Abstract
Combustion of mixed materials during open air burning of refuse or structural fires in the wildland urban interface produces emissions that worsen air quality, contaminate rivers and streams, and cause poor health outcomes including developmental effects. The zebrafish, a freshwater fish, is a useful model for quickly screening the toxicological and developmental effects of agents in such species and elicits biological responses that are often analogous and predictive of responses in mammals. The purpose of this study was to compare the developmental toxicity of smoke derived from the burning of 5 different burn pit-related material types (plywood, cardboard, plastic, a mixture of the three, and the mixture plus diesel fuel as an accelerant) in zebrafish larvae. Larvae were exposed to organic extracts of increasing concentrations of each smoke 6-to-8-hr post fertilization and assessed for morphological and behavioral toxicity at 5 days post fertilization. To examine chemical and biological determinants of toxicity, responses were related to emissions concentrations of polycyclic hydrocarbons (PAH). Emissions from plastic and the mixture containing plastic caused the most pronounced developmental effects, including mortality, impaired swim bladder inflation, pericardial edema, spinal curvature, tail kinks, and/or craniofacial deformities, although all extracts caused concentration-dependent effects. Plywood, by contrast, altered locomotor responsiveness to light changes to the greatest extent. Some morphological and behavioral responses correlated strongly with smoke extract levels of PAHs including 9-fluorenone. Overall, the findings suggest that material type and emissions chemistry impact the severity of zebrafish developmental toxicity responses to burn pit-related smoke.
Keywords: Burn pits, Smoke, Zebrafish, Developmental toxicity, Plastic, Polyaromatic hydrocarbons, Particulate matter (PM), Behavior.
Highlights
-
•
Burn pit-related emissions caused developmental toxicity in zebrafish.
-
•
Emissions from plastic and mixtures containing plastic were most toxic.
-
•
Plywood and cardboard emissions were less toxic than plastic emissions.
-
•
Altered morphology and behavior correlated with emission PAH levels.
1. Introduction
Open burning of mixed materials in refuse or housefires produces emissions that degrade air quality and that are increasingly linked to adverse health effects [1,2]. While the use of military burn pits has been curtailed or eliminated, long term health consequences from past exposures to burn pit emissions continue to be documented. For example, veterans who self-reported burn pit exposures had increased risk of emphysema, chronic bronchitis, and chronic obstructive pulmonary disease [1,[3], [4], [5], [6], [7], [8], [9], [10], [11]]. Homes burned during wildfires also pose a growing risk to human health as the likelihood of wildfires increases due to warmer and drier conditions [12] and growth of the wildland-urban interface [13]. Smoke from wildfires, which often includes emissions from structural fires, is associated with mortality and adverse cardiovascular outcomes [14]. Linkage with other health impacts including developmental effects is less clear. Exposure to ambient air pollution containing particulate matter (PM) and volatile organic compounds (VOC) near industrial areas has been associated with low birth weights and exposure to low levels of urban air pollution has been correlated with low birth weights [15], and higher preterm births [16], although the impacts of burn pit smoke are less certain. For example, one study of pregnant military personnel exposed to burn pit smoke found no link between exposure and congenital defects [17], while a case-control study reported that infants and children had an increased likelihood of congenital abnormalities the closer they lived to active burn pits [18]. The precise developmental effects of emissions from mixed material burns and the role of material type in toxicity responses remain unclear.
Ambient particulate matter also contaminates lakes, rivers, and streams in the form of watershed runoff and ash deposits, with the potential to adversely affect freshwater organisms [19,20]. Assessment of the impacts from exposure to PM from key sources including burn pits is, therefore, necessary to characterize risk in aquatic species. Zebrafish (Danio rerio), a freshwater fish, is a useful model for quickly screening the toxicological and developmental effects of smoke in such species. Moreover, its high degree of functional similarity to mammals, in vitro-like ease of use in the laboratory, quick reproduction and development, and amenability to genetic and pharmacological manipulation [21], have increased its adoption as a higher throughput alternative to mammalian models for predicting toxicity potential. Previous developmental toxicity studies have shown a strong concordance among zebrafish and mammalian models (72 %–92 %) [[22], [23], [24], [25], [26], [27]]. Zebrafish can also absorb chemicals from their environment through their epidermis [28], which contains epithelial cells that are sensitive to chemicals that also irritate mammalian lung epithelium [29,30]. Zebrafish skin also expresses TRPA1 [31,32], which has been linked to oxidative stress in mammalian pulmonary airways [33]. PM mixtures are rich in chemical triggers of oxidative stress, such as oxygenated PAHs like 9-fluorenone [[34], [35], [36]]. Zebrafish also exhibit locomotor responses which we previously used to derive potency determinations of biomass smoke PM [37], responses that were consistent with separate assessments of lung toxicity potential after intra-airway exposure of the same PM samples in mice [38]. Zebrafish also have well-understood and measurable developmental behaviors and biology [39], which are altered by exposure to household [40] and urban PM [41]. The extent to which burn pit PM impacts zebrafish development, however, remains unclear.
We previously determined that inhalation exposure to smoke from various burn pit-related materials generated under smoldering conditions impairs breathing in mice, effects that were largely mitigated with particle filtration [42]. Here we determined whether smoke from the same burn pit material types also burned under smoldering conditions cause developmental toxicity in zebrafish. An automated furnace that generated combustion emissions from different fuels was previously paired with a cryotrap collection system to collect large quantities of smoke condensates from five fuels, including plywood, cardboard, plastics, a mixture of the three, and that same mixture with diesel fuel accelerant to mimic fuel often added to ignite burn pit fires [36,38]. The potential for organic extracts of these condensates, a chemical fraction of PM that contains PAHs and is linked to oxidative stress [43] and mutagenicity [38], to cause developmental toxicity in zebrafish was assessed by measuring malformations using an automated imaging system. We also assessed impacts on behavior by measuring locomotor responses to changes in light. In addition, we performed correlation analyses to examine the relationship between toxicity responses and chemical concentrations of PAHs. Finally, using a fluorescent cyp1a-tagged transgenic line of zebrafish [44], we assessed the impacts of exposure on expression of cyp1a, a key metabolizing enzyme for many toxicants including PAHs. Importantly, PAHs, including those found in PM [45] are known ligands of the aryl hydrocarbon receptor (AhR), a highly conserved ligand-activated transcription factor that regulates expression of phase I enzymes, including cyp1 [46].
2. Materials and methods
2.1. Burn pit materials
The materials burned, which model materials historically disposed of within burn pits [36], consisted of plywood (ActionPak Inc., Bristol, PA), cardboard (ActionPak, Inc., Bristol, PA), plastic (a blend of low-density polyethylene, high-density polyethylene, polyethylene terephthalate, and polystyrene pellets), a mixture of plywood, plastic, and cardboard materials, and the mixture + diesel fuel (10 wt%) [36]. The mixture was designed to mimic burn pits, many of which consist mostly of paper (49 wt%), plastic (27 wt%) and wood (24 wt%) [36], the composition of which was derived from waste stream data from US military bases [36,47,48].
2.2. Combustion and byproduct collection
Combustion and smoke condensate byproduct collection have been previously described [36]. The smoke condensates used in the present study were the same smoldering smoke condensates used in our previous study [36], which described combustion and smoke condensate byproduct collection in greater detail. Briefly, 1 cm long pieces of each fuel type were burned in a quartz tube furnace at 500 °C to create 5 unique smoke samples, which were collected using a multi-phase (−10 °C, −50 °C, and −70 °C) cryotrap system, enabling collection of particulates, and volatile and semi-volatile combustion byproducts. The smoke condensates were then rinsed, concentrated, and solvent exchanged into dimethyl sulfoxide (DMSO; ≥99.9 % purity, Sigma-Aldrich; St. Louis, MO) making a stock of ‘extractable organic material’ stored at 4 °C in the dark. We focused on the organic fraction of the smoke condensates because we have previously shown that this fraction of PM contains many toxic PAHs including oxy- and nitro-PAHs and causes substantial acute toxicity [30,37,38].
