Abstract
People living with HIV are affected by the chronic consequences of neurocognitive impairment (NCI) despite antiretroviral therapies that suppress viral replication, improve health and extend life. Furthermore, viral suppression does not eliminate the virus, and remaining infected cells may continue to produce viral proteins that trigger neurodegeneration. Comorbidities such as diabetes mellitus are likely to contribute substantially to CNS injury in people living with HIV, and some components of antiretroviral therapy exert undesirable side effects on the nervous system. No treatment for HIV-associated NCI has been approved by the European Medicines Agency or the US Food and Drug Administration. Historically, roadblocks to developing effective treatments have included a limited understanding of the pathophysiology of HIV-associated NCI and heterogeneity in its clinical manifestations. This heterogeneity might reflect multiple underlying causes that differ among individuals, rather than a single unifying neuropathogenesis. Despite these complexities, accelerating discoveries in HIV neuropathogenesis are yielding potentially druggable targets, including excessive immune activation, metabolic alterations culminating in mitochondrial dysfunction, dysregulation of metal ion homeostasis and lysosomal function, and microbiome alterations. In addition to drug treatments, we also highlight the importance of non-pharmacological interventions. By revisiting mechanisms implicated in NCI and potential interventions addressing these mechanisms, we hope to supply reasons for optimism in people living with HIV affected by NCI and their care providers.
Introduction
Acquired neurocognitive impairment (NCI) affects 30–50% of the 38 million people living with HIV worldwide1,2. Persistent NCI is a major concern for the ageing population of people living with HIV who are affected by the chronic, negative consequences of this condition3. By highlighting interventions that treat or prevent HIV-associated NCI, we hope to supply reasons for optimism in affected individuals. Among the general mechanisms that underlie HIV-associated NCI are chronic inflammation, oxidative stress and direct neurotoxicity of viral proteins. In subsequent sections, we specifically and precisely describe these potentially targetable mechanisms.
HIV infects and replicates in immune cells, particularly in CD4+ T cells, macrophages and microglia. It causes cellular dysfunction and disease by a variety of mechanisms, such as progressive CD4+ T cell depletion and inflammation. The prognosis for people living with HIV has made great strides forward over the past two decades as effective, well-tolerated antiretroviral therapies (ART) have evolved. In most people living with HIV, these combination drug regimens suppress viral replication, restore CD4+ T cell numbers, slow, stop and even partially reverse disease progression, reduce the likelihood of opportunistic diseases and cancer, and greatly extend lifespan. While ART has not been specifically approved to ameliorate NCI by the European Medicines Agency or the US Food and Drug Administration (FDA), individuals initiating ART often demonstrate substantial, clinically significant improvements in cognitive performance3,4, particularly when ART is initiated early, before substantial immunosuppression occurs5. Nevertheless, some degree of NCI persists in many individuals and does not appear to improve in the long term6. While frank dementia is now very uncommon, milder HIV-associated NCI persists in the context of suppression of viral replication. The remaining lingering mild NCI is associated with poor health outcomes and quality of life in people living with HIV1,7,8. Several ART trials designed specifically to target HIV-associated NCI have shown little effectiveness3,9, and numerous plausible explanations for the lack of effectiveness have been advanced. For example, an intensification trial that added abacavir, a drug with good, predicted CNS penetration, to existing background ART, did not show neurocognitive benefit. Subsequent assays found that two-thirds of patients had baseline resistance to abacavir, probably because of prior exposure to other drugs in the same class (for example, HIV nucleoside reverse transcriptase inhibitors).
The clinical literature on NCI in HIV is characterized by numerous areas of debate, controversy and inconsistent findings. These include concerns about nomenclature and varying reports on the prevalence and incidence of NCI. In our discussion of these various areas of study, we highlight several sources of disagreement. Discrepancies arise from factors such as differences in NCI prevalence depending on the selection of neurocognitive test instruments, variations in cohort composition (for example, proportions of men versus women, different racial or ethnic groups, differential access to care, and specific antiretroviral treatment regimens), the rapid evolution of specific antiretroviral combinations resulting in long-term group trends, and differences in the thresholds for designating impaired performance (for example, one standard deviation in two domains versus two standard deviations).
Nomenclature, epidemiology and clinical impact of HIV-associated NCI
In 2007, a multinational consensus document outlined the terminology for HIV-associated neurocognitive disorders, including HIV-associated dementia, mild neurocognitive disorder and asymptomatic neurocognitive impairment (ANI)10. Diagnosis of these conditions is based on impaired performance in multiple neurocognitive domains in combination with a clinical history including premorbid or ongoing conditions, impact on activities of daily living, medical and neurological examination, and laboratory testing designed to identify conditions other than HIV (‘confounds’) that contribute to or in some cases fully account for NCI and functional impairments. However, with the rapid advancement of ART and the ageing of people living with HIV, the patterns of neurocognitive dysfunction are evolving. The classification terminology is debated, particularly the importance of ANI11–13. Some investigators suggest that isolated test results showing NCI (ANI) might represent false positives or artefacts of inadequate normative corrections that are clinically insignificant, are related to comorbidities rather than HIV itself, and have no basis in neuropathogenesis. Counter arguments include observations that ANI often progresses to symptomatic impairment and is associated with blood, cerebrospinal fluid (CSF) and neuroimaging biomarkers of neuronal injury and neuroinflammation that have plausible connections to HIV itself14–16. Recognizing the continuing debate in this area, we have chosen to use the broader term HIV-associated NCI.
Despite sustained ART and viral suppression, HIV-associated NCI affects 30–50% of people living with HIV1,17–19. Key symptoms are difficulties with attention and sustained focus, forgetfulness, and loss of the ability to perform some cognitive tasks that the person previously managed with relative ease. Additionally, depression often accompanies this condition. HIV-associated NCI adversely impacts ART adherence20, driving20, quality of life21–24, independence in daily activities11,16,17 and life expectancy25. The risk of HIV-associated NCI significantly increases in people living with HIV aged 50 years and older, who now comprise the largest and fastest-growing age group of people living with HIV in Europe and the USA26–29. Legacy effects contribute to the high prevalence of NCI in people living with HIV. This concept is supported by the well-established observation that lower nadir levels of CD4+ T cells, which reflect a history of advanced immunosuppression, are associated with an increased risk of NCI30,31. Additionally, people living with HIV show premature and accelerated neurocognitive ageing23,24, leaving them vulnerable to functional decline25,26 and impaired quality of life27. Unlike Alzheimer disease (AD) and many other neurodegenerative disorders, HIV-associated NCI is not inexorably progressive32, but the cumulative effect of decades of neurocognitive disability is profound.
Ethnic and racial disparities in HIV-associated NCI have received increasing attention over the past few years. In the USA, new HIV infection rates are eight and four times higher, respectively, among Black and Latino people than in non-Latino white people. Black and Latino people account for 65% of the national population of people living with HIV33. These groups are underserved clinically and under-represented in research. Despite inconsistent findings across studies, some have reported that Black people living with HIV have cognitive impairment more frequently than white people living with HIV in the USA34–36. Similarly, Latino people living with HIV of diverse heritage (Mexican and Puerto Rican) and language use (speaking primarily English and Spanish), and who live in different geographical regions in the USA37–40, show more frequent NCI and faster longitudinal neurocognitive decline than non-Latino white people living with HIV.
In 2021, 38.4 million people were living with HIV worldwide, with the vast majority in low-income and middle-income countries, including 53% in eastern and southern Africa2. Although estimates vary according to the viral clade (subtype), regional subpopulations and assessment methods, HIV-associated NCI is quite prevalent in these regions41–43. Notable disparities in access to ART and other medical resources are likely to drive at least part of the increased risk of HIV-associated NCI42, yet there are important gaps in our understanding of the underlying mechanisms and management of HIV-associated NCI in these settings.
Neuropathogenesis of HIV-associated NCI
A variety of molecular mechanisms contribute to HIV-associated NCI; we focus on the direct and indirect effects related to HIV infection. Because the number of neuropathogenic mechanisms is large, we focus on a subset of mechanisms for which robust experimental support exists. Figure 1 presents an overview of some of the key contributing causes of HIV-associated NCI.
Fig. 1 |. Overview of factors contributing to neurocognitive impairment in people living with HIV with viral suppression.
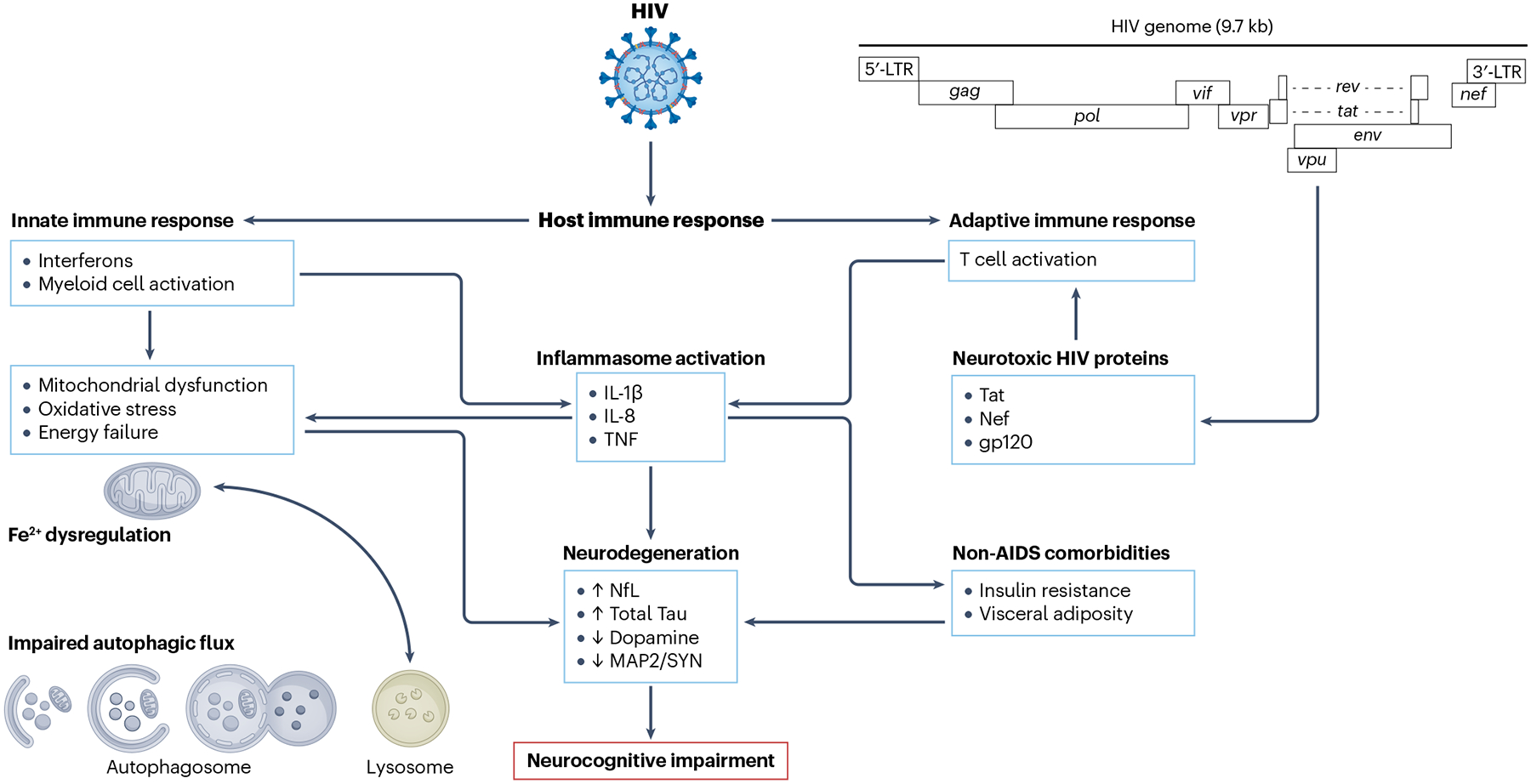
HIV proteins, such as Tat, which continue to be produced even when HIV replication is suppressed by antiretroviral therapy, are toxic to neurons and glial cells. In addition, HIV interacts with the host to produce deleterious immune responses. Particularly important here is the innate immune response, mediated in large part by interferon signalling and myeloid cell activation, which results in the production of neurotoxic inflammatory mediators, mitochondrial dysfunction and oxidative stress, impaired autophagy and ultimately neurodegeneration, leading to neurocognitive impairment. Additional contributing factors are comorbidities, such as insulin resistance, and visceral adiposity as well as toxicities of antiretroviral medications. Addressing these alterations might influence the long-term course of brain disease associated with HIV. LTR, long terminal repeat; NfL, neurofilament light; MAP2, microtubule associated protein 2; SYN, synaptophysin; TNF, tumour necrosis factor.
HIV persistence in the brain
Despite ART-mediated suppression, HIV proviruses are detectable in the brain and CSF of people living with HIV. As such, the brain presents a major challenge for viral eradication44–47, and HIV persistence directly or indirectly contributes to HIV-associated NCI.
Although CSF viral loads (HIV RNA) do not always provide a reliable surrogate for brain viral loads, CSF escape is well described in people living with HIV on ART. CSF escape is a condition in which HIV RNA is detectable in the CSF but undetectable in blood or is significantly higher in CSF than in blood (usually by 0.5 log10 [copies per millilitre]). Most studies report a prevalence of CSF escape of 5–15%, but this prevalence varies depending on the characteristics of the cohort studied and has generally declined over the past 15 years as ART regimens have become increasingly effective, convenient and well tolerated48–50.
Although most instances of CSF escape are asymptomatic, clinical presentations are non-specific and include encephalopathy, seizures and stroke-like symptoms. In some cases, antiretroviral drug resistance mutations have been found in CSF but not in blood, although this finding is confounded by technical limitations in assessing resistance at low viral loads. Reports exist of individuals whose antiretroviral regimens were changed in response to CSF escape and drug resistance profiles, with a subset of these cases showing clinical improvement49,51. However, whether these improvements were spontaneous or related to the regimen changes is unclear.
Intact and defective HIV DNA provirus has been detected in post-mortem brain tissue from people with HIV who had been on ART52,53. Transcriptomic analyses of brain tissue showed increased HIV RNA levels accompanied by elevated interferon signalling compared with uninfected controls. Conversely, the expression of genes linked to synaptic and neuronal function was downregulated compared with uninfected controls54. HIV RNA transcripts have also been measured in the CSF of people living with HIV, predominantly in CD4+ T cells55. High levels of intracellular HIV RNA transcription in CSF CD4+ T cells have been associated with ongoing neuronal injury as measured by proton magnetic resonance spectroscopy56. Another study showed that HIV-infected cells (HIV DNA) persist in the CSF of almost half of individuals on sustained ART and that the presence of these cells is correlated with decreased neurocognitive performance57. Consistently, HIV transcription occurs in infected cells even under sustained ART58,59. Chronic transcription and translation could potentially induce chronic immune activation60. The factors driving HIV transcription despite ART are not well defined. Interestingly, HIV transcripts and proteins, such as Nef, Tat and Env, are present in exosomes — small vesicles carrying proteins and RNAs from the originating cells — secreted from infected cells61–63. Therefore, exosomes continue to be an evolving topic for improved understanding of HIV infection and the associated NCI64,65. Exosomes are being studied for their suitability in providing HIV and NCI biomarkers64,66,67 but also as functional players in the promotion63,64,66,67 of infection and NCI and as delivery vehicles for therapeutics61,62,66–68. Exosomes from HIV-infected cells modulate the gene expression and function of uninfected immune cells, for instance, via activation of NF-κB69,70. Vice versa, exosomes from uninfected cells can modulate the transcription of HIV in infected cells, leading to an increased release of HIV RNA from infected cells71. Comorbidities such as substance use disorders have also been shown to modulate HIV transcription72. Specifically, the use of benzodiazepines, at least in a subset of people living with HIV, is linked to NCI73,74. The benzodiazepine alprazolam increases HIV RNA transcription and might thus indirectly contribute to neuronal dysfunction75. The precise mechanisms by which drugs modulate HIV transcription in T cells and myeloid cells are not well understood.
Long-term HIV infection can induce neuronal damage that could have been triggered before starting ART, commonly referred to as a legacy effect76. Concerted efforts such as the ‘Last Gift’ study, which follows people living with HIV who were diagnosed with a terminal illness during the last months of their lives and agreed to donate their bodies to HIV research, provide the unprecedented opportunity to deeply profile all anatomical niches, including the brain, where HIV plays hide and seek77.
