Abstract
Multi-strain Limosilactobacillus (L.) fermentum is a potential probiotic with reported immunomodulatory properties. This study aimed to evaluate the composition, richness, and diversity of the gut microbiota in male and female rats after treatment with a multi-strain of L. fermentum at different doses. Thirty rats (fifteen male and fifteen female) were allocated into a control group (CTL), a group receiving L. fermentum at a dose of 108 CFU (Lf-108), and a group receiving L. fermentum at a dose of 1010 CFU (Lf-1010) for 13 weeks. Gut microbiota and serum cytokine levels were evaluated after L. fermentum treatment. Male CTL rats had a lower relative abundance of Bifidobacteriaceae and Prevotella and a lower alpha diversity than their female CTL counterparts (p < 0.05). In addition, male CTL rats had a higher Firmicutes/Bacteroidetes (F/B) ratio than female CTL rats (p < 0.05). In female rats, the administration of L. fermentum at 108 CFU decreased the relative abundance of Bifidobacteriaceae and Anaerobiospirillum and increased Lactobacillus (p < 0.05). In male rats, the administration of L. fermentum at 1010 CFU decreased the F/B ratio and increased Lachnospiraceae and the diversity of the gut microbiota (p < 0.05). The relative abundance of Lachnospiraceae and the alpha-diversity of gut microbiota were negatively correlated with serum levels of IL1β (r = −0.44) and TNFα (r = −0.39), respectively. This study identified important changes in gut microbiota between male and female rats and showed that a lower dose of L. fermentum may have more beneficial effects on gut microbiota in females, while a higher dose may result in more beneficial effects on gut microbiota in male rats.
Keywords: probiotics, Limosilactobacillus, gut microbiota, dose–response, sex differences
1. Introduction
Evidence from clinical and animal studies has shown that host sex influences the gut microbiota [1]. In healthy humans, the relative abundance of Bacteroidetes is typically lower in females than in males [2], whereas the relative abundance of the genus Prevotella is higher in males than in females [3]. In mice, the phyla Actinobacteria and Tenericutes were more abundant in males, while the family Lachnospiraceae was more abundant in females [4]. In addition, previous studies have shown that sex differences are associated with several diseases, including colorectal cancer [5], Parkison’s disease [6], essential hypertension [7], and ischemic stroke [8].
It is recognized that the administration of probiotics in adequate amounts can improve the composition, diversity, and function of the gut microbiota and promote host health benefits [9,10], such as in the treatment of cardiometabolic, cancer, inflammatory, and immune diseases [11,12,13,14]. The appropriate or effective dose of probiotics for overall health, gut microbiota, bowel function, and immune strength is a gap in the research [15]. Doses of around 1 × 109 CFU/day (one billion CFU) have been used in studies to prevent or treat disrupted gut microbiota [16,17,18]. Given the interactions between the host and the microbiome, it has been suggested that gender may be a key aspect that can influence how probiotics may exert their effects on the gut microbiota in a host system [19].
A mixed-fruit-derived Limosilactobacillus fermentum developed by our research group has been reported as safe in a series of in vitro and in vivo experiments [20] and as having broad probiotic properties, such as the normalization of disturbed gut microbiota and antioxidant and anti-inflammatory properties when administered at 1 × 109 CFU/day [21,22,23,24]. However, it is unclear whether mixed L. fermentum can modulate the gut microbiota in a dose- and sex-specific manner. Looking to develop a live biotherapeutic product that overcomes the major inconsistencies across studies with probiotic therapy, such as dose, duration of treatment, and male/female mixed population, the main endpoint of this study was to evaluate the dose- and sex-response of the gut microbiota in Wistar rats after the administration of a multi-strain mixture of L. fermentum 139, 263, and 296.
There is increasing evidence that the commensal gut microbiota can regulate local and systemic inflammation [15]. Therefore, we secondarily analyzed the correlation between inflammatory biomarkers and changes in the gut microbiota induced by probiotic administration to expand the available information and enrich the evidence on gut microbiota and inflammation after probiotic therapy.
2. Materials and Methods
2.1. Animals and Ethical Aspects
Thirty Wistar rats (fifteen male and fifteen female) were housed in polypropylene cages with filtered water and chow (Labina, Purina Aribands) ad libitum throughout the experiment, maintained on a 12 h light–dark cycle, with temperature of 22 ± 2 °C and controlled humidity (55 ± 10%). This study was approved by the Animal Experimentation Ethics Committee of the Federal University of Paraiba (CEUA/UFPB), under number 1871160322, and followed the recommendations of the National Council for the Control of Animal Experimentation (CONCEA, Sao Paulo, Brazil) and the International Principles for Biomedical Research Involving Animals.
2.2. Probiotic Strains and Reparation of Cell Suspension
The strains L. fermentum 139, L. fermentum 263, and L. fermentum 296 were kindly provided by the Laboratory of Microbiology, Department of Nutrition, Federal University of Paraíba (João Pessoa, PB, Brazil). Each strain was cultured anaerobically (Anaerobic System Anaerogen, Oxoid, Hampshire, UK) in Mann, Rogosa, and Sharpe (MRS) broth (Himedia, Mumbai, India) at 37 ± 0.5 °C for 20–24 h. To obtain the cell suspension, the cells were collected by centrifugation (8000× g, 10 min, 4 °C), washed twice with sterile PBS solution, resuspended in PBS solution, and homogenized by vortexing (30 s) to obtain standard cell suspensions with optical density (OD) at 625 nm (OD625) of 1.2 and 2.0, corresponding to viable cell counts of approximately 108 colony-forming units per milliliter (CFU/mL) and 1010 CFU/mL, respectively, when plated on MRS agar (HiMedia, Thane, India). In order to increase the specific strain characteristics and to obtain a multi-strain probiotic, mixed cell suspensions were prepared at a ratio of 1:1:1 (v/v). These doses have been tested to achieve 1 log below and 1 log above a dose widely considered therapeutic (109 CFU).
2.3. Experimental Design
Male and female rats were grouped into (i) control group (CTL, n = 5/sex), (ii) L. fermentum receiving a dose of 108 CFU (Lf-108, n = 5/sex), and (iii) L. fermentum receiving a dose of 1010 CFU (Lf-1010, n = 5/sex). The control group received PBS as a placebo vehicle. Placebo or L. fermentum was administered by oral gavage at a dose of 1 mL for 13 weeks. Then, 24 h after the last dose of L. fermentum, blood samples were collected for cytokine analysis and feces were collected for gut microbiota analysis.
2.4. Measurement of Cytokines
At the end of the experimental test, all animals were euthanized with an overdose of anesthetic. Blood samples were collected and centrifuged to separate serum and plasma. Serum samples were stored at −80 °C in a freezer until the time of cytokine analysis.
