Abstract
Introduction
Daratumumab plus lenalidomide and dexamethasone (D-Rd) and bortezomib plus lenalidomide and dexamethasone (VRd) are commonly used treatment combinations for transplant-ineligible (TIE) patients with newly diagnosed multiple myeloma (NDMM). D-Rd and VRd demonstrated superior efficacy relative to lenalidomide and dexamethasone (Rd) in the MAIA and SWOG S0777 trials, respectively, but have not been compared directly in a head-to-head trial. Naïve comparisons of efficacy across the two trials may be biased because MAIA enrolled only TIE patients (median age 73 years), whereas SWOG S0777 enrolled both TIE patients and transplant-eligible patients who chose to defer/refuse frontline stem cell transplantation (median age 63 years). The present study compared progression-free survival (PFS) in TIE patients with NDMM treated with D-Rd versus VRd based on an adjusted indirect treatment comparison (ITC) that leveraged individual patient-level data from MAIA and SWOG S0777.
Methods
Harmonized inclusion/exclusion criteria (including age ≥ 65 years as a proxy for transplant ineligibility) and propensity-score weighting were used to balance the trial populations on measured baseline characteristics. After differences in trial populations were adjusted for, an anchored ITC was performed wherein within-trial PFS hazard ratios (HRs) for D-Rd versus Rd and VRd versus Rd were estimated and used to make indirect inference about PFS for D-Rd versus VRd.
Results
PFS HRs were 0.52 (95% confidence interval [CI] 0.41–0.67) for D-Rd versus Rd based on MAIA data, 0.88 (95% CI 0.63–1.23) for VRd versus Rd based on SWOG S0777 data, and 0.59 (95% CI 0.39–0.90) for the Rd-anchored ITC of D-Rd versus VRd. Sensitivity and subgroup analyses produced results consistent with the primary results.
Conclusion
This anchored ITC demonstrated a greater PFS benefit for D-Rd versus VRd in TIE patients with NDMM. In the absence of head-to-head trials comparing D-Rd and VRd, the present trial may help inform treatment selection in this patient population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02807-y.
Keywords: Daratumumab, Indirect treatment comparison, Newly diagnosed multiple myeloma, Progression-free survival, Transplant ineligible
Plain Language Summary
Multiple drug combinations can be used to treat patients with newly diagnosed multiple myeloma (NDMM) who are not eligible for a stem cell transplant. Two of these combinations—daratumumab plus lenalidomide and dexamethasone (D-Rd) and bortezomib plus lenalidomide and dexamethasone (VRd)—have each been studied in clinical trials (MAIA and SWOG S0777) against the combination of lenalidomide plus dexamethasone (Rd), but D-Rd and VRd have not been compared directly in a head-to-head clinical trial. Our study used data from the MAIA and SWOG S0777 trials to indirectly compare outcomes observed with D-Rd and VRd. For this indirect comparison between D-Rd and VRd, we first made adjustments to the patient populations of each trial to make them more similar to each other; this helped to make sure any differences we saw in treatment outcomes between D-Rd and VRd would not be because of differences in the characteristics of the patients who participated in the trials. After we made these adjustments to the patient populations of each trial, both D-Rd and VRd lowered the risk of disease progression or death compared with Rd alone. However, when indirectly compared in our study, D-Rd lowered the risk of disease progression or death by 41% compared with VRd. As data directly comparing treatment outcomes for D-Rd and VRd are not available, this indirect comparison can contribute to the information used to make treatment decisions for patients with NDMM who are not eligible for a stem cell transplant.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-024-02807-y.
Key Summary Points
| Why carry out this study? |
| Daratumumab plus lenalidomide and dexamethasone (D-Rd) and bortezomib plus lenalidomide and dexamethasone (VRd) have each shown superior efficacy, relative to lenalidomide and dexamethasone alone, in patients with newly diagnosed multiple myeloma (NDMM) in the MAIA and SWOG S0777 trials, respectively. |
| D-Rd and VRd have not been compared directly in a head-to-head trial; therefore, we compared progression-free survival (PFS) with D-Rd and VRd in transplant-ineligible (TIE) patients with NDMM, leveraging individual patient-level data from both trials and adjusting for differences in trial inclusion/exclusion criteria and baseline patient characteristics. |
| What was learned from the study? |
| Results of the adjusted indirect treatment comparison indicate that PFS was superior for D-Rd relative to VRd in TIE patients with NDMM. |
| These results may help to better inform treatment selection in TIE patients with NDMM. |
Introduction
Systemic therapy options for transplant-ineligible (TIE) patients with newly diagnosed multiple myeloma (NDMM) include daratumumab plus lenalidomide and dexamethasone (D-Rd) and bortezomib plus lenalidomide and dexamethasone (VRd), both of which are recommended in key treatment guidelines, such as the European Hematology Association and the European Society for Medical Oncology Clinical Practice Guidelines [1] and the NCCN Clinical Practice Guidelines in Oncology1 (NCCN Guidelines®) for Multiple Myeloma [2]. Evidence supporting these regimens comes from the MAIA and Southwest Oncology Group (SWOG) S0777 trials, respectively. In the updated analysis of the phase 3 MAIA trial, with a median follow-up of 64.5 months, D-Rd showed a significant progression-free survival (PFS) benefit versus lenalidomide plus dexamethasone (Rd) alone (hazard ratio [HR] 0.55; 95% confidence interval [CI] 0.45–0.67; P < 0.0001) [3]. In the phase 3 SWOG S0777 trial, VRd similarly prolonged PFS versus Rd alone (HR 0.712; 96% Wald CI 0.56–0.906; 1-sided P = 0.0018) at a median follow-up of 55 months [4].
