Abstract
Background:
Among its functions, brain-derived neurotrophic factor (BDNF) regulates endothelial and macrophage activation, possibly playing a role in atherosclerotic plaque pathophysiology. Given contradicting reports, this study sought to investigate whether blood levels of BDNF differed between patients with coronary heart disease (CHD) and controls.
Methods:
We explored PubMed, Embase, Web of Science, and Cochrane Library for studies comparing BDNF blood levels in patients with CHD and controls. Random-effect meta-analysis was conducted to calculate the standardized mean differences (SMD) and 95% confidence intervals (CI). The Newcastle-Ottawa scale was used to evaluate the quality of included articles, and statistical analyses were conducted using R version 4.0.4.
Results:
The final analysis comprised 12 investigations covering 1422 CHD cases and 929 controls with mean ages of 59.66±13.56 and 53.78±13.61 years, respectively. The initial analyses revealed a tendency toward low levels of BDNF in the CHD group compared with the control group (SMD= −0.41; 95% CI, −1.12 to 0.30; P=0.26). After the removal of outliers, the difference achieved statistical difference (SMD= −0.56; 95% CI, −0.93 to −0.19; P<0.01). Subgroup analysis demonstrated no significant difference between serum and plasma BDNF levels (P=0.54); however, subgroup analyses of studies investigating plasma BDNF showed that patients with CHD had significantly lower BDNF levels.
Conclusion:
Serum and plasma BDNF concentrations were considerably lower in patients with CHD than in healthy controls. Further studies of higher quality are required on the potential role of BDNF as a biomarker of CHD pathophysiology and severity.
Keywords: Brain-derived neurotrophic factor, Coronary disease, Systematic review, Meta-analysis
Introduction
Coronary heart disease (CHD), also known as ischemic heart disease, is a leading cause of death globally. CHD is caused by a combination of mechanisms, such as endothelial dysfunction and inflammation, resulting in atherosclerotic plaques.1 Diabetes, hypertension, smoking, and obesity all contribute to the acceleration of these events.2 There is great interest in identifying and validating molecular diagnostic and prognostic markers for CHD, including those originating from damaged myocardial tissue, those that have increased levels in blood circulation after coronary events, and the ones with abnormal levels before coronary events.3–5
Brain-derived neurotrophic factor (BDNF) is a neurotrophin expressed in the central nervous system and peripheral tissues.6, 7 Besides its role as a neurotrophic factor, BDNF is involved in the physiology of other tissues, such as the heart.8 BDNF also plays a role in the development of the heart,9 endothelial cells,10 smooth muscle cells,11 macrophages,12 lymphocytes,13 and atherosclerotic vessels.11 Serum BDNF levels were shown to be low in individuals with CHD who had subsequent coronary events.14 According to data from the Framingham Heart Study, a high BDNF concentration is related to a reduced risk of CHD and mortality.14 Previous experimental studies have demonstrated that BDNF promotes endothelial cell survival and neoangiogenesis via the activation of nuclear factor TrκB receptors, inhibits p75-mediated apoptosis of cardiac myocytes, improves endothelial function, modulates blood flow in ischemic myocardium, and enhances left ventricular function following ischemic injury, thereby providing cardioprotective effects.15, 16 Regardless of age, higher BDNF levels are related to higher body mass index (BMI), blood pressure, low-density lipoprotein cholesterol (LDL-C), and cholesterol.17 On the other hand, higher levels of BDNF are statistically linked to a lower risk of cardiovascular disease and death independent of their risk factors, such as BMI or physical activity.18
The present systematic review and meta-analysis sought to synthesize the existing evidence on serum and plasma BDNF levels in patients with CHD compared with controls. In addition, the probable effects of sex, age, sample size, and the quality of included studies on BDNF concentrations were analyzed and discussed.
Methods
The inclusion criteria were as follows: 1) clinical studies with a control group that measured serum and/or plasma BDNF levels in control and CHD groups and 2) cross-sectional and interventional studies in which the BDNF concentration was measured before the intervention.
We excluded studies that were case reports or series, letters, reviews, conference abstracts, or pre-clinical/experimental studies.
A comprehensive search was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)19 statement guidelines in international online databases, including PubMed, Embase, Web of Science, and Cochrane Library, to find studies relevant to the selection criteria in July 2022. The search terms included “BDNF” OR “brain-derived neurotrophic factor” AND “ischemic heart” OR “coronary heart disease” and other related terms. No limitations were applied in the search. The details of the searched queries are available in Supplementary Table 1.
