Abstract
Inflammatory bowel disease (IBD) comprising ulcerative colitis and Crohn’s disease is a chronic immune-mediated disease which affects the gastrointestinal tract with a relapsing and remitting course, causing lifelong morbidity. IBD pathogenesis is determined by multiple factors including genetics, immune and microbial factors, and environmental factors. Although therapy options are expanding, remission rates are unsatisfiable, and together with the disease course, response to therapy remains unpredictable. Therefore, the identification of biomarkers that are predictive for the disease course and response to therapy is a significant challenge. Extrachromosomal circular DNA (eccDNA) fragments exist in all tissue tested so far. These fragments, ranging in length from a few hundreds of base pairs to mega base pairs, have recently gained more interest due to technological advances. Until now, eccDNA has mainly been studied in relation to cancer due to its ability to act as an amplification site for oncogenes and drug resistance genes. However, eccDNA could also play an important role in inflammation, expressed both locally in the- involved tissue and at distant sites. Here, we review the current evidence on the molecular mechanisms of eccDNA and its role in inflammation and IBD. Additionally, the potential of eccDNA as a tissue or plasma marker for disease severity and/or response to therapy is evaluated.
Keywords: circular DNA, inflammatory bowel disease (IBD), ulcerative colitis (UC), Crohn’s disease (CD), colorectal cancer associated with colitis (CAC), extrachromosomal circular DNA (eccDNA)
1. Introduction
Inflammatory bowel disease (IBD) is a group of chronic multifactorial intestinal inflammatory disorders, including Crohn’s disease (CD) and ulcerative colitis (UC). These disorders arise primarily in genetically predisposed individuals and are influenced by a complex interplay of factors such as microbial dysbiosis, aberrant immune responses, and environmental triggers such as psychosocial stress, certain medications, dietary habits (e.g., Western diet), smoking, and the use and abuse of antibiotics [1]. The incidence and prevalence of IBD is increasing dramatically around the world, with a major increase in developing countries and young children [2]. In addition to the intestinal symptoms, these diseases have a serious impact on patients’ quality of life and their productivity [3].
Current medications include biological drugs like anti-tumor necrosis factor (anti-TNF), anti-integrins (vedolizumab), anti-IL12/23 (ustekinumab and risankizumab), and small molecules such as JAK inhibitors (tofacitinib, upadacitinib, and filgotinib). These therapies are associated with high costs, adverse events, and unfortunately rather modest long-term response and remission rates. In addition, almost no indicators are available to assist clinicians in selecting the best therapy for each patient [4]. Therefore, identifying predictive markers for response to therapy represents an urgent clinical need in IBD, allowing for successful treatment and subsequently reducing the risk of exposure to potentially harmful and ineffective therapies.
In the search for relevant biomarkers, cell-free DNA (cfDNA) could be considered. This type of DNA is defined as any DNA fragment free from its origin cell able to circulate freely in the bloodstream or within microvesicles. Previously, we have extensively described the main characteristics of different types of circulating DNAs [5]. Among cfDNA, in particular, extrachromosomal circular DNA (eccDNA) has gained interest as a potential biomarker. Emerging evidence from studying eccDNA might provide insights for understanding the etiopathogenetic and molecular mechanisms involved in several inflammatory and immune-mediated diseases and shed a light on new therapeutic strategies in clinical practice.
In this review, we therefore provide an overview of the recent literature on the relationship between eccDNA and inflammation, and the main molecular mechanisms involved. In addition, we also evaluate the potential use of eccDNA in IBD as a new type of diagnostic biomarker to classify the type of inflammation occurring in various human diseases and its potential to predict response to treatment.
2. Methods
PubMed (https://pubmed.ncbi.nlm.nih.gov, accessed on 2 July 2023) and Scopus (https://www.scopus.com, accessed on 2 July 2023) databases were searched using the following search string: (“IBD”, “ulcerative colitis”, “Crohn’s disease”) AND (“extrachromosomal DNA”, “circular DNA”, “circulating DNA”), of which only articles published between 2019 and 2023 were considered. The “AND” operator was used to create all possible combinations of selected terms. Only English research articles (original and review articles) were included, while articles without full-text reports were excluded.
3. Extrachromosomal Circular DNA
eccDNA is a term used to describe the full spectrum of eukaryotic extrachromosomal circular DNAs, which show a wide heterogeneity in size, ranging from hundreds to millions of base pairs, and have been classified in relation to their size and content, as previously described [6,7]. eccDNAs are double-stranded (ds), circular-shaped DNA fragments that exist independently from the chromosomal DNA in the nuclei of all eukaryotic species studied, such as drosophila [8], yeasts, and humans [9]. eccDNA is derived from nuclear chromosomal DNA and can harbor any type of genomic structure, including full-length and/or trunked genes, intergenic sequences, and repeated sequences. Moreover, eccDNA can be transcribed, giving rise to mRNA coding for full-length and/or truncated proteins and RNA with regulatory functions of gene expression (siRNA, miRNA, lncRNA, etc.) [10]. Moreover, eccDNA is common in both healthy and pathologic human tissue and blood, in sizes large enough to carry one or several complete genes [11]. Several models exist for how eccDNA is formed in human cells. The main mechanisms behind eccDNA production are errors during DNA repair processes (mismatch repair), hypoxia and chromosome shattering (chromothripsis), and errors during DNA replication and active DNA transcription (microdeletions) [6]. However, eccDNA can also be a product of cell apoptosis [9,12,13].
The presence of small circular fragments of extrachromosomal DNA, within the cellular nucleus, was already described in 1964 by Alix Bassel and Yasuo Hotta [14] during the study of Franklin Sthal’s theory. These “circles” were later identified in several types of human cancer cells [15] and recently also in healthy eukaryotic cells [11]. In addition, eccDNA has also been identified as a cell-free DNA (cfDNA) in human tissue [11,16], plasma [17], urine [18], and other biological fluids [19], and therefore, eccDNA could serve as a potential biomarker.
