Abstract
Introduction
Patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL) often require multiple lines of treatment and have a poor prognosis, particularly after failing covalent Bruton tyrosine kinase inhibitor (cBTKi) therapy. Newer treatments such as brexucabtagene autoleucel (brexu-cel, chimeric antigen receptor T cell therapy) and pirtobrutinib (non-covalent BTKi) show promise in improving outcomes.
Methods
Without direct comparative evidence, an unanchored matching-adjusted indirect comparison was conducted to estimate the relative treatment effects of brexu-cel and pirtobrutinib for post-cBTKi R/R MCL. Using logistic propensity score models, individual patient-level data from ZUMA-2 brexu-cel-infused population (N = 68) were weighted to match pre-specified clinically relevant prognostic factors based on study-level data from the BRUIN cBTKi pre-treated cohort (N = 90). The base-case model incorporated the five most pertinent factors reported in ≥ 50% of both trial populations: morphology, MCL International Prognostic Index, number of prior lines of therapy, disease stage, and prior autologous stem cell transplant. A sensitivity analysis additionally incorporated TP53 mutation and Ki-67 proliferation. Relative treatment effects were expressed as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
In the base-case model, brexu-cel was associated with higher rates of objective response (OR 10.39 [95% CI 2.81–38.46]) and complete response (OR 10.11 [95% CI 4.26–24.00]), and improved progression-free survival (HR 0.44 [95% CI 0.25–0.75]), compared to pirtobrutinib. Overall survival and duration of response favored brexu-cel over pirtobrutinib but the differences crossed the bounds for statistical significance. Findings were consistent across the adjusted and unadjusted analyses.
Conclusions
Findings suggest that brexu-cel may offer clinically and statistically significant benefits regarding objective response, complete response, and progression-free survival compared to pirtobrutinib among patients with R/R MCL after prior cBTKi therapy. Given the short follow-up and high degree of censoring in BRUIN, an analysis incorporating updated BRUIN data may provide more definitive overall survival results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02822-z.
Keywords: Brexucabtagene autoleucel, Brexu-cel, Bruton tyrosine kinase inhibitor, CAR T cell therapy, KTE-X19, MAIC, Matching-adjusted indirect comparison, Mantle cell lymphoma, Non-Hodgkin lymphoma, Pirtobrutinib
Key Summary Points
| Why carry out this study? |
| Historical non-comparative and/or observational studies suggest poor outcomes among patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL) who previously failed treatment with a covalent Bruton tyrosine kinase inhibitor (cBTKi), i.e., ibrutinib and acalabrutinib. |
| Brexucabtagene autoleucel (brexu-cel) and pirtobrutinib are approved treatments associated with improved clinical outcomes among patients with R/R MCL in the post-cBTKi setting; the comparative efficacy of these therapies has not been evaluated in a head-to-head trial and, therefore, an unanchored matching-adjusted indirect comparison was conducted to estimate the relative efficacy of brexu-cel and pirtobrutinib in this population using data from the ZUMA-2 and BRUIN single-arm trials, respectively. |
| What was learned from the study? |
| Compared to pirtobrutinib, brexu-cel was associated with statistically significant higher odds of objective response and complete response as well as improved progression-free survival. |
| In terms of duration of response and overall survival, the point estimates trended in favor of brexu-cel over pirtobrutinib, although treatment differences were not statistically significant; it is important to highlight that the comparisons of overall survival were likely influenced by differences in median follow-up time (47.5 months in ZUMA-2 and 23.5 months in BRUIN) and that a more reliable treatment effect could be possible with longer-term BRUIN data. |
| These results provide valuable insights that may help inform treatment decisions for patients with R/R MCL who have previously received cBTKi therapy; an update to the analysis would be of interest when more mature overall survival data from BRUIN becomes available. |
Introduction
Mantle cell lymphoma (MCL) is a rare hematologic B cell malignancy that represents less than 10% of all cases of non-Hodgkin lymphoma (NHL) [1]. Response rates to initial therapy are generally high [2]; however, almost all patients eventually experience disease relapse and require multiple lines of therapy [3–5]. Limited published data from retrospective, non-comparative, observational and real-world evidence indicate that outcomes are particularly poor among patients with relapsed/refractory (R/R) MCL who experience disease relapse after treatment with a covalent Bruton tyrosine kinase inhibitor (cBTKi). In this population, median overall survival (OS) was reported to range from 5.8 to 12.5 months with conventional subsequent treatment [6–10]. As such, until recently, there remained a high unmet need for novel treatment options for patients with R/R MCL in the post-cBTKi setting.
Over the last few years, the treatment landscape for R/R MCL evolved substantially with the introduction of brexucabtagene autoleucel (brexu-cel; KTE-X19), an autologous anti-CD19 chimeric antigen receptor (CAR) T cell therapy. On the basis of positive results of the phase 2 ZUMA-2 clinical trial [11, 12], brexu-cel was first approved in July 2020 by the US Food and Drug Administration (FDA) for the treatment of R/R MCL [13]. Subsequently in December 2020, brexu-cel was approved by the European Medicines Agency for treatment of R/R MCL after two or more lines of systemic therapy, including a cBTKi [14]. In January 2023, the FDA granted accelerated approval of another novel treatment option, the third-generation non-covalent BTKi pirtobrutinib, on the basis of results of the phase 1/2 BRUIN trial [15]. Similar to the European indication for brexu-cel, pirtobrutinib is approved by the FDA for the treatment of R/R MCL after at least two lines of systemic therapy which included a BTKi [16]. Pirtobrutinib also received a conditional market authorization for the treatment of R/R MCL post-BTKi from the European Committee for Medicinal Products for Human Use in October of 2023 [17].
Understanding the relative efficacy of available treatment options is a key component of clinical decision-making. To date, the comparative effects of brexu-cel and pirtobrutinib have not been evaluated in a head-to-head randomized controlled trial. Therefore, the objective of this study was to estimate the relative efficacy of these therapies in the post-cBTKi setting of R/R MCL via an unanchored matching-adjusted indirect comparison (MAIC).
