Abstract
Essential tremor (ET) is a prevalent neurological disorder with a largely unknown underlying biology. In this genome-wide association study meta-analysis, comprising 16,480 ET cases and 1,936,173 controls from seven datasets, we identify 12 sequence variants at 11 loci. Evaluating mRNA expression, splicing, plasma protein levels, and coding effects, we highlight seven putative causal genes at these loci, including CA3 and CPLX1. CA3 encodes Carbonic Anhydrase III and carbonic anhydrase inhibitors have been shown to decrease tremors. CPLX1, encoding Complexin-1, regulates neurotransmitter release. Through gene-set enrichment analysis, we identify a significant association with specific cell types, including dopaminergic and GABAergic neurons, as well as biological processes like Rho GTPase signaling. Genetic correlation analyses reveals a positive association between ET and Parkinson’s disease, depression, and anxiety-related phenotypes. This research uncovers risk loci, enhancing our knowledge of the complex genetics of this common but poorly understood disorder, and highlights CA3 and CPLX1 as potential therapeutic targets.
Subject terms: Genome-wide association studies, Genetic variation, Movement disorders, RNA sequencing, Proteomic analysis
GWAS meta-analysis associates 12 sequence variants with essential tremor, identifies seven candidate causal genes including CA3 with multiomics-based analysis, and reveals key roles of dopaminergic and GABAergic neurons in the pathogenesis.
Introduction
Essential tremor (ET) is one of the most common neurological disorders, affecting up to 5% of the population1. However, epidemiological studies show that the prevalence of the disorder is considerably underestimated as mildly affected individuals may not seek medical care2.
ET is an isolated syndrome of bilateral upper limb postural or kinetic tremor, that may be with or without tremor of head, voice, or lower limbs and without other neurological signs such as dystonia, ataxia, or parkinsonism3. Although not life-threatening, the disorder can severely impact daily activities, reducing quality of life. Increasing age, European descent4–6 and family history7,8 are considered risk factors for ET.
Diagnosing ET can be challenging and often requires subspecialty consultation with a movement disorders neurologist. The diagnosis involves reviewing medical and family history and conducting a thorough neurological examination, as a biomarker or diagnostic test is not available9. Although there is no cure for ET, several treatment options are available to ease the symptoms. These include drug therapy (beta blockers, anti-epileptics, and tranquilizers), deep brain stimulation, and lifestyle modifications, such as avoiding triggers that can increase the severity of the tremors.
The cause of ET is not fully understood, but there is a growing support for the etiology of ET being partly related to abnormalities of the cerebello-thalamo-cortical network, including loss of Purkinje cells and reduced γ-aminobutyric acid (GABA) receptor expression in the dentate nucleus10,11.
Previously, the largest genome-wide association study (GWAS) meta-analysis of ET reported five risk loci using data from 7177 cases and 475,877 controls from European populations12. Here, we more than double the ET case number, combining 16,480 cases and 1,936,173 controls in a GWAS meta-analysis and find 12 independent sequence variants at 11 loci, of which 8 are novel. Our research presents new genetic revelation regarding GABAergic dysfunction in ET, highlights the role of dopaminergic neurons, and provides further insight into the genetics of ET, offering clues that may lead to novel future treatment options.
Results
GWAS meta-analysis
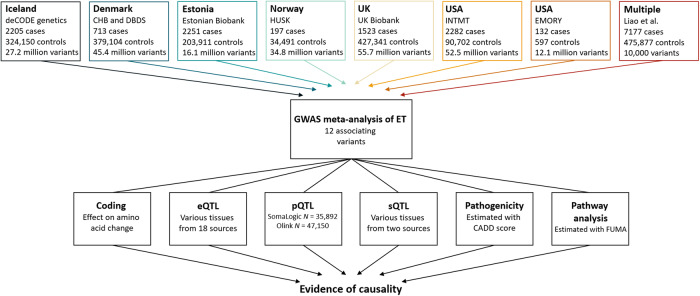
In a meta-analysis of ET, we combined GWAS results from Iceland, Denmark, Estonia, Norway, UK, and USA (seven datasets) with summary statistics from a reported GWAS12, resulting in 16,480 cases and 1,936,173 controls (Fig. 1 and Supplementary Data 1). Using a fixed-effect inverse variance model, we tested for association, under an additive model, between ET and sequence variants with imputation information over 0.8 and minor allele frequency (MAF) over 0.01% in each dataset (except the Estonian dataset and the reported GWAS, where variants with MAF over 1% were included). To account for multiple testing, we used weighted genome-wide significance thresholds based on the predicted functional impacts of the associated variants (Supplementary Data 2).
Fig. 1. Study design.
The first row lists the datasets used in the GWAS meta-analysis, number of ET cases, controls and variants analyzed. We included variants with MAF > 0.01% in all datasets except for the Estonian dataset and the previous GWAS12, where variants with MAF > 1% were included. The summary data from a previous GWAS, only includes the top 10,000 variants. The last row lists the multiomics approaches used to search for potential causal genes. Expression quantitative trait loci (eQTL) data sources are listed in Supplementary Data 15. Plasma protein levels (pQTL) were measured in Icelandic samples using Somalogic platform and in UK samples using the Olink platform. Splicing quantitative loci (sQTL) data were estimated using Icelandic RNA sequencing data, in addition to data imported from GTEx.
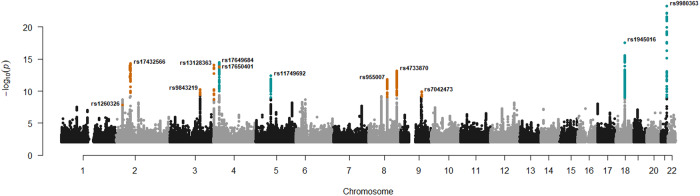
We uncovered association with ET at 11 loci (Supplementary Fig. 1 and Supplementary Data 3). Conditional analysis revealed a secondary signal at one of the loci (Supplementary Data 4). In total, we uncovered 12 independent common variants, 8 of which are novel (Fig. 2). There was no evidence of heterogeneity (all P-het > 0.05), indicating consistency of effects across the datasets (Supplementary Data 3). Five variants have been reported to associate with ET12 and we show supportive evidence for all except one, at chromosome 1p13.1 (Supplementary Data 5). We report these signals directly or through a correlated variant (r2 ≥ 0.8) at the same locus (Supplementary Data 5). At chromosome 4p15.2, we observed a previously reported variant as the primary signal, along with a novel variant at the same locus acting as the secondary signal (r2 = 0.022, Supplementary Fig. 1c, f).
Fig. 2. Manhattan plot showing common variants in the ET meta-analysis.
The -log10P-values (y-axis) are plotted for each variant against their chromosomal position (x-axis). Variants with P-values below their weighted variant-class threshold are highlighted. Novel variants are marked in orange and previously reported variants are marked in blue. P-values are two-sided and derived from a likelihood-ratio test. Manhattan plots for each dataset are shown in Supplementary Fig. 2.