Neuropathogenesis in diverse ethnic and racial groups
An understanding of the sociocultural, behavioural and biological factors that underlie HIV-associated NCI is crucial to achieving treatment benefits. Most human studies in the USA have included primarily non-Latino white people and have not investigated differences in neuropathogenesis across diverse ethnic and racial groups. However, evidence exists that key pathophysiological mechanisms of HIV-associated NCI might differ across ethnic and racial groups. For example, a higher score on the Veterans Aging Cohort Study Index was significantly associated with worse global neurocognition in white and Black people living with HIV but not among Latino people living with HIV in southern California78. Age and lower current CD4 T cell counts were the most important predictors among Black people. For Latino people, age and hepatitis C virus (HCV) status were most important. In a multisite study at six medical centres in the USA78, comorbidity status was a unique and important predictor of higher NCI in participants of Mexican and Puerto Rican heritage. Among those of Mexican heritage, lower nadir CD4 T cell count was also a notable predictor, as was being on ART, in people living with HIV of Puerto Rican origin78. A study of Spanish-speaking people living with HIV in southern California showed that, among those without a history of lifetime substance use disorder, more years of ART exposure were significantly associated with decreased rates of global NCI, while those not meeting criteria for a lifetime substance use disorder showed no such association40. A post-mortem study of people with HIV on ART showed that alterations in triggering receptor expressed on myeloid cells 2 (TREM2) were an important marker of HIV-associated NCI among Latino people and a potential therapeutic target for intervention79. One genetic association study found that a common mitochondrial DNA haplogroup within this population (haplogroup B) was linked with decreased risk for NCI among Latino people living with HIV80.
Evidence of neuronal injury in HIV-associated NCI
The final common cause of HIV-associated NCI is neuronal dysfunction. Comprehensive neuropathological analyses of post-mortem brain samples from people with HIV with NCI report reductions in synapses and dendrites, weakening the functional connectivity between neurons upon which cognition depends81–83. Imaging studies of people living with HIV with NCI suggest that neuronal dysfunction is associated with brain atrophy14 and abnormal brain white matter connections84. Functional measures, such as PET and functional MRI, show additional abnormalities. CSF, serum and plasma levels of neurofilament light and Tau — both major structural elements of large-calibre myelinated axons — increase in many neurodegenerative disorders85–90, including HIV91, indicating progressive axonal and neuronal degeneration. A study conducted in 2022 showed that increasing levels of neurofilament light, total Tau and phosphorylated Tau 181 were associated with worse neurocognitive performance16. These clinical findings reinforce the neuropathological data linking neurodegeneration with NCI in people living with HIV. Again, minority racial and ethnic groups are under-represented in these studies.
HIV neuropathogenic mechanisms can be classified into two broad categories: direct toxic effects of the virus itself and toxic effects related to host response to the virus and its downstream consequences. Because HIV does not infect neurons, most proposed mechanisms of neuronal injury are indirect.
Animal models used in research on HIV in the brain
Numerous animal models of HIV neuropathogenesis have been developed, ranging from chimpanzees and other non-human primates to cats and rodents (rats and mice)92–97. Only non-human primates, cats and rodents have been found to be suitable for the study of HIV neuropathogenesis, neurological sequelae and behavioural impairment (reviewed elsewhere98). Non-human primates, cats and rodents are not permissive to HIV infection; however, some monkey species can contract simian immunodeficiency virus (SIV), and cats can be infected with a lentivirus called feline immunodeficiency virus (FIV)99–101. Both SIV and FIV induce AIDS-like disease with neuropathology or encephalitis, and thus macaques and cats have been used to study the pathogenesis of AIDS and NeuroAIDS99–105. However, there are substantial differences between these viruses and HIV, limiting the translation of the findings into the human system.
As rodents cannot be productively infected with wild-type HIV, two chimeric HIV mutants have been generated in which the viral envelope protein gp120 is replaced with the gp80 of ecotropic murine leukaemia virus (EcoHIV)106. This pseudo-typing approach enabled the establishment of a lasting and neuroinvasive lentiviral infection in mice with an associated immune response, and thus provides a model suitable for research on HIV in the brain107.
An earlier experimental approach for research on HIV in the brain used intracranial injection of HIV-infected human monocyte-derived macrophages into the brains of mice with severe combined immunodeficiency108–111. This model system provided evidence that HIV-infected macrophages cause key features of the neuropathology observed in post-mortem brains from patients with HIV dementia. Alternatively, a human haematopoietic system can be established in some immunocompromised mouse strains, resulting in ‘humanized mice’ that are permissive to HIV infection and provide a small animal model for research on HIV in the brain97,112.
Mice and rats have the important advantage that they can be genetically modified; several transgenic mice and a rat that express an entire HIV genome develop AIDS-like diseases93,113–116. Transgenic mouse models expressing the entire HIV genome or single components of the virus, such as gp120, Tat or Vpr, in the brain develop various degrees of behavioural deficiencies and neuropathology, including loss of synapses, neuronal dendrites and entire neurons, as well as glial activation; these models thus recapitulate key pathological features of people living with HIV who have neurocognitive impairment117–124. Moreover, the brains of HIV-gp120 transgenic mice share a considerable fraction of differential gene regulation with brains from people with HIV or HIV encephalitis who did and did not have NCI when alive125.
Direct neurotoxic mechanisms
Although HIV does not infect neurons, these cells are directly exposed to neurotoxic HIV proteins from infected myeloid cells. Low-level expression of HIV proteins in the brain and periphery continues in the presence of suppressed viral replication in the brain126–128 and might explain the neuronal dysfunction and damage that underlie many of the clinical neuronal complications associated with HIV infection. In vitro, the HIV proteins Tat, gp120, Vpr and Nef trigger neuronal oxidative stress, altered mitochondrial transport and autophagic flux, induction of apoptosis, and abnormal Ca2+ signalling in cellular and animal models129–136. The involvement of HIV proteins in mitochondrial dysfunction in the brain was highlighted in a study that found alterations in the electron transport chain, glycolytic pathways, mitochondrial trafficking proteins and proteins involved in various energy pathways in a rat model of HIV-induced neurotoxicity137.
Indirect mechanisms of neurotoxicity
The concept of indirect mechanisms of neurotoxicity refers to the observations that HIV-infected cells, specifically macrophages and microglia, secrete neurotoxins in conjunction with HIV virions or viral components and host proteins as well as other toxic molecules that damage neurons. The neurotoxicity of macrophages and microglia is in line with the notion that HIV infects and persists in myeloid cells.
Persistence of HIV in brain myeloid cells.
HIV enters the brain within days after infection, infecting myeloid cells through chemokine receptors and sustaining productive infection (replication and assembly of infectious virions)138. The earliest descriptions of HIV encephalitis identified fused macrophages (multinucleated giant cells) and microglial nodules reflecting direct infection139,140. Modern neuropathological studies of decedents who had HIV infection and were virally suppressed find predominantly synaptodendritic pruning141,142. This shift in neuropathology has paralleled the clinical shift from frank dementia to milder forms of NCI. Astrocytes can also be infected but are resistant to productive infection143,144. Brain myeloid cells can constitute a ‘latent’ reservoir and are associated with chronic neuroinflammation145, although viral genes continue to be transcribed and translated128. Latent HIV in brain myeloid cells is associated with chronic neuroinflammation146 and neuronal dysfunction147,148 (Fig. 2). Several lines of evidence suggest that macrophages and microglia can injure neurons by releasing excitotoxic substances that lead to excessive activation of glutamate receptors, primarily of the N-methyl-d-aspartate subtype (NMDARs), including a metabolite of the tryptophan–kynurenine pathway, quinolinic acid149–152. Quinolinic acid concentrations are increased even in the brains of people living with HIV with viral suppression and have been linked to prospective memory impairment151,153, providing a potential explanation for persistent NCI despite viral control. Moreover, neurotoxicity can also be mediated by macrophage-derived and microglial-derived inflammatory cytokines (such as IL-1α and tumour necrosis factor (TNF)), arachidonate and its metabolites (including the closely related platelet-activating factor (PAF), the biosynthesis of which shares a precursor source with arachidonic acid and eicosanoids), certain chemokines, and the release of viral proteins such as gp120, Tat, Nef or Vpr143,149,151,152,154.
Fig. 2 |. Hypothetical model of HIV-mediated myeloid cell and neuronal dysfunction in HIV-associated NCI.
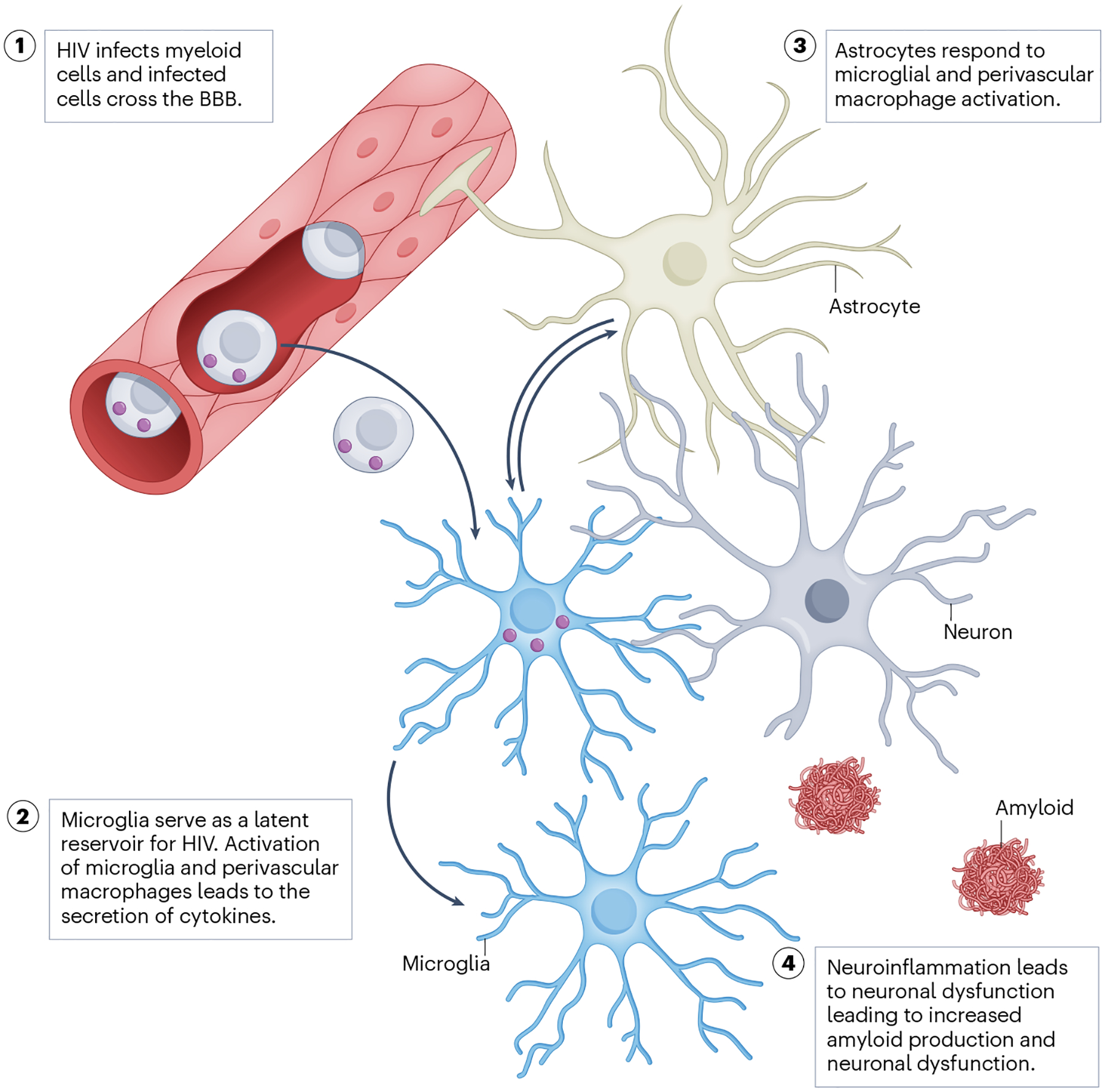
HIV-infected monocytes transmigrate from the peripheral blood to the brain. HIV-infected perivascular macrophages and microglia respond by releasing cytokines that further activate microglia and astrocytes. This eventually leads to neuronal dysfunction, as evidenced by impairment of synaptic transmission, disturbed mitochondrial bioenergetics and, eventually, neurocognitive impairment (NCI). BBB, blood–brain barrier.
HIV-associated NCI is characterized by distinct microglial phenotypes. Microglia respond to pathogens, injury and neurodegeneration by changing morphology, migrating to the site of damage and destroying pathogens. Microglia possess sensing mechanisms, such as TREM2, to detect CNS damage79. Transcriptomic analyses of brain tissue revealed dysregulated genes implicated in immune functioning and synaptic transmission155, and pathway analyses identified genes involved in immune responses, neurotransmission and cell signalling156–158. In vitro, exposure to HIV Tat protein resulted in microglial activation and induced the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome159.
Type I interferons.
In contrast to interferon-α (IFNα), a product of activated microglia and macrophages, IFNβ exerts anti-inflammatory effects and is able to control HIV and SIV infection in the periphery and brain160,161. Studies of SIV-infected macaques show that IFNβ is the main type I interferon that is produced by the brain during acute infection, and its expression is associated with viral control in the brain160.
In the classical model of type I interferon signalling, IFNβ controls the time course of IFNα production162. However, SIV infection induces a protective antiviral IFNβ response in the brain without the production of IFNα163. The suppression of IFNα during SIV infection depends on CCL2, which is predominantly produced by astrocytes upon viral infection164.
Similar to the observation in SIV-infected macaques, transiently increased expression of Ifnb1 mRNA (encoding IFNβ) is seen in the brains of HIV-gp120 transgenic mice165. This transgenic mouse model of HIV in the brain expresses viral gp120 of the HIV isolate lymphadenopathy-associated virus (LAV) under the control of a modified glial fibrillary acidic protein (Gfap) promotor in astrocytes in the CNS and recapitulates key features of brain injury seen in patients living with HIV or AIDS118. Moreover, as mentioned previously, HIV-gp120 transgenic mouse brains share a considerable number of differentially expressed genes with patients with human HIV and HIV encephalitis, including evidence of an endogenous interferon response125,166. Most interestingly, treatment of HIV-gp120 transgenic mice with exogenous recombinant IFNβ via intranasal delivery results in neuroprotection, at least in the cortex and hippocampus165, whereas, in the absence of exogenously supplied IFNβ, IFNAR1 critically contributes to the neurotoxicity of gp120 (ref. 167).
Because of its pronounced antiviral activity168,169, IFNα has been investigated for HIV treatment in several settings: before the introduction of combination ART, as part of a structured treatment interruption strategy, in acute HIV infection, as a component of salvage therapy and, most recently, in attempts to eradicate viral reservoirs170,171. Early attempts at treating established HIV infection were disappointing or inconclusive, in part because of a lack of understanding that the effects of the endogenous interferon system and exogenously supplied interferons depend on the stage of the HIV infection172–174. Moreover, chronically elevated IFNα eventually compromises the immune system and facilitates viral persistence and progression to AIDS171. Accordingly, one study suggested that blocking type I interferon (IFNα) signalling during chronic HIV infection with an antibody against IFNAR2 enables the restoration of immune function175. However, other investigations related to HIV eradication suggest that non-IFNα2 type I interferons, such as IFNε, or type III interferons along with combination ART as well as viral reactivation agents can support the elimination of HIV reservoirs172,173,176–178. As the brain constitutes an HIV reservoir in the majority of people living with HIV, even in those on virally suppressive ART53, it presents a potential challenge for viral eradication44–46. Additionally, it will be important to assess whether therapeutic modulation of the interferon system can remediate NCI. Unfortunately, prior clinical trials of interferon treatment for HIV did not evaluate cognitive endpoints. The timing and duration of interferon treatment might be critical factors for the successful eradication and treatment of NCI179,180. IFNβ induces expression of antiviral C-C motif chemokine receptor 5 (CCR5) ligands165,181 and, as a therapeutic tool, it seems to cause fewer adverse effects than IFNα179. Moreover, owing to its immunomodulatory effect, IFNβ is approved by the FDA for the treatment of the inflammatory neurodegenerative autoimmune disease multiple sclerosis.179,180. Thus, on the basis of the available data, it is reasonable to further develop human IFNβ and IFNα isoforms to therapeutically control chronic HIV infection and delay or prevent the development of HIV-associated NCI.
Lipocalin 2.
Lipocalin 2 (LCN2) is a component of the innate immune system that counteracts bacterial infection by sequestering iron and regulates biological processes such as cellular energy metabolism and apoptosis182–186. LCN2 has been implicated in the regulation of anxiety, emotional and contextual discrimination, and cognition182,187–190. In addition, it regulates inflammation125,129,130 and modulates microglial activation191,192 and possibly neurodegeneration125,193,194. Knockout of the Lcn2 gene in HIV-gp120 transgenic mice reduced behavioural impairment and neuronal damage195. Elevated levels of LCN2 may be neurotoxic and override neuroprotection mediated by CCR5 and its chemokine ligands. Increased plasma and serum levels of LCN2 in people living with HIV correlate with worse processing speed and motor function196. Further studies are needed to clarify whether LCN2 can serve as a therapeutic target for HIV-associated NCI195.
