Abstract
The intact cervicovaginal mucosa is a relative barrier to the sexual transmission of human immunodeficiency virus type 1 (HIV-1). In the simian immunodeficiency virus (SIV) macaque model of HIV infection, seronegative transient viremia (STV; virus isolation positive followed by repeated negative cultures) occurs after intravaginal inoculation of a low dose of pathogenic SIVmac251 (C. J. Miller, M. Marthas, J. Torten, N. Alexander, J. Moore, G. Doncel, and A. Hendrickx, J. Virol. 68:6391–6400, 1994). Thirty-one adult female macaques that had been inoculated intravaginally with pathogenic SIVmac251 became transiently viremic. One monkey that had been culture negative for a year after SIV inoculation became persistently viremic and developed simian AIDS. No other STV monkey developed persistent viremia or disease. Results of very sensitive assays showed that 6 of 31 monkeys had weak SIV-specific antibody responses. SIV-specific antibodies were not detected in the cervicovaginal secretions of 10 STV monkeys examined. Twenty of 26 monkeys had lymphocyte proliferative responses to p55gag and/or gp130env antigens; 3 of 6 animals, including the monkey that became persistently viremic, had detectable cytotoxic T-lymphocyte (CTL) responses to SIV. At necropsy, lymphoid tissues and vaginal mucosa were virus culture negative, but in 10 of 10 animals, SIV provirus was detected by PCR using gag-specific primer pairs. Fifty percent of the PCR-positive tissue samples were also positive for SIV gag RNA by reverse transcriptase PCR. Thus, transient viremia following intravaginal inoculation of pathogenic SIV is associated with persistent, systemic infection, either latent or very low level productive. Atypical immune responses, characterized by lymphocyte proliferation and some CTL responses in the absence of conventionally detectable antibodies, develop in transiently viremic monkeys.
In the last several years, it has become clear that infection with human immunodeficiency virus type 1 (HIV-1) results in a variety of disease patterns. The majority of HIV-1-infected individuals undergo persistent infection with a long, clinically silent interval which leads to AIDS. But the rate of progression to AIDS varies widely. At one extreme, some individuals experience rapid progression to AIDS and fail to seroconvert to HIV (28, 33, 39). At the other extreme are seropositive individuals with long-term HIV infection and no signs of disease (3, 4, 35). Another pattern of infection with HIV-1, silent infection (15), was proposed to explain the case of some individuals exposed to HIV-1 that neither seroconvert nor develop disease but in whom HIV infection had been detected either by culture or by PCR of peripheral blood cells (16). However, this pattern of HIV infection has not been detected in many cases of repeated exposure to HIV in discordant sexual partners (2), and the subject remains controversial (14, 23). Nevertheless, some HIV-exposed individuals who remain seronegative have cellular immune responses to HIV-1 that would suggest that they had been infected earlier or that they have a silent infection (42).
In the animal model of HIV-1 infection and disease, simian immunodeficiency virus (SIV) infection of macaques, the three patterns of persistent infection with rapid, normal, or no disease progression described above have been found (10, 18). In addition, seronegative transient viremia (STV) was detected in female monkeys that had been inoculated intravaginally with low doses of pathogenic SIVmac (30). Similar observations of SIV transient viremia have been reported following inoculation of low doses of pathogenic SIVmac by the intrarectal (36, 46), oral (48), or intravenous (i.v.) (12) route. In all of these studies, the monkeys did not seroconvert to SIV antigens or develop any signs of disease. Proviral DNA could be detected in peripheral blood cells in the absence of virus culture isolation for prolonged periods following inoculation (36) or only during the early stage of infection (12, 30).
There are several possible explanations for transient viremia following intravaginal inoculation of pathogenic SIVmac: (i) the animals had an abortive infection limited to the genital tract that was cleared by the immune response; (ii) the animals possessed some genetic element of resistance to SIV which impeded viral dissemination beyond the genital tract; (iii) the animals were systemically infected with an attenuated variant of SIVmac. To determine which of these possibilities is most likely, we used virus culture and PCR to characterize the distribution of SIV in the tissues of STV monkeys and undertook detailed analyses of SIV-specific immune responses. In this report we show that SIV had disseminated to the systemic lymphoid tissues of STV monkeys and that spontaneous reactivation of productive infection with subsequent disease progression is a possible outcome of STV. Further, atypical immune responses characterized by lymphocyte proliferation and cytotoxic T-lymphocyte (CTL) activity in the absence of conventional antibody responses were common in STV monkeys. Thus, a latent or a very low level productive infection can occur after exposure to SIV, without overt signs of infection. This type of infection might explain the case of humans exposed to HIV-1 who remain seronegative but who have cell-mediated immune responses to the virus.
MATERIALS AND METHODS
Animals and viruses.
All animals used in this study were colony-bred, multiparous, female rhesus macaques (Macaca mulatta) from the California Regional Primate Research Center. The animals were housed in accordance with the standards of the American Association for the Accreditation of Laboratory Animal Care. The animals were housed in individual cages, and strict biosafety level 2 procedures were followed in the daily care of the animals. When necessary, the animals were immobilized with ketamine HCl (10 mg/kg of body weight; Parke-Davis, Morris Plains, N.J.) injected intramuscularly. The investigators adhered to Guide for the Care and Use of Laboratory Animals (7a). Prior to study, the animals were shown to be negative for antibodies to HIV-2, SIV, simian type D retrovirus, and simian T-cell leukemia virus type 1. Two stocks of SIVmac251 were used for intravaginal inoculation of monkeys as previously reported (30). The virus used to produce both stocks was obtained from R. Desrosiers, New England Regional Primate Research Center.
Necropsy collection and preparation of tissue samples.
A complete necropsy examination was performed on all animals that were experimentally euthanized during the course of the study. Tissues collected at necropsy were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 6 μm, stained with hematoxylin and eosin, and examined by light microscopy. Additional fresh tissue samples were placed aseptically into RPMI 1640 medium supplemented with 1% penicillin, streptomycin, and amphotericin (100× antibiotic solution; Sigma Chemical Co., St. Louis, Mo.) and kept on ice. Lymph node mononuclear cells (LNMC) and lymphocytes from the vaginal mucosa were isolated as previously described (22).
