Abstract
Previous studies characterized the third variable (V3) loop of the envelope gp120 as the principal neutralizing determinant for laboratory T-cell-line-adapted (TCLA) strains of human immunodeficiency virus type 1 (HIV-1). However, primary viruses isolated from infected individuals are more refractory to neutralization than TCLA strains, suggesting that qualitatively different neutralizing antibodies may be involved. In this study, we investigated whether the V3 loop constitutes a linear target epitope for antibodies neutralizing primary isolates. By using peptides representative of the V3 regions of various primary isolates, an early, relatively specific and persistent antibody response was detected in sera from HIV-infected patients. To assess the relationship between these antibodies and neutralization, the same peptides were used in competition and depletion experiments. Addition of homologous V3 peptides led to a competitive inhibition in the neutralization of the TCLA strain HIVMN/MT-4 but had no effect on the neutralization of the autologous primary isolate. Similarly, the removal of antibodies that bind to linear V3 epitopes resulted in a loss of HIVMN/MT-4 neutralization, whereas no decrease in the autologous neutralization was measured. The different roles of V3-specific antibodies according to the virus considered were thereby brought to light. This confirmed the involvement of V3 antibodies in the neutralization of a TCLA strain but emphasized a more pronounced contribution of either conformational epitopes or epitopes outside the V3 loop as targets for antibodies neutralizing primary HIV-1 isolates. This result underlines the need to focus on new vaccinal immunogens with epitopes able to induce broadly reactive and efficient antibodies that neutralize a wide range of primary HIV-1 isolates.
Analysis of the immune responses developed within weeks after infection by the human immunodeficiency virus (HIV) suggests that the immune system may control the viral load. Strong cellular responses are detected in patients early in infection and provide evidence of a major role of cytotoxic T cells (CTL) in the decline of the initial burst of viremia (13, 29). Protection by CTL is also suggested by the existence of active and/or memory HIV-specific CTL in long-term nonprogressors infected for more than 10 years without any development of AIDS (19, 53). Furthermore, specific CTL are present in individuals who have been frequently exposed to the virus without being infected (35, 54); whether these CTL are simply markers for exposure to viral antigens or allow resistance to infection remains to be determined. In addition, CD8+ T lymphocytes and macrophages, effector cells of the immune response, can secrete soluble inhibitory factors (10, 32), including the chemokines MIP-1α, MIP-1β, and Rantes (8), interleukin-16 (IL-16) (1), and the macrophage-derived factor (49), which inhibit the replication of HIV-1 primary isolates.
On the other hand, neutralizing antibodies (NAb) are probably involved in the control of viral replication, even if these antibodies are detected only several months after infection (13, 38, 51). This is borne out by the higher titers and broader-reactivity NAb found in long-term nonprogressors (6, 52, 58). In a recent publication, Shan-Lu et al. described host immune responses in two patients infected almost simultaneously from the same source; while one developed the disease extremely rapidly (in less than 2 years), the other had a more average course of progression and remained asymptomatic for 3 years postinfection (62). These authors correlated more vigorous NAb and lymphocyte proliferation responses with a slower disease progression. Interestingly, it has been shown that the presence of NAb to primary HIV-1 isolates, including autologous virus, was related to a lower risk of mother-to-child transmission (56, 57), and it was postulated that a broad cross-reactive NAb response may reduce the risk of transmission of HIV-1 by controlling the maternal viral load. Overall, these observations indicate that NAb found to be able to inhibit viral replication in vitro may very likely play a role in vivo by either preventing infection or reducing the spread of the virus and the progression of the disease. NAb would allow a low viral load to be sustained during the asymptomatic phase and would allow HIV-1 replication that occurs throughout the entire course of infection to be controlled. Nevertheless, despite these data, the correlates of protection and the relative contributions of cellular and humoral responses remain unclear. Understanding them constitutes the first objective in the process of developing an effective vaccine (20); a subsequent objective is the identification of the antigens and target epitopes able to induce such a protective immunity.
Viral proteins of HIV-1 are highly immunogenic, and various sites on the virion give rise to humoral responses. In particular, the envelope glycoproteins gp120 and gp41 constitute the principal targets for NAb (40). They contain both conserved and hypervariable domains described as epitopes recognized by immune sera and monoclonal antibodies (14, 43). Interestingly, the third variable (V3) domain of gp120 forms an exposed, accessible loop on the surface of the viral particles (45) and induces the production of V3 antibodies detectable either after natural infection or following specific immunization (74). Moreover, this region is a determinant for cellular tropism and viral infectivity (21, 22, 63). V3 sequences act upon the interaction with coreceptors CXCR4 or CCR5 (12, 66), and the V3 loop takes part in the initiation of the fusion step necessary for virus entry. Neutralizing V3 antibodies may therefore be able to block either the attachment of the virus to the cell or subsequent postbinding events. For these reasons, attention has been focused on the V3 region of gp120, which has been defined as the principal neutralizing determinant (PND) for T-cell-line-adapted (TCLA) viruses (24, 55). In one study, Spear et al. described V3 loop-specific antibodies as important in mediating a major proportion of immune effector functions, including complement activation and neutralization of the laboratory strain HIV-1MN (64). Because such antibodies neutralized many TCLA strains (23, 64, 73) and because their presence could be correlated with the protection against homologous challenge found in chimpanzees (15), the V3 loop has been used to elaborate vaccine and therapeutic strategies. Unfortunately, this region is hypervariable and is most often the target of type-specific antibodies rather than broad-specificity, cross-reactive NAb. Moreover, subsequent studies have revealed differences between TCLA strains and primary viruses isolated from infected individuals. Indeed, besides differences in tropism (macrophage-tropic versus lymphotropic), replication capacity (slow low versus rapid high), and use of coreceptors (CXR5 versus CXCR4) (2, 12, 44), it is clear that primary wild-type isolates are more refractory to neutralization than TCLA strains (9, 17, 34). Higher antibody quantities, but also qualitatively different antibodies, appear to be necessary to neutralize primary isolates (33), and different epitopes may be recognized by NAb (3, 70). It is therefore crucial to reconsider antibodies involved in the neutralization of field isolates and to determine if the V3 loop is also a PND for primary isolates. A better knowledge of these neutralization epitopes will help in elaborating a vaccine designed to counter primary isolates of HIV-1.
In this study we attempted to determine if, as for TCLA strains, neutralization of primary isolates was mediated by antibodies directed against the autologous V3 loop. The presence of V3-specific antibodies in sera from HIV-infected patients was detected by using V3 loop peptides with sequences similar or close to the amino acid sequences of the V3 regions of four primary isolates. To further assess if the antibodies binding to these V3 loop peptides were involved in the autologous neutralization and in the neutralization of the TCLA strain HIV-1MN, competition and depletion experiments were carried out. The different roles of V3 loop-specific antibodies in the neutralization of TCLA strains versus primary isolates were brought to light.
MATERIALS AND METHODS
Cells, viruses, and sera.