BBB dysfunction in HIV
The blood–brain barrier (BBB) consists of vascular endothelial cells welded together via tight junctions surrounded by pericytes and astrocyte end-feet. The BBB is important in selectively filtering substances from the peripheral blood supply into the brain parenchyma. Increased BBB permeability allows harmful products from the systemic circulation to enter the CNS, including both HIV and activated immune cells197. HIV affects the function, structure and multicellular components of the BBB, including subcellular structures such as mitochondria, all of which contribute to the regulation of tight junctions and BBB permeability197. HIV affects the BBB largely through infection of monocytes and macrophages, which affect the BBB upon crossing198–201. HIV also infects astrocytes202–204 and pericytes205, although to a smaller extent than the aforementioned myeloid-lineage cells, as astrocytes and pericytes are resistant to productive infection143,144. In people living with HIV with viral suppression on ART, the BBB can also be affected by HIV protein expression and ART-induced inflammation198,206 as well as by drugs of abuse207. Studies on the triggers of BBB breakdown consistently report a decrease in tight junction proteins, with a critical role for HIV proteins208–211. Although a comprehensive review of HIV-induced and ART-induced BBB dysfunction is beyond the scope of this Review, mounting evidence suggests that further investigation into this area is warranted.
Neuroinflammation and inflammasome activation
Neuroinflammation is a major indirect contributor to synaptodendritic degeneration. HIV infection induces an inflammatory state that alters the homeostasis of cytokines in both plasma and CSF212–215. Cytokines are immunomodulatory proteins with a variety of functions, including recruiting immune cells to sites of infection and regulating their functions, to resolve infections and repair injured tissues. However, chronic expression of cytokines is harmful. As most neural cells express cytokine receptors216–219, cytokines affect and mostly impair neuronal function. Several key biomarkers of inflammation and their clinical correlations in people living with HIV are described below.
Macrophages secrete TNF, which binds lymphotoxin-α220. Soluble TNF receptor II (sTNFRII) is the cell membrane-bound receptor for TNF and modulates its biological function. Concentrations of sTNFRII in plasma correlate strongly with the clinical stage and progression of disease221,222. Neopterin is a marker of myeloid cell activation and a biochemical product of the guanosine triphosphate pathway. Neopterin is elevated in people living with HIV and strongly predicts disease progression223. Neopterin levels correlate strongly with reactive oxygen species released by myeloid cells, which contribute to oxidative stress and neurodegeneration. In addition, neopterin induces pro-inflammatory signal transduction224. ART reduces CSF neopterin levels; however, neopterin often remains mildly elevated despite suppression of plasma and CSF HIV RNA, raising the important question of whether neopterin elevation is caused by continued CNS infection or persistent CNS injury224.
The NLRP3 inflammasome
Recent studies show that HIV alters NLRP3 inflammasome signalling in CNS microglia, astrocytes and neurons159,225–229, suggesting a role for this pathway in HIV neuropathogenesis. The NLRP3 gene encodes the NALP3 protein (cryopyrin), a member of the NLRP3 inflammasome complex. This complex is an intracellular sensor that detects microbial motifs and endogenous danger signals such as reactive oxygen species and lysosomal damage230, resulting in the assembly and activation of the inflammasome231. Inflammasome activation leads to caspase 1-dependent release of the pro-inflammatory cytokines IL-1β and IL-18 as well as to pyroptosis, a rapid, inflammatory form of lytic programmed cell death. NLRP3 remains activated in people living with HIV with viral suppression232,233. Inflammatory cytokines regulated by NLRP3 (refs. 234–236) are elevated in the blood and CSF of people living with HIV. High IL-1β and IL-18 deplete synaptic serotonin, dopamine and norepinephrine237. Both IL-1β and IL-18 affect dendritic sprouting, synaptic plasticity, long-term potentiation, growth factors and neurogenesis238–240.
mTOR and NCI in HIV
Mammalian target of rapamycin (mTOR) is the catalytic subunit of two structurally distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTORC2 (ref. 241). The functions of mTORC1 include initiation of translation, protein synthesis and autophagy, while mTORC2 orchestrates cytoskeletal organization and cell survival242. mTOR regulates the metabolic machinery necessary for glucose, lipid, amino acid and nucleic acid metabolism243,244. HIV enhances mTORC1 activity to favour its replication, contributing to dysregulated apoptosis, autophagy and inflammation245,246. However, inhibition of mTOR is neuroprotective247,248 and has beneficial effects relevant to HIV, including improved immune function249,250 and metabolism251, preserved insulin sensitivity252, and suppression of T and B cell activation253,254.
AD-like pathogenic mechanisms in HIV
As the mortality rate of HIV has decreased as a result of ART, people living with HIV reach the age when their risk of AD increases. Indeed, histological studies using post-mortem brain tissue suggest that increased interaction of HIV neuropathogenesis with age-dependent neurological diseases might contribute to the pathogenesis of HIV-associated NCI255–257. For example, post-mortem studies of the brains of individuals with HIV-associated NCI revealed that the intracellular and extracellular presence of β-amyloid is a common pathological feature255,257. Dysfunctional clearance of β-amyloid was shown to correlate with increased levels of HIV RNA and neuroinflammation in the brain82. HIV can lead to the accumulation of amyloid in the brain through several mechanisms, including neuroinflammation. Infected microglia release inflammatory cytokines, such as TNF and IL-1β, which increase the production of amyloid precursor protein and its cleavage by β-secretase and γ-secretase, resulting in the release of amyloid that can accumulate in brain tissue258–261. Studies have shown that HIV-associated NCI and AD share common pathogenic pathways, including signs of immune activation, decreased levels of microglial receptors involved in the clearance of protein aggregates and dying brain cells, and compromised neuronal functioning79,82,156,256. These findings have led some researchers to postulate that HIV may promote accelerated ageing of the brain262,263. For example, multiple studies have indicated reduced levels of microglial-bound TREM2 in the CNS of people living with HIV with NCI79,264. This finding might be important because TREM2 dysfunction has been implicated in AD neuropathogenesis, suggesting that HIV neuropathogenesis might share mechanisms with AD, consistent with findings of HIV-associated premature ageing. However, there is evidence that HIV-induced neuropathogenesis is distinct from that of AD. For example, some studies did not detect increased brain β-amyloid in people living with HIV compared with controls265. As will be discussed, disruptions in metabolic homeostasis, including autophagy and mitochondrial dysfunction, are also potential common aetiologies of HIV and AD. The prospect of HIV-associated AD-like pathogenesis is the subject of many ongoing studies. Mackiewicz et al.266 have published a thorough review of the current findings on this topic.
Comorbidities as sources of NCI in HIV
Ageing people living with HIV have an excess burden of comorbidities, such as diabetes mellitus and depression267,268, and these comorbidities have a substantial adverse effect, including frailty269, poor adherence to ART and virological failure270,271, NCI272, poor quality of life22,273–275, and early death276–279. Comorbidities tend to appear at younger ages in people living with HIV than in the general population and accumulate at a faster rate as people living with HIV age, phenomena often referred to as premature and accelerated ageing280–282. Numerous cross-sectional and some short-term longitudinal studies have shown that comorbid medical conditions predispose both people living with HIV and people without HIV to worse neurocognitive outcomes283–286. Additionally, an increased burden of comorbidity in people living with HIV with viral suppression is associated with increased brain white matter abnormalities, reduced grey matter volume and loss of neuronal integrity, illustrating continuing effects on the brain even with virally suppressive ART287. It is plausible that comorbidities are now the chief source of brain injury and NCI in people living with HIV, rather than HIV disease itself or its treatment with ART284.
Various comorbidity indices have been developed to predict the risk of poor cognitive outcomes in people living with HIV288–292. These indices incorporate data such as medical history, medication use, laboratory testing and, in some cases, demographic characteristics. In a study of people living with HIV followed for 7 years on average293, more rapid neurocognitive worsening correlated with worse average cumulative comorbidity scores and lower nadir CD4+ T cell count. Poorer visit-specific neurocognition was related to worse comorbidity scores, detectable HIV viral load and higher CD4+ T cell counts. The impact of comorbidities on neurocognitive decline exceeded that of HIV disease factors. In a second study, a simple comorbidity index composed of the presence or absence of chronic lung disease, hypertension and depression predicted significantly worse cognitive decline over 12 years. HIV infection increases the prevalence of all three of these conditions294–299. Treatments for these comorbidities were at best partially effective, and more successful treatment or prevention of these comorbidities might improve neurocognitive outcomes. Effective treatment of comorbidities in people living with HIV may require different strategies from those for comorbidities in people without HIV. For example, depression in people living with HIV is often refractory to typical anti-depressant medications such as serotonin reuptake inhibitors300–302. Because chronic inflammation persists despite viral suppression, it might be that inflammation drives depression in people living with HIV to a greater extent than in people without HIV. Inflammation in people living with HIV also confers an increased risk of hypertension and chronic lung disease303–306. Improvement in hypertension can be achieved with exercise, dietary interventions and anti-hypertensive medications307,308. Abundant evidence in other clinical situations has shown that successful treatment of comorbidities does indeed benefit neurocognition309,310. Treatment of comorbidities associated with cognitive decline is particularly important in middle age311–314.
Abdominal obesity, metabolic syndrome and HIV-associated NCI
Abdominal obesity is a central component of metabolic syndrome, a very common comorbidity in people living with HIV. Metabolic syndrome was present in 20% of 2,247 antiretroviral-naive individuals entering ART clinical trials and developed de novo in an additional 27% after 2.8 years315. The frequency of the metabolic syndrome increases with age and is associated with increased risk for and severity of NCI in people living with HIV316. The severity of NCI in people living with HIV correlates with waist circumference (a measure of abdominal obesity317), as also occurs in HIV-negative populations318. Abdominal obesity is a major risk factor for systemic inflammation, which is mediated by an influx of M1 pro-inflammatory macrophages that replace resident M2 anti-inflammatory macrophages319 in lipid-laden adipocytes. In older people with obesity and living with HIV, more than 70% had elevated serum TNF, insulin resistance and impaired endothelial function320.
CNS insulin resistance
Preclinical data implicate insulin resistance in the CNS in HIV-associated brain injury and suggest that insulin treatment might be beneficial. Neurons in the hippocampus, cerebellum and cerebral cortex express insulin receptors321–324. Insulin treatment of HIV-infected primary human microglia suppressed HIV p24 levels and reduced CXCL10 and IL6 transcript levels325. Insulin treatment of primary human neurons prevented HIV Vpr-mediated cell process retraction and death325, suggesting that insulin acts as a neurotrophic factor to improve survival. In a mouse model of HIV-associated NCI, NCI correlated with reductions in hippocampal dendritic arbours and downregulation of neuronal function genes; intranasal insulin reversed these changes and completely reversed NCI. Intranasal insulin treatment also reduced brain HIV DNA in mice326. Clinical data supporting the use of insulin for HIV-associated NCI and other conditions, such as mild cognitive impairment, have recently emerged327–329. A randomized, double-blind, placebo-controlled study of intranasal insulin for people living with HIV with mild-to-moderate cognitive impairment (NCT03277222) was recently completed, and the results are being analysed.
Co-infections
People living with HIV frequently present with co-infections. Although some co-infections might not markedly impact HIV disease, others affect the natural history of HIV infection and vice versa330–332. Several common co-infections, such as HCV, tuberculosis, cryptococcal meningitis and malaria, have been linked with worse NCI in people living with HIV333–337. The mechanisms underlying this increased risk of NCI in people with HIV co-infections vary by type of co-infection, and many remain unclear. Evidence is emerging that herpesviruses, including cytomegalovirus (CMV) and Epstein–Barr virus (EBV), can also contribute to NCI in people living with HIV. For example, higher anti-CMV IgG titres were associated with worse HIV-associated NCI338–341. Higher anti-CMV IgG concentrations in blood were associated with higher CSF viral load and higher sCD163 (a marker of activated monocytes), suggesting that the CMV-related immune response at least indirectly influences pathological events in the CNS, perhaps by increased migration of activated monocytes, increased sCD163 or by infected CD4+ T cell-derived HIV RNA into this protected compartment. CMV is likely to contribute to HIV-associated NCI through metabolic317,342,343 and vascular disease284,344 and by replicating in vascular endothelial and smooth muscle cells, leading to macrophage infiltration of vessel walls and promoting atherogenesis345,346. An ongoing clinical trial (NCT04840199) is testing whether the potent anti-CMV drug letermovir can reduce inflammation and atherosclerosis in people living with HIV, with the aim of improving neurocognitive performance. Detection of EBV DNA in CSF was associated with increased levels of biomarkers of neuronal damage and inflammation347.
Mitochondrial dysfunction
Mitochondrial dysfunction has downstream effects on autophagy, metal ion signalling, and other pathways and vice versa; intracellular and intercellular communication must be kept in mind when establishing research paradigms. Thus, targeting metabolism is promising for therapeutic discovery, and such discoveries might not only lead to help for people living with HIV but also for people with other CNS disorders.
Mitochondria in HIV CNS dysfunction
Mitochondrial dysfunction has long been implicated in the pathogenesis of HIV-associated NCI348–351. Neurons require high levels of mitochondrial activity to maintain and form new synapses and are thus highly dependent upon energy substrate from blood and a healthy network of mitochondria. In a healthy brain, astrocytes transport glucose across the BBB into the brain parenchyma and provide an energy substrate to neurons in the form of lactate and glutamine352,353. Perivascular macrophages and microglia primarily rely on oxidative phosphorylation to generate ATP to fuel their functions in immune surveillance of the brain parenchyma. During brain injury, such as occurs with HIV infection, astrocytes and microglia shift their metabolic profiles to oxidative phosphorylation and glycolysis, respectively354,355. These metabolic shifts in glia might lead to metabolic stress and mitochondrial dysfunction in neurons355–362. Thus, the metabolic interplay between neurons, astrocytes and macrophages might impair brain health in HIV-associated NCI.
Almost every aspect of mitochondrial function is negatively affected by HIV or ART. Mitochondrial biogenesis, mitochondrial fission and fusion, and mitophagy are altered in the brains of people living with HIV and HIV-associated NCI358,363,364. Mitochondrial biogenesis proteins are reduced in neurons and increased in astrocytes in the brains of people living with HIV on ART and in in vitro and in vivo models for HIV-induced and ART-induced neurotoxicity358,359. Mitochondrial fission and fusion are compromised by gp120 but possibly also by other HIV proteins, and the trafficking of mitochondrial proteins along neuronal microtubules is also compromised, resulting in damaged and elongated mitochondria in neurons135,136,364,365. Inflammatory cytokines induce increased glycolysis and oxidative phosphorylation in astroglia and confer neurotoxicity358. Several studies have shown that blocking this increase in metabolic activity in stimulated astroglia, for example, by using cannabinoid receptor agonists and caloric restriction mimetics, might be neuroprotective358,366–368. Mitochondria are key regulators of the sequestration and signalling of metal ions, particularly calcium and iron, both of which are dysregulated by HIV proteins, including Tat, nef and VPR369–374. Animal models expressing gp120 in the brain induce mitochondrial biogenesis in astroglia while concomitantly reducing it in neurons359–362. Inflammatory cytokines induce increased glycolysis and oxidative phosphorylation in astroglia and confer neurotoxicity358. Several studies have shown that blocking this increase in metabolic activity in stimulated astroglia might be neuroprotective358,366–368. In line with the neuroprotective properties of caloric restriction mimetics, exercise is a potential intervention (discussed below) that might provide neuroprotection in part through improved mitochondrial function in neurons, which might help to ameliorate HIV-associated NCI375–380.
Autophagy and lysosomal biology
As the molecular processes of autophagy and the lysosomal systems were deciphered in detail381–386, it became clear that HIV might affect this important process for cellular renewal during times of physiological stress129,247. Although autophagy often plays a defensive role in viral infections, some viruses can hijack these cellular processes to facilitate infection and replication. The number of autophagosomes in the brain is increased in people living with HIV compared with control individuals247. HIV gp120 and Tat alter autophagosome, lysosome and endosome function in mouse models and neuronal cultures387–389. Pharmacological compounds, such as rapamycin, an mTOR inhibitor, show neuroprotective properties that coincide with the normalization of autophagy markers and levels of astrogliosis and microgliosis388. Gene delivery of the autophagy nucleation protein, beclin 1, also showed promising results in the gp120 transgenic mouse model, reducing neurodegeneration markers and gliosis while enhancing autophagy proteins387. Thus, targeting autophagy might be worth further investigation as a therapeutic avenue for people living with HIV.
Iron regulation in HIV CNS dysfunction
Several studies have implicated disruptions in iron regulation and signalling as an aetiology of HIV-induced neurological dysfunction373,374,390,391. These findings are particularly important because iron ions regulate the intracellular redox state, which modulates the function of mitochondria, autophagosomes, lysosomes and practically all cellular signalling370,371,392. Intracellular iron levels affect HIV replication by enhancing Tat escape from endolysosomes, which might be a mechanism that promotes neurotoxicity in people living with HIV393.
