Abstract
Activated neutrophils release a range of inflammatory products that represent potential biomarkers, and there is interest in the prognostic value of these in acute coronary syndrome (ACS) patients. We conducted a systematic review to examine neutrophil-enriched biomarkers and the occurrence of major adverse cardiovascular events (MACE) in patients with ACS. We identified twenty-seven studies including 17,831 patients with ACS. The most studied biomarkers were neutrophil gelatinase-associated lipocalin (NGAL) and myeloperoxidase (MPO). Meta-analyses showed that elevated NGAL was associated with higher MACE rates (unadjusted risk ratio (RR) 1.52, 95% CI 1.12–2.06, p = 0.006) as were elevated MPO levels (unadjusted RR 1.61, 95% CI 1.22–2.13, p = 0.01). There was limited data suggesting that increased levels of calprotectin, proteinase-3 and double-stranded DNA were also associated with MACE. These results suggest that higher levels of neutrophil-enriched biomarkers may be predictive of MACE in patients with ACS, although higher-quality studies are needed to confirm these observations.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12265-023-10425-2.
Keywords: Neutrophils, Acute coronary syndromes, Prognosis
Introduction
Acute coronary syndrome (ACS) is one of the leading causes of cardiovascular mortality and morbidity [1]. Inflammation plays a pivotal role in driving the pathology of ACS, leading to the development of clinical trials targeting inflammation either acutely, such as in ASSAIL-MI, or chronically, such as in CANTOS in order to improve clinical outcomes. Alongside this, there has been extensive interest in utilising circulating inflammatory biomarkers for risk prediction post-ACS.
Hallmarks of the early inflammatory response to ACS are the exaggerated release of neutrophils from the bone marrow into circulation and infiltration of circulating neutrophils into the site of injury [2]. Neutrophils are effective phagocytes [3] and exert their antimicrobial and proinflammatory effects through the generation of reactive oxygen species (ROS), secretion of granular proteins and formation of neutrophil extracellular traps (NETs) [4]. In patients with myocardial infarction (MI), infiltrated neutrophils drive an inflammatory response at the site of infarction to facilitate the rapid clearance of necrotic cardiomyocytes and degradation of the surrounding extracellular matrix (ECM) [4, 5]. While these processes are necessary for successful scar deposition and myocardial healing post-MI, excessive neutrophil-driven inflammation has been associated with infarct expansion, maladaptive changes in left ventricular (LV) structure and function and, in turn, adverse outcomes [6].
The differential production and release of soluble granule contents are responsible for many of the phagocytic and oxidative functions of neutrophils in acute inflammation [3, 7]. Granule contents include myeloperoxidase (MPO) [4], serine proteases (proteinase 3 (PR3), neutrophil elastase (NE), and cathepsin G [4], azurocidin [8, 9], neutrophil gelatinase-associated lipocalin (NGAL) [10, 11], matrix metalloproteinases (MMP-8 and MMP-9) [4], calprotectin [12, 13], antimicrobial peptides (α-defensin) [14] and ficolins [15]. Previous studies have shown that these soluble factors are significantly elevated in the circulation of patients with ACS, and, in some instances, their release into circulation precedes the release of established markers of myocardial necrosis [16, 17]. The de novo process of NET formation [18, 19] is another important inflammatory function of neutrophils. NETs are abundantly present in coronary thrombi and have prothrombotic and proinflammatory roles during the development of atherosclerosis and in the acute inflammatory response to ACS [20].
Given the importance of neutrophils in driving a proinflammatory—sometimes excessive—response to ACS, the aim of this systematic review and meta-analysis was to assess the prognostic value of neutrophil-enriched soluble factors in predicting long-term major adverse cardiovascular events (MACE) in patients with ACS. Many circulating inflammatory mediators that are typically released during neutrophil degranulation can concurrently arise from other cellular sources. In this review, we examined markers that are known to be predominantly released by neutrophils including MPO, PR3, NGAL, calprotectin and markers of NETosis. We have termed these ‘neutrophil-enriched’ biomarkers. Other factors in which neutrophils are not considered the most significant source in blood circulation, including MMP-8, MMP-9, LL-37 and IL-8, were not assessed here.
Methods
Systematic Review Search Strategy and Eligibility Criteria
This systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic review and Meta-Analyses) and registered with PROSPERO (The International Prospective Register of Systematic Reviews; ID: CRD42021293391) [21, 22]. Circulating biomarkers which are described in prior literature to be predominantly, though not necessarily exclusively, released by neutrophils were considered ‘neutrophil-enriched’ in this study. These include double-stranded DNA (dsDNA), MPO-DNA, NE-DNA, citrullinated histone H3 (CitH3) (all of which are described as surrogate markers of NETosis), NE, MPO, NGAL, calprotectin, PR3, neutrophil α-defensin, azurocidin, cathepsin G, lactoferrin, ficolin and neutrophil-derived extracellular vesicles (EVs) (Supplementary Table 1). A comprehensive review of studies published from 1946 until October 2021 was conducted using the MEDLINE, EMBASE and EMBASE Classic, Scopus, SCIE (Web of Science) and Cochrane Central Register of Controlled Trials databases. Search terms are given in Supplementary Table 2. Duplicates were removed and additional studies were identified by manual searching of reference lists (Supplementary Table 3). Titles and abstracts were screened, and studies reporting the association of neutrophil-enriched biomarker levels with adverse outcome in patients with ACS were retrieved as full-text articles. Two independent reviewers (JY and AH) examined studies for eligibility using a standardised tool based on the PICOS format (Population, Intervention, Comparison, Outcomes, Studies) (Supplementary Figure 1). Studies were considered for inclusion according to the following criteria: the study population comprised ≥ 70% of patients with confirmed ACS; neutrophil-enriched biomarkers were sampled during hospital admission with ACS; and outcomes included, at minimum, all-cause mortality at ≥ 6 months following admission. Where disagreements arose, consensus was reached through discussion.
Data Extraction and Quality Assessment
Data extraction and quality assessment of eligible studies were conducted following the recommendations of the Cochrane Review Group [23]. When required, authors were contacted to provide further clarification. Data were extracted regarding study design, inclusion criteria, patient characteristics, biomarker measurement (including inter-assay and intra-assay variability) and duration of follow-up. Categorical variables were reported as frequency (percentage), and continuous variables were given as median (interquartile range; IQR). For each reported MACE outcome, we extracted the odds ratio (OR), hazard ratio (HR), risk ratio (RR) or biomarker concentrations (median and IQR) and event rates, as applicable. Study quality (ranging from poor to high) was assessed using tools for cohort and case-control studies (Supplementary Figures 2 and 3), based on existing instruments [24–27]. Studies were excluded from further analysis if the overall quality rating was deemed poor due to lack of consideration of confounding variables in the study design and analysis, or if the minimum data required for meta-analysis was unable to be extracted.
Meta-analyses
We conducted meta-analyses of unadjusted summary statistics for neutrophil-enriched markers that were investigated in n > 3 studies. For studies reporting multiple MACE endpoints, only the outcome comprising the highest number of events was analysed. Different measures (RR, OR, HR) were used to report effect sizes across studies and required transformation prior to meta-analysis. For completeness of data, extracted unadjusted measures were transformed to unadjusted RR (95% CI). Crude event rates were compared across binary groupings of marker concentration (most commonly median) to estimate RR and concentrations given without sufficient event data were approximated to RR from standardised mean differences (Cohen’s d). For studies with rare outcomes (≤ 15%), OR were approximated to RR, while HR were directly pooled with RR. Pooled effect sizes with RR and 95% CI were calculated using the weighted inverse-variance method and restricted maximum likelihood (REML) estimations [28]. A random effects model with Hartung-Knapp adjustment was used to account for residual variability between studies [29, 30]. Heterogeneity was assessed with Cochran’s Q test (high heterogeneity was defined as p < 0.10) and the I2 test (high heterogeneity was defined as I2 > 75%) [29, 31, 32]. Potential publication bias was evaluated by assessment of funnel plot asymmetry with Egger’s regression test [33, 34]. All statistical tests were two-tailed, and significance was determined by p < 0.05. Statistical analyses were performed using R packages “meta”, “metafor” and “MetaUtility” in R Statistical Software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Characteristics
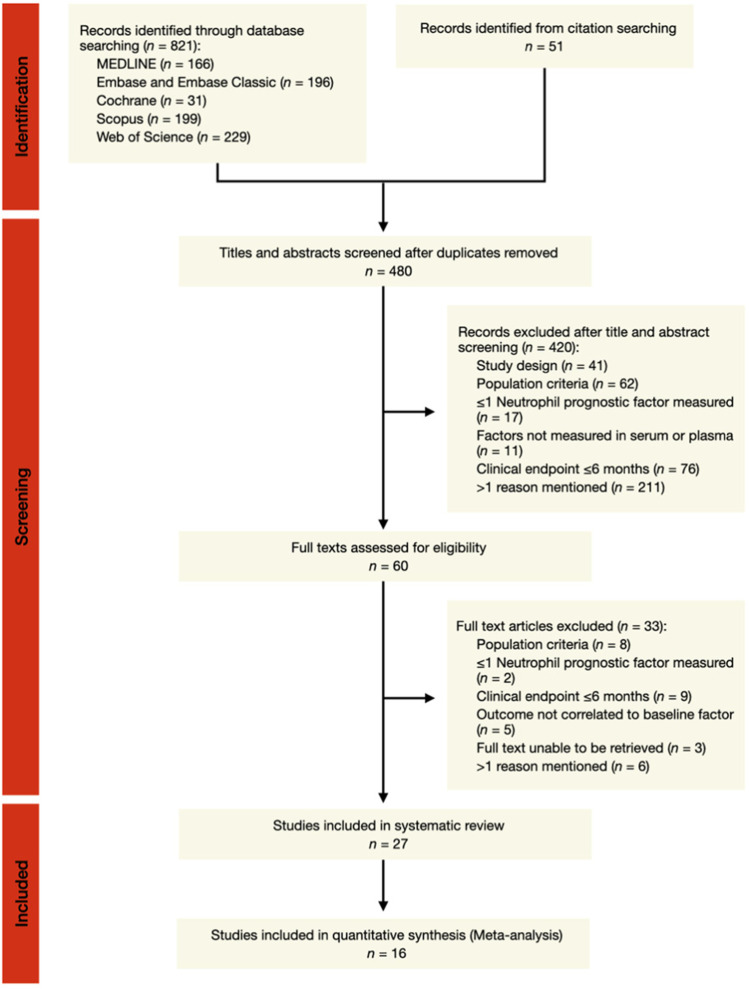
Of 872 articles identified, 27 studies (24 observational cohort and three case-control) were included in the systematic review (Fig. 1). A total of eight neutrophil-enriched soluble factors were measured in 17,831 patients with ACS. These factors were dsDNA, MPO-DNA, NE-DNA and CitH3—all surrogate markers of NETosis—as well as NGAL, MPO, calprotectin and PR3. The study characteristics are presented in Table 1. Patients were predominantly male (74 (67-79)%) with a median age of 62 (61-65) years. ACS encompasses a heterogenous population of STEMI, NSTEMI and unstable angina (UA) diagnoses. For this systematic review, five studies (19%) recruited patients with ACS, while 19 studies (70%) included patients with acute MI only (STEMI and/or NSTEMI); 11 of these studies specified a diagnosis of STEMI. Potential confounders such as renal dysfunction, inflammatory disease or cancer were excluded in a majority of the study populations (19 of 27 studies: 70%).
