Abstract
In this study, we compared different methods—including transmission electron microscopy—and various nucleic acid labeling methods in which we used the fluorochromes 4′,6′-diamidino-2-phenylindole (DAPI), 4-[3-methyl-2,3-dihydro-(benzo-1,3-oxazole)-2-methylmethyledene]-1-(3′-trimethyl ammoniumpropyl)-quinilinium diioide (YOPRO-1), and SYBR Green I, which can be detected by epifluorescence microscopy (EM), for counting viruses in samples obtained from freshwater ecosystems whose trophic status varied and from a culture of T7 phages. From a quantitative and qualitative viewpoint, our results showed that the greatest efficiency for all ecosystems was obtained when we used the EM counting protocol in which YOPRO-1 was the label, as this fluorochrome exhibited strong and very stable fluorescence. A modification of the original protocol in which YOPRO-1 was used is recommended, because this modification makes the protocol faster and allows it to be used for routine analysis of fixed samples. Because SYBR Green I fades very quickly, the use of this fluorochrome is not recommended for systems in which the viral content is very high (>108 particles/ml), such as treated domestic sewage effluents. Experiments in which we used DNase and RNase revealed that the number of viruses determined by EM was slightly overestimated (by approximately 15%) because of interference caused by the presence of free nucleic acids.
For a long time it has been thought that in aquatic ecosystems the bacterioplankton level and bacterioplankton production are controlled mainly by temperature, the availability of resources, and predation (6, 19). However, many attempts at modeling microbial food webs, particularly energy flow and material flow among dissolved organic matter, bacteria, and protozoans, have been unsuccessful, undoubtedly because poorly understood processes are involved in losses that affect pelagic microbial communities (19, 21, 25, 26). This is one of the reasons that biologists have recently become interested in the role of viruses in ecological processes in the pelagic environment. The results that have been obtained have clearly shown that viruses are abundant, active, and ubiquitous in aquatic ecosystems, in which they probably play a dominant role in losses that affect microbial communities (1, 2, 7–9, 12, 24, 28, 30, 32, 38).
The study of viruses in aquatic environments requires a reliable method for estimating the levels of these biological entities. In modern studies of aquatic microbial ecology, the first estimates of viral levels were obtained by concentrating the viruses by ultracentrifugation (1, 3, 27) or by ultrafiltration (24, 39) and then counting them by using transmission electron microscopy (TEM). However, the high costs, the long analysis time required, and the difficulty of this method led to the development of alternative, faster, and less expensive methods based on the use of epifluorescence microscopy (EM); with these methods viruslike particles (VLPs) can be observed and counted after they have been labeled with fluorochromes that are specific for nucleic acids. DAPI (4′,6′-diamino-2-phenylindole), a fluorochrome specific for double-stranded DNA (23), was the first fluorochrome used for labeling and counting of aquatic VLPs by EM (9, 11, 24, 31). Later, Hennes and Suttle (13) developed another VLP counting protocol in which EM was used; they used a fluorochrome that was based on cyanine and is specific for DNA and RNA, YOPRO-1 {4-[3-methyl-2,3-dihydro-(benzo-1,3-oxazole)-2-methylmethyledene]-1-(3′-trimethyl ammoniumpropyl)-quinilinium diioide}. The long time needed to stain samples and the requirement for samples that have not been fixed by aldehydes led Xenopoulos and Bird (40) to propose a modification of the original protocol of Hennes and Suttle (13). This modification consists of staining samples while they are hot, which substantially reduces the time needed to stain the samples. Samples fixed with aldehydes can also be analyzed by this method. More recently, a new fluorochrome that is also specific for nucleic acids and is intended for counting viral particles by EM (SYBR Green I) has been tested by Marie et al. (17) and by Noble and Fuhrman (20).
Some of the methods described above have been compared previously (10, 11, 13, 20, 35, 40). However, most comparisons have been made by using plankton samples obtained from oligotrophic marine environments. To our knowledge, the five protocols described above have not been simultaneously tested previously with samples of viruses obtained from aquatic ecosystems that differ in trophic status. Furthermore, the effects of free nucleic acids (DNA and RNA) on quantitative estimates of numbers of viruses in aquatic environments have received very little attention (5, 13) or no attention (for RNA).
The aim of this study was to compare the five protocols for counting viral particles. We used TEM and EM coupled with fluorescent staining with DAPI, YOPRO-1, and SYBR Green I to determine the most efficient protocol for routine counting of viruses in aquatic ecosystems that differed in trophic status (oligotrophic to hypereutrophic). We also tried to estimate the bias related to fading of fluorescently marked particles and the effects of free DNA and RNA fragments on the total number of viruses determined by TEM and EM.
Our results revealed that the modified EM-YOPRO protocol was the most useful method for reliably counting freely occurring viruses in aquatic ecosystems. Because of the very fast fading time of SYBR Green I, using this fluorochrome is not recommended for systems in which the viral concentration is high (>108 particles/ml), such as domestic sewage treatment plant effluents. Finally, we found that interference caused by free nucleic acids leads to slight overestimation of the number of viruses as determined by EM.
MATERIALS AND METHODS
Sample collection.
