Abstract
Children are the most naturally physically active human beings; reduced physical activity is a cardinal sign of childhood disease, and exercise testing provides mechanistic insights into health and disease that are often hidden when the child is at rest. The physical inactivity epidemic is leading to increased disease risk in children and, eventually, in adults in unprecedented ways. Cardiopulmonary exercise testing (CPET) biomarkers are used to assess disease severity, progress, and response to therapy across an expanding range of childhood diseases and conditions. There is mounting data that fitness in children tracks across the life span and may prove to be an early, modifiable indicator of cardiovascular disease risk later in life. Despite these factors, CPET has failed to fulfill its promise in child health research and clinical practice. A major barrier to more accurate and effective clinical use of CPET in children is that data analytics and testing protocols have failed to keep pace with enabling technologies and computing capacity. As a consequence, biomarkers of fitness and physical activity have yet to be widely incorporated into translational research and clinical practice in child health. In this review, the author re-examines some of the long-held assumptions that mold CPET in children. In particular, the author suggests that current testing strategies that rely predominantly on maximal exercise may, inadvertently, obfuscate novel and clinically useful insights that can be gleaned from more comprehensive data analytics. New pathways to discovery may emanate from the simple recognition that the physiological journey that human beings undertake in response to the challenge of exercise may be far more important than the elusive destination of maximal or peak effort.
The purpose of this review is to focus on several intersecting journeys, new pathways necessary to advance the field of exercise medicine and exercise science in child health. We will cover the journey of concept and discovery in which novel technologies are reshaping how we measure and gauge fitness in critical periods of growth and development in children, and the physiological journey that integrates thermodynamic, biologic, and cognitive mechanisms permitting children to engage in physical activities that vary widely in intensity, duration, and skill. Collectively, these journeys are bringing us to a new and exciting era of discovery for all of child health research and clinical application.
The Nobel Laureate A.V. Hill was the pioneering British exercise scientist who in the 1920s accessed the novel technologies and conceptualized the existence of a maximum level of oxygen intake. In describing a series of exercise tests in several healthy volunteers, Hill et al (48) wrote, “The oxygen intake rises steadily as the [running] speed is increased, attaining a maximum, however, beyond which no bodily effort can drive it.” Hill and his group were inquisitive experimentalists and also noted that the so-called maximal levels of ˙VO2 in a given individual intake were dependent on many conditions such as the type of exercise and the concentration of inspired oxygen.
Hill was acutely aware that his laboratory’s focus on exercise in the context of athletics limited the generalizability of the nascent discipline of exercise physiology. In his introduction to a series of lectures given at Cornell University in 1926–1927, he (47) wrote, “The complaint has been made to me–’Why investigate athletics, why not study the process of industry or of disease?’ The answer is twofold. (i) The processes of athletics are simple and measureable and carried out to a constant degree, namely to the utmost of a man’s powers: those of industry are not; and (ii) athletes themselves, being in a state of health and dynamic equilibrium, can be experimented on without danger and can repeat their performances exactly again and again.” Hill’s prescience in grasping that the approach to exercise science depended on the context in which physical activity (PA) was considered (eg, athletics, presence of disease) was not shared by the majority of physiologists and clinicians over the past century. Historical realities, the cultural zeitgeist, and politics have always shaped scientific research (83), and exercise science has not been an exception. A century ago, the Russian revolution prompted scientists to better understand the physiological limits of physical labor as productivity at that time depended in no small measure on the physical work capacity of laborers (87). In the United States, entry into World War I and institution of mandatory conscription to military service revealed an alarming percentage of young Americans simply unfit for service. It is not surprising that the Harvard Fatigue Laboratory, established in 1927 and housed in the basement of the Harvard School of Business, led the nation in understanding the limits of physical capacity in human beings with a particular focus on industry and the military (94).
The focus on athletic and sports performance has remained the predominant theme in pediatric exercise science. As shown in Figure 1, an exponential increase in research in publications focused on exercise testing in the pediatric age range in the context of sports and athletics has occurred over the past 5 to 6 decades. A far smaller increase has occurred over the same period examining exercise testing and performance in the context of disease or illness. In Figure 2, I present a 10-year snapshot (2007–2017) of the relative focus of research publications in pediatric exercise on relevant diseases and conditions.
Figure 1 —
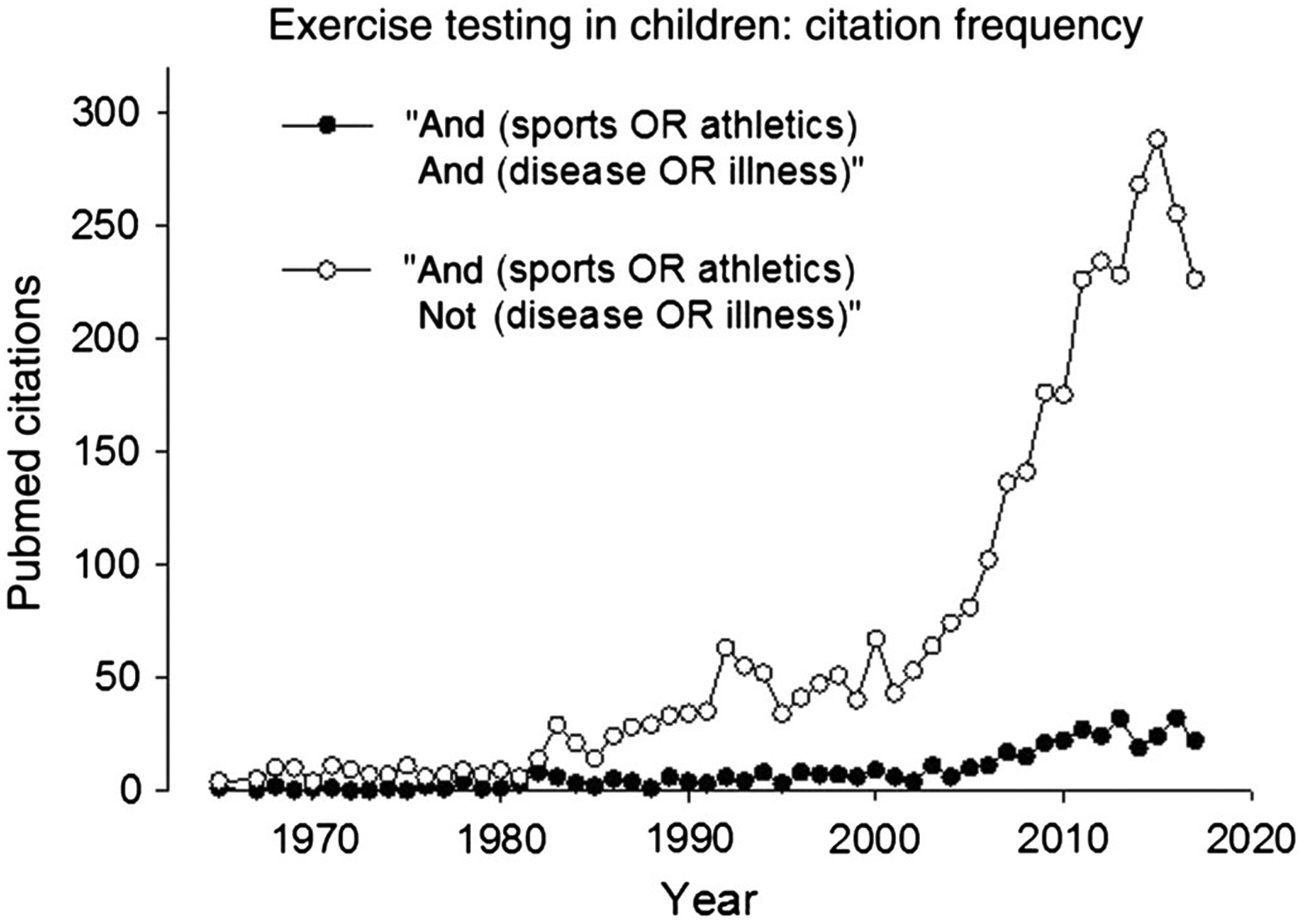
A bibliometrics search examining the use of exercise testing to assess functional aspects of fitness in children in both health and disease.
Figure 2 —
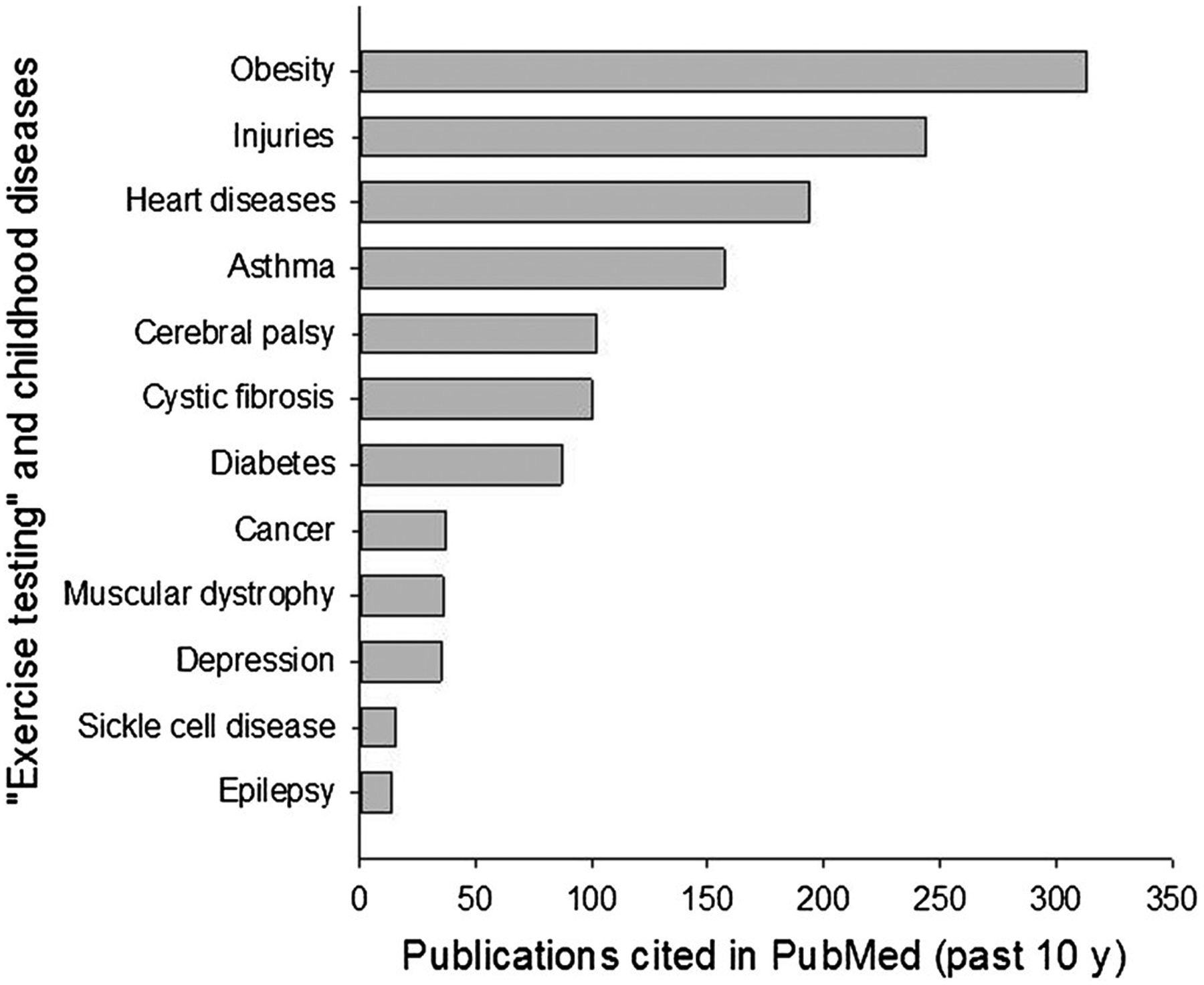
Distribution of publications in exercise testing and childhood conditions, and diseases over the past 10 years.