While D-Rd and VRd have demonstrated superior efficacy relative to Rd alone in patients with NDMM [3–6], they have not been compared in a head-to-head trial. A naïve cross-trial comparison of efficacy across the MAIA and SWOG S0777 trials may be biased as a result of differences in the patient populations enrolled in each trial. Notably, while the MAIA trial enrolled only TIE patients with NDMM (D-Rd group, median age 73 years [interquartile range 70–78]) [5], the SWOG S0777 trial enrolled both patients with NDMM who were TIE, as well as transplant-eligible patients who chose to defer or refuse a frontline transplant (VRd group, median age 63 years [interquartile range 56–70]) [4]. In the present study, individual patient-level data from the MAIA and SWOG S0777 trials were leveraged to perform an anchored indirect treatment comparison (ITC) of D-Rd versus VRd, adjusting for differences in trial inclusion/exclusion criteria and baseline patient characteristics.
Methods
Data Sources and Study Design
Individual patient-level data were obtained from the phase 3, global, randomized MAIA trial (ClinicalTrials.gov identifier NCT02252172) based on a median follow-up of 64.5 months, and from the phase 3, USA-based, randomized SWOG S0777 trial (ClinicalTrials.gov identifier NCT00644228) based on the primary data cut with a median follow-up of 55 months [3, 4]. Relative treatment effects across the two trials were compared using an anchored ITC design (Fig. S1 in the electronic supplementary material) with matched patient eligibility criteria (Fig. 1). Additional details are provided below in the “Statistical Methods” section.
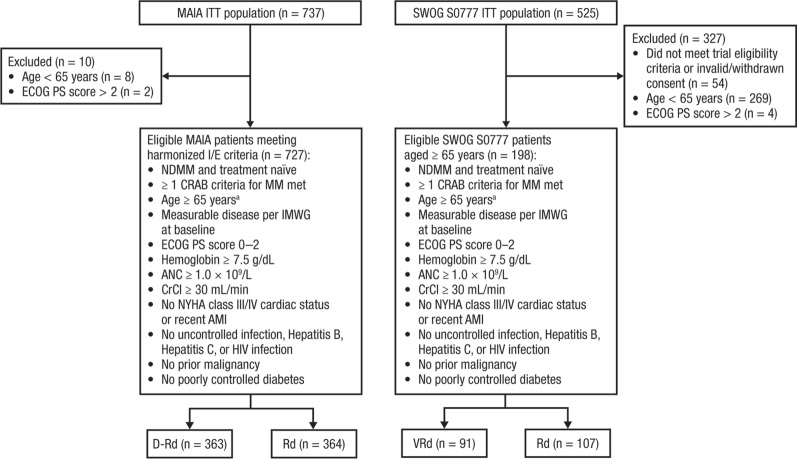
Fig. 1.
Selection of eligible patients from the MAIA and SWOG S0777 trials with TIE NDMM. SWOG Southwest Oncology Group, TIE transplant ineligible, NDMM newly diagnosed multiple myeloma, ITT intent-to-treat, ECOG PS Eastern Cooperative Oncology Group performance status, I/E inclusion/exclusion, CRAB calcium elevation, renal impairment, anemia, bone involvement, MM multiple myeloma, IMWG International Myeloma Working Group, ANC absolute neutrophil count, CrCl creatinine clearance, NYHA New York Heart Association, AMI acute myocardial infarction, HIV human immunodeficiency virus, D-Rd daratumumab plus lenalidomide/dexamethasone, Rd lenalidomide/dexamethasone, VRd bortezomib plus lenalidomide/dexamethasone, Rd lenalidomide/dexamethasone. aAge ≥ 65 years used as a proxy for transplant ineligibility. In the MAIA trial, which enrolled patients considered ineligible for transplant because of age ≥ 65 years or comorbidities precluding transplant, 99% of patients were aged ≥ 65 years at enrollment. In the SWOG S0777 trial, which enrolled patients without intent for immediate transplant (including TIE and transplant-eligible patients), 202 of 471 (43%) patients eligible for analysis were aged ≥ 65 years at enrollment
MAIA Trial
The MAIA trial enrolled patients aged ≥ 18 years with NDMM who were ineligible for high-dose chemotherapy with autologous stem cell transplantation (ASCT) because of age (≥ 65 years) or the existence of adverse comorbidities, satisfied CRAB criteria (C = calcium elevation; R = renal impairment; A = anemia; B = bone involvement) for multiple myeloma, had measurable disease, and had an Eastern Cooperative Oncology Group (ECOG) performance status score of ≤ 2. Patients were enrolled from March 2015 through January 2017, across 176 sites in 14 countries globally [5]. Patients were randomized 1:1 to receive D-Rd or Rd [5]. Patients in both treatment groups received 28-day cycles of orally administered lenalidomide (25 mg on days 1–21 of each cycle) and orally administered dexamethasone (40 mg on days 1, 8, 15, and 22 of each cycle). Patients in the D-Rd group additionally received intravenously administered daratumumab (16 mg/kg, once weekly during cycles 1–2, once every 2 weeks in cycles 3–6, and once every 4 weeks thereafter). Treatment was given until disease progression or unacceptably toxicity [5].
SWOG S0777 Trial
The SWOG S0777 trial enrolled patients with NDMM who were ineligible or without intent to receive an ASCT as part of first-line therapy, satisfied CRAB criteria, had measurable disease, and had an ECOG performance status score of 0 to 3. Patients were enrolled from participating SWOG and National Clinical Trial Network (NCTN) institutions throughout the USA [4]. Patients were randomized 1:1 to receive VRd or Rd alone [4]. The Rd regimen was given as six 28-day cycles, in which patients received orally administered lenalidomide (25 mg on days 1–21 of each cycle) and orally administered dexamethasone (40 mg on days 1, 8, 15, and 22 of each cycle). The VRd regimen was given as eight 21-day cycles, in which patients received intravenously administered bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11 of each cycle) combined with orally administered lenalidomide (25 mg on days 1–14 of each cycle) and dexamethasone (20 mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of each cycle). Treatment was given until disease progression or unacceptable toxicity [4]. Individual patient-level data from the SWOG S0777 trial were obtained from the NCTN/National Cancer Institute Community Oncology Research Program (NCORP) Data Archive of the National Cancer Institute’s (NCI) NCTN.
