Abstract
Upon infection of Japanese encephalitis virus (JEV), baby hamster kidney (BHK-21) and Chinese hamster ovary (CHO) cells were killed by a mechanism involved in apoptosis. While readily established in a variety of cell lines, JEV persistence has never been successfully instituted in BHK-21 and CHO cells. Since stable expression of human bcl-2 in BHK-21 cells has been shown to delay JEV-induced apoptosis, in this study we investigated whether JEV persistence could be established in such cells. When constitutively expressing bcl-2, but not its closest homolog, bcl-XL, following a primary lytic infection, approximately 5 to 10% of BHK-21 and CHO cells became persistently JEV infected during a long-term culture. From the persistent bulks, several independent clones were selected and expanded to form stable cell lines that continuously produced infectious virus without marked cytopathic effects (CPE). Among these stable cell lines, the truncated nonstructural protein 1 (NS1) was also detected and was indistinguishable from the NS1 truncations previously observed in JEV-persistent murine neuroblastoma N18 cells. However, the stable expression of NS1 alone, regardless of whether it was truncated or full length, failed to render the engineered cells persistently infected by JEV, implying that aberrant NS1 proteins were likely a consequence of, rather than a cause for, the viral persistence. Enforced bcl-2 expression, which did not affect virus replication and spread during the early phase of cytolytic infection, appeared to attain JEV persistence by restriction of virus-induced CPE. Our results suggest that it is the antiapoptotic, rather than the antiviral, effect of cellular bcl-2 which plays a role in the establishment of JEV persistence.
A variety of animal DNA and RNA viruses can establish long-term persistent infections. For a cytolytic virus to institute a persistent infection in its host cells, more harmonious interactions between virus and cell must first occur to restrict the virus-induced cytopathic effects (CPE). This persistent status can be achieved by virus infection of nonpermissive cells or of cells under a nonpermissive environment; alternatively, emergence of virus and/or cell variants during the infection course appears to also contribute to persistence (3). True latent infections, which do not yield infectious virions, are commonly associated with DNA viruses when nonpermissive cells are infected; the virus may resume replication as long as the condition remains appropriate. On the other hand, the lack of susceptibility of target cells to virus infection often leads to an abortive replication of RNA viruses. Most RNA viruses can only persist in infected cells where at minimum a reduced level of virus replication is still able to take place, thereby resulting in constant shedding of moderate amounts of infectious virions.
Characteristically, most animal DNA viruses seem to merely play a passive role either in the establishment of latency or in viral reactivation (reviewed in reference 3). In contrast, both viruses and their infected host cells have been shown to actively participate in the establishment of persistence for several RNA viruses, namely reovirus (2), Sindbis virus (25), poliovirus (1, 54), and coxsackie A9 virus (50). Among these viruses, mutations in certain viral genes have been demonstrated to be responsible for turning a lytic infection into a persistent one (reviewed in reference 3). Still, the involvement of cellular genes in viral persistence remains largely unexplored.
Human bcl-2 was the first cellular gene recognized to be capable of blocking apoptosis induced by certain RNA viruses (21, 26, 27, 39, 51). Studies involving Sindbis virus (26), Semiliki Forest virus (44), and influenza virus (21, 38) have further indicated that constitutive bcl-2 expression can not only prevent the infected cells from undergoing apoptosis but also, subsequently, render the cells to be persistently infected. These results suggest that bcl-2 may play a role in determining whether a cytolytic RNA virus can chronically infect its host cells.
Like other mosquito-borne flaviviruses, Japanese encephalitis virus (JEV) is transmitted to humans through persistently infected mosquito vectors. JEV infection is especially prevalent in some East Asian countries and may cause an acute encephalitis in humans which is frequently associated with a high mortality rate (6, 52, 53). The genome of JEV is a single-stranded, positive-sense RNA of approximately 11 kb in length which contains an open reading frame encoding a single polyprotein. In the infected cells this viral polyprotein is proteolytically cleaved into at least 11 proteins. The virus structural proteins, including the capsid (C), membrane (M; precursor M, prM), and envelope (E) proteins, are encoded by the 5′ one-third of the open reading frame, and the nonstructural (NS) proteins, designated NS1 through NS5, are encoded in the remainder (reviewed in references 10 and 42). Among these proteins, prM, E, and NS1 are membrane-associated glycoproteins. With two N-linked glycosylation sites at amino acid positions 130 and 207, the actual molecular size of NS1 detected in JEV-infected cells is approximately 46 kDa. The proteolytic cleavage between E-NS1 ensues the translocation of NS1 into the lumen of the endoplasmic reticulum (ER), and the cleavage between NS1-2A might occur in the lumen of the vesicular compartments (10). JEV is unique among flaviviruses in that an additional NS1-2A-related protein (named NS1′) with a molecular size of about 53 kDa is often observed in the JEV-infected cells (10) and is probably generated by an unknown protease that recognizes an alternative cleavage site within NS2A (32). The biological significance for the existence of both types of NS1 proteins in JEV-infected cells remains unclear.
The natural life cycle of JEV involves complex relationships among arthropods, vertebrate reservoirs, and humans, illustrating the uniqueness of the broad host spectrum for JEV infection (9). In fact, a wide variety of primary and continuous cell cultures from different origins (e.g., monkey, hamster, pig, chicken, and mosquito) can support the productive growth of JEV. JEV is usually cytolytic for susceptible cells, so due to apparent CPE induced by JEV infection, Vero, LLC-MK2 (monkey kidney cells), and BHK-21 (baby hamster kidney cells) are frequently used for virus titration by plaque assays (49). Nevertheless, persistent JEV infection has been demonstrated in cell cultures (11, 45–47), as well as in a mouse model (33, 34). In humans, latent infection of mononuclear cells in the peripheral blood from JEV-infected patients has also been documented (48); moreover, viral persistence in the human nervous system has been shown in approximately 5% of JEV-associated encephalitis cases (41), suggesting that JEV persistence might contribute to neural sequelae after the acute infection phase. One proposed mechanism for other flaviviruses to persist in cell cultures is the production of defective interfering (DI) particles (5, 23, 40, 45). However, the emergence of DI virus during long-term infections has not been universally observed.
The underlying mechanism for JEV persistence in vitro and in vivo is not fully understood. We previously demonstrated that, despite the fact that JEV could be an apoptotic inducer (27), persistent infection of JEV could also be established in different cell types after a primary lytic infection and that such persistence was closely associated with the abnormal expression of truncated JEV NS1 proteins (11). Still, JEV persistence has never been successfully established in BHK-21 and CHO cells, although both of them could support the productive replication of JEV, indicating that it was the cell type difference, rather than the permissiveness, of target cells that determined the achievement of JEV persistence. The present study demonstrates that expression of human bcl-2, which delays JEV-induced apoptosis, can assist in instituting JEV persistence in BHK-21 and CHO cells after the lytic phase of infection. Moreover, we provide evidence to show that for the development of an environment suited to JEV persistence, bcl-2 appears to exert its antiapoptotic, but not antiviral, function to subvert JEV CPE. Thus, bcl-2 becomes the first cellular gene shown to be capable of modulating the outcome of JEV infection in a cultured system.
MATERIALS AND METHODS
Viruses and cell lines.
The Taiwanese local JEV strain RP-9, a plaque-purified strain (11, 12), was employed throughout this study. The propagation of virus was carried out in BHK-21 and CHO cells with RPMI 1640 medium containing 2% fetal calf serum (FCS). Virus titers were determined by plaque-forming assay on BHK-21 cells.
Virus infection and titration.
To infect with JEV, monolayers of the indicated cell lines grown in 6- or 12-well plates were initially adsorbed with JEV at a multiplicity of infection (MOI) of 5 for 1 h at 37°C. After adsorption, the unbound viruses were removed by three gentle washings with serum-free RPMI 1640 medium. Fresh medium containing 2% FCS was added to each plate for further incubation at 37°C. At the end of infection, the culture media were harvested for plaque-forming assay to determine virus titers. Briefly, virus dilution was added onto 80% confluent BHK-21 cells and incubated at 37°C for 1 h. After adsorption, the cells were washed and overlaid with 1% agarose (SeaPlaque; FMC BioProducts) containing RPMI 1640 with 1% FCS. After incubation for 4 days, the resulting cells were fixed with 10% formaldehyde and stained with 0.5% crystal violet for plaque counting. Virus titers were denoted as PFU per milliliter.