2.3. Chemical analysis of burn pit smoke
Chemical analysis of the condensates was conducted as described previously [36]. In brief, gas chromatography-mass spectrometry (GC-MS; Model: 7890/5975B GC/MSD system, Agilent Technologies Inc., Santa Clara, CA) was used to analyze VOC and high-performance liquid chromatography (HPLC) to analyze carbonyl hydrazones. To analyze carbon species within the smoke condensates, PM was extracted using acetone and analyzed for organic and elemental carbon, and for PAHs using GC-MS. The organic carbon sample was then analyzed for sixteen U.S. Environmental Protection Agency (EPA) priority PAHs and 16 nitro-/oxy-PAHs using GC–MS [36].
2.4. Experimental animals
All studies were approved by and followed the guidelines of the Institutional Animal Care and Use Committee at the U.S. EPA's Center for Public Health and Environmental Assessment (Animal Care and Use Protocol: 24-05-001). Zebrafish were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility with a 14:10 h light:dark cycle, with lights on at 7:00 a.m. EST. The mating regimen utilized an in-house line of zebrafish which were annually mixed with wild-type zebrafish to maintain genetic diversity. The wild-type adult zebrafish (Danio rerio) used are an undefined, outbred stock originally obtained from Aquatic Research Organisms, Hampton, NH, 03842 and EkkWill Waterlife Resources Ruskin, FL 33575). Previously, Dr. Seok-Yong Choi's laboratory developed a transgenic cyp1a reporter line of zebrafish (Tg(cyp1a:nls-egfp; background strain TL) using DNA recombineering to reveal target tissues for dioxin and was later used to measure impacts of PAH activity by the Tanguay laboratory who then provided embryos to us [44]. The cyp1a reporter line of zebrafish were kept separately from in-house and wild-type lines on-site and handled in the same protocols [44]. The cyp1a transgenic strain was used only for cyp1a imaging and not for assessment of malformations and behavior and it is unclear whether responses in the cyp1a strain would differ from the wild-type strain. Adult male and female zebrafish were housed together in 8-L tanks, with water temperature maintained at 28 °C. Adult fish populations (80–100 per 15-L static tank) were used as spawning groups the afternoon before collection. Embryos were collected from the spawning tank the following morning, placed in a beaker which was then stored in a 26 °C water bath, and washed according to previously outlined lab practices [37]. The washed embryos were reared in 10 % HBSS (Hanks' balanced salt solution) prior to treatment. Post-assay, larvae were anesthetized via immersion into ice-cold water. Following that, anesthetized larvae were then euthanized via immersion in ice-cold 20 % bleach solution.
2.5. Lethality and malformation assessment
Zebrafish embryos were placed individually with their chorion intact in a 96 well mesh plate (Multiscreen™, Millipore Sigma, Burlington, MA, USA) and exposed to fuel extracts at 6–8 hpf. The embryos were transferred into a dosing plate containing final nominal concentrations of vehicle (0.4 % DMSO), or each fuel type ranging from 40, 12.66, 4.01, 1.27, 0.40, 0.13, 0.04, or 0.01 μg/mL (serial half-log dilutions; Fig. 1). The exposure concentrations were derived from a stock plate containing concentrations 250X the dosage concentrations. One μl of the stock plate solution was added to 249 μl HBSS within each well of the dosage plate using a multichannel pipette. At 4 days post fertilization (dpf), the zebrafish were transferred from the dosing plate to a fresh plate containing 10 % HBSS with no test chemicals added and returned to the 28 °C incubator.
Fig. 1.
Experimental timeline for zebrafish exposures preceding morphological and behavioral assays. Zebrafish larvae were plated at 6–8 hpf and dosed at 12 hpf. The zebrafish were incubated at 28 °C for 4 days, at which point they were transferred from the dosing solution into a fresh plate of 10 % Hanks' balanced salt solution (HBSS) and returned to the incubator. On the fifth day, the plates were transferred into another fresh stock of 10 % HBSS immediately before assessment.
After the fifth day of incubation, a detailed visual assessment of larvae was conducted by researchers blinded to the dosing scheme. At this point, healthy larvae would be hatched and free swimming with their swim bladders inflated. A tally was made of mortality among the larvae on the plate. Deformities were also tallied, listing features such as uninflated swim bladders, abnormalities (craniofacial deformities, slight tail curvatures, fin deformities), and severe abnormalities (deformities imminently harmful to the larvae, such as edemas & gross morphological defects).
Following the visual assessment, wells containing live larvae were assessed using the VAST Bioimager (Union Biometrica Inc., Massachusetts, USA) for automated morphological assessment. In summary, the VAST system can automatically anesthetize the larvae, pick up each larva in a 96-well plate, and move them to a glass capillary tube for imaging. The larvae used in this study were imaged in four orientations: dorsal, ventral, and left/right lateral views. Once imaged, the embryos were deposited into a new 96-well plate loaded with 10 % HBSS. The morphological features of the zebrafish's lateral view were automatically annotated using FishInspector (https://www.ufz.de/index.php?en=44460), a publicly available program designed to recognize and measure features of zebrafish [27]. These automatically annotated images were manually inspected for quality before being analyzed using a KNIME workflow to create numerical outputs for statistical analysis. The KNIME workflow created outputs for “Distance cont-yolk, Head size-eye, Length (mm), Eye size (mm2), Distance cont-yolk (mm), Otolith missing, Bladder size (mm2), Pericardial size (mm2), Yolk sac size (mm2), Otolith-eye distance (mm), Head size (mm2), Tail length (mm), Mandibular arch distance, Angle jaw-eye otolith, Eye-jaw distance (mm), Max tail curvature, and Curvature direction”.
2.6. Behavioral assay
Zebrafish embryos were placed individually in a 96 well mesh plate (Multiscreen™, Millipore Sigma, Burlington, MA, USA) and exposed to fuel extracts at 6–8 hpf. The embryos were transferred into a dosing plate containing concentrations of vehicle (0.4 % DMSO, final nominal concentration), or each fuel type ranging from 1.27, 0.4014, 0.127, 0.0402, 0.0127 μg/mL. Embryos were exposed to a positive control plate using fluoxetine hydrochloride (Sigma Aldrich, Millipore Sigma, Burlington, MA, USA) dosed at 4, 1.2, and 0.4 μM alongside controls in 0.4 % DMSO vehicle. As with the morphological assay, zebrafish embryos remained in the dosing solution until 4 dpf at which point the fish were transferred into a fresh plate containing 10 % HBSS with no test chemical and returned to the 28 °C incubator. At 5 dpf, the larvae were removed for the incubator and transferred into a second fresh plate containing 10 % HBSS and kept in a dark room containing the Viewpoint machine 2 h prior to the start of the assay. Mortalities and fish with abnormal or severely abnormal effects at 120 hpf were grounds for exclusion from the behavioral assay due to their impact on swimming strength.
All behavioral assays were run using the Viewpoint Zebrabox (Viewpoint Life Sciences, Lyon, France) to evaluate photoinduced changes in locomotion in 5 dpf larvae. The testing room was kept at an ambient temperature of 26 °C throughout the assay. The assay began with a 20-min dark period (12 lux) for acclimation, followed by an alternating light (3500 lux) and dark period that lasted 40 min each, then three light and dark alternating periods lasting 2 min each. All light and dark periods were conducted at uniform settings. This accounted for a total of 4 light-dark transitions. These assay conditions were repeated for each plate containing smoke extracts. Each plate contained concentrations of a single extract in addition to vehicle-exposed controls.