Harmonized Inclusion/Exclusion Criteria
Harmonized key inclusion/exclusion criteria were applied to align the trial populations, allowing for treatment effects to be estimated in a similar target patient population. The harmonized inclusion criteria applied for the present study included NDMM, age ≥ 65 years (a proxy for transplant ineligibility), symptomatic disease (≥ 1 CRAB criteria satisfied), measurable disease per International Myeloma Working Group criteria, ECOG performance status score ≤ 2, hemoglobin ≥ 7.5 g/dL, absolute neutrophil count ≥ 1.0 × 109/L, and creatinine clearance ≥ 30 mL/min. Key exclusion criteria included New York Heart Association class III/IV cardiac status or recent acute myocardial infarction, uncontrolled infection, human immunodeficiency virus infection, hepatitis B or C infection, prior cancer, or poorly controlled diabetes (Fig. 1; Table S1 in the electronic supplementary material).
Because the SWOG S0777 trial included a mixed population of patients without intent for immediate ASCT, age ≥ 65 years was used as a proxy for transplant ineligibility [7]. For consistency, the age ≥ 65 years restriction was also applied to the MAIA trial population, 99% of whom were aged ≥ 65 years at enrollment.
Baseline Patient Characteristics
As explained in more detail below in the “Statistical Methods” section, key baseline patient characteristics were identified for cross-trial covariate adjustment based on their potential role as treatment-effect modifiers. These baseline patient characteristics included age, sex, International Staging System disease stage, ECOG performance status score, hemoglobin, estimated glomerular filtration rate, lactate dehydrogenase (LDH), and cytogenetic risk, with high risk defined as the presence of ≥ 1 high-risk cytogenetic abnormality (del17p, t[14;16], or t[4;14]).
Outcomes
The primary outcome was PFS, defined as the time from treatment randomization to disease progression or death, whichever occurred first. Progressive disease was determined in accordance with the International Myeloma Working Group criteria [8, 9]. In the primary analysis of PFS in the MAIA trial, patients were censored if subsequent therapy was initiated prior to disease progression; however, this was not done in the SWOG S0777 trial. To align the censoring rules for the present ITC, no censoring for subsequent therapy was done for PFS analyses in either the MAIA or SWOG S0777 trials.
Statistical Methods
The present study used an adjusted anchored ITC design, a preferred approach for conducting a cross-trial comparison of treatment effectiveness when outcome data are available from two randomized controlled trials in which two different treatments were each compared against the same comparator or “anchor” (Fig. S1). In an anchored ITC, the magnitudes of relative treatment effects versus the common comparator are contrasted to make indirect inference about the relative effectiveness of the two treatment regimens not directly compared in a head-to-head trial [10, 11].
A critical assumption for an anchored ITC is that the two trials being compared need to have enrolled patients who are similar with respect to possible treatment effect modifiers, such as patient age, fitness, or cytogenetic risk [12]. The availability of individual patient-level data from both trials allows for adjustment for cross-trial differences in trial inclusion/exclusion criteria and patient baseline characteristics. As a result of differences in patient populations between the MAIA and SWOG S0777 trials, a harmonized set of inclusion/exclusion eligibility criteria were applied to ensure the balance of potential treatment effect modifiers across both data sources (Fig. 1; Table S1). Propensity-score weighting was then used to balance the two trial populations on key baseline characteristics [13, 14]. Baseline covariate balance after propensity-score weighting was assessed using standardized differences, with an absolute standardized difference > 0.1 considered a meaningful imbalance [15].
For ITCs, Rd was used as the common anchor across the MAIA and SWOG S0777 trials. To determine differences in PFS, Cox proportional hazards regression was used to calculate HRs between treatments relative to the common Rd anchor within each trial (MAIA, D-Rd vs Rd; SWOG S0777, VRd vs Rd). Using these calculated direct HRs, an ITC was then used to indirectly estimate the HR between trials (D-Rd vs VRd). Robust standard errors were used in the Cox regression models to account for the use of propensity-score weighting. Inspection of Kaplan–Meier survival plots and the statistical interactions between treatment and follow-up time were used to evaluate the Cox regression model’s proportional hazard assumption.
Missing baseline covariate data were addressed with multiple imputation. Multiple imputation by chained equations was used to assign missing baseline covariate values and was repeated to create ten complete datasets [16, 17]. Following multiple imputation, each complete dataset was analyzed separately, and the resulting parameter estimates and standard errors were pooled to obtain a summarized parameter estimate and standard error [18].
All statistical analyses were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute, Cary, NC).
Sensitivity and Subgroup Analyses
Three sensitivity analyses analyzing PFS were performed. The first was an unweighted analysis in which harmonized inclusion/exclusion criteria were applied to both trial populations without propensity-score weighting. The second was a doubly robust analysis in which both propensity-score weighting and Cox outcome regression model adjustment for baseline covariates were used. The third was an analysis restricted to only patients with hemoglobin ≥ 9 g/dL. In terms of inclusion criteria, the MAIA trial required a baseline hemoglobin of ≥ 7.5 g/dL and the SWOG S0777 trial required a hemoglobin of ≥ 9 g/dL; however, both trials included patients with hemoglobin < 9 g/dL (14% and 8% of MAIA and SWOG S0777 patients, respectively, aged ≥ 65 years). In contrast, in the primary analysis of PFS, propensity-score weighting was used to adjust for baseline hemoglobin, but no restriction based on hemoglobin was applied (Table S1). Additionally, a subgroup analysis was performed in patients with high cytogenetic risk.
Research Ethics Statements
The MAIA and SWOG S0777 trials were conducted in accordance with the principles of the Declaration of Helsinki, with study protocols approved by independent ethics committees and/or institutional review boards at each site. All patients provided written informed consent.