Infectious center assay.
To determine the exact portion of a cell population capable of producing infectious virus particles, an infectious center assay was carried out. Briefly, persistent cell clones were trypsinized and washed three times with serum-free RPMI 1640 medium to make single-cell suspensions. Cell suspensions were counted, serially diluted with medium, mixed with target BHK-21 cell suspensions, and plated into 96-well microtiter plates. After incubation at 37°C for 4 h, the adherent cells were washed and overlaid with 1% agarose (SeaPlaque) containing RPMI 1640 with 1% FCS for virus titration as described above.
Viral one-step growth curve.
BHK-21 and its derivatives (2 × 106) were infected with viruses at an MOI of 5. After 1 h of adsorption at 37°C, the unbound virus particles were removed from cells by three washes with phosphate-buffered saline (PBS). The infected cells were then grown in RPMI 1640 medium supplemented with 2% FCS at 37°C. At the indicated time points postinfection, the culture supernatants of infected cells were collected and clarified by centrifugation. Next, the virus titers in supernatants were determined by plaque assay on BHK-21 cells.
Monoclonal antibodies.
The specificity of monoclonal antibodies D2/39.1 and JE7/45-2 against JEV NS1 used in this study was confirmed by Western blotting and immunoprecipitation by using the antigen source as previously described (11). In addition to recognizing native forms of NS1, both antibodies can detect denatured forms of NS1 when the lysate is boiled in the presence of 2-mercaptoethanol prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), indicating that the two antibodies recognize a linear epitope of NS1. As characterized with several NS1 deletion mutants expressed in BHK-21 cell (11), the epitope location recognized by D2/39.1 is located in the N-terminal one-third of NS1. By using synthetic peptides for fine mapping, the epitope of D2/39.1 was further mapped to a 16-mer peptide whose sequence was TELRYSWKTWGKAKM; this peptide contained a consensus linear sequence [WK(A/T)WGK] present in the NS1 of JEV, Kunjin virus, and dengue viruses (16, 17).
Cloning of persistently infected cells.
Limiting dilution was used to clone persistently infected cells as previously described (11). Briefly, the bulk of persistently JEV-infected B2-5 cells were trypsinized and washed once with complete RPMI 1640 medium. Cell suspensions were counted, diluted with medium, and distributed into a 96-well microtiter plate. Each well, which contained an average of a single cell, was microscopically observed daily for the presence of cell colonies. Once they became confluent in wells, cells were treated with trypsin and transferred to new tissue culture flasks for further growth.
Indirect immunofluorescence assay (IFA) staining.
Cells were fixed in acetone-methanol (1:1) solution for 5 min and stained with JEV-specific monoclonal antibodies at room temperature for 1 h. The dilutions of antibodies used in this assay were 1:500 or 1:1,000. After being washed with PBS, the cells were reacted with goat anti-mouse fluorescein-conjugated secondary antibody (Cappel) and examined with a Leitz fluorescent microscope.
Cell labeling and immunoprecipitation.
Cell monolayers were starved with methionine-free and cysteine-free RPMI 1640 for 1 h and labeled with 50 μCi of 35S-labeled Pro-Mix (Amersham) per 35-mm dish for 2 h at 37°C. The cells were rinsed and lysed with lysis buffer (1% Nonidet P-40; 150 mM NaCl; 50 mM Tris-HCl, pH 7.5; 1 mM EDTA) containing a cocktail of protease inhibitors, including 20 μg of phenylmethylsulfonyl fluoride, 2 μg of leupeptin, and 2 μg of aprotinin per ml. Aliquots of cell lysates were mixed with monoclonal antibodies captured on staphylococcal protein A-coated Sepharose (Pharmacia) for 1 h at room temperature. The immune complexes were washed with radioimmunoprecipitation assay buffer (10 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA; 0.1% SDS; 1% Triton X-100; 1% sodium deoxycholate), analyzed by SDS–10% PAGE, and fluorographed at −70°C. For endoglycosidase F (endo-F) digestion, the immunoprecipitated proteins were boiled in endo-F boiling buffer (50 mM sodium phosphate, pH 7.5; 0.5% SDS; 1% 2-mercaptoethanol) and then incubated overnight at 37°C with an equal volume of the endo-F incubation buffer (50 mM sodium phosphate, pH 7.5; 2% Nonidet P-40; 0.2% SDS; 1% 2-mercaptoethanol; 25 mM EDTA) with or without endo-F (Boehringer Mannheim).
Western immunoblot analysis.
Cell monolayers were rinsed and lysed with lysis buffer as described above. Cell lysates were mixed with an equal volume of sample buffer (without β-mercaptoethanol), boiled or not boiled, separated by SDS-PAGE, and transferred to nitrocellulose membranes (Hybond-C Super; Amersham). The nonspecific antibody-binding sites were blocked with 5% skimmed milk in PBS and reacted with monoclonal anti-JEV E, NS1, or NS3 antibodies (11). The blots were then treated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Cappel) and developed with an ECL system (Amersham).
RNA preparation and reverse transcriptase (RT)-PCR reaction.
Total RNA of the JEV-infected cell cultures or viral RNA harvested from the media were incubated with lysis buffer (4 M guanidine thiocyanate; 50 mM Tris HCl, pH 8.5; 10 mM EDTA; 0.5% sarcosyl) for 15 min at 4°C and extracted twice with an equal volume of acid phenol-chloroform (14, 28). RNA in the aqueous phase was precipitated with isopropanol. Four different primers were used in the amplification reaction, including primers a (5′-GCGGATCCAGACACTGGATGTGCCA-3′, “+” sense, nucleotides [nt] 2478 to 2493), b (5′-GCGGATCCTAAGCATCAACCTGTGA-3′, “−” sense, nt 3534 to 3519), c (5′-GGAAGGGGAGACAAAGCAGATCAACC-3′, “+” sense, nt 2133 to 2157), and d (5′-CTAGTGACAGATCTGACTCC-3′, “−” sense, nt 2662 to 2643). Primers a and b were used to amplify the full length of NS1, and primers c and d were used to amplify the junction between E and NS1. When primer b or d was used, 5 μg of purified RNA was transcribed into first-strand cDNA at 42°C for 1 h in 20 μl of reaction buffer containing 200 U of Moloney murine leukemia virus RT (Bethesda Research Laboratories), 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, and 1 mM concentrations of dATP, dGTP, dCTP, and dTTP. When primer set a and b or primer set c and d was used, 2 μl of the resulting first-strand cDNA sample were PCR amplified in 100 μl of reaction mixture containing 5 U of Taq polymerase (Perkin-Elmer); 2 mM MgCl2; 1 mM concentrations of dATP, dGTP, dCTP, and dTTP; 20 mM Tris-HCl (pH 8.4); and 50 mM KCl. Thirty-five cycles of amplification were performed with a PCR thermocycler (AG 9600 Thermal Station; AcuGen System). Each cycle consisted of a denaturation step at 94°C for 30 s followed by a primer-annealing step at 55°C for 30 s and a primer extension step at 72°C for 30 s, except for a longer denaturation step (5 min) and a longer primer extension step (10 min) at the first and last cycle.
Construction of plasmid expressing full-length or truncated NS1 proteins.
Plasmid pJNS1, expressing full-length NS1 protein, has been described previously (29). To construct a truncated NS1, pJNS1 was used as a template to perform a PCR cloning. The primers used for PCR were 5′-GCATTCTCTTTGCCCCGGAATTGGC-3′ (+ sense, located at nt 2839 to 2863 in the NS1 region) and 5′-GCGGATCCTAAGCATCAACCTGTGA-3′ (− sense, complementary to nt 3519 to 3534 in the NS1 region and also containing the underlined sequence with an extra BamHI site that was convenient for further subcloning). The PCR products were first TA cloned into a vector pCR3.1 (Invitrogen) and, after BamHI digestion, the properly oriented insert was released and then subcloned into expression vector pSecTag-B (Invitrogen). The resulting plasmid was able to express a truncated NS1 with an arbitrary deletion of the first 120 amino acids, and the N-terminus of this truncated NS1 protein was fused in-frame with a signal sequence derived from mouse immunoglobulin κ chain, which could direct the engineered proteins into the ER during the translation process.