Virus isolation from peripheral blood, lymph nodes, and vaginal mucosa.
For detection of infectious SIV in blood, peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient separation (lymphocyte separation medium; Organon Teknika, West Chester, Pa.) and cocultured with CEMx174 cells (provided by J. A. Hoxie, University of Pennsylvania, Philadelphia) as previously described (20). LNMC from axillary, inguinal, and iliac (genital) lymph nodes were cocultured similarly. Five million PBMC or LNMC were cultured with 2 million CEMx174 cells, and samples of the culture media were assayed regularly for the presence of SIV p27gag by antigen capture enzyme-linked immunosorbent assay (ELISA). Cultures were considered positive if they were antigen positive at two consecutive time points. All cultures were maintained for 8 weeks before being scored as virus negative.
Detection of SIV by PCR amplification.
Nested PCR was carried out on genomic DNA using gag-specific primer pairs in a DNA Thermal Cycler (Perkin-Elmer Cetus, Emeryville, Calif.), using a previously described technique (31). This PCR protocol was found to be more sensitive than the one used in the initial report of STV animals (30). Briefly, cryopreserved PBMC isolated from blood or LNMC obtained at necropsy where washed three times in Tris buffer at 4°C and resuspended at 107 cells/ml; 10 μl of the cell suspension were added to 10 μl of PCR lysis buffer (50 mM Tris HCl [pH 8.3], 0.45% Nonidet P-40, 0.45% Tween 20) with proteinase K 200 μg/ml. The cells were incubated for 3 h at 55°C and then for 10 min at 96°C. Two rounds of 30 cycles of amplification were performed on aliquots of plasmid DNA containing the complete genome of SIVmac1A11 (25) or aliquots of cell lysates, using conditions described elsewhere (47). DNA from uninfected CEMx174 cells was amplified as a negative control in all assays to monitor potential reagent contamination. β-Actin DNA sequences were amplified with two rounds of PCR (30 cycles/round) from all samples to detect potential inhibitors of Taq polymerase in cell lysates. Following the second round of amplification, a 10-μl aliquot of the reaction product was removed and run on a 1.5% agarose gel. Amplified products in the gel were visualized by ethidium bromide staining.
Detection of SIV RNA by RT-PCR.
Selected samples of cryopreserved PBMC or LNMC that were positive or negative for proviral DNA by PCR were further analyzed for viral RNA after extraction of total cellular RNA and reverse transcription using a previously reported method (40). Briefly, 10 μg of cellular RNA was reverse transcribed by using random hexamer primers (Random Primer; Pharmacia Biotech Inc., Piscataway, N.J.) and the reverse transcriptase (RT) enzyme of murine leukemia virus AVM (Boehringer Mannheim Corp., Indianapolis, Ind.). Subsequent PCR amplification with nested gag-specific primers and gel visualization were performed as described above. Controls for contamination were cellular samples from a monkey that was never exposed to SIV.
Serum and vaginal wash antibodies to SIV.
Western blotting of serum samples with SIV antigen was performed as described (45). Vaginal wash samples were collected and assayed for SIV-specific immunoglobulin G (IgG) and IgA as previously described (31). Vaginal washes consist of a mixture of cervical and vaginal secretions and were collected by vigorously infusing 6 ml of sterile phosphate-buffered saline into the vaginal canal and aspirating as much of the instilled volume as possible. Care was taken to ensure that the cervical mucus was bathed in the lavage fluid and that no trauma to the mucosa occurred during the procedure. The samples were immediately snap-frozen on dry ice and stored at −80°C until analysis.
Enhanced chemiluminescence Western blotting of early serum samples was performed as described previously (32), with modifications. A 12% running gel was used with a 6% stacking gel, and 14 μl of SIV antigen was loaded per minigel. One microliter of serum sample was diluted in 1 ml of 4% goat serum in phosphate-buffered saline to react with each blot. The secondary antibody was diluted in 4% goat serum at 1:10,000. This assay is approximately 100 times more sensitive than conventional Western blotting for detection of specific serum antibodies (8). Antibodies to conformational envelope epitopes of SIVmac were measured in a live, infected cell immunofluorescence assay as described elsewhere (38).
Lymphocyte proliferative responses to Gag and Env antigens.
Antigen-specific proliferation was measured in PBMC from fresh blood samples. The cells were suspended at 2 × 106 per ml in RPMI 1640 medium supplemented with 10% fetal calf serum and plated in triplicate at 50 μl per well in 96-well round-bottom microtiter plates. Antigen dilutions or control reagents were plated at 50 μl per well; 100 μl of fresh medium was added after 48 h, and the plates were incubated for 7 days in a CO2 incubator. The wells were pulsed with [3H]thymidine (1 μCi per well; NEN-DuPont Co., Wilmington, Del.) overnight prior to harvest. The plates were aspirated onto fiberglass filters and washed with a cell harvester (Inotech Biosystems International, Lansing, Mich.). The filters were saturated with scintillation cocktail and sealed, and radioactivity in the 3H window was measured with a 96-well scintillation counter (Microbeta 1450; Wallac Biosystems, Gaithersburg, Md.). The SIV antigens were SIVmac239 p55gag produced in baculovirus (provided by S. Ahmad and T. Yilma, University of California—Davis) and gp130env (provided by F. Vogel [National Institutes of Health] via Biomolecular Technology, Frederick, Md.). Because all monkeys had been immunized to tetanus, tetanus toxoid (Connaught Laboratories, Inc., Swiftwater, Pa.) was tested as a positive control antigen. The nucleoprotein of vesicular stomatitis virus, produced in baculovirus (provided by S. Ahmad and T. Yilma), was used as a negative control for p55gag, and medium alone was the control for gp130env and tetanus toxoid. The antigens were tested at 0.1, 1.0, and 10 μg/ml in every assay. A stimulation index (SI), calculated as the mean counts per minute of replicate antigen wells divided by the mean counts per minute of control wells, was scored positive if ≥2.0. This assay was optimized in a series of preliminary experiments. The proliferative responses to SIV antigens of 10 monkeys that had not been exposed to SIV were tested, and it was determined that a level of 200 cpm in the negative control wells was required to eliminate false-positive SIs. Thus, proliferation cultures were excluded from analysis if the negative control counts were <200 cpm. To determine the phenotype of the proliferating cells, CD4+ or CD8+ cell depletion was performed with antibody-coated magnetic beads (Dynabeads-M450 anti-CD4 and anti-CD8; Dynal Inc., Lake Success, N.Y.) immediately prior to culture in two experiments.
