Abstract
Nonalcoholic fatty liver disease (NAFLD) is the liver component of a cluster of conditions, while its subtype, nonalcoholic steatohepatitis (NASH), emerges as a potentially progressive liver disorder that harbors the risk of evolving into cirrhosis and culminating in hepatocellular carcinoma (HCC). NASH and cardiovascular disease (CVD) have common risk factors, but compared to liver-related causes, the most common cause of death in NASH patients is CVD. Within the pharmacological armamentarium, statins, celebrated for their lipid-modulating prowess, have now garnered attention for their expansive therapeutic potential in NASH. Evidence from a plethora of studies suggests that statins not only manifest anti-inflammatory and antifibrotic properties but also impart a multifaceted beneficial impact on hepatic health. In this review, we used “statin”, “NAFLD”, “NASH”, and “CVD” as the major keywords and conducted a literature search using the PubMed and Web of Science databases to determine the safety and efficacy of statins in patients and animals with NASH and NAFLD, and the mechanism of statin therapy for NASH. Simultaneously, we reviewed the important role of the intestinal microbiota in statin therapy for NASH, as it is hoped that statins will provide new insights into modulating the harmful inflammatory microbiota in the gut and reducing systemic inflammation in NASH patients.
Keywords: nonalcoholic steatohepatitis (NASH), statins, cardiovascular risk, drug safety, molecular mechanism
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) has emerged as the pre-eminent chronic hepatic disorder and is now the most rapidly escalating cause of liver-related mortality worldwide. NAFLD affects a quarter of the global adult populace, as the prevalence of NAFLD has soared from 25.3% in the span of 1990–2006 to an alarming 38.0% in the period of 2016–2019, and is expected to be the leading cause of liver transplantation by 2030. Within this cohort, about one-third of patients are grappling with nonalcoholic steatohepatitis (NASH). This condition will impose a substantial fiscal impact on healthcare systems [1,2,3]. Currently, in terms of the progress of research in the field of NASH, Resmetirom has been formally approved by the FDA for the treatment of adults with noncirrhotic NASH with moderate to advanced liver scarring (fibrosis). Although encouraging, the results raise important questions, such as the uncertainty about the overall risks and benefits of Resmetirom [4].
NAFLD is the hepatic manifestation of the metabolic syndrome, delineated by the accrual of hepatic lipids in the absence of excessive alcohol consumption [5]. It is a dynamic and progressive phenomenon that commences with the augmented accretion of fatty acids and triglycerides (>5%) in hepatocytes, leading to hepatic inflammation and culminating in hepatic injury [6]. NASH is a pathologic entity involving hepatocyte ballooning and lobular inflammation [7]. Once hepatic inflammation is triggered in NASH, it is perpetuated by several vicious cycles that occur both outside and inside the hepatic injury, which eventually result in cirrhosis and hepatocellular carcinoma (HCC) [8]. In addition to liver disease and mortality, NAFLD is associated with cardiovascular disease (CVD), metabolic syndrome, type 2 diabetes, and malignant tumors [2]. Therefore, not only is the deleterious impact of NAFLD related to the progression of hepatic deterioration but also heightens the independent risk of the development of atherosclerosis and other CVD-related morbidities [9]. Increased de novo lipogenesis (DNL) promotes liver fat accumulation in NAFLD, contributing to the development of a proatherosclerotic lipid profile and atherosclerotic cardiovascular disease [10]. Interestingly, certain novel NASH drugs, which promote weight reduction and ameliorate dyslipidemia and insulin resistance, may improve long-term clinical outcomes beyond their hepatic benefits by providing extended cardioprotective benefits [11].
There has been a notable rise in the administration of statins to individuals suffering from cardiovascular disease over the past few decades. Statins act by decreasing the cellular cholesterol content by selectively impeding the enzyme 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, curtailing cholesterol biosynthesis and diminishing hepatic cholesterol concentrations [12]. Excessive cholesterol accumulation in the liver or bloodstream can precipitate pathological conditions such as hepatic steatosis and atherosclerosis. Disrupted hepatic cholesterol equilibrium and the aggregation of free cholesterol are intricately associated with the pathogenesis of NASH/NAFLD [13,14,15]. Consequently, impaired hepatic cholesterol homeostasis may represent a common pathogenesis of both NAFLD and atherosclerosis [16]. The lipid-lowering capabilities of statins hold promise for conferring beneficial effects in NAFLD.
In recent years, the intestinal microbiota has emerged as a hot topic of research. The gut–liver axis refers to the bidirectional relationship between the gut and its resident microbiota, involving the transport of gut-derived metabolites via the portal vein to the liver, while bile and antibody secretion from the liver provide feedback to the intestine. A high-fat diet alters the microbiome, which subsequently compromises the integrity of both the intestinal barrier and the gut vascular barrier, and it facilitates the portal influx of bacterial products, exacerbating non-hepatic inflammation and metabolic abnormalities [17]. Moreover, the intestinal microbiota presents potential as a predictive tool for individual responses to statin therapy. Dietary interventions inclusive of probiotics may enhance the efficacy of statins in managing hyperlipidemia, while concurrently mitigating adverse effects such as myopathy [18]. It is worth noting that concurrent administration of antibiotics with lovastatin may attenuate the systemic bioavailability of lovastatin’s active metabolites, thereby diminishing its therapeutic efficacy [19].
In this review, we outline the role of statins in the management of NASH and assess their safety. The mechanism of statins in the treatment of NASH has been demonstrated through Kupffer cells (KCs), hepatic stellate cells (HSCs), liver sinusoidal endothelial cells (LSECs), Paraoxonase 1 (PON1), small guanine triphosphate binding proteins (GTPases), Peroxisome proliferator-activated receptors (PPARs), AMP-activated protein kinase (AMPK) and ferroptosis. We next summarize the most recent advancements in understanding the modulation of NASH and CVD pathogenesis by statins via the intestinal microbiota, which may provide new potential therapeutic targets for NASH.
2. Hypolipidemic and Pleiotropy of Statins
2.1. Hypolipidemic
Statins are used to treat lipid disorders caused by elevated cholesterol in the world and are a hallmark of liposuction therapy. As reductase inhibitors of HMG-CoA, statins curtail cholesterol formation by targeting and impeding a key step in the biosynthesis of isoprenoids and sterols, effectively lowering the serum cholesterol levels, which significantly reduces the morbidity and mortality of CVD [20]. One of the main mechanisms by which statins exert their therapeutic effect is promoting lipoprotein clearance, primarily by upregulating low-density lipoprotein receptor (LDL-R) expression without affecting very low-density lipoprotein (VLDL) synthesis [21], and reducing the content of low-density lipoprotein cholesterol (LDL-C) and the risk associated with CVD [22]. This multifaceted approach places statins at the forefront of interventions combating lipid-related CVD ailments. Research has shown a link between the impact of statins on reducing LDL-C levels and the duration of drug usage. In the case of short-acting statins, there was a notable increase in LDL-C and total cholesterol (TC) reduction during the evening dose when compared to the morning [23]. In addition to lowering LDL-C, statins exhibit a broader metabolic influence by reducing the fasting blood cholesterol and triglyceride (TG) concentrations. Notably, prolonged statin therapy moderates the postprandial TG elevation typically induced by lipid-rich meals [24]. Statins can raise the high-density lipoprotein cholesterol (HDL-C) levels, and cause low levels of HDL-C that are associated with an increased risk of coronary heart disease (CHD) events [25]. Such findings accentuate the relationship between statin administration and lipid profile optimization, supporting their indispensable status in contemporary cardiovascular therapeutics.
2.2. Pleiotropy
Statins are increasingly recognized for their multifaceted roles, which extend beyond their primary function of lowering LDL. This breadth of action, known as ‘statin pleiotropy’, has catalyzed a burgeoning research interest, deepening our understanding of statins’ comprehensive impact on CVD [26]. High-sensitivity C-reactive protein (Hs-CRP) is an inflammatory biomarker that predicts vascular risk with an effect estimate paralleling TC or HDL-C [27]. Statin treatment may reduce vascular inflammation as assessed by Hs-CRP, and higher Hs-CRP levels appeared to increase the risk of recurrent stroke and vascular events [28,29]. HMG-CoA reductase inhibition leads to an upsurge in endothelial nitric oxide (NO) synthase activity, thereby enhancing the bioavailability of NO; thus, statins may stabilize atherosclerotic plaques by increasing the bioavailability of NO [30,31]. Statins have also been demonstrated to enhance endothelial function and to attenuate both platelet reactivity and the production of proinflammatory cytokines, facilitating the recovery of endothelial function after myocardial ischemia–reperfusion injury in a dose-dependent manner [32,33]. These pleiotropic effects may be attributed to the inhibition of synthesis of isoprenoids, which are important lipid attachments for intracellular signaling molecules [34,35]. The accumulating body of research continues to confirm the diverse benefits of statin pleiotropy, progressively illuminating its efficacy far beyond the confines of CVD management.
3. Mechanisms Linking NAFLD and CVD
3.1. Insulin Resistance
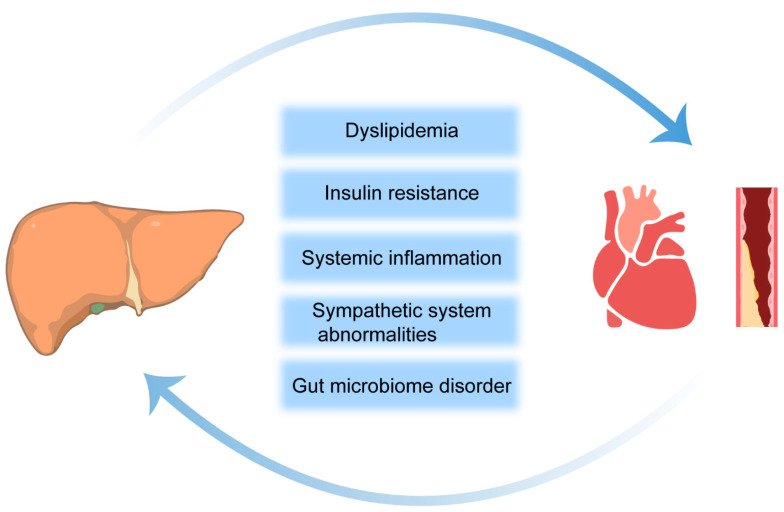
Over the past 30 years, there have been many new insights into the pathogenesis of NAFLD. There is a partial relationship of the etiology between NAFLD and CVD, and the most common cause of death in patients with NAFLD is CVD rather than chronic liver disease [36]. The precise pathophysiological mechanisms that elevate the CVD risk in the context of NASH/NAFLD are yet to be fully elucidated. However, current evidence points to a constellation of potential factors, including dyslipidemia, insulin resistance (IR), systemic inflammation, coagulopathies, anomalies within the sympathetic nervous system, and perturbations in the gut microbiome. Among these, dyslipidemia, IR, and systemic inflammation stand out as conventional risk factors for CVD and as being independent of NASH/NAFLD in terms of exacerbating the CVD risk (Figure 1) [37].
Figure 1.
Potential risk factors linking NAFLD to the development and progression of CVD. The arrows indicate that NAFLD and CVD are interconnected.
