Abstract
Cytosolic double-stranded DNA (dsDNA) is frequently accumulated in cancer cells due to chromosomal instability or exogenous stimulation. Cyclic GMP-AMP synthase (cGAS) acts as a cytosolic DNA sensor, which is activated upon binding to dsDNA to synthesize the crucial second messenger 2’3’-cyclic GMP-AMP (2’3’-cGAMP) that in turn triggers stimulator of interferon genes (STING) signaling. The canonical role of cGAS-cGAMP-STING pathway is essential for innate immunity and viral defense. Recent emerging evidence indicates that 2’3’-cGAMP plays an important role in cancer progression via cell autonomous and non-autonomous mechanisms. Beyond its role as an intracellular messenger to activate STING signaling in tumor cells, 2’3’-cGAMP also serves as an immunotransmitter produced by cancer cells to modulate the functions of non-tumor cells especially immune cells in the tumor microenvironment by activating STING signaling. In this review, we summarize the synthesis, transmission, and degradation of 2’3’-cGAMP as well as the dual functions of 2’3’-cGAMP in a STING-dependent manner. Additionally, we discuss the potential therapeutic strategies that harness the cGAMP-mediated antitumor response for cancer therapy.
Keywords: CDNs, 2’3’-cGAMP, cGAS-STING, tumor microenvironment, ENPP1, TREX1
Introduction
Cyclic dinucleotides (CDNs) serve as crucial second messengers in both prokaryotes and eukaryotes, playing integral roles in diverse biological processes. Stimulator of interferon genes (STING) is a direct receptor for CDNs. Upon CDNs binding, including cyclic di-cGMP, cyclic di-cAMP and cyclic GMP-AMP (cGAMP), STING recruits and activates TANK-binding kinase 1 (TBK1), which in turn phosphorylates STING. Phosphorylation of STING facilitates further recruitment and activation of TBK1, leading to the binding and phosphorylation of interferon regulatory factor 3 (IRF3) [1]. Activated IRF3 stimulates the expression of type I interferon (IFN), which plays critical roles in antiviral and innate immune responses [2]. Different from the classical bacterial second messengers c-di-GMP and c-di-AMP, cGAMP comprises adenosine and guanosine, which are linked via two phosphodiester linkages and exists as multiple isomers, such as 3’3’-cGAMP, 3’2’-cGAMP and 2’3’-cGAMP. 3’3’-cGAMP acts as a bacterial second messenger recognized by STING, which is initially identified in V. cholerae, where a dinucleotide cyclase (DncV) catalyzes the cyclization of AMP and GMP through two 3’-5’ phosphodiester bonds upon double-stranded DNA (dsDNA) stimulation [3]. 3’2’-cGAMP is produced by a double-stranded RNA (dsRNA) sensor cGLR1 in Drosophila upon dsRNA stimulation [4]. 3’2’-cGAMP is also an endogenous natural ligand of STING in insects, activating STING to induce antiviral activity via nuclear factor-kappa B (NF-κB) -dependent signaling [5]. In mammalian cells, the primary form of CDN is 2’3’-cGAMP (hereafter referred to as cGAMP), which possesses a distinct 2’-5’ phosphodiester linkage produced by cGAMP synthase (cGAS) [6]. cGAMP is the endogenous natural ligand of STING in the mammalian innate immune system, and displays a higher affinity with STING than 3’2’-cGAMP and other CDNs, mediating innate immunity in response to microbial invasion and self-DNA leakage [2].
Recently, the intricate role of cGAMP in cancer procession has been gradually elucidated. The presence of aberrant cytosolic DNA in cancer cells activates cGAS, leading to the production of cGAMP and subsequent activation of the STING pathway. Activated STING upregulates type I IFN and other inflammatory factors, thereby exerting tumor-suppressive roles [7]. However, emerging evidence has suggested that chromosomally unstable cancer cells exploited the cGAS-cGAMP-STING pathway to upregulate non-canonical NF-κB signaling, thereby fostering tumor metastasis [8]. In addition, tumor-derived cGAMP is constantly and efficiently exported to the extracellular space to activate STING pathway in host cells within the tumor microenvironment (TME). Myeloid cells, including macrophages and dendritic cells (DCs), respond to tumor-derived cGAMP to induce a robust type I IFN signaling. Consequently, this process promotes anti-tumor immunity of CD8+ T cells or natural killer (NK) cells and enhances the response to immunotherapies with checkpoint inhibitors [9, 10]. However, intrinsic STING activation in T cells stimulated by cGAMP has been demonstrated to induce apoptosis of T cells, thereby contributing to immune evasion and cancer progression [11]. The multifaceted functions of cGAMP in cancer cells and immune cells underscore its significant and intricate roles in tumor growth and metastasis, suggesting that modulating cGAMP could be exploited for tumor therapy.
In this review, we elaborate the synthesis, transmission, and degradation of cGAMP, as well as elucidate its diverse roles in cancer progression through cell-intrinsic and -extrinsic mechanisms. Additionally, the current therapeutic strategies that exploit cGAS-cGAMP-STING pathway for cancer therapy are summarized, which provides novel perspectives for potential therapeutic strategies.
cGAMP as an important second messenger
The synthesis of cGAMP
cGAMP is an important transmitter of immune signaling produced by cGAS in response to pathogenic or aberrant endogenous DNA in the cytoplasm. In eukaryotic cells, DNA is strictly compartmentalized in the nucleus and mitochondria to avoid autoimmunity. The appearance of cytosolic DNA serves as a warning signal indicative of foreign pathogens or self-DNA damage, encompassing various sources such as DNA viruses, retroviruses, bacteria, dying cells, exosomes, and damaged mitochondria. As a member of the nucleotidyltransferase superfamily, cGAS is recognized as a unique pattern recognition receptor responsible for sensing cytosolic dsDNA [6]. Structurally, cGAS is a ~60 kD protein composed of an unstructured, disorder N terminal domain and a C-terminal catalytic domain possessing nucleotidyltransferase (NTase) activity [12]. Under physiological conditions in the absence of cytosolic DNA, cGAS remains auto-inhibited [13]. Upon the presence of aberrant dsDNA in the cytoplasm, cGAS recognizes dsDNA in a sequence-independent manner, and forms a 2:2 complex containing two cGAS and two dsDNA molecules. This complex induces a conformational alteration of the catalytic pocket, leading to activation of cGAS. Activated cGAS simultaneously binds ATP and GTP to generate an intermediate product pppG (2′–5′)pA by utilizing ATP as the donor and the 2’-OH on GTP as the acceptor. Subsequently, it places the GTP moiety at the donor position and the AMP moiety at the acceptor position for the formation of a 3’-5’phosphodiester bond, ultimately generating cGAMP [14]. The efficacy of dsDNA to activate cGAS depends on its length. Longer DNA (>45 bp) that favors cGAS oligomerization exhibits greater potency in activating cGAS compared to shorter DNA (~20 bp) [15]. Cytosolic DNA in cancer cells is typically derived from carcinogens, radiation, and anticancer drugs especially chemotherapeutics [16–19]. Additionally, chromosomal instability (CIN), a hallmark of cancer, promotes chromosome mismatch and leads to the formation of micronuclei. Chromosomes enclosed in micronuclei release dsDNA into the cytoplasm following micronuclei rupture [20]. Accumulated cytosolic DNA activates cGAS to generate cGAMP either stimulating STING-dependent signaling in tumor cells or being exported as an extracellular signal for intercellular communication.
The transmission of cGAMP
cGAMP produced in the cytoplasm can be transmitted outside of cells or to other cells via different transmission modes. Gap junctions, which serve as intercellular channels facilitating the transfer of cytosolic molecules between cells, play a crucial role in the transportation of cGAMP. Gap junctions have been reported to be responsible for transmitting cGAMP from virus-infected cells to bystander cells to induce antiviral immunity [21]. Various connexin proteins, including connexin 43, connexin 45 and connexin 32, are essential components of gap junctions and actively participate in the transfer of cGAMP [22–24]. Recent research by Chen et al. revealed that brain cancer cells exploited gap-junctional networks to transfer cGAMP to astrocyte cells and promoted cancer metastasis [22].
Extracellular cGAMP can also be transported into cells through specialized transporters located on cell membrane. SLC19A1, a member of the solute carrier (SLC) family, has been identified as a direct importer of cGAMP in monocyte-derived cells. Knocking out SLC19A1 impaired the import of cGAMP in THP-1, Nu-DUL-1, and U937 cells [25]. Another SLC family member, SLC46A2 has been recognized as an importer that facilitates the uptake of cGAMP derived from tumor cells into macrophages and CD14+ monocytes [26]. A recent study revealed that the P2X family member P2X7R enabled the entry of cGAMP into host immune cells, thereby enhancing the innate immune response [27].