SIV-specific CTL responses.
The presence of SIV-specific CTL in PBMC, LNMC, or vaginal mucosa was assessed as previously reported (22). Briefly, lymphocytes isolated from the vaginal mucosa were cultured in a limiting dilution format due to the low number of cells recovered, in parallel with lymphocytes from blood, spleen, and lymph nodes. Lymphocytes were diluted threefold serially for three dilutions in complete medium with replicates of 28 to 30 wells per dilution in 96-well round bottom plates (Fisher Scientific Co., Santa Clara, Calif.). The cells were stimulated with concanavalin A (10 μg/ml; Sigma) and supplemented with irradiated human PBMC as feeder cells at a concentration of 105 per well and 5% human interleukin-2 (Schiapparelli Biosystems, Inc., Columbia, Md.). On day 7 of culture, recombinant human interleukin-2 (20 U/ml; donated by Cetus Corp., Emeryville, Calif.) was added. Cytotoxicity was measured on day 14. Individual wells were split three ways and assayed for cytolytic function in a 5-h chromium release assay against autologous target cells. Autologous B lymphocytes were transformed by herpesvirus papio (594Sx1055 producer cell line; provided by M. Sharp, Southwest Foundation for Biomedical Research, San Antonio, Tex.), infected overnight with wild-type vaccinia virus WR or a recombinant vaccinia virus expressing the p55gag or gp160env of SIVmac239 (provided by L. Giavedoni and T. Yilma, University of California—Davis), and then labeled with 50 μCi of chromium-51 (Na2CrO4; Amersham Holdings, Inc. Arlington Heights, Ill.) per 106 cells. Positive wells were scored from supernatant chromium measured in a 96-well scintillation counter (Microbeta 1450; Wallac Biosystems) and had at least 15% specific lysis, based on a bimodal distribution of chromium release.
RESULTS
The natural history of SIV transiently viremic monkeys.
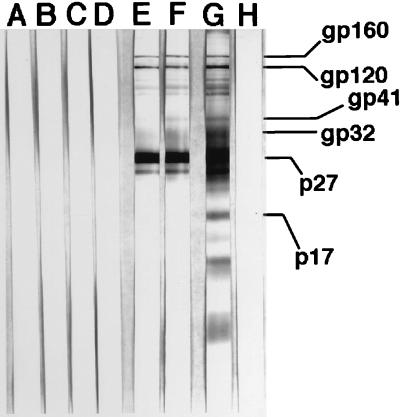
The phenomenon of STV following intravaginal inoculation of pathogenic SIVmac, as initially reported (30), was characterized by (i) intermittent virus isolation from PBMC during the first 10 weeks postinoculation (p.i.; at 30 weeks p.i. in one animal) and (ii) lack of seroconversion to SIV antigens as determined by conventional ELISA and Western blot assays. The prevalence of STV was high, occurring in 31 of 53 vaginally inoculated monkeys, usually in those inoculated with one or more 10-fold dilutions of the challenge virus stock. Following the initial virus isolations, STV monkeys appeared to be uninfected by standard virologic and clinical assessments, including CD8+ T-cell depletion prior to culture of PBMC for SIV and PCR detection of SIV proviral DNA (30). With one exception, they remained healthy until the time of euthanasia, which was performed from 1.2 to 3.4 years p.i. (mean, 2.5 years p.i.). The exception, monkey 20563, was SIV culture negative for more than 1 year, spontaneously became viremic again, and then developed simian AIDS over the next 1.5 years. Around the time of SIV reactivation, this monkey had a relatively strong CTL response but no antibody response to SIV (not shown). As described below, this animal also had a lymphocyte proliferative response to SIV gp130env. Subsequently, antibodies to multiple SIV proteins were detected by Western blotting in the sera of this monkey drawn 12 months prior to euthanasia due to the clinical signs of AIDS (Fig. 1).
FIG. 1.
Seroconversion of transiently viremic monkey 20563 after reactivation of productive infection, determined by Western blotting using whole SIV antigen. Lane E shows the first antibody-positive sample at 31 months p.i. Reactivation of SIV infection was detected as persistent viremia beginning at 24 months p.i. Lanes A to D, 14, 17, 24, and 26 months p.i.; lanes E and F, 31 and 32 months p.i.; lane G, SIV positive control serum; lane H, SIV negative control serum. The positions of the major viral proteins are indicated at the right.
Humoral immunity to SIV in transiently viremic monkeys.
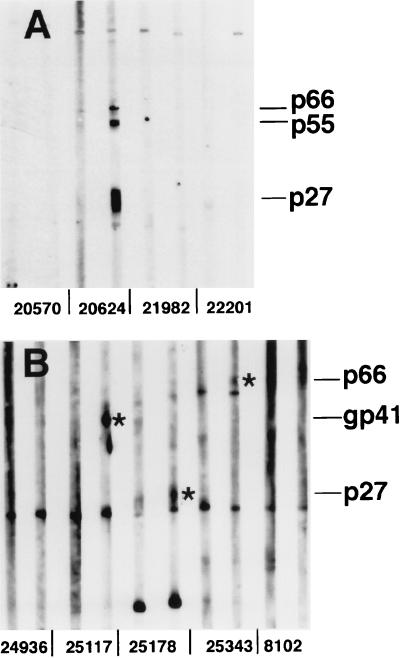
As previously reported, SIV-specific antibodies were not detected in the sera of transiently viremic monkeys by using a conventional whole-virus ELISA or Western blotting or a sensitive ELISA for antibody to gp130env (30). Two additional methods, both of which can detect early seroconversion responses in HIV-1-infected humans (38, 32), were used in an effort to detect SIV-specific antibodies from these animals. Preinoculation and 16 and 24 week p.i., we tested samples from these animals together with samples from SIV-seropositive, persistently viremic monkeys. By 16 or 24 weeks p.i., most persistently infected monkeys had detectable antibodies to SIV (30). By enhanced chemiluminescence Western blotting (32), 5 of 31 transiently viremic monkeys had a detectable antibody response to at least one SIV protein, as shown for 3 STV monkeys in Fig. 2. As shown in Fig. 3 for three other transiently viremic monkeys, there were no detectable antibodies in the sera of a majority of the animals. The sera from 29 animals were also negative for antibodies to SIV in an assay that detects IgG or IgM antibody binding to the surface of live, SIV-infected cells (38). Only one transiently viremic monkey had antibody detectable by this method, at a low titer of 1/20. This animal’s sera were negative for SIV antibody in all other assays. Finally, the vaginal washes of 10 transiently viremic monkeys were tested for IgG and IgA antibodies to SIV by a sensitive ELISA technique (31), and all samples were negative at the low dilutions tested. Thus, there was no evidence for mucosal antiviral antibody production in these STV monkeys inoculated with SIV by the intravaginal route. As noted above, the monkey that had a recurrent productive infection with progression to AIDS, 20563, seroconverted to multiple SIV proteins by Western blotting after the reappearance of SIV viremia, but this animal had no detectable antibodies to SIV at the 16- and 24-week time points tested in the two sensitive antibody assays.