Fig 1.
Overview of study selection for the systematic review and meta-analysis. The selection, screening, and inclusion of studies evaluating the association between neutrophil-enriched soluble markers and major cardiovascular events in ACS patients are detailed in the flow diagram, adapted from the PRISMA 2020 guidelines for systematic review reporting [6]
Table 1.
Summary of the characteristics of the 27 studies included in the systematic review
| Study (year), country | Marker | Sample size (MACE, %) | Age, years | Male, % | Sampling time from symptom onset | Inclusion criteria | Major exclusion criteria | Clinical endpoint | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Akcay (2012), Turkey [50]a | NGAL | 300 (19.8) | 51.4 ± 6.1 (53 patients with NGAL > 46 ng/mL) | 73.58 | 5 ± 2.2 hrs, before angiography | STEMI + PCI | Culprit lesion ≥ 50% stenosis‡, CABG, chronic inflammatory disease, infection or cancer | All-cause mortality and MACE† (mortality, MI, HF, revascularisation) | 1 year |
| Alfakry (2012), Finland [43]a | MPO | 141 (29.1) | 65.2 (IQR 10.2) | 65.2 | ≤ 48 hrs, angiography NR | NSTEMI and UA ± PCI | Thrombolysis ≤ 48 hrs, coronary angioplasty ≤ 6 or CABG ≤ 3 months, chronic renal or hepatic disease or chronic antibiotic use | MACE† (CV mortality, MI, UA, stroke) | Median 519 (138–924) days |
| Avci (2020), Turkey [47]a | NGAL | 68 (20.6) | 61.5 ± 14.7 | 82.4 | 4.6 ± 3 hrs, before angiography | STEMI ± PCI | Culprit lesion ≥ 50% stenosis‡, CABG or MI, LVEF < 55%, creatinine > 1.4 mg/dL, inflammatory disease, infection or cancer | CV death† | 6 months |
| Baldus (2003), multicentre [68]a | MPO | 547 (11.7) | 61.7 ± 10.4 | 61 | 8.7 ± 4.9 hrs, before angiography | ACS with significant culprit lesion + PTCA | Prior MI, persistent ischemia, culprit lesion > 50% stenosis‡; recent surgery, GI bleeding ≤ 6 weeks; anticoagulant or thrombolytic agent; autoimmune disease or platelet count < 100 × 109/L | All-cause mortality and MACE† (CV mortality, MI, UA, stroke) | 6 months |
| Barbarash (2017), Russia [54]a | NGAL | 318 (88.4) | 61.3 (95% CI 59.5–62.6) | 72.3 | 12–14 days, before angiography | STEMI ± PCI | Prior PCI or CABG, autoimmune disease or cancer | MACE† (CV mortality, MI, UA, stroke, acute HF) | 3 years |
| Brügger-Andersen (2008), Norway [102]a | MPO | 298 (27.8) | 64 ± 13 | 79.3 | 4–6 days, after angiography | MI ± PCI | Severe HF (NYHA class IV), life expectancy < 2 years, late-stage cancer, GI bleeding, hepatic disease, thrombocytopenia or platelet count < 100 × 109/L | MACE† (CV mortality, ACS) | Median 45 months |
| Cavusoglu (2007), USA [69]a | MPO | 182 (18.1) | 64.8 ± 10 | 100 | ≥ 12 hrs from admission, before angiography | ACS ± PCI | GI bleeding | MACE† (mortality, MI) | 2 years |
| Hally (2021), New Zealand [41]b | MPO-DNA, NE-DNA, CitH3 | 300 (33) | 68 (57–75) | 67.7 | 3 (2–4) days, before angiography | MI ± PCI | Cardiac arrest, CHF, eGFR < 30, fibrinolytic agent ≤ 24 hrs, inflammatory disease, platelet function disorder or count < 100 × 109/L | MACE† (CV mortality, MI, stroke) | 1 year |
| Helanova (2015), Czech Republic [51]a | NGAL | 673 (6.4) | 61 (46–78) | 76.52 | ≤ 24 hrs, before angiography | STEMI + PCI | Cardiac arrest, inflammatory or connective tissue disease, cancer, life expectancy < 12 months or culprit lesion stenosis < 50%‡ | All-cause mortality† | Median 2.7 years |
| Helseth (2019), Norway [37]a | dsDNA, MPO-DNA | 251 (7.3) | 60 (53–67) | 82 | Median 21.4 hrs, before angiography | STEMI + PCI | Prior MI, renal failure, contraindications to CMR or clinical instability | MACE† (mortality, MI, stroke, HF, revascularisation) | 1 year |
| Jensen (2010), Denmark [36]b | Calprotectin | 141 (9.2) | 68.6 ± 13.4 (13 patients who died at follow-up) | 73.6 | 12.5 days (9.2–31.6) and 14.2 (IQR 9.6–21.5)§§, before angiography | STEMI with LAD occlusion + PCI | Lack of acute LAD occlusion, prior MI, HF, infection or inflammatory disease | All-cause mortality† | 1 year |
| Kaya (2012), Turkey [70]b | MPO | 73 (21.9) | 56.5 ± 11.9 | 76.71 | ≤ 6 hrs, angiography NR | STEMI ± PCI | Valvular heart disease, chronic renal or hepatic disease inflammatory disease or cancer | MACE† (mortality, MI, CHF, revascularisation, cerebrovascular event) | Mean 25 ± 16 months |
| Langseth (2020), Norway [38]a | dsDNA, MPO-DNA, CitH3 | 959 (19.9) | 60.8 (range 24–94) | 80 | Median 24 hrs, after angiography | STEMI + PCI | Oral anticoagulant use | All-cause mortality or MACE† (mortality, MI, revascularisation, HF, stroke) | Median 4.6 years |
| Lindberg (2012), Denmark [35]a | NGAL | 584 (19) | 66 ± 13; > 170.1 μg/L | 73 | 3.2 (2.2–5.2) hrs, before angiography | STEMI + PCI | Troponin I increase ≤ 0.5g/L or no angiographic stenoses | All-cause mortality and MACE† (CV mortality, MI, HF) | Median 23 (20-24) months |
| Liu (2021), China [48]a | NGAL | 633 (6.5) | 72.1 (68.2–79.2); ≥ 102.6 ng/L | 52.92 | ≤ 24 hrs from admission, angiography NR | MI and stable angina ± PCI | Chronic renal or hepatic disease, severe aortic stenosis, cardiomyopathy, significant anaemia or cancer | CV death† | 10 years |
| McCann (2009), multi (UK) [103]a | MPO | 550 (9.8) | 62 ± 13 | 70 | 6 (3.4–12.4) hrs, before angiography | Suspected ACS with chest pain ± PCI | Thrombolytic or anticoagulant use | All-cause mortality, non-fatal MI or MACE† (mortality, MI) | 1 year |
| Mocatta (2007), New Zealand [39]a | MPO | 507 (15.4) | 61.7 ± 11 | 80 | 24–96 hrs from admission, after angiography | MI ± PCI | Cardiogenic shock or in-hospital death ≤ 24 hours after onset | All-cause mortality† | 5 years |
| Morrow (2008), multi [66]b | MPO | 1524 (15.3) | 61 (52–70) | 66.99 | ≤ 24 hrs, before angiography | ACS with survival ≥180 days + tirofiban ± PCI | Prior PCI or CABG ≤ 6 months, persistent STE, LBBB, severe CHF, cardiogenic shock, systemic disease or creatinine > 2.5 mg/dL | Non-fatal ACS† | 6 months |
| Ng (2011), UK [42]a | MPO, PR3 | 900 (22.8) | 64.6 ± 12.4 | 70 | 2–5 days, after angiography | MI ± PCI | Cancer or recent surgery ≤ 1 month | All-cause mortality or hospitalisation with HF† | Mean 347 days |
| Nguyen (2019), France [44]a | NGAL | 701 (11.5) | 62.8 ± 14.4; without CI-AKI (88%) | 74.61 | On admission, before angiography | STEMI + PCI | Chronic haemodialysis or peritoneal dialysis | All-cause mortality | 1 year |
| Nymo (2018), Sweden [52]a | NGAL | 1121 (54.4) | 69 (60–74); > 403 μg/L | 70 | Median 3 days, before angiography | MI ± PCI | Life expectancy < 1 year | All-cause mortality† | Median 167 months |
| Obeid (2020), multi (Switzerland ) [53]a | NGAL | 1832 (10.5) | NR | 79.15 | ≤ 72 hrs (91.3%), before angiography | MI ± PCI | No informed consent | All-cause mortality and MACE† (mortality, MI, revascularisation, cerebrovascular events) | 1 year |
| Peng (2019), China [49]a | NGAL | 422 (32.2) | 61 ± 13.1 | 74.17 | ≤ 24 hrs and 7 days, before angiography | NSTEMI with CTO + PCI | Rescue PCI or CABG, valvular heart disease or cardiomyopathy, CTO < 1 or > 1, life expectancy < 1 year, dialysis or contraindication to antiplatelet or anticoagulation therapy | MACE† (CV mortality, stroke, revascularisation, cardiogenic shock) | 2 years |
| Scirica (2010), multi [71]a | MPO | 352 (6.8) | Mean 64 | 64.9 | Median 22.4 hrs, before angiography | NSTEMI and UA ± PCI | Revascularisation, persistent STE, pulmonary oedema, systolic BP < 90 mmHg, cardiogenic shock, LBBB, LV hypertrophy, chronic hepatic disease, dialysis or life expectancy < 1 year | CV death, MI, HF | Mean 343 days |
| Wang (2018), China [55]a | dsDNA | 142 (18.3) | 59 (range, 28–88) | 79.5 | Mean 6.3 hrs, before angiography | STEMI + PCI | Valvular heart disease or cardiomyopathy, AF, chronic hepatic or renal disease, inflammatory or autoimmune disease or cancer | MACE† (mortality, revascularisation, ACS, stroke) | Mean 24.5-25.71 months§ |
| Wang (2019), China [56]a | Calprotectin | 273 (17.2) | 63.4 ± 8.5 | 62.4 | On admission, before angiography | ACS with diabetes + PCI | Unsuccessful PCI (≤ TIMI grade 3), prior MI or CABG, infection or inflammatory disease, chronic hepatic or renal disease, long-term antiplatelet or anticoagulant use | MACE† (CV mortality, MI, revascularisation) | 1 year |
| Yndestad (2009), multi (Europe) [40]a | NGAL | 236 (13.6) | 67.4 ± 9.8 | 71.2 | 3 (1–7) days, angiography NR | MI with acute HF ± PCI | Planned revascularisation, systolic BP < 100 mmHg, ACEi or Ang II antagonist, UA, valvular heart disease | MACE† (CV mortality, MI, stroke) | Median 2.7 years |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; AF, atrial fibrillation; Ang II, angiotensin II; AP, angina pectoris; BP, blood pressure; CHF, congestive heart failure; CTO, complete total occlusion; CV, cardiovascular; GI, gastrointestinal; HF, heart failure; LAD, left anterior descending artery; LBBB, left bundle-branch block; LV, left ventricular; MI, myocardial infraction; NR, not reported; PTCA, percutaneous transluminal coronary angioplasty, may also refer to PCI; STE, ST-segment elevation; TIMI, Thrombolysis In Myocardial Infarction (risk score for NSTEMI and UA); UA, unstable angina; UK, United Kingdom; USA, United States of America. aRepresents a cohort study; brepresents a case-control study; †indicates the primary study endpoint; ‡refers to the percentage reduction of the intraluminal diameter of coronary arteries with stenosis; §denotes the mean follow-up period in months for low and high dsDNA groups, respectively; §§denotes the median time from symptom onset to sampling for patients who died and who survived at follow-up, respectively
