Abstract
The pathogenesis of human immunodeficiency virus-associated motor and cognitive disorders is poorly understood. In this context both a protective and a harmful role of the immune system has been discussed. This question was addressed in the present study by correlating the occurrence of neurologic disease in simian immunodeficiency virus (SIV)-infected macaques with disease progression and the humoral and cellular intrathecal antiviral immune response. Overt neurologic signs consisting of ataxia and apathy were observed at a much higher frequency in rapid progressor animals (6 of 12) than in slow progressors (1 of 7). Whereas slow progressors mounted a strong antiviral antibody (Ab) response as evidenced by enzyme-linked immunosorbent and immunospot assays, neither virus-specific Ab titers nor Ab-secreting cells could be found in the cerebrospinal fluid (CSF) or brain parenchyma of rapid progressors. Similarly, increased infiltration of CD8+ T cells and cytotoxic T lymphocytes specific for viral antigens were detected only in the CSF of slow progressors. The finding that neurologic signs develop frequently in SIV-infected macaques in the absence of an antiviral immune response demonstrates that the immune system does not contribute to the development of motor disorders in these animals. Moreover, the lower incidence of neurologic symptoms in slow progressors with a strong intrathecal immune response suggests a protective role of the virus-specific immunity in immunodeficiency virus-induced central nervous system disease.
Infection with human immunodeficiency virus (HIV) can cause multiple neurologic complications collectively defined as AIDS dementia complex (ADC) (26). Although the pathogenesis of this disorder remains poorly understood, a number of mechanisms have been proposed, including cytotoxic effects mediated by viral proteins (18) and toxic factors released by infected central nervous system (CNS) cells (22). In addition, the role of the immune system in the pathogenesis of HIV-induced neurologic disease has remained a topic of controversy (6, 11). It has been observed that in most patients neurologic symptoms arise when the immune functions deteriorate, suggesting that the immune response exerts a protective effect within the CNS. On the other hand, both a high intrathecal antibody (Ab) synthesis (31, 35) and a vigorous cytotoxic T-cell response found in the cerebrospinal fluid (CSF) of ADC patients (10) have led to speculations that this virus-specific immune response may harm brain functions. Moreover, several studies (4, 13, 17, 20) have found Abs to cellular antigens of the CNS in CSF samples of HIV-infected individuals, suggesting that autoimmune phenomena may contribute to the development of neurologic complications (16).
Since investigations on the pathogenesis of neurologic disorders are severely hampered by the restrictions in collecting repeated CSF samples for longitudinal studies from HIV-infected individuals with or without neurologic symptoms, it is essential to use animal models to further the understanding about the mechanisms involved in immunodeficiency virus-induced neurologic disease. In this context, infection of macaques with simian immunodeficiency viruses (SIV) mirrors many of the neuropathological changes found in HIV-infected patients (14, 15, 32). In addition, close examination of SIV-infected macaques with a battery of behavioral and electrophysiological tests has shown evidence for early cognition and motor impairment (24) as well as neurophysiological abnormalities (27), thus representing the most suitable animal model (25).
In order to correlate parameters of the intrathecal immune response with the development of neurologic symptoms it was important to increase the percentage of neurologic disease in SIV-infected monkeys. In the present study, this was achieved by using the primary viral isolate SIVmac251 after in vitro passage on monkey peripheral blood mononuclear cells (MPBMC) (40) as inoculum (referred to hereafter in this work as SIVmac251 MPBMC). After infection with this viral strain, overt clinical signs of neurologic disease were induced in about 40% of macaques with AIDS. By following the time course of humoral and cellular immune functions in these animals, we observed development of neurologic disease predominantly in the absence of an intrathecal virus-specific immune response. This finding suggests a possible active role of the immune system in the prevention of immunodeficiency virus-induced neurologic disorders.
MATERIALS AND METHODS
Animals.
Rhesus monkeys (Maccaca mulatta) used for this study were housed at the Deutsche Primatenzentrum, Göttingen, Germany, according to institutional guidelines. They were serologically free of SIV, simian T-cell leukemia, and simian retrovirus. A total of 43 macaques were infected with 100 50% monkey infective doses of SIVmac251 MPBMC (40). All animals were immunized with keyhole limpet hemocyanin (KLH) and received a booster immunization with KLH 2 weeks prior to the infection. Animals were monitored clinically, and blood was sampled and CSF was collected by suboccipital puncture at regular intervals, with the animals under anesthesia. Monkeys were sacrificed when they became moribund or at specified time points according to the experimental schedule. At necropsy, brains from uninfected and SIVmac-infected rhesus monkeys were thoroughly perfused with 2 liters of Hank’s balanced salt solution (HBSS) containing 3% fetal calf serum. Several coronal slices of brain, 0.5 cm thick, were prepared, and replicate slices were processed for isolation of lymphocytes. Additional tissue from lymph node, spleen, bone marrow, and thymus specimens was obtained at necropsy.
Preparation of cells.
Citrated blood samples were subjected to Ficoll-Hypaque (1.077 g/ml) gradient centrifugation (Pharmacia, Freiburg, Germany) in Leucosep tubes (Greiner, Nürtingen, Germany) in order to obtain mononuclear cells. Cells were collected from CSF samples (1 to 3 ml) by centrifugation at 170 × g for 5 min, and after removal of the supernatant for investigations on humoral parameters, cells were resuspended in HBSS. Viable cells were counted in a Neubauer chamber and differentiated by trypan blue exclusion. In order to minimize the influence of iatrogenic blood contamination during puncture on the parameters determined, CSF samples with more than 5 × 105 erythrocytes/ml (equivalent to a contamination of about 1/10,000) were excluded from further analysis. Lymph node, spleen, and bone marrow specimens were forced through a 100-mesh metal sieve, and the single-cell suspension was collected by centrifugation. Brain lymphocytes and microglia were isolated from fresh tissue by a Percoll gradient technique (34) modified for primate brains (38). Briefly, after the meninges were carefully removed in ice-cold HBSS, pieces of CNS tissue were forced through a 100-mesh metal sieve. After centrifugation at 170 × g, the pellet was resuspended in DNase-collagenase buffer (41 mM MgCl2, 23 mM CaCl2, 50 mM KCl, 153 mM NaCl) containing 500 U of collagenase (Sigma, Deisenhofen, Germany) and 400 U of DNase I (Boehringer, Mannheim, Germany) per g of tissue and digested enzymatically for 60 min at 37°C in a rocking water bath. The suspension was mixed with isotonic Percoll (pH 7.4, 1.122 g/ml; Pharmacia), resulting in a density of 1.030 g/ml, and was transferred to 50-ml centrifuge tubes on top of 5 ml of Percoll at 1.088 g/ml. After centrifugation at 1,250 × g for 15 min, cells were collected from the interface, washed, and resuspended in 5 ml of HBSS. This cell suspension was layered onto a second Percoll density gradient, composed of four layers with densities (from the bottom of the tube) of 1.088, 1.077, 1.060, and 1.030 g/ml. This gradient was again centrifuged at 1250 × g for 15 min. Cells were collected from the 1.077-g/ml interface, washed in HBSS, and resuspended in RPMI 1640 medium (Gibco, Eggenstein, Germany) supplemented with 10% fetal calf serum (Gibco), sodium pyruvate (1 mM; Biochrom, Berlin, Germany), l-glutamine (2 mM; Biochrom), nonessential amino acids (1:100; Biochrom), and gentamicin (Boehringer) (this supplemented medium is termed RPMI+) and counted by trypan blue exclusion in a Neubauer chamber. As evidenced by fluorescence-activated cell sorting (FACS) analysis, cells consisted of >90% microglia, with the remaining cells being predominantly T cells. Microglial cells were cultured for 20 h in 24-well plates at a density of 2 × 106/ml.
