Abstract
Purpose
There is an unclear relationship between estradiol levels and fresh embryo transfer (ET) outcomes. We determined the relationship between estradiol on the day of trigger, in fresh ET cycles without premature progesterone elevation, and good birth outcomes (GBO).
Methods
We identified autologous fresh ET cycles from 2015 to 2021 at multiple clinics in the USA. Patients with recurrent pregnancy loss, uterine factor, and elevated progesterone on the day of trigger (progesterone > 2 ng/mL or 3-day area under the curve > 4.5 ng/mL) were excluded. The primary outcome was GBO (singleton, term, live birth with appropriate weight). Log-binomial generalized estimating equations determined the likelihood of outcomes.
Results
Of 17,608 fresh ET cycles, 5025 (29%) yielded GBO. Cycles with estradiol 4000 pg/mL had a greater likelihood of GBO compared to cycles < 1000 pg/mL (aRR = 1.32, 95% CI 1.13–1.54). Pairwise comparisons of estradiol between < 1000 pg/mL versus 1000–1999 pg/mL and 1000–1999 pg/mL versus 2000–2999 pg/mL revealed a higher likelihood of GBO with higher estradiol (aRR 0.83, 95% CI 0.73–0.95; aRR 0.91, 95% CI 0.85–0.97, respectively). Comparisons amongst more elevated estradiol levels revealed that the likelihood of GBO remained similar between groups (2000–2999 pg/mL versus 3000–3999 pg/mL, aRR 1.04, 95% CI 0.97–1.11; 3000–3999 pg/mL versus 4000 pg/mL, aRR 0.96, 95% CI 0.9–1.04).
Conclusion
In fresh ET cycles, higher estradiol levels were associated with an increased prevalence of GBO until estradiol 2000–2999 pg/mL, thereafter plateauing. In fresh ET candidates, elevated estradiol levels should not preclude eligibility though premature progesterone rise, and risk of ovarian hyperstimulation syndrome must still be considered.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-024-03062-4.
Keywords: Fresh embryo transfer, Estradiol, Good birth outcome, Live birth
Introduction
Fresh embryo transfer (ET) versus a freeze-all approach during in vitro fertilization (IVF) remains disputed within our field [1, 2]. A freeze-all approach appears to benefit select patient populations, such as patients at risk for ovarian hyperstimulation syndrome (OHSS) or with elevated progesterone levels [3, 4]. In other patient populations, a fresh ET will shorten the time of pregnancy, be less expensive, and may yield equivalent pregnancy outcomes [5]. The need to understand whether other factors, such as peak estradiol levels, affect outcomes of fresh ETs during stimulation is warranted [6].
Ovarian stimulation during IVF cycles leads to supraphysiologic levels of estradiol, sometimes increased tenfold from natural spontaneous cycles. Clinical studies linking the effects of peak estradiol levels during hyperstimulation on pregnancy rates after fresh ETs are conflicting. A recent systematic review and meta-analysis concluded that there was insufficient data to reach a conclusion linking estradiol levels and pregnancy rates after fresh ETs [6]. Retrospective studies have reported an unclear relationship between peak estradiol levels and pregnancy rates. One study conducted in 1990 reported higher pregnancy rates with estradiol levels > 2777 pg/mL [7], while other studies have demonstrated positive associations between estradiol levels and pregnancy rates suggestive of a possible optimal estradiol range for fresh ETs [8–10]. Notably, none of these studies [7–10] reported or adjusted for an important confounder, serum progesterone at the time of trigger, which also rises with robust stimulations. Thus, the relationship between peak estradiol levels in fresh ET and pregnancy rates should be investigated with progesterone levels in mind.
The need to maximize good perinatal outcomes after fresh ET also requires consideration. Live births from fresh ET are at increased risk for preterm delivery, low birth weight, and small for gestational age compared to frozen ETs [11, 12]. Existing data are conflicting on whether the degree of estradiol elevation impacts the severity of adverse perinatal outcomes with some studies reporting a detrimental effect [13–16] and another reporting no association [17].
Thus, we sought to determine the relationship between estradiol levels on the day of trigger in fresh ET cycles without premature progesterone elevation and good birth outcomes (GBO) defined as singleton, term live births with appropriate weight, and to determine if there is an optimal estradiol level that maximizes GBO.
Materials and methods
Study design
This retrospective cohort study was conducted utilizing data from 2015 and 2021 at US Fertility Centers which included Shady Grove Fertility, Fertility Centers of Illinois, Reproductive Science Center, and IVF Florida.