Cytokine levels in blood serum samples (TNF-α, IL-1β, IL-6, and IL-10) were determined using the Millipore 7-plex kit (Millipore Corp., Billerica, MA, USA). The assay was performed in a 96-well plate with a filter membrane according to the manufacturer’s instructions. Cytokine concentrations in the samples were estimated from a standard curve using a third-order polynomial equation and expressed in pg/mL. Samples below the limit of detection of the assay were recorded as zero, while samples above the highest quantification limit of the standard curve were assigned to the highest value on the curve. Reading was performed using a microplate reader [21].
2.5. DNA Extraction, 16S rRNA Gene Amplicon Library Preparation, and Sequencing
Fecal samples were collected directly from the animals’ colons and stored in a −80 °C freezer for later analysis. Total DNA was extracted using the QIAmp PowerFceal® DNA Kit, and a region of approximately 426 bp encompassing the V3 and V4 hypervariable regions of the 16S rDNA gene was targeted for the sequencing of each sample.
The bacterial diversity was assessed via the high-throughput sequencing of the 16S rRNA V3/V4 region employing 341F (CCTACGGGRSGCAGCA G) and 806R (GGACTACHVGGGTWTCTAAT) primers. The 16S rRNA libraries were sequenced using the MiSeq Sequencing System (Illumina Inc., San Diego, CA, USA) using the standard Illumina primers provided in the kit, with 300 cycles (paired-end sequencing with 200 bp). After sequencing, quality filters were applied to fastq files, including for the removal of truncated and low-quality reads (Phred score < 20) using the Trimmomatic tool [25]. Then, sense and antisense paired reads were merged into contigs, and the singletons and chimeras were removed. The sequences were grouped into Taxonomic Operational Units (OTUs) using Uchime v. 4.2.40 and Vsearch v 2.22.1 [26,27] (97% identity) and assigned taxonomically considering a 97% similarity alignment against sequences from the SILVA database [28]. All 16 s rRNA Illumina amplicon sequencing data provided in this study can be publicly obtained from the Sequence Read Archive (SRA) of The National Center for Biotechnology Information (NCBI) under the accession number PRJNA1004239.
2.6. Statistical Analysis
Data are presented as mean ± standard deviation. The Shapiro–Wilk test was used to assess the normality of the data. Statistical significance was assessed using a two-way analysis of variance ANOVA test with dose (108 and 1010 CFU) and sex (male and female) as factors. The Bonferroni post hoc test was used. Pearson’s correlation test was used. Statistical analysis was performed using GraphPad Prism® (version 6.01) and significance was maintained at p < 0.05. Data were analyzed with GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Effects of Multi-Strain L. fermentum Administration on Bacterial Phyla Composition in Gut Microbiota
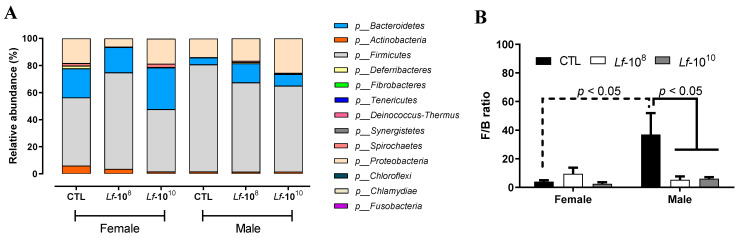
Thirteen phyla were identified by 16S rRNA sequencing (Figure 1A). The most abundant phyla detected were Firmicutes, Bacteroidetes, and Proteobacteria, followed by Actinobacteria (Figure 1A). Female rats of the CTL group had a reduced Firmicutes/Bacteroidetes ratio compared to male CTL rats (p < 0.05, Figure 1B). The administration of L. fermentum at 108 and 1010 CFU doses did not change the Firmicutes/Bacteroidetes ratio in female rats, but significantly reduced the Firmicutes/Bacteroidetes ratio in male rats (p < 0.05, Figure 1B).
Figure 1.
Relative abundance of phylum (A) and Firmicutes/Bacteroides ratio (B) in the gut microbiota of male and female Wistar rats after the administration of Limosilactobacillus fermentum 139, 263, and 296 at different doses for 13 weeks. F/B ratio data are presented as mean ± standard deviation and analyzed using two-way ANOVA. p < 0.05 indicates a significant difference. A dotted line was used to identify significant differences between sex and a solid line was used to identify significant differences in L. fermentum administration. Groups: control group (CTL): PBS (1 mL), L. fermentum receiving a dose of 108 CFU/mL (Lf-108), and L. fermentum receiving a dose of 1010 CFU/mL (Lf-1010).
3.2. Effects of Multi-Strain L. fermentum Administration on Bacterial Family Composition in Gut Microbiota
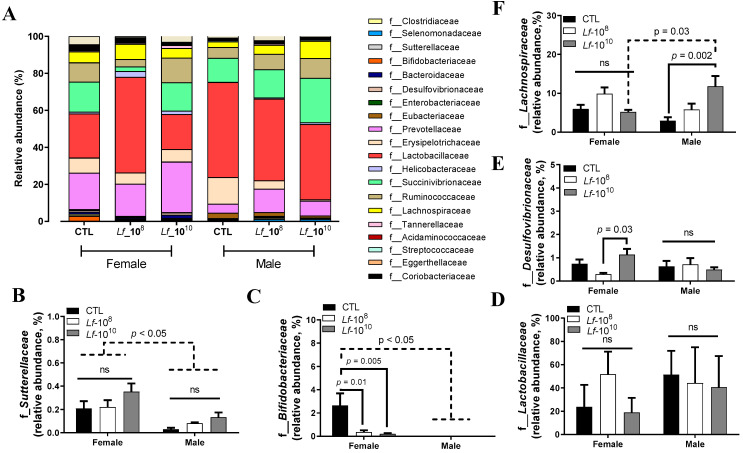
The most abundant phyla detected were Clostridiaceae, Selenomonadaceae, Sutterellaceae, Bifidobacteriaceae, Bacteroidaceae, Desulfovibrionaceae, Enterobacteriaceae, Eubacteriaceae, Prevotellaceae, Erysipelotrichaceae, Lactobacillaceae, Helicobacteraceae, Succinivibrionaceae, Ruminococcaceae, Lachnospiraceae, Tannerellaceae, Acidaminococcaceae, Streptococcaceae, Eggerthellaceae, and Corlobacteriacea (Figure 2A).
Figure 2.
Relative abundance of bacterial families in the gut microbiota of male and female Wistar rats after the administration of Limosilactobacillus fermentum 139, 263, and 296 at different doses for 13 weeks. Evaluation of the most abundant families identified in the gut microbiota of male and female rats (A). Relative abundances of Sutterellaceae (B), Bifidobacteriaceae (C), Lactobacillaceae (D), Desulfovibrionaceae (E), and Lachnospiraceae (F) were analyzed by two-way ANOVA. p < 0.05 indicates a significant difference. A dotted line was used to identify significant difference between sex and a solid line was used to identify significant difference in L. fermentum administration. Groups: control group (CTL): PBS (1 mL), L. fermentum receiving a dose of 108 CFU/mL (Lf-108), and L. fermentum receiving a dose of 1010 CFU/mL (Lf-1010). Not significant: ns.
