Abstract
Partial nephrectomy (PN) is the primary surgical method for renal tumor treatment, typically involving clamping the renal artery during tumor removal, leading to warm ischemia and potential renal function impairment. Off-clamp approaches have been explored to mitigate organ damage, yet few results have emerged about the possible effects on hemoglobin loss. Most evidence comes from retrospective studies using propensity score matching, known to be sensitive to PS model misspecification. The energy balancing weights (EBW) method offers an alternative method to address bias by focusing on balancing all the characteristics of covariate distribution. We aimed to compare on- vs. off-clamp techniques in PN using EB-weighted retrospective patient data. Out of 333 consecutive PNs (275/58 on/off-clamp ratio), the EBW method achieved balanced variables, notably tumor anatomy and staging. No significant differences were observed in the operative endpoints between on- and off-clamp techniques, although off-clamp PNs showed slight reductions in hemoglobin loss and renal function decline, albeit with slightly higher perioperative blood loss. Our findings support previous evidence, indicating comparable surgical outcomes between standard and off-clamp procedures, with the EBW method proving effective in balancing baseline variables in observational studies comparing interventions.
Keywords: partial nephrectomy, hilar clamping, surgical complications, anemia, energy balancing weight, propensity score, minimally invasive surgery
1. Introduction
Renal cancer represents more than 3% of all newly diagnosed tumors worldwide, with an incidence of more than 400,000 cases per year [1]. Obesity, hypertension, and chronic renal disease are just some of the increasingly prevalent risk factors contributing to the global rise in renal cancer incidence [2]. Consequently, recent diagnostic technological advancements have facilitated the earlier detection of renal lesions in many patients [3]. As a result, 75% of newly diagnosed renal masses are asymptomatic, incidental findings, and typically smaller than 7 cm in diameter [4].
Over the last few decades, there has been growing recognition of the long-term consequences of renal cancer surgery in terms of renal function decline, with constant progress in the knowledge of the etiology of the phenomenon, followed by the reporting of various strategies aimed at minimizing the occurrence of postoperative kidney functional impairment [5,6].
With advancements in minimally invasive surgical techniques, partial nephrectomy (PN) has garnered increased popularity and attention to the point of becoming the standard surgical approach for the treatment of renal tumors nowadays [7]. Several studies have shown that in addition to the benefits of preserving long-term renal function [7,8,9,10], PN may reduce the incidence of cardiovascular events and mortality [8,11,12].
Regardless of the minimally invasive surgical approach (laparoscopic or robot-assisted), the traditional technique of inducing renal warm ischemia by clamping the hilar vessels just before tumor excision remains standard practice. Hilar clamping creates an ischemic state within the kidney, reducing intraoperative blood loss and improving the visibility of the surgical area during tumor removal and subsequent renorrhaphy. Numerous studies indicate that inducing warm ischemia can lead to both short-term and long-term kidney function impairment [13,14]. Consequently, surgeons are advised to minimize warm ischemia time (WIT) whenever possible [15], and strategies of selective clamping have been proposed [16,17].
To further reduce organ damage related to tumor resection, completely off-clamp approaches [18,19] have been developed in recent years.
To date, studies comparing the two techniques have shown no clear advantage in terms of short-term renal function for the off- versus on-clamp approach [20,21,22]. Less evidence has emerged regarding the difference in the incidence of surgical complications, particularly in terms of blood loss and anemia [23,24].
Furthermore, most results come from retrospective observational studies that used propensity score matching (PSM) to reduce possible confounding [25,26,27,28,29,30,31,32,33]. Essentially, the propensity score (e.g., the probability, given the specific patient’s characteristics, of receiving the treatment or the control) acts as a balancing metric: when conditioned on the propensity score, the distribution of observed baseline covariates will be similar (balanced) between the two treatment arms [34]. Beyond PSM, there are other propensity score methods such as inverse probability of treatment weighting (IPTW). IPTW uses the propensity score to estimate a “weight” for each patient, to create a synthetic sample where patient contribution to the outcome estimation is weighted based on the probability of receiving the treatment and the distribution of baseline covariates is independent of this probability [35,36]. Applying either of these methods requires the definition of a model for estimating the propensity score to determine the association between baseline characteristics and received treatment. However, it is known that the misspecification of this model can result in substantial bias in estimating the treatment effect [37]. To address this issue, alternative methods to estimate weights have been proposed that, instead of modeling for the propensity score, are focused on finding a set of weights to maximize baseline variable balancing [38]. In particular, recently, the energy balancing weight (EBW) method was proposed, which estimates weights by minimizing the energy distance (a measure of the difference between two multivariate distributions) between distributions of variables in different treatment groups [39]. The main advantage of the EBW method is that all features of the covariate distribution are balanced, not just means, as with other methods like entropy balancing [40].
Therefore, the aim of our study was to compare the on- and. off-clamp techniques in terms of pre- and post-operative serum hemoglobin changes and intraoperative blood loss in a retrospective cohort of patients undergoing PN statistically weighted using the energy balancing methodology.
2. Materials and Methods
2.1. Study Population
Data from all patients diagnosed with primary renal cancer who underwent minimally invasive PN from October 2016 to December 2022 at San Luigi Gonzaga Hospital, University of Turin, Italy, were analyzed. Information on age, gender, BMI, Charlson Comorbidity Index (CCI) adjusted by age, comorbidity status, and tumor’s lesion clinical characteristics was collected. Preoperative and first postoperative day serum Hb, creatinine, and eGFR calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Creatinine equation [41] were recorded. Peri- and postoperative complications, estimated operative blood loss, intraoperative blood transfusion, WIT, surgical time, length of stay, and tumor histotype were collected and analyzed as well. Tumors’ anatomical characteristics were recorded, and the Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score was calculated [42]. Informed consent was obtained from all the participants. All the investigations were performed according to the principles of the Declaration of Helsinki.
2.2. Surgical Technique
All surgical procedures were performed via either robotic or laparoscopic technique by one experienced surgeon, with the choice of transperitoneal or retroperitoneal approaches contingent upon tumor localization, patient demographics, and surgeon discretion.
During the operation, management of the pedicle showed variability based on the specific characteristics of the renal mass and kidney vasculature, encompassing strategies ranging from off-clamp or selective clamping to the adoption of a global clamping approach.
