Abstract
Purpose
To investigate the effects of accommodation on the geometrical parameters of human lens.
Methods
Eight databases from inception to November 2023 were used for the literature search: CNKI, CBM, VIP, Wan-Fang, PubMed, Web of Science, EMBASE, and the Cochrane Library. The Methodological Index for Non-randomized Studies was used to assess the risk of bias. The PRISMA were followed and the following outcomes were taken into consideration: lens diameter (LD), lens thickness (LT), anterior curvature radius (ACR), posterior curvature radius (PCR), lens center position (LCP), and total cross-sectional area (TCSA). This systematic review was registered on an international platform for registered systematic reviews and meta-analysis (INPLASY202260085).
Results
A total of 19 studies were included. LT increased by 0.04 mm/D (18 studies; 95% confidence interval [CI], 0.03–0.06; I2 = 96.6%; P < 0.001). At the same time, LD, ACR, and PCR decreased by 0.06 mm/D (6 studies; 95%CI, −0.07–0.05; I2 = 50.1%; P < 0.001), 0.53 mm/D (8 studies; 95%CI, −0.64–0.41; I2 = 96.5%; P < 0.001), and 0.14 mm/D (9 studies; 95%CI, −0.19–0.09; I2 = 94.7%; P < 0.001) during accommodation, respectively. Moreover, LCP shifted forward by 0.01 mm/D (3 studies; 95%CI, −0.02–0.00; I2 = 0.0%; P < 0.001), and TCSA by 0.58 mm2/D (2 studies; 95%CI, 0.41–1.57; I2 = 97.0%; P = 0.457) during accommodation.
Conclusions
Changes in LT, LD, ACR, PCR and LCP supported Helmholtz's theory. Different apparatuses or measurement methods influenced the measurement of lens geometrical parameters.
Keywords: Accommodation, Biological parameter, Crystalline lens
1. Introduction
Accommodation is a dynamic process that results in adjustment of the optical apparatus of the human eye [1]. The accommodative apparatus adjusts during accommodation to create a sharply focused image. Since Kepler first proposed that accommodation was caused by a change in the lens position in 1611 [2], the accommodation mechanism has been further explored [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. Helmholtz's “lens rebound” hypothesis is the classical and most accepted hypothesis [7]. He posited that during accommodation, the zonular relaxes, the lens rebounds elastically, the diameter of the lens equator decreases, the central thickness increases, and the optical power increases [7]. However, the “lens rebound” theory is imperfect as it cannot answer the questions about the stability of the lens and change in spherical aberration [[13], [14], [15], [16]]. According to Helmholtz's theory, when the zonules relax, the lens is affected by gravity and tends to become unstable. However, physical optics theory states that the closer the object is to the lens, the higher is stability of the optical system so that the image can be formed. The lens tends to be spherical due to its elasticity during accommodation, and this deformation leads to an increase in the spherical aberration. However, the fact that spherical aberration decreases was confirmed by Thomas Young in the 19th century [6]. In 1992, Schachar put forward the hypothesis of “lens stretching,” which explained the issues of “lens stability” and “spherical aberration” to some extent [9,13]. Schachar predicated that equatorial zonules are tense during accommodation and pull the lens equator outward, which leads to increase in the lens diameter (LD) [9,13]. This ultimately results in increase in the lens thickness (LT). Moreover, the peripheral surface flattens, increasing the optical power and reducing the spherical aberration [9,13]. Glasser et al. supported Helmholtz's theory by measuring the eyes of rhesus monkeys in vivo [[17], [18], [19]]. In addition, Glasser et al. reported that the lens center position (LCP) was affected by gravity during accommodation [18].
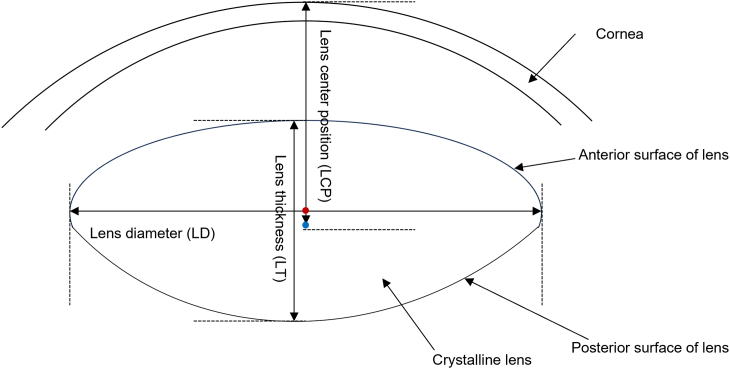
It is well known that the lens is one of the most critical parts of the accommodative apparatus, and revealing its changes during accommodation will deepen the understanding of accommodation mechanism. This accommodation mechanism is closely associated with the pathogeneses of myopia and presbyopia. Myopia affects nearly one-fourth of the world's population [20], and presbyopia is a problem faced by almost every middle-aged and older person [21]. Hence, exploration of the accommodation mechanism is necessary to prevent and treat myopia and presbyopia. However, some geometrical parameters of the lens are controversial. Although the hypotheses regarding accommodation mechanism differ [22], they all have one thing in common: the geometrical parameters of the lens [that is, LT, LD, LCP, distance between the lens equator and ciliary body, anterior curvature radius (ACR), posterior curvature radius (PCR), and total cross-sectional area (TCSA) (Fig. 1)] change during accommodation. Among these, the role of changes in LD, LCP in the direction of gravity, and distance between the lens equator and ciliary body remain controversial in the accommodation mechanism [9,13,[17], [18], [19]].
Fig. 1.
Schematic of lens parameters. Lens thickness (LT), measured from the anterior to posterior lens poles; lens diameter (LD), equatorial diameter of the lens; lens center position (LCP), measured from the anterior pole of the cornea to the center of the lens; anterior curvature radius (ACR), posterior curvature radius (PCR), and total cross-sectional area (TCSA) were automatically calculated using the instrument's built-in software; the red dot represents the midpoint of the equatorial diameter of the lens; the blue dot represents the midpoint of the anterior and posterior poles of the lens. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To the best of our knowledge, no meta-analysis related to changes in the geometrical parameters of the lens during accommodation has been published. We calculated the changes in the most common geometrical parameters of the lens during accommodation by performing a meta-analysis of the available data, such as LT, LD, ACR, PCR, LCP, and TCSA. Unfortunately, some controversial geometrical parameters were difficult to analyze because of the few numbers of studies that were included or even due to lack of relevant studies.
2. Methods
This systematic review and meta-analysis protocol was prospectively registered on the international platform of registered systematic reviews and meta-analysis protocols (INPLASY) as INPLASY202260085.