Potential causal genes
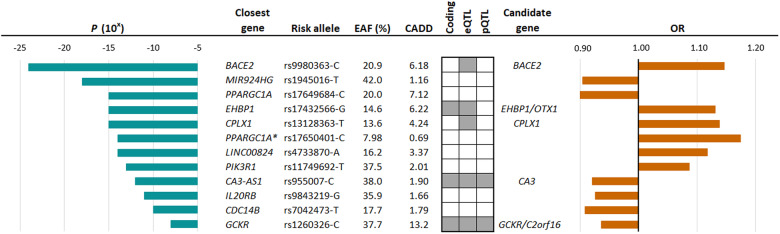
We searched for causal genes at the ET loci by evaluating the affected amino acid sequence of the lead variants and highly correlated variants (r2 ≥ 0.8), mRNA expression (expression quantitative trait loci [eQTLs]), splicing quantitative loci (sQTLs), and plasma protein levels (pQTLs) (Fig. 1). We found coding variants at three of the ET associated loci, in CA3 (p.V31I, r2 = 1.00 with lead variant), EHBP1 (p.K720Q/K755Q, r2 = 0.96 with lead variant), and GCKR (p.L446P, lead variant) (Fig. 3 and Supplementary Data 6). We found eQTLs for BACE2, CPLX1, OTX1, C2orf16, and CA3 (Supplementary Data 7) and pQTLs for CA3 (Supplementary Data 8) and GCKR (Supplementary Data 9).
Fig. 3. Sequence variants that associate with ET and multiomics approaches used to uncover candidate causal genes.
Using multiomics approaches of the lead 12 variants, we identified 7 potential causal genes. Gray boxes indicate where data points to a candidate causal gene. Effects are shown for the minor allele. Combined Annotation Dependent Depletion (CADD)84 score estimates the deleteriousness of sequence variants. Variants are considered pathogenic if CADD > 12.37. *Secondary signal at PPARGC1A.
The lead ET variant at the CA3 locus confers protection against ET (rs955007-C, P = 1.4 × 10−12, OR = 0.92, Fig. 3. and Supplementary Data 3) and is also highly correlated (r2 = 0.97) with the primary cis-eQTL which decreases CA3 expression in skeletal muscles (rs10088136-A, P = 8.7 × 10−13, β = -0.13, Supplementary Data 7). Using COLOC13, we estimated that the posterior probability that the ET association and the eQTL are caused by the same variant is 89%. In addition, the lead variant is highly correlated (r2 = 1.00) with the primary pQTL for carbonic anhydrase III and decreases its plasma levels (chr8:85445533, P = 3.2 × 10−131, β = −0.22, Supplementary Data 8). Furthermore, rs955007-C also associates with lower plasma levels of carbonic anhydrase XIII (located roughly 200KB upstream, P = 6.3 × 10−195, β = −0.23). However, rs955007 is not in high LD (r2 = 0.14) with the primary pQTL at the region (Supplementary Data 8).
The lead intronic ET variant within EHBP1 is highly correlated (r2 = 0.91) with the top cis-eQTLs for OTX1 in whole blood (rs76298426-C, P = 10−1673, β = 1.15) and neutrophiles (rs146236066-CT, P = 1.6 × 10−51, β = 0.98, Supplementary Data 7).
We identified an intronic variant in CPLX1 that confers risk of ET (rs13128363-T, P = 9.0 × 10−15, OR = 1.14, Fig. 3 and Supplementary Data 3) and is the top cis-eQTL for the gene in whole blood (Supplementary Data 7).
There were no sQTLs at the loci in whole blood.
Gene-set enrichment analysis in FUMA14 highlighted cell types in the human embryonic midbrain such as dopaminergic neurons, GABAergic neuroblasts and neurons, and mediolateral neuroblast (Supplementary Data 10). In addition, the analysis revealed enrichment for pathways such as regulation of response to stress, cell adhesion, and Rho GTPase cycles (Supplementary Data 10).
The sex ratio in our study is close to 50% (52.9% females, Supplementary Data 1). When applying sex-specific models to the Icelandic, Danish, Norwegian, UK, and US-INTMT datasets for the 12 ET variants, none of the variants had an effect that significantly differed between the sexes, after accounting for multiple testing (P-het > 0.05/12 = 0.0042, Supplementary Data 11).
Familial clustering and genetic variance explained
A close to complete genealogy exists for the Icelandic dataset. We did not find high-impact variants, including start-lost, stop-gain, stop-lost, splice donor, splice acceptor, or frameshift, segregating among 53 large families with high incidence of ET (N ≥ 5). In addition, we did not see a significant difference in effects of 11 of the 12 lead variants between familial (N = 1153) and sporadic cases (P-het > 0.05/12 = 0.0042, Supplementary Fig. 3). Using the 12 independent variants, we estimated the genetic variance explained to be 4.4% (Supplementary Data 12).
Genetic correlation
Considering the epidemiology and positive genetic correlation that has been reported between ET and Parkinson’s disease (PD) and depression12, we estimated the genetic correlation between ET and these two phenotypes using the most recent GWASs and cross-trait LD score regression. In line with previous reports, we observed a positive genetic correlation between ET and these phenotypes (PD15, rg = 0.28, P = 1.1 × 10−6; depression16, rg = 0.15, P = 3.4 × 10−5, Supplementary Data 13). In addition, we estimated the genetic correlation between ET and summary data from 1142 published GWASs (P-threshold ≤ 0.05/1142 = 4.4 × 10−5) and found that ET correlates most strongly with anxiety-related phenotypes (e.g., feeling nervous, rg = 0.20, P = 2.0 × 10−6, Supplementary Data 13).
Discussion
We report a GWAS meta-analysis of ET that combines 16,480 cases and expands results from previous GWASs by identifying 12 variants at 11 loci, of which 8 are novel. We leveraged mRNA expression, including splicing, plasma protein measurements and predicted coding effects to highlight seven putative causal genes and the biological roles of some of the variants. Through gene-set enrichment analysis, we underscored the involvement of dopaminergic and GABAergic neurons in ET, as well as the biological significance of the Rho GTPase cycle. We did not find high-impact variants segregating in families with high prevalence of ET. We showed a positive genetic correlation between ET and PD, depression, and anxiety-related phenotypes.
Based on functional annotation, we highlighted seven genes that may participate in the pathogenesis of ET. One of the candidate causal genes is CA3 which encodes carbonic anhydrase III and is in close proximity ( >1 Mb) to other carbonic anhydrase genes including CA1, CA2, and CA13. Carbonic anhydrase inhibitors represent a class of drugs that have demonstrated the ability to improve tremors, potentially through modulating brain pH levels. Additionally, acidification facilitates GABA receptor potentiation which may facilitate the effect of the inhibitors, on tremor and seizures17,18. Primidone, an anti-epileptic drug widely used to treat ET patients, has been shown to inhibit carbonic anhydrase II18. The lead variant at the CA3 locus confers protection against ET and is highly correlated with a variant that associates (top cis-eQTLs) with decreased expression of CA3 in skeletal muscles. The variant also associates with decreased plasma levels of carbonic anhydrase III and XIII. The high LD at the locus suggest a potentially shared biological mechanism or pathway through which the variants exert their effects. Further research is needed to identify the most likely causal variants. Carbonic anhydrases are relevant proteins that may have an important role in the biology of ET. This notable finding lends support to the hypothesis that inhibiting carbonic anhydrases could lead to improved tremor control. Thus, targeting the interplay of CA3 and its closely related enzymes might lead to the development of more targeted and effective treatments for individuals suffering from ET.