FIG. 2.
Weak antibody responses to SIV in three transiently viremic monkeys, detected by enhanced chemiluminescence Western blotting. For each monkey, there are paired sera: preinoculation serum (left) and 8- or 16-week-p.i. serum (right). (A) Monkeys 20570, 21982, and 22201 are STV animals, and there is no evidence of seroconversion; monkey 20624 is an SIV productively infected animal. (B) Monkeys 25117, 25178, and 25343 are STV animals, and each has antibody to one viral antigen in the p.i. serum (indicated by an asterisk); monkey 24936 is an STV animal with no evidence of seroconversion, and monkey 8102 was not exposed to SIV. Positions of the major viral proteins are indicated at the right.
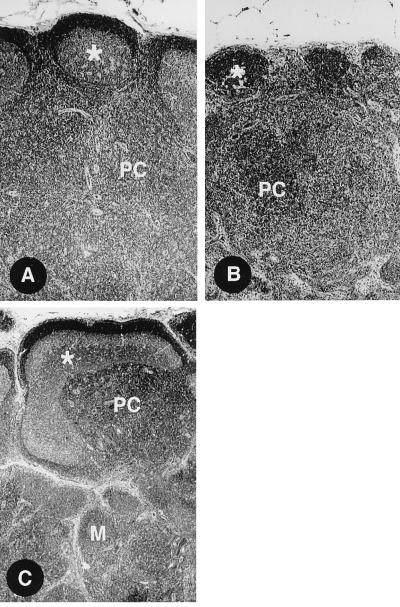
FIG. 3.
Histology of lymph nodes from three STV monkeys at necropsy. These animals were clinically normal and did not appear to be infected. (A) Lymph node with mild paracortical (PC) hyperplasia. The asterisks indicates a follicle germinal center with mild follicular hyperplasia. (B) A collapsed follicle (asterisk) and marked paracortical (PC) hyperplasia. (C) Marked follicular hyperplasia with dysplasia (asterisk) and paracortical (PC) and medullary (M) hyperplasia. All panels are shown at a magnification of ×200.
Lymphocyte proliferative responses to SIV in STV monkeys.
To determine whether transient viremia elicited SIV-specific helper T lymphocytes in STV animals, lymphocyte proliferative responses to SIV Gag and envelope proteins were tested. The assays were performed with multiple blood samples from animals generally taken more than 1 year p.i. and at necropsy. Representative data from five animals are shown in Table 1. All five animals had proliferative responses to tetanus toxoid, the positive control antigen to which all the monkeys had been immunized prior to infection with SIV. In addition, four of five monkeys had proliferative responses to the Gag and/or envelope proteins of SIV. Monkey 24071 responded to both Gag and envelope antigens. With this assay, 20 of 26 STV monkeys tested had a lymphocyte proliferative response to SIV (Table 2). Fifteen monkeys responded to Gag, eight monkeys responded to envelope, and three responded to both antigens. Positive stimulation indices ranged from 2.0 to 25.0 for Gag and from 2.0 to 18.9 for envelope. These responses were similar to or less than the responses to tetanus toxoid (SI range, 2.0 to 98.1). Five of the six animals that had weak antibody responses, as noted above, also had proliferative responses to SIV. The proliferative response for one weakly seropositive monkey was not determined. Proliferation assays were performed with the lymphocytes from blood and lymphoid tissues at necropsy for 10 animals and the results for PBMC were generally the same as for blood samples taken before necropsy. In two experiments which tested responses from purified CD4+ and CD8+ T cells, the proliferative responses of PBMC to SIV antigens were due to expansion of CD4+ T cells.
TABLE 1.