Tumor excision adhered strictly to the principles of enucleation/enucleoresection. Closure of the renal defect was accomplished using one or two running monofilament sutures, employing diverse techniques dictated by individual surgeon practices. In instances where there was a breach of the urinary collecting system, a specialized suture technique was implemented.
2.3. Statistical Analysis
Continuous variables are reported as the mean ± SD or median (IQR) if non-normally distributed, while categorical variables are shown as the frequency (%). To compare baseline data between patients subjected to on- and off-clamp PN, Student’s t-test, the Wilcoxon–Mann–Whitney test, the chi-square test, or the Fisher exact test were used as appropriate.
To estimate the EBW, we used the R package WeightIt (v1.0.0) [43]. The model to calculate the weights included the age, gender, BMI, age-adjusted CCI, tumor side, face, clinical size, PADUA score, cystic features, vascular anatomy, clinical T, preoperative Hb, eGFR, and minimally invasive approach. The balance was evaluated using standardized mean differences (SMD), Kolmogorov–Smirnov (KS) statistics, and the variance ratio (for numeric variables). Variables with SMD between −0.1 and 0.1 were considered balanced. The estimand of interest was the average treatment effect in the treated (ATT), which can be considered as the effect of avoiding the treatment of interest (the off-clamp technique in this case) on those who would otherwise receive it [44]. To estimate the marginal effect of the off- vs. on-clamp strategy, the g-computation method was used [45], and confidence intervals were calculated with a bootstrap procedure (999 bootstrap samples). The endpoints considered were the post—preoperative percentage delta for serum Hb and eGFR, the estimated operative blood loss, the length of hospital stay, the total operative time, and the proportion of positive surgical margins. For continuous outcomes, linear regression models were developed to estimate the difference between the surgical approaches, while logistic models were used to estimate the relative risk in the case of categorical variables. Patients undergoing blood transfusion during the surgical intervention were excluded from the analysis of the Hb delta endpoint.
A p < 0.05 was considered significant. All analyses were conducted using R version 4.3.2 [46].
3. Results
From October 2016 to December 2022, 333 consecutive partial nephrectomies were performed and prospectively registered. Patients’ characteristics at surgery are presented in Table 1. The average patient age was about 64 years, with the majority of patients being males (69%). As expected, on-clamp tumor resections presented a higher PADUA score, with significant differences in lesion size, exophytic rate, and rim location, as well as renal sinus and urinary collecting system (UCS) involvement. Overall, 81% of off-clamp surgeries presented a T1a clinical stage vs. 54% in the on-clamp group (p < 0.001), while no significant differences were observed in preoperative Hb and eGFR. The majority of on-clamp PNs were robot-assisted (79% vs. 21% laparoscopic), while this proportion was almost even in the off-clamp group (48 vs. 52%; p < 0.001). A similar distribution was observed also for trans versus retroperitoneal access.
Table 1.
Patients’ characteristics at baseline.
| Characteristic | Overall n = 333 |
On-Clamp n = 275 |
Off-Clamp n = 58 |
p-Value |
|---|---|---|---|---|
| Age (years) | 63.87 ± 12.45 | 63.49 ± 12.98 | 65.66 ± 9.50 | 0.144 |
| Gender (Male) | 229 (69%) | 185 (67%) | 44 (76%) | 0.2 |
| BMI (Kg/m2) | 25.80 ± 3.96 | 25.81 ± 3.98 | 25.71 ± 3.91 | 0.857 |
| CCI (age-adjusted) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.465 |
| Solitary Kidney | 15 (4.5%) | 11 (4.0%) | 4 (6.9%) | 0.307 |
| Side | 0.99 | |||
| Right | 172 (52%) | 142 (52%) | 30 (52%) | |
| Left | 161 (48%) | 133 (48%) | 28 (48%) | |
| Face | 0.125 | |||
| Anterior | 202 (61%) | 172 (63%) | 30 (52%) | |
| Posterior | 131 (39%) | 103 (37%) | 28 (48%) | |
| Polar location | 0.508 | |||
| Inferior | 90 (27%) | 77 (28%) | 13 (22%) | |
| Middle | 150 (45%) | 120 (44%) | 30 (52%) | |
| Superior | 93 (28%) | 78 (28%) | 15 (26%) | |
| Exophytic rate | 0.004 | |||
| ≥50% | 165 (50%) | 125 (45%) | 40 (69%) | |
| <50% | 125 (38%) | 110 (40%) | 15 (26%) | |
| Endophytic | 43 (13%) | 40 (15%) | 3 (5.2%) | |
| Rim location | 0.006 | |||
| Lateral | 212 (64%) | 166 (60%) | 46 (79%) | |
| Medial | 121 (36%) | 109 (40%) | 12 (21%) | |
| Renal sinus involvement | 70 (21%) | 67 (24%) | 3 (5.2%) | 0.001 |
| UCS involvement | 66 (20%) | 62 (23%) | 4 (6.9%) | 0.007 |
| Tumor size score (cm) | 0.001 | |||
| ≤4 | 194 (58%) | 148 (54%) | 46 (79%) | |
| 4.1–7 | 127 (38%) | 116 (42%) | 11 (19%) | |
| >7 | 12 (3.6%) | 11 (4.0%) | 1 (1.7%) | |
| Padua Score | 8 (7–9) | 8 (7–10) | 7 (7–8) | <0.001 |
| Tumor Size (mm) | 39.13 ± 15.67 | 40.79 ± 15.50 | 31.24 ± 14.11 | <0.001 |
| Cystic tumor | 49 (15%) | 41 (15%) | 8 (14%) | 0.827 |
| Peritumoral pseudocapsule | 318 (95%) | 264 (96%) | 54 (93%) | 0.307 |
| Vascular anatomy | 0.160 | |||
| Regular | 307 (92%) | 251 (91%) | 56 (97%) | |
| Double renal artery | 11 (3.3%) | 9 (3.3%) | 2 (3.4%) | |
| Feeding artery | 15 (4.5%) | 15 (5.5%) | 0 (0%) | |
| Clinical T | <0.001 | |||
| T1a | 195 (59%) | 148 (54%) | 47 (81%) | |
| T1b | 121 (36%) | 113 (41%) | 8 (14%) | |
| T2a | 17 (5.1%) | 14 (5.1%) | 3 (5.2%) | |
| Preoperative Hb (g/dL) | 14.29 ± 1.44 | 14.23 ± 1.48 | 14.56 ± 1.20 | 0.078 |
| Preoperative eGFR (mL/min) | 79.46 ± 21.71 | 80.21 ± 21.73 | 75.88 ± 21.41 | 0.166 |
| Minimally invasive approach | <0.001 | |||
| Robot-assisted | 246 (74%) | 218 (79%) | 28 (48%) | |
| Laparoscopic | 87 (26%) | 57 (21%) | 30 (52%) | |
| Access | <0.001 | |||
| Transperitoneal | 249 (75%) | 219 (80%) | 30 (52%) | |
| Retroperitoneal | 84 (25%) | 56 (20%) | 28 (48%) |
CCI: Charlson Comorbidity Index; UCS: urinary collecting system.