Study population
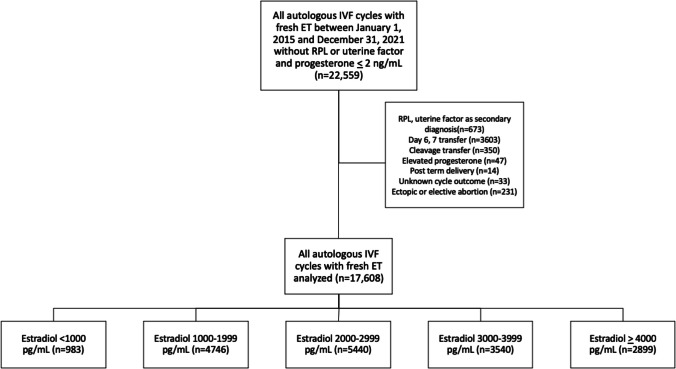
All cycle data from patients who underwent autologous fresh oocyte retrieval resulting in fresh embryo transfer between January 1, 2015, and December 31, 2021, were queried. Our data query excluded uterine factor and recurrent pregnancy loss diagnosis and included only cycles with known estradiol levels on the day of trigger and progesterone levels ≤ 2 ng/mL on the day of trigger. Cycles were further limited to only a fresh day 5 blastocyst transfer. Exclusion criteria included cycles with a 3-day area under the curve (AUC) cumulative serum progesterone > 4.5 ng/mL over the last days of stimulation based on internal institutional data [18]. Additional exclusion criteria included any secondary diagnoses of uterine factor infertility or recurrent pregnancy loss, cycles missing birth outcome data, and post-term deliveries ≥ 42 weeks. A detailed flow chart of study criteria is shown in Fig. 1.
Fig. 1.
Study design and flow chart with inclusion and exclusion criteria. RPL, recurrent pregnancy loss. Elevated progesterone is defined as total progesterone > 4.5 ng/mL over the final 3 days of stimulation
Stimulation protocol
Ovarian stimulation was at physician discretion using the standard clinical protocols at each site. In general, mixed FSH and LH (Gonal-F, Germany; Follistim, USA; Menopur, USA) were administered under GnRH antagonist, GnRH agonist pituitary suppression, and microdose flare protocols. Oral contraceptives were utilized for 10–21 days. For antagonist protocols, the antagonist was started when the dominant follicle was 14 mm in size. Once follicles measured 18 mm, final oocyte maturation was achieved with either hCG (5000–10,000 IUs), GnRH agonist (leuprolide 4 mg), or dual trigger (leuprolide 4 mg with hCG 1000–5000 IUs) at provider discretion with consideration for risk of OHSS. Serum estradiol was obtained on the day of trigger. Conversion from intended fresh ET to a freeze-all approach due to elevated progesterone (either serum progesterone > 2 ng/mL on the day of trigger or a 3-day AUC progesterone > 4.5 ng/mL) or high risk for OHSS occurred at provider discretion, and these cycles were not included. Oocyte retrieval occurred 36 h later. Fertilization was performed with conventional IVF or intracytoplasmic sperm injection as clinically indicated. Sperm was obtained by masturbation or surgical extraction. Embryos were graded as good, fair, or poor according to the simplified SART embryo scoring system [19]. Embryo transfer was performed on day 5 under ultrasound guidance. Luteal support was provided with exogenous estrogen and a combination of vaginal or intramuscular progesterone. Intramuscular progesterone was included in cases of exclusive GnRH agonist trigger. Serum hCG levels were measured at approximately 4 weeks gestational age followed by ultrasonography confirmation of an intrauterine pregnancy in all pregnant patients.
Outcomes
The primary outcome was good birth outcome (GBO) which was defined as a singleton, live birth at term, 37 weeks, with appropriate weight for gestational age (2500–3999 g). A weight of < 4000 g was chosen based on definitions of macrosomia at term [20, 21]. Secondary outcomes were spontaneous abortion rate, clinical pregnancy rate (defined as the presence of cardiac activity), overall live birth rate (24 weeks), term birth rate (37 to < 42 weeks), and preterm birth rate (24 to < 37 weeks). All outcomes were calculated per fresh ET transfer.
Statistical analysis
Differences in intended parent characteristics by estradiol groups were assessed using ANOVA or chi-square tests. Differences in cycle characteristics by estradiol groups were assessed with generalized estimating equation (GEE) models to incorporate data from multiple cycles (i.e., repeated subject-level data). These GEE models included multinomial GEEs with generalized logit links for categorical outcomes with more than 2 levels of response, GEEs assuming a normal distribution and identity links for continuous outcomes, and GEEs assuming a Poisson distribution for count outcomes.
