Abstract
This study aimed to assess the clinical utility of blood lactate-to-bicarbonate (L/B) ratio, as a prognostic factor for 28-day in-hospital mortality in children with dengue shock syndrome (DSS), admitted to the pediatric intensive care unit (PICU). This single-center retrospective study was conducted at a tertiary children hospital in southern Vietnam from 2013 to mid-2022. Prognostic models for DSS mortality were developed, using a predefined set of covariates in the first 24 hours of PICU admission. Area under the curves (AUCs), multivariable logistic and Least Absolute Shrinkage and Selection Operator (LASSO) regressions, bootstrapping and calibration slope were performed. A total of 492 children with DSS and complete clinical and biomarker data were included in the analysis, and 26 (5.3%) patients died. The predictive values for DSS mortality, regarding lactate showing AUC 0.876 (95% CI, 0.807–0.944), and that of L/B ratio 0.867 (95% CI, 0.80–0.934) (P values of both biomarkers < .001). The optimal cutoff point of the L/B ratio was 0.25, while that of lactate was 4.2 mmol/L. The multivariable model showed significant clinical predictors of DSS fatality including severe bleeding, cumulative amount of fluid infused and vasoactive-inotropic score (>30) in the first 24 hours of PICU admission. Combined with the identified clinical predictors, the L/B ratio yielded higher prognostic values (odds ratio [OR] = 8.66, 95% confidence interval [CI], 1.96–38.3; P < .01) than the lactate-based model (OR = 1.35, 95% CI, 1.15–1.58; P < .001). Both the L/B and lactate models showed similarly good performances. Considering that the L/B ratio has a better prognostic value than the lactate model, it may be considered a potential prognostic biomarker in clinical use for predicting 28-day mortality in PICU-admitted children with DSS.
Keywords: dengue shock syndrome, lactate, lactate/bicarbonate ratio, mortality, Vietnam
Key Points:
Dengue shock syndrome (DSS) accounts for a fatality rate of 5.3% among children admitted to the PICU.
Blood lactate and the lactate-to-bicarbonate (L/B) ratio had high predictive values for DSS mortality, with AUCs of 0.874 (95% CI, 0.786–0.961) and 0.867 (95% CI, 0.80–0.934), respectively (P values < .001). The optimal cutoff point of the L/B ratio was 0.25, while that of lactate was 4.2 mmol/L.
The L/B ratio has a better prognostic value than the lactate model; therefore, the L/B ratio may be considered a potential prognostic biomarker in clinical use for predicting in-hospital mortality in PICU-admitted children with DSS.
1. Introduction
Dengue hemorrhagic fever has emerged as a consequential infectious malady, imposing substantial challenges on disease morbidity and mortality in tropical and subtropical regions, and it is a leading cause of mortality among children in both Asia and Latin America.[1,2] From 1990 to 2013, dengue infection was associated with approximately 9221 annual deaths globally, accompanied by an additional 1.14 million disability-adjusted life-years in 2013.[2] Severe dengue has a reported mortality rate of approximately 20%; however, early diagnosis and timely treatment have the potential to curtail this figure to <1%.[2] Complications associated with severe dengue, including prolonged dengue shock syndrome (DSS), severe bleeding, substantial plasma leakage, respiratory failure, and acute liver failure, predominantly contribute to the fatalities observed in hospitalized dengue children.[3–8]
Lactic acidosis is associated with tissue hypoxia and is commonly observed in patients with DSS. The metabolic functions of the liver can be greatly affected by dengue-induced hepatic transaminases, which can manifest as increased serum lactate and decreased bicarbonate levels.[9,10] Several studies have shown that increased serum lactate and decreased bicarbonate levels are associated with dengue-related fatalities.[6,9,11] Likewise, other studies have highlighted the lactate-to-albumin ratio as a crucial predictor in critically ill patients, particularly those with sepsis and septic shock.[12–17]
Currently, there is a lack of robust data on the prognosis of pediatric cohorts with severe dengue.[4,6] The identification of prognostic factors for the risk of death in pediatric patients with DSS upon admission to the pediatric intensive care unit (PICU) is of paramount significance for classification and prioritized interventions, which can potentially improve clinical and survival outcomes. In this respect, we aimed to identify better prognostic clinical and laboratory predictors for early recognition of the high risk of death in children with DSS admitted to the PICU, in order to provide timely and appropriate intervention strategies to reduce the dengue fatality rate. Based on the disease pathogenesis, clinical knowledge and medical literature, we hypothesized that elevated blood lactate levels in parallel with reduced bicarbonate levels, in terms of the blood lactate-to-bicarbonate (L/B) ratio, could generate a higher predictive value for mortality in DSS patients than either serum lactate or bicarbonate alone.[8–11] Therefore, this study aimed to investigate the prognostic value of serum L/B ratio and compare it with blood lactate level when combined with clinical factors in predicting 28-day in-hospital mortality in children with DSS admitted to the PICU.
