Abstract
Progressive multifocal leukoencephalopathy is a demyelinating disease of the human central nervous system that results from lytic infection of oligodendrocytes by the polyomavirus JC (JCV). Originally, JCV was thought to replicate exclusively in human glial cells, specifically oligodendrocytes. However, we have recently shown that JCV can replicate in cells of lymphoid origin such as hematopoietic precursor cells, B lymphocytes, and tonsillar stromal cells. To determine whether tonsils harbor JCV, we tested a total of 54 tonsils, 38 from children and 16 from adult donors. Nested PCRs with primer sets specific for the viral T protein and regulatory regions were used for the detection of JCV DNA. JCV DNA was detected in 21 of 54 tonsil tissues, or 39% (15 of 38 children and 6 of 16 adults) by using regulatory-region primers and in 19 of 54 tonsil tissues, or 35% (13 of 38 children and 6 of 16 adults) by using the T-protein primers. The DNA extracted from children’s nondissected tonsil tissue, isolated tonsillar lymphocytes, and isolated stromal cells that demonstrated PCR amplification of the JCV regulatory region underwent cloning and nucleotide sequencing. Of the regulatory-region sequences obtained, nearly all contained tandem repeat arrangements. Clones originating from nondissected tonsil tissue and tonsillar lymphocytes were found to have sequences predominantly of the Mad-1 prototype strain, whereas the majority of clones from the DNA of tonsillar stromal cells had sequences characteristic of the Mad-8br strain of JCV. A few clones demonstrated structures other than tandem repeats but were isolated only from tonsillar lymphocytes. These data provide the first evidence of the JCV genome in tonsil tissue and suggest that tonsils may serve as an initial site of viral infection.
A high percentage of the world’s population undergoes infection by the polyomavirus JC (JCV) (17, 26). The infection generally occurs during childhood, with specific antibodies to the virus developing subsequent to infection (23, 37, 39). In immunocompromised individuals, JCV lytically infects oligodendrocytes and causes a demyelinating disease of the central nervous system termed progressive multifocal leukoencephalopathy (PML) (26, 31). The incidence of PML has increased worldwide in conjunction with immunosuppression attributed to the human AIDS pandemic and the use of immunosuppressive therapies. Evidence suggests that PML occurs predominantly from reactivation of latent JCV infection in immunocompromised individuals, as well as from primary infection (4, 20, 35).
Originally, JCV was thought to have a very restricted cellular host range, replicating only in oligodendrocytes (32, 38). Because of its capacity to cause demyelination in the central nervous system and multiply chiefly in human glial cells in culture, JCV was considered strictly a neurotropic virus. However, JCV infection has also been described in lymphoid cells of PML patients (21), in peripheral blood lymphocytes from patients with and without PML (5, 36), and in peripheral blood lymphocytes from human immunodeficiency virus-infected patients (13) and from immunocompetent individuals (12). In a recent report we demonstrated that JCV infects and replicates in hematopoietic precursor cells, B lymphocytes, and tonsillar stromal cells (29). We also demonstrated that both tonsillar B lymphocytes and stromal cells are infectible in vitro but that a much higher percentage of tonsillar stromal cells than B lymphocytes become infected (29).
Studies have shown that JCV is present in the urine of pregnant women (6, 8, 18, 27), immunocompetent, healthy individuals (7, 9, 22, 41), and PML patients (11, 40). Consequently, JCV establishes a persistent infection in the kidney. Unlike the Mad-1 regulatory-region sequences found predominantly in JCV isolated from the brains of PML patients (15, 19, 28, 34), JCV isolated from urine has the “archetype” regulatory-region sequence arrangement (14, 40–42). Variations in the genomic sequences within the VP1 structural protein region have also been reported and have been used in some studies to classify different JCV genotypes (1–3, 25).
In this study we identified the JCV genome in tonsils of both pediatric and adult donors and determined the nucleotide sequences of the regulatory regions present not only in the total tonsil tissue of three children but also in their isolated tonsillar lymphocytes and isolated tonsillar stromal cells. The regulatory-region sequences are thought to be the predominant factor controlling viral host range. If primary infection occurs by a common route such as respiratory inhalation, as suggested by viral seroepidemiology, tonsils are in an ideal anatomical position to be a primary site of JCV infection. The presence of the JCV genome in tonsil tissue described here provides the first evidence of where JCV may initiate infection in its human host.
MATERIALS AND METHODS
Source of tonsillar tissue.
