Abstract
Globally, the presence of cyanotoxins in water supplies and food has been widely investigated for over a decade. Cyanotoxins are harmful metabolites produced by toxic cyanobacterial genera. These metabolites belong to diverse chemical classes, with a variety of physicochemical properties, chemical structures, and toxic activities. The present study seeks to investigate the occurrence of cyanotoxins in water supplies destined for food processing and assess the human health risk from exposure to cyanotoxins. To achieve this, a simple, sensitive, and reliable analytical method was developed for the determination of microcystins (MC-RR, MC-LR, MC-YR) in process water, raw maize meal, and cooked maize (porridge) at ppb (parts per billion) levels. These compounds were extracted using Solid Phase Extraction (SPE) with optimized parameters; thereafter, Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) was used for the rapid determination of the analytes selected for the present study. The method developed was applied to samples collected from the meal grinding station located in Mawoni village in South Africa; and was able to detect and quantify all the target cyanotoxins. MC-LR, MC-YR and MC-RR were detected at concentrations ranging from 10 to 11.2 μg/L, 9.1–9.4 μg/L, and 2.3–3.5 μg/L, in water samples, respectively. However, MC-YR was not detected in ground water sample. Moreover, MC-LR, MC-YR, and MC-RR concentrations in maize and porridge samples ranged between 9.2 and 11.2, 5.5–8.6, and 6.3–9.3 μg/kg dry weight, respectively. The hazard quotient index (HQi) levels found in the present study ranged between 2.2 – 8.4 and 0.11–8.9 for adults and children, respectively, representing potential risks to human health. Findings from LC-MS/MS reveal that cyanotoxins can be transferred from water to food during food processing using cyanotoxins contaminated water. Furthermore, the methods developed can be used by environmental and health agencies to strengthen the monitoring of cyanotoxins in water and food.
Keywords: Cyanobacteria, Liquid chromatography-mass spectrometry, Maize meal, Microcystins, Process water, Solid phase extraction
1. Introduction
Human-driven eutrophication has led to the intensification of cyanobacterial blooms in aquatic ecosystems [[1], [2], [3]]. This has been reported in many countries such as China, the USA, South Africa, Botswana, Tanzania, and Zimbabwe [[4], [5], [6], [7], [8], [9]] and has been expected to increase with continued climate change [10,11]. Numerous factors such as low turbulent condition, high water retention time, cycling of nutrients, temperature increase and low nitrate and phosphate (N:P) ratio have been reported to promote cyanobacteria dominance [[12], [13], [14]]. Excessive growth of cyanobacteria changes the taste and odour of water and also increases the costs of water treatment [[15], [16], [17], [18]]. However, of all the effects, the most prominent negative feature of cyanobacterial blooms is their ability to produce toxins (microcystins) that can induce additional health and ecological problems [14,19,20] such as reducing the availability of clean water for human and animal consumption as well as water for crop irrigation [21]. Microcystins transfer to food during irrigation or processing using microcystin-contaminated water has been reported in previous studies [[21], [22], [23], [24]]. Human exposure to cyanotoxins has been associated with skin irritation, primary liver cancer, gastrointestinal illness and possible death caused by renal dialysis [[25], [26], [27]].
Pindihama et al. [28] also reported declines in freshwater resources in South Africa. People in rural regions are reportedly using untreated water directly from the source for domestic use, such as drinking, washing and preparing food [[29], [30]]. However, these sources of water are often contaminated by bacteria such as toxic cyanobacteria [30]. People from Mawoni and the surrounding villages use plastic containers (20–25L) to collect and store water for domestic use. This water can be stored and used for several days. Cyanobacteria may be collected with source water or may develop and proliferate inside the container as some of these containers are light permitting, and therefore, can promote the growth of cyanobacteria, which then poses a health risk to the users. Moreover, some of this cyanobacteria-contaminated water is being used to soften the maize grain during maize meal production, and therefore, the present study suspects contamination of maize by cyanotoxin from the cyanobacteria-contaminated water that is being used during the production process [31]. People from South African countries eat pap porridge made from maize meal as their everyday staple food. Hence, consumption of porridge made of cyanotoxins contaminated maize meal can be a threat to human health and can also be considered one of the major routes of human exposure to cyanotoxins. The health threats caused by cyanotoxins have led the World Health Organization (WHO) to set the standards for drinking water quality for these toxins; for example, a guideline value for total MCs is 1 μg/L in drinking water [[32], [33], [34]] and this value has been adopted by several countries such as South Africa, Spain, Uruguay, New Zealand, Turkey, Brazil, and Singapore [33]. According to Chorus and Welker [33], the guideline values for lifetime drinking water were derived assuming that 80 % of the tolerable daily intake is through drinking water since drinking water is usually the most likely long-term source of exposure, which implies that other sources such as food and recreational water are less significant i.e. contributing to 20 % of the tolerable daily intake.
The present study aims to develop an analytical method for the determination of cyanotoxin microcystins (MC-RR, MC-LR, MC-YR) in diverse matrices (process water, maize meal, and pap porridge) during food processing using the LC-MS with triple quadrupole (QqQ) mass analyzers for a sensitive and accurate quantification, and identification of these analytes. Quantification of cyanotoxins in maize meal (raw and cooked) after the maize grinding process has not been conducted, hence, the present study has realized the necessity of such practice. This study was conducted in Mawoni village located in South Africa, where water contaminated by cyanobacteria is being used during maize meal production. It was therefore hypothesized that such water is likely to contaminate maize during production. Assessment of the human health risk of cyanotoxins in drinking water was also undertaken and discussed, as this practice may provide a basis for the development of further prevention and control measures. The present research will also help to fill an information gap regarding the occurrence of cyanotoxins in process water, maize meal and pap porridge used for food production, as this could be another route for human exposure to these toxins, which are highly toxic and threaten human health.