2. Methods
2.1. Study design, setting and participants
This retrospective, single-center study was carried out at Children Hospital No.2 in Ho Chi Minh City, Vietnam, a distinguished tertiary referral pediatric institution in southern Vietnam with a capacity of approximately 1400 in-hospital beds. We screened all critically ill children with confirmed dengue infection who were admitted to the PICU between January 2013 and mid-2022. Eligibility criteria were defined as age below 18 years, laboratory-confirmed dengue infection, and presence of DSS.[8] Exclusion criteria were lack of serologically confirmed dengue infection and incomplete blood lactate and bicarbonate data.
2.2. Study outcome and candidate predictors
The primary study outcome was 28-day in-hospital dengue-associated mortality rate. A set of predefined covariates in the first 24 hours of PICU admission, included age, sex, dengue severity, respiratory rates, severe bleeding and liver transaminases, platelet cell count and hematocrit level, serum creatinine level, cumulative amount of infused fluid during the first 24 hours of PICU admission, vasoactive-inotropic score and biomarkers of interest (blood lactate level and L/B ratio).
2.3. Study definitions
Dengue infection was delineated in accordance with the World Health Organization (WHO) criteria from 2009, wherein confirmation was established through laboratory testing using the Dengue-IgM antibody or nonstructural 1 (NS1) antigen test.[8] The diagnosis of DSS adhered to the guidelines outlined by the WHO for dengue in 2009.[8]
2.4. Data measurements
Dengue-associated acute liver failure (PALF) was diagnosed according to the 2017 EASL Clinical Practical Guidelines.[18] The vasoactive-inotropic score (VIS) was used to assess hemodynamic support.[19] Serum lactate levels were measured using a machine-Alinity® ci-series (Abbott), and the normal range of serum lactate: 1.8–2.7 mmol/L. Additionally, serum bicarbonate was analyzed by arterial blood gas analysis using the fully automated system-RapidLab®348EX (Siemens Healthcare Diagnostics Inc., UK) with a normal bicarbonate range: 22 to 26 mEq/L.
2.5. Data collection
To uphold adherence to Good Clinical Practice, all patient data were de-identified. Case report forms were used to gather clinical and laboratory data from medical records at the time of PICU admission and 24 hours postadmission. Subsequently, these data were meticulously entered into an electronic database to facilitate comprehensive statistical analysis. The clinical outcomes of patients were scrutinized throughout their tenure in the PICU and at discharge.
2.6. Ethics statement
The authors affirm the absence of ethical issues to disclose regarding this study. Approval for the study was granted by the Institutional Review Board of Children Hospital No.2, Ho Chi Minh City, Vietnam, with the designated approval number 391, signed on 24-03-2022. The study was conducted in strict adherence to the principles of Good Clinical Practice and in accordance with the ethical guidelines of the Declaration of Helsinki.
2.7. Statistical analysis
Continuous variables were described using the median and interquartile range (IQRs), while categorical variables were presented as numerical counts (n) and corresponding percentages (%). Missing data on serum lactate and bicarbonate levels from the retrospective data collection were considered the main study bias. Only participants with complete biomarker data were included in this analysis. The receiver operating characteristic (ROC) curve with area under the curve (AUC) values, sensitivity, specificity, accuracy and Youden index were assessed for each biomarker (serum lactate, bicarbonate, and L/B ratio) in predicting 28-day in-hospital mortality. The Youden index was used to determine the optimal cutoff points for biomarkers.[20] We selected biomarkers with high AUCs combined with clinical and laboratory data to construct predictive models for DSS-associated deaths. We performed univariate and multivariable logistic regressions with complete-case analyses, based on predefined covariates, which were chosen based on disease pathogenesis and clinical experience. The covariate of the cumulative amount of fluid from referral hospitals and within 24 hours of PICU admission was standardized by the square root for regression analysis. We performed backward stepwise model selection based on the Akaike Information Criteria (AIC) for all predetermined covariates. Interactions among the covariates were also examined. The weighted combination of Least Absolute Shrinkage and Selection Operator (LASSO) and ridge regression was used to select clinical predictors of importance. The internal validation of each model was performed using bootstrap resampling (n = 500). The lactate and L/B model performances were assessed using the C-statistic, calibration slope, and Brier score.[21] The Brier score ranges from 0 to 1, with a lower score indicating superior performance. Statistical significance was set at P values < .05 for all comparisons. The R statistical software (version 4.3.1, Boston, MA) was used for all analyses.