Samples were obtained from the following freshwater ecosystems, which differed in trophic status and were located in the Massif Central region of France (46°N, 3°E): (i) an oligotrophic reservoir (the Sep Reservoir), (ii) a moderate-altitude oligomesotrophic lake (Lake Pavin), (iii) a eutrophic reservoir (the Villerest Reservoir), and (iv) a domestic sewage works lagoon (the Roche Blanche plant), which was considered a hypereutrophic environment. A culture of T7 phages (phages with double-stranded DNA) that was grown in tryptone-casein-soy broth was also used. Five replicate water samples were collected at each lake or reservoir site by using an opaque polyvinyl chloride Van Dorn type of bottle, and five replicate samples were collected manually with a sterile container at the entrance to the sewage lagoon. The following two types of samples (five replicates each) were collected from each aquatic ecosystem that was tested: samples that were stored at 4°C without a fixative and were analyzed immediately and samples that were fixed with formaldehyde (final concentration, 2%) and analyzed within 1 week. Five replicates for each protocol were also analyzed for the T7 phage culture. Before analysis, the T7 phage culture was diluted 1:10 with deionized-distilled water (DDW). All working solutions (i.e., stains, DDW, mountant, formalin) were filter sterilized immediately before they were used by using Anotop 10 units (Whatman) equipped with 0.02-μm-pore-size inorganic membranes and sterile syringes.
Virus counts.
For samples examined by TEM, the viruses in 1 ml of each of the five replicates were harvested by ultracentrifugation onto three TEM grids that previously had been fixed to the platform of a polymer resin support and placed at the bottom of an ultracentrifugation tube (27); to do this, we used a Centrikon TST 41.14 Swing-Out rotor that was centrifuged at 120,000 × g for 2 h at 4°C (4, 27, 31). The electron microscope grids were 400-mesh copper grids with carbon-coated Formvar films (catalog no. A03; Pelanne Instruments), which provided the best compromise between strength and electron transparency (31). Immediately before the grids were used, they were soaked for 1 min in 1 drop of 0.1% (wt/vol) poly-l-lysine (catalog no. P8920; Sigma) in order to make the surfaces of the films evenly hydrophilic (31). Following ultracentrifugation, each grid was stained for 30 s with uranyl acetate (2%, wt/wt), and the viruses were counted with a JEOL model 1200EX TEM operated at 80 kV and a magnification of ×40,000. Before counting, each grid was scanned to make sure that the viruses were distributed randomly. A minimum of 20 TEM fields were then randomly selected and the viruses were counted until the total counts exceeded 200 viral particles (31). Taper corrections were used for the final calculations of viral concentrations by using the formulae developed by Suttle (31), who provided more details concerning the protocol used to determine the concentrations of viruses in aqueous solutions by TEM.
EM was also used to count VLPs, which were stained with DAPI, YOPRO-1, or SYBR Green I. For the original EM-YOPRO-1 method (13), a stock solution of YOPRO-1 (Molecular Probes Europe, Leiden, The Netherlands) was diluted to a concentration of 50 μM by using an aqueous 2 mM NaCN solution. The viruses in 1-ml test samples were gently filtered (15-kPa vacuum) onto 0.02-μm-pore-size Al2O3 Anodisc filters (Whatman). While the filters were still damp, they were placed in a petri dish on 80-μl drops of YOPRO-1 (final concentration, 50 μM) for 48 h in the dark. Since fixation with aldehydes interferes with binding of YOPRO, viruses were filtered and stained immediately after they were collected. After staining, the filters were rinsed twice by filtering 800-μl portions of DDW through the membranes. The damp membranes were transferred to glass slides and covered with single drops of a solution containing 50% glycerol, 50% phosphate-buffered saline (0.05 M Na2HPO4, 0.85% NaCl; pH 7.5), and 0.1% p-phenylenediamine (Sigma) (made fresh daily from a frozen 10% aqueous stock solution; Sigma) on 25-mm-square coverslips. This mountant minimized fading (20). The VLPs were counted by using an Olympus microscope (model HB 2) equipped with a 100/1.25 Neofluar objective lens and a blue filter set.
The modified EM-YOPRO method described by Xenopoulos and Bird (40) can be used to stain fixed samples. For this method, 1-ml samples fixed with formalin (final concentration, 2%) were filtered onto 0.02-μm-pore-size Anodisc membrane filters and rinsed three times with 500-μl portions of DDW. The filters were put sample side up on 80-μl drops of YOPRO in petri dishes. Each petri dish was placed in a cardboard container to protect it from light and irradiated in a domestic microwave oven equipped with a turntable for no more than 4 min at a low to intermediate power level (∼400 W). The heated petri dish was allowed to cool for about 10 min, and then the filters were replaced on the filter support and rinsed three times with 800-μl portions of DDW. The filters were transferred to glass slides and VLPs were counted as described above for the original EM-YOPRO method.
For the EM-SYBR Green I method (20), a stock solution of SYBR Green I (Molecular Probes Europe) was diluted 1:10 with DDW. For each new filter, 2.5 μl of the 10% SYBR Green I working solution was added to a 97.5-μl drop of sterile DDW on the bottom of a petri dish (final dilution, 2.5 × 10−3). A 1-ml sample fixed with formalin (final concentration, 2%) was filtered through a 0.02-μm-pore-size Anodisc membrane filter (Whatman). The Anodisc membrane was dried and laid sample side up on 1 drop of the staining solution for 15 min in the dark. The Anodisc filter was mounted on a glass slide and VLPs were counted as described above for the EM-YOPRO methods.