Conversely, ABCC1, a member of the ATP-binding cassette (ABC) transporter family, has been identified as the direct exporter of cGAMP. Inhibition of ABCC1 by MK-571 was found to attenuate the export of cGAMP in bone marrow-derived macrophages (BMDMs) by a systematic screening of the transporter inhibitors [28]. ABCC1 was further revealed to mediate ATP-dependent export of cGAMP to restrict cell-intrinsic STING signaling in both murine and human cells [28].
The volume-regulated anion channels (VRACs), specifically LRRC8 channels, have been identified as bi-directional transporters of cGAMP. LRRC8A, an essential component of VRACs, formed heteromeric channels with LRRC8C/E to regulate cGAMP transport in human endothelial cells and microvascular cells [29]. Moreover, LRRC8C has been revealed to mediate the transport of cGAMP in T cells, resulting in STING activation [30]. However, blocking VRAC activity by ablation of LRRC8A only led to a 50%–70% of reduction in cGAMP uptake in mouse embryonic fibroblasts (MEFs) or BMDMs, suggesting the involvement of other transporters and potential cooperative interactions between SLC19A1, SLC46A2, P2X7R and VRAC channels [31].
Taken together, cGAMP derived from tumor cells or exogenous cGAMP can be transported into cells in the TME through a complex transport network that comprises various channels and transport modes. The transmission of cGAMP between cells, particularly from tumor cells to immune cells, sheds light on its identity as an immunotransmitter.
The degradation of cGAMP
Second messengers are typically degraded by hydrolases to avoid continuous activation. Though poxin, an immune nuclease found in mammalian poxviruses, is able to hydrolyze cGAMP to linear Gp[2’-5’]Ap[3’] [32], the extracellular enzyme ectonucleotide pyrophosphatase/phosphodiesterase1 (ENPP1) is recognized as the primary enzyme to degrade cGAMP with high specificity. ENPP1 belongs to the family of ectonucleotidases, and is a type-II transmembrane glycoprotein present on cell membrane or in blood and body fluids [33]. ENPP1 has also been known to regulate bone mineralization via hydrolyzing ATP to produce AMP and pyrophosphate (PPi) [34]. ENPP1 recognizes the adenine and guanine bases of cGAMP and hydrolyzes the 2’-5’ and 3’-5’ phosphodiester linkage to produce GMP and AMP, the latter is subsequently hydrolyzed into adenosine by CD73 [35]. Elevated expression of ENPP1 has been observed in breast cancer, non-small cell lung cancer, and astrocytic brain tumors [36–38]. Beyond cancer cells, stromal cells and immune cells also express ENPP1 to hydrolyze tumor-derived cGAMP in the TME [39]. Up-regulation of ENPP1 may result in a decreased level of cGAMP in the TME, which attenuates STING signaling to promote tumor progression and metastasis [36].
The cGAMP-mediated signaling
Canonical cGAS-cGAMP-STING pathway
The cGAS-cGAMP-STING pathway plays a pivotal role in the innate immune response. Upon recognition of dsDNA, cGAS produces cGAMP, which subsequently mediates the activation of downstream STING signaling cascades. STING is a transmembrane protein located in the endoplasmic reticulum (ER) membrane and exhibits high affinity toward cGAMP [40]. The CDN-binding domain of STING forms a homodimer with a V-shaped conformation, and subsequently, one molecule of cGAMP binds to the central crevice of a STING dimer [41]. Upon cGAMP binding, STING undergoes a conformational change and translocates from ER to ER-Golgi intermediate compartment (ERGIC) and Golgi [42]. Following this translocation, the carboxy-terminal tail (CTT) of STING is released to recruit TBK1, which phosphorylates the CTT of STING as well as itself. The STING-TBK1 complex recognizes and phosphorylates the transcription factor IRF3, prompting its translocation to the nucleus [43]. In parallel, STING also activates IκB kinase (IKK)- NF-κB signaling. Both IRF3 and NF-κB regulate innate immunity via autocrine and paracrine manners by inducing the expression of interferons and inflammatory cytokines [44].
cGAMP-mediated non-canonical signaling
In addition to its role in the canonical cGAS–cGAMP-STING pathway to regulate innate immunity, emerging evidence suggests that cGAMP participates in non-canonical signaling in a STING-dependent manner.
cGAMP-induced STING activation has been reported to be associated with LC3B lipidation and inflammasome activation [45]. Upon cGAMP stimulation, the activation of STING acted as a proton channel and promoted LC3 lipidation, a crucial step in autophagosome biogenesis and induction of autophagy [46]. STING activation in human monocytes was reported to prime a lysosomal cell death program, and further induced the activation of NLRP3 inflammasome [47]. These findings underscore the significance of cGAMP-induced STING activation in autophagy and inflammasome response independent of IFN induction.
Additionally, cGAMP-activated STING has been found to initiate a non-canonical STING-PKR-like endoplasmic reticulum kinase (PERK)-eIF2α pathway, independent of the canonical STING-TBK1-IRF3 pathway [48]. Upon cGAMP binding, ER-localized STING directly activated PERK through intracellular domain interactions. Subsequently, PERK phosphorylated eIF2α, which mediated translation regulation favoring inflammatory and survival signaling. The STING–PERK–eIF2α pathway preceded the TBK1-IRF3 pathway and this signalosome disassembled when STING translocated to ERGIC and Golgi. This non-canonical pathway may play an important role in cellular senescence and organ fibrosis, implying the diverse functions of cGAMP-STING signaling [48].
As a pivotal second messenger, cGAMP participates in multiple signal transduction pathways and plays a crucial role in innate immunity. Elucidating the mechanisms of cGAMP-mediated signaling is essential for exploiting cGAMP to regulate diverse physiological and pathological processes, which may open up promising avenues for therapeutic interventions.
The autonomous role of cGAMP in tumor cells
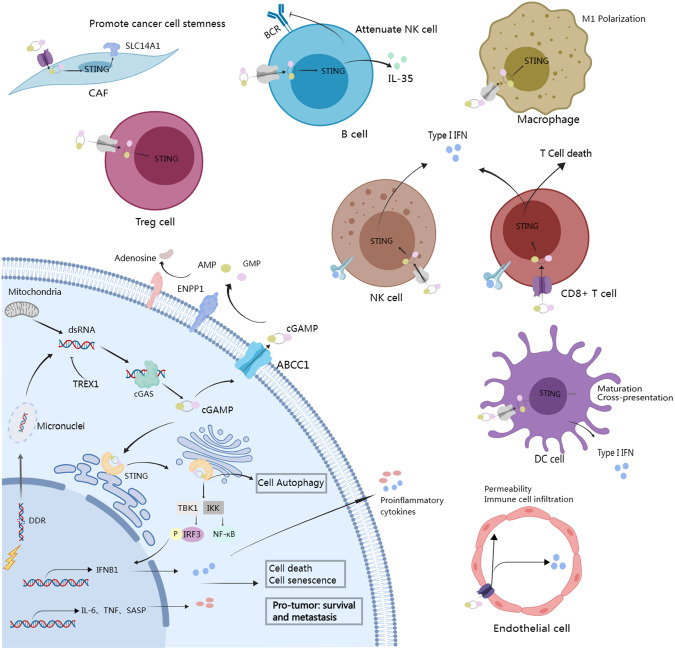
cGAMP exerts significant effects on tumor progression in both autonomous and non-autonomous manners (Fig. 1). Tumor cells constantly produce cGAMP as a consequence of chromosomal instability, DNA damage induced by chemotherapy and radiotherapy, or mitochondrial DNA leakage. Endogenous cGAMP triggers signaling in tumor cells, leading to multiple functional outcomes.
Fig. 1. The multifaceted effects of cGAMP in tumor cells and tumor microenvironment.
cGAS recognizes cytosolic dsDNA in tumor cells, arising from chromosomal instability, DNA damage response, and mitochondrial DNA leakage to generate cGAMP. Upon cGAMP binding, STING in tumor cells activates IRF3 and NF-κB pathways to induce cell autophagy, death, and senescence while triggering non-canonical NF-κB signaling to promote tumor survival and metastasis. cGAMP can also be transmitted into the tumor microenvironment where it is internalized by stromal and immune cells to mediate diverse immune responses.