In Table 2, the surgical and postoperative characteristics are presented. Differences were recorded both for the postoperative Hb and eGFR loss. The proportion of UCS openings was significantly higher in the on-clamp group, as well as the number of hospitalization days (6.9 vs. 5.8 days for the on- and off-clamp techniques, respectively; p = 0.02). In only ten PNs were positive surgical margins recorded, with just one in the off-clamp group. Intraoperative transfusions and other complications were extremely rare, and 94% of the patients were discharged with a Clavien Grade of I or II, with no differences between the different surgical techniques.
Table 2.
Patients’ surgical and postoperative characteristics.
| Characteristic | Overall n = 333 |
On-Clamp n = 275 |
Off-Clamp n = 58 |
p-Value |
|---|---|---|---|---|
| Postoperative Hb (g/dL) | 12.18 ± 1.50 | 12.07 ± 1.51 | 12.70 ± 1.39 | 0.002 |
| Post—Preoperative Hb Delta (%) | −14.46 ± 9.48 | −14.86 ± 9.70 | −12.57 ± 8.18 | 0.065 |
| Postoperative eGFR (mL/min) | 70.94 ± 23.90 | 71.07 ± 24.21 | 70.36 ± 22.60 | 0.831 |
| Post—Preoperative eGFR Delta (%) | −10.84 ± 17.47 | −11.67 ± 17.84 | −6.91 ± 15.15 | 0.038 |
| Warm Ischemia Time (min) | 17.44 ± 7.59 | 18.58 ± 7.38 | 0 ± 0 | <0.001 |
| Imaging support | 125 (38%) | 115 (42%) | 10 (17%) | <0.001 |
| UCS Opening | 62 (19%) | 61 (22%) | 1 (1.7%) | <0.001 |
| Estimated blood loss (mL) | 187.39 ± 187.44 | 189.45 ± 170.39 | 177.61 ± 254.86 | 0.736 |
| Intraoperative transfusion | 4 (1.2%) | 3 (1.1%) | 1 (1.7%) | 0.537 |
| Post-operative complications | 4 (1.2%) | 4 (1.5%) | 0 (0%) | >0.999 |
| Clavien Grade | 0.569 | |||
| I | 286 (86%) | 234 (85%) | 52 (90%) | |
| II | 26 (7.8%) | 21 (7.6%) | 5 (8.6%) | |
| III | 19 (5.7%) | 18 (6.5%) | 1 (1.7%) | |
| IV | 1 (0.3%) | 1 (0.4%) | 0 (0%) | |
| V | 1 (0.3%) | 1 (0.4%) | 0 (0%) | |
| Length of stay (days) | 6.70 ± 3.08 | 6.88 ± 3.23 | 5.84 ± 2.00 | 0.002 |
| Malignant | 272 (82%) | 228 (83%) | 44 (76%) | 0.207 |
| ISUP Grade | 0.622 | |||
| I | 41 (12%) | 35 (13%) | 6 (10%) | |
| II | 187 (56%) | 156 (57%) | 31 (53%) | |
| III | 41 (12%) | 35 (13%) | 6 (10%) | |
| IV | 1 (0.3%) | 1 (0.4%) | 0 (0%) | |
| N.A. | 63 (19%) | 48 (17%) | 15 (26%) | |
| Necrosis | 68 (20%) | 57 (21%) | 11 (19%) | 0.762 |
| Positive Surgical Margins | 10 (3.0%) | 9 (3.3%) | 1 (1.7%) | >0.999 |
| pT | <0.001 | |||
| benign | 35 (11%) | 24 (8.7%) | 11 (19%) | |
| pT1a | 159 (48%) | 124 (45%) | 35 (60%) | |
| pT1b | 91 (27%) | 84 (31%) | 7 (12%) | |
| pT2 | 9 (2.7%) | 8 (2.9%) | 1 (1.7%) | |
| pT3a | 38 (11%) | 35 (13%) | 3 (5.2%) | |
| pT3b | 1 (0.3%) | 0 (0%) | 1 (1.7%) |
UCS: urinary collecting system.