For the EM-DAPI protocol (31), the viruses in 1-ml samples that were fixed with formalin (final concentration, 2%) were stained with DAPI (final concentration, 1 μg ml−1) in the dark for 30 min and then filtered onto 0.02-μm-pore-size filters (Anodisc). The filters were mounted on microscope slides as described above for the other methods, and VLPs were counted by using a UV filter set and the Olympus microscope.
Measuring the fading times of the fluorochromes.
The intensity of fluorescence of stained biological particles decreases as the duration of excitation during EM increases. This phenomenon, known as fading, depends on the nature of the fluorochrome and, therefore, the excitation and observation wavelengths of the fluorochrome. The total number of fluorescent particles determined by direct EM counting, therefore, depends on the rate of fading of the fluorochrome used and the time taken by the observer to count all of the excited particles present in the microscope field. In order to estimate the bias resulting from fading of fluorescently marked viral particles in this study, we measured the speed of fading of each of the fluorochromes used. To do this, we measured the time (in seconds) that it took for the last viral particle to disappear from each microscope field for six samples that were obtained from the different study sites and had been filtered with 0.2-μm-pore-size filters (to eliminate most of the nonviral particles). Fifty microscope fields were analyzed for each sample. The shape of the fluorescence decay curve and the related kinetic parameters might have been more important than the time that it took for the last viral particle to fade during EM, but technically these data were difficult to obtain. Our fading data were thus empirical estimates based on the assumption that the decay of fluorescence is linear.
Estimate of interference caused by free nucleic acids.
The fluorochromes used in this study were specific stains for nucleic acids, which also occur in a free state in aquatic media (34, 37). To estimate the effects of fragments of free DNA and RNA on the total number of viruses counted, samples were treated with two enzymes, RNase-free DNase I (catalog no. D7291; Sigma) and RNase A (catalog no. R4642; Sigma). For DNase I- and RNase A-treated samples, 250 Kunitz units of DNase I per ml and 250 Kunitz units of RNase A per ml were added, and the preparations were incubated for 30 min (13). The phages in triplicate samples treated in this way and in untreated control samples were counted by using both TEM and EM. These experiments were conducted by using five replicate samples from each of the study sites.
The supplier (Sigma) acknowledged that the stock solution of RNase A used in the experiments described above could have contained between 0 and 10% DNase and that boiling this solution to inactivate the residual DNase was not recommended. Therefore, we compared the contaminated RNase A with a DNase-free RNase (catalog no. 1119 915; Boehringer Mannheim) that was obtained from the same source (bovine pancreas). Numbers of viruses were determined for samples collected in February 2000 from the surface waters of Lake Pavin and eutrophic Lake Aydat (also located in the Massif Central region of France) after the samples were treated with the contaminated RNase A and with the DNase-free RNase (see above). These tests were performed with five replicate samples from each lake, and viral concentrations were determined by using the modified EM-YOPRO and TEM methods. As determined by both protocols, the samples treated with DNase-free RNase contained similar to slightly higher (mean, 1.07-fold; range, 0.96- to 1.19-fold) viral concentrations than the samples treated with contaminated RNase A contained. An analysis of variance performed with the results showed that the numbers of viruses in samples treated with both RNase A and DNase-free RNase were significantly (P < 0.05) higher than the numbers of viruses in untreated samples, but the values for the RNase A-treated samples were not significantly different (P > 0.05) than the values for the samples treated with DNase-free RNase. On the basis of this analysis, we concluded that comparisons of the effects of DNase I and RNase A in our original experiments were not affected by DNase contamination in the RNase A stock solution.
Statistical analyses.
The viral concentrations obtained when the various protocols were used were compared by performing a one-way analysis of variance for the differences between treatments. Similar analyses were also performed for the studies in which we examined the effects of free nucleic acids and the fading times of the fluorochromes. Significant differences between the results for fluorochromes or between the results for different treatments were tested by using an a posteriori test, the Fisher least-significant-difference test (29). For all of the analyses of variance, the null hypothesis was that there was not a significant difference between the results obtained with the various treatments or between the results obtained with the different fluorochromes.
RESULTS
Comparison of methods of counting viruses.
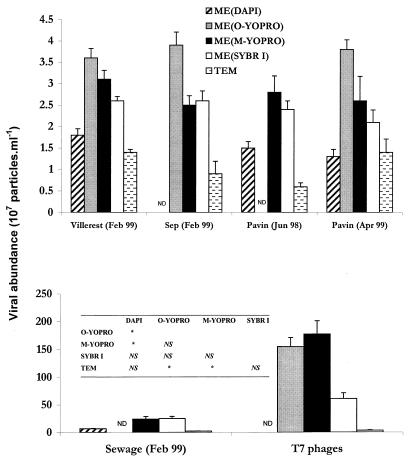
For all of the methods used and all of the samples analyzed, the coefficients of variation (coefficient of variation = standard deviation × 100/mean) for the mean concentrations of viral particles, calculated by using the values for five replicates, were relatively low, between 2.5 and 18.4%. The results which we obtained (Fig. 1) show that the concentrations of VLPs were up to 50 times higher when EM was used than when TEM was used. The difference was much more pronounced for the samples obtained from the lagoon and the T7 phage culture, the samples in which the viral concentrations were the highest. For all of the samples obtained from the lagoon and the T7 phage culture, the viral concentrations obtained by the TEM method were 18 and 3% of the viral concentrations obtained by EM, respectively. For the samples obtained at other sites, at which the viral particle concentrations were lower, the values were between 27 and 65% (Table 1).
