Abstract
Immunosuppressed individuals face a significantly elevated risk of developing aggressive cutaneous malignancies, often surpassing the aggressiveness observed in immunocompetent counterparts. Our patient exhibited several risk factors associated with melanoma development in renal recipients, including skin type, sun exposure, and the duration of immunosuppression. The determination of staging holds paramount importance as it directly influences both prognosis and subsequent management. It is crucial to handle suspected lesions with caution in these patients to facilitate early melanoma detection and enhance overall prognosis.
INTRODUCTION
Amelanotic melanoma, an uncommon and notably aggressive variant, poses distinct challenges in diagnosis and management, especially when occurring in individuals with a history of kidney transplantation [1, 2]. Individuals undergoing kidney transplantation and relying on lifelong immunosuppressive therapy face an elevated vulnerability to various malignancies, including skin cancers [3]. Among these, melanoma stands out as a less common yet formidable threat, warranting careful attention due to its potential for delayed diagnosis and poorer outcomes [2–4]. This case report sheds light on a rare occurrence of amelanotic melanoma in a kidney transplant recipient, highlighting the intricate interplay between immunosuppression and the development of skin cancer. By detailing the clinical presentation, diagnostic challenges, histopathological characteristics, and subsequent management, this report aims to contribute to the broader understanding of the complexities involved in recognizing and treating amelanotic melanoma within the unique context of kidney transplantation. Through an exploration of this distinctive case, we navigate the nuanced landscape of immunocompromised patients, shedding light on the importance of vigilance, early detection, and tailored therapeutic approaches to mitigate the impact of this rare malignancy in a specific clinical setting. In this report, we are presenting a case of a renal transport recipient developing an amelanotic melanoma 14 years after transplantation.
CASE PRESENTATION
A blond 45-year-old man presented to our clinic for a dermatologic consultation with a large nodular lesion, located on the anterior thigh. The onset was 6 months earlier, with a rapid evolution in terms of increasing in size. The lesion was asymptomatic, except for bleeding after minor local trauma. The patient was known as a renal transplant recipient 14 years ago and was placed on a regimen consisting of oral Tacrolimus, Prednisolone, and Mycophenolate Mofetil (MMF). However, chronic rejection of the graft has occurred. He had no personal or familial history of melanoma or NMSC. Physical examination revealed a firm, well-circumscribed large nodule with a diameter of about 3 cm, with a red hue barely noticeable because of the overlying crust resulting from frequent bleeding (Fig. 1). The systems review was unremarkable, with no lymphadenopathy or visceral enlargements. Hematological parameters were normal except for the elevation of serum creatinine and urea values as a cause of chronic rejection (crea:3.3 mg/dl, urea: 78 mmol/dl). All serological viral tests including HIV tests were performed and were negative.
Figure 1.
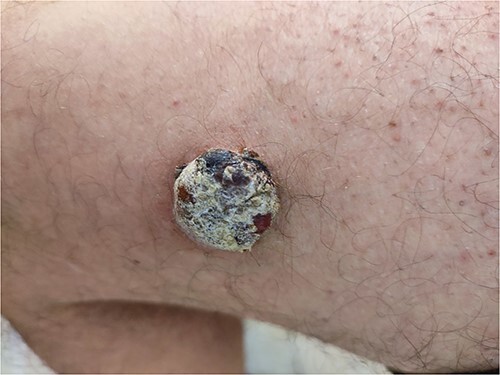
A large non-pigmented crusted nodule on the thigh in a blond 45-year-old man.
According to the patient’s physical examination and medical history, several differential diagnoses were suggested:
Pyogenic Granuloma
Amelanotic Melanoma
Squamous cell carcinoma
Thus, a plan of surgical excision and histopathological examination was made. The pathology revealed proliferating of malignant spindle cells with epidermal ulceration (Fig. 2A). Tumor cells invade sub-cutaneous fatty tissue (Fig. 2B). Pleomorphism, prominent nuclei, and mitotic figures are seen (Fig. 2C and D). An immunohistochemical stain for HMB-45 was performed, and the tumor cells were positive (Fig. 3).
Figure 2.
