Abstract
Vaccinia virus (VV) induces two forms of cell motility: cell migration, which is dependent on the expression of early genes, and the formation of cellular projections, which requires the expression of late genes. The need for viral gene expression prior to cell motility suggests that VV proteins may affect how infected cells interact with the extracellular matrix. To address this, we have analyzed changes in cell-matrix adhesion after infection of BS-C-1 cells with VV. Whereas uninfected cells round up and detach from the culture flask in the presence of EGTA, infected cells remain attached to the culture flask with a stellate morphology. Ca2+-independent cell-matrix adhesion was evident by 10 h postinfection, after the onset of cell motility but before the formation of virus-induced cellular projections. Progression to Ca2+-independent adhesion required the expression of late viral genes but not the formation of intracellular enveloped virus particles or intracellular actin tails. Analyses of specific matrix proteins identified vitronectin and fibronectin as optimal ligands for Ca2+-independent adhesion and the formation of cellular projections. Adhesion to fibronectin was mediated via RGD motifs alone and was not inhibited by 500 μg of heparin/ml. Kistrin, a disintegrin which binds preferentially to the αvβ3 (vitronectin/fibronectin) receptor inhibited the formation of cellular projections without disrupting preformed matrix interactions. Finally, we show that Ca2+-independent cell-matrix adhesion is a dynamic process which mediates changes in the morphology of VV-infected cells and uninfected cells which exhibit a transformed phenotype.
Vaccinia virus (VV) is a member of the Poxviridae family, a group of large, double-stranded DNA viruses which replicate within the cytoplasm of the infected cell. The VV genome contains approximately 200 genes, which can be subdivided into three temporally distinct classes termed early, intermediate, and late (36). Early genes are transcribed immediately after infection by enzymes and transcription factors contained within the infecting virion, while late and intermediate genes are transcribed only after the start of viral DNA replication (36, 56). This cascade of gene expression can be uncoupled by addition of the drug cytosine β-d-arabinofuranoside (ara-C), which inhibits DNA replication and permits only early genes to be expressed.
The assembly of viral particles is a complex process which results in the formation of two infectious forms of the virus (35). Initially the viral genome is surrounded by crescents which extend to form spherical immature virus. These particles then condense to form brick-shaped intracellular mature virus (IMV), which represents most of the infectious progeny. After leaving the viral factory, some IMV particles become wrapped by membranes derived from the tubular endosomes or trans-Golgi network (49, 54) to form an intracellular enveloped virus (IEV). Components within the outer membranes of IEV particles promote the polymerization of actin on one side of the IEV (12, 13), and this assists the intracellular movement of IEV particles in a manner similar to that described for Shigella, Listeria, and Rickettsia spp. (10, 11, 52). When an IEV particle reaches the plasma membrane, its outer membrane fuses with the plasma membrane, exposing infectious cell-associated enveloped virus (CEV) on the cell surface. In the case of the Western Reserve (WR) strain of VV, the majority of enveloped virus particles remain attached to the cell as CEV, and only a small percentage are released from the cell as extracellular enveloped virus (EEV) (5).
The outer membranes of CEV or EEV particles contain several virus-encoded and cellular proteins which are not found in IMV (40, 55). The virus-encoded proteins are gp85, the hemagglutinin (A56R) (40, 50), p37 (F13L) (21), gp42 (B5R) (16, 25), gp22-24 (A34R) (14), p45-50 (A36R) (38), and gp23-28 (A33R) (42). All of these virus genes except A56R are required for the formation of actin tails (12, 33, 43, 45, 57, 58). Nevertheless, actin tails are not essential for the release of infectious EEV particles, as shown by the fact that mutant viruses which form IEV particles but no actin tails still form EEV (19, 33, 43, 45, 57).
Infection with VV induces dramatic changes in cell function, metabolism, and morphology which are collectively termed the cytopathic effect (CPE). The first sign of CPE is a transient increase in plasma membrane permeability which accompanies virus entry. This change is caused by the infecting virus particle and does not require the translation of virus genes (8). In contrast, later forms of CPE require the expression of either early viral genes alone or both early and late viral genes. These changes are interesting because they illustrate how virus genes exert a dominant effect over normal cellular function; they include the inhibition of host cell protein synthesis (3), cell rounding (2), and cell migration (47) (all of which require the expression of early genes), as well as the formation of surface microvilli (20), inclusion bodies (39), or cellular projections (47) (all of which require the expression of late genes). The need for viral gene expression prior to cell motility suggests that VV genes can induce changes in cytoskeletal organization and cell-matrix adhesion. VV-induced changes in actin (4, 12, 13, 20) and microtubule (47) organization have been described previously; however, the effect of VV infection on cell-matrix adhesion has not been described.
Adhesion of cells to the extracellular matrix (ECM) is mediated by integrins, which act as the principal receptors for ECM proteins, including fibronectin, vitronectin, laminin, and collagens (24). In addition, proteoglycans function as coreceptors for several matrix proteins, including fibronectin (23). Integrins are transmembrane glycoproteins which bind to a variety of ECM proteins, including fibronectin, laminin, vitronectin, and various collagens (6, 22, 24, 53). Each integrin molecule is a heterodimer containing one α and one β subunit which are noncovalently linked. Selective pairing of different types of α and β subunits confers binding specificity for different types of matrix protein. The integrin α subunits possess binding sites for divalent cations, which are usually required for function (9, 15, 51). However, there is an important exception to this rule: neuronal crest cells bind to laminin by the α1β1 integrin complex in the absence of extracellular Ca2+ (27, 28). Interestingly, these cells, which exhibit Ca2+-independent matrix adhesion, are more motile then cells which maintain Ca2+-dependent adhesion (27). Binding of integrins to the ECM occurs via RGD motifs contained within matrix proteins (44). This interaction can be disrupted by small RGD-containing proteins called disintegrins (18, 26). Variation in the amino acid residues adjacent to the RGD motif of disintegrin molecules confers selectivity on the type of integrin molecules to which particular disintegrins can bind (48). Consequently, disintegrins can be used as molecular probes to analyze particular integrin-matrix interactions (26).
In this report we show that VV changes how infected cells interact with the ECM. In particular, VV induces a transition from Ca2+-dependent to Ca2+-independent cell-matrix adhesion. Ca2+-independent cell-matrix adhesion is also observed after NRK cells attain a transformed and motile phenotype. The reduced requirement for extracellular Ca2+ during different forms of cell motility is discussed.