Quantitation of IgG and albumin in plasma and CSF.
In order to assess the permeability of the blood brain barrier (BBB) and the intrathecal immunoglobulin G (IgG) production, albumin and IgG concentrations in blood and CSF were quantitated by a standard nephelometric method designed for determination of human albumin and IgG (Beckmann, Munich, Germany) according to the manufacturer’s instructions. As a measure for the integrity of the BBB, a quotient (QAlb) of the albumin concentrations in CSF (AlbCSF) and blood (Albplasma) was calculated (QAlb = AlbCSF/Albplasma). Intrathecal synthesis of immunoglobulin was assumed when the quotient (QIgG) of the IgG concentrations in CSF and blood was greater than the limit 0.8 × √(QAlb2 + 15−6) − 1.3−3 (limQIgG), a formula empirically established for humans (30).
ELISA, ELISPOT, and Western blotting.
Virus-specific titers for the SIV-specific envelope (Env) protein and the group-specific antigens (Gag) were determined in serum and CSF by an indirect solid-phase fluorescence enzyme immunoassay similar to that described previously (39). Recombinant SIVmac structural proteins fused with β-galactosidase and expressed in Escherichia coli provided by A. Rethwilm (Institut für Virologie und Immunbiologie, Würzburg, Germany) were used as solid-phase coupled antigens. The recombinant Env protein represented the complete surface domain, and the Gag proteins consisted of the entire product of the gag region. Control antigen was extracted from bacteria transfected with a plasmid devoid of the SIV-specific insert. The cutoff values were defined by adding two standard deviations to the mean value of fluorescence ratios between viral antigen- and control antigen-coated wells determined in serum specimens from 17 noninfected, healthy macaques. For determination of intrathecal synthesis of antigen-specific IgG, a quotient (Qantigen) was determined according to the following formula: Qantigen = (IgGplasma × titerCSF)/(IgGCSF × titerplasma), where IgGplasma and IgGCSF are amounts of IgG in plasma and CSF, respectively and titerCSF and titerplasma are titers of CSF and plasma, respectively. With reference to the calculated ratios in healthy humans (41), a quotient of >2 was taken as evidence for intrathecal synthesis of virus-specific Abs.
For quantitation of Ab-secreting cells (ASC), the enzyme-linked immunospot assay (ELISPOT) technique was employed (33). Plates with square wells with an area of about 3 cm2 were coated with either rabbit anti-monkey IgG (Sigma) or recombinant viral proteins as described for the enzyme-linked immunosorbent assay (ELISA) and washed with phosphate-buffered saline. Cells isolated from various tissues were plated in the precoated wells in parallel dilutions starting with 2 × 106 cells in 1 ml of RPMI+ and incubated for 18 h at 37°C in a 5% CO2 humidified atmosphere. The plates were vigorously washed with phosphate-buffered saline and incubated for 3 h with rabbit anti-monkey IgG coupled with alkaline phosphatase (Sigma). After extensive washing, 1 ml of bromochloroindolylphosphate (1 mg/ml) (Roth, Karlsruhe, Germany) was added as a substrate in 0.1 M 2-amino-2-methyl-1-propanol buffer (Sigma), pH 10.25, with 0.7 mM MgCl2, 0.1% Triton X-100, and 0.5% low-melting-point agarose (Sigma). After 3 to 4 h, the reaction was stopped by the addition of 200 μl of NaOH (1 M) to each well, and the blue spots which had developed at the sites of Ab production were counted. Comparison of the proportions of virus-specific ASC in different organs was performed by using the paired t test.
In order to detect Abs specific to viral proteins other than Env or Gag in plasma and CSF, we have used Western blot analysis with pelleted virus derived from supernatants of chronically infected C8166 cells loaded on nitrocellulose strips as described previously (40).
Antigenemia was determined in plasma and cell-free CSF as well as in 24-h culture supernatants of isolated microglia by an HIV core antigen capture ELISA (Innogenetics, Zwijndrecht, Belgium) cross-reactive with SIV Gag, performed according to the manufacturer’s instructions. Serial dilutions of a supernatant from persistently SIV-infected C8166 cells with known concentrations of viral antigen were used as standards.
FACS analysis.
All Abs were used at pretitrated concentrations. Controls were performed with the appropriate isotype-matched Abs. In order to quantitate CD4+ and CD8+ T-cell subsets, fluorescein isothiocyanate- and phycoerythrin-conjugated Abs for CD4 (OKT4; Ortho, Neckargemünd, Germany) and CD8 (IOT8 Immunotech, Hamburg, Germany) were combined with the biotinylated anti-monkey CD3 Ab FN18 (M. Jonker [TNO, Rijswijk, The Netherlands]). Bound biotinylated Abs were detected with streptavidin-coupled RED670 (Gibco). Cells were fixed after a final washing step in 3.5% formaldehyde and analyzed within 24 h on a FACScan flow cytometer (Becton-Dickinson, Heidelberg, Germany). If the sample permitted, 104 events were collected for analysis. Otherwise, counting of events was continued until the specimen was exhausted. A minimum of 200 CD3+ T cells in the CSF samples was regarded as necessary for an evaluation of the data.
Cytotoxic T-lymphocyte (CTL) assay.
Cells from blood and CSF were polyclonally stimulated for 48 h with concanavalin A (10 μg/ml; Sigma) and 50% of a supernatant of rhesus MPBMC stimulated with concanavalin A for 48 h in the presence of 105 irradiated (30 Gy), allogeneic herpesvirus papio (HVP)-transformed B-cell lines as feeder cells. Thereafter, the medium was supplemented with 100 U of interleukin-2/ml and 20% ConA supernatant plus α-methyl-d-mannopyranoside (10 mg/ml; Roth) and changed every second to third day. Cells were expanded for the next 2 to 3 weeks in order to obtain a sufficient number for evaluation of virus-specific cytotoxicity.