2.1. Literature search
Two researchers (GZ and JJ) independently searched each database according to the search rules. A systematic electronic literature search of the following databases was conducted: PubMed, Cochrane Library, Web of Science, and EMBASE in English and CNKI (http://www-cnki-net-s.vpn.uestc.edu.cn:8118/), Wan-Fang (http://www-wanfangdata-com-cn-s.vpn.uestc.edu.cn:8118/index.html), VIP (http://qikan.cqvip.com/) and Chinese Biomedical Literature Service System (http://www.sinomed.ac.cn/index.jsp) in Chinese (up to November 2023). The search strategy is presented in Appendix Text 1.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Randomized controlled trials or non-randomized controlled trial (nRCTs), (2) Trials including human participants aged 18–50 years, (3) Intervention: any measures that could cause accommodation, and (4) Outcome measurements: mean change per diopter in LT, LD, ACR, PCR, TCSA, and LCP. For studies with different degrees of accommodation, we only used the maximum degree of accommodation value for the analysis.
The exclusion criteria were as follows: (1) Articles with repeated publications or no full text, and (2) Reviews, comments, letters, animal experiments, case reports, conferences, or meta-analyses.
2.3. Literature screening and data extraction
(1) The retrieved literature was imported into Endnote software, and duplicate literature was deleted. (2) Literature that did not meet the requirements was deleted by screening the titles, abstracts, and full texts by two researchers in accordance with the inclusion and exclusion criteria. (3) Data was extracted (authors, publication year, study type, sample size, age, refractive status, type of apparatus, baseline conditions, interventions, and outcome indicators). The data were integrated and cross-checked after extraction by two researchers. Any disagreement was resolved by a third reviewer reevaluating the article (CQ). The final result was determined by a third reviewer.
2.4. Quality assessment
All studies included in this meta-analysis were nRCTs; therefore, the methodological quality of each included study was assessed using Methodological Index for Non-randomized Studies (MINORS) [23]. The twelve parameters of the MINORS are listed in Appendix Table 1.
Table 1.
Results of the meta-analysis of the effects of accommodation on geometrical parameters of the crystalline lens in humans.
| Group | Subgroup (apparatus) | Studies (n) | Heterogeneity test |
Effect model | Results of meta-analysis |
||
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | MD (95% CI) | P value | ||||
| LT | 18 | 96.60 | 0.000 | Random | 0.04 (0.03, 0.06) | <0.001 | |
| OCT and IOL Master | 12 | 86.30 | 0.000 | Random | 0.04 (0.02, 0.05) | <0.001 | |
| MRI | 5 | 88.10 | 0.000 | Random | 0.06 (0.04, 0.08) | <0.001 | |
| A-scan ultrasonography | 1 | 0.00 | N/A | Random | 0.05 (0.05, 0.05) | <0.001 | |
| LD | 6 | 50.10 | 0.075 | Random | −0.06 (−0.07, −0.05) | <0.001 | |
| MRI | 4 | 12.10 | 0.332 | Random | −0.07 (−0.08, −0.05) | <0.001 | |
| A-scan ultrasonography | 1 | 0.00 | N/A | Random | −0.05 (−0.06, −0.04) | <0.001 | |
| OCT | 1 | 0.00 | N/A | Random | −0.08 (−0.12, −0.05) | <0.001 | |
| ACR | 8 | 96.50 | 0.000 | Random | −0.53 (−0.64, −0.41) | <0.001 | |
| OCT and IOL Master | 7 | 96.70 | 0.000 | Random | −0.58 (−0.71, −0.45) | <0.001 | |
| MRI | 1 | 0.00 | N/A | Random | −0.25 (−0.31, −0.19) | <0.001 | |
| PCR | 9 | 94.70 | 0.000 | Random | −0.14 (−0.19, −0.09) | <0.001 | |
| OCT and IOL Master | 7 | 95.80 | 0.000 | Random | −0.16 (−0.23, −0.08) | <0.001 | |
| MRI | 2 | 0.00 | 0.648 | Random | −0.09 (−0.12, −0.07) | <0.001 | |
| LCP | 3 | 0.00 | 1.000 | Fixed | −0.01 (-0.02, −0.00) | <0.001 | |
| TCSA | 2 | 97.00 | 0.000 | Random | 0.58 (−0.41, 1.57) | >0.05 | |
ACR, anterior curvature radius; LCP, lens center position; LD, lens diameter; LT, lens thickness; MRI, magnetic resonance imaging; N/A, not available; OCT, optical coherence tomography; PCR, posterior curvature radius; TCSA, total cross-sectional area.
2.5. Statistical analysis
Forest and funnel plots were drawn using STATA 15.0 software. Weighted mean difference (WMD) and its 95% confidence interval (CI) were used to estimate the measurement data. P < 0.05 indicated significant difference and vice versa.
Heterogeneity test criteria: I2 ≤50% and P > 0.10 indicated that the heterogeneity was not significant and the fixed-effect model was used to combine the effect size; I2 >50% and P ≤ 0.10 indicated that the heterogeneity was considerable, and random-effect model combined the effect size.
Sensitivity analysis: When there was considerable heterogeneity, it was necessary to explore the sources of heterogeneity through sensitivity analysis. Moreover, verification of stability and reliability of the results of this meta-analysis was also required.
Subgroup analysis: When sufficient data were available, different subgroup categories were formed according to “apparatus.” The meta-analysis results for each subgroup category were analyzed to explore whether relevant factors or characteristics affected the results.
When the number of studies included was ≥10, the symmetry of the visual funnel plot was used to test publication bias. The abscissa of the graph was the effect value, namely the WMD, and the ordinate was the sample size. Symmetry of distribution of points on the funnel plot suggested a lower possibility of publication bias; otherwise, it indicated a particular publication bias. When the number of studies included in each outcome measurement ranged between 2 and 10, publication bias could not be accurately evaluated using funnel plot. Therefore, STATA 15.0 software was used to assess the publication bias through Begger's test. P > 0.05 indicated that there was no publication bias. Publication bias could not be evaluated when the number of included articles was ≤ 2.