In 24 studies (89%), blood samples were obtained within 3 days of symptom onset. Of these, patients in 17 studies (71%) were sampled within 24 h. Blood samples were taken prior to angiography in 18 studies (67%). Biomarkers were measured in plasma or serum samples in roughly equal proportions in 26 studies (46% and 54%, respectively), and only one study did not specify sample type. Nearly all studies measuring NGAL, MPO or NET-related biomarkers (dsDNA, MPO-DNA, NE-DNA and CitH3) used commercial or in-house developed ELISAs (72%, 90% and 100%, respectively) and other platforms included time-resolved immunofluorometric assays (TR-IFA). Notably, all eight studies using in-house developed assays for measuring biomarkers demonstrated intra-assay and inter-assay coefficients of variance of 3.8–10% and 0.63–14.8%, respectively [35–42].
The median length of follow-up was 1 (1–2.7) year from index admission, during which 16 (11-21) % of patients experienced MACE. Endpoints were variably defined among studies, and commonly included all-cause or cardiovascular death, non-fatal MI, stroke, repeat revascularisation and new-onset heart failure.
Quality Assessment
The quality of the 24 cohort studies and the three case-control studies are summarised in Supplementary Table 4 and Supplementary Table 5, respectively. Three (12%) cohort studies were deemed of poor quality, while the remaining 24 studies were deemed of acceptable quality. A poor quality rating was given for three cohort studies due to potential confounders being inadequately addressed [40, 43] or for unclear outcome assessment methods [44]. Only one study was excluded from the final analysis for failure to adequately discuss the possible implications of inadequate adjustment for confounders and the inability to extract the minimum data required to create a risk ratio value [40]. Study objectives, inclusion criteria and methodology were generally well-defined and outcomes for primary endpoints were largely assessed using objective measures. Complete outcome data was provided for all participants in 22 studies, and participants who were reported as lost to follow-up or with incomplete data were generally excluded from analysis from the remaining papers. In total, 22 cohort studies sufficiently controlled for potential confounding factors in multivariate models. All three case-control studies addressed confounders either by sample matching or statistical adjustment based on clinical covariates.
Association of Neutrophil-Enriched Biomarkers and Cardiovascular Outcomes
NGAL
The relationship between NGAL and either mortality and/or MACE was examined in 11 cohort studies ranging in size from 68 to 1832 patients with follow-up ranging from 6 months to more than 13 years (Table 2). Most commonly, patients were categorised into high versus low NGAL groups based on medians, tertiles or quartiles (seven studies; 64%) or based on a threshold derived from ROC analysis (two studies, 18%). In three studies (27%), NGAL was treated as a continuous variable in statistical analysis (note that one study used both categorical and continuous approaches to analysis). A significant univariate association between higher levels of NGAL and adverse outcomes were reported in 10 of the 11 studies. In addition, statistically significant multivariate relationships were reported for NGAL and either mortality or MACE in eight (73%) of the 11 studies.
Table 2.
Association between NGAL and MACE in patients with ACS
| Study | Population | Reporting of effect | Endpoint and follow-up | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| Unadjusted effect (95% CI) | p-value | Adjusted effect (95% CI) | p-value | ||||
| Liu et al. (2021) [48] | n = 633 patients with MI and SA | Per 1 SD increase | CV death at 10 years† | HR 2.74 (1.75–5.37) | < 0.001 | HR 2.62 (1.51–4.96) | < 0.001 |
| Avci et al. (2020) [47] | n = 68 patients with STEMI | Grouped via median | CV death at 6 months† | 357 (71–694) vs. 120 (9–513) ng/mL‡ | < 0.001 | β±SE 0.017 ± 0.007, (5.59)2 | 0.01 |
| Obeid et al. (2020) [53] | n = 1832 patients with MI | Per 1 ng/mL increase | All-cause death† and composite of death, MI, cerebrovascular events or revascularisation at 1 year† | OR 1.01 (1.007–1.013), all-cause death | < 0.001 | HR 1.003 (0.999–1.008), all-cause death | 0.16 |
| OR 1.006 (1.003–1.008), MACE | < 0.001 | HR 1.001 (0.998–1.005), MACE | 0.48 | ||||
| Nguyen et al. (2019) [44] | n = 701 STEMI patients treated with PCI | Grouped via highest vs. lowest tertile | All-cause death at 1 year‡ | OR 2.4 (1.53–3.89) | < 0.0001 | OR NR | NS |
| Peng et al. (2019) [49] | n = 422 NSTEMI patients with CTO | Grouped by ROC threshold (7-day NGAL) | Composite of CV death, cardiogenic shock, ischemic stroke or revascularisation at 2 years† | 2.70 ± 1.11 vs. 2.21 ± 0.83 ng/mL‡ | < 0.001 | OR 2.01 (1.45–2.79) | < 0.001 |
| Nymo et al. (2018) [52] | n = 1121 MI patients | Grouped by highest quartile vs. others | All-cause death during median 13.9 years† | HR 1.93 (1.63–2.28) | < 0.001 | HR 1.63 (1.31–2.03) | < 0.001 |
| Barbarash et al. (2017) [54] | n = 357 patients with STEMI | Grouped by lowest quartile vs. others | CV death† and composite of CV death, MI, and hospitalisation due to UA, stroke or acute HF at 3 years† | 2.36 vs. 1.61 ng/mL‡, CV mortality | 0.02 | OR NR, CV death | NS |
| 2.04 vs. 1.48 ng/mL‡, MACE | 0.01 | OR 2.9 (1.4–6.0), MACE | 0.003 | ||||
| Helanova et al. (2015) [51] | n = 673 patients with STEMI | Grouped by ROC threshold | All-cause death at 1 year during median 2.7 years† | OR 1.939 (1.31–2.86) | < 0.001 | OR 1.616 (1.027–2.543) | 0.038 |
| Akcay et al. (2012) [50] | n = 106 patients with STEMI undergoing PCI | 1Per 1 ng/mL increase; 2grouped via median | All-cause death† and composite of death, non-fatal MI, revascularisation or new CHF at 1 year† | HR 1.13 (1.08–1.25)1; 1.18 (1.09–1.37)2, all-cause death | < 0.0011; < 0.012 | HR 1.10 (1.06–1.22)1, all-cause death | 0.011 |
| HR 1.09 (1.04–1.19)1, MACE | 0.021 | ||||||
| HR 1.11 (1.04–1.24)1; 1.20 (1.12–1.34)2, MACE | < 0.011; < 0.012 | HR 1.19 (1.06–1.22)2, all-cause death | 0.012 | ||||
| HR 1.17 (1.08–1.27)2, MACE | 0.012 | ||||||
| Lindberg et al. (2012) [35] | n = 584 patients with STEMI treated with PCI | Grouped by highest quartile vs. others | All-cause death† and composite of CV death or hospitalisation due to MI or HF during median 23 (20–24) months† | Log rank‡, all-cause death | < 0.001 | HR 2.00 (1.16–3.44), all-cause death | 0.01 |
| Log rank‡, MACE | < 0.001 | HR 1.51 (1–2.3), MACE | 0.05 | ||||
| Yndestad et al. (2009) [40] | n = 236 MI patients with acute HF | Grouped via median | Composite of all-cause or CV death, MI or stroke during median 27 months† | Log rank‡ | < 0.001 | OR NR | S |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; β ± SE, beta coefficient ± standard error; BMI, body mass index; BNP, brain natriuretic protein; BP, blood pressure; CAD, coronary artery disease; CRP, C-reactive protein; cTnI, cardiac Troponin I; cTnT, cardiac Troponin T; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazards ratio; hsCRP, high-sensitivity CRP; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NGAL, neutrophil gelatinase-associated lipocalin; NR, not reported; NS, not significant (p < 0.05); OR, odds ratio; PCI, percutaneous coronary intervention; S, p-value is statistically significant (unspecified); SA, stable angina; 95% CI, 95% confidence interval; TIMI, Thrombolysis in Myocardial Infarction; WBC, white blood cell
†Indicates a primary endpoint; ‡indicates concentration is represented as median (IQR) or mean ± SD for cases versus controls, respectively; 1denotes continuous variables were assessed per unit change in concentration, as specified; 2denotes endpoints were compared in patients stratified according to pre-specified categories of biomarker concentration. Statistical significance was considered as two-tailed p ≤ 0.05 (bolded)
NGAL can also be released into the circulation by injured renal cells and may confound the neutrophil-mediated release of NGAL in ACS patients with renal impairment [45, 46]. To account for this, four of the 11 studies (36%) excluded patients with severe renal impairment as indicated by chronic renal disease, serum creatinine > 1.4 mg/dL, and/or requirement for haemodialysis [44, 47–49]. Though comparable exclusion criteria were not described in the remaining seven NGAL studies, five studies reported levels of serum creatinine (range 0.81–1.37 mg/dL) and eGFR (median range 65–117 mL/min/1.73 m2) within normal ranges [35, 40, 50–52], and only a small proportion of patients were reported as requiring dialysis in three studies (range 0.3–2.0%) [35, 50, 53]. In contrast, Barbarash et al. did not state whether the moderate proportion of patients in this study (29%) with marked renal impairment (eGFR < 60 mL/min/1.73 m2) were accounted for in the analysis [54].