MATERIALS AND METHODS
Cells and viruses.
Monkey kidney BS-C-1 cells and NRK cells were obtained from the Sir William Dunn School of Pathology Cell Bank, and NFL-38 cells were provided by G. Banting (University of Bristol, Bristol, United Kingdom). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS). The sources of VV strains WR, Tian Tan, Lister, Wyeth, and Copenhagen have been described previously (1). VV WR was used unless stated otherwise. All infections were performed at 3 PFU per cell. VV WR mutants that lack the genes A34R (gp22-24) (34) and A36R (p45) (38) or in which expression of A27L (p14) is repressed by the Escherichia coli LacI protein (41) have been described previously. For simplicity, the mutant viruses are referred to here by names indicating the gene which has been deleted (Δ) or repressed (I), e.g., ΔA34R, ΔA36R, or IA27L.
Reagents.
Anti-VV serum was obtained from rabbits previously infected with VV WR. Ara-C, cycloheximide, trypsin, EDTA, EGTA, vitronectin, fibronectin, laminin, collagen II, kistrin, heparin, and ProNectin-F were all obtained from Sigma (Poole, United Kingdom). N1-Isonicotinoyl-N2-3-methyl-4-chloro-benzoylhydrazine (IMCBH) was obtained from Hoechst (Frankfurt, Germany). CELLocate coverslips made by Eppendorf (Hamburg, Germany) were obtained from Merck Ltd., Lutterworth, United Kingdom.
Depletion of extracellular Ca2+ or Ca2+/Mg2+ and quantification of Ca2+-independent cell-matrix adhesion.
Unless indicated otherwise, divalent cations were depleted as follows: BS-C-1 cells were washed three times in phosphate-buffered saline (PBS) and then incubated in PBS containing 1 mM EGTA for 10 min at 37°C. After depletion of divalent cations, the morphology of cells was recorded by using an Olympus CK2 inverted phase-contrast microscope. For quantification of cell-matrix adhesion, three random areas of the glass coverslip, containing approximately 30 cells per field, were photographed with a 20× lens objective. The number of round or adherent cells was then scored from projected images. Standard errors of the means (SEM) were calculated from the variation among three different fields at each time point.
Adhesion to selective matrix proteins.
Stock solutions of laminin, collagen II, fibronectin, and vitronectin were made to 0.5 μg/ml with sterile H2O and stored at −20°C. Prior to use in cell adhesion assays, 10-mm glass coverslips were coated with the desired matrix protein. Stock solutions of matrix proteins were diluted in sterile PBS to a final concentration of 0.1, 1, or 10 μg/ml. Coverslips were then placed onto 200 μl of diluted matrix protein, incubated at 37°C for 2.5 h, and washed five times with PBS prior to the addition of cells. BS-C-1 cells were cultured to confluence in 6-well plates and then either mock infected or infected with VV. Three hours postinfection, cells were detached by depletion of extracellular Ca2+ as described above, and detached cells were harvested by centrifugation, washed with minimal essential medium (MEM), and resuspended in 10 ml of MEM. An aliquot (0.5 ml) of this cell suspension was added to each coverslip. To analyze the morphology of cells incubated in the presence or absence of extracellular Ca2+, BS-C-1 cells were infected with VV and then seeded at low confluence on grid-marked CELLocate coverslips (Eppendorf) coated with vitronectin (10 μg/ml). The morphology and grid reference of 10 cells were recorded at 11 h postinfection (hpi) and again after a further 10-h incubation at 37°C in the presence or absence of Ca2+.
RESULTS
VV-infected cells develop Ca2+-independent cell-matrix adhesion.
BS-C-1 cells are maintained in confluent monolayers via Ca2+-dependent interactions, and consequently the depletion of extracellular Ca2+ by the addition of EGTA for 10 min results in cell dissociation and rounding (compare Fig. 1A and D). In contrast, BS-C-1 cells which were infected with VV for 18 h were resistant to cell rounding induced by depletion of extracellular Ca2+ (compare Fig. 1B and E). Conversion to Ca2+-independent adhesion was also observed after infection of BS-C-1 cells with VV strains Lister, Tian Tan, Wyeth, and Copenhagen (data not shown). To standardize conditions used for cation depletion and to assess the requirement for Mg2+, cells were exposed to increasing concentrations of EGTA or EDTA at 37°C for up to 20 min (Fig. 2A and B). These data show that infected cells require neither Ca2+ nor Mg2+ to maintain adherence and that the maximal difference between the adhesion of mock-infected cells and that of VV-infected cells was evident after 10 min of cation depletion. Similar results were obtained with HeLa cells infected with VV under the same conditions (data not shown).
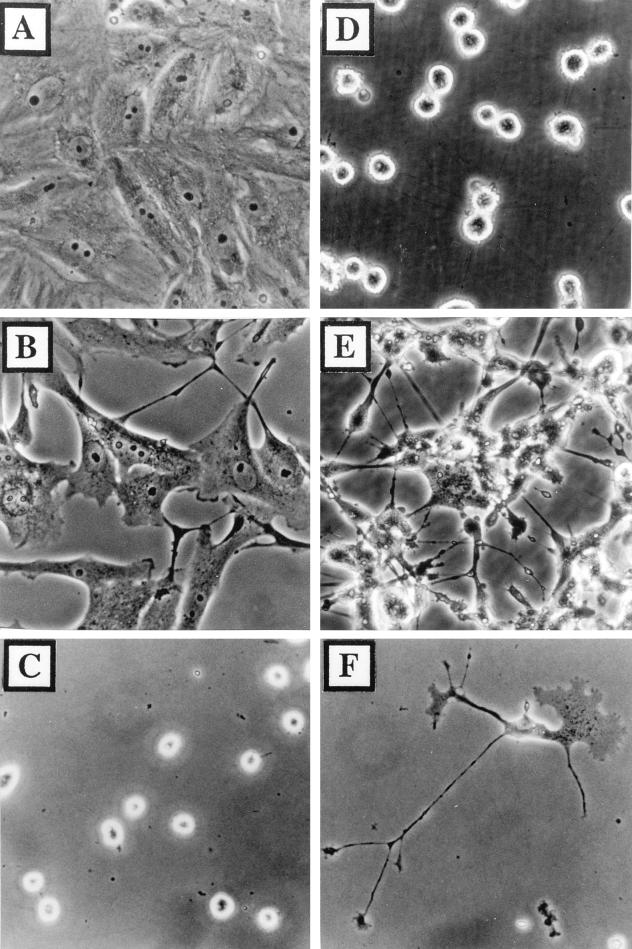
FIG. 1.
