Abstract
Thirty new Bdellovibrio strains were isolated from an agricultural soil and from the rhizosphere of plants grown in that soil. Using a combined molecular and culture-based approach, we found that the soil bdellovibrios included subpopulations of organisms that differed from rhizosphere bdellovibrios. Thirteen soil and seven common bean rhizosphere Bdellovibrio strains were isolated when Pseudomonas corrugata was used as prey; seven and two soil strains were isolated when Erwinia carotovora subsp. carotovora and Agrobacterium tumefaciens, respectively, were used as prey; and one tomato rhizosphere strain was isolated when A. tumefaciens was used as prey. In soil and in the rhizosphere, depending on the prey cells used, the concentrations of bdellovibrios were between 3 × 102 to 6 × 103 and 2.8 × 102 to 2.3 × 104 PFU g−1. A prey range analysis of five soil and rhizosphere Bdellovibrio isolates performed with 22 substrate species, most of which were plant-pathogenic and plant growth-enhancing bacteria, revealed unique utilization patterns and differences between closely related prey cells. An approximately 830-bp fragment of the 16S rRNA genes of all of the Bdellovibrio strains used was obtained by PCR amplification by using a Bdellovibrio-specific primer combination. Soil and common bean rhizosphere strains produced two and one restriction patterns for this PCR product, respectively. The 16S rRNA genes of three soil isolates and three root-associated isolates were sequenced. One soil isolate belonged to the Bdellovibrio stolpii-Bdellovibrio starrii clade, while all of the other isolates clustered with Bdellovibrio bacteriovorus and formed two distantly related, heterogeneous groups.
Bdellovibrio spp. are small, very motile gram-negative bacteria that exhibit a unique and obligate requirement for other gram-negative cells, which they invade and use as substrates (22). These organisms were first isolated from soil (25), where they are commonly encountered. They can also be found in freshwater, brackish water, seawater, sewage, water pipes, and water reservoirs (13, 22). It has been shown that marine Bdellovibrio species preferentially associate with surfaces, where they are components of biofilms (13, 31).
The biphasic growth cycle of Bdellovibrio species includes a free-swimming attack phase and an intraperiplasmic growth phase; this growth cycle distinguishes this group of bacteria from all other bacterial parasites of bacteria (29). Three Bdellovibrio species, Bdellovibrio bacteriovorus, Bdellovibrio stolpii, and Bdellovibrio starrii, were described first on the basis of their G+C contents and DNA relative association data (23, 26) and then on the basis of 16S rRNA analysis data (6). Because of the large distances between the species, Baer et al. (2) recently proposed that the genus should be split and the new genus Bacteriovorax should be created and should include two species, Bacteriovorax starrii and Bacteriovorax stolpii.
Although bdellovibrios survive in nature, the activity of these organisms and their influence on bacterial communities are still controversial. The intrinsic ability of bdellovibrios to parasitize and lyse prey cells makes them attractive potential biocontrol agents which could be used against gram-negative root phytopathogens (9, 28). However, until now, there has been no study in which researchers have compared soil and rhizosphere bdellovibrios and examined the activities of these organisms against agriculturally important microbes.
It has been shown that the structure of the bacterial communities found in soil and the structure of the bacterial communities found in the rhizosphere are different (5, 14, 17). Bdellovibrios use members of these communities as substrates and have specific host ranges, and whether bdellovibrio populations in the rhizosphere environment and in soil are different is not known.
In this study, using collection and newly isolated soil and rhizosphere bdellovibrios in conjunction with molecular and culture-based techniques, we found that coexisting Bdellovibrio soil subpopulations and rhizosphere bdellovibrio populations are different.
MATERIALS AND METHODS
Bacterial strains, media, isolation procedure, and maintenance.
The Bdellovibrio and prey strains used are shown in Tables 1 and 2, respectively; the origins of the strains are also shown. Strains whose designations begin with SRP, SRA, and SRE, strains whose designations begin with BRP, and strains whose designations begin with BEP were isolated from soil, from the rhizosphere, and from the total root extract of common bean in four, two, and two independent isolation events, respectively. Strain TRA2 was isolated from the total root extract of a tomato plant.
TABLE 1.
Bdellovibrio strains used in this study
| Strain(s) | Host | Origin |
|---|---|---|
| Bdellovibrio strains BEP1, BEP2, BEP3, and BEP4 | P. corrugata PC | Total root extract (this study) |
| Bdellovibrio strains BRP3, BRP4, and BRP6 | P. corrugata PC | Rhizosphere (this study) |
| Bdellovibrio strain TRA2 | A. tumefaciens C58 | Total root extract (this study) |
| Bdellovibrio strains SRA9 and SRA10 | A. tumefaciens C58 | Soil (this study) |
| Bdellovibrio strains SRP1, SRP2, SRP3, SRP4, SRP8E, SRP11, SRP15, SRP16, SRP17, SRP18, SRP19, SRP20, and SRP21 | P. corrugata PC | Soil (this study) |
| Bdellovibrio strains SRE2, SRE3, SRE6, SRE7, SRE8, SRE9, and SRE12 | E. carotovora subsp. carotovora 24 | Soil (this study) |
| B. bacteriovorus W (= ATCC 27047) | E. coli ML35 | Soil (American Type Culture Collection) |
| B. bacteriovorus 109J (= ATCC 43826) | E. coli ML35 | Soil (H. N. Williams, University of Maryland) |
| B. stolpii UKi2 (= ATCC 27052) | E. coli K-12 | Soil (J. J. Tudor, St. Joseph's College, Philadelphia, Pa.) |
TABLE 2.