2. Materials and methods
2.1. Reagents and chemicals
Certified analytical standards solutions of microcystins LR, YR, and RR (all were 10 μg/mL in methanol) selected for the present study were supplied by Industrial Analytical, Kyalami, South Africa. All solutions had purity >95 %. Solvents for sample extraction and reconstitution were LC-MS grade. Acetonitrile (ACN) and methanol (MeOH), all ≥99.9 % were supplied by Lasec SA (PTY) LTD, Ndabeni, Cape Town, South Africa. Ultrapure water purchased from Lasec SA (PTY) LTD, Ndabeni, Cape Town, South Africa, was used throughout the study. The Supelco Visiprep SPE Solid Phase Extraction Vacuum Manifold DL 12-port 57044 (California Tech Zone, USA), was used for sample concentration and cleanup. A Diaphragm Vacuum Pump, GM-0.50A, was purchased from Bioevopeak Co., Ltd. Shandong, China and Oasis HLB cartridges were purchased from MicroSep, Sandton, South Africa.
2.2. Samples collection
Water sample (CW) was collected from a 25 L container (used to collect and store water) using Latex Free, Polypropylene Specimens with screw-lid sampling containers. Groundwater (GW) was pumped out and samples were collected (Fig. S1) using similar sampling containers. These water samples (100 mL) were collected from the mill grinding station that was randomly selected for the present study. From these stock water samples, 10 mL of water was then measured for cyanotoxin extraction. Two samples of raw maize meal powder produced from the grinding processes (Fig. S2) were further collected for cyanotoxin analysis. The first sample was collected just after the cyanobacteria-contaminated water was introduced to the maize grain and the second sample was collected after the whole grinding mill process is complete. A pap porridge sample was also prepared for the present study using raw maize meal sample.
2.3. Sample preparation
Samples of raw maize meal powder and processed porridge samples were ground using a pestle and mortar and were then portioned into 1 g aliquots and placed into a glass test tube. Except for the unknown sample, each sample was spiked with microcystins LR, YR, and RR in concentrations ranging from 2 ppb to 5 ppb. Samples were then incubated for 20 h at room temperature in the dark to allow the formation of covalently bound MC complexes as reported by Craig et al. [35]. A tissue probe homogenizer was further used to homogenize samples after spiking and incubation.
2.3.1. Optimisation of extraction conditions (solvent system homogenisation and SPE cleanup procedure)
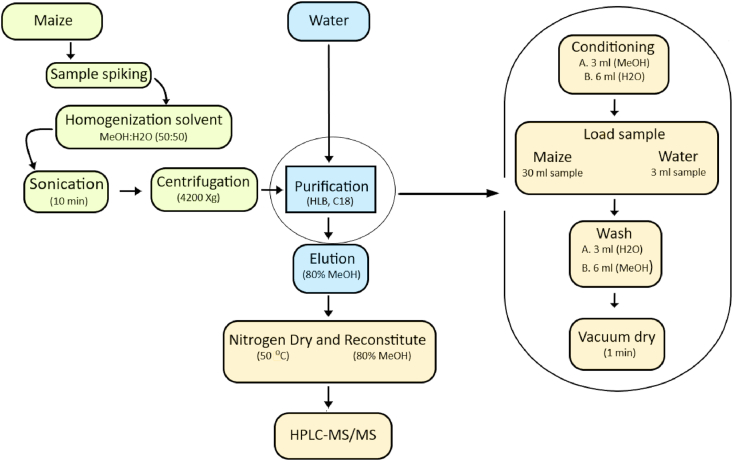
For the determination of total MCs, water samples were sonicated before extraction. For maize and porridge samples, a solvent system (50:50 methanol: water) was used in the present study (Fig. 1) to increase the movement of bound MCs from the homogenate in the solution. MCs were extracted from the homogenate by adding 10 mL of a solvent into homogenized samples followed by 10 min incubation at room temperature in the glass test tube and 5 min sonication of homogenized extracts was performed in a water bath. Afterwards, extracts were centrifuged at 4500×g for 20 min. The extracts supernatant was collected after centrifugation. Thereafter, the samples were ready for SPE cleanup.
Fig. 1.
Schematic showing how water and maize samples were prepared for MCs quantification in the present study.
The SPE was performed using a 12-port SPE vacuum manifold containing large-volume samplers and a diaphragm vacuum pump. As shown in Fig. 1, the Oasis HLB cartridges with a bed size of 500 mg (6 mL capacity) were connected in series and sequentially conditioned with 3 mL of methanol and 6 mL of water. Samples (30 mL of extracts supernatant and 10 mL of water samples) were slowly passed through the assembly and then washed afterwards with 20 % methanol. Before eluting samples with 80 % methanol, cartridges were vacuum dried for 1 min. Water samples and extract supernatant were eluted with 5 mL and 25 mL of methanol, respectively. The eluents were dried under a gentle stream of nitrogen gas. Residues were then re-dissolved with 1 mL of 80 % methanol and filtered into autosampler glass vials using 0.2 μm polytetrafluoroethylene filters (Inqaba Biotec, Pretoria, South Africa). Samples were then ready for LC-MS/MS analyses.
2.4. Liquid chromatograph -mass spectrometer method development
A Liquid Chromatograph Triple Quadrupole Mass Spectrometer (LC-QqQ-MS/MS) (Shimadzu, Japan) equipped with a binary solvent and sampler manager was used to conduct the analysis. For chromatography separation, Shim-pack Velox C18, 2.1 × 100 mm, 2.7 μm with a serial number of 227-32009-03 (COU, MO, USA) was used at a flow rate of 0.4 ml/min. The injection of 1 μ/L analytical volume was found suitable for this analysis. The mobile phase used was A = H2O and B = MeOH in gradient mode. The gradient program started at 95 % A (held for 1.5 min), decreased to 5 % A (from 1.5 to 2.0 min), 5 % A (held from 2.0 to 3.0 min), and increased to 95 % A (from 3.0 to 4.0 min). The temperature of the oven column was maintained at 40 °C. Retention times for each identified cyanotoxin are presented in Table S1.
2.5. Calibration and method validation
Separate 500 μg/L stock solutions of microcystins -LR, -YR, and –RR were prepared in methanol and stored at −20 °C until working standards were prepared. As highlighted in Table S1, to evaluate measurements and calibration range, standard solutions with known concentrations ranging from 2 to 100 μg/L were prepared in 80 % methanol and 20 % water. A linear calibration curve was then generated for each microcystin congener. For specificity assessment, an analysis of blank samples was performed. The precision and trueness of the method were evaluated by recovery experiments, i.e., analyzing samples that are spiked with a known concentration of analytes. The signal:noise (S/N) ratio of 3 for the limit of detection (LOD) was estimated from the chromatograms of the samples spiked at the lowest validated level.