FIG. 1.
Viral concentrations in the different environments tested, as determined by TEM and by EM after staining with various fluorochromes. ND, not determined. The table shows the results obtained by a one-way analysis of variance in which Fisher's least-significant-difference test was used. An asterisk indicates significance at a level of P < 0.05. NS, not significant. ME(DAPI), EM-DAPI protocol; ME(O-YOPRO), original EM-YOPRO protocol; ME(M-YOPRO), modified EM-YOPRO protocol; ME(SYBR I), EM-SYBR Green I protocol; TEM, TEM protocol; O-YOPRO, original YOPRO; M-YOPRO, modified YOPRO; SYBR I, SYBR Green I.
TABLE 1.
Ratios of the viral densities obtained by TEM to the viral densities obtained by EM
| Source of sample | Viral density ratios (%)
|
||||
|---|---|---|---|---|---|
| TEM/EM-DAPI | TEM/original EM-YOPRO | TEM/modified EM-YOPRO | TEM/EM-SYBR Green I | Mean | |
| Villerest Reservoir (February 1999) | 78.33 (4.55)a | 39.28 (3.48) | 45.48 (4.68) | 54.86 (2.01) | 54.49 |
| Sep Reservoir (February 1999) | NDb | 20.45 (7.24) | 32.93 (11.78) | 32.4 (7.54) | 28.59 |
| Lake Pavin (June 1998) | 37.09 (4.59) | ND | 19.86 (3.86) | 23.05 (3.37) | 26.66 |
| Lake Pavin (April 1999) | 106.2 (9.67) | 35.77 (9.58) | 52.9 (1.50) | 64.02 (18.24) | 64.72 |
| Sewage (February 1999) | 34.85 (8.43) | ND | 9.85 (1.06) | 9.46 (3.04) | 18.05 |
| T7 phages | ND | 2.26 (0.41) | 1.97 (0.52) | 5.75 (0.80) | 3.33 |
| Mean | 64.12 | 24.44 | 27.16 | 31.59 | 36.83 |
The values in parentheses are standard deviations based on the data obtained for five replicates.
ND, not determined.
Therefore, the TEM method provided lower viral concentrations with a much higher average coefficient of variation (10.2%) than the methods based on epifluorescence provided (EM-DAPI method coefficient of variation, 7.7%; original EM-YOPRO method, 5.3%; modified EM-YOPRO method, 8.8%; EM-SYBR Green I method, 8.7%). An analysis of variance confirmed that the viral concentrations obtained with the TEM method were significantly lower (P < 0.05) than the concentrations obtained with the two methods in which YOPRO and epifluorescence were used (Fig. 1).
There was, however, considerable variability in the viral concentrations estimated by the epifluorescence methods depending on the fluorochrome used. The original YOPRO staining method resulted in concentrations for samples from all of the sites that were higher than the concentrations obtained with DAPI and SYBR Green I. The modified EM-YOPRO protocol in all cases gave values lower than the values obtained with the original EM-YOPRO protocol, except for the samples obtained from the T7 phage culture, in which the viral concentration was very high. However, the analysis of variance revealed no significant difference (P > 0.05) between the results obtained with these two protocols. The values obtained when DAPI was used were the lowest values obtained with the EM methods. There were significant differences between the results obtained with this method and the results obtained with the two protocols in which YOPRO was used. The values obtained with the EM-SYBR Green I protocol were similar to the values obtained with the modified EM-YOPRO protocol but were lower than the values obtained with the original EM-YOPRO method for all of the samples when both YOPRO protocols were used (Fig. 1).
Fading of the fluorochromes.
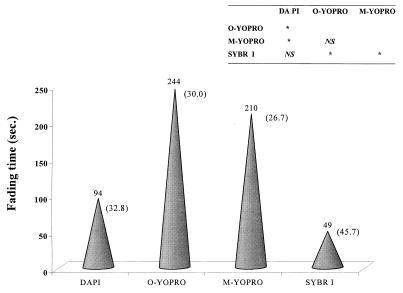
The fluorochrome fading experiment clearly showed that the fluorescence of particles that were stained with the original YOPRO protocol (244 s) and the modified YOPRO protocol (210 s) lasted the longest. An analysis of variance revealed no significant difference (P > 0.05) between the results obtained when these two protocols were used (Fig. 2). DAPI (94 s) and especially SYBR Green I (49 s) had significantly shorter mean fading times than both the original YOPRO and the modified YOPRO. For SYBR Green I, the data were equivalent to a fading rate for fluorescent particles that was five times higher than the fading rate for the original YOPRO. Furthermore, the coefficients of variation for the fading times when DAPI (32.8%) and SYBR Green I (45.7%) were used were much higher than the coefficients of variation obtained when the original EM-YOPRO protocol (30%) and the modified EM-YOPRO protocol (26.7%) were used.
FIG. 2.