Bacterial strains used as potential prey
| Strain | Origina |
|---|---|
| Agrobacterium tumefaciens C58 | L. Chernin (The Hebrew University of Jerusalem) |
| Agrobacterium tumefaciens IDI | L. Chernin (The Hebrew University of Jerusalem) |
| Azospirillum brasilense Cd | Laboratory collection |
| Bacillus megaterium ATCC 13632 | Laboratory collection |
| Chromobacterium violaceum ATCC 31532 | L. Chernin (The Hebrew University of Jerusalem) |
| Enterobacter agglomerans | L. Chernin (The Hebrew University of Jerusalem) |
| Erwinia amylovora 1327 | G. Kritzman (The Volcani Center, ARO) |
| Erwinia carotovora subsp. carotovora 24 | G. Kritzman (The Volcani Center, ARO) |
| Erwinia carotovora subsp. carotovora 2 | G. Kritzman (The Volcani Center, ARO) |
| Escherichia coli ML35 | H. N. Williams (University of Maryland) |
| Pseudomonas corrugata PC | G. Kritzman (The Volcani Center, ARO) |
| Pseudomonas maltophila 7NM1 | Laboratory collection |
| Pseudomonas putida WCS 417 | P. Weisbeck (University of Utrecht) |
| Pseudomonas syringae pv. tomato CL | G. Kritzman (The Volcani Center, ARO) |
| Rhizobium cicer 50/70 | Y. Okon (The Hebrew University of Jerusalem) |
| Rhizobium etli CIAT 632 | Y. Okon (The Hebrew University of Jerusalem) |
| Rhizobium tropici CIAT 899 | Y. Okon (The Hebrew University of Jerusalem) |
| Sinorhizobium meliloti Rm41 | E. Kondorosi (ISV, CNRS, Gif sur Yvette, France) |
| Sinorhizobium meliloti AK 631 | E. Kondorosi (ISV, CNRS, Gif sur Yvette, France) |
| Serratia marcescens | I. Chet (The Hebrew University of Jerusalem) |
| Vibrio fluvialis WS1 | Laboratory collection |
| Xanthomonas campestris pv. vesicatoria 75-3 | U. Bonas (Martin Luther University, Halle, Germany) |
ARO, Agricultural Research Organization; ISV, Institut des Sciences Végétales; CNRS, Centre National de la Recherche Scientifique.
All of the strains were isolated by using the method described by Varon and Shilo (30). A sample (soil or roots with adhering rhizosphere soil) was shaken for 30 min and then centrifuged at 800 × g for 5 min at 4°C. The resulting supernatant was centrifuged at 27,000 × g for 20 min at 4°C, which efficiently concentrated the Bdellovibrio cells in the pellet and eliminated bacteriophages. The pellet was dissolved in a few milliliters of diluted nutrient broth (DNB) (a 1:10 dilution of nutrient broth amended with 3 mM MgCl2 · 6H2O and 2 mM CaCl2 · 2H2O [pH 7.2]) and passed through a 1.2-μm-pore-size Nuclepore filter. The filtrate was then serially diluted, 100 μl of each dilution was mixed with 250 to 300 μl of a suspension (containing approximately 5 × 109 CFU/ml) of a potential prey organism in 0.6% molten diluted nutrient agar, and the mixture was spread onto a diluted nutrient bottom agar plate, which was incubated at 30°C. Typical, regular, and growing Bdellovibrio plaques were detected after 3 to 5 days. Random plaques were also examined to determine whether small, rapidly swimming cells were present. Alternatively, the filtration step was replaced by centrifugation for 20 min at 1,620 × g through a linear 1 to 15% Ficoll gradient. The upper 3 ml was plated as described above. Individual Bdellovibrio plaques were purified by three successive platings.
In order to create a lysate, a plug containing one plaque and the surrounding prey was lifted from a plate and resuspended in DNB. At first the prey cells grew; this was followed by development of the Bdellovibrio predator, which resulted in clearing of the suspension.
Bdellovibrios were stored as plaques or lysates for up to 15 days at 4°C. For long-term storage, aliquots of a 25% glycerol suspension of concentrated fresh lysate were frozen in liquid nitrogen and stored at −80°C.
Plant and soil material and rhizosphere extraction.
The soil used was Rehovot sand, which was obtained from the experimental farm of The Hebrew University of Jerusalem and contained 95% sand, 5% silt, and no clay. The organic matter content was 0.9%, the pH was 7.3, and the electrical conductance was 112 μS · cm−1. Common bean (Phaseolus vulgaris cv. Bulgarian) (Gedera Seeds, Gedera, Israel) and tomato (Lycopersicon esculentum cv. M-82) (Gedera Seeds) were grown in 1-kg pots in a greenhouse for 3 and 4 weeks, respectively. The soil used to grow the tomato plants was amended with 30% sphagnum peat. Rhizosphere samples were obtained by gently rinsing the roots with sterile water, leaving the adhering particles and small clumps, and blotting them to remove excess liquid. The roots (5 g) were shaken for 30 min at 200 rpm in 40 ml of 0.1 M phosphate buffer (pH 7.2) at room temperature, and the resulting suspension was treated as described above. To include the endorhizosphere, washed roots were ground with a mortar and pestle in 0.2% polyvinylpolypyrrolidone in sterile distilled water, which yielded the total root bacteria. Bdellovibrios were isolated from this slurry as described above.
Electron microscopy.
One drop of fresh lysate was placed on a microscope grid, and excess liquid was removed by blotting. The sample was then counterstained with a 1% (wt/vol) solution of uranyl acetate for 30 min and examined with a JEOL model 100-CX transmission electron microscope.
