Abstract
Background: Vibrio is a genus of Gram-negative bacteria found in various aquatic environments, including saltwater and freshwater. Vibrio bacteremia can lead to sepsis, a potentially life-threatening condition in which the immune system enters overdrive in response to the disease, causing widespread inflammation and damage to tissues and organs. V. vulnificus had the highest case fatality rate (39%) of all reported foodborne infections in the United States and a high mortality rate in Asia, including Taiwan. Numerous scoring systems have been created to estimate the mortality risk in the emergency department (ED). However, there are no specific scoring systems to predict the mortality risk of Vibrio bacteremia. Therefore, this study modified the existing scoring systems to better predict the mortality risk of Vibrio bacteremia. Methods: Cases of Vibrio bacteremia were diagnosed based on the results from at least one blood culture in the ED. Patient data were extracted from the electronic clinical database, covering January 2012 to December 2021. The primary outcome was in-hospital mortality.This study used univariate and multivariate analyses to evaluate the mortality risk. Results: This study enrolled 36 patients diagnosed with Vibrio bacteremia, including 23 males (63.9%) and 13 females (36.1%), with a mean age of 65.1 ± 15.7 years. The in-hospital mortality rate amounted to 25% (9/36), with 31.5% in V. vulnificus (6/19) and 17.6% in V. non-vulnificus (3/17). The non-survivors demonstrated higher MEDS (10.3 ± 2.4) than the survivors (6.2 ± 4.1) (p = 0.002). Concerning the qSOFA, the survivors scored 0.3 ± 0.5, and the non-survivors displayed a score of 0.6 ± 0.7 (p = 0.387). The AUC of the ROC for the MEDS and qSOFA was 0.833 and 0.599, respectively. This study modified the scoring systems with other predictive factors, including BUN and pH. The AUC of the ROC for the modified MEDS and qSOFA reached up to 0.852 and 0.802, respectively. Conclusion: The MEDS could serve as reliable indicators for forecasting the mortality rate of patients grappling with Vibrio bacteremia. This study modified the MEDS and qSOFA to strengthen the predictive performance of mortality risk for Vibrio bacteremia. We advocate the prompt initiation of targeted therapeutic interventions and judicious antibiotic treatments to curb fatality rates.
Keywords: Vibrio, bacteremia, mortality risk, scoring systems, seasonal distribution
1. Introduction
Vibrio is a genus of Gram-negative bacteria found in various aquatic environments, including saltwater and freshwater [1,2,3,4]. V. vulnificus can cause severe wound infections and sepsis in people with compromised immune systems [5,6]. People can become infected with V. vulnificus through two main routes: consuming contaminated seafood (particularly raw or undercooked shellfish) or directly exposing open wounds to seawater containing the bacterium. In population-based studies in the United States in the 1980s, the incidence of V. vulnificus infections was approximately 0.5/100,000 people per year [7,8,9].
Other pathogenic Vibrio species include V. cholerae, the causative agent of cholera, a severe diarrheal disease that can be fatal if left untreated [10,11]. V. cholerae non-O1 and non-O139 strains have been increasingly recognized as a cause of gastroenteritis and extraintestinal infections, although they are less commonly associated with the widespread outbreaks typical of the O1 and O139 serogroups. The transmission of non-O1 and non-O139 V. cholerae is typically associated with consuming contaminated water or undercooked seafood, especially in coastal areas [12].
Vibrio bacteremia is a condition in which Vibrio bacteria, usually V. vulnificus or V. cholerae, enter the bloodstream and cause an infection. In a previous study, V. vulnificus had the highest case fatality rate of 39% in all reported foodborne infections in the United States [13]. In Asia, studies from South Korea, Japan, and China have also shown a very high mortality rate from Vibrio infections [14,15,16]. Even in the limited data from Taiwan, the high fatality rate of Vibrio infections is consistently demonstrated [17,18].
Vibrio bacteria, particularly V. vulnificus, thrive in warm seawater temperatures, with optimal growth occurring between 20 °C and 30 °C (68 °F and 86 °F). As a result, Vibrio infections, including Vibrio bacteremia, tend to increase during the warmer months, particularly in areas with warm coastal waters [14,15,19,20]. One study, for example, found that the case fatality rate of V. vulnificus bacteremia was significantly higher during the summer months in the United States [21].
Otherwise, numerous scoring systems have been created to estimate the mortality risk in emergency departments (EDs) [22,23]. Their efficiency has been documented across various scenarios, including cases of infectious disease, length of stay (LOS), and hospital admission. In a literature review, there were no studies that used specific scoring systems to predict the mortality risk of Vibrio bacteremia. This study focused on modifying the existing scoring systems by adding the laboratory variables according to the results of the univariate analysis. The modified scoring systems demonstrated more powerful performance and could help clinicians to provide appropriate antibiotics and intervention as early as possible to lower the mortality of Vibrio bacteremia.
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
The institutional review board at Taichung Veterans General Hospital (TCVGH) granted approval for our research (No. CE22240B), following the ethical guidelines of the Declaration of Helsinki. Nevertheless, the informed consent of the patients was waived because of the retrospective design. This observational research was carried out at a tertiary care center in Taiwan, which accommodates approximately 65,000 ED visits each year. We carried out this hospital-based study on patients with Vibrio bacteremia. Cases of confirmed Vibrio bacteremia were identified through the findings of at least one blood culture in the ED. Patient information was gathered from the electronic clinical database of TCVGH, spanning from January 2012 to December 2021. Data included demographics, laboratory investigations, and clinical outcomes. The primary outcome was in-hospital mortality. This study used univariate and multivariate analyses to evaluate the mortality risk.
2.2. Microbiological Diagnosis
In this study, the microbiological laboratory used VITEK® MS PRIME (bioMérieux, Lyon, France) to identify the microorganisms and VITEK® II for routine antimicrobial susceptibility testing (AST) to provide efficient workflow and faster AST results.
2.3. Scoring Systems
This study used the following clinical scoring systems to predict the clinical outcome and the risk of mortality (Table S1): Mortality in Emergency Department Sepsis (MEDS) Score, Worthing Physiological Scoring (WPS), Rapid Emergency Medicine Score (REMS), and quick Sepsis-related Organ Failure Assessment (qSOFA). According to the results of the univariate analysis, this study modified the systems mentioned above with blood urea nitrogen (BUN) and the potential of hydrogen (pH) to predict the mortality risk of Vibrio bacteremia again.