As target cells, HVP-transformed B-cell lines established from PBMC of each individual animal by infection with supernatant from an HVP-producing cell line (29) were used. These cells were grown in RPMI+ and were infected 16 h before the CTL assay with recombinant vaccinia viruses at a multiplicity of infection of 2. Vaccinia viruses expressing SIV gag, pol, and env genes were provided by F. Bex and A. Burny and obtained via the British Medical Research Council AIDS reagent project. As controls, vaccinia viruses expressing HIV Pol (B. Moss, NIH AIDS reagent program) or measles virus nucleocapsid (B. Bankamp, Institut für Virologie und Immunbiologie) were used. After washing, pelleted target cells were incubated for 90 min at 37°C with Na51CrO4 (10 μCi/105 cells; DuPont, Bad Homburg, Germany) and washed three more times. Meanwhile, effector cells (100 μl) at different concentrations were plated in triplicate in 96-well round-bottom plates, resulting in effector-to-target ratios from 200:1 to 12:1 when 104 target cells in 100 μl were added to each well. Control wells were used to determine spontaneous (target cells alone) and maximum (with addition of 1% Triton X-100) chromium release. After 5 h, the radioactivity in 100 μl of cell supernatant was measured. Specific chromium release was calculated as 100 × (experimental release − spontaneous release)/(total release − spontaneous release). Tests with spontaneous release of more than 25% of maximum release were excluded. Individual values of triplicates differed less than 10%.
RESULTS
Neurologic disease in SIV251 MPBMC-infected monkeys.
For this study we infected a total of 43 macaques with SIVmac251 MPBMC. Of these monkeys, 12 animals (28%) developed simian AIDS within 28 weeks of infection and were termed rapid progressors (Table 1), with a survival time of 18.1 ± 5.6 weeks (unless stated otherwise, data are reported as means ± standard deviations). Seven animals developed AIDS after 7 months of infection (survival time, 58.3 ± 23 weeks) (Table 1). These and another six animals surviving for more than half a year and still clinically asymptomatic at the scheduled time of necropsy were integrated in the slow progressor group. An additional 18 animals were sacrificed within the first 6 months after infection according to the experimental protocol and thus could not conclusively be classified as rapid or slow progressors. However, for this study, all but one of these animals were considered slow progressors according to several virological and immunological markers. All animals were carefully monitored for signs of neurologic disease. Among the 12 rapid progressors, 6 (50%) of the animals developed overt neurologic signs, in some cases even before the onset of other AIDS-defining symptoms such as untreatable diarrhea or pneumonia. These severe clinical signs of neurologic disease included ataxia, opisthotonus, failure at gripping food, and apathy not attributable to weakness. Of the seven slow progressors which were observed until the final stage of AIDS, only one displayed clinical signs of neurologic disease. Neuropathologically, rapid progressors with neurologic symptoms revealed perivascular cuffings, meningitis, glial nodules, and giant cells to a greater extent than those without a neurologic disease in the absence of opportunistic infections and tumors. In contrast, slow progressors primarily showed signs of meningitis and perivascular cuffings (5a).
TABLE 1.
Clinical signs of SIVmac251 MPBMC-infected animals with AIDSa
| Progressor group | Animal no. | Time of necropsy (wpi) | Symptom(s) | Neurologic symptom(s) |
|---|---|---|---|---|
| Rapid | 1 | 12 | Cachexia | Ataxia, seizure |
| 2 | 12 | Ataxia, opistho-tonus | ||
| 3 | 13 | Ataxia, seizure | ||
| 4 | 14 | Dyspnea | Ataxia | |
| 5 | 15 | Anorexia, thrombocytopenia | Apathy | |
| 6 | 26 | Ataxia | ||
| 7 | 15 | Diarrhea, multiple abscesses | ||
| 8 | 17 | Anorexia, thrombocytopenia, diarrhea | ||
| 9 | 20 | Anorexia, thrombocytopenia, diarrhea | ||
| 10 | 21 | Anorexia, thrombocytopenia | ||
| 11 | 24 | Cachexia | ||
| 12 | 28 | Cachexia | ||
| Slow | 13 | 30 | Diarrhea, dyspnea | |
| 14 | 39 | Lymphadenopathy, dyspnea | ||
| 15 | 40 | Dyspnea, pneumocystis carinii | ||
| 16 | 55 | Dyspnea, pneumocystis carinii | ||
| 17 | 58 | Anorexia, cachexia | Ataxia, opistho-tonus | |
| 18 | 73 | Anorexia, cachexia, thrombo-cytopenia | ||
| 19 | 94 | Cachexia, thrombocytopenia |
In addition to the above-listed monkeys, 24 SIV251 MPBMC-infected animals were sacrificed according to the experimental schedule without signs of neurologic disease.
The difference in the incidence of overt neurologic signs in rapid progressors and slow progressors prompted us to compare several parameters of the virus-specific immune response between these two groups of animals in order to determine the role of the immune system for the development of SIV-induced neurologic disorders.
BBB and intrathecal IgG synthesis.
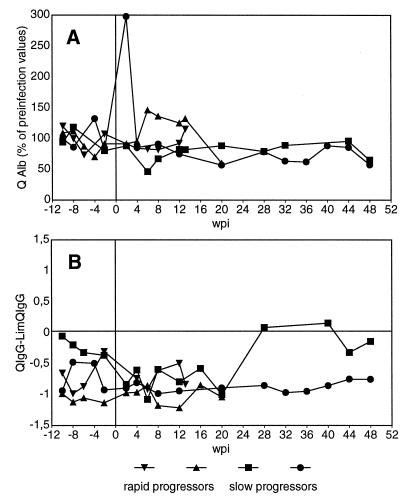
In order to determine the integrity of the BBB, we have measured the AlbIgG and AlbCSF of 17 animals and calculated a quotient (QAlb = AlbCSF/AlbIgG). Since there are considerable differences in the QAlb among individual uninfected animals, we have compared the QAlbs of the individual animals before and after infection with SIV. Representative results for two rapid and two slow progressing animals are shown in Fig. 1A. An increase in the QAlb by more than twice the standard deviation of the individual fluctuations before infection was judged as indicative for breakdown of the BBB. According to this criterion, there was no infection-induced leakage of the BBB in any of the animals investigated, except in one slow progressor (circles in Fig. 1A), which displayed a transient increase in the QAlb at 2 weeks postinfection (wpi). Concomitantly, this animal also showed the highest pleocytosis in the CSF (2 × 105 cells/ml) among more than 50 animals infected with various viral strains studied so far.
FIG. 1.
Integrity of the BBB and intrathecal IgG synthesis. Albumin and IgG concentrations in plasma and CSF were determined. (A) QAlb was calculated. The values for each time point are shown as percentages of the mean preinfection values. (B) QIgG is compared with the limQIgG for intrathecal IgG synthesis. Values of >0 are indicative of intrathecal antibody production. Representative data of two rapid progressors and two slow progressors are depicted.
