Abstract
Introduction
Nearly 60% of patients with non-small cell lung cancer (NSCLC) present with metastatic disease, and approximately 20% have brain metastases (BrMs) at diagnosis. During the disease course, 25–50% of patients will develop BrMs. Despite available treatments, survival rates for patients with NSCLC and BrMs remain low, and their overall prognosis is poor. Even with newer agents for NSCLC, options for treating BrMs can be limited by their ineffective transport across the blood–brain barrier (BBB) and the unique brain tumor microenvironment. The presence of actionable genomic alterations (AGAs) is a key determinant of optimal treatment selection, which aims to maximize responses and minimize toxicities. The objective of this systematic literature review (SLR) was to understand the current landscape of the clinical management of patients with NSCLC and BrMs, particularly those with AGAs.
Method
A Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)-compliant SLR was conducted to identify studies in patients with BrMs in NSCLC. Searches used the EMBASE and MEDLINE® databases, and articles published between January 1, 2017 and September 26, 2022 were reviewed.
Results
Overall, 179 studies were included in the SLR. This subset review focused on 80 studies that included patients with NSCLC, BrMs, and AGAs (19 randomized controlled trials [RCTs], two single-arm studies, and 59 observational studies). Sixty-four of the 80 studies reported on epidermal growth factor receptor (EGFR) mutations, 14 on anaplastic lymphoma kinase (ALK) alterations, and two on both alterations. Ninety-five percent of studies evaluated targeted therapy. All RCTs allowed patients with previously treated, asymptomatic, or neurologically stable BrMs; the percentage of asymptomatic BrMs varied across observational studies.
Conclusions
Although targeted therapies demonstrate systemic benefits for patients with NSCLC, BrMs, and AGAs, there remains a continued need for effective therapies to treat and prevent BrMs in this population. Increased BBB permeability of emerging therapies may improve outcomes for this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02799-9.
Keywords: NSCLC, Brain metastases, Systematic literature review, EGFR mutation
Key Summary Points
| More than half of newly diagnosed patients with lung cancer have advanced or metastatic disease, 10–26% present with brain metastases at the time of diagnosis, and another 30% will develop brain metastases over the course of their disease. |
| Current treatment options, particularly in later lines of therapy, are limited in their ability to pass through the blood–brain barrier, leaving a continuing treatment need in patients with non-small cell lung cancer (NSCLC) who have or develop brain metastases. |
| This study reviewed the current global landscape of clinical management used for patients with NSCLC, brain metastases, and actionable genomic alterations to gain a better understanding of treatment needs and how emerging therapies can fill those gaps. |
| For patients with NSCLC, brain metastases, and actionable genomic alterations, the current standard of care is suboptimal, and even with targeted therapies and local therapies (e.g., radiotherapies), prognosis is generally poor, regardless of the therapeutic regimen. |
| These findings emphasize the need for new therapies and therapeutic approaches that can improve clinical outcomes for this patient population. |
Introduction
Lung cancer is the most common cause of cancer mortality worldwide, with an estimated 1.80 million deaths annually [1]. Non-small cell lung cancer (NSCLC) accounts for 81% of all lung cancers [2]. More than half of newly diagnosed patients with lung cancer have advanced or metastatic disease [3]; 10–26% present with brain metastases at diagnosis, and another 30% will develop brain metastases over the course of their disease [4–6]. Although various treatments are available, including surgery, stereotactic radiotherapy (SRT), stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), systemic therapy, and supportive care, survival rates for patients with NSCLC and brain metastases remain low, with overall poor patient outcomes and prognosis [7].
In the past, treatment strategies had been based primarily on the stage of disease or histologic appearance (squamous vs. non-squamous). Now, in addition to staging and histology, an improved understanding of tumor biology, along with overall advancements in treatment for NSCLC, has facilitated personalization of clinical management. Research has demonstrated that the presence or absence of actionable genomic alterations (AGAs), e.g., alterations in EGFR, ALK, ROS1, and other less common alterations, is a key determinant of optimal treatment selection, which aims to maximize responses and minimize toxicities [8]. To test for AGAs, advanced polymerase chain reaction (PCR)-based methods, such as quantitative PCR (qPCR) and reverse transcriptase PCR (RT-PCR), are used. Since brain metastases genetically diverge from the main tumor in NSCLC, evaluating for AGAs is important to determine therapy. Circulating tumor DNA (ctDNA) from cerebrospinal fluid seems to provide a better representation of brain metastases profiling compared to plasma ctDNA [9]. Tissue sampling of brain metastases poses a particular challenge, as many patients are not candidates for brain resections or have tumors in inaccessible sites. The low availability of tissue samples makes designing comprehensive studies problematic [10]. Still, AGAs that predict response to targeted treatment, including tyrosine kinase inhibitors (TKIs), are only present in approximately 30% of patients with NSCLC [8]. Even with recent advancements in earlier-line treatment, e.g., third-generation epidermal growth factor receptor (EGFR) TKIs, patients with both NSCLC and brain metastases have limited therapeutic options in later lines as many current treatments are unlikely to cross the blood–brain barrier (BBB) because of their molecular size [11].
In addition, patients often develop resistance to treatments, and therapeutic options may be associated with adverse events due to off-target drug activity [12]. For the development of new therapeutic options, such as fourth-generation EGFR TKIs and antibody–drug conjugates (ADCs), it will be important to understand their comparative activity in relation to the current treatments used for patients with NSCLC and brain metastases.
While the overall objective of the systematic literature review (SLR) was to understand the current landscape of clinical characteristics and clinical management for patients with brain metastases in NSCLC, this current review focused on summarizing the subset of studies with patients whose NSCLC harbored AGAs (e.g., EGFR, ALK, ROS1, NTRK, BRAF, MET, RET, KRAS, HER2).
Methods
An SLR was performed following standard methods outlined in the updated Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 and Cochrane guidelines [13, 14]. This article is based on previously published scientific studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria
The criteria are presented according to the PICOTS (Population, Intervention, Comparators, Outcomes, Timing, and Study design) format (Table 1). The inclusion and exclusion criteria are presented in Table 2.
Table 1.
PICOTS eligibility criteria for overall systematic literature review
| Criterion | Description |
|---|---|
| Population |
Adults with brain metastases in NSCLC within the following patient populations With actionable genomic alterations EGFR, ALK, ROS1, NTRK, BRAF, MET, RET, KRAS, HER2 Without actionable genomic alterations |
| Intervention | Any pharmacotherapy, radiotherapy, or surgery |
| Comparators | Any standard-of-care or emerging therapy |
| Outcomes |
Clinical characteristics: signs, symptoms, and pathology in stable and active disease Clinical management: current methodologies of treatment, limitations, evolution of brain metastases post-treatment (including radiotherapy) Unmet needs: frequency of response & non-response, intracranial efficacy or lack thereof, reasons for non-response with standard of care Emerging therapies: clinical activity on brain metastases, bioavailability, trends for emerging agents, or regimens specifically addressing brain metastases |
| Date range | January 1, 2017 to September 26, 2022 (search date) |
| Study design |
Phase 3 or 4 clinical trials (50 or more participants) Observational/real-world studies (100 or more participants) Clinical practice guidelines or preferred practice patterns |
| Other |
Limited to English language only No geographical limit Excluded conference abstracts |
Table 2.
Eligibility criteria amendments
| Areas targeted for scope refinement and prioritization | Original protocol | Protocol amendment after title and abstract screening | Protocol amendment after full-text screening |
|---|---|---|---|
| Time restriction | Last 5 years | Limit to January 1, 2017 to September 26, 2022 | Limit to January 1, 2017 to September 26, 2022 |
| Sample size | – | Exclude studies with ≤ 50 patients |
Include RCTs with ≥ 50 patients Include observational studies with ≥ 100 patients |
| Publication type | – | – | Include only full-text articles; exclude conference abstracts |
| Study type | – | – | Include only phase 3/4 trials; exclude phase 1/2 trials |
RCT randomized controlled trial
During the course of the SLR, amendments were made (December 5, 2022) to the protocol to refine the eligibility criteria and focus on the most relevant and robust information available. All amendments were made prior to the data extraction phase and were applied universally across all records (Table 2).
Databases Searched
The search was conducted in the following databases using the OvidSP® platform:
EMBASE
MEDLINE® Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Medline® Daily, Medline and Versions®
The search strategy was based on a combination of free-text words, indexing terms (e.g., Excerpta Medica database [EMBASE] subject heading [EMTREE] or Medical Subject Headings [MESH] terms) and their relationship using Boolean terms (e.g., and, or, not). Full strategies (including search dates) for all sources searched are included in Tables S1 and S2.
Screening Process
Publications identified through the systematic literature search were evaluated in a stepwise process to assess whether they should be included for data extraction.
Step 1—Title and abstract review: All unique records identified from the searches were reviewed on the basis of the predefined PICOTS criteria described in Table 1. Two reviewers independently screened titles and abstracts and classified each record as either (1) exclude or (2) continue to full-text review. Any discrepancy between reviewers was resolved by a third reviewer, who also confirmed the classifications for all studies marked for full-text review and from a sample of excluded abstracts. Furthermore, artificial intelligence technology was used to screen all excluded records and assign each a probability of likelihood for inclusion. Any study with a probability ranking over 85% was rescreened.
On the basis of the large number of potentially relevant studies identified after title and abstract screening (> 800), the eligibility criteria were amended to prioritize the most relevant and applicable evidence available that would address the research questions of interest. The amendments made following title and abstract screening are shown in Table 2.
Step 2—Full-text review: Full-texts of publications included after title and abstract review, and meeting the amended eligibility criteria, were screened by two reviewers on the basis of the amended PICOTS criteria. A third reviewer resolved any discrepancies.
Following full-text screening, additional amendments were made to better refine the project scope and identify the most relevant studies. The amendments made following full-text screening are shown in Table 2. Studies that met the amended PICOTS criteria after full-text review were included in the SLR.
Records that were excluded after review of the full-text report were documented, along with a clear justification for their exclusion. All references included after completion of the full-text review were retained for quality assessment and data extraction.
Data Extraction
Extraction of data from the included studies was conducted using a standardized Excel-based data extraction template. For each included study and methodological characteristics, selection criteria, study population/patient characteristics, and results were collected. If results for the same study were reported in more than one publication, the relevant records were grouped per study. Data extraction from included sources was conducted by two investigators independently, with discrepancies resolved by a third reviewer.