IR is recognized as a pivotal factor in the evolution from simple fatty liver to NASH and hepatic cirrhosis, which also poses a significant risk of CVD. IR-induced compensatory hyperinsulinemia also engenders a spectrum of metabolic and cellular aberrations, including dyslipidemia, hypertension, endothelial dysfunction, oxidative stress, and alterations in cardiac metabolism [38]. Chronically elevated levels of serum glucose, especially postprandial glucose spikes, instigate sympathetic hyperactivity and the synthesis of advanced glycation end products (AGEs). Through their interaction with specific receptors, AGEs activate proinflammatory signaling cascades and foster oxidative stress, chronic vascular inflammation, endothelial dysfunction, lipoprotein metabolism disorders, and the accumulation of ectopic fat [39,40]. Hyperinsulinemia alters the transport of intracellular free fatty acids (FFAs) and transfers fatty acid uptake from adipose tissue to other metabolic organs, thereby contributing to the association of the ectopic fat distribution with obesity-related metabolic diseases. Furthermore, impaired insulin metabolism in these organs can lead to the exacerbation of IR, which in turn triggers a cascade of cardiometabolic dysfunction in the heart [41]. Consequently, the aggravation of IR in NASH/NAFLD patients creates favorable conditions for atherosclerotic dyslipidemia, inducing cardiomyopathy and arrhythmias, ultimately leading to CVD [42].
3.2. Dyslipidemia
Dyslipidemia encompasses a broad spectrum of lipid perturbations and manifests as increased plasma TG and LDL-C and reduced levels of HDL-C. It may increase the risk of CVD in patients with NASH/NAFLD [43]. Furthermore, the severity of NASH/NAFLD is positively correlated with the concentrations of small dense low-density lipoprotein (sdLDL) and the ratio of sdLDL/LDL. The study also found that the cholesterol content within sdLDL is linked to an elevated risk of CVD outcomes across various vascular territories [44,45]. In NAFLD, the mechanisms of hepatic lipid acquisition, predominantly through increased fatty acid uptake and DNL, are enhanced even in the presence of steatosis. While lipid disposal mechanisms may be upregulated, they are ultimately insufficient to offset the progressive accumulation of intrahepatic fat [46]. These changes lead to lipid dysregulation, which not only causes hepatocyte apoptosis and necrosis but also increases the possibility of CVD in patients with NASH/NAFLD.
3.3. Localized and Systemic Inflammation
NAFLD is understood as a systemic metabolic disorder, with its inflammatory triggers emanating not only from within the hepatic milieu through lipotoxicity, innate immune responses, and cell death pathways but also from external sources such as adipose tissue and the gut [8]. The systemic chronic inflammatory state is fueled by the reciprocal exchange of inflammatory mediators among the liver, adipose tissue, and the gut [47]. Fetuin-A, synthetized in the liver, is associated with endothelial dysfunction and an increased risk of ischemic stroke and carotid atherosclerosis, particularly when it provokes mild inflammation. Furthermore, elevated serum levels of C-reactive protein (CRP) have been correlated with carotid atherosclerosis, attributable to CRP’s role in enhancing plasminogen expression, facilitating the adhesion of molecules to endothelial cells, and augmenting macrophage LDL phagocytosis [48]. Hepatocyte-derived extracellular vesicles (EVs) from fatty livers notably instigate endothelial inflammation. MicroRNA-1 (miR-1), a critical component within these EVs, which triggers endothelial cell inflammation by downregulating Krüppel-like factor 4 (KLF4) and activating the nuclear factor kappa-B (NF-κB) pathway. Interestingly, inhibiting miR-1 not only reduces endothelial inflammation in vitro but also mitigates atherogenesis in ApoE-deficient mice [9]. Due to being central to the nexus between NASH and CVD, the hepatic miRNAs can enter into the bloodstream and promote CVD through alterations in lipid metabolism and/or the promotion of systemic inflammation [49].
4. Statin Therapy in Patients with NAFLD: Safety and Efficacy
4.1. Effects of Statins on NAFLD Patients with CVD
NAFLD and CVD have common risk factors. In NAFLD, the increased liver-related mortality is primarily attributed to complications of advanced liver fibrosis and cirrhosis, such as HCC and decompensated cirrhosis. Compared to liver-related causes, however, most patients with NAFLD face a higher risk of early morbidity and mortality from CVD [50,51]. In addition, the risk of CVD and liver-related mortality rises exponentially rather than linearly as liver fibrosis progresses [52]. Statins, mimicking HMG-CoA, the natural substrate, competitively inhibit the HMG-CoA reductase enzyme, thereby reducing mevalonate production and downstream cholesterol biosynthesis. Considering that most cholesterol synthesis occurs in hepatocytes, HMG-CoA reductase inhibitors primarily target the liver [53]. Dyslipidemia and NAFLD usually coexist, and pooled estimates indicate a 69.16% prevalence of hyperlipidemia/dyslipidemia among NAFLD and NASH patients [36]; therefore, statins can often be used as primary and secondary prophylaxis for CVD in patients with NAFLD. The current statin treatments in NASH/NAFLD clinical trials are summarized in Table 1.
Table 1.
Clinical trials for statins in treating NASH/NAFLD registered at ClinicalTrials.gov.
| Study Title | Agent | Dose/Day (Statins) | Status | Major Inclusion Criteria | Estimated Completion | Trial Number |
|---|---|---|---|---|---|---|
| NAFLD pharmacological treatment: metformin versus atorvastatin | Metformin and atorvastatin | 20 mg | Unknown, N/A | Signs of simple liver steatosis at ultrasonography | 1 June 2015 | NCT01544751 |
| NAFLD influence of statin therapy | Rosuvastatin | / | Withdrawn, N/A | Cardiology patient fatty liver in a cohort of patients | 1 September 2009 | NCT00375349 |
| Combination of obeticholic acid (OCA) and statins for monitoring of lipids (control) [54] | Obeticholic acid and atorvastatin | 10 mg | Completed, phase 2 | Histologic evidence of NASH | 12 March 2018 | NCT02633956 |
| Atorvastatin versus vitamin E in treatment of NAFLD | Atorvastatin | 20 mg | Unknown, N/A | Sign informed consent before involvement in any trial-related activity | 1 December 2016 | NCT01720719 |
| Phase IV study to evaluate the effects of statin monotherapy or statin/ezetimibe combination therapy on hepatic steatosis in patients with hyperlipidemia and NAFLD | Rosuvastatin | 5 mg | Completed, phase 4 | Patients diagnosed with fatty liver or liver fibroscan | 11 September 2019 | NCT03434613 |
| Atorvastatin, L-Carnitine and NASH | Atorvastatin, carnitine and atoral | 20 mg | Unknown, N/A | NASH diagnosed on the basis of established criteria | 1 December 2019 | NCT01617772 |
| Assessment of endothelial function in patients with NAFLD and the impact of statin treatment | Atorvastatin | 20 mg | Withdrawn, N/A | Patients with fatty liver | 1 December 2015 | NCT01987310 |
| Statins for the treatment of NASH | Atorvastatin | 40 mg | Recruiting, phase 2 | NASH or fibrosis stage ≥ 2 | 1 December 2024 | NCT04679376 |
| Effects of pitavastatin on insulin sensitivity and liver fat | Pitavastatin | 4 mg | Completed, Unknown | BMI ≥ 27 kg/m2 and waist circumference ≥ 102 cm, high probability risk factors for NAFLD | 30 April 2018 | NCT02290106 |
| Liver cirrhosis network rosuvastatin efficacy and safety for cirrhosis in the United States | Rosuvastatin | 20 mg | Recruiting, phase 2 | Cirrhosis due to NASH, alcohol-associated liver disease, or chronic viral hepatitis or cryptogenic cirrhosis | 1 November 2026 | NCT05832229 |
| Clinical effects of new approach on patients with NASH | Rosuvastatin, vitamin E, and N-acetyl cysteine | 20 mg | Not yet recruiting, early phase 1 | NASH diagnosis using fibroscan detecting the degree of steatosis and fibrosis | 17 January 2025 | NCT06105060 |
| Comparative clinical study to evaluate the efficacy and safety of rosuvastatin vs CoQ10 on NASH | Coenzyme Q10 and rosuvastatin | 20 mg | Not yet recruiting, phase 3 | Patients have established diagnosis of NASH | 1 April 2024 | NCT05731596 |
A search of the keyword “NAFLD” or “NASH” in the item “Condition or disease” at https://clinicaltrials.gov/ (accessed on 5 November 2023) yielded 1404 listed studies, 12 of which are listed in this table that are related to statins. N/A, not available. NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Three post hoc analyses of randomized controlled trials (n = 1600, n = 1123, n = 8864) indicate that atorvastatin is beneficial for NASH/NAFLD on the basis of a reduction in liver enzymes and improvements in ultrasonography. Moreover, statin treatment halved the CVD morbidity and mortality, as well as reduced the CVD events by two-thirds, compared to NASH/NAFLD patients not receiving statins [55]. Another large-scale cross-sectional analysis within a population-based cohort study found that statin-naive individuals with suspected NAFLD (Fatty Liver Index ≥ 60) and suspected advanced fibrosis (NAFLD fibrosis score > 0.676) face a significantly elevated cardiovascular risk, thereby warranting a higher consideration for statin therapy. The proportion of NASH subjects requiring statin treatment increases with increasing LDL-C levels and higher CVD risk prediction categories [30].
In summary, statin therapy has a significant ameliorative effect in patients with NASH/NAFLD. Most importantly, statins are beneficial for their concurrent CVD. As the disease worsens, statins may have the potential to save the lives of patients with severe liver disease complicated by CVD.
4.2. Safety Assessment of Statins in the Treatment of NASH/NAFLD
Many lipid-lowering drugs have side effects, including elevated liver function and hepatotoxicity. Statins, as lipid-lowering drugs, have also gained significant attention for their potential toxicity. A systematic examination and meta-analysis of over 90,000 participants in randomized studies revealed that statins markedly elevate both the relative and absolute risks of myopathy, kidney, and liver dysfunction [56]. Myotoxic effects caused by statins rank among the most frequent side effects, presenting as exhaustion, muscle pain, muscle weakness, nocturnal cramping, and more, with occurrence rates varying between 7% and 29% [57]. Furthermore, randomized studies indicate that moderate-intensity statins elevate the risk of type 2 diabetes by approximately 11%, and there is a potential additional 12% risk increase when moving to high-intensity statins [58]. Research indicates that statin prescription is sometimes limited in NASH/NAFLD patients due to the concern of physicians about hepatotoxicity [59]. In fact, statins have only a slight effect on aminotransferases, and they even have a decreasing effect on these enzymes. The Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) study revealed that of 437 patients presenting with moderately abnormal liver tests at baseline, potentially indicative of NAFLD, 227 treated with a statin (predominantly atorvastatin) exhibited significant improvements in the liver function tests. In contrast, the 210 patients not receiving statin treatment experienced further elevations in the liver enzyme concentrations [60].
Different clinical studies have consistently demonstrated that the serum aminotransferase levels are reduced significantly in NAFLD patients with dyslipidemia by atorvastatin and simvastatin treatment [61,62]. Although prolonged use of certain drugs, such as rosuvastatin, can cause liver enzyme abnormalities, none of the subjects exhibited more than a threefold increase in these enzymes, and they showed significant improvements in lipid parameters [63]. A recent meta-analysis scrutinized variations in the liver function tests, specifically alanine transaminase (ALT), aspartate transaminase (AST), and gamma-glutamyl transpeptidase (γ-GGT), in 22 studies with 2345 NAFLD patients treated with statins. The results reveal a significant diminution in the liver enzyme levels in the statin-treated NAFLD patients [64]. Another meta-analysis similarly indicated that statins reduced the liver enzyme levels and improved liver histology [65]. The prospective study investigating statin therapy’s impact on liver enzyme in the very elderly (≥80 years old) included 515 patients aged 80 to 98 with hypercholesterolemia (LDL-C levels ≥3.4 and <5.7 mmol/L), atherosclerosis, CHD, or a CHD-risk equivalent. The findings indicate that 24 patients (4.7, 95% CI 2.7–6.6) experienced increased hepatic aminotransferase levels [66], implying that treatment with statins is safe, even in elderly patients. Statins are deemed safe for patients with cirrhosis. A retrospective cohort study of veterans demonstrated that statin therapy reduced the risk of acute-on-chronic liver failure in cirrhotic patients [67]. Furthermore, statin therapy significantly reduces the risk of HCC in patients with diabetes, NASH, and cirrhosis [68,69].