MPO
The prognostic ability of circulating MPO was assessed in 10 studies (eight cohorts and two case-control) that included 9074 patients with ACS (Table 3). Three studies (30%) treated MPO as a continuous variable, while eight (80%) studies grouped patients into high versus low MPO levels (one study did both). A total of seven studies reported an association between increased levels of MPO and adverse clinical outcome on univariate analysis, with statistical significance retained in six studies.
Table 3.
Association between MPO and MACE in patients with ACS
| Study | Population | Reporting of effect | Endpoint and follow-up | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| Unadjusted effect (95% CI) | p-value | Adjusted effect (95% CI) | p-value | ||||
| Alfakry (2012) [43] | n = 141 patients with acute non-Q-wave infarction and UA | Highest quartile of MPO concentration vs. lowest quartiles | Composite of CV mortality, MI, UA or ischaemic stroke at 1 year† | 275.3 (238.4) vs. 160.8 (241.0) ng/mL‡ | 0.017 | RR 3.540 (1.600–7.831) | 0.002 |
| Ng (2011) [42] | n = 900 patients with MI | Treated as continuous variable | All-cause death†, hospitalisation due to HF§† and combined at 1 year† | OR NR1, MACE | NS | HR NR‡, MACE | NS |
| Scirica (2010) [71] | n = 4352 patients with NSTEMI and UA | Grouped by ROC threshold | Composite of CV death and HF†, CV death†, recurrent MI† and hospitalisation due to HF at 1 year† | No univariate analysis | HR 1.49 (1.18–1.88), MACE | 0.001 | |
| HR 1.49 (1.12–1.97), CV death | 0.006 | ||||||
| McCann (2009) [103] | n = 550 patients with chest pain | Grouped by highest quartile vs. others | Composite of all-cause death or MI at 1 year† | OR 0.8 (0.4–1.6) | 0.51 | No multivariate analysis | |
| Brügger-Andersen (2008) [102] | n = 298 MI patients | Grouped by highest quartile vs. others | Recurrent ACS or CV death during median 45 months† | HR NR | S | HR 1.04 (0.59−1.86) | 0.89 |
| Morrow (2008) [66] | n = 1524 patients with MI and UA surviving to 180 days | 1Treated as continuous variable; 2grouped via median | Composite of recurrent MI or hospitalisation due to ACS at 6 months† | Log rank2 | 0.14 | OR 1.15 (0.99–1.34)1; 1.26 (0.95–1.68)2 | 0.0721; 0.0722 |
| Cavusoglu (2007) [66] | n = 182 patients with MI and UA with coronary angiography | Treated as continuous variable | MI-free survival at 2 years† | OR 1.51 (1.05–2.18) | 0.028 | OR 1.6 (1.09–2.36) | 0.017 |
| Mocatta (2007) [39] | n = 507 patients with MI | Grouped via median | All-cause death at 5 years† | Log rank2 | 0.0011 | RR 1.8 (1.1–3.1) | 0.03 |
| Baldus (2003) [68] | n = 547 ACS patients treated with PCI | Grouped by highest tertile vs. others | All-cause death†, composite of CV death, MI, UA, or ischaemic stroke† and combined death and MI at 6 months† | HR NR, mortality and MI | 0.012 | HR 2.25 (1.32–3.82), death and MI | 0.003 |
| HR 2.11 (1.21–3.67), death and MI | 0.008 | ||||||
Abbreviations: ASA, acetylsalicylic acid; BMI, body mass index; BNP, brain natriuretic protein; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; CD40L, CD40 ligand; CRP, C-reactive protein; cTnI, cardiac Troponin I; cTnT, cardiac Troponin T; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazards ratio; hsCRP, high-sensitivity CRP; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NGAL, neutrophil gelatinase-associated lipocalin; NR, not reported; NS, not significant (p < 0.05); OR, odds ratio; PCI, percutaneous coronary intervention; S, p-value is statistically significant (unspecified); SA, stable angina; 95% CI, 95% confidence interval; TIMI, Thrombolysis in Myocardial Infarction; VEGF, x; WBC, white blood cell. †Indicates a primary endpoint; ‡indicates concentration is represented as median (IQR) or mean ± SD for cases vs. controls, respectively; §HF requiring high-dose diuretics, inotropes or intravenous nitrate; §§indicates statistical adjustment for variable identified as univariate predictors of MACE; 1denotes continuous variables were assessed per unit change in concentration, as specified; 2denotes endpoints were compared in patients stratified according to pre-specified categories of biomarker concentration. Statistical significance was considered as two-tailed p ≤ 0.05 (bolded)
Markers of NETosis
The association between markers of NETosis (dsDNA, MPO-DNA, NE-DNA and CitH3) and MACE were examined in 1649 patients across four studies (Table 4). Increased levels of dsDNA were independently predictive of MACE in three studies [37, 38, 55], two of which were conducted by the same institution [37, 38]. Neither MPO-DNA, NE-DNA nor CitH3 were found to predict MACE after ACS in three studies [37, 38, 41].
Table 4.