Lymphocyte proliferative responses to SIV Gag and Env proteins from five representative SIV transiently viremic monkeys
| Monkey | Antigen concn (μg/ml) | p55gag
|
gp130env
|
Tetanus toxoidd
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cont cpma | Test cpm | SIc | Cont cpmb | Test cpm | SI | Cont cpmb | Test cpm | SI | ||
| 18421 | 0.1 | 355 ± 127 | 493 ± 88 | 1.4 | 239 ± 102 | 299 ± 90 | 1.3 | |||
| 1.0 | 527 ± 175 | 403 ± 106 | 0.8 | 163 ± 87 | 0.7 | 239 ± 102 | 752 ± 137 | 3.1 | ||
| 10.0 | 562 ± 14 | 678 ± 192 | 1.2 | 217 ± 25 | 0.9 | 1,042 ± 439 | 4.4 | |||
| 18540 | 0.1 | 829 ± 504 | 802 ± 276 | 1.0 | 456 ± 68 | 610 ± 205 | 1.2 | |||
| 1.0 | 519 ± 138 | 681 ± 353 | 1.3 | 1,341 ± 80 | 2.9 | 456 ± 68 | 3,238 ± 786 | 7.1 | ||
| 10.0 | 5,031 ± 770 | 2,236 ± 594 | 0.4 | 2,589 ± 874 | 5.7 | 4,722 ± 1,511 | 10.4 | |||
| 22995 | 0.1 | 516 ± 98 | 348 ± 34 | 0.7 | 249 ± 80 | 245 ± 65 | 1.0 | |||
| 1.0 | 491 ± 77 | 398 ± 48 | 0.8 | 345 ± 144 | 1.4 | 249 ± 80 | 999 ± 404 | 4.0 | ||
| 10.0 | 585 ± 121 | 1,443 ± 378 | 2.5 | 349 ± 84 | 1.4 | 2,217 ± 709 | 8.9 | |||
| 24071 | 0.1 | 477 ± 130 | 606 ± 264 | 1.3 | 287 ± 239 | 243 ± 79 | 1.0 | |||
| 1.0 | 527 ± 221 | 649 ± 286 | 1.2 | 486 ± 215 | 2.0 | 287 ± 239 | 3,099 ± 511 | 13.0 | ||
| 10.0 | 786 ± 292 | 4,179 ± 1,402 | 5.3 | 722 ± 171 | 3.0 | 5,640 ± 711 | 23.6 | |||
| 25343 | 0.1 | 461 ± 161 | 434 ± 190 | 0.9 | 228 ± 50 | 380 ± 53 | 1.7 | |||
| 1.0 | 627 ± 231 | 1,268 ± 223 | 2.0 | 315 ± 65 | 1.4 | 228 ± 50 | 464 ± 27 | 2.0 | ||
| 10.0 | 671 ± 112 | 2,124 ± 246 | 3.2 | 299 ± 56 | 1.3 | 541 ± 186 | 2.4 | |||
Negative control (cont): baculovirus-expressed nucleoprotein of vesicular stomatitis virus in wells.
Negative control: medium only in wells.
As defined in Materials and Methods. Values of ≥2.0 are underlined and scored positive.
Positive control antigen for proliferative capacity; all monkeys were immunized with tetanus toxoid.
TABLE 2.
Most SIV transiently viremic monkeys have lymphocyte proliferative responses to viral antigens
| Monkey | Response to:
|
||
|---|---|---|---|
| p55gag | gp130env | Tetanus toxoid | |
| 7938 | + | − | + |
| 18421 | − | − | + |
| 18462 | − | − | + |
| 18540 | − | + | + |
| 19103 | + | − | + |
| 19147 | + | − | + |
| 19825 | − | + | + |
| 20563 | − | + | + |
| 20570 | + | − | + |
| 21982 | − | − | + |
| 22201 | − | − | + |
| 22995 | + | − | + |
| 23051 | + | + | + |
| 23205 | − | − | + |
| 23363 | − | + | + |
| 23774 | + | + | + |
| 23545 | + | − | + |
| 23676 | + | − | + |
| 24071 | + | + | + |
| 24300 | + | − | + |
| 24739 | + | − | − |
| 24842 | − | − | + |
| 24936 | + | − | + |
| 25117 | + | − | + |
| 25178 | − | + | + |
| 25343 | + | − | − |
CTL responses to SIV in STV monkeys.
Five transiently viremic monkeys and monkey 20563, serendipitously sampled near the time of recrudescence of her SIV viremia, were selected for studies of the antiviral CTL response. Following antigen-specific restimulation in vitro (21), the PBMC of monkey 20563 had detectable CTL activity targeted to both Gag and envelope proteins (not shown). In addition, three of the five transiently viremic monkeys tested had secondary SIV-specific CTL in their PBMC (not shown). These three STV monkeys also had a lymphocyte proliferative response to SIV antigens. The PBMC of one animal were clearly negative for CTL activity; for another animal, the results were indeterminant due to high background lysis of target cells. In addition to analyzing CTL activity in PBMC, we tested four animals for SIV-specific CTL in tissues. At necropsy, the lymphocytes from multiple lymphoid tissues and from the vaginal epithelium were obtained from monkey 20563 and from three STV monkeys. By limiting dilution analysis, precursor CTL were detected from the vaginal epithelium of monkey 20563 and one of three transiently viremic animals (Table 3). Both monkey 20563 and transiently viremic monkey 23676 had precursor CTL targeted to Gag and/or envelope antigens in peripheral blood and in the vaginal mucosa. The precursor frequencies for antiviral CTL from the STV monkey were low but within the range reported previously for SIV-infected or vaccinated rhesus monkeys (49, 17).
TABLE 3.
SIV-specific CTL in the peripheral blood and vaginal epithelium
| Monkey | Precursor CTL frequency/106 lymphocytes (95% confidence interval)
|
|||
|---|---|---|---|---|
| PBMC
|
Vaginal epithelium
|
|||
| Gag | Env | Gag | Env | |
| 23676 | 45 (23–66) | 29 (12–45) | <1 | 83 (39–127) |
| 20563 | 309 (203–446) | 82 (48–117) | 128 (60–197) | 76 (28–123) |
Persistent, systemic SIV infection.
At necropsy of the STV monkeys, no specific gross pathology was observed. Histologic examination of lymphoid tissues disclosed a variable pattern of lymphoid hyperplasia (Fig. 3), which is not found in the lymph nodes of normal, adult monkeys in the colony. Infectious virus was not isolated from lymphoid tissues or genital mucosa by coculture of mononuclear cells with CEMx174 cells. However, as shown in Table 4, SIV proviral DNA was detected by nested PCR in the PBMC and/or lymphoid tissues of 10 of 10 monkeys tested. SIV DNA was detected more frequently in lymphoid tissues than in blood (Table 4), and in every case of PBMC positivity, proviral DNA was also detected in lymphoid tissues. In addition, viral RNA was detected by RT-PCR in some of the tissues that were SIV provirus positive from four of seven animals (5 of 12 tested samples were positive for viral RNA). Thus, the phenomenon of STV following intravaginal inoculation of SIV is characterized by a latent or very low level productive infection with some activation of the immune system, as suggested by the one case of spontaneous reactivation of SIV productive infection, by PCR evidence of occult infection, and by the moderate to marked lymphoid hyperplasia (Fig. 3).
TABLE 4.