Two reviewers (AK and AHB) independently screened all the studies with titles and abstracts for eligibility. The included studies with the full text were screened by AK and AHB separately, and disagreements were settled through discussions with the third author (PS). In addition, the references of the included studies were screened for any possible relevant study.
Two reviewers (AK and AHB) independently used the data extraction sheet designed by the third reviewer (PS) to extract data, including first author, year, and country of publication; demographic characteristics (number of participants, age, and male percentage in CHD and control groups); and plasma and serum BDNF concentrations in each group. The concentration unit was converted to picograms per milliliter (pg/mL) for all the included studies. We contacted the corresponding authors of the studies in which the absolute values of the serum BDNF level were not mentioned.
Assessment of risk of bias in the included studies was performed using the Newcastle-Ottawa Quality Assessment Scale20 for observational studies. Two reviewers (AHB and AK) independently evaluated the quality of the included studies. Disagreements in the quality evaluation were resolved by the third investigator (PS). Selection, comparability, and outcome were the principal categories of potential bias. Scores of 9–10, 7–8, 5–6, and <5 were considered very good, good, satisfactory, and unsatisfactory studies concerning quality, respectively.
Data analysis was performed using R version 4.0.4 (R Core Team [2020]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria). Due to different measurement methods in the studies, the standardized mean difference (SMD) and the 95% confidence interval (CI) were used to compare CHD patients with controls. Additionally, P values <0.05 were considered the statistical significance cutoff throughout the study.
In the case of the report of the median and interquartile range or the median and range for any needed variable in an article, the mean and standard deviation were calculated based on statistical methods by Luo et al21 and Wan et al.22 In cases where there was a need to combine the groups’ means and standard deviations, formulas suggested by Cochrane’s Handbook23 were utilized.
Statistical heterogeneity was evaluated using Higgins’ I2 test based on Cochrane’s Q. The thresholds used for heterogeneity I2 were ≤25%, 26–75%, and ≥75% for low, moderate, and high heterogeneity, respectively. Given the high heterogeneity between the studies, the random effect model (DerSimonian and Laird) was utilized for SMD analysis. To identify the source of heterogeneity, we performed meta-regression on the mean age and the NOS score of the studies. Possible publication bias was investigated via a visual assessment of funnel plots and Egger’s and Begg’s tests.24, 25 Finally, potential outliers were identified, and a sensitivity analysis was conducted to evaluate the effect of each study.
Results
The initial search yielded 1779 references: 1184 from PubMed, 390 from Embase, 47 from Cochrane Library, and 158 from Web of Science. After the exclusion of 484 duplicate studies and 1295 investigations unrelated to our topic, 73 studies examining peripheral BDNF levels in CHD were discovered and thoroughly evaluated. Subsequently, 61 studies were excluded due to various reasons described in Figure 1. Finally, 12 studies,26–37 including 2351 participants, fulfilled all of the inclusion criteria and were included in the meta-analysis. A detailed flow chart of the search and selection process is presented in Figure 1.
Figure 1.
The flow diagram summarizes the selection of eligible studies based on the PRISMA guidelines.
The characteristics of the 12 selected studies are presented in Table 1. All the articles were published between 2005 and 2022. The included investigations yielded 2351 observations, composed of 1422 CHD cases and 929 controls. The average age of the patients and controls were 59.66±13.56 and 53.78±13.61, respectively.26, 27, 29–37 Biological sources for BDNF analysis were the plasma26, 29, 32, 34, 36 and the serum.27, 28, 30, 31, 33, 35, 37 All 12 included studies assessed BDNF levels using enzyme-linked immunosorbent assay (ELISA) as the analytical procedure. All the studies were of high quality, with an average NOS score of 9.43±0.94 (Table 2).
Table 1.
