Abstract
TAK, a multisubunit cellular protein kinase that specifically associates with the human immunodeficiency virus Tat proteins and hyperphosphorylates the carboxyl-terminal domain of RNA polymerase II, is a cofactor for Tat and mediates its transactivation function. The catalytic subunit of TAK has been identified as cyclin-dependent kinase Cdk9, and its regulatory partner has been identified as cyclin T1; these proteins are also components of positive transcription elongation factor P-TEFb. TAK activity is up-regulated upon activation of peripheral blood lymphocytes and following macrophage differentiation of promonocytic cell lines. We have found that activation of peripheral blood lymphocytes results in increased mRNA and protein levels of both Cdk9 and cyclin T1. Cdk9 and cyclin T1 induction occurred in purified CD4+ primary T cells activated by a variety of stimuli. In contrast, phorbol ester-induced differentiation of promonocytic cell lines into macrophage-like cells produced a large induction of cyclin T1 protein expression from nearly undetectable levels, while Cdk9 protein levels remained at a constant high level. Measurements of cyclin T1 mRNA levels in a promonocytic cell line suggested that regulation of cyclin T1 occurs at a posttranscriptional level. These results suggest that cyclin T1 and TAK function may be required in differentiated monocytes and further show that TAK activity can be regulated by distinct mechanisms in different cell types.
The efficient transcription of human immunodeficiency virus (HIV) genes is dependent on the viral transactivator protein Tat. Tat enhances the processivity of elongation of RNA polymerase II (RNAP II) complexes that initiate in the HIV long terminal repeat region. Tat is unique as a transcriptional activator in that its cis-acting response sequence is a highly structured RNA element, TAR RNA, that is located at the 5′ ends of nascent viral transcripts. The Tat protein contains two regions that are important for its function—an arginine-rich region that mediates the binding to TAR RNA and an activation domain that mediates the interaction with the cellular transcription machinery (24).
Although a number of cellular factors that interact with the Tat activation domain have been identified, recent work indicates that Tat transactivation function is mediated by TAK, the Tat-associated kinase (5, 7, 23). TAK was originally identified as a cellular protein kinase activity that specifically interacts with the activation domain of Tat and hyperphosphorylates the carboxyl-terminal domain (CTD) of RNAP II (18, 19). Because phosphorylation of the CTD is thought to regulate the elongation activity of RNAP II (6), this property of TAK suggested a model for Tat function through recruitment of TAK to the TAR RNA stem-loop (19). TAK would then be positioned to hyperphosphorylate the CTD, promoting the formation of highly processive elongation complexes. Although the activities of other cellular proteins may be regulated through phosphorylation by TAK, experimental evidence has confirmed that the CTD is indeed required for Tat transactivation (2, 30, 41).
TAK is composed of at least two subunits—the catalytic subunit cyclin-dependent kinase Cdk9 (previously named PITALRE) and the regulatory subunit cyclin T1 (39, 40, 43). Both of these proteins are also present in P-TEFb, a positive transcription elongation factor that was originally identified in Drosophila as a DRB-sensitive CTD kinase that is required for efficient elongation of many genes (28, 29, 43). Complexes containing Cdk9 and cyclin T1-related proteins, cyclin T2a or cyclin T2b, are also active for P-TEFb activity (32). It has been demonstrated that direct and specific binding of cyclin T1 to the activation domain of Tat mediates the association of Tat with TAR RNA (39). Therefore, cyclin T1 is a direct cellular target of Tat that is required for specific and high-affinity binding to TAR RNA. There is no evidence that Tat interacts directly with Cdk9; rather, Cdk9 is recruited to TAR RNA via cyclin T1. It has not yet been demonstrated that cyclin T2 can function like cyclin T1 in directing the binding of Tat to TAR RNA.
Support for a critical role of TAK in Tat transactivation comes from several independent lines of investigation. First, there is a precise correlation between the ability of Tat mutant proteins to associate with TAK and their ability to support Tat transactivation (18, 19, 41). Second, there is a strong correlation between the ability of protein kinase inhibitors such as the nucleoside analog DRB to inhibit TAK (and P-TEFb) activity and their ability to block Tat transactivation (19, 27). Third, introduction of cyclin T1 restores Tat transactivation in murine cells that normally do not support Tat transactivation through TAR RNA (39). Finally, dominant negative mutants of Cdk9 selectively inhibit Tat transactivation in vivo and in vitro (12, 27).
Because primary targets of HIV infection are peripheral blood lymphocytes (PBLs) and monocytic cells, we were interested in determining the mechanisms of regulation of cyclin T1 or Cdk9 expression in these cell types, particularly in response to cell activation- or differentiation-associated stimuli. Previously, we showed that TAK activity is up-regulated following activation of PBLs and differentiation of a promonocytic cell line (40). We now report that the mRNA and protein levels of both cyclin T1 and Cdk9 are increased as quiescent T cells are activated. Interestingly, the expression of cyclin T1 is very low in two actively growing promonocytic cell lines but is greatly increased as the cells are induced by phorbol ester to differentiate into macrophage-like cells, concurrent with increased TAK activity. The induction of cyclin T1 expression appears to occur by a posttranscriptional mechanism. Cdk9 is detected at high levels in cycling promonocytic cells and remains high following differentiation. These results indicate that cyclin T1 is limiting in promonocytic cells and that its function may be required in differentiated monocytes. Furthermore, these results show that distinct mechanisms for the regulation of TAK activity exist in PBLs and monocytic cell lines.
MATERIALS AND METHODS
Cells and preparation of cell extracts.
PBLs were obtained from heparinized blood drawn from healthy hepatitis B virus- and HIV-seronegative donors (obtained from the Gulf Coast Regional Blood Center) and purified by centrifugation through Isolymph (Gallard/Schlesinger). Following two rounds of depletion of monocytes by plastic adherence, lymphocytes were consistently ≥91% pure as determined by flow cytometry (Coulter EPICS) with two-color staining for CD14 and CD45. Monocyte contamination was less than 2%. Viability of PBLs as determined by trypan blue exclusion was ≥98%. For activation, cells were adjusted to 1 × 106 to 2 × 106/ml and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics. Where indicated, the medium also contained phytohemagglutinin (PHA) or phorbol 12-myristate 13-acetate (PMA) used at a final concentration of 1 μg/ml or 1 ng/ml, respectively. PHA was dissolved in phosphate-buffered saline, and PMA was dissolved in dimethyl sulfoxide (DMSO); an equal volume of DMSO solvent was added to control cultures. At the times indicated in the figure legends, cells were washed in phosphate-buffered saline and lysed in EBC buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 0.5% Nonidet P-40, 5 mM dithiothreitol) containing protease inhibitors (aprotinin, leupeptin, and phenylmethylsulfonyl fluoride) as described previously (19).