Establishment of cell clones permanently expressing Bcl-2 or Bcl-XL.
All cell lines stably expressing Bcl-2 or Bcl-XL were cloned from single cells by the limiting-dilution method described above. BHK-21 cells permanently expressing human Bcl-2 (B2-5) were as described previously (27). To establish CHO cells stably expressing bcl-2, cells were transfected by lipofectamine (BRL) with human bcl-2 expression plasmid pZipBcl-2 or with a vector control, pZipneo (26). The transfected cells were selected and cloned in the presence of Geneticin (GIBCO). The expression of bcl-2 in cell clones was assessed by Western blotting and IFA by using an antibody specific for the human Bcl-2 protein (Santa Cruz). The resultants were cultured in RPMI 1640 medium containing 5% FCS. Similarly, bcl-XL/pCR3.1 was used to construct BHK-21 cells constitutively expressing human Bcl-XL proteins.
RESULTS
Establishment of JEV persistence in the cells stably expressing bcl-2.
Previously, we demonstrated that following the initial lytic infection, JEV persistence could be readily established in mouse neuroblastoma N18, Vero, mouse astrocytoma DBT, and human neuronal progenitor NT2 cells (11). In contrast, this persistence was never successfully established in BHK-21 and CHO cells. By 3 days postinfection by JEV, both BHK-21 and CHO cells were completely killed through some mechanism, at least in part, involved in apoptosis (27 [also see below]). In addition, we observed that the constitutive expression of human bcl-2, a proto-oncogene, inhibited JEV-induced apoptosis in BHK-21 cells (27). In the present study, we therefore investigated whether JEV persistence could be instituted in the cells that stably expressed either bcl-2 or its antiapoptotic homolog, human bcl-XL.
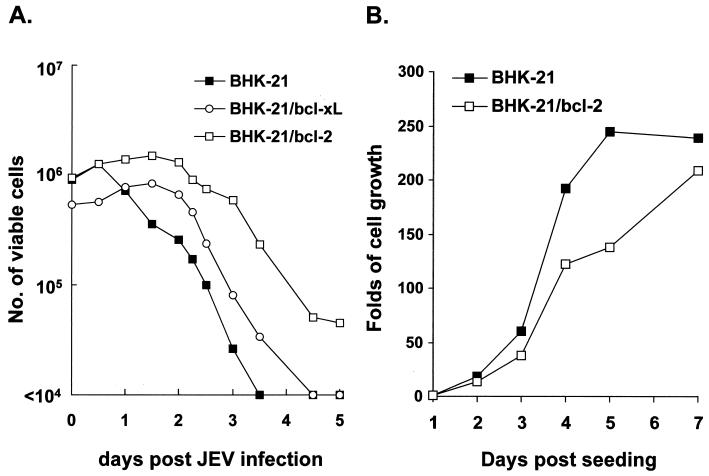
We first established several permanent BHK-21 and CHO cell clones that constitutively expressed bcl-2 or bcl-XL. By using specific mouse antisera, expression of bcl-2 or bcl-XL in these clones was confirmed by IFA (data not shown) and Western blot analysis (Fig. 1A and B). RP-9, a neurovirulent strain of JEV (12), was then used to infect these cells to explore the effect of human Bcl-2 or Bcl-XL protein on the establishment of JEV persistence in cells originally incompetent for such persistence. Representative data presented in Fig. 2 demonstrate how bcl-2 or bcl-XL expression affected the killing kinetics of JEV in different BHK-21 clones. Upon JEV infection at an MOI of 10, wild-type BHK-21 cells were all killed by 3.5 days postinfection, whereas following the initial cytolytic infection, approximately 10% of bcl-2-expressing BHK-21 cells survived (Fig. 2A) and became persistently infected during a long-term culture (see below). In contrast, expression of bcl-XL, by BHK-21 cells was able to slightly prolong the life span of infected cells, although viral persistence failed to be established after the assault from primary JEV infection (Fig. 2A). The outgrowth of JEV-infected BHK-21/bcl-2 cells as shown in Fig. 2A was not due to the fast-growing capability of the cells, because without infection the growth rate of BHK-21/bcl-2 cells appeared to be slower than that of wild-type BHK-21 cells (Fig. 2B). This observation was consistent with the antiproliferation characteristic of Bcl-2 on cell cycle entry (22).
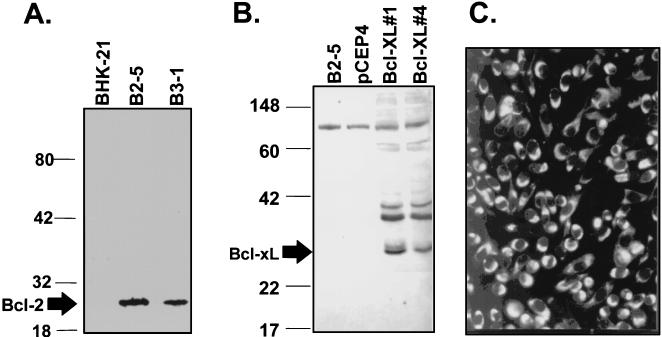
FIG. 1.
(A) Western blot analysis of the expression of human bcl-2 in permanent cell clones derived from BHK-21 cells. The 26-kDa Bcl-2 protein (as indicated by arrow) was expressed only in B2-5 and B3-1 cell clones but not in control BHK-21 cells. Numbers on the left are molecular masses in kilodaltons. (B) Western blot analysis of the expression of human bcl-XL in stable cell clones derived from BHK-21 cells. The 28-kDa Bcl-XL protein (as indicated by arrow) was observed in stable clones Bcl-XL#1 and #4 but not in controls of B2-5 or BHK/pCEP4 cells. (C) IFA staining of persistently JEV infected B2-5/RP9 cells with monoclonal antibodies specific for JEV NS1 protein.
FIG. 2.
(A) Effect of stable bcl-2 or bcl-XL expression on cell growth in response to JEV infection. Cells (106) were infected with JEV RP9 at an MOI of 10, and viable cells were assessed by trypan blue exclusion in three independent experiments at the indicated time intervals postinfection. (B) Growth curves of bcl-2-expressing and wild-type BHK-21 cells in the absence of JEV infection. Growth curves of the two cells were determined by their viability as in panel A at the indicated points after cells were seeded.
The surviving JEV-infected BHK-21/bcl-2 cells shown in Fig. 2A were maintained and grown into a stable cell population without apparent CPE. To determine whether the cell bulk was JEV persistent, IFA with virus-specific antibodies was carried out to detect viral antigens expressed in these cells; about 90% of the cells showed microscopically positive staining in the cytoplasm (data not shown). In addition, a virus titer of about 103 to 105 PFU/ml was continuously detected in the culture media from the bulk cells for several months, indicating that these cells had been persistently infected by JEV. This fluctuation of virus yields suggests a possible existence of DI virus particles generated during persistent infection.
In an attempt to obtain persistently JEV-infected cell clones, the above-noted persistent bulk cells were further cloned by limiting dilution as described in Materials and Methods. Five persistent clones were independently established. During 3-month passages, all five clones continued to release low titers of virus (approximately 103 to 105 PFU/ml) into their media, and nearly 100% of cells in each clone showed positive IFA staining with antibodies against viral proteins (representative data from B2-5/RP9 is shown in Fig. 1C). Nevertheless, as evidenced by infectious center assay, less than 15% of the cell population from each of the five clones was able to actively produce small amounts of infectious virions, indicating that the level of viral replication had been significantly reduced in those cells. It remains to be studied why only a portion of the persistent cell population was able to produce viable virions. We also examined the susceptibility of these persistent clones to superinfection with homologous JEV, and representative results are shown in Table 1. All five clones appeared to be resistant to homologous superinfection with JEV-RP9 at an MOI of 50; no clones displayed severe CPE by 40 h postinfection, and no obvious change in virus yields was observed after superinfection (Table 1). As a control, a primary JEV infection in wild-type BHK-21 cells reached virus yields as high as 5 × 107 PFU/ml (Table 1) and concomitantly caused severe CPE. Together, these data illustrate that the five clones, derived from JEV-infected BHK-21 cells expressing bcl-2, are all bona fide JEV-persistent cell lines. Similarly, we also established JEV persistence in CHO cells expressing bcl-2 and selected one of several independent clones, CHO-bcl2/RP9 (see below), for further study.