Physical Activity: An Essential Element of Growth and Development in Children and Adolescents
Exercise in children and adolescents is not merely play but is an essential component of growth and development (39,57,91). Children are among the most spontaneously physically active human beings (66). It is not surprising that participation in PA is a major determinant of health across the life span and health-related quality of life in both healthy children and in children with chronic diseases (46,64,68,76). Despite this essential biologic role for PA, children have not been spared the relentless reduction in levels of PA, that is, creating a crisis in health care in our nation and throughout the world (58). Recognition of the enormous morbidity and cost of physical inactivity–related diseases, such as atherosclerosis, type 2 diabetes, and osteoporosis, has spurred new policy initiatives targeting preventive medicine early in life (18,23,74). The concept of pediatric origins of adult health and disease is gaining scientific merit (10,13,44,86), highlighting the need to transform existing notions of how to evaluate health in a growing child. A physically inactive (even normal weight) child may have no symptoms of disease, but evidence of deterioration in vascular health may already be present (20,51,92).
Equally worrisome is that the deleterious health effects of physical inactivity and poor fitness are exacerbated in children with chronic disease and/or disabilities (1,49,50,62,67,99) or with environmental–lifestyle conditions like obesity (53). Children with diseases or conditions previously associated with excessive mortality and morbidity during the first 2 decades of life (eg, cystic fibrosis, pulmonary hypertension, sickle cell disease, congenital heart disease, malignancies, etc) are living longer because of remarkable advances in research and care (35,37,43,55,59) but are often unable to achieve levels of PA and fitness associated with health benefits in otherwise healthy children (2,60,89,95). Not surprisingly, the health span (the period of life free from serious chronic diseases and disability [90]) of children with chronic diseases is threatened not only by the underlying disease but also by the compounding effects of insufficient PA and sedentary behavior (15,19,33,40,81). Increasing PA and fitness is feasible but has proven quite challenging to implement in a systematic manner (38).
Once a pattern of physical inactivity and a sedentary lifestyle is established, a vicious cycle ensues (Figure 3), in which constraints on PA harm immediate health and contribute to lifelong health impairment ranging from cardiovascular and metabolic disease to osteoporosis (16,24,32,57,61,63). Exactly what constitutes ideal physical fitness in a child with a chronic condition remains unknown. Finding beneficial levels of PA in children with chronic disease or disability is challenging because the optimal range of exercise is much narrower than in a healthy child (Figure 4).
Figure 3 —
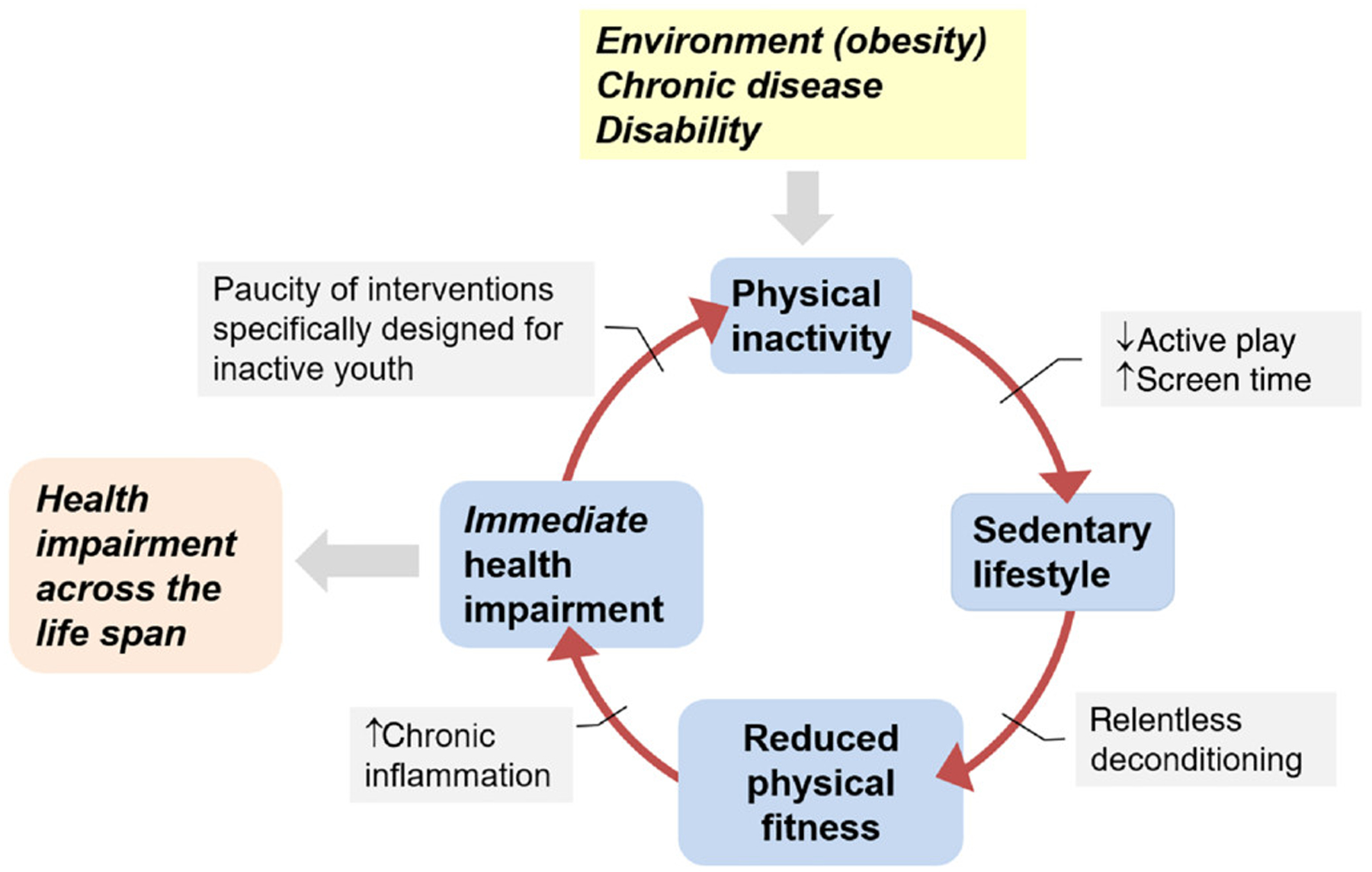
Physical inactivity in childhood, be it imposed by environmental factors or chronic diseases or conditions, leads to a vicious cycle that impairs health across the life span.
Figure 4 —
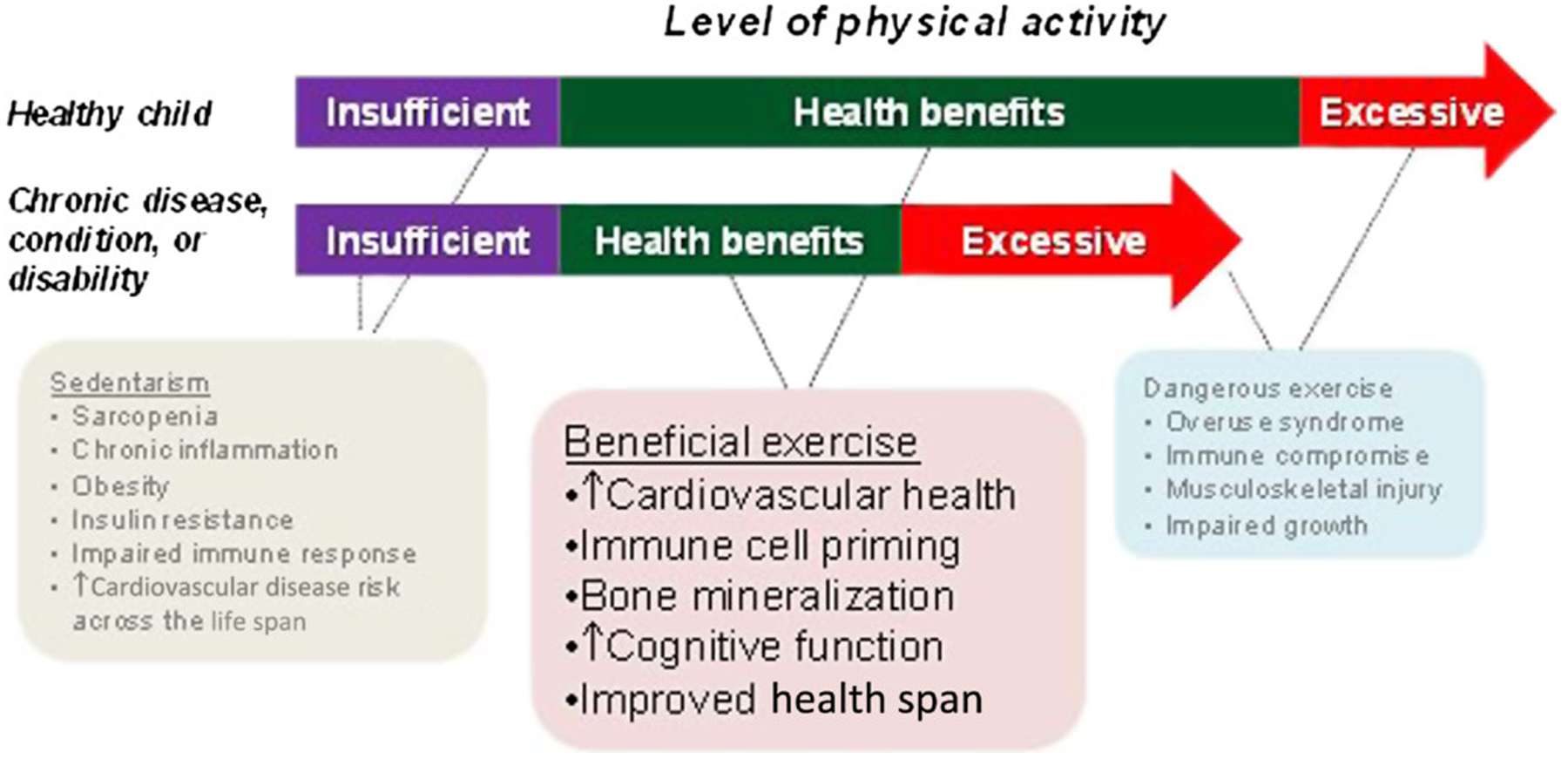
Health benefits of exercise are determined, in part, by the energy expenditure associated with physical activity. Both too much (excessive) and too little (sedentarism) exercise can impair health. As shown, the range of healthy exercise is narrower in the child with chronic disease or disability. CPET and physical fitness testing must be designed to identify biological mechanisms that can be translated into finding the mode, frequency, duration, and intensity of exercise that can benefit health during critical periods of growth. CPET indicates cardiopulmonary exercise testing.