For CD4+-T-cell experiments, following apheresis of healthy donors, PBLs were isolated by Percoll gradient centrifugation and CD4+ T cells were purified as described previously (25). Purified CD4+ cells (90 to 98% pure) were cultured at 106 cells/ml in RPMI 1640 medium with 10% fetal calf serum, 20 mM HEPES, 2 mM glutamine, and 50 μg of gentamicin/ml for 72 h. Cells were stimulated with either 5 μg of PHA and 100 U of interleukin-2 (IL-2) per ml, 1.2 ng of PMA and 80 ng of ionomycin per ml, or Dynal M-450 antibody-coated beads at a ratio of 1 bead per cell. Magnetic beads were coated via tosyl conjugation with equal amounts of anti-CD3 and anti-CD28 monoclonal antibodies (37).
The promonocytic cell lines HL-60 and U937 were maintained in RPMI 1640 medium supplemented with 10% FBS and antibiotics. The density of the cells was maintained between 2 × 105 and 8 × 105 cells/ml. For experiments, cells were adjusted to 2 × 105/ml and treated with 1 ng of PMA/ml (unless otherwise noted in the figure legends) or an equal volume of DMSO solvent (at a concentration not higher than 0.01%). By 24 h in the presence of PMA, cells were adherent and altered morphology was observed. In some experiments, cells were analyzed by flow cytometry following propidium iodide staining (21) and by analysis of Cdk2 kinase activity with histone H1 as an exogenous substrate (21) to monitor changes in the cell cycle. For kinase assays and immunoblots, extracts were prepared as described previously (19). Protein concentrations were determined with a Bio-Rad protein assay, and equal protein amounts, generally 25 μg for kinase assays and 15 μg for immunoblots, were used.
Kinase assays.
Kinase assays were performed as described by Herrmann and Rice (19). Briefly, Tat-2 was expressed in bacteria as a fusion with glutathione S-transferase (GST) and purified by adsorption to glutathione beads. The GST–Tat-2 bead complex was then incubated with extracts prepared from PBLs or the promonocytic cell lines. The complexes were washed extensively and then incubated under kinase reaction conditions (50 mM Tris [pH 7.4], 5 mM MgCl2, 2.5 mM MnCl2, 5 μM ATP, and 5 μCi [γ-32P]ATP) (3,000 Ci/mmol) for 60 min at room temperature in the presence of GST-CTD (200 ng) added as an exogenous substrate. Protein complexes were resolved by electrophoresis on 9% sodium dodecyl sulfate-polyacrylamide gels. CTDo phosphorylation was quantified by PhosphorImager scanning.
Immunoblots and immunoprecipitations.
Immunoblotting (Western blotting) was performed by standard procedures by using enhanced chemiluminescence for detection as described previously (17). Anti-cyclin T1 antibodies (39) were used at a dilution of 1:6,000. Anti-cyclin T2 antibodies were obtained from D. Price (32) and were used at a dilution of 1:2,000. Antibodies directed against Cdk9 (PITALRE), Cdk7, Cdk2, and cyclin H were purchased from Santa Cruz Biotechnology and used at a dilution of 1:5,000. Immunoprecipitations were performed as described previously (41).
Isolation of RNA and Northern blot analysis.
Total cellular RNA was isolated using TRIzol reagent as recommended by the manufacturer (Life Technologies). Poly(A)+ RNA was prepared from HL-60 RNA with a Poly(A)Pure kit and from PBL RNA with a MicroPoly(A)Pure kit as recommended by the manufacturer (Ambion). Poly(A)+ RNA was quantified by ethidium bromide staining relative to a standard of total cellular RNA of known concentration. RNA was electrophoresed through a 1% agarose formaldehyde gel and transferred to nylon membranes. For preparation of 32P-labeled probes, Cdk9, cyclin T1, and β-actin cDNAs were labeled with [α-32P]dCTP with a High Prime system (Boehringer Mannheim) random-primed DNA labeling reaction.
RESULTS
Cdk9 and cyclin T1 protein levels are induced following T-cell activation.
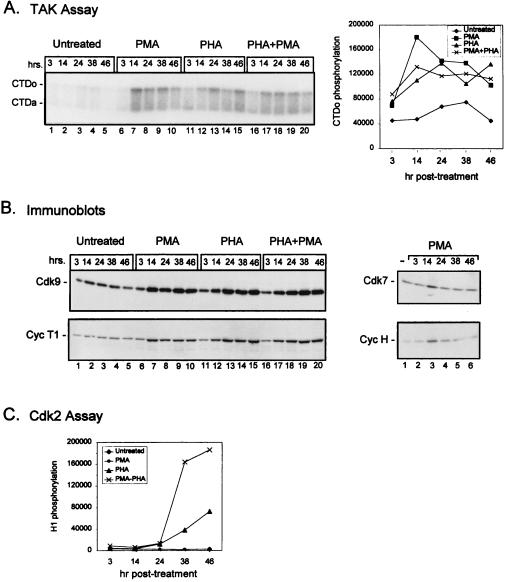
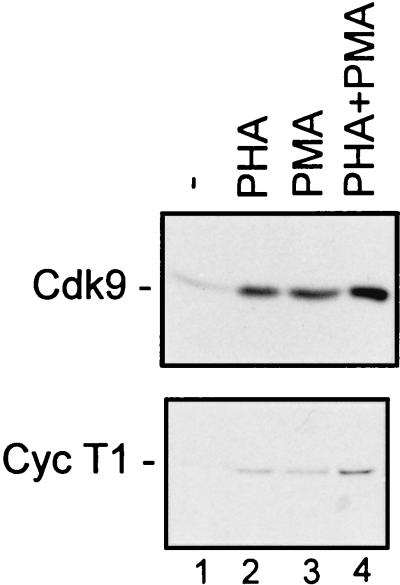
Previously, we showed that TAK activity, as determined by CTD hyperphosphorylation, increases 3- to 5-fold following activation of PBLs by PHA, PMA, or PHA plus PMA (reference 40; see also Fig. 3). To understand the mechanism of regulation of TAK activity, we wanted to determine whether the increase in kinase activity results from changes in the steady-state levels of the two known subunits of TAK, Cdk9 and cyclin T1. Therefore, PBLs purified from healthy uninfected individuals (see Materials and Methods) were cultured with PHA and PMA alone or in combination, and the protein levels of Cdk9 and cyclin T1 were examined by immunoblot analysis (Fig. 1). The levels of both Cdk9 and cyclin T1 were elevated in cells stimulated by either compound alone or used together. By quantitative Western blot analysis with twofold dilutions of extracts, we estimate that Cdk9 levels increased two- to fourfold following treatment with either PHA or PMA alone and four- to eightfold following that with PHA plus PMA (data not shown). Cyclin T1 levels were 4- to 8-fold higher in PHA- or PMA-treated cells and 8- to 16-fold higher in PHA plus PMA-treated cells.