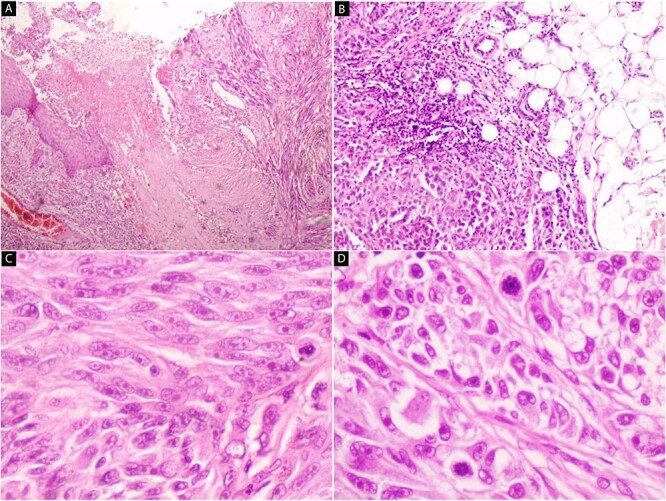
Hematoxylin and eosin stain (A–D) Microscopic images of the lesion. (A) Proliferating of malignant spindle cells with epidermal ulceration (40×). (B) Tumor cells invade subcutaneous fatty tissue (100×). (C and D) Pleomorphism, prominent nuclei, and mitotic figures are seen (200× and 400×).
Figure 3.
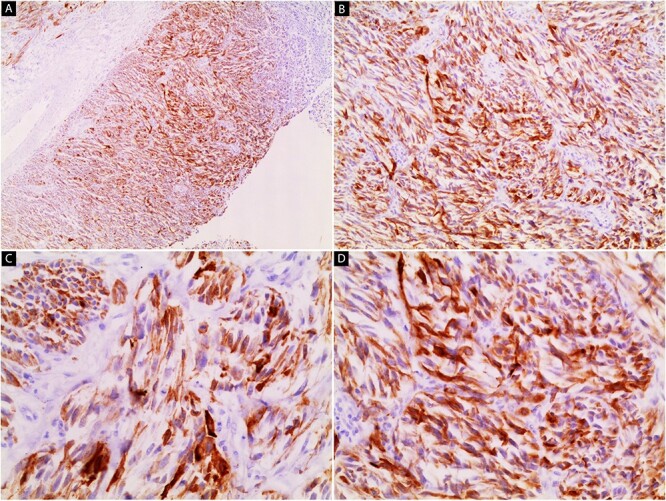
Immunohistochemical stain image (A–D). The tumor cells are positive for HMB-45 (40×, 100×, 200×, 200×).
A CT scan was requested to determine the presence of any distant metastasis and revealed:
Chest: A single parenchymal node in the periphery of the upper right lobe that measures around 4 mm.
Abdomen and pelvis: Few Small nodules left to the aorta that measure around 7 mm.
Otherwise normal.
Due to the unavailability of SLNB, ELND was done to detect any microscopic metastasis and this proved no presence of any metastasis. A wide excision to the depth has been done. According to the TNM classification, our patient was in pathological stage IIb.
After five months of follow-up, the patient developed multiple enlarged left inguinal lymph nodes (Fig. 4), which were confirmed as metastatic melanoma after biopsy and histopathological study. Consequently, a PET scan will be conducted to assess the condition of other lymph nodes and organs, enabling accurate staging and facilitating the selection of appropriate treatment.
Figure 4.
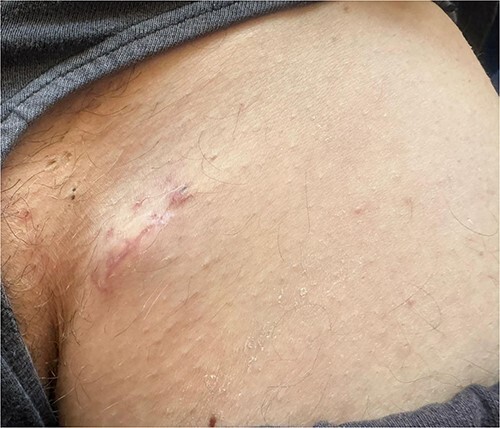
A clinical image shows multiple enlarged left inguinal lymph nodes.