The gut microbiome
The gut is an important reservoir for HIV and acts as a hub for its dissemination310. Some gut microbes produce metabolites such as short-chain fatty acids (for example, acetic acid, propionic acid and butyric acid) and neurotransmitters (for example, acetylcholine, GABA and serotonin). These metabolites can communicate to the brain via the bloodstream and vagus nerve. Changes in gut microbial composition and metabolites have been associated with altered neurotransmitter signalling, neurogenesis, microglial development and response, myelination, neurogenesis, and diseases including Parkinson disease, AD and amyotrophic lateral sclerosis394–396. Interestingly, experimentally induced colitis in rhesus macaques resulted in a neuroinflammatory profile like that observed in SIV-infected rhesus macaques, highlighting the importance of the gut–brain axis397. For example, inflammatory cytokines, including interferon, activate indolamine dioxygenase, an enzyme that metabolizes tryptophan, ultimately lowering serum tryptophan concentrations. Indeed, tryptophan levels are lower in people living with HIV compared to people without HIV398. Further studies investigating treatments targeting the gut microbiota in people living with HIV are needed.
Antiretroviral medication neurotoxicity
Although ART has been transformative in the management of HIV infection, antiretroviral drugs can induce neurotoxicity directly or indirectly, contributing to cognitive impairment399–401. The neurotoxic effects of currently used antiretroviral drugs are summarized in Table 1. Notably, the ‘greying’ (shifting age of the population) of people living with HIV on virally suppressive ART is linked to polypharmacy that includes both ART and non-ART drugs, increasing the risk of drug–drug interactions that could worsen neurotoxicity and NCI402,403. Age-related changes in drug distribution, binding proteins, metabolism and elimination can lead to increased CNS drug exposure in older people living with HIV404–406, further predisposing to NCI. In addition, ageing causes structural and functional changes in the BBB that result in increased BBB permeability, which can affect ART CNS pharmacokinetics407–409. Information on ethnic and sex disparities in this area is scant.
Table 1 |.
Neurotoxicity of currently used antiretrovirals
| Drug class | Examples | Clinical findings | Proposed mechanism of toxicity |
|---|---|---|---|
| NRTIs | Tenofovir alafenamide, abacavir | Psychosis, mania457, chronic kidney disease458,459 | Inhibition of mitochondrial DNA polymerase-γ460, energy depletion and oxidative stress461–463, ER stress464, mitochondrial DNA depletion465,466 |
| NNRTIs | Efavirenz, rilpivirine | Dizziness, insomnia, vivid dreams, headache, hallucinations, mania, depression467, anxiety468 | Increased ER stress and mitochondrial toxicity469 |
| Protease inhibitors | Ritonavir, lopinavir, darunavir | Nausea, insomnia, circumoral paraesthesias470 | Reduced oligodendrocyte maturation471, astrocyte glutamate homeostasis472 and synaptic acetylcholine473, worse small vessel disease474, oxidative stress475 |
| INSTIs | Dolutegravir, bictegravir, raltegravir, elvitegravir | Diarrhoea, nausea, headache476, neural tube defects477, insomnia478,479, depression, anxietya | Increased production of reactive oxygen species in astrocytes480, integrated stress response (elvitegravir)475 |
| Entry inhibitors | Maraviroc, cenicriviroc, ibalizumab | Bronchitis, nasopharyngitis, oesophageal candidiasis481 | No notable CNS toxicity reported482–485 |
Experimental treatments for HIV-associated NCI
Examples of past and current human clinical trials of treatments for HIV-associated NCI — selected to illustrate mechanisms discussed in this Review — are listed in Table 2. Some of these treatments have already been approved for other neurodegenerative diseases (for example, selegiline and memantine)410,411 or represent re-purposing of treatments for other conditions (for example, minocycline, fluconazole, intranasal insulin (NCT03277222), statins (NCT01600170))412–417 and antioxidants (coenzyme Q10, haem oxygenase 1 and dimethyl fumarate)418–420. A comprehensive list is beyond the scope of this Review, but a summary of the interventions that are most relevant to the mechanisms discussed here is also provided (Table 3).
Table 2 |.
Selected past and ongoing human clinical trials for HIV-associated NCI illustrating mechanisms described in this Review
| Drug (NCT number) | Targeted mechanism(s) | Sample and design | Status | Key findings | Ref. |
|---|---|---|---|---|---|
| Memantine (00000867) | Reduce glutamate toxicity | n = 99; placebo controlled | Completed | Ineffective | 490 |
| Selegiline (00013585) | Reduce oxidative stress | n = 128; placebo controlled | Completed | Ineffective | 411 |
| Intranasal insulin (03081117) | Neuroprotection | n = 21; placebo controlled | Completed | Improved performance on neuropsychological tests related to memory and attention | 491 |
| Statins (01600170) | Reduce inflammation | n = 11; crossover | Completed | No normalization of monocyte gene expression patterns; no changes in monocyte surface markers or plasma mediators | 492 |
| Minocycline (00855062) | Reduce inflammation | n = 73; placebo controlled | Terminated | No change in HIV-associated NCI | 413 |
| Fluconazole/paroxetine (01354314) | Neuroprotection | n = 45; placebo controlled | Completed | Fluconazole showed no benefit in cognition and an increase in multiple markers of cellular stress; paroxetine showed improvement in a summary neuropsychological test measure | 415 |
| Tesamorelin (02572323) | Reduce inflammation, neuroprotection | n = 100; crossover | Recruiting and ongoing | Results not yet published. Outcomes: change in neuropsychological performance, change in IGF1, MRS neuroinflammation, hippocampal volume | 423 |
IGF1, insulin-like growth factor 1; NCI, neurocognitive impairment; NCT, National Clinical Trial; MRS, magnetic resonance spectroscopy.
Table 3 |.
Examples of potential treatment strategies for HIV-associated NCI illustrating mechanisms described in this Review
| Intervention | Proposed mechanism | Status of research | Effectiveness |
|---|---|---|---|
| Iptakalim | Reduced neuroinflammation | In vivo studies | Inhibited microglia-mediated neuroinflammation in rats493 |
| Antibody to IFNAR2 | Blocks downstream interferon-stimulated cascade | In vivo studies | Reduced inflammation in animals494 |
| Intranasal IFNβ | IFNβ has antiviral effects and anti-inflammatory effects | In vivo study in model system | Neuroprotection in vivo165 |
| Interferons with or without ART | Type I interferons reduce viral replication and prevent infection of new targets | Human clinical trials (NCT02471430, NCT02767193, NCT01935089, NCT02227277) | Ongoing, aiming at reduced HIV reservoir495 |
| Letermovir | Antiviral against cytomegalovirus, reducing inflammation | Proposed secondary end point in ongoing human clinical trial (NCT04840199) | Ongoing |
| Physical exercise | Improved mitochondrial biogenesis, hippocampal neurogenesisa | In vivo studies | In mice, exercise is associated with mitochondrial biogenesis and hippocampal neurogenesis496 |
| Mindful meditation | Improved function in brain networks supporting attention and emotion regulationa | Human clinical trial (NCT02936401) | Not yet published |
| Ketogenic diet | Antioxidant increases mitochondrial biogenesisa | Human clinical trial | Ketogenic drink improved cognition in mild cognitive impairment497 |
ART, antiretroviral therapy.
Non-pharmacological interventions.
Tesamorelin
Tesamorelin, an analogue of human growth hormone-releasing hormone (hGHRH), has been approved by the FDA to treat HIV-related abdominal obesity but has not been tested for the treatment of NCI in people living with HIV. Tesamorelin stimulates physiological growth hormone (GH) secretion and restores the normally pulsatile nocturnal rhythm of GH release that is seen in GH-deficient conditions such as HIV421. In addition to reducing abdominal obesity, tesamorelin also reduced C-reactive protein (CRP), an inflammatory mediator released from the liver422,423. A second mechanism by which tesamorelin might benefit neurocognition is by stimulating the production of insulin-like growth factor 1 (IGF1), a neurotrophic hormone. IGF1, in turn, stimulates brain blood flow and angiogenesis, improves neurogenesis, neurite out-growth and synaptic complexity, and is neuroprotective against oxidative stress424. Blood IGF1 levels correlate with overall cognitive performance425, including perceptual motor performance, fluid intelligence and processing speed426,427. In animals, when IGF1 levels are increased in the hippocampus by treatment with IGF1, deficits in cognitive functions are corrected428. An ongoing clinical trial (NCT02572323) hypothesizes that tesamorelin will attenuate systemic inflammation and immune activation in people living with HIV with viral suppression and cognitive impairment.
GPLD1
GPLD1, an enzyme also known as phosphatidylinositol-glycan-specific phospholipase D, can mimic the beneficial effects of exercise (discussed below). Levels of GPLD1 are increased by physical activity, improving antioxidant systems429 and anti-inflammatory responses430. Novel mechanistic associations have been reported between physical activity, cognition and GPLD1 levels431. GPLD1 from the liver cleaves glycosylphosphatidylinositol and glycosylphosphatidylinositol-anchored proteins to regulate mitochondrial function and regulate inflammation, clotting and vascular integrity432–434. GPLD1 also ameliorates oxidative stress and inflammation435,436. In mice, systemic administration of GPLD1 recapitulated the neurogenic and cognitive benefits of physical activity431. Furthermore, in mice, higher GPLD1 levels dampened inflammation and normalized coagulation, factors shown to be fundamentally dysregulated in people living with HIV and to predict declines in cognition, mood and other outcomes even in people living with HIV with viral suppression437. By mimicking the effects of physical activity, exogenous administration of GPLD1 or other interventions to modify this pathway might be beneficial for HIV-associated NCI and are currently being tested in animal models.
Effectiveness of pharmacological therapy for HIV-associated NCI
A consistently efficacious treatment or prevention for HIV-associated NCI has not yet been discovered, for multiple reasons. Clinical trials for NCI frequently fail despite promising preclinical results owing to inadequate participant recruitment and/or retention, fundamental differences between animal models and human participants, and unforeseen adverse effects. More specifically, the pathogenesis of HIV-associated NCI remains unclear. HIV-associated NCI can result from legacy effects, inadequate viral suppression in the CSF, persistent inflammation, direct or indirect neurotoxicity from antiretroviral drugs, combinations of these factors, or other uncharacterized factors. Without a clear pathological target, developing specific treatment modalities becomes exceptionally challenging, and the impetus for the many prior clinical trials came either from medications that showed neuroprotection in other diseases or from incidental findings in the clinic. Pathophysiological mechanisms of NCI in people living with HIV might vary between ethnic and/or racial groups, and sociocultural and behavioural factors might interact with the pathophysiological mechanisms underlying NCI. To properly confront this disease entity, more research to provide answers to preclinical questions about NCI is essential. Furthermore, future studies incorporating culturally relevant psychosocial, environmental, biomedical and genetic factors might best inform the identification of therapeutic targets and the development of clinical interventions to promote the neurocognitive health of the diverse population of people living with HIV.
Non-pharmacological interventions
Pharmacological therapeutics are important, but non-pharmacological approaches are also needed These therapies can affect metabolism and inflammatory processes in ways that drugs cannot. Despite advances in our understanding of HIV-associated NCI pathogenesis, the translation of findings into the clinical setting has been disappointing. Medical approaches targeting different mechanisms have not resulted in better primary outcomes. Owing to comorbidities, people living with HIV already require several medications. Thus, attention should be placed on non-pharmacological interventions for HIV-associated NCI, including sleep, physical exercise, diet and cognitive training interventions.
More than half of people living with HIV with NCI report symptoms of insomnia438–441. Compromised sleep quality and duration are often connected to drug or alcohol use and other comorbidities such as depression or obstructive sleep apnoea442,443. Poor sleep health leads to reduced cognitive performance in memory, learning and concentration. In healthy individuals, β-amyloid levels in the brain on PET imaging were elevated after a single night of sleep deprivation444. Reduced sleep can also activate the immune system and increase the production of inflammatory mediators445,446.
The benefits of caloric restriction, ketogenic diets, intermittent fasting and physical activity — all of which improve metabolic function — need to be investigated for therapeutic efficacy. One trial (NCT02936401) is currently assessing the use of mindfulness-based stress reduction as a method to improve function in people living with HIV with NCI who are older than 60 years. Another trial (NCT03483740) is testing cognitive remediation group therapy in a similar cohort of older people living with HIV with NCI.
As mentioned above, physical activity has a plethora of positive effects at the molecular level in the brain by increasing levels of neurotrophins and growth factors and by modulating synaptic plasticity, neurogenesis and blood flow447. Physical activity improves cognition, mood and general health status in the general population, and we and others have published findings showing correlations between greater physical activity and better cognition in people living with HIV448–454.
Cognitive training is another non-pharmacological intervention that has been associated with improvements in cognition and daily function in people living with HIV. Some computerized cognitive training programmes might improve cognition in people living with HIV455. The domains of speed of information processing and attention and/or working memory seem the most likely to show improvement456. However, there are gaps in understanding the type and dose of cognitive training that might be most beneficial. There is a need to consider sociocultural factors in the development of all types of non-pharmacological interventions for people living with HIV to assure their acceptability and utility among diverse people living with HIV, particularly those living in regions of the world where health-care access is limited and resources scarce.
Conclusions
Findings from preclinical and clinical studies suggest that HIV-associated NCI is a heterogeneous disease with a complex multi-system pathogenesis. Several mechanisms can contribute to neurodegeneration and NCI in people living with HIV, including mitochondrial toxicity, endoplasmic reticulum stress, oxidative species and neurotoxicities of specific antiretroviral medications. A substantial number of experimental therapies, including anti-inflammatory drugs, are not beneficial in the treatment of NCI in people living with HIV. We have presented promising avenues for the improvement of cognition in the ageing cohort of people living with HIV. Lastly, we delineated non-pharmacological treatments that could be readily implemented in the daily lives of people living with HIV. We think that, with the current rapid scientific advances in the field of HIV-associated NCI, more treatment options will be available for people living with HIV and NCI.
Key points.
The pathogenesis of neurocognitive impairment (NCI) in people living with HIV is complex, and no disease-modifying therapies are currently available.
Neuroinflammatory and metabolic changes are hallmarks of HIV-associated NCI and are potential therapeutic targets.
The neuropathogenesis of HIV-associated NCI in diverse ethnic and racial groups warrants further exploration.
Examples of promising targets for the treatment of NCI in people living with HIV include human growth hormone-releasing hormone (hGHRH) and the enzyme phosphatidylinositol-glycan-specific phospholipase D (also known as GPLD1).
Non-pharmacological interventions that might enhance cognitive function in people living with HIV include increased physical activity, improved quality of sleep and nutritional treatments.
Acknowledgements
This work was supported by the U.S. National Institutes of Health grants MH128108, AG066215 (Fields); K24AG075240 (Marquine); R01DA052209, R01MH104131, R01MH087332 (Kaul); R01DA056058, R01DA043430, R01MH128869 (Ellis), and R21MH134401 (Schlachetzki).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Heaton RK et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol 17, 3–16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV.gov. Global HIV/AIDS Overview https://www.who.int/data/gho/data/themes/hiv-aids (2023).
- 3.Ellis RJ et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin. Infect. Dis 58, 1015–1022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EJ Neurological disease: the effects of HIV and antiretroviral therapy and the implications for early antiretroviral therapy initiation. Curr. Opin. HIV AIDS 4, 447–452 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Vecchio A et al. Neurocognitive effects of antiretroviral initiation among people living with HIV in rural Uganda. J. Acquir. Immune Defic. Syndr 84, 534–542 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Gao C et al. Antiretroviral therapy improves neurocognitive impairment in people living with HIV? A meta-analysis. Int. J. Nurs. Sci 7, 238–247 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coban H et al. Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS 31, 1565–1571 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simioni S et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24, 1243–1250 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Brew BJ et al. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin. Trials 2, e13 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antinori A et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nightingale S et al. Moving on From HAND: why we need new criteria for cognitive impairment in persons living with human immunodeficiency virus and a proposed way forward. Clin. Infect. Dis 73, 1113–1118 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Nightingale S et al. A new approach to cognitive impairment in people with HIV. Lancet HIV 9, e815–e817 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Nightingale S et al. Cognitive impairment in people living with HIV: consensus recommendations for a new approach. Nat. Rev. Neurol 19, 424–433 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Alakkas A et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J. Neurovirol 25, 32–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eden A et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 11, e0157160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis RJ et al. Higher cerebrospinal fluid biomarkers of neuronal injury in HIV-associated neurocognitive impairment. J. Neurovirol 28, 438–445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaton RK et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75, 2087–2096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chetty L, Cobbing S & Chetty V Physical activity and exercise for older people living with HIV: a protocol for a scoping review. Syst. Rev 9, 60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saloner R & Cysique LA HIV-associated neurocognitive disorders: a global perspective. J. Int. Neuropsychol. Soc 23, 860–869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anuradha S et al. Factors influencing adherence to ART: new insights from a center providing free ART under the national program in Delhi, India. J. Int. Assoc. Provid. AIDS Care 12, 195–201 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Benedict RH, Mezhir JJ, Walsh K & Hewitt RG Impact of human immunodeficiency virus type-1-associated cognitive dysfunction on activities of daily living and quality of life. Arch. Clin. Neuropsychol 15, 535–544 (2000). [PubMed] [Google Scholar]
- 22.Jones JD et al. Changes in cognition precede changes in HRQoL among HIV+ males: longitudinal analysis of the multicenter AIDS cohort study. Neuropsychology 33, 370–378 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinheiro CA et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz. J. Med. Biol. Res 49, e5344 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laverick R et al. Self-reported decline in everyday function, cognitive symptoms, and cognitive function in people with HIV. J. Acquir. Immune Defic. Syndr 76, e74–e83 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Sevigny JJ et al. An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch. Neurol 64, 97–102 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Valcour VG, Shikuma CM, Watters MR & Sacktor NC Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. AIDS 18, S79–86 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendelken LA & Valcour V Impact of HIV and aging on neuropsychological function. J. Neurovirol 18, 256–263 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker JT, Lopez OL, Dew MA & Aizenstein HJ Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 18 (Suppl. 1), S11–18 (2004). [PubMed] [Google Scholar]
- 29.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372, 293–299 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis RJ et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 25, 1747–1751 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz-Moreno JA et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res. Hum. Retroviruses 24, 1301–1307 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Grant I et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82, 2055–2062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Estimated HIV Incidence and Prevalence in the United States, 2015–2019 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (2021).