FIG. 3.
Time course of induction of TAK activity in stimulated PBLs. PBLs were cultured in media containing 10% FBS (lanes 1–5) or in the presence of PMA (1 ng/ml) (lanes 6–10), PHA (1 μg/ml) (lanes 11–15), or PMA plus PHA (lanes 16–20) for the indicated times. Cell lysates were prepared, and equal amounts of protein were assayed for TAK activity by a kinase assay with recombinant CTD as a substrate (A) or for protein levels of Cdk9, cyclin T1 (Cyc T1), Cdk7, or cyclin H (Cyc H) by immunoblotting (B). The numbers at the top of the blots indicate the number of hours (hrs.) post-PMA treatment, while the numbers at the bottom represent lane numbers. The transient increase in expression seen at 14 h in PMA-treated cells was not reproducible. CTDo, hyperphosphorylated form of the CTD; CTDa, underphosphorylated form of the CTD. (C) Equal amounts of protein were also analyzed for Cdk2 kinase activity by using histone H1 as an exogenous substrate. Quantitation of the TAK and Cdk2 assays was performed by measurement of CTDo or histone H1 phosphorylation, respectively, as determined by PhosphorImager scanning.
FIG. 1.
Cdk9 and cyclin T1 protein levels are increased following activation of PBLs. PBLs were cultured in media containing 10% FBS (−) (lane 1) or with PHA at a final concentration of 1 μg/ml (lane 2), PMA at a final concentration of 1 ng/ml (lane 3), or PHA and PMA together (lane 4). After 48 h, cells were lysed and protein concentrations were determined. Equal amounts of protein were analyzed for Cdk9 or cyclin T1 (Cyc T1) levels by immunoblotting. See Materials and Methods for experimental details.
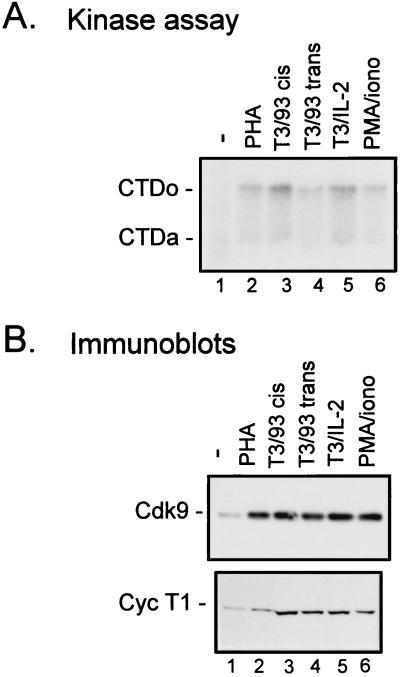
Because the CD4+ subset of T cells is the major target of HIV infection, we wanted to determine whether TAK activity is regulated in CD4+ cells. Primary CD4+ T cells were purified from PBLs by negative selection with magnetic beads coated with antibodies to remove non-CD4+ cells (25). When purified CD4+ cells were stimulated with PHA, an eightfold induction of TAK activity was observed (Fig. 2A, lane 2) and both Cdk9 and cyclin T1 levels were increased (Fig. 2B, lane 2). Induction of TAK activity and increases of Cdk9 and cyclin T1 levels were also seen when cells were cultured with PMA and ionomycin (Fig. 2, lanes 6). To examine TAK activity and protein levels in T cells activated by more physiological stimuli, CD4+ cells were incubated with bead-immobilized antibodies against the CD3 component of the T-cell receptor complex. Costimulatory signals were provided by IL-2 or by antibodies to the costimulatory receptor CD28 present either on the same bead as the anti-CD3 antibody (cis) or on separate beads (trans) (Fig. 2, lanes 3 to 5). All of these signals were capable of up-regulating TAK activity and increasing Cdk9 and cyclin T1 protein levels. Similar results were obtained with primary CD8+ cells (not shown). Thus, TAK activity can be induced by a variety of stimuli in primary CD4+ cells, indicating that TAK induction is not dependent on a single T-cell activation pathway.
FIG. 2.
Cdk9 and cyclin T1 protein levels are increased following activation of CD4+ T cells by a variety of activating stimuli. Primary CD4+ cells were cultured in media containing 10% FBS (−) (lanes 1) or with the addition of PHA (5 μg/ml) (lanes 2), antibodies against CD3 and CD28 (9.3) on the same bead complex (T3/93 cis) (lanes 3) or on separate beads (T3/93 trans; beads used at a ratio of 1 bead/cell) (lanes 4), IL-2 (100 U/ml) and antibody against CD3 (T3/IL-2) (lanes 5), or PMA (1.2 ng/ml) and ionomycin (0.08 μg/ml) (PMA/iono) (lanes 6). After 3 days, cells were lysed and equal amounts of protein were assayed for TAK activity by a kinase assay with recombinant CTD as a substrate (A) or for protein levels of Cdk9 or cyclin T1 (Cyc T1) by immunoblotting (B). CTDo, hyperphosphorylated form of the CTD; CTDa, underphosphorylated form of the CTD.