Determination of prey range and kinetics of lysis.
Cultures of the prey bacteria tested were grown in nutrient broth until the stationary phase and then were pelleted by centrifugation at 4,400 × g for 10 min at 4°C. Each pellet was washed once with 25 mM HEPES buffer containing 2 mM CaCl2 · 2H2O (pH 7.8) and resuspended in the same buffer. Then the optical density at 570 nm of the suspension was adjusted to 0.55 to 0.6, which corresponded to a concentration of 108 to 5 × 108 cells · ml−1 for most strains, by using a cuvette with a 1-cm light path and a Genesis 5 spectrophotometer (Spectronic, Rochester, N.Y.). Fresh lysates of the bdellovibrios which were going to be tested were passed through a 0.45-μm-pore-size filter in order to remove residual prey and cell debris, centrifuged at 10,000 × g for 15 min at 4°C, and resuspended in HEPES to a final concentration of 106 to 5 × 106 cells · ml−1. The supernatant was filtered through a 0.22-μm-pore-size membrane filter and used as a control. Prey suspensions (150 μl) and predator suspensions (60 μl) were added to 96-well microtiter plates. The controls included Bdellovibrio-free prey suspensions amended with buffer and prey suspensions amended with filtered lysates. The plates were incubated overnight at 30°C on a rotary shaker at 200 rpm. Turbidity (cell density) was determined at 570 nm by using a model MR5000 enzyme-linked immunosorbent assay plate reader (Dynatech, Denkerdorf, Germany). Six replicates of each predator-prey combination were prepared, and each experiment was carried out at least twice.
The kinetics of lysis was monitored by using the same setup; Pseudomonas corrugata was used as the prey. Optical densities were determined every 1.5 h.
Primers and PCR conditions.
A Bdellovibrio-specific oligonucleotide sequence designed to represent almost all bdellovibrios, including representative of all species of this bacterial parasitic genus, was constructed by aligning the 12 Bdellovibrio sequences (two complete sequences and 10 partial sequences) present in databases. Seven sequences exhibited no mismatches, three exhibited mismatches caused by undefined bases (indicated by N), and two exhibited one defined base substitution (11). This conserved sequence was analyzed to determine whether it was specific by using CHECKPROBE software (11, 16), and then it was used as a Bdellovibrio-specific primer (primer 842R; 5′-CGWCACTGAAGGGGTCAA-3′) in conjunction with Bacteria-specific primer 63F (18) in PCR. Primer 799F (5′-GGTAGTCCACGCCGTAAACGATG-3′), which was designed on the basis of partial Bdellovibrio sequences, is not specific for this group and was used in PCR along with primer 1492R (1).
Primers 176F (5′-GTGGCTTCAAACGGAGTGGA-3′) and 887R (5′-ACGACTGTGAACGGCAACG-3′), which are specific for the hit locus, were designed based on a previously published nucleotide sequence (4). hit is a genetic locus in B. bacteriovorus 109J which appears to be involved in the ability of this strain to form axenic mutants (4).
The templates used for PCR were either 1 or 10 ng of DNA, purified as described by Tsai and Olson (27), or individual plaques. In the latter case, plaques were resuspended in 100 μl of sterile double-distilled water and vortexed at a high speed. The liquid phase was transferred to a new tube and subjected to three cycles of freezing in liquid nitrogen followed by 3 min of heating in boiling water. The tubes were then cooled on ice, and 10% dimethyl sulfoxide was added.
PCR (50-μl mixtures) were performed by using a programmable cyclic reactor (Ericomp Inc., San Diego, Calif.) or a Mastercycler gradient (Eppendorff, Hamburg, Germany). Each reaction mixture contained 3 mM MgCl2, each deoxyribonucleoside triphosphate at a concentration of 20 μM, 1× reaction buffer, 1.25 U of Taq polymerase, and each primer at a concentration of 1 μM. When the Ericomp cycler was used, the samples were covered with 2 drops of mineral oil (Sigma Chemical Co., St. Louis, Mo.). Amplification was started by using a denaturation cycle consisting of 4 min at 94°C, 50°C for 1 min, and 72°C for 1 min, and this was followed by 34 cycles consisting of 94°C for 1 min, 50°C for 1 min, and 72°C for 1 min and a final elongation step consisting of 72°C for 5 min. Most of the steps were only 45 s long when the Mastercycler gradient was used; the only exceptions were the first denaturation step and the last elongation step.
Restriction analysis.
Products obtained from PCR performed with primers 63F and 842R were purified by using a High Pure PCR purification kit (Roche Molecular Biochemicals, Mannheim, Germany) and then were enzymatically digested by using BamHI, EcoRI, SacII, and XbaI as recommended by the manufacturers. Digests were electrophoresed in 1% agarose gels and stained with ethidium bromide. A computer-assisted restriction analysis of sequences obtained from databases and in this study was performed by using the GCG package (Genetics Computer Group, Madison, Wis.).
DNA sequencing and sequence analysis.
Products obtained from PCR performed with primers 63F and 842R or with primers 799F and 1492R (1) were purified as described above. A Big Dye terminator kit (Perkin-Elmer Inc., Branchburg, N.J.) was used in conjunction with these primer pairs to sequence the 16S ribosomal DNA (rDNA) fragments. The sequences were determined by using a model Prism 377 DNA sequencer (Applied Biosystem Inc., Foster City, Calif.).
Phylogenetic analysis.