Cells infected with VV maintain an adherent morphology after depletion of extracellular Ca2+. BS-C-1 cells cultured as confluent monolayers (A, B, D, and E) or isolated cells (C and F) were mock infected (A, C, and D) or infected with VV at 3 PFU/cell (B, E, and F). Cell morphology was recorded at 18 hpi before (A and B) or after (C through F) incubation in 1 mM EGTA for 10 min at 37°C.
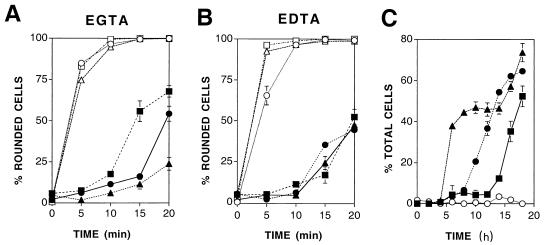
FIG. 2.
Kinetic analysis of Ca2+-independent cell-matrix adhesion. (A and B) Effects of increasing concentrations of EGTA and EDTA on the adhesion of infected or mock-infected cells. Confluent monolayers of BS-C-1 cells were mock infected (open symbols) or infected with VV (filled symbols). At 18 hpi cells were washed in PBS and then incubated in PBS containing 0.1 (circles), 1 (triangles), or 10 (squares) mM EGTA (A) or EDTA (B). The number of rounded cells was determined 0, 5, 10, 15 or 20 min later. Data shown are means ± SEM (n = 3). (C) Rate at which Ca2+-independent adhesion forms relative to the onset of cell migration and cellular projection formation. Isolated BS-C-1 cells were mock infected (open circles) or infected with VV (filled symbols), and the numbers of cells showing signs of migration (triangles), Ca2+-independent adhesion (circles), or cellular projections (squares) were determined at 2-h intervals up to 18 hpi. Data shown are means ± SEM (n = 3).
To analyze the effect of VV infection on cell-matrix rather than cell-cell interactions, BS-C-1 cells were cultured at low confluence. Under these conditions, cell-cell contact is avoided and cellular projection formation can be analyzed more easily. Isolated BS-C-1 cells were either mock infected or infected with VV, and extracellular Ca2+ was depleted at 18 hpi by exposure to 1 mM EGTA for 10 min. Although mock-infected cells rounded up after depletion of extracellular Ca2+ (Fig. 1C), infected cells maintained a stellate morphology (Fig. 1F) as noted previously (47). Taken together, the panels in Fig. 1 show that (i) VV infection changes the mechanism by which BS-C-1 cells interact with the extracellular matrix, (ii) changes in the adhesion properties of VV-infected cells are not regulated by cell-cell contact, and (iii) all parts of VV-infected cells exhibit Ca2+-independent adhesion, including dynamic structures such as lamellipodia and cellular projections (Fig. 1F).
To relate the observed change in cell-matrix adhesion to the two phases of VV-induced cell motility (47), the kinetics of VV-induced cell migration, cellular projection formation, and Ca2+-independent cell-matrix adhesion were analyzed up to 18 hpi. Figure 2C shows that Ca2+-independent cell-matrix adhesion was evident by 10 hpi, after the onset of cell motility, but before the formation of virus-induced cellular projections. Similar results were obtained in two further experiments (data not shown). Therefore, Ca2+-independent cell-matrix adhesion is not required for virus-induced cell migration but may be required for the formation of cellular projections.
Virus components required for the induction of Ca2+-independent cell adhesion.
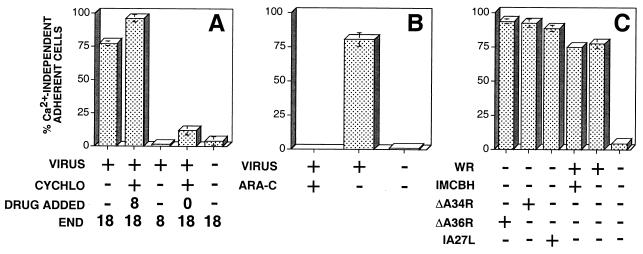
Ca2+-independent cell-matrix adhesion was evident between 10 and 18 hpi. During this period virus particles are assembled and released, intracellular actin tails are formed on IEV particles, and cell surface microvilli are apparent. Each of these events might affect the surface properties and therefore the adhesion of infected cells. To assess the contribution of these events to the development of Ca2+-independent cell-matrix adhesion, drugs or virus mutants which arrest morphogenesis at different stages of infection were analyzed. Cycloheximide (100 μg/ml) severely inhibited the onset of Ca2+-independent cell-matrix adhesion when added immediately after infection but not when added at 8 hpi (Fig. 3A). Similarly, addition of ara-C (40 μg/ml) inhibited the progression to Ca2+-independent cell-matrix adhesion (Fig. 3B). Ca2+-independent cell-matrix adhesion is therefore promoted by late virus genes synthesized by 8 hpi. The requirement for IEV particles, intracellular actin tails, or EEV release was assessed following the infection of BS-C-1 cells with mutant viruses which are defective in IEV formation (IA27L), actin tail nucleation (ΔA34R and ΔA36R), or CEV particle retention (ΔA34R). As an additional control, IEV particle assembly was inhibited by the addition of IMCBH (100 μg/ml). Figure 3C shows that conversion to Ca2+-independent cell adhesion is not dependent on the formation of IEV particles, EEV release, or the nucleation of intracellular actin tails.
FIG. 3.
Viral components required for Ca2+-independent cell-matrix adhesion. (A) Isolated BS-C-1 cells were either mock infected (−) or infected with VV (+). Cycloheximide (CYCHLO) (100 μg/ml) was added to cells (+) at 0 or 8 hpi. At 8 or 18 hpi, extracellular Ca2+ was depleted as indicated (END), and the percentages of adherent and rounded cells were determined. (B) BS-C-1 cells were mock infected (−) or infected with VV (+) in the presence (+) or absence (−) of 40 μg of ara-C/ml, and the percentage of cells binding to the extracellular matrix via Ca2+-independent adhesion was determined at 18 hpi. (C) BS-C-1 cells were mock infected (−) or infected either with VV WR in the presence (+) or absence (−) of IMCBH or with ΔA34R, ΔA36R, or IA27L, and the percentage of Ca2+-independent adherent cells was determined at 18 hpi. Data shown are means ± SEM (n = 3).