3. Results
3.1. Literature results
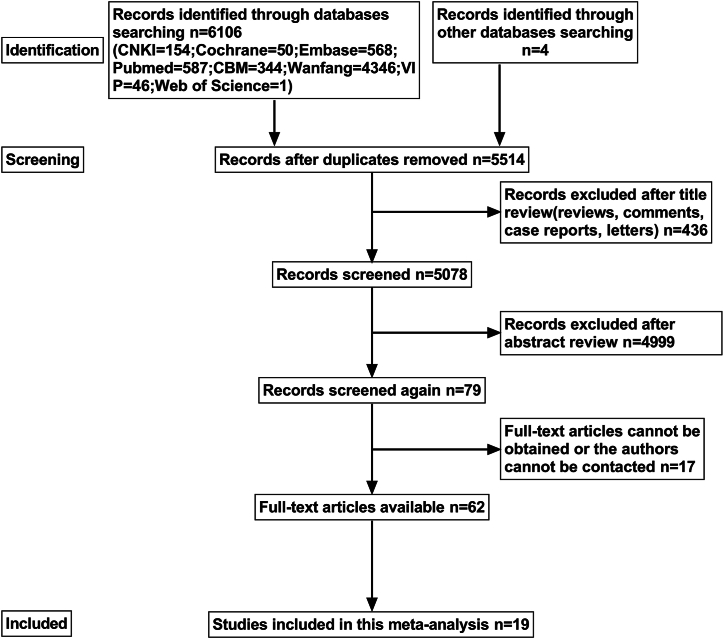
Database searches yielded 6110 potentially relevant entries. After removing 596 duplicate publications, 5514 articles remained. Among the 5514 articles that qualified for title and abstract review, 5435 were excluded because of their apparent irrelevance (i.e., reviews, comments, case reports, animal experiments, and inconsistency with content or intervention). In addition, the full texts of 17 articles could not be obtained, or the authors could not be contacted. In total, 62 articles were assessed for full-text review. Of these, 43 were excluded because no data were available. Thus, 19 articles met the inclusion criteria and were included in this meta-analysis. The search process is illustrated in Fig. 2.
Fig. 2.
Flowchart depicting literature screening.
The 19 studies included in this meta-analysis were nRCTs without a control group; therefore, the quality of the literature was evaluated only according to the first eight items of the MINORS evaluation items. According to the MINORS, studies with a score of 0–8 were of low quality, 9–16 were of medium quality, and 17–24 were of high quality. Studies with scores <12 were excluded from the meta-analysis. The scores of the 19 studies included in this meta-analysis ranged between 14 and 16, all of which were of medium quality (Appendix Table 2). The overall quality of the studies was acceptable.
3.2. Effect of accommodation on LT
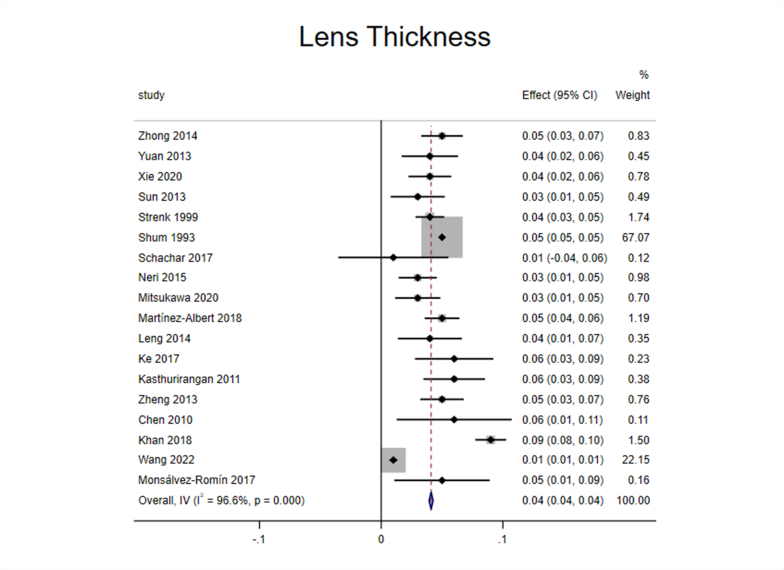
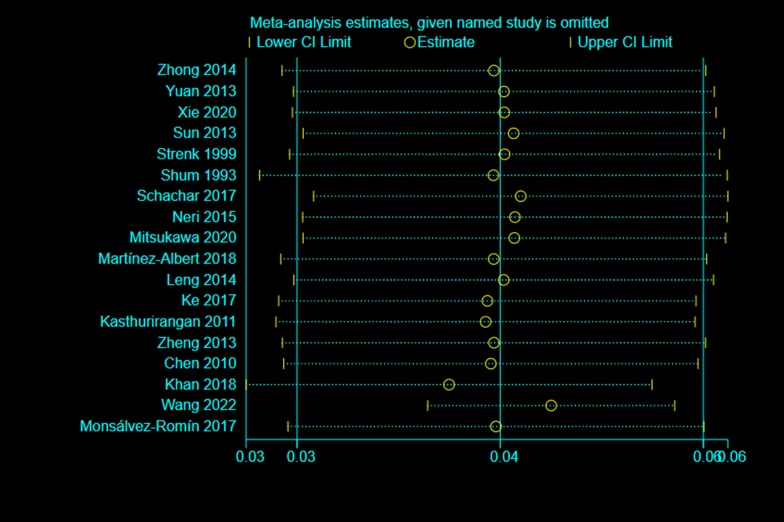
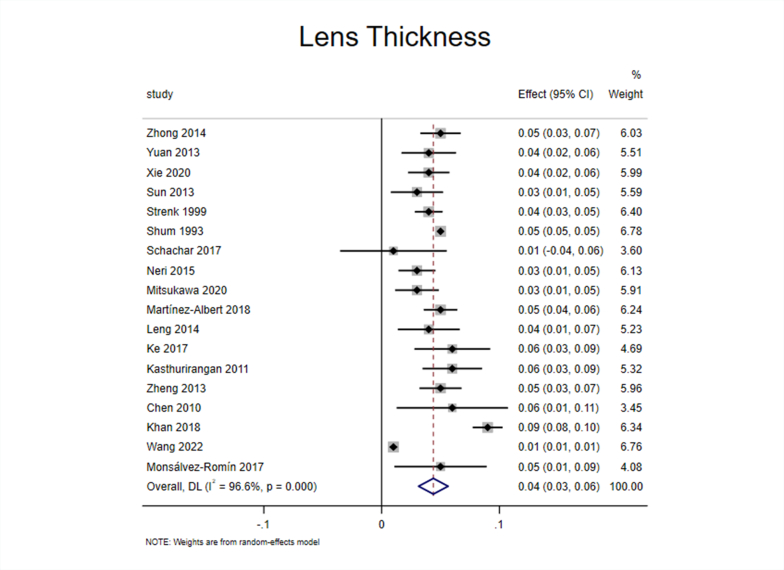
Eighteen studies [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]] reported the effect of accommodation on LT. The 18 included studies were tested for heterogeneity (I2 = 96.6%, P < 0.1), which revealed significant heterogeneity in the included studies (Appendix Fig. 1). Sensitivity analysis showed that none of the articles significantly interfered with this meta-analysis (Appendix Fig. 2). Therefore, the random-effects model was used to combine effect sizes, which showed that LT increased by 0.04 mm/D during accommodation (95% CI, 0.03–0.06, z = 6.865, P < 0.001) (Appendix Fig. 3).