- 34.Manly JJ et al. The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. The HIV Neurobehavioral Research Center (HNRC) Group. J. Int. Neuropsychol. Soc 4, 291–302 (1998). [PubMed] [Google Scholar]
- 35.Vo QT et al. Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. J. Neurovirol 19, 24–31 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winston A et al. Neurocognitive function in HIV Infected patients on antiretroviral therapy. PLoS One 8, e61949 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson CW-M et al. Ethnic/racial disparities in longitudinal neurocognitive decline in people with HIV. J. Acquir. Immune Defic. Syndr 90, 97–105 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan YW, Burgess GH & Green RJ The effects of acculturation on neuropsychological test performance: a systematic literature review. Clin. Neuropsychol 35, 541–571 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Wojna V et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J. Neurovirol 12, 356–364 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Kamalyan L et al. Neurocognitive impairment in Spanish-speaking Latinos living with HIV in the US: application of the neuropsychological norms for the US-Mexico border region in Spanish (NP-NUMBRS). Clin. Neuropsychol 35, 433–452 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhanya V et al. HIV-1 subtype C Vpr amino acid residue 45y and specific conserved fragments are associated with neurocognitive impairment and markers of viral load. AIDS Res. Hum. Retroviruses 39, 166–175 (2023). [DOI] [PubMed] [Google Scholar]
- 42.Aderinto N HIV-associated neurocognitive disorders in Africa: an emerging challenge: a correspondence. IJS Glob. Health 6, e0146 (2023). [Google Scholar]
- 43.Sacktor N, Nakasujja N, Robertson K & Clifford DB HIV-associated cognitive impairment in sub-Saharan Africa–the potential effect of clade diversity. Nat. Clin. Pract. Neurol 3, 436–443 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Gray LR et al. CNS-specific regulatory elements in brain-derived HIV-1 strains affect responses to latency-reversing agents with implications for cure strategies. Mol. Psychiatry 21, 574–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nath A Eradication of human immunodeficiency virus from brain reservoirs. J. Neurovirol 21, 227–234 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellis R & Letendre SL Update and new directions in therapeutics for neurological complications of HIV infections. Neurotherapeutics 13, 471–476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desplats P et al. Molecular and pathologic insights from latent HIV-1 infection in the human brain. Neurology 80, 1415–1423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perez-Valero I et al. Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33, 475–481 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukerji SS et al. Temporal patterns and drug resistance in CSF viral escape among ART-experienced HIV-1 infected adults. J. Acquir. Immune Defic. Syndr 75, 246–255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trunfio M et al. Cerebrospinal fluid HIV-1 escape according to different thresholds and underlying comorbidities: is it time to assess the definitions. AIDS 33, 759–762 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Manesh A et al. Symptomatic HIV CNS viral escape among patients on effective cART. Int. J. Infect. Dis 84, 39–43 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Cochrane CR et al. Intact HIV proviruses persist in the brain despite viral suppression with ART. Ann. Neurol 92, 532–544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliveira MF et al. Evaluation of archival HIV DNA in brain and lymphoid tissues. J. Virol 97, e0054323 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanna PP, Repunte-Canonigo V, Masliah E & Lefebvre C Gene expression patterns associated with neurological disease in human HIV infection. PLoS One 12, e0175316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farhadian SF et al. Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV. JCI Insight 3, e121718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki K et al. Elevation of cell-associated HIV-1 transcripts in CSF CD4+ T cells, despite effective antiretroviral therapy, is linked to brain injury. Proc. Natl Acad. Sci. USA 119, e2210584119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spudich S et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J. Clin. Invest 129, 3339–3346 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lian X et al. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci. Transl. Med 13, eabl4097 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Einkauf KB et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 185, 266–282.e15 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pollack RA et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21, 494–506.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lenassi M et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11, 110–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C & Margolis L Extracellular vesicles carry HIV Env and facilitate Hiv infection of human lymphoid tissue. Sci. Rep 7, 1695 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra PK et al. Latent HIV-exosomes induce mitochondrial hyperfusion due to loss of phosphorylated dynamin-related protein 1 in brain endothelium. Mol. Neurobiol 58, 2974–2989 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pulliam L, Sun B, Mustapic M, Chawla S & Kapogiannis D Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol 25, 702–709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giannessi F, Aiello A, Franchi F, Percario ZA & Affabris E The role of extracellular vesicles as allies of HIV, HCV and SARS viruses. Viruses 12, 571 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahajan SD, Ordain NS, Kutscher H, Karki S & Reynolds JL HIV neuroinflammation: the role of exosomes in cell signaling, prognostic and diagnostic biomarkers and drug delivery. Front. Cell Dev. Biol 9, 637192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ojha CR et al. Interplay between autophagy, exosomes and HIV-1 associated neurological disorders: new insights for diagnosis and therapeutic applications. Viruses 9, 176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrivastava S et al. Exosome-mediated stable epigenetic repression of HIV-1. Nat. Commun 12, 5541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narayanan A et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J. Biol. Chem 288, 20014–20033 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sampey GC et al. Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through Trans-activating response (TAR) RNA. J. Biol. Chem 291, 1251–1266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barclay RA et al. Exosomes from uninfected cells activate transcription of latent HIV-1. J. Biol. Chem 292, 14764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyagi M, Bukrinsky M & Simon GL Mechanisms of HIV transcriptional regulation by drugs of abuse. Curr. HIV Res 14, 442–454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saloner R et al. Benzodiazepine use is associated with an increased risk of neurocognitive impairment in people living with HIV. J. Acquir. Immune Defic. Syndr 82, 475–482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sundermann EE et al. The association between benzodiazepine use and greater risk of neurocognitive impairment is moderated by medical burden in people with HIV. J. Neurovirol 28, 410–421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin A et al. Alprazolam prompts HIV-1 transcriptional reactivation and enhances CTL response through RUNX1 inhibition and STAT5 activation. Front. Neurol 12, 663793 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qu Y et al. Legacy effect on neuropsychological function in HIV-infected men on combination antiretroviral therapy. AIDS 36, 19–27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rawlings SA, Chaillon A, Smith D & Gianella S Scale up rapid research autopsies for tissue immunology. Nature 595, 352 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marquine MJ et al. The impact of ethnicity/race on the association between the Veterans Aging Cohort Study (VACS) index and neurocognitive function among HIV-infected persons. J. Neurovirol 22, 442–454 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fields JA et al. Alterations in brain TREM2 and amyloid-β levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy. J. Neurochem 147, 784–802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hulgan T et al. Mitochondrial DNA haplogroups and neurocognitive impairment during HIV infection. Clin. Infect. Dis 61, 1476–1484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore DJ et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20, 879–887 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Levine AJ et al. Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J. Neurovirol 22, 431–441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masliah E et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol 42, 963–972 (1997). [DOI] [PubMed] [Google Scholar]
- 84.Jernigan TL et al. Clinical factors related to brain structure in HIV: the CHARTER study. J. Neurovirol 17, 248–257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaetani L et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Tortelli R et al. Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur. J. Neurol 22, 215–218 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Tortelli R et al. Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur. J. Neurol 19, 1561–1567 (2012). [DOI] [PubMed] [Google Scholar]
- 88.Petzold A et al. CSF neurofilament levels: a potential prognostic marker in Guillain-Barre syndrome. Neurology 67, 1071–1073 (2006). [DOI] [PubMed] [Google Scholar]
- 89.Petzold A et al. CSF protein biomarkers for proximal axonal damage improve prognostic accuracy in the acute phase of Guillain-Barre syndrome. Muscle Nerve 40, 42–49 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Mariotto S et al. Serum and cerebrospinal neurofilament light chain levels in patients with acquired peripheral neuropathies. J. Peripher. Nerv. Syst 23, 174–177 (2018). [DOI] [PubMed] [Google Scholar]
- 91.Peterson J et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PLoS One 9, e116081 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gardner MB & Luciw PA Animal models of AIDS. FASEB J 3, 2593–2606 (1989). [DOI] [PubMed] [Google Scholar]
- 93.Reid W et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl Acad. Sci. USA 98, 9271–9276 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keppler OT et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med 195, 719–736 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klotman PE & Notkins AL Transgenic models of human immunodeficiency virus type-1. Curr. Top. Microbiol. Immunol 206, 197–222 (1996). [DOI] [PubMed] [Google Scholar]
- 96.Toggas SM & Mucke L Transgenic models in the study of AIDS dementia complex. Curr. Top. Microbiol. Immunol 206, 223–241 (1996). [DOI] [PubMed] [Google Scholar]
- 97.Van Duyne R et al. The utilization of humanized mouse models for the study of human retroviral infections. Retrovirology 6, 76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thaney VE et al. Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research. J. Neurovirol 24, 156–167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ambrose Z, KewalRamani VN, Bieniasz PD & Hatziioannou T HIV/AIDS: in search of an animal model. Trends Biotechnol 25, 333–337 (2007). [DOI] [PubMed] [Google Scholar]
- 100.Clements JE, Anderson MG, Zink MC, Joag SV & Narayan O The SIV model of AIDS encephalopathy. Role of neurotropic viruses in diseases. Res. Publ. Assoc. Res. Nerv. Ment. Dis 72, 147–157 (1994). [PubMed] [Google Scholar]
- 101.Olmsted RA et al. Molecular cloning of feline immunodeficiency virus. Proc. Natl Acad. Sci. USA 86, 2448–2452 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meeker RB, Thiede BA, Hall C, English R & Tompkins M Cortical cell loss in asymptomatic cats experimentally infected with feline immunodeficiency virus. AIDS Res. Hum. Retroviruses 13, 1131–1140 (1997). [DOI] [PubMed] [Google Scholar]
- 103.Jacobson S et al. Cortical neuronal cytoskeletal changes associated with FIV infection. J. Neurovirol 3, 283–289 (1997). [DOI] [PubMed] [Google Scholar]
- 104.Clements JE, Mankowski JL, Gama L & Zink MC The accelerated simian immunodeficiency virus macaque model of human immunodeficiency virus-associated neurological disease: from mechanism to treatment. J. Neurovirol 14, 309–317 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Williams R et al. Nonhuman primate models of NeuroAIDS. J. Neurovirol 14, 292–300 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Potash MJ et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl Acad. Sci. USA 102, 3760–3765 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim BH et al. CCL2 is required for initiation but not persistence of HIV infection mediated neurocognitive disease in mice. Sci. Rep 13, 6577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tyor WR, Power C, Gendelman HE & Markham RB A model of human immunodeficiency virus encephalitis in scid mice. Proc. Natl Acad. Sci. USA 90, 8658–8662 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Persidsky Y et al. Human immunodeficiency virus encephalitis in SCID mice. Am. J. Pathol 149, 1027–1053 (1996). [PMC free article] [PubMed] [Google Scholar]
- 110.Poluektova LY, Munn DH, Persidsky Y & Gendelman HE Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J. Immunol 168, 3941–3949 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Zhang J et al. Human microglia extensively reconstitute in humanized-BLT mice with human interleukin-34 transgene and support HIV-1 brain infection. Front. Immunol 12, 672415 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dash PK et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J. Neurosci 31, 3148–3157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leonard JM et al. Development of disease and virus recovery in transgenic mice containing HIV proviral DNA. Science 242, 1665–1670 (1988). [DOI] [PubMed] [Google Scholar]
- 114.Iwakura Y et al. The induction of cataracts by HIV-1 in transgenic mice. AIDS 6, 1069–1075 (1992). [DOI] [PubMed] [Google Scholar]
- 115.Hanna Z et al. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J. Virol 72, 121–132 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanna Z et al. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell 95, 163–175 (1998). [DOI] [PubMed] [Google Scholar]
- 117.Thomas FP, Chalk C, Lalonde R, Robitaille Y & Jolicoeur P Expression of human immunodeficiency virus type 1 in the nervous system of transgenic mice leads to neurological disease. J. Virol 68, 7099–7107 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toggas SM et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367, 188–193 (1994). [DOI] [PubMed] [Google Scholar]
- 119.Berrada F et al. Neuronal expression of human immunodeficiency virus type 1 env proteins in transgenic mice: distribution in the central nervous system and pathological alterations. J. Virol 69, 6770–6778 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Toneatto S, Finco O, van der Putten H, Abrignani S & Annunziata P Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS 13, 2343–2348 (1999). [DOI] [PubMed] [Google Scholar]
- 121.Kim BO et al. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol 162, 1693–1707 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruce-Keller AJ et al. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 56, 1414–1427 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones GJ et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci 27, 3703–3711 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D’Hooge R, Franck F, Mucke L & De Deyn PP Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur. J. Neurosci 11, 4398–4402 (1999). [DOI] [PubMed] [Google Scholar]
- 125.Maung R et al. CCR5 knockout prevents neuronal injury and behavioral impairment induced in a transgenic mouse model by a CXCR4-using HIV-1 glycoprotein 120. J. Immunol 193, 1895–1910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Imamichi H et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117, 3704–3710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ferdin J et al. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS One 13, e0191613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bachani M, Sacktor N, McArthur JC, Nath A & Rumbaugh J Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. J. Neurovirol 19, 82–88 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dinkins C, Arko-Mensah J & Deretic V Autophagy and HIV. Semin. Cell Dev. Biol 21, 712–718 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nath A, Conant K, Chen P, Scott C & Major EO Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J. Biol. Chem 274, 17098–17102 (1999). [DOI] [PubMed] [Google Scholar]
- 131.Nath A, Padua RA & Geiger JD HIV-1 coat protein gp120-induced increases in levels of intrasynaptosomal calcium. Brain Res 678, 200–206 (1995). [DOI] [PubMed] [Google Scholar]
- 132.Piller SC, Jans P, Gage PW & Jans DA Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc. Natl Acad. Sci. USA 95, 4595–4600 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rozzi SJ, Avdoshina V, Fields JA & Mocchetti I Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov 4, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sawaya BE, Khalili K, Mercer WE, Denisova L & Amini S Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J. Biol. Chem 273, 20052–20057 (1998). [DOI] [PubMed] [Google Scholar]
- 135.Teodorof-Diedrich C & Spector SA Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J. Virol 92, e00993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thangaraj A et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy 14, 1596–1619 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Villeneuve LM et al. HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J. Neurovirol 22, 564–574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gendelman HE, Lipton SA, Tardieu M, Bukrinsky MI & Nottet HS The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol 56, 389–398 (1994). [DOI] [PubMed] [Google Scholar]
- 139.Kato T, Hirano A, Llena JF & Dembitzer HM Neuropathology of acquired immune deficiency syndrome (AIDS) in 53 autopsy cases with particular emphasis on microglial nodules and multinucleated giant cells. Acta Neuropathol 73, 287–294 (1987). [DOI] [PubMed] [Google Scholar]
- 140.Michaels J, Price RW & Rosenblum MK Microglia in the giant cell encephalitis of acquired immune deficiency syndrome: proliferation, infection and fusion. Acta Neuropathol 76, 373–379 (1988). [DOI] [PubMed] [Google Scholar]
- 141.Iskander S, Walsh KA & Hammond RR Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J. Neuroinflammation 1, 7 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bryant AK et al. Antiretroviral therapy reduces neurodegeneration in HIV infection. AIDS 29, 323–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gonzalez-Scarano F & Martin-Garcia J The neuropathogenesis of AIDS. Nat. Rev.Immunol 5, 69–81 (2005). [DOI] [PubMed] [Google Scholar]
- 144.Thompson KA, Cherry CL, Bell JE & McLean CA Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am. J. Pathol 179, 1623–1629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Churchill MJ et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol 12, 146–152 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Ryan SK et al. Neuroinflammation and EIF2 signaling persist despite antiretroviral treatment in an hiPSC tri-culture model of HIV infection. Stem Cell Rep 14, 991 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kraft-Terry SD, Buch SJ, Fox HS & Gendelman HE A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64, 133–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gosselin D et al. An environment-dependent transcriptional network specifies human microglia identity. Science 356, eaal3222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kaul M, Garden GA & Lipton SA Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001). [DOI] [PubMed] [Google Scholar]