Quality Assessment
The quality assessment analyzes the strength and robustness of the available evidence with the aim of evaluating the applicability and internal and external validity of studies.
A quality assessment of individual papers was performed according to the study design. The Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2) [15] was used for randomized controlled trials (RCTs), and the Newcastle–Ottawa Scale (NOS) [16] was used for non-randomized studies. The quality assessment results were recorded in a tabular format in the data extraction file. The quality assessment for studies included in this NSCLC, brain metastases, and AGA subgroup analysis are available in Tables S4 and S5.
Supplemental Search Results
Although not eligible for inclusion in the SLR, the most recent meetings of five conferences (Table S6) and two major clinical trial registries were searched to provide a current view of the evidence landscape (see supplementary materials).
Results
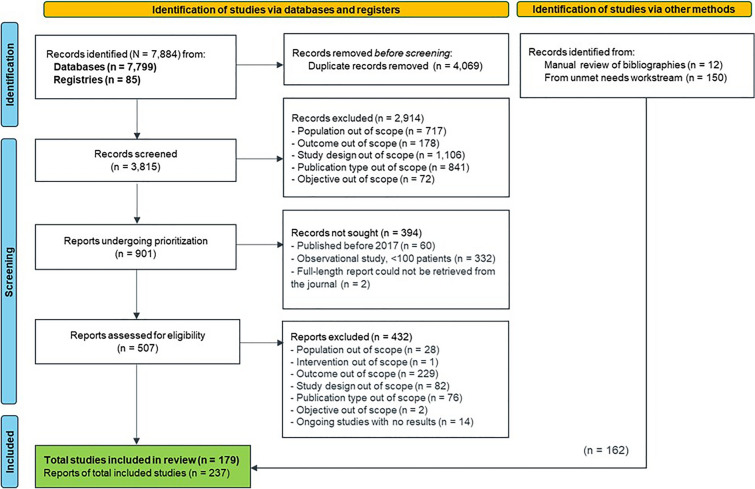
The search and screening process in the SLR is reported in accordance with the PRISMA flow diagram (Fig. 1).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of literature search results. Figure shows the flow of the study identification and selection process. In total, 7884 records were originally identified. After removal of duplicates, 3815 records were screened. Several records were excluded throughout the process for reasons such as the population being out of scope, not having results because of an ongoing study, or the study design being out of scope. Ultimately, 179 studies were included in this review
Overall Systematic Search Output
The database and registry searches identified a total of 7884 records. Following deduplication, 3815 records underwent title and abstract screening, of which 901 records were classified as potentially relevant according to the original PICOTS criteria. After the PICOTS criteria were amended, 394 records were excluded and not sought for full-text review; the full texts of the remaining 507 records were retrieved and reviewed.
After full-text review and amendments to the PICOTS criteria, 432 records were excluded, and 75 records meeting the eligibility criteria were included. An additional 150 records were identified for inclusion, as were an additional 12 records identified from reviewing bibliographies of relevant review articles, based on a concurrent SLR on overall unmet needs in NSCLC with similar eligibility criteria. Overall, 237 publications reporting data on 179 studies were included in the review. The 179 studies included 33 RCTs, two single-arm trials, and 144 observational studies. Most RCTs (n = 17, 52%) were multiregional, whereas most observational studies (n = 75, 52%) were conducted in Asia.
Studies were further characterized as follows:
Studies of patients with AGAs: Of the 80 studies that included patients with AGAs, 19 were RCTs, two were single-arm trials, and 59 were observational studies. These studies are summarized in Tables 3 and S3 [17–98].
Table 3.
Summary of studies for subgroup of patients with NSCLC, brain metastases, and actionable genomic alterations
| Author year | Country/region | Study design | Total patients, N | BrM population, n (%) | Mutation | Line of therapy | Treatment | Arm, n | Asymptomatic BrM, n (%) | CNS-PFS median, months | Intracranial response, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Addeo et al. 2021 [17] | Multinational | OBS | 896 | 332 (37.1) | EGFR | 1L | 1G or 2G EGFR TKI | 332 | – | – | – |
| Bai et al. 2017 [18] | China | OBS | 148 | 148 (100) | EGFR | 2L | GEF | – | 47 (31.8) | – | 33 (34.7) |
| ERL | – | – | 21 (39.6) | ||||||||
| Baldacci et al. 2022 [19] | France | OBS | 208 | 160 (76.9) | ALK | 2L + | LOR | 160 | – | – | – |
| Bilgin et al. 2021 [20] | Turkey | OBS | 283 | 68(24.0) | EGFR | 1L | AFA | 21 | – | – | – |
| ERL or GEF | 47 | – | – | – | |||||||
| Bozorgmehr et al. 2021 [21] | Germany | OBS | 401 | 102 (25.4) | EGFR | 1L to > 3L | EGFR TKI, CT, palliative RT, de novo stage IV | 89 | – | – | – |
| EGFR TKI, CT, palliative RT, secondary stage IV | 13 | – | – | – | |||||||
| Camidge et al. 2018 [22] | Intercontinental | RCT | 275 | 81 (29.4) | ALK | 1L | Brigatinib | 40 | – | 24 | 31 (65.6) |
| CRIZ | 41 | – | 5.6 | 7 (14.0) | |||||||
| Chang et al. 2021 [23] | Taiwan | OBS | 205 | 67 (32.7) | EGFR | 1L | EGFR TKI (GEF, ERL, AFA) | 67 | – | – | – |
| Chen et al. 2020 [24] | China | OBS | 148 | 148 (100) | EGFR | 1L to 2L | EGFR TKI only | 72 | – | 10.2 | – |
| EGFR TKI + WBRT | 76 | – | 11.9 | – | |||||||
| Chen et al. 2019a [25] | Taiwan | OBS | 134 | 134 (100) | EGFR | 1L | GEF | 62 | – | 23.6 | 33 (53.2) |
| ERL | 49 | – | 27.8 | 34 (69.4) | |||||||
| AFA | 23 | – | 17.2 | 15 (65.2) | |||||||
| Chen et al. 2019b [26] | Taiwan | OBS | 141 | 141 (100) | EGFR | 1L + | EGFR TKI + WBRT | 94 | – | – | – |
| EGFR TKI alone | 47 | ||||||||||
| Chen et al. 2018 [27] | China | OBS | 105 | 39 (37.1) | EGFR | 1L or 2L | EGFR TKIs alone | 39 | 9 (23.1) | 6.8 | 26 (66.7) |
| EGFR TKIs + concurrent WBRT | 34 | 8 (23.5) | 12.4 | 29 (85.3) | |||||||
| WBRT followed by EGFR TKIs | 32 | 8 (25.0) | 9.1 | 24 (75.0) | |||||||
| Chiu et al. 2022 [28] | Taiwan | OBS | 310 | 137 (44.2) | EGFR | 1L | EGFR TKI (GEF or ERL) ± BEV | 137 | – | – | – |
| de Marinis et al. 2021 [29] | Intercontinental | SA | 479 | 83 (17.3) | EGFR | 1L + | AFA | 83 | – | – | – |
| Doherty et al. 2017 [30] | Canada | OBS | 184 | 184 (100) | EGFR/ALK | 1L | WBRT + SRS + TKIs | 120 | 58 (48.3) | 50.5 | – |
| SRS + TKIs | 37 | 31 (83.8) | 12 | ||||||||
| TKIs | 27 | 24 (88.9) | 15 | ||||||||
| Duruisseaux et al. 2017 [31] | France | OBS | 318 | 111 (34.9) | ALK | 1L + | CRIZ | 111 | – | – | – |
| El Shafie et al. 2021 [32] | Germany | OBS | 141 | 141 (100) |
EGFR: 76.6% ALK: 23.4% |
1L + | Delayed local therapy | 54 | 45 (88.2) | 10.6 | – |
| Early local therapy | 87 | 41 (48.8) | 19.4 | – | |||||||
| Gijtenbeek et al. 2020 [33] | Netherlands | OBS | 873 | 112 (12.8) | EGFR | 1L | ERL | 65 | – | – | – |
| GEF | 29 | ||||||||||
| AFA | 18 | ||||||||||
| He et al. 2019 [34] | China | OBS | 104 | 104 (100) | EGFR | 1L | EGFR TKI (GEF, ERL, ICO) + WBRT | 56 | 27 (48.2) | 17.7 | – |
| EGFR TKI (GEF, ERL, ICO) | 48 | 29 (60.4) | 11 | – | |||||||
| Horn et al. 2021 [35] | Intercontinental | RCT | 290 | 90 (31.0) | ALK | 1L | Ensartinib | 40 | 40 (100) | – | – |
| CRIZ | 50 | 50 (100) | – | – | |||||||
| Huang et al. 2021 [36] | Taiwan | OBS | 612 | 211 (34.4) | EGFR | 1L + | GEF/ERL | 113 | – | – | – |
| AFA | 98 | ||||||||||
| Huang et al. 2022 [37] | Taiwan | OBS | 516 | 151 (30.3) | EGFR | 1L | AFA | 151 | – | – | – |
| Hyun et al. 2020 [38] | South Korea | OBS | 173 | 173 (100) | EGFR | – | EGFR TKI (GEF, ERL, AFA) | 107 | 98 (91.6) | 10.4 | 67 (62.6) |
| WBRT followed by EGFR TKI (GEF, ERL, AFA) | 36 | 22 (61.1) | 10.8 | 26 (72.2) | |||||||
| SRS followed by EGFR TKI (GEF, ERL, AFA) | 30 | 21 (70.0) | 15.8 | 18 (60.0) | |||||||
| Ito et al. 2021 [39] | Japan | OBS | 160 | 160 (100) | EGFR | 1L | AFA | 75 | – | – | – |
| OSI | 85 | ||||||||||
| Jahanzeb et al. 2020 [40] | USA | OBS | 581 | 160 (27.5) | ALK | 1L and 2L | ALK TKIs (CRIZ, CER, ALEC, BRIG) | 160 | – | – | – |
| Jia et al. 2019 [41] | China | OBS | 114 | 114 (100) | EGFR | 1L | SRS + EGFR TKI (GEF, ERL) | 57 | 9 (15.8) | 12.2 | – |
| WBRT + EGFR TKI (GEF, ERL) | 57 | 5 (8.8) | 11.5 | – | |||||||
| Jiang et al. 2019 [42] | China | OBS | 208 | 208 (100) | EGFR | 1L | EGFR TKI (GEF, ERL, ICO) + BEV | 59 | 38 (64.4) | 14 | 39 (66.1) |
| EGFR TKI (GEF, ERL, ICO) | 149 | 95 (63.8) | 8.2 | 62 (41.6) | |||||||