2.6. Health risk assessment of MCs on human
Human exposure to MCs has been reported to be both directly (consumption of drinking water) and indirectly (consumption of food containing MCs) [36]. According to the carcinogenicity of the contaminant of interest, health risk assessment from exposure to MCs can be divided into carcinogenic and non-carcinogenic risks [36,37]. The HQi was used to assess the non-carcinogenic risks of MCs to humans. To assess the human health risk, the estimation of daily intake (EDI) of MCs through food (maize meal) consumption and drinking water was calculated in the present study and compared with the WHO tolerable daily intake (TDI) limit of 0.04 μg/kg/day for MC-LR [34] for adult and children with weight of 60 and 25 kg, respectively. HQi was determined using the following formula (equation (1)):
| (1) |
Where.
-
-
EDI is an estimation of daily intake.
-
-
RfD is the maximum exposure reference dose (μg/kg/d).
According to Table S2, the non-carcinogenic level of health risk is considered acceptable when HQ is less than 1. The daily exposure to MCs through intake oral and inhalation can be evaluated using the following formula (equation (2)):
| (2) |
Where.
-
-
Cv is concentration of MCs in drinking water (μg/L)
-
-
IR is daily water/food consumption (L/d or g/d)
-
-
EF is frequency of exposure (times/year)
-
-
ED is time of exposure (non-carcinogens, 30 years; carcinogens, 70 years)
-
-
BW is average body weight (kg)
-
-
AT is AT is the average exposure time (noncarcinogens, 30 x 365 = 10 950 d; carcinogens, 70 x 365 = 25 550 d).
The present study assumed that an adult need 20 mouthful piece (morsels) of pap porridge per day, weighing 29 g each, whereas, children need 7 mouthful piece of pap porridge per day, weighing 15 g each. Therefore, in the present study, the non-carcinogenic risk of MCs to human health through drinking water and food (maize meal) was calculated using the following simplified formula (equation (3)):
| (3) |
Where.
-
-
C is the concentration of MCs in maize meal (μg/L)
-
-
IR is the water intake (1.8 L/d for adults and 0.8 L/d for children) or maize meal intake (20 × 29 g of dry pap morsel per day for an adult and 7 × 15 g for children).
-
-
BW is the body weight (60 kg for adults and 25 kg for children).
-
-
Rfd is the reference dose of MCs.
3. Results and discussion
3.1. Liquid chromatography and mass spectrometry
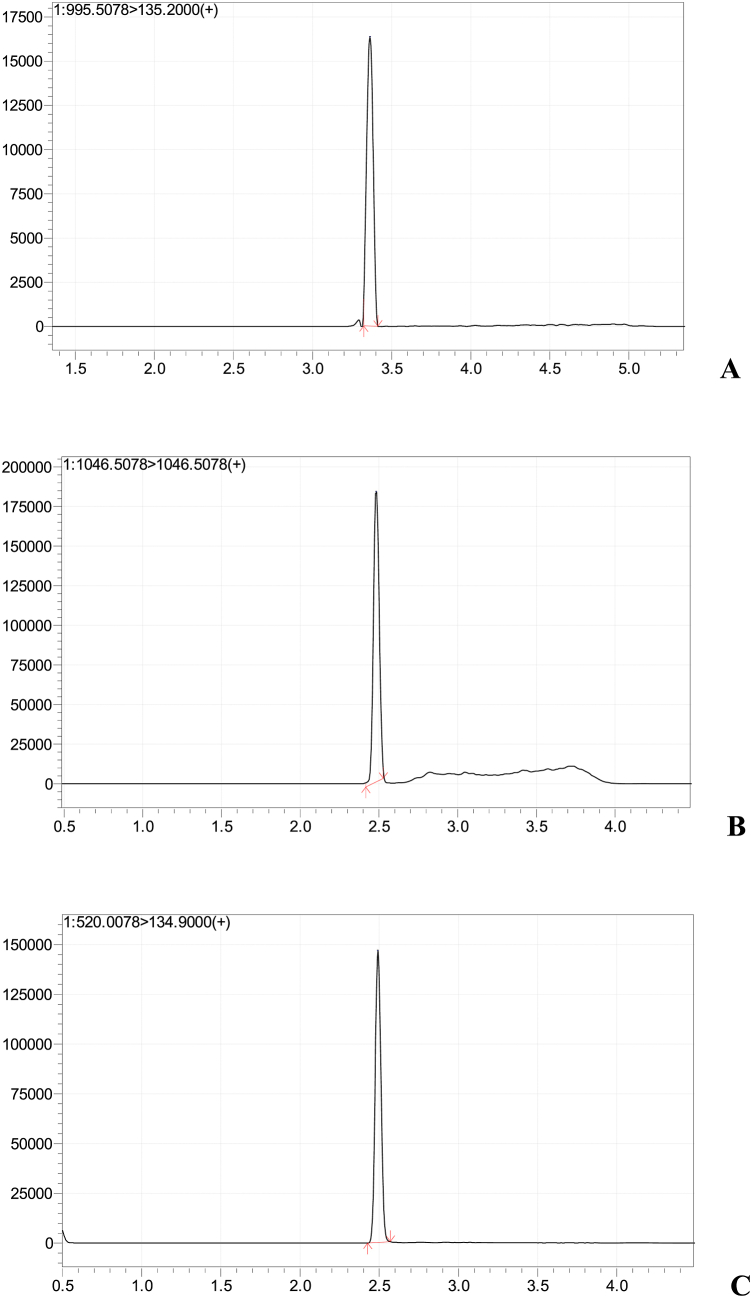
To improve the quality of mass peak separation and maximize the sensitivity of the method, a liquid chromatograph method was developed for the separation of the target toxins. Therefore, few gradient programs were tested in the present study for the chromatographic separation of the toxins (MC-LR, MC-YR, and MC-RR). Compatibility of the column with water/acetonitrile acidified with 0.1 % formic acid in the mobile phase enabled efficient chromatograph separation of the target compounds. Furthermore, quantification data sets (i.e. signal-to-noise (S/N) ratio and peak height) and resolution were compared to select the separation procedures with the highest sensitivity. The optimum chromatographic performance was provided by the Shim-pack Velox SP-C18 (2.7 μm, 2.1 × 100 mm) column for all the targeted compounds. This column displayed increased pressure and thus imposing the use of lower flow rates and increased overall chromatographic time, producing acceptable peak shape and perfect resolution under reproducible retention times as stated by Kuster et al. [38] and Rodrigues et al. [39]. This column allowed the separation of MC-LR, MC-YR, and MC-RR peak pairs as shown in Fig. 2(A–C).