Comparison of fading times for the various fluorochromes used with EM. The numbers in parentheses are the coefficients of variation for the fading times obtained with six samples that originated from the different bodies of water sampled. The table shows the results obtained by a one-way analysis of variance in which Fisher's least-significant-difference test was used. An asterisk indicates significance at a level of P < 0.05. NS, not significant. O-YOPRO, original YOPRO; M-YOPRO, modified YOPRO; SYBR I, SYBR Green I.
Effects of DNase and RNase.
To evaluate the effects of free nucleic acids on estimates of viral concentrations, the viruses in samples obtained from the three lakes which differed in trophic status were counted by using the modified EM-YOPRO protocol; the samples were either treated or not treated with RNase-free DNase I (catalog no. D7291; Sigma) and RNase A (catalog no. R4642; contaminated with 0 to 10% DNase; Sigma). In all cases, the samples treated with DNase I or RNase A contained significantly lower virus concentrations than the untreated samples (Table 2). On average, for all of the samples DNase I treatment resulted in an 18.3% reduction in the total virus level. The reduction after treatment with RNase A was 26.4%. We observed that the action spectrum of the DNase-contaminated RNase A used in these experiments was not significantly different (P > 0.05; analysis of variance) than the action spectrum of pure RNase (i.e., DNase-free RNase; catalog no. 1119 915; Boehringer Mannheim). Accordingly, the cumulative effect of DNase I and RNase A could be estimated by adding the effects of the two enzymes. The combined effects were equivalent to reductions in the mean total viral concentration of 39% for the Villerest Reservoir samples, 53% for the Lake Pavin samples, and 42% for the Sep Reservoir samples.
TABLE 2.
Viral concentrations in untreated samples and samples treated with DNase I and RNase A
| Protocol | Source of sample | Viral concn (107 particles/ml)
|
||
|---|---|---|---|---|
| Untreateda | DNase treated | RNase treated | ||
| Modified EM-YOPRO | Villerest Reservoir, May 1999 | 5.15 (4.53)b | 4.52 (10.06) | 3.77 (1.46) |
| Lake Pavin, May 1999 | 4.23 (9.55) | 3.34 (5.42) | 2.88 (8.13) | |
| Sep Reservoir, May 1999 | 4.47 (6.26) | 3.50 (6.29) | 3.55 (7.14) | |
| TEM | Villerest Reservoir, June 1999 | 2.64 (8.19) | 1.99 (15.18) | 2.23 (7.06) |
| Lake Pavin, June 1999 | 3.05 (6.02) | 2.66 (2.04) | 2.54 (4.18) | |
The values for the untreated samples are significantly higher than the values for the DNase-treated and RNase-treated samples (P < 0.05; one-way analysis of variance; Fisher's least-significant-difference test).
The values in parentheses are coefficients of variation based on the data obtained for three replicates.
After samples were treated with DNase I and RNase A and then TEM counts were determined, we recorded reductions in mean viral concentrations of 18.7 and 16.1%, respectively, for Lake Pavin and the Villerest Reservoir, the only two bodies of water tested. When the effects of the two enzymes were added, the viral concentrations were reduced by 40% for the samples obtained from the Villerest Reservoir and by 30% for the samples obtained from Lake Pavin. The analysis of variance performed with these results showed that the two enzymes had a significant effect (P < 0.05) on total viral concentration as determined by TEM (Table 2).
DISCUSSION
Comparative study of the methods of counting viruses.
Our results show that two microscopic procedures for estimating viral concentrations in aquatic ecosystems gave different results. With only one exception, the viral concentrations determined by EM were higher than the viral concentrations determined by TEM. The values obtained when TEM was used were comparable to the values obtained when we used DAPI staining followed by EM but were significantly lower than the values obtained when the two EM-YOPRO methods were used. The differences between the values obtained when TEM was used and the values obtained when EM was used were always more pronounced for the samples containing higher viral concentrations (i.e., the T7 phage culture and domestic sewage works lagoon samples) (Table 1). These findings agree with the results obtained by Hennes and Suttle (13) and Weinbauer and Suttle (35), who showed that the difference between estimates of viral concentrations obtained by EM and TEM increased as the number of viruses in the medium increased. This is probably due to the high concentrations of particulate matter that adversely affect TEM counting. The underestimation of values and the significantly greater variability of the TEM method (coefficient of variation, 10.2%) than of epifluorescence methods can also be explained by the high magnifications needed for viral counting by TEM (13, 31). Viruses can also be lost during staining of the grids with uranyl acetate or during the rinsing operations. Due to insufficient dilution (dilution ratio, 1:10), our TEM data for the level of T7 phages must be viewed as underestimates (3, 11). However, the underestimation did not really affect the general observation, based on the results of this study, in which different aquatic systems were tested, and the results of previous studies, that the numbers of VLPs determined by using fluorescence are significantly higher than the numbers of VLPs obtained when TEM preparations are used (for a review, see reference 7). Although the reasons for this are not known (7), it is likely that nonviral fluorescent particles are counted together with viruses by EM. In this study, we calculated the overestimate of viral concentration due to interference from free nucleic acids when EM was used (see below).