All cell cultures were maintained at 37°C in 5% CO2.
MT-4 cells (a human T4 lymphoid cell line) were cultured in RPMI 1640 containing 10% fetal calf serum (FCS). This cell line supports the replication of the TCLA strain HIV-1MN, a syncytium-inducing strain obtained through the AIDS Research and Reference Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and amplified on the MT-4 cell line to constitute a viral stock (HIV-1MN/MT-4). The TCLA strain HIV-1MN was also produced on peripheral blood mononuclear cells (PBMC), and a single passage was enough to produce a viral stock of HIV-1MN/PBMC.
PBMC were isolated from buffy coats of healthy HIV-seronegative donors by Ficoll gradient purification. The cells, suspended in RPMI 1640 containing 10% FCS, were stimulated for 3 days with 2 μg of phytohemagglutinin A (Sigma) per ml and then frozen in RPMI containing 20% FCS and 40% dimethyl sulfoxide. One day before their use in the neutralization assay, PBMC from five random donors were thawed, pooled, and cultured for 24 h in RPMI 1640 supplemented with 10% FCS and 20 U of IL-2 (Boehringer Mannheim) per ml.
The HIV-1 primary isolates Bx08, Bx16, Bx17, and Bx26 were isolated early after seroconversion by cocultivation of PBMC from seropositive individuals with PBMC from healthy seronegative donors (51). To constitute viral stocks, we amplified these viruses as described before (38, 39) by one or two passages of the isolates exclusively on PBMC.
The sera studied were collected from the same seropositive patients at various times after seroconversion. Their autologous and heterologous neutralizing activities have been described elsewhere in a longitudinal study of early humoral responses detected during the first years of HIV infection (38). The sera for which we detected an autologous neutralizing activity were selected to further characterize NAb.
V3 loop sequences of primary isolates and V3 peptides.
RNAs isolated from the viral stocks of primary isolates, amplified once on PBMC, were converted into cDNA and then amplified with primer 5′ (V5, 5′-ATGAATTCGCTGTTRAATGGCAGTCTAGGCAGA-3′) and primer 3′ (V3, 5′-ATGAATTCATTTCTGGGTCCCCTCCTGAGGA-3′). Amplified products were digested with the restriction enzyme EcoRI and cloned in an M13mp18 vector. Five to seven clones were sequenced with the dye-primer sequencing kit (Applied Biosystems). A very low intravariability (less than 0.5%) was observed in the C2V3 region, a feature expected for viruses isolated early after seroconversion. The amino acid sequences were derived from the individual nucleotide sequences.
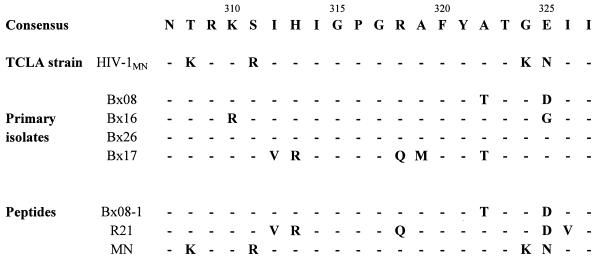
Two 21-mer peptides corresponding to the V3 loops of the HIV-1 primary isolate Bx08 and of the TCLA strain HIV-1MN were synthesized by the solid-phase method of Merrifield with the 9-fluorenylmethoxycarbonyl alternative (36). A third peptide, R21, having the V3 loop sequence of a Rwandese subtype A primary isolate, which is very close to the sequence of the Bx17 isolate, has also been used. Sequences of the peptides and viruses are represented in Fig. 1, aligned and compared to the French V3 loop consensus sequence (7).
FIG. 1.
V3 loop amino acid sequences of peptides and viruses used in the study, aligned with the subtype B (United States/European) consensus sequence.
Detection of anti-V3 loop antibodies by enzyme-linked immunosorbent assay (ELISA).
The V3 loop peptides were immobilized on Nunc Maxisorp microplates by an overnight incubation at 4°C of 100 μl of peptide per well diluted at 2 μg/ml in 50 mM bicarbonate buffer (pH 9.6). Unbound peptides were removed by washing once with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (wash buffer), and the plates were blocked with 200 μl of PBS containing 5% nonfat dry milk. Coated plates were washed four times and dried for 1 h at 37°C. At this stage, the plates could be frozen at −20°C for subsequent use or immediately used with the addition of 100 μl of serum (dilution, 1/20) for 1 h at 37°C. After five washings, bound V3-specific antibodies were detected by the addition of 100 μl of peroxidase–rabbit anti-human immunoglobulin conjugate (Dako) diluted 1/50,000 in PBS containing 5% nonfat dry milk and 0.3% Tween 20. Immune complexes were revealed with tetramethyl benzidine substrate, and the colorimetric reaction was stopped by the addition of H2SO4. The optical density was read at 450 nm against 650 nm (OD450–650), and the binding activity was detected by comparison with the OD450–650 obtained after the incubation of a serum from a consensus negative individual provided by the National Institutes of Health. The cutoff was defined as the OD450–650 of this negative serum plus 0.05.
Depletion of V3 loop-specific antibodies.
In order to remove V3-specific antibodies from the sera, six to eight passages on V3 peptide-coated wells were carried out by using the ELISA binding protocol with some modifications. First, we increased the binding capacity by coating the plates with 200 μl of 20-μg/ml V3 peptide. Second, to avoid the deleterious effects of detergent in subsequent cell cultures, the Tween 20 in the wash solution was replaced by 5% FCS. Third, sequential twofold dilutions of sera were incubated for several (six to eight) successive passages on coated wells until the V3-specific antibodies were eliminated. For this purpose, after 1 h of incubation at 37°C, the sera were removed by pipetting and further incubated on other coated wells for an additional hour. For controls, the sera were also mock depleted by serial passages on plates treated in parallel but not coated with V3 peptides. The antibodies removed from the sera and bound to the peptide-coated wells were then detected as described above with the tetramethyl benzidine chromogene substrate followed by the addition of the stop solution H2SO4. The diminution in OD450–650 throughout the successive passages could be monitored and correlated to the sequential depletion in V3-specific antibodies. For the lowest dilution of serum tested, a depletion percentage was calculated as follows: percent depletion = 100 − [100 × (OD450–650 at the last passage/OD450–650 at the first passage)]. After the last passage, the different depleted fractions of sera were filtered through 0.45-μm-pore-size filters (μstar; Costar) before being tested for their neutralizing activity.
Neutralization assays.
The neutralization of primary isolates and of the TCLA strain HIV-1MN was studied by using two different protocols.