Immediately after tonsillectomy, tonsils from 38 children and 16 adult donors were placed in phosphate-buffered saline. Pediatric donors ranged in age from 3 to 15 years (average, 7.7 years), and adult donors ranged in age from 18 to 57 years (average, 26 years).
DNA extraction from embedded tonsillar tissue.
Tonsillar tissue was dissected into 1-cm3 pieces and fixed in formalin for slide preparation. Fixed, paraffin-embedded tonsillar tissue was deparaffinized by three 5-min treatments with xylene and two 5-min treatments with 100% ethanol and was air dried. Deparaffinized tissue was scraped into 500-μl tubes, resuspended in 100 μl of a solution of 50 mM Tris-HCl (pH 8.2), 1 mM EDTA, 0.5% Tween 20, and 10 μg of proteinase K/μl, and incubated overnight in a 37°C water bath. Heating at 95°C for 8 min stopped enzymatic activity. DNA was extracted twice with phenol and twice with chloroform. Sodium acetate was added to a final concentration of 0.3 M, and the addition of 2.5 volumes of cold ethanol at −20°C overnight precipitated the DNA. This DNA was subsequently used in nested PCR (n-PCR) experiments.
Tonsillar lymphocytes.
The lymphocyte population, consisting of both T and B cells, was isolated from 41 of the 54 tonsils by centrifugation through Ficoll-Hypaque (density, 1.08). Lymphocytes could not be isolated from the remaining 13 samples due to microbial contamination. Some lymphocyte samples were placed in culture medium, RPMI 1640 with 10% fetal bovine serum, before DNA extraction. The lymphocytes were washed, pelleted by centrifugation, and lysed by resuspension in 0.5 ml of 10% sodium dodecyl sulfate plus 10 μg of proteinase K/μl. The DNA from these samples was extracted by the procedure described above.
Tonsillar stromal cells.
The method used to isolate stromal cells from human tonsils was described previously in detail (24). Briefly, tonsillar parenchyma was dissected into small pieces and enzymatically digested by treatment with collagenase and trypsin for 1 h at 37°C. The cells released by enzymatic treatment were washed twice in RPMI 1640 with 10% fetal bovine serum, resuspended in this medium, and seeded into 75-cm2 tissue culture flasks. Nonadherent cells were aspirated from cultures in wash solution, and the adherent stromal cells were expanded by passaging several times.
Stromal cells were cultured in vitro from only 16 of the 54 tonsil samples because, upon receipt, some samples were microbially contaminated and others were not viable when dissociated from tissue and placed in culture. DNA for n-PCR was extracted from in vitro-cultured tonsillar stromal cells by phenol-chloroform treatment as described above.
n-PCR and Southern analysis.
Selected JCV nucleotide sequences present in the DNA from whole tonsillar tissue and from isolated tonsillar lymphocytes and stromal cells were amplified by n-PCR. The primer sets used in the PCRs were specific for the conserved region of the JCV genome coding for T protein or the regulatory region. PCR assays were performed in a final volume of 100 μl by using 1 μg of sample DNA, 2.5 U of AmpliTaq polymerase (Perkin-Elmer, Branchburg, N.J.) and 2 mM MgCl2 in accordance with the manufacturer’s instructions. The nested primer pairs used to amplify a 768-bp external segment (nucleotides [nt] 4231 to 4252 and 4999 to 4979) and a 577-bp internal segment (nt 4301 to 4321 and 4878 to 4858) of a conserved region of the JCV genome coding for the T protein have been reported elsewhere (29).
For amplification of segments of the JCV genome regulatory region, the external primer pair coding for a 586-bp segment was 5′-CCC TAT TCA GCA CTT TGT CC-3′ (nt 4992 to 5011) and 5′-CAA ACC ACT GTG TCT CTG TC-3′ (nt 448 to 428). The internal primer pair coding for a 388-bp segment was 5′-GGG AAT TTC CCT GGC CTC CT-3′ (nt 5060 to 5079) and 5′-ACT TTC ACA GAA GCC TTA CG-3′ (nt 312 to 297). Numbering for nucleotide positions was adopted from Frisque et al. (16). The PCR program used for amplification of the regulatory-region segments with both external and internal primers consisted of 5 cycles of 95°C for 1 min (denaturing), 60°C for 1 min (high-stringency annealing), and 72°C for 2 min (extension), followed by 25 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 2 min. All amplifications were completed with a 7-min, 72°C extension period. A Perkin-Elmer Cetus model 480 Thermal Cycler was used for all DNA amplifications. All n-PCR experiments included a positive control (0.1 ng of the JCV plasmid pM1TC) added to the reaction mixture and a negative control consisting of the reaction mixture without JCV template. Nested PCR (n-PCR) was performed with 10 μl of the reaction products from the first amplification. Fifteen microliters of amplified n-PCR products were examined by gel electrophoresis. All the n-PCR products were analyzed by Southern blot transfer as previously described (29), by using a specific JCV 32P-labeled pM1TC DNA probe (Lofstrand Labs, Gaithersburg, Md.).