While ET may not be directly caused by imbalances in neurotransmitters, certain neurotransmitters have been implicated in its development and severity, such as GABA10,19,20. CPLX1 encodes Complexin-1, a soluble presynaptic protein that specifically enhances transmitter release by increasing fusogenicity of synaptic vesicles21. CPLX1 is overexpressed in substantia nigra from PD patients22 and dysregulation of the gene have been associated with neurogenetic disorders23, including myoclonic epilepsy24. In addition, homozygous Cplx1 knockout mice have the earliest known onset of ataxia seen in a mouse model21,25,26. We identified an intronic variant in CPLX1 that increases the risk of ET and is the top cis-eQTL for CPLX1 in blood. This finding aligns with previous research and strongly suggests the involvement of CPLX1 in the pathogenicity of ET.
OTX1 is a homeodomain transcription factor and is encoded by Orthodenticle homeobox 1. In the mammalian brain, OTX1 is expressed in the forebrain and midbrain during early stages of neural development27 and at later stages and adulthood at high levels in layers 5 and 6, the deepest layers of the cortical plate28,29. Layer 5 neurons convey signals controlling motor behavior via their projections to the colliculi, pons, and spinal cord30–33. Otx1 mutant mice have been reported to show spontaneous epileptic behavior and multiple abnormalities affecting certain brain regions34. Otx1 mutant animal studies suggest that Otx1 is required for the development of normal axonal connectivity and the generation of coordinated motor behavior29. The ET associated variant in EHBP1 is in high LD with a missense variant in the same gene and is, additionally, highly correlated with two intronic variants in EHBP1 and one variant in OTX1, all of which are top cis-eQTLs for OTX1 expression in blood and neutrophiles. Based on these findings, our observations suggest that the intronic variant in EHBP1 may contribute to the risk of ET by potentially upregulating the expression of OTX1. Consequently, OTX1 emerges as a promising candidate gene that could play a significant role in the underlying pathogenic mechanism of ET. However, it is important to note that we did not investigate protein levels of OTX1, as it is neither measured on the Somalogic nor Olink platforms.
GCKR has been associated with high serum uric acid and purine metabolism disorders35. Extrapyramidal signs such as tremor are often observed in these disorders36. Individuals with high serum uric acid might experience tremors attributed to these metabolic issues, yet, due to the lack of routine serum uric acid testing, they could be incorrectly diagnosed with ET. Whether the tremor associated with the GCKR variant is a phenocopy or typical ET needs to be investigated further.
GABAergic dysfunction, consistently observed in ET patients10,19,37, is a focal point of research, but its genetic underpinnings remain unidentified. Our gene-set enrichment analysis reinforces the proposed role of GABA in ET, yet the exact role of GABA in the causative framework requires further elucidation. In addition, our analysis found enrichment for dopaminergic neurons, a crucial regulator of extrapyramidal movement. Given the shared phenotypic and genetic traits between ET and PD, the association with dopaminergic neurons is anticipated, particularly as their selective degeneration in the substantia nigra pars compacta characterizes PD38. Notably, neurologist have long suspected a potential link between ET and PD, but definitive evidence for this relationship has remained elusive39. Furthermore, our analysis underscores the significance of various biological processes, most notably the Rho GTPase cycle. Rho GTPases regulate the actin cytoskeleton of dopaminergic neurons, thus influencing their degeneration40,41, and have been implicated in PD41,42. Interestingly, our findings underscore the significance of stress response regulation, especially given the genetic overlap between ET and anxiety phenotypes. ET patients exhibit intensified tremors under stress, and anti-anxiety medications, such as Clonazepam, prove highly effective in alleviating these tremors.
Familial clustering of ET is well recognized. However, estimates of the proportion with a family history is highly debated and ranges from as low as 17% to as high as 100%43. Linkage studies have identified susceptibility loci on 3q1344 and 2p24.145 but others have shown absence of linkage at these loci46. The absence of a significant difference between the effects of sporadic and familial cases and the lack of high-impact variants segregating in several large Icelandic families, underscores the potential that ET may be predominantly influenced by common variants or a combination of such variants, rather than rare variants. To gain deeper insights into the contribution of common variants on ET risk, the creation of a polygenic risk score would be valuable. A PRS analysis, if conducted with larger and more diverse datasets, could offer further clarity on the polygenic nature of ET and thus, the assembly of larger cohorts in future research holds promise to increasing our understanding of the complex genetics of ET. Another limitation lies in the lack of ethnic diversity within the datasets studied. As an increasing amount of genotypic and phenotypic data becomes available for diverse ethnic backgrounds, the inclusion of greater diversity in future studies could be helpful in uncovering ethnicity-specific genetic contributions and advancing our understanding of the genetic underpinnings of ET.
In the previous GWAS conducted on ET, a subset consisting of 216 cases from the UK Biobank ET data was used. Regrettably, we could not ascertain whether these particular cases overlap with the UK Biobank cases utilized in our current study. It is worth mentioning that even if all of these cases overlap with our data, they represent only 2% of the total 9303 cases (excluding cases from the previous GWAS). Given their small proportion, the exclusion of these cases would not significantly impact the results or conclusions of our study.
To conclude, through a comprehensive GWAS meta-analysis and a multiomics approach using a substantial cohort, we have advanced our understanding of the genetics and pathogenesis of ET. This progress not only enhances our knowledge of this complex and prevalent neurological disorder but can also form the basis for future investigations into treatment strategies and personalized interventions.
Methods
Study sample and ethics statement
In this study, ET cases were defined using International Classification of Diseases 10 (ICD-10) code G25.0 or ICD-9 code 333.1 in all datasets, as described in detail below, in addition to the sample defined by Liao et al.12. All ethical regulations relevant to human research participants were followed. The data used in the GWAS meta-analysis were collected through studies approved by ethics committees governing each dataset and written informed consent was obtained from all participants. Personal identifiers of participants’ data were encrypted for privacy protection purposes in accordance with the regulation in each country. Genetic ancestry quality control was performed for all datasets47–50 and participants were genotypically verified as being of European descent. In total, we studied data from 16,480 ET cases and 1,936,173 controls (Fig. 1 and Supplementary Data 1).
Iceland – deCODE genetics
A large fraction of the Icelandic population has participated in a research program at deCODE genetics. Participants donated blood or buccal samples after signing a broad informed consent allowing the use of their samples and data in various projects approved by the National Bioethics Committee (NBC). The data in this study was approved by the NBC (VSN-17-142-V5; VSNb2017060004/03.01) following review by the Icelandic Data Protection Authority. All personal identifiers of the participants’ data were encrypted in accordance with the regulations of the Icelandic Data Protection Authority. The Icelandic ET cases were identified from medical records, filed from 1985 to 2022, through collaboration with physicians at Landspitali—National University Hospital in Reykjavik, the Registry of Primary Health Care Contacts, and the Registry of Contacts with Medical Specialists in Private Practice.
Denmark – The Copenhagen Hospital Biobank and The Danish Blood Donor Study
The Copenhagen Hospital Biobank (CHB) is a research biobank, which contains samples obtained during diagnostic procedures on hospitalized and outpatients in the Danish Capital Region hospitals. Data analysis was performed under the Developing the basis for personalized medicine in degenerative and episodic brain disorders protocol, approved by the National Committee on Health Research Ethics (H-21058057). The Danish Blood Donor Study (DBDS) Genomic Cohort is a nationwide study of ~160,000 blood donors51. The Danish Data Protection Agency (P-2019-99) and the National Committee on Health Research Ethics (NVK-1700407) approved the studies under which data on DBDS participants were obtained. The DBDS data requested for this study was approved by the DBDS steering committee.