T-cell activation is characterized by a series of changes in gene expression patterns, with genes required for T-cell activation being induced at immediate (<30 min), early, or late (>2 days) times (38). To determine when TAK induction occurs, we stimulated PBLs with PMA, PHA, or PMA plus PHA and harvested cells at various time points following induction. For all of these conditions, there was a slight increase in TAK activity at 3 h but full induction was seen at the 14- or 24-h time points and remained fairly steady thereafter (Fig. 3A). The enhancement of TAK activity was reflected in the levels of Cdk9 and cyclin T1 at the various time points (Fig. 3B). Therefore TAK is induced with early kinetics.
We also analyzed the levels of Cdk7 and cyclin H, another Cdk-cyclin pair that is involved in transcriptional regulation and has been reported to interact with Tat (4, 11, 31). Unlike Cdk9 and cyclin T1, Cdk7 and cyclin H did not show a sustained increase in expression following stimulation with PMA (Fig. 3B). This is consistent with the lack of induction by PMA of Cdk7-associated kinase activity towards the CTD (20, 40). Treatment of PBLs with PHA did result in an increase in cyclin H levels by 38 h and a concomitant increase in Cdk7-associated CTD kinase activity (data not shown). These results imply that the temporal regulation of Cdk7-cyclin H is distinct from that of Cdk9-cyclin T1 and that only expression of Cdk9 and cyclin T1 parallels the sustained increase in TAK activity.
Because Cdks and cyclins are known to be induced following stimulation of quiescent cells, induction of Cdk9 and cyclin T1 expression was not unexpected. As a control for cell cycle entry, we measured the kinase activity of Cdk2 using histone H1 as a substrate. Cdk2, the major cell cycle regulator, becomes active in late G1. Unlike TAK activity, Cdk2 activity was not induced by PMA (Fig. 3C). PHA treatment resulted in stimulation of Cdk2 activity beginning at 24 h and increasing up to 48 h. As expected, the highest level of Cdk2 activity was observed in PBLs activated by both PMA and PHA at the 38- and 48-h time points, because the combination of PMA and PHA fully activates T cells (see Discussion).
Cdk9 and cyclin T1 mRNA levels are induced following T- cell activation.
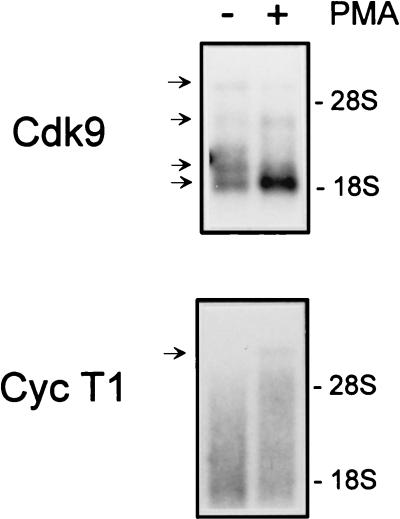
To determine whether the induction of Cdk9 and cyclin T1 protein levels reflects an increase in mRNA levels, poly(A)+-selected RNA was isolated from control and PMA-treated PBLs and Cdk9 and cyclin T1 mRNA levels were analyzed by Northern blotting (Fig. 4). Four distinct Cdk9 transcripts were detected. Ubiquitously expressed transcripts of 3.2 and 2.8 kb as well as larger, tissue-specific transcripts have been observed previously (13). Following T-cell activation by PMA, the abundance of the smallest Cdk9 transcript increased significantly while levels of the other Cdk9 transcripts increased slightly or remained constant. For cyclin T1, a single discrete transcript of ∼8 kb was detected, consistent with previous reports (32, 39). The level of the ∼8-kb RNA in PMA-treated PBLs was elevated relative to that in unstimulated cells. Therefore, regulation of Cdk9 and cyclin T1 expression in activated PBLs occurs at the level(s) of transcription, mRNA processing, and/or mRNA stability.
FIG. 4.
Cdk9 and cyclin T1 mRNA levels are induced following PBL activation. PBLs were cultured in media containing 10% FBS (−) or in the presence of PMA (1 ng/ml) (+) for 24 h. Poly(A)+ RNA was isolated as described in Materials and Methods. Equal amounts of RNA (∼2 μg) were electrophoresed through a 1% agarose formaldehyde gel, transferred to nylon membranes, and probed for Cdk9 or cyclin T1 (Cyc T1). 28S and 18S rRNAs were visualized by ethidium bromide staining of the gel to demonstrate RNA integrity; the positions of these bands are indicated on the right. The blots were exposed to film for 1 day for Cdk9 or 5 days for cyclin T1.
Cyclin T1, but not Cdk9, protein levels are induced following differentiation of monocytic cells.
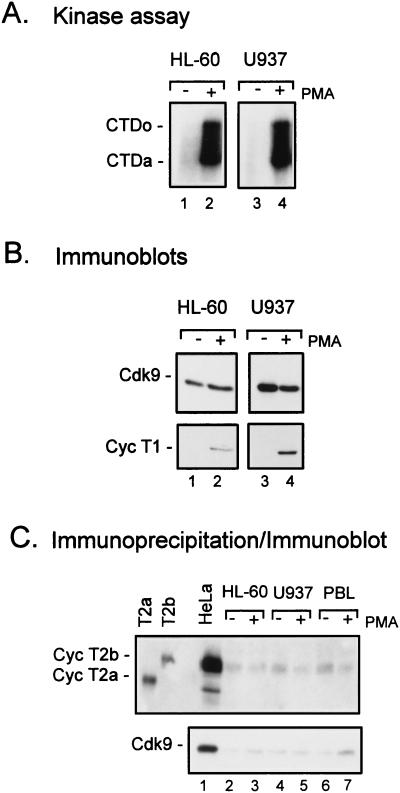
Another major target of HIV infection is cells of the monocyte lineage. Previously, we showed that TAK activity is stimulated when promonocytic cell lines are induced to differentiate into macrophage-like cells (40). To examine levels of Cdk9 and cyclin T1 in monocytic cells, we used two different promonocytic cell lines, HL-60 and U937, that can be induced to differentiate along the monocytic pathway by PMA and other agents (15). PMA treatment of HL-60 and U937 cells resulted in 9- and 11-fold increases in TAK activity, respectively, as measured by CTDo phosphorylation (Fig. 5A). While Cdk9 protein levels were high in actively growing, untreated cells, cyclin T1 was virtually undetectable in both cell lines (Fig. 5B). When cells were treated with PMA, a large induction in cyclin T1 levels was observed in both HL-60 and U937 cells. By quantitative Western blot analysis with twofold dilutions of extract, we estimate that cyclin T1 levels are increased by PMA treatment at least 16-fold in HL-60 and U937 cells. This result suggests that cyclin T1 is limiting for TAK activity in these cell lines.