2.4. Statistic Analysis
In this study, continuous variables were presented as the mean ± standard deviation (SD), and categorical variables as number and percentages. To evaluate differences in categorical variables, chi-squared tests were used, whereas Mann–Whitney–Wilcoxon U tests were employed for continuous variables to compare the mortality risk between survivors and non-survivors. The study conducted univariate and multivariate analyses using the Cox regression model to identify potential mortality predictors, presenting the results as hazard ratios and confidence intervals. The predictive power of different scoring systems was compared using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. Cut-off points were utilized to categorize mortality risks based on sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). The population distribution and mortality risk according to cumulative points was calculated and plotted. Statistical significance was assigned to p-values < 0.05. Data analyses were carried out using the Statistical Package for the Social Science (IBM SPSS version 22.0; International Business Machines Corp., New York, NY, USA) and R (Version 4.1.3, R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Demographics and Clinical Characteristics
This study summarized the demographics, comorbidities, and clinical findings of the 36 patients with Vibrio bacteremia in Table 1, including 23 males (63.9%) and 13 females (36.1%), with a mean age of 65.1 ± 15.7 years and an average LOS of 16.6 ± 12.7 days. The comorbidities for Vibrio bacteremia included liver cirrhosis, which showed the highest proportion (27.8%), followed by congestive heart failure (22.2%) and alcoholism (16.7%). None of the comorbidities showed significant differences in terms of mortality. The 30-day in-hospital mortality rate amounted to 25% (9/36), with 31.5% in V. vulnificus (6/19) and 17.6% in V. non-vulnificus (3/17).
Table 1.
Characteristics, manifestations, clinical course, and management of patients with Vibrio bacteremia.
| General Data | All (n = 36) | Survival (n = 27) | Mortality (n = 9) | p-Value |
|---|---|---|---|---|
| Sex | 0.235 | |||
| Male | 23 (63.9%) | 19 (70.4%) | 4 (44.4%) | |
| Female | 13 (36.1%) | 8 (29.6%) | 5 (55.6%) | |
| Age | 65.1 ± 15.7 | 62.3 ± 15.5 | 73.7 ± 13.6 | 0.081 |
| Pathogens | 0.451 | |||
| Vibrio vulnificus | 19 (52.8%) | 13 (48.2%) | 6 (66.7%) | |
| Vibrio non–vulnificus | 17 (47.2%) | 14 (51.9%) | 3 (33.3%) | |
| Vital signs | ||||
| SBP | 127.72 ± 28.13 | 128.81 ± 27.49 | 124.44 ± 31.46 | 0.865 |
| DBP | 70.58 ± 14.85 | 72.74 ± 15.12 | 64.11 ± 12.61 | 0.195 |
| MAP | 89.6 ± 17.8 | 91.4 ± 18.2 | 84.2 ± 16.3 | 0.433 |
| HR | 104.6 ± 25.4 | 107.6 ± 25.7 | 95.7 ± 23.5 | 0.138 |
| RR | 19.4 ± 2.40 | 19.2 ± 1.7 | 20.1 ± 3.8 | 0.761 |
| BT | 37.7 ± 1.2 | 37.9 ± 1.2 | 37.3 ± 1.2 | 0.195 |
| Symptoms | ||||
| Fever or chills | 21 (58.3%) | 15 (55.6%) | 6 (66.7%) | 0.705 |
| Limb pain or swelling | 10 (27.8%) | 8 (29.6%) | 2 (22.2%) | 1.000 |
| Abdominal pain or diarrhea | 7 (19.4%) | 6 (22.2%) | 1 (11.1%) | 0.652 |
| Comorbidities | ||||
| HCVD | 5 (13.9%) | 4 (14.8%) | 1 (11.1%) | 1.000 |
| CAD | 5 (13.9%) | 4 (14.8%) | 1 (11.1%) | 1.000 |
| CHF | 8 (22.2%) | 6 (22.2%) | 2 (22.2%) | 1.000 |
| CVA | 16 (44.4%) | 12 (44.4%) | 4 (44.4%) | 1.000 |
| DM | 7 (19.4%) | 5 (18.5%) | 2 (22.2%) | 1.000 |
| Alcoholism | 6 (16.7%) | 4 (14.8%) | 2 (22.2%) | 0.627 |
| Liver cirrhosis | 10 (27.8%) | 8 (29.6%) | 2 (22.2%) | 1.000 |
| COPD | 5 (13.9%) | 3 (11.1%) | 2 (22.2%) | 0.581 |
| Transplant | 2 (5.6%) | 0 (0%) | 2 (22.2%) | 0.057 |
| Cancer | 11 (30.6%) | 8 (29.6%) | 3 (33.3%) | 1.000 |
| Clinical course | ||||
| Shock | 7 (19.4%) | 4 (14.8%) | 3 (33.3%) | 0.333 |
| Intubation | 15 (41.7%) | 10 (37.0%) | 5 (55.6%) | 0.443 |
| Urgent hemodialysis | 4 (11.1%) | 1 (3.7%) | 3 (33.3%) | 0.041 * |
| Hypotension | 11 (30.6%) | 5 (18.5%) | 6 (66.7%) | 0.012 * |
| Vasopressor | 10 (27.8%) | 5 (18.5%) | 5 (55.6%) | 0.079 |
| Management | ||||
| Antibiotics | 0.024 * | |||
| Cephalosporins | 19 (52.8%) | 16 (59.3%) | 3 (33.3%) | |
| Cephalosporins+Tetracyclines | 7 (19.4%) | 6 (22.2%) | 1 (11.1%) | |
| Cephalosporins+Quinolone | 5 (13.9%) | 4 (14.8%) | 1 (11.1%) | |
| Others | 5 (13.9%) | 1 (3.7%) | 4 (44.4%) | |
| Surgery | 11 (30.6%) | 7 (25.9%) | 4 (44.4%) | 0.409 |
| Drainage | 6 (16.7%) | 4 (14.8%) | 2 (22.2%) | 0.627 |
| Infection source | 0.169 | |||
| Primary | 12 (33.3%) | 7 (25.9%) | 5 (55.6%) | |
| Wound or Marine | 12 (33.3%) | 9 (33.3%) | 3 (33.3%) | |
| GI tract | 12 (33.3%) | 11 (40.7%) | 1 (11.1%) | |
Chi-squared test. Mann–Whitney U-test. * p < 0.05, statistically significant. Continuous data were expressed as mean ± SD. Categorical data were expressed as number and percentage. Abbreviations: BT, body temperature; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DBP, diastolic blood pressure; DM, diabetes mellitus; GI: gastrointestinal; HCVD, hypertensive cardiovascular disease; HR, heart rate; MAP, mean blood pressure; RR, respiratory rate; SBP, systolic blood pressure.
3.2. Laboratory Data
The laboratory data and scoring systems are shown in Table 2. White blood cell count (WBC) (12,280.7 ± 6517.3 vs. 6018.9 ± 2766.3, p = 0.009), absolute neutrophil count (ANC) (10,829.8 ± 6038.4 vs. 4720.0 ± 2002.0, p = 0.003), BUN (20.4 ± 14.1 vs. 40.0 ± 21.2, p = 0.005), potassium (K) (3.82 ± 0.73 vs. 4.64 ± 1.12, p = 0.038), maximum of creatine kinase (CK) (103.1 ± 92.2 vs. 1126.4 ± 1896.1, p = 0.009), and pH (7.40 ± 0.05 vs. 7.32 ± 0.09, p = 0.016) showed significant differences between the survivors and the non-survivors.