Fig. 2.
LC-MS MRM chromatograms of quantification ions for A: microcystins-LR, B: microcystins-YR, and microcystins-RR at a concentration of 100 ppb.
The behaviour of the mass spectra of the compounds was studied by infusing pure solutions into the mass spectrometer under positive ionization modes. MC-LR and MC-YR showed abundant ionization in positive ion mode forming a single protonated molecular ion [M+H]+ due to the presence of one arginine moiety, known as the protonation site [40]. Contrarily, MC-RR showed double protonated precursor ion [M+2H]2+, as their molecular structure is characterized by the presence of two arginine residues. In the positive mode ions [M+H]+, the base peaks were 995.5 for MC-LR, 1045.5 for MC-YR, and for MC-RR, which has two arginine residues, gave a base peak ions [M+2H]2+ of 519.7. Thus, it forms double-charged ions easier, and its abundance was significantly higher than that of single-charged ions. Observations from the present study are compatible with findings of single and double-charged ions of similar compounds from previous studies [[40], [41], [42]].
3.2. SPE method development
Experiments were conducted using Oasis HLB (as described in section 2.4.1) for the extraction of the targeted microcystins. Various SPE process parameters were evaluated and optimized for effective extraction. These parameters include sample pH, SPE cartridge material, elution solvent composition and volume, reconstitution solvent composition, and sonication time of the final extract. Most toxins are retainable by HLB cartridges at alkaline pH [43,44]; however, in the present study microcystins were effectively retained by Oasis HLB cartridges at a neutral pH. Similar results were observed in previous studies such as Van der Westhuizen and Eloff [45], Filatova et al. [42], and Schenzel et al. [46].
3.3. Method validation
For method validation, Table 1 shows the coefficient of determination (r2) for all the target compounds from the linear regression analysis. The correctness of the method developed was assessed in water and maize meal samples spiked with different concentrations of stock solution of the target analytes. Table 2 shows the concentrations levels of stock solution used to spike different samples. Furthermore, the specificity of the method was evaluated by analysing blank samples and there were no interfering peaks or signals observed close to the retention time of the analytes. Standard solutions were analysed at six different concentrations (from 2 ppb to 200 ppb) to study the linearity of the method and measurement range. A total of 3 maize samples were spiked and analysed, thereafter, mean recoveries were calculated and found satisfactory, ranging between 69 and 100 % for all the target compounds (Table 2).
Table 1.
Microcystin variants and their linear relationship.
| Reference material | Standard curve range (μg/L) | Standard curve regression fit style | Coefficient of determination (r2) |
|---|---|---|---|
| MC-LR | 2–100 | Linear | 0.998 |
| MC-YR | 2–100 | Linear | 0.999 |
| MC-RR | 2–100 | Linear | 0.998 |
Table 2.
Concentration levels of stock solution (1 μg/L, 2 μg/L, and 5 μg/L) used to spike different samples and recoveries (%) for method validation.
| Microcystin variant | Maize 1 |
Maize 2 |
Maize 3 (porridge) |
LOD (μg/L) | LOD (μg/kg) |
|---|---|---|---|---|---|
| 2 μg/L | 1 μg/L | 5 μg/L | |||
| MC-LR | 100 % | 89 % | 98 % | 5 | 5 |
| MC-YR | 80 % | 92 % | 69 % | 4 | 4 |
| MC-RR | 96 % | 92 % | 93 % | 1 | 1 |
Fig. 2(A–C) also shows the chromatograms selected for standards of microcystins at the highest validated concentration of 100 ppb. The limit of detection (LOD) was calculated in the present study for all the target compounds. These values were estimated from the chromatograms of the spiked samples at the lowest validated levels. Signal-to-noise ratios (S/N) for all the target toxins were found higher than 3. Previous studies [40,47,48] obtained similar ratios. Therefore, validation results in the present study indicated that the method is appropriate for the quantitative determination of the target compounds.
3.4. Application of the method to real samples
The presence of microcystins in process water and maize meal samples collected from Mawoni village has been associated with blooms of potentially toxic cyanobacterial species, such as Leptolyngbya, Chroococcus, and Microcystis sp [31]. Target cyanotoxins in process water (tap water and groundwater), maize meal, and pap porridge samples were determined using the analytical method developed for the present study. Extracted samples were filtrated through nylon syringes (0.22 μm) and then injected into the automated LC-MS/MS system. Standards concentration used for calibration curves were injected at the beginning of each sample batch together with two spiked samples that were used as quality controls. Table 3 shows the presence of microcystins in most of the samples. MC-YR was not detected in the groundwater sample; however, MC-LR and RR were detected in this sample at 10.1 and 3.5 μg/L concentration levels, respectively. These findings are compatible with results obtained by previous studies such as Mohamed and Al Shehri [49] and Tian et al. [50], who detected MC in groundwater collected close to cyanobacteria-contaminated surface water in Saudi Arabia and China, respectively. The findings from the present study further indicated that MC-LR was the most abundant and predominant variant among all the detected toxins with concentrations ranging between 9.2 and 12.0 μg/kg and 10.0 – 11.2 μg/L in maize and water samples, respectively.
Table 3.
Concentration of microcystin variants determined in all the samples.
| Microcystin variant | Concentration (μg/L) |
Concentration (μg/kg) |
|||
|---|---|---|---|---|---|
| CW | GW | P1 | P2 | P3 | |
| MC-LR | 11.2 | 10.1 | 11.2 | 12.0 | 9.2 |
| MC-YR | 9.4 | <LOD | 8.6 | 7.5 | 5.5 |
| MC-RR | 2.3 | 3.5 | 9.3 | 8.9 | 6.3 |
*<LOD – not detected. CW (container water), P1 (maize meal 1), P2 (maize meal 2), P3 (porridge), GW (groundwater).