A global intrathecal IgG synthesis was assumed when the QIgG was higher than limQIgG, calculated as 0.8 × √(QAlb2 + 15−6) − 1.3−3, a formula empirically established for humans (30). Representative kinetics for an intrathecal synthesis as calculated by QIgG − limQIgG are shown in Fig. 1B. We found no evidence for an intrathecal synthesis of IgG in any of the nine SIVmac251 MPBMC-infected animals studied longitudinally for up to 2 years. Moreover, there was also no significant increase of the individual values after infection.
Virus-specific humoral immune response.
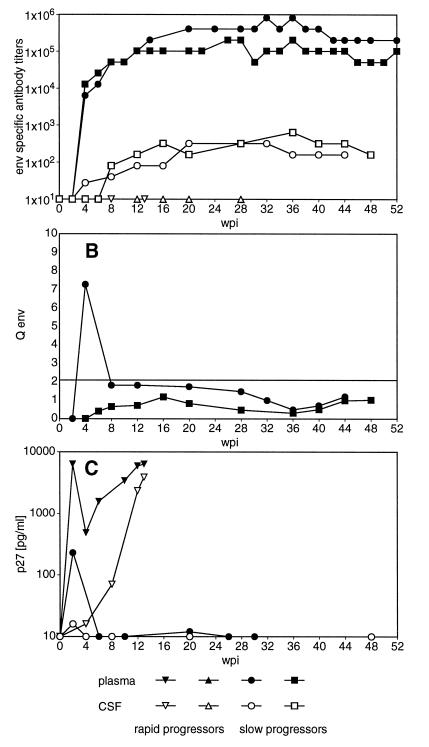
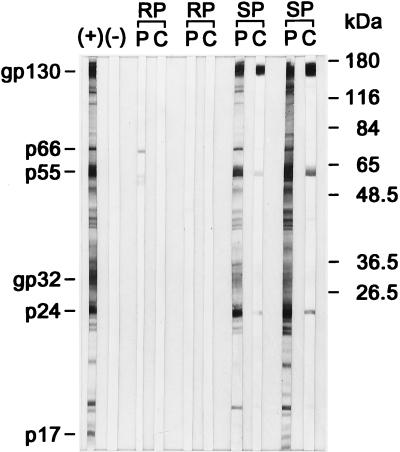
Although we have not found evidence for an overall intrathecal IgG synthesis, virus-specific Abs were found in the CSF. As shown in Fig. 2A, an Env-specific humoral immune response was readily detectable in plasma of slow progressors within 4 weeks of infection. Ab titers specific for Gag showed similar kinetics, though at lower titers (data not shown). In the CSF of slow progressors, both Env- and Gag-specific Abs were found as early as 4 wpi and followed the course of titers in the periphery. In contrast to that, rapid progressors did not reveal any virus-specific Ab titers in plasma or CSF (Fig. 2A). In order to define whether the virus-specific Abs found in the CSF of slow progressors were merely diffused through the BBB or at least in part produced within the CNS, we have compared the virus-specific titers in plasma and CSF according to the formula of Ukkonen et al. (41). By this calculation, no evidence for prolonged intrathecal synthesis of Env- and Gag-specific Abs was revealed in six slow progressors infected with SIVmac251 MPBMC (Fig. 2B) and in only 1 of 14 animals infected with other viral strains (39). Additional Western blot analysis at selected time points demonstrated Abs specific for all major viral polypeptides in the CSF and plasma of all slow progressor animals (Fig. 3). Rapid progressors exhibited only faint bands, either against the transmembrane part of the Env protein or Pol in plasma, but no evidence for virus-specific Abs in undiluted CSF (Fig. 3).
FIG. 2.
Virus specific antibodies and viral antigen in plasma and CSF. (A) Env-specific titers in plasma and CSF. Representative data of two rapid progressors and two slow progressors are shown. (B) Intrathecal synthesis of Env-specific antibodies. Values of >2 are indicative of intrathecal synthesis of antigen-specific antibodies. Representative data of two slow progressors are shown. (C) Representative levels of viral antigen in plasma and CSF of a rapid progressor and a slow progressor. Microglial cells isolated from the brains of SIVmac251 MPBMC-infected animals were cultured for 20 h, and the supernatant was assayed for p27 production. Overall levels of viral antigen produced by microglial cells isolated from SIVmac251 MPBMC-infected animals with AIDS were 9 ± 4 and 8,574 ± 4,155 pg/ml (mean ± SEM) for slow progressors (n = 8) and rapid progressors (n = 6), respectively.
FIG. 3.
Antigen specificity of Abs in plasma and CSF. Paired plasma (P) and CSF (C) samples of two rapid progressors (RP) and two slow progressors (SP) were subjected to Western blot analysis at 1:50 dilutions (plasma) and 1:2 dilutions (CSF). Positive (+) and negative (−) controls are shown in the first two lanes.
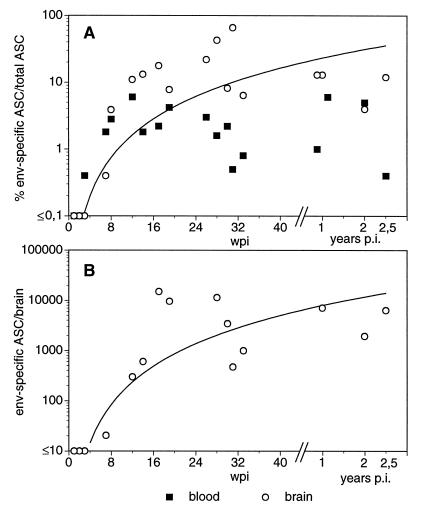
One possible explanation for the failure to detect virus-specific Abs in rapid progressors could be the formation of complexes between these Abs and the abundant viral antigen found both in plasma and CSF of rapid progressing animals (Fig. 2C). Therefore, we tried to detect ASC isolated from blood and brain tissue in vitro by the ELISPOT assay. In order to determine the normal situation within the brain we have first enumerated both the total number of Ig-secreting cells and the frequency of antigen-specific cells in uninfected animals immunized with KLH. In these animals, about 1 in 104 isolated cells from the CNS secreted Ig and none reacted with KLH, even at a time when KLH-specific plasma cells were abundant in blood and lymphoid organs (data not shown). In contrast, ASC specific for the Env protein could be frequently found in brain-derived cells from SIV-infected animals (Fig. 4), indicating intrathecal production of virus-specific IgG. Since we were not able to detect Env-specific ASC in any organ of three rapid progressors tested, we have only statistically compared the percentages of Env-specific ASC isolated from different organs of slow progressing animals. In blood, 2.5% ± 1.9% of the total plasma cells produced Env-specific Abs. Similar proportions of Env-specific ASC among total plasma cells, 2 to 4%, could be observed in lymph node and spleen specimens (data not shown). In contrast, Env-specific ASC account on average for about 15% of all CNS-derived plasma cells, with a proportion of up to 60% in individual infected animals. These values were significantly higher than those for blood, as evaluated by the paired t test (P ≤ 0.01), arguing for a strong intrathecal humoral immune response. Gag-specific ASC were found at lower frequencies in the periphery, though they were never detected in the CNS. In order to determine the time course of the appearance of virus-specific ASC in the CNS, we have plotted the percentage of ASC in blood and CSF of individual animals against the time point after infection when they were sacrificed (Fig. 4A). As early as 3 wpi, the first ASC secreting Ab which reacted with the coated viral proteins were detectable in blood, but the exact number of antigen-specific ASC could not be determined since at this time point, many ASC also reacted with the control antigen. At later time points, no such unspecific reaction was observed. At 4 wpi virus-specific ASC could be identified in blood. Due to the schedule of the experiments, we do not have data for the CNS at this time point. Nevertheless, at 7 wpi virus-specific ASC could be observed in the CNS, although at a low frequency. Thereafter, the proportion as well as the absolute numbers of Env-specific ASC increased in the first 4 months postinfection and seemed to stabilize at about 10%, corresponding to 7,000 Env-specific ASC per brain (Fig. 4B). Since CD20-expressing cells could not be detected by FACS analysis of the isolated cells (data not shown), plasma cells seem to represent the only B-cells within the brain parenchyma.