Table 2.
Laboratory data of patients with Vibrio bacteremia.
| Variables | All (n = 36) | Survival (n = 27) | Mortality (n = 9) | p-Value |
|---|---|---|---|---|
| Complete blood cells | ||||
| WBC | 10,715.3 ± 6392.5 | 12,280.7 ± 6517.3 | 6018.9 ± 2766.3 | 0.009 ** |
| ANC | 9302.3 ± 5933.1 | 10,829.8 ± 6038.4 | 4720.0 ± 2002.0 | 0.003 ** |
| Hb | 11.9 ± 2.2 | 12.0 ± 2.4 | 11.6 ± 1.7 | 0.559 |
| PLT | 162.9 ± 84.3 | 173.9 ± 89.0 | 131.4 ± 62.6 | 0.291 |
| Biochemistry | ||||
| BUN | 25.6 ± 18.2 | 20.4 ± 14.1 | 40.0 ± 21.2 | 0.005 ** |
| Cr | 1.34 ± 0.76 | 1.20 ± 0.66 | 1.76 ± 0.92 | 0.111 |
| Na | 134.2 ± 4.9 | 133.9 ± 5.3 | 135.1 ± 3.8 | 0.621 |
| K | 4.03 ± 0.90 | 3.82 ± 0.73 | 4.64 ± 1.12 | 0.038 * |
| Total bilirubin | 3.24 ± 4.94 | 3.15 ± 5.22 | 3.56 ± 4.23 | 0.820 |
| GPT | 60.4 ± 55.5 | 68.0 ± 57.9 | 36.0 ± 40.5 | 0.062 |
| LDH | 329.4 ± 143.0 | 334.9 ± 151.4 | 307.3 ± 127.5 | 1.000 |
| CRP | 4.59 ± 5.72 | 4.35 ± 5.76 | 5.33 ± 5.89 | 0.407 |
| Lactate | 33.6 ± 20.7 | 34.1 ± 21.4 | 32.5 ± 20.1 | 0.940 |
| Glucose | 142.2 ± 61.1 | 151.17 ± 66.27 | 115.38 ± 31.51 | 0.268 |
| Maximum of CK | 389.6 ± 1060.5 | 103.1 ± 92.2 | 1126.4 ± 1896.1 | 0.009 ** |
| Arterial blood gas | ||||
| pH | 7.38 ± 0.07 | 7.40 ± 0.05 | 7.32 ± 0.09 | 0.016 * |
| PaO2− | 53.05 ± 25.22 | 55.04 ± 24.31 | 46.21 ± 29.03 | 0.397 |
| PaCO2− | 22.79 ± 2.85 | 22.74 ± 2.91 | 22.93 ± 2.89 | 0.728 |
| HCO3− | −2.10 ± 2.62 | −1.83 ± 2.65 | −3.01 ± 2.48 | 0.204 |
Chi-squared test. Mann–Whitney U-test. * p < 0.05, ** p < 0.01, statistically significant. Continuous data were expressed as mean ± SD. Abbreviations: ANC, absolute neutrophil count; BUN, blood urea nitrogen; CK, creatine kinase; CRP, C-reactive protein; Cr, creatinine; GPT, glutamic pyruvic transaminase; Hb, hemoglobin; K, potassium; LDH, lactate dehydrogenase; Na, sodium; PLT, platelet; WBC, white blood cell count.
3.3. Microbiology and Seasonal Distribution of Mortality Cases
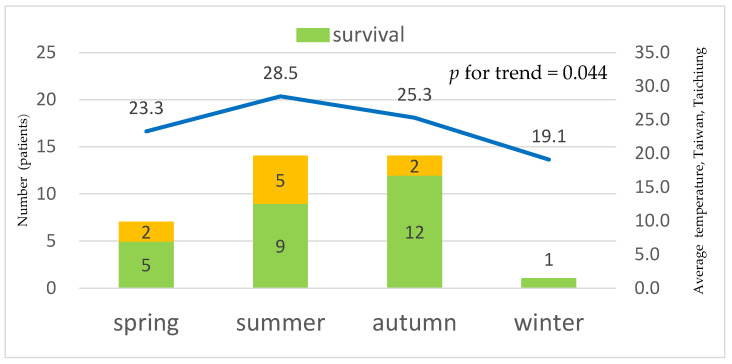
Emergency physicians performed a bacterial culture on individual patients at least once. The microorganisms found in blood culture were distributed between V. vulnificus (n = 19) and V. non-vulnificus (n = 17), including V. cholera, non-O1, non-O139, (n = 10), V. fluvialis (n = 5), V. cholerae O1 (n = 1), and V. alginolyticus (n = 1) (Table 3). There was a trend association between the mortality cases of Vibrio bacteremia and seasonal distribution, with a trend of p = 0.044 (Figure 1).
Table 3.
The microorganisms causing Vibrio bacteremia.
| Microorganisms | Case Numbers (n) |
|---|---|
| Vibrio vulnificus | 19 |
| Vibrio non-vulnificus | 17 |
| Vibrio cholera non-O1, non-O139 | 10 |
| Vibrio fluvialis | 5 |
| Vibrio cholerae O1 | 1 |
| Vibrio alginolyticus | 1 |
Figure 1.
The trend association between the mortality cases of Vibrio bacteremia and seasonal distribution (p = 0.044).
3.4. Scoring Systems
The non-survivors had significantly higher scores in the original MEDS (10.3 ± 2.4) than the survivors (6.2 ± 4.1) (p = 0.002). The remaining scoring systems showed no different significance (Table 4).
Table 4.
The scoring systems to predict the mortality risk of patients with Vibrio bacteremia.
| Scores | All (n = 36) | Survival (n = 27) | Mortality (n = 9) | p-Value |
|---|---|---|---|---|
| MEDS | 7.1 ± 4.2 | 6.2 ± 4.1 | 10.3 ± 2.4 | 0.002 ** |
| WPS | 2.4 ± 2.1 | 2.0 ± 1.6 | 3.4 ± 3.2 | 0.349 |
| qSOFA | 0 ± 0.6 | 0.3 ± 0.5 | 0.6 ± 0.7 | 0.387 |
| REMS | 6.0 ± 2.6 | 5.7 ± 2.2 | 6.9 ± 3.6 | 0.426 |
** p < 0.01, statistically significant. Continuous data were expressed as mean ± SD. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
3.5. Univariateand Multivariate Analyses of Risk Factors
This study conducted univariate analyses for predisposing factors on clinical outcomes and the results are summarized in Table 5. Higher hazard ratios (HRs) were foundin the non-survivors, including hypotension, renal failure, urgent hemodialysis, organ transplant, and elevation of WBC, potassium, BUN, and creatinine. In univariate analysis, the MEDS and WPS showed significantly higher in the non-survivors (Table 6). Higher HR in the non-survivors regarding scores of the original MEDS (p = 0.037) in multivariate analysis was found (Table 6).