Results
Patient Selection and Baseline Patient Characteristics
Following the application of harmonized inclusion/exclusion criteria, 727 patients from the MAIA trial (D-Rd, n = 363; Rd, n = 364) and 198 patients from the SWOG S0777 trial (VRd, n = 91; Rd, n = 107) were eligible for inclusion (Fig. 1). The primary reason patients were excluded was age < 65 years (MAIA, n = 8; SWOG S0777, n = 269), reflecting the enrollment of a younger, transplant-not-intended patient population in the SWOG S0777 trial. Patients with a baseline ECOG performance status score of > 2 were also excluded from the comparative analyses (MAIA, n = 2; SWOG S0777, n = 4). Other key trial eligibility were aligned across the two trials (Fig. 1; Table S1).
Baseline patient characteristics, including age, sex, International Staging System disease stage, ECOG performance status score, hemoglobin, estimated glomerular filtration rate, LDH, and high cytogenetic risk, were well balanced across treatment arms within each trial (absolute standardized mean differences < 0.1 for all covariates; Table 1) following multiple imputation and propensity-score weighting. Baseline patient characteristics were also well balanced across the MAIA and SWOG S0777 trial populations (absolute standardized mean differences < 0.1 for all covariates).
Table 1.
Baseline patient characteristics after multiple imputation and propensity-score weighting in the MAIA and SWOG S0777 trials
| Covariate | MAIA (n = 727) |
SWOG S0777 (n = 198) |
||||
|---|---|---|---|---|---|---|
| D-Rd (n = 363) |
Rd (n = 364) |
Standardized differencea | VRd (n = 91) |
Rd (n = 107) |
Standardized differencea | |
| Age, mean (SD), years | 72.75 (4.77) | 72.65 (4.81) | 0.021 | 72.69 (5.36) | 72.65 (5.13) | 0.009 |
| Female | 40.4% | 41.9% | − 0.031 | 39.0% | 39.8% | − 0.017 |
| ISS disease stage | ||||||
| I | 20.2% | 20.6% | − 0.010 | 20.1% | 19.7% | 0.010 |
| II | 44.0% | 45.3% | − 0.026 | 44.5% | 44.8% | − 0.006 |
| III | 35.8% | 34.1% | 0.036 | 35.4% | 35.5% | − 0.002 |
| ECOG PS score | ||||||
| 0 | 42.5% | 40.9% | 0.032 | 40.1% | 40.1% | 0.001 |
| 1 | 48.9% | 50.4% | − 0.030 | 51.2% | 51.0% | 0.004 |
| ≥ 2 | 8.6% | 8.7% | − 0.003 | 8.7% | 8.9% | − 0.009 |
| Hemoglobin < 10 g/dL | 31.0% | 30.4% | 0.013 | 31.9% | 32.1% | − 0.004 |
| eGFR < 60 mL/min/1.73 m2 | 47.3% | 47.0% | 0.005 | 47.2% | 46.9% | 0.007 |
| LDH ≥ 190 U/L | 38.6% | 39.9% | − 0.027 | 39.4% | 39.0% | 0.009 |
| High cytogenetic riskb | 18.1% | 19.1% | − 0.024 | 16.4% | 16.6% | − 0.005 |
SWOG Southwest Oncology Group, D-Rd daratumumab plus lenalidomide/dexamethasone, Rd lenalidomide/dexamethasone, VRd bortezomib plus lenalidomide/dexamethasone, SD standard deviation, ISS International Staging System, ECOG PS Eastern Cooperative Oncology Group performance status, eGFR estimated glomerular filtration rate, LDH lactate dehydrogenase
aStandardized mean differences shown in the table reflect the magnitude of the difference in baseline covariate means across trial arms within each trial. Cross-trial standardized mean differences for the MAIA versus SWOG S0777 trials, not shown in the table, were < 0.1 for all baseline covariates
bHigh cytogenetic risk was defined in the MAIA and SWOG S0777 trials as the presence of ≥ 1 high-risk cytogenetic abnormality (del17p, t[14;16], or t[4;14])
In the MAIA trial, data were complete for all baseline patient characteristics except for LDH (D-Rd, 4.7% [n = 17/363]; Rd, 2.5% [n = 9/364]) and cytogenetic risk (D-Rd, 13.2% [n = 48/363]; Rd, 12.6% [n = 46/364]; Table S2 in the electronic supplementary material). In the SWOG S0777 trial, data were complete for all baseline covariates except for LDH (VRd, 1.1% [n = 1/91]; Rd, 0%) and cytogenetic risk (VRd, 40.7% [n = 37/91]; Rd, 36.4% [n = 39/107]; Table S3 in the electronic supplementary material). As described in the “Methods” section, missing baseline data were addressed with multiple imputation by chained equations prior to propensity-score weighting. Distributions of baseline patient characteristics before multiple imputation and propensity-score weighting are shown for the MAIA and SWOG S0777 trials in Tables S2 and S3, respectively.