Characteristics of the included studies
| Author | Year | Design | Country | Analytical Technology | Source | Population | Control | n total | n CHD | Age | % Male |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amadio et al | 2021 | Cross-Sectional | Italy | ELISA | Plasma | >50% stenosis by visual estimate at angiography | No history of CAD, no MI symptoms, no CV therapy | 76 | 55 | 61.89±11.62 | 86.84 |
| Aslan et al | 2020 | Cross-Sectional | Turkey | ELISA | Serum | Presence of typical exertional angina, positive stress test or reversible myocardial perfusion defects during stress imaging, and angiographically normal coronary arteries (MVA) | Normal coronary arteries, inconclusive findings in stress tests or functional imaging, and no CAD in angiography | 152 | 91 | 57.28±29.11 | 44.74 |
| Bai et al | 2019 | Cross-Sectional | China | ELISA | Serum | Chronic CHD according to the clinical guidelines by the ACC/AHA | NR | 211 | 108 | NR | NR |
| Ejiri et al | 2005 | Cross-Sectional | Japan | Sandwich ELISA | Plasma(Peripheral Vein) | >75% stenotic lesions in the LCA determined by myocardial perfusion scintigraphy to be the culprit lesion (UA or SA) | No significant coronary artery stenosis >25% luminal diameter | 107 | 83 | 64.46±25.86 | 65.42 |
| Esmaeili et al | 2021 | Cross-Sectional | Iran | ELISA | Serum | At least one major coronary artery with > 50% stenosis confirmed by angiography (CAD) | Normal angiogram and no coronary stenosis | 146 | 84 | 53.50±9.41 | 60.96 |
| Forent et al | 2022 | Cross-Sectional | Canada | ELISA | Plasma | Documented CHD | Without cardiac disease and aortic stenosis symptoms | 59 | 39 | 64.92±8.07 | 71.19 |
| Han et al | 2019 | Cross-Sectional | China | DAS-ELISA | Serum | At least one of the three major coronary arteries or major branches significant coronary artery stenosis ≥50% | Normal clinical, physical, radiographic, and ECG examinations | 257 | 156 | 59.58±9.83 | 58.37 |
| Jin et al | 2018 | Cross-Sectional | China | ELISA | Plasma | >50% stenosis of the primary coronary artery or its major branches | <20% stenosis | 234 | 143 | 65.92±10.54 | 65.38 |
| Monisha et al | 2020 | Cross-Sectional | India | ELISA | Serum | Lesion >30% in the primary coronary artery or its major branches | Lesion ≤30% | 221 | 116 | 55.94±10.97 | NR |
| Sustar et al | 2019 | Cross-Sectional | Croatia | ELISA | Plasma | >50% stenotic lesions in at least one major coronary vessel, MI, coronary stent implantation, and coronary artery bypass surgery. (CHD=SA/UA) | Screened during routine physical check-ups in the same hospital | 364 | 208 | 50.72±11.14 | 53.30 |
| Wu et al | 2022 | Cross-Sectional | China | ELISA | Serum | Criteria of coronary heart disease in “Diagnostic Criteria for Coronary Atherosclerotic Heart Disease”, and coronary angiography shows that the inner diameter of any one of the left main trunks, left circumflex artery, left anterior descending artery, and right coronary artery stenosis degree ≥50% or left main stem stenosis >30% | NR | 277 | 132 | 51.35±10.23 | 42.60 |
| Xia et al | 2022 | Cross-Sectional | China | ELISA | Serum | Diagnosed as CHD by coronary angiography | Non-CHD by coronary angiography | 247 | 207 | NR | NR |
SA, Stable angina; UA, Unstable angina; MI, Myocardial infarction; CAD, Coronary artery disease; CHD, Coronary heart disease; ACS, Acute coronary syndrome; CV, Cardiovascular; MVA, Microvascular Angina; LCA, Left coronary artery; ECG, Electrocardiogram; ACC, American College of Cardiology; AHA, American Heart Association; NR, Not reported
Table 2.
Quality assessment of the included studies based on the Newcastle-Ottawa scale (NOS)
| Study | Selection | Comparability | Outcome | Overall Score | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Representation | Sample Size | Non-Respondents | Exposure | Outcome | Statistical Test | |||
| Amadio et al | * | * | * | ** | ** | ** | * | 10 |
| Aslan et al | * | * | * | ** | 0 | ** | * | 8 |
| Bai et al | * | * | * | ** | ** | ** | * | 10 |
| Ejiri et al | * | * | * | ** | 0 | ** | * | 8 |
| Esmaeili et al | * | * | * | ** | ** | ** | * | 10 |
| Forent et al | * | * | * | ** | ** | ** | * | 10 |
| Han et al | * | * | * | ** | ** | ** | * | 10 |
| Jin et al | * | * | * | ** | ** | ** | * | 10 |
| Monisha et al | * | * | * | ** | 0 | ** | * | 8 |
| Sustar et al | * | * | * | ** | ** | ** | * | 10 |
| Wu et al | * | * | * | ** | ** | ** | * | 10 |
| Xia et al | * | * | * | ** | 0 | ** | * | 8 |
The estimates pooled from the 12 studies showed a tendency toward lower BDNF levels in patients with CHD than in controls (SMD= −0.4089; 95% CI, −1.1168 to 0.2990; P=0.2576) (Figure 2). However, there was significant statistical heterogeneity across the studies (I2, 98.2%; P<0.0001).