Table 5.
Hazard ratios and 95% confidence interval of univariate analysis for patients with Vibrio bacteremia.
| Variables | Hazard Ratios | 95% Confidence Interval | p-Value |
|---|---|---|---|
| WBC | 1.00 | 1.00–1.00 | 0.016 * |
| BUN | 1.04 | 1.01–1.07 | 0.009 ** |
| Cr | 2.05 | 1.03–4.08 | 0.041 * |
| K | 2.11 | 1.23–3.64 | 0.007 ** |
| CK | 1.00 | 1.00–1.00 | 0.011 * |
| pH | 0.79 | 0.68–0.91 | 0.001 ** |
| Transplant | 11.41 | 2.19–59.39 | 0.004 ** |
| Urgent hemodialysis | 5.96 | 1.48–24.08 | 0.012 * |
| Hypotension | 5.35 | 1.33–21.51 | 0.018 * |
| Renal failure | 0.25 | 0.07–0.94 | 0.040 * |
* p < 0.05, ** p < 0.01, statistically significant. Abbreviations: BUN, blood urea nitrogen; CK, creatine kinase; Cr, creatinine; K, potassium; WBC, white blood cell count.
Table 6.
Hazard ratios and 95% confidence interval of univariate and multivariate analysesfor patients with Vibrio bacteremia.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| MEDS | 1.23 | 1.05–1.44 | 0.011 * | 1.28 | 1.02–1.62 | 0.037 * |
| WPS | 1.38 | 1.03–1.84 | 0.030 * | 1.08 | 0.45–2.61 | 0.863 |
| qSOFA | 1.88 | 0.74–4.72 | 0.182 | 2.21 | 0.13–38.98 | 0.588 |
| REMS | 1.22 | 0.96–1.56 | 0.100 | 1.48 | 0.91–2.40 | 0.111 |
* p < 0.05, statistically significant. Abbreviations: CI, conference interval; HR, hazard ratios; MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
3.6. The Receiver Operating Characteristic Curve (ROC)
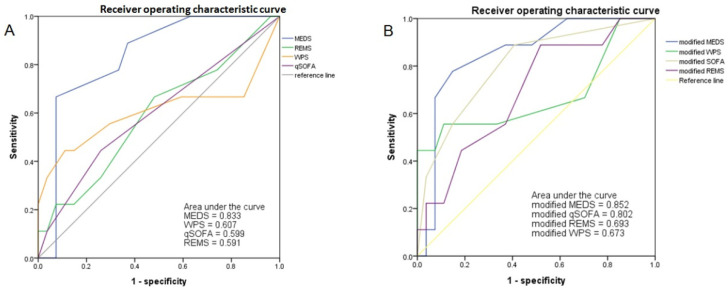
The ROC of the original MEDS, WPS, qSOFA, and REMS for accuracy in predicting the mortality riskswas analyzed, and the results are shown in Figure 2 and Table 7. The cut-off point of the MEDS was 10, and the AUC of the ROC measured up to 0.833, which had a sensitivity of 66.7% and a specificity of 92.6% (p = 0.003).
Figure 2.
The AUC of ROC of the MEDS, WPS, qSOFA, and REMS in predicting the mortality risks of patients with Vibrio bacteremia (Panel A). The AUC of ROC of the modified MEDS, WPS, qSOFA, and REMS in predicting the mortality risks of patients with Vibrio bacteremia (Panel B). AUC = area under the curve; ROC = receiver operating characteristic curve.
Table 7.
The AUC of the ROC, cut-off point (COP), sensitivity specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and standard error (SE) of the original MEDS, WPS, qSOFA, and REMS to predict mortality risk.
| Scores | AUC | COP | Sensitivity | Specificity | PPV | NPV | Accuracy | SE | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| MEDS | 0.833 | 10 | 66.7% | 92.6% | 75.0% | 89.3% | 86.1% | 0.07 | 0.003 ** |
| WPS | 0.607 | 5 | 44.4% | 88.9% | 57.1% | 82.8% | 77.8% | 0.14 | 0.342 |
| qSOFA | 0.599 | 1 | 44.4% | 74.1% | 36.4% | 80.0% | 66.7% | 0.12 | 0.381 |
| REMS | 0.591 | 6 | 66.7% | 51.9% | 31.6% | 82.4% | 55.6% | 0.11 | 0.422 |
** p < 0.01, statistically significant. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
This study modified the original scoring systems with other predictive factors, including BUN (if BUN > 25, the modified score had one point added) and pH (if pH < 7.36, the modified score had one point added). We reveal the results in Figure 2 and Table 8. The cut-off point of the modified MEDS was 10, and the AUC of the ROC increased to 0.852 with a sensitivity of 77.8% and a specificity of 85.2% (p = 0.002). The cut-off point of the modified qSOFA was 1, and the AUC of the ROC reached up to 0.802, with a sensitivity of 88.9% and a specificity of 59.3% (p = 0.007).
Table 8.
The AUC of ROC, cut-off point (COP), sensitivity specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and standard error (SE) of the modified MEDS, WPS, qSOFA, and REMS to predict the mortality risk.