Cell death
cGAMP triggers STING-dependent downstream signaling, which has been shown to activate cell-autonomous death. The activation of the cGAS-cGAMP-STING cascade facilitates the secretion of type I IFN, which subsequently binds the heterodimer IFN receptor IFNAR1/IFNAR2 in tumor cells. Type I IFN signaling activates the Janus kinase 1 (JAK1) and phosphorylates the signal transducer and activator (STAT) family, which forms a homodimer or binds STAT2 for nuclear translocation, thereby inducing the expression of the IFN-stimulated genes (ISGs). IFNAR1 and STAT1 play an essential role in cGAS-cGAMP-STING-induced cell death, as evidenced by their depletion attenuating cGAS-induced CXCL10 expression and cell death in multiple lines of lung cancer cells [49, 50]. In tumor cells experiencing mitotic arrest induced by Taxol, cGAS bound mitotic chromosomes to produce cGAMP, which activated downstream STING signaling and led to gradual accumulation of IRF3 phosphorylation. Phosphorylated IRF3 promoted cell apoptosis by suppressing Bcl-XL independently of its transcriptional capacity, thereby enhancing the response of mouse xenograft tumors to antimitotic agents [51]. In addition, Banerjee et al. reported that cGAMP-activated TBK1 stimulated the autophosphorylation of the protein kinase ataxia telangiectasia mutated (ATM), which then activated cell cycle checkpoint kinase 2 (CHK2), leading to G1 cell cycle arrest and cell death [52]. Hence, harnessing cell death mediated by intrinsic cGAMP signaling holds potential for cancer therapy.
Cell autophagy
Emerging evidence suggests that cGAMP is closely associated with the activation of autophagy in a STING-dependent manner. Autophagy plays a dichotomous role in tumor suppression or promotion, dependent on cell type, tumor microenvironment, disease stage, and external stimuli. Activated STING by cGAMP translocates to the ERGIC and triggers autophagy by directly interacting with LC3 to induce its lipidation and autophagosome formation independent of TBK1 and IRF3 activation [46, 53]. Recently, Tan et al. have discovered that STING functioned as a conserved ion channel and triggered non-canonical autophagy in human osteosarcoma cells [54]. It also has been revealed that the cGAS-cGAMP-STING pathway engaged in macroautophagy formation, which was important for inhibiting the proliferation of tumor cells undergoing replicative crisis [55]. Overall, these findings suggest that cGAMP-mediated autophagy could be a potential barrier to tumorigenesis. However, due to the paradoxical nature of autophagy in tumor progression, it is possible that cGAMP could have a dual role in tumorigenesis. Mitochondrial dysfunction-induced mtDNA stress has been found to increase cGAMP production and STING activation, stimulating autophagic vesicles and ultimately fostering esophageal squamous cell carcinoma survival [56]. Additionally, STING-induced autophagy in turn regulated STING degradation to form a negative feedback loop [57]. Further studies are necessary to understand the molecular mechanisms underlying cGAMP-induced autophagy and its role in tumor progression.
Cell senescence
Senescence, characterized by an irreversible cell-cycle arrest, serves as a protective mechanism against the proliferation of damaged cells, which occurs in response to both intracellular and extracellular stress signals, such as oncogene activation and radiation, and is closely associated with cancer progression. Recent studies have revealed a robust association between cGAS-cGAMP-STING signaling and cellular senescence in tumor cells. Stress signals in cancer cells led to an increase in cytosolic dsDNA, which triggered the cGAS-cGAMP-STING pathway and subsequently activated downstream NF-κB signaling. The activation of NF-κB signaling promoted the senescence-associated secretory phenotype (SASP) and resulted in the release of inflammatory cytokines, chemokines, and growth factors [58]. Loss of cGAS attenuated cellular senescence and retarded immortalization [59]. However, chronic SASP induced by CIN in cancer exhibited pro-tumor function that differs from acute STING-induced SASP [20]. Despite the unclear mechanism underlying the pro-tumor effects of chronic SASP, it is noteworthy that cGAMP may play multifaceted roles in tumor cell senescence.
Pro-tumor role of cGAMP via tumor cell-intrinsic mechanisms
cGAMP-STING signaling is widely acknowledged for its role in inhibiting tumor progression. Cancer cell-autonomous signaling mediated by STING has been demonstrated to suppress the reactivation of dormant metastasis and prevent spontaneous metastasis outbreaks [60]. However, it should be noted that a limited number of tumors exhibit downregulation of cGAS and STING, suggesting the potential in promoting tumor progression [20]. CIN in cancer cells creates a preponderance of micronuclei, which releases DNA into the cytoplasm after rupture, resulting in the continuous activation of cGAS-cGAMP-STING signaling. This sustained activation could trigger non-canonical NF-κB signaling instead of canonical NF-κB or type I IFN signaling, as evidenced by up-regulation of p52 and phosphorylated p100, along with decreased expression of NF receptor-associated factor 2 (TRAF2), an inhibitor of non-canonical NF-κB pathway [8, 50]. Activation of non-canonical NF-κB signaling via the cGAMP-STING pathway mediated metastasis in a tumor cell-autonomous manner. Depletion of STING in CIN-high cells attenuated epithelial-mesenchymal transition (EMT) and inflammatory pathways. Conversely, the addition of cGAMP increased invasion and migration of CIN-low cells, revealing the roles of cGAMP-STING in regulating EMT and metastasis via non-canonical NF-κB pathway [8]. In consistency with these findings, triple-negative breast cancer cells exhibiting CIN triggered cGAS–cGAMP-STING signaling followed by non-canonical NF-κB pathway, which induced IL-6-STAT3 axis and counteracted cell death signaling mediated by type I IFN-STAT1 signaling. Thus, IL-6-STAT3 signaling drives the survival of CIN-high cancer cells, suggesting their dependence on cGAS-cGAMP-STING mediated signaling for survival [50]. Additionally, CIN-induced chronic activation of STING has been shown to induce type I IFN tachyphylaxis and cancer cell-derived ER stress response, which facilitated an immunosuppressive microenvironment and prompted tumor metastasis [61]. The intricate roles of cGAMP may pose challenges for its regulation to maximize antitumor efficacy.
The non-autonomous role of cGAMP in the tumor microenvironment
The TME is highly heterogeneous composing of diverse cell types, including cancer cells, immune cells, stromal cells and endothelial cells. The dynamic interaction between cancer cells and non-cancerous host cells within the TME plays an important role from tumor initiation to metastasis. Cancer cells regulate host cells through cell-cell contact and paracrine signaling to establish an immunosuppressive environment, while non-neoplastic cells exploit this communication to exert influence on cancer progression. Recent studies have suggested that cGAMP not only functions in a tumor cell-autonomous fashion but also plays a vital role in a tumor cell-non-autonomous manner. On one hand, cGAMP acts as an intracellular second messenger to activate type I IFN signaling in a STING-dependent manner in tumor cells and regulate the infiltration of neighboring immune cells. On the other hand, cancer cells continuously export cGAMP, with extracellular cGAMP serving as an immunotransmitter. Extracellular cGAMP could be imported by immune cells and other target cells, subsequently activating STING signaling to modulate tumor immune microenvironment.
Macrophages
Tumor-associated macrophages (TAMs), broadly categorized as M1 macrophages and M2 macrophages, play pivotal but dual roles in cancer [62]. In general, M1 macrophages exhibit anticancer properties by fostering robust anti-tumoral responses of T and NK cells, whereas M2 macrophages are associated with tumor progression through multiple mechanisms such as angiogenic regulation and immune suppression. Upon secretion of cGAMP into the TME, macrophages have been observed to directly respond to extracellular cGAMP [26]. CD14+ monocytes and M1 macrophages predominantly utilized SLC46A2 as the cGAMP importer and produced high levels of type I IFN upon exposure to extracellular cGAMP [26]. cGAMP significantly increased TNFα via STING activation in tumor-associated myeloid cells and induced robust apoptosis in tip-like and proliferative tumor cells [63]. In addition, intratumoral administration of cGAMP promoted the accumulation of M1 macrophages in tumor tissue, which exhibited cytotoxic activity by producing TNFα and T cell-recruiting chemokines [64]. Macrophages possess the ability to clear apoptotic tumor cells via efferocytosis, a process potentially linked to immune tolerance. It has been demonstrated that cGAMP-mediated STING signaling contributed to the potentiation of anti-tumor immunity by blocking the phagocytic receptor MerTK [27]. Overall, these findings highlight the potential to modulate the cGAMP-STING pathway in macrophages as a strategy to enhance anti-tumor immunity.
Dendritic cells
DCs are specialized antigen-presenting cells (APCs) responsible for the uptake of tumor antigens and presentation of antigens to T cells, facilitating innate and adaptive immune responses [65]. Several studies have revealed that DCs served as the primary extracellular cGAMP-sensing cells within the TME. cGAMP stimulated STING to trigger type I IFN signaling in tumor-infiltrating DCs, making them the major cellular source of IFN-β, thereby contributing to immune surveillance [66]. It has been demonstrated that cGAMP derived from cancer cells was transferred to DCs via gap junctions and promoted infiltration of protective CD8+ T cells, resulting in improved response to genotoxic agents or immune checkpoint inhibitors [9]. In addition, STING activation promoted the maturation and functional capability of DCs to cross-present antigens [67, 68]. Irradiated tumor cells exhibited increased cGAMP production and augmented antigen presentation of DCs compared with non-irradiated tumor cells, which ultimately enhanced tumor immunogenicity and antitumor efficacy [68].