In addition, the present study observed that the retention time for real samples was identical to the one for reference standards. Furthermore, all recoveries to the quality control results were within the accepted range (60–140) as stipulated by Beltran et al. [51] and Romera-Garcia et al. [52]. The presence of MCs in food (rice, tomatoes, carrots, fish) has been reported in previous studies such as Chen et al. [53], Drobac et al. [54], Machado et al. [55], and Trung et al. [56]. However, the method developed in the present study was able to detect and quantify targeted MCs for the first time in maize meal processed using cyanotoxins-contaminated water. These findings further show that all the microcystins concentrations quantified in all the samples exceeded the maximum permitted concentration value (1 μg/L) established by the World Health Organization [34].
Cyanobacteria may be collected with source water or may develop and proliferate inside containers due to nutrient availability [31]. Moreover, these containers are light permitting, an important consideration as light availability inside these containers could influence the growth of cyanobacteria species including Microcystis and therefore possibly influence the level of cyanotoxins. Cyanobacteria could increase in numbers in containers, be it through growth or sustained accumulation by growth factors in the container biofilm, especially light-permitting containers. Maize seed is softened with the contaminated process water and then passes through the grinding mill process. Due to high temperatures or extremely warm air within the grinding machines, cyanobacteria cells rupture leading to the release of cyanotoxins microcystins into maize meal. Thereafter, Mutoti et al. [31] reported that the maize meal was then cooked on an electric stove into pap porridge. The high temperatures during the cooking of pap porridge destroyed the remaining proportion of cyanobacteria that were available in maize meal and then cyanotoxins microcystins were released into pap porridge. These findings agree with what O'Keeffe [57] reported. O'Keeffe [57] reported that cyanobacteria die at high temperatures. At boiling point, cells of cyanobacteria burst and cyanotoxins are released into the water which may increase toxin levels [57] as these toxins do not degrade at the highest temperatures. Sklenar et al. [58] and Spoof et al. [59] reported that MCs do not degrade or break down during boiling. Morais et al. [60] also reported in their study that boiling does not significantly change the MCs content after being boiled at 100 °C.
The presence of cyanotoxins microcystins in groundwater has been documented [49,61]. In China, Yang et al. [61] reported the presence of MCs in groundwater collected from a borehole located next to a eutrophic lake; and it was reported that groundwater-surface water interaction was the cause of MCs occurrence in groundwater. It was further reported in a study by Chatziefthimiou et al. [62] and Bouaicha and Corbel [63] that cyanobacteria occur in soils and earth crusts in semi-arid regions and their toxins can infiltrate the ground during rainy events, which then indicates that MCs can be present in groundwater system [64]. MCs are known to adsorb onto soils and other biological crusts [49,63]. Although their adsorption is low, there could be high bioavailability for soil plants and a higher possibility of infiltration into groundwater during irrigation and precipitation events. In the present study groundwater sample was collected from a borehole located closer (190 m away) to Mawoni River and Makhado Oxidation ponds (Fig. S1). The Makhado oxidation ponds have been reported to discharge their effluent into the Mawoni River, making it a nutrient-rich environment bringing forth risks of eutrophication, which contributes to the development of harmful algal blooms [65]. Therefore, it can be assumed that groundwater-surface water interaction within this area has led to the occurrence of MCs in groundwater. Moreover, it was observed during sampling that the surface soils where the borehole is located have higher blue-green algae content and therefore their toxins can infiltrate and percolate the ground and contaminate groundwater.
3.5. Health risk assessment of MCs on human
The HQi was estimated to assess the potential influence of MCs on human health from consuming the water and porridge meal cooked using cyanotoxin microcystins-contaminated water. The HQi levels found in the present study ranged between 2.26 and 2.75 for adults, both of which were within the high-risk degree according to Table S2; whereas for children the HQi levels ranged between 0.84 and 1.02, meaning they were within the average and high-risk degree (Table 4). The HQi levels recorded in container water, groundwater and porridge samples exceeded the acceptable limit of 1 and could represent a health risk for adults. However, for children, the recorded HQi levels were high in container water and moderate in both groundwater and porridge samples. These findings indicate that porridge cooked using cyanotoxins-contaminated maize meal could represent a risk to human health. Therefore, these findings clearly show that higher MCs concentrations in process water may result in greater MCs concentration in porridge meal, as well as increased human health risks.
Table 4.
Human health risk assessment related to MCs in water and maize meal.
| Adults | Children | |||
|---|---|---|---|---|
| Sample | HQi | Risk level | HQi | Risk level |
| Container Water | 8.4 | Extremely High | 8.9 | Extremely High |
| Groundwater | 7.5 | Extremely High | 8.0 | Extremely High |
| Porridge | 2.2 | High | 0.11 | Low |
4. Conclusion
An effective, fast, and simple method was developed in the present study to determine a class of cyanobacterial toxins MC. The method developed was applied to samples collected in Mawoni Village to determine the occurrence of total MCs. These samples were extracted using an SPE method, thereafter, detection and quantification of target cyanotoxins were carried out using the LC-MS/MS in MRM mode. For the first time, MCs were found in water (tap and groundwater) used for maize production and in maize meal processed with cyanotoxins-contaminated water. Concentration levels of MC-LR, MC-YR, and MC-RR were found ranging from 10 to 11.2 μg/L, 9.1–9.4 μg/L, and 2.3–3.5 μg/L, in water samples, respectively, except for MC-YR, which was not detected in groundwater. Moreover, MC-LR, MC-YR, and MC-RR concentrations in maize and porridge samples ranged between 9.2 and 11.2, 5.5–8.6, 6.3–9.3 μg/kg dry weight, respectively. The HQi levels found in the present study ranged between 2.2 – 8.4 and 0.11–8.9 for adults and children, respectively, representing potential risks to human health. The fact that these cyanotoxins were found at levels that are way higher than the recommended guideline values, further reveals that maize meal processed with cyanotoxins contaminated water could be another potential route of human chronic exposure to cyanotoxins. Therefore, local health and drinking water management agencies or authorities need to take precautionary steps by employing proactive strategies to inhibit the growth of the algal bloom and prevent the toxin from reaching drinking water sources and food supplies. Moreover, the ability of the developed method to detect and quantity toxins of various classes can significantly add value and promote further research on the presence of a wide range of cyanotoxins in water supplies and maize samples.