| Modified Scores | AUC | COP | Sensitivity | Specificity | PPV | NPV | Accuracy | SE | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Modified MEDS | 0.852 | 10 | 77.8% | 85.2% | 63.6% | 92.0% | 83.3% | 0.07 | 0.002 ** |
| Modified qSOFA | 0.802 | 1 | 88.9% | 59.3% | 42.1% | 94.1% | 66.7% | 0.09 | 0.007 ** |
| Modified REMS | 0.693 | 6 | 88.9% | 48.1% | 36.4% | 92.9% | 58.3% | 0.10 | 0.086 |
| Modified WPS | 0.673 | 5 | 55.6% | 88.9% | 62.5% | 85.7% | 80.6% | 0.13 | 0.125 |
** p < 0.01, statistically significant. If BUN > 25, the modified score had one point added; if pH < 7.36, the modified score had one point added. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
3.7. Cumulative Survival Rates obtained by Kaplan–Meier and Discrimination Plot
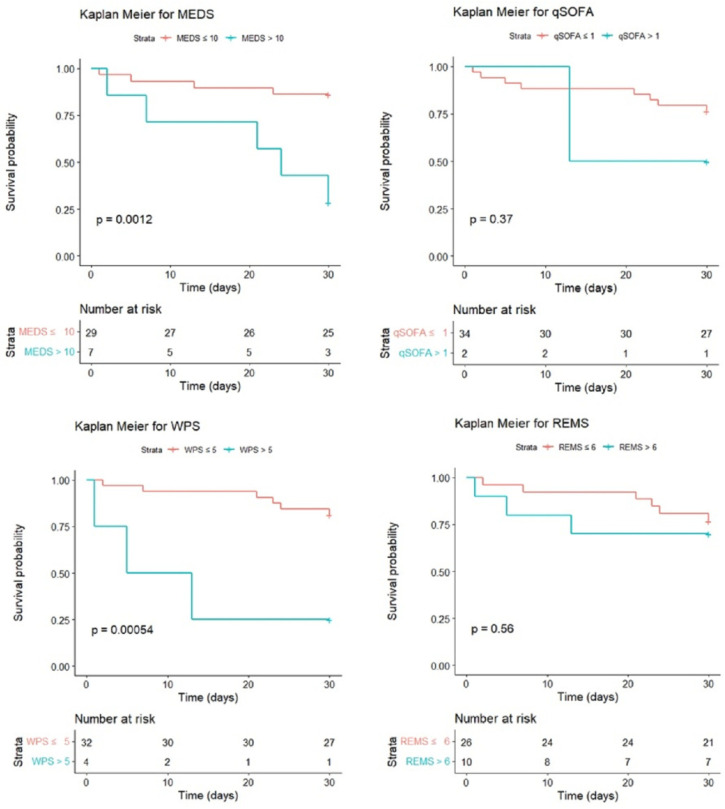
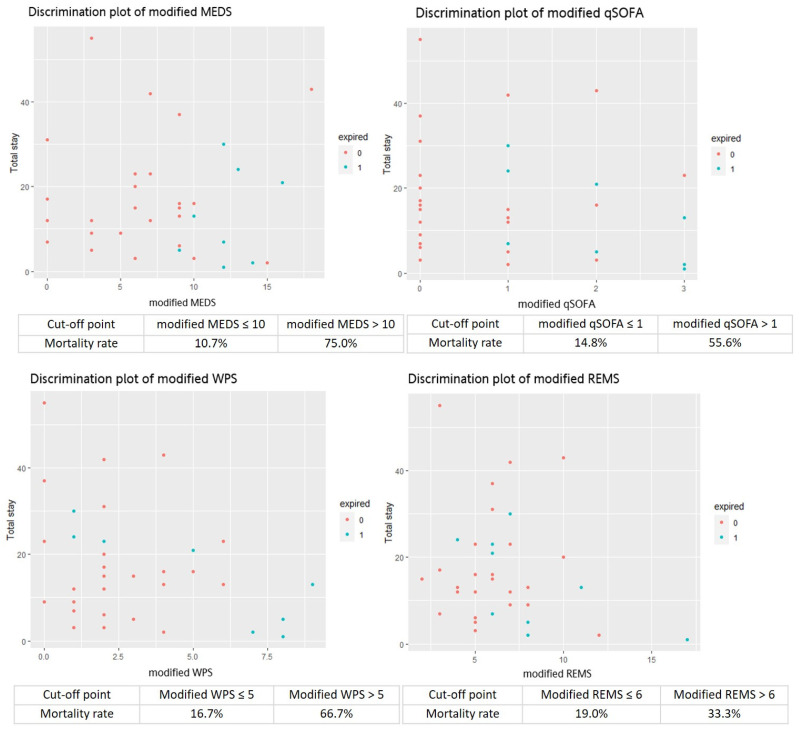
This study analyzed the cumulative survival rates of patients with Vibrio bacteremia using Kaplan–Meier. The original MEDS and WPS demonstrated significant differences between the survivors and non-survivors (p = 0.0012 and p < 0.0001) if the cut-off points of the original MEDS and WPS were 10 and 5. Otherwise, the original qSOFA and REMS demonstrated no significant differences between the survivors and non-survivors (p = 0.37 and p = 0.56) if the cut-off points of the qSOFA and REMS were 1 and 6 (Figure 3). However, the modified MEDS, WPS, and qSOFA demonstrated significant differences between the survivors and non-survivors (p < 0.0001, p = 0.00044, and p = 0.0034) if the cut-off points of the modified MEDS, WPS, and qSOFA were 10, 5, and 1. Otherwise, the modified REMS demonstrated no significant differences between the survivors and non-survivors (p = 0.28) if the cut-off point of the REMS was 6 (Figure 4).The discrimination plots of patients with Vibrio bacteremia show that the mortality rates of the original MEDS, WPS, qSOFA, and REMS were 71.4%, 75.0%, 50.0%, and 30.0% if the cut-off points were more than 10, 5, 1, and 6, respectively (Figure 5). The mortality rates of the modified MEDS, WPS, qSOFA, and REMS were 75.0%, 66.7%, 55.6%, and 33.3% if the cut-off points were more than 10, 5, 1, and 6, respectively (Figure 6).
Figure 3.
The cumulative 30-day survival rates of patients with Vibrio bacteremia were calculated by Kaplan–Meier. The cut-off points of the original MEDS, WPS, qSOFA, and REMS were 10, 5, 1, and 6. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
Figure 4.
The cumulative 30-day survival rates of patients with Vibrio bacteremia were calculated by Kaplan–Meier. The cut-off points of the modified MEDS, WPS, qSOFA, and REMS were 10, 5, 1, and 6. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
Figure 5.
Discrimination plots showing the mortality rates of 71.4%, 75.0%, 50.0%, and 30.0% in the original scoring systems of the MEDS, WPS, qSOFA, and REMS if the cut-off points were more than 10, 5, 1, and 6, respectively. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
Figure 6.
Discrimination plots showing the mortality rates of 75.0%, 66.7%, 55.6%, and 33.3% in the scoring systems of the modified MEDS, WPS, qSOFA, and REMS if the cut-off points were more than 10, 5, 1, and 6, respectively. Abbreviations: MEDS, Mortality in Emergency Department Sepsis; REMS, Rapid Emergency Medicine Score; qSOFA, quick Sepsis-related Organ Failure Assessment; WPS, Worthing Physiological Scoring system.
4. Discussion
Our study showed an overall mortality rate of 25%, with 31.5% due to V. vulnificus (n = 6/19). The reported mortality rate of Vibrio infection was about 19~39%, and 37% in another study in medical centers in Taiwan [6,13,16,24]. V. vulnificus was the most common cause of Vibrio-related illness and demonstrated high mortality; about 36% in a previous study in the United States [25].
In the previous studies, the ratio of male to female patients was 2:1 in Taiwan [26] and 3.6:1 (84.8%) in mainland China [27]. The results of these studies indicated that Vibrio infection is more likely to occur in males. Our study also demonstrated that cirrhosis was the most common comorbidity, accounting for 27.8%, and chronic liver disease represented 36.1%, similar to the previous research [8,25,28,29]. Liver diseases appeared more common in males [30], which might explain why the proportion of males with Vibrio bacteremia had a higher prevalence rate.
In the clinical course, hypotension was an unfavorable prognostic factor [31]. Hypotension indicated a more severe state of septic shock and was associated with an increased mortality rate [32,33]. Additionally, a significant increase in mortality was observed in cases when urgent hemodialysis was required. Previous studies already supported this finding [34,35]. Other interventions, such as intubation and vasopressor use, did not differ significantly.