Adhesion of cells to individual matrix proteins.
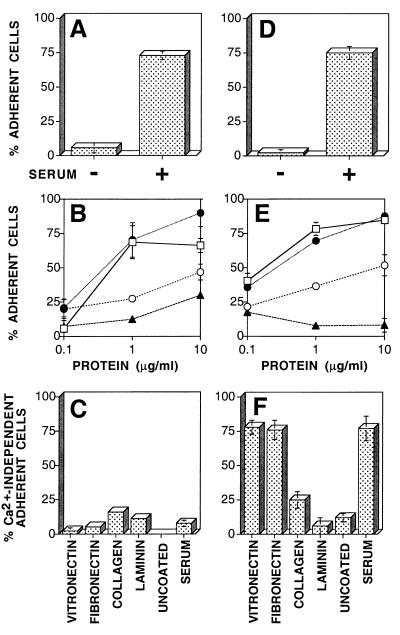
To identify matrix proteins which support Ca2+-independent adhesion, BS-C-1 cells were either mock infected or infected with VV. To avoid the synthesis of nascent matrix proteins, cells were removed from tissue culture plastic at 3 hpi (after virus-induced shutoff of host protein synthesis) by depletion of extracellular Ca2+ and transferred to glass coverslips that had been treated with FBS, PBS alone, or PBS containing 0.1, 1, or 10 μg of vitronectin, fibronectin, collagen II, or laminin/ml. Both mock- and VV-infected cells adhered well to coverslips coated with serum but not to uncoated (PBS) coverslips (Fig. 4A and D). This requirement for exogenous matrix proteins showed that Ca2+-independent adhesion was not mediated by factors secreted from the transferred cells. Both mock- and VV-infected cells adhered well to coverslips coated with vitronectin or fibronectin at concentrations of ≥1 μg/ml but less well to collagen II or laminin (Fig. 4B and E). Depletion of extracellular Ca2+ 15 h after transfer of cells disrupted the adhesion of mock-infected cells to all matrix proteins (Fig. 4C), showing that adhesion to each of these substrates is mediated by Ca2+-dependent interactions. In contrast, VV-infected cells maintained adhesion to vitronectin and fibronectin even after depletion of extracellular Ca2+ at 15 h after transfer. These data show that VV infection changes the mechanism by which BS-C-1 cells bind to vitronectin and fibronectin (Fig. 4C and F) but not their inherent preference for adhesion to different matrix proteins (Fig. 4B and E).
FIG. 4.
Adhesion of cells to specific matrix proteins. Isolated BS-C-1 cells were either mock infected (A through C) or infected with VV (D through F). At 3 hpi, cells were detached by depletion of Ca2+ and transferred to glass coverslips that had been treated with PBS (−) or serum (A and D) or with PBS containing the indicated concentrations of vitronectin (filled circles), fibronectin (open squares), collagen II (open circles), or laminin (filled triangles) (B and E). At 18 hpi the percentage of adherent cells was determined before (A, B, D, and E) or after (C and F) depletion of extracellular Ca2+. Data shown are means ± SEM (n = 3).
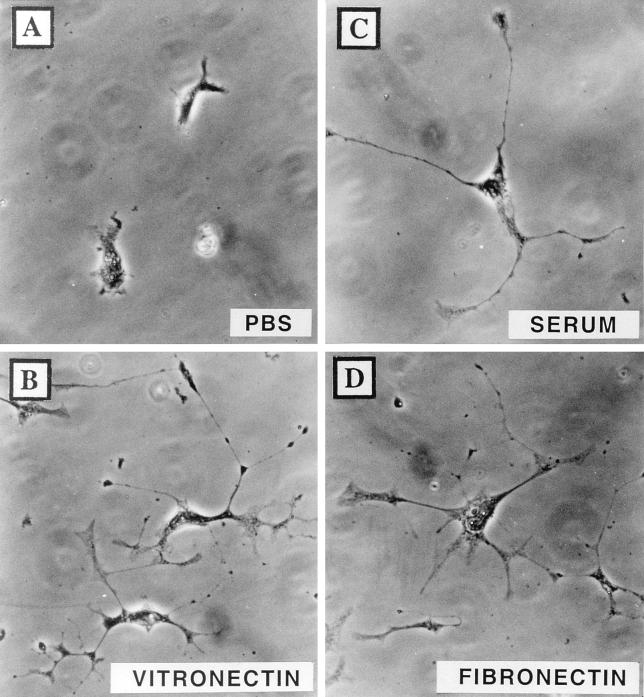
Analyses of the morphology of infected cells cultured on vitronectin or fibronectin showed that both matrix proteins promoted the formation of long cellular projections (Fig. 5B and D), which were not formed in the absence of matrix proteins added exogenously (Fig. 5A). Evidently, matrix proteins which support Ca2+-independent adhesion also promote the formation of VV-induced cellular projections.
FIG. 5.
Vitronectin and fibronectin permit the formation of VV-induced cellular projections. BS-C-1 cells were infected with VV and were transferred to glass coverslips pretreated with PBS (A), 10 μg of vitronectin/ml (B), FBS (C), or 10 μg of fibronectin/ml (D). The morphology of cells was recorded at 18 hpi after fixation for 1 h in 0.1% crystal violet.
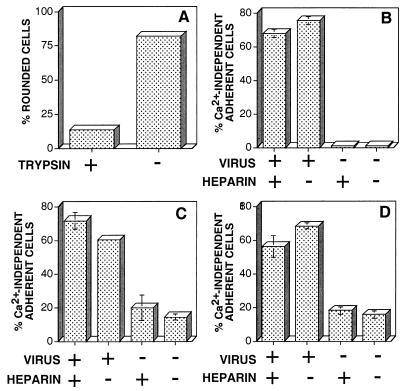
Ca2+-independent adhesion to fibronectin can occur via cell surface proteoglycans which bind to the heparin-binding domains of fibronectin (23) or via integrins that bind to the RGD-containing cell-binding domain of fibronectin (27). To determine which form of matrix interaction mediates VV-induced Ca2+-independent adhesion, BS-C-1 cells were infected with VV and cultured on glass coverslips coated with fibronectin (10 μg/ml) or ProNectin-F (a synthetic molecule which contains 13 fibronectin cell-binding domains interspersed with structural segments derived from fibrin). VV-infected cells developed Ca2+-independent adhesion to both fibronectin and ProNectin-F, suggesting that integrins and not proteoglycans were the surface receptors mediating Ca2+-independent matrix adhesion. This observation was confirmed by showing that high concentrations of heparin (500 μg/ml) did not inhibit the development of Ca2+-independent adhesion to serum-treated tissue culture plastic, fibronectin, or ProNectin-F (Fig. 6B through D).