In MT-4 cells, the replication of the TCLA strain HIV-1MN induces a cytopathogenic effect characterized by the formation of syncytia followed by cellular death. The neutralizing activity of the serum was detected as an inhibition of this virus-induced cytopathogenicity. The neutralization assay was performed in 96-well flat-bottom tissue culture plates (Costar 3596) by incubating 50 μl of an appropriate dilution of virus with 50 μl of serial dilutions of sera. After 1 h at 37°C, 4 × 104 MT-4 cells were added and the cultures were maintained for 5 days. Cell viability was then measured as described previously (50) with a colorimetric reaction based on the capacity for mitochondrial dehydrogenase of living cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide into formazan. The quantity of formazan produced was measured by OD540 and was correlated with the number of living cells (50). For each serum dilution the experiment was done in triplicate, and the mean value was used to calculate the percentage of protection according to Pauwels’ formula (50). The neutralization titer of the serum was defined as the serum dilution that allowed 50% protection against the cytopathogenic effect.
To detect the neutralizing activities of sera against primary isolates, the neutralization assay was performed with PBMC as target cells. This previously described test (39) combines serial dilutions of virus with serial dilutions of serum and is based on detecting a 10-fold virus titer reduction in the presence of the immune serum. Briefly, 50 μl of four fourfold dilutions of virus was incubated for 1 h at 37°C with 50 μl of serial serum dilutions in a 96-well filtration plate (Durapor-Dv 0.65μ; Millipore, Molsheim, France) before addition of 105 phytohemagglutinin A-stimulated PBMC (a pool from five seronegative donors). After 2 h at 37°C, extensive washings were done (three times with 200 μl of RPMI 1640), and cells were cultured in 200 μl of RPMI 1640 containing 10% FCS and 20 U of IL-2 per ml. Half of the medium was changed at 4 days postinfection, and viral production was measured at 7 days postinfection. For each serum dilution the assay was performed in quadruplicate, and the HIV-positive wells were identified by the quantity of p24 antigen (ELISA kit from Du Pont or Innogenetics) in the culture supernatants. This allowed the viral titer (50% tissue culture infective dose) to be calculated in the presence (Vn) and in the absence (Vo) of the serum, according to the Reed-Muench method. The neutralization titer of the serum was defined as the serum dilution that resulted in a 10-fold decrease in the viral titer (Vn/Vo = 0.1). For a given dilution of serum, a neutralization percentage could also be defined as follows: percent neutralization = 100 − (Vn/Vo × 100).
Competition assays.
In order to evaluate the participation of V3-specific antibodies in neutralization, the neutralization assays were adapted. For this purpose, V3 loop peptides were preincubated for 1 h at 37°C with the serum before the neutralization test was performed as described above. The competition assay combined serial concentrations of peptide (0.4 to 50 μM) and serial twofold dilutions of serum. Data obtained with the serum dilution whose reciprocal was twofold the neutralization titer were chosen for the presentation of the results. A competition was observed when the addition of peptide resulted in a decrease in the neutralizing capacity of the serum.
RESULTS
Neutralization of primary isolates and of the TCLA strain HIV-1MN.
Viruses from four early-stage HIV-1-infected individuals and the corresponding autologous sera were analyzed in this study. Neutralizing activities of these sera collected at different times after seroconversion have been determined and described previously (38). The sera, obtained about 1 1/2 years after seroconversion in the cases of patients Bx16, Bx17, and Bx26 and 3 years after seroconversion in the case of patient Bx08, vary in their efficiency of neutralization of the autologous virus isolated early (2 to 8 months) after seroconversion (Table 1). For example, serum Bx17(1.5 years) has a neutralization titer of 500, whereas a titer of only 10 was measured for serum Bx08(3 years). Heterologous neutralizing activities against several primary isolates have also been studied (38). Among the sera for which data are presented here, serum Bx26(1.5 years) was the only one to display a cross-neutralizing activity, given that 4 of the 17 primary isolates tested were neutralized. Nevertheless, the heterologous neutralization titers, ranging from 10 to 40 (data not shown), were lower than the autologous titer (140). Moreover, the four sera neutralized the TCLA strain HIV-1MN on the MT-4 cell line (Table 1). The relatively high neutralization observed [neutralization titers ranging from 150 for Bx16(1.5 years) to 870 for Bx26(1.5 years)] was consistent with other studies, as the neutralizing activity against the laboratory strain HIV-1MN was usually strong and easy to detect compared with the neutralization of primary viruses.
TABLE 1.
Neutralizing activities detected in sera from HIV-positive patients
| Serum | Time after seroconversion | Neutralization of:
|
||
|---|---|---|---|---|
| Primary isolates on PBMC
|
TCLA strain HIV-1MN/MT-4c | |||
| Autologousa | Heterologousb | |||
| Bx08 | 8 mo | <10 | 0/6 | NDd |
| 3 yr | 10e | 0/14 | 350 | |
| Bx16 | 7 mo | <10 | 0/6 | ND |
| 1.5 yr | 15 | 0/14 | 150 | |
| Bx17 | 5 mo | <10 | 0/6 | <20 |
| 1.5 yr | 500 | 0/16 | 220 | |
| Bx26 | 6 mo | <10 | 0/6 | 160 |
| 1.5 yr | 140 | 4/17 | 870 | |
Reciprocal serum dilution for which a 10-fold decrease in virus titer was measured. Values are means from at least two separate experiments.
Number of isolates neutralized/number tested.
Reciprocal serum dilution that allowed 50% inhibition of the virus-induced cytopathogenicity on MT-4 cells. Values are means from at least four independent experiments.
ND, not done.
Boldface indicates neutralizing activities.
Detection of V3 loop-specific antibodies.
Serial serum samples obtained at various times after seroconversion were analyzed for the presence of specific antibodies directed against the hypervariable V3 region of the envelope protein gp120. These V3 loop-specific antibodies were detected by ELISA, using peptides with sequences identical or close to those of the viruses used in the neutralization assays (Fig. 1). The peptides Bx08-1 and MN are homologous to the United States/European consensus V3 sequence and mimic the V3 loops of the primary isolates Bx08, Bx16, and Bx26, classified as subtype B viruses. In particular, the peptide Bx08-1 is identical to Bx08’s V3 loop. The peptide R21 contains several substitutions compared to the consensus sequence and is very close to the V3 loop of the primary isolate Bx17. In particular, it shares with this isolate the amino acids V(312), R(313), and Q(318) at the top (crown) of the V3 loop. Given their positions, these nonsynonymous substitutions have been described as playing an important role in determining the properties of the viruses, particularly their tropism (63), antibody binding (31), and sensitivity to neutralization (27). Consequently, peptide R21 can be considered quite different from peptides MN and Bx08-1.