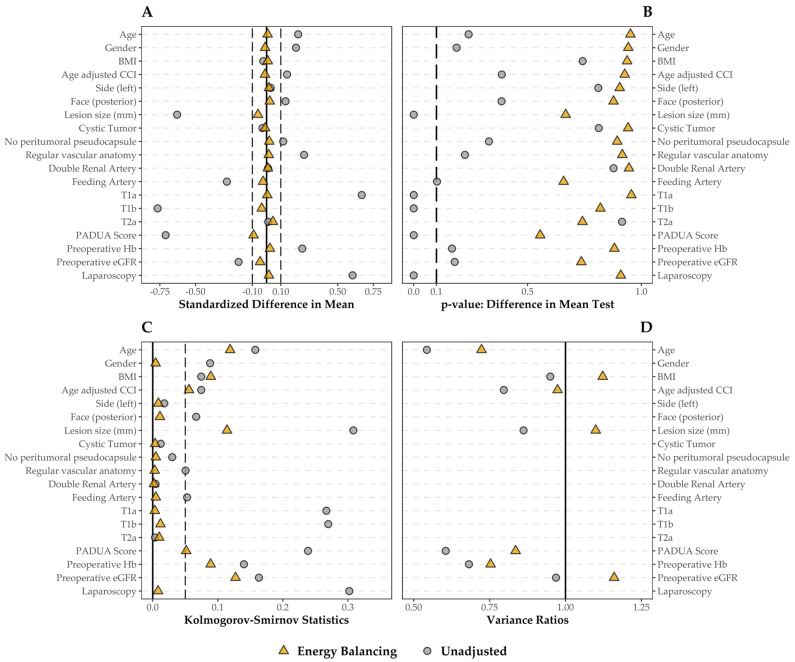
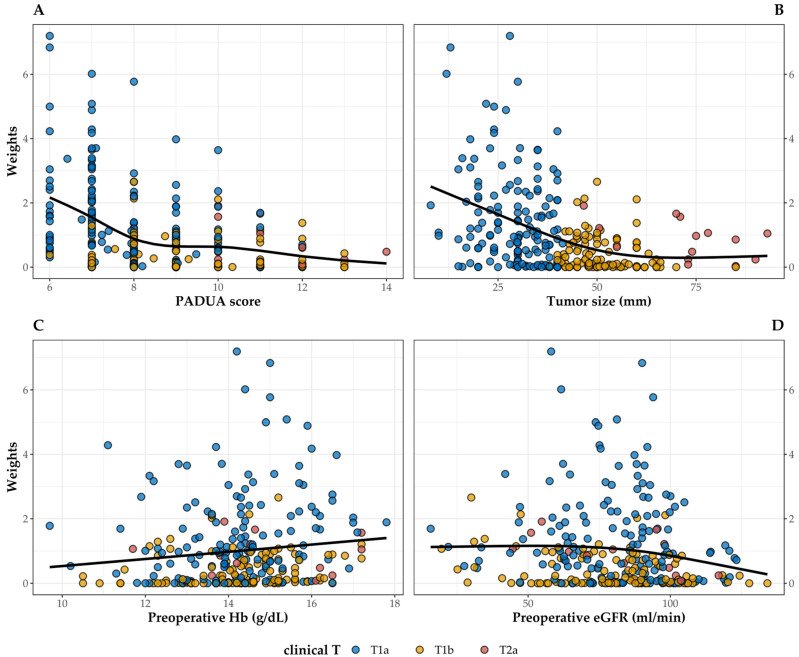
To reduce the selection bias and balance the covariates between the two groups, the EBW was calculated, and the derived balancing was checked via SMD. Figure 1 shows the results of the balancing step. All of the variables were balanced, particularly those concerning the tumor’s anatomy and clinical staging presenting a larger SMD before weighting. As it was the minority subgroup, the EBW procedure assigned a weight equal to 1 to all patients in the off-clamp group. To check for possible extreme weights that may have inflated the variance and confidence intervals of the effect estimate, we graphically checked the distribution of the weights assigned to patients in the on-clamp group (median = 0.602, IQR = 0.065–1.379, range = 0–7.195) against the main variables influencing the choice of surgical procedure (Figure 2). As expected, there was a negative significant association between weights and PADUA score (R2 = 0.138, p < 0.001), as well as tumor size (R2 = 0.164, p < 0.001). The associations with preoperative Hb (R2 = 0.011, p = 0.041) and preoperative eGFR (R2 = 0.009, p = 0.059) were weaker.
Figure 1.
Covariate balance before and after the application of the EBW method; all the categorical covariates included were transformed, creating a binary variable for each category. (A) Standardized mean difference: values closer to 0 (solid line) have better balance, values between −0.1 and 0.1 (dashed line) are considered balanced. (B) p-value for a covariate-by-covariate t-test for the differences in means. (C) Kolmogorov–Smirnov statistics: the closer to 0 (solid line), the better the balancing, and the 0.05 threshold is indicated as well (dashed line). (D) Variance ratios only for numeric variables: optimal value is 1 (solid line), but a value below 2 is considered well balanced.
Figure 2.
Association between weights assigned by the EBW procedure and PADUA score (A), tumor size (B), preoperative Hb (C), and preoperative eGFR (D) in patients in the on-clamp group classified by tumor T staging. The black line shows a smooth cubic spline curve.
None of the analyzed endpoints showed statistically significant differences between the different surgical procedures (Table 3). The estimated average effect of the off-clamp technique was a 2.3% reduced Hb loss (95% CI −1.4–6.2) and a 6.7% reduced eGFR loss (−3.9–17.4). Similarly, off-clamp PNs resulted in a higher average operative blood loss of 25.1 mL (−39.7–88.0), −0.54 hospitalization days (−1.5–0.5), and essentially no difference in the average risk of positive surgical margins (RR 1.16, 95% CI 0.16–5.50).
Table 3.
ATTs of the outcomes analyzed.
| ATT | 95% CI | p-Value | |
|---|---|---|---|
| Post—Preoperative Hb Delta (%) *‡ | 2.347 | −1.375–6.195 | 0.210 |
| Post—Preoperative eGFR Delta (%) * | 6.731 | −3.861–17.369 | 0.213 |
| Perioperative estimated blood loss (mL) * | 25.124 | −39.73–88.023 | 0.433 |
| Total Operative time (min) | −8.369 | −19.77–3.176 | 0.152 |
| Length of stay (days) * | −0.541 | −1.501–0.484 | 0.270 |
| Positive Surgical Margins † | 1.157 | 0.163–5.507 | 0.873 |
* ATT expressed as mean difference between the off- and on-clamp techniques; † ATT expressed as relative risk of off- with respect to on-clamp techniques; ‡ analysis of 329 patients with no intraoperative transfusion.
4. Discussion
Taken together, our results highlight how there are no significant differences between on- and off-clamp PN in terms of perioperative complications and confirm the EBW method as a reliant statistical technique for covariate balance in the context of causal effect estimation in observational studies.
In our study, no differences in Hb loss were observed between the two surgical procedures. Even though hemorrhagic complications have been an important focus in the literature on the matter, few studies have presented data on comparing postoperative Hb decline. We found a non-significant difference of around 2% Hb delta in favor of off-clamp PNs, similar to that presented in previous studies. In a two-center retrospective study, Bertolo et al. [31] analyzed 600 patients from two distinct high-volume centers and found a 0.2 g/dL difference in Hb loss after PS matching, though the authors did not estimate the endpoint via statistical modeling. More similarly to our finding, the CLOCK II trial [22] found no difference in post-operative Hb between on- and off-clamp laparoscopic PN, although interestingly, this difference was larger on the fifth postoperative day than on the first.