We assessed whether estradiol groups were associated with clinical outcomes (dichotomous) using log-binomial generalized estimating equations. Two models were included for each outcome: one including estradiol groups only (unadjusted) and one adjusting for risk factors associated with estradiol or clinical outcomes (including age, body mass index [BMI], progesterone on trigger day, reason for assisted reproductive technology, protocol, smoking status, number of transferred embryos, trigger medication, number of retrieved oocytes, and number of good and poor embryos from the retrieval). We did not have luteal support regimen data available, so we included trigger medication as an alternative marker. In addition, we attempted to control for the underlying prognostic differences that are associated between estradiol levels at the end of stimulation (e.g., younger patient with higher ovarian reserve) by including the number of oocytes retrieved and the number of good and poor embryos from the retrieval. Missing was included as an additional category for smoking status and trigger medication to reduce the potential for bias. Concerns for multi-collinearity for risk factors such as estradiol levels, trigger medication, the number of oocytes retrieved were assessed by evaluating the variance inflation factors of covariates, as well as by assessing whether the parameter estimates and confidence intervals changed when potentially correlated covariates were removed. In the model for clinical pregnancy, we excluded trigger medication, number of good embryos, and number of poor embryos due to convergence issues which were not seen in our models for other outcomes. Endometrial thickness was not adjusted for in the models due to convergence issues. We looked to evaluate estradiol continuously with GBO and live birth; however, there was not enough of a linear relationship between estradiol values and these outcomes to do so. Adjusted risk ratios (aRR) and 95% confidence limits are presented. Power/sample size calculations were conducted assuming 2700 women in the highest estradiol category, repeated measures correlation of 0.1, and two-sided type I error of 5% to compare this group to equal-sized groups with incidence of GBO of 30%. Based on these assumptions, this study has 80% or better power to detect a difference in GBO of ± 3.6%.
A sensitivity analysis was performed for GBO, using similar unadjusted and adjusted GEE models, using estradiol categories that included < 1000, 1000–3999, 4000–5999, and ≥ 6000 pg/mL.
Ethical approval
Ethical approval for this study was granted by Advarra institutional review board (No. Pro00058669).
Results
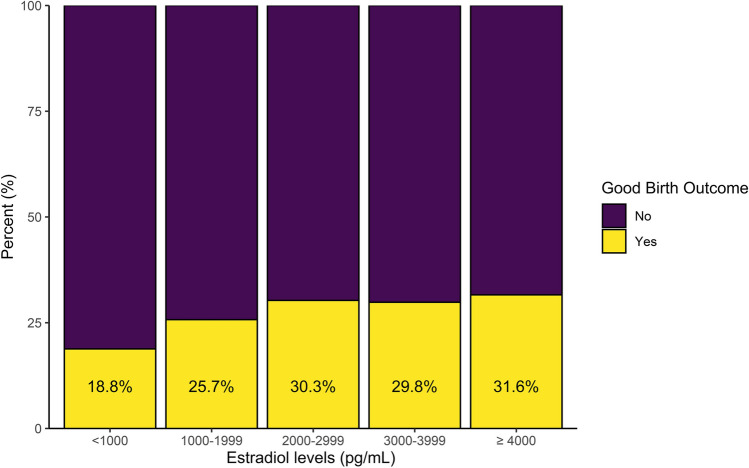
Data from 17,608 cycles were included for 14,708 women (Fig. 1). Most women (83%) contributed one cycle. For women with more than one cycle (17%), the range was 2–7 cycles. Patients were, on average, 34.1 (SD, 4.3) years old, with an average BMI of 27.0 (5.9) kg/m2. Patient and cycle characteristics are included in Table 1. Frequencies of clinical outcomes by estradiol levels are shown in Fig. 2 and Table 2.
Table 1.
Baseline patient and cycle demographics
| Estradiol levels on day of trigger | All | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| < 1000 pg/mL | 1000–1999 pg/mL | 2000–2999 pg/mL | 3000–3999 pg/mL | ≥ 4000 pg/mL | ||||
| Total number of cycles | 983 | 4746 | 5440 | 3540 | 2899 | 17,608 | ||
| Total number of patients | 876 | 4212 | 5046 | 3373 | 2741 | 14,708 | ||
| Intended parent characteristics (n = 14,708) | ||||||||
| Age | 36.7 (4.3) | 34.9 (4.3) | 34.0 (4.1) | 33.4 (4.1) | 33.0 (4.1) | 34.1 (4.3) | < 0.001a | |