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Mulalo I. Mutoti: Writing – review & editing, Writing – original draft, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Afam I.O. Jideani: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization. Ntakadzeni E. Madala: Writing – review & editing, Validation, Resources, Formal analysis, Data curation. Jabulani R. Gumbo: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project was supported by the National Research Foundation through Doctoral Scholarship (UID 102072) for MIM.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29882.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Fernandez-Figueroa E.G. Doctoral Dissertation. Auburn University; 2021. From drones to Daphnia: exploring eutrophication and climate change impacts on algal blooms at various scales. [Google Scholar]
- 2.Sukenik A., Kaplan A. Cyanobacterial harmful algal blooms in aquatic ecosystems: a comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms. 2021;9:1472. doi: 10.3390/microorganisms9071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kazmi S.S.U.H., Yapa N., Karunarathna S.C., Suwannarach N. Perceived intensification in harmful algal blooms is a wave of cumulative threat to the aquatic ecosystems. Biology. 2022;11:852. doi: 10.3390/biology11060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paerl H.W., Xu H., McCarthy M.J., Zhu G., Qin B., Li Y., Gardner W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res. 2011;45:1973–1983. doi: 10.1016/j.watres.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Gumbo J.R., Dzaga R.A., Nethengwe N.S. Impact on water quality of Nandoni water reservoir downstream of municipal sewage plants in Vhembe District, South Africa. Sustainability. 2016;8:597. [Google Scholar]
- 6.Kimambo O.N., Gumbo J.R., Msagati T.A., Chikoore H. The unusual reddish-bloom appearance in a freshwater fishpond at kingolwira national fish farming center, morogoro, Tanzania. Int. J. Environ. 2020;9:204–216. [Google Scholar]
- 7.Hamandishe V.R., Saidi P.T., Gumbo J., Nhiwatiwa T. Diet composition of Oreochromis niloticus from selected impoundments of different states, with special focus on toxigenic cyanobacteria identified using molecular techniques. Sci. Afr. 2021;13 [Google Scholar]
- 8.Wang H., Xu C., Liu Y., Jeppesen E., Svenning J.C., Wu J., Zhang W., Zhou T., Wang P., Nangombe S., Ma J. From unusual suspect to serial killer: cyanotoxins boosted by climate change may jeopardize megafauna. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruk C., Segura A., Pineiro G., Baldassini P., Perez‐Becona L., Garcia‐Rodriguez F., Perera G., Piccini C. Rise of toxic cyanobacterial blooms is promoted by agricultural intensification in the basin of a large subtropical river of South America. Glob. Change Biol. 2023;29:1774–1790. doi: 10.1111/gcb.16587. [DOI] [PubMed] [Google Scholar]
- 10.Nazari-Sharabian M., Ahmad S., Karakouzian M. Climate change and eutrophication: a short review. ETASR. 2018;8:3668. [Google Scholar]
- 11.Mardones J.I., Paredes-Mella J., Flores-Lenero A., Yarimizu K., Godoy M., Artal O., Corredor-Acosta A., Marcus L., Cascales E., Espinoza J.P., Norambuena L. Extreme harmful algal blooms, climate change, and potential risk of eutrophication in Patagonian fjords: insights from an exceptional Heterosigma akashiwo fish-killing event. Prog. Oceanogr. 2023;210 [Google Scholar]
- 12.Giannuzzi L. Cyanobacteria growth kinetics. ALGAE. 2019:1–17. doi: 10.5772/intechopen.81545. [DOI] [Google Scholar]
- 13.Zhou B., Cai X., Wang S., Yang X. Analysis of the causes of cyanobacteria bloom: a review J. Resour. Ecol. 2020;11:405–413. [Google Scholar]
- 14.Mutoti M., Gumbo J., Jideani A.I.O. Occurrence of cyanobacteria in water used for food production: a review. Phys. Chem. Earth, Parts A/B/C. 2022;125 [Google Scholar]
- 15.Golshan A., Evans C., Geary P., Morrow A., Maeder M., Tauler R. Patterns of cyanobacterial abundance in a major drinking water reservoir: what 3 years of comprehensive monitoring data reveals? Environ. Monit. Assess. 2020;192:1–11. doi: 10.1007/s10661-020-8090-z. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Giesy P.J., Adamovsky O., Svirčev Z., Meriluoto J., Codd G.A., Mijovic B., Shi T., Tuo X., Li S., Pan B., Chen J., Xie P. Challenges of using blooms of Microcystis spp. in animal feeds: a comprehensive review of nutritional, oxicological and microbial health evaluation. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142319. [DOI] [PubMed] [Google Scholar]
- 17.Adams H., Smith S.A., Reeder S., Appleton E., Leinweber B., Forbes S., Barrowman P., Ford G., Ikehata K., Southard M. Characterizing and mitigating cyanobacterial blooms in drinking water reservoirs. J. Am. Water Work. Assoc. 2022;114:26–38. [Google Scholar]
- 18.Zhang W., Liu J., Xiao Y., Zhang Y., Yu Y., Zheng Z., Liu Y., Li Q. The Impact of cyanobacteria blooms on the aquatic environment and human health. Toxins. 2022;14:658. doi: 10.3390/toxins14100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmichael W.W., Boyer G.L. Health impacts from cyanobacteria harmful algae blooms Implications for the North American Great Lakes. Harmful Algae. 2016;54:194–212. doi: 10.1016/j.hal.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Zi J., Pan X., MacIsaac H.J., Yang J., Xu R., Chen S., Chang X. Cyanobacteria blooms induce embryonic heart failure in an endangered fish species. Aquat. Toxicol. 2018;194:78–85. doi: 10.1016/j.aquatox.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Melaram R., Newton A.R., Chafin J. Microcystin contamination and toxicity: implications for agriculture and public health. Toxins. 2022;14:350. doi: 10.3390/toxins14050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdullahi H., Tanimu Y., Akinyemi S.A., do Carmo Bittencourt-Oliveira M., Chia M.A. Assessment of microcystins in surface water and irrigated vegetables in Kwaru stream, Hayin Danmani, Kaduna-Nigeria. Environ. Sci. Pollut. Control Ser. 2022;29(52):78303–78313. doi: 10.1007/s11356-022-21381-w. [DOI] [PubMed] [Google Scholar]