DNA sequences.
PCR-amplified regulatory-region segments of JCV DNA from whole tonsillar tissue, isolated lymphocytes, and stromal cells were cloned directly into a pCR2.1 plasmid vector by using the Original TA Cloning Kit (Invitrogen, Carlsbad, Calif.) in accordance with the manufacturer’s instructions. Briefly, n-PCR products positive for JCV regulatory-region sequences (inserts) were directly ligated into the pCR2.1 plasmid vector with DNA polymerase at 14°C overnight. Ligations were then transformed into competent bacteria and plated on LB plates with 50 mg of kanamycin (Life Technologies Gibco, BRL, Grand Island, N.Y.)/ml and 40 mg of X-Gal (5-bromo-4-chloro-3-indolyl β-d-galactopyraminoside; Sigma, St. Louis, Mo.)/ml.
Transformed bacterial clones were detected by blue/white screening. Positive white clones were confirmed by excision of inserts by treatment for 1 h at 37°C with the restriction enzyme EcoRI (New England Biolabs Inc., Beverly, Mass.). Cultures of positive clones were grown in order to obtain enough DNA (Midi Prep; Qiagen Inc., Chatsworth, Calif.) for sequencing.
The nucleotide sequencing reactions were performed with Amersham ET primers and Thermosequenase in accordance with the manufacturer’s instructions (Amersham Corp., Arlington Heights, Ill.). Reaction product sequences were then determined by using an ABI 373 DNA sequencer with stretch modification.
RESULTS
PCR amplification of JCV-specific sequences in DNA isolated from tonsils.
DNA isolated from nondissected tonsillar tissue from 54 individuals was analyzed by n-PCR for the presence of nucleotide sequences homologous to the conserved region of the JCV T protein and variable regulatory regions (Fig. 1). Southern blot hybridization of the n-PCR product from agarose gels was used to assess the number of amplified tonsillar DNA samples which were positive for JCV sequences. Positive results were obtained in 19 of the 54 (35%) samples amplified with primers specific to the nonstructural JCV T protein, while 21 of 54 (39%) samples were positive with primers specific for the JCV regulatory region (Table 1). Among the 16 tonsil samples assayed from adult donors, 6 positives were equally divided between males and females. In contrast, 11 of 23 tonsil samples from male children were positive, while only 4 of 15 from female children were positive.
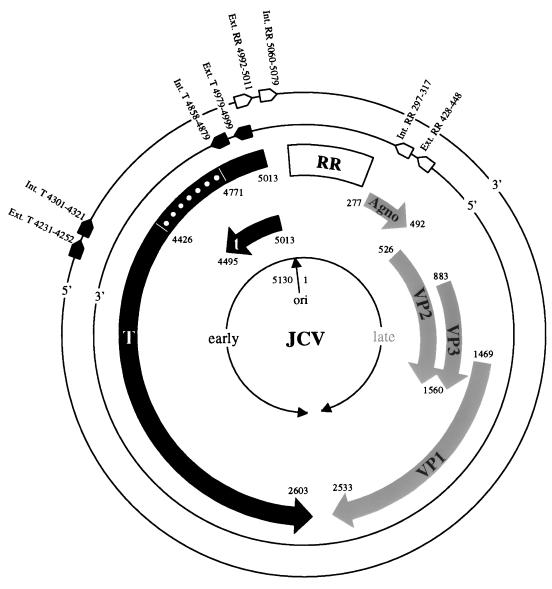
FIG. 1.
Schematic diagram of the JCV genome, including the nucleotide positioning and 5′-to-3′ conformation of primer pairs used for n-PCR amplification of the T protein and the regulatory region (RR). Broad arrows represent early (dark shading) and late (light shading) protein coding regions roughly divided by the origin of DNA replication (ori). Dots represent the noncoding region of the large T protein. Numbering is adopted from the prototype Mad-1 sequence (16). Int., internal primer; Ext., external primer.
TABLE 1.