| Reason for ART | DOR | 273 (37%) | 809 (21%) | 471 (10%) | 181 (6%) | 91 (4%) | 1825 (12%) | < 0.001b |
| Endometriosis | 40 (5%) | 200 (5%) | 199 (4%) | 129 (4%) | 86 (3%) | 654 (4%) | ||
| Male | 153 (20%) | 995 (26%) | 1362 (30%) | 927 (31%) | 773 (31%) | 4210 (29%) | ||
| Other | 61 (8%) | 300 (8%) | 361 (8%) | 249 (8%) | 188 (8%) | 1159 (8%) | ||
| PCOS | 55 (7%) | 438 (11%) | 624 (14%) | 523 (17%) | 524 (21%) | 2164 (15%) | ||
| Tubal | 72 (10%) | 411 (11%) | 508 (11%) | 320 (11%) | 293 (12%) | 1604 (11%) | ||
| Unexplained | 93 (12%) | 723 (19%) | 1032 (23%) | 694 (23%) | 550 (22%) | 3092 (21%) | ||
| BMI (kg/m2) | 28.5 (6.2) | 27.3 (6.0) | 27.0 (5.9) | 26.6 (5.7) | 26.4 (5.6) | 27.0 (5.9) | < 0.001a | |
| Ever smoker | No | 489 (65%) | 2686 (69%) | 3239 (71%) | 2122 (70%) | 1674 (67%) | 10,210 (69%) | < 0.001b |
| Yes | 37 (5%) | 178 (5%) | 218 (5%) | 102 (3%) | 83 (3%) | 618 (4%) | ||
| Unknown | 221 (30%) | 1012 (26%) | 1100 (24%) | 799 (26%) | 748 (30%) | 3880 (26%) | ||
| Cycle characteristics (n = 17,608) | ||||||||
| Year of cycle | 2015–2017 | 452 (46%) | 2756 (58%) | 2709 (50%) | 1994 (56%) | 1345 (46%) | 9256 (53%) | < 0.001c |
| 2018–2019 | 278 (28%) | 1140 (24%) | 1471 (27%) | 805 (23%) | 791 (27%) | 4485 (25%) | ||
| 2020–2021 | 253 (26%) | 850 (18%) | 1260 (23%) | 741 (21%) | 763 (26%) | 3867 (22%) | ||
| Stimulation protocol | Luteal phase Lupron | 27 (3%) | 245 (5%) | 393 (7%) | 383 (11%) | 237 (8%) | 1285 (7%) | < 0.001c |
| Microdose flare | 176 (18%) | 555 (12%) | 428 (8%) | 195 (6%) | 87 (3%) | 1441 (8%) | ||
| Other | 75 (8%) | 299 (6%) | 215 (4%) | 91 (3%) | 52 (2%) | 732 (4%) | ||
| Antagonist | 705 (72%) | 3647 (77%) | 4404 (81%) | 2871 (81%) | 2523 (87%) | 14,150 (80%) | ||
| Trigger medications | HCG and Lupron | 20 (2%) | 82 (2%) | 130 (2%) | 111 (3%) | 73 (3%) | 416 (2%) | < 0.001c |
| Lupron | 21 (2%) | 484 (10%) | 1413 (26%) | 1581 (45%) | 2103 (73%) | 5602 (32%) | ||
| Missing | 21 (2%) | 90 (2%) | 77 (1%) | 34 (1%) | 24 (1%) | 246 (1%) | ||
| HCG | 921 (94%) | 4090 (86%) | 3820 (70%) | 1814 (51%) | 699 (24%) | 11,344 (64%) | ||
| P4 on trigger day (ng/mL) | 0.7 (0.3) | 0.9 (0.4) | 1.1 (0.4) | 1.1 (0.4) | 1.2 (0.4) | 1.0 (0.4) | < 0.001d | |
| 3d AUC P4 prior to trigger day (ng/mL) | 1.7 (0.7) | 1.9 (0.8) | 2.2 (0.8) | 2.3 (0.8) | 2.4 (0.8) | 2.2 (0.8) | < 0.001d | |
| No. of oocytes retrieved | 4.8 (3.3) | 9.2 (4.9) | 13.4 (6.0) | 17.4 (7.3) | 21.7 (9.0) | 14.0 (8.1) | < 0.001d | |
| No. of transferred blastocysts | 1 | 589 (60%) | 2822 (59%) | 3685 (68%) | 2470 (70%) | 2214 (76%) | 11780 (67%) | < 0.001c |
| 2 | 394 (40%) | 1924 (41%) | 1755 (32%) | 1070 (30%) | 685 (24%) | 5828 (33%) | ||
| Good | 0 | 435 (44%) | 1371 (29%) | 1033 (19%) | 552 (16%) | 309 (11%) | 3700 (21%) | < 0.001c |
| 1 | 460 (47%) | 2798 (59%) | 3697 (68%) | 2508 (71%) | 2233 (77%) | 11,696 (66%) | ||
| 2 + | 88 (9%) | 577 (12%) | 710 (13%) | 480 (14%) | 357 (12%) | 2212 (13%) | ||
| Fair | 0 | 830 (84%) | 3939 (83%) | 4774 (88%) | 3121 (88%) | 2678 (92%) | 15,342 (87%) | < 0.001c |
| 1 | 131 (13%) | 636 (13%) | 512 (9%) | 327 (9%) | 173 (6%) | 1779 (10%) | ||
| 2 + | 22 (2%) | 171 (4%) | 154 (3%) | 92 (3%) | 48 (2%) | 487 (3%) | ||
| Poor | 0 | 543 (55%) | 3428 (72%) | 4490 (83%) | 3063 (87%) | 2618 (90%) | 14,142 (80%) | < 0.001c |
| 1 | 293 (30%) | 762 (16%) | 522 (10%) | 254 (7%) | 150 (5%) | 1981 (11%) | ||
| 2 + | 147 (15%) | 556 (12%) | 428 (8%) | 223 (6%) | 131 (5%) | 1485 (8%) | ||
| Total blastocyst yield | 1.9 (1.1) | 2.8 (1.8) | 3.8 (2.6) | 4.6 (3.2) | 5.7 (4.0) | 3.9 (3.0) | < 0.001e | |
aANOVA
bChi-square test
cGEE with multinomial distribution and generalized logit link, Wald type 3 p-values are presented
dGEE with normal distribution
eGEE with Poisson distribution
Fig. 2.