Analysis of larval response to light stimuli (photo-locomotion) was tracked from videos using Ethovision XT (Noldus Information Technology) software Version 13 by a blinded researcher according to previous work in the lab [37]. The Ethovision's tracking rate was 5 samples/s (5 images taken a second). Objects darker than the plate's background were detected using dynamic subtraction using a lower detection threshold of 25 pixels. The outputs were analyzed for total distance moved in centimeters, per every 2-min integration period. System noise was removed during analysis via an input filter of 0.2 cm (minimum distance moved). All photo-locomotion data were expressed as distance moved (cm)/2 min interval.
2.7. Cyp1a reporter line
The cyp1a-tagged transgenic zebrafish embryos were placed individually in a 96-well clear-bottomed plate (Corning® 96 Well Special Optics Microplate, Millipore Sigma, Burlington, MA, USA) and exposed to fuel extracts at 6–8 hpf (Fig. 7). The embryos were dosed with vehicle (0.4 % DMSO, final nominal concentration), or individual fuel types at either 0.40 or 1.27 μg/mL. Beta-napthoflavone served as a positive control at a concentration of 1 μM in a 0.4 % DMSO vehicle. Zebrafish embryos were incubated at 28 °C in the dosing solution until 4 dpf. To assess cyp1a levels in exposed embryos, images were collected of live specimens at 24 and 96 hpf using a Nikon Ti inverted fluorescence microscope (Nikon Instruments, Melville, NY). Images were obtained with a Photometrics HQ2 CCD camera using an eGFP filter via a 2x objective. The software NIS-Elements (version 4.13, Nikon Instruments, Melville, NY) was used to designate a whole embryo region of interest, and then an intensity threshold was established indicating cyp1a-positive cells and a binary image layer was created from pixels exceeding that intensity. Mean binary intensity for each sample was calculated using an automated macro (total binary intensity/binary area). Fish displaying mortality or abnormal or severely abnormal effects were excluded from the fluorescence assay.
Fig. 7.
Correlations between morphological changes (A–C) and, separately, locomotor responses (D–F) and concentrations of total PAHs in the smoke condensates of each fuel type were analyzed by comparing mean distance moved per 2-min interval using AUC analysis per preliminary lighting conditions in GraphPad Prism 9. The resulting AUC values were compared to PAH concentrations using simple linear regressions and Pearson r correlations to determine if the slopes were significant (*p < 0.05).
2.8. Statistics
All statistical analyses were performed using GraphPad Prism software (version 9.0, GraphPad Software Inc., San Diego, CA) & StatView (version 5.0, SAS Institute Inc., Cary, NC). The data from the morphological assay were not normally distributed (D'Agostino & Pearson omnibus normality test) and were, therefore, analyzed by the nonparametric Kruskal-Wallis test, followed by correction for multiple comparisons using Dunn's test. All values are reported as mean ± standard error of the mean (SEM). Behavioral data was analyzed using a repeated measures 2-way ANOVA using Statview for each individual fuel type. The mean movement per concentration of each light phase was calculated, and if a significant interaction between factors was found, a second ANOVA was run on each phase compared to control. Fisher's PLSD was run as a post-hoc test in the event a significant difference was found. Additionally, Graphpad Prism 9 was used to analyze individual mean distance moved per 2-min intervals using repeated measures 2-way ANOVA with the Geisser-Greenhouse correction, along with Dunnett's multiple comparisons post-hoc test comparing all concentrations to vehicle controls. The cyp1a reporter line fluorescence was analyzed using a mixed effects model to handle missing values. Sphericity was not assumed, and a Geisser-Greenhouse correction was applied to the analysis. All treatments were compared to the DMSO-vehicle control. Holm-Sidak was used to test for multiple comparisons. In all tests, a p value < 0.05 was considered statistically significant.
Morphological correlations between fuel type and chemical class were analyzed using area under the curve (AUC) analysis according to fuel type in GraphPad Prism (Fig. 5, Fig. 6). The resulting AUC values were compared to PAH concentrations using simple linear regressions and Pearson r correlations to determine if the slopes were significant (p < 0.05). Locomotor response correlations between fuel type and chemical class were analyzed by comparing mean distance moved per 2-min interval using AUC analysis per individual lighting condition in GraphPad Prism 9. The resulting AUC values were compared to PAH concentrations using simple linear regressions and Pearson r correlations to determine if the slopes were significant (p < 0.05). The figure legends contain the number of independent observations.
Fig. 5.
Correlations between morphological changes and concentrations of oxy-PAHs in the smoke condensates of each fuel type. Data were analyzed using area under the curve (AUC) analysis according to fuel type in GraphPad Prism 9. The resulting AUC values were compared to PAH concentrations using simple linear regressions and Pearson r correlations to determine if the slopes were significant (*p < 0.05).
Fig. 6.
Correlations between locomotor responses and concentrations of oxy-PAHs in the smoke condensates of each fuel type were analyzed by comparing mean distance moved per 2-min interval using AUC analysis per preliminary lighting conditions in GraphPad Prism 9. The resulting AUC values were compared to PAH concentrations using simple linear regressions and Pearson r correlations to determine if the slopes were significant (*p < 0.05).
3. Results
3.1. Burn pit related smoke exposure effects on lethality and morphology
In this study, the effects of early burn pit-related smoke extract exposure on zebrafish development were assessed. To do this, gastrulation-stage embryos (from 5.25 to 10 hpf) were exposed to concentrations of burn-pit related smoke ranging from 0.01 to 40 μg/mL (Fig. 1). The concentration range varied in half log steps, serving as a range finding experiment for lethality and morphological changes among the zebrafish. This concentration range was decided upon to determine effect levels per fuel type and avoid lethal concentrations in future assays.
Prior to imaging with the VAST system, researchers blinded to the exposure regimen reviewed plates under dissecting microscopes, finding that exposure to smoke condensates at the highest dose of 40 μg/mL was generally lethal across all fuel types (Fig. 2). All non-surviving fish were excluded from the subsequent VAST morphological assay. Morphological changes, a major sign of overt toxicity, occurred across varying concentrations per fuel type, with effects being more pronounced in plastic-exposed larvae at lower concentrations compared to other fuel types. At a concentration of 12.66 μg/mL across all fuel types, 100 % of larvae failed to inflate their swim bladders and showed signs of abnormal development (Fig. 2). The plastic and mixture smoke condensates continued this trend to a lesser extent at 4.01 μg/mL (Fig. 2). 25 % of the larvae exposed to 1.26 μg/mL of plastic smoke condensate had an uninflated swim bladder. About 12.5 % of the larvae exposed to the mixture plus diesel condensate at the highest concentration of 40 μg/mL were rated as severely abnormal (Fig. 2).
Fig. 2.
Visual determinations of lethality and malformation responses to extracts. Assay blinded researchers inspected zebrafish embryos immediately prior to morphological assessment in the VAST Bioimager. The tally assessed swim bladder inflation status (Swim Bladder), Abnormalities (minor edema or tail curvature), Severe Abnormalities (deformities likely to affect larval survival such as major edemas, tail curvatures, craniofacial abnormalities, and other gross morphological defects), and Mortality. N = 8 per concentration.