This result is confirmed by the absence of any significant difference in estimated blood loss in our cohort. Previous observational studies found significantly higher blood loss in off-clamp PNs [26,30], although the average difference in both cases was around 50 mL. On the other hand, all the RCTs presented to date confirm our results [20,21,22].
Even though the main objects of our work were surgical complications, we evaluated the difference in postoperative renal function as well. As expected, the eGFR loss was similar in the two subgroups, as emerged in the most recent literature. The systematic review by Shrivastava and colleagues [47], pulling results from two RCTs and nine PS-matched observational studies and a total of 2483 patients undergoing robotic PN, found no difference whatsoever in early renal function decline between the two surgical procedures, and similar findings were reported by Bertolo et al. [22] in laparoscopic PNs. Recent evidence highlights how the association between ischemia and postoperative eGFR is influenced by baseline renal function [48], and only values of WIT longer than thirty minutes seem to be predictive of a transition to acute kidney disease [49]. On the other hand, there is a growing body of literature about the role of residual parenchymal volume as the predominant factor in the conservation of kidney functionality [50,51], and the recent work by Xiong and colleagues, which found no association between ischemia time and histological modification in healthy renal parenchyma, seems to confirm these observations.
The only discrepancies between our results and those of the meta-analysis by Shrivastava concern the total operative time and the incidence of positive margins [47]. In their systematic review, these authors found a significant difference of almost twenty-two minutes in favor of off-clamp PNs, but it should be noted that this result seems to be highly influenced by a study limited to cT2 tumors which reported a massive difference of 80 vs. 190 operative minutes in favor of the off-clamp technique [32]. Regarding the incidence of positive margins, our results are in line with previous PS-matched studies where no significant effects were registered, while the pulled results evidenced a slight, but significant, reduction in the incidence in the off-clamp group. In this case, additional data are necessary to assess whether the meta-analysis results were affected by the heterogeneity of the selected studies or whether there is an actual beneficial effect in not clamping the renal pedicle. Nevertheless, both the latter outcomes discussed are highly dependent on the experience of the operative surgeons, and comparisons between different cohorts might be inaccurate.
It is worth noting that the classification system for assessing the surgical complexity of renal masses relies on outdated nephrometric scores that fail to incorporate the latest advancements in diagnostic imaging technology. For example, the emergence of new three-dimensional reconstructions derived from 2D images enables the precise characterization of the kidney’s vasculature, facilitating the planning of selective clamping or clampless procedures with enhanced safety measures. As a result, certain renal masses may be deemed less surgically complex than indicated by traditional classification methods. Looking ahead, there is a need to overhaul nephrometric scores to better capture the true surgical complexity of renal neoplasms [52,53,54].
In our study, we used a novel statistical technique to balance the distribution of covariates across treatments and control for potential bias. In their work, Huling and Mak demonstrated how the main source of bias for the estimation of average effects in the context of observational study is covariate imbalance [39]. Unlike other methods, the advantage of their EBW method lies in balancing all characteristics of the variable distribution, not only mean and variance. In line with their findings, EBW application in our cohort resulted in a good covariate balance between on- and off-clamp groups, in particular for those variables known to be highly influential for the choice of the surgical approach, such as the PADUA score, clinical tumor staging, and baseline eGFR. In particular, we ran balance checking diagnostics using SMD, KS statistics, and the variance ratio for continuous variables as per best practice in studies based on the propensity score [55]. Comparison with previous analyses is difficult, since ours is the first using weighting instead of PS matching [25,26,27,28,29,30,31,32,33], and in these studies, balance checking, when reported, is often based on hypothesis testing (e.g., t-test, Mann–Whitney test, or chi-square test), which has been demonstrated to be potentially misleading [56,57].
We chose ATT as the estimand to investigate. Since the focus of our work was the evaluation of differences in surgical complications between the two techniques, our primary research question was whether patients undergoing off-clamp partial nephrectomy would benefit from the “classical” procedure in terms of perioperative safety. The use of g-computation for the ATT estimate [58] allowed for the evaluation of the “marginal” effect of off-clamp PN, namely the average effect of treatment on the whole population and not on a single patient, while with the bootstrap procedure, we were able to obtain robust standard errors and confidence intervals [59]. Given the reliability of the statistical methods applied, we think that the reported results could be generalized to the population from which our cohort was derived, which is represented by patients who are candidates for minimally invasive PN, with relatively small, non-complex tumors and preserved renal function.
This study has limitations worth considering. First, the observational nature of the study does not allow us to exclude the existence of additional confounding not accounted for, even if the statistical methodology used assures the minimization of potential bias. In addition, this study lacks the evaluation of markers more indicative of kidney damage than the eGFR. As evidenced in a recent study [60], serum and urinary biomarkers could help in the identification of patients at a higher risk of long-term renal dysfunction. In addition, our patients were enrolled over a relatively long time range (six years); therefore, the surgeons’ increasing level of experience may have influenced the incidence of operative complications.
5. Conclusions
In conclusion, through the implementation of a different statistical method than the majority of the published contributions, we confirmed how the off-clamp approach for partial nephrectomy has no effect in terms of the incidence of major intraoperative complications when compared to the “traditional” technique, and at the same time no advantages in terms of the early recovery of kidney functionality. Future studies should focus on identifying factors affecting renal function and chronic renal disease onset in the long term to define the best overall treatment approach for each specific patient.