Good birth outcome by estradiol levels
Table 2.
Frequency of cycle outcomes by estradiol levels
| Estradiol levels on day of trigger | All | |||||
|---|---|---|---|---|---|---|
| < 1000 pg/mL | 1000–1999 pg/mL | 2000–2999 pg/mL | 3000–3999 pg/mL | ≥ 4000 pg/mL | ||
| Cycles (n) | 983 | 4746 | 5440 | 3540 | 2899 | 17,608 |
| Women (n) | 876 | 4212 | 5046 | 3373 | 2741 | 16,248 |
| Spontaneous abortion | 104 (11%) | 481 (10%) | 477 (9%) | 309 (9%) | 260 (9%) | 1631 (9%) |
| Clinical pregnancy | 404 (41%) | 2373 (50%) | 2899 (53%) | 1900 (54%) | 1584 (55%) | 9160 (52%) |
| Livebirth | 294 (30%) | 1872 (39%) | 2401 (44%) | 1575 (44%) | 1310 (45%) | 7452 (42%) |
| Term birth | 219 (22%) | 1421 (30%) | 1867 (34%) | 1218 (34%) | 1031 (36%) | 5756 (33%) |
| Pre-term birth | 75 (8%) | 451 (10%) | 534 (10%) | 357 (10%) | 279 (10%) | 1696 (10%) |
| Good birth outcome | 185 (19%) | 1221 (26%) | 1647 (30%) | 1056 (30%) | 916 (32%) | 5025 (29%) |
In the unadjusted models, there were significant associations between estradiol groups and clinical pregnancy, live birth, and good birth outcome (all p-values < 0.001). Cycles with higher estradiol levels had a significantly higher prevalence of clinical pregnancy, live birth, and good birth outcomes after adjustment for covariates. Notably, all cycles with higher estradiol levels had higher clinical pregnancy rates, live births, and good birth outcomes when compared to those with estradiol < 1000 pg/mL (all p < 0.019) (Supplemental Table 1). For those with estradiol levels ≥ 4000 pg/mL, clinical pregnancy was 18% higher (aRR = 1.18; 95% CI 1.08, 1.29) compared to those with estradiol < 1000 pg/mL. Live birth was 16% higher (aRR = 1.16; 95% CI 1.04, 1.29), and the likelihood of good birth outcomes was 32% higher (aRR = 1.32; 95% CI 1.13, 1.54) for cycles with estradiol ≥ 4000 pg/mL compared with those with estradiol < 1000 pg/mL. Further evaluation of pairwise comparisons of estradiol groups did not demonstrate differences in the prevalence of GBO when 2000–2999 pg/mL was compared with 3000–3999 pg/mL (aRR 1.04, 95% CI 0.97–1.11), or when 3000–3999 pg/mL was compared with 4000 pg/mL (aRR 0.96, 95% CI 0.9–1.04). All significant pairwise comparisons from the adjusted model are depicted in Fig. 3.
Fig. 3.
Predicted prevalence of GBO, clinical pregnancy, and live birth from adjusted models by estradiol levels. Pairwise comparisons performed with log-binomial generalized estimating equations adjusted for A, C age, BMI, progesterone on trigger day, reason for ART, protocol, smoking status, number of transferred embryos, trigger medication, number of retrieved oocytes, and number of good and poor embryos and B age, BMI, progesterone on trigger day, reason for ART, protocol, smoking status, number of transferred embryos, number of retrieved oocytes. Asterisk (*) indicates p-value < 0.05. GBO, good birth outcome; CP, clinical pregnancy; LB, live birth
Our sensitivity analysis revealed that cycles with estradiol levels ≥ 6000 pg/mL (n = 282) compared to 4000–5999 pg/mL (n = 2617) had decreased prevalence of GBO (aRR 0.78, 95% CI 0.63–0.97) which was not seen with additional pairwise comparisons to lower estradiol levels (Supplemental Table 2).
There were no statistically significant associations between estradiol groups and spontaneous abortion, term birth (amongst live births), or preterm birth (amongst live births) in both unadjusted and adjusted models (Supplemental Table 1). Finally, while differences in GBO by network site were observed, the addition of network site to our model was not a confounder. Due to some small sample sizes for individual network sites, these data have not been included in our report.
Discussion
In this study of fresh ETs in cycles without premature progesterone elevation, we saw a clinically meaningful rise in overall GBO (defined as a singleton, term, live birth with appropriate weight) prevalence in rising estradiol groups from 19% amongst the lowest estradiol group, < 1000 pg/mL, up to 30% for estradiol levels between 2000 and 2999 pg/mL. After adjusting for important confounders, higher estradiol levels on the day of trigger were associated with an increased prevalence of a GBO. Specifically, the higher likelihood of GBO was observed with increasing estradiol levels until 2000–2999 pg/mL; thereafter, outcomes amongst higher estradiol levels plateau.