FIG. 5.
Cyclin T1, but not Cdk9 or cyclin T2, protein levels are increased upon PMA treatment of promonocytic cell lines. The promonocytic cell lines HL-60 and U937 were cultured in media containing 10% FBS (−) or with the addition of PMA (1 ng/ml) (+) for 48 h. Cell lysates were prepared, and equal amounts of protein were assayed for TAK activity by a kinase assay with recombinant CTD as a substrate (A) or for protein levels of Cdk9 or cyclin T1 (Cyc T1) by immunoblotting (B). CTDo, hyperphosphorylated form of the CTD; CTDa, underphosphorylated form of the CTD. (C) Cyclin T2 was detected by immunoprecipitation of Cdk9-containing complexes with an antibody directed against Cdk9 followed by immunoblotting with an antibody directed against cyclin T2. Recombinant cyclin T2a (Cyc T2a) and cyclin T2b (Cyc T2b) were used as standards.
Although Cdk9 is expressed at equivalent levels in control and PMA-treated cells, there is little detectable TAK activity in untreated cells. Cdk9 has been shown to associate with two cyclin-T1-related proteins, cyclin T2a and cyclin T2b (32). The absence of TAK activity in normal HL-60 and U937 cells suggests that cyclins T2a and T2b cannot functionally substitute for cyclin T1 to generate TAK activity in these cell lines. However, we wanted to examine the regulation of cyclin T2 in the promonocytic cell lines. Extracts from the same experiment as that shown in Fig. 5B were analyzed for cyclin T2 levels. Because of the low level of cyclin T2 in these cell lines, to detect cyclin T2 it was necessary to immunoprecipitate Cdk9-containing complexes with a Cdk9 antibody and then analyze cyclin T2 levels by immunoblotting using a cyclin T2 antibody, as was done in a previous study with HeLa cells (32). For cyclin T2b, there was no change in protein level following PMA induction in HL-60 or U937 cells (Fig. 5C, lanes 2 to 5). No regulation of cyclin T2b levels was observed in PBLs either (Fig. 5C, lanes 6 and 7). We were not able to detect cyclin T2a in HL-60 or U937 cells or in PBLs. Cyclin T2a was detected in HeLa cells, although at a much lower level than cyclin T2b (Fig. 5C, lane 1). These results argue that the increased levels of TAK activity observed in PMA-treated cells cannot be explained by changes in cyclin T2 levels, at least for cyclin T2b, but rather induction of cyclin T1 parallels changes in TAK activity.
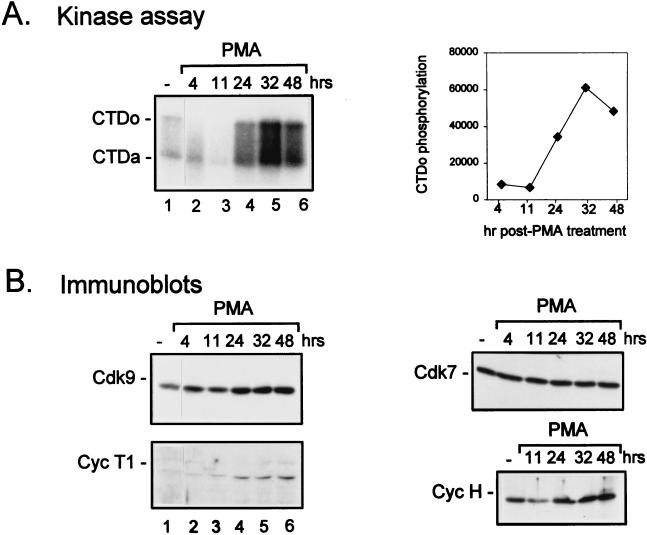
To examine the kinetics of TAK up-regulation in promonocytic cell lines, HL-60 cells were treated with PMA and extracts were prepared at various time points. TAK activity, as measured by CTD hyperphosphorylation, was elevated by 24 h following PMA treatment, peaked at 32 h, and began to decline by 48 h (Fig. 6A), perhaps due to cell death as implied from flow cytometry analysis (data not shown). Analysis of protein levels revealed that Cdk9 remained fairly constant, while cyclin T1 was elevated by 24 h (Fig. 5B), consistent with the enhancement of TAK activity at that time point. The induction of cyclin T1 was specific, in that neither cyclin T2 level (Fig. 5C) nor cyclin H level (Fig. 6B) was increased as a result of PMA treatment. The increases in cyclin T1 protein levels and TAK activity suggest that cyclin T1 and TAK may play a role during monocyte differentiation and/or in terminally differentiated macrophages.
FIG. 6.
Time course of induction of cyclin T1 protein levels following PMA treatment of HL-60 cells. HL-60 cells were cultured in media containing 10% FBS (−) or with the addition of PMA (0.3 ng/ml) for the indicated times. Cell lysates were prepared, and equal amounts of protein were assayed for TAK activity by a kinase assay with recombinant CTD as a substrate (A) or for protein levels of Cdk9 or cyclin T1 (Cyc T1) by immunoblotting (B). Quantitation of the TAK assay was performed by measurement of CTDo phosphorylation as determined by PhosphorImager scanning. CTDo, hyperphosphorylated form of the CTD; CTDa, underphosphorylated form of the CTD; hr and hrs, hours.
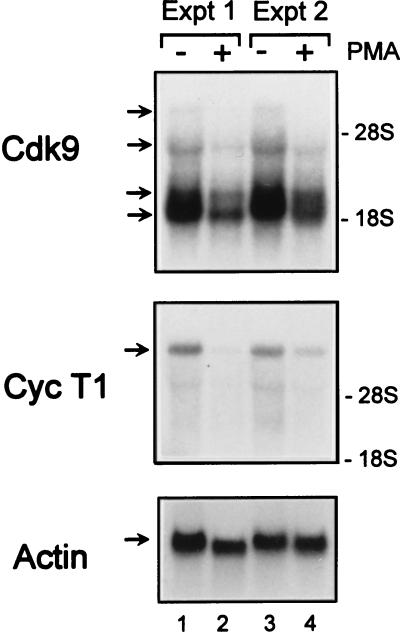
Cyclin T1 is induced by a posttranscriptional mechanism following differentiation of monocytic cells.