- 23.Redouane E.M., Tazart Z., Lahrouni M., Mugani R., Elgadi S., Zine H., Zerrifi S.E.A., Haida M., Martins J.C., Campos A., Oufdou K. Health risk assessment of lake water contaminated with microcystins for fruit crop irrigation and farm animal drinking. Environ. Sci. Pollut. Control Ser. 2023:1–11. doi: 10.1007/s11356-023-27914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tazart Z., Lahrouni M., Mugani R., Elgadi S., Zine H., Zerrifi S.E.A., Haida M., Martins J.C., Campos A., Oufdou K., Vasconcelos V. Health risk assessment of lake water contaminated with microcystins for fruit crop irrigation and farm animal drinking. Environ. Sci. Pollut. Res. Int. 2023;30(33) doi: 10.1007/s11356-023-27914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svircev Z., Lalic D., Bojadzija Savic G., Tokodi N., Drobac B.D., Chen L., Meriluoto J., Codd G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019;93:2429–2481. doi: 10.1007/s00204-019-02524-4. [DOI] [PubMed] [Google Scholar]
- 26.Codd G.A., Testai E., Funari E., Svircev Z. Cyanobacteria, cyanotoxins, and human health. Water treatment for purification from cyanobacteria and cyanotoxins. 2020;11:37–68. [Google Scholar]
- 27.Chorus I., Welker M. vol. 858. Taylor & Francis; 2021. (Toxic Cyanobacteria in Water: a Guide to Their Public Health Consequences, Monitoring and Management). [Google Scholar]
- 28.Pindihama G.K., Gumbo J.R., Oberholster P.J. Evaluation of a low-cost technology to manage algal toxins in rural water supplies. Afr. J. Biotechnol. 2011;10:9883–9889. [Google Scholar]
- 29.Obi C.L., Potgieter N., Bessong P.O., Matsaung G. Assessment of the microbial quality of river water sources in rural Venda communities in South Africa. WaterSA. 2002;28:287–292. [PubMed] [Google Scholar]
- 30.Fosso-Kankeu E., Jagals P., Du Preez H. Exposure of rural households to toxic cyanobacteria in container-stored water. WaterSA. 2008;34:631–636. [Google Scholar]
- 31.Mutoti M.I., Jideani A.I., Gumbo J.R. Using FlowCam and molecular techniques to assess the diversity of Cyanobacteria species in water used for food production. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-23818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrer D., Counter M., Hillwig R., Cude C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins. 2015;7:457–477. doi: 10.3390/toxins7020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chorus I., Welker M. Toxic Cyanobacteria in Water. CRC Press; 2021. Exposure to cyanotoxins: understanding it and short-term interventions to prevent it; pp. 295–400. [Google Scholar]
- 34.World Health Organization (WHO) World Health Organization; Geneva: 2017. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. [PubMed] [Google Scholar]
- 35.Craig M., Luu H.A., McCready T.L., Holmes C.F., Williams D., Andersen R.J. Molecular mechanisms underlying the interaction of motuporin and microcystins with type-1 and type-2A protein phosphatases. Biochem. Cell. Biol. 1996;74:569–578. doi: 10.1139/o96-061. [DOI] [PubMed] [Google Scholar]
- 36.Ge S., Qiao X., Zhao X., Li X., Liu Y. Microcystin in source water: pollution characteristics and human health risk assessment. RSC adv. 2021;11:6415–6422. doi: 10.1039/d0ra08983d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T.A.D., Nguyen L.T., Enright A., Pham L.T., Tran H.Y.T., Tran T.T., Nguyen V.H.T., Tran D.N. Health risk assessment related to cyanotoxins exposure of a community living near Tri an Reservoir, Vietnam. Environ. Sci. Pollut. Res. 2021;28:56079–56091. doi: 10.1007/s11356-021-14545-7. [DOI] [PubMed] [Google Scholar]
- 38.Kuster M., de Alda M.L., Barcelo D. Analysis of pesticides in water by liquid chromatography‐tandem mass spectrometric techniques. Mass Spectrom. Rev. 2006;25:900–916. doi: 10.1002/mas.20093. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues M.A., Reis M.P., Mateus M.C. Liquid chromatography/negative electrospray ionization ion trap MS2 mass spectrometry application for the determination of microcystins occurrence in Southern Portugal water reservoirs. Toxicon. 2013;74:8–18. doi: 10.1016/j.toxicon.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Zervou S.K., Christophoridis C., Kaloudis T., Triantis T.M., Hiskia A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard Mater. 2017;323:56–66. doi: 10.1016/j.jhazmat.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Kleinteich J., Puddick J., Wood S.A., Hildebrand F., Laughinghouse I.V., Pearce D.A., Dietrich D.R., Wilmotte A. Toxic cyanobacteria in Svalbard: chemical diversity of microcystins detected using a liquid chromatography-mass spectrometry precursor ion screening method. Toxins. 2018;10:147. doi: 10.3390/toxins10040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filatova D. University of Barcelona; 2021. Origin and Release of Cyanotoxins in Surface Water Reservoirs. Doctoral Thesis. [Google Scholar]
- 43.Gerssen A., McElhinney M.A., Mulder P.P., Bire R., Hess P., de Boer J. Solid phase extraction for removal of matrix effects in lipophilic marine toxin analysis by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2009;394:1213–1226. doi: 10.1007/s00216-009-2790-0. [DOI] [PubMed] [Google Scholar]
- 44.Tran N.H., Li Y., Reinhard M., He Y., Gin K.Y.H. A sensitive and accurate method for simultaneous analysis of algal toxins in freshwater using UPLC-MS/MS and 15N-microcystins as isotopically labelled internal standards. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.139727. [DOI] [PubMed] [Google Scholar]