3. Results
3.1. Baseline characteristics of patients’ cohort on PICU admission
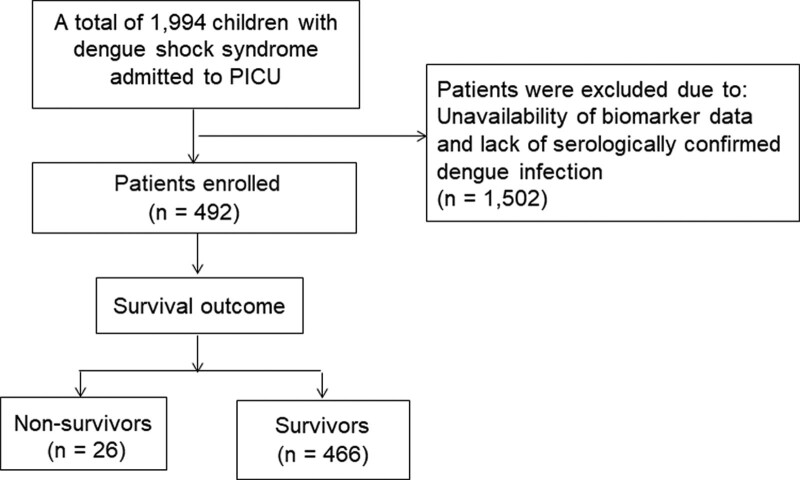
We identified 1994 children with DSS admitted to the PICU over the study period, of whom 492 patients with available clinical and biomarker data of interest, retrieved from hospital paper-based medical records were enrolled and analyzed (as shown in Fig. 1). Clinical and laboratory data of the study participants on admission are presented in Table 1. The median patient age was 7.6 (interquartile range, IQR: 5–10) years, and females accounted for approximately 51.6% of the study participants. The median body mass index was 18.4 (IQR, 15.8–21.5) kg/m2. Forty-eight participants (9.8%) had underlying diseases. Notably, 428 (87%) children were diagnosed with compensated DSS and the remaining 64 (13%) patients had decompensated DSS. Severe bleeding was observed in 67 (13.6%) patients. On PICU admission, the median respiratory rate was 27 breaths/min (IQR, 24–30) breaths per minute. The median systolic shock index was 1.33 (IQR, 1.12–1.56) bpm/mm Hg and diastolic shock index was 1.75 (IQR, 1.47–2.15) bpm/mm Hg. The median high vasoactive-inotropic score (VIS) was 20 (IQR, 0–30). Full blood counts revealed a marked increase in hematocrit and decrease in platelet cell count. Severe hepatic transaminases (aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥ 1000 IU/L) were observed in 85 (17.3%) DSS patients. The median serum creatinine level was 51 (IQR, 43–60) µmol/L. A total of 119 (24.2%) children with DSS experienced severe respiratory failure and were indicated for mechanical ventilation (MV) support; 43 (8.7%) DSS-experiencing children developed dengue-associated PALF. The median length of PICU stay was 3 (IQR, 2–4) days, and that of a hospital stay was 5 (IQR, 3–11) days.
Figure 1.
The study flow-chart.
Table 1.
Clinical and laboratory characteristics of study participants in the first 24h of PICU admission and associated outcomes at discharge (N = 492).
| Characteristics | Summary statistics |
|---|---|
| On PICU admission | |
| Age (yr) | 7.6 (5–10) |
| Female sex, n (%) | 254 (51.6) |
| Body mass index (kg/m2) | 18.4 (15.8–21.5) |
| Underlying diseases, yes | 48 (9.8) |
| Day of occurrence of dengue shock since onset of fever (d) |
5 (4–5) |
| Grading of dengue severity Compensated DSS, n (%) Decompensated DSS, n (%) |
428 (87) 64 (13) |
| Severe bleeding, n (%) | 67 (13.6) |
| Respiratory rate (breaths/min) | 27 (24–30) |
| Systolic shock index (bpm/mm Hg) | 1.33 (1.12–1.56) |
| Diastolic shock index (bpm/mm Hg) | 1.75 (1.47–2.15) |
| Vasoactive-inotropic score | 20 (0–30) |
| White blood cell count (×109/L) | 5.46 (3.76–8.0) |
| Hemoglobin (g/dL) | 14.5 (12.9–15.7) |
| Peak hematocrit, n (%) | 48 (44–51) |
| Nadir hematocrit, n (%) | 37 (34–40) |
| Platelet cell count (×109/L) | 33 (20–52) |
| AST (IU/L) | 191 (102–564) |
| ALT (IU/L) | 87 (40–263) |
| Severe hepatic transaminases, n (%) | 85 (17.3) |
| International normalized ratio | 1.29 (1.13–1.63) |
| Serum creatinine (µmol/L) | 51 (43–60) |
| Troponin I (ng/mL) | 0.04 (0.01–0.14) |
| Serum lactate (mmol/L) | 2.3 (1.6–3.4) |
| Arterial blood gas analysis | |
| pH | 7.45 (7.41–7.48) |
| PCO2 (mm Hg) | 26.2 (22.2–30) |
| PO2 (mm Hg) | 123 (97–159) |
| Fraction of inspired oxygen (FiO2, %) | 32 (28–40) |
| Bicarbonate (mEq/L) | 17.6 (15–20.3) |
| At 24h after PICU admission | |
| Mechanical ventilation requirement, n (%) | 119 (24.2) |
| Dengue-associated PALF, n (%) | 43 (8.7) |
| Outcomes of patients at discharge | |
| Length of PICU stay (d) | 3 (2–4) |
| Length of hospital stay (d) | 5 (3–11) |
| Fatal outcome, n (%) | 26 (5.3) |
Summary statistics are presented as median (interquartile range, IQR) for continuous variables and frequency (%) for categorical variables.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; DSS = dengue shock syndrome; PALF = dengue-associated acute liver failure; PICU = pediatric intensive care unit.