Figure 2.
The image illustrates the forest plot of the subgroup meta-analysis of BDNF levels in the CHD group compared with the control group.
BDNF, Brain-derived neurotrophic factor; CHD, Coronary heart disease
In the 12 studies included in this meta-analysis, BDNF levels were investigated in the serum by 7 and in the plasma by 5. Lower BDNF levels were found in CHD compared with controls in both subgroups of studies. The pooled SMD was −0.4148 (95% CI, −1.6426 to 0.8130; P=0.51) for BDNF levels assessed in the serum and −0.4042 (95% CI, −0.6589 to −0.1494; P<0.01) for BDNF levels assessed in the plasma (Figure 2). Subgroup comparisons found no significant difference between BDNF concentration SMD reported in the serum and plasma between CHD and controls (P=0.9867).
The funnel plot was symmetric (Figure 3). Moreover, Begg’s and Egger’s tests did not reveal significant evidence of publication bias among the included studies (Begg’s test: P=1.00; Egger’s test: P=0.98).
Figure 3.
A) The funnel plot shows no evidence of publication bias, statistically supported by Egger’s regression test. B) The image presents the counter-enhanced funnel plot.
Meta-regression analyses were applied to investigate heterogeneity sources between the studies and the influence of modifiers. Univariable meta-regression models showed no relationships between sample size, age, NOS score, sex, and BDNF levels (Table 3 & Figure 4). Moreover, age accounted for 17.83%, and male sex accounted for 11.18% of observed heterogeneity, while the others could not justify the heterogeneity.
Table 3.
Meta-regression of BDNF levels in patients with CHD and healthy controls
| Moderator | No. of comparisons | No. of Subjects | Meta-Regression | P | R2 Analog (proportion of the variance explained) | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| CHD | HC | Slope | Lower 95% | Upper 95% | ||||
| CI | CI | |||||||
| Sample Size | 12 | 1422 | 929 | −0.005 | −0.019 | 0.010 | 0.527 | 0% |
| Age (mean, y) | 11 | 1314 | 826 | −0.118 | −0.258 | 0.021 | 0.096 | 17.8% |
| NOS score | 12 | 1422 | 929 | 0.387 | −0.367 | 1.141 | 0.315 | 0% |
| Sex (male, %) | 10 | 1198 | 721 | −0.034 | −0.082 | 0.014 | 0.161 | 11.2% |
NOS, Newcastle-Ottawa scale; CHD, Coronary heart disease; HC, Healthy control; CI, Confidence interval
Figure 4.
The images present the bubble plots of the meta-regression.
A) mean age, B) NOS score, C) sample size, and D) sex (male)
Studies by Han et al,30 Monisha et al,31 and Esmaeili et al33 were identified as outliers. Accordingly, we repeated the meta-analysis of the remaining 6 studies. The results showed an SMD of −0.5595 (95% CI, −0.9293 to −0.1896; P=0.0030) (Figure 5), indicating statistically significantly lower BDNF levels in CHD participants than in controls. (The P value dropped from 0.2576 to 0.003.) However, considerable statistical heterogeneity remained across the studies (I2, 89.7%; P<0.0001).
Figure 5.
The forest plot of the meta-analysis of BDNF levels in patients with CHD after the removal of outliers is presented herein.
BDNF, Brain-derived neurotrophic factor; CHD, Coronary heart disease
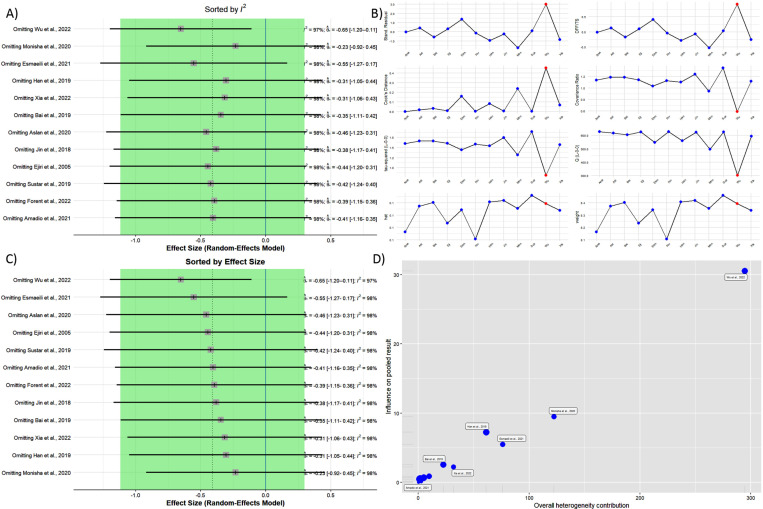
The impact of each study on the overall estimate was determined by systematically omitting studies and comparing the pooled estimate from the remaining 11 studies to the pooled estimate from all 12 studies. The results indicated that patients with CHD had lower peripheral BDNF levels than controls in all the studies except for that by Wu et al,37 which changed the results statistically significantly after being removed (SMD= −0.65; 95% CI, −1.20 to −0.111) (Figure 6).