- 45.Van der Westhuizen A.J., Eloff J.N. Toxin extraction from the blue-green alga Microcystis aeruginosa by different extraction media. J. Lim. Soc. SA. 1982;8:76–77. [Google Scholar]
- 46.Schenzel J., Schwarzenbach R.P., Bucheli T.D. Multi-residue screening method to quantify mycotoxins in aqueous environmental samples. J. Agric. Food Chem. 2010;58:11207–11217. doi: 10.1021/jf102737q. [DOI] [PubMed] [Google Scholar]
- 47.Lajeunesse A., Segura P.A., Gelinas M., Hudon C., Thomas K., Quilliam M.A., Gagnon C. Detection and confirmation of saxitoxin analogues in freshwater benthic Lyngbya wollei algae collected in the St. Lawrence River (Canada) by liquid-chromatography–tandem mass spectrometry. J. Chromatogr. A. 2012;1219:93–103. doi: 10.1016/j.chroma.2011.10.092. [DOI] [PubMed] [Google Scholar]
- 48.Van Hassel W.H.R., Ahn A.C., Huybrechts B., Masquelier J., Wilmotte A., Andjelkovic M. LC-MS/MS Validation and quantification of cyanotoxins in algal food supplements from the Belgium market and their molecular origins. Toxins. 2022;14:513. doi: 10.3390/toxins14080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed Z.A., Shehri M.A. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard Mater. 2009;172:310–315. doi: 10.1016/j.jhazmat.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Tian D.W., Zheng X., Wei X., Sun L., Liu X., Chen H., Zhang Y., Zhou H., Chen H., Wang Z.X. Dissolved microcystins in surface and ground waters in regions with high cancer incidence in the Huai River Basin of China. Chemosphere. 2013;91:1064–1071. doi: 10.1016/j.chemosphere.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 51.Beltran E., Ibanez M., Sancho J.V., Hernandez F. Determination of six microcystins and nodularin in surface and drinking waters by on-line solid phase extraction–ultra high-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2012;1266:61–68. doi: 10.1016/j.chroma.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 52.Romera-Garcia E., Helmus R., Ballesteros-Gomez A., Visser P.M. Multi-class determination of intracellular and extracellular cyanotoxins in freshwater samples by ultra-high-performance liquid chromatography coupled to high-resolution mass spectrometry. Chemosphere. 2021;274 doi: 10.1016/j.chemosphere.2021.129770. [DOI] [PubMed] [Google Scholar]
- 53.Chen W., Jia Y., Li E., Zhao S., Zhou Q., Liu L., Song L. Soil-based treatments of mechanically collected cyanobacterial blooms from Lake Taihu: efficiencies and potential risks. Environ. Sci. Technol. 2012;46:13370–13376. doi: 10.1021/es3027902. [DOI] [PubMed] [Google Scholar]
- 54.Drobac D., Tokodi N., Kiprovski B., Malencic D., Vazic T., Nybom S., Meriluoto J., Svircev Z. Microcystin accumulation and potential effects on antioxidant capacity of leaves and fruits of Capsicum annuum. J. Toxicol. Environ. Health. 2017;80:145–154. doi: 10.1080/15287394.2016.1259527. [DOI] [PubMed] [Google Scholar]
- 55.Machado J., Azevedo J., Freitas M., Pinto E., Almeida A., Vasconcelos V., Campos A. Analysis of the use of microcystin-contaminated water in the growth and nutritional quality of the root-vegetable, Daucus carota. Environ. Sci. Pollut. Res. 2017;24:752–764. doi: 10.1007/s11356-016-7822-7. [DOI] [PubMed] [Google Scholar]
- 56.Trung B., Dao T.S., Faassen E., Lurling M. Cyanobacterial blooms and microcystins in southern vietnam. Toxins. 2018;10:471. doi: 10.3390/toxins10110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Keeffe J. 2019. Cyanobacteria - Tapping into the Risks to Drinking Water.https://ncceh.ca/content/blog/cyanobacteria-tapping-risks-drinking-water Sep. 2022. [Google Scholar]
- 58.Sklenar K., Westrick J., Szlag D. AWWARF; Denver, CO, USA: 2016. Managing Cyanotoxins in Drinking Water: A Technical Guidance Manual for Drinking Water Professionals. [Google Scholar]
- 59.Spoof L., Jaakkola S., Vazic T., Haggqvist K., Kirkkala T., Ventela A.M., Kirkkala T., Svircev Z., Meriluoto J. Elimination of cyanobacteria and microcystins in irrigation water—effects of hydrogen peroxide treatment. Environ. Sci. Pollut. Res. 2020;27:8638–8652. doi: 10.1007/s11356-019-07476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morais J., Augusto M., Carvalho A.P., Vale M., Vasconcelos V.M. Cyanobacteria hepatotoxins, microcystins: bioavailability in contaminated mussels exposed to different environmental conditions. Eur. Food Res. Technol. 2008;227:949–952. [Google Scholar]
- 61.Yang Z., Kong F., Zhang M. Groundwater contamination by microcystin from toxic cyanobacteria blooms in Lake Chaohu, China. Environ. Monit. Assess. 2016;188:280. doi: 10.1007/s10661-016-5289-0. [DOI] [PubMed] [Google Scholar]
- 62.Chatziefthimiou A.D., Metcalf J.S., Glover W.B., Banack S.A., Dargham S.R., Richer R.A. Cyanobacteria and cyanotoxins are present in drinking water impoundments and groundwater wells in desert environments. Toxicon. 2016;114:75–84. doi: 10.1016/j.toxicon.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Bouaicha N., Corbel S. Cyanobacterial toxins emerging contaminants in soils: a review of sources, fate and impacts on ecosystems, plants and animal and human health. InTech Rijeka. 2016;21:105–126. [Google Scholar]
- 64.Abesh B.F., Liu G., Vazquez-Ortega A., Gomezdelcampo E., Roberts S. Cyanotoxin transport from surface water to groundwater: simulation scenarios for Lake Erie. J. Great Lakes Res. 2022;48:695–706. [Google Scholar]
- 65.Shibambu C.S., Gumbo J.R. Assessing the water quality improvement of sewage effluent passage in a natural wetland: a case study of Makhado oxidation ponds. SETWM-20. 2020;18:115–120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.