Next, we asked whether burn pit-related smoke exposure during development could quantifiably affect the larvae's morphological features. We found that treatment with each of the five fuel types resulted in significant morphological changes in 12 of 15 studied endpoints (Supplemental Fig. 1). Zebrafish larvae exposed to plastic exhibited significant changes from control at concentrations ≥1.27 μg/mL in three of these endpoints (tail length, tail curvature, and bladder size) (Fig. 3). An interesting effect of exposure to the smoke condensates happened regarding tail length. Larvae exposed to plastic displayed morphological changes at lower concentrations, while plywood and cardboard were less affected. As the concentration of plastic condensate increased from 1.27 to 4.01 μg/mL, the tail length of the exposed larvae increased from control significantly (Fig. 3). However, this trend reversed at a concentration of 12.66 μg/mL, the highest concentration observable by the VAST Bioimager, leading to a collapse in the overall tail length of the tested larvae (Fig. 3). This trend appeared to be mirrored in the mixture and mixture plus diesel condensates to a lesser extent. In those condensates, we noted a staggered overall increase at similar concentrations that appears to be indicative of a biphasic dose response to a component of plastic condensate (Fig. 3).
Fig. 3.
Morphological responses to extracts using the VAST Bioimager. Morphological changes induced by exposures to smoke extracts at concentrations ranging from 0.0127 to 12.66 μg/mL from 6 to 8 hpf to 96 hpf across 3 endpoints as recorded through the VAST Bioimager. Images were taken at 5 dpf. Concentration-response curves shown on the top row, while corresponding heat maps are included below to further visualize the change in endpoints. Tail Length, Max Tail Curvature, and Bladder Size endpoints are included. N = 8 per treatment group.
Next, we looked at the change in tail curvature based on the VAST's images of the larval notochord analyzed using FishInspector [27]. Tail curvature is a highly variable feature of development in zebrafish, and therefore a large degree of difference is necessary to show a significant difference from controls. Strikingly, the only significant changes in tail curvature from control doses occurred in larvae exposed to high doses of plastic smoke condensate. Starting at 4.01 μg/mL and continuing to 12.66 μg/mL, the maximum tail curvature increased to 0.14 and 0.20 mm, respectively (Fig. 3). We also looked for the effects of exposure on the size of the swim bladder in zebrafish larvae. Here we found that exposure to high concentrations of smoke condensates during development led to the larvae's failure to inflate their swim bladders, regardless of fuel type. However, animals exposed to plastic and the mixture containing plastic had significantly smaller swim bladders at 4.01 μg/mL, which was more potent than the other fuel types which showed changes at concentrations of 12.66 μg/mL (Fig. 3). It is important to note that significant changes in many endpoints such as swim bladder size were mirrored across all fuel types, with plastic leading the trend at lower concentrations than the others (Fig. 3). Plastic smoke condensate alone caused significant changes from control including decreased eye size, increased tail curvature, decreased pericardium size, and decreased cont-yolk distance (Supplemental Fig. 1).
3.2. Burn pit related smoke exposure effects on behavior
Next, behavioral effects from exposure to smoke condensates during development on the zebrafish's response to stimuli were assessed in the photo-locomotion assay. During the first dark period, the lowest dose of plywood smoke displayed significantly higher activity compared to control doses (Fig. 4C). In the second light phase, the lowest plywood dose was once again significantly hyperactive. No significant effect across lighting phases occurred until the third dark phase, in which there was an overall dose effect and the lowest dose mean movement was significantly hyperactive. On the fourth dark phase, plywood-exposed zebrafish displayed an overall dose effect, and mean movement was significantly hyperactive with the three lower doses (Fig. 4C). No overall effects of dose or interactions during light periods were discovered in other fuel types (Fig. 4 A, B, D, & E).
Fig. 4.
Effects of darkness and visible light on locomotion in larval zebrafish (n = 28 per treatment group) exposed to concentrations of smoke condensates of various fuel types, or vehicle. Photo-locomotor activity recorded for Plastic (A), Mixture (B), Plywood (C), Mixture + Diesel (D), and Cardboard (E). Larval response to light stimuli from 20 to 60 min preceding the larval startle response and adaptation to sudden darkness at 60–100 min. Three short light-dark transitions follow from 100 to 112 min. Data are presented as mean distance moved (in cm) ± S.E.M. in 2-min intervals. Movement during each 2-min interval was compared to vehicle via ANOVA indicated significant differences (*P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001) according to fuel types. Multiple comparisons were corrected for via Dunnett tests using GraphPad Prism software (version 9.0, GraphPad Software Inc., San Diego, CA). N = 28–32 per treatment group.
When 2-min interval periods were compared to controls, we found that the second highest dose (0.40 μg/mL) of plywood moved significantly more than controls at 60 min, the transition point from light to darkness (2.5950 mean difference; p = 0.0326). Interestingly, from minutes 74–80, zebrafish exposed to the highest dose (1.27 μg/mL) of plywood condensate moved significantly less than controls as the rest of the concentrations returned to baseline from the sudden light-dark transition (−1.9640, −1.6760, −1.5650, −1.5500 mean difference; p = 0.0453, 0.0073, 0.0208, 0.0015 respectively). The same effect happened at 86 min in zebrafish exposed to 0.04 and 1.27 μg/mL of plywood smoke (−0.7184, −0.7884 mean difference; p = 0.0463, 0.0280 respectively). The third and fourth dark phases recorded significantly higher movement among plywood-exposed zebrafish in the lowest concentration, confirming our results in Statview (3.2660, 2.5760; p = 0.0038, 0.0172) (Fig. 4C).
3.3. Correlations of EPA priority PAHs, oxy-PAHs, and total PAHs with morphological and behavioral effects
Linear regressions of morphological effects AUC per condensate or behavioral responses (distance traveled AUC per condensate) vs. concentrations of the EPA priority PAHs naphthalene, fluorene, and phenanthrene, the oxy-PAHs 9-flourenone, 1-naphthalenecarboxaldehyde, and 9,10-anthraquinone, and total PAHs per condensate were carried out to determine if there was a relationship between the morphological (maximum tail curvature, tail length, and bladder size) or behavior change observed and PAH constituent concentration within the smoke condensate (Supplementary Table 1). None of the morphological changes (Supplementary Fig. 2) or locomotor responses (Supplementary Fig. 3) significantly correlated with any of the EPA priority PAHs.
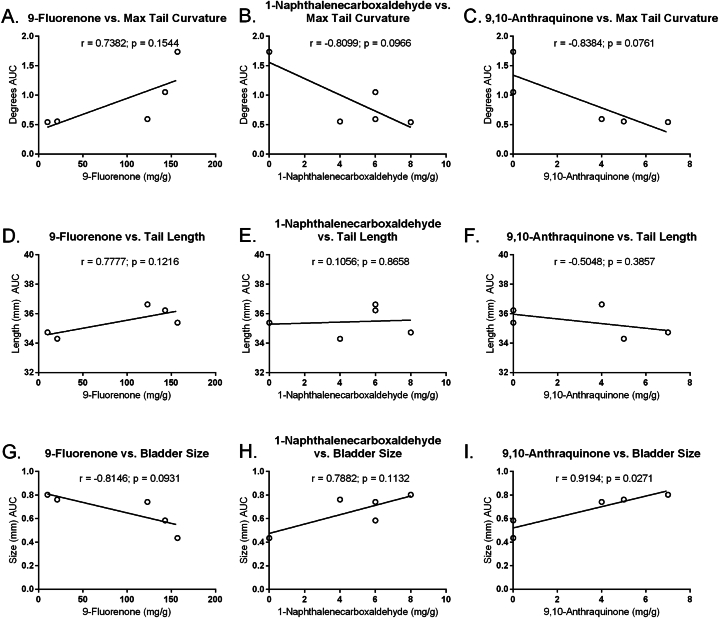
Maximum tail curvature did not correlate with the oxy-PAHs 9-flourenone (Fig. 5A) and had tendencies towards significant negative correlations with 1-naphthalenecarboxaldehyde (r = −0.8099; p = 0.0966; Figs. 5B), and 9 and 10-anthraquinone concentration (r = −0.8384; p = 0.0761; Fig. 5C). Tail length did not significantly correlate with any of the oxy-PAHs (Fig. 5D–F). Bladder size significantly positively correlated with 9,10-anthraquinone (r = 0.9194; p = 0.0271; Fig. 5I), had a tendency towards a significant negative correlation with 9-flourenone (r = −0.8146; p = 0.0931; Fig. 5G), and did not correlate with 1-naphthalenecarboxaldehyde (Fig. 5H). Mean distance moved was significantly positively correlated with 9-flourenone concentration in the dark period (r = 0.8499, p = 0.0321; Fig. 6G), had a tendency towards a significant positive correlation during acclimation (r = 0.7894; p = 0.0619; Fig. 6A), but no correlation during the light period (Fig. 6D). Mean distance moved had tendencies towards significant positive correlations with 1-naphthalenecarboxaldehyde during the acclimation (r = 0.7581; p = 0.0807; Fig. 6B) and dark (r = 0.7924; p = 0.0602; Fig. 6H) periods, but not in the light period (Fig. 6E). Mean distance moved was not significantly correlated with 9,10-anthraquinone concentration during any period.