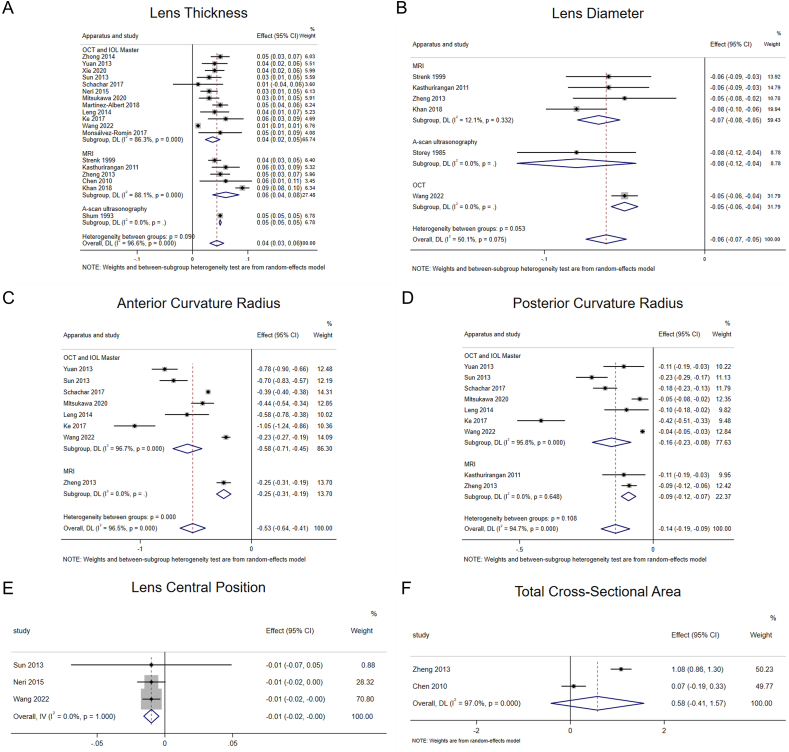
Fig. 3.
Forest plots of the effects of accommodation on geometrical parameters of the human lens. MRI, magnetic resonance imaging; OCT, optical coherence tomography.
The 18 articles were divided into three subgroups according to the “apparatus” for analysis (Fig. 3A). The results showed considerable heterogeneity among the three subgroups. The effect size in optical coherence tomography (OCT) and intraocular lens (IOL) Master subgroup [[24], [25], [26], [27],[30], [31], [32], [33], [34], [35],40,41] reached 0.04 (95% CI, 0.02–0.05; z = 5.434; P < 0.001), which meant that LT increased during accommodation when measured with OCT or IOL Master. There was considerable heterogeneity in the magnetic resonance imaging (MRI) subgroup [28,[36], [37], [38], [39]], and the effect size reached 0.06 (95% CI, 0.04–0.08; z = 4.958; P < 0.001), which meant that LT increased during accommodation when measured with MRI. Although A-scan ultrasonography subgroup [29] was included in only one study, from a numerical point of view, LT also increased during accommodation. In general, the different measurement equipment demonstrated differences in measuring LT, in which the values measured by OCT or IOL Master were the smallest, and the values measured by MRI were the largest.
Based on the above analysis, it can be inferred that the LT increased during accommodation.
3.3. Effect of accommodation on LD
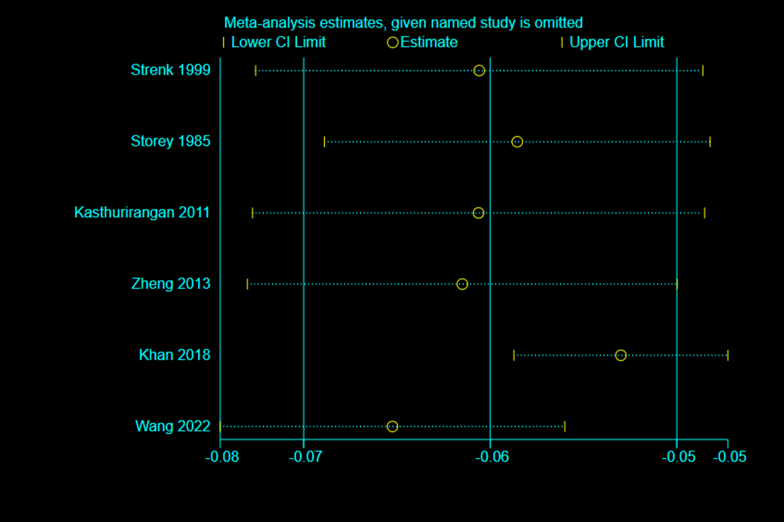
Six studies [28,36,37,39,40,42] reported the effects of accommodation on LD. The heterogeneity test (I2 = 50.1%, and P < 0.1) showed significant heterogeneity among the selected studies (Appendix Fig. 4).
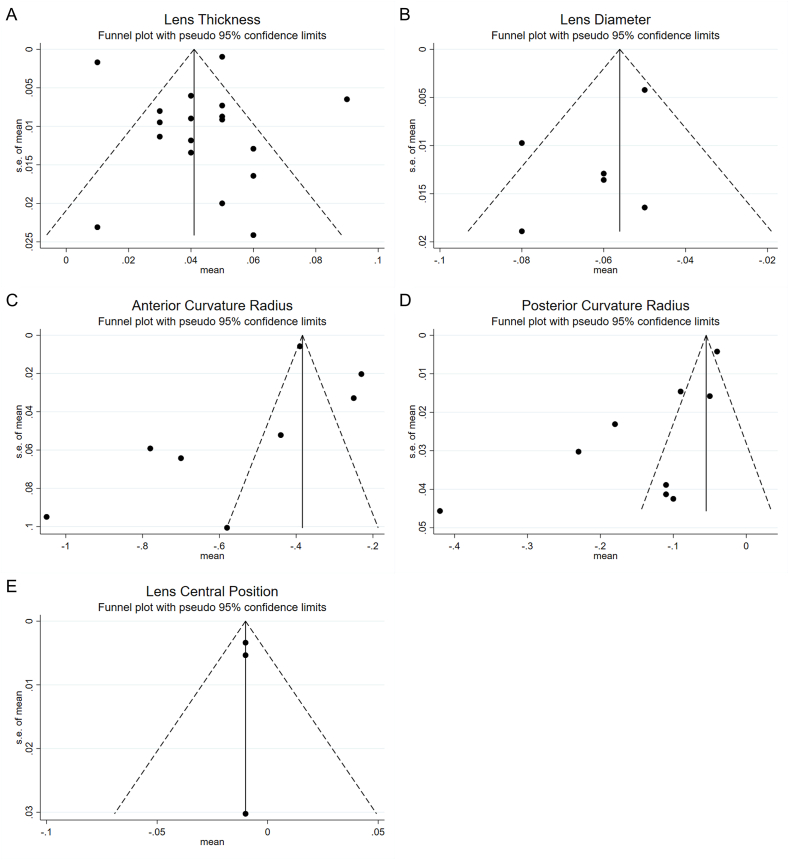
Fig. 4.
Funnel plots of the effect of accommodation on geometrical parameters of the human lens.