Association between NETosis components and MACE in patients with ACS
| Study | Population | Reporting of effect | Endpoint and follow-up | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| Unadjusted effect (95% CI) | p-value | Adjusted effect (95% CI) | p-value | ||||
| dsDNA | |||||||
| Langseth et al. (2020) [38] | n = 956 patients with STEMI |
2 Grouped by median 3Grouped by highest quartile vs. others |
All-cause death† and composite of death, MI, stroke, hospitalisation due to HF, or revascularisation > 3 months at median 4.6 years† | HR 3.36 (1.95–5.78)3; log rank2; 460 (407–508) vs. 411 (370–466) ng/mL‡, all-cause death | < 0.0013; < 0.0012; < 0.001‡ | HR 2.28 (1.19–4.36) | 0.013 |
| Log rank2; 429 (317–481) vs. 412 (372–466) ng/mL‡, MACE | 0.0522; 0.255‡ | HR 2.06 (1.08-3.93) | 0.029 | ||||
| Helseth et al. (2019) [37] | n = 251 STEMI patients treated with PCI | Grouped by median | Composite of all-cause mortality, MI, revascularisation ≥3 months, stroke, or hospitalisation due to HF at 1 year† | HR 5.9 (1.7–20.3) | 0.005 | HR 6.7 (1.9–23.2) | 0.003 |
| Wang et al. (2018) [55] | n = 142 STEMI patients treated with PCI | Grouped by ROC threshold | All-cause† and CV death† & composite of death, ACS, PCI or CABG, or stroke at mean 24.5 months† | Log rank2, MACE | 0.04 | OR 7.43 (1.25–4.07), MACE | 0.027 |
| MPO-DNA | |||||||
| Hally et al. (2021) [41] | n = 100 patients with MI |
2 Grouped by median §Median z-score §§Median z-score with sP-selectin |
Composite of CV death, non-fatal MI, or ischemic stroke at 1 year† | OR 1.13 (0.7–1.83)2; OR 1.28 (0.72–2.1)§; OR 1.86 (1.13–3.08)§§ | 0.62; 0.33§; 0.015§§ | No multivariate analysis | |
| OR 1.94 (1.16–3.25)§§ | 0.011 | ||||||
| Langseth et al. (2020) [38] | n = 956 patients with STEMI | 2Grouped by median | See Langseth et al. (2020) | Log rank2; 0.167 (0.14–0.25) vs. 0.18 (0.14–0.26) OD‡, all-cause death | 0.362; 0.91‡ | No multivariate analysis | |
| Log rank2; 0.17 (0.14–0.24) vs. 0.18 (0.14–0.27) OD‡, MACE | 0.162; 0.33‡ | ||||||
| Helseth et al. (2019) [37] | n = 251 STEMI patients treated with PCI | Grouped by median | See Helseth et al. (2019) | HR NR | NS | No multivariate analysis | |
| NE-DNA | |||||||
| Hally et al. (2021) [41] | n = 100 patients with MI | Grouped by median | See Hally et al. (2021) | OR 1.06 (0.66–1.72) | 0.81 | No multivariate analysis | |
| Citrullinated histone 3 | |||||||
| Hally et al. (2021) [41] | n = 100 patients with MI | Grouped by median | See Hally et al. (2021) | OR 1.43 (0.89–2.33) | 0.14 | No multivariate analysis | |
| Langseth et al. (2020) [38] | n = 956 patients with STEMI | 2 Grouped by median | See Langseth et al. (2020) | Log rank2; 10.25 (4.96–17.32) vs. 9.07 (4.83–17.24) ng/mL‡, all-cause death | 0.0922; 0.6‡ | No multivariate analysis | |
| Log rank2; 92 (4.48–16.52) vs. 9.32 (4.91–17.30) ng/mL‡, MACE | 0.882; 0.46‡ | ||||||
Abbreviations: BMI, body mass index; BNP, brain natriuretic protein; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; CitH3, citrullinated histone H3; H3(cit), see CitH3; CRP, C-reactive protein; cTnI, cardiac Troponin I; cTnT, cardiac Troponin T; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GOT, glutamic oxalacetic transaminase; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; hsCRP, high-sensitivity CRP; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NE, neutrophil elastase; NGAL, neutrophil gelatinase-associated lipocalin; NR, not reported; NS, not significant (p < 0.05); OR, odds ratio; PCI, percutaneous coronary intervention; S, p-value is statistically significant (unspecified); SA, stable angina; 95% CI, 95% confidence interval; TIMI, Thrombolysis in Myocardial Infarction; WBC, white blood cell. †Indicates a primary endpoint; ‡indicates concentration is represented as median (IQR) or mean ± SD for cases vs. controls, respectively; 1denotes continuous variables assessed per unit change in concentration, as specified; 2denotes endpoints compared in patients stratified according to median biomarker concentration; 3denotes endpoints compared in patients stratified according to pre-specified categories of biomarker concentration. §Denotes effect size was calculated using the composite NET z-score (comprising z-scores of MPO-DNA% of NET standard, NE-DNA% of pooled serum standard and H3 (cit)% of NET standard) with platelet count; §§denotes effect parameter was calculated using composite NET z-score with platelet count and sP-selectin. Statistical significance was considered as two-tailed p ≤ 0.05 (bolded)
Calprotectin
Adjusted HRs were extracted from two studies evaluating the prognostic significance of calprotectin in 207 patients with ACS [36, 56] (Table 5). All-cause mortality was reported in 9.2% of patients in Jensen et al. [36]and in 17.2% of patients in Wang et al [56] within the first year after coronary revascularisation. Both studies observed a statistically significant independent association between elevated calprotectin levels and MACE.
Table 5.
Association between calprotectin and proteinase-3 and MACE in patients with ACS
| Study | Population | Reporting of effect | Endpoint and follow-up | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|---|
| Unadjusted effect (95% CI) | p-value | Adjusted effect (95% CI) | p-value | ||||
| Calprotectin | |||||||
| Wang et al. (2019) [56] | n = 273 ACS patients with diabetes treated with PCI | Grouped by ROC threshold | Composite of CV death, non-fatal MI or unplanned revascularisation at 1 year† | HR 1.56 (1.08–4.62) | 0.01 | HR 2.11 (1.14–6.65) | < 0.01 |
| Jensen et al. (2010) [36] | n = 141 STEMI patients with acute LAD occlusion | 1Continuous variable; 2 Grouped by ROC threshold | All-cause death at 1 year† | HR 1.30 (1.1–1.5)1; 6.28 (0.4–28.1)2 | < 0.0011; 0.02 | HR 1.28 (1.1–1.5)1, 7.28 (1.6–32.9)2 | < 0.0011; 0.01 |
| Proteinase-3 | |||||||
| Ng et al. (2011) [42] | n = 900 patients with MI |
1Continuous variable 2Grouped by median |
All-cause death, hospitalisation due to HF§, and composite of death and HF at 1 year† | OR 6.42 (2.25–18.3)1; NR2 | 0.0011; < 0.0012 | HR 3.80 (1.78–8.14)2 | 0.0012 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; BMI, body mass index; BNP, brain natriuretic protein; BP, blood pressure; CABG, coronary artery bypass graft; CAD, coronary artery disease; CitH3, citrullinated histone H3; H3(cit), see CitH3; CRP, C-reactive protein; cTnI, cardiac Troponin I; cTnT, cardiac Troponin T; CV, cardiovascular; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FBG, fasting blood glucose; GOT, glutamic oxalacetic transaminase; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NR, not reported; NS, not significant (p < 0.05); OR, odds ratio; PCI, percutaneous coronary intervention; S, p-value is statistically significant (unspecified); SA, stable angina; 95% CI, 95% confidence interval; TIMI, Thrombolysis in Myocardial Infarction; WBC, white blood cell. †Indicates a primary endpoint; ‡indicates concentration is represented as median (IQR) or mean ± SD for cases vs. controls, respectively; §HF requiring high-dose diuretics, inotropes or intravenous nitrate. 1Denotes continuous variables assessed per unit change in concentration, as specified; 2denotes endpoints compared in patients stratified according to pre-specified categories of biomarker concentration. Statistical significance was considered as two-tailed p ≤ 0.05 (bolded)
PR3
Ng et al. [42] demonstrated a significant independent association between MACE at 1 year and PR3 (per 10-fold increase in log concentration) in 900 patients with ACS (Table 5). MACE was defined as all-cause mortality, recurrent MI and hospitalisation with HF and was reported in 16% of patients.
Meta-analysis of NGAL
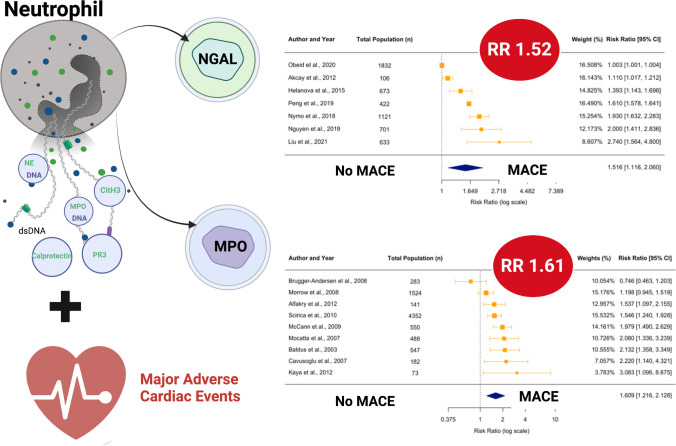
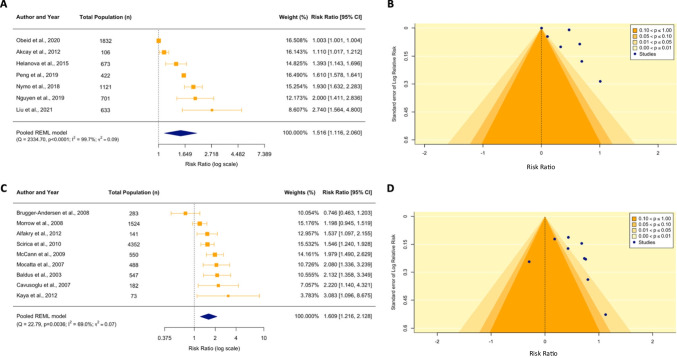
Of the 11 studies investigating circulating NGAL levels, risk ratio data from 7 studies (64%) was extracted and pooled in a random effects model [44, 48–53]. Within these studies, a total of 1384 MACE events were recorded for 5488 patients with ACS. Four studies were excluded from meta-analysis due to incomplete study data. Composite MACE [49, 50, 53] and all-cause mortality endpoints were each assessed in three studies [44, 51, 52], while CV mortality was assessed in one study [48]. Meta-analysis demonstrates that, when dichotomized by high or low baseline NGAL levels, high levels were associated with a 51.6% increased risk of MACE (unadjusted RR 1.52, 95% CI 1.16–2.06, p = 0.016) compared to low levels (used as the reference) (Fig. 2A). There was evidence of substantial statistical heterogeneity in the model, with τ2 of 0.09 (95% CI 0.03–0.57, p < 0.0001) and I2 of 99.7% (95% CI 99.1–100.0%). Observation of a non-uniform distribution of study p-values in the contour funnel plot alongside a significant Egger’s test (z = 2.65, p = 0.04) indicates likely publication bias resulting from small study effects (Fig. 2B).
Fig. 2.