Methods
Data Sources
Two single-arm clinical trials were considered relevant for the indirect comparison: ZUMA-2 and BRUIN. Study details have been previously published and described briefly below. Comparison of key trial eligibility criteria in ZUMA-2 and BRUIN can be found in the supplementary appendix Table S1.
Evidence for Brexu-cel
For brexu-cel, individual patient-level data were available from the ZUMA-2 trial (NCT02601313) [11, 12]. In brief, ZUMA-2 is an ongoing, single-arm, multicenter, phase 2 trial in adult patients at least 18 years of age with histologically confirmed MCL that is R/R to up to five prior regimens including (1) an anthracycline-containing or bendamustine-containing chemotherapy, (2) an anti-CD20 monoclonal antibody, and (3) a BTKi therapy. The intention-to-treat population (ITT, N = 74) included all enrolled/leukapheresed patients, of whom six underwent leukapheresis but did not receive brexu-cel infusion. The modified ITT population (mITT, N = 68), the primary focus of this study, included all patients who received brexu-cel infusion at a target dose of 2 × 106 anti-CD19 CAR T cells/kg. The latest data cutoff date for objective response rate (ORR), complete response (CR) rate, duration of response (DOR), and progression-free survival (PFS) as assessed by an independent radiologic review committee, per Lugano 2014 criteria [18], was July 24, 2021 (median potential follow-up time from brexu-cel infusion of 35.6 months) [12], while longer-term data for OS were available from the July 23, 2022 cutoff date (median follow-up time 47.5 months).
Evidence for Pirtobrutinib
BRUIN (NCT03740529) is an ongoing, multicenter, open-label, phase 1/2 study of pirtobrutinib in adult patients aged at least 18 years with NHL and who had received at least two previous lines of therapy, irrespective of prior cBTKi use [15, 19–21]. As of the data cutoff date of July 29, 2022, a total of 166 patients with MCL were enrolled. Published aggregate data from BRUIN pertaining to the cBTKi pretreated patients with MCL (N = 90 in the primary efficacy cohort) were identified and used for the current analysis. Reported efficacy outcomes of interest were ORR, CR, DOR, and PFS as assessed by an independent review committee, per Lugano 2014 criteria [18], as well as OS (median OS follow-up time 23.5 months). The primary efficacy cohort (hereafter referred simply as the BRUIN population) comprised more than 90% of responders to be followed for a least 9 months from onset of initial response to the data cutoff date and included patients enrolled to either the phase 1 dose-escalation portion (25 mg to 300 mg once daily) or phase 2 dose-expansion portion (200 mg once daily) of the trial, with 85.6% of patients (N = 77/90) receiving at least one dose of pirtobrutinib at 200 mg once daily.
Statistical Analysis
The relative treatment effects of brexu-cel and pirtobrutinib were estimated by comparing data from ZUMA-2 and BRUIN using both a pairwise naïve (unadjusted) approach and via a MAIC. The MAIC aimed to reduce the bias in the treatment effect estimates inherent in a naïve comparison by adjusting for differences in patient characteristics between the trials. The primary outcomes for analysis were ORR and CR as assessed by an independent (radiologic) review committee assessment, aligning with the primary endpoints of ZUMA-2 and BRUIN. Secondary outcomes of interest were PFS and DOR, both assessed by an independent (radiologic) review committee, as well as OS. Outcome definitions were comparable between trials (see supplementary appendix Table S2). The main analyses focused on the ZUMA-2 mITT population and scenario analyses were based on the ZUMA-2 ITT population. Within ZUMA-2, outcomes were measured from the date of brexu-cel infusion for the mITT population and from the date of trial enrollment for the ITT population. Data for pirtobrutinib were obtained from published reports of the BRUIN trial and Kaplan–Meier (KM) curves for OS, PFS, and DOR were digitized (DigitizeIt; http://www.digitizeit.de/). The underlying individual survival and censoring times for these outcomes were recreated from the extracted curves using a commonly accepted estimation algorithm [22].
For the MAIC, a logistic propensity score model was applied to estimate weights for the individual patients in ZUMA-2, such that the weighted mean of select baseline characteristics matched those observed in the BRUIN population. These weights were then applied to the ZUMA-2 population prior to estimating relative treatment effects. The degree of precision after weighting was expressed with the effective sample size statistic, which relates to the amount of overlap in the distribution of the covariates between the two trial populations [23]. Prior to the MAIC, a comprehensive list of baseline characteristics was ranked according to their prognostic relevance in the R/R MCL population by clinical experts in MCL. On the basis of this pre-specified ranking and data availability across both trials, the base-case model incorporated the top five most pertinent variables that were reported for at least 50% of patients in both ZUMA-2 and BRUIN: blastoid morphology, simplified MCL International Prognostic Index (sMIPI; a prognostic stratification tool specific to patients with advanced-stage MCL), number of prior lines of therapy, disease stage, and prior autologous stem cell transplant (auto-SCT). The sMIPI score stratifies patients into low, intermediate, and high-risk groups based on four independent prognostic factors (age, performance status, lactate dehydrogenase levels, and white blood cell counts); therefore, the inclusion of sMIPI in the model aimed to reduce the between-study differences across all four individual components (including lactate dehydrogenase levels and white blood cell counts which were not reported in BRUIN). A sensitivity analysis additionally incorporated TP53 mutation status (which was missing for approximately 50% of patients in ZUMA-2 and 60% of patients in BRUIN) and Ki-67 proliferation index status (missing for 62% of patients in BRUIN), for a total of seven prognostic variables. Several relevant prognostic variables (i.e., response to prior cBTKi therapy, response to last therapy, and duration on prior BTKi therapy) could not be included in the model as these were not reported in the BRUIN trial.
For ORR and CR, relative treatment effects were estimated using a logistic regression analysis applied to weighted ZUMA-2 individual patient-level data and observed BRUIN aggregate level data, with results expressed as odd ratios (OR) along with 95% confidence intervals (CIs). For OS, PFS, and DOR, a Cox proportional hazards model was applied to weighted ZUMA-2 individual patient-level data and reconstructed individual patient-level data from BRUIN with results expressed as hazard ratios (HR) with 95% CIs. Note the proportional hazards assumption was assessed visually using plots of the log cumulative hazards and Schoenfeld residuals as well as with the Grambsch and Therneau test [24, 25].