16S rDNA sequence analyses were performed by using the program package ARB (http://www.biol.chemie.tu-muenchen.de/pub/ARB/). Sequences were aligned by using the implemented ARB automated alignment tool, and the alignment was refined manually by visual inspection and by secondary-structure analysis. Phylogenetic analyses were performed by using ARB parsimony, distance matrix, and maximum-likelihood methods. To determine the robustness of phylogenetic trees, analyses were performed both with the original data set and with a data set from which highly variable positions were removed by using a 50% conservation filter for members of the genus Bdellovibrio in order to reduce potential tree artifacts that may have resulted from multiple base changes. The consistency of the phylogenic tree was also verified by bootstrapping (n = 100).
Southern blot analysis.
A Southern blot analysis was performed by using total DNA from strains SRP1, BEP2, BRP4, TRA2, and 109J after enzymatic digestion with SauIIIA and with a hit probe obtained by PCR digoxigenin labelling (Roche Molecular Biochemicals, Mannheim, Germany). Prehybridization was performed at 68°C, and hybridization was performed at 60°C. Detection was performed by using CSPD as recommended by the manufacturer (Roche Molecular Biochemicals, Mannheim, Germany) after low-stringency washing (0.5 × SSC, 60°C) and high-stringency washing (0.1 × SSC, 68°C) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Nucleotide sequence accession numbers.
Sequences obtained in this study have been deposited in the GenBank database under accession no. AF148938, AF148939, AF148940, and AF148941.
RESULTS
Isolation and quantification of soil and root-associated bdellovibrios.
Thirty strains were isolated from soil and from the root systems of common bean and tomato plants. The isolation schemes used resulted in efficient separation of bdellovibrios and other plaque-forming organisms, such as bacteriophages and bacterium-consuming protozoans. Microscopic observations and the presence of very active, swimming, small, usually vibrioid cells established that lysates obtained from all of the plaques tested resulted from bdellovibrio activity. Nine soil strains isolated by using P. corrugata PC as prey (strains SRP8E to SRP21 [Table 2]), three soil strains isolated by using Erwinia carotovora subsp. carotovora 24 as prey (strains SRE8, SRE9, and SRE12), and two soil strains isolated by using Agrobacterium tumefaciens C58 as prey (strains SRA9 and SRA10), all of which originated from the same isolation event, were tested to determine whether they exhibited activity with each of these three substrate organisms in liquid culture. While the SRP and SRE isolates induced lysis of both P. corrugata PC and E. carotovora subsp. carotovora 24, they did not utilize A. tumefaciens C58 as prey. The SRA isolates were inactive when strain PC or strain 24 was used as a substrate. Moreover, strains SPR1 to SPR4 did not prey on E. carotovora subsp. carotovora. Table 3 shows the numbers of Bdellovibrio plaques retrieved from soil, from the rhizosphere, and from a total extract of washed roots of common bean.
TABLE 3.
Quantification of Bdellovibrio plaques retrieved from soil, from the rhizosphere, and from total extracts of washed rootsa
| Origin | Substrate cells | 103 PFU · g−1b | Total bacterial counts on nutrient agar (CFU · g−1)b |
|---|---|---|---|
| Soil | P. corrugata PC | 5.2 ± 0.85 | 2.3 × 107 |
| Soil | E. carotovora subsp. carotovora 24 | 5.8 ± 0.52 | 2.3 × 107 |
| Soil | A. tumefaciens C58 | 0.3 ± 0.09 | 2.3 × 107 |
| Rhizosphere of common bean | P. corrugata PC | 23 ± 1 | 1 × 108 |
| Extract of washed roots of common beanc | P. corrugata PC | 0.28 ± 0.05 | 5 × 107 |
Representative results of at least two experiments.
PFU and CFU are expressed per gram (dry weight) of soil and per gram (fresh weight) of roots.
Roots were thoroughly shaken and washed prior to grinding.
Determination of prey range and kinetics of prey lysis.
Prey cell lysis data were validated by performing experiments in which the changes in the optical densities of mixed suspensions containing P. corrugata PC or Escherichia coli ML35 and strain SRP1 were determined both in microtiter plates and in Erlenmeyer flasks. The cultures behaved similarly; P. corrugata PC was cleared within 30 h, while E. coli ML35 was resistant to the predator (data not shown). The bdellovibrios examined (strains BEP2, BRP4, and SRP1, which were isolated from a common bean rhizosphere, a total root extract, and soil, respectively, by using P. corrugata; TRA2, which originated from tomato roots when A. tumefaciens was used as prey; and collection strain 109J) lysed a wide range of selected gram-negative bacteria, including both bacteria that enhance plant growth and phytopathogens (Table 4). In some instances, increases in optical density were observed in control wells that were not inoculated with bdellovibrios or in prey suspensions that were not attacked by the predator; these increases may have been due to utilization of capsule or storage materials. Controls, which were prepared by using cell-free lysate of each of the Bdellovibrio strains tested and each type of prey, were always negative; i.e., lysis did not occur. Strains BEP2 and BRP4 exhibited the same prey range and grew on 9 of the 22 potential prey species tested, while SRP1 and TRA2 grew on six different prey species. The most versatile predator was strain 109J, which grew at the expense of 10 of the 22 prey cell suspensions tested. None of the substrate cell suspensions tested supported growth of all five Bdellovibrio strains, while Azospirillum brasilense, Vibrio fluvialis, E. carotovora subsp. carotovora 2, and the gram-positive organism Bacillus megaterium were resistant to attack by each of the predators (Table 4). Large differences were observed in this lysis fingerprinting analysis, even when closely related substrate strains were used; while E. carotovora subsp. carotovora 24 was preyed upon by strains BEP2, BRP4, and 109J, E. carotovora subsp. carotovora 2 was not. Similarly, the Bdellovibrio strains exhibited particular abilities to grow at the expense of various rhizobia and fluorescent pseudomonads. A. tumefaciens C58 was resistant to all of the bdellovibrios except TRA2, but A. tumefaciens IDI was lysed by bdellovibrio strains BEP2 and BRP4. Although strain TRA2 produced relatively large plaques on A. tumefaciens C58, it was not able to lyse cell suspensions of this bacterium in HEPES and only partially lysed such suspensions in DNB. However, efficient lysis was observed when Sinorhizobium meliloti (which is phylogenetically closely related to A. tumefaciens) was used as prey in HEPES preparations. This bacterium, as well as Rhizobium cicer and Xanthomonas campestris pv. vesicatoria, produces copious amounts of extracellular material; however, as previously reported, this material did not protect the cells against Bdellovibrio attack (15).