FIG. 4.
Kinetics of Env-specific ASC in blood and brain samples of SIV-infected animals. Numbers of total plasma cells and Env-specific ASC were determined by ELISPOT. (A) Percent Env-specific ASC among total plasma cells in blood and brain samples. (B) Absolute numbers of Env-specific ASC per brain. Each point represents ASC isolated from one sacrificed animal. In addition, the line of best fit is shown for the values of the brain. p.i., postinfection.
Virus-specific cellular immune response.
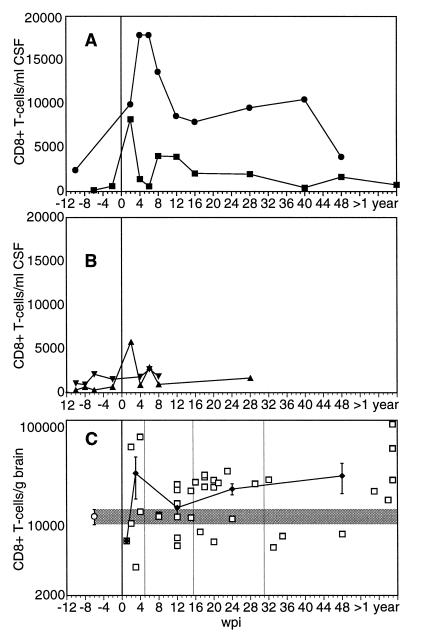
As a first measure for a cellular immune response within the CNS, we determined the number and phenotype of infiltrating leucocytes in the CSF of individual animals by flow cytometry. In uninfected animals, CSF cells consisted almost exclusively of T lymphocytes at <5,000 cells/ml. Between 30 and 50% of these infiltrating T cells expressed CD8. The absolute counts as well as the percentages of CD8+ cells differed between individual monkeys but remained stable in the single animals. Representative kinetics of the absolute numbers of CD8+ T cells in CSF of two slow and two rapid progressing animals are shown in Fig. 5. Whereas slow progressors experienced a strong and sustained infiltration of CD8+ T cells as early as 2 wpi (Fig. 5A), the absolute count of CD8+ T lymphocytes in CSF of rapid progressors was changed only transiently and to a lesser degree (Fig. 5B). Similar kinetics of increased numbers of infiltrating CD8+ T cells were found in the brain parenchyma of slow progressors as determined with cells isolated with the same procedure as for ASC (Fig. 5C). For rapid progressors, data could only be obtained in the final stage of AIDS. At that time point, only two of nine rapid progressors investigated had increased CD8+ T-cell numbers in the brain tissue.
FIG. 5.
Kinetics of CD8+ T cells in CSF and brain tissue. CSF cells and gradient-purified brain cells were counted, and the number of CD8+ T cells was calculated according to the percentage among total cells as determined by flow cytometry. Representative data of CSF from two slow progressors (A) and two rapid progressors (B) are shown. (C) The number of CD8+ T cells per gram of brain tissue of individual SIV-infected slow progressors (□) is shown. In addition, the mean values for uninfected (○ and shaded area) and infected (⧫) animals sacrificed between 2 and 4 wpi, 5 and 15 wpi, 16 and 31 wpi, and after 32 wpi are depicted. Error bars, standard errors of the means.
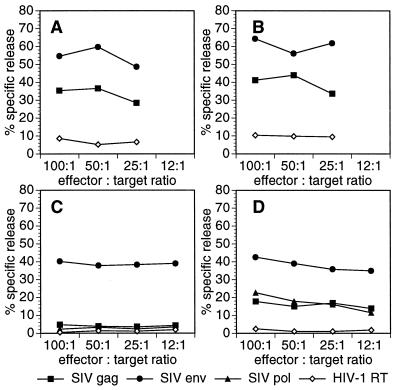
Since the phenotypical analysis of cells in the CSF revealed an increase in the number of CD8+ T cells in slow progressors, we determined the virus-specific cytolytic capacity of these infiltrating cells from three slow and two rapid progressors after polyclonal expansion in vitro. CSF cells isolated from slow progressors displayed cytolytic capacities against autologous cells expressing various viral antigens comparable to those of PBMC investigated in parallel (Fig. 6A and B). In contrast, in vitro-stimulated PBMC of rapid progressors (Fig. 6C) did not show evidence for major histocompatibility complex (MHC)-restricted virus specific cytotoxicity as did PBMC from slow progressors isolated at the same time point after infection (Fig. 6D). The Env-specific lysis of the rapid progressor shown in Fig. 6C is not MHC restricted, because allogeneic cells expressing the Env protein were lysed to a similar degree. Rather, this finding of unrestricted lysis may be explained by fusion-like events of CD4+ cells present in the effector cell population with target cells expressing the Env protein at their surface as described recently (8).
FIG. 6.
Virus-specific cytotoxicity in blood and CSF. Blood (A, B, and C) and CSF (D) cells were polyclonally stimulated, and cytotoxic capacities against autologous target cells expressing the SIV antigens Gag, Env, Pol, or HIV-1 reverse transcriptase (RT) were determined in a chromium release assay. Representative data of a rapid progressor (C) and slow progressors (A, B, and D) are shown.