Notably, Moller et al. in 2018 revealed that large parts of the human genome can be detected in eccDNAs. This could potentially be explained by random mutational processes of repetitive sequences with extra space allowing for circularization [11]. In previous studies, short eccDNAs were detected with <2k bp (microDNAs), and even those as limited as only 100–200 bp were found in nuclei from muscle samples and leukocytes.
Previous findings have shown a high abundance of smaller cell-free eccDNAs in the plasma during healthy, inflammatory, and neoplastic conditions [16,17]. Interestingly, Pang et al. showed that healthy controls had significantly longer eccDNA sequences in plasma than patients with gouty arthritis [12].
4. Molecular Mechanisms of eccDNA and Its Role in Inflammatory Processes
eccDNA performs several functions within the eukaryotic cells, including gene amplification for both oncogenes and drug resistance genes in neoplastic cells [13,20], as well as having a role in intercellular heterogeneity and genomic instability [21,22], cellular aging [23], genetic compensation [24], intercellular communication [25], the restoration of telomere length (t-circles) [26,27], the production of short regulatory RNA, molecular sponges (microDNA), and immune regulation [10,28,29].
Cell-free circulating eccDNA could play a driving role in both systemic and tissue-specific inflammatory processes. For example, tissue-purified eccDNA, or synthetic circular DNA, but not their linear counterparts, have significant immunostimulatory activity, especially in the activation of the innate immune system. eccDNA has been shown to dramatically induce the production of type I Interferons (IFN-1α and IFN-1β), interleukin IL-6, and tumor necrosis factor (TNF-α) [29].
eccDNA activates innate immunity through the activation of several DNA-sensing pathways, including stimulator of interferon protein (STING) and a protein called absent in melanoma 2 (AIM2) [30].
A major source of eccDNA is mitochondrial DNA (mtDNA) which is released to the cytosol in response to apoptotic stimuli, next to cytochrome C. In the cytosol, this mtDNA could activate cyclic guanosine–adenosine monophosphate synthase (cGAS) to trigger inflammation [31]. Conversely, Wang et al. found that apoptosis could increase eccDNA generation, depending on apoptotic DNA fragmentation mediated by DNaseγ and ligation by DNA ligase 3, probably occurring in the late stage of apoptosis. These eccDNAs (<3 kb) were shown to have strong immunostimulatory activity mediated by the cGAS-STING pathway, depending on their circular shape rather than their sequence [29]. Extracellular vesicles (EVs) carrying double-stranded (ds) DNA, responsible for intercellular communication among intestinal cells, are significantly increased in murine colitis and active human CD, and positively correlated with disease activity via the activation of the STING pathway in macrophages [32].
Few hypotheses are available on the mechanisms of DNA damage inducing type I IFNs and other immune-regulatory cytokines [33,34]. DNA normally resides in the nucleus and mitochondria; hence, its presence in the cytoplasm serves as a DAMP to trigger immune responses. Cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS) detects DNA as a DAMP and induces type I IFNs and other cytokines [31,35,36,37]. In addition to NF-κB, MAPK and signal transducer and activator of transcription 6 (STAT6) are activated upon recognition of cytosolic dsDNA. Subsequently, STING stimulates autophagosome formation by facilitating the formation of puncta of microtubule-associated protein 1A/1B-light chain 3 (LC3) and autophagy protein 9a (ATG) [38]. In addition, beclin-1 (BECN1) inhibits the interaction between cGAS and dsDNA, thus restricting cGAMP formation in response to cytosolic dsDNA. The interplay between cGAS and BECN1 induces autophagy and the elimination of cytosolic dsDNA [39]. One of the main functions of autophagy is to eliminate and recycle cellular components, including cfDNA, in order to avoid inflammatory damage. Defects in the autophagy process enhance the recognition of cfDNA by various cytosolic pattern recognition receptors (PRRs) and enhance the response of the immune system [39].
IBD is characterized by defects in the autophagic machinery [40]. This is evident in the prevalence of polymorphisms within genes such as NOD2/CARD15, ATG16L1, and IRGM, which are linked to an increased disease risk. Therefore, it is reasonable to assert that eccDNA fulfils a major role in driving and enhancing intestinal and systemic inflammation in IBD.
In addition, IBD features an abnormal mucosal immune response against luminal bacterial products, including unmethylated double-stranded DNA, single-stranded RNA, and broad DAMPs. Due its ability to bind to TRL9, human endogenous eccDNA could also act as an endogenous DAMP, particularly due to the curvatures or U-shaped structures of eccDNA, and therefore could have an important pro-inflammatory role that is expressed both locally and systemically. The latter could be obtained by its ability to move in plasma within phospholipid vesicles or to move freely. Accordingly, eccDNA within vesicles can be assumed to be detected by innate immune cells, be phagocytosed, and activate intracellular pathways, such as cGAS-cGAMP-STING, which leads to the transcription of pro-inflammatory cytokine genes and thereby actively contributes to intestinal inflammation.
Chromothripsis, a complex phenomenon, involves the abrupt shattering of one or more chromosomes due to a sudden genomic instability event. Subsequent to this event, the shattered fragments undergo repair in a random order and orientation, potentially resulting in various outcomes, including DNA circularization [41,42,43]. In an inducible model of chromothripsis, the pharmacological inhibition of DNA ligase IV has been demonstrated to impede the reassembly of shattered fragments [44,45]. Additionally, sequence analysis of the junctions of rearranged chromosomes has indicated minimal or absent microhomology [46]. Consequently, levels of peripheral and oxidative DNA damage are elevated compared to those in healthy subjects, with distinct differences observed between patients with UC and CD patients [47].