- 150.Saylor D et al. HIV-associated neurocognitive disorder — pathogenesis and prospects for treatment. Nat. Rev. Neurol 12, 309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heyes MP et al. Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J 12, 881–896 (1998). [DOI] [PubMed] [Google Scholar]
- 152.Smith DG et al. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J. Neurovirol 7, 56–60 (2001). [DOI] [PubMed] [Google Scholar]
- 153.Anderson AM et al. HIV, prospective memory, and cerebrospinal fluid concentrations of quinolinic acid and phosphorylated Tau. J. Neuroimmunol 319, 13–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Giulian D et al. Study of receptor-mediated neurotoxins released by HIV-1-infected mononuclear phagocytes found in human brain. J. Neurosci 16, 3139–3153 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Borjabad A et al. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog 7, e1002213 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Levine AJ et al. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer’s disease. BMC Med. Genomics 6, 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Borjabad A & Volsky DJ Common transcriptional signatures in brain tissue from patients with HIV-associated neurocognitive disorders, Alzheimer’s disease, and multiple sclerosis. J. Neuroimmune Pharmacol 7, 914–926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ginsberg SD et al. Expression profiling suggests microglial impairment in HIV neuropathogenesis. Ann. Neurol 83, 406–417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chivero ET et al. HIV-1 tat primes and activates microglial NLRP3 inflammasome-mediated neuroinflammation. J. Neurosci 37, 3599–3609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barber SA, Herbst DS, Bullock BT, Gama L & Clements JE Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol 10, 15–20 (2004). [DOI] [PubMed] [Google Scholar]
- 161.Veazey RS et al. Prevention of SHIV transmission by topical IFN-β treatment. Mucosal Immunol 9, 1528–1536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Honda K, Takaoka A & Taniguchi T Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360 (2006). [DOI] [PubMed] [Google Scholar]
- 163.Alammar L, Gama L & Clements JE Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J. Immunol 186, 4008–4018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zaritsky LA, Gama L & Clements JE Canonical type I IFN signaling in simian immunodeficiency virus-infected macrophages is disrupted by astrocyte-secreted CCL2. J. Immunol 188, 3876–3885 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thaney VE et al. IFNβ protects neurons from damage in a murine model of HIV-1 associated brain injury. Sci. Rep 7, 46514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gelman BB et al. The National NeuroAIDS Tissue Consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One 7, e46178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Singh H et al. A pivotal role for interferon-α receptor-1 in neuronal injury induced by HIV-1. J. Neuroinflammation 17, 226 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thaney VE & Kaul M Type I interferons in NeuroHIV. Viral Immunol 32, 7–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bourke NM et al. Control of HIV infection by IFN-α: implications for latency and a cure. Cell Mol. Life Sci 75, 775–783 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rivero-Juarez A, Frias M & Rivero A Current views on interferon therapy for HIV. Expert Opin. Biol. Ther 16, 1135–1142 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Utay NS & Douek DC Interferons and HIV infection: the good, the bad, and the ugly. Pathog. Immun 1, 107–116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Noel N et al. Interferon-associated therapies toward HIV control: the back and forth. Cytokine Growth Factor. Rev 40, 99–112 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Sugawara S, Thomas DL & Balagopal A HIV-1 infection and type 1 interferon: navigating through uncertain waters. AIDS Res. Hum. Retroviruses 35, 25–32 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gondim MVP et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl. Med 13, eabd8179 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhen A et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J. Clin. Invest 127, 260–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sandstrom TS, Ranganath N & Angel JB Impairment of the type I interferon response by HIV-1: potential targets for HIV eradication. Cytokine Growth Factor. Rev 37, 1–16 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Hua S et al. Pegylated interferon-α-induced natural killer cell activation is associated with human immunodeficiency virus-1 DNA decline in antiretroviral therapy-treated HIV-1/hepatitis C virus-coinfected patients. Clin. Infect. Dis 66, 1910–1917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.George J & Mattapallil JJ Interferon-α subtypes as an adjunct therapeutic approach for human immunodeficiency virus functional cure. Front. Immunol 9, 299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Paul S, Ricour C, Sommereyns C, Sorgeloos F & Michiels T Type I interferon response in the central nervous system. Biochimie 89, 770–778 (2007). [DOI] [PubMed] [Google Scholar]
- 180.Markowitz CE Interferon-beta: mechanism of action and dosing issues. Neurology 68 (Suppl. 4), S8–11 (2007). [DOI] [PubMed] [Google Scholar]
- 181.Kitai R, Zhao ML, Zhang N, Hua LL & Lee SC Role of MIP-1β and RANTES in HIV-1 infection of microglia: inhibition of infection and induction by IFNβ. J. Neuroimmunol 110, 230–239 (2000). [DOI] [PubMed] [Google Scholar]
- 182.Ferreira AC et al. From the periphery to the brain: Lipocalin-2, a friend or foe? Prog. Neurobiol 131, 120–136 (2015). [DOI] [PubMed] [Google Scholar]
- 183.Flo TH et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432, 917–921 (2004). [DOI] [PubMed] [Google Scholar]
- 184.Yang J et al. An iron delivery pathway mediated by a lipocalin. Mol. Cell 10, 1045–1056 (2002). [DOI] [PubMed] [Google Scholar]
- 185.Bao G et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol 6, 602–609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bachman MA, Miller VL & Weiser JN Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin. PLoS Pathog 5, e1000622 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jha MK et al. Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci. Biobehav. Rev 49, 135–156 (2015). [DOI] [PubMed] [Google Scholar]
- 188.Ferreira AC et al. Lipocalin-2 is involved in emotional behaviors and cognitive function. Front. Cell Neurosci 7, 122 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ferreira AC et al. Lipocalin-2 regulates adult neurogenesis and contextual discriminative behaviours. Mol. Psychiatry 23, 1031–1039 (2018). [DOI] [PubMed] [Google Scholar]
- 190.Mucha M et al. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proc. Natl Acad. Sci. USA 108, 18436–18441 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee S et al. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. J. Immunol 179, 3231–3241 (2007). [DOI] [PubMed] [Google Scholar]
- 192.Xing C et al. Neuronal production of lipocalin-2 as a help-me signal for glial activation. Stroke 45, 2085–2092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bi F et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl Acad. Sci. USA 110, 4069–4074 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dekens DW et al. Lipocalin 2 as a link between ageing, risk factor conditions and age-related brain diseases. Ageing Res. Rev 70, 101414 (2021). [DOI] [PubMed] [Google Scholar]
- 195.Ojeda-Juarez D et al. Lipocalin-2 mediates HIV-1 induced neuronal injury and behavioral deficits by overriding CCR5-dependent protection. Brain Behav. Immun 89, 184–199 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Williams ME, Ipser JC, Stein DJ, Joska JA & Naude PJW The association of immune markers with cognitive performance in South African HIV-positive patients. J. Neuroimmune Pharmacol 14, 679–687 (2019). [DOI] [PubMed] [Google Scholar]
- 197.Ivey NS, MacLean AG & Lackner AA Acquired immunodeficiency syndrome and the blood-brain barrier. J. Neurovirol 15, 111–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Maclean AG, Belenchia GE, Bieniemy DN, Moroney-Rasmussen TA & Lackner AA Simian immunodeficiency virus disrupts extended lengths of the blood–brain barrier. J. Med. Primatol 34, 237–242 (2005). [DOI] [PubMed] [Google Scholar]
- 199.Lu TS et al. Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J. Immunol 181, 6406–6416 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eugenin EA et al. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J. Leukoc. Biol 79, 444–452 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Persidsky Y, Zheng J, Miller D & Gendelman HE Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. J. Leukoc. Biol 68, 413–422 (2000). [PubMed] [Google Scholar]
- 202.Valdebenito S, Castellano P, Ajasin D & Eugenin EA Astrocytes are HIV reservoirs in the brain: a cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. J. Neurochem 158, 429–443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Churchill MJ et al. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann. Neurol 66, 253–258 (2009). [DOI] [PubMed] [Google Scholar]
- 204.Wahl A & Al-Harthi L HIV infection of non-classical cells in the brain. Retrovirology 20, 1 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bertrand L, Cho HJ & Toborek M Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 142, 502–511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hao Y et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fattakhov N, Torices S, Stangis M, Park M & Toborek M Synergistic impairment of the neurovascular unit by HIV-1 infection and methamphetamine use: implications for HIV-1-associated neurocognitive disorders. Viruses 13, 1883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Toborek M et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem 84, 169–179 (2003). [DOI] [PubMed] [Google Scholar]
- 209.Avraham HK, Jiang S, Lee TH, Prakash O & Avraham S HIV-1 Tat-mediated effects on focal adhesion assembly and permeability in brain microvascular endothelial cells. J. Immunol 173, 6228–6233 (2004). [DOI] [PubMed] [Google Scholar]
- 210.Andras IE et al. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res 74, 255–265 (2003). [DOI] [PubMed] [Google Scholar]
- 211.Andras IE et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J. Cereb. Blood Flow Metab 25, 1159–1170 (2005). [DOI] [PubMed] [Google Scholar]
- 212.de Almeida SM et al. Biomarkers of chemotaxis and inflammation in cerebrospinal fluid and serum in individuals with HIV-1 subtype C versus B. J. Neurovirol 22, 715–724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang Z, Shang H & Jiang Y Chemokines and chemokine receptors: accomplices for human immunodeficiency virus infection and latency. Front. Immunol 8, 1274 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Anderson AM et al. Cerebrospinal fluid CXCL10 is associated with the presence of low level CSF HIV during suppressive antiretroviral therapy. J. Neuroimmunol 353, 577493 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Letendre SL et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J. Neurovirol 17, 63–69 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alves de Lima K et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol 21, 1421–1429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Prieto GA & Cotman CW Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Growth Factor Rev 34, 27–33 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Harden LM, du Plessis I, Poole S & Laburn HP Interleukin (IL)-6 and IL-1β act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav. Immun 22, 838–849 (2008). [DOI] [PubMed] [Google Scholar]
- 219.Bourgognon JM & Cavanagh J The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv 4, 2398212820979802 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stein DS et al. Predicting clinical progression or death in subjects with early-stage human immunodeficiency virus (HIV) infection: a comparative analysis of quantification of HIV RNA, soluble tumor necrosis factor type II receptors, neopterin, and β2-microglobulin. J. Infect. Dis 176, 1161–1167 (1997). [DOI] [PubMed] [Google Scholar]
- 221.Diez-Ruiz A et al. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol 54, 1–8 (1995). [DOI] [PubMed] [Google Scholar]
- 222.Savès M et al. Prognostic value of plasma markers of immune activation in patients with advanced HIV disease treated by combination antiretroviral therapy. Clin. Immunol 99, 347–352 (2001). [DOI] [PubMed] [Google Scholar]
- 223.Suh J et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J. Neuroinflammation 11, 199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hagberg L et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther 7, 15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Walsh JG et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 11, 35 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mamik MK et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J. Neuroimmune Pharmacol 12, 233–248 (2017). [DOI] [PubMed] [Google Scholar]
- 227.Mamik MK & Power C Inflammasomes in neurological diseases: emerging pathogenic and therapeutic concepts. Brain 140, 2273–2285 (2017). [DOI] [PubMed] [Google Scholar]
- 228.Sil S et al. Role of inflammasomes in HIV-1 and drug abuse mediated neuroinflammaging. Cells 9, 1857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Periyasamy P, Thangaraj A, Bendi VS & Buch S HIV-1 Tat-mediated microglial inflammation involves a novel miRNA-34a-NLRC5-NFκB signaling axis. Brain Behav. Immun 80, 227–237 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kelley N, Jeltema D, Duan Y & He Y The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci 20, 3328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Swanson KV, Deng M & Ting JP The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol 19, 477–489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mullis C & Swartz TH NLRP3 inflammasome signaling as a link between HIV-1 infection and atherosclerotic cardiovascular disease. Front. Cardiovasc. Med 7, 95 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Andrade-Santos JL et al. IL18 gene polymorphism and its influence on CD4+ T-cell recovery in HIV-positive patients receiving antiretroviral therapy. Infect. Genet. Evol 75, 103997 (2019). [DOI] [PubMed] [Google Scholar]
- 234.Howren MB, Lamkin DM & Suls J Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med 71, 171–186 (2009). [DOI] [PubMed] [Google Scholar]
- 235.Dowlati Y et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457 (2010). [DOI] [PubMed] [Google Scholar]
- 236.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H & Kivimaki M Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun 49, 206–215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Miller AH, Haroon E, Raison CL & Felger JC Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30, 297–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ding Y & Dai J Advance in stress for depressive disorder. Adv. Exp. Med. Biol 1180, 147–178 (2019). [DOI] [PubMed] [Google Scholar]
- 239.Juruena MF, Eror F, Cleare AJ & Young AH The role of early life stress in HPA axis and anxiety. Adv. Exp. Med. Biol 1191, 141–153 (2020). [DOI] [PubMed] [Google Scholar]
- 240.Smith SM & Vale WW The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci 8, 383–395 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Polak P & Hall MN mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol 21, 209–218 (2009). [DOI] [PubMed] [Google Scholar]
- 242.Laplante M & Sabatini DM mTOR signaling at a glance. J. Cell Sci 122, 3589–3594 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Boden G Interaction between free fatty acids and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 5, 545–549 (2002). [DOI] [PubMed] [Google Scholar]
- 244.Snodgrass RG, Huang S, Choi IW, Rutledge JC & Hwang DH Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol 191, 4337–4347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Akbay B, Shmakova A, Vassetzky Y & Dokudovskaya S Modulation of mTORC1 signaling pathway by HIV-1. Cells 9, 1090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Cinti A et al. HIV-1 enhances mTORC1 activity and repositions lysosomes to the periphery by co-opting Rag GTPases. Sci. Rep 7, 5515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Alirezaei M, Kiosses WB & Fox HS Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy 4, 963–966 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Alirezaei M, Kiosses WB, Flynn CT, Brady NR & Fox HS Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One 3, e2906 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Papadopoli D et al. mTOR as a central regulator of lifespan and aging. F1000Research 8, 998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mannick JB et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 251.Westendorp RG et al. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J. Am. Geriatr. Soc 57, 1634–1637 (2009). [DOI] [PubMed] [Google Scholar]
- 252.Barzilai N, Gabriely I, Gabriely M, Iankowitz N & Sorkin JD Offspring of centenarians have a favorable lipid profile. J. Am. Geriatr. Soc 49, 76–79 (2001). [DOI] [PubMed] [Google Scholar]
- 253.Zheng Y & Jiang Y mTOR inhibitors at a glance. Mol. Cell Pharmacol 7, 15–20 (2015). [PMC free article] [PubMed] [Google Scholar]
- 254.Avila CL et al. mTOR inhibition suppresses posttransplant alloantibody production through direct inhibition of alloprimed B cells and sparing of CD8+ antibody-suppressing T cells. Transplantation 100, 1898–1906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Achim CL et al. Increased accumulation of intraneuronal amyloid β in HIV-infected patients. J. Neuroimmune Pharmacol 4, 190–199 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Soontornniyomkij V et al. Cerebral β-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE ε4 carriers. AIDS 26, 2327–2335 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Green DA et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 19, 407–411 (2005). [DOI] [PubMed] [Google Scholar]
- 258.Ortega M & Ances BM Role of HIV in amyloid metabolism. J. Neuroimmune Pharmacol 9, 483–491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hategan A, Masliah E & Nath A HIV and Alzheimer’s disease: complex interactions of HIV-Tat with amyloid β peptide and Tau protein. J. Neurovirol 25, 648–660 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bae M et al. Activation of TRPML1 clears intraneuronal Aβ in preclinical models of HIV infection. J. Neurosci 34, 11485–11503 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Andras IE & Toborek M Amyloid beta accumulation in HIV-1-infected brain: the role of the blood brain barrier. IUBMB Life 65, 43–49 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Breen EC et al. Accelerated aging with HIV begins at the time of initial HIV infection. iScience 25, 104488 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sehl ME et al. Increased rate of epigenetic aging in men living with HIV prior to treatment. Front. Genet 12, 796547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Gisslen M et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol. Neuroimmunol. Neuroinflamm 6, e512 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Murray J et al. Frontal lobe microglia, neurodegenerative protein accumulation, and cognitive function in people with HIV. Acta Neuropathol. Commun 10, 69 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mackiewicz MM, Overk C, Achim CL & Masliah E Pathogenesis of age-related HIV neurodegeneration. J. Neurovirol 25, 622–633 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rodriguez-Penney AT et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS 27, 5–16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Allavena C et al. Antiretroviral exposure and comorbidities in an aging HIV-infected population: the challenge of geriatric patients. PLoS One 13, e0203895 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Morgello S et al. Frailty in medically complex individuals with chronic HIV. AIDS 33, 1603–1611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Becker BW, Thames AD, Woo E, Castellon SA & Hinkin CH Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS Behav 15, 1888–1894 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kamal S et al. The presence of human immunodeficiency virus-associated neurocognitive disorders is associated with a lower adherence to combined antiretroviral treatment. Open Forum Infect. Dis 4, ofx070 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tedaldi EM, Minniti NL & Fischer T HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed. Res. Int 2015, 641913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ijoma UN et al. Health-related quality of life in people with chronic diseases managed in a low-resource setting — a study from South East Nigeria. Niger. J. Clin. Pract 22, 1180–1188 (2019). [DOI] [PubMed] [Google Scholar]