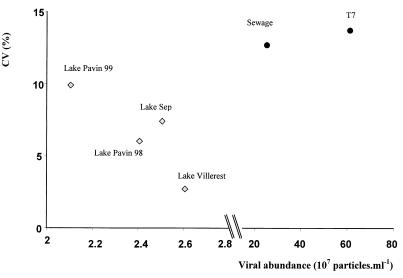
The virus counting protocol in which TEM is used is more expensive and difficult, requiring highly specialized training. With the exception of the original EM-YOPRO method, in which samples that have not been fixed are used and a staining time of 2 days is required, the protocols in which EM is used are much simpler and faster than the TEM protocol. Of the fluorochromes used for EM, DAPI gave the lowest concentrations. Similar results have been reported by several authors (10, 35), which supports the hypothesis that VLP concentrations are underestimated when DAPI is used; this finding is attributed to the lower DAPI light intensity (compared to other fluorochromes), the affinity of DAPI for only double-stranded DNA, and a relatively short fading time (94 s) (Fig. 2). The concentrations of viruses stained with SYBR Green I and examined by EM were similar to the concentrations obtained when the YOPRO protocol was used; the only exception was the value for the sample obtained from the T7 phage culture, which contained a very high viral concentration. In the latter case, the viral concentration obtained with the EM-SYBR Green I protocol was much lower (Fig. 1). Noble and Fuhrman (20) and Marie et al. [17] have recently tested SYBR Green I with EM by using seawater samples. Our results obtained with freshwater samples agree with the results of these researchers; the virus concentrations determined by the TEM protocol were lower than the concentrations determined by the EM-SYBR Green I protocol, and the concentrations determined by the EM-DAPI and EM-SYBR Green I protocols did not differ significantly from one another. Nevertheless, despite a fluorescence intensity that was similar to that of YOPRO, SYBR Green I had a shorter fading time (∼50 s). In samples treated with SYBR Green I in which the viral concentration was less than 5 × 107 particles ml−1, the coefficients of variation were lower at higher viral concentrations. However, in samples in which the viral concentration was more than 5 × 107 particles ml−1, the coefficient of variation increased as the viral concentration increased (Fig. 3). Such a relationship was not found with the other fluorochromes. These results suggest that the precision of the EM-SYBR Green I method decreases with samples containing high viral concentrations because of the very short fading time. This protocol is therefore not recommended for quantitative studies of viruses that do not include an image analyzer for aquatic systems containing high viral concentrations, such as sewage effluents. It has recently been shown that in a pelagic oligotrophic environment, using SYBR Green I with flow cytometry results in effective counting of VLPs (17).
FIG. 3.
EM-SYBR Green I protocol: relationship between the mean viral concentrations and the corresponding coefficients of variation (CV) for the six environments tested.
In all of the samples with which it was tested, YOPRO staining yielded viral densities that were higher than the densities obtained with the other methods tested. The results of the analysis of variance showed that the reduction in the staining time from 48 h (original EM-YOPRO method) to a few minutes when YOPRO was hot (modified EM-YOPRO method) did not have a significant effect on the quality of staining (similar intensities and fading times) and therefore did not have a significant effect on the estimate of viral concentration. On the other hand, the average coefficients of variation for all of the samples tested were 5.3% for the original EM-YOPRO protocol and 8.9% for the modified EM-YOPRO protocol. However, the latter value is still relatively low and represents an acceptable level of precision and reproducibility. There has been only a single study of the use of the modified EM-YOPRO protocol (40), and only a very few samples were tested in that study. The authors did not find a significant difference in viral concentrations when they compared the modified EM-YOPRO protocol with the original protocol of Hennes and Suttle (13).
On the basis of our results, we recommend using the modified EM-YOPRO protocol to count viruses in aquatic systems, since this protocol is much faster than the original EM- YOPRO protocol, can be used with samples that are fixed with aldehydes, and has satisfactory precision and efficiency. Examining TEM preparations in which phages are recognizable with much more confidence than on EM slides is not precluded when it is possible, at least for occasional checks. The TEM method is necessary for describing the morphological diversity and functional importance of viruses in aquatic systems (27, 28).
Interference from free nucleic acids.
An alternative or additional explanation for the differences in viral concentrations observed when EM and TEM are used could be that nonviral fluorescent particles are counted by EM. These particles could be very small bacteria (<100 nm), free nucleic acids, or even free mitochondria or ribosomes that are stained by the nucleic acid-specific fluorochromes. Since free ribosomes and mitochondria cannot survive for a long time in the aquatic environment and since the concentration of very small bacteria in the planktonic environment is negligible (35), only free nucleic acids can interfere significantly with the viral concentrations determined by EM. In aquatic ecosystems, free DNA generally originates from cell lysis (22) and from grazing activity by consumers (33).
To estimate this interference, we treated samples with DNase and RNase. The viral concentrations in samples treated separately with these two enzymes were significantly lower than the concentrations in untreated samples (Table 2). Based on our results, 39 to 53% of the particles considered to be viruses by EM were in fact free nucleic acids. These results differ from those of Hennes and Suttle (13) and Drake et al. (5), who observed no significant difference between marine plankton samples treated with DNase and untreated samples. In contrast, Hara et al. (11) reported that about one-third of the small VLPs stained by DAPI in the marine environment are not in fact viruses. It should be mentioned that to our knowledge, our study is the first study to consider the effect of RNase on free aquatic virus counts.
