Highlights
-
•
Non-puerperal uterine inversion can be associated with uterine sarcomas.
-
•
Adenosarcoma is a tumor composed of benign epithelium and malignant stroma.
-
•
If malignancy is suspected or confirmed treatment of uterine inversion with hysterectomy is advised.
Keywords: Adenosarcoma, Uterine Inversion, Non-Puerperal, Inversion
1. Introduction
Non-puerperal uterine inversion is a rare complication where the fundus of the uterus turns inside out and prolapses through the cervix [1]. The incidence of non-pleural uterine inversion is unknown and is most commonly caused by benign leiomyomas, while it is rarely associated with uterine sarcomas [1], [2]. Uterine adenosarcoma is a biphasic tumor composed of benign epithelium and malignant stroma that accounts for 5–10 % of uterine sarcomas [1], [2]. It is hypothesized that rapid growth of tumor and softening of uterine wall due to tumor enlargement can cause the rare complication of uterine inversion [1].
2. Case description
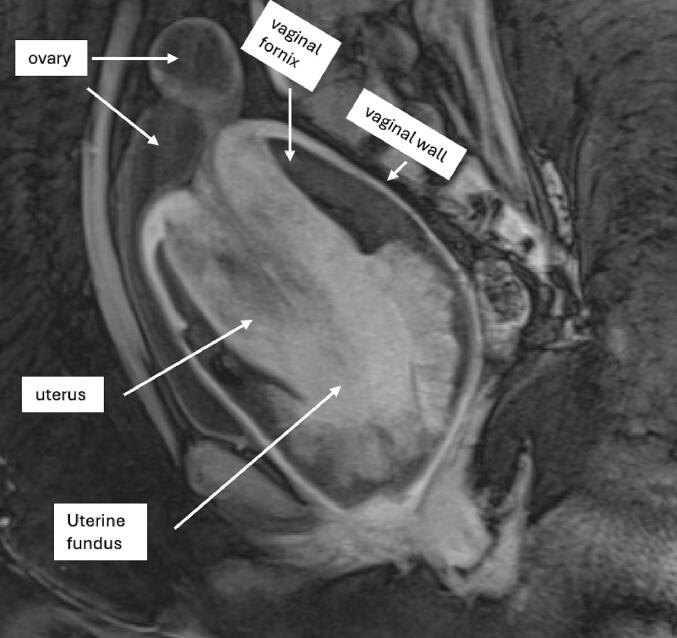
A 35-year-old G1P0010 female presented with pelvic mass and acute blood loss anemia. The patient had no significant past medical history and past surgical history of dilation and curettage after spontaneous abortion. Laboratory findings on admission notable for a hemoglobin of 5.1 ultimately requiring a total of six units of packed red blood cells prior to being transferred to our tertiary care center. Exam under anesthesia with biopsy was also performed prior to transfer with a large mass protruding from and encompassing the cervix with active bleeding. Computed Tomography scan of abdomen and pelvis was notable for distended vagina containing fluid and gas with abnormal configuration of uterus and apparent uterine inversion into the vagina. Magnetic Resonance Imaging (MRI) of the Pelvis confirmed the uterus to be inverted with associated distension of the vaginal canal and the left ovary with partial intussusception within the inverted uterus [Fig. 1].
Fig. 1.
MRI pelvis.