DISCUSSION
ADC is one of the most devastating complications of HIV infection because of its poor prognosis and the severity of the patient’s functional impairment. In order to study the pathogenesis of this syndrome, we have employed the infection of macaques by SIV as the most suitable animal model. However, in this model neurologic disease mostly has been defined by either morphological criteria, such as SIV encephalitis or vacuolar leukencephalopathy (2, 7, 14, 43), or by subtle cognitive and motor impairments detectable only with sophisticated and laborious behavioral tests (24). In the present study, we observed clinically manifested signs of neurologic disease at a relatively high frequency in macaques infected with the SIVmac251 MPBMC (40). Of a total of 19 animals observed until the development of AIDS, 7 (37%) became neurologically symptomatic. In contrast to 50% of the 12 animals that rapidly developed AIDS, only 1 of the 7 animals which survived longer than 7 months after infection showed clinical signs of neurologic disease. As shown for asymptomatic animals with sophisticated behavioral tests (24), it is very likely that the other animals of our cohort also displayed subclinical neurologic deficits. However, in the study of Murray and colleagues (24), those animals which developed AIDS first also had the lowest performance on cognitive and motor tasks, which, together with the relatively insensitive definition of clinical neurologic signs employed in our study, clearly demonstrates more severe neurologic disorders in rapid progressors. Moreover, the higher incidence of neurologic signs within the group of rapid progressors is in line with previous studies in which a lower mean time of survival among animals with brain parenchymal lesions than in those without such lesions was reported (2, 46). It is interesting that some animals of our cohort presented neurologic disease as the first and only symptom of AIDS. This finding further adds to the similarities of this animal model with HIV infection in humans, as ADC was reported in 7% of the patients at the onset of AIDS and in about 3% of the patients was the only AIDS-defining illness (9).
In a first attempt to explain the difference in the incidence of neurologic disease among the groups with different rates of progression to AIDS, we investigated the antiviral immune response. We found that rapid progressor animals were able to induce a measurable virus-specific immune response neither in the periphery nor in the CNS. Lack of virus-specific Abs in the blood and its correlation with rapid disease progression in HIV-infected patients (23, 28) and SIV-infected rhesus monkeys has already been described by several studies (3, 12, 45). However, by employing the ELISPOT assay for detection of single Ab-producing cells in blood and lymphoid organs ex vivo, we can now exclude the previously discussed possibility that the inability to detect Abs is due to complexation of virus-specific Abs under conditions of antigen excess. In contrast, our results show that lack of virus-specific Ab synthesis results in unrestricted viral replication, leading to early mortality. Moreover, the present report emphasizes a possible role of the cytotoxic immune response in curtailing immunodeficiency virus replication, as rapid progressors did not display MHC-restricted cytolytic activity against several viral antigens. However, since we could not determine the cellular immune response within the first 6 wpi, we cannot rule out the possibility of a temporary virus-specific cytotoxic immune response very early in the course of the disease even in rapid progressors. As shown in other viral infections and discussed as a possible mechanism for the pathogenesis of AIDS (48), this early cytolytic response might have resulted in destruction of infected cells and contributed to the early immunosuppression in these animals. However according to our results, the pattern of expression of several surface markers such as MHC II and CD29 on CD8+ T cells (data not shown) in the course of infection of rapid progressors rather argues against this hypothesis. Alternatively, tolerance might have been induced by the quick spread of the virus in these animals, leading to early exhaustion of the cellular immune response. In contrast to virus-host interactions with noncytopathic viruses, this situation leads to complete destruction of CD4+ T cells, immunosuppression, opportunistic infections, wasting, and neurologic signs in SIV-infected macaques. Thus, the results of our study support the hypothesis of a protective function of CTLs in immunodeficiency virus infection. Experiments to deplete CD8+ T cells in the initial phase of disease, as reported recently (19), have to be carried out to formally prove the role of virus-specific CTLs in SIV-infected macaques.
The status of the immune response in the periphery is also reflected in the brain. Animals without virus-specific Abs and CTLs in the blood showed no evidence of an intrathecal antiviral immune response, whereas longer surviving animals revealed a humoral and cellular immune response in the CNS similar in both kinetics and strength to that found in the blood. The higher incidence of neurologic disease among animals without SIV-specific Abs extends previous observations which found a correlation between lack of virus-specific IgG in the CSF and SIV encephalitis (37). Thus, SIV-specific Abs may play a protective role in the pathogenesis of SIV-associated neurologic disease. Although we have not experimentally excluded the presence of Abs directed against CNS antigens, our findings raise arguments against a possible contribution of autoantibodies in the pathogenesis of ADC (16). Thus, there was no evidence of intrathecal IgG synthesis, and the number of IgG-secreting cells isolated from the brains of rapid progressors was not increased compared to those from both uninfected and asymptomatic SIV-infected animals. In addition as shown previously, rapid progressors rather developed hypogammaglobulinemia (data not shown and reference 7) and showed a selective loss of CD20+ B cells (data not shown and reference 3) instead of increased B-cell activation. Similarly, the occurrence of motor disorders among animals without a detectable virus-specific CTL response indicates that an excessive cellular immune response was not the cause of neurologic disease in these animals as suggested previously (11, 47). According to our results, SIV induced neurologic disease can also develop in the absence of increased BBB permeability. This is in line with previous observations of an intact BBB in SIV- and chimeric simian-human immunodeficiency virus-infected macaques (5, 36).
In slow progressors, we observed a strong antiviral immune response both in the periphery and in the CNS. Several studies investigated the kinetics of the intrathecal humoral immune response in SIV-infected macaques (36, 37, 39). With conventional serological tests, an intrathecal synthesis of virus-specific Abs is only found in some animals late in the course of infection (37, 39). In contrast, ELISPOT analysis revealed the presence of virus-specific plasma cells in the brain parenchyma of every single animal tested, indicative of a strong intrathecal production of SIV-specific Abs. By demonstrating a spontaneous activity and higher frequency of virus-specific B-cells in the CNS, our results extend previous findings of HIV-specific Abs in the supernatants of mitogen-activated CSF cells from six of six HIV-infected individuals (1). However, it remains unclear why the intrathecal synthesis of Abs by these cells is not detectable in the CSF. One explanation could be the lower sensitivity of the titration of specific Abs by ELISA. However, cultivation of isolated brain cells as for the ELISPOT assay yielded Env-specific Ab titers in the supernatant (data not shown). It remains to be demonstrated that this amount of Ab produced is sufficient to establish measurable concentrations in vivo. Additional, more-sensitive serological tests, such as visualization of oligoclonal bands by isoelectric focusing, also did not show evidence for intrathecal Ab synthesis (data not shown), arguing for a similar clonal distribution of plasma cells in CNS and periphery. As SIV-infected cells of the monocyte/macrophage lineage, which are the main reservoir for SIV infection within the brain, express high levels of Env at their surface (21), it is possible that the lack of evidence of intrathecal synthesis in the CSF is due to binding of parenchymally synthesized Abs to their antigens. However, information about the expression of gp160 in the brains of SIV-infected monkeys has not yet been published. Alternatively, it is possible that the CSF represents a separate compartment within the CNS and does not exactly reflect the situation within the parenchyma.