However, direct observations by TEM allowed us to demonstrate that DNase and RNase also break down true viral particles. As determined by TEM, a technique that results in certain identification of phages, the viral concentrations calculated after samples from two different environments were treated with DNase and RNase were significantly lower than the viral concentrations in untreated samples (Table 2). In the two environments studied, the average reductions in viral concentrations were 18.7% after treatment with DNase and 16.1% after treatment with RNase. The sensitivity of phages (viral DNA) to DNase has been demonstrated previously by several authors, especially for the marine environment. For example, Jiang and Paul (14) showed that in a culture containing T2 phages and plankton samples from the Gulf of Mexico 33 to 48% of the encapsulated viral DNA was digested by DNase. Maruyama et al. (18) found that in Tokyo Bay about 10% of the <0.2-μm fraction of DNA, which could be assumed to be viruses (i.e., “coated DNA”), was sensitive to DNase. These authors used a lower concentration of DNase (20 Kunitz units/ml) than we used in this study (250 Kunitz units/ml), which could explain the greater sensitivity of viral DNA in our experiments (18.7% reduction as determined by TEM and 18.3% reduction as determined by EM, on average). The results of this comparison also suggest that DNA is more likely to resist the action of DNase in seawater than in freshwater. The ability of viruses to be adsorbed quickly onto suspended particles is well known (36), and this process is undoubtedly more pronounced in the marine environment, where the ratio of virus density to sensitive host density is lower (16). It is known that such adsorption occurs with nucleic acids in a combined form, which are more refractory to the action of DNase (22). The degradation of viral RNA by RNase in our experiments led to losses similar to those obtained with DNase, and on average, 16.1% and 26.4% of the bacteriophages present in our samples were destroyed, as determined by TEM and EM, respectively. To our knowledge, these estimates are the first data concerning the sensitivity of planktonic viruses to RNase. The vertical distribution of RNA concentrations in the North Atlantic Ocean has been described by Karl and Bailiff (15), but there is still great uncertainty concerning the origins of the RNA and its role in aquatic ecosystems.
We concluded that the apparent destruction of viruses by DNase and RNase makes it impossible to use these enzymes routinely in virus-counting protocols in which EM is used. However, by comparing the viral losses due to the cumulative effects of DNase and RNase as determined by EM (ca. 45%) and TEM (ca. 35%), we were able to quantify the interference due to free nucleic acids by using the following formula: 0.65A = 0.55(A+B), where the sum of the fraction of viruses (A) and the fraction of free nucleic acids (B) in the samples analyzed by EM is equal to 1. By assuming that the use of DNase and RNase leads to the destruction of all of the free nucleic acids, we were then able to calculate that the overestimate of the viral concentration determined by EM due to interference from nucleic acids in all of our samples was 15.4%.
ACKNOWLEDGMENTS
The field assistance and technical assistance of G. Coffe, C. Doniol, S. Fournet-Fayard, C. Portelli, D. Sargos, and B. Vigues are greatly appreciated. We are also grateful for valuable comments and suggestions on an earlier version of the manuscript by two anonymous reviewers.
This study was supported by an MRT graduate fellowship to Y.B.. Funding by CNRS grant 9507004, ACC-SV 7 (Diversité fonctionnelle des réseaux trophiques microbiens dans les écosystèmes aquatiques), also facilitated the investigation.
REFERENCES
- 1.Bergh O, Børsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature (London) 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 2.Børsheim K Y. Native marine bacteriophages. FEMS Microbiol Ecol. 1993;102:141–159. [Google Scholar]
- 3.Børsheim K Y, Bratbak G, Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990;56:352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratbak G, Heldal M. Total count of viruses in aquatic environments. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 135–138. [Google Scholar]
- 5.Drake L A, Choi K H, Haskell A G E, Dobbs F C. Vertical profiles of virus-like particles and bacteria in the water column and sediments of Chesapeake Bay, USA. Aquat Microb Ecol. 1998;16:17–25. [Google Scholar]
- 6.Fenchel T. Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar Ecol Prog Ser. 1982;9:35–42. [Google Scholar]
- 7.Fuhrman J A. Marine viruses and their biogeochemical and ecological effects. Nature (London) 1999;399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman J A, Noble R T. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr. 1995;40:1236–1242. [Google Scholar]
- 9.Fuhrman J A, Suttle C A. Viruses in marine planktonic systems. Oceanography. 1993;6:51–63. [Google Scholar]