Female rats of all groups had a higher abundance of Sutterellaceae and Bifidobacteriaceae when compared to male rats (p < 0.05, Figure 2B,C). The administration of L. fermentum at 108 or 1010 CFU did not alter the abundance of Sutterellaceae in male and female rats (p > 0.05, Figure 2B). The administration of L. fermentum at 108 or 1010 CFU reduced the relative abundance of Bifidobacteriaceae in female rats (p < 0.05, Figure 2C), but did not change that of Bifidobacteriaceae in male rats (p > 0.05, Figure 2C). The relative abundance of Lactobacillaceae was similar between female and male rats and the administration of L. fermenutm at 108 or 1010 did not alter the relative abundance of Lactobacillaceae in male and female rats (p > 0.05, Figure 2D). The relative abundance of Desulfovibrionaceae was similar between female and male rats (p > 0.05, Figure 2E). Female rats receiving L. fermentum at 1010 CFU had a higher relative abundance of Desulfovibrionaceae than female rats receiving 108 CFU (p < 0.05, Figure 2E). The administration of L. fermentum at 108 or 1010 CFU did not alter the relative abundance of Desulfovibrionaceae in male rats (p > 0.05, Figure 2E). The administration of L. fermentum at 108 or 1010 CFU did not alter the relative abundance of Lachnospiraceae in female rats (p > 0.05, Figure 2F). Male rats receiving L. fermentum at 1010 CFU had a higher relative abundance of Lachnospiraceae than male CTL rats (p < 0.05, Figure 2F). Male rats receiving L. fermentum at 1010 CFU had a higher relative abundance of Lachnospiraceae than female rats receiving the same dose (p< 0.05, Figure 2F).
3.3. Effects of Multi-Strain L. fermentum Administration on Bacterial Gender Composition in Gut Microbiota
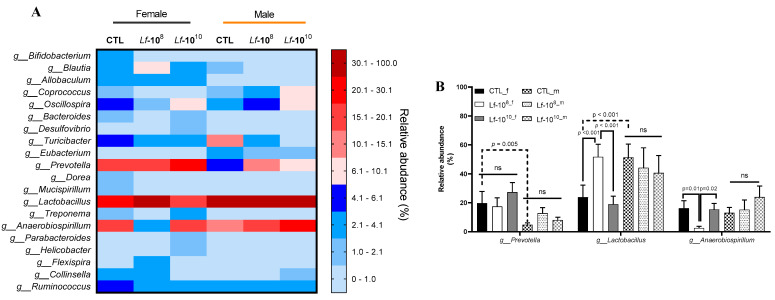
The most abundant genera detected in the gut microbiota are shown in Figure 3A. The genera Lactobacillus, Prevotella, and Anaerobiospirillum showed higher relative abundance in female and male rats (Figure 3A). The female CTL group had a higher relative abundance of Prevotella than the male CTL group (p < 0.05, Figure 3B). The administration of L. fermentum at 108 or 1010 CFU did not alter the relative abundance of Prevotella in female and male rats (p > 0.05, Figure 3B). The female CTL group had a lower relative abundance of Lactobacillus than the male CTL group (p < 0.05, Figure 3B). The administration of L. fermentum at 108 or 1010 CFU did not alter the relative abundance of Lactobacillus in male rats (p > 0.05, Figure 3B). In female rats, the administration of L. fermentum at 108 CFU increased the relative abundance of Lactobacillus when compared to the CTL group. However, the administration of L. fermentum at 1010 CFU promoted deleterious effects on Lactobacillus abundance when compared to female rats receiving L. fermentum at 108 CFU (p < 0.05, Figure 3B). The relative abundance of Anaerobiospirillum was similar between female and male rats (p > 0.05, Figure 3B). The administration of L. fermentum at 108 or 1010 CFU did not alter the relative abundance of Anaerobiospirillum in male rats (p > 0.05, Figure 3B). In female rats, the administration of L. fermentum at 108 CFU decreased the relative abundance of Anaerobiospirillum when compared to the CTL group (p < 0.05, Figure 3B). The administration of L. fermentum at 1010 CFU increased the abundance of Anaerobiospirillum when compared to female rats receiving L. fermentum at 108 CFU (p < 0.05, Figure 3B).
Figure 3.
Heat map of the most abundant bacterial genera in male and female Wistar rats after administration of Limosilactobacillus fermentum 139, 263, and 296 at different doses for 13 weeks. Evaluation of the heat map of the most abundant genera (A) in the gut microbiota of male and female rats. Relative abundances of Prevotella, Lactobacillus, and Anaerobiospirillum (B) were analyzed by two-way ANOVA. p < 0.05 indicates a significant difference. A dotted line was used to identify significant difference between sex and a solid line was used to identify significant difference in L. fermentum administration. Groups: control group (CTL): PBS (1 mL), L. fermentum receiving a dose of 108 CFU/mL (Lf-108), and L. fermentum receiving a dose of 1010 CFU/mL (Lf-1010). not significant (ns).
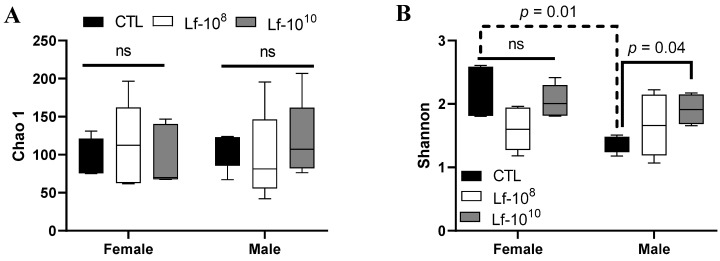
3.4. Effects of Multi-Strain L. fermentum Administration on the Richness and Diversity of the Gut Microbiota
Species richness was estimated using the Chao 1 index and the alpha diversity was assessed using the Shannon index (Figure 4A,B). Species richness was similar between male and female rats, and the administration of L. fementum did not alter the Chao 1 index in either sex (p > 0.05, Figure 4A). Male rats of the CTL group had lower alpha diversity than their counterpart CTL female rats (p < 0.05, Figure 4B). The administration of L. fermentum at 1010 CFU increased alpha diversity in male rats compared to the CTL group (p < 0.05, Figure 4B), but had no effect in female rats (p > 0.05, Figure 4B).
Figure 4.
Richness and alpha diversity of male and female Wistar rats after administration of Limosilactobacillus fermentum 139, 263, and 296 at different doses for 13 weeks. Evaluation of the Chao 1 index (A) and Shannon index (B) in in the gut microbiota of male and female rats. Data were analyzed by two-way ANOVA. P < 0.05 indicates a significant difference. A dotted line was used to identify significant difference between sex and a solid line was used to identify significant difference in L. fermentum administration. Groups: control group (CTL): PBS (1 mL), L. fermentum receiving a dose of 108 CFU/mL (Lf-108), and L. fermentum receiving a dose of 1010 CFU/mL (Lf-1010). not significant (ns).