The approach used for analysis was consistent with the methodological guidance provided in the National Institute of Health and Care Excellence Decision Support Unit Technical Support Document 18 [26]. In the MAIC, robust estimators of variance were employed to account for uncertainty in weighting. All analyses were performed using R version 4.2.1 (http://www.r-project.org/), with the ‘survival’ package used for comparisons of time-to-event outcomes.
Ethical Approval
Results presented in this article are based on a retrospective analysis of data from published studies, and therefore no institutional board review was required. Review boards at participating institutions approved the ZUMA-2 and BRUIN trials.
Results
Study Populations
Prior to matching, ZUMA-2 and BRUIN study populations were generally comparable at baseline in terms of the proportion of patients with more than three prior lines of therapy, bone marrow involvement, and male sex. Relative to the BRUIN study population, the ZUMA-2 study population had a higher proportion of patients (more than 5% difference) in each the following categories: blastoid morphology, low-risk sMIPI, stage IV disease, received prior auto-SCT, TP53 wild-type tumor, Ki-67 ≥ 30%, bulky disease, presence of extranodal disease, and received ibrutinib as prior cBTKi treatment (Table 1). Of note, TP53 mutation status was missing in 32/68 (47%) ZUMA-2 mITT patients, 38/74 (51%) ZUMA-2 ITT patients, and 54/90 (60%) BRUIN patients. Ki-67 proliferation index status was missing in 16/68 (24%) ZUMA-2 mITT patients, 22/74 (30%) ZUMA-2 ITT patients, and 56/90 (62%) BRUIN patients. Proportions of patients with Ki-67 proliferation and TP53 mutation used for weighted analyses were estimated on the basis of non-missing data.
Table 1.
Baseline characteristics of ZUMA-2 (mITT population) before and after matching to BRUIN
| Characteristic | Observed ZUMA-2 mITT (N = 68) | BRUIN (N = 90) | MAIC-adjusted ZUMA-2 mITT | ||
|---|---|---|---|---|---|
| Base-case modela ESS = 39.1 |
Sensitivity analysisb ESS = 16.5 |
||||
| ESS reduction (% of original sample size) | – | – | 42.5 | 75.8 | |
| Morphology | Blastoid | 25 | 9 | 9 | 9 |
| sMIPI | High risk | 14 | 22 | 22 | 22 |
| Intermediate risk | 44 | 56 | 56 | 56 | |
| Prior lines of therapy | > 3 | 37 | 34 | 34 | 34 |
| Disease stage | IV | 85 | 78 | 78 | 78 |
| Prior auto-SCT | Yes | 43 | 19 | 19 | 19 |
| TP53 mutation | Yes | 17c | 47c | 18* | 47 |
| Ki-67 index | ≥ 30% | 83 | 74d | 82* | 74 |
| Bulky disease | ≥ 10 cm | 10 | 3 | 11* | 18* |
| Bone marrow involvement | Yes | 55 | 51 | 59* | 70* |
| Extranodal disease | Yes | 56 | 39 | 56* | 65* |
| Prior ibrutinib | Yes | 85 | 66 | 90* | 91* |
| Sex | Male | 84 | 80 | 84* | 89* |
All values reported in percentages. Variables with an asterisk were not included in the indicated model
auto-SCT autologous stem cell transplant, ESS effective sample size, ITC indirect treatment comparison, MAIC matching-adjusted indirect comparison, mITT modified intention-to-treat, sMIPI simplified Mantle Cell Lymphoma International Prognostic Index
aIncluded five prognostic variables (blastoid morphology, sMIPI, number of prior lines of therapy, disease stage, and prior auto-SCT)
bIncluded seven prognostic variables (blastoid morphology, sMIPI, number of prior lines of therapy, disease stage, prior auto-SCT, TP53 mutation status, and Ki-67 proliferation index status)
cData missing for a high proportion of patients in both trials (47% of patients in ZUMA-2 and 60% of patients in BRUIN)
dData missing for a high proportion of patients in BRUIN (62% of patients)
After weights from the MAIC model were applied, all prognostic variables considered in the base case and sensitivity analysis were well balanced between the ZUMA-2 mITT population and the BRUIN population (Table 1). The effective sample size of the ZUMA-2 mITT population after weighting was 39.1 for the base case, a reduction of 42.5% of the original sample size. For the sensitivity analysis, the mITT population after weighting was 16.5, a reduction of 75.8% from the original sample size; this low effective sample size had an impact on the precision of the estimates and resulted in wider 95% CI, and therefore results from the sensitivity analysis should be interpreted with caution. Baseline characteristics for the ZUMA-2 ITT population before and after weighting as well as the effective sample size after weighting are provided in the Supplementary material Table S3.
Comparison of Outcomes
Relative treatment effects of brexu-cel (ZUMA-2 mITT) versus pirtobrutinib from the naïve indirect comparison and MAIC are illustrated in Fig. 1 for ORR and CR, and in Fig. 2 for DOR, PFS, and OS (also see Supplementary material Tables S4 and S5). Results were consistent between the naïve and adjusted comparisons. The proportional hazards assumption did not appear to be violated in any of the time-to-event outcome comparisons, suggesting that the estimated HRs for DOR, PFS, and OS of brexu-cel versus pirtobrutinib were constant over time.
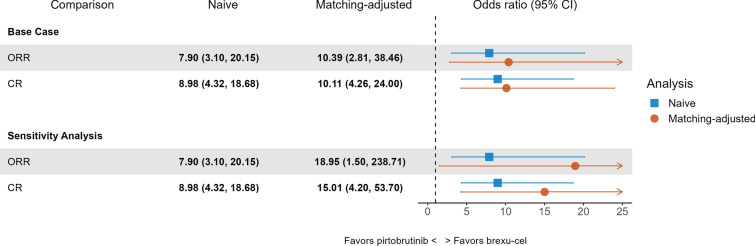
Fig. 1.