Figure 6.
- A) Sorted by I2.
- B) The influence analysis plot shows influence diagnostics, including externally standardized residuals, the DFFITS value, Cook’s distance, covariance ratio, leave-one-out τ2, Q value, hat value, and study Weight.
- C) Sorted by effect size
- D) Baujat’s plot is illustrated herein.
DFFITS, Difference in fits
Discussion
To our knowledge, this is the first meta-analysis of studies to compare BDNF peripheral levels between patients with chronic CHD and healthy controls. CHD was associated with significantly lower levels of BDNF. Moreover, subgroup analysis revealed statistically significantly lower plasma BDNF levels in patients with CHD and not serum levels.
Previous studies have suggested a significant role for BDNF in cardiovascular development, functions, risk factors, and disorders.38–41 In mice, BDNF perfusion increased cardiac contraction force and systolic pressure but decreased diastolic pressure, not affecting coronary blood flow.42 In a prospective cohort, a lower plasma level of BDNF was associated with a higher risk of major coronary events in angina pectoris patients with a median follow-up of 48 months.43 There is also an inverse relation between BDNF and atherogenic index (total cholesterol/HDL-C), high-sensitivity C-reactive protein, and oxidized LDL.44 The higher levels of BDNF in multiple sclerosis patients with higher activities may be attributable to the normal population and can be another possible mechanism indirectly affecting CHD.45 Conversely, higher BDNF levels were related to higher BMI, blood pressure, LDL-C, and cholesterol17 (ie, risk factors for CHD). In this scenario, BDNF might increase as a response to risk factors of disease progression (eg, high cholesterol), exerting protective roles.
Several studies have suggested the pathophysiology of the association between cardiovascular disease and circulating BDNF. BDNF is expressed in vascular endothelial cells, the smooth muscle of atherosclerotic coronary arteries, and macrophages, in addition to the neural system.29, 46 Thus, a study suggested that BDNF might affect plaque stability, although the clinical relevance of this hypothesis is still uncertain.47 In contrast with this local effect, the effect of BDNF on the integrity and maintenance of cardiac vasculature may be more critical. In an in vivo study, low BDNF levels in mice resulted in less survival of the endothelial cells in myocardial arteries and, finally, postnatal death.48
As serum and plasma BDNF levels have a strong positive correlation,49 we used both sources of BDNF (plasma and serum) in this meta-analysis as subgroup analysis. The average serum BDNF level is higher (100-fold in a study by Radka et al49 and 14-fold in a study by Yoshimura et al50) than plasma levels. As human platelets contain large amounts of BDNF, this difference is due to the clotting process in which platelets degranulate.51, 52 Serum BDNF levels, in contrast with plasma levels, are primarily dependent on clotting time, which varies between studies.53 These disparities in serum levels may be why our results in the plasma subgroup were statistically significant. We found no relevant study that evaluated both serum and plasma levels of BDNF in patients with CHD and controls. Further research may help investigate the difference we mentioned in terms of meaningfulness.
The results of the present study suggest the possibility of the diagnostic value of BDNF for CHD, but more importantly, it highlights the possible pathophysiological pathway from BDNF to CHD, which can assist in designing and using novel therapies for CHD. Until now, the most therapeutic applications of BDNF have been in neurological diseases, with an oral BDNF agonist from the plant flavonoids showing effectiveness on stroke and Parkinson’s disease in mice.54 Further trials and studies are needed to investigate such effects for the prevention of cardiovascular disease.
