with a TCRζ-kinase complex could result in tyrosine phosphorylation of nef, perhaps on a conserved YXXL sequence near the amino terminus which resembles an SH2 binding domain, and this could in turn result in recruitment of other tyrosine kinases through the phosphorylated SH2 binding domain. Thus, the picture that emerges from this scenario is that both HIV-1 nef and SIVmac, SIVsm, and HIV-2 nef may affect signaling through tyrosine kinases but that they may rely on a slightly different combination of cellular partners and binding domains to achieve these ends.
ACKNOWLEDGMENTS
We thank Dean Regier and Kim Deary for assistance in the DNA sequencing, Joanne Newton for manuscript preparation, and Kristen Toohey for photography support.
This work was supported by PHS grants AI25328, AI38559, and RR00168.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander L, Du Z, Rosenzweig M, Jung J J, Desrosiers R C. A role for natural SIV and HIV nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baniyash M, Garcia-Morales P, Bonifacino J S, Samelson L E, Klausner R D. Disulfide linkage of the ζ and η chains of the T cell receptor. J Biol Chem. 1988;263:9874–9878. [PubMed] [Google Scholar]
- 5.Baur A S, Sass G, Laffert B, Willbold D, Cheng-Mayer C, Peterlin B M. The N-terminus of nef from HIV-1/SIV associates with a protein complex containing lck and a serine kinase. Immunity. 1997;6:283–291. doi: 10.1016/s1074-7613(00)80331-3. [DOI] [PubMed] [Google Scholar]
- 6.Baur A S, Sawai E T, Dazin P, Fanti W J, Cheng-Mayer C, Peterlin B M. HIV-1 nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 7.Benichou S, Bomsel M, Bodeus M, Durand H, Doute M, Letourneur F, Camonis J, Benarous R. Physical interaction of the HIV-1 nef protein with β-cop, a component of non-clathrin-coated vesicles essential for membrane traffic. J Biol Chem. 1994;269:30073–30076. [PubMed] [Google Scholar]
- 8.Cambier J C. Antigen and Fc receptor signaling: the awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 9.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 10.Carrera A C, Rodriguez-Borlado L, Martinez-Alonso C, Merida I. T cell receptor associated alpha phosphatidylinositol 3-kinase becomes activated by T cell receptor cross linking and requires pp56(lck) J Biol Chem. 1994;269:19435–19440. [PubMed] [Google Scholar]
- 11.Chan A C, Desai D M, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 12.Chan A C, Iwashima M, Turck C W, Weiss A. ZAP-70: A 70 kd protein-tyrosine kinase that associates with TCR ζ chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 13.Clevers H, Alacron B, Willeman Y, Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- 14.Collete Y, Dutartre H, Benziane A, Ramos-Morales F, Benarous R, Harris M, Olive D. Physical and functional interaction of nef with lck. J Biol Chem. 1996;71:6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 15.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 16.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, Pchee D A, Greenway A L, Ellett A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cuuningham A, Dwyer D, Downton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 16a.Du, Z., L. Alexander, A. Y. M. Howe, and R. C. Desrosiers. Unpublished data.
- 17.Du Z, Ilysinskii P O, Sasseville V G, Lackner A A, Desrosiers R C. Requirements for lymphocyte activation by unusual strains of simian immunodeficiency virus. J Virol. 1996;70:4157–4161. doi: 10.1128/jvi.70.6.4157-4161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J J, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 19.Durfee T, Becherer K, Chen P L, Yeh S H, Yang Y, Kilburn A E, Lee W H, Elledge S J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- 20.Frank S J, Samelson L E, Klausner R D. The structure and signaling function of the invariant T cell receptor components. Semin Immunol. 1990;2:89–97. [PubMed] [Google Scholar]
- 21.Garcia J A, Miller A D. Serine phosphorylation independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–551. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith M A, Weiss A. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc Natl Acad Sci USA. 1987;84:6879–6883. doi: 10.1073/pnas.84.19.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenway A, Azad A, McPhee D. Human immunodeficiency virus type 1 nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J Virol. 1995;69:1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenway A, Azad A, Mills J, McPhee D. Human immunodeficiency virus type 1 nef binds directly to lck and mitogen-activated protein kinase, inhibiting kinase activity. J Virol. 1996;70:6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch V M, Olmstead R A, Murphu-Corb M, Purcell R M, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 26.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irving B A, Chan A C, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor ζ chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irving B A, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 29.Iwashima M, Irving B A, van Oers N S C, Chan A C, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 30.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of lck-binding elements in tip of herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 31.