Detection of nucleotide sequences for JCV T and regulatory region by n-PCR
| Sample | No. of positivea samples/no. tested (% positive) for the:
|
|
|---|---|---|
| T region | Regulatory region | |
| Nondissected tissue | 19/54 (35) | 21/54 (39) |
| Lymphocytes | 12/41 (29) | 11/41 (27) |
| Stromal cellsb | 3/16 (19) | 4/16 (25) |
Samples were considered positive if nucleotide sequences specific for either the T protein or the regulatory region of the JCV genome, or both, were amplified by n-PCR. By using the positive signal for the regulatory region as a reference, 17 of 21 samples from the tonsil tissue, 9 of 11 samples from the tonsillar lymphocytes, and 3 of 4 samples from the tonsillar stromal cells were also positive for the T region.
Stromal cells were placed in culture for 2 weeks prior to PCR analysis.
PCR amplification of specific JCV sequences in DNA isolated from tonsillar lymphocytes and stromal cells.
Of the 54 total tonsils studied, lymphocytes were obtained from 41 tonsils, whereas stromal cells were obtained from only 16 tonsils. This lower number resulted from the inability to culture stromal cells in vitro from nondissected tonsil tissue, in part due to bacterial contamination. n-PCR was performed on DNA from the tonsillar-lymphocyte (Fig. 2) and stromal-cell (Fig. 3) samples by using primers specific for the JCV T protein and regulatory region. As shown in Table 1, 12 of 41 (29%) lymphocyte DNA samples were positive for T-protein sequences, and 11 of 41 (27%) were positive for regulatory-region sequences. Among the DNA samples from tonsillar stromal cells, 3 of 16 (19%) were positive for T-protein sequences and 4 of 16 (25%) were positive for regulatory-region sequences.
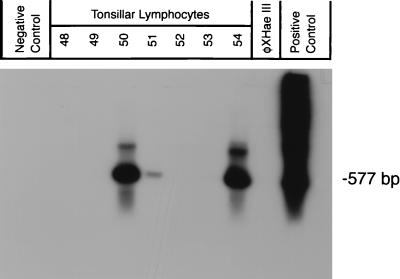
FIG. 2.
Detection of the JCV genome by n-PCR amplification and Southern blot hybridization from tonsillar lymphocytes. The primer sets used in this experiment were specific for the T-protein region of the JCV genome. The internal primers amplified a 577-bp segment. Lanes marked 48 to 54 contained DNA extracted from tonsillar lymphocytes isolated from tonsil tissue of healthy donors. Negative control, PCR amplification without template. HaeIII-digested φX, DNA molecular weight markers. Positive control, JCV plasmid pM1TC.
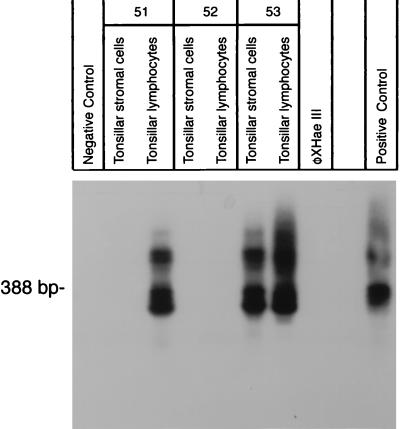
FIG. 3.
Detection of the JCV genome by n-PCR amplification and Southern blot analysis of tonsillar lymphocytes and stromal cells isolated from tonsil tissue. For amplification of segments of the JCV genome regulatory region, primer pairs which amplified a 388-bp internal segment were used. Lanes marked 51 through 53 contain DNA extracted from isolated and cultured tonsillar lymphocytes and DNA extracted from stromal cells from three different donors. Positive and negative controls are similar to those in Fig. 2.
Characterization of regulatory-region sequences found in clones from tonsil tissue.