TABLE 1.
Properties of cell clones established from bcl-2-expressing BHK-21 cell bulks persistently infected by JEV
| Clone | Without superinfection
|
With superinfection, virus titer (PFU/ml) | ||
|---|---|---|---|---|
| Virus titer (PFU/ml) | IFA | RT-PCR | ||
| B2-5/RP9 | 9 × 104 | + | + | 105 |
| B1-1.1/RP9 | 2 × 104 | + | + | 2 × 104 |
| BHK-21 | −a | − | − | 5 × 107b |
The negative results were from mock-infected BHK-21 cells.
The virus yield was derived from primarily JEV-infected BHK-21 cells.
Effects of bcl-2 expression on JEV replication and spread.
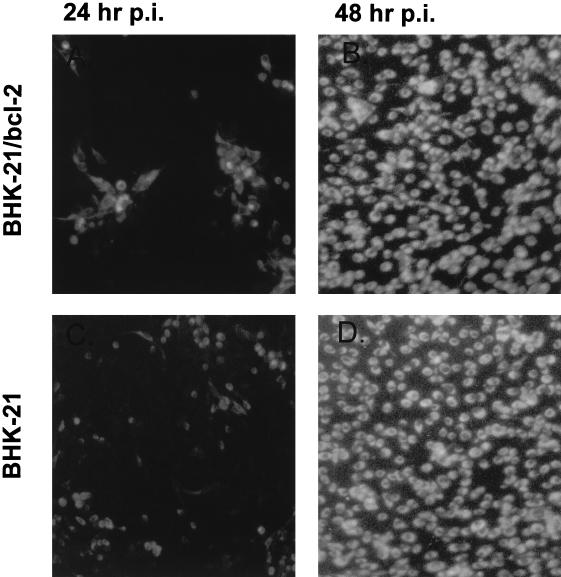
We next examined the mechanism by which bcl-2 enabled the cells to become persistently infected with JEV. As shown by a one-step growth curve of the virus, the overexpression of bcl-2 failed to suppress the virus replication in B2-5 cells infected with JEV. Both the virus growth curves and the virus yields from infection appeared to be comparable to those from a primary JEV infection in wild-type BHK-21 cells (27). To further study the effect of bcl-2 on JEV infection during multiple rounds of replication, wild-type BHK-21 and B2-5 cells were grown to confluence and then infected with RP9 at an MOI of 0.001. At 24 and 48 h postinfection, the cells were stained by immunofluorescent labeling with monoclonal antibodies specific for JEV to monitor infection status. As Fig. 3 shows, the JEV infection in B2-5 cells (top panels) could scatter around as readily as in the wild-type BHK-21 cells (bottom panels) at 48 h postinfection, as evidenced by nearly 100% of both infected cells showing positive IFA staining (right panels). These results illustrate that the bcl-2 expression did not restrict the spread of JEV in the cultured cells. Since bcl-2 expression did not influence JEV replication in a primary infection, these data thus suggest that Bcl-2 proteins may primarily play an antiapoptotic, rather than an antiviral, role that assists in the establishment of JEV persistence in B2-5 cells.
FIG. 3.
The effect of stable bcl-2 expression on multiple rounds of JEV infection in BHK-21 cells. The bcl-2-expressing cells (top panels) and wild-type BHK-21 cells (bottom panels) were infected with RP9 at a low MOI (0.001). At 24 or 48 h postinfection, the cells were fixed and stained by IFA with JEV-specific antibodies.
Examination of Bcl-2 cleavage products in Bcl-2-overexpressing cells either persistently or primarily infected with JEV.
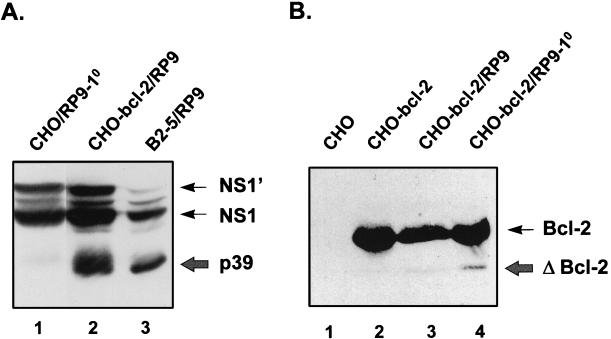
At the early phase of the persistence process, JEV infection killed more than 90% of the bcl-2-expressing cells (Fig. 2A), indicating that Bcl-2 could not completely protect the cells from virus-induced cell death. Hardwick and associates (13) demonstrated that conversion of Bcl-2 to a Bax-like death effector by caspases might occur in the systems where Bcl-2 failed to suppress apoptosis. By Western blot analysis with antibody against Bcl-2, we next sought to examine the Bcl-2 cleavage products in the CHO-bcl2 cells either primarily or persistently infected with JEV. As Fig. 4B indicates, when persistently infected by RP9, the CHO-bcl2 cells displayed no truncated Bcl-2 proteins (lane 3), whereas the primarily infected cells exhibited a cleaved species of Bcl-2 protein that was 23 kDa (lane 4); as a control, no cleavage product of Bcl-2 was detected in CHO-bcl2 cells in the absence of JEV infection (lane 2). These data suggest that there might be a Bcl-2 cleavage-resistant cell population being selected out from infected CHO-bcl2 cells during establishment of JEV persistence which was able to suppress virus-induced cell death.
FIG. 4.
(A) The truncated NS1 proteins derived from the persistently JEV-infected CHO cells expressing bcl-2. Cell lysates from the primary infection of CHO cells (lane 1) and from the persistent infection of CHO-bcl-2/RP9 (lane 2) and B2-5/RP9 cells (lanes 3) were separated by SDS–10% PAGE and immunoblotted with anti-NS1 monoclonal antibody JE7/45-2. The wild-type NS1 proteins are indicated by thin arrows, and the truncated ones (p39) are marked by a thick arrow. (B) Examination of Bcl-2 cleavage products in the CHO-bcl-2 cells either persistently or primarily infected with JEV. The cell lysates from CHO-bcl-2 alone (lane 2) and from CHO-bcl-2 either persistently (lane 3) or primarily (lane 4) (at 24 h postinfection) infected by RP9 were immunoblotted with anti-Bcl-2 antibody 100 (Santa Cruz). The wild-type Bcl-2 is indicated by a thin arrow, and its cleavage product is marked by a thick arrow, along with ΔBcl-2.
A similar experiment was also performed with B2-5 cells. The results revealed that both persistently and primarily JEV- infected B2-5 cells displayed a cleaved species of Bcl-2 that was 23 kDa, results contrasting with what we observed with CHO-bcl2 cells in Fig. 4B, where the persistent cells exhibited no truncated Bcl-2. This clearly indicates that besides the Bcl-2 cleavage-resistant mechanism proposed above for CHO-bcl2 cells, another, as-yet-unknown mechanism apparently also operates in B2-5 cells for JEV persistence to be established.
Characterization of the truncated NS1 proteins from JEV persistent cell lines.