Specifically, the study by Pereira et al. has highlighted that CD patients exhibit significantly higher levels of peripheral DNA damage compared to UC patients, whereas oxidative DNA damage is more pronounced in UC patients than in those with CD [48]. This variance may stem from the downregulation of DNA repair systems observed in UC patients, resulting in defects in the repair of 8-oxo-7,8-dihydro-2′-deoxyguanosine [8-oxo-dG]. This, in turn, leads to the accumulation of endogenously produced oxidized DNA bases, thereby increasing susceptibility to cancer development [49].
DNA transcription has the potential to modulate the mechanism of DNA circularization; however, the exact mechanism remains to be elucidated. Nonetheless, it has been proposed that the formation of R-loops during transcription could facilitate the direct repeats on the unpaired strand that form a loop which can subsequently be excised and ligated into a circular structure [50]. Transcription stress may render DNA more susceptible to damage [51], potentially leading to the generation of errors in DNA repair processes that could contribute to eccDNA formation. These observations establish a link between changes in the environmental conditions and eccDNA profiles, underscoring again the pivotal role of eccDNA in facilitating rapid adaptation to varying environments. Sequencing eccDNA in IBD holds promise for identifying the genes involved in the pathogenesis and mediation of the intestinal damage, as well as characterizing inflammation at the different disease stages.
Recently, apoptosis has emerged as a novel mechanism of biogenesis of cell-free eccDNA [29]. Apoptosis inducers have been shown to increase cell-free eccDNA generation, a process dependent on apoptotic oligonucleosomal DNA fragmentation mediated by DNaseγ, followed by ligation by DNA ligase 3, independent of both DNA ligase 1 and 4 [29]. Apoptosis is also a major source of cell-free DNA (cfDNA) [52,53], suggesting that cell-free eccDNA is a component of the cfDNA pool in the human body. Similarly, other mechanisms of cellular death, such as necrosis or pyroptosis [54], may also contribute to the pool of cfDNA. Pyroptosis, a pro-inflammatory cell death mechanism executed by gasdermin family proteins, including gasdermin B (GSDMB) and D (GSDMD), has been implicated in IBD susceptibility, regulating intestinal inflammation by promoting GSDMD-mediated pyroptosis [55].
Overall, these findings suggest the possibility of an increased amount of eccDNA resulting from inflammatory processes characterized by cell death, the accumulation of reactive oxygen species, and genomic instability [31,56,57,58,59,60].
5. Clinical Evidence regarding eccDNA in Inflammatory Bowel Disease
Only a few clinical publications have studied cfDNA in inflammatory bowel disease, and to the best of our knowledge, none have focused on eccDNA. In a pioneering study in 2003, Rauh et al. demonstrated the presence of cfDNA in the serum of UC patients, identifying a microsatellite alteration previously detected in mucosal cells from UC patients [61]. Building further on this, two different studies revealed a significant increase in cfDNA concentration in the plasma of mice with DSS-induced colitis compared to controls [62,63]. Recent findings further highlighted a progressive rise in total cfDNA levels in the plasma of mice with DSS-induced colitis [64,65], peaking at day seven of DSS administration and correlating with indicators of disease activity, such as neutrophil extracellular traps (NETs) [64,65]. Notably, the elevation in total plasma cfDNA did not correspond with an increase in cf-ncDNA or cf-mtDNA, indicating the potential involvement of other cfDNA types, such as eccDNA [65].
In contrast, while the total amount of cfDNA originating from colon tissue was increased only in the early stages of the disease, it was primarily associated with the increase in cf-ncDNA and cf-mtDNA subtypes [65]. These data highlight the importance of colonic inflammation in the early stage of the disease, subsequently leading to increased intestinal permeability and the release of cfDNA into systemic circulation, thereby contributing to the systemic immune activation.
Furthermore, IBD patients were shown to exhibit elevated levels of both ncDNA and mtDNA compared to healthy controls, suggesting that these types of DNAs could be used as biomarkers for IBD diagnosis, but not for monitoring disease activity [66]. Boyapati et al. additionally demonstrated that active IBD patients displayed higher levels of both circulating cfDNA and cell-free mtDNA, suggesting the use of mtDNA as a mechanistic biomarker of disease activity [67].
In the context of IBD, cfDNA can be generated by different types of programmed cell death, such as apoptosis, but also pyroptosis and NETosis [5,68,69]. As potential biomarkers, eccDNAs provide several advantages compared to linear DNAs. First, the circularly formed DNAs are resistant to exonuclease digestion due to their close circular structure, thereby being more stable than their linear counterparts [70]. Second, some eccDNAs found in the systemic circulation are much longer than their linear counterparts, which are normally 160–170 bp in size, facilitating their detection and dynamic monitoring [16]. Third, eccDNAs seem to have lineage specificity in some human cells, such as lymphoid cell lines, fibroblasts, and granulocytes [71]. Several studies have already demonstrated the potential application of circular extrachromosomal DNA elements in body fluids as biomarker candidates for the diagnosis, monitoring, and prognosis of various types of cancers and chronic kidney disease [18,72,73,74,75,76,77,78,79]. The growing evidence is suggestive of the potential of eccDNA as a biomarker in different types of diseases, including autoimmune or autoinflammatory diseases, such as IBD, particularly for monitoring disease progression and the severity of inflammation over time. Such insights may enable the implementation of aggressive therapeutic strategies for those patients with a high risk of severe inflammation. By serving as a mechanistic biomarker, eccDNA could aid in stratifying patients for relevant personalized therapeutic interventions, thereby advancing the field of personalized medicine in complex diseases such as IBD.