Progression-Free Survival
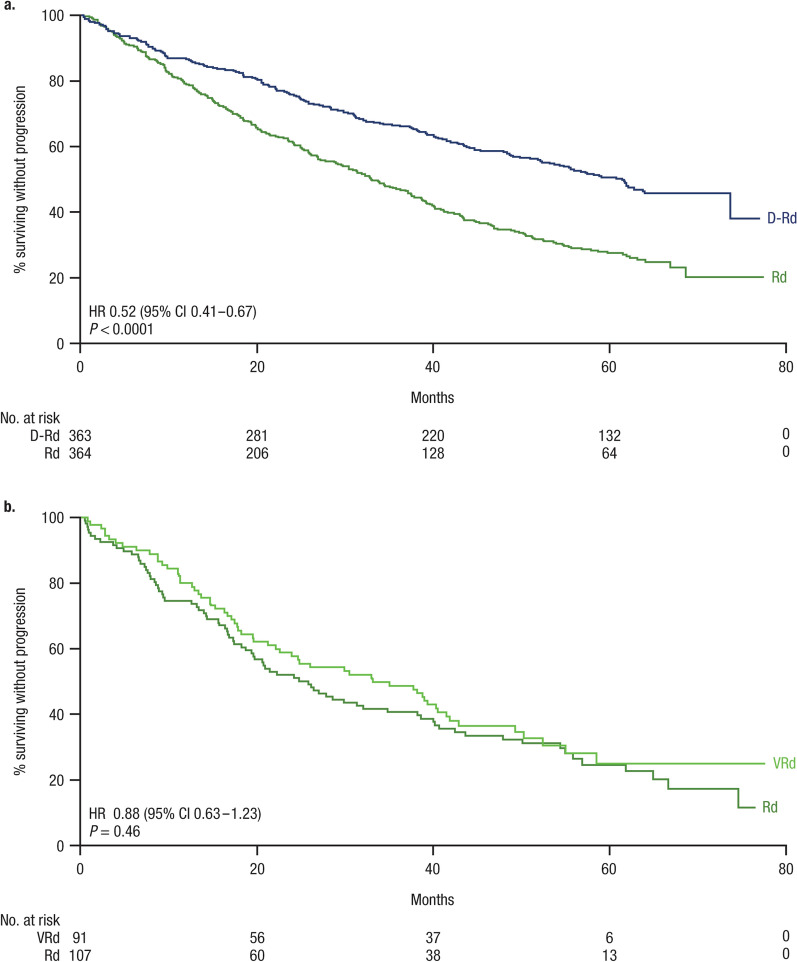
In the MAIA trial, after harmonization of the eligibility criteria, the PFS HR was 0.52 (95% CI 0.41–0.67; P < 0.0001; Fig. 2a; Table 2) for D-Rd versus Rd, demonstrating a significant improvement in PFS for patients receiving D-Rd, with a 48% reduction in the risk of disease progression or death versus Rd alone. For the SWOG S0777 trial, after harmonization of the eligibility criteria, the PFS HR was 0.88 (95% CI 0.63–1.23; P = 0.46; Fig. 2b; Table 2) for VRd versus Rd, demonstrating a nonsignificant improvement in PFS for patients receiving VRd, with a 12% reduction in risk of disease progression or death relative to Rd alone. No evidence for a significant violation of the proportional hazards assumption was found in either the MAIA or SWOG S0777 analyses.
Fig. 2.
PFS results from a MAIA (D-Rd vs Rd) and b SWOG S0777 (VRd vs Rd). PFS Kaplan–Meier plots reflect results after propensity-score weighting for patients in the MAIA trial (n = 727) and SWOG S0777 trial (n = 198) who met the harmonized inclusion/exclusion criteria. HRs for PFS reflect comparisons after application of harmonized inclusion/exclusion criteria and propensity-score weighting. PFS progression-free survival, D-Rd daratumumab plus lenalidomide/dexamethasone, Rd lenalidomide/dexamethasone, SWOG Southwest Oncology Group, VRd bortezomib plus lenalidomide/dexamethasone, HR hazard ratio, CI confidence interval
Table 2.
HRs reflecting of primary, sensitivity, and subgroup comparative analyses of PFS
| Direct within-study treatment comparisons | ITCs | ||
|---|---|---|---|
| MAIA D-Rd vs MAIA Rd | SWOG S0777 VRd vs SWOG S0777 Rd | MAIA D-Rd vs SWOG S0777 VRd | |
| Primary analysis: adjusted (aligned I/E criteria and propensity-score weighted) | 0.52 (0.41–0.67); P < 0.0001 | 0.88 (0.63–1.23); P = 0.46 | 0.59 (0.39–0.90); P = 0.01 |
| Sensitivity analyses | |||
| Aligned I/E criteria but no propensity-score weighting | 0.54 (0.45–0.66); P < 0.0001 | 0.84 (0.60–1.17); P = 0.31 | 0.65 (0.44–0.95); P = 0.03 |
| Doubly robust analysis | 0.51 (0.40–0.65); P < 0.0001 | 0.89 (0.63–1.25); P = 0.50 | 0.57 (0.38–0.87); P = 0.01 |
| Restricted to patients with hemoglobin > 9 g/dL | 0.51 (0.39–0.66); P < 0.0001 | 0.91 (0.64–1.30); P = 0.61 | 0.56 (0.36–0.86); P = 0.01 |
| Subgroup analysis | |||
| High cytogenetic riska | 0.58 (0.35–0.94); P = 0.03 | 1.02 (0.30–3.20); P = 0.97 | 0.57 (0.16–1.98); P = 0.37 |
Data reported as HR (95% CI)
HR hazard ratio, PFS progression-free survival, ITCs indirect treatment comparisons, D-Rd daratumumab plus lenalidomide/dexamethasone, Rd lenalidomide/dexamethasone, SWOG Southwest Oncology Group, VRd bortezomib plus lenalidomide/dexamethasone, I/E inclusion/exclusion, CI confidence interval
aHigh cytogenetic risk was defined in the MAIA and SWOG S0777 trials as the presence of ≥ 1 high-risk cytogenetic abnormality (del17p, t[14;16], or t[4;14]). Note that the high cytogenetic risk subgroup analysis was based on small sample sizes (MAIA, n = 91; SWOG S0777, n = 17)
Based on the derived effect estimates of the MAIA and SWOG S0777 trials, the anchored ITC estimated that D-Rd treatment was associated with a statistically significant improvement in PFS, with a 41% reduction in the risk of disease progression or death compared with VRd treatment (HR 0.59; 95% CI 0.39–0.90; P = 0.01; Table 2). Consistent with the primary ITC analysis, D-Rd demonstrated a statistically significant improvement in PFS versus VRd in all sensitivity analyses (unweighted but aligned to inclusion and exclusion criteria [HR 0.65; 95% CI 0.44–0.95; P = 0.03], a doubly robust analysis [HR 0.57; 95% CI 0.38–0.87; P = 0.01], and an analysis restricted to patients with hemoglobin ≥ 9 g/dL [HR 0.56; 95% CI 0.36–0.86; P = 0.01]).