Odds ratios for ORR and CR for brexu-cel (ZUMA-2 mITT) versus pirtobrutinib (BRUIN). Across all comparisons, odds ratio estimates were statistically significant at a 0.05 level, indicating that brexu-cel was associated with higher odds of achieving ORR and CR compared with pirtobrutinib. Dashed vertical line indicates an odds ratio of 1. CI confidence interval, CR complete response, MAIC matching-adjusted indirect comparison, mITT modified intention-to-treat, ORR overall response rate
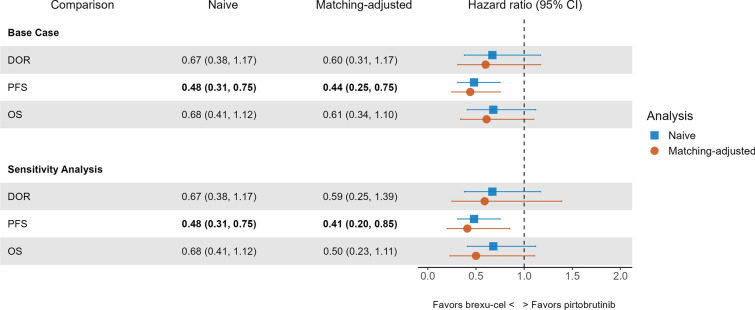
Fig. 2.
Hazard ratios for OS, PFS, and DOR for brexu-cel (ZUMA-2 mITT) versus pirtobrutinib (BRUIN). For PFS, hazard ratio estimates were statistically significant at a 0.05 level, indicating that brexu-cel improved PFS compared with pirtobrutinib. CI confidence interval, DOR duration of response, MAIC matching-adjusted indirect comparison, mITT modified intention-to-treat, OS overall survival, PFS progression-free survival
Primary Outcomes
The unadjusted ORR and CR rates were 91% and 68% respectively for brexu-cel compared to 57% and 19% respectively for pirtobrutinib. After weights from the base case model were applied, the adjusted ORR and CR rates were 93% and 70% respectively for brexu-cel. Results from the unadjusted and adjusted comparison suggested that brexu-cel was more efficacious than pirtobrutinib in terms of response rates with adjusted ORs of 10.39 (95% CI 2.81–38.46; p < 0.01) for ORR and 10.11 (95% CI 4.26–24.00; p < 0.01) for CR. Similarly, estimates from the sensitivity analysis which adjusted for seven prognostic variables suggested brexu-cel to be more efficacious than pirtobrutinib in terms of achieving a response with adjusted ORs of 18.95 (95% CI 1.50–238.71; p = 0.02) for ORR and 15.01 (95% CI 4.20–53.70; p < 0.01) for CR.
Secondary Outcomes
The relative treatment effect estimates obtained from the naïve comparison and MAIC for DOR had point estimates which trended in favor of brexu-cel when compared to pirtobrutinib, but the 95% CI crossed the bounds for statistical significance. The DOR KM curves for brexu-cel before and after matching the ZUMA-2 mITT population and the reconstructed DOR KM curves from pirtobrutinib are shown in Fig. 3a (see Fig. S1A for the sensitivity analysis). The adjusted DOR HR was 0.60 (95% CI 0.31–1.17; p = 0.13) in the base-case analysis and 0.59 (95% CI 0.25–1.39; p = 0.23) in the sensitivity analysis (Fig. 2).
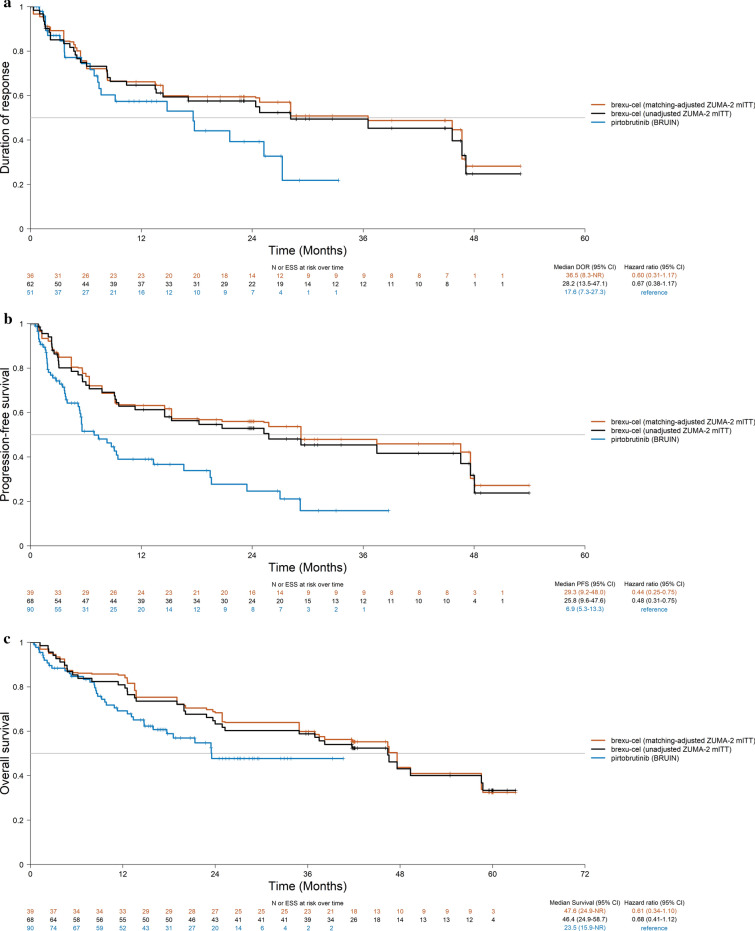
Fig. 3.