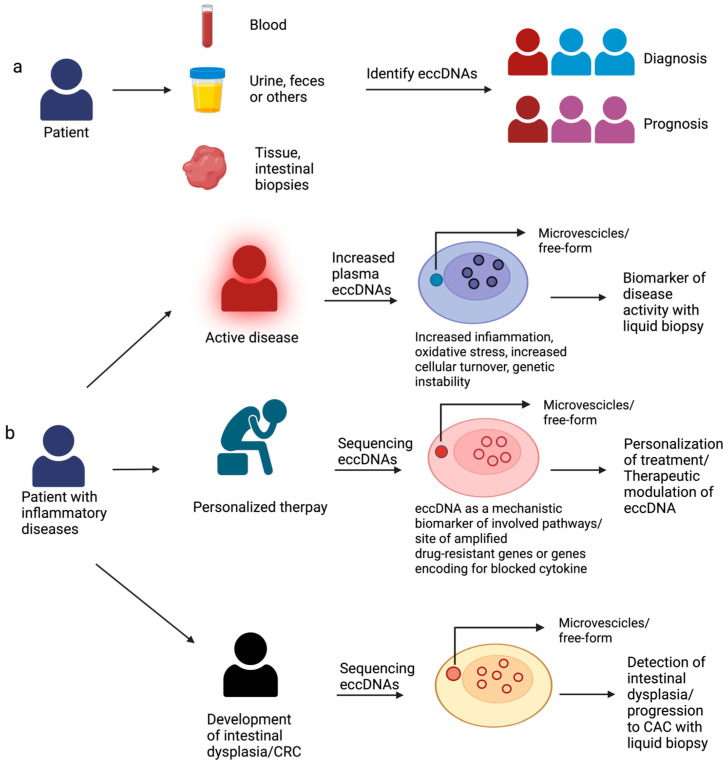
Regarding IBD pathogenesis, eccDNA could play a major role in exacerbating mucosal inflammation by activating innate immunity and thereby establishing a vicious circle in affected patients. Consequently, the level of eccDNA may correlate with the disease severity and the extent of intestinal and systemic inflammation (Figure 1). This finding could revolutionize the management of IBD patients by offering a non-invasive means of tracking mucosal healing and assessing disease activity through the analysis of cell-free eccDNA. Such an approach would obviate the need for invasive endoscopic examinations, enhancing patient care and comfort. Additionally, there is a potential role for the use of cell-free eccDNA in the screening and detection of pre-IBD in high-risk groups, such as relatives of affected patients (Figure 1). Nuclear eccDNAs, capable of harboring entire genes or gene fragments, may encode modified proteins, as observed in cancer (16). Consequently, resistance to biological therapy in IBD may arise due to the amplification of specific genes or fragments within eccDNA molecules.
Figure 1.
eccDNAs as potential biomarkers in inflammatory bowel disease. The clinical value of eccDNAs in IBD. (a) eccDNAs can be found in patient samples, such as blood, urine, feces, or intestinal biopsies. The identification of eccDNAs would be helpful in the diagnosis of IBD and prognosis. (b) The amount of eccDNAs in the intestinal mucosa and in the serum could correlate with the degree of intestinal inflammation, making it possible to assess the patient’s disease activity non-invasively. The sequencing of eccDNA will allow us to detect whether there are genes related to any non-response mechanisms, such as amplification of the targeted cytokine, enabling us to personalize the therapy and possibly even improve its efficacy due to therapeutic modulation of eccDNA. The sequencing of circulating eccDNA in serum could allow us to detect at an early stage the presence of genes (e.g., oncogenes) indicative of the development of intestinal dysplasia, without the need for continuous invasive testing.
Finally, an intriguing novel clinical application (Figure 1) of eccDNA profiling lies in the surveillance for colorectal cancer associated with colitis (CAC). The profiling and sequencing of both plasma-circulating eccDNA and mucosal nuclear eccDNA could allow us to detect specific DNA mutations, oncogene amplification, or other eccDNA signatures which precede the development of detectable high-grade dysplasia, or which predict the evolution of low-grade dysplasia to CAC, thereby aiding in early intervention and management.
6. Conclusions and Future Perspectives
In conclusion, eccDNA profiling represents a revolutionary approach to monitor and guide therapeutic decisions in IBD. Rapid advancements in this field highlight the pivotal role of eccDNA in intracellular homeostasis and its pathological implications in various diseases. eccDNA as a biomarker in IBD may reduce the number of invasive endoscopic examinations that IBD patients must undergo over a lifelong period for the surveillance for CAC [80], allowing for the use of more rapid non-invasive tests, for example, with blood or even with fecal material. Large-scale prospective cohort studies are recommended to fully elucidate the diagnostic, prognostic, and predictive accuracy of eccDNA in immune-mediated disorders. Yet, the stability of eccDNA in tissue and blood holds promise for its integration as a prognostic biomarker in clinical practice.
Furthermore, understanding the mechanisms underlying eccDNA-mediated immune responses may unveil novel treatment strategies, ultimately paving the way for personalized medicine in IBD. Future research endeavors should aim to validate the potential of eccDNA as a biomarker and develop predictive models incorporating eccDNA analysis for personalized medicine in IBD.
Acknowledgments
The authors are grateful to Fondazione Roma for the continuous support in their scientific research.