Estonia – Estonian Biobank
The Estonian Biobank is a population-based cohort of approximately 210,000 participants, each accompanied by a variety of phenotypic and health-related data52. Upon recruitment, participants granted permission through signed consents for subsequent linkage to their electronic health records, enabling the longitudinal accumulation of phenotypic details. The Estonian Biobank facilitates access to the records from the National Health Insurance Fund Treatment Bills (since 2004), Tartu University Hospital (since 2008), and North Estonia Medical Center (since 2005). For each participant, data is available on diagnoses coded in ICD-10 and drug dispensing records, including ATC codes, prescription statuses, and purchase dates (when available). The activities of the EstBB are regulated by the Human Genes Research Act, which was adopted in 2000 specifically for the operations of the EstBB. Analysis of individual level data from the EstBB was carried out under ethical approval 1.1-12/624 from the Estonian Committee on Bioethics and Human Research (Estonian Ministry of Social Affairs), using data according to release application [6-7/GI/29 977] from the Estonian Biobank.
Norway – The Hordaland Health Study
The Hordaland Health Study (HUSK) is a community-based study in Western Norway conducted as a collaboration between the University of Bergen, the Norwegian Health Screening Service and the Municipal Health Service in Hordaland (https://husk-en.w.uib.no/)53. In 1992–93 and 1997–99 participants were invited based on year of birth and site of residence. Residents from Hordaland County born 1950–52 and residents from Bergen and three neighboring municipalities born 1925–27, in addition to a random sample born 1926–49 were invited in 1992–93. In 1997–99, previous participants born 1950–51 and 1925–27 were reinvited, in addition to all residents in Hordaland County born 1953–57. In total, approximately 36,000 individuals participated in the study, 18,000 in 1992–93 and 26,000 in 1997–99, with some participating at both times. ET cases were identified through diagnostic codes reported in the patient registry during 2008 to 2021. The HUSKment study is approved by the Regional Committee for Medical Research Ethics Western Norway, reference 2018/915.
The UK – The UK Biobank
The UK Biobank resource has collected extensive phenotype and genotype data from ~500,000 participants in the age range 40–69, from across the UK after signing an informed consent for the use of their data in genetic studies54. The North West Research Ethics Committee reviewed and approved UK Biobank’s scientific protocol and operational procedures (REC Reference Number: 06/MRE08/65). This study was conducted using the UK Biobank resource under application number 42256. ET cases were identified in General Practice clinical event records (Field ID 42040) and UK hospital diagnoses (Field ID 41270 and 41271).
The US – Intermountain Healthcare
Participants, voluntary US residents over the age of 18 years, were recruited by The Intermountain Inspire Registry and The HerediGene: Population study55, a large-scale collaboration between Intermountain Healthcare, deCODE genetics, and Amgen, Inc (https://intermountainhealthcare.org). The Intermountain Healthcare Institutional Review Board approved this study, and all participants provided written informed consent prior to enrollment.
The US – Emory General Clinical Research Center
The Clinical Research in Neurology (CRIN) provides an umbrella structure for subject enrollment in observational and genetic studies in neurology, consent-approved data sharing across studies and disorders, and consistent sample processing. Participants were recruited under the CRIN protocol through support from Emory Clinical Research Center NIH/NCRR M01 RR00039 (CRIN Infrastructure support). The study was approved by the Emory Institutional Review Board (IRB) and informed consent was obtained from all subjects. ET genotyping work was done under specific IRB protocols. Samples were drawn from either review of previously enrolled subjects in the CRIN database, or prospective enrollment of ET subjects into CRIN/ET observational and genetics work. All subjects underwent a basic structured interview for demographics and family history. A Folstein Mini Mental Status Exam was administered to all CRIN subjects by trained CRIN personnel supervised by a neuropsychologist per published guidelines. All CRIN database subjects enrolled prior to January 2007 with a reported diagnosis of 333.1 were reviewed. ET subjects were called in for full in-person assessments whenever possible. ET subjects mid-2006 onward were recruited through IRB-approved ads in the Emory Movement Disorders and Neurosurgery deep brain stimulation group clinics, and ET community education events. ET subjects and family members were examined directly by at least one movement disorders specialist; two independent exams were obtained whenever possible (a tremor rating scale derived from the Fahn-Tolosa-Marin scale and Tremor Research Group scale items, the motor United Parkinson Disease Rating Scale, Tinetti gait and balance scales56, tandem gait57, and assessment for dystonia). Semi-structured interviews included ET specific questions derived from the Fahn-Tolosa-Marin scale and WHIGET studies58. CRIN review and new enrollment subjects were given a research diagnosis of ET using Movement Disorders Society and Tremor Research Group criteria. ET cases with either Parkinson’s disease or dystonia were excluded. Subjects were excluded based on a number of criteria; if an in-person exam and re-interview determined a different diagnosis, if movement disorders clinical notes listed an uncertain or different final diagnosis (i.e., medication induced tremor), if there was an incomplete examination, lack of medication response, or other data to clearly establish an ET research diagnosis.
Genotyping and imputation
Iceland – deCODE genetics
The genomes of 63,460 Icelanders were whole genome sequenced (WGS)47,59 using GAIIx, HiSeq, HiSeqX, and NovaSeq Illumina technology to a mean depth of 38×. Genotypes of single nucleotide polymorphisms (SNPs) and insertions/deletions (indels) were identified and called jointly with Graphtyper60,61. Over 173,000 Icelanders (including all WGS Icelanders) were genotyped using various Illumina SNP arrays47,59. The genotypes were long-range phased62, which allows for improving genotype calls using haplotype sharing information. Subsequently, extensive encrypted genealogic information was used to impute variants into the chip-typed Icelanders, as well as ungenotyped close relatives63 to increase the sample size and power for association analysis.
Denmark – The Copenhagen Hospital Biobank and The Danish Blood Donor Study
The Danish samples from the CHB and DBDS were genotyped using Illumina Global Screening Array, and long-range phased together with 270,627 genotyped samples from North-western Europe using Eagle264. Samples and variants with less than 98% yield were excluded. A haplotype reference panel was prepared in the same manner as for the Icelandic data47,62 by phasing genotypes of 25,215 WGS individuals (sequenced with NovaSeq Illumina technology to a mean depth of 20×) from North-western Europe, including 8,360 Danes, using the phased chip data. Graphtyper60,61 was used to call the genotypes which were subsequently imputed into the phased chip data. WGS, chip-typing, quality control, long-range phasing, and imputation from which the data for this analysis were generated was performed at deCODE genetics.