3.2. In-hospital mortality rate of PICU-admitted children with DSS
Overall, 26/492 (5.3%) DSS patients died during the PICU stay. The main causes of death among the studied patients were PALF, multiple organ failure, acute respiratory distress syndrome (ARDS), pulmonary and cerebral hemorrhage, and severe PICU-associated infections (ventilator-associated pneumonia and sepsis).
3.3. Predicting mortality outcome by biomarkers, including serum lactate, bicarbonate and lactate/bicarbonate (L/B) ratio during the first 24 hours of PICU admission
On PICU admission, the median serum lactate was 2.3 (IQR, 1.6–3.4) mmol/L, and that of bicarbonate was 17.6 (IQR, 15–20.3) mEq/L (Table 1). The performance of biomarkers in predicting the deaths of DSS-experiencing children with cutoff points is presented in Table 2. Serum lactate levels were predictive of DSS mortality, with an AUC of 0.876 (95% CI, 0.807–0.944, P < .001), and the AUC of the L/B ratio was closely equivalent to lactate, with values of 0.867 (95% CI, 0.80–0.934, P < .001). However, serum bicarbonate had an AUC of 0.672 (95% CI, 0.567–0.777, P < .001), which was markedly inferior to the blood lactate and L/B values. Further analyses showed the optimal cutoff points for serum lactate and L/B ratio were 4.2 (mmol/L) and 0.25, corresponding to Youden indices of 0.60 and 0.56, respectively.
Table 2.
Area under the receiver operating characteristic curve (AUC) for blood lactate, bicarbonate and lactate/bicarbonate (L/B) ratio and cutoffs for 28-d mortality
| Parameters | AUC of 28-d mortality (95% confidence interval) |
P value | ||
|---|---|---|---|---|
| Lactate (mmol/L) | 0.876 | 0.807–0.944 | <.001 | |
| Bicarbonate (mEq/L) | 0.672 | 0.567–0.777 | <.01 | |
| L/B ratio | 0.867 | 0.80–0.934 | <.001 | |
| Optimal cutoff points | Sensitivity | Specificity | Accuracy | Youden Index |
| Lactate ≥ 4.2 (mmol/L) | 0.731 | 0.867 | 0.86 | 0.60 |
| L/B ratio ≥ 0.25 | 0.730 | 0.831 | 0.83 | 0.56 |
AUC = area under the curve (%); L/B = lactate-to-bicarbonate ratio.
3.4. Logistic regression analyses for clinical predictors for in-hospital DSS mortality among children with DSS
As presented in Table 3, the multivariable logistic regression showed that the significant clinical predictors of in-hospital mortality in patients with DSS were severe bleeding, high cumulative infused fluid from referral hospitals and 24 hours of PICU admission, and a high VIS (>30). No significant interactions were found among the covariates.
Table 3.