To determine whether the PMA-induced increase in cyclin T1 protein abundance is due to elevated levels of cyclin T1 mRNA, Northern blot analysis was performed. A predominant cyclin T1 transcript of ∼8 kb was observed. Unlike the case in PBLs, no increase in cyclin T1 mRNA abundance was observed (Fig. 7). Rather, cyclin T1 mRNA levels were lower following PMA treatment. There were also reductions in the levels of the four Cdk9 transcripts in PMA-treated cells relative to control cells. The blots were reprobed for actin mRNA levels to control for equal loading, and although actin levels were slightly lower in PMA-treated cells in experiment 1 (Fig. 7, lanes 1 and 2), actin levels were equivalent in experiment 2 (Fig. 7, lanes 3 and 4). These results imply that regulation of cyclin T1 expression in monocytic cell lines occurs by a posttranscriptional mechanism.
FIG. 7.
Cdk9 and cyclin T1 mRNA levels are not induced by PMA treatment of HL-60 cells. HL-60 cells were cultured in media containing 10% FBS (−) or with the addition of PMA (1 ng/ml) (+) for 24 h. Poly(A)+ RNA was isolated as described in Materials and Methods. Equal amounts of RNA (∼10 μg) were electrophoresed through a 1% agarose formaldehyde gel, transferred to nylon membranes, and probed for Cdk9, cyclin T1 (Cyc T1), or β-actin. The blots were exposed to film for 2.5 h (Cdk9), 5 h (Cyc T1), or 2 min (β-actin). The positions of 28S and 18S rRNAs, visualized by ethidium bromide staining of marker RNA run on the gel, are indicated on the right. The results of two independent experiments (Expt) are shown.
DISCUSSION
Cdk9 and cyclin T1 are regulated by distinct mechanisms in PBLs and monocytic cells.
Because TAK is a critical cellular cofactor for Tat, it is important to understand the regulation of expression of TAK subunits in cell types that are susceptible to infection by HIV. The two major targets of HIV infection are CD4+ T cells and monocytes/macrophages. We have shown here that mRNA and protein expression of both Cdk9 and cyclin T1 are induced when quiescent T cells are activated. Therefore, both Cdk9 and cyclin T1 are regulated at the mRNA level, during either the process of synthesis, processing, or stability. Because not all Cdk9 transcripts are up-regulated, Cdk9 mRNA expression may be subject to a complex mechanism of regulation.
In contrast to the expression patterns of Cdk9 and cyclin T1 seen in PBLs, when monocytic cell lines are induced to differentiate into macrophage-like cells, Cdk9 levels remain constant, while cyclin T1 protein levels are up-regulated from nearly undetectable levels. The increase in cyclin T1 protein levels does not parallel cyclin T1 mRNA levels, indicating that regulation of cyclin T1 expression occurs at the protein level. To begin to determine whether cyclin T1 induction results from increased protein stability or increased protein synthesis, HL-60 cells were metabolically labeled with [35S]methionine for 1 h (pulse) and then were incubated in the presence of excess unlabeled methionine for 3 h (chase). Although cyclin T1 was difficult to detect in uninduced cells, the level of cyclin T1 appeared unchanged in both control and PMA-treated cells during the 3-h chase period (20). Although further work is required to elucidate the mechanism of this regulation, our data are most consistent with regulation of cyclin T1 expression at the translational level. While regulation of protein stability by ubiquitin-mediated proteolysis is a common regulatory mechanism for cyclins and other proteins involved in cell cycle control, precedents for translational regulation of cyclins exist. For example, expression of the yeast cyclin Cln3 protein is repressed by a translational mechanism during growth arrest resulting from nutrient deprivation conditions (9, 35). Positive control of translation has been observed for the Cdk inhibitor p27 in growth-arrested mammalian cells (16).
The difference in the regulation patterns of Cdk9 and cyclin T1 expression in T cells and monocytic cell lines may reflect inherent differences between lymphocytes and monocytes or the fact that PBLs are primary cells while HL-60 and U937 cells are established cell lines. Alternatively, the difference may be due to the fact that PBLs are quiescent cells that must be activated in order to replicate while the promonocytic cell lines undergo active replication and are induced to withdraw from the cell cycle to undergo terminal differentiation into macrophage-like cells. Nevertheless, these results show that distinct mechanisms for the regulation of TAK activity exist.
Cdk9 and cyclin T1 expression in activated T cells.
Complete T-cell activation in vivo generally requires two stimuli; these signals can be mimicked in vitro by PHA and PMA. In PBLs, Cdk9 and cyclin T1 levels were induced by PMA or PHA alone with a resulting increase in TAK activity (Fig. 1). Although either PMA or PHA allows quiescent T cells to enter the cell cycle, PMA-treated cells progress only into the G1 phase of the cell cycle, while PHA-treated cells can traverse the G1/S boundary. Hence, Cdk2 activity, which is activated in late G1, is activated by PHA but not PMA (Fig. 3C). PMA was also not sufficient to activate Cdk7 activity (20, 40) or protein levels (Fig. 3B). The induction of TAK by PMA implies that TAK becomes active in the G1 phase of the cell cycle prior to activation of Cdk2 or Cdk7 and suggests that TAK may play a role in T-cell activation. In fact, Cdk9 has recently been shown to be able to negatively regulate IL-2 promoter activity in a T-cell line (42).
In addition to up-regulation by PHA and PMA, TAK activity can be up-regulated by a variety of combinations of T-cell activation stimuli, which provide the signals to fully activate T cells (Fig. 2). At this point, it is difficult to distinguish whether TAK activation is dependent on the entry of cells into the cell cycle or whether TAK induction is dependent on the activation of specific T-cell signaling pathways. For example, all the methods of T-cell costimulation employed here act through protein kinase C, raising the possibility that TAK is a downstream target of protein kinase C.
Cyclin T1 induction in differentiated monocytic cell lines.