While being the first meta-analysis to investigate blood BDNF levels in CHD, this study had some limitations. Firstly, the lack of proper matching for confounders in the studies could influence their results and, therefore, our analysis. We only included cross-sectional, case-control, or cohort studies that had both CHD and control groups. The low number of included studies and the low number of studies in our subgroup analysis were also considerable limitations. We excluded studies that mentioned the BDNF levels only graphically and/or did not mention the quantitative data. The authors did not respond to our email requests for their data, and we had to exclude the studies due to not having the required data for meta-analysis. Finally, the statistical methods used for converting the median and the interquartile range to the mean and standard deviation, as suggested by Luo et al21 and Wan et al,22 although they are used in many meta-analysis studies, may affect the results due to the possible skewness of a variable distribution. Future studies with larger sample sizes investigating the association between the severity of chronic CHD and BDNF levels might help identify the pathophysiology of our findings.
Conclusion
To summarize, peripheral concentrations of BDNF were significantly lower in patients with CHD than in healthy controls, principally because of studies that measured plasma BDNF.
Notes:
This paper should be cited as: Shobeiri P, Behnoush AH, Khalaji A, Teixeira AL, Rezaei N. Peripheral Levels of the Brain-Derived Neurotrophic Factor in Coronary Artery Disease: A Systematic Review and Meta-Analysis. J Teh Univ Heart Ctr 2023;18(4):244-255.
Supplementary Material
Table 1.
Search queries used for each database and the search results
| Query | Results (No.) | ||
|---|---|---|---|
| PubMed | |||
| #1 | “Brain-Derived Neurotrophic Factor”[Mesh] OR “BDNF” OR “brain-derived neurotrophic factor” | 29,312 | |
| #2 | “Myocardial Ischemia”[Mesh] OR “Myocardial ischemia” OR “Coronary Artery Disease”[Mesh] OR “Coronary Atherosclerosis” OR “Coronary Disease”[Mesh] OR “Coronary Heart Disease*” OR “ischemic heart” OR “ischemia” | 707,485 | |
| #3 | #1 AND #2 | 1,184 | |
| Embase | |||
| #1 | ‘brain derived neurotrophic factor’/exp OR ‘bdnf’ OR ‘brain derived neurotrophic factor’ OR ‘brain-derived neurotrophic factor’ | 50,031 | |
| #2 | (‘ischemic heart disease’/exp OR ‘coronary heart disease’ OR ‘ischemia heart disease’ OR ‘ischemic cardiac disease’ OR ‘ischemic cardiopathy’ OR ‘ischemic heart disease’) | 807,302 | |
| #3 | #1 AND #2 | 390 | |
| Web of Science | |||
| #1 | TS=“brain-derived neurotrophic factor” OR TS=“BDNF” OR TS=“brain derived neurotrophic factor” | 33,432 | |
| #2 | TS=“myocardial ischemia” OR TS=“coronary artery disease” OR TS=“coronary atherosclerosis” OR TS=“coronary diseases” OR (TS=“heart disease” AND TS=“coronary”) OR (TS=“heart disease” AND TS=“ischemic”) OR TS=“ischemic cardiac disease” | 307,020 | |
| #3 | #1 AND #2 | 158 | |
| Cochrane Library | |||
| #1 | “BDNF” OR “brain-derived neurotrophic factor” | 1,802 | |
| #2 | “Myocardial Ischemia” OR “Coronary Artery Disease” OR “ischemia” OR “ischemic heart” OR “heart disease, coronary” OR “ischemic cardiac disease” | 44,993 | |
| #3 | #1 AND #2 | 47 | |
References
- 1.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Postgrad Med J 2008;84:368–371. [DOI] [PubMed] [Google Scholar]
- 2.Munnur RK, Nerlekar N, Wong DT. Imaging of coronary atherosclerosis in various susceptible groups. Cardiovasc Diagn Ther 2016:382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasipoularides A. Genomic translational research: Paving the way to individualized cardiac functional analyses and personalized cardiology. Int J Cardiol 2017;230:384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Pan N, An Y, Xu M, Tan L, Zhang L. Diagnostic and Prognostic Biomarkers for Myocardial Infarction. Front Cardiovasc Med 2021;7:617277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raygan F, Etminan A, Mohammadi H, Akbari H, Nikoueinejad H. Serum Levels of Growth Differentiation Factor-15 as an Inflammatory Marker in Patients with Unstable Angina Pectoris. J Tehran Heart Cent 2021;16:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 2015;11:1164–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scalzo P, Kümmer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 2010;257:540–545. [DOI] [PubMed] [Google Scholar]