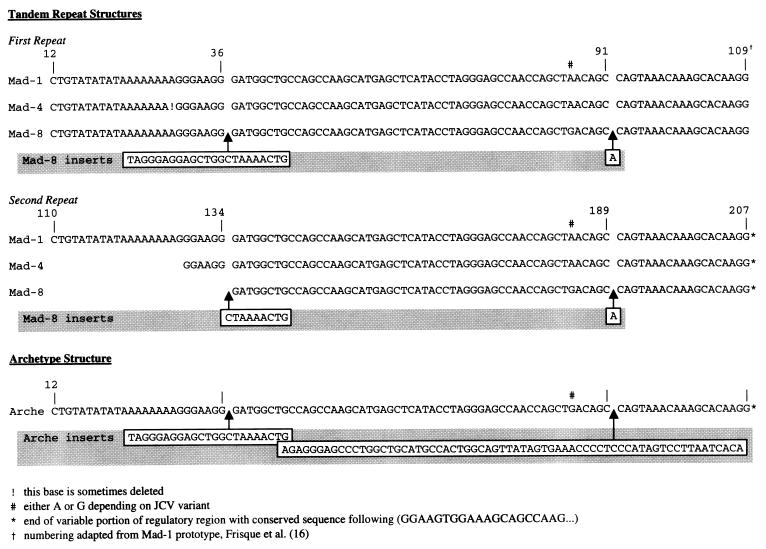
Some of the n-PCR products obtained by using JCV regulatory-region primer pairs were cloned directly into a plasmid vector. Positive clones were confirmed by excision of the insert with the restriction enzyme EcoRI (Fig. 4). Successive nucleotide sequence analysis was performed on a total of 59 clones, which were obtained from nondissected tonsils, tonsillar lymphocytes, and stromal cells from three children’s tonsils (Table 2). These samples were selected because all three compartments were PCR positive for JCV DNA. None of the adult tonsil tissue samples resulted in PCR detection of JCV DNA in all three compartments. The majority of the clones (33 of 59) were found to have the regulatory region sequence of the JCV prototype strain Mad-1. The Mad-1 regulatory region, generally referred to as a 98-bp repeat, consists of two direct 98-bp units, each having a complete TATA box (Fig. 5). Six clones had a deletion of the TATA sequence from the later 98 bp that is characteristic of the Mad-4 strain (Fig. 5). Twelve clones from tonsillar stromal cells showed a regulatory-region sequence identical to that of the Mad-8br strain, an isolate first found in the brain of a PML patient (28). The regulatory region of the Mad-8br strain has the tandem repeat structure of Mad-4 but also contains a 23-bp insert in the first unit of the 98-bp repeat at nucleotide 37 and a 9-bp insert, corresponding to the last 9 bp of the 23-bp insert, in the second unit of the 98-bp repeat at nucleotide 109 (Fig. 5). Among the 21 clones isolated from the tonsillar lymphocytes, 8 demonstrated the regulatory region characteristic of the archetype structure which can be isolated from urine samples of both PML and non-PML patients (14, 40–42). This sequence arrangement does not contain repeats, having only one 98-bp unit from the tandem repeat structure of Mad-1, but has two inserted nucleotide sequences of 23 bp (after nt 36 of Mad-1) and 66 bp (after nt 91 of Mad-1 [Fig. 5]).
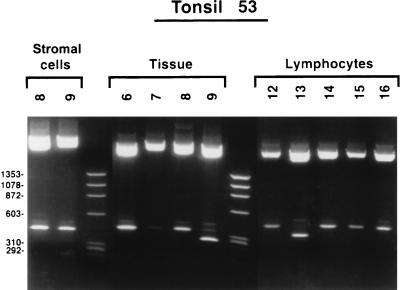
FIG. 4.
EcoRI restriction enzyme digests of JCV regulatory-region clones from nondissected tonsil tissue, tonsillar lymphocytes, and stromal cells isolated from tonsil 53. DNA fragments were visualized after staining with ethidium bromide and were photographed with UV light. The upper bands represent vector pCR2.1 (Invitrogen). The lower bands are excised inserts that were subsequently sequenced. The different molecular weights of the insert bands correspond to different viral regulatory-region sequences.
TABLE 2.
Nucleotide sequence arrangements of the regulatory regions of JCV clones isolated from tonsil specimensa
| Specimen | Sequence(s)b (no. of clones) in:
|
||
|---|---|---|---|
| Nondissected tissue | Lymphocytes | Stromal cells | |
| 49T91 | Mad-1 (8) | Arche (7) | Mad-1 (1) |
| Mad-4 (4) | Mad-8 (4) | ||
| 53T90 | Mad-1 (9) | Mad-1 (9) | Mad-8 (8) |
| Mad-4 (1) | Mad-4 (1) | ||
| Arche (1) | |||
| 54T90 | Mad-1 (1) | Mad-1 (3) | Mad-1 (2) |
Specimens were obtained from one 6-year-old child and two 7-year-old children. These samples were selected because all three compartments were positive for JCV DNA.
Expressed as JCV strains; for example, eight clones from nondissected tonsillar tissue of specimen 49T91 had the regulatory-region sequence of the Mad-1 strain. Arche, archetype structure.
FIG. 5.
Representative sequences of four variations in the regulatory region of JCV, derived from 59 different clones sequenced from pediatric and adult donor tonsil tissue, tonsillar lymphocytes, and stromal cells (adapted from reference 28). See Table 2.