Clinical predictors of 28-d in-hospital mortality in DSS children from univariable and multivariable logistic regressions with covariates in the first 24h of PICU admission
| Factors | Nonsurvivors (n = 26) |
Survivors (n = 466) |
Unadjusted OR (95% CI), P value |
Adjusted OR (95% CI), P value |
|---|---|---|---|---|
| Age (yr) | 7.5 (5.9–10) | 7.6 (5–10) | 1.02 (0.92–1.14), P = .69 |
- |
| Gender | ||||
| Male | 15 (58) | 223 (48) | 0.67 (0.3–1.5), P = .33 |
- |
| Female | 11 (42) | 243 (52) | ||
| Dengue severity* | ||||
| Compensated DSS | 18 (69) | 410 (88) | 3.95 (1.73–9.0), P = .001 |
- |
| Decompensated DSS | 8 (31) | 56 (12) | ||
| Respiratory rate (breaths/min) | 30 (24–35) | 26 (24–30) | 1.03 (0.98–1.08), P = .19 |
- |
| Severe bleeding* | ||||
| No | 7 (27) | 418 (90) | 23.6 (9.5–59.1), P < .001 |
4.14 (1.25–13.7), |
| Yes | 19 (73) | 48 (10) | P = .02 | |
| Severe liver transaminases* | ||||
| No | 8 (31) | 399 (86) | 13.4 (5.6–32.1), P < .001 |
- |
| Yes | 18 (69) | 67 (14) | ||
| Peak hematocrit level (%) | 48 (42–52) | 48 (45–51) | 0.97 (0.91–1.04), P = .41 |
- |
| Low platelet cell count, (< 20,000 cells × 109/L) |
||||
| No | 12 (46) | 361 (77) | 4.0 (1.8–8.91), P < .001 |
- |
| Yes | 14 (54) | 105 (23) | ||
| Serum creatinin (µmol/L) | 68 (58–123) | 50 (42–58) | 1.03 (1.02–1.05), P < .001 |
- |
| High VIS (> 30) No Yes |
5 (19) 21 (81) |
452 (97) 14 (3) |
135 (45–412), P < .001 |
36.2 (10.7–122.3), P < .001 |
| Square root of cumulative amount of infusion fluid from referral hospitals and 24h PICU admission (mL/kg)† | 16 (14–18) | 11 (9–13) | 1.36 (1.21–1.52), P < .001 |
1.18 (1.04–1.34), P = .01 |
Summary statistics are median (IQR) for continuous variables and frequency (%) for categorical data.
CI = confidence interval; DSS = dengue shock syndrome; OR = odds ratio, PICU = pediatric intensive care unit; VIS = vasoactive-inotropic score, WHO = World Health Organization.
These factors were defined by the WHO dengue guidelines in 2009.
Standardized covariate of the cumulative amount of fluid from referral hospitals and within 24h PICU admission by square root.
3.5. Selecting candidate predictors by LASSO regression
All predefined clinical covariates and biomarkers were analyzed using LASSO regression. The most significant predictors were identified by LASSO analyses, including severe bleeding, large volume of fluid infused, high VIS score, blood lactate level and L/B ratio.
3.6. Prognostic models for DSS mortality with combined clinical factors and biomarkers
Table 4 compares the prognostic values generated by the lactate and L/B ratio predictive models for DSS-associated fatality during the first 24 hours of PICU admission. The independent clinical predictors were cumulative fluid infusion and high VIS (>30). Markedly, the L/B model showed better prognostic values than the lactate model. Severe bleeding was not statistically significant in the final models (both lactate and L/B) However, based on its clinical importance in disease pathogenesis and literature, it was retained in the final prognostic models.
Table 4.
Multivariable logistic models for predicting 28-d in-hospital mortality among DSS children with clinical predictors and biomarkers in the first 24h of PICU admission.
| Prognostic factors | Lactate model | Lactate-to-bicarbonate model | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Severe bleeding (yes) | 3.16 (0.89–11.3) | .07 | 3.4 (0.97–11.9) | .05 |
| Square root of cumulative amount of infusion fluid from referral hospitals and during 24h of PICU admission (mL/kg) | 1.22 (1.06–1.41) | <.01 | 1.18 (1.03–1.36) | .02 |
| High VIS score > 30 (yes) | 25.9 (7.2–93.9) | <.001 | 31.3 (8.73–112.2) | <.001 |
| Blood lactate (mmol/L) | 1.35 (1.15–1.58) | <.001 | - | - |
| Blood L/B ratio | - | - | 8.66 (1.96–38.3) | <.01 |
DSS = dengue shock syndrome, L/B = lactate/bicarbonate ratio; OR = odds ratio; PICU = pediatric intensive care unit, VIS = vasaoactive-inotropic score.
3.7. Performance of predictive models for DSS mortality and internal validation
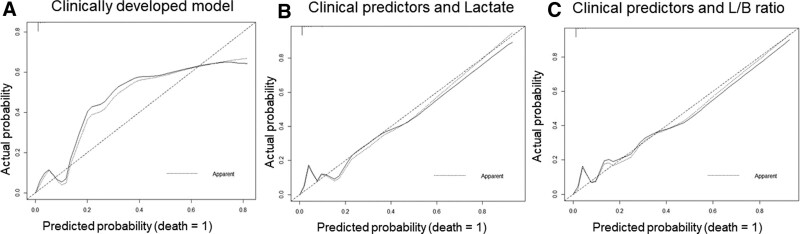
The predictive model performance parameters with discrimination (C-statistic), calibration slope, and Brier score for the training and test sets are shown in Table 5. The calibration plots are shown in Figure 2. Notably, the clinically developed model with identified predictors (severe bleeding, cumulative amount of fluid infused, and VIS > 30) had the highest C-index and Brier score values in the training and test sets; however, the calibration plot showed poor performance, with a significantly skewed distribution between the observed and predicted values (Fig. 2A). When combined with the identified clinical predictors, the L/B prognostic model had a mostly equivalent C-statistic and Brier score to those of the lactate model. Nevertheless, the L/B model showed a better slope calibration plot (Table 5, Fig. 2B and C).