In 2006, the Liver Expert Panel of the National Lipid Association (NLA), in a series of special reports on the safety of statins, indicated that elevated liver enzymes do not necessarily lead to hepatotoxicity. Additionally, patients with suspected NAFLD and elevated liver enzymes do not exhibit an increased risk of developing liver injury when using statins compared to those with normal liver enzymes prior to recruitment [70]. The 2020 Evidence-Based Clinical Practice Guidelines for NAFLD/NASH also advocate for the consideration of statins in NAFLD/NASH patients with hypercholesterolemia [71]. A large amount of evidence in recent years suggests that statin therapy for NASH has a low risk of causing serious hepatotoxic reactions, prompting an increase in the use of statins for primary prevention over time [59,72].
5. Effects of Statins on Liver Histology—From Animal Models to Human Studies
5.1. Improvement of Steatosis
NAFLD is typified by hepatic steatosis, marked by over 5% parenchymal fat accumulation, without hepatocyte injury [7]. Statins, as efficacious lipid-lowering agents, reduce LDL-C by 20–60%, TG by 10–33%, and increase HDL-C by 5–10% in NAFLD patients [73]. Statins may potentially contribute to blocking NAFLD progression by controlling lipid status. Specifically, atorvastatin has been shown to effectively ameliorate NAFLD-related hyperlipidemia in rats, reducing liver steatosis and modulating the expression of lipid metabolism-regulating genes [74]. Likewise, the treatment of NAFLD rats with simvastatin and fluvastatin reduced their liver weight, hepatic index, ALT, AST, and regulated abnormal lipid metabolism [75,76].
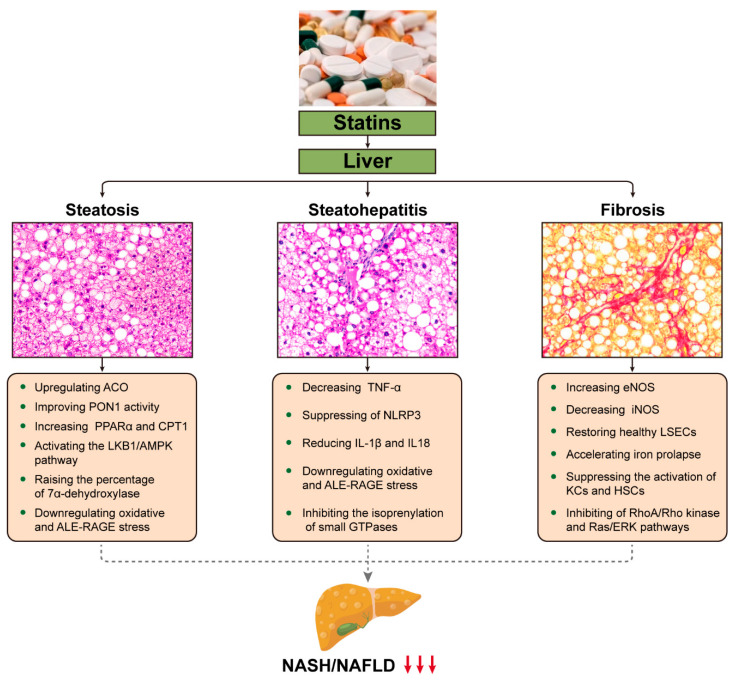
In an open-label trial, 70 participants with NAFLD were randomized to receive either ezetimibe combined with rosuvastatin or rosuvastatin alone for a duration of up to 24 weeks, and the effectiveness of ezetimibe plus rosuvastatin was assessed compared to rosuvastatin alone in reducing hepatic fat by utilizing magnetic resonance imaging-derived proton density fat fraction (MRI-PDFF) in patients. The results demonstrated that hepatic steatosis was reduced by the combination or individual use of rosuvastatin [77]. Another study found that during 12 weeks of continuous treatment with either pitavastatin (2–4 mg/day) or atorvastatin (10–20 mg/day), CT scans showed no significant elevation in ALT and a significant reduction in hepatic fat accumulation (pitavastatin group: p = 0.014; atorvastatin group: p = 0.021) during treatment [78]. In 2015, Kargiotis et al. examined patients treated with rosuvastatin (10 mg/d) monotherapy for 12 months, with repeat liver biopsy and ultrasound at the end of treatment. It was found that the NASH was improved in 95% of the patients [79]. Several studies have indicated that statins can reduce hepatic steatosis in patients [80,81,82]; however, partial statin use, such as pitavastatin, has no significant effect on liver steatosis [83]. Long-term treatment with statins (18 months) did not translate into a histologic improvement in the NAFLD Activity Score (NAS), despite a reduction in liver fat (13 ± 2 vs. 8 ± 2%, p < 0.001) [84]. The results and potential hepatoprotective mechanisms of statins used in NASH/NAFLD are listed below (Figure 2 and Table 2).
Figure 2.
Potential mechanisms by which statins may favorably affect liver histology in NASH/NAFLD. This cartoon emphasizes the pleiotropic effects of statins on the liver and the potential mechanisms that may combine to ameliorate the primary pathological conditions of NASH/NAFLD, such as steatosis, inflammation, and fibrosis. The downward pointing arrow signifies downregulation. ACO, acyl-CoA oxidase; PON1, paraoxonase 1; PPAR, peroxisome proliferator-activated receptor; CPT, carnitine palmitoyltransferase; LKB1/AMPK, AMP-activated protein kinase; ALE-RAGE, advanced lipoxidation end product-receptors of advanced glycation end products; TNF, tumor necrosis factor; NLRP3, NOD-like receptor family pyrin domain-containing 3; IL-1β, cytokines interleukin (IL)-1β; GTPases, small guanine triphosphate-binding proteins; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; LSECs, liver sinusoidal endothelial cells; KCs, Kupffer cells; HSCs, hepatic stellate cells; RAS/ERK; Ras-extracellular signal-regulated kinase.
Table 2.
Summary of studies evaluating the effects of statin use in NASH/NAFLD.
| Type of Statin | Study Design |
Diseases | Dose/Day | Results | Comments | Reference |
|---|---|---|---|---|---|---|
| Atorvastatin | Rat | NAFLD | 30 mg/kg | Atorvastatin up-regulated the expression of PPARα, liver fatty acid β-oxidation, and reduced the liver TG | Atorvastatin treatment effectively improved NAFLD-related hyperlipidemia and inhibited liver steatosis, accompanied by modulating the expression of genes for regulating lipid metabolism | [74] |
| Mice | NAFLD-NASH | 4.5 mg/kg | Atorvastatin prevents cholesterol crystal formation, thereby precluding NLRP3 inflammasome activation to prevent further development of NAFLD | Atorvastatin prevents development of hepatic steatosis, inflammation and fibrosis in mice | [85] | |
| Human | Hypercholesteremia | 10 mg | Atorvastatin reduced LDL-C concentrations and the severity of hepatic steatosis | Atorvastatin effectively and safely reduces elevated hepatic enzyme concentrations in hypercholesterolemic patients | [78] | |
| Fluvastatin | Rat | NASH | 5 mg/kg or 10 mg/kg | Fluvastatin reduced steatosis and fibrosis scores, α-SMA protein expression, mRNA expression of pro-inflammatory and pro-fibrogenic genes in livers | Fluvastatin alleviated steatosis-induced HSCs activation and hepatic fibrogenesis through mitigating inflammation and oxidative stress | [75] |
| Rosuvastatin | Human | NASH | 10 mg | Rosuvastatin resulted in complete resolution of NASH in 19 patients, and lipid values were normalized | Rosuvastatin could ameliorate biopsy-proven NASH and reduce the risk of vascular and liver morbidity and mortality in NASH patients | [79] |
| Simvastatin | Mice | NAFLD | 20 mg/kg | Simvastatin restored antioxidant enzyme activity and decreased lipid peroxidation and ALE-RAGE pathway activation | Simvastatin improved microcirculatory function in NAFLD by downregulating oxidative and ALE-RAGE stress and attenuated steatosis, inflammation and fibrosis. | [86] |
NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; TG, triglycerides; NLRP3, NOD-like receptor family pyrin domain-containing 3; LDL-C, low-density lipoprotein cholesterol; α-SMA, alpha-smooth muscle actin; HSCs, hepatic stellate cells; ALE-RAGE, advanced lipoxidation end product-receptors of advanced glycation end products.
5.2. Reduction of Inflammation
Statins have some anti-inflammatory activity, with evidence showing their ability to attenuate the inflammatory and pathogenic activities of T cells via KLF2-dependent mechanisms [87]. Simvastatin prevented microcirculatory dysfunction and NAFLD by downregulating oxidative stress and advanced lipoxidation end product-receptors of advanced glycation end product (ALE-RAGE) stress, thus ameliorating steatosis, fibrosis, and inflammatory parameters [86]. Atorvastatin notably inhibits the activation of the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome pathway triggered by cholesterol crystals, subsequently reducing the expression of pro-inflammatory cytokines IL-1β and IL-18, which are implicated in NAFLD progression. Atorvastatin has been found to inhibit the activation of the NOD-like receptor family, NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome pathway induced by cholesterol crystals, thereby reducing the expression of pro-inflammatory cytokines interleukin (IL)-1β and IL-18, which are implicated in NAFLD progression [85]. Additionally, atorvastatin also impedes NASH progression, partly by reducing tumor necrosis factor-α (TNF-α). The study enrolled 42 biopsy-proven nonalcoholic steatohepatitis patients with dyslipidemia. A 12-month treatment with atorvastatin (10 mg/day) resulted in significant decreases in the levels of AST, γ-GGT, LDL-C, TG, type IV collagen, and TNF-α, and it revealed that the extent of the LDL-C reduction by atorvastatin was independently associated with the increase in the liver to spleen density ratio [88]. Another study similarly found that NAFLD patients taking statins had a significant reduction in the necro-inflammatory phase of the liver [72]. Long-term statin therapy has been shown to improve steatosis, hepatocellular ballooning degeneration, and diffuse lobular mixed acute and chronic inflammation in 95% of patients based on repeat liver biopsy [79].
5.3. Improvement of Fibrosis
Advanced liver fibrosis serves as a pivotal prognostic determinant for end-stage liver disease, cardiovascular, and overall mortality [89]. Some studies have found that statins can be an effective antifibrotic agent in hepatic fibrosis [90,91,92]. For instance, simvastatin has been observed to enhance the prognosis of NASH-related fibrosis by modulating the expression of endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) while inhibiting the activation of HSCs [93]. Fluvastatin alleviates steatosis-induced activation of HSCs and hepatic fibrogenesis by mitigating inflammation and oxidative stress in vitro and in vivo [75]. In addition, a clinical investigation, utilizing liver stiffness measurements (LSM) as an index of fibrosis, discerned that statin administration correlates with a reduced likelihood of advanced liver fibrosis (OR 0.35, 95% CI 0.13–0.90, p = 0.03) [94].
In a cross-sectional analysis involving 346 diabetics with biopsy-confirmed NAFLD, statin use was independently and inversely correlated with both NASH (OR 0.57, 95% CI 0.32 to 1.01, p = 0.055) and significant fibrosis (SF) (OR 0.47, 95% CI 0.26 to 0.84, p = 0.011) [95]. Sfikas et al. studied 604 military personnel with NASH/NAFLD to assess the effects of diet–exercise, and one year of treatment with atorvastatin, rosuvastatin, or pitavastatin, on NASH/NAFLD. The results showed that all three statins reduced NASH and the Fibrosis-4 score (FIB-4) [96]. Previous studies have indicated that statins may not improve liver fibrosis [81,97,98]; however, a recent study found that statin use reduced the risk of significant liver fibrosis (AOR 0.43; 95% CI 0.42–0.44) in a national case-control study [99], and the risk of HCC was lower in patients with NASH and advanced fibrosis after statin use [100,101].