To ensure the accuracy and stability of the meta-analysis, we continued to perform a sensitivity analysis, and the results showed that none of the articles significantly interfered with the meta-analysis (Appendix Fig. 5). Therefore, a random-effects model was used to combine effect sizes, which showed that LD decreased by 0.06 mm/D during accommodation (95% CI, −0.07–0.05, z = −9.585, P < 0.001) (Fig. 3B).
The six articles were divided into three subgroups according to “apparatus” for analysis (Fig. 3B). The results showed significant heterogeneity among the three subgroups. There was insignificant heterogeneity in the MRI subgroup [28,37,39] (I2 = 12.1%, P > 0.1), and the effect size reached −0.07 (95% CI, −0.08–0.05; z = −9.829; P < 0.001), which meant that LD decreased during accommodation when measured with MRI. Although A-scan ultrasonography [42] and OCT subgroups [40] were included in only one study, from a numerical perspective, LD also decreased during accommodation.
Based on the above analysis, it can be concluded that LD decreased during accommodation.
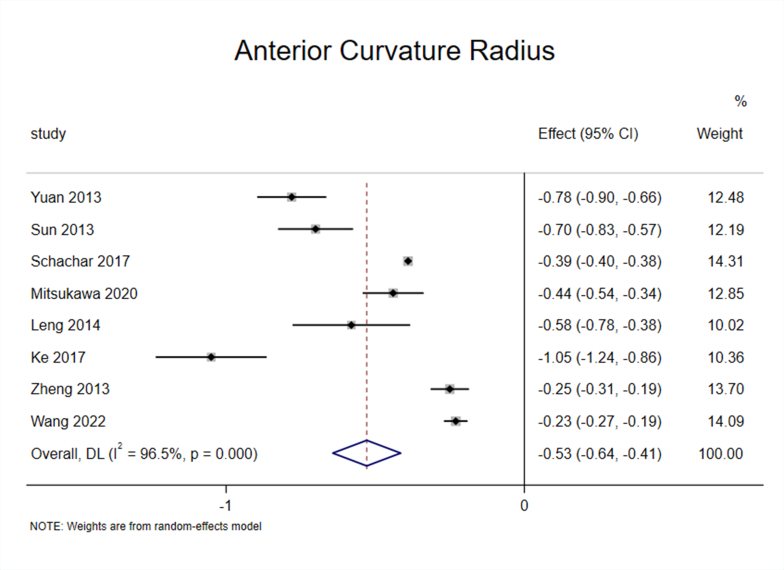
3.4. Effect of accommodation on ACR of the lens
Eight studies [25,27,30,32,34,35,37,40] reported the impact of accommodation on ACR, and the results suggested significant heterogeneity among the studies (I2 = 96.5%, P < 0.1) (Appendix Fig. 6).
To ensure the accuracy and stability of the meta-analysis, we continued to perform sensitivity analysis, and the results showed that none of the articles significantly interfered with the meta-analysis (Appendix Fig. 7). Therefore, a random-effects model was used to combine the effect sizes, which showed that ACR decreased by 0.53 mm/D during accommodation. (95% CI, −0.64–0.41, z = −9.103, P < 0.001) (Appendix Fig. 8).
Subgroup analysis of the eight studies based on “apparatus” showed significant heterogeneity within the OCT ang IOL Master subgroup [25,27,30,32,34,35,40] (I2 = 96.7%, P < 0.1), and the effect size reached −0.58 (95% CI, −0.71–0.45) and was statistically significant (z = −8.671, P < 0.001). Although MRI subgroup [37] was included in only one study, from a numerical point of view, the ACR also decreased during accommodation (Fig. 3C).
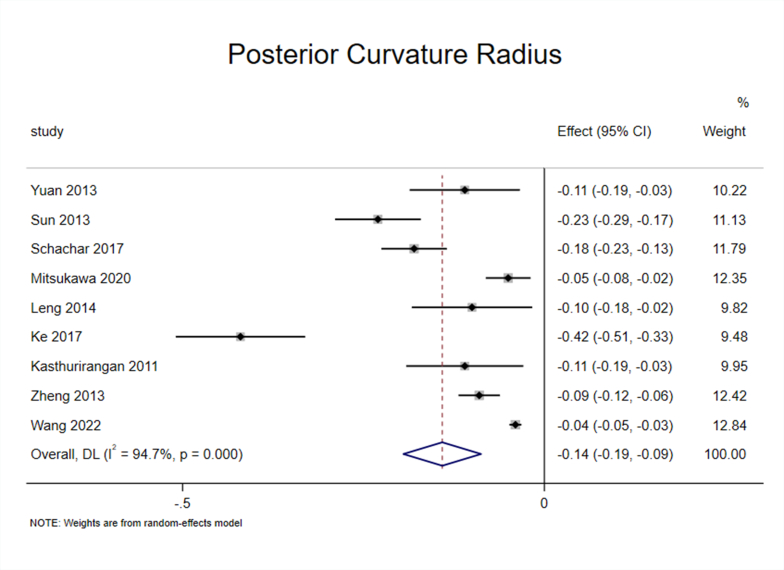
3.5. Effect of accommodation on PCR of the lens
Nine studies [25,27,30,32,[34], [35], [36], [37],40] reported the effect of accommodation on PCR, and the heterogeneity test (I2 = 94.7%, P < 0.1) suggested significant heterogeneity among the selected studies (Appendix Fig. 9). To ensure the accuracy and stability of the meta-analysis, we continued to perform sensitivity analysis, and the results showed that none of the articles significantly interfered with the meta-analysis (Appendix Fig. 10). Therefore, a random-effects model was used to combine effect sizes, which showed that the PCR decreased by 0.14 mm/D during accommodation (95% CI, −0.19–0.09, z = −9.103, P < 0.001) (Appendix Fig. 11).
Subgroup analysis of the nine studies based on “apparatus” showed significant heterogeneity within the OCT and IOL Master subgroup [25,27,30,32,34,35,40] (I2 = 95.8%, P < 0.1), and the effect size reached −0.16 (95% CI, −0.23–0.08) and was statistically significant (z = −4.215, P < 0.001). In addition, there was no heterogeneity within the MRI subgroup [36,37] (I2 = 0.0%, P > 0.1), and the effect size reached −0.09 (95% CI, −0.12–0.07) and was statistically significant (z = −6.697, P < 0.001) (Fig. 3D).
3.6. Effect of accommodation on LCP
Three studies [27,31,40] reported the effect of accommodation on LCP. The heterogeneity test suggested no heterogeneity among the studies (I2 = 0.0%, P > 0.1). Therefore, a fixed-effects model was selected for meta-analysis. The results showed that the lens shifted forward by 0.01 mm/D (95% CI, −0.02-0.00) during accommodation and the difference was statistically significant (z = −3.516, P < 0.001) (Fig. 3E).
3.7. Effect of accommodation on TCSA of the lens
The heterogeneity test (I2 = 97.0%, P < 0.1) indicated significant heterogeneity within the studies [37,38] included, and a random-effects model was used for meta-analysis. The results showed that the TCSA during accommodation was larger in comparison to non-accommodation; however, the difference was not statistically significant (z = 1.143, P > 0.05) (Fig. 3F).