Though we did see a reduced likelihood of GBO in our exploratory sensitivity analysis of the highest estradiol levels (≥ 6000 pg/mL compared to 4000–5999 pg/mL), the small number of cycles in this group (n = 218) and a confidence interval that approaches the null preclude definitive conclusions. Higher estradiol levels were also associated with increased likelihood of pregnancy and live birth while no association was found with spontaneous abortion and preterm delivery.
Our results provided reassuring outcomes and perinatal data in fresh ET cycles that occur after robust stimulations with high estradiol levels. Peak estradiol levels are intrinsically tied to underlying ovarian reserve and are byproducts of stimulation, not necessarily the target, and these data do not suggest that higher estradiol levels should be the goal. Rather, if fresh ETs were to be considered in cycles with a robust response, high estradiol level itself is not associated with a detrimental effect on outcomes. Thus, our data suggests that disqualification from fresh ET based solely on estradiol levels should be reconsidered.
Our study controlled for an important confounder in fresh ET cycles; premature rise of progesterone during the follicular phase which prior studies did not include [7–10, 13]. Imudia et al.’s study suggested an optimal estradiol range of 1882–2465 pg/mL for pregnancy rates [8]; thereafter, a non-significant declining trend was seen. Other studies comparing pregnancy rates based on estradiol exposure throughout stimulation also found positive associations with estradiol levels up to a certain point, beyond which pregnancy outcomes declined [10]. However, elevated progesterone in patients could explain this inverted U-shaped relationship beyond those optimal estradiol thresholds. Ovarian stimulation that leads to robust estradiol levels and multifollicular growth also results in higher progesterone production in the follicular phase [22] which can lead to detrimental embryo-endometrial asynchrony [4, 23]. Indeed, Kyrou et al. found that amongst high responders (> 2446 pg/mL), those who achieved pregnancy were more likely to have low progesterone on the day of trigger [24]. Overall, they found that estradiol was not predictive of pregnancy outcomes while low progesterone on the day of trigger was predictive of outcomes [24]. Our study was limited to a subset of patients with normal progesterone levels (defined as ≤ 2 ng/mL or 3-day AUC ≤ 4.5 ng/mL) on the day of trigger to explore the relationship between estradiol and pregnancy outcomes in the absence of this additional concerning factor. Our practice uses a threshold of ≤ 2 ng/mL as a qualifier for fresh ET based off historical data from our centers [23]. Since other studies suggest an even lower threshold for detrimental effects for progesterone [4], we included progesterone as a continuous variable in our model to account for possible subtle effects. Our results suggest that prior studies’ results may have been confounded by elevated progesterone, as we saw no detrimental effect on clinical pregnancy, live birth, or GBO with increasing estradiol levels.
In vitro studies show that supraphysiologic levels of estradiol are harmful and may induce morphologic and biochemical alterations in the endometrium which could compromise embryo implantation and receptivity [25, 26]. Mouse studies have demonstrated that high estradiol concentrations impaired mouse embryo adhesion via in vitro assays [26]. However, we did not find a detrimental association between outcomes and cycles with estradiol levels 3000–3999 pg/mL and ≥ 4000 pg/mL in our study. These findings support those of smaller retrospective studies [27, 28] that found no correlation between high estradiol and live birth rate or adverse pregnancy outcomes. Similarly, a large multicenter cohort found no statistically significant difference in the clinical live birth rate following fresh ET across six subgroups on increasing E2 on the day of trigger [29]. These large cohort analyses may suggest that the effect of a high estradiol environment on the endometrium may not translate into a reduction in good clinical outcomes. An additional explanation for our findings is that patients with more robust estradiol responses may intrinsically reflect patients with a better prognosis since ovarian reserve is inversely related to age. Though we attempted to control for this by including age, reason for assisted reproductive technology, the number of oocytes retrieved, and embryo quality in our model, the limitations of a retrospective study with its lack of randomization precluded the complete elimination of confounders.
We chose GBO as our primary outcome and limited our analysis to only singleton pregnancies to avoid the confounding effects of multiple gestations on preterm birth and birth weight outcomes. GBO has been used as an outcome in other recent studies [30–32] and has been advocated as the most relevant parameter in fertility treatment as it incorporates both perinatal morbidity and mortality [32]. Fresh ETs are associated with an increased risk of small for gestational age neonates [11, 12] compared to frozen ETs. The exact mechanism for this is unknown but perhaps is related to supraphysiologic estradiol levels that affect early implantation and placental development. Murine studies demonstrated that fresh mouse blastocysts transferred into a superovulated uterine environment resulted in impaired protein synthesis that may contribute to fetal growth restriction [33]. Surprisingly, our main results showed no detrimental association between high estradiol levels and GBO. A recent placental study showed that high responders (estradiol > 8700 pg/mL) had no difference in low birth weight, rate of preterm delivery, or placental abnormalities including placental weight or maturation on histology than those with estradiol levels below that threshold [34].