Detection of SIV proviral DNA in lymphoid tissues of transiently viremic rhesus macaques by PCR
| Monkey | Detection of SIV DNA in:
|
||||
|---|---|---|---|---|---|
| PBMC | Genital node | Axillary node | Mesenteric node | Spleen | |
| 17562 | + | + | + | + | − |
| 19103 | − | − | + | − | − |
| 19147 | − | + | − | + | + |
| 20570 | + | − | + | − | + |
| 21982 | − | − | + | − | − |
| 22201 | − | − | − | + | − |
| 22995 | + | + | − | − | + |
| 23061 | − | − | − | + | − |
| 23363 | − | + | − | − | + |
| 24936 | − | − | − | − | + |
DISCUSSION
Without rigorous cocultures of PBMC at regular, frequent time points p.i., the STV monkeys in this report would have been judged to be uninfected (30). However, we were able to detect subtle immune responses in a majority of the animals. Occult infection was not confined to the reproductive tract in those animals examined at necropsy; rather, the virus had disseminated to systemic lymphoid tissues. In one STV monkey, occult infection progressed and the animal became persistently viremic and developed simian AIDS. Thus, the phenomenon of seronegative transient viremia in rhesus macaques following intravaginal inoculation of pathogenic SIVmac is more likely due to latent or very low level productive infection rather than to an abortive infection or immune viral clearance. Similar observations were reported for cats infected with feline immunodeficiency virus (9, 34) and for chimpanzees infected with HIV-1 (44). Whether latent infection of nonpermissive cells is a strategy for lentivirus persistence is not known (1), and there is currently no operational definition of latent infection in vivo for HIV or SIV.
We initially hypothesized that transient viremia following intravaginal inoculation of SIVmac was due to one of two mechanisms: (i) infection confined primarily to the reproductive tract or (ii) infection with an attenuated variant of SIVmac251. Because transient viremia usually occurred after inoculation of a 10-fold or greater dilution of the challenge virus stock (30), either a limited number of infected cells would allow the host immune response to clear the infection with minimal opportunity for viral dissemination to systemic lymphoid tissues or, by chance alone, an attenuated virus present at relatively high frequency in the inoculum was transmitted. SIVmac1A11 is an infectious, attenuated molecular clone that was derived from a stock of SIVmac251 (25). SIVmac1A11 causes transient viremia without disease in rhesus monkeys (26). However, three observations make it unlikely that transient viremia following intravaginal inoculation of SIVmac251 is the result of infection with an attenuated virus with the exact phenotype of SIVmac1A11. First, one transiently viremic monkey in this report, 20563, developed a productive infection and simian AIDS. A similar pattern of recrudescence of productive infection following transient viremia after intrarectal inoculation of a different stock of pathogenic SIVmac251 was reported by other investigators (46). Reversion of an attenuated clone of SIVmac to virulence in vivo is possible (50), but no recurrence of productive infection or disease has been observed in monkeys infected with SIVmac1A11 for up to 9 years (24). Second, animals infected with SIVmac1A11 by i.v. inoculation develop humoral immune responses, including neutralizing antibody (25, 27), that were not observed in transiently viremic monkeys after intravaginal inoculation of SIVmac251. Third, SIVmac1A11 is not efficiently transmitted by intravaginal inoculation (29). Despite these observations, infection with an attenuated variant of SIVmac251 that is distinct from SIVmac1A11 remains a possible explanation for STV. Rescue and animal passage of viral isolates from STV animals will be required to demonstrate this. Another possible explanation for transient viremia following low-dose inoculation of pathogenic stocks of SIVmac is that viral latency may be favored (5, 19) in the case of an infection of only a few target cells that do not release sufficient cytokines to activate T cells and macrophages (51).
In this study, STV monkeys failed to make antiviral antibody responses detectable by conventional methods. However, 6 of 31 animals studied with more sensitive assays had weak antibody responses to infection. In contrast, the majority of STV monkeys had lymphocyte proliferative responses to SIV antigens, as noted by other investigators (11, 46), and two of five monkeys tested had antiviral CTL, including CTL in the vaginal mucosa. We propose that the subtle immune responses to SIV observed in transiently viremic monkeys comprise a state of helper T-cell priming that would progress to a fully differentiated immune response had viral replication and viral antigen production continued above a threshold level. In fact, monkey 20563 developed antibodies to all the major viral proteins once her infection had reactivated (Fig. 1).
It has been proposed that cellular immunity, in the absence of humoral immunity, could be protective in SIV and HIV infections (7). In one report, two monkeys that did not develop persistent, productive infection following a low-dose rectal inoculation were protected from infection following a second intrarectal challenge (6). Similarly, four monkeys that failed to develop persistent viremia following a low-dose intrarectal inoculation of SIVmac were protected from a subsequent high-dose challenge (43). These same animals were not protected from a subsequent i.v. challenge (46). In a third report, two of four monkeys that had been exposed to HIV-2 by i.v. inoculation and that were seronegative were protected from a subsequent rectal challenge with pathogenic SIVmac (37). In contrast, six STV monkeys that had been exposed to two low doses of SIV by the i.v. route were not protected from infection following a third high-dose i.v. challenge (12). Based on these four reports of protection from pathogenic virus challenge, one could propose that the partial immunity induced by transient SIV viremia would protect against a subsequent mucosal but not a parenteral virus challenge. In contrast, other results suggest that protection from a mucosal challenge with pathogenic SIVmac239 requires persistent low-level infection with an attenuated virus (31).
In the spectrum of infection and disease induced by pathogenic strains of SIV in the rhesus monkey, the condition of seronegative transient viremia is one extreme. Animals with occult SIV infection appear to be uninfected, using conventional antibody assays. Does this condition model the case of humans at high risk for HIV infection who are persistently seronegative? While the infection status of HIV exposed individuals is controversial, some HIV-exposed individuals have CD8+ T-cell responses to the virus that usually require productive infection for their induction (41). If occult HIV infection does occur, then reactivation of productive infection would likely manifest as seroconversion without an identified exposure to HIV, or as the onset of symptoms and progression to AIDS very many years after exposure and infection (13). There is clearly a need to recognize (and define) or repudiate latent infection in vivo by HIV.