Author Contributions
Conceptualization: M.D.D., F.P. and D.C.; methodology: D.L. and A.P. (Anna Perri); software: D.L.; validation: D.A., M.D.D., V.R. and F.P.; investigation: D.L., V.Z., M.M., C.B., P.P.S. and A.P. (Alberto Piana); resources: D.C. and F.P.; data curation: D.A. and A.P. (Alberto Piana); writing—original draft preparation: D.L.; writing—review and editing: M.D.D., V.R., D.A., A.P. (Anna Perri) and F.P.; visualization: D.L.; supervision: M.D.D., A.P. (Anna Perri), D.C. and F.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethics Committee of San Luigi Hospital, Orbassano (Turin) was notified of this study as required by Italian regulations in case of observational studies.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data can be shared up on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bukavina L., Bensalah K., Bray F., Carlo M., Challacombe B., Karam J.A., Kassouf W., Mitchell T., Montironi R., O’Brien T., et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022;82:529–542. doi: 10.1016/j.eururo.2022.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Huang J., Leung D.K.W., Chan E.O.T., Lok V., Leung S., Wong I., Lao X.Q., Zheng Z.J., Chiu P.K.F., Ng C.F., et al. A Global Trend Analysis of Kidney Cancer Incidence and Mortality and Their Associations with Smoking, Alcohol Consumption, and Metabolic Syndrome. Eur. Urol. Focus. 2022;8:200–209. doi: 10.1016/j.euf.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Diana P., Klatte T., Amparore D., Bertolo R., Carbonara U., Erdem S., Ingels A., Kara O., Marandino L., Marchioni M., et al. Screening Programs for Renal Cell Carcinoma: A Systematic Review by the EAU Young Academic Urologists Renal Cancer Working Group. World J. Urol. 2023;41:929–940. doi: 10.1007/s00345-022-03993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J.H., Shim S.R., Lee H.Y., Park J.J., Khandwala Y., Jeong I.G., Chung B.I. Prevalence of Benign Pathology after Partial Nephrectomy for Suspected Renal Tumor: A Systematic Review and Meta-Analysis. Int. J. Surg. 2020;84:161–170. doi: 10.1016/j.ijsu.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Mir M.C., Ercole C., Takagi T., Zhang Z., Velet L., Remer E.M., Demirjian S., Campbell S.C. Decline in Renal Function after Partial Nephrectomy: Etiology and Prevention. J. Urol. 2015;193:1889–1898. doi: 10.1016/j.juro.2015.01.093. [DOI] [PubMed] [Google Scholar]
- 6.Volpe A., Blute M.L., Ficarra V., Gill I.S., Kutikov A., Porpiglia F., Rogers C., Touijer K.A., Van Poppel H., Thompson R.H. Renal Ischemia and Function after Partial Nephrectomy: A Collaborative Review of the Literature. Eur. Urol. 2015;68:61–74. doi: 10.1016/j.eururo.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Campbell S.C., Clark P.E., Chang S.S., Karam J.A., Souter L., Uzzo R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021;206:199–208. doi: 10.1097/JU.0000000000001911. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Wang G., Xia Q., Shang Z., Yu X., Wang M., Jin X. Partial Nephrectomy vs. Radical Nephrectomy for Renal Tumors: A Meta-Analysis of Renal Function and Cardiovascular Outcomes. Urol. Oncol. Semin. Orig. Investig. 2016;34:533.e11–533.e19. doi: 10.1016/j.urolonc.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Huang W.C., Levey A.S., Serio A.M., Snyder M., Vickers A.J., Raj G.V., Scardino P.T., Russo P. Chronic Kidney Disease after Nephrectomy in Patients with Renal Cortical Tumours: A Retrospective Cohort Study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fergany A.F., Hafez K.S., Novick A.C. Long-Term Results of Nephron Sparing Surgery for Localized Renal Cell Carcinoma: 10-Year Followup. J. Urol. 2000;163:442–445. doi: 10.1016/S0022-5347(05)67896-2. [DOI] [PubMed] [Google Scholar]
- 11.Huang W.C., Elkin E.B., Levey A.S., Jang T.L., Russo P. Partial Nephrectomy Versus Radical Nephrectomy in Patients with Small Renal Tumors—Is There a Difference in Mortality and Cardiovascular Outcomes? J. Urol. 2009;181:55–62. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan H.-J., Norton E.C., Ye Z., Hafez K.S., Gore J.L., Miller D.C. Long-Term Survival Following Partial vs Radical Nephrectomy Among Older Patients with Early-Stage Kidney Cancer. JAMA. 2012;307:1629–1635. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonelli A., Cindolo L., Sandri M., Veccia A., Annino F., Bertagna F., Maida Di F., Celia A., D’Orta C., De Concilio B., et al. The Role of Warm Ischemia Time on Functional Outcomes after Robotic Partial Nephrectomy: A Radionuclide Renal Scan Study from the Clock Randomized Trial. World J. Urol. 2023;41:1337–1344. doi: 10.1007/s00345-023-04366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funahashi Y., Hattori R., Yamamoto T., Sassa N., Fujita T., Gotoh M. Effect of Warm Ischemia on Renal Function During Partial Nephrectomy: Assessment with New 99mTc-Mercaptoacetyltriglycine Scintigraphy Parameter. Urology. 2012;79:160–164. doi: 10.1016/j.urology.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 15.Thompson R.H., Lane B.R., Lohse C.M., Leibovich B.C., Fergany A., Frank I., Gill I.S., Blute M.L., Campbell S.C. Every Minute Counts When the Renal Hilum Is Clamped during Partial Nephrectomy. Eur. Urol. 2010;58:340–345. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman L., Barod R., Dalela D., Diaz-Insua M., Abaza R., Adshead J., Ahlawat R., Challacombe B., Dasgupta P., Gandaglia G., et al. Use of Main Renal Artery Clamping Predominates over Minimal Clamping Techniques during Robotic Partial Nephrectomy for Complex Tumors. J. Endourol. 2017;31:149–152. doi: 10.1089/end.2016.0678. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L., Wei X., Sun W.J., Liu Q., Jian Z.Y., Li H., Wang K.J. Selective Versus Hilar Clamping during Minimally Invasive Partial Nephrectomy: A Systematic Review and Meta-Analysis. J. Endourol. 2015;29:855–863. doi: 10.1089/end.2014.0878. [DOI] [PubMed] [Google Scholar]
- 18.Guillonneau B., Bermúdez H., Gholami S., El Fettouh H., Gupta R., Adorno Rosa J., Baumert H., Cathelineau X., Fromont G., Vallancien G. Laparoscopic Partial Nephrectomy for Reanl Tumor: Single Center Experience Comparing Clamping and No Clamping Techniques of the Renal Vasculature. J. Urol. 2003;169:483–486. doi: 10.1016/S0022-5347(05)63939-0. [DOI] [PubMed] [Google Scholar]