3.8. Publication bias
More than ten studies were included in the LT, and funnel plots were used to evaluate publication bias. The symmetrical funnel plot suggested no publication bias (Fig. 4A). The number of studies included in the outcomes, such as LD, ACR, PCR, and LCP, was less than 10, and none of the outcomes could effectively evaluate publication bias through funnel plots. Therefore, Begger tests were used to assess publication bias. The results showed no publication bias (P > 0.05; P > 0.05; P > 0.05; P > 0.05) (Fig. 4B, C, 4D, and 4E). Publication bias assessments could not be performed because only two studies included TCSA.
4. Discussion
Although different scholars have different understandings of the accommodation mechanism, there is no doubt that the human eye is an integrated accommodation mechanism [[2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. The light reflected by the object enters the refractive system of the anterior segment and the retinal visual transformation system of the posterior segment, and then the visual information is finally transmitted to the visual center of the cerebral cortex through the visual pathway for more advanced processing. The integrity of this pathway is the basis for vision formation. Scientists have explored the accommodation mechanism for hundreds of years and determined that the cornea and axis oculi are not involved in eye accommodation [[2], [3], [4], [5], [6], [7], [8],10,12,[43], [44], [45], [46], [47]]. Since then, scholars have mainly concentrated on the refractive system of the anterior segment, especially the “ciliary body-zonules-lens” apparatus [6,7,10,12,47]. Changes in the lens shape and position are significant for changes in the refractive power of the eye. However, due to the sample size being too small or detection technology being limited, changes in some geometrical parameters of the lens during accommodation remain controversial. In this meta-analysis, 19 articles were identified, including six outcome measurements (LT, LD, ACR, PCR, TCSA, and LCP). This meta-analysis showed that LT increased, whereas LD, ACR, PCR, and LCP decreased during accommodation, and these differences were statistically significant. Moreover, TCSA increased during accommodation, but the difference was not statistically significant (Table 1).
In the included studies, the LT of all the participants increased during accommodation. The results measured by OCT and IOL Master were the lowest, whereas the results measured by MRI were the highest. We speculate that this may be related to the following reasons: (1) Different measurement principles. The measurement principles of OCT and IOL Master are based on the coherent interference of light, whereas MRI typically exploits the phenomenon of nuclear magnetic resonance, whereby atomic nuclei, which are exposed to a strong magnetic field, absorb and reemit electromagnetic waves at characteristic or “resonant” frequency, falling into the radiofrequency range [48]. Imaging structures as small as the crystalline lens using MRI presents a number of challenges, including resolution limitations, signal-to-noise ratio limitations, image acquisition time constraints, difficulty of presenting accommodative stimuli within the confines of the imager, and the need to limit head and eye movements during the scans [28]. (2) Different resolutions. MRI is a relatively time-consuming technique and has a lower resolution than optical and ultrasound techniques [36]. Among the included studies, except for the one by Khan (2018), 1.5T MRI was used, which has a lower resolution. (3) Strict image registration method. Schachar believed that accommodation induces cyclotorsion and convergence of the eye. These non-random changes in ocular alignment can systematically bias lens measurements if not carefully guarded using precise image registration techniques [30]. Accordingly, before analyzing the data measured by IOL Master, Schachar et al. used predetermined positional references, which did not change position during accommodation, to align multiple images to find an acceptable pair for comparison [30]. By using this image registration method, the effects of ocular alignment and/or microsaccadic movements on the measurement can be avoided, which may be the reason for measurement value to be smaller.
Several studies have reported that the central part of the lens thickens during accommodation [[24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. However, whether the thickness of the periphery of the lens also increases remains controversial. Helmholtz [7] believed that the lens rebounded during accommodation, indicating that the periphery thickened accordingly. However, this does not explain the decrease in spherical aberration. Therefore, Schachar put forward the hypothesis of “lens stretching” [10,13]. He posited that the anterior radial muscle contracts towards the sclera during accommodation, pulling the equatorial zonules to increase its tension. The lens equator stretches, resulting in the deformation of the central part of the lens, wherein becomes convex and peripherally flattened. This deformation increases the refractive power, reduces spherical aberration, and simultaneously maintains lens stability [10,13]. Luo's “lens push” theory [12] postulated that the lens is simultaneously subjected to centripetal pressure from ciliary body-zonules, forward compression of the vitreous body, and blocking of the iris to the periphery of the lens during accommodation. Under the action of these three forces, the protrusion of the lens center and flattening of the lens periphery results in aspheric deformation. In summary, the increase in LT and decrease in spherical aberration during accommodation are clear. However, the causes of aspheric deformation require further exploration.
Since the lens equator is obscured by the iris and is limited by detection technology, few studies have investigated LD changes in vivo during accommodation. Among the six studies included in this meta-analysis, four used MRI [28,36,37,39], whereas the others used improved A-scan ultrasonography [42] or OCT [40]. Sensitivity analysis showed that the stability of the three methods was good, indicating that the different measurement equipment had little effect on the results. The meta-analysis results showed that LD decreased during accommodation, which is consistent with the results of majority of the studies. Nevertheless, Schachar still argued to the contrary, and the reason was that the above experiments did not use a strict image registration method [9,10,[13], [14], [15], [16],30,[49], [50], [51]]. Wendt et al. [52] measured the LD of 33 monkeys under general anesthesia. Subsequently, accommodation was pharmacologically stimulated using 2% pilocarpine via the perfusion lens in 21 monkeys and LD was measured online. Measurements were recorded automatically every 10 s, using a custom written Optimas macros. The measurement terminated when no further decrease in LD was observed in the online recording [17]. General anesthesia avoided the movement between the monkey eye and measuring instrument. On the other hand, the lens image was calibrated by the inner diameter of the perfusion lens located in the conjunctival sac. Therefore, after strict image registration, the result of the study still showed that LD decreased during accommodation. In summary, LD decreased during accommodation. However, the extent of this decrease needs to be measured more accurately to deepen the understanding of accommodation mechanism.
The results of the meta-analysis showed that ACR and PCR decreased during accommodation, and the former was more obvious; that is, lens deformation mainly occurred on the anterior surface. This conclusion is consistent with the findings of most previous studies.