DISCUSSION
We previously showed that, in addition to human glial cells, JCV could infect tonsillar lymphocytes and stromal cells in vitro (29). The anatomical location of tonsils and the susceptibility of lymphocytes and stromal cells, both of which are present in tonsils, to JCV infection make it plausible that JCV infection can occur via a respiratory route. We used n-PCR amplification to determine whether DNA from nondissected tonsillar tissue, isolated tonsillar lymphocytes, and isolated stromal cells of healthy individuals contained JCV-specific nucleotide sequences. Of 54 tonsils from immunocompetent children and adult donors, 19 (35%) were positive for the JCV T-protein sequences and 21 (39%) were positive for the regulatory-region sequences. We also showed by n-PCR amplification that DNA of isolated tonsillar lymphocytes and stromal cells contained JCV-specific T-protein and regulatory-region sequences (Table 1). The lower percentage of n-PCR products found in tonsillar stromal cells could be explained by the loss of infected and/or susceptible stromal cells during the cultivation procedures. After enzymatic treatment, small pieces of tonsil tissue were cultured for 1 to 2 weeks to allow for the separation of adherent stromal cells from the other cell populations present in tonsil tissue, ensuring a pure population of stromal cells. It is possible that during the period of in vitro cultivation, selection for noninfected stromal cells occurred while the JCV-infected cells died. As we noted in Materials and Methods, some stromal-cell cultures were not viable after dissociation from the tonsil tissue when cultures were held for several weeks.
The reported differences in regulatory-region sequences of JCV isolated from different tissues, such as brain and urine, prompted us to investigate the regulatory-region sequences of JCV in tonsillar tissue and the specific cell types isolated from this tissue. The prototype Mad-1 brain isolate of JCV includes a 98-bp tandem repeat regulatory region. However, isolates from urine, referred to as the archetype, have one of the two 98-bp structures deleted and have 23- and 66-bp structures inserted as shown in Fig. 5.
The majority of the regulatory-region clones derived from the nondissected tonsillar-tissue samples and from the isolated lymphocytes were found to have nucleotide sequences characteristic of the Mad-1 prototype (Table 2). In contrast, 12 of the 15 clones from tonsillar stromal cells had regulatory-region sequences characterized by the presence of a 23-bp insert in the first unit of the 98-bp repeat but only the last 9 nt of the same insert in the second unit, which also lacked the TATA box. These regulatory regions also contained a single-base pair insert where the archetype has a 66-bp sequence. An isolate found previously from the brain of a PML patient (28), MAD-8br, has the same regulatory-region sequence as the clones derived from tonsillar stromal-cell DNA. However, this sequence arrangement was found in stromal cells from only two tonsil tissues. Additional stromal-cell cultures from other tonsil tissue samples are needed to determine if the Mad-8br regulatory sequences are the most common in these cells. No in vitro biological activity has ever been demonstrated for the MAD-8br strain (10). This observation may explain our inability to identify JCV-specific proteins by immunocytochemistry in the samples that were positive for JCV DNA. Of the 21 total clones derived from tonsillar lymphocytes, 12 had prototype Mad-1 regulatory-region sequences (Table 2). Eight clones presented an archetype structure, characterized by the presence of one copy of the 98-bp structure found in Mad-1 along with a 23- and a 66-bp insert. One clone showed a Mad-4 sequence, characterized by the absence of inserts and a deletion of the TATA box sequence in the second 98-bp unit. Because lymphocytes circulate freely between the blood and other tissue, they may be responsible for transporting JCV with the archetype sequence from the kidney to the tonsil tissue. The presence of Mad-1 and Mad-8br strains of JCV in tissues other than brain has already been demonstrated (30).
It has been postulated that JCV strains with the archetype regulatory-region sequence could be the origin of the “rearranged” prototype, Mad-1. This would be accomplished by the loss of the 23- and 66-bp insert structures, followed by a duplication of the remaining 98-bp structure. Regardless of which, if any, of these mechanisms are viable, our results show that the predominant JCV strain in tonsillar tissue is Mad-1. Alternatively, others have proposed that immunosuppression creates the conditions that allow JCV to replicate and foster change in its regulatory-region sequence. This may permit JCV replication in specific organs and cell types (33).