Natural killer cells
As the cytotoxic lymphocytes of the innate immune system, NK cells are responsible for recognizing and eliminating virally infected and malignant cells [69]. The expression of NKG2D, a co-stimulatory receptor in NK cells [70], was associated with the response to extracellular cGAMP. It has been found that NK cells with low NKG2D expression directly responded to extracellular cGAMP, leading to STING activation and IFN-β production. Conversely, NKG2Dhigh NK cells induced IFN-β production without STING activation by extracellular cGAMP [26], which might be attributed to the active state of NK cells. Additionally, NK cells could be indirectly activated by interferons produced by myeloid cells and B cells, where STING pathway was triggered by cGAMP derived from tumor cells [10, 71].
T cells
As the primary component of the adaptive immune system, T cell activation stands as a crucial determinant in the anti-tumor immunity of cGAMP-STING signaling. cGAMP-induced STING activation in non-tumor cells releases type I IFN and immune-stimulated cytokines, subsequently activating effector CD8+ T cells to execute immune surveillance [47, 72, 73]. However, recent studies have found that the intrinsic STING signaling could induce cell death in T cells [72, 74]. Wu et al. revealed that STING signaling in T cells was predominantly IFN-independent. T cells responded to cGAMP in the TME, and tumor cells exploited this activity to induce STING-mediated T cell death as a means of evading immune surveillance [11]. STING activation was also involved in modulating regulatory T cell (Treg)-mediated immunosuppression. In HPV-positive cervical cancer, cGAMP stimulation enhanced the efficiency of induction of Treg from CD4+-naïve T cells, accompanied by activation of STING signaling and elevated levels of forkhead box P3 (FOXP3) [73].
Overall, cGAMP-induced STING activation may exert dual effects on T cells. The activation of STING in the TME results in the accumulation of type I IFN, which enhances T cell infiltration and cytotoxic effect to improve anti-tumor effect. On the other hand, STING activation in T cells predominantly induces cell death and tumor immune evasion. Further studies are required to maximize the antitumor effects in T cells by modulating cGAMP.
B cells
B cell is a population of adaptive immune cells with complex and heterogeneous functions in the TME due to diverse subsets. Multiple studies have highlighted the double-faced roles of B cells in cancer progression. Regulatory B (Breg) cell is an important subset of B cells, which produce immunosuppressive cytokines of IL-10 and IL-35 to inhibit anti-tumor immunity and promote tumor growth [75]. Recent research has delineated the unexpected impact of cGAMP on Breg cells. cGAMP induced expression of immunosuppressive factor IL-10 and IL-35 by Breg cells in an IRF3-dependent but IFN-independent manner. Furthermore, the STING-IL35 axis in Breg cells reduced the proliferation of NK cells and attenuated NK cell response, which could be relieved by blocking B cell-intrinsic STING signaling [76]. In addition, cGAMP-mediated STING activation in B cells down-regulated the BCR signaling and restrained the formation of antigen-specific plasma cells and antibodies [77]. This phenomenon might explain why STING was down-regulated in some cases of malignant chronic lymphocytic leukemia, where BCR activation and survival may confer a more advantageous milieu for the disease. Overall, these findings underscore the distinct role of the cGAMP-STING axis in B cells in regulating immune responses, and further research is needed to fully understand the mechanisms underlying these effects and to develop strategies to modulate STING activation in B cells for therapeutic purposes.
Endothelial cells
Endothelial cells are important stromal cells that play a critical role in tumor angiogenesis [78]. The presence of STING in endothelial cells enables them to respond to cGAMP stimulation. In B16F10 melanoma allografts injected with cGAMP, endothelial cells were identified as the principal source of type I IFN in the TME [79]. This observation shed light on the potential role of the tumor vasculature in regulating anti-tumor immunity through STING. Extracellular cGAMP derived from cancer cells can activate STING signaling in endothelial cells, amplifying the production of IFN-β and increasing vascular permeability as well as upregulating the expression of specific adhesion molecules including E-selectin, vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1(ICAM-1). Effector immune cells need to adhere and extravagate from the vasculature in order to infiltrate the TME. Therefore, cGAMP-mediated up-regulation of microvascular permeability and adhesion molecules could facilitate T cell adhesion to the endothelium and extravasation, ultimately leading to immune cell infiltration to inhibit tumor progression [80, 81]. As one of the barriers to both immune cell infiltration and tumor metastasis, endothelial cells have profound effects on tumor angiogenesis and tumor growth. Consequently, the increased vascular permeability caused by STING activation should be considered with caution and more evidence is needed to clarify STING function in endothelial cells.
Cancer-associated fibroblasts (CAFs)
Cancer-associated fibroblasts (CAFs) are a crucial component of the TME and serve as a substantial source of growth factors and cytokines, playing important roles in tumor progression and therapeutic responses [82]. cGAMP-activated STING signaling has been reported to induce the differentiation of SLC14A1+ CAFs, which in turn promoted the stemness of bladder cancer cells [83]. The level of cGAMP in bladder cancer was correlated with SLC14A1+ CAFs abundance, indicating poor prognosis and resistance to immunotherapy and chemotherapy [83]. Moreover, cGAMP produced by cancer cells could also be transmitted to CAFs by transcytosis of cytoplasm, leading to STING activation in CAFs and subsequent IFN-β expression. IFN-β triggered interferon-stimulated transcriptional programs in both cancer cells and CAFs, fundamentally compromising the efficacy of oncolytic viruses [84]. In light of the available evidence, cGAMP-induced STING activation in CAF may negatively affect the efficacy of tumor therapy. The negative impact of STING activation by cGAMP in the CAF is worthy of attention in tumors abundant of CAFs.
In conclusion, STING activation triggered by cGAMP stimulates diverse signaling and cellular processes dependent on cellular contexts, underscoring the complexity of cGAMP-STING pathway in the TME. Given the importance of cGAMP, it is vital to distinguish between the pro-cancer and anticancer effects of cGAMP to maximize its capacity for cancer treatment.
Modulation of cGAMP for cancer therapy
As an important immunotransmitter, modulation of cGAMP displays promising potential for tumor therapy. In recent years, interventions targeting cGAMP levels by supplementation of cGAMP or analogs as well as blocking cGAMP degradation by inhibiting ENPP1 have demonstrated remarkable antitumor efficacy. Clinical and preclinical studies to modulate cGAMP for cancer treatment have been summarized in Table 1.
Table 1.
Modulation of cGAMP for cancer therapies.
| Direct cGAMP and cGAMP analogs | ||||
| Compound | Route of administration | Cancer type | Status | Reference |
| 2'3'-cGAMP | Intratumoral | Colon cancer, breast cancer, and melanoma | Preclinical | [64, 87] |
| 3'3'-cGAMP | Intraperitoneal | Lymphocytic leukemia | Preclinical | [122] |
| ADU-S100 | Intratumoral | Solid tumors and lymphomas | Phase I | NCT03172936 |
| Head and neck cancer | Phase II | NCT03937141 | ||
| MK-1454 | Intratumoral | Lymphoma | Phase I | NCT03010176 |
| SB-11285 | Intravenous | Advanced solid tumor | Phase I | NCT04096638 |
| IMSA-101 | Intratumoral | Solid tumor | Phase II | NCT04020185 |
| TAK-676 | Intravenous | Solid tumor | Phase I | NCT04420884 |
| BI-1387446 | Intratumoral | Solid tumor | Phase I | NCT04147234 |
| SR-717 | Intraperitoneal | Melanoma | Preclinical | [123] |
| MAS-2 | Oral | Colon cancer, breast cancer, and melanoma | Preclinical | [67, 124] |
| ENPP1 inhibitors | ||||
| Name | Advent delivery | Cancer type | Status | Reference |
| RBS2418 | Oral | Advanced cancer | Phase I | NCT05270213 |
| TXN10128 | Oral | Advanced cancer | Phase I | NCT05978492 |
| MV-626 | Intraperitoneal | Pancreatic cancer and colon cancer | Preclinical | [104] |
| AVA-NP-695 | Oral | Breast cancer | Preclinical | [102] |
| ZX-8177 | Oral | Pancreatic cancer and colon cancer | Preclinical | [103] |
| STF1623 | Intraperitoneal | Pancreatic cancer and breast cancer | Preclinical | [99, 101] |
| SR8314 | Intraperitoneal | Colon cancer and melanoma | Preclinical | [105] |
| Emerging strategies | ||||
| Name | Advent delivery | Cancer type | Status | Reference |
| Manganese | Intratumoral | Melanoma and lymphoma | Preclinical | [109] |
| STACT-TREX1 | Intravenous | Colon cancer and melanoma | Preclinical | [125] |
Supplementation of cGAMP and cGAMP analogs
CDNs, including c-di-GMP, c-di-AMP, 2’3’-cGAMP, 3’3’-cGAMP, and 3’2’-cGAMP, are natural ligands to activate STING and exhibit high affinity for human STING [85, 86]. Intratumoral injection of cGAMP activated tumor STING signaling and regulated the immune response in the TME, achieving tumor inhibition [64, 87]. However, cGAMP is a poor drug candidate due to the poor membrane permeability and hydrolytic degradation by ENPP1 [88].