3.5. Correlation between the Gut Microbiota Parameters of Inflammatory Cytokines
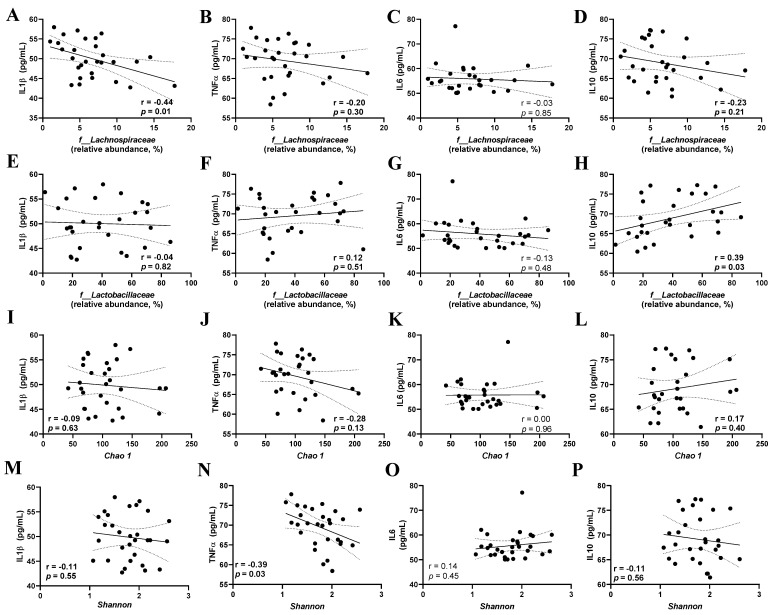
The relative abundance of Lachnospiraceae and Lactobacillaceae and the Chao 1 and Shannon indices were used to assess the correlation between gut microbiota parameters and inflammatory cytokines. The relative abundance of Lachnospiraceae was negatively correlated with IL-1β levels (r = 0.44, p = 0.01, Figure 5A), but not with TNF-α, IL-6, and IL-10 levels (p > 0.05, Figure 5B–D). The relative abundance of Lactobacillaceae was positively correlated with IL-10 levels (r = 0.39, p = 0.03, Figure 5H), but not with IL-1β, TNF-α, and IL-6 levels (p > 0.05, Figure 5E–G). The Chao 1 index did not correlate with IL-1β, TNF-α, IL-6, and IL-10 levels (p > 0.05, Figure 5I–L). The Shannon index was negatively correlated with TNF-α levels (p < 0.05, Figure 5N), but not with IL1β, IL-6, and IL-10 levels (p > 0.05, Figure 5M,O,P).
Figure 5.
Correlation between cytokine serum levels and gut microbiota parameters in male and female Wistar rats after the administration of Limosilactobacillus fermentum 139, 263, and 296 at different doses for 13 weeks. Evaluation of the correlation of Lachnospiraceae (A–D), Lactobacillaceae (E–H), Chao 1 Index (I–L), and Shannon Index (M–P) with serum levels of Interleukin 1 beta (IL1β), Tumor Necrosis Factor alpha (TNF-α), Interleukin-6 (IL–6), and Interleukin-10 (IL–10). Pearson’s correlation test was used, and significant difference was considered when p < 0.05.
4. Discussion
This study showed changes in the gut microbiota composition of male and female Wistar rats after treatment with L. fermentum 139, 263, and 296 at different doses for 13 weeks. The results showed that multi-strain L. fermentum treatment altered the relative abundance of bacteria at the phylum, family, and genus levels. The relative abundance of Lachnospiraceae was negatively associated with serum IL1β levels, while the relative abundance of Lactobacillaceae was positively associated with serum IL-10 levels. In addition, alpha diversity was negatively correlated with TNFα. It has been suggested that the composition of the gut microbiota is sex-dependent [1,29] and may respond differently to probiotic treatment [19,30] and that lactobacilli can alter the population of microorganisms that make up the gut microbiota and control the functioning of the gut microbiota ecosystem [10].
The most abundant bacterial phyla in the healthy gut microbiota are represented by Firmicutes and Bacteroidetes [31]. The Firmicutes/Bacteroidetes ratio has been used as a potential biomarker for obesity and associated disorders [32]. An increased Firmicutes/Bacteroidetes ratio has been reported in several diseases, such as obesity, diabetes mellitus, inflammatory bowel disease, and cardiovascular disease [33,34,35]. On the other hand, a low Firmicutes/Bacteroidetes ratio has been associated with a lean phenotype, younger age, cardiovascular health, and an improved immune system [32]. Treatment with Lf-108 or Lf-1010 decreased the Firmicutes/Bacteroidetes ratio in male rats compared to the CTL group, while no change was observed in female rats. These results suggest that treatment with L. fermentum 139, 263, and 296 can positively modulate the gut microbiota composition at the phyla level.
Lactobacillaceae was the family with the highest relative abundance in the gut microbiota of Wistar rats. The Lactobacillaceae family can be found in different environments, such as the gastrointestinal tract and urinary and genital systems [34]. Although the treatment with L. fermentum 139, 263, and 296 did not alter the relative abundance of Lactobacillaceae in male and female rats, many Lactobacillus species are used as probiotics due to strain-specific properties, such as cholesterol-lowering activity, immunomodulatory effects, and antioxidant properties [36,37,38,39].
Lachnospiraceae is a family of anaerobic bacteria in the Clostridiales order within the phylum Firmicutes, and they are obligate members of the gut microbiota in healthy humans [40]. An increased abundance in short-chain fatty acid (SCFA)-producing bacteria belonging to the Lachnospiraceae family has been reported in subjects fed a high-fiber diet or treated with omega-3 polyunsaturated fatty acids (PUFA), and has been associated with host health benefits [41]. On the other hand, Lachnospiraceae-enriched gut microbiota have been reported in patients with chronic and inflammatory diseases [42]. Treatment with L. fermentum 139, 263, and 296 increased the relative abundance of Lachnospiraceae in male rats receiving Lf-1010 when compared to the CTL group. However, the treatment with L. fermentum 139, 263, and 296 did not change the relative abundance of Lachnospiraceae in female rats. The reason for this is not explained and reinforces the idea that probiotic therapy may have a sex-specific effect on the gut microbiota.
Bifidobacteriaceae are a family of bacteria with fermentative metabolism that inhabit the gastrointestinal tract of humans and animals [43]. A previous meta-analysis suggested that high populations of Bifidobacteriaceae may be involved in the pathogenesis of Parkinson’s disease [44], while a systematic review indicated a higher abundance of Bifidobacteriaceae in individuals with depression [45]. Our results showed a decrease in the relative abundance of Bifidobacteriaceae in male rats when compared with female rats. In addition, the treatment with Lf-108 and Lf-1010 decreased the relative abundance of Bifidobacteriaceae in female rats when compared to their CTL group.