FIG. 6.
Ca2+-independent adhesion is mediated by protein receptors which bind to the RGD motif of fibronectin. BS-C-1 cells were infected with VV and were cultured on tissue culture plastic in MEM containing 2.5% FBS (A and B) or on glass coverslips that had been precoated with 10 μg of fibronectin/ml (C) or 10 μg of ProNectin-F/ml (D), both in the absence of serum. (A) To determine if matrix adhesion was mediated by a protein receptor, at 16 hpi VV-infected cells were incubated in PBS containing 1 mM EGTA with (+) or without (−) 1 mg of trypsin/ml, and the percentage of rounded cells was determined after 10 min at 37°C. (B through D). To assess the contribution of matrix-encoded heparin-binding domains in Ca2+-independent cell adhesion, 500 μg of heparin/ml was added where indicated to mock-infected (−) or VV-infected (+) cells at 8 hpi. In each case the percentage of cells exhibiting Ca2+-independent matrix adhesion was determined at 18 hpi after the depletion of extracellular Ca2+.
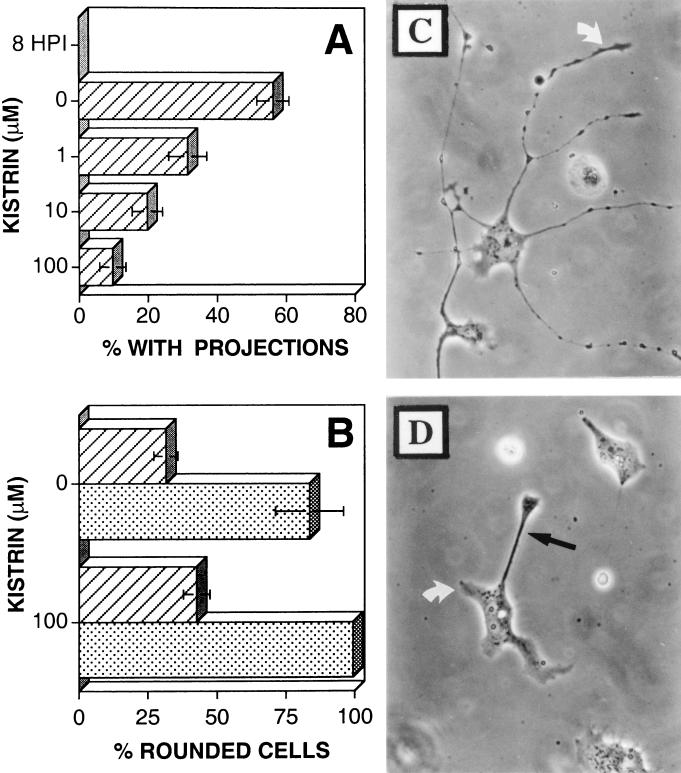
The ability of infected cells to bind to vitronectin and fibronectin in a Ca2+-independent manner is not surprising, as both matrix proteins can be recognized by common integrin heterodimers, including αvβ1 and αvβ3. The αvβ3 integrin heterodimer is of particular interest, as it promotes cell motility (26, 30). To assess the involvement of αv-containing integrins in VV-induced Ca2+-independent adhesion and cellular-projection formation, infected cells were cultured on vitronectin in the presence of increasing concentrations of kistrin, a disintegrin molecule which binds selectively to the αvβ3 vitronectin/fibronectin receptor (26). VV-infected BS-C-1 cells were cultured on coverslips coated in vitronectin (5 μg/ml), and their morphology was recorded at 8 hpi, immediately before the addition of kistrin (0 to 100 μM), and again at 18 hpi, before the depletion of extracellular Ca2+. Concentrations of kistrin of ≥1 μM suppressed the formation of VV-induced cellular projections (Fig. 7A and D), although Ca2+-independent matrix interaction was preserved (Fig. 7B). Concentrations of kistrin of >100 μM destabilized all matrix interactions and induced cell rounding (data not shown). These data show that binding of a kistrin-sensitive receptor to vitronectin is essential for the formation of VV-induced cellular projections and that Ca2+-independent matrix adhesion alone is not sufficient to induce the formation of cellular projections.
FIG. 7.
Effect of kistrin on cell morphology and adhesion. BS-C-1 cells were either mock infected (stippled bars) or infected with VV (striped bars). At 3 hpi cells were detached by depletion of extracellular Ca2+ and transferred to glass coverslips coated with vitronectin (10 μg/ml). At 8 hpi kistrin was added at the indicated concentration, and the percentage of cells which had developed multiple (≥2) projections (A) or Ca2+-independent adhesion (B) was determined at 18 hpi. (C and D) Cells were infected with VV for 3 h before being detached and transferred to glass coverslips coated with vitronectin (10 μg/ml). At 8 hpi kistrin (100 μM) was added to cells shown in panel D but not to cells shown in panel C. Cells were photographed under phase-contrast microscopy at 18 hpi. White arrows, virus-induced cellular projections; solid arrow, a trailing extension formed by cell migration.
Ca2+-independent adhesion is a dynamic process.
Previously it was shown that the migration of VV-infected cells is retarded just before the formation of cellular projections (47). Because the onset of Ca2+-independent adhesion also occurs at this time (Fig. 2C), it was of interest to determine if adhesion to vitronectin in the absence of extracellular Ca2+ is a dynamic process or if Ca2+-independent interactions are permanent points of contact which act to stabilize preformed structures. To investigate these possibilities, changes in the morphology of infected cells that were cultured in the presence or absence of extracellular Ca2+ were analyzed. BS-C-1 cells were infected with VV and were cultured at low confluence on vitronectin (5 μg/ml)-coated CELLocate coverslips. At 11 hpi, cells were either washed in PBS and then incubated in spinner culture-modified Eagle’s medium (without serum or Ca2+) or maintained in Ca2+-containing serum-free MEM. The morphology of infected cells changed significantly between 11 and 18 hpi (data not shown). Of the 10 cells analyzed in the absence of extracellular Ca2+, 5 had rounded up or detached by 18 hpi. This result is consistent with Fig. 2C, which shows that only 40% of VV-infected cells have attained Ca2+-independent adhesion by 11 hpi. Therefore Ca2+-independent adhesion is a dynamic process which mediates changes in the morphology of VV-infected cells.