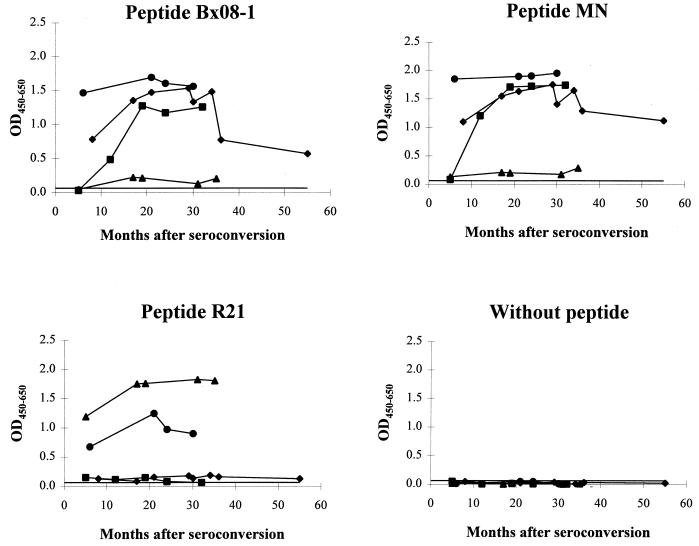
As represented in Fig. 2, antibodies of the sera Bx08, Bx16, and Bx26 bound to the related peptides Bx08-1 and MN. They were already detected at the first time point analyzed, or at 7 months postseroconversion for Bx16, and remained detectable throughout the period studied (until 3 years after seroconversion). Nevertheless, for patient Bx08, the antibody binding to V3 peptides decreased after 35 months. In contrast, in the serum from patient Bx17, we found a very low level of antibodies able to react with the heterologous peptides Bx08-1 and MN. When peptide R21 was used in the ELISA, binding of antibodies from patient Bx17 could be detected throughout the period of infection considered (from 5 months to 3 years after seroconversion). Only a very weak reactivity, close to the cutoff level, was detected in the sera from patients Bx08 and Bx16, but significant binding to this heterologous peptide was found in the serum from patient Bx26. Among those tested, serum Bx26(1.5 years) was also the only one to display a cross-reactive neutralizing activity (Table 1).
FIG. 2.
Temporal development of immunoglobulin reactivity to peptides representative of different V3 loops of gp120. V3-specific antibodies were detected by ELISA in sequential sera of seropositive individuals at various times after seroconversion. The OD450–650 values are the means from three separate experiments performed with the sera from patients Bx08 (⧫), Bx16 (■), Bx17 (▴), and Bx26 (•). Solid line, cutoff value.
This binding assay allowed the antibodies directed towards the homologous V3 peptide to be detected in all of the sera tested, except in serum Bx16 collected very early (5 months) after seroconversion. Accordingly, these results confirm the immunogenicity of the gp120 V3 domain and the capacity of the immune system to produce an early, relatively specific, and persistent antibody response to the V3 loop of the autologous primary isolate.
Competition experiments.
To examine a possible correlation between V3-specific antibodies and neutralization, we investigated the functional importance of these antibodies in the neutralization of primary isolates. For this purpose, we first performed competition experiments by adding 0.4 to 50 μM V3 loop peptides in the neutralization assays. In this way we examined the effects of the capture of V3 loop-specific antibodies on neutralization.
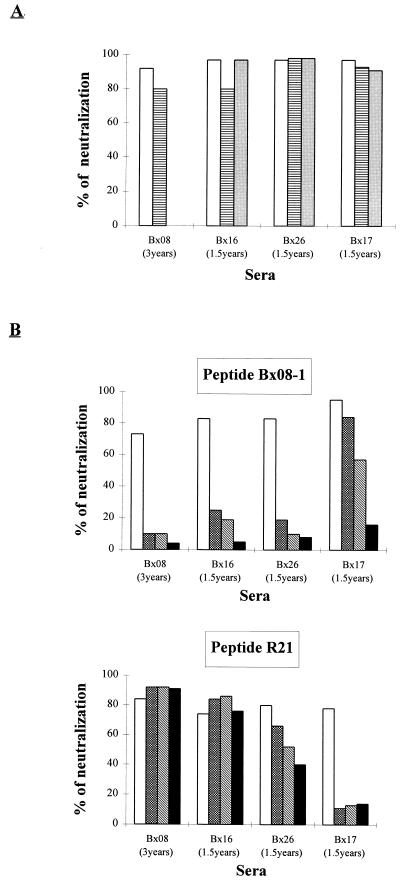
For all of the sera tested, the addition of up to 50 μM peptide had no effect on the neutralization of the autologous primary isolate (Fig. 3A). In other words, whatever the dilutions of serum tested, the percentages of neutralization were equivalent in the presence or absence of peptide, and this was true whether the peptide was homologous or heterologous (data not shown and Fig. 3A).
FIG. 3.
Effect of the addition of V3 loop peptides in
neutralization assays involving autologous primary isolates (A) or
the TCLA strain HIV-1MN/MT-4 (B). Peptide solutions at
concentrations ranging from 0.4 to 50 μM were preincubated with
serial dilutions of serum before the neutralization test was performed.
The reciprocal dilution of serum corresponding to twice the
neutralization titer of the serum was chosen for presenting the
results. Values representative of those from at least three separate
experiments with the MT-4 cell line and two separate experiments
with PBMC are shown. □, no peptide; ▤, 50
μM Bx08-1;
 , 50
μM R21;
, 2 μM peptide; ▧, 10 μM peptide;
■, 50 μM peptide.
, 50
μM R21;
, 2 μM peptide; ▧, 10 μM peptide;
■, 50 μM peptide.
As these results were not consistent with those of previously published competition studies with TCLA strains (64), we analyzed the addition of the same peptides in neutralization tests involving the TCLA strain HIV-1MN/MT-4. In these assays, a competitive inhibition of neutralization was obtained. Peptide Bx08-1, added at a concentration as low as 2 μM, inhibited the neutralization of HIV-1MN/MT-4 by the sera Bx08(3 years), Bx16(1.5 years), and Bx26(1.5 years) (Fig. 3B). Addition of a lower concentration of peptide (0.4 μM) did not result in a significant drop in neutralization (data not shown), and at the highest peptide concentration tested (50 μM), losses of protection of 87, 94, and 88% were measured for sera Bx08(3 years), Bx16(1.5 years), and Bx26(1.5 years), respectively. An inhibition of HIV-1MN/MT-4 neutralization was also observed in the presence of 2 μM peptide MN, resulting in a similar loss of protection (data not shown). However, it was only at the highest concentration (50 μM) of peptides Bx08-1 and MN that neutralization of HIV-1MN/MT-4 by serum Bx17(1.5 years) was significantly inhibited (Fig. 3B and data not shown).
The same competition experiments have been carried out with the peptide R21, which is quite different from peptides Bx08-1 and MN and has a sequence close to that of the V3 loop of the Bx17 isolate. The neutralization of HIV-1MN/MT-4 by serum Bx17(1.5 years) was inhibited by a peptide concentration of 50 to 2 μM (∼80% inhibition). Preincubation of this peptide with the Bx08(3 years) and Bx16(1.5 years) sera had no effect on the neutralization of HIV-1MN/MT-4. However, this heterologous peptide competitively inhibited the neutralizing activity of serum Bx26(1.5 years) on this TCLA strain. It is noteworthy that the serum from patient Bx26 already shows a binding activity to both homologous and heterologous peptides.