Abbreviations
8-oxo-dG = 8-oxo7,8-dihydro-2′-deoxyguanosine; AID = autoimmune diseases; AIM2 = protein called absent in melanoma 2; ALT = alternative lengthening of telomers; AMP = adenosine monophosphate; ATG = autophagy protein 9a; BECN1 = Beclin-1; CAC = colorectal cancer associated with colitis; CD = Crohn’s disease; cfDNA = cell-free DNA; cf-mtDNA = cell-free mitochondrial DNA; cf-ncDNA = cell-free nuclear DNA; cGAMP = cyclic GMP-AMP; cGAS = cyclic guanosine–adenosine monophosphate synthase; DAMP = damage-associated molecular pattern; DNaseγ = apoptotic DNA fragmentation; DSB = double-stranded breaks; dsDNA = double-stranded DNA; DSS = dextran sulfate sodium; eccDNA = extrachromosomal circular DNA; EV = extracellular vesicles; GMP = guanosine monophosphate; GSDMB = gasdermin B; GSDMD = gasdermin D; HR = homologous recombination; IBD = inflammatory bowel disease; IFN-α = interferon α; IFN-β = interferon β; IL-10 = interleukin 10; IL-17° = interleukin 17°; IL-17F = interleukin 17F; IL-18 = interleukin 18; IL-1β = interleukin 1 beta; IL-6 = interleukin 6; INF = interferon; LC3 = microtubule-associated protein 1A/1B-light chain 3; lncRNA = long non-coding RNA; MAPK = mitogen-activated protein kinase; MDP = muramyl dipeptide; miRNA = microRNA; MMEJ = microhomology-mediated end joining; MMR = mismatch repair; mtDNA = mitochondrial DNA; ncDNA = nuclear DNA; NETs = neutrophil extracellular traps; NF-κB = nuclear factor kappa B; NHEJ = non-homologous end joining; NIPT = non-invasive prenatal testing; PI3K = class III phosphatidylinositol 3-kinase; PRR = pattern recognition receptor; siRNA = small interfering RNA; spcDNA = small polydispersed circular DNA; STAT6 = signal transducer and activator of transcription 6; STING = stimulator of interferon genes; Th17 = T-helper 17; TLR-9 = toll-like receptor 9; TLRs = toll-like receptors; TNF = tumor necrosis factor; TNF-α = tumor necrosis factor α; UC = ulcerative colitis; ucf-eccDNAs = urinary cell-free extrachromosomal circular DNA; UTRs = untranslated regions.
Author Contributions
V.P. (conceptualization: equal; data curation: lead; methodology: equal; writing—original draft: lead). F.D.V. (conceptualization: equal; data curation: lead; methodology: equal; writing—original draft: lead). L.P. (formal analysis: supporting; investigation: equal; validation: equal). M.T.A. (conceptualization: supporting; methodology: supporting; supervision: supporting). B.R. (formal analysis: supporting; investigation: equal; validation: equal; funding acquisition: lead). A.G. (conceptualization: supporting; supervision: supporting; funding acquisition: equal). F.S. (conceptualization: lead; funding acquisition: equal; supervision: lead; writing—review and editing: lead). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no conflicts of interest or competing financial interests.
Funding Statement
This work is funded by the European Union’s Horizon 2020 Research and Innovation Programme (899417).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ananthakrishnan A.N., Bernstein C.N., Iliopoulos D., Macpherson A., Neurath M.F., Ali R.A.R., Vavricka S.R., Fiocchi C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018;15:39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 2.Ramos G.P., Papadakis K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parra R.S., Chebli J.M.F., Amarante H., Flores C., Parente J.M.L., Ramos O., Fernandes M., Rocha J.J.R., Feitosa M.R., Feres O., et al. Quality of life, work productivity impairment and healthcare resources in inflammatory bowel diseases in Brazil. World J. Gastroenterol. 2019;25:5862–5882. doi: 10.3748/wjg.v25.i38.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atreya R., Neurath M.F. Biomarkers for personalizing IBD therapy: The quest continues. Clin. Gastroenterol. Hepatol. 2024 doi: 10.1016/j.cgh.2024.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Di Vincenzo F., Yadid Y., Petito V., Emoli V., Masi L., Gerovska D., Arauzo-Bravo M.J., Gasbarrini A., Regenberg B., Scaldaferri F. Circular and Circulating DNA in Inflammatory Bowel Disease: From Pathogenesis to Potential Molecular Therapies. Cells. 2023;12:1953. doi: 10.3390/cells12151953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noer J.B., Horsdal O.K., Xiang X., Luo Y., Regenberg B. Extrachromosomal circular DNA in cancer: History, current knowledge, and methods. Trends Genet. 2022;38:766–781. doi: 10.1016/j.tig.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Arrey G., Keating S.T., Regenberg B. A unifying model for extrachromosomal circular DNA load in eukaryotic cells. Semin. Cell Dev. Biol. 2022;128:40–50. doi: 10.1016/j.semcdb.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Stanfield S.W., Lengyel J.A. Small circular DNA of Drosophila melanogaster: Chromosomal homology and kinetic complexity. Proc. Natl. Acad. Sci. USA. 1979;76:6142–6146. doi: 10.1073/pnas.76.12.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaubatz J.W. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat. Res. 1990;237:271–292. doi: 10.1016/0921-8734(90)90009-G. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen T., Shibata Y., Kumar P., Dillon L., Dutta A. Small extrachromosomal circular DNAs, microDNA, produce short regulatory RNAs that suppress gene expression independent of canonical promoters. Nucleic Acids Res. 2019;47:4586–4596. doi: 10.1093/nar/gkz155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller H.D., Mohiyuddin M., Prada-Luengo I., Sailani M.R., Halling J.F., Plomgaard P., Maretty L., Hansen A.J., Snyder M.P., Pilegaard H., et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat. Commun. 