Maximum tail curvature (Fig. 7A) and bladder size (Fig. 7C) were not significantly correlated with total PAH concentration while tail length was significantly positively correlated with total PAH concentration (r = 0.9442; p = 0.0157; Fig. 7B). Mean distance moved was not significantly correlated with total PAH concentration during any period (Fig. 7D–F).
3.4. Burn pit related smoke exposure effects on cyp1a fluorescence
Cyp1a induction by the extracts was studied using a transgenic reporter line and compared to a positive and negative control. At a high dose of 1.27 μg/mL, plywood and cardboard emissions condensates significantly increased cyp1a related fluorescence compared to DMSO vehicle control at 24 hpf, although these responses were far less than the responses to the positive control BNF (p = 0.0001, p = 0.0241, and p = 0.0488 respectively) (Supplemental Fig. 4A). This trend disappeared at 96 hpf, where only BNF, significantly increased cyp1a related fluorescence compared to DMSO vehicle control (p < 0.0001) (Supplemental Fig. 4B). The lower dose yielded similar results. At a dose of 0.40 μg/mL, plywood, cardboard, the mixture containing all three extracts, and BNF also significantly increased cyp1a related fluorescence compared to DMSO vehicle control at 24 hpf (p < 0.0001, p < 0.0001, p = 0.0140, and p < 0.0001 respectively) (Supplemental Fig. 4C). Similarly, significant increases in fluorescence disappeared when tested at 96 hpf except for the positive control (p = 0.0001) (Supplemental Fig. 4D).
4. Discussion
Developmental exposure to burn pit-related smoke condensates caused overt toxicity characterized by malformations at high concentrations of all material types, while toxicity at lower concentrations was largely limited to emissions from plastic or mixtures containing plastic. Only the condensate from burning plastic increased tail length at 1.27 μg/mL and 4.01 μg/mL compared to controls, but interestingly this effect was reversed at a dose of 12 μg/mL, perhaps reflecting mechanisms linked to overt toxicity. Typically, shortened length is reflective of overt toxicity and commonly takes place after exposure to high concentrations of PAHs [49,50]. Oxy-PAHs, like 9- fluorenone, have been implicated in causing shortened body length in zebrafish through impairment of pancreatic endocrine development [51]. Failure to inflate the swim bladder was also evident in zebrafish exposed to emissions from plastic or the mixture containing plastic at lower concentrations relative to other fuels. Inflation of the swim bladder is crucial for survival in teleost fish by minimizing the energy required to float in the water column [52]. While the zebrafish's swim bladder is evolutionarily homologous to the mammalian lung [53], it is not clear how impaired swim bladder inflation relate to developmental processes in mammals. Inflation of the swim bladder relies on blood circulation and has been theorized to be a secondary effect of disrupted vascularization following exposure to chemicals affecting heart rate [54,55]. Tail curvature was most sensitive to developmental exposure to emissions from plastic or the mixture containing plastic. Compounds that caused tail or body curvature in zebrafish were also previously found to cause neural tube defects in mammals [27]. Interestingly, samplings of PAH- and oxy-PAH contaminated waters linked oxy-PAHs with notochord defects [56]. In aggregate, these findings are consistent with our previous findings in mice exposed to smoldering and flaming burn pit materials, indicating that inhalation of smoldering plastic emissions caused the largest decrement in breathing relative to other burn pit material types & temperatures [42].
At concentrations below those that elicited overt toxicity, plywood, but not plastic smoke, altered behavior. While the precise reason for this divergence is unclear, we previously demonstrated that smoldering plywood smoke alone caused significant increases in lung neutrophils after exposure in mice [36]. Larvae exposed to smoldering plywood condensate were hyperactive compared to controls during the light period consistent with evidence that wood smoke, rather than plastic smoke, inhibited the generation of reactive oxygen species which despite having innate toxic properties, also play an important role in neurotransmission [57]. By contrast, plywood-smoke exposed zebrafish were less responsive to the light:dark transition. This result is in line with research showing that exposure to wood smoke from open-fire cooking was associated with lower scores on memory tests and cognitive performance in children [58]. Embryonic exposure to certain PAHs can decrease whole animal oxygen consumption and total cellular respiration in the mitochondria of organs such as the brain, liver, and heart, impacting behavior by reducing neuronal mitochondrial function which could potentially explain the observed changes in zebrafish locomotor behavior [59]. Exposure to an environmentally relevant mixture of PAH's that was linked to decreased learning and startle stimulus responses was also linked with decreased mitochondrial function [60], which may be one possible explanation for the lack of responses in the light:dark transition to emissions from the mixture plus diesel fuel. This trend was not sustained throughout the assay and could possibly be a result of the total PAH mixture overall, rather than one chemical or chemical class specifically. Exposure to PAHs from oil contaminated aquatic ecosystems has also been linked to similar locomotor responses [61].
Morphological and behavioral toxicity responses to organic extracts of the smoke condensates correlated with oxy-PAH content in the whole condensates. PAHs in general belong to a class of chemicals generated from the incomplete combustion of organic materials, and have been shown to have carcinogenic [14], mutagenic [62], and pro-inflammatory effects [63,64], and have been linked to developmental toxicity in fish [[65], [66], [67]], increased metabolizing enzymatic activity [68], and developmental effects stemming from exposure to PM2.5 [69]. Despite the high levels of the EPA priority PAHs naphthalene, fluorene, and phenanthrene in the extracts from plastic and mixtures containing plastic in the present study, their levels did not correlate with toxicity responses, potentially indicating that they played minimal roles in the responses observed. By contrast, locomotor responses were strongly positively correlated and bladder size to a lesser extent negatively correlated with the oxy-PAH, 9-fluorenone. In addition, tail length positively correlated with total PAH levels, responses perhaps driven by the high levels of 9-fluorenone, although tail length did not significantly correlate with 9-fluorenone levels suggesting other PAHs or chemical classes influenced this endpoint. Interestingly, morphological and behavioral toxicity responses were negatively correlated with the oxy-PAHs 1-napthalenecarboxaldehyde and 9,10, anthraquinone, compounds that have previously been found in ambient PM [70] and biomass combustion emissions [62], respectively. The precise reasons for this are unclear. These two compounds, however, were both present at low levels compared to 9-fluorenone and likely had less influence. Oxy-PAHs are water soluble and often deposit from airborne particulate matter [71]. 9-fluorenone is both a transformation product resulting from the oxidation of fluorene and is also generated from the combustion of solid fuels [72]. Exposure to 9-fuorenone has previously been linked to decreased heart rate, increased incidence of craniofacial deformities, and increased body curvature in zebrafish [51]. Exposure to mixtures of oxy-PAHs derived from soil samples of industrial sites has previously been linked to malformations and mortality in embryonic zebrafish [71]. Developmental toxicity responses caused by 9-fluorenone could be indicative of an increased anxious response, a result that has been replicated in similar studies in zebrafish after embryonic exposure to PAHs and photo-locomotor responses [65]. Hyperactive swimming has also been reported in larval killifish in response to increasing concentrations of PAHs, until overt toxicity occurred at the maximum concentration [67].