According to these results, V3 peptides able to fix and capture antibodies present in immune sera inhibit the neutralization of the TCLA strain HIV-1MN/MT-4, and this confirms the role of V3-specific antibodies in the neutralization of TCLA strains. In contrast, whether recognized or not by antibodies present in the sera, none of the peptides had an effect on the autologous neutralization of the four primary isolates tested. The involvement of antibodies specific for V3 linear epitopes in the neutralization of primary isolates could thus not be demonstrated.
Evaluation of the effect of experimental conditions on competition results.
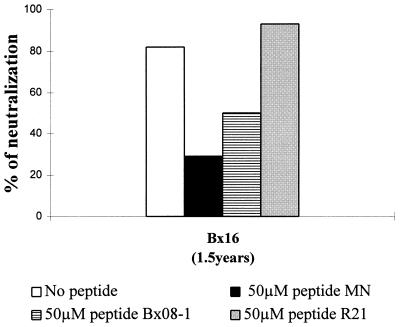
To make sure that the differential effect produced by the addition of V3 peptides in the neutralization of TCLA strains versus primary isolates did not result from experimental conditions, several experimental parameters were modified. In particular, for the TCLA virus, peptides and sera were always present in the test with the MT-4 cell line whereas they were removed by washing in the test involving primary isolates and PBMC. By including washing after adsorption of the virus in the former test, similar results were obtained (data not shown), demonstrating that the observed competitive effects on the neutralization of HIV-1MN/MT-4 were not due to the continual presence of peptide.
On the other hand, besides the virus (TCLA strain versus primary isolate), the type of cell used either to constitute the viral stock or as target in the neutralization assay represents an important parameter that may strongly influence the results of neutralization and competition (17). We could not grow these primary isolates on T-cell lines, but multiplication of the HIV-1MN strain on PBMC was obtained, and after a single passage on these primary cells, a new virus stock, HIV-1MN/PBMC, was constituted. Neutralization and competition experiments were then carried out with this virus, using exactly the same protocol as for primary isolates. Under these experimental conditions, only sera Bx16(1.5 years) and Bx26(1.5 years) neutralized the HIV-1MN/PBMC strain, though less efficiently, as neutralization titers were 10 and 40, respectively. The addition of 50 μM peptide MN and peptide Bx08-1 led to 60 and 50% inhibition in neutralization by serum Bx16(1.5 years), respectively, while peptide R21 did not diminish the neutralizing activity (Fig. 4). Although the percentage of inhibition was lower than that for HIV-1MN/MT-4, the neutralization of the HIV-1MN/PBMC strain was still, at least partially, inhibited by V3 loop peptides.
FIG. 4.
Competition experiments with the TCLA strain HIVMN/PBMC. HIVMN/MT-4 was passaged on PBMC and amplified on these primary cells before neutralization and competition experiments with exactly the same experimental conditions as for primary isolates. Results with the addition of 50 μM peptide are indicated. Values are representative of those from two separate experiments.
Overall, these data reveal a difference in the functional activity of the V3-specific antibodies according to the virus involved in the neutralization assay, i.e., whether it is a TCLA strain or a primary isolate.
Depletion experiments.
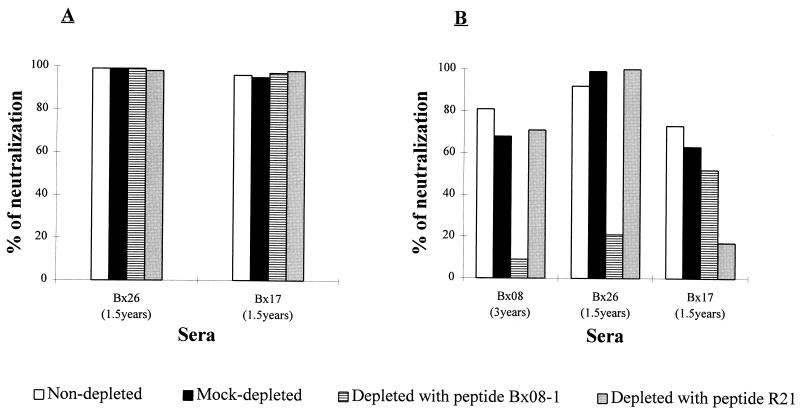
In order to further evaluate the role of V3-specific antibodies in neutralization, a depletion assay which allowed antibodies that bound to the V3 peptides to be removed from the sera was set up. This depletion method, based on successive passages of the sera on V3 peptide-coated plates, permitted over a 94% depletion in the V3-specific antibodies, which reach levels undetectable by ELISA (Table 2). Depleted sera were then tested in both neutralization assays for a residual neutralizing activity. Like for the competition experiments, results obtained with the serum dilution whose reciprocal corresponds to twice the neutralization titer of the undepleted sera are presented (Fig. 5B); similar effects were obtained for the other dilutions tested (data not shown). We observed that the removal of V3-specific antibodies from serum Bx08(3 years) with the homologous peptide Bx08-1 led to a drop in HIV-1MN/MT-4 neutralization. However, depletion experiments using the heterologous peptide R21 or no peptide did not show a notable reduction in the neutralizing activity. The depletion procedure was thus specific and did not adversely affect the antibody-neutralizing capacity. For serum Bx26(1.5 years), which had antibodies to both peptides, only the removal of antibodies directed towards the homologous peptide Bx08-1 resulted in an inhibition in HIV-1MN/MT-4 neutralization. A significant decrease in the neutralizing activity against HIV-1MN/MT-4 was detected in serum Bx17(1.5 years) depleted with the related peptide R21, with a slight decrease being also measured for this serum when it was depleted with the heterologous peptide Bx08-1, which corroborates the competitive effect of this peptide at 50 μM. In contrast, whatever the peptide used in the depletion protocol, the depleted sera retained their neutralizing activities against the autologous primary isolate (Fig. 5A).
TABLE 2.
Depletion of V3-specific antibodies from immune seraa
| Serum | % Depletionb with
peptide:
|
|
|---|---|---|
| Bx08-1 | R21 | |
| Bx083 years | 100 | —c |
| Bx261.5 years | 98 | 94 |
| Bx171.5 years | — | 96 |
Antibodies captured on V3 peptide-coated plates and thus removed from the sera were detected by ELISA. The depletion was monitored by the decrease in OD450–650 through the successive passages.
Calculated for the lowest dilution of serum tested (1/20) as 100 − [100 × (OD450–650 at last passage/OD450–650 at first passage)].
—, no binding to the V3 peptide was detected.
FIG. 5.
Consequences of the removal of V3-specific antibodies from immune sera. Neutralization was measured in sera not depleted, mock depleted, and depleted with the autologous and heterologous V3 peptides (peptide Bx08-1 and peptide R21). The capacities of the sera to neutralize the autologous primary isolate (A) and the TCLA strain HIV-1MN/MT-4 (B) were determined. Values are representative of those from two separate experiments.
