Abstract
In monarchE, adjuvant abemaciclib significantly improved invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS), with sustained benefit beyond the 2-year treatment period. Abemaciclib dose reductions were allowed to proactively manage adverse events. Exploratory analyses to investigate the impact of dose reductions on efficacy were conducted. Across the three patient subgroups as defined by relative dose intensity (≤66%, 66–93%, ≥93%), the estimated 4-year IDFS rates were generally consistent (87.1%, 86.4%, and 83.7%, respectively). In the time-dependent Cox proportional hazard model, the effect of abemaciclib was consistent at the full dose compared to being reduced to a lower dose (IDFS hazard ratio: 0.905; 95% confidence interval: 0.727, 1.125; DRFS hazard ratio: 0.942; 95% confidence interval: 0.742, 1.195). These analyses showed that the efficacy of adjuvant abemaciclib was not compromised by protocol mandated dose reductions for patients with node positive, hormone receptor positive, human epidermal growth factor 2-negative, high-risk early breast cancer.
Subject terms: Breast cancer, Targeted therapies
Introduction
The addition of 2 years of abemaciclib to standard adjuvant endocrine therapy (ET) for high-risk, hormone receptor-positive (HR+), human epidermal growth factor receptor negative (HER2-) early-stage breast cancer (EBC) resulted in significant improvements in invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS), which were further strengthened after the 2-year treatment period1,2. Notably, at a median follow-up of 42 months, improvements in IDFS and DRFS represented a relative risk reduction of 34% for disease recurrence or distant metastases in the abemaciclib plus ET arm compared to the ET alone arm2. Based on these results, abemaciclib is currently the only cyclin-dependent kinase 4/6 dual inhibitor approved as adjuvant therapy for node-positive, HR+, HER2-, high-risk EBC3–5, with a National Comprehensive Cancer Network Category 1 rating6 and a maximum score (A) from the European Society for Medical Oncology on the Magnitude of Clinical Benefit Scale7.
The goal of adjuvant treatment is to eliminate micro-metastatic disease to prevent recurrence; thus, the failure to retain patients on abemaciclib for the full 2-year treatment period could compromise treatment efficacy. It has also been established previously, that abemaciclib dose modifications improve tolerability without negatively affecting progression-free survival (PFS) in the metastatic breast cancer setting8.
Similar to the safety profile of abemaciclib plus ET in the advanced and metastatic breast cancer setting8, most adverse events in the EBC setting were reversible and manageable with supportive medications and/or dose modifications9. In monarchE, dose reductions due to adverse events occurred in 43.4% of patients treated with abemaciclib, most frequently in response to diarrhea, neutropenia, and/or fatigue9. Although dose reductions occurred at different timepoints, the majority took place within the first 6 months9. Abemaciclib dose reductions were shown to effectively manage adverse events, with only a small proportion of patients discontinuing after a dose reduction (8.9%), indicating that early dose reductions may improve treatment adherence9. In contrast, 52% of patients who discontinued abemaciclib due to an adverse event did not have a prior dose reduction, including 88% of patients who discontinued during the first month of treatment9.
However, the patient disease characteristics associated with dose reductions, as well as the impact of abemaciclib dose reductions on efficacy during adjuvant EBC treatment have not been previously described. In this post hoc analysis we evaluated baseline patient disease characteristics to identify patients who could benefit from more frequent monitoring of side effects and, if required, earlier dose reductions. More importantly, we report exploratory analyses to determine the impact of dose modifications, specifically dose reductions, on the efficacy of adjuvant abemaciclib for the treatment of high-risk, node-positive, HR+, HER2- EBC.
Results
Patient characteristics by the number of dose reductions
In the monarchE trial, 2791 patients were treated with adjuvant abemaciclib plus ET. Of these, 1221 (43.7%) had dose reductions, including 832 (29.8%) with one and 389 (13.9%) with two dose reductions. Dose reductions were most commonly required for diarrhea (17.3%), neutropenia (8.1%), or fatigue (4.5%)9.
Table 1 shows patient demographics and clinical characteristics by the number of dose reductions. For all reported demographics and clinical characteristics except age and pre-existing comorbidities, distributions of characteristics were similar across the dose reduction groups. Higher proportions of patients ≥65 years old or with ≥4 pre-existing comorbidities were observed among patients with dose reductions. In addition, the likelihood of dose reductions within each patient characteristic factor was assessed: 55.8% of older patients and 49.9% of patients with ≥4 comorbidities had at least one dose reductions.