| Jung et al. 2020 [43] | South Korea | OBS | 559 | 198 (35.4) | EGFR | 1L | GEF | 68 | – | – | 22 (64.7) |
| ERL | 58 | – | – | 15 (68.2) | |||||||
| AFA | 72 | – | – | 27 (72.9) | |||||||
| Jung et al. 2022 [44] | South Korea | OBS | 737 | 287 (38.9) | EGFR | 1L and 2L | 1L AFA + 2L OSI | 54 | 42 (77.8) | – | 71 (24.7) |
| 1L AFA + 2L CT or other treatments | 61 | 40 (65.6) | |||||||||
| 1L AFA + 2L systemic treatment or SC | 46 | 37 (80.4) | |||||||||
| 1L AFA only | 126 | 96 (76.2) | |||||||||
| Ko et al. 2022 [45] | Taiwan | OBS | 400 | 140 (35.0) | EGFR | 1L | GEF or ERL or AFA ± denosumab | 140 | – | – | – |
| Kong et al. 2021 [46] | USA | OBS | 502 | 222 (100) | EGFR | – | EGFR TKI (AFA, ERL, GEF) | 222 | – | – | – |
| Lee et al. 2021 [47] | South Korea | OBS | 422 | 168 (39.8) | EGFR | 1L to 2L | AFA | 168 | – | – | – |
| Lee et al. 2019a [48] | Taiwan | OBS | 100 | 100 (100) | EGFR | 1L + | EGFR TKI + brain surgery + WBRT | 40 | 6 (15.0) | – | – |
| EGFR TKI + WBRT | 60 | 16 (26.7) | – | – | |||||||
| Lee et al. 2019b [49] | Taiwan | OBS | 198 | 198 (100) | EGFR | – | WBRT | 75 | – | – | – |
| SRS | 21 | ||||||||||
| Delayed radiation | 27 | ||||||||||
| Never cranial irradiation | 75 | ||||||||||
| Lee et al. 2020 [50] | South Korea | OBS | 351 | 351 (100) | EGFR | – | With or without OSI | 351 | – | – | – |
| Li et al. 2017 [51] | China | OBS | 104 | 104 (100) | EGFR | – | EGFR TKI (GEF or ERL) or EGFR TKI (GEF or ERL) + WBRT | 104 | – | – | – |
| Li et al. 2019 [52] | China | OBS | 195 | 195 (100) | EGFR | 1L | WBRT followed by EGFR TKI (GEF, ERL, ICO) | 67 | 51 (76.1) | – | – |
| EGFR TKI (GEF, ERL, ICO) + WBRT | 64 | 40 (68.8) | – | – | |||||||
| EGFR TKI (GEF, ERL, ICO) followed by WBRT | 64 | 46 (71.8) | – | – | |||||||
| Lin et al. 2019 [53] | Taiwan | OBS | 125 | 125 (100) | EGFR | 1L | GEF | 28 | – | – | – |
| ERL | 54 | – | – | – | |||||||
| AFA | 43 | – | – | – | |||||||
| Liu et al. 2017 [54] | China | OBS | 11 | 113 (100) | EGFR | 1L to 2L | EGFR TKI (GEF, ERL, ICO) + early RT (WBRT, SRS) | 49 | 10 (20.4) | 21.4 | – |
| EGFR TKI (GEF, ERL, ICO) | 37 | 32 (86.4) | 24.4 | – | |||||||
| EGFR TKI (GEF, ERL, ICO) + salvage RT (WBRT, SRS) | 27 | 18 (66.7) | 23.6 | – | |||||||
| Liu et al. 2020 [55] | USA | OBS | 365 | 145 (39.7) | EGFR | 1L to > 4L | OSI | 124 | – | – | – |
| OSI + ASA | 21 | – | – | – | |||||||
| Lu et al. 2022a [56] | China | RCT | 429 | 115 (26.8) | EGFR | 1L | Aumolertinib | 56 | – | – | – |
| GEF | 59 | – | – | – | |||||||
| Lu et al. 2022b [57] | China | RCT | 444 | 160 (36.0) | EGFR | 2L and 3L | Sintilimab + IBI305 + CT | 53 | 53 (100) | – | – |
| Sintilimab + CT | 52 | 52 (100) | – | – | |||||||
| CT alone | 55 | 55 (100) | – | – | |||||||
| Magnuson et al. 2017 [58] | USA | OBS | 351 | 351 (100) | EGFR | 1L | ERL followed by WBRT or SRS | 131 | 115 (87.7) | 17 | – |
| WBRT followed by ERL | 120 | 69 (57.5) | 24 | – | |||||||
| SRS followed by ERL | 100 | 51 (51.0) | 23 | – | |||||||
| Masuda et al. 2018 [59] | Japan | OBS | 496 | 496 (100) | ALK | 1L + | ALEC | 496 | – | – | – |
| Mehlman et al. 2019 [60] | France | OBS | 226 | 121 (53.5) | EGFR | 1L and > 2L | OSI (≥ 2L with T790M) | 92 | – | – | – |
| OSI (≥ 2L without T790M) | 26 | ||||||||||
| OSI (1L) | 3 | ||||||||||
| Miyawaki et al. 2019 [61] | Japan | OBS | 176 | 176 (100) | EGFR | 1L | EGFR TKI | 107 | 97 (90.6) | 12 | – |
| Local therapy | 69 | 42 (60.9) | 22 | – | |||||||
|
Mok et al. 2017 [62] Wu 2017 [63] |
Intercontinental | RCT | 419 |
144 (34.4) n with CNS metastases |
EGFR T790M | 2L | OSI | 93 | 93 (100) | – | – |
| PBC + PEM | 51 | 51 (100) | |||||||||
|
116 (27.7) n with CNS lesions on BL brain scan (BICNR) |
OSI | 75 | – | 11.7 | 30 (40.0) | ||||||
| PBC + PEM | 41 | 5.6 | 7 (17.1) | ||||||||
| Nadler et al. 2020 [64] | USA | OBS | 402 | 201 (50.0) | EGFR | 1L + | ERL | 201 | – | – | – |
| Patel et al. 2017 [65] | USA | OBS | 189 | 78 (41.3) | EGFR | 1L + | ERL | 78 | – | – | – |
| Peters et al. 2017 [66] | Intercontinental | RCT | 303 | 122 (40.3) | ALK | 1L | ALEC | 64 | – | – | – |
| CRIZ | 58 | – | – | – | |||||||
| Ramotar et al. 2020 [67] | Canada | OBS | 198 | 198 (100) | EGFR | 1L | SRS | 43 | – | – | – |
| WBRT | 121 | ||||||||||
| TKI | 34 | ||||||||||
| Saida et al. 2019 [68] | Japan | OBS | 104 | 104 (100) | EGFR | 1L | EGFR TKI without upfront brain RT | 65 | 55 (84.6) | 11.1 | 24 (36.9) |
| EGFR TKI with upfront brain RT | 39 | 19 (48.7) | 15.6 | 14 (35.6) | |||||||
| Saito et al. 2019 [69] | Japan | RCT | 228 | 72 (31.6) | EGFR | 1L | ERL + BEV | 36 | 36 (100) | – | – |
| ERL | 36 | 36 (100) | – | – | |||||||
| Shaw et al. 2017 [70] | Intercontinental | RCT | 231 | 134 (58.0) | ALK | 2L/2L + | CER | 65 | – | – | – |
| CT | 69 | – | – | – | |||||||
| Shaw et al. 2020 [71] | Intercontinental | RCT | 296 | 78 (26.4) | ALK | 1L | LOR | 38 | – | – | 23 (60.5) |
| CRIZ | 40 | – | 6 (15.0) | ||||||||
| Shi et al. 2017 [72] | China | RCT | 296 | 81 (27.3) | EGFR | 1L | ICO | 41 | – | – | – |
| CT | 40 | – | – | – | |||||||
| Shi et al. 2022 [73] | China | RCT | 358 | 127 (35.4) | EGFR | 1L | Furmonertinib | 65 | – | 20.8 | – |
| GEF | 62 | – | 9.8 | – | |||||||
| Solomon et al. 2018 [74] | Intercontinental | RCT | 343 | 92 (26.8) | ALK | 1L | CRIZ | 45 | – | – | – |
| CT | 47 | – | – | – | |||||||
| Soria et al. 2017 [75] | Intercontinental | RCT | 376 | 121 (32.2) | ALK | 1L | CER | 59 | – | – | 25 (46.3) |
| PBC | 62 | – | – | 11 (21.2) | |||||||
| Soria et al. 2018 [76] and Reungwetwattana et al. 2018 [77] | Intercontinental | RCT | 556 | 116 (21.0) | EGFR | 1L | OSI | 53 | – | – | 40 (65.6) |
| GEF or ERL | 63 | – | – | 29 (43.3) | |||||||
| Tang et al. 2021 [78] | China | OBS | 351 | 132 (37.6) | EGFR T790M | – | OSI | 132 | – | – | – |
| Teocharoen et al. 2021 [79] | Thailand | OBS | 304 | 149 (49.0) | EGFR | 1L to > 2L | EGFR TKI | 149 | – | – | – |
| Tu et al. 2022 [80] | Asia | SA | 541 | 103 (19.0) | EGFR | 1L to 3L + | AFA | 103 | 103 (100) | – | – |
| Wang et al. 2018 [81] | China | OBS | 181 | 181 (100) | EGFR | 1L to > 2L |
Asymptomatic pts EGFR TKI ± RT (WBRT, SRS) |
132 | 132 (72.9) | iPFS in 181 pts | B/C RT n = 91:51 (55.6) |
| B/C RT n = 91: 11.7 | |||||||||||
|
Symptomatic pts EGFR TKI ± RT (WBRT, SRS) |
49 |
Upfront RT n = 90: 9.7 |
Upfront RT n = 90: 56 (62.6) |
||||||||
| Wang et al. 2020 [82] | China | OBS | 113 | 113 (100) | EGFR | – | None | 18 | 67 (59.3) | – | |
| RT (WBRT, SRS) | 27 | 12 | |||||||||
| EGFR TKIs in TKI-naïve | 14 | 7 | |||||||||
| CT | 15 | 10 | |||||||||
| EGFR TKIs + RT (WBRT, SRS) | 39 | 21 | |||||||||
| Wolf et al. 2022 [83] | Intercontinental | RCT | 119 | 76 (63.9) | ALK | 3L | ALEC | 50 | – | 9.6 | – |
| PEM or DOC | 26 | – | 1.4 | – | |||||||
| Wu et al. 2018 [84] | Intercontinental | RCT | 207 | 53 (25.6) | ALK | 1L | CRIZ | 21 | – | NR | – |
| CT | 32 | – | 16 | – | |||||||
| Yang et al. 2017a [85] | China | OBS | 228 | 228 (100) | EGFR | – | BEV + GEF + WBRT | 77 | – | – | – |
| WBRT | 75 | ||||||||||
| Yang et al. 2017b [86] | China | RCT | 176 | 176 (100) | EGFR | 1L to 2L | ICO | 85 | – | 10 | – |
| WBRT ± CT | 91 | – | 4.8 | – | |||||||
| Yang et al. 2021a [87] | China | OBS | 124 | 124 (100) | EGFR | 2L | OSI | 60 | – | – | – |
| AFA | 64 | – | – | – | |||||||
| Yang et al. 2021b [88] | China | OBS | 198 | 198 (100) | EGFR | – | Delayed RT | 94 | 73 (77.7) | 11.1 | 38 (40.4) |
| Upfront RT | 104 | 45 (43.3) | 19.9 | 79 (76.0) | |||||||
| Yomo et al. 2018 [89] | Japan | OBS | 133 | 133 (100) | EGFR | 1L + | SRS ± EGFR TKI (GEF, ERL, AFA, OSI) | 133 | – | – | – |
| Yu et al. 2019 [90] | China | OBS | 261 | 261 (100) | EGFR | 1L + | EGFR TKIs (ICO, GEF, ERL) | 261 | 114 (43.7) | – | – |