A preclinical study showed that the consumption of ground beef and sucrose stimulated an expansion of the Desulfovibrionaceae family in the colonic microbiome, which was associated with oxidative stress and cardiac hypertrophy [46]. High-fat diet consumption increased the relative abundance of Desulfovibrionaceae in mice [47]. In the present study, the administration of Lf-1010 increased the relative abundance of the Desulfovibrionaceae family in female rats compared to the dose of 108 CFU/mL and the CTL group, although no difference was found when compared to male rats.
It has been shown that the relative abundance of Sutterellaceae was increased in fecal samples from patients with irritable bowel syndrome [48]. In the present study, the relative abundance of Sutterellaceae was lower in male rats when compared with female rats. Further studies may be conducted to determine whether females have a higher risk of developing irritable bowel syndrome. The administration of L. fermentum did not alter the relative abundance of Sutterellaceae in either sex. It has been demonstrated that oats, a soluble fiber used as a prebiotic, decreased the relative abundance of Sutterellaceae in a Chinese population with mild hypercholesterolemia [49]. In addition, the abundance of Sutterellaceae was negatively correlated with quercetin concentration in healthy elderly humans [50]. Our research group has developed a novel nutraceutical product containing prebiotics, polyphenols, and L. fermentum strains [51,52], and further studies will be conducted to understand their effects on gut microbiota composition in health and disease.
The composition of the gut microbiota was also assessed at the genus level. In the present study, the genera with the highest relative abundance were Lactobacillus, Prevotella, and Anaerobiospirillum. The genus Anaerobiospirillum is understudied in the literature. An early study showed Anaerobiospirillum succiniproducens-induced bacteremia in a healthy man [53]. Here, we have shown that the administration of L. fermentum at 108 CFU decreased the relative abundance of Anaerobiospirillum in female rats, but such an effect was absent when 1010 CFU of L. fermentum was administered.
Increased Lactobacillus counts in the feces of rats treated with L. fermentum strains have previously been documented [23,39]. This study showed that females have a lower relative abundance of Lactobacillus than males, and the administration of L. fermentum at 108 CFU may be more beneficial to Lactobacillus abundance in females than 1010 CFU. In males, L. fermentum treatment did not alter the relative abundance of Lactobacillus. The benefits of Lactobacillus when used as a probiotic have been associated with improvements in metabolic, immunological, and cardiovascular parameters and may be a promising alternative for the management of inflammatory bowel diseases and cardiometabolic disorders [54,55,56].
Prevotella was one of the genera with the highest increase due to our treatment with L. fermentum. Females have a higher relative abundance of Prevotella than males, and the administration of L. fermentum did not alter Prevotella abundance in either sex. This genus belongs to the family Prevotellaceae, and compared to other genera, Prevotella has received less attention [57]. Prevotella species can have different characteristics between and within species, but their functions and host relationships are still unclear [58]. Although the abundance of this bacterial genus is evident in the healthy microbiota, studies have suggested that some members may be associated with diseases, including bacterial vaginosis and inflammatory autoimmune diseases, but the direct causes are still uncertain [59,60]. There are conflicting reports in the literature regarding the effects of the genus Prevotella on glucose homeostasis [57,61]. A previous study using germ-free mice transplanted with microbiota from human donors and subjected to the consumption of barley-based bread showed an improvement in glucose metabolism associated with greater Prevotella abundance, possibly related to increased hepatic glycogen storage [62].
Probiotics are generally effective at oral doses greater than 106 CFU, but the most commonly used doses in experiments are 108 to 1010 CFU/mL [63]. Regarding dose–response, it was demonstrated in female rats that treatment with L. fermentum 139, 263, and 296 promoted a greater relative abundance of the Lactobacillus genus in the Lf-108 group compared to the Lf-1010 groups. A previous study found that the dose–response effects of Bifidobacterium infantilum 35624 were effective in reducing irritable bowel syndrome in adult women at the 108 CFU/day dose, with no significant difference between the 106 and 1010 CFU/day doses and placebo [64]. The combination of these results suggests that identifying an appropriate dose is very important for probiotic therapy and that further studies should be conducted to evaluate the dose–response effects of potential probiotics.
Another critical parameter evaluated in this study was the effect of the administration of L. fermentum 139, 263, and 296 on the richness and alpha diversity of the gut microbiota in male and female rats. Increased microbial diversity has been associated with improved microbiota stability, with implications for host health benefits [65]. The study showed that although richness was similar between male and female rats, alpha diversity was higher in female rats than in male rats. The administration of L. fermentum did not change the species richness in either sex. Treatment with the higher dose of L. fermentum 139, 263, and 296 increased the alpha diversity of the gut microbiota in male rats. A previous study showed that the administration of L. fermentum did not increase alpha diversity in rats fed a diet high in fat and cholesterol [55]. Reduced gut microbiota diversity may be associated with clinical conditions such as obesity and inflammatory bowel disease [66,67]. These findings suggest that the response of gut microbiota diversity to probiotic treatment may vary depending on the health status of the host.
Physiologically, the gut microbiota is directly linked to the immune system in maintaining homeostasis in the host gut [68]. In addition, it has been suggested that the consumption of specific probiotic strains modulates the immune response via the innate and adaptive immune systems, the regulation of intestinal epithelial permeability, mucus secretion, and competition within the bacterial ecosystem via the secretion of antimicrobial compounds [69]. However, the mechanistic effects of L. fermentum 139, 263, and 296 on the host immune system remain to be elucidated. This study showed that the relative abundance of Lachnospiraceae was negatively correlated with IL-1β levels, the relative abundance of Lactobacillaceae was positively correlated with IL-10 levels, and the alpha diversity of the gut microbiota was negatively correlated with TNF-α levels.
The health-promoting effects of members of the Lachnospiraceae and Lactobacillaceae families have been described in the literature, including the production of SCFA, the conversion of primary bile acids into secondary bile acids, and the protection of the intestinal barrier by resisting the colonization of drug-resistant pathogens [40,42,70]. Another characteristic associated with members of these families is the modulation of the immune system [71]. The relationship between Lachnospiraceae and the immune system has previously been described, showing that colonization with Lachnospiraceae in mdr2 −/− mice pre-treated with antibiotics caused a reduction in liver fibrosis, inflammation, and pathobiont translocation, which could be mediated by Lachnospiraceae metabolites, such as SCFA [72].
The positive correlation between the relative abundance of Lactobacillaceae and IL-10 levels suggests that supplementation with these potentially probiotic strains may be associated with a beneficial modulation of the immune response. Species of the Lactobacillaceae family have been used as probiotics to improve human or animal health [15], including for anti-inflammatory properties [73]. The effects of probiotics on the immune system are mainly explained by their ability to increase SCFA production [74]. Among the SCFA, butyrate has typically been associated with these anti-inflammatory effects, in addition to providing energy to colonic epithelial cells and regulating the expression of intestinal barrier junction proteins [75].