TABLE 4.
Prey ranges of Bdellovibrio strains BEP2, BRP4, SRP1, TRA2, and 109J
| Prey | Prey range of:
|
||||
|---|---|---|---|---|---|
| BEP2 | BRP4 | SRP1 | TRA2 | 109J | |
| Agrobacterium tumefaciens C58a | − | − | − | +b | − |
| Agrobacterium tumefaciens IDIa | + | + | − | −c | − |
| Azospirillum brasilensed | − | − | − | − | − |
| Bacillus megaterium | − | − | − | − | − |
| Chromobacterium violaceumd | + | + | + | − | + |
| Enterobacter agglomeransd | + | + | − | + | + |
| Erwinia amylovoraa | + | + | − | − | − |
| Erwinia carotovora subsp. carotovora 24a | + | + | − | − | + |
| Erwinia carotovora subsp. carotovora 2a | − | − | − | − | − |
| Escherichia coli | + | + | − | − | + |
| Pseudomonas corrugataa | + | + | + | − | + |
| Pseudomonas maltophila | − | − | − | − | + |
| Pseudomonas putidad | − | − | + | − | + |
| Pseudomonas syringae pv. tomatoa | + | + | + | − | + |
| Rhizobium cicerd | − | − | + | − | + |
| Rhizobium etlid | − | − | − | + | − |
| Rhizobium tropicid | − | − | − | + | − |
| Sinorhizobium meliloti Rm41d | − | − | − | + | − |
| Sinorhizobium meliloti AK 631d | − | − | − | + | − |
| Serratia marcescensd | − | − | − | − | + |
| Vibrio fluvialis | − | − | − | − | − |
| Xanthomonas campestris pv. vesicatoriaa | + | + | + | − | − |
Phytopathogen.
Determined in DNB.
−, no lysis occurred in HEPES and not determined in DNB.
Plant growth-enhancing bacterium.
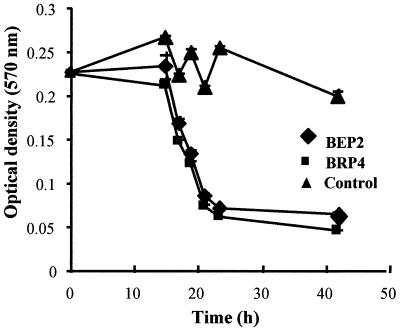
We determined the kinetics of lysis of P. corrugata by strains BEP2 and BRP4. As shown in Fig. 1, these two strains behaved similarly. They also had the same prey range, were identical in size, and were placed in the same ribotype (see below). When growing at the expense of P. corrugata PC cells in agar plates, strains BEP2 and BRP4 developed plaques more slowly than strain SRP1; when these strains were used, the lysis zones were up to 1 mm in diameter after 2.5 days, while the SPR1 plaques were 2.5 to 4 mm in diameter. However, we observed no differences in the kinetics of lysis for the strains in microtiter plates (data not shown).
FIG. 1.
Kinetics of lysis of P. corrugata by strains BEP2 and BRP4. The control contained prey cells without bdellovibrios. Bars indicate standard errors.
Electron microscopy.
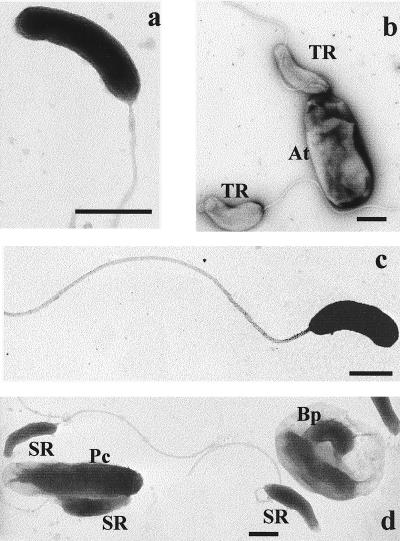
Transmission electron microscopy of negatively stained preparations of attack cells was used to measure the sizes of isolate BEP2, BRP4, SRP1, and TRA2 cells. As shown in Fig. 2, BEP2 and BRP4 cells were the smallest and the same size (0.85 by 0.2 μm), and SRP1 and TRA2 cells were larger (1 by 0.25 and 1.15 by 0.4 μm, respectively). A Bdellovibrio-containing bdelloplast is evident in Fig. 2d.
FIG. 2.
Electron micrographs of Bdellovibrio isolates. (a) BEP2 attack-phase cell. (b) TRA2 attack-phase cells (TR), near an A. tumefaciens substrate cell (At). (c) BRP4 attack-phase cell. (d) SRP1 attack-phase cells (SR). Pc, P. corrugata prey cell; Bp, bdelloplast. Bars = 0.5 μm.