2018;9:1069. doi: 10.1038/s41467-018-03369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang J., Pan X., Lin L., Li L., Yuan S., Han P., Ji X., Li H., Wang C., Chu Z., et al. Characterization of Plasma Extrachromosomal Circular DNA in Gouty Arthritis. Front. Genet. 2022;13:859513. doi: 10.3389/fgene.2022.859513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koche R.P., Rodriguez-Fos E., Helmsauer K., Burkert M., MacArthur I.C., Maag J., Chamorro R., Munoz-Perez N., Puiggros M., Dorado Garcia H., et al. Extrachromosomal circular DNA drives oncogenic genome remodeling in neuroblastoma. Nat. Genet. 2020;52:29–34. doi: 10.1038/s41588-019-0547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta Y., Bassel A. Molecular Size and Circularity of DNA in Cells of Mammals and Higher Plants. Proc. Natl. Acad. Sci. USA. 1965;53:356–362. doi: 10.1073/pnas.53.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao Z., Jiang W., Ye L., Li T., Yu X., Liu L. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA (ecDNA) in tumor heterogeneity and progression. Biochim. Biophys. Acta Rev. Cancer. 2020;1874:188392. doi: 10.1016/j.bbcan.2020.188392. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P., Dillon L.W., Shibata Y., Jazaeri A.A., Jones D.R., Dutta A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the Circulation. Mol. Cancer Res. 2017;15:1197–1205. doi: 10.1158/1541-7786.MCR-17-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J., Zhang F., Du M., Zhang P., Fu S., Wang L. Molecular characterization of cell-free eccDNAs in human plasma. Sci. Rep. 2017;7:10968. doi: 10.1038/s41598-017-11368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv W., Pan X., Han P., Wang Z., Feng W., Xing X., Wang Q., Qu K., Zeng Y., Zhang C., et al. Circle-Seq reveals genomic and disease-specific hallmarks in urinary cell-free extrachromosomal circular DNAs. Clin. Transl. Med. 2022;12:e817. doi: 10.1002/ctm2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen K., Zhang L., Cai Y., Teng H., Liang J., Yue Y., Li Y., Huang Y., Liu M., Zhang Y., et al. Identification and characterization of extrachromosomal circular DNA in patients with high myopia and cataract. Epigenetics. 2023;18:2192324. doi: 10.1080/15592294.2023.2192324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M., Chen X., Yu F., Ding H., Zhang Y., Wang K. Extrachromosomal Circular DNAs: Origin, formation and emerging function in Cancer. Int. J. Biol. Sci. 2021;17:1010–1025. doi: 10.7150/ijbs.54614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanda T., Sullivan K.F., Wahl G.M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr. Biol. 1998;8:377–385. doi: 10.1016/S0960-9822(98)70156-3. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg G., Rosengren A.H., Hakanson U., Stewenius H., Jin Y., Stewenius Y., Pahlman S., Gisselsson D. Binomial mitotic segregation of MYCN-carrying double minutes in neuroblastoma illustrates the role of randomness in oncogene amplification. PLoS ONE. 2008;3:e3099. doi: 10.1371/journal.pone.0003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunisada T., Yamagishi H., Ogita Z., Kirakawa T., Mitsui Y. Appearance of extrachromosomal circular DNAs during in vivo and in vitro ageing of mammalian cells. Mech. Ageing Dev. 1985;29:89–99. doi: 10.1016/0047-6374(85)90050-8. [DOI] [PubMed] [Google Scholar]
- 24.Libuda D.E., Winston F. Amplification of histone genes by circular chromosome formation in Saccharomyces cerevisiae. Nature. 2006;443:1003–1007. doi: 10.1038/nature05205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soltesz B., Urbancsek R., Pos O., Hajas O., Forgacs I.N., Szilagyi E., Nagy-Balo E., Szemes T., Csanadi Z., Nagy B. Quantification of peripheral whole blood, cell-free plasma and exosome encapsulated mitochondrial DNA copy numbers in patients with atrial fibrillation. J. Biotechnol. 2019;299:66–71. doi: 10.1016/j.jbiotec.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Reddel R.R. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 2003;194:155–162. doi: 10.1016/S0304-3835(02)00702-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S., Wang F., Liu L. Alternative Lengthening of Telomeres (ALT) in Tumors and Pluripotent Stem Cells. Genes. 2019;10:1030. doi: 10.3390/genes10121030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yerlici V.T., Lu M.W., Hoge C.R., Miller R.V., Neme R., Khurana J.S., Bracht J.R., Landweber L.F. Programmed genome rearrangements in Oxytricha produce transcriptionally active extrachromosomal circular DNA. Nucleic Acids Res. 2019;47:9741–9760. doi: 10.1093/nar/gkz725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Wang M., Djekidel M.N., Chen H., Liu D., Alt F.W., Zhang Y. eccDNAs are apoptotic products with high innate immunostimulatory activity. Nature. 2021;599:308–314. doi: 10.1038/s41586-021-04009-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T., Chen Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018;215:1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao F., Zheng T., Gong W., Wu J., Xie H., Li W., Zhang R., Liu P., Liu J., Wu X., et al. Extracellular vesicles package dsDNA to aggravate Crohn’s disease by activating the STING pathway. Cell Death Dis. 2021;12:815. doi: 10.1038/s41419-021-04101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erdal E., Haider S., Rehwinkel J., Harris A.L., McHugh P.J. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 2017;31:353–369. doi: 10.1101/gad.289769.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H., Wang H., Ren J., Chen Q., Chen Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA. 2017;114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu Q., Man S.M., Gurung P., Liu Z., Vogel P., Lamkanfi M., Kanneganti T.D. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 2014;193:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saitoh T., Fujita N., Hayashi T., Takahara K., Satoh T., Lee H., Matsunaga K., Kageyama S., Omori H., Noda T., et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. USA. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Q., Seo G.J., Choi Y.J., Kwak M.J., Ge J., Rodgers M.A., Shi M., Leslie B.J., Hopfner K.P., Ha T., et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–238. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowdell A.S., Colgan S.P. Metabolic Host-Microbiota Interactions in Autophagy and the Pathogenesis of Inflammatory Bowel Disease (IBD) Pharmaceuticals. 2021;14:708. doi: 10.3390/ph14080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoshani O., Brunner S.F., Yaeger R., Ly P., Nechemia-Arbely Y., Kim D.H., Fang R., Castillon G.A., Yu M., Li J.S.Z., et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature. 2021;591:137–141. doi: 10.1038/s41586-020-03064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umbreit N.T., Zhang C.Z., Lynch L.D., Blaine L.J., Cheng A.M., Tourdot R., Sun L., Almubarak H.F., Judge K., Mitchell T.J., et al. Mechanisms generating cancer genome complexity from a single cell division error. Science. 2020;368:6488. doi: 10.1126/science.aba0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C.Z., Spektor A., Cornils H., Francis J.M., Jackson E.K., Liu S., Meyerson M., Pellman D. Chromothripsis from DNA damage in micronuclei. Nature. 2015;522:179–184. doi: 10.1038/nature14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ly P., Cleveland D.W. Rebuilding Chromosomes After Catastrophe: Emerging Mechanisms of Chromothripsis. Trends Cell Biol. 2017;27:917–930. doi: 10.1016/j.tcb.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly P., Teitz L.S., Kim D.H., Shoshani O., Skaletsky H., Fachinetti D., Page D.C., Cleveland D.W. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat. Cell Biol. 2017;19:68–75. doi: 10.1038/ncb3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stephens P.J., Greenman C.D., Fu B., Yang F., Bignell G.R., Mudie L.J., Pleasance E.D., Lau K.W., Beare D., Stebbings L.A., et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Risques R.A., Lai L.A., Brentnall T.A., Li L., Feng Z., Gallaher J., Mandelson M.T., Potter J.D., Bronner M.P., Rabinovitch P.S. Ulcerative colitis is a disease of accelerated colon aging: Evidence from telomere attrition and DNA damage. Gastroenterology. 2008;135:410–418. doi: 10.1053/j.gastro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira C., Coelho R., Gracio D., Dias C., Silva M., Peixoto A., Lopes P., Costa C., Teixeira J.P., Macedo G., et al. DNA Damage and Oxidative DNA Damage in Inflammatory Bowel Disease. J. Crohns Colitis. 2016;10:1316–1323. doi: 10.1093/ecco-jcc/jjw088. [DOI] [PubMed] [Google Scholar]
- 49.Obtulowicz T., Swoboda M., Speina E., Gackowski D., Rozalski R., Siomek A., Janik J., Janowska B., Ciesla J.M., Jawien A., et al. Oxidative stress and 8-oxoguanine repair are enhanced in colon adenoma and carcinoma patients. Mutagenesis. 2010;25:463–471. doi: 10.1093/mutage/geq028. [DOI] [PubMed] [Google Scholar]
- 50.Dillon L.W., Kumar P., Shibata Y., Wang Y.H., Willcox S., Griffith J.D., Pommier Y., Takeda S., Dutta A. Production of Extrachromosomal MicroDNAs Is Linked to Mismatch Repair Pathways and Transcriptional Activity. Cell Rep. 2015;11:1749–1759. doi: 10.1016/j.celrep.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lans H., Hoeijmakers J.H.J., Vermeulen W., Marteijn J.A. The DNA damage response to transcription stress. Nat. Rev. Mol. Cell Biol. 2019;20:766–784. doi: 10.1038/s41580-019-0169-4. [DOI] [PubMed] [Google Scholar]
- 52.Mehanna P., Gagne V., Lajoie M., Spinella J.F., St-Onge P., Sinnett D., Brukner I., Krajinovic M. Characterization of the microDNA through the response to chemotherapeutics in lymphoblastoid cell lines. PLoS ONE. 2017;12:e0184365. doi: 10.1371/journal.pone.0184365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lui Y.Y., Chik K.W., Chiu R.W., Ho C.Y., Lam C.W., Lo Y.M. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin. Chem. 2002;48:421–427. doi: 10.1093/clinchem/48.3.421. [DOI] [PubMed] [Google Scholar]
- 54.Zhang S., Liang Y., Yao J., Li D.F., Wang L.S. Role of Pyroptosis in Inflammatory Bowel Disease (IBD): From Gasdermins to DAMPs. Front. Pharmacol. 2022;13:833588. doi: 10.3389/fphar.2022.833588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Q., Shi P., Wang Y., Zou D., Wu X., Wang D., Hu Q., Zou Y., Huang Z., Ren J., et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell Biol. 2019;11:496–508. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimizu T., Marusawa H., Endo Y., Chiba T. Inflammation-mediated genomic instability: Roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–1206. doi: 10.1111/j.1349-7006.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Techer H., Pasero P. The Replication Stress Response on a Narrow Path between Genomic Instability and Inflammation. Front. Cell Dev. Biol. 2021;9:702584. doi: 10.3389/fcell.2021.702584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuschak T.I., Kuschak B.C., Taylor C.L., Wright J.A., Wiener F., Mai S. c-Myc initiates illegitimate replication of the ribonucleotide reductase R2 gene. Oncogene. 2002;21:909–920. doi: 10.1038/sj.onc.1205145. [DOI] [PubMed] [Google Scholar]
- 59.Kuschak T.I., Taylor C., McMillan-Ward E., Israels S., Henderson D.W., Mushinski J.F., Wright J.A., Mai S. The ribonucleotide reductase R2 gene is a non-transcribed target of c-Myc-induced genomic instability. Gene. 1999;238:351–365. doi: 10.1016/S0378-1119(99)00341-8. [DOI] [PubMed] [Google Scholar]