Although oxy-PAH levels correlated with some toxicity endpoints, PAHs comprised only a small percentage of the mixture by mass [36] and several endpoints of developmental toxicity did not correlate with either total PAHs or 9-flourenone content of the condensates, suggesting that there were other chemical classes that contributed to toxicity. This is supported by recent findings that indicated that while oxy-PAHs within stream-collected samples were implicated in causing notochord defects, other chemical classes were likely also at play in causing morphological toxicity [56]. Furthermore, combustion conditions influence emissions chemistry as we previously determined that condensates derived from high temperature flaming conditions, which were not tested in the present study, caused greater lung toxicity in mice than those generated by smoldering conditions, effects likely driven by the greater production of oxy- and nitro-PAHs with flaming combustions [36]. Also, chemical constituents may interact with other factors in the environment to cause adversity. For example, wildfires have been shown to cause hypoxic conditions in aquatic environments following a burn, possibly precipitating an interaction between hypoxia and PAH mixtures to cause pericardial edema and mortality in zebrafish [73].
The mechanisms by which the developmental toxicity responses to the plastic extract took place in the present study are unclear. Oxy-PAHs, including 9-flourenone have been linked to increased production of reactive oxygen species and oxidative stress [71] and inhibition of cyp1a [72]. PAH binding to the AhR, promotes transcription of cyp1, enabling swift clearance of pro-carcinogenic and teratogenic PAHs [46]. Urban and rural PM-induced malformations in zebrafish have previously been associated with pronounced induction of AhR signaling, which correlated very strongly with PAH concentration [69]. Interestingly, PAHs within the plastic smoke extract and the mixtures containing plastic have been shown to have cyp1a inhibiting properties (fluoranthene, 9-fluorenone) while others are AhR agonists (phenanthrene, beta-napthoflavone) [74]. It has been found that exposure to PAH mixtures containing cyp1 inhibitors and AhR agonists causes synergistic, dioxin-like developmental effects in fish [75]. The plastic extract, which had the highest concentration of oxy-PAHs, did not increase cyp1a fluorescence in the transgenic fish in the present study, contrasting with the small but significant increases caused by the plywood and cardboard extracts. However, the net increase in whole fish fluorescence was very small, and the conclusions from these assessments are rather limited given the absence of accompanying measures of cyp1a activity, protein levels, and/or gene expression. Furthermore, future studies would need to confirm the role of any specific pathway or mechanism of action by use of inhibitors and/or genetic manipulation.
5. Limitations
Several limitations of the present study need to be considered. Hydrophobic organic components of PM, such as PAHs, are readily absorbed by zebrafish [76] and can trigger inflammatory responses once internalized [77]. This study is therefore limited by lack of measures of internal concentration, as it is unclear to what extent responses resulted from mechanisms at the skin's surface or through internalization of the organic material. Zebrafish in the present study were exposed to smoke condensates through immersion rather than inhalation or via airway exposure as in our previous work in mice that examined the impacts of these smoke condensates on lung toxicity responses [36]. While this difference increases the complexity in extrapolating findings to the human condition, functional conservation and similarities in biological responses suggest potential for utility in the predictivity of toxicity potential across species. Importantly, the exposure levels in this study are comparable to those used in previously reported in vitro human lung cell studies [76,77]. Also, this study only examined impacts of organic extracts of the smoke samples, and not the whole sample, thus the extent to which toxicity may be driven by other fractions of the sample is unclear. Finally, the PAH data was obtained from measures of the original smoke condensates and not from the organic extracts used in the zebrafish assays. These extracts were obtained by solvent-exchanging with DMSO, a process that likely influences and potentially alters chemical composition. Thus, future studies should assess the composition of such extracts to better link chemistry with toxicity.
6. Conclusions
In conclusion, exposure to emissions of disparate burn pit-related fuel types and their mixture has significant adverse impacts on development in zebrafish. The pronounced effects evident after developmental exposure suggest that the biological potency of these combustion byproducts is especially acute during this life stage in aquatic species and perhaps mammals. Although plywood emissions elicited adverse behavioral responses, emissions from plastic and the mixtures containing plastic caused the greatest developmental toxicity pointing to the variability in responsiveness among fuel types, likely driven by emissions chemistry. Further studies should be undertaken to identify the specific chemical components and biological mechanisms responsible for such effects, especially those caused by plastic emissions. Taken together, these findings illustrate the potential dangers associated with smoke from the open burning of refuse and structural fires, and the need for increased awareness of the exaggerated toxicity potential of certain material types.
Disclaimer
This manuscript has been reviewed by the Center for Public Health and Environmental Assessment, United States Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Declarations
●The ethics approval number for our studies was 24-05-001, which was approved by the Institutional Animal Care and Use Committee of the US EPA.
●The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability statement
All data used to generate the figures and tables are located in Supplementary File 2.