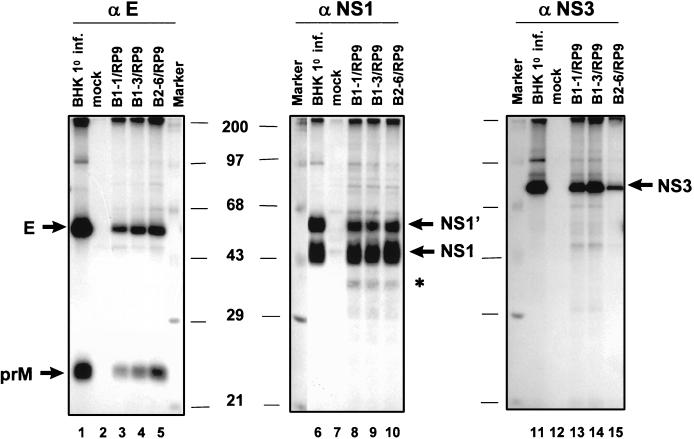
To study the expression profiles of viral proteins, [35S]methionine-labeled cell lysates from primarily infected BHK-21 cells and from JEV-persistent cell clones were precipitated by JEV-specific antibodies and separated by SDS-PAGE (Fig. 5). When monoclonal antibodies specific for either E (Fig. 5, lanes 1 to 5) or NS3 (Fig. 5, lanes 11 to 15) were used, the expression patterns were comparable between primary and persistent JEV infections, although the amounts of viral proteins varied. In contrast, as monoclonal antibodies against JEV NS1 (Fig. 5, lanes 6 to 10) were used, in addition to normal NS1 and NS1′ proteins, other protein bands with faster mobilities were observed in the persistently infected clones B1-1/RP9 (lane 8), B1-3/RP9 (lane 9), and B2-6/RP9 (lane 10), but not in the primarily infected BHK-21 cells (lanes 6).
FIG. 5.
Profiles of viral protein expression between primary and persistent JEV infections. [35S]methionine-labeled lysates from the JEV-infected cells were immunoprecipitated with monoclonal antibodies specific for E (αE, lanes 1 to 5), NS1 (αNS1, lanes 6 to 10), or NS3 (αNS3, lanes 11 to 15). Results from primary infection of BHK-21 cells with RP9 are shown in lanes 1, 6, and 11; results from persistent clones are in lanes 3 to 5, 8 to 10, and 13 to 15. The positions of each viral protein as determined by SDS–10% PAGE are indicated by arrows. Note that additional protein bands, marked by an asterisk, were found only in the cell lysates from JEV-persistent clones when precipitated by anti-NS1 monoclonal antibody (lanes 8 to 10).
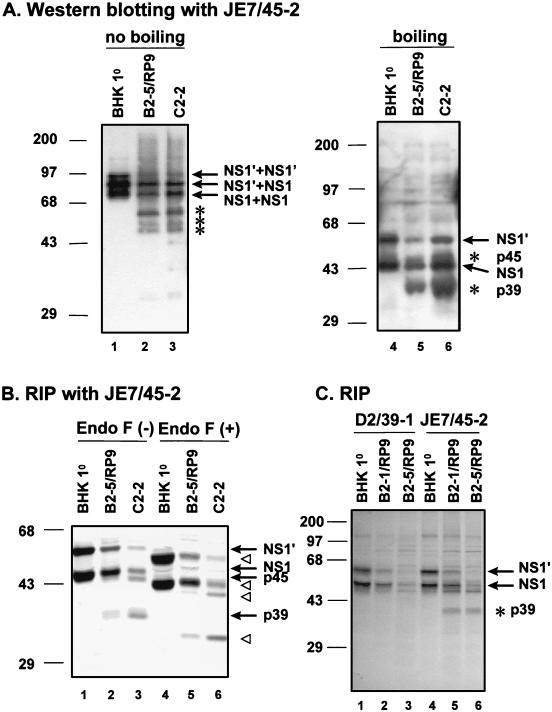
To ascertain whether the faster-migrating proteins detected in the persistently infected clones were truncated NS1 or simply cellular proteins associated with NS1, we carried out immunoblotting analysis with a monoclonal antibody, JE7/45-2, specific for JEV NS1. An example of the results from this analysis is shown in Fig. 6A. When protein samples were heat denatured prior to electrophoresis, in addition to the normal NS1 and NS1′ proteins, the monomeric form of truncated NS1 p39 was detected in persistent clone B2-5/RP9 (Fig. 6A, lane 5), as well as in C2-2 cells (lane 6), a JEV-persistent clone derived from a mouse neuroblastoma N18 cell line (11). No abnormal NS1 proteins could be detected in the primarily JEV-infected BHK-21 cells (Fig. 6A, lane 4). In the absence of heat denaturation, however, both the normal (Fig. 6A, lane 1) and the truncated (lanes 2 and 3) NS1 forms began to migrate slowly, probably due to the formation of homo- or heterodimers among different NS1 proteins (12, 18, 19, 24). These results clearly indicate that, like C2-2 cells, the JEV-persistent clones derived from BHK-21 cells stably expressing bcl-2 were capable of displaying the truncated NS1 proteins. In fact, the generation of truncated NS1 protein in JEV-persistent cells could be observed as early as the first cell passage after being established from primary infection (data not shown).
FIG. 6.
(A) Immunoblot analysis of NS1 and its derivatives from primary or persistent JEV infections. Cell lysates from primary infection of BHK-21 cells (lanes 1 and 4) and from persistent infection of B2-5/RP9 (lanes 2 and 5) and C2-2 cells (lanes 3 and 6) were separated by SDS–10% PAGE and immunoblotted with anti-NS1 monoclonal antibody JE7/45-2. Protein samples were treated either with boiling (lanes 4 to 6) or without boiling (lanes 1 to 3). The wild-type NS1 proteins are indicated by arrows. The truncated NS1 and NS1′ are marked by asterisks. Molecular mass markers (kilodaltons) are on the left of the figure. (B) Glycosylation analysis of NS1 proteins by endo-F digestion. Cell lysates from JEV primary infection of BHK-21 (lanes 1 and 4) and from persistent infection of B2-5/RP9 (lanes 2 and 5) and C2-2 cells (lanes 3 and 6) were immunoprecipitated by anti-NS1 monoclonal antibodies and then were treated either without (lanes 1 to 3) or with (lanes 4 to 6) endo-F at 37°C for 16 h. The deglycosylated NS1 and NS1′, as well as their truncated proteins p45 and p39 (lanes 4 to 6), are indicated by open triangles; their counterparts without endo-F digestion (lanes 1 to 3) are marked by arrows. (C) Localization of the truncation region to the N termini of NS1 and NS1′ proteins derived from the persistently JEV-infected cells by immunoprecipitation with anti-NS1 monoclonal antibodies. 35S-labeled lysates from primarily infected BHK-21 (lanes 1 and 4) and persistently infected B2-1/RP9 (lanes 2 and 5) and B2-5/RP9 (lanes 3 and 6) cells were precipitated by anti-NS1 D2/39.1 (lanes 1 to 3) or JE7/45-2 (lanes 4 to 6) and then separated by SDS–10% PAGE. The wild-type NS1 and NS1′ are marked with arrows, and the truncated protein p39 is marked by an asterisk. Note that only antibody JE7/45-2 recognized the truncated NS1 (lanes 5 and 6).
Wild-type NS1 contains two predicted glycosylation sites at the amino acid positions 130 and 207 (Fig. 7A), and one glycosylated moiety is estimated to be about 3 kDa. Consistent with the observations from our previous study (11), we demonstrated (Fig. 6B) that after complete digestion by endoglycosylase F (endo-F), the size of NS1 decreased, as expected, from 48 to 42 kDa (compare lanes 2 with 5); similarly, the size of p39, the truncated NS1, was also reduced from 39 to 33 kDa (Fig. 6B, compare lanes 2 and 3 with 5 and 6, respectively) after complete endo-F digestion. These data clearly illustrate that NS1 proteins, irrespective of whether wild type or truncated, had been properly glycosylated at both predicted sites in the persistent B2-5/RP9 cells. Such results also suggest that the truncated NS1s, like their wild-type counterparts, are translocated into the ER and are glycosylated during the biosynthesis process. In agreement with the results from our earlier study (11), the truncation was located in the N terminus of NS1 proteins derived from B2-5/RP9 cells (Fig. 6C) because the monoclonal antibody D2/39-1, which has been characterized to recognize a linear epitope located at the amino acid positions 115 to 120 in the N terminus of JEV NS1 (see Materials and Methods; also see Fig. 7A), precipitated only the wild-type NS1 and NS1′ but not the truncated NS1 proteins (compare lanes 2 and 3 with 5 and 6). Similar truncation (p39) in NS1 proteins was also observed in persistent CHO-bcl-2/RP9 cells (Fig. 4A, lane 2), a result resembling the truncation derived from B2-5/RP9 cells (lane 3). Together, these results indicate that truncated NS1 proteins from the JEV-persistent cells expressing bcl-2 were indistinguishable from those derived from C2-2 cells.