Restriction enzyme profiles of 16S rDNA sequences amplified with a Bdellovibrio-specific primer pair.
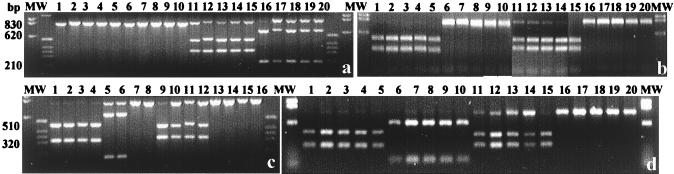
A PCR product was obtained with all of the isolates, as well as with the collection strains, when we used primers 63F and 842R and Bdellovibrio DNA or individual plaques as templates. No products were obtained when plugs of prey cell lawns or prey cell DNA was used as the template (data not shown). The PCR products of soil isolates yielded two restriction patterns, one identical to the restriction pattern of B. stolpii UKi2 (strains SRP 1 to SRP4 [Fig. 3a]) and one that was represented by 18 other SRP, SRE, and SRA strains (five of which are shown in Fig. 3b) and was identical to the restriction pattern of collection strain B. bacteriovorus 109J. Most of the rhizosphere strains produced a third pattern, which was identical to the restriction pattern of the cyst-forming organism B. bacteriovorus W (Fig. 3c and d); the only exception was strain TRA2, whose pattern was like that of B. bacteriovorus 109J.
FIG. 3.
Restriction digestion of PCR-amplified 16S rDNA fragments of soil and rhizosphere Bdellovibrio strains obtained by using Bacteria-specific primer 63F and Bdellovibrio-specific primer 842R. (a) Soil strains SRP1, SRP2, SRP3, SRP4, and UKi2. Lanes 1 through 5, BamHI; lanes 6 through 10, EcoRI; lanes 11 through 15, SacII; lanes 16 through 20, XbaI. (b) Soil strains SRE6, SRE7, SRE8, SRE9, and SRE12. Lanes 1 through 5, BamHI; lanes 6 through 10, EcoRI; lanes 11 through 15, SacII; lanes 16 through 20, XbaI. (c) Rhizosphere strains BEP2, BRP4, TRA2, and soil strain 109J. Lanes 1 through 4, BamHI; lanes 5 through 8, EcoRI; lanes 9 through 12, SacII; lanes 13 through 16, XbaI. (d) Rhizosphere strains BEP1, BEP3, BEP4, BRP3, and W. Lanes 1 through 5, BamHI; lanes 6 through 10, EcoRI; lanes 11 through 15, SacII; lanes 16 through 20, XbaI. Lanes MW contained the molecular weight marker.
Sequence analysis and phylogenetic tree.
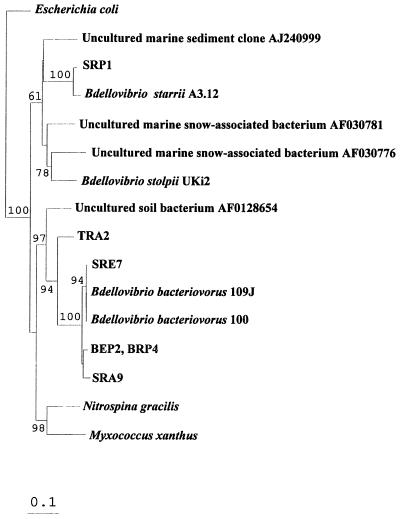
The almost complete 16S rRNA sequences of four Bdellovibrio strains are available from databases; these sequences include two very recently completed collection strain sequences (2). We also determined that sequences of two clones obtained from marine snow, one clone that originated from cold sediments, and an unidentified soil bacterium exhibited homology to the 16S rRNA gene of Bdellovibrio spp. Only short fragments, mostly of poor quality, were available for the other strains. Therefore, we decided to use only the four long previously described Bdellovibrio sequences and the data obtained in this study to construct a reliable phylogenetic tree based on the almost complete 16S rRNA gene sequence. Our phylogenetic analysis confirmed that the bdellovibrios are members of the δ subclass of the class Proteobacteria (32) and that these organisms are related to Myxococcus xanthus (6) and Nitrospina gracilis. Strains BEP2 and BRP4 were found to be identical. Along with soil strains SAR9 and SRE7 and the unidentified soil bacterial sequence whose accession number is AF0128654, they clustered with B. bacteriovorus 109J (Fig. 4). Although isolate TRA2 belonged to the B. bacteriovorus group, it was more distantly related, exhibiting 92.4% identity with strain 109J. This bacterium and type strain 100 are almost identical; they differ only in one substitution and in a small number of undefined bases. Isolate SRP1 was found to be very similar to B. starrii; the sequences of these organisms exhibited 99.2% identity. Along with two sequences of uncultured bacteria that originated from marine snow in the Adriatic Sea and one uncultured clone obtained from marine sediment, the B. stolpii-B. starrii cluster formed a rather heterologous group, which was distantly related to B. bacteriovorus. Our sequence analysis confirmed that B. stolpii, B. starrii, and SRP1 produce the same restriction profile. Restriction analysis of the sequenced isolates clustering with B. bacteriovorus was also confirmed.
FIG. 4.
16S rRNA-based phylogenetic tree for Bdellovibrio isolates and previously published sequences. The tree was produced by using the neighbor-joining algorithm and sequence positions at which the residues in at least 50% of all available complete or almost complete 16S rRNA sequences from members of this subgroup were identical. The orders of branching were similar for all of the treeing approaches used (see Materials and Methods). Bar = estimated 10% sequence divergence. The bootstrap values at the nodes are percentages based on 100 iterations; only values greater than 50% are shown.