Estonia – Estonian Biobank
The samples from the Estonian Biobank were genotyped at the Genotyping Core Facility of the Institute of Genomics at the University of Tartu, using the Illumina Global Screening Array. In total, 212,955 samples passed quality control. Samples were excluded from the analysis if their call-rate was below 95% or if the gender, identified by the heterozygosity of the X chromosome, did not align with the gender documented in phenotype data. Variants were excluded if the call-rate was below 95% or if the HWE p-value was less than 1e-4 (only autosomal variants). In addition, SNPs that showed potential traces of batch bias were removed. Two batch bias control steps were performed: 1) SNPs that showed poor cluster separation results among any of Estonian Biobank genotyping experiments were removed. The threshold for SNP removal was Illumina GenTrain score <0.6 and/or cluster separation score <0.4. 2) SNPs that showed inconsistent allele frequency among genotyping experiments were excluded. First, allele frequency was calculated for each SNP for each genotyping experiment with more than 10,000 samples. Next, mean allele frequency was calculated. Finally, if SNP allele frequency was more than 5% away from the mean in any of genotyping experiments, the SNP was excluded from the merged dataset. Prior to imputation, variants with MAF less than 1% and indels were removed. The Eagle v2.4.164 was used for prephasing and imputation was executed using Beagle v5.4 (beagle.22Jul22.46e)65. An imputation reference, specific to the Estonian population, consisting of 2056 WGS samples was used66. Participants with non-European assigned group ancestry were removed, leaving a total of 206,162 samples.
Norway – The Hordaland Health Study
The Norwegian dataset was genotyped using Illumina SNP arrays (either OmniExpress or Global Screening Array). The chip-genotyping quality control and imputation were performed at deCODE genetics, where the same methods used for the Danish sample were applied. The imputation process relied on the same haplotype reference panel as the Danish sample, a panel composed of phased genotypes of 25,215 WGS samples of European ancestry, including 3,336 samples of Norwegian origin.
The UK – The UK Biobank
The samples from the UK Biobank were genotyped using two different Affymetrix chips – the UK BiLEVE Axiom in the first 50,000 individuals67, and the Affymetrix UK Biobank Axiom array68 in the remaining participants. In total, 428,864 participants have been genotyped and 131,272 WGS. Samples with variant yield below 98% were filtered out and any duplicate samples were removed. High-quality sequence variants and indels to a mean depth of at least 20× were identified using Graphtyper60,61. Quality-controlled chip-genotype data were phased using Shapeit469 and variants where at least 50% of the samples had a genotype quality score above 0 were used to prepare a haplotype reference panel using in-house tools and the long-range phased chip data. The variants in the haplotype reference panel were imputed into the chip-genotyped samples using the same in-house tools and methods described for the Icelandic data47,62.
The US – Intermountain Healthcare
The Intermountain dataset was genotyped using Illumina Global Screening Array chips (N = 76,660) and WGS with NovaSeq Illumina technology (N = 20,632). The samples were filtered on 98% variant yield and duplicates removed. High-quality sequence variants and indels with at least a mean depth of 20× were identified with Graphtyper60,61. Quality-controlled genotype data were phased with Shapeit469. A phased haplotype reference panel was prepared with the same in-house tools and methods described for the Icelandic data47,62.
The US – Emory General Clinical Research Center
The genotyping of the Emory dataset has been described previously50. In short, the Emory dataset was genotyped using three types of chips from Illumina (HumanHap300, HumanHap300-Duo and HumanCNV370-Duo). These chips have 314,125 SNPs in common. Prior to analysis, certain SNPs were excluded based on the following criteria; being monomorphic, having less than 95% yield in either cases or controls, deviating from Hardy-Weinberg equilibrium, or displaying divergent allele frequencies between the chips. Additionally, samples with a call-rate less than 98% were excluded.
Statistics and reproducibility
We applied logistic regression assuming an additive model using the expected allele counts as covariates, and combined the results with the available GWAS summary statistics of 10,000 variants12 to test for association between sequence variants and ET. The covariates we used in the association analysis are described in Supplementary Data 14 for the datasets. We used LD score regression to account for distribution inflation due to cryptic relatedness and population stratification70 and used the intercepts as correction factors.
We combined the results from the association analysis of all of the datasets together with the summary statistics (only the top 10,000 variants)12 using a fixed-effects inverse variance method71 based on effect estimates and standard errors in which each dataset was assumed to have a common OR but allowed to have different population frequencies for alleles and genotypes. Sequence variants were mapped to NCBI Build38 and matched on position and alleles to harmonize the datasets. The genome-wide significance threshold was corrected for multiple testing using a weighted Bonferroni adjustment that controls for the family-wise error rate. Variants were weighted based on predicted functional impact72 (Supplementary Data 2).
In a random-effects method, a likelihood ratio test was performed in all genome-wide associations to test the heterogeneity of the effect estimate in the datasets; the null hypothesis is that the effects are the same in all datasets and the alternative hypothesis is that the effects differ between datasets.
The primary signal at each locus was defined as the sequence variant with the lowest Bonferroni-adjusted P-value using the adjusted significance thresholds (Supplementary Data 2). To identify secondary signals at each locus (defined as 1 Mb from the index variants), we performed conditional association analyses using the true imputed genotype data of each dataset except the Estonian and US-EMORY datasets and the summary statistics where an approximate conditional analysis implemented in the GCTA software73 was used. LD between variants was estimated using a set of 5,000 WGS Icelanders. After adjusting for all variants in high LD (r2 > 0.8) and vice versa, the P-values were combined for all datasets to identify the most likely causal variant at each locus and any secondary signals. Based on the number of variants tested, we chose a conservative P-value threshold of <5 × 10−8 for secondary signals.
Manhattan plots were generated using the qqman package in R74.
Functional data
To highlight potential causal genes associating with ET, we annotated the variants associating with ET or variants in high LD (r2 ≥ 0.8 and within ± 1 Mb) that are predicted to affect coding or splicing of a protein (variant effect predictor using Refseq gene set), mRNA expression (top local expression quantitative trait loci [cis-eQTL]) in multiple tissues from deCODE, GTEx (https://gtexportal.org), and other public datasets (Supplementary Data 15), and/or plasma protein levels (top protein quantitative trait loci [pQTL]) in large proteomic datasets from Iceland and the UK.
RNA sequencing was performed on whole blood (N = 17,848) and subcutaneous adipose tissue (N = 769). RNA isolation was performed using RNAzol RT according to manufacturer’s protocol (Molecular Research Center RN 190). We isolated RNA using Chemagic Total RNA Kit special (PerkinElmer) in whole blood and RNAzol RT in adipose tissue, according to the manufacturer’s instructions (Molecular Research Center, RN190). The concentration and quality of the RNA were determined with an Agilent 2100 Bioanalyzer (Agilent Technologies). RNA was prepared and sequenced on the Illumina HiSeq 2500 and Illumina Novaseq systems according to the manufacturer’s recommendation. RNA-seq reads were aligned to personalized genomes using the STAR software package v.2.5.3 with Ensembl v.87 gene annotations75,76. Gene expression was computed based on personalized transcript abundances using kallisto77. Association between sequence variants and gene expression (cis-eQTL) was estimated using a generalized linear regression, assuming additive genetic model and quantile-normalized gene expression estimates, adjusting for measurements of sequencing artefacts, demographic variables, blood composition, and PCs78. The gene expression PCs were computed per chromosome using a leave-one-chromosome-out method.
Quantification of alternative RNA splicing in whole blood was done using LeafCutter79. The cis association between sequence variants and quantified splicing (cis-sQTL) was estimated using linear regression assuming an additive genetic model and quantile-normalized percentage-spliced-in (PSI) values of each splice junction, adjusting for measurements of sequencing artefacts, demography variables, and 15 leave-one-chromosome-out PCs of the quantile-normalized PSI matrix. All variants with MAF > 0.2% within 30 Kb of each LeafCutter cluster were tested.