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Schgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 32.Kirchoff F, Greenoug T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with non-progressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 33.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Lee C-H, Leung B, Lemmon M A, Zheng J, Cowburn D, Kuriyan J, Saksela K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 nef protein. EMBO. 1995;14:5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. FASEB. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- 36.Liu L X, Margottin F, Le Gall S, Schwartz O, Selig L, Benarous R, Benichou S. Binding of HIV-1 nef to a novel thioesterase enzyme correlates with nef-mediated CD4 down-regulation. J Biol Chem. 1997;272:13779–13785. doi: 10.1074/jbc.272.21.13779. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Wu X, Plemenitas A, Yu H, Sawai E T, Abo A, Peterlin B M. CDC42 and Rac1 are implicated in the activation of the nef-associated kinase and replication of HIV-1. Curr Biol. 1996;12:1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 38.Miller M D, Warmerdam M T, Gaston I, Green W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niederman T M, Hastings W R, Luria S, Ratner L. Human immunodeficiency virus type 1 nef protein inhibits NF-kB induction in human T cells. Virology. 1993;194:338–344. doi: 10.1006/viro.1993.1264. [DOI] [PubMed] [Google Scholar]
- 40.Northrop J P, Ho S N, Chen L, Thomas D J, Timmerman L A, Nolan G P, Admon A, Crabtree G R. NF-AT components define a family of transcription factor targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 41.Ravichandran K S, Lee K K, Songyang Z, Cantley L C, Burn P, Burakoff S J. Interaction of Shc with the zeta chain of the T-cell receptor upon T cell activation. Science. 1993;262:902–905. doi: 10.1126/science.8235613. [DOI] [PubMed] [Google Scholar]
- 42.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 43.Rhee S S, Marsh J W. Human immunodeficiency virus type 1 nef-induced down-modulation of CD4 is due to rapid internalization and degradation of surface CD4. J Virol. 1994;68:5156–5163. doi: 10.1128/jvi.68.8.5156-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T cell antigen Fc receptor ζ chain. Cell. 1992;68:889–897. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- 45.Rossi F, Gallina A, Milanes G. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology. 1996;217:397–403. doi: 10.1006/viro.1996.0130. [DOI] [PubMed] [Google Scholar]
- 46.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for downregulation of CD4. EMBO. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Samelson L E, Klausner R D. Tyrosine kinases and tyrosine-based activation motifs. Current research on activation via the T cell antigen receptor. J Biol Chem. 1992;267:24913–24916. [PubMed] [Google Scholar]
- 49.Samuel K, Hodge D R, Chen Y-M A, Papas T S. Nef proteins of the human immunodeficiency viruses (HIV-1 and HIV-2) and simian immunodeficiency virus (SIV) are structurally similar to leucine zipper transcriptional activation factors. AIDS Res Hum Retroviruses. 1991;7:697–706. doi: 10.1089/aid.1991.7.697. [DOI] [PubMed] [Google Scholar]
- 50.Sanfridson A, Cullen B R, Doyle C. The simian immunodeficiency virus nef protein promotes degradation of CD4 in human T cells. J Biol Chem. 1994;269:3917–3920. [PubMed] [Google Scholar]
- 51.Sawai E T, Baur A, Struble H, Peterlin B M, Levy J A, Cheng-Mayer C. Human immunodeficiency virus type I nef associates with a cellular serine kinase in T lymphocytes. Proc Natl Acad Sci USA. 1994;91:1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of MHC-1 molecules is induced by HIV-1 nef. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 53.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO. 1993;12:703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith B L, Krushelnycky B W, Mochly-Rosen D, Berg P. The HIV nef protein associates with protein kinase C theta. J Biol Chem. 1996;271:16753–16757. doi: 10.1074/jbc.271.28.16753. [DOI] [PubMed] [Google Scholar]
- 56.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terwilliger E, Sodroski J G, Rosen C A, Haseltine W A. Effects of mutations with the 3′ orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986;60:754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thome M, Duplay P, Guttinger M, Acuto O. Syk and ZAP70 mediate recruitment of p56lck/CD4 to the activated T cell receptor/CD3/ζ complex. J Exp Med. 1997;181:1997–2006. doi: 10.1084/jem.181.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 60.Weissman A M, Baniyash M, Hou D, Samelson L E, Burgess W H, Klausner R D. Molecular cloning of the zeta chain of the T cell antigen receptor. Science. 1988;239:1018–1021. doi: 10.1126/science.3278377. [DOI] [PubMed] [Google Scholar]
- 61.Weissman A M, Hou D, Orloff D G, Modi W S, Seuanez H, O’Brien S J, Klausner R D. Molecular cloning and chromosomal localization of the human T-cell receptor ζ chain: distinction from the molecular CD3 complex. Proc Natl Acad Sci USA. 1988;85:9709–9713. doi: 10.1073/pnas.85.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]