| Yu et al. 2021a [91] | China | OBS | 205 | 205 (100) | EGFR | 1L to 2L | OSI with upfront cranial RT | 48 | – | 24.1 | – |
| OSI without upfront cranial RT | 157 | – | 17.7 | – | |||||||
| Yu et al. 2021b [92] | China | OBS | 571 | 571 (100) | EGFR | 1L to > 2L | EGFR TKI (GEF, ICO, ERL, AFA, OSI), local brain therapies (surgery, WBRT, SRS) | 571 | – | – | – |
| Zeng et al. 2022 [93] | China | OBS | 1081 | 293 (27.1) | EGFR | 1L | EGFR TKI | 293 | – | – | – |
| Zhao et al. 2021 [94] | China | RCT | 313 | 92 (29.4) | EGFR | 1L | APA + GEF | 51 | – | – | – |
| PBO + GEF | 41 | ||||||||||
| Zhao et al. 2019 [95] | China | OBS | 344 | 344 (100) | EGFR | 2L + | WBRT (TKI-naïve group) | 207 | 0 | 7.7 | – |
| WBRT (TKI-resistant group) | 137 | 0 | 5.4 | – | |||||||
| Zhao et al. 2022 [96] | China | OBS | 367 | 367 (100) | EGFR | 1L | 1G EGFR TKI (GEF or ERL) | 265 | 117 (44.1) | – | 133 (50.0) |
| OSI | 102 | 57 (55.8) | – | 69 (68.3) | |||||||
| Zhou et al. 2019 [97] | Asia | RCT | 187 | 67 (35.8) | ALK | 1L | ALEC | 44 | – | – | 32 (72.7) |
| CRIZ | 23 | – | 5 (21.7) | ||||||||
| Zhu et al. 2017 [98] | China | OBS | 133 | 133 (100) | EGFR | – | 1G EGFR TKI + RT | 67 | – | 16 | – |
| 1G EGFR TKI | 66 | – | 11.5 | – |
Dash ( -) not reported; 1L/2L/3L/4L first-/second-/third-/fourth-line, 1G/2G first-/second-generation, AFA afatinib, ALEC alectinib, ALK anaplastic lymphoma kinase, APA apatinib, ASA aspirin, B/C before or concurrent, BEV bevacizumab, BICNR blinded independent central neuroradiology review, BL baseline, BRIG brigatinib, BrM brain metastasis, CER ceritinib, CNS central nervous system, CRIZ crizotinib, CT chemotherapy, DOC docetaxel, EGFR epidermal growth factor receptor, ERL erlotinib, GEF gefitinib, ICO icotinib, iPFS intracranial progression-free survival, LOR lorlatinib, NE not evaluable, NR not reached, OBS observational study, OSI osimertinib, PBC platinum-based chemotherapy, PBO placebo, PEM pemetrexed, PFS progression-free survival, pts patients, RCT randomized controlled trial, RT radiotherapy, SA single-arm, SC supportive care, SRS stereotactic radiosurgery, TKI tyrosine kinase inhibitor, USA United States of America, WBRT whole-brain radiation therapy, Y yes
Studies of patients without AGAs: Of the 18 studies of patients with no actionable mutations, nine were RCTs and nine were observational studies.
Studies of patients with or without AGAs: Of the 51 studies of patients with or without actionable mutations, five were RCTs and 46 were observational studies.
Studies of patients with not specified/unknown alterations: All 30 studies of patients with mutation status not specified or unknown were observational studies.
Results for Studies of Subgroup of Patients with NSCLC, Brain Metastases, and AGAs
Of the 19 RCTs, 10 were multiregional and nine were conducted in Asia (China, Japan, South Korea, Hong Kong, Malaysia, Taiwan, and Thailand). Only three trials enrolled fewer than 200 participants, 15 enrolled 200–500 participants, and one enrolled more than 500 participants (median 296 patients; range 119–556 patients). In the four RCTs reporting age and sex for the brain metastasis population, median age was 58.5 years (range 56–63), and median percentage of female patients was 60.5% (range 54–62%).
Of the two single-arm trials, one was multiregional and included 479 participants with EGFR mutations treated with targeted therapy (afatinib) for first-line or later-line therapy. The other study was conducted in multiple countries in Asia and included 541 participants with EGFR mutations treated with targeted therapy (afatinib) for first-line or later-line therapy. Of the 59 observational studies, 75% (n = 44) were conducted in Asia (China, Japan, South Korea, Taiwan, Thailand, and Turkey), eight in North America (USA and Canada), and seven in Europe (France, Germany, the Netherlands, and multi-country). Twenty-seven studies included fewer than 200 patients, 22 included between 200 and 500 patients, and 10 included more than 500 patients (median 208 patients; range 100 to 1081 patients). In the 34 observational studies that reporting age and sex for the brain metastasis population, median age was 58.5 years (range 54–68), and median percentage of female patients was 60.5% (range 37–73%).
Clinical Characteristics
Across the 80 studies that included patients with AGAs, 64 reported data for EGFR mutations (with the majority when reported being exon 19 deletions or exon 21 L858R), 14 reported data for patients with ALK alterations, and two reported data for both EGFR mutations and ALK alterations (Table 3). Still, across all 179 of the publications reviewed in the SLR, only a minority of patients with NSCLC and brain metastases (20–30%) had any actionable mutation. No study reported biomarkers specific to brain metastases.
For patients who had NSCLC and brain metastases, most RCTs included only those patients who were asymptomatic and/or neurologically stable at baseline. Similarly, on the basis of 23 observational studies, a range of 12.3–81.5% patients were reported to have asymptomatic brain metastases at baseline (Table 3). Among the three observational studies that reported symptoms, headache, nausea, and mental changes were the most frequently reported [30, 46, 64]. Patients who were asymptomatic were more likely to have been treated with EGFR TKIs only or EGFR TKIs plus SRS compared with patients who were treated with WBRT alone or in combination with another type of therapy.
Brain metastases were more often multisite than single site. The majority of brain metastases reported were located at the cerebral hemispheres and cerebellum. Few studies reported the median time interval between the diagnosis of NSCLC and brain metastases; among those studies that did, the average time to diagnosis was between 1 and 2 years (this average does not include patients who had brain metastases at NSCLC diagnosis) [45, 82, 88]. One Japanese retrospective study noted that the rate and frequency of developing brain metastases were rapid and higher in patients with EGFR mutations than in patients without EGFR mutations [99]. Similarly, in a Canadian cohort study, patients with EGFR mutations were reported to be at higher risk of developing brain metastases than patients without EGFR mutations [100].
Clinical Management
Overall, the clinical management reported by studies included in this SLR typically followed respective clinical practice guidelines. In brief, of the 80 total studies, 76 evaluated targeted therapy; 15 evaluated chemotherapy; six evaluated immune checkpoint inhibitors (ICIs)/monoclonal antibodies (mAbs), including bevacizumab, sintilimab, and denosumab; and 29 evaluated radiotherapy. Table 3 provides details on therapeutic regimens evaluated in each study.
Nine RCTs reported data for EGFR mutations only, eight of which evaluated EGFR TKIs. Six RCTs evaluated first-line targeted therapy, including aumolertinib, furmonertinib, osimertinib, apatinib, icotinib, gefitinib, and erlotinib plus bevacizumab. One RCT evaluated first- and second-line therapies, including icotinib versus WBRT with or without chemotherapy, and one evaluated second-line therapy with osimertinib versus platinum and pemetrexed-based chemotherapy. One RCT evaluated sintilimab plus a bevacizumab biosimilar plus chemotherapy versus chemotherapy only in patients who had unsuccessful treatment with an EGFR TKI.
The remaining 10 RCTs evaluated patients with ALK alterations. Eight RCTs evaluated first-line targeted therapies (alectinib, brigatinib, ensartinib, lorlatinib, crizotinib, and ceritinib). One RCT evaluated second-line and later-line therapy with ceritinib versus chemotherapy, and one RCT evaluated alectinib versus chemotherapy for third-line therapy.