Icelandic plasma samples were collected through two main projects: the Icelandic Cancer Project (52% of participants; samples collected from 2001 to 2005) and various genetic programs at deCODE genetics, mainly the population-based deCODE Health study. The average participant age was 55 years (SD = 17 years) and 57% were women. In the case of repeated samples for an individual, one was randomly selected. This left measurements for 39,155 individuals. Of these, 35,892 Icelanders were used in the protein GWASs, because they also had genotype information80. The plasma samples were measured with SomaScan v4 assay (SomaLogic®). The assay scanned 4,907 aptamers that measure 4719 proteins. Plasma protein levels were standardized and adjusted for year of birth, sex, and year of sample collection.
The plasma levels of a subset of 47,150 individuals in the UK Biobank were measured with the Olink Explore 1536 platform as a part of the UKB–Pharma Proteomics Project (UK Biobank application no. 65851)81 at Olink’s facilities in Uppsala, Sweden. The majority of the samples were randomly selected across the UK Biobank. Plasma protein levels were standardized to a normal distribution.
We performed gene-based enrichment analysis using the GENE2FUNC tool in FUMA14. The genes on the loci (closest protein coding gene per locus was prioritized) that met traditional genome-wide significance (P ≤ 5 × 10−8) in the ET meta-analysis were tested for over-representation in different gene sets, including Gene Ontology biological processes (MsigDB c5), Reactome (MsigDB c2) and Cell type signatures (MsigDB c8). A Bonferroni test was used for multiple comparison correction.
Familial clustering
We used family-based method to test rare coding variants for segregation within a pedigree. We focused our search on rare (carried by <30 whole-genome-sequenced individuals), high-penetrance coding variants that could account for the familial clustering. To test for association, we created a scoring function based on the coding effect of the variant and its cosegregation with ET, inside and outside of the pedigrees, and used genome-wide simulations to estimate the significance. This method has been described in detail elsewhere82.
Estimation of genetic variance explained
We calculated the variance explained (h2) using the β and EAF from the ET meta-analysis of each of the independent and significant variant with the formula h2 = β2 × (1-EAF) × 2EAF83.
Genetic correlation
Cross-trait LD score regression70 was used to estimate the genetic correlation between the ET meta-analysis and GWAS meta-analyses of other neurological phenotypes, namely Parkinson’s disease and major depressive disorder. We also estimated the genetic correlation between the ET meta-analysis and 1152 previously published GWAS traits (P ≤ 3.8 × 10−5) each with an effective sample size over 5,000 for an unbiased estimate of genetic correlation and heritability. To avoid bias due to sample overlap, we excluded the UK dataset from the ET meta-analysis. We used results for about 1.2 million well imputed variants, and for LD information we used precomputed LD scores for European populations (downloaded from: https://data.broadinstitute.org/alkesgroup/LDSCORE/eur_w_ld_chr.tar.bz2).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the participants in this study for their valuable contribution to research. We thank all investigators and colleagues who contributed to data collection, phenotypic characterization of clinical samples, genotyping, and analysis of the whole-genome association data. The Estonian dataset was funded by European Union through the European Regional Development Fund Project No. 2014-2020.4.01.15-0012 GENTRANSMED and by Estonian Research Council grant PRG1291. Data analysis of the Estonian dataset was carried out in part in the High-Performance Computing Center of University of Tartu. This research was conducted using the UK Biobank Resource (application number 42256).
Author contributions
A.T.S., H.S., and K.S. designed the study. A.T.S., L.S., G.H.H., O.A.S., H.S., and K.S. analyzed the data and interpreted the results. Data collection and subject ascertainment and recruitment was carried out by A.T.S., A.B., P.J., V.P., and H.S. for the Icelandic dataset, L.A.M. and A.O. for the Estonian dataset, K.U.K., D.A.J., and L.D.N. for the US-INTMT dataset, J.I., J.H., and O.A.A. for the Norwegian dataset, D.R. for the US-EMORY dataset, and S.R.O., O.B.P., M.D., S.B., K.B., T.F.H., C.E. for the Danish dataset. A.T.S. drafted the manuscript with input and comments from G.H.H., A.B., G.B.W., R.S.G., G.B., T.E.T., G.A.J., P.S., D.F.G., S.R.O., L.A.M., J.H., O.A.A., J.I., H.S., and K.S. All authors read the final version of the manuscript.
Data availability
The GWAS summary statistics for the ET meta-analysis are available at https://www.decode.com/summarydata/. Other data generated or analyzed in this study are included in the article and Supplementary data and information.
Code availability
GraphTyper (v2.0-beta, GNU GPLv3 license) at https://github.com/DecodeGenetics/graphtyper
Svimmer (v0.1, GNU GPLv3 license), the structural variant merging software at https://github.com/DecodeGenetics/svimmer
SHAPEIT4 (v4.2.2) at https://odelaneau.github.io/shapeit4/
Eagle2 (v2.4.1) at http://www.hsph.harvard.edu/alkes-price/software/
Beagle (v5.4) at https://faculty.washington.edu/browning/beagle/beagle.html
GCTA (v1.93.3beta2) at https://yanglab.westlake.edu.cn/software/gcta/#Overview
STAR (v2.5.3) at http://star.mit.edu/
Kallisto at https://pachterlab.github.io/kallisto/
LeafCutter at https://davidaknowles.github.io/leafcutter/
LD score regression (first release) at https://github.com/bulik/ldsc
qqman package (v0.1.6) at https://github.com/stephenturner/qqman
Axiom genotyping algorithm (v1) at https://www.thermofisher.com/is/en/home.html
FUMA at https://fuma.ctglab.nl/
Competing interests
A.T.S., L.S., G.H.H., O.A.S., G.B.W., R.S.G., G.B., T.E.T., G.A.J., P.S., D.F.G., H.S., and K.S. are employees of deCODE genetics/Amgen Inc. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lists of authors and their affiliations appear at the end of the paper.
Contributor Information
Astros Th. Skuladottir, Email: astros.skuladottir@decode.is
Kari Stefansson, Email: kstefans@decode.is.