The human promyelomonocytic HL-60 cell line and myelomonocytic U937 cell line can be induced to terminally differentiate into cells exhibiting monocyte/macrophage characteristics by PMA, vitamin D3, and other agents; HL-60 cells can additionally be induced to differentiate into granulocyte cells by DMSO (3). As shown previously for U937 cells and shown here for HL-60 cells, PMA treatment produced a large induction of TAK activity (40) (Fig. 5A), accompanied by a large increase in the protein levels of cyclin T1. The expression pattern of cyclin T1 following PMA treatment of HL-60 cells is unlike that of other cyclins (for example, see Fig. 5C and 6B). While most cyclins and Cdks involved in cell cycle regulation are down-regulated following differentiation (1), some cyclins that appear not to play a role in cell cycle regulation are preferentially expressed in postmitotic cells (10). The preferential expression of cyclin T1 in PMA-treated promonocytic cell lines suggests that cyclin T1 may play a role in differentiated cells. It is possible that cyclin T1 may be involved in the differentiation program of monocytes or it may perform a function in terminally differentiated cells.
Regulation of TAK and P-TEFb activity.
In addition to being involved in TAK activity, Cdk9 and cyclin T1 are also components of P-TEFb, an activity that is thought to facilitate the transition from abortive to productive elongation (28, 43). Both cyclin T1 and T2 can independently associate with Cdk9 to form distinct P-TEFb complexes (32). It is presently unclear whether cyclin T2-containing complexes function as TAK. To date, we have been unable to demonstrate a specific interaction between Tat and cyclin T2 (20). Until the complete molecular compositions of TAK and P-TEFb are determined, it remains unresolved as to whether TAK and P-TEFb are identical protein complexes or whether TAK is a subset of P-TEFb complexes.
It is intriguing that the regulation of cyclin T2 expression differs from that of cyclin T1. While the cyclin box regions of cyclins T1 and T2 have 81% identity, their carboxyl-terminal regions are significantly less well conserved (32). Although we did not examine P-TEFb activity in PBLs or monocytic cell lines, the control of cyclin T1 expression described here raises the possibility of a mechanism of regulation of P-TEFb activity such that P-TEFb complexes comprised of Cdk9 and cyclin T2 may be active under different conditions from those of Cdk9- and cyclin-T1-containing P-TEFb complexes. This also raises the possibility that the different P-TEFb complexes might perform specialized functions in the cell.
While TAK activity is clearly low in unstimulated PBLs and monocytic cells, it is possible that Cdk9/cyclin T2 complexes could provide sufficient P-TEFb activity for transcriptional elongation of genes that require its function. Although P-TEFb is apparently required for efficient elongation of many genes, based on an analysis of a number of promoters in in vitro transcription extracts derived from Drosophila (29), this study suggests that actively growing HL-60 cells appear not to require high levels of TAK activity. The large induction of TAK activity following PMA treatment suggests that TAK activity is required during differentiation, perhaps to allow the efficient transcription of genes involved in monocyte differentiation or necessary for the specialized function of terminally differentiated cells. It is unclear whether this function is equivalent to P-TEFb activity or whether TAK acquires novel substrates and, consequently, additional functions in differentiated cells. Although Cdk9 is the only known Cdk partner of cyclin T1, we cannot exclude the possibility that cyclin T1 could associate with additional Cdk partners.
Possible implications of TAK regulation for HIV replication.
How might the regulation of TAK activity influence the course of infection by HIV? A speculative hypothesis is that the HIV Tat protein may have evolved to utilize TAK as a cellular cofactor because the cellular state in which TAK is active may be favorable to HIV replication. Perhaps environmental stimuli that lead to Cdk9 and cyclin T1 induction and resultant TAK activation allow the virus to escape from a transcriptionally silent state. Conversely, under conditions where TAK is inactive, Tat may fail to stimulate viral gene expression, causing the virus to enter into a transcriptionally latent state and escape from immune surveillance, as suggested by Emerman and Malim (7).
Promonocytic cell lines have served as a useful model system for HIV transcriptional latency. In a derivative of U937 cells that contain stably integrated HIV provirus and express very low levels of HIV mRNA and protein, HIV gene expression is dramatically enhanced by PMA (36). Transcriptional activation of HIV can also be induced by a number of cytokines, including tumor necrosis factor alpha and IL-6 (14, 33, 34). It has been shown that PMA and tumor necrosis factor alpha lead to the induction of nuclear factor κB, which contributes to, but is not sufficient for, HIV activation in this cell system (14). Activation of TAK may be an additional event that is required for PMA-induced activation of HIV gene expression. It will be interesting to assess the effects of cytokines on cyclin T1 expression and TAK activity. Stimulatory effects of cytokines on HIV gene expression have also been observed in primary monocytes/macrophages (26). Important areas for future research will be examination of Cdk9 and cyclin T1 expression in primary monocytes/macrophages and elucidation of the molecular mechanism of this regulation.
A major challenge in the treatment of HIV-infected patients is the eradication of transcriptionally latent-infected CD4+ memory T cells (8, 22). In this regard, an understanding of signals that induce TAK activity and result in activation of viral expression following latency may be useful. Furthermore, identification of the upstream regulators of TAK activity may provide additional targets for antiviral agents.
ACKNOWLEDGMENTS
We thank Jeff Milton and David Price for cyclin T2 reagents, Phuc Nyugen for technical assistance, and the Baylor Center for AIDS Research flow cytometry core staff for flow cytometry analysis. We are grateful to Wade Harper, Dorothy Lewis, and Xinzhen Yang for helpful discussions.
This work was supported by grants AI42558 (C.H.H.) and AI35381 (A.P.R.) from the National Institutes of Health. P.W. and K.A.J. were supported by grants from the NIH and the Universitywide AIDS Research Program.