In an exploratory subgroup analysis of PFS among patients with high cytogenetic risk, D-Rd demonstrated a statistically significant improvement in PFS versus Rd (HR 0.58; 95% CI 0.35–0.94; P = 0.03) in the MAIA trial. In contrast, there was no significant difference between treatment arms observed in the SWOG S0777 trial (HR 1.02; 95% CI 0.30–3.20; P = 0.97). Following the ITC analysis, D-Rd demonstrated a numerically similar but statistically nonsignificant improvement in PFS compared with VRd in the high cytogenetic risk subgroup (HR 0.57; 95% CI 0.16–1.98; P = 0.37).
Discussion
To our knowledge, no clinical trial has directly compared the efficacy of the D-Rd and VRd treatment regimens in TIE patients with NDMM. Therefore, the present study was conducted to address this evidence gap. On the basis of the anchored ITC, treatment with D-Rd was associated with a statistically significant improvement in PFS, with a 41% reduction in the risk of disease progression or death versus VRd treatment in TIE patients with NDMM. Additionally, PFS results from sensitivity and subgroup analyses remained consistent with the primary ITC analysis. In the subgroup of patients with high cytogenetic risk, PFS benefit favored D-Rd versus Rd in the MAIA trial and D-Rd versus VRd in the current anchored ITC; however, neither analysis reached significance, likely as a result of the limited number of patients in this subgroup. Results in this current study provide insight into the efficacy of D-Rd and VRd treatments, both of which are recommended for the treatment of NDMM in TIE patients [1]. These findings suggest a superior benefit of D-Rd versus VRd and could therefore contribute to more informed decision-making in the real-world setting regarding treatment regimen choice for TIE patients with NDMM. Of interest, the phase 3 SWOG S2209 study (ClinicalTrials.gov identifier NCT05561387) will provide definitive head-to-head efficacy and safety data for D-Rd followed by lenalidomide ± daratumumab maintenance versus VRd-lite followed by lenalidomide maintenance in frail or intermediate-fit TIE patients with NDMM; currently, this trial is actively recruiting [19].
These findings should be put in the context of other relevant clinical trial data. In this study, within-trial comparison of the SWOG S0777 treatments, VRd versus Rd, demonstrated no significant difference in PFS between treatments (P = 0.46; Fig. 2b). However, in the primary analysis of the SWOG S0777 trial, median PFS was significantly improved for patients receiving VRd versus Rd alone (HR 0.712; 96% Wald CI 0.56–0.906; 1-sided P = 0.0018) [4]. The differences in HR estimates in this current study versus the SWOG S0777 trial are likely due to variations in patient populations and the alignment of inclusion/exclusion criteria required for this ITC, in particular, the restriction to SWOG S0777 participants aged ≥ 65 years (accounted for 43% of the SWOG S0777 population) [4]. In a prior post hoc analysis of the SWOG S0777 study stratified by age, the estimated PFS benefit for VRd versus Rd was smaller in magnitude for patients aged ≥ 65 years (median PFS 33.1 vs 25.8 months, respectively; HR 0.83; 95% CI 0.60–1.16) relative to those aged < 65 years (55.4 vs 36.6 months; HR 0.63; 95% CI 0.46–0.87) [20]. This supports our finding that VRd may not be as effective in older TIE patients with NDMM versus other treatment combinations, such as D-Rd.
The SWOG S0777 trial enrolled a mixed population of patients without intent for immediate transplant, inclusive of TIE patients and those who chose to defer or refuse frontline transplant, and reported a median age of 63 years [4]. Conversely, MAIA only enrolled patients with NDMM who were TIE and reported a median age of 73 years [3]. Given the cross-trial variation in age and transplant criteria, both trial populations were restricted to a patient age of ≥ 65 years, which simultaneously served as a proxy for transplant ineligibility [7]. Application of harmonized eligibility criteria led to a noticeable reduction in the number of SWOG S0777 patients eligible for inclusion in this ITC (SWOG S0777: primary, n = 525; ITC, n = 198), as opposed to a slight reduction in MAIA patients (MAIA: primary, n = 737; ITC, n = 727). While advanced aged (≥ 65 years) may preclude many patients from receiving stem cell transplant, hence its use as a proxy for TIE status, it is important to note that stem cell transplant is feasible in some patients aged 65 to 70 years without substantial comorbidities [1]; thus, use of this proxy is a limitation of the current analysis.
Results from the current study are similar to another previously reported ITC. The PEGASUS trial utilized an ITC to investigate the efficacy of D-Rd in comparison to other standard-of-care regimens, including VRd and bortezomib plus dexamethasone, for TIE patients with NDMM, with a comparable Rd anchor [21]. Patient-level data for individuals treated with D-Rd were acquired from the MAIA trial, as done in the current analysis, whereas data for those treated with VRd were acquired from the Flatiron Health electronic health record–derived database, which is a real-world, nationwide, demographically and geographically diverse database. On the basis of the resulting ITC, D-Rd was associated with a significantly longer PFS in comparison to VRd (HR 0.68; 95% CI 0.48–0.98), consistent with our findings.
In addition to ITCs based on individual patient-level data, network meta-analyses are additional, well-accepted avenues to compare clinical effectiveness data across multiple clinical trials [22]. As reported in a recent network meta-analysis exploring the efficacy of varying standard-of-care therapies for the treatment of TIE patients with NDMM across 45 unique randomized controlled trials, D-Rd had the highest probability of offering the best PFS compared to Rd (HR 0.53; 95% credible interval 0.30–0.92) and was superior to VRd versus Rd (HR 0.77; 95% credible interval 0.42–1.41) [23]. While the results are consistent with our findings, slight differences are likely due to the varied statistical adjustments—the prior network meta-analysis used aggregated results for the SWOG S0777 subgroup of patients aged ≥ 65 years as a proxy for TIE status, whereas the current ITC used patient-level data with harmonization of the enrollment criteria and propensity-score weighting for balance between the study populations.