Table 1.
Patient demographics and clinical characteristics by number of abemaciclib dose reductions
| Characteristics | No dose reduction N = 1570 | One dose reduction N = 832 | Two dose reductions N = 389 |
|---|---|---|---|
| Age group | |||
| <65 years old | 1380 (87.9) | 683 (82.1) | 298 (76.6) |
| ≥65 years old | 190 (12.1) | 149 (17.9) | 91 (23.4) |
| Prior chemotherapy | |||
| Neoadjuvant chemotherapy | 580 (36.9) | 315 (37.9) | 137 (35.2) |
| Adjuvant chemotherapy | 922 (58.7) | 483 (58.1) | 228 (58.6) |
| No chemotherapy | 68 (4.3) | 34 (4.1) | 24 (6.2) |
| Pre-existing comorbidities | |||
| None | 294 (18.7) | 117 (14.1) | 49 (12.6) |
| 1–3 comorbidities | 796 (50.7) | 396 (47.6) | 181 (46.5) |
| ≥4 comorbidities | 480 (30.6) | 319 (38.3) | 159 (40.9) |
| Number of positive nodesa | |||
| 1–3 nodes | 616 (39.2) | 323 (38.8) | 176 (45.2) |
| ≥4 nodes | 949 (60.4) | 507 (60.9) | 213 (54.8) |
| Pathological tumor sizeb | |||
| <20 mm | 444 (28.3) | 221 (26.6) | 110 (28.3) |
| ≥20 mm | 1094 (69.7) | 599 (72.0) | 275 (70.7) |
| Histological tumor gradec | |||
| Grade 1 | 126 (8.0) | 50 (6.0) | 33 (8.5) |
| Grade 2 | 791 (50.4) | 413 (49.6) | 167 (42.9) |
| Grade 3 | 580 (36.9) | 329 (39.5) | 166 (42.7) |
Data cutoff date: July 01, 2022 N, number of patients; n, number of patients in the group aPatients with no nodes were not included in this table, bPatients with missing tumor size information were not included in this table, cPatients with missing on non-assessable tumor grade were not included in this table.
Abemaciclib exposure by the number of dose reductions
The median duration of abemaciclib treatment was 23.7 months, regardless of dose reductions. Compared to those with no dose reduction, a greater proportion of patients with one or two reductions completed the first 6 months of treatment (Table 2). At later time points, treatment retention was comparable, or even improved, in patients with dose reductions (Table 2).
Table 2.
Abemaciclib exposure by the number of dose reductions
| No dose reduction N = 1570 | One dose reduction N = 832 | Two dose reductions N = 389 | |
|---|---|---|---|
| Treatment duration, months | |||
| Median | 23.7 | 23.7 | 23.7 |
| Q1–Q3 | 14.9–23.8 | 20.6–23.8 | 13.2–23.8 |
| >3 months, n (%) | 1349 (85.9) | 787 (94.6) | 367 (94.3) |
| >6 months, n (%) | 1276 (81.3) | 750 (90.1) | 333 (85.6) |
| >12 months, n (%) | 1200 (76.4) | 677 (81.4) | 297 (76.3) |
| >18 months, n (%) | 1146 (73.0) | 637 (76.6) | 274 (70.4) |
| Cumulative dose, mg | |||
| Median | 192,450 | 137,475 | 77,200 |
| Q1–Q3 | 112,900–210,900 | 98,825–151,950 | 50,100–96,500 |
| Relative dose intensitya, % | |||
| Median | 94.6 | 66.5 | 40.2 |
| Q1–Q3 | 83.4–99.0 | 59.5–74.4 | 34.5–50.7 |
Data cutoff date: July 01, 2022.
N, number of patients; n, number of patients in the group; Q1–Q3, quartile 1 to quartile 3 range aRelative dose intensity was defined as the average daily dose of abemaciclib received by each patient over the treatment duration, relative to the full dose (150 mg twice per day). Dose reductions of up to two 50-mg dose levels (100 or 50 mg) were permitted during the on-study treatment period.
Patients with dose reductions had a lower cumulative dose and RDI compared to those without (median RDI: 94.6%, 66.5%, and 40.2% in the no, one, and two dose reduction subgroups, respectively; Table 2).