CRediT authorship contribution statement
Jacob Smoot: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Stephanie Padilla: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yong Ho Kim: Writing – review & editing, Resources, Methodology, Investigation. Deborah Hunter: Writing – review & editing, Methodology, Formal analysis, Data curation. Alan Tennant: Writing – review & editing, Software, Methodology, Investigation, Formal analysis, Data curation. Bridgett Hill: Writing – review & editing, Methodology, Data curation. Morgan Lowery: Software, Methodology, Data curation. Bridget R. Knapp: Writing – review & editing, Methodology, Data curation. Wendy Oshiro: Writing – review & editing, Resources, Investigation. Mehdi Hazari: Writing – review & editing, Resources, Investigation. Michael D. Hays: Writing – review & editing, Methodology, Data curation. William T. Preston: Methodology, Formal analysis, Data curation. Ilona Jaspers: Methodology, Investigation, Funding acquisition, Data curation. M. Ian Gilmour: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Conceptualization. Aimen K. Farraj: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr. John Cowden and Dr. James Samet of the US EPA for their thorough review of this manuscript prior to publication. We would also like to thank Dr. Seok-Yong Choi of Chonnam National University of Korea and Dr. Robyn Tanguay of Oregon State Univeristy for providing their transgenic cyp1a reporter zebrafish. This work was funded in part by U.S. Department of Defense Award No. W81XWH-18-1-0731 (IJ).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29675.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Liu J., et al. Burn pit emissions exposure and respiratory and cardiovascular conditions among airborne hazards and open burn pit registry participants. J. Occup. Environ. Med. 2016;58(7):e249–e255. doi: 10.1097/JOM.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 2.McLean J., et al. The potential effects of burn pit exposure on the respiratory tract: a systematic review. Mil. Med. 2021;186(7–8):672–681. doi: 10.1093/milmed/usab070. [DOI] [PubMed] [Google Scholar]
- 3.Korzeniewski K., et al. Respiratory tract infections in the military environment. Respir. Physiol. Neurobiol. 2015;209:76–80. doi: 10.1016/j.resp.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallon C.T., et al. Introduction to department of Defense research on burn pits, biomarkers, and health outcomes related to deployment in Iraq and Afghanistan. J. Occup. Environ. Med. 2016;58(8 Suppl 1):S3–S11. doi: 10.1097/JOM.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 5.Masiol M., et al. Airborne dioxins, furans, and polycyclic aromatic hydrocarbons exposure to military personnel in Iraq. J. Occup. Environ. Med. 2016;58(8 Suppl 1):S22–S30. doi: 10.1097/JOM.0000000000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrbeck P., Hu Z., Mallon C.T. Assessing health outcomes after environmental exposures associated with open pit burning in deployed US service members. J. Occup. Environ. Med. 2016;58(8 Suppl 1):S104–S110. doi: 10.1097/JOM.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szema A., et al. Proposed Iraq/Afghanistan war-lung injury (IAW-LI) clinical practice recommendations: national academy of Sciences' Institute of medicine burn pits workshop. Am. J. Men's Health. 2017;11(6):1653–1663. doi: 10.1177/1557988315619005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thatcher T.H., et al. Analysis of postdeployment serum samples identifies potential biomarkers of exposure to burn pits and other environmental hazards. J. Occup. Environ. Med. 2019;61(Suppl 12):S45–S54. doi: 10.1097/JOM.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wauters R.H., Foster B.E., Banks T.A. Environmental exposures and asthma in active duty service members. Curr. Allergy Asthma Rep. 2019;19(9):43. doi: 10.1007/s11882-019-0873-3. [DOI] [PubMed] [Google Scholar]
- 10.Woeller C.F., et al. MicroRNAs as novel biomarkers of deployment status and exposure to polychlorinated dibenzo-p-dioxins/dibenzofurans. J. Occup. Environ. Med. 2016;58(8 Suppl 1):S89–S96. doi: 10.1097/JOM.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia X., et al. Polycyclic aromatic hydrocarbons and polychlorinated dibenzo-p-dioxins/dibenzofurans in microliter samples of human serum as exposure indicators. J. Occup. Environ. Med. 2016;58(8 Suppl 1):S72–S79. doi: 10.1097/JOM.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abatzoglou J.T., Williams A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc Natl Acad Sci U S A. 2016;113(42):11770–11775. doi: 10.1073/pnas.1607171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radeloff V.C., et al. Rapid growth of the US wildland-urban interface raises wildfire risk. Proc Natl Acad Sci U S A. 2018;115(13):3314–3319. doi: 10.1073/pnas.1718850115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straif K., et al. Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006;7(12):977–978. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 15.Melody S., et al. Adverse birth outcomes in Victoria, Australia in association with maternal exposure to low levels of ambient air pollution. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109784. [DOI] [PubMed] [Google Scholar]
- 16.Laurent O., et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001-2008. Environ. Health Perspect. 2016;124(9):1479–1486. doi: 10.1289/ehp.1510133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conlin A.M., et al. Birth outcomes among military personnel after exposure to documented open-air burn pits before and during pregnancy. J. Occup. Environ. Med. 2012;54(6):689–697. doi: 10.1097/JOM.0b013e31824fe154. [DOI] [PubMed] [Google Scholar]
- 18.Savabieasfahani M., Basher Ahamadani F., Mahdavi Damghani A. Living near an active U.S. military base in Iraq is associated with significantly higher hair thorium and increased likelihood of congenital anomalies in infants and children. Environ Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113070. [DOI] [PubMed] [Google Scholar]
- 19.Gomez Isaza D.F., Cramp R.L., Franklin C.E. Fire and rain: a systematic review of the impacts of wildfire and associated runoff on aquatic fauna. Glob Chang Biol. 2022;28(8):2578–2595. doi: 10.1111/gcb.16088. [DOI] [PubMed] [Google Scholar]
- 20.Ruppel M.M., et al. Spatial and temporal patterns in black carbon deposition to dated fennoscandian arctic lake sediments from 1830 to 2010. Environ. Sci. Technol. 2015;49(24):13954–13963. doi: 10.1021/acs.est.5b01779. [DOI] [PubMed] [Google Scholar]
- 21.Howe K., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brannen K.C., et al. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res B Dev Reprod Toxicol. 2010;89(1):66–77. doi: 10.1002/bdrb.20223. [DOI] [PubMed] [Google Scholar]
- 23.Hermsen S.A., et al. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol. Vitro. 2011;25(3):745–753. doi: 10.1016/j.tiv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Selderslaghs I.W., et al. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod. Toxicol. 2009;28(3):308–320. doi: 10.1016/j.reprotox.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Van den Bulck K., et al. Zebrafish developmental toxicity assay: a fishy solution to reproductive toxicity screening, or just a red herring? Reprod. Toxicol. 2011;32(2):213–219. doi: 10.1016/j.reprotox.2011.06.119. [DOI] [PubMed] [Google Scholar]
- 26.Krupp E. Screening of developmental toxicity–Validation and predictivity of the zebrafish embryotoxicity assay (ZETA) and strategies to optimize de-risking developmental toxicity of drug candidates. Toxicol. Lett. 2016;258:S39. [Google Scholar]
- 27.Teixido E., et al. Automated morphological feature assessment for zebrafish embryo developmental toxicity screens. Toxicol. Sci. 2019;167(2):438–449. doi: 10.1093/toxsci/kfy250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morikane D., Zang L., Nishimura N. Evaluation of the percutaneous absorption of drug molecules in zebrafish. Molecules. 2020;25(17) doi: 10.3390/molecules25173974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prober D.A., et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 2008;28(40):10102–10110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens J.S., et al. Zebrafish locomotor responses reveal irritant effects of fine particulate matter extracts and a role for TRPA1. Toxicol. Sci. 2018;161(2):290–299. doi: 10.1093/toxsci/kfx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroll F., et al. A simple and effective F0 knockout method for rapid screening of behaviour and other complex phenotypes. Elife. 2021;10 doi: 10.7554/eLife.59683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khara L.S., Ali D.W. The endocannabinoid system's involvement in motor development relies on cannabinoid receptors, TRP channels, and Sonic Hedgehog signaling. Physiol Rep. 2023;11(1) doi: 10.14814/phy2.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bessac B.F., et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 2008;118(5):1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel A.B., et al. Polycyclic aromatic hydrocarbons: sources, toxicity, and remediation approaches. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.562813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.H., et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part. Fibre Toxicol. 