6. Mechanisms of Statins in the Treatment of NASH/NAFLD
6.1. KCs, HSCs and LSECs
In atherosclerosis and NASH, the inflammatory response is primarily driven by lysosomal cholesterol accumulation in macrophages rather than the overall amount of intracellular lipids [102]. In animal models, dietary cholesterol can activate hepatic injury by promoting the M1 phenotype in KCs, leading to the activation of HSCs, oxidative stress, and inflammation [103]. The KCs can be activated by free cholesterol (FC), cholesteryl esters and TG around dead hepatocytes to form coronary structures, and a liver with these activated KCs progresses into inflammation and fibrosis distinct from simple steatosis [104]. Paracrine signaling from various resident and inflammatory cells, including hepatocytes, LSECs, hepatic macrophages, natural killer/natural killer T cells, biliary epithelial cells, hepatic progenitor cells, and platelets, exerts a direct or indirect regulatory influence on the differentiation and activation of HSCs. Activated HSCs are pivotal in the initiation and progression of liver fibrosis [105]. Furthermore, altered LSECs contribute to hepatic angiogenesis, inflammation, fibrosis and HCC during the stages of NASH. Multiple pieces of evidence indicate that the dysfunction of LSECs in fatty livers is associated with increased intrahepatic vascular resistance related to steatosis [106].
Statins reduce hepatocellular cholesterol uptake, effectively preventing or reversing cholesterol accumulation in hepatocytes, and they inhibit the activation of KCs and HSCs, which slows down liver fibrosis [107]. A study showed that atorvastatin reduced hepatic steatosis and inflammation induced by a high-saturated fat, high-fructose, and high-cholesterol diet in animals with NASH and significantly regulated bile acid metabolism by altering intestinal reabsorption and hepatic synthesis in NASH mice [108]. The separation of the components in an atherogenic diet has revealed that liver damage and fibrosis may be attributed to the initial accumulation of bile acid. Bile acids play a role in the transdifferentiation of HSCs into myofibroblasts, ultimately resulting in fibrosis, which suggests that hepatic cholesterol and bile acid homeostasis may influence fibrosis caused by HSCs proliferation [109].
Moreover, combination therapy with lipid-lowering drugs has proven more efficacious in treating NASH compared to individual drug use. In multiple animal model studies, investigators have evaluated the roles of different interventions in NASH, confirming that an ezetimibe/atorvastatin combination normalized the hepatic FC levels, although it had a minimal impact on saturated FFAs and other lipids. Lipid-lowering drugs can break down cholesterol crystals, crown-like structures of activated KCs and improve fibrosis. In contrast, ezetimibe and atorvastatin alone yield similar but less pronounced effects [110,111]. Increased activation of KCs and HSCs due to the dysfunction of LSECs may be modulated by statins. A study of rats treated with simvastatin or atorvastatin demonstrated that statins not only improve NASH histology but also restore healthy LSECs and HSCs phenotype, leading to decreased portal pressure and, consequently, an improved prognosis for the disease [112].
In summary, statins counteract hepatic lipotoxicity by processing cholesterol crystals and KCs with a coronal structure, further preventing inflammation and fibrosis formation, restoring healthy LSECs, and inhibiting HSCs activation, thus antagonizing the development of fibrosis in NASH.
6.2. PON1
PON1 is an antioxidant enzyme of hepatic origin. The structure activity of three groups of known substrates of PON1 (phosphotriesters, esters, and lactones) has been verified and PON1 is, in fact, a lactonase [113]. PON1 hinders oxidative modification of LDL through the elimination of lipid peroxides from LDL. PON1 is recognized as an essential component of the antioxidant and anti-inflammatory functions of HDL. The serum activity of PON1 correlates with systemic lipid peroxidation (LPO) stress and potential cardiovascular risk [114,115]. A meta-analysis found that paraoxonase activity, which is related to PON1, was markedly diminished in patients with NAFLD compared to those without the condition. This suggests that the paraoxonase levels may impact the pathophysiology of NASH/NAFLD and could serve as a useful biomarker for the condition [116].
Statins have been demonstrated to restore the serum PON1 levels in NASH/NAFLD patients. Increased PON1 activity attenuates NASH/NAFLD-induced LPO. In a clinical trial, 50 NAFLD patients were bifurcated into 2 cohorts, 1 with an 8-month intervention of 40 mg tablet atorvastatin and 1 without atorvastatin. Various investigations were conducted, including abdominal ultrasonography, serum PON1 activity level, liver function tests, serum lipid profile, and serum levels of malondialdehyde (MDA), an indicator of oxidative stress. The results showed that after atorvastatin treatment, the serum PON1 activity was significantly increased, the blood lipids, especially LDL-C, were decreased, hepatic oxidative stress was reduced and the progression of NAFLD was reversed [117].
However, whether statins can ameliorate NASH by increasing serum PON1 activity has not been reported in animals, and there are only a few studies even in clinical trials. If it is found in animal models that PON1 is the therapeutic target of statin action in NASH, it will be better established.
6.3. GTPases
The Rab family GTPases are quintessential controllers of vesicle transport and membrane trafficking in eukaryotic cells. They interact with various effectors, such as molecular motors, scaffolding proteins, and lipid kinases, orchestrating virtually every aspect of vesicular trafficking across cellular compartments [118]. The regulation of intracellular trafficking and the interactions with regulators and effectors of Rho proteins rely heavily on the post-translational isoprenylation of small G proteins. Statins impede the production of isoprenoid intermediates by restraining mevalonate synthesis, thus hindering the isoprenylation of small GTPases and consequently inhibiting their signaling. Statins also attenuate hepatic inflammation and fibrosis by inhibiting signaling downstream of the Rho family small G proteins (RhoA, RAS). The cause of this effect can be attributed to the multiple actions of statins rather than their ability to lower cholesterol [119,120,121,122].
Researchers fed 12-week-old ApoE−/− mice a Western-style diet for 7 weeks to induce NASH, while treating the mice with simvastatin for 6 weeks. The results revealed that the antifibrotic and anti-inflammatory effects of simvastatin were dependent on the inhibition of two specific pathways: the Ras/ERK1/2 (Ras-extracellular signal-regulated kinase) pathway and the RhoA/Rho kinase pathway. These findings highlight the potential therapeutic benefits of statins in the treatment of NASH and provide insights into the underlying mechanisms of its action [123].
6.4. PPARs
Peroxisome proliferator-activated receptors (PPARs), which bind fatty acids and their metabolites and control inflammatory and metabolic pathways, are also essential regulators of lipid and glucose metabolism, inflammation and fibrogenesis in various tissues [124]. PPARα is a key factor in regulating fatty acid metabolism and ketogenesis. It has the ability to regulate the transportation of fatty acids, oxidation of peroxisomes or mitochondria, and lipolysis, ultimately impacting the production of apolipoproteins [125]. Deletion of hepatocyte PPARα in NAFLD mice disrupted liver and systemic fatty acid homeostasis, leading to hepatic lipid accumulation [126]. Mice treated with different PPAR agonists improved both their steatosis and hepatitis [127]. Previously, statins have been found to exert anti-inflammatory effects via PPARα. The study found that the liver PPARα, acyl-CoA oxidase (ACO), and carnitine palmitoyltransferase I (CPTI) mRNA levels and hepatic fatty acid β-oxidation activity were increased by atorvastatin at 30 mg/kg [128]. Rosuvastatin treatment attenuated hepatic steatosis in NAFLD mice by modulating PPAR homeostasis. This effect was accompanied by a reduction in insulin resistance, an improvement in the anti-inflammatory adipokine profile, and a decrease in HSC activation [129]. Different types of statins (fluvastatin, pravastatin, simvastatin, atorvastatin, and rosuvastatin) can prevent NASH by inducing PPARα and increasing mitochondrial and peroxisomal fatty acid oxidation (FAO) [130].
6.5. AMPK
AMPK plays an essential role in controlling a variety of metabolic pathways. Its significance lies in its ability to regulate glucose uptake, uphold mitochondrial equilibrium, avert lysosomal harm, and facilitate autophagy. Furthermore, AMPK hinders the production of fatty acids and cholesterol [131,132]. AMPK, adept at inhibiting various enzymes and transcription factors pivotal for lipid biosynthesis, plays a critical role in metabolic regulation. Diminished AMPK activity impairs FAO and augments adipogenesis by attenuating ACC phosphorylation [133]. Fluvastatin was found to act as an SIRT6 agonist and inhibit lipid synthesis in hepatocytes by activating the LKB1/AMPK pathway to regulate the transcriptional factor Srebp1 [134]. In another study, atorvastatin also mitigated hepatic fat accumulation through AMPK-dependent upregulation of PPARα and PGC1α in the high-fat diet-induced NAFLD hamster model [135]. Additionally, simvastatin treatment led to the suppression of oncoproteins STAT3 and Skp2 and activation of the energy sensor AMPK, culminating in reduced tumor growth in HepG2 xenograft mice [136].
6.6. Ferroptosis
Ferroptosis represents an iron-dependent, non-apoptotic mode of cell death, characterized by large levels of LPO [137]. The liver plays a pivotal role in regulating the systemic iron balance through the synthesis and secretion of hepcidin, a major regulator of iron homeostasis [138]. Ferroptosis has been proposed to be implicated in the development and progression of NASH/NAFLD [139]. Knockout of the iron chaperone protein PCBP1 in hepatocytes resulted in defects in liver iron homeostasis and led to reactive oxygen species (ROS) production, which caused LPO and hepatic steatosis in mice [140]. Statins can induce ferroptosis and suppress tumor growth [141]. Subsequent research has elucidated that simvastatin could suppress the expression of HMG-CoA, consequently downregulating the mevalonate pathway and glutathione peroxidase 4 (GPX4), which in turn triggers ferroptosis in cancer cells [142]. Simvastatin also mediates the activation of HSCs through the mevalonate pathway, reducing the intracellular cholesterol levels in HSCs and inducing iron prolapse, and may be an potential therapeutic agent for treating hepatic fibrosis [143]. Although the effects of statins on the iron death pathways have been reported [144], whether one of the pathological mechanisms by which statins are favorable to the progression of NASH/NAFLD is linked to the inhibition of iron death requires further investigation.
6.7. Intestinal Microbiota
In 2011, Arumugam M and colleagues first proposed the concept of ‘enterotypes’ [145]. Later, scientists further proposed that the microbial composition of the human gut can be divided into four distinct enterotypes, such as Bacteroidetes1 (Bact1), Bacteroidetes2 (Bact2), Prevotella (Prev) and Ruminococcaceae (Rum) [146,147,148]. Sara et al. [148] indicated that less than 6% of obese individuals treated with statins had a Bact2 gut phenotype, which is comparable to non-obese individuals. It also suggests that statins may play a role in modulating the harmful inflammatory microbiota of the gut and alleviating the systemic inflammation levels in obese patients. These findings indicate that commonly used cholesterol-lowering statin drugs may be a potential therapeutic option for microbiota modulation (Table 3).
Table 3.