Efficacy by patient subgroups defined by relative dose intensity
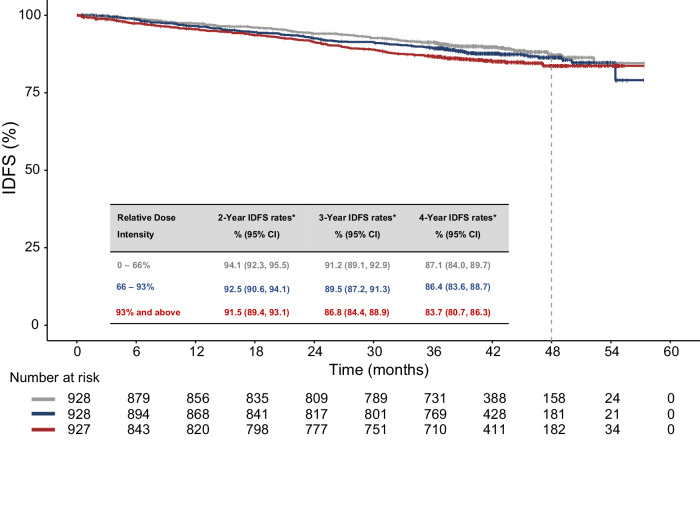
According to the Kaplan-Meier plots of IDFS by RDI subgroups (Fig. 1), the effect of abemaciclib was generally consistent across RDI subgroups with no clinically meaningful differences in the estimated 4-year IDFS rates between the RDI subgroups (4-year rates [95% CI]: 87.1% [84.0%, 89.7%], 86.4% [83,6%, 88.7%], and 83.7% [80.7%, 86.3%], respectively; Supplementary Table 1). Similar findings were observed in abemaciclib-treated patients in Cohort 1 (Supplementary Table 1 and Supplementary Figure 2).
Fig. 1. Invasive disease-free survival by relative dose intensity subgroup in patients treated with abemaciclib.
RDI was defined as the average daily dose of abemaciclib received by each patient over the treatment duration, relative to the full dose (150 mg twice per day). Among the 2791 abemaciclib-treated patients, 2783 had complete treatment exposure information for RDI calculation and thus were included in this analysis. *Estimated by the Kaplan-Meier method. For efficacy analyses, patients were divided into three equal-sized subgroups according to their abemaciclib RDI. Data cutoff date: July 01, 2022. CI confidence interval, IDFS invasive disease-free survival, RDI relative dose intensity.
Impact of dose reductions on efficacy using a time-dependent model
According to the time-dependent Cox proportional hazards model that included dose levels with their start and end time as the only variable, the abemaciclib benefit was consistent at the 150 mg full dose, compared to the reduced doses of 100 mg or 50 mg (unadjusted hazard ratio [95% CI] IDFS: 0.905 [0.727, 1.125]; DRFS: 0.942 [0.742, 1.195]; Table 3). These results were further supported by a time-dependent Cox PH model adjusted by baseline age, stratification factors, key disease characteristics, and pre-existing co-morbidities (Table 3). Similar findings were observed in Cohort 1 patients for both adjusted and unadjusted estimates (Table 3).
Table 3.
Time-dependent Cox PH model for the impact of dose reductions on Invasive disease-free survival and distant relapse-free survival
| Efficacy Endpoint | Assessment of Efficacy Staying at full dose vs Being reduced to a lower dose | |
|---|---|---|
| Unadjusted Hazard Ratio (95% CI)a | Adjusted Hazard Ratio (95% CI)b | |
| Patients treated with abemaciclib in Intent-to-treat population | ||
| IDFS | 0.905 (0.727, 1.125) | 0.922 (0.740, 1.148) |
| DRFS | 0.942 (0.742, 1.195) | 0.954 (0.751, 1.212) |
| Patients treated with abemaciclib in Cohort 1c | ||
| IDFS | 0.899 (0.718, 1.125) | 0.918 (0.732, 1.150) |
| DRFS | 0.958 (0.750, 1.223) | 0.972 (0.76, 1.243) |
Data cutoff date: July 01, 2022.