Strengths of our study included our large sample size (n = 17,608) from multiple centers across the USA. While previous studies have limited subjects with extremely high estradiol levels, our study was able to include 2899 cycles with estradiol levels greater than 4000 pg/mL. We attempted to limit confounders by excluding cycles with premature progesterone elevation and limit heterogeneity by including transfers of the same stage of embryonic development, day 5 blastocyst transfers. Furthermore, we looked beyond live birth rates to incorporate the overall likelihood of an optimal birth outcome. Study limitations include the retrospective study design. Our study could not account for OHSS; however, a prior national data analysis found an incidence of OHSS in 1.2% of patients who underwent fresh ET and had a positive hCG [35]. While there may be an association between OHSS and risk of preterm delivery and low birth weight, clinical differences are likely very small [35]. Additionally, parity and history of prior preterm birth, both of which can impact the likelihood of future adverse pregnancy outcomes, were not accounted for since this information could not be extracted from the database consistently.
In conclusion, our study of fresh ET cycles without premature progesterone elevation demonstrated that elevated estradiol levels were not detrimental to GBO, live birth rates, or pregnancy rates. Our data suggests there may not be an optimal estradiol range for pregnancy outcomes in fresh ET cycles though prospective studies are needed especially in cycles with very elevated levels, e.g., ≥ 6000 pg/mL, during ovarian stimulation. In addition, future studies comparing GBO between fresh and frozen ET cycles would be of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Heather Aldrich for proofreading our manuscript.
Author contribution
I.L.L., K.D., and A.J.P. were involved in the study conceptualization. I.L.L., L.G., K.D., S.M.P., A.N.I., M.D.S., and A.J.P. designed the study. I.L.L. and S.J. performed data collection. L.G. and M.D.S. performed statistical analyses. I.L.L. and L.Z. drafted the manuscript. All other authors were involved in the review and editing of the manuscript. All authors interpreted the data, critically revised the article, and approved the final version.
Data availability
The data that support the findings of this study are available from the corresponding author, IL, upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahart E, Luevano G, Marsh C. To freeze or not to freeze, what is the answer? Fertil Steril. 2023;119(2):195. doi: 10.1016/j.fertnstert.2022.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Eapen A, Sparks A. Improved outcomes following frozen embryo transfer does not provide a “universal license to chill”. Fertil Steril. 2018;110(5):847–848. doi: 10.1016/j.fertnstert.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z-J, Legro RS. Fresh versus frozen embryos in polycystic ovary syndrome. N Engl J Med. 2016;375(20):e42. doi: 10.1056/NEJMc1611871. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Santistevan A, Hunter Cohn K, et al. Freeze-only versus fresh embryo transfer in a multicenter matched cohort study: contribution of progesterone and maternal age to success rates. Fertil Steril. 2017;108(2):254–261.e4. doi: 10.1016/j.fertnstert.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Acharya KS, Acharya CR, Bishop K, Harris B, Raburn D, Muasher SJ. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: an analysis of 82,935 cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2018;110(5):880–887. doi: 10.1016/j.fertnstert.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Karatasiou GI, Bosdou JK, Venetis CA, et al. Is the probability of pregnancy after ovarian stimulation for IVF associated with serum estradiol levels on the day of triggering final oocyte maturation with hCG? A systematic review and meta-analysis. J Assist Reprod Genet. 2020;37(7):1531–1541. doi: 10.1007/s10815-020-01829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenette PE, Sauer MV, Paulson RJ. Very high serum estradiol levels are not detrimental to clinical outcome of in vitro fertilization. Fertil Steril. 1990;54(5):858–863. doi: 10.1016/s0015-0282(16)53946-6. [DOI] [PubMed] [Google Scholar]
- 8.Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet. 2014;31(1):65–71. doi: 10.1007/s10815-013-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93(2):442–446. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 10.Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86(3):588–596. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 11.Pelkonen S, Koivunen R, Gissler M, et al. Perinatal outcome of children born after frozen and fresh embryo transfer: the Finnish cohort study 1995–2006. Hum Reprod. 2010;25(4):914–923. doi: 10.1093/humrep/dep477. [DOI] [PubMed] [Google Scholar]