Summary of the effects of statins on the intestinal microbiota in different diseases.
| Disease | Type of Statin | Study Design | Dose/Day | Intestinal Microbial Changes | Results | Reference |
|---|---|---|---|---|---|---|
| Hypercholesteremia | Atorvastatin | Rat | 10, 15, 20 mg/kg | Proteobacteria increased, Firmicutes decreased | Intestinal microbial diversity increased | [149] |
| Human | 20 mg |
Faecalibacterium prausnitzii, Akkermansia muciniphilaa and Oscillospira increased Desufovibrio decreased |
Reduced pro-inflammatory bacteria and taxa associated with atherosclerosis formation and CVD progression | [150] | ||
| Ischemic stroke | Atorvastatin | Mice | 20 mg/kg | Firmicutes and Lactobacillus increased, Bacteroidetes decreased | Increased fecal butyrate level, promoted intestinal barrier function | [151] |
| Acute coronary syndrome |
Statins | Human | / |
Parabacteroides merdae decreased Bifidobacterium longum subsp. longum, Anaerostipes hadrus and Ruminococcus obeum increased |
Specific changes in bacterial taxa were associated with disease severity or outcomes either directly or by mediating metabolites such as fatty acids and prenol lipids | [152] |
| Obesity | Atorvastatin | Mice | 10 mg/kg | Bacteroides, Butyricimonas, and Mucispirillum increased | Statins improved the inflammation associated microbiota in elderly obese mice | [153] |
| Rosuvastatin | 3 mg/kg 9.3 mg/kg |
Lachnospiraceae, Rikenella and Coprococcus increased Akkermansiaceae, Proteobacteria decreased |
[153,154] | |||
| NASH/NAFLD | Atorvastatin | Mice | 20 mg/kg | Mucispirillum, Desulfovibrio, Anaerotruncus and Desulfovibrionaceae decreased | Increased serum TCA and depleted 3-IPA | [155] |
| 20 mg/kg | Ruminococcaceae increased | Promoted the formation of deoxycholic acid and lithocholic acid | [156] |
CVD, cardiovascular disease; NASH, nonalcoholic steatohepatitis; NAFLD, nonalcoholic fatty liver disease; TCA, taurocholic acid; IPA, indolepropionic acid.
Cholesterol is oxidized in hepatocytes via cytochrome P450 to synthesize bile acids. The intestinal microbiota can biotransform these BAs into their unconjugated forms via bile salt hydrolase (BSH) activity, subsequently producing secondary BAs through reactions such as 7α-dehydroxylation or isopropylation [157]. Several bacterial genera, including Bacteroides, Bifidobacterium, Clostridium, Lactobacillus, and Listeria, have been identified as containing BSHs [158]. Increasingly, studies are focusing on the regulatory effects of statins on cardiovascular and other diseases by the intestinal microbiota. Such research found that the treatment of hypercholesterolemic mice with atorvastatin resulted in elevated levels of the anti-inflammatory bacteria [149]. Similarly, statins can significantly modify inflammation-associated bacterial flora, such as Butyricimonas and Mucispirillum [153]. Diminished populations of intestinal Lactobacillus and Bifidobacterium, leading to transport defects, may significantly impair the efficacy of rosuvastatin in reducing the serum levels of TC and LDL-C in rats [159]. Studies have also found that the hypolipidemic effect of simvastatin was related to the composition of the intestinal microbiota. Simvastatin stimulates hepatic Farnesoid X receptor (FXR) and increases Cyp7a1 gene expression in mice on an HFD diet, which was impaired by antibiotic treatment [160]. This further supports the therapeutic effect of statins on disease via the intestinal microbiota. The same was found in studies of ischemic stroke, showing that atorvastatin not only increased abundance of Firmicutes and Lactobacillus and increased the fecal butyrate level but also regulated intestinal immune function, significantly reduced the level of circulating endotoxin, and promoted intestinal barrier function [151]. Cross-sectional studies have also shown that long-term, regular oral administration of aspirin in conjunction with atorvastatin modulates the human gut microbiota, contributing to the prevention of ischemic stroke [161].
Development of fibrosis, cirrhosis and HCC in NASH/NAFLD patients is strongly associated with hepatic cholesterol metabolism [107,162]. Statins can prevent further progression of NAFLD by modulating the disorders of the intestinal microbiota and its metabolites, improving bile acid metabolism. A study proposed that an interaction of the intestinal microbiota and cholesterol metabolism leads to increased levels of adiposity in mice [163], and the presence of cholesterol-metabolizing bacteria correlates with reduced levels of TC in both the stool and serum in human cohorts [164]. There have been researchers who have associated statins with NAFLD through the intestinal microbiota [155]. They have found that high-fat/high-cholesterol (HFHC) led to intestinal microbiota in mice, such as Mucispirillum, Desulfovibrio, Anaerotruncus and Desulfovibrionaceae increased sequentially, while Bifidobacterium and Bacteroides were depleted, and impaired microbial tryptophan metabolism. Atorvastatin treatment reversed the abundance of disturbed intestinal microbiota, with increased serum taurocholic acid (TCA) and depleted 3-indolepropionic acid (IPA). The results revealed that atorvastatin completely prevented HFHC diet-induced NAFLD-HCC development [155]. In another study, atorvastatin was also found to be effective in reducing hepatic fat deposition in NASH mice by enhancing the percentage of 7α-dehydroxylase-expressing bacteria (Ruminococcaceae) in the intestine, which promotes the formation of deoxycholic acid and lithocholic acid, both of which are GPBAR1 agonists [156].
Despite this, there is still a deficiency of articles investigating the link between statins and NASH via the intestinal microbiota, and how statins ameliorate NASH through the intestinal microbiota and its metabolites still needs to be further explored.
7. Conclusions
NASH/NAFLD form part of a multisystemic disease that is considered to be the hepatic manifestation of metabolic syndrome and is a significant contributor to hepatic cirrhosis and HCC. Resmetirom received FDA approval on 14 March 2024. This marks it as the first medication approved for the treatment of NASH with liver fibrosis, providing a treatment option for these patients, in addition to diet and exercise. It also serves as a crucial reference for creating additional medications to treat NASH. As a lipid-lowering drug, statins have consistently demonstrated in several clinical trials having no significant effect on hepatotoxicity in patients, further encouraging the use of statins in NASH patients. Here, we propose that statins may be a core coordinator in orchestrating the lipids, inflammation, and fibrosis implicated in conditions associated with NASH. Multiple in vivo and in vitro research studies suggest that statins might prevent or treat NASH via HSCs, LSECs, PON1, GTPase, PPARs, AMPK, and ferroptosis. An increasing number of studies show that statins can improve CVD through the intestinal microbiota and have a protective effect on NASH (Figure 3). In this context, we need more clinical trials to explore the impact of statins on NASH patients and the cardiovascular risk mediated by the intestinal microbiota. The mechanism of statins in the treatment of NASH needs to be further delineated in view of the fact that more and more statin therapies have recently been identified.
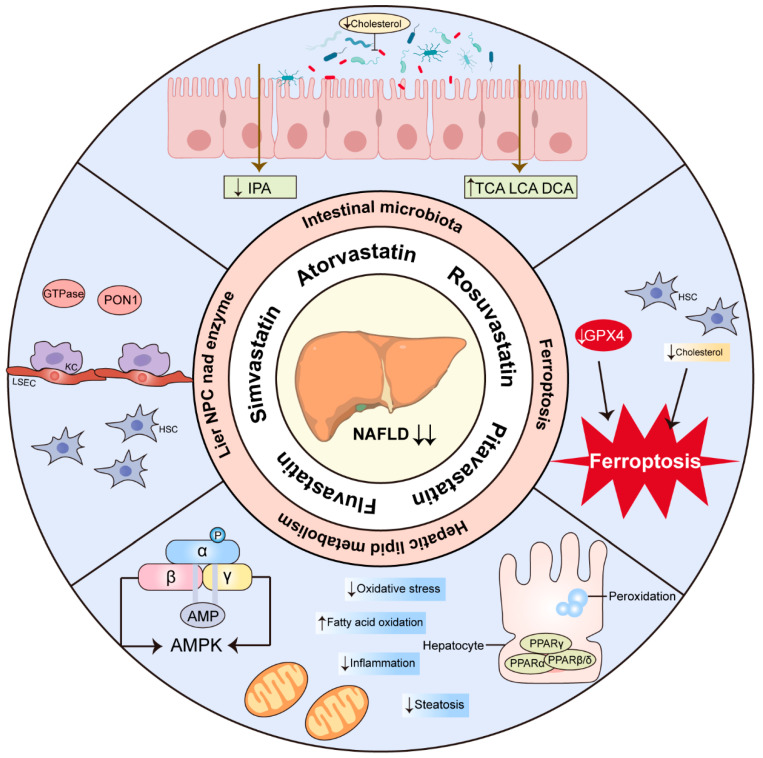
Figure 3.
Overview of the proposed mechanisms of statin therapy for NASH. The arrows pointing up indicate upregulation and the pointing down indicate downregulation. IPA, indolepropionic acid; TCA, taurocholic acid; LCA, lithocholic acid; DCA, deoxycholic acid; GPX4, glutathione peroxidase 4; PPAR, peroxisome proliferator-activated receptor; AMPK, AMP-activated protein kinase; HSC, hepatic stellate cell; KC, Kupffer cells; LSEC, liver sinusoidal endothelial cells; PON1, paraoxonase 1; GTPase, small guanine triphosphate binding proteins; NPC, non-parenchymal cell.