Overall, these results confirm the previous observations made with competition experiments and strengthen the differential role of V3-specific antibodies in the neutralization of TCLA strains versus primary isolates.
DISCUSSION
The identification of epitopes implicated in the neutralization of HIV-1 primary isolates remains one of the fundamental goals of HIV vaccine development. Until a few years ago, the V3 loop of gp120 was considered the major target for NAb and was defined as the PND for laboratory TCLA strains. Since then, through analysis of the neutralization of viruses isolated directly from infected individuals, the role of the V3 loop as the PND has become controversial. On one hand, reports describing the neutralization of primary isolates by V3 monoclonal antibodies furthered the idea that the V3 region is the target for NAb (11, 46). On the other hand, large amounts of V3-specific antibodies detectable in human sera are not predictive of neutralization (41). Moreover, a conflicting argument is provided by results of cross-clade neutralization studies that indicate a lack of correlation between genetic subtypes and neutralization serotypes (28, 42, 71). Another source of controversy over the role of V3 antibodies in controlling HIV-1 is the fact that such antibodies can display neutralizing but also nonneutralizing and even enhancing activities (27).
In this paper, we have shown that antibodies present in immune sera of infected individuals bind to V3 loop peptides, and we have thereby confirmed the immunogenicity of the V3 region. The antibodies detected were highly specific to the V3 loop representative of the autologous primary isolates, except for the serum from patient Bx26. In this case, antibodies bound to a related peptide (Bx08-1) as well as to a much more divergent peptide (R21). It should be noted that the serum from this patient was also the one that displayed cross-neutralizing activity. However, V3-specific antibodies were detected very early after seroconversion, i.e., before the detection of autologous and heterologous neutralizing activities (Table 1) (38). There is thus a lack of correlation between the presence of V3 antibodies and NAb at early stages of infection. This was also described previously (41) and raises the question as to whether V3-specific antibodies are involved in the neutralization of primary isolates.
In competition experiments, the addition of a related peptide (able to bind V3-specific antibodies) resulted in a loss of the capacity of the sera to neutralize the TCLA strain HIV-1MN/MT-4. Conversely, although they bound antibodies, the same peptides did not competitively inhibit the neutralization of the autologous primary isolate. This dual effect of the V3 peptides suggested that the V3-specific antibodies play different roles in the neutralization of TCLA strains versus primary isolates. To confirm this, we removed antibodies that bind to V3 linear epitopes from the sera and measured the residual neutralizing activities after depletion. Similar conclusions could be drawn. Indeed, depletion with a homologous peptide led to a loss in neutralizing activity against the TCLA strain HIV-1MN/MT-4, while depleted sera neutralized their autologous primary isolate with exactly the same efficiency as undepleted sera. It is noteworthy that for serum Bx26(1.5 years), depletion with the heterologous peptide R21 did not modify neutralization of the TCLA strain HIV-1MN/MT-4, while a 50% drop in neutralizing activity was measured when 50 μM peptide was added in competition experiments. Thus, slight differences were observed in competition and depletion experiments, but the conclusions remained similar.
As neutralization results are highly dependent on the assays used (13, 17), we verified that the different capacities of V3-specific antibodies for neutralizing TCLA strains versus primary isolates were not the consequences of experimental parameters. Using exactly the same experimental conditions as for primary isolates, we studied the neutralization of the HIV-1MN strain grown on PBMC. This neutralization was inhibited by the addition of a V3 peptide recognized by the immune serum. However, the percentage of inhibition was lower than that of HIV-1MN/MT-4, suggesting that after the passage on PBMC, HIV-1MN had acquired certain characteristics of field isolates.
Overall, these data confirm previous results implicating V3-specific antibodies in the neutralization of TCLA strains such as HIV-1MN. However, this does not apply for primary isolates, as antibodies that bind to linear epitopes of the V3 loop do not appear to be involved in the neutralization of primary isolates. We therefore corroborate and extend two studies (3, 70) in which a similar differential role of V3-specific antibodies has been pointed out. In the first study, by depleting V3-specific antibodies, Vancott et al. (70) showed that the contribution of these antibodies in the neutralization of primary isolates is less pronounced than that in the neutralization of a TCLA strain. However, the 20% loss of total antibodies caused by their depletion procedure could account for part of the inhibitory effect observed. Moreover, whereas they studied only heterologous neutralization, we took into account the autologous neutralization of primary viruses. In the second study, Beddows et al. (3) carried out competition experiments and obtained similar results, i.e., that a V3 peptide was able to inhibit the neutralization of a TCLA strain but not that of field isolates. However, the 35-amino-acid MN V3 peptide used contained as many as nine substitutions compared to the V3 loop of the primary isolates studied, and this may limit interpretation of the inhibition data, as pointed out by the authors. By using several 21-amino-acid V3 peptides, we have shown that a sequence-specific competition can indeed be observed in three of four sera tested. Taken together, these complementary studies lead to the same conclusions and suggest a predominant role of antibodies with specificities outside the V3 loop and/or a contribution of antibodies directed towards complex epitopes, including conformational V3 determinants not mimicked by V3 peptides.
A lower accessibility of the V3 epitopes on the oligomeric form of gp120 at the surface of the primary virions could hinder the recognition and the function of NAb (4, 26). Quantitative and qualitative differences have indeed been proposed to explain the relative sensitivity to neutralization of TCLA strains, compared to the apparent resistance of primary isolates. Along with others, we have shown that a larger amount of gp120 could be detected at the surface of primary isolates than at that of TCLA strains (39, 48). This higher density of glycoproteins could hamper the interactions between the antibody and the virus. However, Karlsson et al. reported that neither the high envelope spike density nor its stability could explain the relative neutralization resistance of primary viruses (25). Recently, several authors ruled out the possibility that this difference in neutralization sensitivity between TCLA and primary viruses could be attributable to coreceptor use (30, 37, 67). Nevertheless, Trkola et al. (67) suggest that antibodies to the V3 loop could interfere more effectively with CXCR4 interaction of HIV-1 than with CCR5 interaction. V3-specific antibodies may therefore be better able to neutralize TCLA strains that use CXCR4, which could account for the neutralizing activity associated with V3-specific antibodies in our study.
In addition, the affinity of the antibody for its epitope may be an important parameter that could affect the capacity of the antibody to neutralize the virus. Nevertheless, V3-specific antibodies of high affinity had more chance of being removed during the depletion procedure than low-affinity antibodies, and this depletion had no influence on the autologous neutralizing activity. This would therefore tend to exclude the implication of V3 linear epitopes in the neutralization of primary isolates. An alternative explanation of the absence of neutralizing capacity associated with V3-specific antibodies was provided by Schreiber et al., who showed that these antibodies were directed towards noninfectious virions but not towards cell-free infectious viruses (59, 60). On the other hand, those authors recently suggested that conformational rather than linear V3 epitopes could be preferentially involved in the neutralization of primary isolates (61). They described conserved V3 discontinuous epitopes formed by the GPGRAF motif and its adjacent amino and carboxy sides as targets for highly specific antibodies detectable in infected individuals. In contrast, as suggested by Garrity et al. (16), the V3 region may have hypervariable, immunodominant epitopes that serve to misdirect or dysregulate the ability of the immune system to focus on more protective targets. By masking the V3 region on a recombinant gp120 used to immunized guinea pigs, they shifted the dominant antibody response away from V3 to neutralizing epitopes in the V1 variable domain of gp120. However, these experiments suffer from the facts that the recombinant gp120 was derived from the TCLA strain HXB2 and that NAb have been characterized against TCLA viruses only. They should accordingly be performed with primary isolates.