Gullstrand [53] postulated that external changes in the crystalline lens cannot provide sufficient refractive power to the eye during accommodation. Therefore, specific changes occurred within the lens, causing an increase in the total index of the crystalline lens. Arne Huggert [47], in 1947, observed that the distance between the adult nucleus and anterior lens surface increased during accommodation, providing evidence of displacements within the crystalline lens layers. However, the existence of an intracapsular accommodation mechanism remains to be fully proven. Luo [12] pointed out that in addition to deformation, the lens may also undergo small displacement during accommodation. Luo [12] compared the human eye to a camera lens and reported that lens deformation was equivalent to zooming, and lens displacement was equal to focusing. When deformation occurred, the change in refractive power caused by the lens deformation was not sufficient to meet the actual demand. The lens only required to move forward slightly to achieve clear vision. This meta-analysis showed that with an average increase of one diopter, the LCP moved forward by 0.01 mm (95% CI, −0.02–0.00), which supported Luo's theory. In summary, determining whether there is an intracapsular accommodation mechanism or a lens displacement during accommodation requires evidence-based support.
Lens deformation can be inferred by measuring the anterior, posterior, and total cross-sectional area changes during accommodation [37,38], but the related research is limited. The results of two studies showed that the TCSA increased by 0.58 mm2/D during accommodation; however, the result was not statistically significant. Although both studies employed MRI as the image capture device, the results differed because the different measurement methods were used. Zheng et al. [37] used Autocad 2010 software to measure the TCSA, and the results showed that the TCSA increased by (1.11 ± 0.39) mm2/D during accommodation. However, Chen et al. [38] magnified the image twice on the MRI workstation and measured it manually, and the results showed that the TCSA increased by (0.28 ± 1.75) mm2/D during accommodation.
In conclusion, LT increased, and LD, ACR, PCR, and LCP decreased, which supported the Helmholtz's theory. Second, different detection equipment or measurement methods influence the measurement of the geometric parameters of the lens. In addition, it is essential to select an appropriate positional reference before analyzing image data.
This study had a few limitations that need consideration. First, the study was limited by the technology of measuring the geometrical parameters of the lens in vivo. There was no study on change in the LCP in the direction of gravity and distance between the lens equator and ciliary body; therefore, it was impossible to discuss the changes in these two critical parameters. Second, the number of studies on LCP and TCSA was comparatively less, and the results of these two outcome measurements should be interpreted cautiously. Third, the included studies were nRCTs, and the selection and measurement biases were inevitable due to the limitations of the research design. Fourth, we ignored the effects of different types of OCTs on the measurements, thus increasing bias. Fifth, the span of both refractive status and age were too large in some studies, while other did not report this, which increased the bias to a certain extent. In future, we need to use a completely random method to conduct large-sample experiments to verify this conclusion.
Ethics approval
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China under Grant [Grant No. 82171026].
Data availability statement
The authors confirm that data supporting the findings of this study are available in the article [and/or] its supplementary materials.
CRediT authorship contribution statement
Guanghong Zhang: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jun Jiang: Writing – review & editing, Software, Methodology, Data curation, Conceptualization. Qian Wei: Writing – review & editing, Methodology, Data curation, Conceptualization. Chao Qu: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29298.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
figs11.
References
- 1.Atchison D.A. Accommodation and presbyopia. Ophthalmic Physiol. Opt. 1995;15(4):255–272. [PubMed] [Google Scholar]
- 2.Kepler J. Dioptrice. Augsburg1611..
- 3.Scheiner C. Oculus. Innsbruck1619..
- 4.Descartes R. Traité de l’homme. Paris1677..
- 5.Lobé JP. Dissertatio de oculo humano. Lugd. Batav1742. p. 119..
- 6.Young T. On the mechanism of the eye. Philos. Trans. 1801;92:23–88. [Google Scholar]
- 7.von Helmholtz H. Über die akommodation des auges. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1855;(1):1–89. [Google Scholar]
- 8.Tscherning M. Keystone; Philadelphia: 1904. Physiological Optics; pp. 160–189. [Google Scholar]
- 9.Schachar R.A. The mechanism of accommodation and presbyopia. Int. Ophthalmol. Clin. 2006;46(3):39–61. doi: 10.1097/00004397-200604630-00006. [DOI] [PubMed] [Google Scholar]
- 10.Schachar R.A., Bax A.J. Mechanism of accommodation. Int. Ophthalmol. Clin. 2001;41(2):17–32. doi: 10.1097/00004397-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Wilson R.S. A new theory of human accommodation: cilio-zonular compression of the lens equator. Trans. Am. Ophthalmol. Soc. 1993;91:401–416. discussion 16-9. [PMC free article] [PubMed] [Google Scholar]
- 12.Fu-ming L. A novel concept of accommodation: human eyes optical system based on hyperfocal distance-micro zoom. Chin. J. Exp. Ophthalmol. 2013;(7):701–710. [Google Scholar]
- 13.Schachar R.A. The correction of presbyopia. Int. Ophthalmol. Clin. 2001;41(2):53–70. doi: 10.1097/00004397-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Schachar R.A., Davila C., Pierscionek B.K., Chen W., Ward W.W. The effect of human in vivo accommodation on crystalline lens stability. Br. J. Ophthalmol. 2007;91(6):790–793. doi: 10.1136/bjo.2006.110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schachar R.A., Cudmore D.P. The effect of gravity on the amplitude of accommodation. Ann. Ophthalmol. 1994;26(3):65–70. [PubMed] [Google Scholar]
- 16.Schachar R.A., Lewis F.L. Error tolerance in helmholtzian accommodation. Ophthalmology. 2003;110(10):2066–2067. doi: 10.1016/s0161-6420(03)00910-2. [DOI] [PubMed] [Google Scholar]
- 17.Glasser A., Wendt M., Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest. Ophthalmol. Vis. Sci. 2006;47(1):278–286. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft M.A., Glasser A., Heatley G., McDonald J., Ebbert T., Nadkarni N.V., et al. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1087–1095. doi: 10.1167/iovs.04-1524. [DOI] [PubMed] [Google Scholar]
- 19.Croft M.A., Glasser A., Heatley G., McDonald J., Ebbert T., Dahl D.B., et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- 20.Holden B.A., Fricke T.R., Wilson D.A., Jong M., Naidoo K.S., Sankaridurg P., et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Holden B.A., Fricke T.R., Ho S.M., Wong R., Schlenther G., Cronjé S., et al. Global vision impairment due to uncorrected presbyopia. Arch. Ophthalmol. 2008;126(12):1731–1739. doi: 10.1001/archopht.126.12.1731. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G., Wei Q., Lu L., Lin A.L., Qu C. The evolution of mechanism of accommodation and a novel hypothesis. Graefes Arch. Clin. Exp. Ophthalmol. 2023 doi: 10.1007/s00417-023-06045-w. [DOI] [PubMed] [Google Scholar]