Author Contributions
S.Z.: Writing—original draft, writing—review and editing, investigation, visualization. X.R.: Writing—original draft, writing—review and editing, investigation, visualization. B.Z.: Investigation. T.L.: Funding acquisition, project administration, supervision. B.L.: Project administration, supervision. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (82070590).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Paternostro R., Trauner M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022;292:190–204. doi: 10.1111/joim.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell E.E., Wong V.W., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. doi: 10.1016/S0140-6736(20)32511-3. [DOI] [PubMed] [Google Scholar]
- 3.Wong V.W.-S., Ekstedt M., Wong G.L.-H., Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023;79:842–852. doi: 10.1016/j.jhep.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 4.The Lancet Gastroenterology H. Resmetirom for NASH: Balancing promise and prudence. Lancet Gastroenterol. Hepatol. 2024;9:273. doi: 10.1016/S2468-1253(24)00049-9. [DOI] [PubMed] [Google Scholar]
- 5.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Sodum N., Kumar G., Bojja S.L., Kumar N., Rao C.M. Epigenetics in NAFLD/NASH: Targets and therapy. Pharmacol. Res. 2021;167:105484. doi: 10.1016/j.phrs.2021.105484. [DOI] [PubMed] [Google Scholar]
- 7.Kanwar P., Kowdley K.V. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2016;20:225–243. doi: 10.1016/j.cld.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Schuster S., Cabrera D., Arrese M., Feldstein A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 9.Jiang F., Chen Q., Wang W., Ling Y., Yan Y., Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2020;72:156–166. doi: 10.1016/j.jhep.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S., Distrutti E. Linking liver metabolic and vascular disease via bile acid signaling. Trends Mol. Med. 2022;28:51–66. doi: 10.1016/j.molmed.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Harrison S.A., Allen A.M., Dubourg J., Noureddin M., Alkhouri N. Challenges and opportunities in NASH drug development. Nat. Med. 2023;29:562–573. doi: 10.1038/s41591-023-02242-6. [DOI] [PubMed] [Google Scholar]
- 12.Sirtori C.R. The pharmacology of statins. Pharmacol. Res. 2014;88:3–11. doi: 10.1016/j.phrs.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Min H.K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., Kellum J., Warnick R., Contos M.J., Sanyal A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maretti-Mira A.C., Salomon M.P., Hsu A.M., Kanel G.C., Golden-Mason L. Hepatic damage caused by long-term high cholesterol intake induces a dysfunctional restorative macrophage population in experimental NASH. Front. Immunol. 2022;13:968366. doi: 10.3389/fimmu.2022.968366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieghs V., Hendrikx T., van Gorp P.J., Verheyen F., Guichot Y.D., Walenbergh S.M., Jeurissen M.L., Gijbels M., Rensen S.S., Bast A., et al. The cholesterol derivative 27-hydroxycholesterol reduces steatohepatitis in mice. Gastroenterology. 2013;144:167–178.e161. doi: 10.1053/j.gastro.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Li H., Yu X.H., Ou X., Ouyang X.P., Tang C.K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021;83:101109. doi: 10.1016/j.plipres.2021.101109. [DOI] [PubMed] [Google Scholar]
- 17.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Sun B., Li L., Zhou X. Comparative analysis of the gut microbiota in distinct statin response patients in East China. J. Microbiol. 2018;56:886–892. doi: 10.1007/s12275-018-8152-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo D.-H., Kim I.S., Van Le T.K., Jung I.-H., Yoo H.H., Kim D.-H. Gut Microbiota-Mediated Drug Interactions between Lovastatin and Antibiotics. Drug Metab. Dispos. 2014;42:1508–1513. doi: 10.1124/dmd.114.058354. [DOI] [PubMed] [Google Scholar]
- 20.Istvan E.S., Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 21.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., Bhala N., Peto R., Barnes E.H., Keech A., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 23.Awad K., Serban M.C., Penson P., Mikhailidis D.P., Toth P.P., Jones S.R., Rizzo M., Howard G., Lip G.Y.H., Banach M., et al. Effects of morning vs evening statin administration on lipid profile: A systematic review and meta-analysis. J. Clin. Lipidol. 2017;11:972–985 e979. doi: 10.1016/j.jacl.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Mora-Rodriguez R., Ortega J.F., Morales-Palomo F., Ramirez-Jimenez M., Moreno-Cabanas A. Effects of statin therapy and exercise on postprandial triglycerides in overweight individuals with hypercholesterolaemia. Br. J. Clin. Pharmacol. 2020;86:1089–1099. doi: 10.1111/bcp.14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenson R.S. Low HDL-C: A secondary target of dyslipidemia therapy. Am. J. Med. 2005;118:1067–1077. doi: 10.1016/j.amjmed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Yu D., Liao J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2022;118:413–423. doi: 10.1093/cvr/cvab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridker P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagawa K., Hosomi N., Nagai Y., Kagimura T., Ohtsuki T., Origasa H., Minematsu K., Uchiyama S., Nakamura M., Matsumoto M., et al. Reduction in High-Sensitivity C-Reactive Protein Levels in Patients with Ischemic Stroke by Statin Treatment: Hs-CRP Sub-Study in J-STARS. J. Atheroscler. Thromb. 2017;24:1039–1047. doi: 10.5551/jat.39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg E.H., Wolters A.A.B., Dullaart R.P.F., Moshage H., Zurakowski D., de Meijer V.E., Blokzijl H. Prescription of statins in suspected non-alcoholic fatty liver disease and high cardiovascular risk, a population-based study. Liver Int. 2019;39:1343–1354. doi: 10.1111/liv.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almeida S.O., Budoff M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019;29:451–455. doi: 10.1016/j.tcm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Di Napoli P., Taccardi A.A., Grilli A., De Lutiis M.A., Barsotti A., Felaco M., De Caterina R. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc. Res. 2005;66:462–471. doi: 10.1016/j.cardiores.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Liu P.Y., Liu Y.W., Lin L.J., Chen J.H., Liao J.K. Evidence for statin pleiotropy in humans: Differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oesterle A., Laufs U., Liao J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C.Y., Liu P.Y., Liao J.K. Pleiotropic effects of statin therapy: Molecular mechanisms and clinical results. Trends Mol. Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 37.Li M., Wang H., Zhang X.J., Cai J., Li H. NAFLD: An Emerging Causal Factor for Cardiovascular Disease. Physiology. 2023;38:255–265. doi: 10.1152/physiol.00013.2023. [DOI] [PubMed] [Google Scholar]
- 38.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuniga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechner K., McKenzie A.L., Krankel N., Von Schacky C., Worm N., Nixdorff U., Lechner B., Scherr J., Weingartner O., Krauss R.M. High-Risk Atherosclerosis and Metabolic Phenotype: The Roles of Ectopic Adiposity, Atherogenic Dyslipidemia, and Inflammation. Metab. Syndr. Relat. Disord. 2020;18:176–185. doi: 10.1089/met.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus–Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W., Okunade A., Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc. Natl. Acad. Sci. USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Targher G., Corey K.E., Byrne C.D. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021;47:101215. doi: 10.1016/j.diabet.2020.101215. [DOI] [PubMed] [Google Scholar]
- 43.Kopin L., Lowenstein C. Dyslipidemia. Ann. Intern. Med. 2017;167:Itc81–Itc95. doi: 10.7326/AITC201712050. [DOI] [PubMed] [Google Scholar]
- 44.Hwang H.W., Yu J.H., Jin Y.J., Suh Y.J., Lee J.W. Correlation between the small dense LDL level and nonalcoholic fatty liver disease: Possibility of a new biomarker. Medicine. 2020;99:e21162. doi: 10.1097/MD.0000000000021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duran E.K., Aday A.W., Cook N.R., Buring J.E., Ridker P.M., Pradhan A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020;75:2122–2135. doi: 10.1016/j.jacc.2020.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ipsen D.H., Lykkesfeldt J., Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrucci L., Fabbri E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdallah L.R., de Matos R.C., YPDM E.S., Vieira-Soares D., Muller-Machado G., Pollo-Flores P. Non-alcoholic Fatty Liver Disease and Its Links with Inflammation and Atherosclerosis. Curr. Atheroscler. Rep. 2020;22:7. doi: 10.1007/s11883-020-0820-8. [DOI] [PubMed] [Google Scholar]
- 49.Braza-Boils A., Mari-Alexandre J., Molina P., Arnau M.A., Barcelo-Molina M., Domingo D., Girbes J., Giner J., Martinez-Dolz L., Zorio E. Deregulated hepatic microRNAs underlie the association between non-alcoholic fatty liver disease and coronary artery disease. Liver Int. 2016;36:1221–1229. doi: 10.1111/liv.13097. [DOI] [PubMed] [Google Scholar]
- 50.Targher G., Marra F., Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51:1947–1953. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 51.Johnston M.P., Patel J., Byrne C.D. Causes of Mortality in Non-Alcoholic Fatty Liver Disease (NAFLD) and Alcohol Related Fatty Liver Disease (AFLD) Curr. Pharm. Des. 2020;26:1079–1092. doi: 10.2174/1381612826666200128094231. [DOI] [PubMed] [Google Scholar]
- 52.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z., Sebastiani G., Ekstedt M., Hagstrom H., Nasr P., et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egom E.E.A., Hafeez H. Biochemistry of Statins. Adv. Clin. Chem. 2016;73:127–168. doi: 10.1016/bs.acc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Pockros P.J., Fuchs M., Freilich B., Schiff E., Kohli A., Lawitz E.J., Hellstern P.A., Owens-Grillo J., Van Biene C., Shringarpure R., et al. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 55.Athyros V.G., Boutari C., Stavropoulos K., Anagnostis P., Imprialos K.P., Doumas M., Karagiannis A. Statins: An Under-Appreciated Asset for the Prevention and the Treatment of NAFLD or NASH and the Related Cardiovascular Risk. Curr. Vasc. Pharmacol. 2018;16:246–253. doi: 10.2174/1570161115666170621082910. [DOI] [PubMed] [Google Scholar]
- 56.Yebyo H.G., Aschmann H.E., Kaufmann M., Puhan M.A. Comparative effectiveness and safety of statins as a class and of specific statins for primary prevention of cardiovascular disease: A systematic review, meta-analysis, and network meta-analysis of randomized trials with 94,283 participants. Am. Heart J. 2019;210:18–28. doi: 10.1016/j.ahj.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 57.du Souich P., Roederer G., Dufour R. Myotoxicity of statins: Mechanism of action. Pharmacol. Ther. 2017;175:18–28. doi: 10.1016/j.pharmthera.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 58.Sattar N. Statins and diabetes: What are the connections? Best Pract. Res. Clin. Endocrinol. Metab. 2023;37:101749. doi: 10.1016/j.beem.2023.101749. [DOI] [PubMed] [Google Scholar]
- 59.Del Ben M., Baratta F., Polimeni L., Pastori D., Loffredo L., Averna M., Violi F., Angelico F. Under-prescription of statins in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2017;27:161–167. doi: 10.1016/j.numecd.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Athyros V.G., Tziomalos K., Gossios T.D., Griva T., Anagnostis P., Kargiotis K., Pagourelias E.D., Theocharidou E., Karagiannis A., Mikhailidis D.P. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 61.Abel T., Fehér J., Dinya E., Eldin M.G., Kovács A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with non-alcoholic fatty liver disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009;15:MS6–MS11. [PubMed] [Google Scholar]