Base-case MAICs of brexu-cel (ZUMA-2 mITT) and pirtobrutinib (BRUIN) for a DOR, b PFS, and c OS. For ZUMA-2, the Kaplan–Meier curves were based on individual patient data whereas for BRUIN, published Kaplan–Meier curves for BRUIN were digitized and individual patient data were reconstructed using the Guyot et al. 2012 algorithm. Tick marks (+) indicate data censoring. CI confidence interval, DOR duration of response, ESS effective sample size, MAIC matching-adjusted indirect comparison, mITT modified intention-to-treat, OS overall survival, PFS progression-free survival
The PFS KM curves for brexu-cel before and after matching the ZUMA-2 mITT population and the reconstructed PFS KM curves from pirtobrutinib are shown in Fig. 3b (see Supplementary material Fig. S1B for the sensitivity analysis). HR estimates obtained from both the unadjusted and MAIC analyses suggested that brexu-cel was associated with a significant improvement in PFS compared to pirtobrutinib. The adjusted HR obtained was 0.44 (95% CI 0.25–0.75; p < 0.01) in the base-case analysis and 0.41 (95% CI 0.20–0.85; p = 0.02) in the sensitivity analysis (Fig. 2).
The OS KM curves for brexu-cel before and after matching in the ZUMA-2 mITT population and the reconstructed OS KM curves from pirtobrutinib are shown in Fig. 3c (see Supplementary material Fig. S1C for the sensitivity analysis). HR point estimates obtained from both the naïve comparison and MAIC trended in favor of brexu-cel over pirtobrutinib, but the confidence intervals crossed the bounds for statistical significance. The adjusted HR obtained was 0.61 (95% CI 0.34–1.10; p = 0.10) in the base-case analysis and 0.50 (95% CI 0.23–1.11; p = 0.09) in the sensitivity analysis (Fig. 2). OS rates at landmark time points (6-month intervals) are reported in Table 2. The 24-month OS rate for ZUMA-2 mITT population treated with brexu-cel in the base case analysis was 73.5% compared to 47.7% in BRUIN MCL population treated with pirtobrutinib.
Table 2.
Overall survival rates of brexu-cel (ZUMA-2 mITT population) and pirtobrutinib at landmark time-points
| Time-points | Overall survival rate, % (95% CI) | |||
|---|---|---|---|---|
| ZUMA-2 mITT unadjusted | ZUMA-2 mITT base case | ZUMA-2 mITT sensitivity analysis | BRUINa | |
| Month 6 | 86.4 (76.4–97.6) | 87.4 (73.5–100) | 85.3 (77.3–94.1) | 84.7 (77.3–92.7) |
| Month 12 | 85.3 (75.3–96.6) | 86.3 (72.3–100) | 80.9 (72.1–90.8) | 69.1 (59.7–80.0) |
| Month 18 | 75.3 (63.2–89.6) | 80.7 (65.7–99.3) | 73.5 (63.8–84.8) | 58.9 (48.7–71.2) |
| Month 24 | 68.3 (55.6–84.0) | 73.5 (57.4–94.2) | 63.2 (52.8–75.8) | 47.7 (36.6–62.2) |
| Month 30 | 63.9 (50.7–80.7) | 71.8 (55.5–92.9) | 60.3 (49.7–73.1) | 47.7 (36.6–62.2) |
| Month 36 | 59.8 (46.3–77.4) | 63.3 (44.7–89.6) | 58.8 (48.2–71.8) | 47.7 (36.6–62.2) |
CI confidence interval, mITT modified intention-to-treat
aOverall survival Kaplan–Meier curve for BRUIN were digitized and individual patient data were reconstructed using the Guyot et al. 2012 algorithm. Overall survival rates for BRUIN were estimated from the reconstructed Kaplan–Meier curve
Scenario Analysis
For the scenario using the ZUMA-2 ITT population, findings were consistent with those observed in the main analysis using the ZUMA-2 mITT population for all outcomes (Supplementary material Tables S6, S7, Figs. S4 and S5). Brexu-cel was associated with statistically significant benefits in ORR, CR, and PFS compared to pirtobrutinib on the basis of both the base-case and sensitivity analysis models. Although point estimates continued to trend in favor of brexu-cel for DOR and OS, statistical significance was not met.
Discussion
In the pre-CAR T cell therapy era, the prognosis of patients with R/R MCL who discontinued cBTKi therapy as a result of disease progression or intolerance was poor following treatment with conventional subsequent therapies. Use of these non-curative interventions aimed to palliate and prolong survival, though median OS typically remained around 1 year or less in the post-BTKi setting [10, 27, 28]. Advancements in treatment options such as CAR T cell therapy and non-covalent BTKi therapy have substantially improved patient outcomes. In the ZUMA-2 trial evaluating patients with MCL who failed prior BTKi, brexu-cel therapy for the mITT cohort was associated with a median OS of 46.4 months (95% CI 24.9–58.7) at 4-year follow-up. At 2-year follow-up in the BRUIN trial evaluating patients with MCL who failed or were intolerant to prior BTKi, median OS was 23.5 months (95% CI 15.9–NE) in the post-cBTKi therapy patient cohort [20]. Given the therapeutic needs experienced by patients with R/R MCL post-cBTKi therapy and the potential benefits in clinical outcomes associated with these new treatment options, it is important to understand their relative clinical efficacy.
In the absence of direct comparative evidence, this study presents an indirect comparison of the treatment effects of brexu-cel and pirtobrutinib using MAIC methodology to adjust for study-level differences between the ZUMA-2 and BRUIN single-arm trials where possible. In the base-case MAIC, the odds of ORR and CR were significantly better for brexu-cel-infused patients than those treated with pirtobrutinib therapy. Brexu-cel was also associated with significant improvements in PFS compared to pirtobrutinib. Although HR point estimates trended in favor of brexu-cel regarding DOR and OS, differences in both outcomes crossed the boundary for statistical significance. DOR among patients achieving a CR (n = 46 [67.6%] complete responders in ZUMA-2 vs n = 17 [18.9%] complete responders in BRUIN) or duration of CR was not included for analysis as data were not reported in BRUIN. It is important to highlight the relatively shorter median follow-up for OS in BRUIN (23.5 months in BRUIN versus 47.5 months in ZUMA-2). As an MAIC cannot be considered equivalent to a randomized controlled study, the ability to detect statistically significant differences for outcomes with low starting sample sizes and small number of events is limited. As such, given the high degree of censoring in BRUIN, an updated analysis incorporating longer follow-up data containing more events from BRUIN could provide more reliable treatment effect estimates. Note, outcomes for a larger sample of patients from BRUIN than used for this analysis were recently presented at ASH; however, the median follow-up was only 14.7 months [21].