The main limitation of this study is the lack of hormonal parameters that could be used to explain some changes related to probiotic therapy in male and female rats. We also pointed out as a limitation of the study that the environment in which the animals were housed did not correspond to the Specific Pathogen Free (SPF) barrier environment.
This study identified important changes in gut microbiota between male and female rats and showed that a lower dose of L. fermentum may have more beneficial effects on the gut microbiota in females, while a higher dose may result in more beneficial effects on the gut microbiota in males. The study showed that a higher relative abundance of Lachnospiraceae and gut microbiota diversity was negatively correlated with pro-inflammatory cytokines, while a higher relative abundance of Lactobacillaceae was positively correlated with serum levels of anti-inflammatory cytokines. Despite the evidence indicating these strains as novel candidates for probiotic use, there is a need to confirm their health benefits through a translational approach by developing randomized, double-blind, placebo-controlled trials to investigate their health-promoting effects in humans [76].
Author Contributions
Conceptualization: J.L.d.B.A. Data curation: L.A.C.d.S., J.L.d.B.A., and J.P.R.C.N. Formal analysis: J.L.d.B.A. and R.D.d.O.C. Funding acquisition: E.L.d.S. and J.L.d.B.A.; investigation: L.A.C.d.S., R.D.d.O.C., K.Á.R.d.O., K.B.S., M.O.d.L.F., and J.L.d.B.A. methodology: L.A.C.d.S., R.D.d.O.C., K.B.S., E.L.d.S., and J.L.d.B.A. project administration: J.L.d.B.A. Supervision: J.L.d.B.A. Validation: L.A.C.d.S. and J.L.d.B.A. Visualization, writing—original draft: L.A.C.d.S., J.P.R.C.N., DEAL, M.O.d.L.F., and J.L.d.B.A. Writing—review and editing: R.D.d.O.C., K.Á.R.d.O., F.F.A., D.E.d.A.L., K.B.S., V.A.d.C.A., E.L.d.S., and J.L.d.B.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no competing interests.
Funding Statement
This study was funded by CNPq (Brazil) (Grant 404353/2021–5) and CAPES (Brazil) (Finance code 001).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kim Y.S., Unno T., Kim B.Y., Park M.S. Sex Differences in Gut Microbiota. World J. Men’s Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominianni C., Sinha R., Goedert J.J., Pei Z., Yang L., Hayes R.B., Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE. 2015;10:e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding T., Schloss P.D. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Org E., Mehrabian M., Parks B.W., Shipkova P., Liu X., Drake T.A., Lusis J.L. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Li P., Qu Z., Zhuang J., Wu Y., Wu W., Wei Q. Gut bacteria and sex differences in colorectal cancer. J. Med. Microbiol. 2023;72:001706. doi: 10.1099/jmm.0.001706. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz G.B., Kayacan Z.C., Karacan I., Sumbul B., Elibol B., Gelisin O., Akgul O. Altered gut microbiota in patients with idiopathic Parkinson’s disease: An age-sex matched case-control study. Acta Neurol. Belg. 2023;123:999–1009. doi: 10.1007/s13760-023-02195-0. [DOI] [PubMed] [Google Scholar]
- 7.Virwani P.D., Qian G., Hsu M.S.S., Pijarnvanit T.K.K.T.S., Cheung C.N.-M., Chow Y.H., Tang L.K., Tse Y.-H., Xian J.-W., Lam S.S.-W., et al. Sex Differences in Association Between Gut Microbiome and Essential Hypertension Based on Ambulatory Blood Pressure Monitoring. Hypertension. 2023;80:1331–1342. doi: 10.1161/HYPERTENSIONAHA.122.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lledós M., Prats-Sánchez L., Llucià-Carol L., Cárcel-Márquez J., Muiño E., Cullell N., Gallego-Fabrega C., Martín-Campos J.M., Aguilera-Simón A., Guasch-Jiménez M., et al. Ischaemic stroke patients present sex differences in gut microbiota. Eur. J. Neurol. 2023;30:3497–3506. doi: 10.1111/ene.15931. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad M.A., Sarker M., Li T., Yin J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018;2018:9478630. doi: 10.1155/2018/9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J.S., Choi J.W., Jhun J., Kwon J.Y., Lee B.I., Yang C.W., Park S.H., Cho M.L. Lactobacillus acidophilus Improves Intestinal Inflammation in an Acute Colitis Mouse Model by Regulation of Th17 and Treg Cell Balance and Fibrosis Development. J. Med. Food. 2018;21:215–224. doi: 10.1089/jmf.2017.3990. [DOI] [PubMed] [Google Scholar]
- 12.Barteneva N.S., Baiken Y., Fasler-Kan E., Alibek K., Wang S., Maltsev N., Ponomarev E.D., Sautbayeva Z., Kauanova S., Moore A., et al. Extracellular vesicles in gastrointestinal cancer in conjunction with microbiota: On the border of Kingdoms. Biochimica et biophysica acta. Rev. Cancer. 2017;1868:372–393. doi: 10.1016/j.bbcan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Saez-Lara M.J., Gomez-Llorente C., Plaza-Diaz J., Gil A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015;2015:505878. doi: 10.1155/2015/505878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Visitación N., Robles-Vera I., Toral M., Duarte J. Protective Effects of Probiotic Consumption in Cardiovascular Disease in Systemic Lupus Erythematosus. Nutrients. 2019;11:2676. doi: 10.3390/nu11112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 16.Barkhidarian B., Roldos L., Iskandar M.M., Saedisomeolia A., Kubow S. Probiotic Supplementation and Micronutrient Status in Healthy Subjects: A Systematic Review of Clinical Trials. Nutrients. 2021;13:3001. doi: 10.3390/nu13093001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Moreno A., Suárez A., Avanzi C., Monteoliva-Sánchez M., Aguilera M. Probiotic Strains and Intervention Total Doses for Modulating Obesity-Related Microbiota Dysbiosis: A Systematic Review and Meta-analysis. Nutrients. 2020;12:1921. doi: 10.3390/nu12071921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva Costa N., de Araujo J.R., da Silva Melo M.F., da Costa Mota J., Almeida P.P., Coutinho-Wolino K.S., Da Cruz B.O., Brito M.L., de Souza Carvalho T., Barreto-Reis E., et al. Effects of Probiotic-Enriched Minas Cheese (Lactobacillus acidophilus La-05) on Cardiovascular Parameters in 5/6 Nephrectomized Rats. Probiotics Antimicrob. Proteins. 2023 doi: 10.1007/s12602-023-10173-4. [DOI] [PubMed] [Google Scholar]
- 19.Snigdha S., Ha K., Tsai P., Dinan T.G., Bartos JD Shahid M. Probiotics: Potential novel therapeutics for microbiota-gut-brain axis dysfunction across gender and lifespan. Pharmacol. Ther. 2022;231:107978. doi: 10.1016/j.pharmthera.2021.107978. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Neto JP R., de Oliveira A.M., de Oliveira K.Á.R., Sampaio K.B., da Veiga Dutra M.L., de Luna Freire M.O., de Souza E.L., de Brito Alves J.L. Safety Evaluation of a Novel Potentially Probiotic Limosilactobacillus fermentum in Rats. Probiotics Antimicrob. Proteins. 2023 doi: 10.1007/s12602-023-10077-3. [DOI] [PubMed] [Google Scholar]