The Physiological Journey of the Exercise Response: Rethinking the Guideposts
As previously noted, a major driving concept in exercise science research in both children and adults over the past century has been maximal oxygen intake (V̇O2max). Substantial controversy still surrounds this term, and many researchers prefer to employ V̇O2peak, particularly when there is no clear plateau of V̇O2 as work rate (WR) increases. There is no doubt that this approach has enormously advanced our understanding of the mechanisms involved in human PA. Maximal exercise tests in children are highly dependent on the willingness of each participant to continue exercise at relatively high WRs when dyspnea, muscle fatigue, and other stress sensations are commonly experienced. These tests involve exercise intensities that are infrequently attained in natural PA (7,12,41) and are not particularly enjoyable for many children (Figure 5). As a result, a fair amount of cheerleading by the laboratory personnel is often necessary to help the child achieve a maximal effort in cardiopulmonary exercise testing (CPET). Despite its proven clinical and research utility, true V̇O2max (finding an unambiguous plateau in V̇O2) only occurs in a minority of exercise tests even in healthy children and adults and maybe less so in children with chronic disease or disability (17,29,34).
Figure 5 —

“Found Art” in the pediatric exercise laboratory. Maximal exercise testing is uncomfortable and unpleasant for many children. A 9-year-old girl left this note for us. It is time to rethink exercise testing in children.
The conundrum of V̇O2max testing is highlighted in the current controversy surrounding the idea that in order to “verify” V̇O2max, one must perform traditional progressive exercise tests to the limit of the participant’s tolerance followed within minutes by a constant WR “supramaximal” test at a higher WR, again, to the limit of the participant’s tolerance. The very name of the additional test, supramaximal, highlights the conceptual difficulty. A maximum is, after all, the greatest or highest amount possible or attained. “Supramax” does not exist. The data clearly indicate that V̇O2 in the supramaximal constant workload test is often higher than the “V̇O2max” obtained moments before in the progressive test. This is a fascinating observation but in no way indicates the discovery of the real V̇O2max. A much more reasonable explanation is that the elusive V̇O2max is dynamic that the prior exercise through well-established impact on time constants, blood flow, lung function, cognitive factors, and so on, (9,14,65,103) altered physiological processes and permitted a greater capacity to uptake atmospheric O2.
One of the transformative discoveries over the past several decades has been the ability of exercise to stimulate systemic immune, stress, and both proinflammatory and anti-inflammatory mediators and cytokines. The seminal observations by Ostrowski et al (75) in adults have been corroborated in children and adolescents (72,73). In retrospect, of course, a stress, “danger” type signaling by exercise is not surprising; even brief bouts of sufficiently intense PA lead to a metabolic acidosis, an outpouring of catecholamines, and a profound perturbation of cellular homeostasis (28,71). The initial acute increase in circulating leukocytes and cytokines such as interleukin-6 is often balanced by anti-inflammatory mechanisms, and exercise training, such as other short-term stresses, may ultimately prove to be, in balance, anti-inflammatory. Nonetheless, exercise stimulus of stress and inflammation can be dangerous. A number of studies have demonstrated that high-intensity exercise is aversive or unpleasant in both children and adults (36,88), and one is reminded of the popular mantra about exercise, “no pain, no gain.”
When a child performs standard CPET but does not reach an exercise level that can be classified as maximal, the whole test may be deemed a failure despite the wealth of data successfully collected. For example, in a large study of children and adolescents (mean age: 12.3 y) who had undergone the Fontan correction for congenital heart disease during childhood, only 40% achieved an acceptable V̇O2max using current criteria (77). Many clinically oriented researchers are hesitant about pushing certain patient populations during high-intensity exercise when acidosis can ensue and stress mediators are elevated. Salvadego et al (85) studied exercise in a group of obese, otherwise healthy, adolescents and stopped exercise when the participant achieved a heart rate (HR) of 180 bpm. The authors noted, “A true maximal test was not performed to avoid the cardiovascular risks associated with maximal exercise in obese subjects.”
The value of alternatives to maximal testing strategies have been recognized for many years, but predominantly focused on using data obtained from submaximal portions of exercise tests to estimate a predicted ˙VO2max rather than on the value of the submaximal data itself. Åstrand and Rhyming (6) recognized over 50 years ago that V̇O2max in adults could be predicted by HR measured during submaximal exercise. Fitness tests that require HR data alone are more feasible for large cohorts than studies in which gas exchange is measured in each participant. For example, Pate et al (78) used HR data collected in the National Health and Nutrition Examination Survey (70) to characterize fitness in 3287 children and adolescents (aged 12–19 y). One must balance the loss of accuracy introduced when ˙VO2 is estimated from HR (often through complex formulas whose parameters are based on many assumptions) with the increased cost and complexity associated with actual breath-by-breath measurements of gas exchange.
New Technology and New Pathways: Impact of Breath-By-Breath Measurement of Gas Exchange
The breakthrough maximal exercise studies in the early 1900s were made possible by technological advances in measurement of oxygen concentration. However, large volumes of gas were required, limiting the number of data points in which variables such as V̇O2 or V̇CO2 could be obtained during an exercise protocol. Progress in gas concentration and gas flow analytics proceeded rapidly with the advent of mass spectrometry and rapid detection of flow rates and vectors from small quantities of gas, and by the 1970s, breath-by-breath measurement of gas exchange became possible (104). The early devices were limited by computing capacity; for example, the then most advanced HP-1000 computer had only 64kb of memory and required clever programming and data manipulation to facilitate the calculations necessary for breath-to-breath analysis. Breath-by-breath CPET became commercially available in the late 1980s and permitted quantification of gas exchange response time over minutes and in adult studies led to a revolution in research focused on “gas exchange kinetics,” an engineering and integrative systems approach to the adaptive biology of PA. Between 1976–1981 and 2011–2015, a PubMed search revealed an increase from 5 to 71 publications in adults using breath-by-breath exercise analytics in clinical trials. Over the same interval, studies in children increased only from 2 to 11. The time is opportune to apply enabling and new technologies to PA biomarkers in pediatric health.
In a study of 169 children and adolescents (27), we demonstrated a set of remarkably strong relationships among submaximal slopes (obtained from the “forgotten” data of progressive exercise CPET), body weight, lean body mass (obtained from dual x-ray absorptiometry [DXA]), and the traditional peak ˙VO2 (Figures 6 and 7). These observations hold the promise of greatly expanding the clinical and translational research utility of CPET. Earlier studies from our group have demonstrated the power of slope analysis in children with specific diseases to identify unique pathophysiological biomarkers associated with the underlying condition. For example, we found abnormal slopes of Δ˙VE/Δ˙VCO2 (the change in ventilations relative to the change in CO2 output) in CF, consistent with increased ventilation/perfusion mismatching and dead space (67). In children who had undergone the Fontan surgical correction for a variety of congenital heart diseases, we noted reduced slopes of Δ˙VO2/ΔWR quantifying the degree of impaired oxygen delivery (99). More recently, abnormal ˙VO2/WR, ˙VE/˙VO2, ˙VO2/HR, and ˙VE/˙VCO2 slopes were observed in patients with sickle cell disease (60). These observations were made during submaximal phases of maximal work CPET in sickle cell disease patients in whom caution should be exercised when encouraging PA at high intensities, characterized by elevated catecholamines and stress and inflammatory factors.