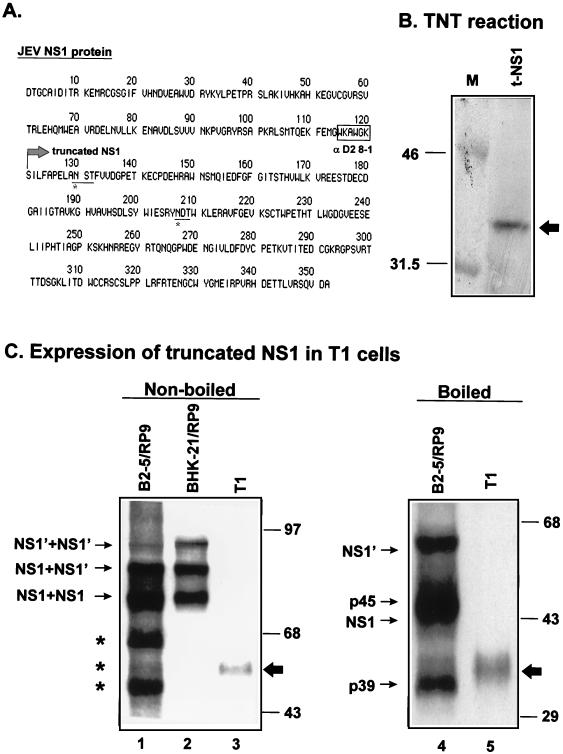
FIG. 7.
Construction of the truncated NS1 in expression vector pSecTag. (A) Full length of the amino acid sequence for JEV NS1 protein. The boxed amino acids are the sequence recognized by monoclonal antibody D2/39.1, as described in Materials and Methods. Amino acids underlined and marked with an asterisk are the predicted sequences for glycosylation. The truncated NS1, marked with an arrow at the start position 121, was created by artificial deletion of the first 120 amino acids of NS1 and PCR cloned into expression vector pSecTag (Invitrogen). (B) Expression of the truncated NS1 protein from plasmid t-NS1, indicated by an arrow, was confirmed by an in vitro coupled transcription-translation assay (TNT; Promega). (C) Expression of truncated NS1 proteins in a stable cell line, T1. Plasmid t-NS1 was used to transfect BHK-21 cells to establish a permanent cell line (T1) stably expressing truncated NS1. Cell lysates from B2-5/RP9 cells (lanes 1 and 4), primarily infected BHK-21 cells (lane 2), and T1 cells (lanes 3 and 5) treated with (lanes 4 and 5) or without (lanes 1 to 3) heat denaturation were immunoblotted with anti-NS1 JE7/45-2.
We next determined whether the truncation of the NS1 protein was an inherent property genetically associated with the viral variants released from the B2-5/RP9 cells. The results revealed that the primary infection of BHK-21 cells with the viruses released from B2-5/RP9 still resulted in a lytic infection after a 30-h incubation and that no abnormal forms of NS1 proteins were detected in the infected cells (data not shown). In addition, when analyzed by RT-PCR with primer sets specific for the NS1 region or the junction between E and NS1 (11; also see Materials and Methods), no truncated PCR products were detected in the total RNA extracted from B2-5/RP9 cells (data not shown; Table 1). These results suggest that the aberrant NS1 expression was not an inborn property of the viruses released from B2-5/RP9 cells but was most likely the outcome of virus-cell interaction associated with the establishment of JEV persistence (11).
Effects of overexpression of truncated NS1 on JEV persistence.
Truncated NS1 proteins have been demonstrated to be strongly associated with JEV persistence in the present (Fig. 4 to 6) and earlier (11) studies. To further determine whether the generation of truncated NS1 proteins is a cause for or an effect of JEV persistence in cell cultures, we performed experiments to determine how a stable expression of truncated JEV NS1 might influence JEV persistence in BHK-21 cells. The truncated NS1, obtained by arbitrary deletion of amino acids 1 to 120 from the N terminus of JEV NS1 (Fig. 7A), was cloned into the expression vector pSecTag under the control of the T7 and CMV promoters, and the plasmid t-NS1 resulted. Once confirmed by in vitro expression for NS1 (Fig. 7B), plasmid t-NS1 was used to establish stable cell lines that constitutively expressed truncated NS1. Representative cell clone T1 is shown in Fig. 7C, in which truncated NS1 expressed in the cell lysates could be detected by immunoblotting (lanes 3 and 5). Without heat denaturing, truncated NS1 from T1 cells appeared to form dimers in the absence of the N terminus of normal NS1 (Fig. 7C, lane 3). The distribution of truncated NS1 in T1 cells was primarily cytoplasmic (data not shown), resembling that of normal NS1 proteins in JEV-infected cells. Endo-F digestion analysis showed that the truncated NS1 proteins expressed from T1 cells were properly glycosylated in the ER (data not shown). Moreover, the amount of truncated NS1 expressed from T1 cells appeared to be comparable to that from JEV-persistent clone B2-5/RP9 cells (data not shown). Following a primary infection by JEV, T1 cells were all killed by 4 days postinfection; this survival pattern was similar to that of infected wild-type BHK-21 cells by the second day postinfection, whereas the positive control B2-5 cells exhibited a slow killing kinetic in response to JEV infection and, as a result, JEV persistence was eventually established in B2-5 cells during long-term culture. Moreover, the one-step growth curves of JEV were found to be comparable among T1, B2-5, and BHK-21 cells (data not shown), indicating that the expression of truncated JEV NS1 alone did not affect JEV replication. Taken together, these results imply that truncated NS1 was probably not a cause for, but was instead a consequence of, the establishment of JEV persistence in cell cultures.
DISCUSSION
In this study we demonstrated that the constitutive expression of human bcl-2 allowed JEV persistence to be established in the BHK-21 and CHO cells that were originally unbearable to such a chronic infection. At the initial stage of persistence, JEV killed virtually 90% of the infected cells (Fig. 2A), even though they were actually expressing bcl-2 (Fig. 1A). This finding indicated that conversion from a lytic infection to a persistent infection must be under tremendous selection pressures for both virus and host cells. By contrast, for the persistence of Sindbis and Semliki Forest viruses to be established, bcl-2 expression seemed to protect the infected cells more effectively (26, 44) than it did the JEV-infected cells in this study. In order for JEV to achieve persistence, the infected cells must be able to adapt themselves to sustain virus replication without being lysed, and JEV itself may also need to change so the virus imposes no damage to its infected cells. Yet, the JEV released from the persistently infected B2-5 cells neither caused persistent infection nor generated truncated NS1 proteins upon a new round of primary infection in BHK-21 cells (data not shown), indicating that the virus itself is not sufficient to establish persistent infection. On the other hand, with the aid of bcl-2 expression, the surviving BHK-21 and CHO cells from the lytic infection phase appeared to have evolved a secure, as-yet-unclear ability to accommodate JEV persistence.