PCR and Southern analysis of the hit locus.
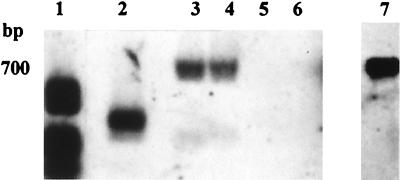
hit is a genetic locus in B. bacteriovorus 109J which appears to be involved in the ability of this organism to form axenic mutants (4). No PCR product was obtained from strain BEP2, BRP4, SRP1, or TRA2 under the various amplification conditions tested (different temperatures and Mg concentrations) when we used hit-specific primers, while an 810-bp fragment was amplified from B. bacteriovorus 109J and was sequenced in order to confirm its identity. The hit locus was then subjected to Southern analysis by using strains SRP1, BEP2, BRP4, and TRA2. No signal was obtained with any of these strains after hybridization with a hit-derived probe when the blot was washed under high-stringency conditions. Under lower-stringency conditions, signals that were clearly different from the 109J signal were obtained with strains BEP2 and BRP4 (Fig. 5).
FIG. 5.
Southern analysis of the hit locus following SauIIIA restriction of total DNA and hybridization with a digoxigenin-labelled hit probe obtained from B. bacteriovorus 109J. Lane 1, molecular weight markers; lane 2, strain 109J; lane 3, strain BEP2; lane 4, strain BRP4; lane 5, strain SRP1; lane 6, strain TRA2; lane 7, homologous probe.
DISCUSSION
New Bdellovibrio strains isolated from an agricultural soil, from plant roots grown in the same soil, and from various sources were examined. Based on restriction, phylogenetic, and prey range analyses, we found that the root bdellovibrios and the rhizosphere bdellovibrios were different. Moreover, we found that various populations coexisted in the soil at different levels.
The bdellovibrios accounted for only a small fraction of the total bacterial population in an agricultural soil and in the rhizosphere of common bean, and the numbers of Bdellovibrio plaques per gram of sample were similar to values reported previously (29). The bdellovibrios are a genetically heterologous group of bacteria that must invade bacterial hosts to survive (22). Therefore, they can be isolated and grown only in two-member cultures with selected prey organisms. This characteristic makes estimating Bdellovibrio population sizes and isolating large numbers of isolates difficult. We obtained a PCR fragment from all of the strains isolated in this study and from collection strains by using a Bdellovibrio-specific oligonucleotide (11) and primer 63F, which targets the domain Bacteria (18). Restriction analysis of the products revealed that the soil isolates belonged to two different ribotypes, one represented by B. stolpii UKi2 and the other represented by B. bacteriovorus 109J; SRP strains (isolated by using P. corrugata cells) belong to both ribotypes. Almost all of the rhizosphere bdellovibrios belonged to a different ribotype identical to the ribotype of B. bacteriovorus W.
Members of a group of strains that were isolated on and preyed on both P. corrugata and E. carotovora subsp. carotovora 24 and members of another group of strains that were isolated on A. tumefaciens C58 produced the same restriction pattern but could not utilize the prey used to isolate members of the other group. A phylogenetic analysis of isolates SRE7 and SRA9, which belonged to the former group and the latter group, respectively, revealed that these organisms clustered with B. bacteriovorus but were different from each other. Also, the number of bdellovibrios that were active on A. tumefaciens C58 and were retrieved from the soil was significantly lower than the number of bdellovibrios that were able to grow at the expense of P. corrugata PC or E. carotovora subsp. carotovora 24. These findings (prey range, ribotype, phylogenetic affiliation, and level in soil samples) together suggest that the soil strains which we isolated belonged to at least three distinct populations.
Strains BEP2 and BRP4, which were analyzed further, were indistinguishable; their 16S rDNA sequences, their low levels of homology to the hit locus, their growth kinetics in liquid and solid media, and their prey ranges were identical. Since they originated from the same root system, they probably were clones of the same population.
The heterogeneity of the genus Bdellovibrio is reflected by the distance between the B. stolpii-B. starrii group and the B. bacteriovorus groups (Fig. 5), as well as the distances within these groups (6; this study). On the basis of the great phylogenetic distances between Bdellovibrio species, Baer et al. (2) recently proposed that the new genus Bdellovorax should be formed and should include B. stolpii and B. starrii. The results presented in this study support this proposal.
Both the B. bacteriovorus branch and the B. stolpii-B. starrii branch contain distantly related organisms, based on sequences, such as isolate TRA2 (which is 92.4% identical to the strain 109J sequence). We also detected one uncultured soil bacterium and three uncultured marine clones which clustered with B. bacteriovorus and B. stolpii, respectively, and increased the heterogeneity in these groups. However, whether these uncultured bacteria are true bacterial parasites is not known.