Figure 6 —
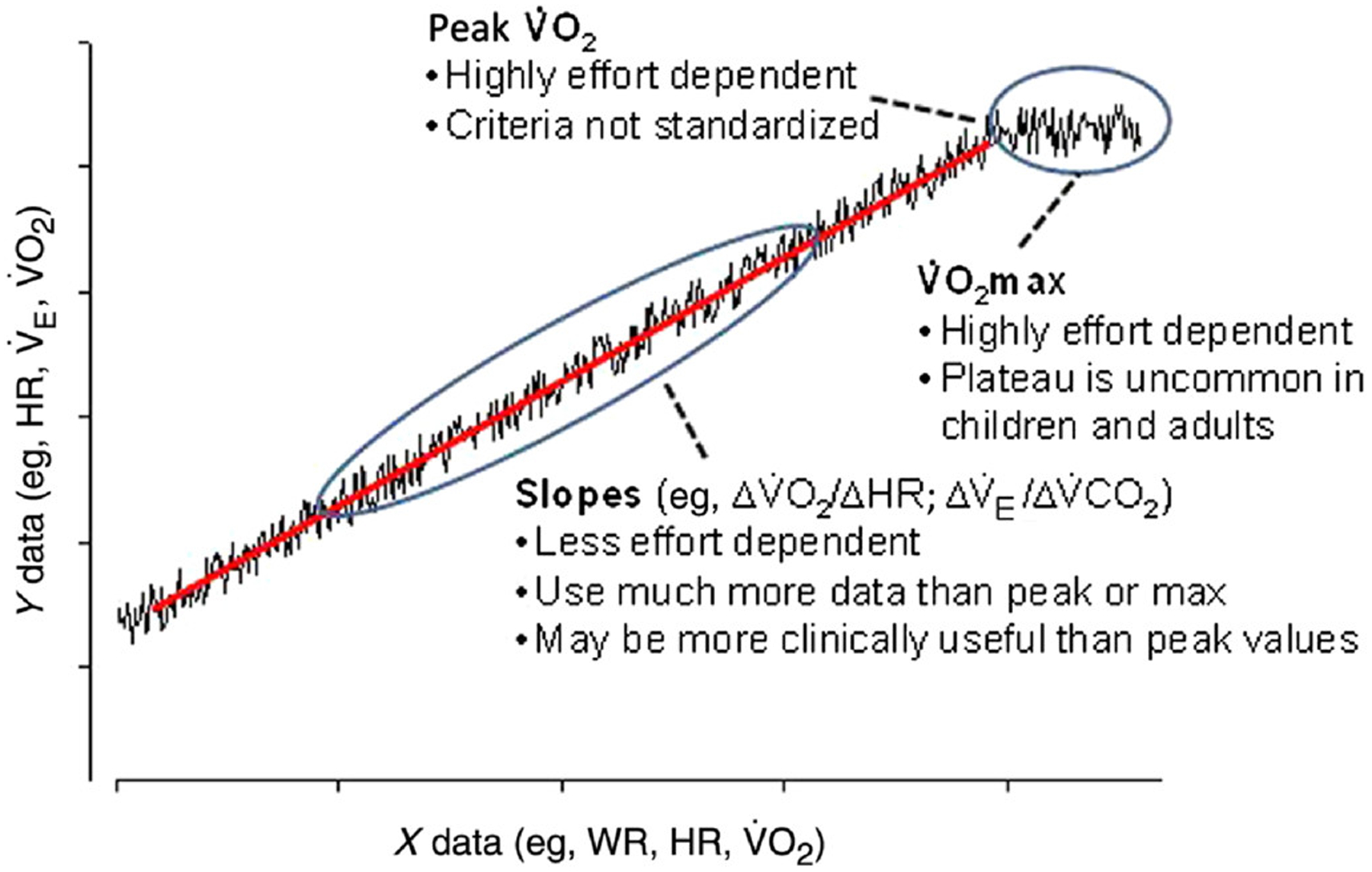
Enhancing the value of CPET. Shown here is a schematic of the type of data derived from progressive exercise in children and adolescents. Traditional CPET relies on maximal or peak values. We propose to better capture dynamic relationships of breath-by-breath data during submaximal phases of progressive CPET and transform how we measure the physiologic response to acute exercise. CPET indicates cardiopulmonary exercise testing; WR, work rate; HR, heart rate; ˙VO2, oxygen uptake; ˙VO2max, maximal ˙VO2 uptake.
Figure 7 —
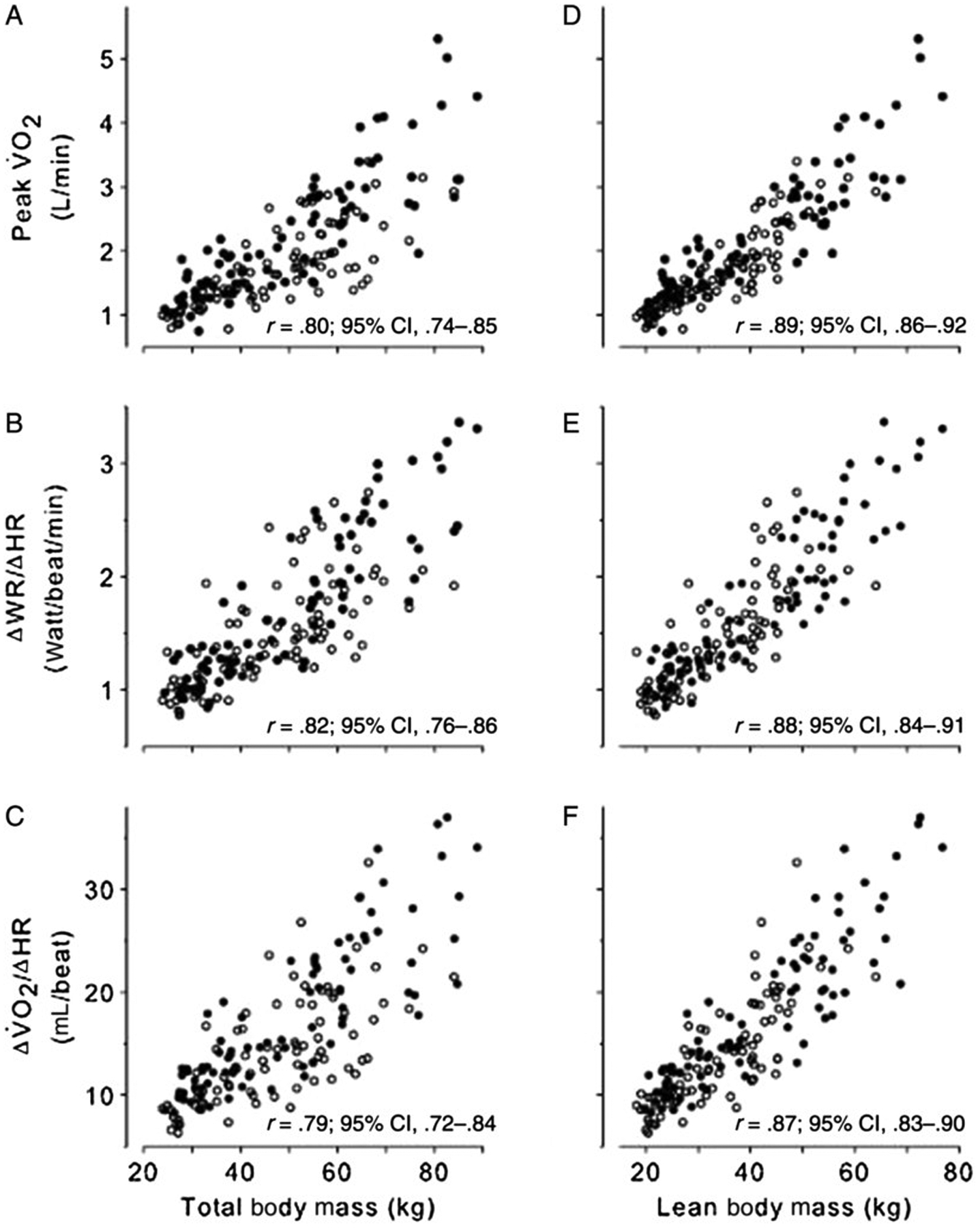
Scatterplots of CPET variables predicted to be relatively body size dependent versus TBM determined by standard scale (A, B, and C) and LBM determined by DXA (D, E, and F). Females are shown as open circles and males as closed circles. The correlation and the corresponding 95% CI are shown for each plot. In all cases, the correlations with TBM were high but were significantly improved with LBM. CPET indicates cardiopulmonary exercise testing; CI, confidence interval; TBM, total body mass; LBM, lean body mass; ˙VO2, oxygen uptake.
Taking full advantage of enabling technologies and computing capacity, new testing strategies have emerged over the past several decades. As valuable as traditional progressive-exercise CPET has proven, it tends to overlook one of the critical components of successful PA in children, namely the ability to rapidly respond to and recover from brief bouts of exercise. The evolutionary pressures of successfully gathering or hunting food and/or escaping predation led to integrated physiological systems in which (in response to exercise) cardiac output increased rapidly, oxygen was delivered quickly to working muscles, and blood flow maintained to key nonexercising organs, such as the brain, so that executive function could be maintained and wise decisions (where to run; when to hide) made. Testing strategies have emerged that accentuate the ability to gauge impairment in the critical onset and recovery phases of the exercise response.
Interestingly, one of A.V. Hill’s most important contributions was the concept of oxygen debt and deficit, an idea that arose from observations that ˙VO2 increased exponentially, not immediately, in response to a constant WR perturbation. Hill et al surmised the existence of stores and energy mechanisms that provided the immediate requirements of muscular work and were not immediately dependent on oxygen uptake from the atmosphere. The deficit incurred was then “paid back” as evidenced by the delayed (exponential) return of ˙VO2 to baseline after the termination of physical work.
Pioneering work from a number of investigators is emerging to examine gas exchange dynamics in children in health and disease (3,4,26). For example, Barker et al (9) combined breath-by-breath measurements of gas exchange with near-infrared spectroscopy to simultaneously measure muscle O2 delivery, O2 utilization, and muscle activity following priming exercise to better understand the factors limiting ˙VO2 kinetics during high-intensity exercise tolerance in youth. Their findings were intriguing (Figure 8); despite an enhanced aerobic energy provision following the priming intervention, exercise tolerance was reduced between exercise bouts due, perhaps, to inadequate recovery of the muscle metabolic status before the subsequent exercise perturbation commenced. Earlier work in otherwise healthy children has demonstrated key differences in gas exchange kinetics between children and adults, most notably in the dynamics of ventilation and ˙VCO2 (26,69).
Figure 8 —
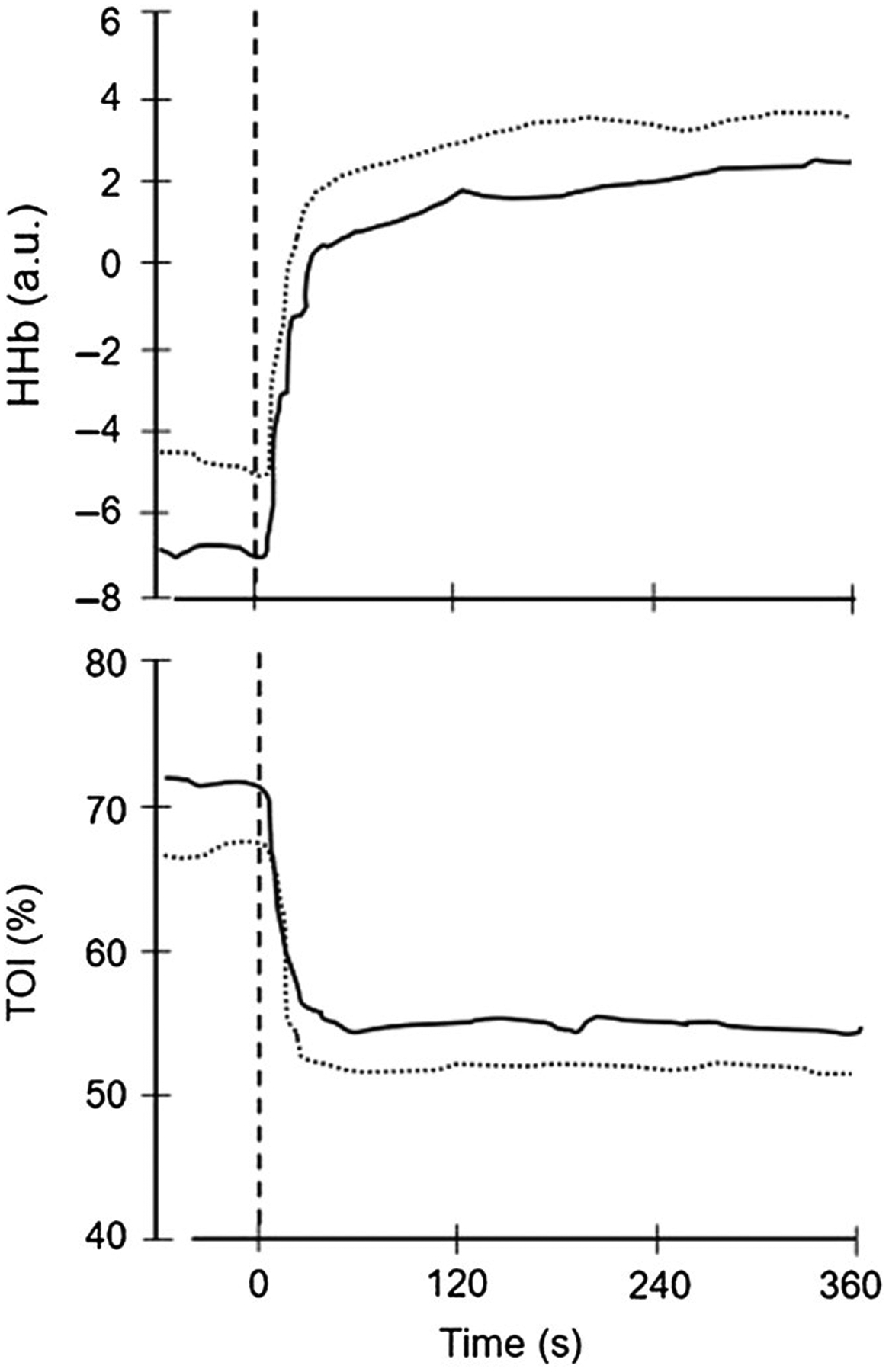
Mean HHb (top panel) and TOI (bottom panel) dynamics obtained from near-infrared spectroscopy during an initial exercise bout (dotted line) rapidly followed by a repeat bout (solid line). The vertical dotted line signifies the onset of exercise. Note that in the second bout, the TOI is elevated at baseline and throughout the exercise transition. Despite this, exercise tolerance was not improved by the priming bout. Reprinted with permission from Barker et al (9). TOI indicates tissue oxidation index; HHb, deoxyhemoglobin.