ALN axillary lymph node, CI confidence interval, DRFS distant relapse-free survival, IDFS invasive disease-free survival.
aHazard ratio (95% CI) was estimated using a time-dependent Cox proportional hazards model to assess the impact of dose levels over time on IDFS and DRFS.
bAdjusted by confounding baseline factors individually associated with risk of recurrence, including age, stratification factors, key disease characteristics, and pre-existing co-morbidities.
cCohort 1 included patients with ≥4 positive pathologic ALNs or 1-3 positive ALNs plus tumor size ≥5 cm and/or tumor grade 3.
In monarchE, 25.8% of patients discontinued abemaciclib due to reasons other than recurrence, including 18.5% due to AEs9. Additionally, the multivariate analysis of TTD identified that age ≥65 years, postmenopausal status, ≥4 pre-existing comorbidities, enrolled in North America or Europe, baseline Eastern Cooperative Oncology Group performance status (ECOG PS) of 1, and presence 1–3 positive ALN were independently associated with greater risk of abemaciclib discontinuation (Table 4). Older age was associated with the greatest increase in risk of treatment discontinuation. For the selected factors, discontinuation rates between subgroups within each factor diverged early and continued to separate during the 2-year treatment period with the highest rates of early discontinuation occurring in those aged ≥65 years old and/or with ≥4 pre-existing comorbidities (Supplementary Table 2; Supplementary Figure 3).
Table 4.
Multivariate analysis of factors associated with the risk of discontinuation in abemaciclib-treated patients
| Factors | Hazard ratio (95% CI) | Multivariate Modela,b P value | |
|---|---|---|---|
| Geographic region | Asia vs NA/Europe | 0.671 (0.541, 0.834) | <0.0001 |
| Other vs NA/Europe | 0.672 (0.557, 0.811) | ||
| Menopausal status | Post- vs premenopausal | 1.514 (1.268, 1.806) | <0.0001 |
| Age group | ≥65 years vs <65 years | 1.879 (1.566, 2.256) | <0.0001 |
| Baseline ECOG PS | 0 vs 1 | 0.801 (0.662, 0.971) | 0.0236 |
| Number of positive nodes | 4–9 vs 1–3 | 0.806 (0.685, 0.949) | <0.0001 |
| ≥10 vs 1–3 | 0.635 (0.514, 0.784) | ||
| Number of unique pre-existing comorbidities | 1–3 vs 0 | 1.213 (0.940, 1.566) | 0.0004 |
| ≥4 vs 0 | 1.563 (1.203, 2.032) | ||
Data cutoff date: July 01, 2022.
CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, NA North America; vs, versus.
aIncluded factors with P value < 0.05 in univariate analyses, selected in a stepwise fashion based on a multivariate Cox model, with an entry and retaining P value threshold of 0.05.
bWald’s P value.
To further explore the impact of dose reductions on efficacy in the adjuvant setting, any IDFS events occurring beyond the abemaciclib treatment period were censored at the time of abemaciclib completion as a sensitivity analysis. In a time-dependent Cox proportional hazards model adjusted by the factors associated with an increased discontinuation, the effect of abemaciclib during the 2-year treatment period was consistent at 150 mg, compared to the reduced doses of 100 mg or 50 mg (hazard ratio [95% CI] IDFS: 0.821 [0.597, 1.129]; DRFS: 0.804 [0.564, 1.145]).
Discussion
In the monarchE trial, adjuvant abemaciclib plus ET significantly improved IDFS and DRFS compared to adjuvant ET alone in patients with high-risk, HR+, HER2- EBC with sustained benefit beyond the 2-year abemaciclib treatment. The well-established safety profile of adjuvant abemaciclib is considered predictable, manageable, and acceptable in the high-risk EBC patient population8. Here, we provide comprehensive analyses assessing the impact of abemaciclib dose modifications, which are essential in the management of toxicities to maximize treatment adherence and retain patients on treatment to achieve optimal benefit. Notably, consistent with the findings in the metastatic setting8, our analyses suggest that the efficacy of adjuvant abemaciclib in high-risk EBC is not compromised by dose reductions.