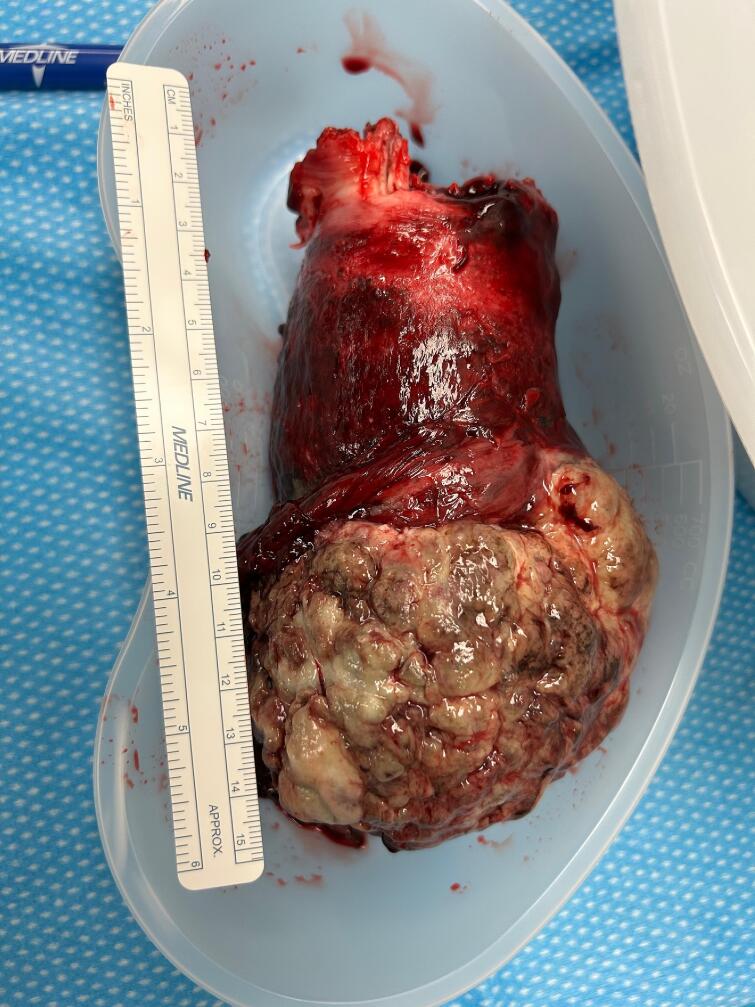
The patient was then transferred for higher level of care and gynecologic oncology consultation. Decision made to proceed with emergent surgery due to continued bleeding, ongoing blood transfusion requirements, and concern for superimposed infection. A total abdominal hysterectomy with bilateral salpingectomy was performed. On bimanual exam a 5 × 8 × 8 cm exophytic mass within the vagina was noted with no evidence of invasion into vaginal tissue or bilateral sidewalls. Intra-abdominal findings were notable for complete uterine inversion with distorted fundal contour and ovaries protruding cephalad from inverted uterine fundus. No evidence of extrauterine pelvic disease noted at time of surgery. An unsuccessful attempt was made to reduce uterus to proper anatomic location. Given altered anatomy, an anterior colpotomy was created using handheld malleable to delineate anterior vaginal fornix, and a retrograde hysterectomy was performed [gross specimen shown in Fig. 2]. Frozen pathology showed extensive necrosis and concern for sarcomatous growth, therefore no lymph node procedures were performed given normal nodal evaluation on preop MRI. The ovaries were left in situ given normal appearance, presumed sarcoma, and the patient’s young age.
Fig. 2.
Endometrial adenosarcoma non-pleural uterine inversion.
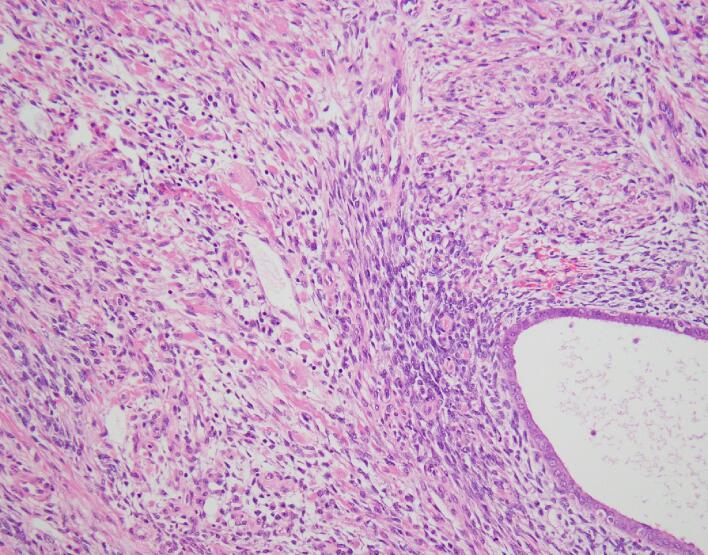
Final histopathologic examination revealed a FIGO Stage IB endometrial adenosarcoma with sarcomatous overgrowth and heterologous rhabdomyosarcoma. Acute inflammation and necrosis involving tumor surface and extensive edema within the tumor was noted. The mass was composed of benign endometrial glands and malignant stroma composed of cells with spindled nuclei arranged in sheets and nests [Fig. 3]. Other areas of stromal cells showed features of strap cells with cytoplasmic striation and cells with eccentric nuclei and bright eosinophilic cytoplasm. CD10 positive stain noted in some peri-glandular stroma and desmin/myogenin noted positive in many of the malignant stromal cells. Estrogen receptor was 20 % positive and progesterone receptor was 30 % positive. Myometrial invasion was present to the depth of 12 mm and lymphovascular invasion was negative. All margins were noted to be negative for sarcoma.