Dynamic exercise testing has also been done to elucidate disease mechanisms in childhood illnesses. For example, Hebestreit et al (45) used oxygen uptake kinetics to demonstrate an impairment of oxygen delivery in children with cystic fibrosis. A number of years ago, our group was able to demonstrate large differences in gas exchange responses to exercise in children who had undergone the Fontan surgical correction for congenital heart diseases. We used a relatively simple approach, the recovery from a 1-minute bout of constant WR exercise (99) (Figure 9).
Figure 9 —
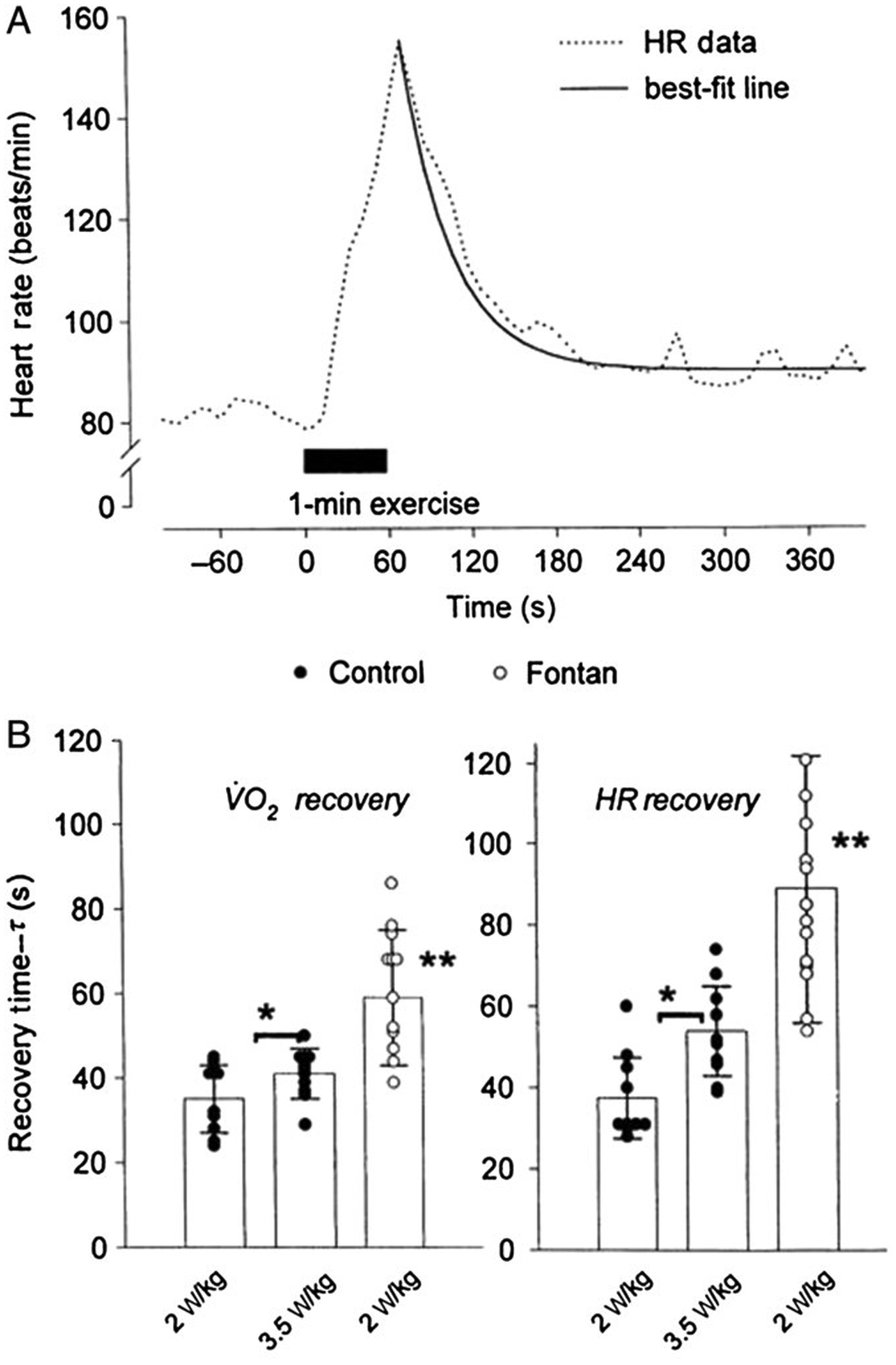
(A) HR response before, during, and after 1 minute of exercise in a 14-year-old Fontan group subject. The recovery kinetics were quantified using a single exponential, as shown. (B) HR and ˙VO2 recovery times for control and Fontan group subjects. In control subjects, recovery times were longer after the higher work rate protocols (*P < .05). In Fontan group subjects, recovery times were prolonged compared with the same absolute (2 W/kg) and relative (3.5 W/kg) protocols in control subjects (**P < .001). HR indicates heart rate; ˙VO2, oxygen consumption.
The Journey From “Noise” to “Signal”: Modern Approaches to CPET and Habitual PA Data Analysis Using Biologic Variability and Machine Learning
Cardiopulmonary exercise testing produces large data sets comprised of a variety of measures of breath-by-breath gas exchange and heart rate. Advanced computing has revolutionized analysis of large data sets, and many physiological data sets previously considered too noisy for predictive analysis can now yield valuable signals in biomedicine with statistics and machine learning expertise. In CPET, for example, Beltrame et al (11) recently found that it is possible to predict ˙VO2max from steady-state and exercise transitions based on easy-to-obtain inputs and machine learning paradigms. Variability (and its related volatility and entropy) is increasingly used as biomarkers in assessing physiologic data in exercise and in adult diseases (8,21,30,100,105). Early work in children has proved promising as well. Reybrouck et al (82) found that increased interbreath variability with oscillatory changes of ˙VO2 during exercise in children with heart disease was predictive of peak ˙VO2. In our own data (unpublished), as shown in Figure 10, we found increased CPET variability in younger compared with older children and as the intensity of exercise progressed.
Figure 10 —
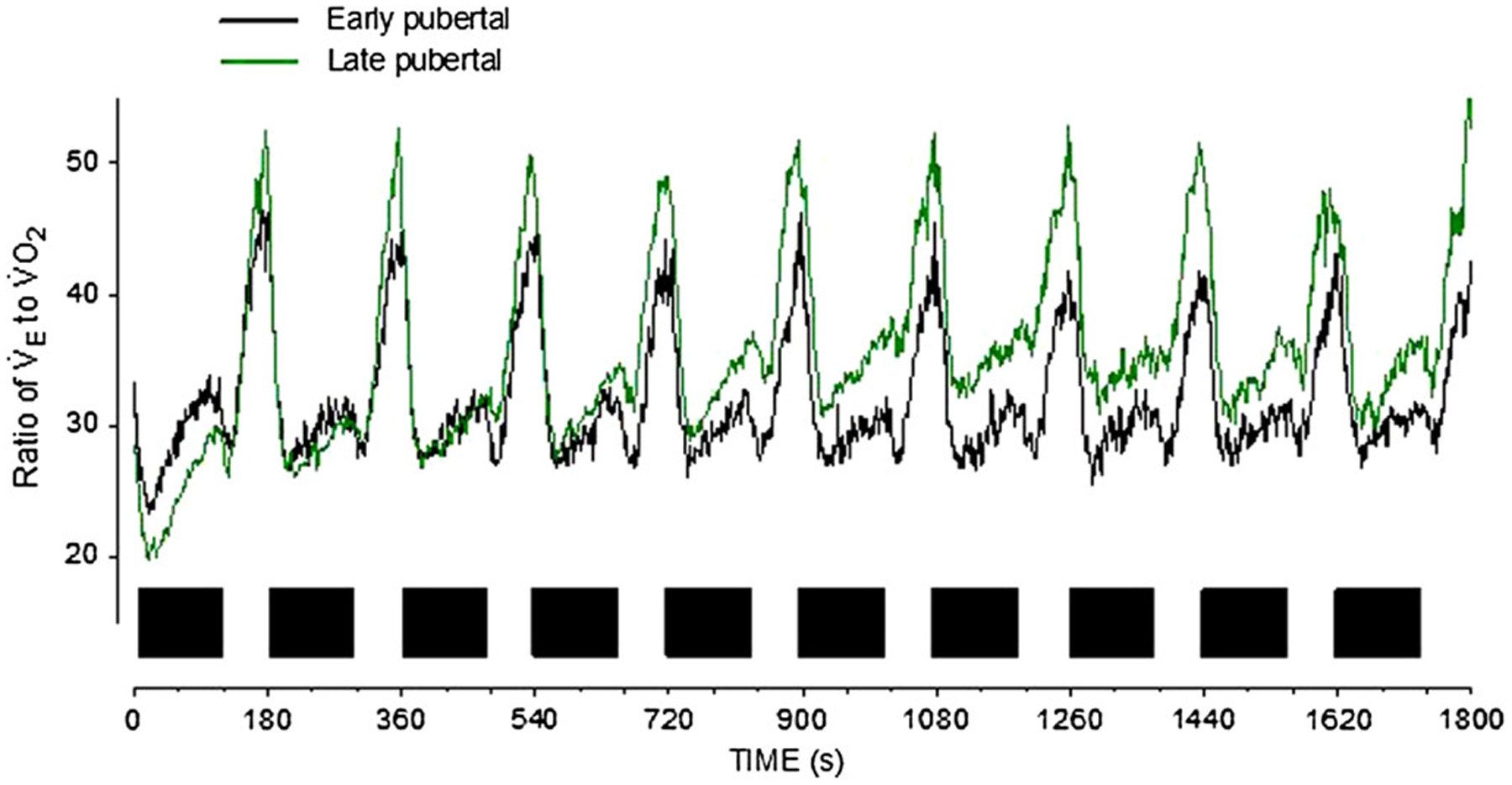
A series of constant work rate exercise bouts (closed squares) reveals striking maturational differences in the relationship between ˙VE and ˙VO2. This ratio (related to the oxygen uptake efficiency slope in standard CPET) is proving useful as a biomarker in a variety of cardiac and pulmonary diseases. Note the value of a test that uncovers data during both the onset and recovery from a brief exercise bout. CPET indicates cardiopulmonary exercise testing; ˙VO2, oxygen consumption.