Treatment with CAR T cell therapy involves a multistep process that begins with leukapheresis to obtain leukocytes for the manufacturing of brexu-cel. Although understanding treatment efficacy among patients who received brexu-cel infusion (the mITT population) is important, it is similarly critical to understand efficacy among those who initiate leukapheresis (the ITT population) as hazard rates in the pre-infusion period may not be comparable between the mITT and ITT populations. A total of six patients who underwent leukapheresis did not received infusion (n = 3 deaths, n = 1 full consent withdrawal, n = 1 adverse event, and n = 1 not meeting inclusion criteria for infusion). Findings from the main analysis (based on ZUMA-2 mITT population) and scenario analysis (based on ZUMA-2 ITT population) were consistent, suggesting that the results were not sensitive to which ZUMA-2 population sets were used for analysis. In addition, subgroup analyses restricted to BRUIN patients (85.6%) who received the recommended phase 2 pirtobrutinib dose of 200 mg at study start were not performed as subgroup data by treatment dose were not reported in BRUIN.
Considered collectively, the results of the MAIC provide important insights to clinical decision makers when determining the optimal approach to management of R/R MCL post-cBTKi therapy. Efficacy and toxicity outcomes in real-world settings identified from a systematic literature review and the US Lymphoma CAR T Consortium reported findings that are consistent with those of ZUMA-2 [28–33]. In an adjusted comparison using inverse probability weighting between ZUMA-2 and SCHOLAR-2, brexu-cel was associated with improved OS compared to non-CAR T cell standard of care (HR 0.38, 95% CI 0.23–0.61) [34]. Efficacy of CAR T cell therapy should be considered alongside potential class-specific toxicities, such as immunologic effector cell-associated cytokine release syndrome and neurotoxicity, among other potential constraints to successful CAR T cell therapy [35]. To our knowledge, pirtobrutinib has yet to be evaluated in the real-world setting; however, the therapy is currently undergoing phase 3 evaluation in the cBTKi-naive setting of MCL, with comparison to ibrutinib, acalabrutinib, or zanubrutinib (BRUIN MCL-321 trial; NCT04662255). The findings of this trial will provide further information relevant for clinical treatment decision-making for R/R cBTKi-treated MCL.
Although outside the scope of the current study, the impact of brexu-cel and pirtobrutinib on health-related quality of life and economic outcomes is also of interest from a decision-making perspective. Such analyses would therefore be of value in the future should sufficiently detailed data be made available to facilitate them. In addition, efficacy of CAR T cell therapy should be considered alongside potential class-specific toxicities, such as immunologic effector cell-associated cytokine release syndrome and neurotoxicity, among other potential constraints to successful CAR T cell therapy. In ZUMA-2, 14.7% of patients experienced grade ≥ 3 cytokine release syndrome, 30.9% of patients experienced grade ≥ 3 neurological events, and 85.3% of patients experienced neutropenia while grade ≥ 3 adverse events were less frequent with pirtobrutinib in BRUIN, with infections (17.1%) and neutropenia (13.4%) being the most frequent grade ≥ 3 adverse events.
Limitations
Some potential limitations that may influence the findings of this study should be recognized. In general, analysis of data from single-arm or non-comparative studies is associated with uncertainty regarding any unknown or unmeasured prognostic factors and effect modifiers that are not included in the model. As such, although every effort was taken to ensure a robust approach to prognostic factor selection, the possibility of residual confounding variables cannot be ruled out. Similarly, in the absence of individual patient-level data for the BRUIN trial, it was challenging to quantify the extent of residual bias in the treatment effect estimates; therefore, some confounding variables may remain unbalanced. Per clinician input, TP53 mutation status, Ki-67 proliferation index, response to prior cBTKi, response to last therapy, and duration on prior cBTKi therapy were identified as covariates of high importance. However, given data availability, these variables could not be evaluated in the base-case analysis and only TP53 mutation status and Ki-67 proliferation index ≥ 30% were explored in a sensitivity analysis.
It is also important to highlight that other observed differences between the two phase 2 trials could not be adjusted for in the MAIC. Mostly notably, reasons for prior BTKi discontinuation varied across trials. While 95.6% of patients in ZUMA-2 had previously discontinued BTKi as a result of disease progression and 4.4% because of adverse events, 82.2% of BRUIN patients had discontinued prior BTKi to disease progression, with the remaining discontinuing as a result of intolerance/toxicity (13.3%) or other reasons (4.4%) without disease progression [36]. As patients who discontinued BTKi as a result of intolerance may be associated with better clinical outcomes than those who discontinued because of progression, the treatment effect estimates from the current analyses are considered conservative [36, 37]. Additionally, differences in subsequent therapy between the two trials may also impact OS results as 17 (18.9%) of patients in BRUIN went on to receive subsequent CAR T cell therapy after pirtobrutinib. In the BRUIN trial, 4.4% of patients received prior allogeneic stem cell transplant and 4.4% received prior CAR T cell therapy; in ZUMA-2, such patients were ineligible for enrollment. Previous SCT and CAR T cell therapy are unknown prognostic factors in the R/R MCL, post-BTKi setting. Still, given the small numbers of such patients in this study, it is unlikely that treatment effect estimates were impacted. Other discrepancies in the patient selection criteria that may have introduced bias into the trial comparisons were the exclusion of patients with possible need for urgent oncological therapy in ZUMA-2 which was not mentioned as an exclusion criterion in BRUIN, inclusion criteria of Eastern Cooperative Oncology Group performance status score of 0–1 in ZUMA-2 compared to 0–2 in BRUIN (although only one patient with a score of 2 was enrolled), inclusion criteria of creatine clearance being ≥ 60 cc/min in ZUMA-2 and ≥ 30 mL/min in BRUIN, exclusion of patients with atrial fibrillation in ZUMA-2 but not in BRUIN, and exclusion criteria of patients with history of clinically significant cardiac disease within 12 months in ZUMA-2 versus within 6 months in BRUIN.