To overcome these limitations, cGAMP-based analogs are exploited in clinical trials, such as ADU-S100, MK-1454, etc. ADU-S100 is the first CDN drug in clinical trials for solid tumors and lymphomas as monotherapy or in combination with immune checkpoint inhibitors [89]. Intratumoral injection of ADU-S100 has demonstrated the ability to prime CD8+ T cell response and inhibit tumor growth and metastasis in multiple tumor models [86, 90, 91]. A phase I dose-escalation trial revealed that intratumoral administered ADU-S100 was well tolerated in patients with advanced/metastatic cancers. Although systemic immune response was activated after monotherapy of ADU-S100, its clinical efficacy was limited [92]. The combination of ADU-S100 with PD-1 antibody Spartalizumab in 106 patients with advanced solid tumors or lymphomas displayed minimal therapeutic efficacy, despite inducing tumor regression in murine models [93]. The differential outcomes of cGAMP and CDN-based analogs in murine models and clinical trials may partly be attributed to the different responses of certain cells to STING activation in mice and humans. Notably, the poor pharmacokinetic properties of these drugs, including low permeability, limited drug distribution and rapid clearance, constrain their anti-tumor effects. Hence, various drug-delivery methods including liposomes, nanoparticles, and extracellular vesicles have been investigated to enhance the efficacy of cGAMP and CDNs [94–96].
Overall, supplementation of cGAMP and cGAMP analogs to stimulate STING in the TME shows potential anti-tumor activity. However, cGAMP and cGAMP analogs as direct STING agonists are facing several challenges. Firstly, cGAMP indistinguishably activates STING in diverse cells, limiting the administration mode as intratumoral injection. In addition, although cGAMP and its analogs have been extensively studied and entered the clinical stage, their efficacy remains unsatisfactory. The transport of cGAMP into tumor cells activates non-canonical NF-κB signaling to promote cancer metastasis, which may compromise its efficacy. Further studies are warranted to improve drug-like properties and specificity, as well as to overcome immune suppression by combinatorial regimens.
Blocking cGAMP degradation by inhibiting ENPP1
Due to its ability to hydrolyze cGAMP and frequent overexpression in tumors, ENPP1 has been identified as a potential target for cancer therapy. ENPP1 hydrolyzes extracellular cGAMP into GMP and AMP, which is subsequently hydrolyzed into immunosuppressive adenosine by CD73 [97]. Consequently, the expression and activity of ENPP1 are correlated with decreased concentration of extracellular cGAMP and enhancement of adenosine signaling [39]. The high expression level of ENPP1 in multiple solid tumors was associated with tumor metastasis, poor prognosis, and stem cell characteristics [36, 37, 98]. ENPP1 overexpression promoted immune evasion and cancer metastasis by selectively degrading cGAMP. Knocking out ENPP1 in tumor cells and host cells could enhance CD8+ T cell infiltration and improve antitumor response to therapies associated with upstream or downstream of the cGAMP-STING pathway [36, 39, 99]. Therefore, inhibiting ENPP1 to prevent cGAMP hydrolysis and enhance immune circuit has emerged as a novel strategy for cancer therapy.
Analogs of adenine nucleotide were designed as a class of ENPP1 inhibitors. These nucleotide derivatives, including α,β-metADP, α,β-metATP, 2-MeSADP, 2-MeSATP and bzATP, exhibit competitive inhibition against ENPP1, owing to their structural resemblance to the natural substrates of ENPP1 [100]. Although nucleotide-based analogs have shown the ability to inhibit ENPP1 in vitro, their drug property is hindered by low bioavailability. As a result, non-nucleotide ENPP1 inhibitors have emerged as the primary focus of research with several inhibitors being reported. A selective ENPP1 inhibitor STF1623, which chelates Zn2+ at the catalytic site of ENPP1, effectively inhibited cGAMP hydrolysis and demonstrated significant antitumor activity as a monotherapy or in combination with radiotherapy in Panc02 tumor [101]. Another orally available ENPP1 inhibitor, AVA-NP-695, was shown to block EMT process and eliminate tumor metastasis in 4T1 model [102]. These inhibitors have proved the concept of inhibiting tumor growth and metastasis by targeting ENPP1 activity. Ongoing advancements in the cGAMP-STING pathway and ENPP1’s molecular mechanism have prompted further preclinical and clinical exploration of ENPP1 inhibitors. RBS2418 and TXN10128 have reached phase I clinical trials to target advanced or metastatic solid tumors. Moreover, multiple ENPP1 inhibitors, such as ZX-8177 [103], MV-626 [104], and SR-8134 [105], are undergoing preclinical studies, covering a wide range of tumor types. Compared with the direct STING agonists, ENPP1 inhibitors could convert anti-inflammatory effect of adenosine into a pro-inflammatory antitumor effect and evade the adverse impact of STING signaling activation in tumor cells. However, cautions are required to avoid hyperactivation of the immune response and unwanted effects on ectopic calcifications due to the role of ENPP1 in skeletal mineralization.
Emerging strategies to increase endogenous cGAMP
cGAS agonists could be an alternative approach to increase cGAMP levels, whereas it has been challenging because the activation of cGAS is a complex process that requires liquid-phase microreactors [106]. Small molecules are typically unable to create such microreactors, posing a primary obstacle to developing cGAS agonists. So far, no cGAS agonists have been identified. Recent research suggested that metal ions could directly activate cGAS in both dsDNA-dependent and dsDNA-independent manners, thereby enhancing the catalytic activity of cGAS and accelerating cGAMP synthesis [107, 108]. As a cGAS potentiator, Mn2+ promoted cGAS-cGAMP-STING signaling and effectively dampened tumor growth in tumor-bearing mice [109, 110]. However, it remains unclear whether Mn2+ could be used as a monotherapy or combined with immune checkpoint inhibitors in clinical settings. Although cGAS agonists have not been developed yet, they may represent a promising avenue for regulating the STING pathway. Further research is needed to comprehensively understand the mechanisms involved in cGAS activation and to discover safe and effective cGAS agonists for clinical applications.
Three prime repair exonuclease 1 (TREX1) is a DNA 3’ → 5’ exonuclease that protects against chronic cGAS activation and autoimmunity by degrading cytosolic DNA [111]. Thus, TREX1 plays a crucial role in restricting cGAS activation and cGAMP accumulation in tumors. The expression of TREX1 was reported to be strongly induced in chemoresistant small cell lung cancer (SCLC). Depletion of TREX1 in SCLC cells re-sensitized chemoresistant SCLC cells to chemotherapy by accumulation of dsDNA and activation of cGAS to generate cGAMP and type I IFN as well as other pro-inflammatory cytokines [112]. In addition, irradiation has been proven to induce the expression of TREX1 in a dose-dependent manner. TREX1 reduced tumor immunogenicity and dampened antitumor efficacy at high radiation doses by degrading DNA that accumulated in the cytosol, thereby reducing the activation of cGAS-cGAMP-STING pathway and the production of IFN-β [113]. Accordingly, targeting TREX1 has emerged as a promising strategy for cancer immunotherapy. Several preclinical studies have demonstrated that inhibiting TREX1 could enhance the antitumor efficacy of immune checkpoint inhibitors and other immunotherapeutic agents [114, 115]. The development of TREX1 inhibitors is still in the early stages, and more efforts are needed to identify effective inhibitors with acceptable safety profiles.
Chemotherapies (e.g. cisplatin [116, 117], paclitaxel [118], doxorubicin [119]), radiotherapies [68], and therapies that target DNA damage response (e.g., PARP inhibitors [19, 120]) have been shown to activate cGAS-mediated production of cGAMP by promoting micronuclei or chromatin bridge formation. These findings illuminate the reliance of conventional therapies on STING. However, the activation of STING via iatrogenic means is limited and its effectiveness varies depending on the tumor type and microenvironment. Therefore, combining conventional therapies with cGAMP or ENPP1 inhibitors presents a viable strategy for enhancing cGAS-cGAMP-STING signaling.