One of the key challenges in assessing aerobic function in children and adolescents in health and disease is to link seemingly diverse testing strategies: formal CPET and measures of habitual PA (HPA) in the real lives of human beings. Surprisingly, in many studies, the correlation between CPET results and HPA metrics in both children and adults is poor. Either we are measuring phenomenon that is biologically minimally related, or alternatively, the way we are measuring these phenotypes is suboptimal. Earlier studies from this and other laboratories had suggested, for example, that the pattern of PA (eg, its tempo and intensity) might be useful in characterizing real-life PA in children (7,12), but these studies involved labor-intensive techniques, such as direct observation and manual recording of PA in field settings. Technological advances in wearable monitoring has led to an outpouring of new research on HPA in child health, and the actual measurement of HPA using techniques such as accelerometry, in contrast to recall or survey methodologies, may lead to a better understanding of the mechanisms that link laboratory CPET testing and HPA (106). Specific patterning of sedentary behaviors may also prove of benefit in linking HPA to cardiovascular disease risk in children and adolescents (31).
A number of researchers are rethinking how to analyze data obtained from wearable accelerometers in the quantification of HPA. Although the application of cut points continues to be common research practice, there is growing recognition that the relationship between proprietary accelerometer counts and energy expenditure is highly activity dependent and that a single regression equation cannot accurately determine energy expenditure across a wide range of activities. Validation studies involving independent samples indicate that regression-based cut-point approaches misclassify the true intensity of HPA 35% to 45% of the time (93,97). An alternative approach to accelerometer data reduction that has significantly improved sensor-based measurement of HPA and sedentary behavior is pattern recognition through machine learning. A number of researchers have demonstrated that machine learning algorithms, such as artificial neural networks (22,42,52,80,98), provide highly accurate predictions of activity type and intensity from accelerometer data collected in children and adolescents. While the potential for data science advanced analytics in quantifying HPA in adults and children is great, much work needs to be done to achieve consensus and harmonization before this promise can be realized (56).
Physical Fitness as a Right of Children: The Journey Toward Robust Metrics Physical inactivity as a leading cause of chronic illness is a global phenomenon, distributed across both low- and high-income nations (25,54,79). In a remarkable report card on the PA of children and youth in 38 countries from 6 continents (representing 60% of the world’s population), Tremblay et al (96) recently noted, “The wide distribution of [PA for children] grades results in global average grades for all indicators being D or C [and] shows that the challenge of enhancing PA behaviors and opportunities for children and youth around the world remains unresolved.”
Two transformative concepts highlight the need to provide clinicians with metrics of physical fitness in children that are accurate, cost-effective, and reproducible. First, there is growing recognition that “social determinants of health,” defined by the World Health Organization (108) as “the conditions in which people are born, grow, live, work and age,” must be identified if we are to mitigate health disparities. While it is evident that physical fitness and PA interact with social determinants of child and adolescent health in many ways (102), research is needed to identify, prioritize, and develop interventions that most effectively use exercise as medicine to address health disparities during critical periods of growth and development.
Second, the notion that children have unique rights was codified in the 1989 United Nations Convention on the Rights of the Child (101). The history of the United Nations Convention indicates the strong connection to child health (107). Among, the rights specified are as follows:
The right of the child to the enjoyment of the highest attainable standard of health and to facilities for the treatment of illness and rehabilitation of health;
The right of the child to develop the fullest potential of mental and physical abilities.
Although it is clear that the adoption the Convention on the Rights of the Child has benefited the lives of children and adolescents (84), no mention is made of the profound role that physical fitness during childhood has on health. It would seem timely that the community of pediatric exercise scientists and clinicians promotes a clear role for physical fitness as a right of children throughout the world. A key challenge facing the community of pediatric exercise clinicians and scientists is the development of testing strategies that can be used to gauge physical fitness robustly, repeatedly, and cost effectively.
Much work needs to be done to achieve this goal. Over a 3-year period, a group of key stakeholders in pediatric exercise science consisting of research scientists, clinicians, and industry partners convened a working group to identify challenges facing optimal utilization of exercise testing in child health research (5). The major recommendations of that group were as follows:
Build a formal framework for data harmonization and terminology interoperability in child health exercise science. This is essential to support the hoped-for expansion of clinical trials using CPET.
- Create a network of child health–focused clinical and research exercise laboratories with the ultimate goal of establishing a data consortium. The network will
- Work to establish robust reference values in child health CPET;
- Begin to more precisely define the impact of disease and therapy on exercise responses during growth and development in children.
Engage and collaborate with existing child health– and adult exercise–focused groups and organizations to support life span research, global health initiatives, and create economies of scale in the data harmonization efforts.
Promote formal programs to train child health care professionals in essential areas of exercise physiology, PA assessment, and relevant concepts of fitness in children.
Utilize the network to establish common protocols for CPET to ensure, in particular, that data obtained from multicenter trials are truly comparable.
Empower the network to become a resource for review, data sharing, and innovation across the broad spectrum of PA research and clinical application in child health.
These challenges can guide our ultimate journey: To improve child health and health across the life span through PA research and its clinical application.
Acknowledgments
This work was supported in part by National Institutes of Health P01HD-048721 grant, Pediatric Exercise and Genomic Research Center Systems Biology Research Fund, National Center for Advancing Translational Sciences (NCATS) UL1TR001414 grant and NCATS CTSA Collaboration Innovation Award, Project REACH (Revamping Exercise Assessments in Child Health), UL1TR002004.
References
- 1.Akber A, Portale AA, Johansen KL. Pedometer-assessed physical activity in children and young adults with CKD. Clin J Am Soc Nephrol. 2012;7:720–26. doi: 10.2215/CJN.06330611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarado AM, Ward KM, Muntz DS, et al. Heart rate recovery is impaired after maximal exercise testing in children with sickle cell anemia. J Pediatr. 2015;166:389–93.e1. doi: 10.1016/j.jpeds.2014.10.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armon Y, Cooper DM, Zanconato S. Maturation of ventilatory responses to 1-minute exercise. Pediatr Res. 1991;29:362–68. doi: 10.1203/00006450-199104000-00007 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong N, Barker AR. Oxygen uptake kinetics in children and adolescents: a review. Pediatr Exerc Sci. 2009;21:130–47. doi: 10.1123/pes.21.2.130 [DOI] [PubMed] [Google Scholar]
- 5.Ashish N, Bamman MM, Cerny FJ, et al. The clinical translation gap in child health exercise research: a call for disruptive innovation. Clin Transl Sci. 2015;8:67–76. doi: 10.1111/cts.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Åstrand PO, Rhyming I. A nomogram for calculation of aerobic capacity from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218–21. doi: 10.1152/jappl.1954.7.2.218 [DOI] [PubMed] [Google Scholar]
- 7.Bailey RC, Olson J, Pepper SL, Barstow TJ, Porszsasz J, Cooper DM. The level and tempo of children’s physical activities: an observational study. Med Sci Sports Exerc. 1995;27:1033–41. doi: 10.1249/00005768-199507000-00012 [DOI] [PubMed] [Google Scholar]
- 8.Bansal T, Haji GS, Rossiter HB, Polkey MI, Hull JH. Exercise ventilatory irregularity can be quantified by approximate entropy to detect breathing pattern disorder. Respir Physiol Neurobiol. 2018;255:1–6. doi: 10.1016/j.resp.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Barker AR, Trebilcock E, Breese B, Jones AM, Armstrong N. The effect of priming exercise on O2 uptake kinetics, muscle O2 delivery and utilization, muscle activity, and exercise tolerance in boys. Appl Physiol Nutr Metab. 2014;39:308–17. doi: 10.1139/apnm-2013-017 [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C, Forsén TJ, Thornburg KL, Kajantie E, Eriksson JG. Foetal and childhood growth and asthma in adult life. Acta Paediatr. 2013;102:732–38. doi: 10.1111/apa.12257 [DOI] [PubMed] [Google Scholar]
- 11.Beltrame T, Amelard R, Villar R, Shafiee MJ, Wong A, Hughson RL. Estimating oxygen uptake and energy expenditure during treadmill walking by neural network analysis of easy-to-obtain inputs. J Appl Physiol. 2016;121:1226–33. doi: 10.1152/japplphysiol.00600.2016 [DOI] [PubMed] [Google Scholar]
- 12.Berman N, Bailey RC, Barstow TJ, Cooper DM. Spectral and bout detection analysis of physical activity patterns in healthy, perpubertal boys and girls. Am J Hum Biol. 1998;10:289–97. doi: [DOI] [PubMed] [Google Scholar]
- 13.Blaisdell CJ, Weinmann GG. NHLBI viewpoint: lung health and disease prevention research starting in childhood. Pediatr Pulmonol. 2015;50:604–6. doi: 10.1002/ppul.23198 [DOI] [PubMed] [Google Scholar]