Various other epitopes with different specificities could also be implicated in the neutralization of primary isolates. Among them are linear epitopes, such as the conserved domain ELDKWA of gp41 recognized by the neutralizing monoclonal antibody 2F5 (47, 68). Additional epitopes in the V1 and V2 regions of gp120 have also been identified as targets of antibodies that neutralize either TCLA strains or primary isolates (72). In particular, a surprising V2-specific antibody that neutralizes primary isolates but not TCLA strains has been described (18). Conformational epitopes encompassing the CD4 binding site and other sites created by amino acids spaced over several regions of gp120 have also been proposed and appear to be crucial (43, 65). Indeed, one of the most efficient monoclonal antibodies described up to now, IgG1b12, recognizes the conformational CD4 binding site and shows a strong cross-clade neutralizing activity (5, 68). Furthermore, antibodies directed towards discontinuous sites appear to be prevalent in the immune sera of naturally infected individuals (43), while such antibodies are lacking in sera of immunized individuals and animals (33, 69). This may explain the inability of sera from vaccinated individuals to neutralize primary isolates even though they neutralize TCLA strains efficiently (9). A qualitative rather than a quantitative defect in the antibodies seems therefore to be implicated, as high quantities of antibodies of different specificities are present, but these are not NAb.
Identifying the antibodies found in infected patients, and especially in long-term nonprogressors, would help to elaborate vaccinal antigens with neutralization epitopes able to induce efficient NAb. Such antigens should display the specificities of a wide range of primary isolates in order to generate a strong cross-reactive antibody response.
ACKNOWLEDGMENTS
We are indebted to Sandrine Haessig and Virginie Roques for technical assistance.
This work is supported by a grant from Synthélabo and has been carried out under the project Action coordonnée 1 of l’Agence Nationale de Recherches sur le SIDA.
REFERENCES
- 1.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 2.Bates P. Chemokine receptors and HIV-1: an attractive pair? Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 3.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easterbrook P J, Simmonds P, Weber J. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol. 1998;79:77–82. doi: 10.1099/0022-1317-79-1-77. [DOI] [PubMed] [Google Scholar]
- 4.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 7.Chaix M-L, Chappey C, Couillin I, Rozenbaum W, Levy J-P, Saragosti S. Diversity of the V3 region of HIV in Paris, France. AIDS. 1993;7:1199–1204. doi: 10.1097/00002030-199309000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Jitters jeopardize AIDS vaccine trials. Science. 1993;262:6574–6578. doi: 10.1126/science.8235635. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J. Likely HIV cofactor found. Science. 1996;272:809–810. doi: 10.1126/science.272.5263.809. [DOI] [PubMed] [Google Scholar]
- 11.Conley A J, Gorny M K, Kessler J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberg D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doms R, Peipert S. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza M P, Mathieson B J. Early phases of HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:1–9. doi: 10.1089/aid.1996.12.1. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza M P, Milman G, Bradac J A, McPhee D, Hanson C V, Hendry R M collaborating investigators. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS. 1995;9:867–874. doi: 10.1097/00002030-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Emini E A, Scleif W A, Numberg J H, Conley A J, Eda Y, Tokiyoshi S, Putney S D, Matsushita S, Cobb K E, Jett C M, Eichberg J W, Murthy K K. Prevention of HIV-1 infection in chimpanzees by gp120 V3 monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 16.Garrity R R, Rimmelzwaan G, Minassian A, Tsai W-P, Lin G, De Jong J J, Goudsmit J, Nara P L. Refocussing neutralizing antibody response by targeted dampening of an immunodominant epitope. J Immunol. 1997;159:279–289. [PubMed] [Google Scholar]
- 17.Golding H, D’Souza P, Bradac J, Mathieson B, Fast P. Neutralization of HIV-1. AIDS Res Hum Retroviruses. 1994;10:633–643. doi: 10.1089/aid.1994.10.633. [DOI] [PubMed] [Google Scholar]
- 18.Gorny M K, Moore J P, Conley A J, Karwowska S, Sodroski J, Williams C, Burda S, Boots L J, Zolla-Pazner S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of human immunodeficiency virus type 1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrer T, Harrer E, Kalams S A, Elbeik T, Staprans S I, Feinberg M B, Cao Y, Ho D D, Yilma T, Caliendo A M, Johnson R P, Buchbinder S P, Walker B D. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable non progressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–592. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 20.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 21.Hwang S S, Boyle T J, Lyverly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 22.Ivanoff L A, Dubay J W, Morris J F, Roberts S J, Gutshall L, Sternberg E J, Hunter E, Matthews T J, Petteway S R. V3 loop region of the HIV-1 gp120 envelope protein is essential for virus infectivity. Virology. 1992;187:423–432. doi: 10.1016/0042-6822(92)90444-t. [DOI] [PubMed] [Google Scholar]
- 23.Jahaverian K, Langlois A J, La Rossa G J, Profy A T, Bolognesi D P, Herlihy W C, Putney S D, Matthews T J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990;250:1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- 24.Jahaverian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson G B, Gao F, Robinson J, Hahn B, Sodroski J. Increased envelope spike density and stability are not required for the neutralization resistance of primary human immunodeficiency viruses. J Virol. 1996;70:6136–6142. doi: 10.1128/jvi.70.9.6136-6142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klasse P J, Moore J P. Quantitative model of antibody- and soluble CD4-mediated neutralization of primary isolates and T-cell line-adapted strains of human immunodeficiency virus type 1. J Virol. 1996;70:3668–3677. doi: 10.1128/jvi.70.6.3668-3677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliks S C, Shioda T, Haigwood N L, Levy J A. V3 variability can influence the ability of an antibody to neutralize or enhance infection by diverse strains of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:11518–11522. doi: 10.1073/pnas.90.24.11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korstrikis L G, Cao Y, Ngai H, Moore J P, Ho D D. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol. 1996;70:445–458. doi: 10.1128/jvi.70.1.445-458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Numberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langedijk J P M, Zwart G, Goudsmitt J, Meloen R H. Fine specificity of antibody recognition may predict substitution in the third variable region of gp120 during HIV type 1 infection. AIDS Res Hum Retroviruses. 1995;10:1153–1162. doi: 10.1089/aid.1995.11.1153. [DOI] [PubMed] [Google Scholar]
- 32.Mackewicz C, Levy J A. CD8+cell anti-HIV activity: noncytolytic suppression of virus replication. AIDS Res Hum Retroviruses. 1992;6:1039–1050. doi: 10.1089/aid.1992.8.1039. [DOI] [PubMed] [Google Scholar]