- 62.Gomez-Dominguez E., Gisbert J.P., Moreno-Monteagudo J.A., Garcia-Buey L., Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment. Pharmacol. Ther. 2006;23:1643–1647. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 63.Rana H., Yadav S.S., Reddy H.D., Singhal S., Singh D.K., Usman K. Comparative Effect of Insulin Sensitizers and Statin on Metabolic Profile and Ultrasonographical Score in Non Alcoholic Fatty Liver Disease. J. Clin. Diagn. Res. 2016;10:OC19–OC23. doi: 10.7860/JCDR/2016/19887.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pastori D., Pani A., Di Rocco A., Menichelli D., Gazzaniga G., Farcomeni A., D’Erasmo L., Angelico F., Del Ben M., Baratta F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 2021;88:441–451. doi: 10.1111/bcp.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutari C., Pappas P.D., Anastasilakis D., Mantzoros C.S. Statins’ efficacy in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Nutr. 2022;41:2195–2206. doi: 10.1016/j.clnu.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Guo M., Zhao J., Zhai Y., Zang P., Lv Q., Shang D. A prospective study of hepatic safety of statins used in very elderly patients. BMC Geriatr. 2019;19:352. doi: 10.1186/s12877-019-1361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahmud N., Chapin S., Goldberg D.S., Reddy K.R., Taddei T.H., Kaplan D.E. Statin exposure is associated with reduced development of acute-on-chronic liver failure in a Veterans Affairs cohort. J. Hepatol. 2022;76:1100–1108. doi: 10.1016/j.jhep.2021.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim G., Jang S.-Y., Nam C.M., Kang E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018;68:476–484. doi: 10.1016/j.jhep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 69.Zou B., Odden M.C., Nguyen M.H. Statin Use and Reduced Hepatocellular Carcinoma Risk in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2022;21:435–444.e6. doi: 10.1016/j.cgh.2022.01.057. [DOI] [PubMed] [Google Scholar]
- 70.Cohen D.E., Anania F.A., Chalasani N. An assessment of statin safety by hepatologists. Am. J. Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Tokushige K., Ikejima K., Ono M., Eguchi Y., Kamada Y., Itoh Y., Akuta N., Yoneda M., Iwasa M., Yoneda M., et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J. Gastroenterol. 2021;56:951–963. doi: 10.1007/s00535-021-01796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fatima K., Moeed A., Waqar E., Atif A.R., Kamran A., Rizvi H., Suri N.F., Haider H., Shuja S.H., Khalid M., et al. Efficacy of statins in treatment and development of non-alcoholic fatty liver disease and steatohepatitis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2021;46:101816. doi: 10.1016/j.clinre.2021.101816. [DOI] [PubMed] [Google Scholar]
- 73.Krishan S. Correlation between non-alcoholic fatty liver disease (NAFLD) and dyslipidemia in type 2 diabetes. Diabetes Metab. Syndr. 2016;10:S77–S81. doi: 10.1016/j.dsx.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 74.Ji G., Zhao X., Leng L., Liu P., Jiang Z. Comparison of dietary control and atorvastatin on high fat diet induced hepatic steatosis and hyperlipidemia in rats. Lipids Health Dis. 2011;10:23. doi: 10.1186/1476-511X-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chong L.-W., Hsu Y.-C., Lee T.-F., Lin Y., Chiu Y.-T., Yang K.-C., Wu J.-C., Huang Y.-T. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15:22. doi: 10.1186/s12876-015-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu Y.-W., Zhu Y.-C., Zhang L., Li P., Yang J., Wen X.-D. Ilexgenin A enhances the effects of simvastatin on non-alcoholic fatty liver disease without changes in simvastatin pharmacokinetics. Chin. J. Nat. Med. 2018;16:436–445. doi: 10.1016/S1875-5364(18)30077-3. [DOI] [PubMed] [Google Scholar]
- 77.Cho Y., Rhee H., Kim Y.E., Lee M., Lee B.W., Kang E.S., Cha B.S., Choi J.Y., Lee Y.H. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: An open-label randomized controlled trial (ESSENTIAL study) BMC Med. 2022;20:93. doi: 10.1186/s12916-022-02288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han K.H., Rha S.W., Kang H.J., Bae J.W., Choi B.J., Choi S.Y., Gwon H.C., Bae J.H., Hong B.K., Choi D.H., et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage) J. Clin. Lipidol. 2012;6:340–351. doi: 10.1016/j.jacl.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Kargiotis K. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J. Gastroenterol. 2015;21:7860–7868. doi: 10.3748/wjg.v21.i25.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura Y., Hyogo H., Yamagishi S.-i., Takeuchi M., Ishitobi T., Nabeshima Y., Arihiro K., Chayama K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: Clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J. Gastroenterol. 2010;45:750–757. doi: 10.1007/s00535-010-0203-y. [DOI] [PubMed] [Google Scholar]
- 81.Hyogo H., Tazuma S., Arihiro K., Iwamoto K., Nabeshima Y., Inoue M., Ishitobi T., Nonaka M., Chayama K. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–1718. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 82.Dongiovanni P., Petta S., Mannisto V., Mancina R.M., Pipitone R., Karja V., Maggioni M., Kakela P., Wiklund O., Mozzi E., et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 2015;63:705–712. doi: 10.1016/j.jhep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Braun L.R., Feldpausch M.N., Czerwonka N., Weiss J., Branch K., Lee H., Martinez-Salazar E.L., Torriani M., Sponseller C.A., Grinspoon S.K., et al. Effects of Pitavastatin on Insulin Sensitivity and Liver Fat: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2018;103:4176–4186. doi: 10.1210/jc.2018-01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bril F., Portillo Sanchez P., Lomonaco R., Orsak B., Hecht J., Tio F., Cusi K. Liver Safety of Statins in Prediabetes or T2DM and Nonalcoholic Steatohepatitis: Post Hoc Analysis of a Randomized Trial. J. Clin. Endocrinol. Metab. 2017;102:2950–2961. doi: 10.1210/jc.2017-00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inia J.A., Stokman G., Pieterman E.J., Morrison M.C., Menke A.L., Verschuren L., Caspers M.P.M., Giera M., Jukema J.W., van den Hoek A.M., et al. Atorvastatin Attenuates Diet-Induced Non-Alcoholic Steatohepatitis in APOE*3-Leiden Mice by Reducing Hepatic Inflammation. Int. J. Mol. Sci. 2023;24:7818. doi: 10.3390/ijms24097818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pereira E., Araujo B.P., Rodrigues K.L., Silvares R.R., Martins C.S.M., Flores E.E.I., Fernandes-Santos C., Daliry A. Simvastatin Improves Microcirculatory Function in Nonalcoholic Fatty Liver Disease and Downregulates Oxidative and ALE-RAGE Stress. Nutrients. 2022;14:716. doi: 10.3390/nu14030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bu D.-X., Tarrio M., Grabie N., Zhang Y., Yamazaki H., Stavrakis G., Maganto-Garcia E., Pepper-Cunningham Z., Jarolim P., Aikawa M., et al. Statin-induced Krüppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J. Clin. Investig. 2010;120:1961–1970. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hyogo H., Yamagishi S.-I., Maeda S., Kimura Y., Ishitobi T., Chayama K. Atorvastatin improves disease activity of nonalcoholic steatohepatitis partly through its tumour necrosis factor-α-lowering property. Dig. Liver Dis. 2012;44:492–496. doi: 10.1016/j.dld.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 89.Polyzos S.A., Kechagias S., Tsochatzis E.A. Review article: Non-alcoholic fatty liver disease and cardiovascular diseases: Associations and treatment considerations. Aliment. Pharmacol. Ther. 2021;54:1013–1025. doi: 10.1111/apt.16575. [DOI] [PubMed] [Google Scholar]
- 90.Yang J.I., Yoon J.H., Bang Y.J., Lee S.H., Lee S.M., Byun H.J., Myung S.J., Kim W., Lee H.S. Synergistic antifibrotic efficacy of statin and protein kinase C inhibitor in hepatic fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G126–G132. doi: 10.1152/ajpgi.00299.2009. [DOI] [PubMed] [Google Scholar]
- 91.Bosch J., Gracia-Sancho J., Abraldes J.G. Cirrhosis as new indication for statins. Gut. 2020;69:953–962. doi: 10.1136/gutjnl-2019-318237. [DOI] [PubMed] [Google Scholar]
- 92.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., Mills P.R., Keach J.C., Lafferty H.D., Stahler A., et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397.e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez-Ortigosa C.M., Wang W., Zhao C., Zhou J., Zhen Z., Wang Y., Shen C. Simvastatin Ameliorates Liver Fibrosis via Mediating Nitric Oxide Synthase in Rats with Non-Alcoholic Steatohepatitis-Related Liver Fibrosis. PLoS ONE. 2013;8:e76538. doi: 10.1371/journal.pone.0076538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciardullo S., Perseghin G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism. 2021;121:154752. doi: 10.1016/j.metabol.2021.154752. [DOI] [PubMed] [Google Scholar]
- 95.Nascimbeni F., Aron-Wisnewsky J., Pais R., Tordjman J., Poitou C., Charlotte F., Bedossa P., Poynard T., Clément K., Ratziu V. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. doi: 10.1136/bmjgast-2015-000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sfikas G., Psallas M., Koumaras C., Imprialos K., Perdikakis E., Doumas M., Giouleme O., Karagiannis A., Athyros V.G. Prevalence, Diagnosis, and Treatment with 3 Different Statins of Non-alcoholic Fatty Liver Disease/Non-alcoholic Steatohepatitis in Military Personnel. Do Genetics Play a Role? Curr. Vasc. Pharmacol. 2021;19:572–581. doi: 10.2174/1570161118666201015152921. [DOI] [PubMed] [Google Scholar]
- 97.Nelson A., Torres D.M., Morgan A.E., Fincke C., Harrison S.A. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J. Clin. Gastroenterol. 2009;43:990–994. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 98.Oni E.T., Sinha P., Karim A., Martin S.S., Blaha M.J., Agatston A.S., Blumenthal R.S., Meneghelo R.S., Conceiçao R.D., Santos R.D., et al. Statin Use Is Not Associated with Presence of and Severity of Nonalcoholic Fatty Liver Disease. Arch. Med. Res. 2014;45:52–57. doi: 10.1016/j.arcmed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Lee J.I., Lee H.W., Lee K.S., Lee H.S., Park J.-Y. Effects of Statin Use on the Development and Progression of Nonalcoholic Fatty Liver Disease: A Nationwide Nested Case-Control Study. Am. J. Gastroenterol. 2021;116:116–124. doi: 10.14309/ajg.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 100.Pinyopornpanish K., Al-Yaman W., Butler R.S., Carey W., McCullough A., Romero-Marrero C. Chemopreventive Effect of Statin on Hepatocellular Carcinoma in Patients with Nonalcoholic Steatohepatitis Cirrhosis. Am. J. Gastroenterol. 2021;116:2258–2269. doi: 10.14309/ajg.0000000000001347. [DOI] [PubMed] [Google Scholar]
- 101.German M.N., Lutz M.K., Pickhardt P.J., Bruce R.J., Said A. Statin Use is Protective Against Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2020;54:733–740. doi: 10.1097/MCG.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 102.Hendrikx T., Walenbergh S.M.A., Hofker M.H., Shiri-Sverdlov R. Lysosomal cholesterol accumulation: Driver on the road to inflammation during atherosclerosis and non-alcoholic steatohepatitis. Obes. Rev. 2014;15:424–433. doi: 10.1111/obr.12159. [DOI] [PubMed] [Google Scholar]
- 103.Vinué Á., Herrero-Cervera A., González-Navarro H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients. 2018;10:939. doi: 10.3390/nu10070939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ioannou G.N., Haigh W.G., Thorning D., Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 2013;54:1326–1334. doi: 10.1194/jlr.M034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai X., Wang J., Wang J., Zhou Q., Yang B., He Q., Weng Q. Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacol. Res. 2020;155:104720. doi: 10.1016/j.phrs.2020.104720. [DOI] [PubMed] [Google Scholar]
- 106.Hammoutene A., Rautou P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Horn C.L., Morales A.L., Savard C., Farrell G.C., Ioannou G.N. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol. Commun. 2022;6:12–35. doi: 10.1002/hep4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lastuvkova H., Faradonbeh F.A., Schreiberova J., Hroch M., Mokry J., Faistova H., Nova Z., Hyspler R., Igreja Sa I.C., Nachtigal P., et al. Atorvastatin Modulates Bile Acid Homeostasis in Mice with Diet-Induced Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2021;22:6468. doi: 10.3390/ijms22126468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaminsky-Kolesnikov Y., Rauchbach E., Abu-Halaka D., Hahn M., García-Ruiz C., Fernandez-Checa J.C., Madar Z., Tirosh O. Cholesterol Induces Nrf-2- and HIF-1α-Dependent Hepatocyte Proliferation and Liver Regeneration to Ameliorate Bile Acid Toxicity in Mouse Models of NASH and Fibrosis. Oxid. Med. Cell. Longev. 2020;2020:5393761. doi: 10.1155/2020/5393761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Rooyen D.M., Gan L.T., Yeh M.M., Haigh W.G., Larter C.Z., Ioannou G., Teoh N.C., Farrell G.C. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J. Hepatol. 2013;59:144–152. doi: 10.1016/j.jhep.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 111.Ioannou G.N., Van Rooyen D.M., Savard C., Haigh W.G., Yeh M.M., Teoh N.C., Farrell G.C. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J. Lipid Res. 2015;56:277–285. doi: 10.1194/jlr.M053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bravo M., Raurell I., Hide D., Fernández-Iglesias A., Gil M., Barberá A., Salcedo M.T., Augustin S., Genescà J., Martell M. Restoration of liver sinusoidal cell phenotypes by statins improves portal hypertension and histology in rats with NASH. Sci. Rep. 2019;9:20183. doi: 10.1038/s41598-019-56366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khersonsky O., Tawfik D.S. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry. 2005;44:6371–6382. doi: 10.1021/bi047440d. [DOI] [PubMed] [Google Scholar]