- 274.Morgan EE et al. Synergistic effects of HIV infection and older age on daily functioning. J. Acquir. Immune Defic. Syndr 61, 341–348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tozzi V et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int. J. STD AIDS 15, 254–259 (2004). [DOI] [PubMed] [Google Scholar]
- 276.Duffau P et al. Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS 32, 1651–1660 (2018). [DOI] [PubMed] [Google Scholar]
- 277.Banerjee N, McIntosh RC & Ironson G Impaired neurocognitive performance and mortality in HIV: assessing the prognostic value of the HIV-dementia scale. AIDS Behav 23, 3482–3492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Erlandson KM et al. Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin. Infect. Dis 68, 131–138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Naveed Z et al. Neurocognitive status and risk of mortality among people living with human immunodeficiency virus: an 18-year retrospective cohort study. Sci. Rep 11, 3738 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.De Francesco D et al. Do people living with HIV experience greater age advancement than their HIV-negative counterparts? AIDS 33, 259–268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Smith RL, de Boer R, Brul S, Budovskaya Y & van Spek H Premature and accelerated aging: HIV or HAART. Front. Genet 3, 328 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Guaraldi G et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis 53, 1120–1126 (2011). [DOI] [PubMed] [Google Scholar]
- 283.Siangphoe U, Archer KJ, Nguyen C & Lee KR Associations of antiretroviral therapy and comorbidities with neurocognitive outcomes in HIV-1-infected patients. AIDS 34, 893–902 (2020). [DOI] [PubMed] [Google Scholar]
- 284.Becker JT et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 73, 1292–1299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wright EJ et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 75, 864–873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ellis RJ, Paolillo E, Saloner R & Heaton RK Higher comorbidity burden predicts worsening neurocognitive trajectories in people with HIV. Clin. Infect. Dis 74, 1323–1328 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Saloner R et al. Effects of comorbidity burden and age on brain integrity in HIV. AIDS 33, 1175–1185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Charlson ME, Pompei P, Ales KL & MacKenzie CR A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis 40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 289.Justice AC et al. Veterans Aging Cohort Study (VACS): overview and description. Med. Care 44 (Suppl. 2), S13–24 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Assmann G & Schulte H The Prospective Cardiovascular Munster (PROCAM) study: prevalence of hyperlipidemia in persons with hypertension and/or diabetes mellitus and the relationship to coronary heart disease. Am. Heart J 116, 1713–1724 (1988). [DOI] [PubMed] [Google Scholar]
- 291.Wong ND & Levy D Legacy of the Framingham Heart Study: rationale, design, initial findings, and implications. Glob. Heart 8, 3–9 (2013). [DOI] [PubMed] [Google Scholar]
- 292.Ridker PM, Buring JE, Rifai N & Cook NR Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297, 611–619 (2007). [DOI] [PubMed] [Google Scholar]
- 293.Ellis RJ, Paolillo E, Saloner R & Heaton RK Higher comorbidity burden predicts worsening neurocognitive trajectories in people with human immunodeficiency virus. Clin. Infect. Dis 74, 1323–1328 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bing EG et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry 58, 721–728 (2001). [DOI] [PubMed] [Google Scholar]
- 295.Turner BJ, Laine C, Cosler L & Hauck WW Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J. Gen. Intern. Med 18, 248–257 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bigna JJ, Kenne AM, Asangbeh SL & Sibetcheu AT Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob. Health 6, e193–e202 (2018). [DOI] [PubMed] [Google Scholar]
- 297.Antoniou T, Yao Z, Raboud J & Gershon AS Incidence of chronic obstructive pulmonary disease in people with HIV in Ontario, 1996–2015: a retrospective population-based cohort study. CMAJ Open 8, E83–E89 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Davis K et al. Association between HIV infection and hypertension: a global systematic review and meta-analysis of cross-sectional studies. BMC Med 19, 105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.van Zoest RA, van den Born BH & Reiss P Hypertension in people living with HIV. Curr. Opin. HIV AIDS 12, 513–522 (2017). [DOI] [PubMed] [Google Scholar]
- 300.Cholera R et al. Depression and engagement in care among newly diagnosed HIV-infected adults in Johannesburg, South Africa. AIDS Behav 21, 1632–1640 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pence BW et al. Assessing the effect of measurement-based care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemp. Clin. Trials 33, 828–838 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Pence BW, O’Donnell JK & Gaynes BN Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 26, 656–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Manner IW et al. Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med 14, 354–361 (2013). [DOI] [PubMed] [Google Scholar]
- 304.Masenga SK et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr. Hypertens. Rep 21, 56 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ellis RJ et al. Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV. Brain Behav. Immun. Health 7, 100121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Morris A et al. HIV and chronic obstructive pulmonary disease: is it worse and why. Proc. Am. Thorac. Soc 8, 320–325 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lee CJ et al. The effects of diet alone or in combination with exercise in patients with prehypertension and hypertension: a randomized controlled trial. Korean Circ. J 48, 637–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Miller ER III et al. Results of the diet, exercise, and weight loss intervention trial (DEW-IT). Hypertension 40, 612–618 (2002). [DOI] [PubMed] [Google Scholar]
- 309.Bliss ES, Wong RH, Howe PR & Mills DE Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab 41, 447–470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Yurko-Mauro K Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr. Alzheimer Res 7, 190–196 (2010). [DOI] [PubMed] [Google Scholar]
- 311.Coca A, Monteagudo E, Domenech M, Camafort M & Sierra C Can the treatment of hypertension in the middle-aged prevent dementia in the elderly. High Blood Press. Cardiovasc. Prev 23, 97–104 (2016). [DOI] [PubMed] [Google Scholar]
- 312.Rouch L et al. Blood pressure and cognitive performances in middle-aged adults: the aging, health and work longitudinal study. J. Hypertens 37, 1244–1253 (2019). [DOI] [PubMed] [Google Scholar]
- 313.Gupta A et al. Treatment of hypertension reduces cognitive decline in older adults: a systematic review and meta-analysis. BMJ Open 10, e038971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tadic M, Cuspidi C & Hering D Hypertension and cognitive dysfunction in elderly: blood pressure management for this global burden. BMC Cardiovasc. Disord 16, 208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Krishnan S et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J. Acquir. Immune Defic. Syndr 61, 381–389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Yu B et al. Metabolic syndrome and neurocognitive deficits in HIV infection. J. Acquir. Immune Defic. Syndr 81, 95–101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.McCutchan JA et al. Role of obesity, metabolic variables, and diabetes in HIV-associated neurocognitive disorder. Neurology 78, 485–492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Panza F et al. Metabolic syndrome, mild cognitive impairment, and dementia. Curr. Alzheimer Res 8, 492–509 (2011). [DOI] [PubMed] [Google Scholar]
- 319.Morgan PK et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J. Biol. Chem 297, 101341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bourgeois C et al. Contribution of adipose tissue to the chronic immune activation and inflammation associated with HIV infection and its treatment. Front. Immunol 12, 670566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Werther GA et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121, 1562–1570 (1987). [DOI] [PubMed] [Google Scholar]
- 322.Marks JL, Porte D Jr., Stahl WL & Baskin DG Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127, 3234–3236 (1990). [DOI] [PubMed] [Google Scholar]
- 323.Doré S, Kar S, Rowe W & Quirion R Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience 80, 1033–1040 (1997). [DOI] [PubMed] [Google Scholar]
- 324.Schulingkamp RJ, Pagano TC, Hung D & Raffa RB Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci. Biobehav. Rev 24, 855–872 (2000). [DOI] [PubMed] [Google Scholar]
- 325.Mamik MK et al. Insulin treatment prevents neuroinflammation and neuronal injury with restored neurobehavioral function in models of HIV/AIDS neurodegeneration. J. Neurosci 36, 10683–10695 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Kim BH et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 33, 973–984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.de la Monte SM Intranasal insulin therapy for cognitive impairment and neurodegeneration: current state of the art. Expert Opin. Drug Deliv 10, 1699–1709 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hallschmid M Intranasal insulin for Alzheimer’s disease. CNS Drugs 35, 21–37 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Craft S et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol 77, 1099–1109 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Chang CC et al. HIV and co-infections. Immunol. Rev 254, 114–142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Brites C, Borges AH, Sprinz E & Page K Editorial: HIV and viral co-infections. Front. Microbiol 12, 731337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Di Gennaro F, Vergori A & Bavaro DF HIV and co-infections: updates and insights. Viruses 15, 1097 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Fialho R et al. Cognitive impairment in HIV and HCV co-infected patients: a systematic review and meta-analysis. AIDS Care 28, 1481–1494 (2016). [DOI] [PubMed] [Google Scholar]
- 334.Bharti AR et al. Asymptomatic malaria co-infection of HIV-infected adults in nigeria: prevalence of and impact on cognition, mood, and biomarkers of systemic inflammation. J. Acquir. Immune Defic. Syndr 86, 91–97 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Hestad KA et al. Cognitive impairment in Zambians with HIV infection and pulmonary tuberculosis. J. Acquir. Immune Defic. Syndr 80, 110–117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Ramlall S et al. Neurocognitive functioning in MDR-TB patients with and without HIV in KwaZulu-Natal, South Africa. Trop. Med. Int. Health 25, 919–927 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Carlson RD et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab. Brain Dis 29, 269–279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Letendre S et al. Higher anti-CMV IgG concentrations are associated with worse neurocognitive performance during suppressive antiretroviral therapy. Clin. Infect. Dis 67, 770–777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Brunt SJ et al. Short communication: do cytomegalovirus antibody levels associate with age-related syndromes in HIV patients stable on antiretroviral therapy? AIDS Res. Hum. Retroviruses 32, 567–572 (2016). [DOI] [PubMed] [Google Scholar]
- 340.Gianella S & Letendre S Cytomegalovirus and HIV: a dangerous Pas de Deux.J. Infect. Dis 214, S67–S74 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Roberts ET, Haan MN, Dowd JB & Aiello AE Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol 172, 363–371 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wu M et al. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 32, 1803–1810 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Vance DE et al. The synergistic effects of HIV, diabetes, and aging on cognition: implications for practice and research. J. Neurosci. Nurs 46, 292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Huck DM et al. Carotid artery stiffness and cognitive decline among women with or at risk for HIV infection. J. Acquired Immune Defic. Syndromes 78, 338–347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Freeman ML et al. CD8 T-cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin. Infect. Dis 62, 392–396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sacre K et al. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 26, 805–814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Lupia T et al. Presence of Epstein-Barr virus DNA in cerebrospinal fluid is associated with greater HIV RNA and inflammation. AIDS 34, 373–380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.van der Walt JM et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am. J. Hum. Genet 72, 804–811 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang DB et al. Declines in Drp1 and parkin expression underlie DNA damage-induced changes in mitochondrial length and neuronal death. J. Neurosci 33, 1357–1365 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ye X, Tai W & Zhang D The early events of Alzheimer’s disease pathology: from mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol. Aging 33, 1122. e1–10 (2012). [DOI] [PubMed] [Google Scholar]
- 351.Tsunemi T et al. PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med 4, 142ra197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cotto B, Natarajaseenivasan K & Langford D Astrocyte activation and altered metabolism in normal aging, age-related CNS diseases, and HAND. J. Neurovirol 25, 722–733 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yellen G Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol 217, 2235–2246 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Van den Bossche J, O’Neill LA & Menon D Macrophage immunometabolism: where are we (going). Trends Immunol 38, 395–406 (2017). [DOI] [PubMed] [Google Scholar]
- 355.Devanney NA, Stewart AN & Gensel JC Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol 329, 113310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Yin F, Sancheti H, Patil I & Cadenas E Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med 100, 108–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jiang T & Cadenas E Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13, 1059–1067 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Swinton MK et al. Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: implications for therapeutic targeting of astroglia. Neurobiol. Dis 130, 104502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Fields JA et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci. Rep 9, 17158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Natarajaseenivasan K et al. Astrocytic metabolic switch is a novel etiology for cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis 9, 415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Samikkannu T, Atluri VS & Nair MP HIV and cocaine impact glial metabolism: energy sensor AMP-activated protein kinase role in mitochondrial biogenesis and epigenetic remodeling. Sci. Rep 6, 31784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sivalingam K, Cirino TJ, McLaughlin JP & Samikkannu T HIV-Tat and cocaine impact brain energy metabolism: redox modification and mitochondrial biogenesis influence NRF transcription-mediated neurodegeneration. Mol. Neurobiol 58, 490–504 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Fields JA & Ellis RJ HIV in the cART era and the mitochondrial: immune interface in the CNS. Int. Rev. Neurobiol 145, 29–65 (2019). [DOI] [PubMed] [Google Scholar]
- 364.Fields JA et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol. Dis 86, 154–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Avdoshina V et al. The HIV protein gp120 alters mitochondrial dynamics in neurons. Neurotox. Res 29, 583–593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Vallee KJ & Fields JA Caloric restriction mimetic 2-deoxyglucose reduces inflammatory signaling in human astrocytes: implications for therapeutic strategies targeting neurodegenerative diseases. Brain Sci 12, 308 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Fields JA, Swinton MK, Montilla-Perez P, Ricciardelli E & Telese F The cannabinoid receptor agonist, WIN-55212–2, suppresses the activation of pro-inflammatory genes induced by interleukin 1 beta in human astrocytes. Cannabis Cannabinoid Res 7, 78–92 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Sheng WS et al. Synthetic cannabinoid WIN55,212–2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia 49, 211–219 (2005). [DOI] [PubMed] [Google Scholar]
- 369.Halcrow PW et al. HIV-1 gp120-induced endolysosome de-acidification leads to efflux of endolysosome iron, and increases in mitochondrial iron and reactive oxygen species. J. Neuroimmune Pharmacol 17, 181–194 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Halcrow PW et al. Heterogeneity of ferrous iron-containing endolysosomes and effects of endolysosome iron on endolysosome numbers, sizes, and localization patterns. J. Neurochem 161, 69–83 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Halcrow PW, Lynch ML, Geiger JD & Ohm JE Role of endolysosome function in iron metabolism and brain carcinogenesis. Semin. Cancer Biol 76, 74–85 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kallianpur AR et al. Hemochromatosis (HFE) gene mutations and peripheral neuropathy during antiretroviral therapy. AIDS 20, 1503–1513 (2006). [DOI] [PubMed] [Google Scholar]
- 373.Fennema-Notestine C et al. Iron-regulatory genes are associated with Neuroimaging measures in HIV infection. Brain Imaging Behav 14, 2037–2049 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Kallianpur AR et al. Genetic variation in iron metabolism is associated with neuropathic pain and pain severity in HIV-infected patients on antiretroviral therapy. PLoS One 9, e103123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Huang J et al. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther 25, 796–807 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Bernardo TC et al. Physical exercise and brain mitochondrial fitness: the possible role against Alzheimer’s disease. Brain Pathol 26, 648–663 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ruegsegger GN et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 4, e130681 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Cheng A et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab 23, 128–142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Park HS et al. Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain. Neuropharmacology 133, 451–461 (2018). [DOI] [PubMed] [Google Scholar]
- 380.Steiner JL, Murphy EA, McClellan JL, Carmichael MD & Davis JM Exercise training increases mitochondrial biogenesis in the brain. J. Appl. Physiol 111, 1066–1071 (2011). [DOI] [PubMed] [Google Scholar]
- 381.Ichimura Y et al. A ubiquitin-like system mediates protein lipidation. Nature 408, 488–492 (2000). [DOI] [PubMed] [Google Scholar]
- 382.Kametaka S, Okano T, Ohsumi M & Ohsumi Y Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J. Biol. Chem 273, 22284–22291 (1998). [DOI] [PubMed] [Google Scholar]