Conclusion
The results of naïve and MAIC analyses suggest that brexu-cel is more efficacious for ORR, CR, and PFS than pirtobrutinib in the treatment of R/R MCL post-cBTKi therapy, providing clinical benefits for these patients. The differences in OS and DOR crossed the bounds for statistical significance although point estimates trended in favor of brexu-cel. The consistency across these analyses and high concordance in sensitivity analyses provide compelling evidence for the validity and robustness of the findings. Given the need for model assumptions, limitations related to covariate adjustment, and the relatively modest size of the trial cohorts, some caution should be used in interpreting the results. Still, the outcomes suggest that brexu-cel remains an important standard treatment option and may be the preferred therapy for patients with R/R MCL who have previously received cBTKi therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance.
The authors wish to thank Dana L. Anger of WRITRIX Medical Communications, Inc., for medical writing support. Support for this assistance was provided by Kite, a Gilead Company.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICJME) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contribution
Study design and conceptualization: Jenny M.H. Chen, Ina Zhang, James J. Wu, Sally W. Wade, and Sam Keeping; Data analysis: Jenny M.H. Chen, Ina Zhang, Dylan Maciel, Keith Chan, and Sam Keeping; Data interpretation: All authors. All authors reviewed, revised, and approved the final manuscript for submission.
Funding
This study, including the journal’s Rapid Service and Open Access Fees, was sponsored by Kite, a Gilead Company.
Data Availability
The datasets generated and analyzed during this study can be made available upon reasonable request to the corresponding author, and decisions regarding data sharing will be made on a case-by-case basis considering data protection and other applicable regulations.
Declarations
Conflict of Interest
Gilles Salles has received consulting fees from Abbvie, Celgene/BMS, Epizyme, Genmab, Incyte, Janssen, Kite, a Gilead Company, Loxo, Milteniy, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, and Takeda; has received payment or honoraria for speaking in symposium from Bayer, Epizyme, and Regeneron; has participated on a Data Safety Monitoring board or Advisory Board for Beigene; and has stock or stock options from Owkin. Jenny M.H. Chen, Ina Zhang, Dylan Maciel, Keith Chan, and Sam Keeping are employees of PRECISIONheor, funded by Kite, a Gilead Company for this study. Fabio Kerbauy declares no competing interests. James J. Wu is an employee of Kite, a Gilead Company; has received honoraria from the Patient-Centered Outcomes Research Institute (PCORI), both as a member of the Rare Disease Advisory Panel, and as a grants reviewer for the Improving Methods Program; has received travel/meeting support from Kite, a Gilead Company, and Amgen; owns stock in Gilead Sciences, Amgen, Abbott, AbbVie, Pfizer, Roche, Curis, Avid Biosciences, Evofem, Lensar, VBI Vaccines, and Viracta Therapeutics. Sally W. Wade has received consulting fees from Kite, a Gilead Company, Abbvie, and Johnson & Johnson. Fabio R. Kerbauy declares no competing interests. Ana Nunes is an employee of Gilead Sciences Europe; own stocks in Gilead Sciences and Amgen; and has received support for attending meetings and/or travel from Kite, a Gilead Company. Chaoling Feng is an employee of Kite, A Gilead Company; own stocks in Gilead Sciences, Boston Scientific, and has received support for attending meetings and/or travel from Kite, a Gilead Company. Ioana Kloos is an employee of Kite, A Gilead Company; own stocks in Gilead Sciences; and has been compensated for a leadership role (such as officer or member of a board of directors) for Kite, A Gilead Company. Weimin Peng is an employee of Kite, a Gilead Company; and owns stock in Gilead Sciences. Julia T. Snider is an employee of Kite, a Gilead Company; owns stock in Gilead Sciences. Bijal Shah has received consultant and education fees from Amgen, Pfizer, Novartis, BMS/Celgene/Juno, Kite, a Gilead company, Precision Biosciences, Jazz, Beigene, Adaptive, Century Therapeutics, and Autolus; and clinical trial grants from Kite, a Gilead company, Jazz, and Servier.
Ethical Approval
Results presented in this article are based on a retrospective analysis of data from published studies, and therefore no institutional board review was required. Review boards at participating institutions approved the ZUMA-2 and BRUIN trials.
References
- 1.American Cancer Society. Types of B-cell lymphoma 2019 [updated January 29, 2019]. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/b-cell-lymphoma.html. Accessed 2023 Aug 27.
- 2.Inwards DJ, Witzig TE. Initial therapy of mantle cell lymphoma. Ther Adv Hematol. 2011;2(6):381–392. doi: 10.1177/2040620711412418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26(2):e233–e240. doi: 10.3747/co.26.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Schuster SJ, Phillips T, et al. Observational study of lenalidomide in patients with mantle cell lymphoma who relapsed/progressed after or were refractory/intolerant to ibrutinib (MCL-004) J Hematol Oncol. 2017;10(1):171. doi: 10.1186/s13045-017-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKay P, Leach M, Jackson B, Robinson S, Rule S. Guideline for the management of mantle cell lymphoma. Br J Haematol. 2018;182(1):46–62. doi: 10.1111/bjh.15283. [DOI] [PubMed] [Google Scholar]
- 6.Eyre TA, Walter HS, Iyengar S, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104(2):e68–e71. doi: 10.3324/haematol.2018.198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCulloch R, Visco C, Eyre TA, et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br J Haematol. 2020;189(4):684–688. doi: 10.1111/bjh.16416. [DOI] [PubMed] [Google Scholar]
- 8.Jain P, Kanagal-Shamanna R, Zhang S, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578–587. doi: 10.1111/bjh.15567. [DOI] [PubMed] [Google Scholar]
- 9.Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 10.Hess G, Dreyling M, Oberic L, et al. Real-world experience among patients with relapsed/refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor failure in Europe: the SCHOLAR-2 retrospective chart review study. Br J Haematol. 2022;202(4):749–759. doi: 10.1111/bjh.18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Munoz J, Goy A, et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. 2023:JCO2102370. [DOI] [PMC free article] [PubMed]
- 13.US Food and Drug Administration. TECARTUS (brexucabtagene autoleucel) prescribing information 2021 [updated October 2021]. https://www.fda.gov/media/140409/download?attachment. Accessed 2023 Aug 27.