- 8.Numakawa T, Odaka H, Adachi N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int J Mol Sci 2018;19:3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg DO, Large TH, Bodary SC, Reichardt LF. Regulation of nerve growth factor mRNA levels in developing rat heart ventricle is not altered by sympathectomy. Dev Biol 1989;134:30–37. [DOI] [PubMed] [Google Scholar]
- 10.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett 2000;470:113–117. [DOI] [PubMed] [Google Scholar]
- 11.Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 12.Barouch R, Appel E, Kazimirsky G, Brodie C. Macrophages express neurotrophins and neurotrophin receptors. Regulation of nitric oxide production by NT-3. J Neuroimmunol 2001;112:72–77. [DOI] [PubMed] [Google Scholar]
- 13.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 1999;189:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaess BM, Preis SR, Lieb W, Beiser AS, Yang Q, Chen TC, Hengstenberg C, Erdmann J, Schunkert H, Seshadri S, Vasan RS, CARDIoGRAM. Assimes TL, Deloukas P, Holm H, Kathiresan S, König IR, McPherson R, Reilly MP, Roberts R, Samani NJ, Stewart AF. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J Am Heart Assoc 2015. Mar;4:e001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000;127:4531–4540. [DOI] [PubMed] [Google Scholar]
- 16.László A, Lénárt L, Illésy L, Fekete A, Nemcsik J. The role of neurotrophins in psychopathology and cardiovascular diseases: psychosomatic connections. J Neural Transm (Vienna) 2019;126:265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, Driscoll I, Ferrucci L, Martin B, Mattson MP. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore Longitudinal Study of Aging. PLoS One 2010;5:e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaess BM, Preis SR, Lieb W, Beiser AS, Yang Q, Chen TC, Hengstenberg C, Erdmann J, Schunkert H, Seshadri S, Vasan RS, CARDIoGRAM. Assimes TL, Deloukas P, Holm H, Kathiresan S, König IR, McPherson R, Reilly MP, Roberts R, Samani NJ, Stewart AF. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J Am Heart Assoc. 2015. Mar 11;4(3):e001544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 21.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 22.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amadio P, Cosentino N, Eligini S, Barbieri S, Tedesco CC, Sandrini L, Zarà M, Fabiocchi F, Niccoli G, Magnani G, Fracassi F, Crea F, Veglia F, Marenzi G, Barbieri SS. Potential Relation between Plasma BDNF Levels and Human Coronary Plaque Morphology. Diagnostics (Basel) 2021;11:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aslan G, Polat V, Bozcali E, Şahin MH, Çetin N, Ural D. Evaluation of serum platelet-derived growth factor receptor-ß and brain-derived neurotrophic factor levels in microvascular angina. Anatol J Cardiol 2020;24:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai HL, Lu ZF, Zhao JJ, Ma X, Li XH, Xu H, Wu SG, Kang CM, Lu JB, Xu YJ, Xiao L, Wu Q, Ye S, Wang Q, Zheng L, Hu YW. Microarray profiling analysis and validation of novel long non-coding RNAs and mRNAs as potential biomarkers and their functions in atherosclerosis. Physiol Genomics 2019;51:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ejiri J, Inoue N, Kobayashi S, Shiraki R, Otsui K, Honjo T, Takahashi M, Ohashi Y, Ichikawa S, Terashima M, Mori T, Awano K, Shinke T, Shite J, Hirata K, Yokozaki H, Kawashima S, Yokoyama M. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation 2005;112:2114–2120. [DOI] [PubMed] [Google Scholar]
- 30.Esmaeili F, Mansouri E, Emami MA, Montazerghaem H, Hosseini Teshnizi S, Kheirandish M, Koochakkhani S, Eftekhar E. Association of Serum Level and DNA Methylation Status of Brain-Derived Neurotrophic Factor with the Severity of Coronary Artery Disease. Indian J Clin Biochem 2022;37:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han W, Zhang C, Wang H, Yang M, Guo Y, Li G, Zhang H, Wang C, Chen D, Geng C, Jiang P. Alterations of irisin, adropin, preptin and BDNF concentrations in coronary heart disease patients co-morbid with depression. Ann Transl Med 2019;7:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin H, Chen Y, Wang B, Zhu Y, Chen L, Han X, Ma G, Liu N. Association between brain-derived neurotrophic factor and von Willebrand factor levels in patients with stable coronary artery disease. BMC Cardiovasc Disord 2018;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monisha KG, Prabu P, Chokkalingam M, Murugesan R, Milenkovic D, Ahmed SSSJ. Clinical utility of brain-derived neurotrophic factor as a biomarker with left ventricular echocardio-graphic indices for potential diagnosis of coronary artery disease. Sci Rep 2020;10:16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sustar