- 33.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCuthan F E, Burke D S for the National Institute of Allergy and Infectious Diseases AIDS Evaluation Group. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 34.Matthews T J. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses. 1994;6:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoli S, Trabattoni D, Lo Caputo S, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 36.Merrifield R B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;•••:2149. [Google Scholar]
- 37.Montefiori D C, Collman R G, Fouts T R, Ying Zhou J, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A-M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moog C, Spenlehauer C, Fleury H J A, Heshmati F, Saragosti S, Letourneur F, Kirn A, Aubertin A-M. Neutralization of primary human immunodeficiency virus type 1 isolates: a study of parameters implicated in neutralization in vitro. AIDS Res Hum Retroviruses. 1997;13:19–27. doi: 10.1089/aid.1997.13.19. [DOI] [PubMed] [Google Scholar]
- 40.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore J P, Cao Y, Leu J, Quin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;68:1342–1349. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore J P, Ho D D. Antibodies to discontinuous or conformational sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore J P, Ho D D. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T-cells. AIDS. 1995;9:S117–S136. [PubMed] [Google Scholar]
- 45.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore J P, Trkola A, Korber B, Boots L J, Kessler J A, McCutchan F E, Mascola J, Ho D D, Robinson J, Conley A J. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J Virol. 1995;69:122–130. doi: 10.1128/jvi.69.1.122-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Rüker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien W A, Mao S-H, Cao Y, Moore J P. Macrophage-tropic and T-cell line-adapted chimeric strains of human immunodeficiency virus type 1 differ in their susceptibilities to neutralization by soluble CD4 at different temperatures. J Virol. 1994;68:5264–5269. doi: 10.1128/jvi.68.8.5264-5269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the β-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 50.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herderwijn P, Desmyter J, De Clerck E. Rapid and automated tetrazolium-based colorometric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 51.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J-L, Ragnaud J-M, Bernard N, Fleury H J A. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquired Immune Defic Syndr. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 52.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long term non progressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 53.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1 long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowland-Jones S, Sutton J, Ariyoshi K, Gotch F, Whitby D, Sabally A, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 55.Rusche J R, Jahaverian K, McDanal C, Petro J, Lynn D L, Grimaila R, Langlois A, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scarlatti G, Albert J, Rossi P, Hodara V, Biraghi P, Muggiasca L, Fenyö E M. Mother-to-child transmission of human immunodeficiency virus type 1: correlation with neutralizing antibodies against primary isolates. J Infect Dis. 1993;168:207–210. doi: 10.1093/infdis/168.1.207. [DOI] [PubMed] [Google Scholar]
- 57.Scarlatti G, Leitner T, Hodara V, Jansson M, Karlsson A, Wahlberg J, Rossi P, Uhlén M, Fenyö E M, Albert J. Interplay of HIV-1 phenotype and neutralizing antibody response in pathogenesis of AIDS. Immunol Lett. 1996;51:23–28. doi: 10.1016/0165-2478(96)02550-3. [DOI] [PubMed] [Google Scholar]
- 58.Schonning K, Nielsen C, Iversen J, Nielsen J O, Hansen J-E S. Neutralizing antibodies in slowly progressing HIV-1 infection. J Acquired Immune Defic Syndr. 1995;10:400–407. doi: 10.1097/00042560-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Schreiber M, Petersen H, Wachsmuth C, Müller H, Hufert F, Schmitz H. Antibodies of symptomatic human immunodeficiency virus type 1-infected individuals are directed to the V3 domain of noninfectious and not of infectious virions present in autologous serum. J Virol. 1994;68:3908–3916. doi: 10.1128/jvi.68.6.3908-3916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiber M, Wachsmuth C, Müller H, Hagen C, Schmitz H, Van Lunzen J. Loss of antibody reactivity directed against the V3 domain of certain human immunodeficiency virus type 1 variants during disease progression. J Gen Virol. 1996;77:2403–2414. doi: 10.1099/0022-1317-77-10-2403. [DOI] [PubMed] [Google Scholar]
- 61.Schreiber M, Wachsmuth C, Müller H, Odemuyiwa S, Schmitz H, Meyer S, Meyer B, Schneider-Mergener J. The V3-directed immune response in natural human immunodeficiency virus type 1 infection is predominantly directed against a variable, discontinuous epitope presented by the gp120 V3 domain. J Virol. 1997;71:9198–9205. doi: 10.1128/jvi.71.12.9198-9205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shan-Lu L, Schacker T, Musey L, Shriner D, McElrath J M, Corey L, Mullins J I. Divergent patterns of progression to AIDS after infection from the same source: human immunodeficiency virus type 1 evolution and antiviral responses. J Virol. 1997;71:4284–4295. doi: 10.1128/jvi.71.6.4284-4295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shioda T, Levy J A, Cheng-Meyer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1992;89:9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spear G T, Takefman D M, Sharpe S, Ghassemi M, Zolla-Pazner S. Antibodies to the HIV-1 V3 loop in serum from infected persons contribute a major proportion of immune effector functions including complement activation, antibody binding, and neutralization. Virology. 1994;204:609–615. doi: 10.1006/viro.1994.1575. [DOI] [PubMed] [Google Scholar]
- 65.Steimer K S, Scandella C J, Skiles P V, Haigwood N L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to gp120. Science. 1991;254:105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- 66.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Meyer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 67.Trkola A, Ketas T, Kewalramani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-immunoglobulin G. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vancott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoproteins by candidate AIDS vaccines. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 70.Vancott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retroviruses. 1995;11:1379–1391. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 71.Weber J, Fenyö E-M, Beddows S, Kaleebu P, Björndal A the WHO Network for HIV Isolation and Characterization. Neutralization serotypes of human immunodeficiency virus type 1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wyatt R, Moore J P, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zwart G, Back N K T, Ramautarsing C, Valk M, Van der Hoek L, Goudsmit J. Frequent and early HIV-1MNneutralizing capacity in sera from Dutch HIV-1 seroconverters is related to antibody reactivity to peptides from the gp120 V3 domain. AIDS Res Hum Retroviruses. 1994;3:245–251. doi: 10.1089/aid.1994.10.245. [DOI] [PubMed] [Google Scholar]
- 74.Zwart G, Langedijk H, Van der Hoek L, De Jong J-J, Wolfs T F W, Ramautarsing C, Bakker M, De Ronde A, Goudsmit J. Immunodominance and antigenic variation of the principal neutralizing domain of HIV-1. Virology. 1991;181:481–489. doi: 10.1016/0042-6822(91)90880-k. [DOI] [PubMed] [Google Scholar]