Among the 59 observational studies, 22 reported first-line therapy, nine reported first-line or second-line therapy, two reported second-line or later-line therapy, 14 reported first-line or later-line therapy, and 10 did not report the line of therapy. For first-line therapy, treatments included targeted therapy (EGFR and ALK TKIs), chemotherapy (platinum- and pemetrexed-based), and radiotherapy (WBRT, SRS, and gamma knife radiotherapy). For second-line therapy, treatments included targeted therapy (gefitinib, erlotinib, and osimertinib). For other lines of therapy and studies that did not report line of therapy, treatments included targeted therapy (EGFR and ALK TKIs), chemotherapy (platinum- and pemetrexed-based), radiotherapy (WBRT, SRS, and gamma knife radiotherapy), and surgery.
CNS Clinical Outcomes
Median central nervous system–progression-free survival (CNS-PFS) and intracranial response (ICR) rates were reported in a minority of studies (n = 24). Studies of EGFR-mutated NSCLC and brain metastases continued to assess first- and second-generation EGFR TKIs, often in combination with other agents or radiotherapy. In studies of first- and second-generation EGFR TKI monotherapy, where reported, CNS-PFS and ICR rate did not vary greatly across agents within each study [18, 25, 43]. In the first-line setting, treatment with upfront WBRT with or without concomitant TKIs resulted in the more favorable clinical outcomes compared with treatment with TKIs only or upfront TKIs followed by WBRT. Three observational studies found that median CNS-PFS was longer in patients who had received earlier or upfront versus no or delayed radiotherapy [32, 68, 88]. Additional observational studies found that EGFR TKIs in combination or sequenced with radiotherapy (WBRT and/or SRS) had longer median CNS-PFS than with EGFR TKI monotherapy [24, 27, 30, 34, 38, 82, 98]. Several of these combination studies were utilizing first- or second-generation EGFR TKIs. One study by Yu et al., which compared osimertinib with and without upfront radiotherapy, also resulted in the combination having a longer median CNS-PFS [91].
In one RCT, second-generation icotinib resulted in a CNS-PFS of 10 months compared with 4.8 months with WBRT plus chemotherapy [86]. In one RCT, CNS-PFS with first-line use of third-generation furmonertinib was 20.8 months versus 9.8 months with first-generation TKIs [73]. In another RCT, second-line osimertinib resulted in a CNS-PFS of 11.7 months versus 5.6 months with chemotherapy [63]. In a first-line RCT, the ICR rate with osimertinib was 66% compared with 43% with first-generation TKIs [77]. Similarly, in a first-line observational study, the ICR rate with osimertinib was 68% compared with 50% with first-generation TKIs [96]. In a second-line RCT, the ICR rate with osimertinib was 40% versus 17% with chemotherapy [63].
Eight RCTs evaluated treatment for patients with NSCLC, brain metastases, and ALK alterations. Crizotinib continues to be the comparator for the second- and third-generation ALK TKIs. Where reported, these second- and third-generation ALK TKIs consistently demonstrated higher CNS-PFS and ICR than did crizotinib or chemotherapy. CNS-PFS with brigatinib was 24 months compared with 5.6 months with crizotinib [22], and 9.6 months with alectrinib compared with 1.4 months with chemotherapy [81]. ICR rates reached 73% (range 46–73%) with second- and third-generation ALK TKIs versus up to 22% with crizotinib and 21% with chemotherapy [71, 75, 97].
Discussion
Trends in Clinical Management
In terms of clinical management for patients with NSCLC, brain metastases, and AGAs, TKIs were described as potentially exhibiting higher penetration rates through the BBB as they are small molecules and have a good lipid–water partition coefficient. For patients with EGFR-mutated NSCLC, EGFR TKIs are the established first-line standard of care. In the treatment of brain metastases, while some countries may continue to rely on first- or even second-generation EGFR TKIs for first-line therapy, evidence has demonstrated that afatinib and osimertinib, as well as other third-generation EGFR TKIs, have better CNS penetration and superior CNS efficacy compared with first-generation options. Similarly, second- and third-generation ALK TKIs are also showing significantly improved CNS efficacy over the previous standard of care, crizotinib. The CNS-PFS and ICR results from both RCTs and observational studies related to these TKIs are continually assessed and reflected in updates across practice guidelines and recommendations globally.
Radiotherapy was found to positively affect the BBB by increasing permeability and the concentration of TKIs in cerebrospinal fluid. Adjuvant radiotherapy, when administered with TKIs, facilitated the TKIs’ capacity to cross the BBB, and thus demonstrated favorable anticancer effect. Additionally, patients who were asymptomatic were more likely to have been treated with EGFR TKIs only or EGFR TKIs plus SRS compared with patients who were treated with WBRT alone or in combination with another type of therapy.
Continuing Need for Optimal Management of NSCLC and Brain Metastases
Optimal management of brain metastases in NSCLC remains a high priority with continuing unmet needs. There were limited actionable targets evaluated among the included studies (79 of 80 studies evaluated EGFR or ALK, and one study evaluated EGFR, ALK, RET, MET, or ROS1). Still, results favored targeted therapy, as well as a combination of localized therapy and targeted therapy, over chemotherapy. Although there is evidence of the effectiveness of systemic therapies and targeted therapies for treatment of brain metastases, many studies of potentially effective anticancer therapies continue to exclude patients with active brain metastases [101].
Though some benefit was observed, the WBRT studies reviewed in this SLR are likely reflective of outdated practice patterns. In the current treatment landscape, conventional WBRT is generally avoided because it causes more neurocognitive problems than SRS [102, 103]. WBRT is frequently reported as an independent prognostic factor of overall survival along with extracranial metastases and performance score. Overall, WBRT is associated with serious harm, does not prolong survival, and yields poorer quality of life; thus, SRS or SRT has been suggested for treating patients with brain metastases when feasible. Lower incidence of radiotherapy-induced brain damage with SRS versus WBRT can be attributed to SRS’s ability to target the high-dose radioactive ray directly at the metastatic brain lesion, resulting in less damage to surrounding normal brain tissues and mitigating the radiotherapy-induced adverse reactions [41, 52]. Of note, in developing countries, first-generation EGFR TKIs and WBRT remain the primary treatment in patients with NSCLC and brain metastases, further highlighting the need for a consistent standard of care for this population [52].
In a retrospective study by Rakshit et al., the authors noted that patients with NSCLC with driver mutations had a high incidence of brain metastases at diagnosis; however, no statistically significant differences in survival outcomes were observed between patients with brain metastases and those without brain metastases [104]. These favorable outcomes for patients with brain metastases were surmised to be related to the use of potent active targeted therapies with good CNS penetration for patients with AGAs. For example, osimertinib has exhibited a protective effect against developing brain metastases, demonstrating an advancement over its first- and second-generation EGFR TKI predecessors [105].
Another study by Julian et al. found that patients with KRAS G12C-positive NSCLC had a higher prevalence of brain metastases compared with patients with KRAS wild-type tumors. This finding suggests that more research should be performed to evaluate whether KRAS G12C inhibitors can be beneficial for patients with brain metastases [106]. A systematic review concluded that TKI alone resulted in superior results in comparison with TKI plus radiotherapy in patients with NSCLC and brain metastases [107].
Emerging Therapies
Although the BBB remains the primary focus of emerging therapies for patients with NSCLC and brain metastases, it must be acknowledged that primary and metastatic brain tumors can disrupt the structure of the BBB and form a blood–tumor barrier (BTB) [108, 109]. This BTB permeability appears to aid in the successful transport of not only targeted therapies but also some chemotherapies. Thus, emerging therapies for patients with NSCLC and brain metastases with or without AGAs focus on maximizing opportunities to cross the BBB and BTB.
As such, EGFR TKIs were reported to exhibit higher penetration rates than other systemic therapies. Some studies also suggested that radiotherapy, such as WBRT, demonstrated favorable effects in increasing the permeability and concentration of TKIs in cerebrospinal fluid. Current emerging targeted therapies being evaluated in patients with brain metastases include almonertinib, anlotinib, apatinib, dacomitinib, icotinib, lazertinib, lenvatinib, neratinib, osimertinib, zorifertinib, D-0316 (InventisBio), and TY-9591. Emerging therapies for other actionable alterations include alectinib, crizotinib, ensartinib, and lorlatinib for patients with ALK alterations; crizotinib and entrectinib for patients with ROS1 mutations; sotorasib and adagrasib for patients with KRAS mutations; tepotinib and capmatinib for patients with MET exon 14 mutations; pralsetinib and selpercatinib for patients with RET fusions; and dabrafenib plus vemurafenib and dabrafenib plus trametinib for patients with BRAF-V600E mutations.
While targeted therapies continue to emerge for those with de novo alterations, as patients move into later lines, therapies no longer work for these AGAs. Thus, it is also important to consider how to treat patients with NSCLC and brain metastases who no longer harbor AGAs. For patients with NSCLC, brain metastases, and no AGAs, immunotherapy has emerged as a new first-line standard of care mostly in combination with or following platinum-based chemotherapy. Emerging therapies for patients without AGAs include immunotherapies targeting programmed death cell (ligand) 1 (PD-1/PD-L1), which are thought to be able to penetrate the BBB, including atezolizumab, camrelizumab, cemiplimab, nivolumab, pembrolizumab, sintilimab, tislelizumab, and zimberelimab. Other emerging treatments noted for patients without AGAs include datopotamab deruxtecan (Dato-DXd; a trophoblast cell-surface antigen 2 [TROP2]-directed antibody–drug conjugate [ADC]), bevacizumab, Endostar (an endostatin), ipilimumab (a CTLA-4 inhibitor), temozolomide, 4-demethyl-4-cholesteryloxycarbonyl-penclomedine, OSE-2101 (a neoepitope vaccine restricted to HLA-A2-positive patients), and patritumab deruxtecan (HER3-DXd; a HER3-targeted ADC).
ADCs are emerging as an effective AGA-agnostic therapeutic option across tumor types and treatment lines. By synergistically combining the specificity of mAbs with the antitumor activity of cytotoxic agents, ADCs selectively bind to cancer cells and deliver their cytotoxic payload directly into cancer cells. Along with US Food and Drug Administration (FDA)-approved T-DXd, which targets HER2 in breast cancer and NSCLC, telisotuzumab-vedotin, Dato-DXd, and HER3-DXd are among several ADCs being investigated in NSCLC. Eight patients with brain metastases experienced a best overall intracranial response of partial or complete response. These findings demonstrate the potential of ADCs to effectively treat patients with brain metastases in later lines of therapy. Further recent data suggest that HER3 may be more abundantly expressed in brain metastases in patients with NSCLC than in extracranial metastases [110]. On the basis of these data and positive results of ADCs in extracranial disease, brain metastases-specific trials with HER3-targeting agents are warranted.