JCV DNA with an archetype regulatory region is mainly detected by PCR in kidney tissue and urine (41, 42). These data support the possibility that the archetype is a variant strain that the cells in different organs can select in order to survive after primary JCV infection. Daniel et al. (10) showed how the deficit of archetype replication in primary human fetal glial cells was due to a failure of the early promoter to express adequate levels of mRNA to support T-protein-mediated archetype DNA replication. Moreover, they demonstrated that the removal of the 23- or the 66-bp insert, or both, from the archetype structure increased its capacity for replication. In our study, different clones were isolated from immunocompetent individuals; the majority of these clones had nucleotide sequences characteristic of the Mad-1 prototype, which is the predominant strain found in uncultured, nondissected tonsil tissue. If the viral genome is rearranged during active replication at the primary site of infection, we can hypothesize that the Mad-1 prototype strain, the form which is more represented in the tonsil tissue and brain, can rearrange to generate the multiple genotypes observed in other infected host cells. Moreover, it is important to consider that in the process of culturing the tonsillar lymphocytes and stromal cells, lytically infected cell types may have been lost and only cells supporting nonproductive viral variants remained. In conclusion, this paper shows the presence of the JCV genome in tonsil tissue from immunocompetent, healthy donors and supports the hypothesis that tonsils can be the site of initial infection.
ACKNOWLEDGMENTS
We gratefully acknowledge B. Curfman and M. Gravell for editing the manuscript. We thank S. Frye for helpful discussion. We also thank Jim W. Nagle of the NINDS DNA facility for performing the sequence analysis and B. Curfman for providing excellent technical assistance. We thank J. Barton and G. Fuller, Department of Pathology, Shady Grove Hospital, Rockville, Md., for supplying tonsils. We thank P. Ballew for preparation of the manuscript.
REFERENCES
- 1.Agostini H T, Ryschkewitsch C F, Brubaker G R, Shao J, Stoner G L. Five complete genomes of JC virus type 3 from Africans and African Americans. Arch Virol. 1997;142:637–655. doi: 10.1007/s007050050108. [DOI] [PubMed] [Google Scholar]
- 2.Agostini H T, Ryschkewitsch C F, Singer E J, Stoner G L. Co-infection with two JC virus genotypes in brain, cerebrospinal fluid or urinary tract detected by direct cycle sequencing of PCR products. J Neurovirol. 1996;2:259–267. doi: 10.3109/13550289609146889. [DOI] [PubMed] [Google Scholar]
- 3.Agostini H T, Ryschkewitsch C F, Stoner G L. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–164. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur R R, Shah K V, Charache P, Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988;158:563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- 5.Azzi A, De Santis R, Ciappi S, Leoncini F, Sterrantino G, Marino N, Mazzotta F, Laszlo D, Fanci R, Bosi A. Human polyomavirus DNA detection in peripheral blood leukocytes from immunocompetent and immunocompromised individuals. J Neurovirol. 1996;2:411–416. doi: 10.3109/13550289609146907. [DOI] [PubMed] [Google Scholar]
- 6.Chang D, Wang M, Ou W, Lee M, Ho H, Tsai R. Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J Med Virol. 1996;48:95–101. doi: 10.1002/(SICI)1096-9071(199601)48:1<95::AID-JMV15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Chesters P M, Heritage J, McCance D J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 8.Coleman D V, Wolfendale M R, Daniel R A, Dhanjal N K, Gardner S D, Gibson P E, Field A M. A prospective study of a human polyomavirus infection in pregnancy. J Infect Dis. 1980;142:1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Coleman D V, Gardner S D, Mulholland C, Fridiksdo V, Porter A A, Lilford R, Valdimarsson H. Human polyomavirus in pregnancy. A model for the study of defense mechanisms to virus reactivation. Clin Exp Immunol. 1983;53:289–296. [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel A M, Swenson J J, Reddy Mayreddy R P, Khalili K, Frisque R J. Sequences within the early and the late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology. 1996;216:90–101. doi: 10.1006/viro.1996.0037. [DOI] [PubMed] [Google Scholar]
- 11.Dörries K, ter Meulen V. Progressive multifocal leukoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11:307–317. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- 12.Dörries K, Vogel E, Günther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 13.Dubois V, Lafon M E, Ragnaud J M, Pellegrin J L, Damasio F, Baudouin C, Michaud V, Feury H J A. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS. 1996;10:353–358. doi: 10.1097/00002030-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Flaegstad T, Sundsfjord A, Arthur R R, Pedersen M, Traavik T, Subramani S. Amplification and sequencing of the control regions of BK and JC virus from human urine by polymerase chain reaction. Virology. 1991;180:553–560. doi: 10.1016/0042-6822(91)90069-n. [DOI] [PubMed] [Google Scholar]