While clinical trials are vital for assessing treatment efficacy and patient outcomes, they are often rigorously controlled, which may not be representative of real-world practice [24]. For example, while the SWOG S0777 trial reported a PFS benefit following VRd treatment (median of 43 months) [4], PFS observed in the real-world setting has been notably shorter (median of 26.5 months) [25]. In a recently published real-world multicenter chart review study of TIE patients with NDMM, patients receiving D-Rd had a 65% lower risk of disease progression or death compared to those receiving VRd (adjusted HR 0.35; 95% CI 0.17–0.73; P = 0.005) [26]. The greater risk reduction in progression or death with D-Rd in the chart review study, as well as the variance in PFS observed following VRd in clinical versus real-world studies, could in part be attributed to the use of a VRd-lite regimen in TIE patients in the real-world setting. This modified regimen is often characterized by lower lenalidomide doses and reduced frequency of bortezomib administration [26, 27]. Thus, an understanding of real-world evidence is important to further support informed clinical decisions.
However, ITCs are not without limitations and can be biased by the presence of both observed and unobserved cross-trial differences. For example, residual differences in prognostic factors may remain as a result of the lack of patient reporting or data availability across trials [11]. To address this, a common comparator, in this case the Rd treatment arm shared between the MAIA and SWOG S0777 trials, was used as an anchor to help minimize the impact of residual confounding factors. In an anchored ITC, only relevant treatment effect modifiers must be adjusted for; residual cross-trial differences in unobserved prognostic factors will not bias the result [11].
A limitation of the present study was that data were missing for two baseline covariates, cytogenetic risk and LDH, with high missingness (VRd, 40.7%; Rd, 36.4%) for cytogenetic risk in the SWOG S0777 trial (Table S3). Multiple imputation was used to address the missing baseline covariate data before the application of propensity-score weighting. While the degree of missingness for cytogenetic risk was high in the SWOG S0777 trial, the subgroup analysis restricted to patients with known high cytogenetic risk was consistent with results in the overall TIE NDMM study population, providing reassurance that cytogenetic risk status was not a critical treatment effect modifier and source of bias in the present ITC.
Conclusion
Through the application of harmonized inclusion criteria and propensity-score weighting, treatment effects of D-Rd and VRd were compared in a similar population of TIE patients with NDMM using an anchored ITC design. On the basis of individual patient-level data from the MAIA and SWOG S0777 trials, D-Rd treatment was associated with a significant 41% reduction in the risk of disease progression or death in comparison to VRd. In the absence of a head-to-head clinical trial between two relevant comparators, methods such as ITCs can provide timely and reliable cross-trial comparative data. Results from this ITC of D-Rd versus VRd could provide valuable insight to health care professionals and contribute to more clinically informed treatment decisions for TIE patients with NDMM.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This manuscript was prepared using SWOG S0777 trial data from Dataset NCT00644228-D1 from the NCTN/NCORP Data Archive of the NCI’s NCTN. Data were originally collected from ClinicalTrials.gov identifier NCT00644228. All analyses and conclusions in this manuscript are the sole responsibility of the authors and do not necessarily reflect the opinions or views of the clinical trial investigators, the NCTN, the NCORP, or the NCI.
Medical Writing and Editorial Assistance
Editorial and medical writing support were provided by Holly Clarke, PhD, of Lumanity Communications Inc., and were funded by Janssen Global Services, LLC.
Author Contribution
Brian G.M. Durie contributed to the study conception and design; to the acquisition, analysis, and interpretation of the data; enrolled patients; and provided general supervision of a research group. Shaji K. Kumar contributed to study conception and design; to the acquisition, analysis, and interpretation of the data; and enrolled patients. Eric M. Ammann contributed to study conception and design and to the acquisition, analysis, and interpretation of the data. Alex Z. Fu contributed to study conception and design and to the acquisition, analysis, and interpretation of the data. Shuchita Kaila contributed to study conception and design. Annette Lam contributed to the study conception and design and to the interpretation of the data. Saad Z. Usmani contributed to the interpretation of data and enrolled patients. Thierry Facon contributed to the acquisition, analysis, and interpretation of the data and enrolled patients. All authors contributed to the drafting and/or critical revision of the manuscript, approved the final version for publication, and agreed to be accountable for all aspects of the work.
Funding
This study and the journal’s Rapid Service and Open Access fees were funded by Janssen Global Services, LLC.
Data Availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the MAIA trial data (ClinicalTrials.gov identifier NCT02252172) can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu. SWOG S0777 trial data were sourced from Dataset NCT00644228-D1 from the NCTN/NCORP Data Archive of the NCI’s NCTN. Data were originally collected from ClinicalTrials.gov identifier NCT00644228. Requests for access to the SWOG S0777 trial data can be made through the NCTN/NCORP data archive at http://nctn-data-archive.nci.nih.gov.
Declarations
Conflict of Interest
Brian G.M. Durie is a consultant for Amgen, Celgene, Johnson & Johnson, and Takeda. Shaji K. Kumar received research funding from AbbVie, Allogene, Amgen, AstraZeneca, Bristol Myers Squibb, CARsgen, GSK, Janssen, Novartis, Roche-Genentech, Takeda, Regeneron, and Molecular Templates; served as a consultant or on an advisory board (with no personal payments) for AbbVie, Amgen, Bristol Myers Squibb, Janssen, Roche-Genentech, Takeda, AstraZeneca, bluebird bio, Epizyme, Secura Bio, Monte Rosa Therapeutics, Trillium, Loxo Oncology, K36, Sanofi, and Arcellx; and served as a consultant or on an advisory board (with personal payments) for Oncopeptides, BeiGene, Antengene, and GLH Pharma. Eric M. Ammann, Alex Z. Fu, Shuchita Kaila, and Annette Lam are employees of Janssen and hold equity in a publicly traded company. Saad Z. Usmani received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx, and Takeda; served as a consultant for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Edo Pharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda, and TeneoBio; and served as a speaker for Amgen, Bristol Myers Squibb, Janssen, and Sanofi. Thierry Facon served as a consultant or advisor for Janssen, Bristol Myers Squibb, Roche, and Takeda; and served on a speakers bureau for Janssen and Bristol Myers Squibb.