- 19.Gill I.S., Eisenberg M.S., Aron M., Berger A., Ukimura O., Patil M.B., Campese V., Thangathurai D., Desai M.M. “Zero Ischemia” Partial Nephrectomy: Novel Laparoscopic and Robotic Technique. Eur. Urol. 2011;59:128–134. doi: 10.1016/j.eururo.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Anderson B.G., Potretzke A.M., Du K., Vetter J.M., Bergeron K., Paradis A.G., Figenshau R.S. Comparing Off-Clamp and On-Clamp Robot-Assisted Partial Nephrectomy: A Prospective Randomized Trial. Urology. 2019;126:102–109. doi: 10.1016/j.urology.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 21.Antonelli A., Cindolo L., Sandri M., Veccia A., Annino F., Bertagna F., Carini M., Celia A., D’orta C., De Concilio B., et al. Is Off-Clamp Robot-Assisted Partial Nephrectomy Beneficial for Renal Function? Data from the CLOCK Trial. BJU Int. 2022;129:217–224. doi: 10.1111/bju.15503. [DOI] [PubMed] [Google Scholar]
- 22.Bertolo R., Bove P., Sandri M., Celia A., Cindolo L., Cipriani C., Falsaperla M., Leonardo C., Mari A., Parma P., et al. Randomized Clinical Trial Comparing On-Clamp Versus Off-Clamp Laparoscopic Partial Nephrectomy for Small Renal Masses (CLOCK II Laparoscopic Study): A Intention-to-Treat Analysis of Perioperative Outcomes. Eur. Urol. Open Sci. 2022;46:75–81. doi: 10.1016/j.euros.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Huang Y., Zhang L., Duan L., Qian S. Perioperative Anemia Predicts Kidney Injury after Partial Nephrectomy. Investig. Clin. Urol. 2022;63:514–522. doi: 10.4111/icu.20220160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonelli A., The AGILE Group (Italian Group for Advanced Laparo-Endoscopic Surgery) Cindolo L., Sandri M., Bertolo R., Annino F., Carini M., Celia A., D’orta C., De Concilio B., et al. Safety of On- vs off-Clamp Robotic Partial Nephrectomy: Per-Protocol Analysis from the Data of the CLOCK Randomized Trial. World J. Urol. 2020;38:1101–1108. doi: 10.1007/s00345-019-02879-4. [DOI] [PubMed] [Google Scholar]
- 25.Tanagho Y.S., Bhayani S.B., Sandhu G.S., Vaughn N.P., Nepple K.G., Figenshau R.S. Renal Functional and Perioperative Outcomes of Off-Clamp versus Clamped Robot-Assisted Partial Nephrectomy: Matched Cohort Study. Urology. 2012;80:838–844. doi: 10.1016/j.urology.2012.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaczmarek B.F., Tanagho Y.S., Hillyer S.P., Mullins J.K., Diaz M., Trinh Q.D., Bhayani S.B., Allaf M.E., Stifelman M.D., Kaouk J.H., et al. Off-Clamp Robot-Assisted Partial Nephrectomy Preserves Renal Function: A Multi-Institutional Propensity Score Analysis. Eur. Urol. 2013;64:988–993. doi: 10.1016/j.eururo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Mari A., Morselli S., Sessa F., Campi R., Di Maida F., Greco I., Siena G., Tuccio A., Vittori G., Serni S., et al. Impact of the Off-Clamp Endoscopic Robot-Assisted Simple Enucleation (ERASE) of Clinical T1 Renal Tumors on the Postoperative Renal Function: Results from a Matched-Pair Comparison. Eur. J. Surg. Oncol. 2018;44:853–858. doi: 10.1016/j.ejso.2018.01.093. [DOI] [PubMed] [Google Scholar]
- 28.Anderson B.G., Potretzke A.M., Du K., Vetter J., Figenshau R.S. Off-Clamp Robot-Assisted Partial Nephrectomy Does Not Benefit Short-Term Renal Function: A Matched Cohort Analysis. J. Robot. Surg. 2018;12:401–407. doi: 10.1007/s11701-017-0745-6. [DOI] [PubMed] [Google Scholar]
- 29.Peyronnet B., Khene Z.-E., Pradère B., Seisen T., Verhoest G., Masson-Lecomte A., Grassano Y., Roumiguié M., Beauval J.-B., Baumert H., et al. Off-Clamp versus On-Clamp Robotic Partial Nephrectomy: A Multicenter Match-Paired Case-Control Study. Urol. Int. 2017;99:272–276. doi: 10.1159/000471772. [DOI] [PubMed] [Google Scholar]
- 30.Rosen D.C., Paulucci D.J., Abaza R., Eun D.D., Bhandari A., Hemal A.K., Badani K.K. Is off Clamp Always Beneficial during Robotic Partial Nephrectomy? A Propensity Score-Matched Comparison of Clamp Technique in Patients with Two Kidneys. J. Endourol. 2017;31:1176–1182. doi: 10.1089/end.2017.0450. [DOI] [PubMed] [Google Scholar]
- 31.Bertolo R., Simone G., Garisto J., Nakhoul G., Armanyous S., Agudelo J., Costantini M., Tuderti G., Gallucci M., Kaouk J. Off-Clamp vs on-Clamp Robotic Partial Nephrectomy: Perioperative, Functional and Oncological Outcomes from a Propensity-Score Matching between Two High-Volume Centers. Eur. J. Surg. Oncol. 2019;45:1232–1237. doi: 10.1016/j.ejso.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Brassetti A., Cacciamani G.E., Mari A., Garisto J.D., Bertolo R., Sundaram C.P., Derweesh I., Bindayi A., Dasgupta P., Porter J., et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for CT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers. 2022;14:4431. doi: 10.3390/cancers14184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma G., Shah M., Ahluwalia P., Dasgupta P., Challacombe B.J., Bhandari M., Ahlawat R., Rawal S., Buffi N.M., Sivaraman A., et al. Off-Clamp Versus On-Clamp Robot-Assisted Partial Nephrectomy: A Propensity-Matched Analysis. Eur. Urol. Oncol. 2023;6:525–530. doi: 10.1016/j.euo.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan S.L., Todd J.J. A Diagnostic Routine for the Detection of Consequential Heterogeneity of Causal Effects. Sociol. Methodol. 2008;38:231–282. doi: 10.1111/j.1467-9531.2008.00204.x. [DOI] [Google Scholar]
- 36.Rosenbaum P.R., Rubin D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 37.Kang J.D.Y., Schafer J.L. Demystifying Double Robustness: A Comparison of Alternative Strategies for Estimating a Population Mean from Incomplete Data. Stat. Sci. 2007;22:523–539. doi: 10.1214/07-STS227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y., Zeng D., Rush A.J., Kosorok M.R. Estimating Individualized Treatment Rules Using Outcome Weighted Learning. J. Am. Stat. Assoc. 2012;107:1106–1118. doi: 10.1080/01621459.2012.695674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huling J.D., Mak S. Energy Balancing of Covariate Distributions. arXiv. 20222004.13962 [Google Scholar]
- 40.Hainmueller J. Entropy Balancing for Causal Effects: A Multivariate Reweighting Method to Produce Balanced Samples in Observational Studies. Political Anal. 2012;20:25–46. doi: 10.1093/pan/mpr025. [DOI] [Google Scholar]
- 41.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., III, Feldman H.I., Kusek J.W., Eggers P., Lente F.V., Greene T., et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ficarra V., Novara G., Secco S., Macchi V., Porzionato A., De Caro R., Artibani W. Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) Classification of Renal Tumours in Patients Who Are Candidates for Nephron-Sparing Surgery. Eur. Urol. 2009;56:786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 43.Greifer N. WeightIt: Weighting for Covariate Balance in Observational Studies. 2023. [(accessed on 7 November 2023)]. Available online: https://cran.r-project.org/package=WeightIt.