- 23.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhong J., Tao A., Xu Z., Jiang H., Shao Y., Zhang H., et al. Whole eye axial biometry during accommodation using ultra-long scan depth optical coherence tomography. Am. J. Ophthalmol. 2014;157(5) doi: 10.1016/j.ajo.2014.01.016. 1064-9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan Y., Shao Y., Tao A., Shen M., Wang J., Shi G., et al. Ocular anterior segment biometry and high-order wavefront aberrations during accommodation. Invest. Ophthalmol. Vis. Sci. 2013;54(10):7028–7037. doi: 10.1167/iovs.13-11893. [DOI] [PubMed] [Google Scholar]
- 26.Xie X., Sultan W., Corradetti G., Lee J.Y., Song A., Pardeshi A., et al. Assessing accommodative presbyopic biometric changes of the entire anterior segment using single swept-source OCT image acquisitions. Eye. 2022;36(1):119–128. doi: 10.1038/s41433-020-01363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y., Fan S., Zheng H., Dai C., Ren Q., Zhou C. Noninvasive imaging and measurement of accommodation using dual-channel SD-OCT. Curr. Eye Res. 2014;39(6):611–619. doi: 10.3109/02713683.2013.860991. [DOI] [PubMed] [Google Scholar]
- 28.Strenk S.A., Semmlow J.L., Strenk L.M., Munoz P., Gronlund-Jacob J., DeMarco J.K. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest. Ophthalmol. Vis. Sci. 1999;40(6):1162–1169. [PubMed] [Google Scholar]
- 29.Shum P.J., Ko L.S., Ng C.L., Lin S.L. A biometric study of ocular changes during accommodation. Am. J. Ophthalmol. 1993;115(1):76–81. doi: 10.1016/s0002-9394(14)73528-7. [DOI] [PubMed] [Google Scholar]
- 30.Schachar R.A., Mani M., Schachar I.H. Image registration reveals central lens thickness minimally increases during accommodation. Clin. Ophthalmol. 2017;11:1625–1636. doi: 10.2147/OPTH.S144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neri A., Ruggeri M., Protti A., Leaci R., Gandolfi S.A., Macaluso C. Dynamic imaging of accommodation by swept-source anterior segment optical coherence tomography. J. Cataract Refract. Surg. 2015;41(3):501–510. doi: 10.1016/j.jcrs.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsukawa T., Suzuki Y., Momota Y., Suzuki S., Yamada M. Anterior segment biometry during accommodation and effects of cycloplegics by swept-source optical coherence tomography. Clin. Ophthalmol. 2020;14:1237–1243. doi: 10.2147/OPTH.S252474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Albert N., Esteve-Taboada J.J., Montés-Micó R. Repeatability assessment of anterior segment biometric measurements under accommodative and nonaccommodative conditions using an anterior segment OCT. Graefes Arch. Clin. Exp. Ophthalmol. 2018;256(1):113–123. doi: 10.1007/s00417-017-3832-5. [DOI] [PubMed] [Google Scholar]
- 34.Leng L., Yuan Y., Chen Q., Shen M., Ma Q., Lin B., et al. Biometry of anterior segment of human eye on both horizontal and vertical meridians during accommodation imaged with extended scan depth optical coherence tomography. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ke B., Mao X., Jiang H., He J., Liu C., Li M., et al. The relationship between high-order aberration and anterior ocular biometry during accommodation in young healthy adults. Invest. Ophthalmol. Vis. Sci. 2017;58(13):5628–5635. doi: 10.1167/iovs.17-21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasthurirangan S., Markwell E.L., Atchison D.A., Pope J.M. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J. Vis. 2011;11(3) doi: 10.1167/11.3.19. [DOI] [PubMed] [Google Scholar]
- 37.Zheng S.L., Zhang A., Shi J.J., Zhou Y.X. Magnetic resonance imaging study of effects of accommodation on human lens morphological characters. Natl. Med. J. China (Peking) 2013;93(41):3280–3283. [PubMed] [Google Scholar]
- 38.Chen Q.H., Wang X.B., Liu A.Z., Wang Z.C. The initial study of morphological changes of human eyes before and after accommodation by MRI. Ophthalmology. 2010;19(2):97–99. [Google Scholar]
- 39.Khan A., Pope J.M., Verkicharla P.K., Suheimat M., Atchison D.A. Change in human lens dimensions, lens refractive index distribution and ciliary body ring diameter with accommodation. Biomed. Opt. Express. 2018;9(3):1272–1282. doi: 10.1364/boe.9.001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Jin G., Ruan X., Gu X., Chen X., Wang W., et al. Changes in crystalline lens parameters during accommodation evaluated using swept source anterior segment optical coherence tomography. Ann. Eye Sci. 2022;7:33. [Google Scholar]
- 41.Monsálvez-Romín D., Moulakaki A.I., Esteve-Taboada J.J., Ferrer-Blasco T., Montés-Micó R. In vivo OCT assessment of anterior segment central axial lengths with accommodation. Arq. Bras. Oftalmol. 2017;80(6):364–368. doi: 10.5935/0004-2749.20170089. [DOI] [PubMed] [Google Scholar]
- 42.Storey J.K., Rabie E.P. Ultrasonic measurement of transverse lens diameter during accommodation. Ophthalmic Physiol. Opt. 1985;5(2):145–148. doi: 10.1016/0275-5408(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 43.Brücke v. Uber das verhalter der optischen medien des auges gegen die Sonnenstrahlen. der Gesellschaft der Naturforscheden Freunde. 1846;21:379–382. [Google Scholar]
- 44.Listing JB. Wagner’s handwőrterbuch. d Physiologie. Braunschweig 1853.p. 498..
- 45.Cramer A.Het Accommodatie-Vermogen Physiologisch Toegelicht. Haarlem 1853..
- 46.Donders F.C. New Sydenham Society; London: 1864. Accommodation and Refraction of the Eye; pp. 204–215. [Google Scholar]
- 47.Huggert A. The intracapsular mechanism of accommodation. Acta Ophthalmol. 1964;42:389–397. doi: 10.1111/j.1755-3768.1964.tb03627.x. [DOI] [PubMed] [Google Scholar]
- 48.Yousaf T., Dervenoulas G., Politis M. Advances in MRI methodology. Int. Rev. Neurobiol. 2018;141:31–76. doi: 10.1016/bs.irn.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Schachar R.A. Letter to the editor: the lens is stable during accommodation. Ophthalmic Physiol. Opt. 2007;27(5):520–521. doi: 10.1111/j.1475-1313.2007.00514.x. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 50.Schachar R.A., Fygenson D.K. Topographical changes of biconvex objects during equatorial traction: an analogy for accommodation of the human lens. Br. J. Ophthalmol. 2007;91(12):1698–1703. doi: 10.1136/bjo.2006.094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schachar R.A., Schachar I.H. Intralenticular hydrostatic pressure increases during ciliary muscle contraction: a finding consistent with the schachar mechanism of accommodation. Invest. Ophthalmol. Vis. Sci. 2020;61(6) doi: 10.1167/IOVS.61.6.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wendt M., Croft M.A., McDonald J., Kaufman P.L., Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008;86(5):746–752. doi: 10.1016/j.exer.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gullstrand A. How I found the intracapsular mechanism of accommodation. Les Prix Nobel en. 1911:414–431. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that data supporting the findings of this study are available in the article [and/or] its supplementary materials.