ACKNOWLEDGMENTS
The expert technical support of Linda Antipa, David Bennet, Paul Brosio, and Steven Joye was crucial for the successful completion of this work.
Support for this work was provided by NIH grants RR00169 and AI35545.
REFERENCES
- 1.Ahmed R, Morris L A, Knipe D M. Persistence of viruses. In: Fields B N, Knipe D M, Howley P, et al., editors. Fields Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 219–249. [Google Scholar]
- 2.Brettler D, Somasundaran M, Forsberg A, Krause E, Sullivan J. Silent human immunodeficiency virus type 1 infection: a rare occurrence in a high-risk heterosexual population. Blood. 1992;80:2396–2400. [PubMed] [Google Scholar]
- 3.Buchbinder S, Katz M, Hessol N, O’Malley P, Holmberg S. Long term HIV-1 infection without immunologic progression. AIDS. 1994;8:1179–1182. doi: 10.1097/00002030-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Qin L, Zhang L, Safrit J, Ho D. Virologic and immunologic characterization of long term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Saksela K, Andino R, Baltimore D. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. JVirol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerici M, Clark E, Polacino P, Axberg I, Kuller L, Casey N, Morton W, Shearer G, Benveniste R. T-cell proliferation to subinfectious SIV correlates with lack of infection after challenge of macaques. AIDS. 1994;8:1391–1395. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Clerici M, Shearer G. Correlates of protection in HIV infection and the progression of HIV infection to AIDS. Immunol Lett. 1996;51:69–73. doi: 10.1016/0165-2478(96)02557-6. [DOI] [PubMed] [Google Scholar]
- 7a.Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington, D.C: Institute of Laboratory Animal Resources, National Research Council; 1996. [Google Scholar]
- 8.Dalessio J, Ashley R. Highly sensitive enhanced chemiluminescence immunodetection method for herpes simplex virus type 2 Western immunoblot. J Clin Microbiol. 1992;30:1005–1007. doi: 10.1128/jcm.30.4.1005-1007.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandekar S, Beebe A, Barlough J, Phillips T, Elder J, Torten M, Pedersen N. Detection of feline immunodeficiency virus (FIV) nucleic acids in FIV-seronegative cats. J Virol. 1992;66:4040–4049. doi: 10.1128/jvi.66.7.4040-4049.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel M, Letvin N, Sehgal P, Hunsmann G, Schmidt D, King N, Desrosiers R. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987;68:3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- 11.Dittmer U, Nisslein T, Bodemer W, Petry H, Sauermann U, Stahl-Hennig C, Hunsmann G. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology. 1995;212:392–397. doi: 10.1006/viro.1995.1496. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer U, Stahl-Hennig S, Coulibaly C, Nisslein T, Luke W, Fuchs D, Bodemer W, Petry H, Hunsmann G. Repeated exposure of rhesus macaques to low doses of simian immunodeficiency virus (SIV) did not protect them against the consequence of high-dose SIV challenge. J Gen Virol. 1995;76:1307–1315. doi: 10.1099/0022-1317-76-6-1307. [DOI] [PubMed] [Google Scholar]
- 13.El-Sadr W, Gettler J. Unrecognized human immunodeficiency virus infection in the elderly. Arch Intern Med. 1995;155:184–186. [PubMed] [Google Scholar]
- 14.Frenkel L, Mullins J, Learn G, Manns-Arcuino L, Herring B, Kalish M, Steketee R, Thea D, Nichols J, Liu S, Harmache A, He X, Muthui D, Madan A, Hood L, Haase A, Zupancic M, Staskus K, Wolinsky S, Krogstad P, Zhao J, Chen I, Koup R, Ho D, Korber B, Apple R, Coombs R, Pahwa S, Roberts N. Genetic evaluation of suspected cases of transient HIV-1 infection in infants. Science. 1998;280:1073–1077. doi: 10.1126/science.280.5366.1073. [DOI] [PubMed] [Google Scholar]
- 15.Haseltine W. Silent HIV infections. N Engl J Med. 1989;320:1487–1489. doi: 10.1056/NEJM198906013202210. [DOI] [PubMed] [Google Scholar]
- 16.Imagawa D T, Lee M H, Wolinsky S M, Sano K, Morales F, Kwok S, Sninsky J J, Nishanian P G, Giorgi J, Fahey J L, Dudley J, Visscher B R, Detels R. Human immunodeficiency virus type 1 infection in homosexual men who remain seronegative for prolonged periods. N Engl J Med. 1989;320:1458–1462. doi: 10.1056/NEJM198906013202205. [DOI] [PubMed] [Google Scholar]
- 17.Le Borgne S, Mancini M, Le Grand R, Schleef M, Dormont D, Tiollais P, Riviere Y, Michel M L. In vivo induction of specific cytotoxic T lymphocytes in mice and rhesus macaques immunized with DNA vector encoding an HIV epitope fused with hepatitis B surface antigen. Virology. 1998;240:340–315. doi: 10.1006/viro.1997.8942. [DOI] [PubMed] [Google Scholar]
- 18.Letvin N, King N. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquired Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 19.Li X D, Moore B, Cloyd M W. Gradual shutdown of virus production resulting in latency is the norm during the chronic phase of human immunodeficiency virus replication and differential rates and mechanisms of shutdown are determined by viral sequences. Virology. 1996;225:196–212. doi: 10.1006/viro.1996.0588. [DOI] [PubMed] [Google Scholar]
- 20.Lohman B, Higgins J, Marthas M, Marx P, Pedersen N. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991;29:2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman B, McChesney M, Miller C, McGowan E, Joye S, Van Rompay K, Reay E, Antipa L, Pedersen N, Marthas M. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance of pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohman B L, Miller C J, McChesney M B. Antiviral cytotoxic T lymphocytes in vaginal mucosa of simian immunodeficiency virus-infected rhesus macaques. J Immunol. 1995;155:5855–5860. [PMC free article] [PubMed] [Google Scholar]
- 23.MacGregor R, Dublin G, Frank I, Hodinka R, Friedman H. Failure of culture and polymerase chain reaction to detect human immunodeficiency virus HIV) in seronegative steady sexual partners of HIV infected individuals. Clin Infect Dis. 1995;21:122–127. doi: 10.1093/clinids/21.1.122. [DOI] [PubMed] [Google Scholar]
- 24.Marthas, M. (University of California—Davis). 1998. Personal communication.