Estonian Biobank:
Tõnu Esko, Reedik Mägi, Mari Nelis, and Georgi Hudjashov
DBDS Genomic Consortium:
Jakob Bay, Jens Kjærgaard Boldsen, Thorsten Brodersen, Kristoffer Burgdorf, Mona Ameri Chalmer, Khoa Manh Dinh, Joseph Dowsett, Bjarke Feenstra, Frank Geller, Daniel Gudbjartsson, Lotte Hindhede, Henrik Hjalgrim, Rikke Louise Jacobsen, Gregor Jemec, Bitten Aagaard Jensen, Katrine Kaspersen, Bertram Dalskov Kjerulff, Lisette Kogelman, Margit Anita Hørup Larsen, Ioannis Louloudis, Agnete Lundgaard, Susan Mikkelsen, Christina Mikkelsen, Ioanna Nissen, Mette Nyegaard, Ole Birger Pedersen, Alexander Pil Henriksen, Palle Duun Rohde, Klaus Rostgaard, Michael Schwinn, Hreinn Stefánsson, Erik Sørensen, Unnur Þorsteinsdóttir, Lise Wegner Thørner, Mie Topholm Bruun, Henrik Ullum, Thomas Werge, and David Westergaard
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06207-4.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz D, Poremba C, Papengut F, Schreiber S, Deuschl G. The psychosocial burden of essential tremor in an outpatient- and a community-based cohort. Eur. J. Neurol. 2011;18:972–979. doi: 10.1111/j.1468-1331.2010.03295.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia KP, et al. Consensus Statement on the Classification of Tremors. From the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018;33:75. doi: 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haerer AF, Anderson DW, Schoenberg BS. Prevalence of Essential Tremor: Results From the Copiah County Study. Arch. Neurol. 1982;39:750–751. doi: 10.1001/archneur.1982.00510240012003. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED, et al. Differences in the prevalence of essential tremor among elderly African Americans, whites, and Hispanics in northern Manhattan, NY. Arch. Neurol. 1995;52:1201–1205. doi: 10.1001/archneur.1995.00540360079019. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Fried LP, Fitzpatrick AL, Longstreth WT, Newman AB. Regional and racial differences in the prevalence of physician-diagnosed essential tremor in the United States. Mov. Disord. 2003;18:1035–1040. doi: 10.1002/mds.10492. [DOI] [PubMed] [Google Scholar]
- 7.Tanner CM, et al. Essential tremor in twins: an assessment of genetic vs environmental determinants of etiology. Neurology. 2001;57:1389–1391. doi: 10.1212/WNL.57.8.1389. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED. Etiology of essential tremor: should we be searching for environmental causes? Mov. Disord. 2001;16:822–829. doi: 10.1002/mds.1183. [DOI] [PubMed] [Google Scholar]
- 9.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 10.Paris-Robidas S, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–116. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 12.Liao C, et al. Association of Essential Tremor With Novel Risk Loci: A Genome-Wide Association Study and Meta-analysis. JAMA Neurol. 2022;79:185–193. doi: 10.1001/jamaneurol.2021.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giambartolomei C, et al. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017;8:1–11. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalls MA, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–1102. doi: 10.1016/S1474-4422(19)30320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard DM, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ondo W. Essential Tremor: What We Can Learn from Current Pharmacotherapy. Tremor Other Hyperkinetic Mov. 2016;6:356. doi: 10.5334/tohm.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magheru C, et al. Antiepileptic Drugs and Their Dual Mechanism of Action on Carbonic Anhydrase. J. Clin. Med. 2022;11:2614. doi: 10.3390/jcm11092614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouginot D, Gähwiler BH. Presynaptic GABAB receptors modulate IPSPs evoked in neurons of deep cerebellar nuclei in vitro. J. Neurophysiol. 1996;75:894–901. doi: 10.1152/jn.1996.75.2.894. [DOI] [PubMed] [Google Scholar]
- 20.Shill HA, et al. Brain biochemistry in autopsied patients with essential tremor. Mov. Disord. 2012;27:113. doi: 10.1002/mds.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang S, et al. Complexin stabilizes newly primed synaptic vesicles and prevents their premature fusion at the mouse calyx of held synapse. J. Neurosci. 2015;35:8272–8290. doi: 10.1523/JNEUROSCI.4841-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso M, et al. Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics. 2004;4:3943–3952. doi: 10.1002/pmic.200400848. [DOI] [PubMed] [Google Scholar]
- 23.Karaca E, et al. Genes that affect brain structure and function identified by rare variant analyses of Mendelian neurologic disease. Neuron. 2015;88:499. doi: 10.1016/j.neuron.2015.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redler S, et al. Variants in CPLX1 in two families with autosomal-recessive severe infantile myoclonic epilepsy and ID. Eur. J. Hum. Genet. 2017;25:889. doi: 10.1038/ejhg.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn D, Drew CJ, Reim K, Brose N, Morton AJ. Profound ataxia in complexin I knockout mice masks a complex phenotype that includes exploratory and habituation deficits. Hum. Mol. Genet. 2005;14:2369–2385. doi: 10.1093/hmg/ddi239. [DOI] [PubMed] [Google Scholar]
- 26.Glynn D, Sizemore RJ, Morton AJ. Early motor development is abnormal in complexin 1 knockout mice. Neurobiol. Dis. 2007;25:483–495. doi: 10.1016/j.nbd.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- 28.Frantz GD, Weimann JM, Levin ME, McConnell SK. Otx1 and Otx2 define layers and regions in developing cerebral cortex and cerebellum. J. Neurosci. 1994;14:5725–5740. doi: 10.1523/JNEUROSCI.14-10-05725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weimann JM, et al. Cortical Neurons Require Otx1 for the Refinement of Exuberant Axonal Projections to Subcortical Targets. Neuron. 1999;24:819–831. doi: 10.1016/S0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 30.O’Leary DDM, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-W. [DOI] [PubMed] [Google Scholar]
- 31.Bates CA, Killackey HP. The emergence of a discretely distributed pattern of corticospinal projection neurons. Dev. Brain Res. 1984;13:265–273. doi: 10.1016/0165-3806(84)90161-5. [DOI] [PubMed] [Google Scholar]
- 32.Wise SP, Jones EG. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J. Comp. Neurol. 1977;175:129–157. doi: 10.1002/cne.901750202. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert CD, Kelly JP. The projections of cells in different layers of the cat’s visual cortex. J. Comp. Neurol. 1975;163:81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- 34.Acampora D, et al. Epilepsy and brain abnormalities in mice lacking the Otx1 gene. Nat. Genet. 1996;14:218–222. doi: 10.1038/ng1096-218. [DOI] [PubMed] [Google Scholar]
- 35.Sandoval-Plata G, Morgan K, Abhishek A. Variants in urate transporters, ADH1B, GCKR and MEPE genes associate with transition from asymptomatic hyperuricaemia to gout: results of the first gout versus asymptomatic hyperuricemia GWAS in Caucasians using data from the UK Biobank. Ann. Rheum. Dis. 2021;80:1220–1226. doi: 10.1136/annrheumdis-2020-219796. [DOI] [PubMed] [Google Scholar]
- 36.Kamatani, N., Jinnah, H. A., Hennekam, R. C. M. & Van Kuilenburg, A. B. P. Purine and Pyrimidine Metabolism. Emery Rimoin’s Princ. Pract. Med. Genet. Genomics Metab. Disord. 183–234. 10.1016/B978-0-12-812535-9.00006-6 (2021).