DISCUSSION
Amelanotic melanoma represents a scarce and aggressive subtype of melanoma, accounting for approximately 5% of nodular melanomas and 2%–8% of all melanoma cases [2, 5]. Its unique feature, the absence of pigment, results in a varied and atypical appearance, frequently confusing with other benign and malignant lesions. This characteristic often leads to delayed diagnoses and subsequently, a poorer prognosis [6]. Whereas renal transplantation is considered the only long-term curative treatment for ESKD, it requires life-long immunosuppressive agents, rendering the recipients more susceptible to the development of malignancies especially skin cancers [7]. While SCC is the most common skin cancer post-transplantation (with a 50 to 250-fold increased risk), melanoma is less common (with a risk of 2–8-fold according to most studies) [8, 9], particularly the amelanotic type, which is very rare. Consequently, there is a lack of published data on its prevalence in renal transplant patients. To date, only two studies have reported on the histopathological types of melanoma in renal transplant patients. The first study, conducted by Le Mire et al. in Oxford in 2006, involved 1874 transplanted patients. Among them, ten patients developed 12 melanomas, with the most common histopathological type being SSM, and no cases of amelanotic melanoma were found [10]. The second study, based on the London experience in 2006, also did not record this rare type [11]. From this perspective, the significance of this rare case becomes apparent as it is documented for the first time. Apart from immunosuppression itself, the immunosuppressive agents are inherently carcinogenic, with an impact related to both the cumulative dose and the type of regimen used [9]. Our patient underwent kidney transplantation 14 years ago and was placed on Calcineurin inhibitors (Tacrolimus), which are cornerstones in most immunosuppressive regimens. Several studies highlighted the carcinogenic effects of this agent which include reducing immune surveillance, increasing vascularization and tumor invasive capacity, and enhancing DNA damage (e.g.: after exposure to UVB radiation) or inhibiting its repair [9]. There are several risk factors associated with the development of post-transplantation melanoma, including older age, skin phenotype (such as blue/green eyes, blond or red hair, and fair skin, which pose a greater risk), cumulative sun exposure, a high number of nevi, and the duration of immunosuppression [3, 12]. Our patient has most of them, as he has blond hair and fair skin with a phenotype II/III according to Fitzpatrick classification, and he worked as a fisherman for several years which indicates chronic high levels of sun exposure. In addition to the long duration of immunosuppression for 14 years. Stage at diagnosis is one of the main prognostic factors in patients with melanoma, and Breslow thickness is the most significant histological prognostic factor [9]. The poor prognostic factors in our case are: Breslow thickness is around 14 mm (very high), with a high rate of mitosis, the spindle cell pattern, and the presence of immunosuppression. The initial approach to treating melanoma in solid organ transplant (SOT) recipients should mirror that of immunocompetent patients, involving simple excision and margin widening based on Breslow thickness, with consideration for sentinel lymph node biopsy (SLNB) as necessary [8, 13]. Due to high Breslow thickness and unavailability of SLNB, ELND to regional lymph nodes was done, so we could put the accurate pathological staging which is essential for management and expect the prognosis. Immunosuppressive therapy should be revised in SOT recipients who develop melanoma [8]. Reduction of immunosuppression may be achieved by dose reduction or withdrawal of one of the immunosuppressive agents, but it is important to balance between the risk of tumor burden and the risk of metastasis versus the increasing risk of graft rejection [8]. Despite the limited evidence, we should consider an mTOR inhibitor (Sirolimus), which has antiproliferative properties, in the immunosuppressive regimen of patients who develop skin cancers [14]. So, after renal consultation, Tacrolimus will be excluded from the regimen and Levrolimus will be added. In this case, we wanted to highlight the importance of handling any suspected lesions with caution in SOT patients, to early detection of melanomas, and thus improve the prognosis.
CONCLUSION
This case underscores the importance of vigilance in handling suspected lesions in renal transplant recipients to facilitate early melanoma detection and enhance prognosis. Management strategies, including revising immunosuppressive therapy, are critical for balancing cancer risk and graft function in these patients.