- 383.Baba M, Takeshige K, Baba N & Ohsumi Y Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol 124, 903–913 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Takeshige K, Baba M, Tsuboi S, Noda T & Ohsumi Y Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol 119, 301–311 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Brier LW et al. Regulation of LC3 lipidation by the autophagy-specific class III phosphatidylinositol-3 kinase complex. Mol. Biol. Cell 30, 1098–1107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ge L, Baskaran S, Schekman R & Hurley JH The protein-vesicle network of autophagy. Curr. Opin. Cell Biol 29, 18–24 (2014). [DOI] [PubMed] [Google Scholar]
- 387.Fields J et al. Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J. Neurovirol 19, 89–101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Fields J et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J. Neurosci 35, 1921–1938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Hui L, Chen X, Haughey NJ & Geiger JD Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro 4, 243–252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Patton SM et al. Cerebrospinal fluid (CSF) biomarkers of iron status are associated with CSF viral load, antiretroviral therapy, and demographic factors in HIV-infected adults. Fluids Barriers CNS 14, 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Abioye AI, Andersen CT, Sudfeld CR & Fawzi WW Anemia, iron status, and HIV: a systematic review of the evidence. Adv. Nutr 11, 1334–1363 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Paul BT, Manz DH, Torti FM & Torti SV Mitochondria and iron: current questions. Expert Rev. Hematol 10, 65–79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Khan N et al. Endolysosome iron restricts Tat-mediated HIV-1 LTR transactivation by increasing HIV-1 Tat oligomerization and β-catenin expression. J. Neurovirol 27, 755–773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Abdel-Haq R, Schlachetzki JCM, Glass CK & Mazmanian SK Microbiome-microglia connections via the gut-brain axis. J. Exp. Med 216, 41–59 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Fang P, Kazmi SA, Jameson KG & Hsiao EY The microbiome as a modifier of neurodegenerative disease risk. Cell Host Microbe 28, 201–222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Ma Q et al. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation 16, 53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Byrnes SJ et al. Chronic immune activation and gut barrier dysfunction is associated with neuroinflammation in ART-suppressed SIV+ rhesus macaques. PLoS Pathog 19, e1011290 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Fuchs D et al. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J. Acquir. Immune Defic. Syndr 3, 873–876 (1990). [PubMed] [Google Scholar]
- 399.Underwood J, Robertson KR & Winston A Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 29, 253–261 (2015). [DOI] [PubMed] [Google Scholar]
- 400.Lanman T, Letendre S, Ma Q, Bang A & Ellis R CNS neurotoxicity of antiretrovirals. J. Neuroimmune Pharmacol 16, 130–143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Bertrand L, Velichkovska M & Toborek M Cerebral vascular toxicity of antiretroviral therapy. J. Neuroimmune Pharmacol 16, 74–89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Alonso-Villaverde C et al. Host–pathogen interactions in the development of metabolic disturbances and atherosclerosis in HIV infection: the role of CCL2 genetic variants. Cytokine 51, 251–258 (2010). [DOI] [PubMed] [Google Scholar]
- 403.Tarr PE & Telenti A Genetic screening for metabolic and age-related complications in HIV-infected persons. F1000 Med. Rep 2, 83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Klotz U Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev 41, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
- 405.Mangoni AA & Jackson SH Age‐related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. Pharmacol 57, 6–14 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Winston A et al. Effects of age on antiretroviral plasma drug concentration in HIV-infected subjects undergoing routine therapeutic drug monitoring. J. Antimicrob. Chemother 68, 1354–1359 (2013). [DOI] [PubMed] [Google Scholar]
- 407.Bertrand L, Nair M & Toborek M Solving the blood-brain barrier challenge for the effective treatment of HIV replication in the central nervous system. Curr. Pharm. Des 22, 5477–5486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Nwogu JN et al. Pharmacokinetic, pharmacogenetic, and other factors influencing CNS penetration of antiretrovirals. AIDS Res. Treat 2016, 2587094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Decloedt EH, Rosenkranz B, Maartens G & Joska J Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin. Pharmacokinet 54, 581–598 (2015). [DOI] [PubMed] [Google Scholar]
- 410.Schifitto G et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS 21, 1877–1886 (2007). [DOI] [PubMed] [Google Scholar]
- 411.Schifitto G et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 69, 1314–1321 (2007). [DOI] [PubMed] [Google Scholar]
- 412.Nakasujja N et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 80, 196–202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Sacktor N et al. Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77, 1135–1142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Meulendyke KA et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J. Neurovirol 20, 591–602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Sacktor N et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J. Neurovirol 24, 16–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Rezaie-Majd A et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol 22, 1194–1199 (2002). [DOI] [PubMed] [Google Scholar]
- 417.Gerena Y et al. Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS One 7, e37358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Velichkovska M, Surnar B, Nair M, Dhar S & Toborek M Targeted mitochondrial coq10 delivery attenuates antiretroviral drug-induced senescence of neural progenitor cells. Mol. Pharm 16, 724–736 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Cross SA et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J. Immunol 187, 5015–5025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Louboutin J-P & Strayer DS in HIV/AIDS 107–123 (Elsevier, 2018). [Google Scholar]
- 421.Rochira V & Guaraldi G Growth hormone deficiency and human immunodeficiency virus. Best Pract. Res. Clin. Endocrinol. Metab 31, 91–111 (2017). [DOI] [PubMed] [Google Scholar]
- 422.Stanley TL et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA 312, 380–389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Stanley TL et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin. Infect. Dis 54, 1642–1651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Wrigley S, Arafa D & Tropea D Insulin-like growth factor 1: at the crossroads of brain development and aging. Front. Cell Neurosci 11, 14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rui-Hua C, Yong-de P, Xiao-Zhen J, Chen J & Bin Z Decreased levels of serum IGF-1 and vitamin D are associated with cognitive impairment in patients with type 2 diabetes. Am. J. Alzheimers Dis. Other Demen 34, 450–456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Aleman A et al. Insulin-like growth factor-I and cognitive function in healthy older men. J. Clin. Endocrinol. Metab 84, 471–475 (1999). [DOI] [PubMed] [Google Scholar]
- 427.Kalmijn S, Janssen JA, Pols HA, Lamberts SW & Breteler MM A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J. Clin. Endocrinol. Metab 85, 4551–4555 (2000). [DOI] [PubMed] [Google Scholar]
- 428.de la Monte SM & Wands JR Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J. Alzheimers Dis 7, 45–61 (2005). [DOI] [PubMed] [Google Scholar]
- 429.Nazem F, Farhangi N & Neshat-Gharamaleki M Beneficial effects of endurance exercise with rosmarinus officinalis labiatae leaves extract on blood antioxidant enzyme activities and lipid peroxidation in streptozotocin-induced diabetic rats. Can. J. Diabetes 39, 229–234 (2015). [DOI] [PubMed] [Google Scholar]
- 430.Gleeson M et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol 11, 607–615 (2011). [DOI] [PubMed] [Google Scholar]
- 431.Horowitz AM et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Abdolmaleki F & Heidarianpour A Endurance exercise training restores diabetes-induced alteration in circulating Glycosylphosphatidylinositol-specific phospholipase D levels in rats. Diabetol. Metab. Syndr 12, 43 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Qin W, Liang YZ, Qin BY, Zhang JL & Xia N The clinical significance of glycoprotein phospholipase D levels in distinguishing early stage latent autoimmune diabetes in adults and type 2 diabetes. PLoS One 11, e0156959 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Baker SK et al. Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 115, E9687–E9696 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Deeg MA et al. Increased expression of GPI-specific phospholipase D in mouse models of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab 281, E147–154 (2001). [DOI] [PubMed] [Google Scholar]
- 436.O’Brien KD, Pineda C, Chiu WS, Bowen R & Deeg MA Glycosylphosphatidylinositol-specific phospholipase D is expressed by macrophages in human atherosclerosis and colocalizes with oxidation epitopes. Circulation 99, 2876–2882 (1999). [DOI] [PubMed] [Google Scholar]
- 437.Montoya JL et al. Coagulation imbalance and neurocognitive functioning in older HIV-positive adults on suppressive antiretroviral therapy. AIDS 31, 787–795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Lee KA et al. Types of sleep problems in adults living with HIV/AIDS. J. Clin. Sleep. Med 8, 67–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Rubinstein ML & Selwyn PA High prevalence of insomnia in an outpatient population with HIV infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol 19, 260–265 (1998). [DOI] [PubMed] [Google Scholar]
- 440.Wiegand M, Moller AA, Schreiber W, Krieg JC & Holsboer F Alterations of nocturnal sleep in patients with HIV infection. Acta Neurol. Scand 83, 141–142 (1991). [DOI] [PubMed] [Google Scholar]
- 441.Nokes KM & Kendrew J Correlates of sleep quality in persons with HIV disease. J. Assoc. Nurses AIDS Care 12, 17–22 (2001). [DOI] [PubMed] [Google Scholar]
- 442.Mahmood Z, Hammond A, Nunez RA, Irwin MR & Thames AD Effects of sleep health on cognitive function in HIV+ and HIV− adults. J. Int. Neuropsychol. Soc 24, 1038–1046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Gamaldo CE et al. Evaluating sleep and cognition in HIV. J. Acquir. Immune Defic. Syndr 63, 609–616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Shokri-Kojori E et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl Acad. Sci. USA 115, 4483–4488 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Kardassis D, Grote L, Sjostrom L, Hedner J & Karason K Sleep apnea modifies the long-term impact of surgically induced weight loss on cardiac function and inflammation. Obesity 21, 698–704 (2013). [DOI] [PubMed] [Google Scholar]
- 446.Wirth MD et al. Association of markers of inflammation with sleep and physical activity among people living with HIV or AIDS. AIDS Behav 19, 1098–1107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Vecchio LM et al. The neuroprotective effects of exercise: maintaining a healthy brain throughout aging. Brain Plast 4, 17–52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Dufour CA et al. A longitudinal analysis of the impact of physical activity on neurocognitive functioning among HIV-infected adults. AIDS Behav 22, 1562–1572 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Fazeli PL et al. Physical activity is associated with better neurocognitive and everyday functioning among older adults with HIV disease. AIDS Behav 19, 1470–1477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Montoya JL, Henry B & Moore DJ Behavioral and physical activity interventions for HAND. Curr. Top. Behav. Neurosci 50, 479–501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Montoya JL et al. Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS 33, 931–939 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Dufour CA et al. Physical exercise is associated with less neurocognitive impairment among HIV-infected adults. J. Neurovirol 19, 410–417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Monroe AK et al. The association between physical activity and cognition in men with and without HIV infection. HIV Med 18, 555–563 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Henry BL & Moore DJ Preliminary findings describing participant experience with iSTEP, an mHealth intervention to increase physical activity and improve neurocognitive function in people living with HIV. J. Assoc. Nurses AIDS Care 27, 495–511 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Vance DE et al. Computerized cognitive training for the neurocognitive complications of HIV infection: a systematic review. J. Assoc. Nurses AIDS Care 30, 51–72 (2019). [DOI] [PubMed] [Google Scholar]
- 456.Wei J et al. Evaluation of computerized cognitive training and cognitive and daily function in patients living with HIV: a meta-analysis. JAMA Netw. Open 5, e220970 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Abers MS, Shandera WX & Kass JS Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 28, 131–145 (2014). [DOI] [PubMed] [Google Scholar]
- 458.Scherzer R & Shlipak MG Risk factors: individual assessment of CKD risk in HIV-positive patients. Nat. Rev. Nephrol 11, 392 (2015). [DOI] [PubMed] [Google Scholar]
- 459.Rodriguez-Nóvoa S, Alvarez E, Labarga P & Soriano V Renal toxicity associated with tenofovir use. Expert Opin. Drug Saf 9, 545–559 (2010). [DOI] [PubMed] [Google Scholar]
- 460.Kakuda TN Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther 22, 685–708 (2000). [DOI] [PubMed] [Google Scholar]
- 461.Kohler JJ & Lewis W A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Env. Mol. Mutagen 48, 166–172 (2007). [DOI] [PubMed] [Google Scholar]
- 462.Lewis W, Day BJ & Copeland WC Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov 2, 812–822 (2003). [DOI] [PubMed] [Google Scholar]
- 463.Nooka S & Ghorpade A Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis 9, 317 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Nagiah S, Phulukdaree A & Chuturgoon AA Lon protease and eiF2α are involved in acute, but not prolonged, antiretroviral induced stress response in HepG2 cells. Chem. Biol. Interact 252, 82–86 (2016). [DOI] [PubMed] [Google Scholar]
- 465.Stankov MV, Lucke T, Das AM, Schmidt RE & Behrens GM Mitochondrial DNA depletion and respiratory chain activity in primary human subcutaneous adipocytes treated with nucleoside analogue reverse transcriptase inhibitors. Antimicrob. Agents Chemother 54, 280–287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Young MJ Off-target effects of drugs that disrupt human mitochondrial DNA maintenance. Front. Mol. Biosci 4, 74 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Allen Reeves A et al. Neurotoxicities in the treatment of HIV between dolutegravir, rilpivirine and dolutegravir/rilpivirine: a meta-analysis. Sex. Transm. Infect 97, 261–267 (2021). [DOI] [PubMed] [Google Scholar]
- 468.Apostolova N et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J. Antimicrob. Chemother 70, 2693–2708 (2015). [DOI] [PubMed] [Google Scholar]
- 469.Blas-García A et al. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: a comparison with efavirenz. J. Antimicrob. Chemother 69, 2995–3000 (2014). [DOI] [PubMed] [Google Scholar]
- 470.Markowitz M et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N. Engl. J. Med 333, 1534–1540 (1995). [DOI] [PubMed] [Google Scholar]
- 471.Jensen BK et al. Altered oligodendrocyte maturation and myelin maintenance: the role of antiretrovirals in HIV-associated neurocognitive disorders. J. Neuropathol. Exp. Neurol 74, 1093–1118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Vivithanaporn P, Asahchop EL, Acharjee S, Baker GB & Power C HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS 30, 543–552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Ekins S et al. α7-Nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease. AIDS 31, 1083–1089 (2017). [DOI] [PubMed] [Google Scholar]
- 474.Soontornniyomkij V et al. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS 28, 1297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Stern AL et al. Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J. Neuroimmune Pharmacol 13, 64–76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.del Mar Gutierrez M, Mateo MG, Vidal F & Domingo P Drug safety profile of integrase strand transfer inhibitors. Expert Opin. Drug Saf 13, 431–445 (2014). [DOI] [PubMed] [Google Scholar]
- 477.Abrams E & Myer L Lessons from dolutegravir and neural tube defects. Lancet HIV 8, e3–e4 (2021). [DOI] [PubMed] [Google Scholar]
- 478.Gray J & Young B Acute onset insomnia associated with the initiation of raltegravir: a report of two cases and literature review. AIDS Patient Care STDS 23, 689–690 (2009). [DOI] [PubMed] [Google Scholar]
- 479.Capetti A et al. Morning dosing for dolutegravir-related insomnia and sleep disorders. HIV Med 838, e62–e63 (2017). [DOI] [PubMed] [Google Scholar]
- 480.Latronico T et al. In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J. Neurochem 144, 271–284 (2018). [DOI] [PubMed] [Google Scholar]
- 481.Reust CE Common adverse effects of antiretroviral therapy for HIV disease. Am. Fam. Physician 83, 1443–1451 (2011). [PubMed] [Google Scholar]
- 482.Treisman GJ & Soudry O Neuropsychiatric effects of HIV antiviral medications. Drug Saf 39, 945–957 (2016). [DOI] [PubMed] [Google Scholar]
- 483.Manfredi R & Sabbatani S A novel antiretroviral class (fusion inhibitors) in the management of HIV infection. Present features and future perspectives of enfuvirtide (T-20). Curr. Med. Chem 13, 2369–2384 (2006). [DOI] [PubMed] [Google Scholar]
- 484.LaBonte J, Lebbos J & Kirkpatrick P Enfuvirtide. Nat. Rev. Drug Discov 2, 345–346 (2003). [DOI] [PubMed] [Google Scholar]
- 485.Oldfield V, Keating GM & Plosker G Enfuvirtide: a review of its use in the management of HIV infection. Drugs 65, 1139–1160 (2005). [DOI] [PubMed] [Google Scholar]
- 486.Curtis L et al. Dolutegravir: clinical and laboratory safety in integrase inhibitor–naive patients. HIV Clin. Trials 15, 199–208 (2014). [DOI] [PubMed] [Google Scholar]
- 487.Harris M, Larsen G & Montaner JS Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS 22, 1890–1892 (2008). [DOI] [PubMed] [Google Scholar]
- 488.Fettiplace A et al. Psychiatric symptoms in patients receiving dolutegravir. J. Acquir. Immune Defic. Syndr 74, 423–431 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Harris M What did we learn from the bictegravir switch studies? Lancet HIV 5, e336–e337 (2018). [DOI] [PubMed] [Google Scholar]
- 490.Zhao Y et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin. Trials 11, 59–67 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Yacoub AD et al. Intranasal Insulin Improves Attention and Memory in People with HIV https://www.natap.org/2021/CROI/croi_91.htm (2021).
- 492.Yadav A et al. Lack of atorvastatin effect on monocyte gene expression and inflammatory markers in HIV-1-infected ART-suppressed Individuals at risk of non-AIDS comorbidities. Pathog. Immun 6, 1–26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Zhou F et al. Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 32, 2570–2580 (2007). [DOI] [PubMed] [Google Scholar]
- 494.Cheng L et al. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. J. Clin. Invest 127, 269–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Azzoni L et al. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J. Infect. Dis 207, 213–222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Lazi E, Burns JM & Swerdlow RH Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 35, 2574–2583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Fortier M et al. A ketogenic drink improves cognition in mild cognitive impairment: results of a 6-month RCT. Alzheimers Dement 17, 543–552 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