Table 5.
Performance of predictive models with internal validation.
| Predictive models | C-statistic | Calibration slope | Brier score |
|---|---|---|---|
| Clinical predictors-based model (1)* | 0.954 (0.946) | 1 (0.961) | 0.023 (0.024) |
| (1) + Blood lactate | 0.950 (0.940) | 1 (0.915) | 0.026 (0.029) |
| (1) + Blood L/B ratio | 0.942 (0.924) | 1 (0.925) | 0.027 (0.029) |
Statistics are summarized for training (test) sets performed with bootstrap and internal validation
L/B = serum lactate/bicarbonate ratio, PICU = pediatric intensive care unit.
Based on clinically developed model, with identified predictors including severe bleeding, cumulative amount of fluid infused from referral hospitals and 24h PICU admission, and high vasoactive-inotropic score (>30).
Figure 2.
Calibration plots for: (1) clinically developed model with identified predictors (Fig. 2A), (2) clinical predictors and the blood lactate model (Fig. 2B), and (3) clinical predictors and lactate-to-bicarbonate (L/B) ratio model (Fig. 2C). The lactate and L/B prognostic models for DSS fatality showed good consistency between predicted data (x-axis) and observed values (y-axis). However, the model with only clinical predictors showed poor consistency between predicted and actual values.
4. Discussion
The mortality rate of hospitalized children presenting with DSS has been reported to range from 1% to 36%.[2–4,7,8,22] In particular, a dramatically higher in-hospital mortality rate has been observed in patients with prolonged DSS, severe respiratory failure requiring MV, and PALF despite undergoing extracorporeal therapies.[4,5,7] In this study, we showed that the in-hospital fatality rate of all-type DSS-experiencing children was 5.3%. Several prognostic models with potential clinical features and biomarkers for predicting dengue severity and mortality have been developed to improve the disease prognosis and management.[4,6,9,23] Therefore, we examined and compared the prognostic value of the blood L/B ratios and lactate levels in patients with DSS during the first 24 hours of PICU admission. Our findings indicate that the L/B ratio yields a better prognostic value and may be clinically used as an important predictor of mortality in PICU-admitted children with DSS.
To date, many studies have advocated serum lactate elevation and persistent hyperlactatemia as crucial prognostic biomarkers for predicting the risk of death in patients admitted to the PICU.[23–25] Additionally, the lactate-to-albumin ratio (L/A) has been proposed as a significant predictor of in-hospital mortality in critically ill patients.[12–17] Elevated L/A ratios are correlated with 28-day in-hospital and ICU mortality in patients with sepsis.[14] Recently, Gupta et al highlighted the use of arterial blood gas indicators in patients with severe dengue and showed that nonsurvivors had significantly lower serum bicarbonate and higher lactate levels than survivors did.[11,26] Importantly, elevated blood lactate levels are a significant determinant of dengue-related mortality.[6] In pathogenesis, lactate is primarily metabolized by the liver and kidneys acting a secondary role, whereas bicarbonate levels are mainly regulated by the lungs and kidneys. Lactic acidosis is associated with tissue hypoxia, which commonly occurs in DSS patients. Dengue-induced hepatocyte injury can significantly affect the metabolic function of the liver, which can manifest as increased serum lactate and decreased bicarbonate levels.[9,10] In this study, severe hepatic transaminases and respiratory failure requiring MV were observed in 17.3% and 24.2% of the patients with DSS, respectively. In addition, Preeprem et al reported that acute respiratory failure was observed in > 40% of DSS patients, contributing to a high fatality rate of 10%.[4] Notably, in our study, the kidney function on admission and during hospitalization was normal in patients with DSS. On this basis, the L/B ratio is more appropriate, considering the pathogenesis of dengue-associated shock syndrome, acute respiratory failure, dengue-induced severe hepatitis and acute liver failure. In this context, we examined the blood L/B as a potential prognostic marker in patients with DSS, rather than the lactate-to-albumin ratio, which has been used in patients with sepsis and/or septic shock.
To the best of our knowledge, this is the first study to examine L/B ratio as a prognostic marker for patients with DSS. This study identified the most significant clinical predictors of dengue-related fatalities, including severe bleeding, large amounts of cumulative infused fluids, and high VIS (>30). Although severe bleeding was not statistically significant in the L/B predictive model, we retained it in the final prognostic model based on disease pathogenesis and evidence from the previous medical literature.[5,22,27] The clinically developed model showed good discrimination performance, with C-statistics in the training and test sets of 0.954 and 0.946, respectively; however, this model had poor calibration with a skewed distributed curve in the calibration plot (Fig. 2A). Therefore, prognostic models that combine clinical covariates and biomarkers are required. We further pointed out that the on PICU admission blood L/B ratio (cutoff value ≥ 0.25) and blood lactate level (cutoff value ≥ 4.2 mmol/L) generated markedly high predictive values for DSS mortality. When combined with the identified clinical predictors, both the L/B ratio and lactate prognostic models showed good performance in terms of high discrimination and calibration slope and low Brier score. The calibration plots of the L/B and lactate models showed better performance in terms of minimal skew distribution (Fig. 2B and C). Most notably, the L/B ratio yielded higher prognostic values (OR = 8.66, 95% CI, 1.96–38.3, P < .01) than the lactate-based prognostic model (OR = 1.35, 95% CI, 1.15–1.58, P < .001). Both L/B and lactate models showed similarly good performance. Because the L/B ratio has a better prognostic value than the lactate model, it may be considered a potential prognostic biomarker in clinical use for predicting 28-day mortality in PICU-admitted children with DSS.