- 10.Guixa-Boixareu N, Lysnes K, Pedros-Alio C. Viral lysis and bacterivory during a phytoplankton bloom in a coastal water microcosm. Appl Environ Microbiol. 1999;65:1949–1958. doi: 10.1128/aem.65.5.1949-1958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara S, Terauchi K, Koike I. Abundance of viruses in marine waters: assessment by epifluorescence and transmission electron microscopy. Appl Environ Microbiol. 1991;57:2731–2734. doi: 10.1128/aem.57.9.2731-2734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennes K P, Simon M. Significance of bacteriophages for controlling bacterioplankton growth in a mesotrophic lake. Appl Environ Microbiol. 1995;61:333–340. doi: 10.1128/aem.61.1.333-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennes K P, Suttle C A. Direct counts of viruses in natural waters and laboratory cultures by epifluorescence microscopy. Limnol Oceanogr. 1995;40:1050–1055. [Google Scholar]
- 14.Jiang S C, Paul J H. Viral contribution to dissolved DNA in the marine environment as determined by differential centrifugation and kingdom probing. Appl Environ Microbiol. 1995;61:317–325. doi: 10.1128/aem.61.1.317-325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karl D M, Bailiff M D. The measurement and distribution of dissolved nucleic acids in aquatic environments. Limnol Oceanogr. 1989;34:543–558. [Google Scholar]
- 16.Maranger R, Bird D. Viral abundance in aquatic systems: a comparison between marine and freshwaters. Mar Ecol Prog Ser. 1995;121:217–226. [Google Scholar]
- 17.Marie D, Brussaard C P D, Thyrhaug R, Bratbak G, Vaulot D. Enumeration of marine viruses in culture and natural samples by flow cytometry. Appl Environ Microbiol. 1999;65:45–52. doi: 10.1128/aem.65.1.45-52.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruyama A, Oda M, Higashihara T. Abundance of virus-sized non-DNase-digestible DNA (coated DNA) in eutrophic seawater. Appl Environ Microbiol. 1993;59:712–717. doi: 10.1128/aem.59.3.712-717.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McManus G B, Fuhrman J A. Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia. 1988;159:51–62. [Google Scholar]
- 20.Noble R T, Fuhrman J A. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol. 1998;14:113–118. [Google Scholar]
- 21.Pace M L. Bacterial mortality and the fate of bacterial production. Hydrobiologia. 1988;159:41–49. [Google Scholar]
- 22.Paul J H, Jiang S C, Rose J B. Concentration of viruses and dissolved DNA from aquatic environments by vortex flow filtration. Appl Environ Microbiol. 1991;57:2197–2204. doi: 10.1128/aem.57.8.2197-2204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter K, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 24.Proctor L M, Fuhrman J A. Viral mortality of marine bacteria and cyanobacteria. Nature (London) 1990;343:60–62. [Google Scholar]
- 25.Servais P, Billen G, Vives-Rego J. Rate of bacterial mortality in aquatic environments. Appl Environ Microbiol. 1985;49:1448–1454. doi: 10.1128/aem.49.6.1448-1454.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr B F, Sherr E B, Pedros-Alio C. Simultaneous measurement of bacterioplankton production and protozoan bacterivory in estuarine waters. Mar Ecol Prog Ser. 1989;54:209–219. [Google Scholar]
- 27.Sime-Ngando T, Mignot J-P, Amblard C, Bourdier G, Desvilettes C, Quiblier-Lloberas C. Characterization of planktonic virus-like particles in a French mountain lake: methodological aspects and preliminary results. Ann Limnol. 1996;32:1–5. [Google Scholar]
- 28.Sime-Ngando T. Viruses in aquatic ecosystems. A review. Ann Biol. 1997;36:181–210. [Google Scholar]
- 29.Sokal R R, Rolf F J. Biometry. W. H. New York, N.Y: Freeman and Co.; 1981. [Google Scholar]
- 30.Suttle C A, Chan A M, Cottrell M T. Infection of phytoplankton by viruses and reduction of primary productivity. Nature (London) 1990;347:647–649. [Google Scholar]
- 31.Suttle C A. Enumeration and isolation of virus. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 121–134. [Google Scholar]
- 32.Suttle C A. The significance of virus to mortality in aquatic microbial communities. Microb Ecol. 1994;28:237–243. doi: 10.1007/BF00166813. [DOI] [PubMed] [Google Scholar]
- 33.Turk V, Rehnstam A S, Lundberg E, Hagström A. Release of bacterial DNA by marine nanoflagellates, an intermediate step in phosphorous regeneration. Appl Environ Microbiol. 1992;58:3744–3750. doi: 10.1128/aem.58.11.3744-3750.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinbauer M G, Fuks D, Puskaric S, Peduzzi P. Diel, seasonal and depth-related variability of viruses and dissolved DNA in the northern Adriatic Sea. Microb Ecol. 1995;30:25–41. doi: 10.1007/BF00184511. [DOI] [PubMed] [Google Scholar]
- 35.Weinbauer M G, Suttle C A. Comparison of epifluorescence and transmission electron microscopy for counting viruses in natural marine waters. Aquat Microb Ecol. 1997;13:225–232. [Google Scholar]
- 36.Wells M, Goldberg E D. Occurrence of small colloids in sea water. Nature (London) 1991;353:342–344. [Google Scholar]
- 37.Wilhelm S W, Weinbauer M G, Suttle C A, Pledger R J, Mitchell D L. Measurements of DNA damage and photoreactivation imply that most viruses in marine surface waters are infective. Aquat Microb Ecol. 1998;14:215–222. [Google Scholar]
- 38.Wilhelm S W, Suttle C A. Viruses and nutrient cycles in the sea. BioScience. 1999;49:781–788. [Google Scholar]
- 39.Wommack K E, Hill R T, Kessel M, Russek-Cohen E, Colwell R R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992;31:415–422. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xenopoulos M, Bird D F. Virus à la sauce Yo-Pro: microwave enhanced staining for counting viruses by epifluorescence microscopy. Limnol Oceanogr. 1997;42:1648–1650. [Google Scholar]