Conclusions and perspectives
cGAMP serves as a crucial second messenger that participates in regulations of multiple signaling pathways, particularly within the cGAS-STING pathway. When cytosolic dsDNA undergoes abnormalities due to internal and external factors, cGAS generates cGAMP to initiate the activation of various downstream signaling. Recent studies have demonstrated that tumor cells are more prone to form micronuclei with a high amount of aberrant dsDNA, which activates cGAS and produces cGAMP [20]. As a pivotal regulatory factor, cGAMP potently activates STING in tumor cells, and at the same time, it can be transmitted into the TME, where it modulates signals of host cells. The multifaceted functions of cGAMP in tumor cells and non-tumor cells create the intricate signal network to modulate cancer progression.
Attributing to the important role of cGAMP in tumor growth and metastasis, targeting the cGAMP axis, encompassing generation, accumulation, and degradation, has emerged as a promising approach to improve antitumor immune response. However, modulating cGAMP for cancer therapy faces a few challenges. The low bioavailability of cGAMP and its analogs necessitates intratumoral injection, restricting their clinical application. Therefore, current research has shifted focus to alternative targets, including ENPP1 and TREX1, to accumulate cGAMP in tumors. Although systemic administration of these inhibitors resolves the inconvenience of intratumoral injection, concerns arise regarding their lack of tumor cell specificity and potential for acute inflammatory and toxic reactions [121]. The improvement of these inhibitors and in-depth understanding of inhibitory mechanisms as well as optimal drug combinations will improve their antitumor efficacy and expand their clinical application. Additionally, as discussed in this review, the roles of cGAMP are intricate and vary among different cell types, exhibiting a dual function in cancer progression. Further studies are required to avoid the pro-metastatic and immunosuppressive functions induced by cGAMP and optimize its role as a tumor suppressor.
Acknowledgements
This work was supported by State Key Laboratory of Chemical Biology.
Competing interests
The authors declare no competing interests.
References
- 1.Chen Q, Sun LJ, Chen ZJJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17:1142–9. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 2.Du XX, Su XD. Detection of cyclic dinucleotides by STING. Methods Mol Biol. 2017;1657:59–69. doi: 10.1007/978-1-4939-7240-1_6. [DOI] [PubMed] [Google Scholar]
- 3.Davies BW, Bogard RW, Young TS, Mekalanos JJ. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell. 2012;149:358–70. doi: 10.1016/j.cell.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slavik KM, Morehouse BR, Ragucci AE, Zhou W, Ai XL, Chen YQ, et al. cGAS-like receptors sense RNA and control 3'2'-cGAMP signalling in Drosophila. Nature. 2021;597:109–13. doi: 10.1038/s41586-021-03743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holleufer A, Winther KG, Gad HH, Ai XL, Chen YQ, Li LH, et al. Two cGAS-like receptors induce antiviral immunity in Drosophila. Nature. 2021;597:114–8. doi: 10.1038/s41586-021-03800-z. [DOI] [PubMed] [Google Scholar]
- 6.Wu JX, Sun LJ, Chen X, Du FH, Shi HP, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13:81. doi: 10.1186/s13045-020-00916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schadt L, Sparano C, Schweiger NA, Silina K, Cecconi V, Lucchiari G, et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 2019;29:1236–48.e7. doi: 10.1016/j.celrep.2019.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49:754–63.e4. doi: 10.1016/j.immuni.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Dobbs N, Yang K, Yan N. Interferon-independent activities of mammalian STING mediate antiviral response and tumor immune evasion. Immunity. 2020;53:115–26.e5. doi: 10.1016/j.immuni.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civril F, Deimling T, de Oliveira Mann CC, Ablasser A, Moldt M, Witte G, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498:332–7. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Shu C, Yi GH, Chaton CT, Shelton CL, Diao JS, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39:1019–31. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Bai XC, Chen ZJ. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53:43–53. doi: 10.1016/j.immuni.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Whiteley AT, de Oliveira Mann CC, Morehouse BR, Nowak RP, Fischer ES, et al. Structure of the human cGAS-DNA complex reveals enhanced control of immune surveillance. Cell. 2018;174:300–11.e11. doi: 10.1016/j.cell.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn J, Xia TL, Konno H, Konno K, Ruiz P, Barber GN. Inflammation-driven carcinogenesis is mediated through STING. Nat Commun. 2014;5:5166. [DOI] [PMC free article] [PubMed]
- 17.Fu GH, Wu YF, Zhao GN, Chen XY, Xu ZJ, Sun JJ, et al. Activation of cGAS-STING signal to inhibit the proliferation of bladder cancer: the immune effect of cisplatin. Cells. 2022;11:3011. [DOI] [PMC free article] [PubMed]
- 18.Constanzo J, Faget J, Ursino C, Badie C, Pouget JP. Radiation-induced immunity and toxicities: the versatility of the cGAS-STING pathway. Front Immunol. 2021;12:680503. [DOI] [PMC free article] [PubMed]
- 19.Pantelidou C, Sonzogni O, Taveira MD, Mehta AK, Kothari A, Wang D, et al. PARP inhibitor efficacy depends on CD8+ T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9:722–37. doi: 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakhoum SF, Cantley LC. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell. 2018;174:1347–60. doi: 10.1016/j.cell.2018.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503:530–4. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–8. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepin G, De Nardo D, Rootes CL, Ullah TR, Al-Asmari SS, Balka KR, et al. Connexin-dependent transfer of cGAMP to phagocytes modulates antiviral responses. mBio. 2020;11:e03187–19. [DOI] [PMC free article] [PubMed]
- 24.Luther J, Khan S, Gala MK, Kedrin D, Sridharan G, Goodman RP, et al. Hepatic gap junctions amplify alcohol liver injury by propagating cGAS-mediated IRF3 activation. Proc Natl Acad Sci USA. 2020;117:11667–73. doi: 10.1073/pnas.1911870117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol Cell. 2019;75:372–81.e5. doi: 10.1016/j.molcel.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordova AF, Ritchie C, Bohnert V, Li LY. Human SLC46A2 is the dominant cGAMP importer in extracellular cGAMP-sensing macrophages and monocytes. ACS Cent Sci. 2021;7:1073–88. doi: 10.1021/acscentsci.1c00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Fei M, Zhang G, Liang WC, Lin W, Wu Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020;52:357–73.e9. doi: 10.1016/j.immuni.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Maltbaek JH, Cambier S, Snyder JM, Stetson DB. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity. 2022;55:1799–812.e4. doi: 10.1016/j.immuni.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahey LJ, Mardjuki RE, Wen XL, Hess GT, Ritchie C, Carozza JA, et al. LRRC8A:C/E heteromeric channels are ubiquitous transporters of cGAMP. Mol Cell. 2020;80:578. doi: 10.1016/j.molcel.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Concepcion AR, Wagner LE, Zhu JJ, Tao AY, Yang J, Khodadadi-Jamayran A, et al. The volume-regulated anion channel LRRC8C suppresses T cell function by regulating cyclic dinucleotide transport and STING-p53 signaling. Nat Immunol. 2022;23:287–302. doi: 10.1038/s41590-021-01105-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Chen X, Planells-Cases R, Chu JC, Wang L, Cao LM, et al. Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity. 2020;52:767–U305. doi: 10.1016/j.immuni.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Eaglesham JB, Pan Y, Kupper TS, Kranzusch PJ. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature. 2019;566:259–63. doi: 10.1038/s41586-019-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onyedibe KI, Wang MD, Sintim HO. ENPP1, an old enzyme with new functions, and small molecule inhibitors—a STING in the tale of ENPP1. Molecules. 2019;24: 4192. [DOI] [PMC free article] [PubMed]