2021;18(1):45. doi: 10.1186/s12989-021-00435-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin W.K., et al. Zebrafish irritant responses to wildland fire-related biomass smoke are influenced by fuel type, combustion phase, and byproduct chemistry. J. Toxicol. Environ. Health. 2021;84(16):674–688. doi: 10.1080/15287394.2021.1925608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim Y.H., et al. Mutagenicity and lung toxicity of smoldering vs. Flaming emissions from various biomass fuels: implications for health effects from wildland fires. Environ. Health Perspect. 2018;126(1) doi: 10.1289/EHP2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stednitz S.J., Washbourne P. Rapid progressive social development of zebrafish. Zebrafish. 2020;17(1):11–17. doi: 10.1089/zeb.2019.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roper C., et al. Workflow for comparison of chemical and biological metrics of filter collected PM(2.5) Atmos. Environ. 1994;2020:226. doi: 10.1016/j.atmosenv.2020.117379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roper C., Simonich S.L.M., Tanguay R.L. Development of a high-throughput in vivo screening platform for particulate matter exposures. Environ Pollut. 2018;235:993–1005. doi: 10.1016/j.envpol.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vance S.A., et al. Contributions of particulate and gas phases of simulated burn pit smoke exposures to impairment of respiratory function. Inhal. Toxicol. 2023;35(5–6):129–138. doi: 10.1080/08958378.2023.2169416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju S., et al. Oxidative stress generated by polycyclic aromatic hydrocarbons from ambient particulate matter enhance vascular smooth muscle cell migration through MMP upregulation and actin reorganization. Part. Fibre Toxicol. 2022;19(1):29. doi: 10.1186/s12989-022-00472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K.H., et al. Cyp1a reporter zebrafish reveals target tissues for dioxin. Aquat. Toxicol. 2013;134–135:57–65. doi: 10.1016/j.aquatox.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 45.O'Driscoll C.A., et al. Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Billiard S.M., et al. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006;92(2):526–536. doi: 10.1093/toxsci/kfl011. [DOI] [PubMed] [Google Scholar]
- 47.Aurell J., Gullett B.K., Yamamoto D. Emissions from open burning of simulated military waste from forward operating bases. Environ. Sci. Technol. 2012;46(20):11004–11012. doi: 10.1021/es303131k. [DOI] [PubMed] [Google Scholar]
- 48.VA, U.S.A.L.I.A.F.B. vols. 03–31. S. Army Central (USARCENT) Area of Responsibility; 2013. (USARCENT AOR Contingency Base Waste Stream Analysis: an Analysis of Solid Waste Streams at Five Bases in the U). [Google Scholar]
- 49.Yang F., Li G., Sang N. Embryonic exposure to soil samples from a gangue stacking area induces thyroid hormone disruption in zebrafish. Chemosphere. 2019;236 doi: 10.1016/j.chemosphere.2019.07.068. [DOI] [PubMed] [Google Scholar]
- 50.Corrales J., et al. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat. Toxicol. 2014;148:16–26. doi: 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun Y., et al. Embryonic exposure to oxy-polycyclic aromatic hydrocarbon interfere with pancreatic beta-cell development in zebrafish via altering DNA methylation and gene expression. Sci. Total Environ. 2019;660:1602–1609. doi: 10.1016/j.scitotenv.2018.12.476. [DOI] [PubMed] [Google Scholar]
- 52.Alexander R.M. The energetics of vertical migration by fishes. Symp. Soc. Exp. Biol. 1972;26:273–294. [PubMed] [Google Scholar]
- 53.Zheng W., et al. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yue M.S., Peterson R.E., Heideman W. Dioxin inhibition of swim bladder development in zebrafish: is it secondary to heart failure? Aquat. Toxicol. 2015;162:10–17. doi: 10.1016/j.aquatox.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bittner L., et al. Influence of pH on the uptake and toxicity of beta-blockers in embryos of zebrafish, Danio rerio. Aquat. Toxicol. 2018;201:129–137. doi: 10.1016/j.aquatox.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Rude C.I., et al. Coupling environmental whole mixture toxicity screening with unbiased RNA-seq reveals site-specific biological responses in zebrafish. Toxics. 2023;11(3) doi: 10.3390/toxics11030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarasenko A., et al. A comparative study of wood sawdust and plastic smoke particulate matter with a focus on spectroscopic, fluorescent, oxidative, and neuroactive properties. Environ. Sci. Pollut. Res. Int. 2022;29(25):38315–38330. doi: 10.1007/s11356-022-18741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munroe R.L., Gauvain M. Exposure to open-fire cooking and cognitive performance in children. Int. J. Environ. Health Res. 2012;22(2):156–164. doi: 10.1080/09603123.2011.628642. [DOI] [PubMed] [Google Scholar]
- 59.Hawkey A.B., et al. Embryonic exposure to benzo[a]pyrene causes age-dependent behavioral alterations and long-term metabolic dysfunction in zebrafish. Neurotoxicol. Teratol. 2022;93 doi: 10.1016/j.ntt.2022.107121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geier M.C., et al. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol. Appl. Pharmacol. 2018;354:115–125. doi: 10.1016/j.taap.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vignet C., et al. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in zebrafish--part II: behavior. Environ. Sci. Pollut. Res. Int. 2014;21(24):13818–13832. doi: 10.1007/s11356-014-2762-6. [DOI] [PubMed] [Google Scholar]
- 62.de Oliveira Galvao M.F., et al. Biomass burning particles in the Brazilian Amazon region: mutagenic effects of nitro and oxy-PAHs and assessment of health risks. Environ Pollut. 2018;233:960–970. doi: 10.1016/j.envpol.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 63.Knecht A.L., et al. Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013;271(2):266–275. doi: 10.1016/j.taap.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayasundara N., et al. AHR2-Mediated transcriptomic responses underlying the synergistic cardiac developmental toxicity of PAHs. Toxicol. Sci. 2015;143(2):469–481. doi: 10.1093/toxsci/kfu245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knecht A.L., et al. Developmental benzo[a]pyrene (B[a]P) exposure impacts larval behavior and impairs adult learning in zebrafish. Neurotoxicol. Teratol. 2017;59:27–34. doi: 10.1016/j.ntt.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knecht A.L., et al. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish. Toxicol. Appl. Pharmacol. 2017;329:148–157. doi: 10.1016/j.taap.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown D.R., et al. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naive Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol. Teratol. 2016;53:55–63. doi: 10.1016/j.ntt.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivares A., et al. Developmental effects of aerosols and coal burning particles in zebrafish embryos. Environ Pollut. 2013;178:72–79. doi: 10.1016/j.envpol.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 69.Mesquita S.R., et al. Toxicity assessment of atmospheric particulate matter in the Mediterranean and Black Seas open waters. Sci. Total Environ. 2016;545–546:163–170. doi: 10.1016/j.scitotenv.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 70.Niu X., et al. Atmospheric levels and cytotoxicity of polycyclic aromatic hydrocarbons and oxygenated-PAHs in PM(2.5) in the Beijing-Tianjin-Hebei region. Environ Pollut. 2017;231(Pt 1):1075–1084. doi: 10.1016/j.envpol.2017.08.099. [DOI] [PubMed] [Google Scholar]
- 71.Idowu O., et al. Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 2019;123:543–557. doi: 10.1016/j.envint.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 72.Niu Y., et al. Enhancing the water solubility of 9-fluorenone using cyclodextrin inclusions: a green approach for the environmental remediation of OPAHs. Crystals. 2023;13(5):775. [Google Scholar]
- 73.Fleming C.R., Di Giulio R.T. The role of CYP1A inhibition in the embryotoxic interactions between hypoxia and polycyclic aromatic hydrocarbons (PAHs) and PAH mixtures in zebrafish (Danio rerio) Ecotoxicology. 2011;20(6):1300–1314. doi: 10.1007/s10646-011-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wincent E., Le Bihanic F., Dreij K. Induction and inhibition of human cytochrome P4501 by oxygenated polycyclic aromatic hydrocarbons. Toxicol. Res. 2016;5(3):788–799. doi: 10.1039/c6tx00004e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Billiard S.M., et al. Nonadditive effects of PAHs on Early Vertebrate Development: mechanisms and implications for risk assessment. Toxicol. Sci. 2008;105(1):5–23. doi: 10.1093/toxsci/kfm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuhnert A., et al. The internal concentration of organic substances in fish embryos--a toxicokinetic approach. Environ. Toxicol. Chem. 2013;32(8):1819–1827. doi: 10.1002/etc.2239. [DOI] [PubMed] [Google Scholar]
- 77.Bai N., van Eeden S.F. Systemic and vascular effects of circulating diesel exhaust particulate matter. Inhal. Toxicol. 2013;25(13):725–734. doi: 10.3109/08958378.2013.844749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used to generate the figures and tables are located in Supplementary File 2.