Since dose reductions are common, it is critical to understand any potential impact of receiving a reduced adjuvant abemaciclib dose on efficacy. This unanswered question presents multiple challenges. First, the timing, number, and duration of dose reductions vary between patients. Second, dose reductions and treatment durations are positively correlated as patients with dose reductions were likely to remain on treatment longer and patients remaining on treatment were more likely to have had a dose reduction. Thus, direct comparisons of efficacy between patients who did and did not have dose reductions could be biased and were not conducted here. To take into account the timing and duration of the dose reduction data, several statistical analyses were applied. In the first instance, given the decreasing trend of RDI by the number of dose reductions, an efficacy analysis by patient subgroups defined by RDI was considered an indirect way to assess the impact of dose modifications on efficacy. Next, to evaluate the relationship between efficacy and dose levels more directly, a time-dependent Cox proportional hazards model was implemented. This more complex analytical approach incorporated the start and end time of each dose level and assessed the effect of staying at full dose in comparison with being reduced to a lower dose. Furthermore, additional sensitivity analyses using the time-dependent Cox model were performed to adjust for confounding effects of baseline characteristics that were potentially associated with dose reductions.
Importantly, multiple analyses assessing the impact of dose reductions on efficacy reached the same conclusion, confirming that the benefit of abemaciclib is consistent whether at the full 150 mg dose or reduced to 100 mg or 50 mg. Of note, the efficacy analysis by RDI subgroups showed numerically higher 4-year IDFS rates among patients with lower RDI for abemaciclib. However, as the confidence intervals around the estimates overlap across these subgroups, there is no evidence suggesting different efficacy across RDI subgroups. These results provide evidence that the treatment benefit is not compromised by dose reductions (made in accordance with the protocol) and is consistent with previous observations in the metastatic breast cancer8.
These observations are clinically relevant as early discontinuation and non-adherence rates for adjuvant ET are high and often unrecognized10,11. Treatment adherence in the adjuvant ET setting has also been reported to be critical to ensure benefit12. In monarchE, approximately 25% of patients discontinued abemaciclib before completing 2-years of treatment for reasons other than tumor recurrence (18.5% due to AEs)9. Most discontinuations occurred early in the treatment period and usually in the first months. Various factors were identified as independently prognostic of discontinuation from the 2-year treatment period in abemaciclib-treated patients, including ≥4 pre-existing comorbidities, age ≥65 years, baseline ECOG PS of 1, and postmenopausal status. Patients with any of these features should be closely monitored for symptoms with early interventions. Instead of discontinuing patients from treatment due to toxicity, dose reductions should be considered to manage side effects and improve treatment adherence.
With the goal of improving tolerability and retaining patients on treatment, abemaciclib dose reductions were commonly implemented to manage side effects particularly in patients ≥65 years old or with ≥4 pre-existing comorbidities. We have previously reported that patients with dose reductions generally completed the 2-year abemaciclib treatment. Only 8.9% of patients discontinued due to adverse events following dose reduction9. Furthermore, two-thirds of abemaciclib discontinuations due to adverse events were in response to low-grade events9, indicating a need for improved and earlier management of the symptoms with concomitant medications, patient education and/or dose modifications to achieve a tolerable dose and treatment persistence.
These exploratory analyses have limitations. First, it should be noted that dose modifications in clinical trials are made in a controlled manner per protocol and in accordance with recommendations to manage hematological and non-hematological toxicities. It is also important to recognize that the monarchE trial was not designed to investigate the impact of dose reductions on efficacy. Following randomization, patients assigned to the treatment arm started abemaciclib at 150 mg, with dose reductions implemented as a measure to manage toxicity for patients who could not tolerate the full dose. Therefore, it was not possible to directly compare different dosing strategies. As a result, the large variability in the number of dose reductions, timing and the treatment duration at each dose level necessitated more sophisticated statistical techniques and several sensitivity analyses that adjusted for confounding factors. Conversely, the process of exploring different analytical approaches also constitutes a strength of these exploratory analyses as they all led to consistent findings, thereby providing confidence in the robustness of the results.
In summary, patients receiving adjuvant abemaciclib should be carefully monitored for possible side effects during treatment, especially if they have features that are associated with higher risk of treatment discontinuation such as age ≥65 years old or ≥4 co-morbidities. Importantly, abemaciclib dose modifications effectively managed side effects and retained more patients on treatment, including those at higher risk for treatment discontinuation. Based on the multiple analyses presented, the efficacy of adjuvant abemaciclib in monarchE was not compromised by dose reductions. Therefore, when required, dose modifications improve tolerability and support the goal of maximizing adherence to maintain the benefit from adjuvant abemaciclib in combination with endocrine therapy for high-risk HR+, HER2- EBC.