REFERENCES
- 1.Burger C, Wick M, Muller R. Lineage-specific regulation of cell cycle gene expression in differentiating myeloid cells. J Cell Sci. 1994;107:2047–2054. doi: 10.1242/jcs.107.7.2047. [DOI] [PubMed] [Google Scholar]
- 2.Chun R F, Jeang K T. Requirements for RNA polymerase II carboxyl-terminal domain for activated transcription of human retroviruses human T-cell lymphotropic virus I and HIV-1. J Biol Chem. 1996;271:27888–27894. doi: 10.1074/jbc.271.44.27888. [DOI] [PubMed] [Google Scholar]
- 3.Collins S J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 4.Cujec T P, Okamoto H, Fujinaga K, Meyer J, Chamberlin H, Morgan D O, Peterlin B M. The HIV transactivator TAT binds to the CDK-activating kinase and activates the phosphorylation of the carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:2645–2657. doi: 10.1101/gad.11.20.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 6.Dahmus M E. Phosphorylation of the C-terminal domain of RNA polymerase II. Biochim Biophys Acta. 1995;1261:171–182. doi: 10.1016/0167-4781(94)00233-s. [DOI] [PubMed] [Google Scholar]
- 7.Emerman M, Malim M H. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science. 1998;280:1880–1884. doi: 10.1126/science.280.5371.1880. [DOI] [PubMed] [Google Scholar]
- 8.Finzi D, Siliciano R F. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 9.Gallego C, Gari E, Colomina N, Herrero E, Aldea M. The Cln3 cyclin is down-regulated by translational repression and degradation during the G1 arrest caused by nitrogen deprivation in budding yeast. EMBO J. 1997;16:7196–7206. doi: 10.1093/emboj/16.23.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao C Y, Zelenka P S. Cyclins, cyclin-dependent kinases and differentiation. Bioessays. 1998;19:307–315. doi: 10.1002/bies.950190408. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez L F, Mavankal G, Neveu J M, Lane W S, Ivanov D, Gaynor R B. Purification of a Tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold M O, Yang X, Herrmann C H, Rice A P. PITALRE, the catalytic subunit of TAK, is required for human immunodeficiency virus Tat transactivation in vivo. J Virol. 1998;72:4448–4453. doi: 10.1128/jvi.72.5.4448-4453.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grana X, De Luca A, Sang N, Fu Y, Claudio P P, Rosenblatt J, Morgan D O, Giordano A. PITALRE, a nuclear CDC2-related protein kinase that phosphorylates the retinoblastoma protein in vitro. Proc Natl Acad Sci USA. 1994;91:3834–3838. doi: 10.1073/pnas.91.9.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffin G E, Leung K, Folks T M, Kunkel S, Nabel G J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989;339:70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- 15.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 16.Hengst L, Reed S I. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann C H, Gold M O, Rice A P. Viral transactivators specifically target distinct cellular protein kinases that phosphorylate the RNA polymerase II C-terminal domain. Nucleic Acids Res. 1996;24:501–508. doi: 10.1093/nar/24.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann C H, Rice A P. Specific interaction of the human immunodeficiency virus Tat proteins with a cellular protein kinase. Virology. 1993;197:601–608. doi: 10.1006/viro.1993.1634. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann, C. H., and A. P. Rice. Unpublished data.
- 21.Herrmann C H, Su L K, Harlow E. Adenovirus E1A is associated with a serine/threonine protein kinase. J Virol. 1991;65:5848–5859. doi: 10.1128/jvi.65.11.5848-5859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho D D. Toward HIV eradication or remission: the tasks ahead. Science. 1998;280:1866–1867. doi: 10.1126/science.280.5371.1866. [DOI] [PubMed] [Google Scholar]
- 23.Jones K A. Taking a new TAK on tat transactivation. Genes Dev. 1997;11:2593–2599. doi: 10.1101/gad.11.20.2593. [DOI] [PubMed] [Google Scholar]
- 24.Jones K A, Peterlin B M. Control of RNA initiation and elongation at the HIV-1 promoter. Annu Rev Biochem. 1994;63:717–743. doi: 10.1146/annurev.bi.63.070194.003441. [DOI] [PubMed] [Google Scholar]
- 25.June C H, Ledbetter J A, Gillespie M M, Lindsten T, Thompson C B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyanagi Y, O’Brien W A, Zhao J Q, Golde D W, Gasson J C, Chen I S. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 27.Mancebo H S, Lee G, Flygare J, Tomassini J, Luu P, Zhu Y, Peng J, Blau C, Hazuda D, Price D, Flores O. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall N F, Peng J, Xie Z, Price D H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J Biol Chem. 1996;271:27176–27183. doi: 10.1074/jbc.271.43.27176. [DOI] [PubMed] [Google Scholar]
- 29.Marshall N F, Price D H. Control of formation of two distinct classes of RNA polymerase II elongation complexes. Mol Cell Biol. 1992;12:2078–2090. doi: 10.1128/mcb.12.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto H, Sheline C T, Corden J L, Jones K A, Peterlin B M. Trans-activation by human immunodeficiency virus Tat protein requires the C-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 1996;93:11575–11579. doi: 10.1073/pnas.93.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parada C A, Roeder R G. Enhanced processivity of RNA polymerase II triggered by Tat-induced phosphorylation of its carboxy-terminal domain. Nature. 1996;384:375–378. doi: 10.1038/384375a0. [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Zhu Y, Milton J T, Price D H. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755–762. doi: 10.1101/gad.12.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poli G, Bressler P, Kinter A, Duh E, Timmer W C, Rabson A, Justement J S, Stanley S, Fauci A S. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–158. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poli G, Kinter A, Justement J S, Kehrl J H, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polymenis M, Schmidt E V. Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast. Genes Dev. 1997;11:2522–2531. doi: 10.1101/gad.11.19.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomerantz R J, Trono D, Feinberg M B, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 37.Riley J L, Carroll R G, Levine B L, Bernstein W, St. Louis D C, Weislow O S, June C H. Intrinsic resistance to T cell infection with HIV type 1 induced by CD28 costimulation. J Immunol. 1997;158:5545–5553. [PubMed] [Google Scholar]
- 38.Ullman K S, Northrop J P, Verweij C L, Crabtree G R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 39.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 40.Yang X, Gold M O, Tang D N, Lewis D E, Aguilar-Cordova E, Rice A P, Herrmann C H. TAK, an HIV Tat-associated kinase, is a member of the cyclin-dependent family of protein kinases and is induced by activation of peripheral blood lymphocytes and differentiation of promonocytic cell lines. Proc Natl Acad Sci USA. 1997;94:12331–12336. doi: 10.1073/pnas.94.23.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Herrmann C H, Rice A P. The human immunodeficiency virus Tat proteins specifically associate with TAK in vivo and require the carboxyl-terminal domain of RNA polymerase II for function. J Virol. 1996;70:4576–4584. doi: 10.1128/jvi.70.7.4576-4584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, X., D. E. Lewis, and A. P. Rice. Unpublished data.
- 43.Zhu Y, Pe’ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]