- 14.European Medicines Agency. Tecartus: EPAR—Product information 2023 [updated November 28, 2023]. https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus. Accessed 2023 Aug 27.
- 15.Wang ML, Jurczak W, Zinzani PL, et al. Pirtobrutinib in covalent BTK-inhibitor pre-treated mantle cell lymphoma. J Clin Oncol. 2023:101200JCO2300562. [DOI] [PMC free article] [PubMed]
- 16.US Food and Drug Administration. JAYPIRCA (pirtobrutinib) prescribing information 2023 [updated January 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216059Orig1s000Corrected_lbl.pdf. Accessed 2023 Aug 27.
- 17.European Medicines Agency. Jaypirca : EPAR—Public assessment report 2023 [updated November 20, 2023; cited 2023 December 4]. https://www.ema.europa.eu/en/medicines/human/EPAR/jaypirca.
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for Initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ML, Shah NN, Jurczak W, et al. Efficacy of pirtobrutinib in covalent BTK-inhibitor pre-treated relapsed/refractory mantle cell lymphoma: additional patients and extended follow-up from the phase 1/2 BRUIN study. Blood. 2022;140(Supplement 1):9368–9372. doi: 10.1182/blood-2022-159425. [DOI] [Google Scholar]
- 20.Shah NN, Jurczak W, Zinzani PL, et al. Pirtobrutinib in covalent BTK-inhibitor (cBTKi) pre-treated mantle cell lymphoma (MCL): updated results and subgroup analysis from the phase 1/2 BRUIN study with 2 years of survival follow-up. In: Presented at American Society of clinical oncology 59th annual meeting; Chicago, IL; June 2–6, 2023. 2023.
- 21.Cohen JB, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed/refractory (R/R) mantle cell lymphoma (MCL) patients with prior cBTKi: safety and efficacy including high-risk subgroup analyses from the phase 1/2 BRUIN study. Blood. 2023;142:981. doi: 10.1182/blood-2023-181627. [DOI] [Google Scholar]
- 22.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Dec Mak. 2018;38(2):200–211. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 25.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990;77(1):147–160. doi: 10.1093/biomet/77.1.147. [DOI] [Google Scholar]
- 26.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE. 2016.
- 27.Dreyling M, Shah B, Wu JJ, et al. SA29 efficacy outcomes following treatment with Bruton tyrosine kinase inhibitors (BTKI) for relapsed/refractory mantle cell lymphoma (R/R MCL): a literature-based meta-analysis. Value Health. 2022;25(7):S609–S610. doi: 10.1016/j.jval.2022.04.1695. [DOI] [Google Scholar]
- 28.Dreyling M, Shah B, Wu J, et al. MCL-373 unmet need in relapsed/refractory (RR) mantle cell lymphoma (MCL) post-Bruton tyrosine kinase inhibitor (BTKi): systematic literature review and meta-analysis. Clin Lymphoma Myeloma Leuk. 2023;23:S461–S462. doi: 10.1016/S2152-2650(23)01377-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Jain P, Locke FL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-care practice: results from the US Lymphoma CAR T Consortium. J Clin Oncol. 2023;41(14):2594–2606. doi: 10.1200/JCO.22.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambhampati S, Ahmed N, Hamadani M, et al. Real-world outcomes of brexucabtagene autoleucel (brexu-cel) for relapsed or refractory (R/R) mantle cell lymphoma (MCL): a CIBMTR subgroup analysis of high-risk characteristics. Blood. 2023;142:107. doi: 10.1182/blood-2023-179269. [DOI] [Google Scholar]
- 31.Herbaux C, Bret C, Di Blasi R, et al. Kte-X19 in relapsed or refractory mantle-cell lymphoma, a “real-life” study from the Descar-T Registry and Lysa Group. Blood. 2021;138:743. doi: 10.1182/blood-2021-148626. [DOI] [Google Scholar]
- 32.Hess G, Vucinic V, Rejeski K, et al. Real world results of brexucabtagene autoleucel for patients with relapsed/refractory mantle cell lymphoma-first German/Swiss analysis. Blood. 2023;142:4394. doi: 10.1182/blood-2023-182415. [DOI] [Google Scholar]
- 33.O'Reilly MA, Sanderson R, Wilson W, et al. Brexucabtagene autoleucel for relapsed/refractory mantle cell lymphoma: real-world outcomes In the United Kingdom. Blood. 2022;140(Suppl 1):7519–7521. doi: 10.1182/blood-2022-165031. [DOI] [Google Scholar]
- 34.Hess G, Dreyling M, Oberic L, et al. Indirect treatment comparison of brexucabtagene autoleucel (ZUMA-2) versus standard of care (SCHOLAR-2) in relapsed/refractory mantle cell lymphoma. Leukemia Lymphoma. 2024;65(1):14–25. [DOI] [PubMed]
- 35.Huang Z, Chavda VP, Bezbaruah R, Dhamne H, Yang D-H, Zhao H-B. CAR T-cell therapy for the management of mantle cell lymphoma. Mol Cancer. 2023;22(1):1–19. doi: 10.1186/s12943-023-01755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen JB, Shah NN, Jurczak W, et al. 981 Pirtobrutinib in relapsed/refractory (R/R) mantle cell lymphoma (MCL) patients with prior cBTKi: safety and efficacy including high-risk subgroup analyses from the phase 1/2 BRUIN Study. American Society of Hematology Annual Meeting; 2023.
- 37.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during this study can be made available upon reasonable request to the corresponding author, and decisions regarding data sharing will be made on a case-by-case basis considering data protection and other applicable regulations.