Ethical Approval
The MAIA and SWOG S0777 trials were conducted in accordance with the principles of the Declaration of Helsinki, with study protocols approved by independent ethics committees and/or institutional review boards at each site. All patients provided written informed consent.
Footnotes
NCCN = National Comprehensive Cancer Network® (NCCN®).
Prior Presentation: This manuscript is based upon data presented previously at the American Society of Clinical Oncology (ASCO) Annual Meeting, held on June 2–6, 2023, in Chicago, IL, USA [poster presentation; abstract available at https://ascopubs.org/doi/abs/10.1200/JCO.2023.41.16_suppl.8037] and at the Society of Hematologic Oncology (SOHO) Annual Meeting, held on September 6–9, 2023, in Houston, TX, USA [poster presentation].
References
- 1.Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Hemasphere. 2021;5(2):e528. doi: 10.1097/HS9.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V2.2024. © National Comprehensive Cancer Network, Inc. 2023. All rights reserved. Accessed Mar 5, 2024. To view the most recent and complete version of the guidelines, go online to NCCN.org. NCCN makes no warranties or any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- 3.Kumar SK, Moreau P, Bahlis N, et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in transplant ineligible patients with newly diagnosed multiple myeloma (NDMM): updated analysis of the phase 3 MAIA study. Presented at: 64th American Society of Hematology (ASH) Annual Meeting & Exposition; December 10–13, 2022; New Orleans, LA.
- 4.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519–527. doi: 10.1016/S0140-6736(16)31594-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596. doi: 10.1016/S1470-2045(21)00466-6. [DOI] [PubMed] [Google Scholar]
- 6.Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT) Blood Cancer J. 2020;10(5):53. doi: 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mian H, Mian OS, Rochwerg B, Foley R, Wildes TM. Autologous stem cell transplant in older patients (age ≥65) with newly diagnosed multiple myeloma: a systematic review and meta-analysis. J Geriatr Oncol. 2020;11(1):93–99. doi: 10.1016/j.jgo.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. NICE DSU Technical Support Document 18: Methods for population-adjusted indirect comparisons in submissions to NICE. http://www.nicedsu.org.uk. Accessed Jun 5, 2023.
- 11.Signorovitch J, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;2012(15):940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Reken S, Sturtz S, Kiefer C, Bohler YB, Wieseler B. Assumptions of mixed treatment comparisons in health technology assessments—challenges and possible steps for practical application. PLoS ONE. 2016;11(8):e0160712. doi: 10.1371/journal.pone.0160712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. doi: 10.1161/CIRCOUTCOMES.113.000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Xin Yu AY, Vyas MV, Kapral MK. Applying propensity score methods in clinical research in neurology. Neurology. 2021;97(18):856–863. doi: 10.1212/WNL.0000000000012777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horton NJ, Kleinman KP. Much ado about nothing: a comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61(1):79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. doi: 10.1080/01621459.1996.10476908. [DOI] [Google Scholar]
- 19.SWOG Cancer Research Network, National Cancer Institute (NCI). Comparing combinations of drugs to treat newly diagnosed multiple myeloma (NDMM) when a stem cell transplant is not a medically suitable treatment. ClinicalTrials.gov Identifier: NCT05561387. Updated Oct 25, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05561387. Accessed Jan 2, 2024.
- 20.Durie BGM, Lam A, Ngo M, Pei H, Ammann EM. Post-hoc analysis of efficacy and safety in the SWOG S0777 trial stratified by age. Blood. 2022;140(suppl 1):10009–10010. doi: 10.1182/blood-2022-165591. [DOI] [Google Scholar]
- 21.Durie BGM, Kumar SK, Usmani SZ, et al. Daratumumab-lenalidomide-dexamethasone vs standard-of-care regimens: efficacy in transplant-ineligible untreated myeloma. Am J Hematol. 2020;95(12):1486–1494. doi: 10.1002/ajh.25963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laws A, Kendall R, Hawkins N. A comparison of national guidelines for network meta-analysis. Value Health. 2014;17(5):642–654. doi: 10.1016/j.jval.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Facon T, San-Miguel J, Dimopoulos MA, et al. Treatment regimens for transplant-ineligible patients with newly diagnosed multiple myeloma: a systematic literature review and network meta-analysis. Adv Ther. 2022;39(5):1976–1992. doi: 10.1007/s12325-022-02083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109. doi: 10.1038/s41408-018-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medhekar R, Ran T, Fu AZ, Patel S, Kaila S. Real-world patient characteristics and treatment outcomes among nontransplanted multiple myeloma patients who received bortezomib in combination with lenalidomide and dexamethasone as first line of therapy in the United States. BMC Cancer. 2022;22(1):901. doi: 10.1186/s12885-022-09980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordan L, Tan CR, Vescio R, et al. Progression-free survival of daratumumab vs. bortezomib triplet combination with lenalidomide and dexamethasone in transplant ineligible patients with newly diagnosed multiple myeloma: TAURUS chart review study. Clin Lymphoma Myeloma Leuk. 2024;24(1):55–63. doi: 10.1016/j.clml.2023.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Ran T, Medhekar R, Fu AZ, Patel S, Kaila S. Patient characteristics associated with dose modifications for VRd among newly diagnosed multiple myeloma patients. Future Oncol. 2022;18(36):3983–3991. doi: 10.2217/fon-2022-0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the MAIA trial data (ClinicalTrials.gov identifier NCT02252172) can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu. SWOG S0777 trial data were sourced from Dataset NCT00644228-D1 from the NCTN/NCORP Data Archive of the NCI’s NCTN. Data were originally collected from ClinicalTrials.gov identifier NCT00644228. Requests for access to the SWOG S0777 trial data can be made through the NCTN/NCORP data archive at http://nctn-data-archive.nci.nih.gov.