- 60.Kuttler F., Mai S. Formation of non-random extrachromosomal elements during development, differentiation and oncogenesis. Semin. Cancer Biol. 2007;17:56–64. doi: 10.1016/j.semcancer.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Rauh P., Rickes S., Fleischhacker M. Microsatellite alterations in free-circulating serum DNA in patients with ulcerative colitis. Dig. Dis. 2003;21:363–366. doi: 10.1159/000075361. [DOI] [PubMed] [Google Scholar]
- 62.Koike Y., Uchida K., Tanaka K., Ide S., Otake K., Okita Y., Inoue M., Araki T., Mizoguchi A., Kusunoki M. Dynamic pathology for circulating free DNA in a dextran sodium sulfate colitis mouse model. Pediatr. Surg. Int. 2014;30:1199–1206. doi: 10.1007/s00383-014-3607-6. [DOI] [PubMed] [Google Scholar]
- 63.Maronek M., Gromova B., Liptak R., Klimova D., Cechova B., Gardlik R. Extracellular DNA is Increased in Dextran Sulphate Sodium-Induced Colitis in Mice. Folia Biol. 2018;64:167–172. doi: 10.14712/fb2018064050167. [DOI] [PubMed] [Google Scholar]
- 64.Maronek M., Gromova B., Liptak R., Konecna B., Pastorek M., Cechova B., Harsanyova M., Budis J., Smolak D., Radvanszky J., et al. Extracellular DNA Correlates with Intestinal Inflammation in Chemically Induced Colitis in Mice. Cells. 2021;10:81. doi: 10.3390/cells10010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubiritova Z., Radvanszky J., Gardlik R. Cell-Free Nucleic Acids and their Emerging Role in the Pathogenesis and Clinical Management of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019;20:3662. doi: 10.3390/ijms20153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vrablicova Z., Tomova K., Tothova L., Babickova J., Gromova B., Konecna B., Liptak R., Hlavaty T., Gardlik R. Nuclear and Mitochondrial Circulating Cell-Free DNA Is Increased in Patients with Inflammatory Bowel Disease in Clinical Remission. Front. Med. 2020;7:593316. doi: 10.3389/fmed.2020.593316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boyapati R.K., Dorward D.A., Tamborska A., Kalla R., Ventham N.T., Doherty M.K., Whitfield P.D., Gray M., Loane J., Rossi A.G., et al. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released during Active IBD. Inflamm. Bowel Dis. 2018;24:2113–2122. doi: 10.1093/ibd/izy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fuchs T.A., Kremer Hovinga J.A., Schatzberg D., Wagner D.D., Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012;120:1157–1164. doi: 10.1182/blood-2012-02-412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sin S.T.K., Jiang P., Deng J., Ji L., Cheng S.H., Dutta A., Leung T.Y., Chan K.C.A., Chiu R.W.K., Lo Y.M.D. Identification and characterization of extrachromosomal circular DNA in maternal plasma. Proc. Natl. Acad. Sci. USA. 2020;117:1658–1665. doi: 10.1073/pnas.1914949117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shoura M.J., Gabdank I., Hansen L., Merker J., Gotlib J., Levene S.D., Fire A.Z. Intricate and Cell Type-Specific Populations of Endogenous Circular DNA (eccDNA) in Caenorhabditis elegans and Homo sapiens. G3. 2017;7:3295–3303. doi: 10.1534/g3.117.300141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen S., Regev A., Lavi S. Small polydispersed circular DNA (spcDNA) in human cells: Association with genomic instability. Oncogene. 1997;14:977–985. doi: 10.1038/sj.onc.1200917. [DOI] [PubMed] [Google Scholar]
- 73.Smith C.A., Vinograd J. Small polydisperse circular DNA of HeLa cells. J. Mol. Biol. 1972;69:163–178. doi: 10.1016/0022-2836(72)90222-7. [DOI] [PubMed] [Google Scholar]
- 74.Krolewski J.J., Schindler C.W., Rush M.G. Structure of extrachromosomal circular DNAs containing both the Alu family of dispersed repetitive sequences and other regions of chromosomal DNA. J. Mol. Biol. 1984;174:41–54. doi: 10.1016/0022-2836(84)90364-4. [DOI] [PubMed] [Google Scholar]
- 75.Krolewski J.J., Bertelsen A.H., Humayun M.Z., Rush M.G. Members of the Alu family of interspersed, repetitive DNA sequences are in the small circular DNA population of monkey cells grown in culture. J. Mol. Biol. 1982;154:399–415. doi: 10.1016/S0022-2836(82)80003-X. [DOI] [PubMed] [Google Scholar]
- 76.Cohen S., Lavi S. Induction of circles of heterogeneous sizes in carcinogen-treated cells: Two-dimensional gel analysis of circular DNA molecules. Mol. Cell Biol. 1996;16:2002–2014. doi: 10.1128/MCB.16.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sanborn J.Z., Salama S.R., Grifford M., Brennan C.W., Mikkelsen T., Jhanwar S., Katzman S., Chin L., Haussler D. Double minute chromosomes in glioblastoma multiforme are revealed by precise reconstruction of oncogenic amplicons. Cancer Res. 2013;73:6036–6045. doi: 10.1158/0008-5472.CAN-13-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogt N., Gibaud A., Lemoine F., de la Grange P., Debatisse M., Malfoy B. Amplicon rearrangements during the extrachromosomal and intrachromosomal amplification process in a glioma. Nucleic Acids Res. 2014;42:13194–13205. doi: 10.1093/nar/gku1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu K., Ding L., Chang T.C., Shao Y., Chiang J., Mulder H., Wang S., Shaw T.I., Wen J., Hover L., et al. Structure and evolution of double minutes in diagnosis and relapse brain tumors. Acta Neuropathol. 2019;137:123–137. doi: 10.1007/s00401-018-1912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Na S.Y., Moon W. Recent advances in surveillance colonoscopy for dysplasia in inflammatory bowel disease. Clin. Endosc. 2022;55:726–735. doi: 10.5946/ce.2022.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.