Generalizability
Most studies included in this review were observational studies, which effectively represent the NSCLC population in a real-world setting and reflect the generalizability of the study population. Additionally, studies in all mutation status subgroups were eligible for inclusion in this review, and results were summarized by patient subgroups, type of therapy, and line of therapy. There were no restrictions on interventions or geography. The outcomes included in this review covered a wide range of topics; as such, the findings could provide a comprehensive understanding of the current landscape of clinical characteristics, clinical management, and emerging therapies for patients with NSCLC and brain metastases with and without AGAs.
Strengths and Limitations
The strengths of this review include following the PRISMA and Cochrane guidelines, using independent reviewers with a process for resolving discrepancies, and utilizing artificial intelligence technology to screen excluded records. The inclusion criteria related to interventions and comparators were left broad to increase generalizability. Furthermore, amendments were made to the protocol in order to focus on the most relevant and robust information available. Most studies focused on EGFR mutations or ALK alterations, and few on other actionable driver alterations; however, these are the most common AGAs in this patient population. Although the search period started only in 2017, the purpose was to summarize and interpret the most recent findings on this topic on the basis of the latest treatment landscape. While the trials and trial designs differed between studies, all trials included followed the PICOTS eligibility criteria. Overall, this review provides a comprehensive overview of the clinical characteristics, clinical management, and emerging therapies for patients with NSCLC and brain metastases.
Of note, in the studies included in this SLR, a majority of patients were treated with first- and second-generation EGFR TKIs in the first-line setting. With osimertinib as the current standard of care in first-line EGFR-mutated NSCLC with brain metastases, this may be an important confounder, given its superior CNS activity in comparison to first-generation TKIs. On the basis of the quality assessment, although the majority of all studies were at low risk of bias, it must be acknowledged that eight of the 19 RCTs included in this subset review were categorized as high risk, and all for deviations from the intended interventions (e.g., non-protocol interventions, non-adherence by patient to assigned intervention) (Table S4). Many of the results from all types of studies supported current practice guidelines and continued to highlight the key treatment gaps for patients with NSCLC and brain metastases.
Conclusion
Brain metastases are a poor prognostic factor and are common in NSCLC. This review underscores the continued needs of patients with brain metastases in NSCLC, even in those who have AGAs, likely due to the lack of clear understanding regarding effective transport of therapeutic agents across the BBB. The results of this SLR emphasize the need for therapies that can improve clinical outcomes for this patient population. More data are still needed to confirm these findings, given the differences in trial designs of the trials evaluated in this SLR.
Given the recent advancement in targeted therapies, such as fourth-generation EGFR TKIs, new options may continue to improve CNS-related outcomes. Similarly, with an array of ADCs demonstrating their ability to deliver cytotoxic payload to tumors bearing the target antigen, it may be valid to hypothesize that ADCs might have strong activity in the CNS. Furthermore, brain metastases may increase the permeability of the BBB, allowing a more efficient passage of these drugs into the brain. It is important to evaluate therapies in patients with active, untreated brain metastases as these patients are often excluded from phase 3 trials because of logistical challenges and higher risks for toxicities [111]. Several approaches are being evaluated to overcome the challenges of the BBB [112]. Further clinical validation and transfer of these strategies to ADCs is planned. Aside from NSCLC, tumor regressions and prolongation of survival have been observed with ado-trastuzumab emtansine (T-DM1) in preclinical mouse models of HER2-positive breast cancer and brain metastases. Given the success of these agents in other tumor types, it is hypothesized that they may also prove to be successful in NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance
Project management was provided by Alexandra Vasile (Daiichi Sankyo, Inc.). Writing and editorial assistance was provided by Sara Thier, PhD, MPH, and Ebenezer M. Awuah-Yeboah (Ashfield Medcomms, an Inizio Company) and funded by Daiichi Sankyo, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Dr. Mustafa Khasraw, Dr. Priyanka Yalamanchili, Dr. Anu Santhanagopal, Dr. Chuntao Wu, Dr. Maribel Salas, Ms. Jie Meng, Dr. Maha Karnoub, Dr. Stephen Esker, and Dr. Enriqueta Felip equally contributed to all aspects of the development of this manuscript, including study conception and design, interpretation of the data, drafting, and critical revision of the manuscript.
Funding
This systematic literature review was funded by Daiichi Sankyo, Inc., including the Rapid and Open service fees for publication.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
Mustafa Khasraw: Received grants or contracts from BioNTech, CNS Pharmaceuticals, Daiichi Sankyo, Inc., Immorna Therapeutics, Immvira Therapeutics, and Personalis, Inc.; received consulting fees from AnHeart Therapeutics, Berg Pharma, George Clinical, Manarini Stemline, and Servier; received honoraria from GSK; and participated on an advisory board for Berg Pharmaceuticals. Priyanka Yalamanchili, Chuntao Wu, Jie Meng are employees of Daiichi Sankyo, Inc. Maha Karnoub, Anu Santhanagopal, Maribel Salas, Stephen Esker are employees of Daiichi Sankyo, Inc. and are shareholders in the company. Enriqueta Felip: received consulting fees from AbbVie, Amgen, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Inc., Eli Lilly, F. Hoffmann-La Roche, Gilead, GSK, Janssen, Merck Serono, Merck Sharp & Dohme, Novartis, Peptomyc, Pfizer, Regeneron, Sanofi, Takeda, and Turning Point; received payment or honoraria from Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, F. Hoffmann–La Roche, Genentech, Janssen, Medical Trends, Medscape, Merck Serono, Merck Sharp & Dohme, Peervoice, Pfizer, Sanofi, Takeda, and Touch Oncology; and received support for attending meetings from AstraZeneca, Janssen, and Roche.
Ethical Approval
This article is based on previously published scientific studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Global Cancer Observatory: Cancer Today. IARC: lung fact sheet; 2020. https://gco.iarc.fr/today/data/factsheets/cancers/15-Lung-fact-sheet.pdf. Accessed 20 June 2023.
- 2.Cancer.net. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell. Accessed 20 Mar 2023.
- 3.National Cancer Institute/Surveillance, Epidemiology, and End Results Program. SEER 22. Adenocarcinoma of the Lung and Bronchus. Stage distribution of SEER incidence cases, 2011–2020. By sexes, all races/ethnicities, all ages. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 22 June 2023.
- 4.Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 6.Waqar SN, Samson PP, Robinson CG, et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. 2018;19(4):e373–e379. doi: 10.1016/j.cllc.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JH, Um S-W. The risk factors for brain metastases in patients with non-small cell lung cancer. Ann Transl Med. 2018;6(Suppl 1):S66. 10.21037/atm.2018.10.27. [DOI] [PMC free article] [PubMed]
- 8.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Liu Z, Huang T, Wang Y, et al. Cerebrospinal fluid circulating tumor DNA depicts profiling of brain metastasis in NSCLC. Mol Oncol. 2023;17(5):810–824. doi: 10.1002/1878-0261.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali S, Górska Z, Duchnowska R, Jassem J. Molecular profiles of brain metastases: a focus on heterogeneity. Cancers (Basel) 2021;13(11):2645. doi: 10.3390/cancers13112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M, Kizilbash SH, Laramy JK, et al. Barriers to effective drug treatment for brain metastases: a multifactorial problem in the delivery of precision medicine. Pharm Res. 2018;35(9):177. doi: 10.1007/s11095-018-2455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudmann DG. On-target and off-target-based toxicologic effects. Toxicol Pathol. 2013;41:310–314. doi: 10.1177/0192623312464311. [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane, 2020. http://www.training.cochrane.org/handbook. Accessed 14 Aug 2023.
- 15.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 12 Jan 2021.