A, Perkovic MN, Erjavec GN, Strac DS, Pivac N. Association between reduced brain-derived neurotrophic factor concentration & coronary heart disease. Indian J Med Res 2019;150:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia F, Zeng Q, Chen J. Circulating brain-derived neurotrophic factor dysregulation and its linkage with lipid level, stenosis degree, and inflammatory cytokines in coronary heart disease. J Clin Lab Anal 2022;36:e24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florent B, Maxime B, Catherine-Alexandra G, Christine G, Nathalie TT, Eric T, Anil N, Martin J, Jonathan T, Mathieu G, Louis B. Differences in cognitive function, cardiorespiratory fitness and BDNF concentration in physically active CHD patients vs healthy controls. Brain Res 2022;1793:148019. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Wang L, Zhan Y, Zhang Z, Chen D, Xiang Y, Xie C. The expression of SAH, IL-1β, Hcy, TNF-α and BDNF in coronary heart disease and its relationship with the severity of coronary stenosis. BMC Cardiovasc Disord 2022;22:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bahls M, Könemann S, Markus MRP, Wenzel K, Friedrich N, Nauck M, Völzke H, Steveling A, Janowitz D, Grabe HJ, Felix SB, Dörr M. Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci Rep 2019;9:15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kermani P, Hempstead B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front Physiol 2019;10:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalaji A, Behnoush AH, Shobeiri P, Saeedian B, Teixeira AL, Rezaei N. Association between brain-derived neurotrophic factor levels and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath 2023;27:829–841. [DOI] [PubMed] [Google Scholar]
- 41.Hang PZ, Zhu H, Li PF, Liu J, Ge FQ, Zhao J, Du ZM. The Emerging Role of BDNF/TrkB Signaling in Cardiovascular Diseases. Life (Basel) 2021;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fulgenzi G, Tomassoni-Ardori F, Babini L, Becker J, Barrick C, Puverel S, Tessarollo L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J Cell Biol 2015;210:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H, Liu Y, Zhang Y, Chen ZY. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem Biophys Res Commun 2011;415:99–103. [DOI] [PubMed] [Google Scholar]
- 44.Zembron-Lacny A, Dziubek W, Rynkiewicz M, Morawin B, Woźniewski M. Peripheral brain-derived neurotrophic factor is related to cardiovascular risk factors in active and inactive elderly men. Braz J Med Biol Res 2016;49:e5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shobeiri P, Karimi A, Momtazmanesh S, Teixeira AL, Teunissen CE, van Wegen EEH, Hirsch MA, Yekaninejad MS, Rezaei N. Exercise-induced increase in blood-based brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis: A systematic review and meta-analysis of exercise intervention trials. PLoS One 2022;17:e0264557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, Sun B. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett 2000;470:113–117. [DOI] [PubMed] [Google Scholar]
- 47.Ejiri J, Inoue N, Kobayashi S, Shiraki R, Otsui K, Honjo T, Takahashi M, Ohashi Y, Ichikawa S, Terashima M, Mori T, Awano K, Shinke T, Shite J, Hirata K, Yokozaki H, Kawashima S, Yokoyama M. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation 2005;112:2114–2120. [DOI] [PubMed] [Google Scholar]
- 48.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibañez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000;127:4531–4540. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimura R, Sugita-Ikenouchi A, Hori H, Umene-Nakano W, Hayashi K, Katsuki A, Ueda N, Nakamura J. A close correlation between plasma and serum levels of brain-derived neurotrophic factor (BDNF) in healthy volunteers. Int J Psychiatry Clin Pract 2010;14:220–222. [DOI] [PubMed] [Google Scholar]
- 50.Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res 1996;709:122–301. [DOI] [PubMed] [Google Scholar]
- 51.Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002;87:728–734. [PubMed] [Google Scholar]
- 52.Pliego-Rivero FB, Bayatti N, Giannakoulopoulos X, Glover V, Bradford HF, Stern G, Sandler M. Brain-derived neurotrophic factor in human platelets. Biochem Pharmacol 1997;54:207–209. [DOI] [PubMed] [Google Scholar]
- 53.Gejl AK, Enevold C, Bugge A, Andersen MS, Nielsen CH, Andersen LB. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci Rep 2019;9:9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A 2010;107:2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]