- 34.Roberts F, Zhu DX, Farquharson C, Macrae VE. ENPP1 in the regulation of mineralization and beyond. Trends Biochem Sci. 2019;44:616–28. doi: 10.1016/j.tibs.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Kato K, Nishimasu H, Oikawa D, Hirano S, Hirano H, Kasuya G, et al. Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat Commun. 2018;9:4424. doi: 10.1038/s41467-018-06922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Duran MA, Dhanota N, Chatila WK, Bettigole SE, Kwon J, et al. Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov. 2021;11:1212–27. doi: 10.1158/2159-8290.CD-20-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bageritz J, Puccio L, Piro RM, Hovestadt V, Phillips E, Pankert T, et al. Stem cell characteristics in glioblastoma are maintained by the ecto-nucleotidase E-NPP1. Cell Death Differ. 2014;21:929–40. doi: 10.1038/cdd.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu M, Guo W, Liao Y, Xu D, Sun B, Song H, et al. Dysregulated ENPP1 increases the malignancy of human lung cancer by inducing epithelial-mesenchymal transition phenotypes and stem cell features. Am J Cancer Res. 2019;9:134–44. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Böhnert V, Joseph AJ, Sudaryo V, Swinderman J, Yu FB, et al. ENPP1 is an innate immune checkpoint of the anticancer cGAMP-STING pathway. bioRxiv. 10.1101/2023.06.01.543353. [DOI] [PMC free article] [PubMed]
- 40.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–U111. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YH, Liu XY, Du XX, Jiang ZF, Su XD. The structural basis for the sensing and binding of cyclic di-GMP by STING. Nat Struct Mol Biol. 2012;19:728–30. doi: 10.1038/nsmb.2333. [DOI] [PubMed] [Google Scholar]
- 42.Dobbs N, Burnaevskiy N, Chen DD, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe. 2015;18:157–68. doi: 10.1016/j.chom.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka Y, Chen ZJJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–U74. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu BX, Carlson RJ, Pires IS, Gentili M, Feng EL, Hellier Q, et al. Human STING is a proton channel. Science. 2023;381:508–14. doi: 10.1126/science.adf8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–6. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaidt MM, Ebert TS, Chauhan D, Ramshorn K, Pinci F, Zuber S, et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–24.e18. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang D, Liu Y, Zhu Y, Zhang Q, Guan H, Liu S, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022;24:766–82. doi: 10.1038/s41556-022-00894-z. [DOI] [PubMed] [Google Scholar]
- 49.Kitajima S, Tani T, Springer BF, Campisi M, Osaki T, Haratani K, et al. MPS1 inhibition primes immunogenicity of KRAS-LKB1 mutant lung cancer. Cancer Cell. 2022;40:1128–44.e8. doi: 10.1016/j.ccell.2022.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong C, Schubert M, Tijhuis AE, Requesens M, Roorda M, van den Brink A, et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature. 2022;607:366–73. doi: 10.1038/s41586-022-04847-2. [DOI] [PubMed] [Google Scholar]
- 51.Zierhut C, Yamaguchi N, Paredes M, Luo JD, Carroll T, Funabiki H. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell. 2019;178:302–15.e23. doi: 10.1016/j.cell.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee D, Langberg K, Abbas S, Odermatt E, Yerramothu P, Volaric M, et al. A non-canonical, interferon-independent signaling activity of cGAMP triggers DNA damage response signaling. Nat Commun. 2021;12:6207. doi: 10.1038/s41467-021-26240-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu D, Wu H, Wang CG, Li YJ, Tian HB, Siraj S, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26:1735–49. doi: 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xun J, Zhang Z, Lyu B, Lu D, Yang H, Shang G, et al. A conserved ion channel function of STING mediates non-canonical autophagy and cell death. bioRxiv. 10.1101/2023.08.26.554976. [DOI] [PMC free article] [PubMed]
- 55.Nassour J, Radford R, Correia A, Fuste JM, Schoell B, Jauch A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–63. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41:76. doi: 10.1186/s13046-022-02262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prabakaran T, Bodda C, Krapp C, Zhang BC, Christensen MH, Sun C, et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37:e97858. [DOI] [PMC free article] [PubMed]
- 58.Dou Z, Ghosh K, Vizioli MG, Zhu J, Sen P, Wangensteen KJ, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–6. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H, Wang HZ, Ren JY, Chen Q, Chen ZJJ. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA. 2017;114:E4612–20. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu J, Sánchez-Rivera FJ, Wang Z, Johnson GN, Ho Y-J, Ganesh K, et al. STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature. 2023;616:806–13. doi: 10.1038/s41586-023-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Hubisz MJ, Earlie EM, Duran MA, Hong C, Varela AA, et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620:1080–8. doi: 10.1038/s41586-023-06464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong SH, Yang MJ, Choi S, Kim J, Koh GY. Refractoriness of STING therapy is relieved by AKT inhibitor through effective vascular disruption in tumour. Nat Commun. 2021;12:4405. [DOI] [PMC free article] [PubMed]
- 64.Ohkuri T, Kosaka A, Ishibashi K, Kumai T, Hirata Y, Ohara K, et al. Intratumoral administration of cGAMP transiently accumulates potent macrophages for anti-tumor immunity at a mouse tumor site. Cancer Immunol Immunother. 2017;66:705–16. doi: 10.1007/s00262-017-1975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardner A, Pulido AD, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. [DOI] [PMC free article] [PubMed]
- 66.Andzinski L, Spanier J, Kasnitz N, Kroger A, Jin L, Brinkmann MM, et al. Growing tumors induce a local STING dependent type I IFN response in dendritic cells. Int J Cancer. 2016;139:1350–7. doi: 10.1002/ijc.30159. [DOI] [PubMed] [Google Scholar]
- 67.Yi M, Niu M, Wu Y, Ge H, Jiao D, Zhu S, et al. Combination of oral STING agonist MSA-2 and anti-TGF-beta/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol. 2022;15:142. doi: 10.1186/s13045-022-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng LF, Liang H, Xu M, Yang XM, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–5. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 71.Nicolai CJ, Wolf N, Chang IC, Kirn G, Marcus A, Ndubaku CO, et al. NK cells mediate clearance of CD8(+) T cell-resistant tumors in response to STING agonists. Sci Immunol. 2020;5:eaaz2738. [DOI] [PMC free article] [PubMed]
- 72.Larkin B, Ilyukha V, Sorokin M, Buzdin A, Vannier E, Poltorak A. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J Immunol. 2017;199:397–402. doi: 10.4049/jimmunol.1601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ni H, Zhang H, Li L, Huang H, Guo H, Zhang L, et al. T cell-intrinsic STING signaling promotes regulatory T cell induction and immunosuppression by upregulating FOXP3 transcription in cervical cancer. J Immunother Cancer. 2022;10:e005151. [DOI] [PMC free article] [PubMed]
- 74.Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun. 2017;8:427. doi: 10.1038/s41467-017-00573-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–12. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Li S, Mirlekar B, Johnson BM, Brickey WJ, Wrobel JA, Yang N, et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature. 2022;610:373–80. doi: 10.1038/s41586-022-05254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang CA, Lee AC, Chang S, Xu Q, Shao A, Lo Y, et al. STING regulates BCR signaling in normal and malignant B cells. Cell Mol Immunol. 2021;18:1016–31. doi: 10.1038/s41423-020-00552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hida K, Maishi N, Annan DA, Hida Y. Contribution of tumor endothelial cells in cancer progression. Int J Mol Sci. 2018;19:1272. [DOI] [PMC free article] [PubMed]
- 79.Demaria O, De Gassart A, Coso S, Gestermann N, Di Domizio J, Flatz L, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA. 2015;112:15408–13. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Campisi M, Sundararaman SK, Shelton SE, Knelson EH, Mahadevan NR, Yoshida R, et al. Tumor-derived cGAMP regulates activation of the vasculature. Front Immunol. 2020;11:2090. doi: 10.3389/fimmu.2020.02090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang H, Lee WS, Kong SJ, Kim CG, Kim JH, Chang SK, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest. 2019;129:4350–64. doi: 10.1172/JCI125413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Z, Li X, Mao Y, Wei C, Huang Z, Li G, et al. Interferon-dependent SLC14A1+ cancer-associated fibroblasts promote cancer stemness via WNT5A in bladder cancer. Cancer Cell. 2022;40:1550–65.e7. doi: 10.1016/j.ccell.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Arwert EN, Milford EL, Rullan A, Derzsi S, Hooper S, Kato T, et al. STING and IRF3 in stromal fibroblasts enable sensing of genomic stress in cancer cells to undermine oncolytic viral therapy. Nat Cell Biol. 2020;22:908. doi: 10.1038/s41556-020-0544-6. [DOI] [PubMed] [Google Scholar]
- 85.Danilchanka O, Mekalanos JJ. Cyclic dinucleotides and the innate immune response. Cell. 2013;154:962–70. doi: 10.1016/j.cell.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–30. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li TJ, Cheng H, Yuan H, Xu QM, Shu C, Zhang YF, et al. Antitumor activity of cGAMP via stimulation of cGAS-cGAMPSTING-IRF3 mediated innate immune response. Sci Rep. 2016;6:19049. [DOI] [PMC free article] [PubMed]