The antibiotic treatment for Vibrio bacteremia typically involves a third-generation cephalosporin combined with tetracycline or fluoroquinolone [36,37,38]. Vibrio bacteremia often exhibited poor responsiveness to the treatment of penicillin. In our study, most cases received treatment with cephalosporins, including ceftriaxone or cefepime, upon arrival at the ED. However, there were six cases where an immediate assessment of the potential source of infection was not feasible due to clinical presentations or patient history inquiries. Consequently, these cases were treated with other broad-spectrum antibiotics—five with piperacillin and one with oxacillin. Notably, the mortality rate among this group of patients was significantly higher.
In microorganisms, V. vulnificus was the most common species causing Vibrio bacteremia [2,39]. This pathogen was prevalent in estuarine waters, aligning with the geographical environment of Taiwan—a seafood-rich island surrounded by the sea on all sides. The second most common species was V.cholerae, non-O1 and non-O139, predominant among V. non-vulnificus. V. cholerae, non-O1 and non-O139, was often associated with infectious diarrhea or contaminated water [40]. Taiwan, situated in the subtropics, possesses geographical features conducive to the growth of these bacterial strains.
A number of clinical scoring systems exist to quickly stratify patients and identify potentially severe conditions in both the ED and intensive care unit based on variable physiological parameters [22,41]. These simple and user-friendly clinical scoring systems enable physicians to quickly decide on the treatment plans for patients and start early goal-directed therapies, including the administration of suitable antibiotics.
The original MEDS score, developed by Shapiro et al. in 2003, incorporates various clinical parameters such as terminal disease, respiratory difficulty, septic shock, platelet count, band proportion, age, lower respiratory infection, nursing home residence, and altered mental status [42]. This scoring system has been shown to accurately estimate the risk of mortality in emergency department patients with suspected infectious conditions [43]. In Taiwan, the MEDS score is commonly utilized for predicting mortality among patients suffering from community-acquired bacteremia [44]. Higher original MEDS scores were found in the non-survivors in this single-center retrospective study. Moreover, the application of multivariate logistic regression revealed that the AUC of ROC for the original MEDS score was 0.833, alongside a sensitivity of 66.7% and a specificity of 92.6%. This highlights its capability to predict mortality in Vibrio bacteremia cases, using a cut-off point of 10.
This study modified the scoring systems by choosing the predictive factors, including BUN and pH, according to univariate and multivariate analyses. Previous studies have highlighted the predictive capability of BUN or the BUN-to-albumin ratio for the mortality rate in bacteremia [45,46,47]. A study in South Korea also suggested that pH levels can aid in estimating the mortality rate of Vibrio infections [48].
The original MEDS and qSOFA were designed for simplicity and ease of calculation, often excluding blood test data [49,50,51]. However, this simplicity came at the cost of some accuracy. In cases where blood test data were available, we enhanced these commonly used scoring systems with laboratory data (BUN and pH) to improve their predictive accuracy, specifically for Vibrio bacteremia. Although they lead to a few minutes’ delay for the blood test results, the modified MEDS and qSOFA will significantly benefit from advancements in testing technologies—making such waits considerably shorter than before. This enhances our ability to predict a patient’s condition’s severity accurately. We believe that a few minutes’ delay can bring advantages, such as more precise diagnoses, and can avoid unnecessary treatment expenses and the risks associated with delayed treatment, ultimately leading to significant long-term savings in healthcare costs.
5. Limitation
There were several limitations in this study. First, this was a single-center retrospective study with a relatively small sample size. This may have led to some analyses showing no significant difference and a selection bias or confounding variables not accounted for in the analysis. Second, Vibrio bacteremia is a rare clinical entity, so finding a control group without Vibrio bacteremia in this retrospective study was challenging. Third, compared to previous studies, we did not document or analyze data related to the source or site of infection in these Vibrio bacteremia patients. Fourth, our study modified those existing scoring systems, and while we did see improvements in sensitivity and specificity, it may still need to catch up to our ideal expectations.
6. Conclusions
The original MEDS could serve as reliable indicators for forecasting the mortality rate of patients grappling with Vibrio bacteremia. This study modified the MEDS and qSOFA by increasing the laboratory variables, including BUN and pH, to strengthen the predictive performance for the mortality risk of Vibrio bacteremia. It is advocated to promptly initiate targeted therapeutic interventions and judicious antibiotic treatments to curb fatality rates. Substantive, expansive investigations are requisite to engender deeper insights into the malady and ensure maximal patient well-being.
Acknowledgments
We thank the Clinical Informatics Research and Development Center and Biostatistics Task Force of Taichung Veterans General Hospital. We thank Yu-Ju Chen, M.T., Center of Microbiological Laboratory, Department of Pathology and Laboratory Medicine, Taichung Veterans General Hospital, for providing the methodology of identification for Vibrio species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm14040385/s1, Table S1: scoring systems. Refs [41,42,43,44,51] are cited in Supplementary Materials.
Author Contributions
Conceptualization, S.-Y.H. and S.-C.H.; methodology, M.-S.H. and S.-Y.H.; data curation, S.-Y.H., C.-H.S. and Y.-C.T.; writing—original draft preparation, C.-M.H. and S.-Y.H.; writing—review and editing, M.-S.H. and S.-Y.H.; project administration, S.-Y.H.; funding acquisition, S.-Y.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The institutional review board of Taichung Veterans General Hospital approved this study on 17 June 2022. (The study period ranged from 1 July 2021 to 30 June 2022.) (IRB file number: CE22240B).
Informed Consent Statement
Patient consent was waived because this study was retrospective, observational, and anonymous.