- 44.Greifer N., Stuart E.A. Choosing the Causal Estimand for Propensity Score Analysis of Observational Studies. arXiv. 20232106.10577 [Google Scholar]
- 45.Snowden J.M., Rose S., Mortimer K.M. Practice of Epidemiology Implementation of G-Computation on a Simulated Data Set: Demonstration of a Causal Inference Technique. Am. J. Epidemiol. 2011;173:731–738. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2023. [Google Scholar]
- 47.Shrivastava N., Sharma G., Ahluwalia P., Gautam G., Erdem S., Amparore D., Marchioni M., Pavan N., Marandino L., Roussel E., et al. Off-Clamp Versus On-Clamp Robot-Assisted Partial Nephrectomy: A Systematic Review and Quantitative Synthesis by the European Association of Urology Young Academic Urologists Renal Cancer Study Group. Eur. Urol. Open Sci. 2023;58:10–18. doi: 10.1016/j.euros.2023.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X., Jin D., Zhang Y., Zhang S. Limited Non-Linear Impact of Warm Ischemia Time on Renal Functional Decline after Partial Nephrectomy: A Propensity Score-Matched Study. Int. Urol. Nephrol. 2023;55:1699–1708. doi: 10.1007/s11255-023-03630-0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S., Jin D., Zhang Y., Wang T. Risk Factors and Predictive Model for Acute Kidney Injury Transition to Acute Kidney Disease in Patients Following Partial Nephrectomy. BMC Urol. 2023;23:156. doi: 10.1186/s12894-023-01325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmons M.N., Fergany A.F., Campbell S.C. Effect of Parenchymal Volume Preservation on Kidney Function After Partial Nephrectomy. J. Urol. 2011;186:405–410. doi: 10.1016/j.juro.2011.03.154. [DOI] [PubMed] [Google Scholar]
- 51.Ginzburg S., Uzzo R., Walton J., Miller C., Kurz D., Li T., Handorf E., Gor R., Corcoran A., Viterbo R., et al. Residual Parenchymal Volume, Not Warm Ischemia Time, Predicts Ultimate Renal Functional Outcomes in Patients Undergoing Partial Nephrectomy. Urology. 2015;86:300–306. doi: 10.1016/j.urology.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 52.Amparore D., Piramide F., Checcucci E., Verri P., De Cillis S., Piana A., Volpi G., Busacca G., Colombo M., Fiori C., et al. Three-Dimensional Virtual Models of the Kidney with Colored Perfusion Regions: A New Algorithm-Based Tool for Optimizing the Clamping Strategy During Robot-Assisted Partial Nephrectomy. Eur. Urol. 2023;84:418–425. doi: 10.1016/j.eururo.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Amparore D., Pecoraro A., Piramide F., Verri P., Checcucci E., De Cillis S., Piana A., Burgio M., Di Dio M., Manfredi M., et al. Three-Dimensional Imaging Reconstruction of the Kidney’s Anatomy for a Tailored Minimally Invasive Partial Nephrectomy: A Pilot Study. Asian J. Urol. 2022;9:263–271. doi: 10.1016/j.ajur.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piana A., Amparore D., Sica M., Volpi G., Checcucci E., Piramide F., De Cillis S., Busacca G., Scarpelli G., Sidoti F., et al. Automatic 3D Augmented-Reality Robot-Assisted Partial Nephrectomy Using Machine Learning: Our Pioneer Experience. Cancers. 2024;16:1047. doi: 10.3390/cancers16051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Austin P.C., Stuart E.A. Moving towards Best Practice When Using Inverse Probability of Treatment Weighting (IPTW) Using the Propensity Score to Estimate Causal Treatment Effects in Observational Studies. Stat. Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai K., King G., Stuart E.A. Misunderstandings between Experimentalists and Observationalists about Causal Inference. J. R. Statist. Soc. 2008;171:481–502. doi: 10.1111/j.1467-985X.2007.00527.x. [DOI] [Google Scholar]
- 57.Austin P.C. Propensity-Score Matching in the Cardiovascular Surgery Literature from 2004 to 2006: A Systematic Review and Suggestions for Improvement. J. Thorac. Cardiovasc. Surg. 2007;134:1128–1135.e3. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 58.Wang A., Nianogo R.A., Arah O.A. G-Computation of Average Treatment Effects on the Treated and the Untreated. BMC Med. Res. Methodol. 2017;17:3. doi: 10.1186/s12874-016-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austin P.C. Bootstrap vs Asymptotic Variance Estimation When Using Propensity Score Weighting with Continuous and Binary Outcomes. Stat. Med. 2022;41:4426–4443. doi: 10.1002/sim.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allinovi M., Sessa F., Villa G., Cocci A., Innocenti S., Zanazzi M., Tofani L., Paparella L., Curi D., Cirami C.L., et al. Novel Biomarkers for Early Detection of Acute Kidney Injury and Prediction of Long-Term Kidney Function Decline after Partial Nephrectomy. Biomedicines. 2023;11:1046. doi: 10.3390/biomedicines11041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be shared up on reasonable request.