Transformed cells also exhibit Ca2+-independent matrix interactions.
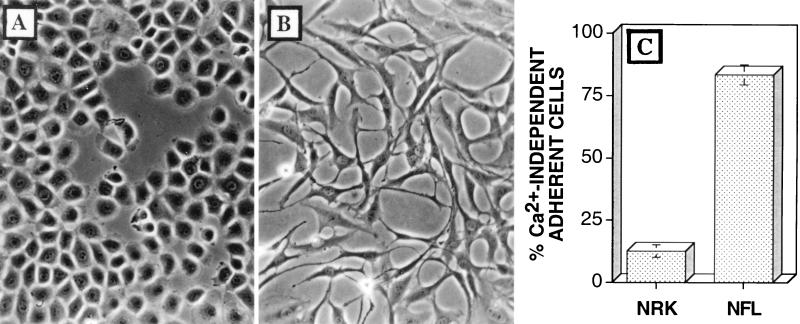
There are several similarities between the CPE induced by VV infection and a transformed cell phenotype. For example, VV infection induces cell-cell dissociation and cell migration, which are comparable with the loss of contact inhibition observed after transformation. Given these phenotypic similarities, it was intriguing that a motile, transformed cell line also exhibited Ca2+-independent adhesion. NFL-38 cells are a transformed cell line derived from NRK cells (17). Unlike NRK cells, which are contact inhibited and take on a cobblestone appearance (Fig. 8A), NFL-38 cells have a fibroblastic morphology (Fig. 8B) which resembles that of VV-infected BS-C-1 cells (Fig. 1B). To test the mechanism of cell-matrix adhesion exhibited by these two cell lines, NRK and NFL-38 cells were cultured at low confluence prior to depletion of extracellular Ca2+. Unlike the parental NRK cells, NFL-38 cells remained adherent after depletion of extracellular Ca2+ (Fig. 8C). Thus, Ca2+-independent adhesion is also exhibited by cells which have lost contact inhibition and exhibit a transformed phenotype.
FIG. 8.
Cells with a transformed phenotype exhibit Ca2+-independent cell-matrix interactions. (A) NRK cells are contact inhibited and exhibit a cobblestone morphology when cultured on tissue culture plastic. (B) Transformed NFL-38 cells have a fibroblastic morphology. (C) Mean percentage of each cell type which remained adherent after the depletion of extracellular Ca2+ ± SEM (n = 3).
DISCUSSION
This paper shows that VV changes how cells bind to components of the extracellular matrix. Specifically, late virus genes induce a transition from Ca2+-dependent to Ca2+-independent cell-matrix adhesion, so that infected cells, but not uninfected cells, remain adherent after depletion of Ca2+. These data define another aspect of VV-induced CPE and identify similarities among the adhesion phenotypes of VV-infected cells, uninfected transformed cells, and embryonic neuronal cells.
With respect to VV-induced CPE, it was shown previously that VV infection induces two forms of cell motility: cell migration, which requires the expression of early viral genes, and the formation of cellular projections, which requires late viral genes (47). Here we show that Ca2+-independent cell-matrix adhesion is apparent after the onset of cell migration but before the formation of cellular projections (Fig. 2C). Therefore, during VV infection, Ca2+-independent matrix adhesion is not linked directly to cell migration. However, the reduced requirement for extracellular Ca2+ may reflect functional changes which regulate other aspects of cell movement, such as the rate of cell migration, the way infected cells interact with neighboring cells, or the formation of cellular projections. Several observations suggest that conversion to Ca2+-independent cell-matrix adhesion may be linked to the formation of cellular projections. First, VV-induced cellular projections are maintained via Ca2+-independent adhesion (Fig. 1F). Second, both phenotypes require the expression of late virus genes (Fig. 3B) (47). Third, matrix proteins which support Ca2+-independent cell-matrix adhesion also promote the formation of cellular projections (Fig. 4 and 5). Finally, the onset of Ca2+-independent adhesion immediately precedes the formation of cellular projections (Fig. 2C). While Ca2+-independent adhesion may be necessary for projection formation, it is not sufficient, as shown by the fact that addition of kistrin (1 to 10 μM) to VV-infected cells inhibited the formation of cellular projections even though the cells still maintained Ca2+-independent adhesion to vitronectin (Fig. 7). These data can be interpreted in two ways. One possibility is that at concentrations below 10 μM, kistrin can competitively inhibit the formation of new Ca2+-independent linkages, while higher concentrations of kistrin (>100 μM) are needed to disrupt preformed matrix interactions.
The rate of cell movement may be linked inversely to the affinity with which cells bind to the extracellular matrix. Consequently, if Ca2+-independent matrix adhesion reflects an increased affinity for matrix components, this could explain the reduced rate of cell migration observed during the period of Ca2+-independent adhesion. Alternatively, projection formation may depend equally on two forms of matrix adhesion, one which induces cytoskeletal reorganization and one which mediates matrix adhesion. In this scenario, kistrin would block adhesion, leading to cytoskeletal reorganization but not Ca2+-independent adhesion. As yet no distinction can be made between these two possibilities; however, there is evidence that kistrin-sensitive integrins do control cell motility in other systems (26).
With respect to the mechanism of Ca2+-independent cell-matrix adhesion, the data show that VV infection changes the way cells bind to specific matrix proteins but not the preference for these proteins. Because fibronectin supported Ca2+-independent cell-matrix adhesion, it was possible to assess the requirement for both integrin-mediated binding to RGD-containing motifs and the binding of proteoglycan to heparin-binding domains. Addition of heparin (500 μg/ml) did not inhibit Ca2+-independent adhesion to fibronectin, and therefore it is clear that the heparin-binding domains of fibronectin are not responsible for the observed Ca2+-independent adhesion. In addition, VV-infected cells developed Ca2+-independent adhesion when cultured on ProNectin-F, a synthetic matrix protein which contains multiple RGD cell-binding domains but no heparin-binding motifs of fibronectin. Together, these data suggest that Ca2+-independent cell-matrix adhesion is, at least in part, dependent on integrin-mediated adhesion.