Data Availability Statement
Readers can access the data and material supporting the study’s conclusion by contacting Sung-Yuan Hu at song9168@pie.com.tw.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported by grants from the Taichung Veterans General Hospital (TCVGH), Taichung, Taiwan (TCVGH-1127203C, TCVGH-T1127801, TCVGH-1137203C, and TCVGH-T1137801), and the Taipei Veterans General Hospital, Taoyuan branch, Taoyuan, Taiwan (TYVH-10808, TYVH-10809, and TYVH-10902). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Morris J.G., Jr., Black R.E. Cholera and other vibrioses in the United States. N. Engl. J. Med. 1985;312:343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- 2.Baker-Austin C., Oliver J.D., Alam M., Ali A., Waldor M.K., Qadri F., Martinez-Urtaza J. Vibrio spp. infections. Nat. Rev. Dis. Primers. 2018;4:8. doi: 10.1038/s41572-018-0005-8. [DOI] [PubMed] [Google Scholar]
- 3.Sampaio A., Silva V., Poeta P., Aonofriesei F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity. 2022;14:97. doi: 10.3390/d14020097. [DOI] [Google Scholar]
- 4.Ina-Salwany M.Y., Al-Saari N., Mohamad A., Mursidi F.A., Mohd-Aris A., Amal M.N.A., Kasai H., Mino S., Sawabe T., Zamri-Saad M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health. 2019;31:3–22. doi: 10.1002/aah.10045. [DOI] [PubMed] [Google Scholar]
- 5.Tacket C.O., Brenner F., Blake P.A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J. Infect. Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 6.Coerdt K.M., Khachemoune A. Vibrio vulnificus: Review of Mild to Life-threatening Skin Infections. Cutis. 2021;107:E12–E17. doi: 10.12788/cutis.0183. [DOI] [PubMed] [Google Scholar]
- 7.Klontz K.C., Lieb S., Schreiber M., Janowski H.T., Baldy L.M., Gunn R.A. Syndromes of Vibrio vulnificus infections: Clinical and epidemiologic features in Florida cases, 1981–1987. Ann. Intern. Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 8.Johnston J.M., Becker S.F., McFarland L.M. Vibrio vulnificus: Man and the sea. JAMA. 1985;253:2850–2853. doi: 10.1001/jama.1985.03350430062026. [DOI] [PubMed] [Google Scholar]
- 9.Hoge C.W., Watsky D., Peeler R.N., Libonati J.P., Israel E., Morris J.G., Jr. Epidemiology and spectrum of Vibrio infections in a Chesapeake Bay community. J. Infect. Dis. 1989;160:985–993. doi: 10.1093/infdis/160.6.985. [DOI] [PubMed] [Google Scholar]
- 10.Satitsri S., Pongkorpsakol P., Srimanote P., Chatsudthipong V., Muanprasat C. Pathophysiological mechanisms of diarrhea caused by the Vibrio cholerae O1 El Tor variant: An in vivo study in mice. Virulence. 2016;7:789–805. doi: 10.1080/21505594.2016.1192743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero D.A., Vidal R.M., Velasco J., George S., Lucero Y., Gómez L.A., Carreño L.J., García-Betancourt R., O’Ryan M. Vibrio cholerae, classification, pathogenesis, immune response, and trends in vaccine development. Front. Med. 2023;10:1155751. doi: 10.3389/fmed.2023.1155751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshayes S., Daurel C., Cattoir V., Parienti J.J., Quilici M.L., de La Blanchardière A. Non-O1, non-O139 Vibrio cholera bacteraemia: Case report and literature review. Springerplus. 2015;4:575. doi: 10.1186/s40064-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mead P.S., Slutsker L., Dietz V., McCaig L.F., Bresee J.S., Shapiro C., Griffin P.M., Tauxe R.V. Food-related illness and death in the United States. Emerg. Infect. Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J.S., Lee E.G., Chun B.C. Epidemiologic Characteristics and Case Fatality Rate of Vibrio vulnificus Infection: Analysis of 761 Cases From 2003 to 2016 in Korea. J. Korean Med. Sci. 2022;37:e79. doi: 10.3346/jkms.2022.37.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leng F., Lin S., Wu W., Zhang J., Song J., Zhong M. Epidemiology, pathogenetic mechanism, clinical characteristics, and treatment of Vibrio vulnificus infection: A case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1999–2004. doi: 10.1007/s10096-019-03629-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H., Xu L., Dong H., Hu J., Gao H., Yang M., Zhang X., Chen X., Fan J., Ma W. Correlations between Clinical Features and Mortality in Patients with Vibrio vulnificus Infection. PLoS ONE. 2015;10:e0136019. doi: 10.1371/journal.pone.0136019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsao C.H., Chen C.C., Tsai S.J., Li C.R., Chao W.N., Chan K.S., Lin D.B., Sheu K.L., Chen S.C., Lee M.C., et al. Seasonality, clinical types and prognostic factors of Vibrio vulnificus infection. J. Infect. Dev. Ctries. 2013;7:533–540. doi: 10.3855/jidc.3008. [DOI] [PubMed] [Google Scholar]
- 18.Chen S.C., Chan K.S., Chao W.N., Wang P.H., Lin D.B., Ueng K.C., Kuo S.H., Chen C.C., Lee M.C. Clinical outcomes and prognostic factors for patients with Vibrio vulnificus infections requiring intensive care: A 10-yr retrospective study. Crit. Care Med. 2010;38:1984–1990. doi: 10.1097/CCM.0b013e3181eeda2c. [DOI] [PubMed] [Google Scholar]
- 19.DePaola A., Hopkins L.H., Peeler J.T., Wentz B., McPhearson R.M. Incidence of Vibrio parahaemolyticus in US coastal waters and oysters. Appl. Environ. Microbiol. 1990;56:2299–2302. doi: 10.1128/aem.56.8.2299-2302.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Archer E.J., Baker-Austin C., Osborn T.J., Jones N.R., Martínez-Urtaza J., Trinanes J., Oliver J.D., González F.J.C., Lake I.R. Climate warming and increasing Vibrio vulnificus infections in North America. Sci. Rep. 2023;13:3893. doi: 10.1038/s41598-023-28247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro R.L., Altekruse S., Hutwagner L., Bishop R., Hammond R., Wilson S., Ray B., Thompson S., Tauxe R.V., Griffin P.M. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. J. Infect. Dis. 1998;178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- 22.Wei X., Ma H., Liu R., Zhao Y. Comparing the effectiveness of three scoring systems in predicting adult patient outcomes in the emergency department. Medicine. 2019;98:e14289. doi: 10.1097/MD.0000000000014289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang S.H., Hsieh C.H., Weng Y.M., Hsieh M.S., Goh Z.N.L., Chen H.Y., Chang T., Ng C.J., Seak J.C., Seak C.K., et al. Performance assessment of the mortality in emergency department sepsis score, modified early warning score, rapid emergency medicine score, and rapid acute physiology score in predicting survival outcomes of adult renal abscess patients in the emergency department. Biomed. Res. Int. 2018;2018:6983568. doi: 10.1155/2018/6983568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuang Y.C., Yuan C.Y., Liu C.Y., Lan C.K., Huang A.H. Vibrio vulnificus infectionin Taiwan: Report of 28 cases and review of clinical manifestations and treatment. Clin. Infect. Dis. 1992;15:271–276. doi: 10.1093/clinids/15.2.271. [DOI] [PubMed] [Google Scholar]