- 14.Blanchfield AW, Hardy J, De Morree HM, Staiano W, Marcora SM. Talking yourself out of exhaustion. Med Sci Sports Exerc. 2014;46:998–1007. doi: 10.1249/MSS.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 15.Bohr AH, Fuhlbrigge RC, Pedersen FK, de Ferranti SD, Müller K. Premature subclinical atherosclerosis in children and young adults with juvenile idiopathic arthritis. A review considering preventive measures. Pediatr Rheumatol Online J. 2016;14:3. doi: 10.1186/s12969-015-0061-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breithaupt P, Adamo KB, Colley RC. The HALO submaximal treadmill protocol to measure cardiorespiratory fitness in obese children and youth: a proof of principle study. Appl Physiol Nutr Metab. 2012;37:308–14. doi: 10.1139/h2012-003 [DOI] [PubMed] [Google Scholar]
- 18.Briggs AM, Cross MJ, Hoy DG, et al. Musculoskeletal health conditions represent a global threat to healthy aging: a report for the 2015 World Health Organization world report on ageing and health. Gerontologist. 2016;56(suppl 2):243–55. doi: 10.1093/geront/gnw002 [DOI] [PubMed] [Google Scholar]
- 19.Caughey MC, Loehr LR, Key NS, et al. Sickle cell trait and incident ischemic stroke in the Atherosclerosis Risk in Communities study. Stroke. 2014;45:2863–7. doi: 10.1161/STROKEAHA.114.006110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cayres SU, de Lira FS, Machado-Rodrigues AM, Freitas IF Junior, Barbosa MF, Fernandes RA. The mediating role of physical inactivity on the relationship between inflammation and artery thickness in prepubertal adolescents. J Pediatr. 2015;166:924–9. doi: 10.1016/j.jpeds.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 21.Chang JS, Kim EY, Jung D, et al. Altered cardiorespiratory coupling in young male adults with excessive online gaming. Biol Psychol. 2015;110:159–66. doi: 10.1016/j.biopsycho.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury AK, Tjondronegoro D, Chandran V, Trost SG. Ensemble methods for classification of physical activities from wrist accelerometry. Med Sci Sports Exerc. 2017;49:1965–73. doi: 10.1249/MSS.0000000000001291 [DOI] [PubMed] [Google Scholar]
- 23.Chu P, Pandya A, Salomon JA, Goldie SJ, Hunink MG. Comparative effectiveness of personalized lifestyle management strategies for cardiovascular disease risk reduction. J Am Heart Assoc. 2016;4:e002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: a systematic review. Obes Rev. 2015;17:276–95. doi: 10.1111/obr.12360 [DOI] [PubMed] [Google Scholar]
- 25.Cominato L, Di Biagio GF, Lellis D, Franco RR, Mancini MC, de Melo ME. Obesity prevention: strategies and challenges in Latin America. Curr Obes Rep. 2018;7:97–104. doi: 10.1007/s13679-018-0311-1 [DOI] [PubMed] [Google Scholar]
- 26.Cooper DM, Kaplan MR, Baumgarten L, Weiler-Ravell D, Whipp BJ, Wasserman K. Coupling of ventilation and CO2 production during exercise in children. Pediatr Res. 1987;21:568–72. doi: 10.1203/00006450-198706000-00012 [DOI] [PubMed] [Google Scholar]
- 27.Cooper DM, Leu SY, Galassetti P, Radom-Aizik S. Dynamic interactions of gas exchange, body mass, and progressive exercise in children. Med Sci Sports Exerc. 2014;46:877–86. doi: 10.1249/MSS.0000000000000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper DM, Radom-Aizik S, Schwindt C, Zaldivar F Jr. Dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol. 2007;103:700–9. doi: 10.1152/japplphysiol.00225.2007 [DOI] [PubMed] [Google Scholar]
- 29.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–34. doi: 10.1152/jappl.1984.56.3.628 [DOI] [PubMed] [Google Scholar]
- 30.Costa M, Goldberger A. Generalized multiscale entropy analysis: application to quantifying the complex volatility of human heartbeat time series. Entropy. 2015;17:1197–203. doi: 10.3390/e17031197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cristi-Montero C, Chillón P, Labayen I, et al. Cardiometabolic risk through an integrative classification combining physical activity and sedentary behavior in European adolescents: HELENA study. J Sport Heal Sci. 2019;8:55–62. doi: 10.1016/j.jshs.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370:403–11. doi: 10.1056/NEJMoa1309753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahhan A Coronary artery ectasia in atherosclerotic coronary artery disease, inflammatory disorders, and sickle cell disease. Cardiovasc Ther. 2015;33:79–88. doi: 10.1111/1755-5922.12106 [DOI] [PubMed] [Google Scholar]
- 34.Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. J Appl Physiol. 2003;95:1901–7. doi: 10.1152/japplphysiol.00024.2003 [DOI] [PubMed] [Google Scholar]
- 35.Dodge JA. A millennial view of cystic fibrosis. Dev period Med. 2015;19:9–13. [PubMed] [Google Scholar]
- 36.Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Sports Med. 2011;41:641–71. Available from: http://link.springer.com/10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Ephrem G, Hebson C, John A, et al. Frontiers in Fontan failure: innovation and improving outcomes: a conference summary. Congenit Heart Dis. 2019; 14:128–137. [DOI] [PubMed] [Google Scholar]
- 38.Foster GD, Linder B, Baranowski T, et al. A school-based intervention for diabetes risk reduction. N Engl J Med. 2010;363:443–53. doi: 10.1056/NEJMoa1001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gates PE, Strain WD, Shore AC. Human endothelial function and microvascular ageing. Exp Physiol. 2009;94:311–6. doi: 10.1113/expphysiol.2008.043349 [DOI] [PubMed] [Google Scholar]
- 40.Gibson TM, Ehrhardt MJ, Ness KK. Obesity and metabolic syndrome among adult survivors of childhood leukemia. Curr Treat Options Oncol. 2016;17:17. doi: 10.1007/s11864-016-0393-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilliam TB, Freedson PS, Geenen DL, Shahraray B. Physical activity patterns determined by heart rate monitoring in 6–7 year-old children. Med Sci Sports Exerc. 1981;13:65–7. [PubMed] [Google Scholar]
- 42.Hagenbuchner M, Cliff DP, Trost SG, Van Tuc N, Peoples GE. Prediction of activity type in preschool children using machine learning techniques. J Sci Med Sport. 2015;18:426–31. doi: 10.1016/j.jsams.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 43.Hansmann G Pulmonary hypertension in infants, children, and young adults. J Am Coll Cardiol. 2017;69:2551–69. doi: 10.1016/j.jacc.2017.03.575 [DOI] [PubMed] [Google Scholar]
- 44.Hardy R, Lawlor DA, Kuh D. A life course approach to cardiovascular aging. Future Cardiol. 2015;11:101–13. doi: 10.2217/fca.14.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hebestreit H, Hebestreit A, Trusen A, Hughson RL. Oxygen uptake kinetics are slowed in cystic fibrosis. Med Sci Sports Exerc. 2005;37:10–7. [DOI] [PubMed] [Google Scholar]
- 46.Herman KM, Hopman WM, Sabiston CM. Physical activity, screen time and self-rated health and mental health in Canadian adolescents. Prev Med. 2015;73:112–6. doi: 10.1016/j.ypmed.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 47.Hill AV. Muscular Movement in Man. New York, NY: McGraw-Hill; 1927. [Google Scholar]
- 48.Hill AV, Long CNH, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen. Proc R Soc B Biol Sci. 1924;97:155–76. http://rspb.royalsocietypublishing.org/cgi/doi/10.1098/rspb.1924.0048. [Google Scholar]
- 49.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31:2799–805. doi: 10.1200/JCO.2012.47.8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Houghton KM, Tucker LB, Potts JE, McKenzie DC. Fitness, fatigue, disease activity, and quality of life in pediatric lupus. Arthritis Rheum. 2008;59:537–45. doi: 10.1002/art.23534 [DOI] [PubMed] [Google Scholar]
- 51.Idler N, Teuner CM, Hunger M, et al. ; GINIplus and LISAplus Study Groups. The association between physical activity and healthcare costs in children—results from the GINIplus and LISAplus cohort studies. BMC Public Health. 2015;15:437. doi: 10.1186/s12889-015-1721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang Y, Kim S, Kim K, Lee D. Deep learning-based classification with improved time resolution for physical activities of children. PeerJ. 2018;6:e5764. doi: 10.7717/peerj.5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiménez-Pavón D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: systematic review. Int J Pediatr Obes. 2010;5:3–18. doi: 10.3109/17477160903067601 [DOI] [PubMed] [Google Scholar]
- 54.Juma PA, Mohamed SF, Matanje Mwagomba BL, et al. Non-communicable disease prevention policy process in five African countries authors. BMC Public Health. 2018;18:961. doi: 10.1186/s12889-018-5825-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–16. doi: 10.1002/cncr.29869 [DOI] [PubMed] [Google Scholar]
- 56.Kerr J, Marinac CR, Ellis K, et al. Comparison of accelerometry methods for estimating physical activity. Med Sci Sports Exerc. 2017;49:617–24. doi: 10.1249/MSS.0000000000001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klentrou P Influence of exercise and training during critical stages of bone growth and development. Pediatr Exerc Sci. 2016;28(2):178–86. doi: 10.1123/pes.2015-0265 [DOI] [PubMed] [Google Scholar]
- 58.Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380:294–305. http://www.sciencedirect.com/science/article/pii/S0140673612608988. [DOI] [PubMed] [Google Scholar]
- 59.Lanzkron S, Carroll CP, Haywood C. Mortality rates and age at death from sickle cell disease: U.S., 1979–2005. Public Health Rep. 2013;128:110–116. doi: 10.1177/003335491312800206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liem RI, Reddy M, Pelligra SA, et al. Reduced fitness and abnormal cardiopulmonary responses to maximal exercise testing in children and young adults with sickle cell anemia. Physiol Rep. 2015;3:pii: e12338. doi: 10.14814/phy2.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobstein T, Jackson-Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385:2510–20. doi: 10.1016/S0140-6736(14)61746-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lunt D, Briffa T, Briffa NK, Ramsay J. Physical activity levels of adolescents with congenital heart disease. Aust J Physiother. 2003;49:43–50. doi: 10.1016/S0004-9514(14)60187-2 [DOI] [PubMed] [Google Scholar]
- 63.Magnussen CG, Smith KJ, Juonala M. When to prevent cardiovascular disease? As early as possible: lessons from prospective cohorts beginning in childhood. Curr Opin Cardiol. 2013;28:561–68. doi: 10.1097/HCO.0b013e32836428f4 [DOI] [PubMed] [Google Scholar]