The observation that kistrin-sensitive receptors are involved in the progression of VV-induced CPE led us to analyze the possible role of a 32-kDa glycoprotein, encoded by gene A38L (37), with amino acid similarity to integrin-associated protein (IAP [31]; also known as OA3 [7] or CD47 [32]). IAP binds the αvβ3 integrin heterodimer, which also binds to kistrin (26), and therefore it was possible that the A38L protein could be responsible for aspects of CPE observed during VV infection. However, a virus lacking the A38L gene (46) still induced Ca2+-independent cell-matrix adhesion and cellular projections (data not shown).
As conversion to Ca2+-independent matrix adhesion occurred during the period of VV-induced motility, it was interesting to know if similar changes in matrix adhesion were observed within other motile cell systems. Two examples were found. First, NFL-38 cells exhibit a transformed cell phenotype that is quite distinct from that of the parental NRK cells. Unlike NRK cells, NFL-38 cells are not contact inhibited and have overlapping processes. In addition, NFL-38 cells exhibited Ca2+-independent cell-matrix adhesion, while the parental NRK cells did not (Fig. 8). Second, neuronal crest cells bind to ECM components via integrin-mediated Ca2+-independent adhesion (27–29). In this case Ca2+-independent adhesion was observed primarily on laminin and, under certain conditions, on fibronectin and collagen (29). Neuronal crest cells that bound to laminin in the absence of extracellular Ca2+ were more motile then those which exhibited Ca2+-dependent adhesion (28). Although all of these cells are motile and all exhibit Ca2+-independent adhesion, it is clear that there is no simple correlation between the existence of Ca2+-independent cell-matrix adhesion and a motile phenotype. For example, in the case of neuronal crest cells, Ca2+-independent cell-matrix adhesion enhanced migration (28). In contrast, VV-infected cells exhibit Ca2+-independent adhesion during a period of reduced cell migration when projections are being formed (47). Clearly, the reduced requirement for extracellular cations is only one aspect of cell motility. Also, the functional consequences of conversion to Ca2+-independent adhesion may be dictated by the particular receptor complex involved. Therefore, although the mechanism of matrix adhesion may change in the same way, different signals, which in turn result in different motile phenotypes, may be generated. Similarities in the behavior of malignant cancer cells and developing cells are well established (24). Consequently, it is perhaps not surprising that NFL-38 cells that have a transformed phenotype exhibit some similarities to embryonic neuronal crest cells. Less predictable are similar effects induced by the nontransforming poxvirus VV. This observation raises the intriguing possibility that VV has evolved mechanisms of controlling cell function similar to those operative in transformed or embryonic cells. Due to its ease of genetic manipulation, VV provides a good system in which to analyze molecular mechanisms controlling cell motility and focal adhesion. Further analyses of VV-induced CPE may lead to a better understanding of how viruses control cells but may also provide insights into events occurring during malignancy and embryonic development.
ACKNOWLEDGMENTS
The work was supported by Programme Grant PG8901790 from the Medical Research Council and by Equipment Grant 039155/Z/93/1.2 from The Wellcome Trust.
We thank Michael Hollinshead for helpful discussions and George Banting (University of Bristol) for NFL-38 cells.
REFERENCES
- 1.Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 2.Bablanian R, Baxt B, Sonnabend J A, Esteban M. Studies on the mechanisms of vaccinia virus cytopathic effects. II. Early cell rounding is associated with virus polypeptide synthesis. J Gen Virol. 1978;39:403–413. doi: 10.1099/0022-1317-39-3-403. [DOI] [PubMed] [Google Scholar]
- 3.Bablanian R, Coppola G, Scribani S, Esteban M. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology. 1981;112:13–24. doi: 10.1016/0042-6822(81)90607-3. [DOI] [PubMed] [Google Scholar]
- 4.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco R, Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992;66:4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck C A, Shea E, Duggan K, Horwitz A F. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J Cell Biol. 1986;103:2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell I G, Freemont P S, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–5420. [PubMed] [Google Scholar]
- 8.Carrasco L, Esteban M. Modification of membrane permeability in vaccinia virus-infected cells. Virology. 1982;117:62–69. doi: 10.1016/0042-6822(82)90507-4. [DOI] [PubMed] [Google Scholar]
- 9.Cheresh D A, Pytela R, Pierschbacher M D, Klier F G, Ruoslahti E, Reisfeld R A. An Arg-Gly-Asp-directed receptor on the surface of human melanoma cells exists in a divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 1987;105:1163–1173. doi: 10.1083/jcb.105.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart P. Actin-based bacterial motility. Curr Opin Cell Biol. 1995;7:94–101. doi: 10.1016/0955-0674(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 11.Cossart P, Kocks C. The actin-based motility of the facultative intracellular pathogen Listeria monocytogenes. Mol Microbiol. 1994;13:395–402. doi: 10.1111/j.1365-2958.1994.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 12.Cudmore S, Cossart P, Griffiths G, Way M. Actin-based motility of vaccinia virus. Nature. 1995;378:636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- 13.Cudmore S, Reckmann I, Griffiths G, Way M. Vaccinia virus: a model system for actin-membrane interactions. J Cell Sci. 1996;109:1739–1747. doi: 10.1242/jcs.109.7.1739. [DOI] [PubMed] [Google Scholar]
- 14.Duncan S A, Smith G L. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J Virol. 1992;66:1610–1621. doi: 10.1128/jvi.66.3.1610-1621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards J G, Robson R T, Campbell G. A major difference between serum and fibronectin in the divalent cation requirement for adhesion and spreading of BHK21 cells. J Cell Sci. 1987;87:657–665. doi: 10.1242/jcs.87.5.657. [DOI] [PubMed] [Google Scholar]
- 16.Engelstad M, Howard S T, Smith G L. A constitutively expressed vaccinia virus gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 17.Girotti M, Banting G. TGN38-green fluorescent protein hybrid proteins expressed in stably transfected eukaryotic cells provide a tool for the real time, in vivo study of membrane traffic pathways and suggest a possible role for rat TGN38. J Cell Sci. 1996;109:2915–2926. doi: 10.1242/jcs.109.12.2915. [DOI] [PubMed] [Google Scholar]