Although we could not test CSF cells for their virus-specific cytolytic capacity before 8 wpi, the kinetics and the phenotype of infiltrating CD8+ T cells provide evidence for an early induction of a CTL response within the CSF of slow progressors. This is in line with a previous study which found virus-specific CTLs in the CSF of an SIV-infected macaque as early as 1 wpi (42). According to our results, the occurrence of neurologic disease is tightly associated with high intrathecal viral loads. Yet in our collective, the viral loads of the two groups of animals were not different during the primary viremia. Only later in the course of the disease did the differences become apparent, as the developing immune response of slow progressors was able to curtail the amounts of viral antigen in both plasma and CNS to undetectable levels by 4 wpi. Similarly in a recent report, the viral RNA levels in plasma before 4 wpi did not correlate with the length of survival (44). Thus, our data provide evidence for a protective role of the intrathecal immune response as one of the host factors to delay or prevent neurologic disease in immunodeficiency virus infection.
Although the SIV infection of macaques provides an excellent model to study the interaction between viral and host factors, the use of this model in the pathogenesis of neurologic alterations has been hampered by the expenditure required to test neurologic functions and the low frequency of neurologic deficits (24). The high incidence and the short incubation time of clinically overt neurologic signs induced by the viral strain SIVmac251 MPBMC among animals with rapid disease progression now renders studies on the pathogenesis and therapy of APC feasible.
ACKNOWLEDGMENTS
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany, and in part by the Max Plank Forschungspreis (to V.t.M.).
We thank A.-M. Aubertin for supplying the viral strain SIVmac251 MPBMC, A. Rethwilm for providing the procaryotic expression systems of SIV proteins, and F. Bex, A. Burny, B. Moss, and B. Bankamp for supplying the recombinant vaccinia viruses. We are indebted to S. Czub and F. J. Kaup, who performed histopathological examinations of the animals. We thank C. Jassoy and I. C. D. Johnston for helpful discussion of the manuscript.
REFERENCES
- 1.Amadori A, De-Rossi A, Gallo P, Tavolato B, Chieco-Bianchi L. Cerebrospinal fluid lymphocytes from HIV-infected patients synthesize HIV-specific antibody in vitro. J Neuroimmunol. 1988;18:181–186. doi: 10.1016/0165-5728(88)90065-3. [DOI] [PubMed] [Google Scholar]
- 2.Baskin G, Murphey-Corb M, Roberts E, Didier P, Martin L. Correlates of SIV encephalitis in rhesus monkeys. J Med Primatol. 1992;21:59–63. [PubMed] [Google Scholar]
- 3.Bohm R, Martin L, Davison-Fairburn B, Baskin G, Murphey-Corb M. Neonatal disease induced by SIV infection of the rhesus monkey (Macaca mulatta) AIDS Res Hum Retroviruses. 1993;9:1131–1137. doi: 10.1089/aid.1993.9.1131. [DOI] [PubMed] [Google Scholar]
- 4.Brey R, Arroyo R, Boswell R. Cerebrospinal fluid anti-cardiolipin antibodies in patients with HIV-1 infection. J Acquired Immune Defic Syndr. 1991;4:435–441. [PubMed] [Google Scholar]
- 5.Coe C, Reyes T, Pauza D, Reinhard J. Quinolinic acid and lymphocyte subsets in the intrathecal compartment as biomarkers of SIV infection and simian AIDS. AIDS Res Hum Retroviruses. 1997;13:891–897. doi: 10.1089/aid.1997.13.891. [DOI] [PubMed] [Google Scholar]
- 5a.Czub, S. Personal communication.
- 6.DalCanto M. AIDS and the nervous system: current status and future perspectives. Hum Pathol. 1989;20:410–418. doi: 10.1016/0046-8177(89)90004-x. [DOI] [PubMed] [Google Scholar]
- 7.Dean A, Montgomery M, Baskerville A, Cook R, Cranage M, Sharpe S, Dennis M, Luthert P, Hou S, Lantos P. Different patterns of neuropathological disease in rhesus monkeys infected by simian immunodeficiency virus, and their relation to the humoral immune response. Neuropathol Appl Neurobiol. 1993;19:336–345. doi: 10.1111/j.1365-2990.1993.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 8.Heinkelein M, Sopper S, Jassoy C. Contact of human immunodeficiency virus type 1-infected and uninfected CD4+ T lymphocytes is highly cytolytic for both cells. J Virol. 1995;69:6925–6931. doi: 10.1128/jvi.69.11.6925-6931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen R, Nwanyanwu O, Selik R, Stehr-Green J. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- 10.Jassoy C, Johnson R, Navia B, Worth J, Walker B. Detection of a vigorous HIV-1-specific T lymphocyte response in cerebrospinal fluid from infected persons with AIDS dementia complex. J Immunol. 1992;149:3113–3119. [PubMed] [Google Scholar]
- 11.Kalams S, Walker B. Cytotoxic T-cells in HIV-induced neurological disease. Curr Top Microbiol Immunol. 1995;202:79–88. doi: 10.1007/978-3-642-79657-9_6. [DOI] [PubMed] [Google Scholar]
- 12.Kannagi M, Kiyotaki M, Desrosiers R, et al. Humoral immune response to T cell tropic retrovirus STLV-III in monkeys with experimentally induced AIDS-like syndrome. J Clin Investig. 1986;78:1229–1236. doi: 10.1172/JCI112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar M, Resnick L, Lowenstein D, Berger J, Eisendorfer C. Brain-reactive antibodies and the AIDS dementia complex. J Acquired Immune Defic Syndr. 1989;2:469–471. [PubMed] [Google Scholar]
- 14.Lackner A, Dandekar S, Gardner M. Neurobiology of simian and feline immunodeficiency virus infections. Brain Pathol. 1991;1:201–212. doi: 10.1111/j.1750-3639.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 15.Letvin N, King N. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquired Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 16.Levy J. HIV neuropathogenesis. J Neurovirol. 1997;3:S14–S15. [PubMed] [Google Scholar]
- 17.Maimone D, Annunziata P, Cioni C, Leonardi A, Guazzi G. Intrathecal synthesis of anti-myelin basic protein IgG in HIV-1+ patients. Acta Neurol Scand. 1994;90:285–292. doi: 10.1111/j.1600-0404.1994.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 18.Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- 19.Matano T, Shibata R, Siemon C, Connors M, Lane H, Martin M. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathisen T, Sönnerborg A, Wahren B. Detection of antibodies against myelin basic protein and increased levels of HIV-IgG antibodies and HIV antigen after solubilization of immune complexes in sera and CSF of HIV infected patients. Viral Immunol. 1989;2:1–9. doi: 10.1089/vim.1989.2.1. [DOI] [PubMed] [Google Scholar]