- 25.Marthas M, Banapour B, Sutjipto S, Siegel M, Marx P, Gardner M, Pedersen N, Luciw P. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J Med Primatol. 1989;18:311–319. [PubMed] [Google Scholar]
- 26.Marthas M, Ramos R, Lohman B, Van Rompay K, Unger R, Miller C, Banapour B, Pedersen N, Luciw P. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67:6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marthas M, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw P, Marx P, Pedersen N. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michael N, Brown A, Voigt R, Frankel S, Mascola J, Brothers K, Louder M, Birx D, Cassol S. Rapid disease progression without seroconversion following primary human immunodeficiency virus type 1 infection—evidence for highly susceptible human hosts. J Infect Dis. 1997;175:1352–1359. doi: 10.1086/516467. [DOI] [PubMed] [Google Scholar]
- 29.Miller C, Marthas M, Greenier J, Lu D, Dailey P, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV) in rhesus macaques. J Virol. 1998;72:3248–3258. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller C, Marthas M, Torten J, Alexander N, Moore J, Doncel G, Hendrickx A. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller C, McChesney M, Lu X, Dailey P, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohamed O, Ashley R, Goldstein A, McElrath J, Dalessio J, Corey L. Detection of rectal antibodies to HIV-1 by a sensitive chemiluminescent western blot immunodetection method. J Acquired Immune Defic Syndr. 1994;7:375–380. [PubMed] [Google Scholar]
- 33.Montagnier L, Brenner C, Chamaret S, Guetard D, Blanchard A, Martin J, Pialou G, Gougeon M. Human immunodeficiency virus infection and AIDS in a person with negative serology. J Infect Dis. 1997;175:955–959. doi: 10.1086/513999. [DOI] [PubMed] [Google Scholar]
- 34.O’Neil L L, Burkhard M J, Obert L A, Hoover E A. Regression of feline immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1997;13:713–718. doi: 10.1089/aid.1997.13.713. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O, Demarest J, Montefiori D, Orenstein J, Fox C, Schrager L, Margolick J, Buchbinder S, Giorgi J, Fauci A. Studies in subjects with long term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 36.Pauza C, Emau P, Salvato M, Trivedi P, MacKenzie D, Schultz K, Malkovsky M. Pathogenesis of SIVmac251 after atraumatic inoculation of the rectal mucosa in rhesus monkeys. J Med Primatol. 1993;22:154–161. [PubMed] [Google Scholar]
- 37.Putkonen P, Makitalo B, Bottinger D, Biberfeld G, Thorstensson R. Protection of human immunodeficiency virus type 2-exposed seronegative macaques from mucosal simian immunodeficiency virus transmission. J Virol. 1997;71:4981–4984. doi: 10.1128/jvi.71.7.4981-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Race E, Ramsey K, Lucia H, Cloyd M. Human immunodeficiency virus infection elicits early antibody not detected by standard tests: implications for diagnostics and viral immunology. Virology. 1991;184:716–722. doi: 10.1016/0042-6822(91)90441-d. [DOI] [PubMed] [Google Scholar]
- 39.Reimer L, Mottice S, Schable C, Sullivan P, Nakashima A, Rayfield M, Den R, Brokopp C. Absence of detectable antibody in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1997;25:98–100. doi: 10.1086/514491. [DOI] [PubMed] [Google Scholar]
- 40.Rota P A, Khan A S, Durigon E, Yuran T, Villamarzo Y S, Bellini W J. Detection of measles virus RNA in urine specimens from vaccine recipients. J Clin Microbiol. 1995;33:2485–2488. doi: 10.1128/jcm.33.9.2485-2488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowland-Jones S, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Curr Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 42.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 43.Salvato M, Emau P, Malkovsky M, Schultz K, Johnson E, Pauza C. Cellular immune responses in rhesus macaques infected rectally with low dose simian immunodeficiency virus. J Med Primatol. 1994;23:125–130. doi: 10.1111/j.1600-0684.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 44.Shibata R, Siemon C, Rizvi T, Matano T, Satterfield W, Lane H, Martin M. Reactivation of HIV type 1 in chronically infected chimpanzees following xeno stimulation with human cells or with pulses of corticosteroid. AIDS Res Hum Retroviruses. 1997;13:377–381. doi: 10.1089/aid.1997.13.377. [DOI] [PubMed] [Google Scholar]
- 45.Sutjipto S, Pedersen N C, Miller C J, Gardner M B, Hanson C V, Gettie A, Jennings M, Higgins J, Marx P A. Inactivated simian immunodeficiency virus vaccine failed to protect rhesus macaques from intravenous or genital mucosal infection but delayed disease in intravenously exposed animals. J Virol. 1990;64:2290–2297. doi: 10.1128/jvi.64.5.2290-2297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trivedi P, Horejsh D, Hinds S, Wu M, Salvato M, Pauza C. Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: selective amplification and host responses to transient or persistent viremia. J Virol. 1996;70:6876–6883. doi: 10.1128/jvi.70.10.6876-6883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unger R, Marthas M, Pratt-Lowe E, Padrid P, Luciw P. The nef gene of simian immunodeficiency virus SIVmacA11. J Virol. 1992;66:5432–5442. doi: 10.1128/jvi.66.9.5432-5442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Rompay K, Marthas M, Lifson J, Berardi C, Vasquez G, Agatep E, Dehqanzada Z, Cundy K, Bischofberger N, Pedersen N. Administration of 9-{2-(phosphonomethoxy)-propyl}adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998;14:761–773. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 49.Venet A, Bourgault I, Aubertin A, Kieny M, Levy J. Cytotoxic T lymphocyte response against multiple simian immunodeficiency virus (SIV) in SIV infected macaques. J Immunol. 1992;148:2899–2908. [PubMed] [Google Scholar]
- 50.Whatmore A, Cook N, Hall G, Sharpe S, Rud E, Cranage M. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W, Lackner A, Simon M, Durand-Gasselin I, Galanaud P, Desrosiers R, Emilie D. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]