Meta-analysis for neutrophil gelatinase-associate lipocalin (NGAL) and myeloperoxidase (MPO). A, C The forest plots illustrate the unadjusted individual and summary risk ratios (RR) for MACE among ACS patients with high and low baseline levels of NGAL (A) and MPO (C) during hospital admission. The summary RR is indicated by the diamond and was calculated based on random effects meta-analysis using REML estimation and Hartung-Knapp adjustment. Weightings for each of the studies included in the model are proportionally reflected by the size of the box. The widths of the intersecting horizontal lines indicate the 95% confidence intervals. B, D Evidence used for publication bias assessment for NGAL (B) and MPO (D) is illustrated in the funnel plots, which present the logRR of studies investigating either NGAL or MPO against the inverse standard error. The shaded contours represent varying levels of statistical significance as indicated by the key. The null effect is denoted by the vertical dotted line
Meta-analysis of MPO
Of the 10 studies describing the association of baseline MPO levels with MACE, risk ratio data was extracted from nine studies and pooled in a random effects meta-analysis. Study effect estimates could not be approximated to RR for Ng et al. due to incomplete study data [42]. A total of 1577 MACE events were captured in 8174 patients with ACS, ranging from 6 months to 5 years after index admission. Elevated circulating levels of MPO were significantly associated with an increased risk of MACE (unadjusted RR 1.61, 95% CI 1.22–2.13, p = 0.004) compared to low MPO levels (Fig. 2C). Moderate heterogeneity was noted in the model (τ2 0.07, 95% CI 0.01–0.52; p = 0.004 and I2 70.0%, 95% CI 25.8–94.1). The funnel plot for MPO indicates the presence of asymmetry with a non-uniform distribution of p-values for the studies in the contour funnel plot; however, Egger’s test was non-significant suggesting small study effects are not driving publication bias (z = 1.11, p = 0.3) (Fig. 2D).
Discussion
We identified 27 studies investigating the prognostic association of either NGAL, MPO, calprotectin, PR3 or markers of NETosis (dsDNA, MPO-DNA, NE-DNA, CitH3) with MACE in patients with ACS. NGAL was the most studied marker, with 11 studies reporting associations between NGAL and MACE. Ten of these found higher levels of NGAL were associated with worse outcomes. Seven studies contained sufficient information to be incorporated into a random effects meta-analysis, which showed that increased levels of circulating NGAL during hospital admission were associated with a 52% increase in risk for long-term MACE. MPO was investigated in 10 studies, 9 of which could be incorporated into a meta-analysis. This analysis demonstrated that increased MPO levels at presentation with ACS were associated with a 61% increased risk of long-term MACE. The other markers were less well studied, but there was some evidence of an association between increased circulating levels of calprotectin, PR3 and dsDNA (used as a surrogate marker of NETosis) and long-term MACE.
Both the multivariate outcomes reported within the individual studies, and the meta-analysis of the unadjusted outcomes support the view that elevated levels of NGAL are associated with worse clinical outcomes. NGAL is an acute-phase glycoprotein contained within specific granules of neutrophils and elicits antimicrobial and chemotactic functions at sites of inflammation [51, 52, 57]. Within the post-ischemia setting, NGAL acts to enhance MMP-9 activation by forming the stable NGAL/MMP-9 complex which, in turn, can amplify and prolong ECM degradation during infarct remodelling [57]. Within the coronary and systemic circulation, neutrophils are the principal source of circulating NGAL [10, 11]. However, NGAL is also an early biomarker of acute kidney injury as a result of secretion from renal tubular cells [58, 59]. It is possible that the release of NGAL by injured renal cells in ACS patients that also have poor kidney function may confound the prognostic association of neutrophil-derived NGAL [40].
Eight of the studies included in this systematic review noted that increased levels of NGAL at baseline may reflect pre-existing renal dysfunction in addition to neutrophil activation during acute inflammation [35, 40, 44, 47, 48, 50, 52, 53]. Due to this interaction [60–63], it is important to consider the ways in which potential confounding effects of renal-mediated NGAL. Chronic kidney disease was an exclusion criterion in four of 11 studies (37%) [44, 47–49]. In addition, all but two study [40, 47] outcomes were adjusted for creatinine or eGFR in multivariate analysis. Most of the studies (8 of 11; 72%) measured NGAL levels in pre-angiography blood samples prior to any potential renal injury caused by the administration of contrast [35, 40, 44, 47, 50–53]. One study did not address renal function at all [40].
MPO is a haemoprotein released from the azurophilic granules of mature neutrophils and is also released, to a lesser degree, by other immune cells such as monocytes and tissue-associated macrophages [64]. Within atherosclerotic lesions, MPO plays a prominent role in plaque destabilisation [65]. The oxidation of ROS substrates such as nitric oxide (NO) and protein and lipid components in the vascular endothelium serves as important mechanisms of MPO-mediated endothelial dysfunction [66]. As an early participant in acute inflammation, MPO induces proteolytic changes in tissue mediators such as MMPs and plasminogen activator inhibitor-1 (PAI-1) to promote ECM degradation in the infarct [67]. Seven out of 10 studies (70%) reported a significant univariate association between increased circulating levels of MPO and long-term MACE, in which MPO remained an independent predictor of MACE in six studies [39, 43, 68–71]. Meta-analysis of the unadjusted outcomes supports this association between higher levels of MPO and increased risk.
NETs are extracellular scaffolds composed of decondensed chromatin, citrullinated histones and granular proteins such as MPO and NE [72–76]. Beyond their antimicrobial role, NETs accumulate in coronary thrombi in ACS to exert prothrombotic functions [77, 78]. For example, NETs act to promote fibrin deposition and thrombin generation through the activation of tissue factors and platelet aggregation [79, 80]. Much of the literature investigating NETs as novel predictors of cardiovascular risk in ACS has focused on circulating dsDNA as a surrogate marker of NETosis [20, 38]. In this review, increased levels of circulating dsDNA in MI patients were found to be a significant predictor of MACE in three studies [37, 38, 55]. A caveat in ACS patients is that dsDNA is not specific to NETosis but can also be released from dying cardiomyocytes. Other surrogate NET markers (namely, MPO-DNA, NE-DNA and CitH3) have greater specificity for NETosis than dsDNA [81–83]. Yet, based on the studies identified in this review, there was no evidence supporting their individual utility for predicting MACE [37, 38, 41].
We found less literature on PR3 and calprotectin. Like MPO, PR3 is a neutrophil-derived serine protease [84]. PR3 has been associated with promoting neutrophil recruitment through its ability to activate certain chemokines and cytokines [85, 86]. In Ng et al., increased plasma levels of a PR3 complex were significantly associated with long-term risk of MACE post-MI [42]. Calprotectin is predominantly released by neutrophils [12, 17, 87, 88] and is involved in a myriad of inflammatory functions including phagocytosis [89], neutrophil and monocyte recruitment [90, 91] and cytokine and chemokine production [92]. Both Wang et al. [56] and Jensen et al. [36] reported calprotectin was associated with long-term MACE after adjustment for confounding variables.
Publication bias is an issue to be mindful of when interpreting the results from any meta-analysis. In our meta-analyses of NGAL and MPO, we observed some clustering of the studies in the p = 0.01–0.05 significance bands in the contour funnel plots. Furthermore, a significant Egger’s test result for NGAL suggests that publication bias due to small study effects is likely to be present. Therefore, it is possible that studies are more likely to be published if reporting a significant association between NGAL and MACE. In the case of MPO, the presence of publication bias is not as clear cut. Despite the presence of asymmetry in the standard funnel plot and the observation of a non-uniform distribution of p-values in the contour funnel plot, the Egger’s test was not significant. An Egger’s test is often used as an objective measure of asymmetry for funnel plots and is useful for assessing the risk of small study bias inside a meta-analysis, reflecting the fact that greater variance is often observed in smaller studies [34]. However, publication bias should only be one factor when considering the presence of asymmetry in a funnel plot. Other important sources of selection bias include outcome reporting bias, clinical heterogeneity and poor methodological design, all of which are often associated with smaller studies [93]. It is difficult to ascertain the definitive source of bias that may exist in our forest plots for the NGAL and MPO studies, although we present some plausible reasoning in our limitations. It must be highlighted that these meta-analyses were conducted on 7 and 9 studies respectively, which is below the minimum number of 10 studies recommended for analysing publication bias [93]. Therefore, the results of bias must be interpreted cautiously from our study as the Egger’s test may not be powered to distinguish real asymmetry from chance. We would like to highlight that small studies, or large effect sizes reported by small studies, are not problematic in themselves. There is valuable information to be gathered from these types of studies. However, it is the selective publication of results which favours the publication of smaller studies more commonly than from larger studies that then causes bias to become an issue.
Limitations
These studies discussed in this review are cohort and case-control designs, none of which constitute high-quality evidence for evaluating the prognostic utility of the biomarkers studied. In addition, for the most studied biomarkers, NGAL and MPO, there is evidence that publication bias may contribute to the effect observed in both meta-analyses.
The heterogeneity of timing of blood sampling among studies included within this review is a potential confounder. The exact timing of peak neutrophil activity is unclear in humans [4, 94–97]. There is currently no consensus regarding an optimal timepoint to measure biomarkers in the acute stage of myocardial infarction, nor whether biomarkers measured at a single moment in time can sufficiently capture their contribution to MACE risk. Studies examining biomarkers at multiple timepoints may provide insight into the impact of timing on prognostic utility.
Length of follow-up and definitions of MACE varied between studies, contributing to the variance in MACE rates observed (from 6.4 [51] to 88.4% [54]). In Barbarash et al. [54], the high MACE rate of 88.4% may likely reflect the higher baseline risk of the population as well as the inclusion of unstable angina as a MACE endpoint. The variance in MACE rates, as well as differences in study populations and length of follow-up, are likely contributors to the high heterogeneity observed in meta-analysis of MPO and NGAL.
For clinically practical reasons, the studies included here examined biomarkers exclusively in peripheral blood samples. However, soluble mediators may exhibit biological compartmentalisation that cannot be captured in peripheral samples. Previous studies have revealed a significantly higher expression of markers of neutrophil degranulation and NETosis in coronary thrombi than in peripheral plasma [98], and in infarct-related arteries, but not in samples taken from non-infarct-related coronaries [79, 99]. By contrast, peripheral and coronary dsDNA are reported to be highly intercorrelated [55]. It is possible that, compared to peripheral blood, coronary sinus blood may offer a better reflection of these biomarkers within the ischaemic microenvironment. However, questions concerning the clinical practicality, safety and invasiveness of coronary sinus sampling remain.