- 88.Li L, Yin Q, Kuss P, Maliga Z, Millan JL, Wu H, et al. Hydrolysis of 2'3’-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat Chem Biol. 2014;10:1043–8. doi: 10.1038/nchembio.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ding C, Song Z, Shen A, Chen T, Zhang A. Small molecules targeting the innate immune cGAS‒STING‒TBK1 signaling pathway. Acta Pharm Sin B. 2020;10:2272–98. doi: 10.1016/j.apsb.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berger G, Knelson EH, Jimenez-Macias JL, Nowicki MO, Han S, Panagioti E, et al. STING activation promotes robust immune response and NK cell-mediated tumor regression in glioblastoma models. Proc Natl Acad Sci USA. 2022;119:e2111003119. doi: 10.1073/pnas.2111003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaidi AH, Kelly RJ, Gorbunova A, Omstead AN, Salvitti MS, Zheng P, et al. Intratumoral immunotherapy with STING agonist, ADU-S100, induces CD8+ T-cell mediated anti-tumor immunity in an esophageal adenocarcinoma model. Oncotarget. 2021;12:292–303. doi: 10.18632/oncotarget.27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meric-Bernstam F, Sweis RF, Hodi FS, Messersmith WA, Andtbacka RHI, Ingham M, et al. Phase I dose-escalation trial of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced/metastatic solid tumors or lymphomas. Clin Cancer Res. 2022;28:677–88. doi: 10.1158/1078-0432.CCR-21-1963. [DOI] [PubMed] [Google Scholar]
- 93.Meric-Bernstam F, Sweis RF, Kasper S, Hamid O, Bhatia S, Dummer R, et al. Combination of the STING agonist MIW815 (ADU-S100) and PD-1 inhibitor spartalizumab in advanced/metastatic solid tumors or lymphomas: an open-label, multicenter, phase Ib study. Clin Cancer Res. 2023;29:110–21. doi: 10.1158/1078-0432.CCR-22-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koshy ST, Cheung AS, Gu L, Graveline AR, Mooney DJ. Liposomal delivery enhances immune activation by STING agonists for cancer immunotherapy. Adv Biosyst. 2017;1:1600013. [DOI] [PMC free article] [PubMed]
- 95.Jang SC, Economides KD, Moniz RJ, Sia CL, Lewis N, McCoy C, et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun Biol. 2021;4:497. doi: 10.1038/s42003-021-02004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–78. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Linden J, Koch-Nolte F, Dahl G. Purine release, metabolism, and signaling in the inflammatory response. Annu Rev Immunol. 2019;37:325–47. doi: 10.1146/annurev-immunol-051116-052406. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi RU, Miyazaki H, Takeshita F, Yamamoto Y, Minoura K, Ono M, et al. Loss of microRNA-27b contributes to breast cancer stem cell generation by activating ENPP1. Nat Commun. 2015;6:7318. [DOI] [PMC free article] [PubMed]
- 99.de Cordoba BRF, Moreno H, Valencia K, Perurena N, Ruedas P, Walle T, et al. Tumor ENPP1 (CD203a)/haptoglobin axis exploits myeloid-derived suppressor cells to promote post-radiotherapy local recurrence in breast cancer. Cancer Discov. 2022;12:1356–77. doi: 10.1158/2159-8290.CD-21-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SY, Muller CE. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm. 2017;8:823–40. doi: 10.1039/C7MD00015D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carozza JA, Bohnert V, Nguyen KC, Skariah G, Shaw KE, Brown JA, et al. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nat Cancer. 2020;1:184–96. doi: 10.1038/s43018-020-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goswami A, Deb B, Goyal S, Gosavi A, Mali M, Martis AM, et al. AVA-NP-695 selectively inhibits ENPP1 to activate STING pathway and abrogate tumor metastasis in 4T1 breast cancer syngeneic mouse model. Molecules. 2022;27:6721. [DOI] [PMC free article] [PubMed]
- 103.Qin X, Li Y-Y, Sun S, Li X, Peng Z, Kong D, et al. Abstract 577: ENPP1 inhibitor ZX-8177 in combination with chemotherapy or radiation exhibits synergistic anti-tumor effects via STING-mediated response. Cancer Res. 2023;83:577. doi: 10.1158/1538-7445.AM2023-577. [DOI] [Google Scholar]
- 104.Baird J, Dietsch G, Florio V, Gallatin M, Knox C, Odingo J, et al. MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo. Society for Immunotherapy of Cancer 2018 Annual Meeting; 2018; Posters. 7.
- 105.Weston A, Thode T, Munoz R, Daniel S, Soldi R, Kaadige M, et al. Abstract 3077: preclinical studies of SR-8314, a highly selective ENPP1 inhibitor and an activator of STING pathway. Cancer Res. 2019;79:3077. doi: 10.1158/1538-7445.AM2019-3077. [DOI] [Google Scholar]
- 106.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–9. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang CG, Guan YK, Lv MZ, Zhang R, Guo ZY, Wei XM, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48:675–87.e7. doi: 10.1016/j.immuni.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Zhao Z, Ma ZX, Wang B, Guan YK, Su XD, Jiang ZF. Mn2+ directly activates cGAS and structural analysis suggests Mn2+ induces a noncanonical catalytic synthesis of 2',3'-cGAMP. Cell Rep. 2020;32:108053. [DOI] [PubMed]
- 109.Lv MZ, Chen MX, Zhang R, Zhang W, Wang CG, Zhang Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–79. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cai L, Wang Y, Chen Y, Chen H, Yang T, Zhang S, et al. Manganese(ii) complexes stimulate antitumor immunity via aggravating DNA damage and activating the cGAS-STING pathway. Chem Sci. 2023;14:4375–89. doi: 10.1039/D2SC06036A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mohr L, Toufektchan E, von Morgen P, Chu K, Kapoor A, Maciejowski J. ER-directed TREX1 limits cGAS activation at micronuclei. Mol Cell. 2021;81:724. doi: 10.1016/j.molcel.2020.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murayama T, Mahadevan NR, Tani T, Ma X, Watanabe H, Barbie DA, et al. Abstract 5145: Targeting TREX1 induces innate immune response in chemoresistant small cell lung cancer. Cancer Res. 2023;83:5145. doi: 10.1158/1538-7445.AM2023-5145. [DOI] [Google Scholar]
- 113.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen V, Francica B, Hsieh D, Freund D, Holtz A, Samanta S, et al. Abstract 1636: Generation of novel potent human TREX1 inhibitors facilitated by crystallography. Cancer Res. 2023;83:1636. doi: 10.1158/1538-7445.AM2023-1636. [DOI] [Google Scholar]
- 115.Francica B, Burdette D, Clark R, Cope J, Freund D, Holtz A, et al. Abstract 2075: Systemic small molecule TREX1 inhibitors to selectively activate STING in the TME of metastatic disease. Cancer Res. 2022;82:2075. doi: 10.1158/1538-7445.AM2022-2075. [DOI] [Google Scholar]
- 116.Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene. 2019;38:2380–93. doi: 10.1038/s41388-018-0581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Della Corte CM, Sen T, Gay CM, Ramkumar K, Diao L, Cardnell RJ, et al. STING pathway expression identifies NSCLC with an immune-responsive phenotype. J Thorac Oncol. 2020;15:777–91. doi: 10.1016/j.jtho.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu Y, Manasrah BK, McGregor SM, Lera RF, Norman RX, Tucker JB, et al. Paclitaxel induces micronucleation and activates pro-inflammatory cGAS-STING signaling in triple-negative breast cancer. Mol Cancer Ther. 2021;20:2553–67. doi: 10.1158/1535-7163.MCT-21-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luthra P, Aguirre S, Yen BC, Pietzsch CA, Sanchez-Aparicio MT, Tigabu B, et al. Topoisomerase II inhibitors induce DNA damage-dependent interferon responses circumventing Ebola virus immune evasion. mBio. 2017;8:e00368-17. [DOI] [PMC free article] [PubMed]
- 120.Chabanon RM, Muirhead G, Krastev DB, Adam J, Morel D, Garrido M, et al. PARP inhibition enhances tumor cell-intrinsic immunity in ERCC1-deficient non-small cell lung cancer. J Clin Invest. 2019;129:1211–28. doi: 10.1172/JCI123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Garland KM, Sheehy TL, Wilson JT. Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chem Rev. 2022;122:5977–6039. doi: 10.1021/acs.chemrev.1c00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang CHA, Zundell JA, Ranatunga S, Lin C, Nefedova Y, Del Valle JR, et al. Agonist-mediated activation of STING induces apoptosis in malignant B cells. Cancer Res. 2016;76:2137–52. doi: 10.1158/0008-5472.CAN-15-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chin EN, Yu C, Vartabedian VF, Jia Y, Kumar M, Gamo AM, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369:993–9. doi: 10.1126/science.abb4255. [DOI] [PubMed] [Google Scholar]
- 124.Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369:eaba6098. [DOI] [PubMed]
- 125.Makarova AM, Iannello A, Rae CS, King B, Besprozvannaya M, Faulhaber J, et al. Abstract 5016: STACT-TREX1: a systemically-administered STING pathway agonist targets tumor-resident myeloid cells and induces adaptive anti-tumor immunity in multiple preclinical models. Cancer Res. 2019;79:5016. doi: 10.1158/1538-7445.AM2019-5016. [DOI] [Google Scholar]