- 17.Addeo A, Hochmair M, Janzic U, et al. Treatment patterns, testing practices, and outcomes in the pre-FLAURA era for patients with EGFR mutation-positive advanced NSCLC: a retrospective chart review (REFLECT) Ther Adv Med Oncol. 2021;13:17588359211059874. doi: 10.1177/17588359211059874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai H, Xiong L, Han B. The effectiveness of EGFR-TKIs against brain metastases in EGFR mutation-positive non-small-cell lung cancer. Onco Targets Ther. 2017;10:2335–2340. doi: 10.2147/OTT.S129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldacci S, Besse B, Avrillon V, et al. Lorlatinib for advanced anaplastic lymphoma kinase-positive non-small cell lung cancer: results of the IFCT-1803 LORLATU cohort. Eur J Cancer. 2022;166:51–59. doi: 10.1016/j.ejca.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Bilgin B, Sendur MAN, Yucel S, et al. Real-life comparison of the afatinib and first-generation tyrosine kinase inhibitors in non-small cell lung cancer harboring EGFR exon 19 deletion: a Turk Oncology Group (TOG) study. J Cancer Res Clin Oncol. 2021;147(7):2145–2152. doi: 10.1007/s00432-020-03501-6. [DOI] [PubMed] [Google Scholar]
- 21.Bozorgmehr F, Kazdal D, Chung I, et al. De novo versus secondary metastatic EGFR-mutated non-small-cell lung cancer. Front Oncol. 2021;11:640048. doi: 10.3389/fonc.2021.640048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camidge D, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New Engl J Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 23.Chang CY, Chen CY, Chang SC, et al. Efficacy and prognosis of first-line EGFR-tyrosine kinase inhibitor treatment in older adults including poor performance status patients with EGFR-mutated non-small-cell lung cancer. Cancer Manag Res. 2021;13:7187–7201. doi: 10.2147/CMAR.S322967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Wu Y, Liu BL, et al. Whole-brain radiotherapy can improve the survival of patients with multiple brain metastases from non-small cell lung cancer treated by epidermal growth factor receptor-tyrosine kinase inhibitors. Cancer Manag Res. 2020;12:11333–11340. doi: 10.2147/CMAR.S279096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YH, Chen YF, Chen CY, et al. Clinical factors associated with treatment outcomes in EGFR mutant non-small cell lung cancer patients with brain metastases: a case-control observational study. BMC Cancer. 2019;19(1):1006. doi: 10.1186/s12885-019-6140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CH, Lee HH, Chuang HY, et al. Combination of whole-brain radiotherapy with epidermal growth factor receptor tyrosine kinase inhibitors improves overall survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Cancers (Basel) 2019;11(8):1092. doi: 10.3390/cancers11081092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Wu A, Tao H, et al. Concurrent versus sequential whole brain radiotherapy and TKI in EGFR-mutated NSCLC patients with brain metastasis: a single institution retrospective analysis. Medicine (Baltimore) 2018;97(44):e13014. doi: 10.1097/MD.0000000000013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu TH, Tung PH, Huang CH, et al. The different overall survival between single-agent EGFR-TKI treatment and with bevacizumab in non-small cell lung cancer patients with brain metastasis. Sci Rep. 2022;12(1):4398. doi: 10.1038/s41598-022-08449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Marinis F, Laktionov KK, Poltoratskiy A, et al. Afatinib in EGFR TKI-naïve patients with locally advanced or metastatic EGFR mutation-positive non-small cell lung cancer: interim analysis of a phase 3b study. Lung Cancer. 2021;152:127–134. doi: 10.1016/j.lungcan.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Doherty MK, Korpanty GJ, Tomasini P, et al. Treatment options for patients with brain metastases from EGFR/ALK-driven lung cancer. Radiother Oncol. 2017;123(2):195–202. doi: 10.1016/j.radonc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8(13):21903–21917. 10.18632/oncotarget.15746. [DOI] [PMC free article] [PubMed]
- 32.El Shafie RA, Seidensaal K, Bozorgmehr F, et al. Effect of timing, technique and molecular features on brain control with local therapies in oncogene-driven lung cancer. ESMO Open. 2021;6(3):100161. doi: 10.1016/j.esmoop.2021.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gijtenbeek RGP, Damhuis RAM, Groen HJM, van der Wekken AJ, van Geffen WH. Nationwide real-world cohort study of first-line tyrosine kinase inhibitor treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer. Clin Lung Cancer. 2020;21(6):e647–e653. doi: 10.1016/j.cllc.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 34.He ZY, Li MF, Lin JH, Lin D, Lin RJ. Comparing the efficacy of concurrent EGFR-TKI and whole-brain radiotherapy vs EGFR-TKI alone as a first-line therapy for advanced EGFR-mutated non-small-cell lung cancer with brain metastases: a retrospective cohort study. Cancer Manag Res. 2019;11:2129–2138. doi: 10.2147/CMAR.S184922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horn L, Wang Z, Wu G, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):1617–1625. doi: 10.1001/jamaoncol.2021.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang AC, Huang CH, Ju JS, et al. First- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors in a large, real-world cohort of patients with non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:17588359211035710. doi: 10.1177/17588359211035710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang CH, Ju JS, Chiu TH, et al. Afatinib treatment in a large real-world cohort of non-small cell lung cancer patients with common and uncommon epidermal growth factor receptor mutation. Int J Cancer. 2022;150(4):626–635. doi: 10.1002/ijc.33821. [DOI] [PubMed] [Google Scholar]
- 38.Hyun DG, Choi CM, Lee DH, et al. Outcomes according to initial and subsequent therapies following intracranial progression in patients with EGFR-mutant lung cancer and brain metastasis. PLoS ONE. 2020;15(4):e0231546. doi: 10.1371/journal.pone.0231546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito K, Morise M, Wakuda K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6(3):100115. doi: 10.1016/j.esmoop.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jahanzeb M, Lin HM, Pan X, et al. Real-world treatment patterns and progression-free survival associated with anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor therapies for ALK+ non-small cell lung cancer. Oncologist. 2020;25(10):867–877. doi: 10.1634/theoncologist.2020-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia F, Cheng X, Zeng H, Miao J, Hou M. Clinical research on stereotactic radiosurgery combined with epithermal growth factor tyrosine kinase inhibitors in the treatment of brain metastasis of non-small cell lung cancer. J BUON. 2019;24(2):578–584. [PubMed] [Google Scholar]
- 42.Jiang T, Zhang Y, Li X, et al. EGFR-TKIs plus bevacizumab demonstrated survival benefit than EGFR-TKIs alone in patients with EGFR-mutant NSCLC and multiple brain metastases. Eur J Cancer. 2019;121:98–108. doi: 10.1016/j.ejca.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Jung HA, Woo SY, Lee SH, et al. The different central nervous system efficacy among gefitinib, erlotinib and afatinib in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer. Transl Lung Cancer Res. 2020;9(5):1749–58. 10.21037/tlcr-20-379. [DOI] [PMC free article] [PubMed]
- 44.Jung HA, Hong MH, Lee HW, et al. Totality outcome of afatinib sequential treatment in patients with EGFR mutation-positive non-small cell lung cancer in South Korea (TOAST): Korean Cancer Study Group (KCSG) LU-19–22. Transl Lung Cancer Res. 2022;11(7):1369–79. 10.21037/tlcr-22-79. [DOI] [PMC free article] [PubMed]
- 45.Ko HW, Chiu CT, Wang CL, et al. Overall survival improvement in patients with epidermal growth factor receptor-mutated non-small cell lung cancer and bone metastasis treated with denosumab. Cancers (Basel) 2022;14(14):3470. doi: 10.3390/cancers14143470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong AM, Pavilack M, Huo H, et al. Real-world impact of brain metastases on healthcare utilization and costs in patients with non-small cell lung cancer treated with EGFR-TKIs in the US. J Med Econ. 2021;24(1):328–338. doi: 10.1080/13696998.2021. [DOI] [PubMed] [Google Scholar]
- 47.Lee SY, Choi CM, Chang YS, et al. Real-world experience of afatinib as first-line therapy for advanced EGFR mutation-positive non-small cell lung cancer in Korea. Transl Lung Cancer Res. 2021;10(12):4353–67. 10.21037/tlcr-21-501. [DOI] [PMC free article] [PubMed]
- 48.Lee HH, Chen CH, Chuang HY, Huang YW, Huang MY. Brain surgery in combination with tyrosine kinase inhibitor and whole brain radiotherapy for epidermal growth factor receptor-mutant non-small-cell lung cancer with brain metastases. Sci Rep. 2019;9(1):16834. doi: 10.1038/s41598-019-53456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JH, Chen HY, Hsu FM, et al. Cranial irradiation for patients with epidermal growth factor receptor (EGFR) mutant lung cancer who have brain metastases in the era of a new generation of EGFR inhibitors. Oncologist. 2019;24(12):e1417–e1425. doi: 10.1634/theoncologist.2019-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, Choi Y, Han J, et al. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15(11):1758–1766. doi: 10.1016/j.jtho.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 51.Li H, Lian J, Jin H, et al. Assessment of prognostic scores of brain metastases from lung adenocarcinoma with EGFR mutations. J Neurooncol. 2017;133(1):129–135. doi: 10.1007/s11060-017-2411-2. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Guo J, Zhao L, et al. Upfront whole brain radiotherapy for multiple brain metastases in patients with EGFR-mutant lung adenocarcinoma. Cancer Manag Res. 2019;11:3433–3443. doi: 10.2147/CMAR.S196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin CY, Chang CC, Su PL, et al. Brain MRI imaging characteristics predict treatment response and outcome in patients with de novo brain metastasis of EGFR-mutated NSCLC. Medicine (Baltimore) 2019;98(33):e16766. doi: 10.1097/MD.0000000000016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Deng L, Zhou X, et al. Concurrent brain radiotherapy and EGFR-TKI may improve intracranial metastases control in non-small cell lung cancer and have survival benefit in patients with low DS-GPA score. Oncotarget. 2017;8(67):111309–17. 10.18632/oncotarget.22785. [DOI] [PMC free article] [PubMed]
- 55.Liu X, Hong L, Nilsson M, et al. Concurrent use of aspirin with osimertinib is associated with improved survival in advanced EGFR-mutant non-small cell lung cancer. Lung Cancer. 2020;149:33–40. doi: 10.1016/j.lungcan.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu S, Dong X, Jian H, et al. AENEAS: a randomized phase III trial of aumolertinib versus gefitinib as first-line therapy for locally advanced or metastatic non-small-cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40(27):3162–3171. doi: 10.1200/JCO.21.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(9):1167–1179. doi: 10.1016/S1470-2045(22)00382-5. [DOI] [PubMed] [Google Scholar]
- 58.Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. doi: 10.1200/JCO.2016.69.7144. [DOI] [PubMed] [Google Scholar]