- 21.De Luna Freire M.O., do Nascimento L.C., De Oliveira K.Á., de Oliveira A.M., dos Santos Lima M., Napoleão T.H., da Costa Silva J.H., Lagranha C.J., De Souza E.L., de Brito Alves J.L. Limosilactobacillus fermentum strains with claimed probiotic properties exert anti-oxidant and anti-inflammatory properties and prevent cardiometabolic disorder in female rats fed a high-fat diet. Probiotics Antimicrob. Proteins. 2021;15:601–613. doi: 10.1007/s12602-021-09878-1. [DOI] [PubMed] [Google Scholar]
- 22.De Luna Freire M.O., do Nascimento L.C.P., de Oliveira K.Á.R., de Oliveira A.M., Napoleão T.H., Lima MD S., Lagranha C.J., de Souza E.L., de Brito Alves J.L. Effects of a Mixed Limosilactobacillus fermentum Formulation with Claimed Probiotic Properties on Cardiometabolic Variables, Biomarkers of Inflammation and Oxidative Stress in Male Rats Fed a High-Fat Diet. Foods. 2021;10:2202. doi: 10.3390/foods10092202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Oliveira Y., Cavalcante RG S., Cavalcanti Neto M.P., Magnani M., Braga V.A., de Souza E.L., de Brito Alves J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020;11:5581–5594. doi: 10.1039/D0FO00514B. [DOI] [PubMed] [Google Scholar]
- 24.De Albuquerque TM R., Garcia E.F., de Oliveira Araújo A., Magnani M., Saarela M., de Souza E.L. In Vitro Characterization of Lactobacillus Strains Isolated from Fruit Processing By-Products as Potential Probiotics. Probiotics Antimicrob. Proteins. 2018;10:704–716. doi: 10.1007/s12602-017-9318-2. [DOI] [PubMed] [Google Scholar]
- 25.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina Sequence Data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rognes T., Flouri T., Nichols B., Quince C., Mahé F. VSEARCH: A versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Opens external link in new window. Nucl. Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valeri F., Endres K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocrinol. 2021;61:100912. doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.Y., Kim N., Nam R.H., Sohn S.H., Lee S.M., Choi D., Yoon H., Kim Y.S., Lee H.S., Lee D.H. Probiotics reduce repeated water avoidance stress-induced colonic microinflammation in Wistar rats in a sex-specific manner. PLoS ONE. 2017;12:e0188992. doi: 10.1371/journal.pone.0188992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomaa E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 32.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai H.J., Tsai W.C., Hung W.C., Hung W.W., Chang C.C., Dai C.Y., Tsai Y.C. Gut Microbiota and Subclinical Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus. Nutrients. 2021;13:2679. doi: 10.3390/nu13082679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodiño-Janeiro B.K., Vicario M., Alonso-Cotoner C., Pascua-García R., Santos J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv. Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathur R., Barlow G.M. Obesity and the microbiome. Expert Rev. Gastroenterol. Hepatol. 2015;9:1087–1099. doi: 10.1586/17474124.2015.1051029. [DOI] [PubMed] [Google Scholar]
- 36.Paulino do Nascimento L.C., Lacerda D.C., Ferreira DJ S., de Souza E.L., de Brito Alves J.L. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob. Proteins. 2022;14:960–979. doi: 10.1007/s12602-022-09943-3. [DOI] [PubMed] [Google Scholar]
- 37.Frappier M., Auclair J., Bouasker S., Gunaratnam S., Diarra C., Millette M. Screening and Characterization of Some Lactobacillaceae for Detection of Cholesterol-Lowering Activities. Probiotics Antimicrob. Proteins. 2022;14:873–883. doi: 10.1007/s12602-022-09959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archer A.C., Muthukumar S.P., Halami P.M. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High Fat Diet Fed and Streptozotocin Induced Diabetic Rats. Probiotics Antimicrob Proteins. 2023;15:1078. doi: 10.1007/s12602-023-10122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalcante RG S., de Albuquerque TM R., de Luna Freire M.O., Ferreira GA H., Carneiro Dos Santos L.A., Magnani M., Cruz J.C., Braga V.A., de Souza E.L., de Brito Alves J.L. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr. Metab. Cardiovasc. Dis. NMCD. 2019;29:1408–1417. doi: 10.1016/j.numecd.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Sorbara M.T., Littmann E.R., Fontana E., Moody T.U., Kohout C.E., Gjonbalaj M., Eaton V., Seok R., Leiner I.M., Pamer E.G. Functional and Genomic Variation between Human-Derived Isolates of Lachnospiraceae Reveals Inter- and Intra-Species Diversity. Cell Host Microbe. 2020;28:134–146.e4. doi: 10.1016/j.chom.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes. 2021;13:1897212. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lugli G.A., Milani C., Turroni F., Duranti S., Mancabelli L., Mangifesta M., Ferrario C., Modesto M., Mattarelli P., Jiří K., et al. Comparative genomic and phylogenomic analyses of the Bifidobacteriaceae family. BMC Genom. 2017;18:568. doi: 10.1186/s12864-017-3955-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo S., Wang H., Zhao Q., Tang J., Wang M., Zhang Y., Sang M., Tian J., Wang P. High levels of Bifidobacteriaceae are associated with the pathogenesis of Parkinson’s disease. Front. Integr. Neurosci. 2023;16:1054627. doi: 10.3389/fnint.2022.1054627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barandouzi Z.A., Starkweather A.R., Henderson W.A., Gyamfi A., Cong X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry. 2020;11:541. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Hecke T., De Vrieze J., Boon N., De Vos W.H., Vossen E., De Smet S. Combined Consumption of Beef-Based Cooked Mince and Sucrose Stimulates Oxidative Stress, Cardiac Hypertrophy, and Colonic Outgrowth of Desulfovibrionaceae in Rats. Mol. Nutr. Food Res. 2019;63:e1800962. doi: 10.1002/mnfr.201800962. [DOI] [PubMed] [Google Scholar]
- 47.Tachon S., Lee B., Marco M.L. Diet alters probiotic Lactobacillus persistence and function in the intestine. Environ. Microbiol. 2014;16:2915–2926. doi: 10.1111/1462-2920.12297. [DOI] [PubMed] [Google Scholar]