It must be noted that this systematic review and meta-analyses focused on studies reporting a correlation between neutrophil biomarkers and clinical outcome in ACS patients. These studies did not investigate a causative association between the investigated biomarker and MACE, such that interpretation of these relationships must be viewed with caution when considering what is driving adverse outcomes in these patients. However, clinical trials such as CANTOS [100] and ASSAIL-MI [101] have presented compelling results to suggest that the level of inflammation in patients with cardiovascular disease is linked with cardiac outcomes. Both CANTOS and ASSAIL-MI show that targeting specific inflammatory cytokines with a monoclonal antibody therapy can reduce cardiovascular events (CANTOS) and limit infarct expansion (ASSAIL-MI). These results give rise to a plausible mechanistic connection between the burden of inflammation during the acute phase of an MI and MACE at 1 year. Whether neutrophil biomarkers, like the ones discussed in this study, are involved in this mechanistic process remains unclear.
Conclusion
This systematic review and meta-analysis examined the prognostic utility of eight neutrophil-enriched biomarkers in patients with ACS. In a meta-analysis, increased levels of circulating NGAL and MPO were found to be significantly associated with long-term adverse cardiovascular outcomes in patients with ACS, supporting the possibility that neutrophil-mediated inflammation may play an important role in myocardial injury processes. For the remaining markers, promising data indicates the association of dsDNA, calprotectin and PR3 with long-term MACE post-ACS. However, no such association was found for MPO-DNA, NE-DNA or CitH3 (all surrogate markers of NETosis). While these eight circulating biomarkers are predominantly produced by neutrophils, the release of some of these (dsDNA and NGAL) are likely to be confounded by other physiological processes in patients with ACS (cardiomyocyte necrosis and renal injury, respectively).
Supplementary Information
(DOCX 39312 kb)
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Declarations
No human studies were carried out by the authors for this article.
Conflict of Interest
Author JY received a University of Otago Doctoral Scholarship for her PhD studies (2019–2021). The authors have no conflicts of interest to declare that are relevant to the content of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JMO, Warnica JW, et al. Predictors of late development of heart failure in stable survivors of myocardial infarction: The CARE study. J Am Coll Cardiol. 2003;42(8):1446–1453. doi: 10.1016/S0735-1097(03)01057-X. [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;24(1). 10.1111/micc.12305. [DOI] [PubMed]
- 3.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Y. Role of neutrophils in cardiac injury and repair following myocardial infarction. Cells. 2021;10(7):1676. 10.3390/cells10071676. [DOI] [PMC free article] [PubMed]
- 5.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Hiasa Y, Ohara Y, Miyazaki S-i, Ogura R, Suzuki N, et al. Relationship of admission neutrophil count to microvascular injury, left ventricular dilation, and long-term outcome in patients treated with primary angioplasty for acute myocardial infarction. Circ J. 2008;72(6):867–872. doi: 10.1253/circj.72.867. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, et al. Association between myeloperoxidase levels and risk of coronary artery disease. Jama. 2001;286(17):2136–2142. doi: 10.1001/jama.286.17.2136. [DOI] [PubMed] [Google Scholar]
- 8.Tapper H, Karlsson A, Mörgelin M, Flodgaard H, Herwald H. Secretion of heparin-binding protein from human neutrophils is determined by its localization in azurophilic granules and secretory vesicles. Blood. 2002;99(5):1785–1793. doi: 10.1182/blood.V99.5.1785. [DOI] [PubMed] [Google Scholar]
- 9.Soehnlein O, Lindbom L. Neutrophil-derived azurocidin alarms the immune system. J Leukoc Biol. 2009;85(3):344–351. doi: 10.1189/jlb.0808495. [DOI] [PubMed] [Google Scholar]
- 10.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26(1):136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 11.te Boekhorst BC, Bovens SM, Hellings WE, van der Kraak PH, van de Kolk KW, Vink A, et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc Res. 2010;89(3):680–688. doi: 10.1093/cvr/cvq340. [DOI] [PubMed] [Google Scholar]
- 12.Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, et al. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation. 2020;141(13):1080–1094. doi: 10.1161/CIRCULATIONAHA.119.043833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8, 14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266(12):7706–7713. doi: 10.1016/S0021-9258(20)89506-4. [DOI] [PubMed] [Google Scholar]
- 14.Brook M, Tomlinson GH, Miles K, Smith RWP, Rossi AG, Hiemstra PS, et al. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci. 2016;113(16):4350–4355. doi: 10.1073/pnas.1601831113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Endo Y, Iwaki D, Nakata M, Matsushita M, Wada I, et al. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J Immunol. 2005;175(5):3150–3156. doi: 10.4049/jimmunol.175.5.3150. [DOI] [PubMed] [Google Scholar]
- 16.Goldmann BU, Rudolph V, Rudolph TK, Holle A-K, Hillebrandt M, Meinertz T, et al. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic Biol Med. 2009;47(1):79–83. doi: 10.1016/j.freeradbiomed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, et al. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28(8):941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30(11):513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Mangold A, Hofbauer TM, Ondracek AS, Artner T, Scherz T, Speidl WS, et al. Neutrophil extracellular traps and monocyte subsets at the culprit lesion site of myocardial infarction patients. Sci Rep. 2019;9(1):16304. doi: 10.1038/s41598-019-52671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, Shamseer L, Tricco AC. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst Rev. 2018;7(1):32. doi: 10.1186/s13643-018-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T HJ, Deeks JJ. Chapter 5: Collecting data. 2021 updated February 2021. In: Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Cochrane, 2021. version 6.2. Available from: http://www.training.cochrane.org/handbook.
- 24.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis 2000 [updated 01/01. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 25.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 26.SIGN. Scottish Intercollegiate Guidelines Network (SIGN) Methodology Checklist 3: cohort studies Scotland: Scottish intercollegiate guidelines network; 2011 [updated 2012. Available from: https://www.sign.ac.uk/what-we-do/methodology/checklists/.
- 27.SIGN. Scottish Intercollegiate Guidelines Network (SIGN) Methodology Checklist 4: case-control studies Scotland: Scottish intercollegiate guidelines network; 2011 [updated 2012. Available from: https://www.sign.ac.uk/what-we-do/methodology/checklists/.
- 28.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, Altman DG, McKenzie JE, Shamseer L, Ahmadzai N, Wolfe D, et al. Flaws in the application and interpretation of statistical analyses in systematic reviews of therapeutic interventions were common: a cross-sectional analysis. J Clin Epidemiol. 2018;95:7–18. doi: 10.1016/j.jclinepi.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 33.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindberg SP, Pedersen SH, Mogelvang R, Jensen JS, Flyvbjerg A, Galatius S, Magnusson NE. Prognostic utility of neutrophil gelatinase-associated lipocalin in predicting mortality and cardiovascular events in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2012;60(4):339–345. doi: 10.1016/j.jacc.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Jensen LJNP S, Bjerre M, Mogelvang R, Jensen JS, Flyvbjerg A. Plasma calprotectin predicts mortality in patients with ST segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Interv Cardiol. 2010;23(2):123–129. doi: 10.1111/j.1540-8183.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- 37.Helseth R, Shetelig C, Andersen GØ, Langseth MS, Limalanathan S, Opstad TB, et al. Neutrophil extracellular trap components associate with infarct size, ventricular function, and clinical outcome in STEMI. Mediators Inflamm. 2019;2019:7816491. doi: 10.1155/2019/7816491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langseth MSH R, Ritschel V, Hansen CH, Andersen GO, Eritsland J, Halvorsen S, Fagerland MW, Solheim S, Arnesen H, Seljeflot I, Opstad TB. Double-stranded DNA and NETs components in relation to clinical outcome after ST-elevation myocardial infarction. Sci Rep. 2020;10(1):5007. doi: 10.1038/s41598-020-61971-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007;49(20):1993–2000. doi: 10.1016/j.jacc.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 40.Yndestad A, Landrø L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30(10):1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 41.Hally KEP OM, Brunton-O'sullivan MM, Harding SA, Larsen PD. Linking neutrophil extracellular traps and platelet activation: a composite biomarker score for predicting outcomes after acute myocardial infarction. Thromb Haemost. 2021;121(12):1637-1649. 10.1055/s-0041-1728763. [DOI] [PubMed]
- 42.Ng LLK SQ, Narayan H, Quinn P, Squire IB, Davies JE. Proteinase 3 and prognosis of patients with acute myocardial infarction. Clin Sci. 2011;120(6):231–238. doi: 10.1042/CS20100366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfakry H, Sinisalo J, Paju S, Nieminen MS, Valtonen V, Tervahartiala T, Pussinen PJ, Sorsa T. The association of serum neutrophil markers and acute coronary syndrome. Scand J Immunol. 2012;76(2):181–187. doi: 10.1111/j.1365-3083.2012.02718.x. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen LSS V, Kerneis M, Hauguel-Moreau M, Barthélémy O, Collet JP, Montalescot G, Silvain J. Evaluation of neutrophil gelatinase-associated lipocalin and cystatin C as biomarkers of acute kidney injury after ST-segment elevation myocardial infarction treated by percutaneous coronary intervention. Arch Cardiovasc Dis. 2019;112(3):180–186. doi: 10.1016/j.acvd.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Shrestha K, Borowski AG, Troughton RW, Thomas JD, Klein AL, Tang WH. Renal dysfunction is a stronger determinant of systemic neutrophil gelatinase-associated lipocalin levels than myocardial dysfunction in systolic heart failure. J Card Fail. 2011;17(6):472–478. doi: 10.1016/j.cardfail.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poniatowski B, Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res. 2009;32(2):77–80. doi: 10.1159/000208989. [DOI] [PubMed] [Google Scholar]