Methods
Study design and patients
The study design, treatments, and procedures for the monarchE trial (ClinicalTrials.gov identifier: NCT03155997) have previously been published in detail1,2,9,13. Patients enrolled to one of two cohorts based on high-risk clinicopathological features. Cohort 1 included patients with ≥4 positive pathologic axillary lymph nodes (ALNs) or 1-3 positive ALNs plus ≥1 of the following: tumor size ≥5 cm or tumor grade 3. Cohort 2 included patients with 1-3 positive ALNs, tumor size <5 cm, tumor grade <3, and centrally assessed Ki-67 ≥ 20%.
Patients were randomized 1:1 to receive standard-of-care adjuvant ET for 5-10 years (physician’s choice) alone or in combination with abemaciclib (150 mg BID) orally for 2 years (on-study treatment period). Abemaciclib dose suspensions and/or up to two 50 mg dose reductions were allowed to manage hematological and non-hematological (diarrhea, increased alanine aminotransferase and/or aspartate aminotransferase, and interstitial lung disease) toxicities, based on toxicity type, severity, persistence, and recurrence. These recommendations have been published previously9 and are referenced in Supplementary Table 3.
The monarchE trial was conducted in accordance with the Declaration of Helsinki (1964) and its amendments, the Council for International Organizations of Medical Sciences International Ethical Guidelines, International Conference on Harmonization Good Clinical Practice Guidelines, and all applicable local laws and regulations. The study protocol14 was approved by institutional review boards at each site. All patients provided written, informed consent.
Statistical analyses
Details of the statistical analyses for the monarchE study have been published previously1,2,9,13. Data in the current analyses are from a prespecified overall survival interim analysis (data cutoff date: July 1, 2022; median follow-up 42 months)2. All patients were off abemaciclib treatment. Patients treated with at least one dose of abemaciclib were included in the analyses.
Among abemaciclib-treated patients, disease characteristics and patient demographics were summarized by the number of dose reductions (0, 1, or 2 dose reductions).
To assess the associations between abemaciclib exposure and the number of dose reductions, treatment duration, cumulative dose, and relative dose intensity (RDI) were summarized by the number of dose reductions. RDI was defined as the average daily dose over the treatment duration each patient received, relative to the full dose.
As dose reductions were expected to be associated with lower RDI, efficacy assessment by RDI-defined patient subgroups was performed as an indirect evaluation of dose reduction impact on efficacy. Patients were divided into three equal-sized subgroups according to their abemaciclib RDI (≤66%, 66–93%, and ≥93%). IDFS, as defined by the STEEP (Standardized Definitions for Efficacy End Points) criteria15, was estimated within each subgroup using the Kaplan-Meier method16.
To formally assess the impact of dose reductions on IDFS and DRFS, a time-dependent Cox proportional hazards model17 was fitted to account for the start and end time of each dose level received. The model assumed that the effect beyond the 2-year abemaciclib treatment period was the same as the last abemaciclib dose received by the patient. Hazard ratios comparing the effect of staying at the full dose versus a reduced dose to 100 or 50 mg were generated with 95% confidence intervals (CI). Additionally, an adjusted model was applied using inverse probability weighting to account for potentially confounding baseline factors that were individually associated with the risk of recurrence, including age, stratification factors, key disease characteristics, and pre-existing co-morbidities.
As a sensitivity analysis to test the assumption on the effect beyond abemaciclib treatment, any IDFS events that occurred beyond completing the 2-year treatment period were censored at the time of discontinuation in a time-dependent Cox proportional hazards model. As treatment discontinuations did not occur randomly, inverse probability censoring weighting was conducted to account for informative censoring wherein hazard ratios were adjusted by potentially confounding baseline factors as well as by factors selected as independently associated with the time to abemaciclib discontinuation due to reasons other than recurrence (TTD). To identify factors independently associated with an increased risk of early abemaciclib discontinuations, patient and disease characteristics were fitted in a multivariate Cox proportional hazards model for time to abemaciclib discontinuations due to reasons other than recurrence, using a stepwise variable selection with entry and retaining a P-value threshold of 0.05. The discontinuation rates at 3, 6, 12 and 24 months were estimated using the Kaplan-Meier method16 within each subgroup of the selected factors that were independently prognostic of treatment discontinuation.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Updated Supplementary information - Impact of dose reductions on the efficacy of adjuvant abemaciclib for high-risk EBC: analyses from the monarchE study
Acknowledgements
This work was supported by Eli Lilly and Company. Eli Lilly had a role in the design of the study and collection, analysis, and interpretation of data and in writing. We thank the 5637 patients and their families/caregivers from 603 sites in 38 countries for participating in this trial. We would like to generously thank the investigators and their support staff who participated in this work. We are also very grateful for the time and efforts of the monarchE Executive and Global Steering Committees. Dr. Goetz is the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. Writing and editorial support were provided by Preethi Govindarajan and Adrienne Schreiber of Syneos health.