ACKNOWLEDGEMENTS
None.
Contributor Information
Yara Melhem, Department of Dermatology, Tishreen University Hospital, Lattakia, Syria.
Seham Khattab, Department of Dermatology, Tishreen University Hospital, Lattakia, Syria.
Moatasem Hussein Al-janabi, Department of Pathology, Cancer Research Center, Tishreen University Hospital, Lattakia, Syria.
Hussein Saeid, Department of Nephrology, Tishreen University Hospital, Lattakia, Syria.
Issa Ahmad, Tishreen University and Al Andulus Private University for Medical Sciences/Faculty of Medicin/Department of Pathology, Tishreen University Hospital, Lattakia, Syria.
Fouz Hasan, Department of Dermatology, Tishreen University Hospital, Lattakia, Syria.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING
There was no funding for this publication.
ETHICAL APPROVAL
No ethical approval was required for this publication.
CONSENT
Informed and written consent from the patient was taken prior to publication.
GUARANTOR
Fouz Hasan.
REFERENCES
- 1. Collins L, Quinn A, Stasko T. Skin cancer and immunosuppression. Dermatol Clin 2019;37:83–94. [DOI] [PubMed] [Google Scholar]
- 2. Stojkovic-Filipovic J, Kittler H. Dermatoscopy of amelanotic and hypomelanotic melanoma. J Dtsch Dermatol Ges 2014;12:467–72. [DOI] [PubMed] [Google Scholar]
- 3. Ascha M, Ascha MS, Tanenbaum J, Bordeaux JS. Risk factors for melanoma in renal transplant recipients. JAMA Dermatol 2017;153:1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson J, Alvey N, Bowman L, Schulte J, Segovia MC, McDermott J. et al. Consensus recommendations for use of maintenance immunosuppression in solid organ transplantation: endorsed by the American College of Clinical Pharmacy, American Society of Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy 2022;42:599–633. [DOI] [PubMed] [Google Scholar]
- 5. Nalamwar R, Kharkar V, Mahajan S, Chikhalkar S, Khopkar U. Nodular amelanotic melanoma. Indian J Dermatol Venereol Leprol 2010;76:273–5. [DOI] [PubMed] [Google Scholar]
- 6. Griffiths CEM, Bleiker TO, Creamer D, Ingram JR, Simpson RC. Rook’s Dermatology Handbook. John Wiley & Sons, 2022. [Google Scholar]
- 7. Bieryło A, Brzósko S, Laudańska E, Naumnik B. Wiadomosci lekarskie (Warsaw, Poland : 1960), 2017, 70(1), 68–73. [PubMed] [Google Scholar]
- 8. Ali FR, Lear JT. Melanoma in organ transplant recipients: incidence, outcomes and management considerations. J Skin Cancer 2012;2012:404615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. González‐Cruz C, Ferrándiz-Pulido C, Briones VG. Melanoma en pacientes receptores de un trasplante de órgano sólido. Actas Dermo-Sifiliográficas, 2021;112(3):216–224. 10.1016/j.ad.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 10. Le Mire L, Hollowood K, Gray D, Bordea C, Wojnarowska F. Melanomas in renal transplant recipients. Br J Dermatol 2006;154:472–7. [DOI] [PubMed] [Google Scholar]
- 11. Brown VL, Matin RN, Cerio R, Leedham-Green ME, Proby CM, Harwood CA. Melanomas in renal transplant recipients: the London experience, and invitation to participate in a European study. Br J Dermatol 2007;156:163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao X, Lai W, Xia X, Xu J, Wu Y, Lv C. et al. Skin cancer outcomes and risk factors in renal transplant recipients: analysis of organ procurement and transplantation network data from 2000 to 2021. Front Oncol 2022;12:1017498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zwald FO, Christenson LJ, Billingsley EM, Zeitouni NC, Ratner D, Bordeaux J. et al. Melanoma in solid organ transplant recipients. Am J Transplant 2010;10:1297–304. [DOI] [PubMed] [Google Scholar]
- 14. Geissler EK. Skin cancer in solid organ transplant recipients: are mTOR inhibitors a game changer? Transplant Res 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