- 48.Lee S.M., Kim N., Yoon H., Kim Y.S., Choi S.I., Park J.H., Lee D.H. Compositional and Functional Changes in the Gut Microbiota in Irritable Bowel Syndrome Patients. Gut Liver. 2021;15:253–261. doi: 10.5009/gnl19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu D., Feng M., Chu Y., Wang S., Shete V., Tuohy K.M., Liu F., Zhou X., Kamil A., Pan D., et al. The Prebiotic Effects of Oats on Blood Lipids, Gut Microbiota, and Short-Chain Fatty Acids in Mildly Hypercholesterolemic Subjects Compared With Rice: A Randomized, Controlled Trial. Front. Immunol. 2021;12:787797. doi: 10.3389/fimmu.2021.787797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamura M., Hoshi C., Kobori M., Takahashi S., Tomita J., Nishimura M., Nishihira J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE. 2017;12:e0188271. doi: 10.1371/journal.pone.0188271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampaio K.B., de Brito Alves J.L., Mangueira do Nascimento Y., Fechine Tavares J., Sobral da Silva M., Dos Santos Nascimento D., Dos Santos Lima M., Priscila de Araújo Rodrigues N., Fernandes Garcia E., Leite de Souza E. Nutraceutical formulations combining Limosilactobacillus fermentum, quercetin, and or resveratrol with beneficial impacts on the abundance of intestinal bacterial populations, metabolite production, and antioxidant capacity during colonic fermentation. Food Res. Int. 2022;161:111800. doi: 10.1016/j.foodres.2022.111800. [DOI] [PubMed] [Google Scholar]
- 52.Sampaio K.B., de Brito Alves J.L., do Nascimento Y.M., Tavares J.F., da Silva M.S., Dos Santos Nascimento D., de Araújo Rodrigues N.P., Monteiro M.C., Garcia E.F., de Souza E.L. Effects of Simulated Gastrointestinal Conditions on Combined Potentially Probiotic Limosilactobacillus fermentum 296, Quercetin, and/or Resveratrol as Bioactive Components of Novel Nutraceuticals. Probiotics Antimicrob. Proteins. 2023;16:308–319. doi: 10.1007/s12602-023-10046-w. [DOI] [PubMed] [Google Scholar]
- 53.Inokuchi R., Ishida T., Maeda J., Nakajima S., Yahagi N., Matsumoto A. Anaerobiospirillum succiniciproducens-induced bacteremia in a healthy man. Am. J. Emerg. Med. 2014;32:812.e1–812.e8123. doi: 10.1016/j.ajem.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 54.Kong C., Gao R., Yan X., Huang L., Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira GA H., Magnani M., Cabral L., Brandão L.R., Noronha M.F., de Campos Cruz J., de Souza E.L., de Brito Alves J.L. Potentially Probiotic Limosilactobacillus fermentum Fruit-Derived Strains Alleviate Cardiometabolic Disorders and Gut Microbiota Impairment in Male Rats Fed a High-Fat Diet. Probiotics Antimicrob. Proteins. 2022;14:349–359. doi: 10.1007/s12602-021-09889-y. [DOI] [PubMed] [Google Scholar]
- 56.Lacerda D.C., Trindade da Costa P.C., Pontes P.B., Santos LA C., Cruz Neto JP R., Silva-Luis C.C., Sousa Brito V.P., de Brito Alves J.L. Potential role of Limosilactobacillus fermentum as a probiotic with anti-diabetic properties: A review. World J. Diabetes. 2022;13:717–728. doi: 10.4239/wjd.v13.i9.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tett A., Pasolli E., Masetti G., Ercolini D., Segata N. Prevotella diversity, niches and interactions with the human host. Nature reviews. Microbiology. 2021;19:585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Si J., You H.J., Yu J., Sung J., Ko G. Prevotella as a Hub for Vaginal Microbiota under the Influence of Host Genetics and Their Association with Obesity. Cell Host Microbe. 2017;21:97–105. doi: 10.1016/j.chom.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Ley R.E. Gut microbiota in 2015: Prevotella in the gut: Choose carefully. Nature reviews. Gastroenterol. Hepatol. 2016;13:69–70. doi: 10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]
- 62.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Ouwehand A.C. A review of dose-responses of probiotics in human studies. Benef. Microbes. 2017;8:143–151. doi: 10.3920/BM2016.0140. [DOI] [PubMed] [Google Scholar]
- 64.Whorwell P.J., Altringer L., Morel J., Bond Y., Charbonneau D., O’Mahony L., Kiely B., Shanahan F., Quigley E.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006;101:1581–1590. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 65.Elderman M., Hugenholtz F., Belzer C., Boekschoten M., van Beek A., de Haan B., Savelkoul H., de Vos P., Faas M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018;9:26. doi: 10.1186/s13293-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norman J.M., Handley S.A., Baldridge M.T., Droit L., Liu C.Y., Keller B.C., Kambal A., Monaco C.L., Zhao G., Fleshner P., et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sergeev I.N., Aljutaily T., Walton G., Huarte E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients. 2020;12:222. doi: 10.3390/nu12010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.La Fata G., Weber P., Mohajeri M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins. 2018;10:11–21. doi: 10.1007/s12602-017-9322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng H., Larson K.J., Cheng W.H., Bukowski M.R., Safratowich B.D., Liu Z., Hakkak R. Advanced liver steatosis accompanies an increase in hepatic inflammation, colonic, secondary bile acids and Lactobacillaceae/Lachnospiraceae bacteria in C57BL/6 mice fed a high-fat diet. J. Nutr. Biochem. 2020;78:108336. doi: 10.1016/j.jnutbio.2019.108336. [DOI] [PubMed] [Google Scholar]
- 71.Huynh U., Zastrow M.L. Metallobiology of Lactobacillaceae in the gut microbiome. J. Inorg. Biochem. 2023;238:112023. doi: 10.1016/j.jinorgbio.2022.112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Awoniyi M., Wang J., Ngo B., Meadows V., Tam J., Viswanathan A., Lai Y., Montgomery S., Farmer M., Kummen M., et al. Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC. Gut. 2023;72:671–685. doi: 10.1136/gutjnl-2021-326500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han S.K., Shin Y.J., Lee D.Y., Kim K.M., Yang S.J., Kim D.S., Choi J.W., Lee S., Kim D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021;21:146. doi: 10.1186/s12866-021-02192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fusco W., Lorenzo M.B., Cintoni M., Porcari S., Rinninella E., Kaitsas F., Lener E., Mele M.C., Gasbarrini A., Collado M.C., et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients. 2023;15:2211. doi: 10.3390/nu15092211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J., Li M., Chen Q., Li X., Chen L., Dong Z., Zhu W., Yang Y., Liu Z., Chen Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022;13:3432. doi: 10.1038/s41467-022-31171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Luna Freire M.O., Cruz-Neto JP R., Albuquerque Lemos D.E., Albuquerque TM R., Garcia E.F., Souza E.L., de Brito Alves J.L. Limosilactobacillus fermentum Strains as Novel Probiotic Candidates to Promote Host Health Benefits and Development of Biotherapeutics: A Comprehensive Review. Probiotics Antimicrob. Proteins. 2024 doi: 10.1007/s12602-024-10235-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.