Author contributions
M.P.G., S.R.D.J., P.R., N.H., V.A., and H.S.R. contributed to the study conception and design. M.P.G., I.C., L.T., S.M.T., J.H., V.G., S.R.D.J., M.M., P.R., N.H., A.S., R.W., V.A., H.S.R., and J.O. contributed to the conduct or collection, data analysis and interpretation. M.P.G., I.C., L.T., S.M.T., J.H., V.G., S.R.D.J., M.M., P.R., N.H., A.S., R.W., V.A., H.S.R., and J.O. contributed to the drafting of the manuscript and critical revisions. All authors gave their final approval of the manuscript to be submitted.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available in order to protect patient privacy but are available from the corresponding author on reasonable request.
Code availability
Data analyses were performed using SAS (enterprise guide 7.1) and R studio server (R version 4.1.2). The code cannot be accessed but may be available from the corresponding author on reasonable request.
Competing interests
MPG reports financial Interests, Institutional, Advisory Board: ARC Therapeutics, Biotheranostics, Biotheryx, Blueprint Medicines, Novartis, Rna Diagnostics, Sanofi Genzyme; Financial Interests, Institutional, Other, Consulting: AstraZeneca, Seattle Genetics; Financial Interests, Institutional, Other, General Consulting: Lilly; Financial Interests, Personal, Invited Speaker, CME Activity: Research to Practice, Clinical Education Alliance, Medscape, MJH Life Sciences; Financial Interests, Personal, Invited Speaker, CME Panel Discussant: Total Health Conferencing; Financial Interests, Personal, Other, Moderator for CME Activity: Curio Science; Financial Interests, Institutional, Local PI: Lilly, Pfizer, LOXO, ATOSSA Therapeutics, AstraZeneca, Sermonix; travel support from Lilly. IC does not report any competing interests. LT is a speaker for Novartis, Roche, Pfizer, Zodiac, Lilly, Merck and Co., Daiichi-Sankyo, and Astra Zeneca. She is on the Advisory Boards for Eli Lilly and Company, Novartis, MSD, Amgen, Daiichi-Sankyo, AstraZeneca, and Pfizer. She receives educational support from Pfizer, Libbs Farmaceutica Ltd, United Medical, Lilly, Zodiac, Daichi-Sankyo, and Gilead. She receives grants from Novartis. She is also on the monarchE steering committee. SMT reports institutional research funding from Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, Gilead, NanoString Technologies, Seattle Genetics, and OncoPep; and consultant/advisory roles for Novartis, Pfizer, Merck, Eli Lilly, AstraZeneca, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, CytomX Therapeutics, Daiichi-Sankyo, Gilead, Ellipses Pharma, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Myovant, Zetagen, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Biopharma, Bayer, Incyte Corp, and Jazz Pharmaceuticals. JH receives honoraria from Eli Lilly and Company, Novartis, Roche, Pfizer, AstraZeneca, Seagen, Gilead, amd Daiichi-Sankyo. She consults with Eli Lilly and company, Novartis, Roche, Pfizer, AstraZeneca, Gilead, and Daiichi-Sankyo. She reports travel expenses from Roche, Novartis, Daiichi-Sankyo, and Gilead. VG reports personal fees for advisory board membership for AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly and company, Exact Sciences, Gilead, Merck Serono, MSD, Novartis, Pfizer, Olema Oncology, Pierre Fabre; personal fees as an invited speaker for AstraZeneca, Daiichi Sankyo, Eli Lilly and company, Exact Sciences, Gilead, GSK, Novartis, Roche and Zentiva; personal fees for expert testimony for Eli Lilly. SRDJ reports grants or contracts from Pfizer, Puma Biotechnology, Eli Lilly, AstraZeneca, Novartis, and Roche–Genentech for research funding to institute for laboratory studies and clinical trials; consulting fees from Eli Lilly, AstraZeneca, Puma Biotechnology, Pfizer, Novartis, and Sanofi Genzyme for consulting or an advisory role; and payment or honoraria from Pfizer, Eisai, AstraZeneca, and Roche–Genentech for speaker’s bureau. MM has received research grants from Roche, PUMA and Novartis, consulting/advisory fees from AstraZeneca, Amgen, Taiho Oncology, Roche/Genentech, Novartis, PharmaMar, Eli Lilly, PUMA, Taiho Oncology, Daiichi Sankyo, Menarini/Stemline, and Pfizer and speakers’ honoraria from AstraZeneca, Lilly, Amgen, Roche/Genentech, Novartis, and Pfizer. PR does not report any competing