- 21.McEntee M, Sharma D, Zink M, Adams R, Flexner C, Clements J, Narayan O. Rhesus monkey macrophages infected with simian immunodeficiency virus cause rapid lysis of CD4-bearing lymphocytes. J Gen Virol. 1991;72:317–324. doi: 10.1099/0022-1317-72-2-317. [DOI] [PubMed] [Google Scholar]
- 22.Merrill J, Chen I. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2392–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- 23.Michael N, Brown A, Voigt R, Frankel S, Mascola J, Brothers K, Louder M, Birx D, Cassol S. Rapid disease progression without seroconversion following primary human immunodeficiency virus type 1 infection—evidence for highly susceptible human hosts. J Infect Dis. 1997;175:1352–1359. doi: 10.1086/516467. [DOI] [PubMed] [Google Scholar]
- 24.Murray E, Rausch D, Lendvay J, Sharer L, Eiden L. Cognitive and motor impairments associated with SIV infection in rhesus monkeys. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- 25.Persidsky Y, Nottet H, Sasseville V, Epstein L, Gendelman H. The development of animal model systems for HIV-1 encephalitis and its associated dementia. J Neurovirol. 1995;1:229–243. doi: 10.3109/13550289509114019. [DOI] [PubMed] [Google Scholar]
- 26.Price R, Brew B, Sidtis J, Rosenblum M, Scheck A, Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:549–553. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- 27.Prospero-Garcia O, Gold L, Fox H, Polis I, Koob G, Bloom F, Henriksen S. Microglia-passaged simian immunodeficiency virus induces neurophysiological abnormalities in monkeys. Proc Natl Acad Sci USA. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pyun K, Ochs H, Dufford M, Wedgewood R. Perinatal infection with human immunodeficiency virus. Specific antibody response by the neonate. N Engl J Med. 1987;317:611–614. doi: 10.1056/NEJM198709033171006. [DOI] [PubMed] [Google Scholar]
- 29.Rabin H, Neubauer R, Hopkins R, Dzhikidze E, Shevtsova Z, Lapin B. Transforming activity and antigenicity of an Epstein-Barr-like virus from lymphoblastoid cell lines of baboons with lymphoid disease. Intervirology. 1977;8:240–249. doi: 10.1159/000148899. [DOI] [PubMed] [Google Scholar]
- 30.Reiber H, Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta. 1987;163:319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 31.Resnick L, diMarzo-Veronese F, Schupbach J, Tourtellotte W, Ho D, Muller F, Shapshak P, Vogt M, Groopman J, Markham P, et al. Intra-blood-brain-barrier synthesis of HTLV-III-specific IgG in patients with neurologic symptoms associated with AIDS or AIDS related complex. N Engl J Med. 1985;313:1498–1504. doi: 10.1056/NEJM198512123132402. [DOI] [PubMed] [Google Scholar]
- 32.Ringler, D., R. Hunt, R. Desrosiers, M. Daniel, M. Chalifoux, and M. King. 1988. Simian immunodeficiency virus induced meningoencephalitis: natural history and retrospective study. Ann. Neurol. 23(Suppl.):S108–S112. [DOI] [PubMed]
- 33.Sedgwick J, Holt P. A solid phase immunoenzymatic technique for the enumeration of specific antibody secreting cells. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 34.Sedgwick J, Schwender S, Imrich H, Dörries R, Butcher G, ter Meulen V. Isolation and direct characterization of resident microglia cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singer E, Syndulko K, Fahy-Chandon B, Shapshak P, Resnick L, Schmid P, Conrad A, Tourtellotte W. Cerebrospinal fluid p24 antigen levels and intrathecal cognitive disease severity in HIV-1. AIDS. 1994;8:197–204. doi: 10.1097/00002030-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Smith M, Heyes M, Lackner A. Early intrathecal events in rhesus macaques (macaca mulatta) infected with pathogenic or nonpathogenic molecular clones of simian immunodeficiency virus. Lab Investig. 1995;72:547–558. [PubMed] [Google Scholar]
- 37.Smith M, Sutjipto S, Lackner A. Intrathecal synthesis of IgG in simian immunodeficiency virus (SIV)-infected rhesus macaques (Macacca mulatta) AIDS Res Hum Retroviruses. 1994;10:81–89. doi: 10.1089/aid.1994.10.81. [DOI] [PubMed] [Google Scholar]
- 38.Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, ter Meulen V. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 1996;220:320–329. doi: 10.1006/viro.1996.0320. [DOI] [PubMed] [Google Scholar]
- 39.Sopper S, Hemm S, Meixensberger J, Coulibaly C, Stahl-Hennig C, Hunsmann G, Fleckenstein B, ter Meulen V, Dörries R. Dynamics of the immune system response in cerebrospinal fluid and blood of SIVmac-infected rhesus monkeys. J Med Primatol. 1993;22:138–146. [PubMed] [Google Scholar]
- 40.Stahl-Hennig C, Voss G, Dittmer U, Coulibaly C, Petry H, Makoschey B, Cranage M, Aubertin A, Luke W, Hunsmann G. Protection of monkeys by a split vaccine against SIVmac depends upon biological properties of the challenge virus. AIDS. 1993;7:787–795. doi: 10.1097/00002030-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ukkonen P, Ganström M, Ränsänen J, Salonen E, Penttinen K. Local production of mumps IgG and IgM antibodies in the CSF of meningitis patients. J Med Virol. 1981;8:257–265. doi: 10.1002/jmv.1890080406. [DOI] [PubMed] [Google Scholar]
- 42.von Herrath M, Oldstone M, Fox H. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995;154:5582–5589. [PubMed] [Google Scholar]
- 43.Watry D, Lane T, Streb M, Fox H. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995;146:914–923. [PMC free article] [PubMed] [Google Scholar]
- 44.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P, Hu S, Haigwood N. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Martin L, Watson E, Montelaro R, West M, Epstein L, Murphey-Corb M. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1988;1988:1277–1286. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]
- 46.Zink M, Amedee-Martin A, Mankowsky J, Craig L, Munoz A, Spelman J, Didier P, Murphey-Corb M, Carter D, Clements J. Pathogenesis of SIV encephalitis. Selection of neurovirulent strains of virus by the CNS. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- 47.Zink M, Spelman J, Bedno-Robinson R, Clements J. SIV infection of macaques—modeling the progression to AIDS dementia. J Neurovirol. 1998;4:249–259. doi: 10.3109/13550289809114526. [DOI] [PubMed] [Google Scholar]
- 48.Zinkernagel R, Hengartner H. T-cell-mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol Today. 1994;15:262–268. doi: 10.1016/0167-5699(94)90005-1. [DOI] [PubMed] [Google Scholar]