- 15.Frisque R J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983;46:170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frisque R J, Bream G L, Cannella M T. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisque R J, White F A. The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy. In: Roos R, editor. Molecular neurovirology. Totowa, N.J: Humana Press; 1992. pp. 25–158. [Google Scholar]
- 18.Gibson P E, Field A M, Gardner S D, Coleman D V. Occurrence of IgM antibodies against BK and JC polyomaviruses during pregnancy. J Clin Pathol. 1981;34:674–679. doi: 10.1136/jcp.34.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinnell B W, Padgett B L, Walker D L. Comparison of infectious JC virus DNAs cloned from human brain. J Virol. 1983;45:299–308. doi: 10.1128/jvi.45.1.299-308.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogan T F, Borden E C, McBain J A, Padgett B L, Walker D L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980;92:373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- 21.Houff S A, Major E O, Katz D A, Kufta C V, Sever J L, Pittalunga S, Roberts J R, Gitt J, Saini N, Lux W. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318:301–305. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T, Aso Y, Kuniyoshi N, Hara K, Yogo Y. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J Infect Dis. 1990;161:1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- 23.Knight R S, Hyman N M, Gardner S D, Gibson P E, Esiri M M, Warlow C P. Progressive multifocal leukoencephalopathy and viral antibody titers. J Neurol. 1988;235:458–461. doi: 10.1007/BF00314247. [DOI] [PubMed] [Google Scholar]
- 24.Lisignoli G, Monaco M C G, Facchini A, Toneguzzi S, Cattini L, Hilbert D M, Lavaroni S, Belvedere O, Degrassi A. In vitro cultured stromal cells from human tonsils display a distinct phenotype and induce B cell adhesion and proliferation. Eur J Immunol. 1996;26:17–27. doi: 10.1002/eji.1830260104. [DOI] [PubMed] [Google Scholar]
- 25.Loeber G, Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988;62:1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Major E O, Amemiya K, Tornatore C S, Houff S A, Berger J R. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowitz R, Eaton B A, Kubik M F, Latorra D, McGregor J A, Dynan W S. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991;65:4515–4519. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin J D, King D M, Slauch J M, Frisque R J. Differences in regulatory sequences of naturally occurring JC virus variants. J Virol. 1985;53:306–311. doi: 10.1128/jvi.53.1.306-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaco M C G, Atwood W J, Gravell M, Tornatore C S, Major E O. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and stromal cells: implications for viral latency. J Virol. 1996;70:7004–7012. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers C, Frisque R J, Arthur R R. Direct isolation and characterization of JC virus from urine samples of renal and bone marrow transplant patients. J Virol. 1989;63:4445–4449. doi: 10.1128/jvi.63.10.4445-4449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padgett B L, ZuRhein G, Walker D, Echroade R, Dessel B. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 32.Padgett B L, Rogers C M, Walker D L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977;15:656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy Mayreddy R P, Safak M, Razmara M, Zoltick P, Khalili K. Transcription of the JC virus archetype late genome: importance of the κB and the 23-base-pair motifs in late promoter activity in glial cells. J Virol. 1996;70:2387–2393. doi: 10.1128/jvi.70.4.2387-2393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rentier-Delrue F, Lubiniecki A, Howley P M. Analysis of JC virus DNA purified directly from human progressive multifocal leukoencephalopathy brains. J Virol. 1981;38:761–769. doi: 10.1128/jvi.38.2.761-769.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidbauer M, Budka H, Shah K V. Progressive multifocal leukoencephalopathy (PML) in AIDS and in the pre-AIDS era. Acta Neuropathol. 1990;80:375–380. doi: 10.1007/BF00307690. [DOI] [PubMed] [Google Scholar]
- 36.Tornatore C, Berger J R, Houff S A, Curfman B, Meyers K, Winfield D, Major E O. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31:454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 37.Walker D L, Padgett B L. The epidemiology of human polyomaviruses. In: Sever J L, Madden D L, editors. Polyomaviruses and human neurological diseases. New York, N.Y: Alan R. Liss; 1983. pp. 99–106. [PubMed] [Google Scholar]
- 38.Walker D L, Padgett B L. Progressive multifocal leukoencephalopathy. Comp Virol. 1983;18:161–193. [Google Scholar]
- 39.Walker D L, Frisque R J. The biology and molecular biology of JC virus. In: Salzam N P, editor. The Papovaviridae. New York, N.Y: Plenum Press; 1986. pp. 327–377. [Google Scholar]
- 40.White F A, III, Ishaq M, Stoner G L, Frisque R J. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992;66:5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yogo Y, Kitamura T, Sugimoto C, Hara K, Iida T, Taguchi F, Tajima A, Kawabe K, Aso Y. Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol. 1991;65:2422–2428. doi: 10.1128/jvi.65.5.2422-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]