- 59.Masuda N, Ohe Y, Gemma A, et al. Safety and effectiveness of alectinib in a real-world surveillance study in patients with ALK-positive non-small-cell lung cancer in Japan. Cancer Sci. 2019;110(4):1401–1407. doi: 10.1111/cas.13977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehlman C, Cadranel J, Rousseau-Bussac G, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: a multicentric retrospective French study. Lung Cancer. 2019;137:149–156. doi: 10.1016/j.lungcan.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Miyawaki E, Kenmotsu H, Mori K, et al. Optimal sequence of local and EGFR-TKI therapy for EGFR-mutant non-small-cell lung cancer with brain metastases stratified by number of brain metastases. Int J Radiat Oncol Biol Phys. 2019;104(3):604–613. doi: 10.1016/j.ijrobp.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 62.Mok TS, Wu Y-L, Ahn M-J, et al. AURA3 Investigators. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40. 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed]
- 63.Wu Y-L, Ahn M-J, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36(26):2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 64.Nadler E, Espirito JL, Pavilack M, Baidoo B, Fernandes A. Real-world disease burden and outcomes of brain metastases in EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2020;16(22):1575–1584. doi: 10.2217/fon-2020-0280. [DOI] [PubMed] [Google Scholar]
- 65.Patel SH, Rimner A, Foster A, et al. Patterns of initial and intracranial failure in metastatic EGFR-mutant non-small cell lung cancer treated with erlotinib. Lung Cancer. 2017;108:109–114. doi: 10.1016/j.lungcan.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 67.Ramotar M, Barnes S, Moraes F, et al. Neurological death is common in patients with EGFR mutant non-small cell lung cancer diagnosed with brain metastases. Adv Radiat Oncol. 2019;5(3):350–357. doi: 10.1016/j.adro.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saida Y, Watanabe S, Abe T, et al. Efficacy of EGFR-TKIs with or without upfront brain radiotherapy for EGFR-mutant NSCLC patients with central nervous system metastases. Thorac Cancer. 2019;10(11):2106–2116. doi: 10.1111/1759-7714.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 70.Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886. doi: 10.1016/S1470-2045(17)30339-X. [DOI] [PubMed] [Google Scholar]
- 71.Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 72.Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 73.Shi Y, Chen G, Wang X, et al. Furmonertinib (AST2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. 2022;10(11):1019–1028. doi: 10.1016/S2213-2600(22)00168-0. [DOI] [PubMed] [Google Scholar]
- 74.Solomon BJ, Kim DW, Wu YL, et al. Final overall survival analysis from a study comparing first-line crizotinib versus chemotherapy in ALK-mutation-positive non-small-cell lung cancer. J Clin Oncol. 2018;36(22):2251–8. 10.1200/JCO.2017.77.4794. [DOI] [PubMed]
- 75.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389(10072):917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 76.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 77.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;JCO2018783118. 10.1200/JCO.2018.78.3118. [DOI] [PubMed]
- 78.Tang X, Li Y, Qian WL, et al. A comprehensive prognostic analysis of osimertinib treatment in advanced non-small cell lung cancer patients with acquired EGFR-T790M mutation: a real-world study. J Cancer Res Clin Oncol. 2022;148(9):2475–2486. doi: 10.1007/s00432-021-03797-y. [DOI] [PubMed] [Google Scholar]
- 79.Teocharoen R, Ruangritchankul K, Vinayanuwattikun C, Sriuranpong V, Sitthideatphaiboon P. Vimentin expression status is a potential biomarker for brain metastasis development in EGFR-mutant NSCLC patients. Transl Lung Cancer Res. 2021;10(2):790–801. 10.21037/tlcr-20-1020. [DOI] [PMC free article] [PubMed]
- 80.Tu H-Y, Feng J, Shi M, et al. A phase IIIb open-label, single-arm study of afatinib in EGFR TKI-naïve patients with EGFRm+ NSCLC: final analysis, with a focus on patients enrolled at sites in China. Target Oncol. 2022;17(1):1–13. doi: 10.1007/s11523-021-00859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Song Z, Zhang Y. Efficacy of brain radiotherapy plus EGFR-TKI for EGFR-mutated non-small cell lung cancer patients who develop brain metastasis. Arch Med Sci. 2018;14(6):1298–1307. doi: 10.5114/aoms.2018.78939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Liu Z, Pang Q, et al. Prognostic analysis of patients with non-small cell lung cancer harboring exon 19 or 21 mutation in the epidermal growth factor gene and brain metastases. BMC Cancer. 2020;20(1):837. doi: 10.1186/s12885-020-07249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolf J, Helland Å, Oh IJ, et al. Final efficacy and safety data, and exploratory molecular profiling from the phase III ALUR study of alectinib versus chemotherapy in crizotinib-pretreated ALK-positive non-small-cell lung cancer. ESMO Open. 2022;7(1):100333. doi: 10.1016/j.esmoop.2021.100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Y-L, Lu S, Lu Y, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(10):1539–1548. doi: 10.1016/j.jtho.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 85.Yang RF, Yu B, Zhang RQ, et al. Bevacizumab and gefitinib enhanced whole-brain radiation therapy for brain metastases due to non-small-cell lung cancer. Braz J Med Biol Res. 2017;51(1):e6073. doi: 10.1590/1414-431X20176073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang J-J, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5(9):707–716. doi: 10.1016/S2213-2600(17)30262-X. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y, Liu Q, Cao L, et al. Osimertinib versus afatinib in patients with T790M-positive, non-small-cell lung cancer and multiple central nervous system metastases after failure of initial EGFR-TKI treatment. BMC Pulm Med. 2021;21(1):172. doi: 10.1186/s12890-021-01539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang S, Xiao J, Liu Q, et al. The sequence of intracranial radiotherapy and systemic treatment with tyrosine kinase inhibitors for gene-driven non-small cell lung cancer brain metastases in the targeted treatment era: a 10-year single-center experience. Front Oncol. 2021;11:732883. doi: 10.3389/fonc.2021.732883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yomo S, Oda K. Impacts of EGFR-mutation status and EGFR-TKI on the efficacy of stereotactic radiosurgery for brain metastases from non-small cell lung adenocarcinoma: a retrospective analysis of 133 consecutive patients. Lung Cancer. 2018;119:120–126. doi: 10.1016/j.lungcan.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Yu X, Fan Y. Real-world data on prognostic factors for overall survival in EGFR-mutant non-small-cell lung cancer patients with brain metastases. J Cancer. 2019;10(15):3486–3493. doi: 10.7150/jca.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu F, Ni J, Zeng W, et al. Clinical value of upfront cranial radiation therapy in osimertinib-treated epidermal growth factor receptor-mutant non-small cell lung cancer with brain metastases. Int J Radiat Oncol Biol Phys. 2021;111(3):804–815. doi: 10.1016/j.ijrobp.2021.05.125. [DOI] [PubMed] [Google Scholar]
- 92.Yu X, Sheng J, Pan G, Fan Y. Real-world utilization of EGFR TKIs and prognostic factors for survival in EGFR-mutated non-small cell lung cancer patients with brain metastases. Int J Cancer. 2021;149(5):1121–1128. doi: 10.1002/ijc.33677. [DOI] [PubMed] [Google Scholar]
- 93.Zeng Y, Guo T, Zhou Y, et al. Clinical outcomes of advanced non-small cell lung cancer patients harboring distinct subtypes of EGFR mutations and receiving first-line tyrosine kinase inhibitors: brain metastasis and de novo T790M matters. BMC Cancer. 2022;22(1):198. doi: 10.1186/s12885-022-09245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao H, Yao W, Min X, et al. First-line treatment in advanced EGFR-mutant NSCLC: the phase III active study (CTONG1706) J Thorac Oncol. 2021;16(9):1533–1546. doi: 10.1016/j.jtho.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Zhao L, Cai X, Chen D, et al. Therapeutic effect of whole brain radiotherapy on advanced NSCLC between EGFR TKI-naïve and TKI-resistant. Radiat Oncol. 2019;15(1):3. doi: 10.1186/s13014-019-1454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao Y, Li S, Yang X, et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant non-small cell lung cancer with brain metastasis. Int J Cancer. 2022;150(8):1318–1328. doi: 10.1002/ijc.33904. [DOI] [PubMed] [Google Scholar]
- 97.Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019;7(5):437–446. doi: 10.1016/S2213-2600(19)30053-0. [DOI] [PubMed] [Google Scholar]
- 98.Zhu Q, Sun Y, Cui Y, et al. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC). Oncotarget. 2017;8(8):13304–11. 10.18632/oncotarget.14515. [DOI] [PMC free article] [PubMed]
- 99.Fujita Y, Kinoshita M, Ozaki T, et al. The impact of EGFR mutation status and single brain metastasis on the survival of non-small-cell lung cancer patients with brain metastases. Neurooncol Adv. 2020;2(1):vdaa064. 10.1093/noajnl/vdaa064. [DOI] [PMC free article] [PubMed]
- 100.Moraes FY, Mansouri A, Dasgupta A, et al. Impact of EGFR mutation on outcomes following SRS for brain metastases in non-small cell lung cancer. Lung Cancer. 2021;155:34–39. doi: 10.1016/j.lungcan.2021.02.036. [DOI] [PubMed] [Google Scholar]
- 101.Tan AC, Boggs DH, Lee EQ, Kim MM, Mehta MP, Khasraw M. Clinical trial eligibility criteria and recently approved cancer therapies for patients with brain metastases. Front Oncol. 2022;11:780379. doi: 10.3389/fonc.2021.780379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinkham MB, Sanghera P, Wall GK, Dawson BD, Whitfield GA. Neurocognitive effects following cranial irradiation for brain metastases. Clin Oncol. 2015;27:630–639. doi: 10.1016/j.clon.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 103.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 104.Rakshit E, Bansai R, Desai A, Leventakos K. Brain metastases in non-small cell lung cancer in era of molecularly driven therapy. J Thorac Oncol. 2021;16(4S):38P. 10.1016/S1556-0864(21)01880-3.
- 105.Liam CK. Central nervous system activity of first-line osimertinib in epidermal growth factor receptor-mutant advanced non-small cell lung cancer. Ann Transl Med. 2019;7(3):61. 10.21037/atm.2018.12.68. [DOI] [PMC free article] [PubMed]
- 106.Julian C, Pal N, Gershon A, et al. Overall survival in patients with advanced non-small cell lung cancer with KRAS G12C mutation with or without STK11 and/or KEAP1 mutations in a real-world setting. BMC Cancer. 2023;23:352. doi: 10.1186/s12885-023-10778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tancherla A, Wijovi F, Hariyanto TI, Kurniawan A, Giselvania A. EGFR-TKI plus radiotherapy versus EGFR-TKI only in non-small cell lung cancer patients with brain metastasis: a systematic review and meta-analysis of observational studies. J Thorac Oncol. 2021;16(4S):142P. doi: 10.1016/S1556-0864(21)01984-5. [DOI] [Google Scholar]
- 108.Steeg PS. The blood–tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol. 2021;18:696–714. doi: 10.1038/s41571-021-00529-6. [DOI] [PubMed] [Google Scholar]
- 109.Ye L-Y, Sun L-X, Zhong X-H, et al. The structure of blood–tumor barrier and distribution of chemotherapeutic drugs in non-small cell lung cancer brain metastases. Cancer Cell Int. 2021;21:556. doi: 10.1186/s12935-021-02263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mair MJ, Bartsch R, Le Rhun E, et al. Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours. Nat Rev Clin Oncol. 2023;20:372–389. doi: 10.1038/s41571-023-00756-z. [DOI] [PubMed] [Google Scholar]
- 111.Mair M, Preusser M. Antibody drug conjugates in brain tumors—new kids on the block? A review on the intracranial activity of ADCs in primary and secondary brain tumors. 2023. https://cancercommunity.nature.com/posts/antibody-drug-conjugates-in-brain-tumors-new-kids-on-the-block. Accessed 25 Aug 2023.
- 112.Wu D, Chen Q, Chen X, et al. The blood–brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther. 2023;8:217. doi: 10.1038/s41392-023-01481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.