- 37.Málly J, Baranyi M, Vizi ES. Change in the concentrations of amino acids in CSF and serum of patients with essential tremor. J. Neural Transm. 1996;103:555–560. doi: 10.1007/BF01273153. [DOI] [PubMed] [Google Scholar]
- 38.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 39.Tarakad A, Jankovic J. Essential Tremor and Parkinson’s Disease: Exploring the Relationship. Tremor Other Hyperkinetic Mov. 2018;8:589. doi: 10.5334/tohm.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall, A. & Lalli, G. Rho and Ras GTPases in Axon Growth, Guidance, and Branching. Cold Spring Harb. Perspect. Biol. 2, a001818 (2010). [DOI] [PMC free article] [PubMed]
- 41.Zhou Z, et al. Rho GTPase regulation of α-synuclein and VMAT2: Implications for pathogenesis of Parkinson’s disease. Mol. Cell. Neurosci. 2011;48:29–37. doi: 10.1016/j.mcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musilli M, et al. Therapeutic effects of the Rho GTPase modulator CNF1 in a model of Parkinson’s disease. Neuropharmacology. 2016;109:357–365. doi: 10.1016/j.neuropharm.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Louis ED, Ottman R. How familial is familial tremor? Neurology. 1996;46:1200. doi: 10.1212/WNL.46.5.1200. [DOI] [PubMed] [Google Scholar]
- 44.Gulcher JR, et al. Mapping of a familial essential tremor gene, FET1, to chromosome 3q13. Nat. Genet. 1997;17:84–87. doi: 10.1038/ng0997-84. [DOI] [PubMed] [Google Scholar]
- 45.Higgins JJ, Loveless JM, Jankovic J, Patel PI. Evidence that a gene for essential tremor maps to chromosome 2p in four families. Mov. Disord. 1998;13:972–977. doi: 10.1002/mds.870130621. [DOI] [PubMed] [Google Scholar]
- 46.Kovach MJ, et al. Genetic heterogeneity in autosomal dominant essential tremor. Genet. Med. 2001;3:197–199. doi: 10.1097/00125817-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Gudbjartsson DF, et al. Large-scale whole-genome sequencing of the Icelandic population. Nat. Genet. 2015;47:435–444. doi: 10.1038/ng.3247. [DOI] [PubMed] [Google Scholar]
- 48.Skuladottir AT, et al. A genome-wide meta-analysis uncovers six sequence variants conferring risk of vertigo. Commun. Biol. 2021;4:1–9. doi: 10.1038/s42003-021-02673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skuladottir AT, et al. A meta-analysis uncovers the first sequence variant conferring risk of Bell’s palsy. Sci. Rep. 2021;11:4188. doi: 10.1038/s41598-021-82736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stefansson H, et al. Variant in the sequence of the LINGO1 gene confers risk of essential tremor. Nat. Genet. 2009;41:277. doi: 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erikstrup C, et al. Cohort Profile: The Danish Blood Donor Study. Int. J. Epidemiol. 2023;52:e162. doi: 10.1093/ije/dyac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leitsalu L, Alavere H, Tammesoo ML, Leego E, Metspalu A. Linking a Population Biobank with National Health Registries—The Estonian Experience. J. Pers. Med. 2015;5:96–106. doi: 10.3390/jpm5020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Refsum H, et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 2006;136:1731S–1740S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 54.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azriel E, et al. Utilizing Public Health Frameworks and Partnerships to Ensure Equity in DNA-Based Population Screening. Front. Genet. 2022;13:886755. doi: 10.3389/fgene.2022.886755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tinetti ME. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 57.Kieburtz K. Unified Huntington’s disease rating scale: Reliability and consistency. Mov. Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 58.Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic Criteria for Essential Tremor: A Population Perspective. Arch. Neurol. 1998;55:823–828. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- 59.Jónsson H, et al. Data Descriptor: Whole genome characterization of sequence diversity of 15,220 Icelanders. Sci. Data. 2017;4:1–9. doi: 10.1038/sdata.2017.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eggertsson HP, et al. Graphtyper enables population-scale genotyping using pangenome graphs. Nat. Genet. 2017;49:1654–1660. doi: 10.1038/ng.3964. [DOI] [PubMed] [Google Scholar]
- 61.Eggertsson HP, et al. GraphTyper2 enables population-scale genotyping of structural variation using pangenome graphs. Nat. Commun. 2019;10:5402. doi: 10.1038/s41467-019-13341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong A, et al. Detection of sharing by descent, long-range phasing and haplotype imputation. Nat. Genet. 2008;40:1068–1075. doi: 10.1038/ng.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gudbjartsson DF, et al. Sequence variants from whole genome sequencing a large group of Icelanders. Sci. Data. 2015;2:1–11. doi: 10.1038/sdata.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loh PR, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016;48:1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Browning SR, Browning BL. Rapid and Accurate Haplotype Phasing and Missing-Data Inference for Whole-Genome Association Studies By Use of Localized Haplotype Clustering. Am. J. Hum. Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitt M, et al. Improved imputation accuracy of rare and low-frequency variants using population-specific high-coverage WGS-based imputation reference panel. Eur. J. Hum. Genet. 2017;25:869–876. doi: 10.1038/ejhg.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wain LV, et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): A genetic association study in UK Biobank. Lancet Respir. Med. 2015;3:769–781. doi: 10.1016/S2213-2600(15)00283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Welsh S, Peakman T, Sheard S, Almond R. Comparison of DNA quantification methodology used in the DNA extraction protocol for the UK Biobank cohort. BMC Genomics. 2017;18:26. doi: 10.1186/s12864-016-3391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delaneau O, Zagury JF, Robinson MR, Marchini JL, Dermitzakis ET. Accurate, scalable and integrative haplotype estimation. Nat. Commun. 2019;10:1–10. doi: 10.1038/s41467-019-13225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulik-Sullivan B, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 72.Sveinbjornsson G, et al. Weighting sequence variants based on their annotation increases power of whole-genome association studies. Nat. Genet. 2016;48:314–317. doi: 10.1038/ng.3507. [DOI] [PubMed] [Google Scholar]
- 73.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turner S. qqman: an R package for visualizing GWAS results using Q-Q. Manhattan plots. J. Open Source Softw. 2018;3:731. doi: 10.21105/joss.00731. [DOI] [Google Scholar]
- 75.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Howe KL, et al. Ensembl 2021. Nucleic Acids Res. 2021;49:D884–D891. doi: 10.1093/nar/gkaa942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 78.Stegle O, Parts L, Piipari M, Winn J, Durbin R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat. Protoc. 2012;7:500–507. doi: 10.1038/nprot.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li YI, et al. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 2018;50:151–158. doi: 10.1038/s41588-017-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferkingstad E, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat. Genet. 2021;53:1712–1721. doi: 10.1038/s41588-021-00978-w. [DOI] [PubMed] [Google Scholar]
- 81.Sun, B. B. et al. Genetic regulation of the human plasma proteome in 54,306 UK Biobank participants. Prepr. 10.1101/2022.06.17.496443v1 20, (2022)
- 82.Steinberg S, et al. Truncating mutations in RBM12 are associated with psychosis. Nat. Genet. 2017 498. 2017;49:1251–1254. doi: 10.1038/ng.3894. [DOI] [PubMed] [Google Scholar]
- 83.Alves AC, et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 2019;5:1–17. doi: 10.1126/sciadv.aaw3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kircher M, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The GWAS summary statistics for the ET meta-analysis are available at https://www.decode.com/summarydata/. Other data generated or analyzed in this study are included in the article and Supplementary data and information.
GraphTyper (v2.0-beta, GNU GPLv3 license) at https://github.com/DecodeGenetics/graphtyper
Svimmer (v0.1, GNU GPLv3 license), the structural variant merging software at https://github.com/DecodeGenetics/svimmer
SHAPEIT4 (v4.2.2) at https://odelaneau.github.io/shapeit4/
Eagle2 (v2.4.1) at http://www.hsph.harvard.edu/alkes-price/software/
Beagle (v5.4) at https://faculty.washington.edu/browning/beagle/beagle.html
GCTA (v1.93.3beta2) at https://yanglab.westlake.edu.cn/software/gcta/#Overview
STAR (v2.5.3) at http://star.mit.edu/
Kallisto at https://pachterlab.github.io/kallisto/
LeafCutter at https://davidaknowles.github.io/leafcutter/
LD score regression (first release) at https://github.com/bulik/ldsc
qqman package (v0.1.6) at https://github.com/stephenturner/qqman
Axiom genotyping algorithm (v1) at https://www.thermofisher.com/is/en/home.html
FUMA at https://fuma.ctglab.nl/