- 47.Avci A, Ozturk B, Demir K, Akyurek F, Altunkeser BB. The prognostic utility of plasma NGAL levels in ST segment elevation in myocardial infarction patients. Adv Prev Med. 2020;2020:4637043. doi: 10.1155/2020/4637043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Wan X, Shi Y, Huang F, Shu H, Huang R, Gu L. Neutrophil gelatinase-associated lipocalin contributes to increased risk of cardiovascular death after acute coronary syndrome. Int J Gen Med. 2021;14:4887–4895. doi: 10.2147/IJGM.S328022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng W, Zhang C, Wang Z, Yang W, Luo H, Li X, Fu D, Yu C, Zhou Y. Prognostic value of neutrophil gelatinase-associated lipocalin and glycosylated hemoglobin for non-ST-segment elevation myocardial infarction patients with single concomitant chronic total occlusion following primary percutaneous coronary intervention: A prospective observational study. Medicine (Baltimore). 2019;98(39):e16982. 10.1097/MD.0000000000016982. [DOI] [PMC free article] [PubMed]
- 50.Akcay AB, Ozlu MF, Sen N, Cay S, Ozturk OH, Yalıcn F, Bilen P, Kanat S, Karakas MF, Isleyen A, Demir AD, Sogut S. Covic A. Kanbay M. Prognostic significance of neutrophil gelatinase-associated lipocalin in ST-segment elevation myocardial infarction. J Invest Med. 2012;60(2):508–513. [DOI] [PubMed]
- 51.Helanova K, Littnerova S, Kubena P, Ganovska E, Pavlusova M, Kubkova L, Jarkovsky J, Pavkova Goldbergova M, Lipkova J, Gottwaldova J, Kala P, Toman O, Dastych M, Spinar J, Parenica J. Prognostic impact of neutrophil gelatinase-associated lipocalin and B-type natriuretic in patients with ST-elevation myocardial infarction treated by primary PCI: a prospective observational cohort study. BMJ Open. 2015;5(10):e006872. 10.1136/bmjopen-2014-006872. [DOI] [PMC free article] [PubMed]
- 52.Nymo SHH M, Ueland T, Yndestad A, Lorentzen E, Truvé K, Karlsson T, Ravn-Fischer A, Aukrust P, Caidahl K. Serum neutrophil gelatinase-associated lipocalin (NGAL) concentration is independently associated with mortality in patients with acute coronary syndrome. Int J Cardiol. 2018;262:79–84. doi: 10.1016/j.ijcard.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 53.Obeid S, Yousif N, Davies A, Loretz R, Saleh L, Niederseer D, Noor HA, Amin H, Mach F, Gencer B, Raber L, Windecker S, Templin C, Nanchen D, Rodondi N, Muller O, Matter CM, von Eckardstein A, Luscher TF. Prognostic role of plasma galectin-3 levels in acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2020;9(8):869–878. [DOI] [PubMed]
- 54.Barbarash OLB IS, Kashtalap VV, Zykov MV, Hryachkova ON, Kalaeva VV, Shafranskaya KS, Karetnikova VN, Kutikhin AG. Serum neutrophil gelatinase-associated lipocalin has an advantage over serum cystatin C and glomerular filtration rate in prediction of adverse cardiovascular outcome in patients with ST-segment elevation myocardial infarction. BMC Cardiovasc Disord. 2017;17(1):81 [DOI] [PMC free article] [PubMed]
- 55.Wang X, Yang D, Liu J, Fan X, Ma A, Liu P. Prognostic value of culprit artery double-stranded DNA in ST-segment elevated myocardial infarction. Sci Rep. 2018;8(1):9294. doi: 10.1038/s41598-018-27639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang CJK Y, Ding YY, Sun JZ, Chen T. Serum calprotectin levels and outcome following percutaneous coronary intervention in patients with diabetes and acute coronary syndrome. Med Sci Monit. 2019;25:9517–9523. doi: 10.12659/MSM.918126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL): modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276(40):37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 58.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 59.Bolignano D, Coppolino G, Lacquaniti A, Buemi M. From kidney to cardiovascular diseases: NGAL as a biomarker beyond the confines of nephrology. Eur J Clin Invest. 2010;40(3):273–276. doi: 10.1111/j.1365-2362.2010.02258.x. [DOI] [PubMed] [Google Scholar]
- 60.Passov A, Petäjä L, Pihlajoki M, Salminen US, Suojaranta R, Vento A, et al. The origin of plasma neutrophil gelatinase-associated lipocalin in cardiac surgery. BMC Nephrol. 2019;20(1):182. doi: 10.1186/s12882-019-1380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passov A, Ilmakunnas M, Pihlajoki M, Hermunen K, Lempinen M, Helanterä I, et al. Neutrophil gelatinase-associated lipocalin does not originate from the kidney during reperfusion in clinical renal transplantation. Intensive Care Med Exp. 2021;9(1):56. doi: 10.1186/s40635-021-00422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96(16):1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10(10):997–1000. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Brennan M-L, Hazen SL. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr Opin Lipidol. 2003;14(4):353–359. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Baldus S, Rudolph V, Roiss M, Ito WD, Rudolph TK, Eiserich JP, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113(15):1871–1878. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- 66.Morrow DA, Sabatine MS, Brennan M-L, de Lemos JA, Murphy SA, Ruff CT, et al. Concurrent evaluation of novel cardiac biomarkers in acute coronary syndrome: myeloperoxidase and soluble CD40 ligand and the risk of recurrent ischaemic events in TACTICS-TIMI 18. Eur Heart J. 2008;29(9):1096–1102. doi: 10.1093/eurheartj/ehn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, et al. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197(5):615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108(12):1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- 69.Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT, et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol. 2007;99(10):1364–1368. doi: 10.1016/j.amjcard.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 70.Kaya MG, Yalcin R, Okyay K, Poyraz F, Bayraktar N, Pasaoglu H, et al. Potential role of plasma myeloperoxidase level in predicting long-term outcome of acute myocardial infarction. Tex Heart Inst J. 2012;39(4):500–506. [PMC free article] [PubMed] [Google Scholar]
- 71.Scirica BM, Sabatine MS, Jarolim P, Murphy SA, de Lemos JL, Braunwald E, et al. Assessment of multiple cardiac biomarkers in non-ST-segment elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 Trial. Eur Heart J. 2010;32(6):697–705. doi: 10.1093/eurheartj/ehq468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33(8):2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLOS Pathogens. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montecucco F, Liberale L, Bonaventura A, Vecchiè A, Dallegri F, Carbone F. The Role of inflammation in cardiovascular outcome. Curr Atheroscler Rep. 2017;19(3):11. doi: 10.1007/s11883-017-0646-1. [DOI] [PubMed] [Google Scholar]
- 78.Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9(1):231. doi: 10.3390/cells9010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–1414. doi: 10.1093/eurheartj/ehv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perdomo J, Leung HH, Ahmadi Z, Feng Y, Passam FH, Chong BH. Neutrophil activation and netosis are the key drivers of thrombosis in heparin-induced thrombocytopenia. Blood. 2018;132(Supplement 1):378. doi: 10.1182/blood-2018-99-116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramirez GA, Manfredi AA, Rovere-Querini P, Maugeri N. Bet on NETs! Or on how to translate basic science into clinical practice. Front Immunol. 2016;7:417. 10.3389/fimmu.2016.00417. [DOI] [PMC free article] [PubMed]
- 82.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117(3):953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, et al. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost. 2013;109(2):290–297. doi: 10.1160/TH12-06-0425. [DOI] [PubMed] [Google Scholar]
- 84.Witko-Sarsat V, Cramer EM, Hieblot C, Guichard J, Nusbaum P, Lopez S, et al. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94(7):2487–2496. doi: 10.1182/blood.V94.7.2487.419k07_2487_2496. [DOI] [PubMed] [Google Scholar]
- 85.Bank U. Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. J Leukoc Biol. 2001;69(2):197–206. doi: 10.1189/jlb.69.2.197. [DOI] [PubMed] [Google Scholar]
- 86.Kessenbrock K, Dau T, Jenne DE. Tailor-made inflammation: how neutrophil serine proteases modulate the inflammatory response. J Mol Med. 2011;89(1):23–28. doi: 10.1007/s00109-010-0677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Djurdjevic PM, Arsenijevic NN, Baskic DD, Djukic AL, Popovic S, Samardzic G. Systemic response of peripheral blood leukocytes and their phagocytic activity during acute myocardial infarction. Exp Clin Cardiol. 2001;6(3):159–166. [PMC free article] [PubMed] [Google Scholar]
- 88.Miyamoto S, Ueda M, Ikemoto M, Naruko T, Itoh A, Tamaki S, et al. Increased serum levels and expression of S100A8/A9 complex in infiltrated neutrophils in atherosclerotic plaque of unstable angina. Heart. 2008;94(8):1002–1007. doi: 10.1136/hrt.2007.121640. [DOI] [PubMed] [Google Scholar]
- 89.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J. Calcium-dependent Tetramer Formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359(4):961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: Proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170(6):3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 91.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, et al. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8(3):R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med. 2013;188(9):1137–1146. doi: 10.1164/rccm.201304-0803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–2900. doi: 10.1161/01.CIR.0000042674.89762.20. [DOI] [PubMed] [Google Scholar]
- 95.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61(3):481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 96.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/S0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 98.Mangold A, Alias S, Scherz T, Hofbauer M, Jakowitsch J, Panzenböck A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–1192. doi: 10.1161/CIRCRESAHA.116.304944. [DOI] [PubMed] [Google Scholar]
- 99.Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347(1):5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 100.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 101.Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, et al. Randomized trial of Interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77(15):1845–1855. doi: 10.1016/j.jacc.2021.02.049. [DOI] [PubMed] [Google Scholar]
- 102.Brügger-Andersen T, Aarsetøy H, Grundt H, Staines H, Nilsen DWT. The long-term prognostic value of multiple biomarkers following a myocardial infarction. Thromb Res. 2008;123(1):60–66. doi: 10.1016/j.thromres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 103.McCann CJ, Glover BM, Menown IBA, Moore MJ, McEneny J, Owens CG, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol. 2009;103(1):22–28. doi: 10.1016/j.amjcard.2008.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 39312 kb)