- 18.Haas T A, Plow E F. Integrin-ligand interactions: a year in review. Curr Opin Cell Biol. 1994;6:652–662. doi: 10.1016/0955-0674(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Herrera E, del Mar Lorenzo M, Blasco R, Isaacs S N. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72:294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiller G, Weber K, Schneider L, Parajsz C, Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979;98:142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- 21.Hirt P, Hiller G, Wittek R. Localization and fine structure of a vaccinia virus gene encoding an envelope antigen. J Virol. 1986;58:757–764. doi: 10.1128/jvi.58.3.757-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz A F, Duggan K, Greggs R, Decker C, Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985;101:2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes R O. Fibronectin. New York, N.Y: Springer-Verlag; 1990. [Google Scholar]
- 24.Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 25.Isaacs S N, Wolffe E J, Payne L G, Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones J I, Prevette T, Gockerman A, Clemmons D R. Ligand occupancy of the αVβ3 integrin is necessary for smooth muscle cells to migrate in response to insulin-like growth factor I. Proc Natl Acad Sci USA. 1996;93:2482–2487. doi: 10.1073/pnas.93.6.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lallier T, Bronner-Fraser M. α1β1 Integrin on neuronal crest cells recognizes some laminin substrata in a Ca2+-independent manner. J Cell Biol. 1992;119:1335–1345. doi: 10.1083/jcb.119.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lallier T, Bronner-Fraser M. Avian neural crest cell attachment to laminin: involvement of divalent cation dependent and independent integrins. Development. 1991;113:1069–1084. doi: 10.1242/dev.113.4.1069. [DOI] [PubMed] [Google Scholar]
- 29.Lallier T, Leblanc G, Artinger K B, Bronner-Fraser M. Cranial and trunk neural crest cells use different mechanisms for attachment to extracellular matrices. Development. 1992;116:531–541. doi: 10.1242/dev.116.3.531. [DOI] [PubMed] [Google Scholar]
- 30.Leavesley D I, Ferguson G D, Wayner E A, Cheresh D A. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibronectin. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberg F P, Gresham H D, Schwarz E, Brown E J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in αvβ3-dependent ligand binding. J Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg F P, Lublin D M, Telen M J, Veile R, Miller Y E, Donis-Keller H, Brown E J. Rh-related antigen CD47 is the signal transducer integrin-associated protein. J Biol Chem. 1994;269:1567–1570. [PubMed] [Google Scholar]
- 33.Mathew E, Sanderson C M, Hollinshead M, Smith G L. The extracellular domain of vaccinia virus protein B5R affects plaque formation, EEV release, and intracellular actin tail formation. J Virol. 1998;72:2429–2438. doi: 10.1128/jvi.72.3.2429-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh A A G, Smith G L. Vaccinia virus glycoprotein A34R is required for infectivity of extracellular enveloped virus. J Virol. 1996;70:272–281. doi: 10.1128/jvi.70.1.272-281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. New York, N.Y: Lippincott Raven Press; 1996. pp. 2637–2671. [Google Scholar]
- 36.Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- 37.Parkinson J E, Sanderson C M, Smith G L. The vaccinia virus A38L gene product is a 33-kDa integral membrane glycoprotein. Virology. 1995;214:177–188. doi: 10.1006/viro.1995.9942. [DOI] [PubMed] [Google Scholar]
- 38.Parkinson J E, Smith G L. Vaccinia virus gene A36R encodes a Mr 43–50K protein on the surface of extracellular enveloped virus. Virology. 1994;204:376–390. doi: 10.1006/viro.1994.1542. [DOI] [PubMed] [Google Scholar]
- 39.Patel D D, Pickup D J, Joklik W K. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology. 1986;149:174–189. doi: 10.1016/0042-6822(86)90119-4. [DOI] [PubMed] [Google Scholar]
- 40.Payne L G, Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976;32:63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez J F, Smith G L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and egress. Nucleic Acids Res. 1990;18:5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roper R L, Payne L G, Moss B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roper R L, Wolffe E J, Weisberg A, Moss B. The envelope protein encoded by the A33R gene is required for formation of actin-containing microvilli and efficient cell-to-cell spread of vaccinia virus. J Virol. 1998;72:4192–4204. doi: 10.1128/jvi.72.5.4192-4204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruoslahti E, Pierschbacher M. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson C M, Frischknecht F, Way M, Hollinshead M, Smith G L. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J Gen Virol. 1998;79:1415–1425. doi: 10.1099/0022-1317-79-6-1415. [DOI] [PubMed] [Google Scholar]
- 46.Sanderson C M, Parkinson J E, Hollinshead M, Smith G L. Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca2+ influx into infected cells. J Virol. 1996;70:905–914. doi: 10.1128/jvi.70.2.905-914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanderson C M, Way M, Smith G L. Virus-induced cell motility. J Virol. 1998;72:1235–1243. doi: 10.1128/jvi.72.2.1235-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scarborough R M, Rose J W, Naughton M A, Phillips D R, Nannizzi L, Arfsten A, Campbell A M, Charo I F. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J Biol Chem. 1993;268:1058–1065. [PubMed] [Google Scholar]
- 49.Schmelz M, Sodeik B, Ericsson M, Wolffe E J, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shida H. Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology. 1986;150:451–462. doi: 10.1016/0042-6822(86)90309-0. [DOI] [PubMed] [Google Scholar]
- 51.Smith J W, Cheresh D A. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61–203 of the β subunit. J Biol Chem. 1988;263:18726–18731. [PubMed] [Google Scholar]
- 52.Tilney L G, Tilney M S. The wily ways of a parasite: induction of actin assembly by Listeria. Trends Microbiol. 1993;1:25–31. doi: 10.1016/0966-842x(93)90021-i. [DOI] [PubMed] [Google Scholar]
- 53.Tomaselli K J, Damskyl C H, Reichardt F. Purification and characterization of mammalian integrins expressed by a rat neural cell line (PC12): evidence that they function as alpha/beta heterodimeric receptors for laminin and type II collagen. J Cell Biol. 1988;107:1241–1252. doi: 10.1083/jcb.107.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia viruses and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1993;60:163–178. [PubMed] [Google Scholar]
- 55.Vanderplasschen A, Hollinshead M, Mathew E, Sim R B, Smith G L. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of cellular complement control proteins into its envelope. Proc Natl Acad Sci USA. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vos J C, Stunnenberg H G. Derepression of a novel class of vaccinia virus genes upon DNA replication. EMBO J. 1988;7:3487–3492. doi: 10.1002/j.1460-2075.1988.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolffe E, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3905–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolffe E J, Weisberg A S, Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;244:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]