- 64.Maher CA, Toohey M, Ferguson M. Physical activity predicts quality of life and happiness in children and adolescents with cerebral palsy. Disabil Rehabil. 2016;38:865–69. doi: 10.3109/09638288.2015.1066450 [DOI] [PubMed] [Google Scholar]
- 65.Mattioni Maturana F, Peyrard A, Temesi J, Millet GY, Murias JM. Faster V ˙ O 2 kinetics after priming exercises of different duration but same fatigue. J Sports Sci. 2018;36:1095–102. doi: 10.1080/02640414.2017.1356543 [DOI] [PubMed] [Google Scholar]
- 66.Mielgo-Ayuso J, Aparicio-Ugarriza R, Castillo A, et al. Physical activity patterns of the spanish population are mostly determined by sex and age: findings in the ANIBES study. PLoS ONE. 2016;11:e0149969. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0149969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moser C, Tirakitsoontorn P, Nussbaum E, Newcomb R, Cooper DM. Muscle size and cardiorespiratory response to exercise in cystic fibrosis. Am J Respir Crit Care Med. 2000;162(5):1823–7. doi: 10.1164/ajrccm.162.5.2003057 [DOI] [PubMed] [Google Scholar]
- 68.Mutlu EK, Mutlu C, Taskiran H, Ozgen IT. Association of physical activity level with depression, anxiety, and quality of life in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2015;28:1273–8. [DOI] [PubMed] [Google Scholar]
- 69.Nagano Y, Baba R, Kuraishi K, et al. Ventilatory control during exercise in normal children. Pediatr Res. 1998;43:704–7. doi: 10.1203/00006450-199805000-00021 [DOI] [PubMed] [Google Scholar]
- 70.National Health and Nutrition Examination Survey (NHANES). National Youth Fitness Survey (NYFS) Treadmill Examination Manual. 2012. https://www.cdc.gov/nchs/data/nnyfs/treadmill.pdf
- 71.Nemet D, Hong S, Mills PJ, Ziegler MG, Hill M, Cooper DM. Systemic vs. local cytokine and leukocyte responses to unilateral wrist flexion exercise. J Appl Physiol. 2002;93:546–54. [DOI] [PubMed] [Google Scholar]
- 72.Nemet D, Oh Y, Kim HS, Hill M, Cooper DM. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–89. doi: 10.1542/peds.110.4.681 [DOI] [PubMed] [Google Scholar]
- 73.Nemet D, Pontello AM, Rose-Gottron C, Cooper DM. Cytokines and growth factors during and after a wrestling season in adolescent boys. Med Sci Sports Exerc. 2004;36:794–800. [DOI] [PubMed] [Google Scholar]
- 74.Nishi A, Milner DA, Giovannucci EL, et al. Integration of molecular pathology, epidemiology and social science for global precision medicine. Expert Rev Mol Diagn. 2016;16:11–23. doi: 10.1586/14737159.2016.1115346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999;515(pt 1):287–91. doi: 10.1111/j.1469-7793.1999.287ad.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pacheco DR, Silva MJ, Alexandrino AM, Torres RM. Exercise-related quality of life in subjects with asthma: a systematic review. J Asthma. 2012;49:487–95. doi: 10.3109/02770903.2012.680636 [DOI] [PubMed] [Google Scholar]
- 77.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081 [DOI] [PubMed] [Google Scholar]
- 78.Pate RR, Wang CY, Dowda M, Farrell SW, O’Neill JR. Cardiorespiratory fitness levels among US youth 12 to 19 years of age: findings from the 1999–2002 National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2006;160:1005–12. doi: 10.1001/archpedi.160.101005 [DOI] [PubMed] [Google Scholar]
- 79.Patton GC, Azzopardi P, Kennedy E, Coffey C, Mokdad A. Global measures of health risks and disease burden in adolescents. In: Bundy DA, Silva ND, Horton S, Jamison DT, Patton GC, eds. Child and Adolescent Health and Development. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/the World Bank; 2017: 57–72. [PubMed] [Google Scholar]
- 80.Pavey TG, Gilson ND, Gomersall SR, Clark B, Trost SG. Field evaluation of a random forest activity classifier for wrist-worn accelerometer data. J Sci Med Sport. 2017;20:75–80. doi: 10.1016/j.jsams.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 81.Reverri EJ, Morrissey BM, Cross CE, Steinberg FM. Inflammation, oxidative stress, and cardiovascular disease risk factors in adults with cystic fibrosis. Free Radic Biol Med. 2014;76:261–77. doi: 10.1016/j.freeradbiomed.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 82.Reybrouck T, Vangesselen S, Mertens L, Gewillig M. Increased interbreath variability of gas exchange during exercise in children with cardiomyopathy. Heart. 2007;93:377–8. doi: 10.1136/hrt.2006.094508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rörsch A The progress of science—Past, present and future. Humanities. 2014;3:442–516. http://www.mdpi.com/2076-0787/3/4/442. [Google Scholar]
- 84.Ruck MD, Keating DP, Saewyc EM, Earls F, Ben-Arieh A. The United Nations convention on the rights of the child: its relevance for adolescents. J Res Adolesc. 2016;26:16–29. http://doi.wiley.com/10.1111/jora.12172. [Google Scholar]
- 85.Salvadego D, Lazzer S, Busti C, et al. Gas exchange kinetics in obese adolescents. Inferences on exercise tolerance and prescription. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1298–305. doi: 10.1152/ajpregu.00038.2010 [DOI] [PubMed] [Google Scholar]
- 86.Sata F Developmental origins of health and disease (DOHaD) and epidemiology. Nihon Eiseigaku Zasshi. 2016;71:41–46. doi: 10.1265/jjh.71.41 [DOI] [PubMed] [Google Scholar]
- 87.Scheffler RW. The power of exercise and the exercise of power: the Harvard Fatigue Laboratory, distance running, and the disappearance of work, 1919–1947. J Hist Biol. 2015;48:391–423. doi: 10.1007/s10739-014-9392-1 [DOI] [PubMed] [Google Scholar]
- 88.Schneider M, Dunn A, Cooper D. Affect, exercise, and physical activity among healthy adolescents. J Sport Exerc Psychol. 2009;31;706–23. doi: 10.1123/jsep.31.6.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schneiderman JE, Wilkes DL, Atenafu EG, et al. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. Eur Respir J. 2014;43:817–23. doi: 10.1183/09031936.00055513 [DOI] [PubMed] [Google Scholar]
- 90.Seals DR, Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging. 2014;6:718–30. doi: 10.18632/aging.100694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, Lloyd-Jones DM. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation. 2013;127:1369–76. doi: 10.1161/CIRCULATIONAHA.113.001559 [DOI] [PubMed] [Google Scholar]
- 92.Stary HC. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am J Clin Nutr. 2000;72:1297S–306S. doi: 10.1093/ajcn/72.5.1297s [DOI] [PubMed] [Google Scholar]
- 93.Staudenmayer J, Pober D, Crouter S, Bassett D, Freedson P. An artificial neural network to estimate physical activity energy expenditure and identify physical activity type from an accelerometer. J Appl Physiol. 2009;107:1300–7. doi: 10.1152/japplphysiol.00465.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tipton CM. Contemporary exercise physiology: fifty years after the closure of Harvard Fatigue Laboratory. Exerc Sport Sci Rev. 1998;26:315–39. doi: 10.1249/00003677-199800260-00014 [DOI] [PubMed] [Google Scholar]
- 95.Tirakitsoontorn P, Nussbaum E, Moser C, Hill M, Cooper DM. Fitness, acute exercise, and anabolic and catabolic mediators in cystic fibrosis. Am J Respir Crit Care Med. 2001;164:1432–7. doi: 10.1164/ajrccm.164.8.2102045 [DOI] [PubMed] [Google Scholar]
- 96.Tremblay MS, Barnes JD, González SA, et al. Global Matrix 2.0: report card grades on the physical activity of children and youth comparing 38 countries. J Phys Act Health. 2016;13:S343–66. doi: 10.1123/jpah.2016-0594 [DOI] [PubMed] [Google Scholar]
- 97.Trost SG, Wong WK, Pfeiffer KA, Zheng Y. Artificial neural networks to predict activity type and energy expenditure in youth. Med Sci Sports Exerc. 2012;44:1801–9. doi: 10.1249/MSS.0b013e318258ac11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trost SG, Zheng Y, Wong WK. Machine learning for activity recognition: hip versus wrist data. Physiol Meas. 2014;35:2183–9. doi: 10.1088/0967-3334/35/11/2183 [DOI] [PubMed] [Google Scholar]
- 99.Troutman WB, Barstow TJ, Galindo AJ, Cooper DM. Abnormal dynamic cardiorespiratory responses to exercise in pediatric patients after Fontan procedure. J Am Coll Cardiol. 1998;31(3):668–73. doi: 10.1016/S0735-1097(97)00545-7 [DOI] [PubMed] [Google Scholar]
- 100.Usemann J, Xu B, Delgado-Eckert E, et al. Dynamics of respiratory symptoms during infancy and associations with wheezing at school age. ERJ Open Res. 2018;4:00037–2018. doi: 10.1183/23120541.00037-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verhellen E Convention on the Rights of the Child: Background, Motivation, Strategies, Main Themes. Leuven, Belgium: Garant; 2000. https://eric.ed.gov/?id=ED454987. [Google Scholar]
- 102.Viner RM, Ozer EM, Denny S, et al. Adolescence and the social determinants of health. Lancet. 2012;379:1641–52. https://www.sciencedirect.com/science/article/pii/S0140673612601494. [DOI] [PubMed] [Google Scholar]
- 103.Wallace PJ, Mckinlay BJ, Coletta NA, et al. Effects of motivational self-talk on endurance and cognitive performance in the heat. Med Sci Sports Exerc. 2017;49:191–9. doi: 10.1249/MSS.0000000000001087 [DOI] [PubMed] [Google Scholar]
- 104.Wasserman K, Whipp BJ. Breath-by-breath analysis of pulmonary gas exchange and the hyperpnea of exercise under non-steady-state and steady-state conditions. Chest. 1972;61:46S–7S. doi: 10.1016/S0012-3692(15)32698-2 [DOI] [PubMed] [Google Scholar]
- 105.Weippert M, Behrens M, Gonschorek R, Bruhn S, Behrens K. Muscular contraction mode differently affects autonomic control during heart rate matched exercise. Front Physiol. 2015;6:156. doi: 10.3389/fphys.2015.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wittekind SG, Edwards NM, Khoury PR, et al. Association of habitual physical activity with cardiovascular risk factors and target organ damage in adolescents and young adults. J Phys Act Health. 2018;15:176–82. doi: 10.1123/jpah.2017-0276 [DOI] [PubMed] [Google Scholar]
- 107.Woltanowski P, Wincewicz A, Sulkowski S. Protection of children’s human rights and health: a legacy of Julian Kramsztyk, Janusz Korczak, and Ludwik Rajchman. Glob Pediatr Health. 2018;5:2333794X1775415. doi: 10.1177/2333794X17754157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.World Health Organization. About social determinants of health. 2017. http://www.who.int/social_determinants/sdh_definition/en/.