Considering the results of our study, the L/B ratio has the potential for the prognostication of patients with DSS. First, the 2 biomarkers (elevated blood lactate and decreased bicarbonate levels) independently predicted mortality of DSS patients with DSS. Second, the L/B ratio is more pertinent to the pathogenesis of severe dengue with hepatic, renal, and pulmonary dysfunctions, hyperlactatemia and reduced bicarbonate. Thus, a comprehensive combination of both parameters, that is the L/B ratio, can increase the prognostic value of predicting DSS-related mortality. However, the main limitation of our study was that it was a single-center retrospective cohort study. Other limitations include unstandardized collections of clinical and laboratory data, particularly the biomarker data of the participants. Further investigations in prospective cohorts are essential to confirm our findings.
5. Conclusions
Both blood lactate and L/B ratio had high predictive values for DSS mortality. The L/B ratio has a better prognostic value than the lactate model, and should be considered a potential prognostic biomarker in clinical use for predicting in-hospital mortality of PICU-admitted children with DSS.
Acknowledgments
We are grateful to the patients and administrative staffs for their support with this study.
Author contributions
Conceptualization: Thanh Tat Nguyen, Luan Thanh Vo.
Data curation: Nhu Hoang-Thien Vo, Dat Tat Nguyen, Phuc Hoang Nguyen, Lien Thi Ho, Duong Hung Doan, Dung Tuan Phan, Yen Nguyen-Hoang Duong, Truc Huynh Nguyen, Tuyet Kim Nguyen, Ha Thi-Thu Dinh, Thuy Thi-Diem Dinh, Anh Thi-Mai Pham, Luan Thanh Vo.
Formal analysis: Thanh Tat Nguyen.
Funding acquisition: Viet Chau Do, Luan Thanh Vo.
Investigation: Thanh Tat Nguyen, Dat Tat Nguyen, Thuy Thi-Diem Dinh, Luan Thanh Vo.
Methodology: Thanh Tat Nguyen, Luan Thanh Vo.
Supervision: Luan Thanh Vo.
Writing – original draft: Thanh Tat Nguyen, Nhu Hoang-Thien Vo.
Writing – review & editing: Thanh Tat Nguyen, Viet Chau Do, Tung Huu Trinh, Luan Thanh Vo.
Abbreviations:
- DSS
- dengue shock syndrome
- L/B
- lactate-to-bicarbonate ratio
- PALF
- dengue-associated pediatric acute liver failure
- PICU
- pediatric intensive care unit
- WHO
- World Health Organization
TNT and LVT contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Nguyen Tat T, Vo Hoang-Thien N, Nguyen Tat D, Nguyen PH, Ho LT, Doan DH, Phan DT, Duong YN-H, Nguyen TH, Nguyen TK, Dinh HT-T, Dinh TT-D, Pham AT-M, Do Chau V, Trinh TH, Vo Thanh L. Prognostic values of serum lactate-to-bicarbonate ratio and lactate for predicting 28-day in-hospital mortality in children with dengue shock syndrome. Medicine 2024;103:17(e38000).
Contributor Information
Phuc Hoang Nguyen, Email: tuyet1706@gmail.com.
Lien Thi Ho, Email: Lienho1421@gmail.com.
Duong Hung Doan, Email: hungduong365@gmail.com.
Dung Tuan Phan, Email: dungphan12121966@gmail.com.
Yen Nguyen-Hoang Duong, Email: Yenduong160691@gmail.com.
Truc Huynh Nguyen, Email: tuyet1706@gmail.com.
Tuyet Kim Nguyen, Email: tuyet1706@gmail.com.
Ha Thi-Thu Dinh, Email: dtdiemthuy@yahoo.com.vn.
Thuy Thi-Diem Dinh, Email: dtdiemthuy@yahoo.com.vn.
Anh Thi-Mai Pham, Email: maianh2406@gmail.com.
Tung Huu Trinh, Email: trinhhuutung@gmail.com.