Emerging evidence suggests that the balance of genes in the bcl-2 family known to regulate the cell death pathway could be crucial for the establishment of alphavirus persistence (reviewed in reference 20;0). In addition to its antiapoptotic capability, Bcl-2 may also assist viruses to achieve persistence by blocking virus replication in the infected cells, as suggested by previous studies of Sindbis virus (51) and Semliki Forest virus (44). In contrast, our data revealed that the overexpression of bcl-2 did not appear to restrict JEV replication and spread during the primary infection in BHK-21 cells (Fig. 3). A similar phenomenon was also previously documented from a reovirus study (43). Conceivably, it is the antiapoptotic, rather than the antiviral, function of Bcl-2 that modulates the outcome of JEV infection progressing from the initial cytolytic phase toward the ultimate virus persistence. Intriguingly, however, the expression of bcl-2 has been proven unable to completely suppress JEV-induced cell death in either BHK-21 or CHO cells. Thus, exactly what renders some, but not all, of the bcl-2-expressing cells to become persistently infected by JEV remains elusive. One plausible mechanism, which is supported by the results derived from CHO-bcl2 cells (Fig. 4B), is that there is a cell population resistant to Bcl-2 cleavage being selected out during the JEV persistence process. In this scenario, slowdown of the turnover rate for Bcl-2 proteins may make the infected cells competent for developing JEV persistence. In fact, conversion of Bcl-2 to its BH4-domain-deleted protein has been attributed to the inability of Bcl-2 to block apoptotic cell death in some systems, whereas the caspase-cleavage-resistant constructs of Bcl-2 seemed to be more capable of preventing the cells from apoptotic attacks than the wild-type Bcl-2 (13). Nevertheless, it is unclear whether the resistance to Bcl-2 being proteolytically cut, as shown in Fig. 4B, was due to any mutation(s) occurring in bcl-2, caspase-3, or both. Whether the observed Bcl-2 cleavage-resistant phenotype was the cause or the effect of development of JEV persistence also needs to be investigated further. It should be noted that to unequivocally address this issue, an experiment employing a cell line expressing mutant Bcl-2 resistant to caspase cleavage (13) should be performed following JEV infection. Even more puzzling, in contrast to bcl-2, its close antiapoptotic homolog bcl-XL failed to assist in development of JEV persistence in BHK-21 cells (Fig. 2A), indicating that only bcl-2 expression can give the cells a unique microenvironment suitable for the establishment of JEV persistence. What dictates such cellular difference by the two antiapoptotic effectors is of interest and needs to be explored in the future. It is conceivable that other cellular and viral gene products may also participate in the process of JEV persistence.
Characteristically, the truncated NS1 proteins were again observed in bcl-2-expressing cells persistently infected by JEV (Fig. 4 to 6); the biological properties of these NS1 proteins closely resembled the ones previously documented from the persistent murine neuroblastoma N18, as well as other persistent cells (11). This supports the notion that the aberrant NS1 expression is a molecular signature for JEV persistence in cultured cells (11). The alteration of virus structural gene products has been shown to be responsible for the persistent infections by several RNA viruses, including poliovirus (7), measles virus (4, 8), Sindbis virus (25), and lymphocytic choriomeningitis virus (35). However, in contrast to these viruses, JEV persistence was demonstrated to be intimately associated with its modified nonstructural protein NS1. While the precise role of NS1 in JEV persistence remains to be further explored, recent results from several studies implicate the involvement of NS1 in flavivirus RNA replication (15, 30, 31, 36, 37). In addition, flaviviral NS1 protein has also been suggested to play a role in virion maturation, a process which also utilizes the cellular secretory pathway (18, 32). These interpretations seem to account for our findings that nearly all of the persistent cells exhibited positive staining for viral antigens and yet less than 15% of them actually released viable virions in the infectious center assay. Conceivably, the generation of aberrant forms of NS1 proteins may reflect the reduction of viral replication and the subversion of virus-induced CPE during the establishment of JEV persistence. However, our data revealed that the stable expression of NS1 protein alone, regardless of whether truncated or full length, was incapable of influencing replication of the virus, which was thereby unable to persistently infect the engineered cells. Despite the close association with JEV persistence, the occurrence of aberrant NS1 proteins per se is insufficient for the creation of JEV persistence. This seems to imply that the generation of truncated NS1 (p39) proteins is merely an inevitable outcome of JEV persistence rather than a cause for its establishment in cultured cells. Since two forms of truncated NS1 proteins (p45 and p39), along with wild-type NS1 and NS1′, coexist in B2-5/RP9 cells (Fig. 4 to 6), one may conjecture that homodimer p39-p39 and/or heterodimer p39-NS1 or p39-NS1′ makes no contribution to the establishment of JEV persistence. Yet, the possible role of truncated NS1′ (p45), homodimer p45-p45, and heterodimer p45-p39 or p45-NS1 in JEV persistence remains to be further investigated.
In summary, we have illustrated that the enforced expression of bcl-2 assisted JEV-infected cells to reach a critical balance point between virus replication and virus-induced cell death, thereby leading to a stable virus persistence state. Although there was no abrogation of virus replication and spread during the early cytolytic phase, bcl-2 overexpression appeared to allow some infected cells to be selected out during the persistence process. Together, the results here suggest that it is the antiapoptotic capability rather than the antiviral capability of bcl-2 that mediates the establishment of JEV persistence in cultured cells.
ACKNOWLEDGMENTS
The kind gifts of plasmids pZipbcl-2 and pZipneo from D. E. Griffin and the Taiwanese local JEV strain NT109 obtained from the National Institute of Preventive Medicine (Taiwan, Republic of China [ROC]) are deeply appreciated.
C.-L.L. was supported by a grant (NSC 87-2314-B-016-088) from the National Science Council (NSC) and a grant (DD01-861X-CR-501-P) from the National Health Research Institute (NHRI) of the ROC. Y.-L.L. was supported by a grant from the NSC (87-2314-B016-090) and one (DOH87-TD-1002) from Department of Health of the ROC. L.-K.C. was supported by a grant (NSC 86-2314-B-016-043 M07) from the NSC and two (DD01-86IX-CR-501-P) from the NHRI.
REFERENCES
- 1.Ackermann W W, Kurtz H. Observations concerning a persisting infection of HeLa cells with poliomyelitis virus. J Exp Med. 1955;102:555–565. doi: 10.1084/jem.102.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, Fields B N. Role of the S4 gene in the establishment of persistent reovirus infection in L cells. Cell. 1982;28:605–612. doi: 10.1016/0092-8674(82)90215-x. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Morrison L A, Knipe D M. Persistence of viruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 219–249. [Google Scholar]
- 4.Baczko K, Lampe J, Liebert U G, Brinckmann U, ter Meulen V, Pardowitz I, Budka H, Cosby S L, Isserte S, Rima B K. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology. 1993;197:188–195. doi: 10.1006/viro.1993.1579. [DOI] [PubMed] [Google Scholar]
- 5.Brinton M A. Replication of flaviviruses. In: Schlesinger S, Schlesinger M J, editors. The Togaviridae and Flaviviridae. New York, N.Y: Plenum Press, Inc.; 1986. pp. 350–359. [Google Scholar]
- 6.Burke D S, Leake C J. Japanese encephalitis. In: Monath T P, editor. The arboviruses: epidemiology and ecology. III. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 63–92. [Google Scholar]
- 7.Calvez V, Pelletier I, Borzakian S, Colbére-Garapin F. Identification of a region of poliovirus genome involved in persistent infection of Hep-2 cells. J Virol. 1993;67:4432–4435. doi: 10.1128/jvi.67.7.4432-4435.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo R, Schmid A, Eschle D, Baczko K, ter Meulen V, Billeter M A. Biased hypermutation and other genetic changes in defective measles viruses in human brain infection. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain R W. Epidemiology of arthropod-borne togaviruses: the role of arthropods as hosts and vectors and of vertebrate hosts in natural transmission cycles. In: Schlesinger R W, editor. The togaviruses: biology, structure, replication. New York, N.Y: Academic Press, Inc.; 1980. pp. 175–228. [Google Scholar]