- 25.Dechet A.M., Yu P.A., Koram N., Painter J. Nonfoodborne Vibrio infections: An important cause of morbidity and mortality in the United States, 1997–2006. Clin. Infect. Dis. 2008;46:970–976. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Wu Y., Sun X., Ma J., Li X., Liu C., Xie H. Non-O1/non-O139 Vibrio cholera bacteraemia in mainland China from 2005 to 2019: Clinical, epidemiological and genetic characteristics. Epidemiol. Infect. 2020;148:e186. doi: 10.1017/S0950268820001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Lu Y., Qian H., Liu G., Mei Y., Jin F., Xia W., Ni F. Non-O1, Non-O139 Vibrio cholerae (NOVC) bacteremia: Case report and literature review, 2015–2019. Infect. Drug Resist. 2020;13:1009–1016. doi: 10.2147/IDR.S245806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blake P.A., Merson M.H., Weaver R.E., Hollis D.G., Heublein P.C. Disease caused by a marine Vibrio: Clinical characteristics and epidemiology. N. Engl. J. Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 29.Kitaura S., Okamoto K., Wakabayashi Y., Okada Y., Okazaki A., Ikeda M., Hakuta R., Nakai Y., Okugawa S., Koike K., et al. Vibrio fluvialis liver abscess and bacteremia in a sashimi lover: A case report and review of the literature. Open Forum Infect. Dis. 2020;7:ofaa212. doi: 10.1093/ofid/ofaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., Landt C.L., Harrison S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Liu J.W., Lee I.K., Tang H.J., Ko W.C., Lee H.C., Liu Y.C., Hsueh P.R., Chuang Y.C. Prognostic factors and antibiotics in Vibrio vulnificus septicemia. Arch. Intern. Med. 2006;166:2117–2123. doi: 10.1001/archinte.166.19.2117. [DOI] [PubMed] [Google Scholar]
- 32.Rangel-Frausto M.S., Pittet D., Costigan M., Hwang T., Davis C.S., Wenzel R.P. The natural history of the systemic inflammatory response syndrome (SIRS): A prospective study. JAMA. 1995;273:117–123. doi: 10.1001/jama.1995.03520260039030. [DOI] [PubMed] [Google Scholar]
- 33.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerstloompleephunt N., Tantawichien T., Sitprija V. Renal failure in Vibrio vulnificus infection. Ren. Fail. 2000;22:337–343. doi: 10.1081/JDI-100100877. [DOI] [PubMed] [Google Scholar]
- 35.Neveu H., Kleinknecht D., Brivet F., Loirat P., Landais P. Prognostic factors in, acute renal failure due to sepsis. Results of a prospective multicentre study. Nephrol. Dial. Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 36.daSilva L.V., Ossai S., Chigbu P., Parveen S. Antimicrobial and genetic profiles of Vibrio vulnificus and Vibrio parahaemolyticus isolated from the Maryland Coastal Bays, United States. Front. Microbiol. 2021;12:676249. doi: 10.3389/fmicb.2021.676249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S.C., Lee Y.T., Tsai S.J., Chan K.S., Chao W.N., Wang P.H., Lin D.B., Chen C.C., Lee M.C. Antibiotic therapy for necrotizing fasciitis causedby Vibrio vulnificus: Retrospective analysis of an 8 year period. J. Antimicrob. Chemother. 2012;67:488–493. doi: 10.1093/jac/dkr476. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.E., Shin S.U., Oh T.H., Kim U.J., Darboe K.S., Kang S.J., Jang H.C., Jung S.I., Shin H.Y., Park K.H. Outcomes of third-generation cephalosporin plus ciprofloxacin or doxycycline therapy in patients with Vibrio vulnificus septicemia: A propensity score-matched analysis. PLoS Negl. Trop. Dis. 2019;13:e0007478. doi: 10.1371/journal.pntd.0007478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson N., Edwards F., Harris P.N.A., Laupland K.B. Vibrio species bloodstream infections in Queensland, Australia. Intern. Med. J. 2024;54:157–163. doi: 10.1111/imj.16187. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y.T., Tang H.J., Chao C.M., Lai C.C. Clinical manifestations of non-O1 Vibrio cholerae infections. PLoS ONE. 2015;10:e0116904. doi: 10.1371/journal.pone.0116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruangsomboon O., Boonmee P., Limsuwat C., Chakorn T., Monsomboon A. The utility of the rapid emergency medicine score (REMS) compared with SIRS, qSOFA and NEWS for Predicting in-hospital Mortality among Patients with suspicion of Sepsis in an emergency department. BMC Emerg. Med. 2021;21:2. doi: 10.1186/s12873-020-00396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro N.I., Wolfe R.E., Moore R.B., Smith E., Burdick E., Bates D.W. Mortality in Emergency Department Sepsis (MEDS) score: A prospectively derived and validated clinical prediction rule. Crit. Care Med. 2003;31:670–675. doi: 10.1097/01.CCM.0000054867.01688.D1. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G., Zhang K., Zheng X., Cui W., Hong Y., Zhang Z. Performance of the MEDS score in predicting mortality among emergency department patients with a suspected infection: A meta-analysis. Emerg. Med. J. 2020;37:232–239. doi: 10.1136/emermed-2019-208901. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh C.C., Yang C.Y., Lee C.H., Chi C.H., Lee C.C. Validation of MEDS score in predicting short-term mortality of adults with community-onset bacteremia. Am. J. Emerg. Med. 2020;38:282–287. doi: 10.1016/j.ajem.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Zou X.L., Feng D.Y., Wu W.B., Yang H.L., Zhang T.T. Blood urea nitrogen to serum albumin ratio independently predicts 30-day mortality and severity in patients with Escherichia coli bacteraemia. Med. Clin. 2021;157:219–225. doi: 10.1016/j.medcli.2020.06.060. [DOI] [PubMed] [Google Scholar]
- 46.Chiang H.Y., Chen T.C., Lin C.C., Ho L.C., Kuo C.C., Chi C.Y. Trend and Predictors of Short-term Mortality of Adult Bacteremia at Emergency Departments: A14-Year Cohort Study of 14625 Patients. Open Forum Infect. Dis. 2021;8:ofab485. doi: 10.1093/ofid/ofab485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X., Zheng R., Zhang T., Zeng Z., Li H., Liu J. Association between blood urea nitrogen and 30-day mortality in patients with sepsis: A retrospective analysis. Ann. Palliat. Med. 2021;10:11653–11663. doi: 10.21037/apm-21-2937. [DOI] [PubMed] [Google Scholar]
- 48.Yun N.R., Kim D.M., Lee J., Han M.A. pH level as a marker for predicting death among patients with Vibrio vulnificus infection, South Korea, 2000–2011. Emerg. Infect. Dis. 2015;21:259–264. doi: 10.3201/eid2102.131249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y.H., Hsieh M.S., Hu S.Y., Huang S.C., Tsai C.A., Tsai Y.C. Scoring Systems to Evaluate the Mortality Risk of Patients with Emphysematous Cystitis: A Retrospective Observational Study. J. Pers. Med. 2023;13:318. doi: 10.3390/jpm13020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., et al. Assessment of clinical criteria for sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.vanderWulp I., Rullmann H.A., Leenen L.P., vanStel H.F. Associations of the Emergency Severity Index triage categories with patients’; vital signs at triage: A prospective observational study. Emerg. Med. J. 2011;28:1032–1035. doi: 10.1136/emj.2010.096172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Readers can access the data and material supporting the study’s conclusion by contacting Sung-Yuan Hu at song9168@pie.com.tw.