- 12.Sha T, Yin X, Cheng W, Massey IY. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. 2018;109(2):330–342.e9. doi: 10.1016/j.fertnstert.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Imudia AN, Awonuga AO, Doyle JO, et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril. 2012;97(6):1374–1379. doi: 10.1016/j.fertnstert.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Pereira N, Elias RT, Christos PJ, et al. Supraphysiologic estradiol is an independent predictor of low birth weight in full-term singletons born after fresh embryo transfer. Hum Reprod. 2017;32(7):1410–1417. doi: 10.1093/humrep/dex095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira N, Reichman DE, Goldschlag DE, Lekovich JP, Rosenwaks Z. Impact of elevated peak serum estradiol levels during controlled ovarian hyperstimulation on the birth weight of term singletons from fresh IVF-ET cycles. J Assist Reprod Genet. 2015;32(4):527–532. doi: 10.1007/s10815-015-0434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30(6):1473–1480. doi: 10.1093/humrep/dev076. [DOI] [PubMed] [Google Scholar]
- 17.Bourdon M, Ouazana M, Maignien C, et al. Impact of supraphysiological estradiol serum levels on birth weight in singletons born after fresh embryo transfer. Reprod Sci. 2020;27(9):1770–1777. doi: 10.1007/s43032-020-00174-x. [DOI] [PubMed] [Google Scholar]
- 18.Royster GDI, Hill MJ, Zarek SM, et al. J. Three day progesterone area under the curve: a better test than progesterone on the day of hCG? Fertil Steril. 2014;102(3):85–86. doi: 10.1016/j.fertnstert.2014.07.290. [DOI] [Google Scholar]
- 19.Heitmann RJ, Hill MJ, Richter KS, DeCherney AH, Widra EA. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J Assist Reprod Genet. 2013;30(4):563–567. doi: 10.1007/s10815-013-9932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison W, Goodman D. Epidemiologic trends in neonatal intensive care, 2007-2012 [published correction appears in JAMA Pediatr. 2015 Sep;169(9):878] JAMA Pediatr. 2015;169(9):855–862. doi: 10.1001/jamapediatrics.2015.1305. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Kort J, Baker VL. Embryo biopsy and perinatal outcomes of singleton pregnancies: an analysis of 16,246 frozen embryo transfer cycles reported in the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System. Am J Obstet Gynecol. 2021;224(5):500.e1–500.e18. doi: 10.1016/j.ajog.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Al-Azemi M, Kyrou D, Kolibianakis EM, et al. Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online. 2012;24(4):381–388. doi: 10.1016/j.rbmo.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Healy MW, Patounakis G, Connell MT, et al. Does a frozen embryo transfer ameliorate the effect of elevated progesterone seen in fresh transfer cycles? Fertil Steril. 2016;105(1):93–9.e1. doi: 10.1016/j.fertnstert.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Kyrou D, Popovic-Todorovic B, Fatemi HM, et al. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24(11):2902–2909. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- 25.Ullah K, Rahman TU, Pan HT, et al. Serum estradiol levels in controlled ovarian stimulation directly affect the endometrium. J Mol Endocrinol. 2017;59(2):105–119. doi: 10.1530/JME-17-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–968. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 27.Dunne C, Cho K, Shan A, et al. Peak serum estradiol level during controlled ovarian stimulation is not associated with lower levels of pregnancy-associated plasma protein-A or small for gestational age infants: a cohort study. J Obstet Gynaecol Can. 2017;39(10):870–879. doi: 10.1016/j.jogc.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Zavy MT, Craig LB, Wild RA, Kahn SN, O'Leary D, Hansen KR. In high responding patients undergoing an initial IVF cycle, elevated estradiol on the day of hCG has no effect on live birth rate. Reprod Biol Endocrinol. 2014;12:119. doi: 10.1186/1477-7827-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Jin L, Shi J, et al. Estradiol on trigger day: irrelevant to live birth rates of fresh cycles but positively associated with cumulative live birth rates. Int J Gynaecol Obstet. 2023;163(2):627–638. doi: 10.1002/ijgo.14887. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Qin Y, Zhao H, et al. Live birth with or without preimplantation genetic testing for aneuploidy. N Engl J Med. 2021;385(22):2047–2058. doi: 10.1056/NEJMoa2103613. [DOI] [PubMed] [Google Scholar]
- 31.Roeca C, Johnson RL, Truong T, Carlson NE, Polotsky AJ. Birth outcomes are superior after transfer of fresh versus frozen embryos for donor oocyte recipients. Hum Reprod. 2020;35(12):2850–2859. doi: 10.1093/humrep/deaa245. [DOI] [PubMed] [Google Scholar]
- 32.Eaton JL, Truong T, Li YJ, Polotsky AJ. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135(3):709–716. doi: 10.1097/AOG.0000000000003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roeca C, Silva E, Barentsen C, Powell TL, Jansson T. Effects of vitrification and the superovulated environment on placental function and fetal growth in an IVF mouse model. Mol Hum Reprod. 2020;26(8):624–635. doi: 10.1093/molehr/gaaa047. [DOI] [PubMed] [Google Scholar]
- 34.Ganer Herman H, Volodarsky-Perel A, Ton Nu TN, et al. The effect of higher estradiol levels during stimulation on pregnancy complications and placental histology. Placenta. 2022;126:114–118. doi: 10.1016/j.placenta.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Schirmer DA, 3rd, Kulkarni AD, Zhang Y, Kawwass JF, Boulet SL, Kissin DM. Ovarian hyperstimulation syndrome after assisted reproductive technologies: trends, predictors, and pregnancy outcomes. Fertil Steril. 2020;114(3):567–578. doi: 10.1016/j.fertnstert.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, IL, upon reasonable request.