Fig. 3.
Endometrial adenosarcoma pathology.
3. Discussion
Surgical management is the mainstay of treatment for non-puerperal uterine inversion once the patient is stabilized; if malignancy is suspected, then a hysterectomy should be performed [2]. The diagnosis of uterine inversion is often made with imaging, with MRI thought to be the most sensitive for diagnosis. However, if the diagnosis is unclear, laparoscopy can be used to confirm inversion [1], [2]. As demonstrated in this case report, patients with non-puerperal uterine inversion can present in unstable condition with significant risk for bleeding and infection. Adenosarcoma most commonly presents with symptoms of abnormal vaginal bleeding [3], [4]. However, more uncommon presentations include uterine enlargement, pelvic mass and protruding cervical tumors [4].
When non-puerperal uterine inversion is encountered attempt to reduce uterus to obtain normal anatomy can be performed. As demonstrated in this case when normal anatomy cannot be restored retrograde abdominal hysterectomy can be utilized as a surgical technique. Retrograde abdominal hysterectomy is a technique that can be used to perform hysterectomy when cervicovaginal junction is not clearly identified or a large vaginal tumor is present [5]. This technique involves initial bladder mobilization and colpotomy creation at the vesicouterine junction [5]. The uterine arteries and cardinal ligament are then clamped and divided at the level of the internal cervical os [5]. The incision at the anterior vaginal wall is extended and the bilateral uterosacral ligaments are ligated [5]. A retrograde hysterectomy is performed without visualization of the ureters. Ureter injury is avoided by close contact with the portio vaginalis uteri when transecting the parametrium and uterosacral ligaments [5].
4. Conclusion
This case report demonstrates a rare presentation of an adenosarcoma in a pre-menopausal person presenting with a similarly rare uterine inversion who ultimately required an abdominal hysterectomy due ongoing vaginal bleeding, instability, and high suspicion of malignancy.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
CRediT authorship contribution statement
Caitlin Witt: . Chelsey Vranes: Writing – review & editing, Writing – original draft, Conceptualization. Leslie H. Clark: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Occhionero M., Restaino G., Ciuffreda M., Carbone A., Sallustio G., Ferrandina G. Uterine inversion in association with uterine sarcoma: A case report with MRI findings and review of the literature. Gynecol Obstet Invest. 2012;73(3):260–264. doi: 10.1159/000334311. [DOI] [PubMed] [Google Scholar]
- 2.Herath R.P., Patabendige M., Rashid M., Wijesinghe P.S. Nonpuerperal Uterine Inversion: What the Gynaecologists Need to Know? Obstet Gynecol Int. 2020 doi: 10.1155/2020/8625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto A., Howitt B. Uterine Adenosarcoma. Arch Pathol Lab Med. 2016;140(3):286–290. doi: 10.5858/arpa.2014-0523-RS. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q., Sun S., Cai J., Yang L., Lv G., Yang Q. Uterine Adenosarcoma: A Case Report and Review of the Literature. 2023;13 [PMC free article] [PubMed] [Google Scholar]
- 5.Hiramatsu Y. Retrograde abdominal hysterectomy. Surg. J. 2019;05(S 01):S27–S32. doi: 10.1055/s-0039-1683919. [DOI] [PMC free article] [PubMed] [Google Scholar]