- 114.Chistiakov D.A., Melnichenko A.A., Orekhov A.N., Bobryshev Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie. 2017;132:19–27. doi: 10.1016/j.biochi.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 115.Soran H., Younis N.N., Charlton-Menys V., Durrington P. Variation in paraoxonase-1 activity and atherosclerosis. Curr. Opin. Lipidol. 2009;20:265–274. doi: 10.1097/MOL.0b013e32832ec141. [DOI] [PubMed] [Google Scholar]
- 116.Kotani K., Watanabe J., Miura K., Gugliucci A. Paraoxonase 1 and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis. Molecules. 2021;26:2323. doi: 10.3390/molecules26082323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Samy W., Hassanian M.A. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab. J. Gastroenterol. 2011;12:80–85. doi: 10.1016/j.ajg.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 118.Goud B., Liu S., Storrie B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases. 2018;9:66–75. doi: 10.1080/21541248.2017.1384087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laufs U., Wassmann S., Schackmann S., Heeschen C., Böhm M., Nickenig G. Beneficial effects of statins in patients with non-ischemic heart failure. Z. Kardiol. 2004;93:103–108. doi: 10.1007/s00392-004-1005-0. [DOI] [PubMed] [Google Scholar]
- 120.Rikitake Y., Hirata K. Inhibition of RhoA or Rac1? Mechanism of cholesterol-independent beneficial effects of statins. Circ. J. 2009;73:231–232. doi: 10.1253/circj.CJ-08-1078. [DOI] [PubMed] [Google Scholar]
- 121.Rikitake Y., Liao J.K. Rho GTPases, statins, and nitric oxide. Circ. Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Laufs U., Kilter H., Konkol C., Wassmann S., Böhm M., Nickenig G. Impact of HMG CoA reductase inhibition on small GTPases in the heart. Cardiovasc. Res. 2002;53:911–920. doi: 10.1016/S0008-6363(01)00540-5. [DOI] [PubMed] [Google Scholar]
- 123.Schierwagen R., Maybüchen L., Hittatiya K., Klein S., Uschner F.E., Braga T.T., Franklin B.S., Nickenig G., Strassburg C.P., Plat J., et al. Statins improve NASH via inhibition of RhoA and Ras. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;311:G724–G733. doi: 10.1152/ajpgi.00063.2016. [DOI] [PubMed] [Google Scholar]
- 124.Francque S., Szabo G., Abdelmalek M.F., Byrne C.D., Cusi K., Dufour J.-F., Roden M., Sacks F., Tacke F. Nonalcoholic steatohepatitis: The role of peroxisome proliferator-activated receptors. Nat. Rev. Gastroenterol. Hepatol. 2020;18:24–39. doi: 10.1038/s41575-020-00366-5. [DOI] [PubMed] [Google Scholar]
- 125.Bougarne N., Weyers B., Desmet S.J., Deckers J., Ray D.W., Staels B., De Bosscher K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018;39:760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 126.Montagner A., Polizzi A., Fouché E., Ducheix S., Lippi Y., Lasserre F., Barquissau V., Régnier M., Lukowicz C., Benhamed F., et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lefere S., Puengel T., Hundertmark J., Penners C., Frank A.K., Guillot A., de Muynck K., Heymann F., Adarbes V., Defrêne E., et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 2020;73:757–770. doi: 10.1016/j.jhep.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Roglans N., Sanguino E., Peris C., Alegret M., Vázquez M., Adzet T., Díaz C., Hernández G., Laguna J.C., Sánchez R.M. Atorvastatin treatment induced peroxisome proliferator-activated receptor alpha expression and decreased plasma nonesterified fatty acids and liver triglyceride in fructose-fed rats. J. Pharmacol. Exp. Ther. 2002;302:232–239. doi: 10.1124/jpet.302.1.232. [DOI] [PubMed] [Google Scholar]
- 129.Marinho T.d.S., Kawasaki A., Bryntesson M., Souza-Mello V., Barbosa-da-Silva S., Aguila M.B., Mandarim-de-Lacerda C.A. Rosuvastatin limits the activation of hepatic stellate cells in diet-induced obese mice. Hepatol. Res. 2017;47:928–940. doi: 10.1111/hepr.12821. [DOI] [PubMed] [Google Scholar]
- 130.Park H.S., Jang J.E., Ko M.S., Woo S.H., Kim B.J., Kim H.S., Park H.S., Park I.S., Koh E.H., Lee K.U. Statins Increase Mitochondrial and Peroxisomal Fatty Acid Oxidation in the Liver and Prevent Non-Alcoholic Steatohepatitis in Mice. Diabetes Metab. J. 2016;40:376–385. doi: 10.4093/dmj.2016.40.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Day E.A., Ford R.J., Steinberg G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017;28:545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Steinberg G.R., Hardie D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2022;24:255–272. doi: 10.1038/s41580-022-00547-x. [DOI] [PubMed] [Google Scholar]
- 133.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.P., O’Neill H.M., Ford R.J., Palanivel R., O’Brien M., et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim J.-H., Lee J.M., Kim J.-H., Kim K.R. Fluvastatin activates sirtuin 6 to regulate sterol regulatory element-binding proteins and AMP-activated protein kinase in HepG2 cells. Biochem. Biophys. Res. Commun. 2018;503:1415–1421. doi: 10.1016/j.bbrc.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 135.Zhang B., Zhang C., Zhang X., Li N., Dong Z., Sun G., Sun X. Atorvastatin promotes AMPK signaling to protect against high fat diet-induced non-alcoholic fatty liver in golden hamsters. Exp. Ther. Med. 2020;19:2133–2142. doi: 10.3892/etm.2020.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang S.-T., Ho H.J., Lin J.-T., Shieh J.-J., Wu C.-Y. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017;8:e2626. doi: 10.1038/cddis.2016.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascón S., Hatzios S.K., Kagan V.E., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang C.Y., Babitt J.L. Liver iron sensing and body iron homeostasis. Blood. 2019;133:18–29. doi: 10.1182/blood-2018-06-815894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J., Li X., Ge C., Min J., Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. 2022;29:467–480. doi: 10.1038/s41418-022-00941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Protchenko O., Baratz E., Jadhav S., Li F., Shakoury-Elizeh M., Gavrilova O., Ghosh M.C., Cox J.E., Maschek J.A., Tyurin V.A., et al. Iron Chaperone Poly rC Binding Protein 1 Protects Mouse Liver from Lipid Peroxidation and Steatosis. Hepatology. 2021;73:1176–1193. doi: 10.1002/hep.31328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 142.Yao X., Xie R., Cao Y., Tang J., Men Y., Peng H., Yang W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021;19:311. doi: 10.1186/s12951-021-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kitsugi K., Noritake H., Matsumoto M., Hanaoka T., Umemura M., Yamashita M., Takatori S., Ito J., Ohta K., Chida T., et al. Simvastatin inhibits hepatic stellate cells activation by regulating the ferroptosis signaling pathway. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2023;1869:166750. doi: 10.1016/j.bbadis.2023.166750. [DOI] [PubMed] [Google Scholar]
- 144.Sahebkar A., Foroutan Z., Katsiki N., Jamialahmadi T., Mantzoros C.S. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy? Metabolism. 2023;146:155659. doi: 10.1016/j.metabol.2023.155659. [DOI] [PubMed] [Google Scholar]
- 145.Arumugam M., Raes J., Pelletier E., Le Paslier D., Yamada T., Mende D.R., Fernandes G.R., Tap J., Bruls T., Batto J.M., et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ding T., Schloss P.D. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Costea P.I., Hildebrand F., Arumugam M., Bäckhed F., Blaser M.J., Bushman F.D., de Vos W.M., Ehrlich S.D., Fraser C.M., Hattori M., et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2017;3:8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vieira-Silva S., Falony G., Belda E., Nielsen T., Aron-Wisnewsky J., Chakaroun R., Forslund S.K., Assmann K., Valles-Colomer M., Nguyen T.T.D., et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–315. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 149.Khan T.J., Ahmed Y.M., Zamzami M.A., Mohamed S.A., Khan I., Baothman O.A.S., Mehanna M.G., Yasir M. Effect of atorvastatin on the gut microbiota of high fat diet-induced hypercholesterolemic rats. Sci. Rep. 2018;8:662. doi: 10.1038/s41598-017-19013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Khan T.J., Ahmed Y.M., Zamzami M.A., Siddiqui A.M., Khan I., Baothman O.A.S., Mehanna M.G., Kuerban A., Kaleemuddin M., Yasir M. Atorvastatin Treatment Modulates the Gut Microbiota of the Hypercholesterolemic Patients. OMICS A J. Integr. Biol. 2018;22:154–163. doi: 10.1089/omi.2017.0130. [DOI] [PubMed] [Google Scholar]
- 151.Zhang P., Zhang X., Huang Y., Chen J., Shang W., Shi G., Zhang L., Zhang C., Chen R. Atorvastatin alleviates microglia-mediated neuroinflammation via modulating the microbial composition and the intestinal barrier function in ischemic stroke mice. Free Radic. Biol. Med. 2021;162:104–117. doi: 10.1016/j.freeradbiomed.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 152.Hu X., Li H., Zhao X., Zhou R., Liu H., Sun Y., Fan Y., Shi Y., Qiao S., Liu S., et al. Multi-omics study reveals that statin therapy is associated with restoration of gut microbiota homeostasis and improvement in outcomes in patients with acute coronary syndrome. Theranostics. 2021;11:5778–5793. doi: 10.7150/thno.55946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim J., Lee H., An J., Song Y., Lee C.-K., Kim K., Kong H. Alterations in Gut Microbiota by Statin Therapy and Possible Intermediate Effects on Hyperglycemia and Hyperlipidemia. Front. Microbiol. 2019;10:1947. doi: 10.3389/fmicb.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nolan J.A., Skuse P., Govindarajan K., Patterson E., Konstantinidou N., Casey P.G., MacSharry J., Shanahan F., Stanton C., Hill C., et al. The influence of rosuvastatin on the gastrointestinal microbiota and host gene expression profiles. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G488–G497. doi: 10.1152/ajpgi.00149.2016. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X., Coker O.O., Chu E.S.H., Fu K., Lau H.C.H., Wang Y.-X., Chan A.W.H., Wei H., Yang X., Sung J.J.Y., et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. doi: 10.1136/gutjnl-2019-319664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchianò S., Biagioli M., Roselli R., Zampella A., Di Giorgio C., Bordoni M., Bellini R., Morretta E., Monti M.C., Distrutti E., et al. Atorvastatin protects against liver and vascular damage in a model of diet induced steatohepatitis by resetting FXR and GPBAR1 signaling. FASEB J. 2021;36:e22060. doi: 10.1096/fj.202101397R. [DOI] [PubMed] [Google Scholar]
- 157.Jia W., Wei M., Rajani C., Zheng X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell. 2021;12:411–425. doi: 10.1007/s13238-020-00804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen J., Thomsen M., Vitetta L. Interaction of gut microbiota with dysregulation of bile acids in the pathogenesis of nonalcoholic fatty liver disease and potential therapeutic implications of probiotics. J. Cell. Biochem. 2019;120:2713–2720. doi: 10.1002/jcb.27635. [DOI] [PubMed] [Google Scholar]
- 159.Wang L., Wang Y., Wang H., Zhou X., Wei X., Xie Z., Zhang Z., Wang K., Mu J. The influence of the intestinal microflora to the efficacy of Rosuvastatin. Lipids Health Dis. 2018;17:151. doi: 10.1186/s12944-018-0801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He X., Zheng N., He J., Liu C., Feng J., Jia W., Li H. Gut Microbiota Modulation Attenuated the Hypolipidemic Effect of Simvastatin in High-Fat/Cholesterol-Diet Fed Mice. J. Proteome Res. 2017;16:1900–1910. doi: 10.1021/acs.jproteome.6b00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen G., Wang Z., Song W., Liao Y., Wang X., Chen C., Ming J., Cui J., Xu K. Effects of long-term regular oral aspirin combined with atorvastatin to prevent ischemic stroke on human gut microbiota. Eur. J. Pharmacol. 2023;951:175800. doi: 10.1016/j.ejphar.2023.175800. [DOI] [PubMed] [Google Scholar]
- 162.Ioannou G.N. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol. Metab. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 163.Kubeck R., Bonet-Ripoll C., Hoffmann C., Walker A., Muller V.M., Schuppel V.L., Lagkouvardos I., Scholz B., Engel K.H., Daniel H., et al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol. Metab. 2016;5:1162–1174. doi: 10.1016/j.molmet.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kenny D.J., Plichta D.R., Shungin D., Koppel N., Hall A.B., Fu B., Vasan R.S., Shaw S.Y., Vlamakis H., Balskus E.P., et al. Cholesterol Metabolism by Uncultured Human Gut Bacteria Influences Host Cholesterol Level. Cell Host Microbe. 2020;28:245–257 e246. doi: 10.1016/j.chom.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]