References
- [1].World Health Organization. Global strategy for dengue prevention and control 2012-2020. World Health Organization, 2012. https://www3.paho.org/hq/dmdocuments/2014/01-2014-cha-global-dengue-situation-strategy-2012-2020.pdf. Accessed December 4, 2023.
- [2].WHO Dengue and severe dengue, 2019. https://www.who.int/health-topics/dengue-and-severe-dengue#tab=tab_1. Accessed December 4, 2023.
- [3].Laoprasopwattana K, Khantee P, Saelim K, et al. Mortality rates of severe dengue viral infection before and after implementation of a revised guideline for severe dengue. Pediatr Infect Dis J. 2022;41:211–6. [DOI] [PubMed] [Google Scholar]
- [4].Preeprem N, Phumeetham S. Paediatric dengue shock syndrome and acute respiratory failure: a single-centre retrospective study. BMJ Paediatr Open. 2022;6:e001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vo LT, Do VC, Trinh TH, et al. Combined therapeutic plasma exchange and continuous renal replacement therapy in children with dengue-associated acute liver failure and shock syndrome: single-center cohort from Vietnam. Pediatr Crit Care Med. 2023;24:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sachdev A, Pathak D, Gupta N, et al. Early predictors of mortality in children with severe dengue fever: a prospective study. Pediatr Infect Dis J. 2021;40:797–801. [DOI] [PubMed] [Google Scholar]
- [7].Vo LT, Nguyen DT, Tran TN, et al. Pediatric profound dengue shock syndrome and use of point-of-care ultrasound during mechanical ventilation to guide treatment: single-center retrospective study, 2013-2021. Pediatr Crit Care Med. 2023;25:e177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- [9].Sirikutt P, Kalayanarooj S. Serum lactate and lactate dehydrogenase as parameters for the prediction of dengue severity. J Med Assoc Thai. 2014;97(Suppl 6):S220–31. [PubMed] [Google Scholar]
- [10].Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gupta M, Agrawal N, Sharma SK, et al. Study of utility of basic arterial blood gas parameters and lactate as prognostic markers in patients with severe dengue. Cureus. 2022;14:e24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang B, Chen G, Cao Y, et al. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Crit Care. 2015;30:271–5. [DOI] [PubMed] [Google Scholar]
- [13].Lichtenauer M, Wernly B, Ohnewein B, et al. The Lactate/Albumin Ratio: a valuable tool for risk stratification in septic patients admitted to ICU. Int J Mol Sci . 2017;18:1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shin J, Hwang SY, Jo IJ, et al. Prognostic value of the lactate/albumin ratio for predicting 28-day mortality in critically ILL sepsis patients. Shock. 2018;50:545–50. [DOI] [PubMed] [Google Scholar]
- [15].Gharipour A, Razavi R, Gharipour M, et al. Lactate/albumin ratio. An early prognostic marker in critically ill patients. Am J Emerg Med. 2020;38:2088–95. [DOI] [PubMed] [Google Scholar]
- [16].Bou Chebl R, Jamali S, Sabra M, et al. Lactate/Albumin Ratio as a predictor of in-hospital mortality in septic patients presenting to the emergency department. Front Med (Lausanne). 2020;7:550182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Erdoğan M, Findikli HA. Prognostic value of the lactate/albumin ratio for predicting mortality in patients with pneumosepsis in intensive care units. Medicine (Baltim). 2022;101:e28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wendon J, et al.; European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical practice guidelines panel. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81. [DOI] [PubMed] [Google Scholar]
- [19].McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the vasoactive-inotropic score in pediatric sepsis. Pediatr Crit Care Med. 2017;18:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruopp MD, Perkins NJ, Whitcomb BW, et al. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nguyen TT, Nguyen DT, Vo TT, et al. Associations of obesity and dengue-associated mortality, acute liver failure and mechanical ventilation in children with dengue shock syndrome. Medicine (Baltim). 2023;102:e36054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gorgis N, Asselin JM, Fontana C, et al. Evaluation of the association of early elevated lactate with outcomes in children with severe sepsis or septic shock. Pediatr Emerg Care. 2019;35:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Koliski A, Cat I, Giraldi DJ, et al. Lactato sérico como marcador prognóstico em crianças gravemente doentes [Blood lactate concentration as prognostic marker in critically ill children]. J Pediatr (Rio J). 2005;81:287–92. [PubMed] [Google Scholar]
- [25].Hatherill M, McIntyre AG, Wattie M, et al. Early hyperlactataemia in critically ill children. Intensive Care Med. 2000;26:314–8. [DOI] [PubMed] [Google Scholar]
- [26].Padyana M, Karanth S, Vaidya S, et al. Clinical profile and outcome of dengue fever in multidisciplinary Intensive Care Unit of a tertiary level hospital in India. Indian J Crit Care Med. 2019;23:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sam SS, Omar SF, Teoh BT, et al. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLoS NeglTrop Dis. 2013;7:e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]