interest. NH reports honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi-Sankyo, EPG Communication, Gilead, Lilly, MEDSCAPE, MSD, Novartis, Pierre-Fabre, Pfizer, Roche, Sandoz, Sanofi, Seagen; Springer, Viatris, Zuelligpharma. She reports a minority ownership Co-Director West German Study Group (WSG). AS, RW, and VA are employees of Eli Lilly and company and minor shareholders. HSR reports institutional research support from AstraZeneca; Daiichi Sankyo, Inc.; F. Hoffmann-La Roche AG/Genentech, Inc.; Gilead Sciences, Inc.; GlaxoSmithKline; Lilly; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; OBI Pharma; Pfizer; Sermonix Pharmaceuticals Inc.; and Stemline. She reports consultancy/advisory support from Puma, NAPO, Scorpion Therapeutics, Mylan, Daiichi Sankyo. JO discloses honoraria from AbbVie Inc, Agendia, Amgen Biotechnology, Aptitude Health, AstraZeneca, Bayer, Bristol-Myers Squibb, Carrick Therapeutics, Celgene Corporation, Daiichi Sankyo, Eisai, G1 Therapeutics, GlaxoSmithKline, Genentech, Genzyme, Gilead Sciences, Immunomedics, Lilly, Merck, Novartis, Ontada, Pfizer, Pharmacyclics, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Prime Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, Stemline Therapeutics, Theralink, and Synthon.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-024-00639-1.
References
- 1.Johnston SRD, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J. Clin. Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston SRD, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77–90. doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Medicines Agency. VERZENIOS™ (abemaciclib). https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios (2022).
- 4.Food and Drug Administration (FDA). FDA D.I.S.C.O. Burst Edition: FDA approval of Verzenio (abemaciclib) with endocrine therapy for patients with HR-positive, HER2-negative, node-positive, early breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-verzenio-abemaciclib-endocrine-therapy-patients-hr-positive (2023).
- 5.Pharmaceuticals and Medical Devices Agency (PMDA). VERZENIO™ (abemaciclib). https://www.pmda.go.jp/drugs/2018/P20181004001/530471000_23000AMX00808_A100_1.pdf (2021).
- 6.National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Breast cancer. Version 4. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (2023). [DOI] [PubMed]
- 7.European Society for Medical Oncology (ESMO). ESMO-MCBS scorecards: abemaciclib. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-scorecards/scorecard-371-1 (2023).
- 8.Rugo HS, et al. Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: safety analysis of MONARCH 2 and MONARCH 3. Oncologist. 2021;26:e53–e65. doi: 10.1002/onco.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugo HS, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 2022;33:616–627. doi: 10.1016/j.annonc.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Hadji P, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann. Oncol. 2013;24:1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 11.Ziller V, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann. Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbeck N, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Eli Lilly and Company. monarchE Protocol I3Y-MC-JPCF(e). https://classic.clinicaltrials.gov/ProvidedDocs/97/NCT03155997/Prot_000.pdf (2019).
- 15.Hudis CA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J. Clin. Oncol. 2007;25:2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation of incomplete observation. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 17.Therneau T. M. & Grambsch P. M. Modeling Survival Data: Extending the Cox Model. 10.1007/978-1-4757-3294-8 (Springer, 2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Updated Supplementary information - Impact of dose reductions on the efficacy of adjuvant abemaciclib for high-risk EBC: analyses from the monarchE study
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available in order to protect patient privacy but are available from the corresponding author on reasonable request.
Data analyses were performed using SAS (enterprise guide 7.1) and R studio server (R version 4.1.2). The code cannot be accessed but may be available from the corresponding author on reasonable request.