- 10.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome, organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 11.Chen L K, Liao C L, Lin C G, Lai S C, Liu C I, Ma S H, Huang Y Y, Lin Y L. Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology. 1996;217:220–229. doi: 10.1006/viro.1996.0109. [DOI] [PubMed] [Google Scholar]
- 12.Chen L K, Lin Y L, Liao C L, Lin C G, Huang Y L, Yeh C T, Lai S C, Jan J T, Chin C. Generation and characterization of organ-tropism mutants of Japanese encephalitis virus in vivo and in vitro. Virology. 1996;223:79–88. doi: 10.1006/viro.1996.0457. [DOI] [PubMed] [Google Scholar]
- 13.Cheng E H Y, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Chu P W G, Westaway E G. Molecular and ultrastructural analysis of heavy membrane fractions associated with the replication of Kunjin virus RNA. Arch Virol. 1992;125:177–191. doi: 10.1007/BF01309636. [DOI] [PubMed] [Google Scholar]
- 16.Falconar A K I, Young P R. Production of dimer-specific and dengue virus group cross-reactive mouse monoclonal antibodies to the dengue 2 virus nonstructural glycoprotein NS1. J Gen Virol. 1991;72:961–965. doi: 10.1099/0022-1317-72-4-961. [DOI] [PubMed] [Google Scholar]
- 17.Falconar A K I, Young P R, Miles M A. Precise location of sequential dengue virus subcomplex and complex B cell epitopes on the nonstructural-1 glycoprotein. Arch Virol. 1994;137:315–326. doi: 10.1007/BF01309478. [DOI] [PubMed] [Google Scholar]
- 18.Fan W F, Mason P W. Membrane association and secretion of the Japanese encephalitis virus NS1 protein from cells expressing NS1 cDNA. Virology. 1990;177:470–476. doi: 10.1016/0042-6822(90)90511-o. [DOI] [PubMed] [Google Scholar]
- 19.Flamand M, Deubel V, Girard M. Expression and secretion of Japanese encephalitis virus nonstructural protein NS1 by insect cells using a recombinant baculovirus. Virology. 1992;191:826–836. doi: 10.1016/0042-6822(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 20.Griffin D E, Hardwick J M. Regulators of apoptosis on the road to persistent alphavirus infection. Annu Rev Microbiol. 1997;51:565–592. doi: 10.1146/annurev.micro.51.1.565. [DOI] [PubMed] [Google Scholar]
- 21.Hinshaw V S, Olsen C W, Dybdahl-Sissoko N, Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994;68:3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang D C S, O’Reilly L A, Strasser A, Cory S. The anti-apoptosis function of Bcl-2 can be genetically separated from its inhibitory effect on cell cycle entry. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster M U, Hodgetts S I, Mackenzie J S, Urosevic N. Characterization of defective viral RNA produced during persistent infection of Vero cells with Murray Valley encephalitis virus. J Virol. 1998;72:2474–2482. doi: 10.1128/jvi.72.3.2474-2482.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee T, Watanabe K, Aizawa C, Nomoto A, Hashimoto H. Preparation of Japanese encephalitis virus nonstructural protein NS1 obtained from culture fluid of JEV-infected Vero cells. Arch Virol. 1991;116:253–260. doi: 10.1007/BF01319246. [DOI] [PubMed] [Google Scholar]
- 25.Levine B, Griffin D E. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J Virol. 1993;67:6872–6875. doi: 10.1128/jvi.67.11.6872-6875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Huang Q, Isaacs J T, Reed J C, Griffin D E, Hardwick J M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 27.Liao C L, Lin Y L, Wang J J, Huang Y L, Yeh C T, Ma S H, Chen L K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y L, Huang Y L, Ma S H, Yeh C T, Chiou S Y, Chen L K, Liao C L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y L, Chen L K, Liao C L, Yeh C T, Ma S H, Chen J L, Huang Y L, Chen S S, Chiang H Y. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol. 1998;72:191–200. doi: 10.1128/jvi.72.1.191-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenbach B D, Rice C M. trans-complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol. 1997;71:9608–9617. doi: 10.1128/jvi.71.12.9608-9617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 32.Mason P W. Maturation of Japanese encephalitis virus glycoproteins produced by infected mammalian and mosquito cells. Virology. 1989;169:354–364. doi: 10.1016/0042-6822(89)90161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur A, Arora K L, Rawat S, Chaturvedi U C. Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol. 1986;67:381–385. doi: 10.1099/0022-1317-67-2-381. [DOI] [PubMed] [Google Scholar]
- 34.Mathur A, Bharadwaj M, Kulshreshtha R, Rawat S, Jain A, Chaturvedi U C. Immunopathological study of the spleen during Japanese encephalitis virus infection in mice. Br J Exp Pathol. 1988;69:423–432. [PMC free article] [PubMed] [Google Scholar]
- 35.Matloubian M, Somasundaram T, Kolhekar S R, Selvakumar R, Ahmed R. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J Exp Med. 1990;172:1043–1048. doi: 10.1084/jem.172.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muylaert I R, Galler R, Rice C M. Mutagenesis of N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology. 1996;222:159–168. doi: 10.1006/viro.1996.0406. [DOI] [PubMed] [Google Scholar]
- 37.Muylaert I R, Galler R, Rice C M. Genetic analysis of yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J Virol. 1997;71:291–298. doi: 10.1128/jvi.71.1.291-298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen C W, Kehren J C, Dybdahl-Sissoko N R, Hinshaw V S. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol. 1996;70:663–666. doi: 10.1128/jvi.70.1.663-666.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pekosz A, Phillips J, Pleasure D, Merry D, Gonzalez-Scarano F. Induction of apoptosis by La Crosse virus infection and role of neuronal differentiation and human bcl-2 expression in its prevention. J Virol. 1996;70:5329–5335. doi: 10.1128/jvi.70.8.5329-5335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poidinger M, Coelen R J, Mackenzie J S. Persistent infection of Vero cells by the flavivirus Murray Valley encephalitis virus. J Gen Virol. 1991;72:573–578. doi: 10.1099/0022-1317-72-3-573. [DOI] [PubMed] [Google Scholar]
- 41.Ravi V, Desai A S, Shenoy P K, Satishchandra P, Chandramuki A, Gourie-Devi M. Persistence of Japanese encephalitis virus in the human nervous system. J Med Virol. 1993;40:326–329. doi: 10.1002/jmv.1890400412. [DOI] [PubMed] [Google Scholar]
- 42.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 931–959. [Google Scholar]
- 43.Rodgers S E, Barton E S, Oberhaus S M, Pike B, Gibson C A, Tyler K L, Dermody T S. Reovirus-induced apoptosis of MDCK cells is not linked to viral yield and is blocked by Bcl-2. J Virol. 1997;71:2540–2546. doi: 10.1128/jvi.71.3.2540-2546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scallan M F, Allsopp T E, Fazakerley J K. bcl-2 acts early to restrict Semliki Forest virus replication and delays virus-induced programmed cell death. J Virol. 1997;71:1583–1590. doi: 10.1128/jvi.71.2.1583-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmaljohn C S, Blair C D. Persistent infection of cultured mammalian cells by Japanese encephalitis virus. J Virol. 1977;24:580–589. doi: 10.1128/jvi.24.2.580-589.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmaljohn C S, Blair C D. Clonal analysis of mammalian cell cultures persistently infected with Japanese encephalitis virus. J Virol. 1979;31:816–822. doi: 10.1128/jvi.31.3.816-822.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah P S, Gadkari D A. Persistent infection of porcine kidney cells with Japanese encephalitis virus. Indian J Med Res. 1987;85:481–491. [PubMed] [Google Scholar]
- 48.Sharma S, Mathur A, Prakash V, Kulshreshta R, Kumar R, Chaturvedi U C. Japanese encephalitis virus latency in peripheral blood lymphocytes and recurrence of infection in children. Clin Exp Immunol. 1991;85:85–89. doi: 10.1111/j.1365-2249.1991.tb05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stim T B. Arbovirus plaquing in two simian kidney cell lines. J Gen Virol. 1969;5:329–338. [Google Scholar]
- 50.Takemoto K K, Habel K. Virus-cell relationship in a carrier culture of HeLa cells and coxsackie A9 virus. Virology. 1959;7:28–44. doi: 10.1016/0042-6822(59)90175-8. [DOI] [PubMed] [Google Scholar]
- 51.Ubol S, Tucker P C, Griffin D E, Hardwick J M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci USA. 1994;91:5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umenai T, Krzysko R, Bektimirov T A, Assaad F A. Japanese encephalitis: current worldwide status. Bull W H O. 1985;63:625–631. [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughan D W, Hoke C H., Jr The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 54.Vogt M, Dulbecco R. Properties of HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958;5:425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]