The 959-bp hit locus, which is associated with the host-independent phenotype in strain 109J (4), is the only genetic locus that has been defined in members of the genus Bdellovibrio. No PCR product was obtained with strains BEP2, BRP4, SRP1, and TRA2 under any of the amplification conditions tested when hit-specific primers were used. The results of Southern analysis suggest that a heterologous, cross-hybridizing sequence is present in strains BEP2 and BRP4, which are closely related to strain 109J, but not in TRA2, a more distantly related strain in the B. bacteriovorus cluster, or in SRP1, which is related to B. stolpii UKi2. Since axenic mutants can be obtained readily from isolated bdellovibrios (24), there may be divergent but functionally equivalent loci in other strains. Therefore, this tool may become a useful tool for characterization of bdellovibrios. Phylogenetic affiliations cannot be inferred on the basis of prey range, which also depends on the experimental conditions used (26). However, such information still provides a useful tool for characterizing and distinguishing between bdellovibrios obtained from a particular environment and for assessing the impact of these bacterial predators on microbial communities. In this study, common bean rhizosphere isolates BEP2 and BRP4 and the two groups of soil SRP strains exhibited different prey spectra, in spite of the fact that they were originally isolated with the same substrate cells. Also, strain TRA2, which was isolated by using A. tumefaciens as prey, utilized five of the seven members of the family Rhizobiaceae tested and differed substantially from all of the other isolates. The biological basis for prey specificity is not known, although it has been suggested that the R antigen is involved (29). However, Rhizobium etli and S. meliloti differ markedly in this respect (12), although both of these organisms are lysed by strain TRA2. Moreover, the rough mutant AK 631 and the smooth wild-type strain S. meliloti Rm41 were also attacked by strain TRA2, in spite of the fact that this kind of phenotype may result from a change in the molecular structure of the R antigen (3).
Soil strain SRP1 and rhizosphere strains BEP2 and BRP4 behaved differently; while the former grew faster, forming larger plaques in double-agar plates, and swam freely in wet mounts, the latter grew more slowly and adhered to the glass. It has been shown that bdellovibrios are components of biofilms (13, 31) and exhibit different tendencies to adhere to glass (21), which may reflect adaptation to specific biotas. In a recent study, Markelova and Kerzhentsev showed that a rhizosphere Bdellovibrio isolate adhered to a plastic surface and completed its growth cycle when it was presented with adsorbed prey cells (19).
Bacterial rhizosphere and soil populations differ in relative composition or in structure, as recently shown by a denaturing gradient gel electrophoresis analysis of samples obtained from both microenvironments (7, 14). Rice et al. (20) showed that marine bdellovibrios obtained from various biotas are able to attack up to 85% of isolated autochthonous gram-negative bacteria. Therefore, a shift in bacterial composition from one biota to another, such as the rhizosphere to bulk soil, may provide an advantage to a subpopulation of bdellovibrios best adapted to use the potential prey found in the root microhabitat. This may reflect adaptations to different niches. Moreover, when cabbage was inoculated with fluorescent pseudomonads, an increase in the number of recoverable rhizosphere bdellovibrios able to use this group of bacteria was detected (8). The possibility that the root-associated bdellovibrios examined in this study originated from the seeds and developed along with the root bacterial populations cannot be ruled out as members of the genus Bdellovibrio have been shown to survive in dry soil for extended periods (10), probably in association with soil particles. However, in view of the lengthy storage the seeds used and no previous reports of seed colonization by bdellovibrios, we assumed that the root-associated bdellovibrios originated in the soil and that the bacterial substrates present were a determining factor for selecting rhizosphere-competent bdellovibrios.
ACKNOWLEDGMENTS
We thank R. Guvrin for technical assistance. We also thank Y. Okon and the participants in our weekly discussion group for their helpful remarks and suggestions.
This work was supported by grants from the Israeli Ministry of Agriculture (grant 823-0138-98) and the Israel Science Foundation (grant 132/99).
REFERENCES
- 1.Amann R, Snaidr J, Wagner M, Ludwig W, Schleifer K H. In situ visualization of high genetic diversity in a natural microbial community. J Bacteriol. 1996;178:3496–3500. doi: 10.1128/jb.178.12.3496-3500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer M L, Ravel J, Chun J, Hill R T, Williams W N. A proposal for the reclassification of Bdellovibrio stolpii and Bdellovibrio starrii into a new genus, Bacteriovorax stolpii comb. nov. and Bacteriovorax starrii comb. nov., respectively. Int J Syst Evol Microbiol. 2000;50:219–224. doi: 10.1099/00207713-50-1-219. [DOI] [PubMed] [Google Scholar]
- 3.Carlson R W, Rheus B, Chen T B, Bhat U R, Noel K D. Lipopolysaccharide core structures in Rhizobium etli and mutants deficient in O antigen. J Biol Chem. 1995;270:11783–11788. doi: 10.1074/jbc.270.20.11783. [DOI] [PubMed] [Google Scholar]
- 4.Cotter T W, Thomashow M F. Identification of a Bdellovibrio bacteriovorus genetic locus, hit, associated with the host-independent phenotype. J Bacteriol. 1992;174:6018–6024. doi: 10.1128/jb.174.19.6018-6024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curl E A, Truelove B. The rhizosphere. Berlin, Germany: Springer-Verlag; 1986. [Google Scholar]
- 6.Donze D, Mayo J A, Diedrich D L. Relationship among bdellovibrios revealed by partial sequences of 16S ribosomal RNA. Curr Microbiol. 1991;23:115–119. [Google Scholar]
- 7.Duineveld B M, Rosado A S, van Elsas J D, van Veen J A. Analysis of the dynamics of bacterial communities in the rhizosphere of the chrysanthemum via denaturating gradient gel electrophoresis and substrate utilization pattern. Appl Environ Microbiol. 1998;64:4950–4957. doi: 10.1128/aem.64.12.4950-4957.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsharif M, Grosman F. Role of biotic factors in the control of soil-borne fungi by fluorescent pseudomonads. Microbiol Res. 1996;151:351–357. [Google Scholar]
- 9.Epton H A S, Walker N M, Sigee D C. Bdellovibrio: a potential control agent for soft rot and black leg of potato. In: Klement Z, editor. Plant pathogenic bacteria. Budapest, Hungary: Akademia Kiado; 1989. pp. 207–212. [Google Scholar]
- 10.Germinda J J. Isolation of Bdellovibrio spp. that prey on Azospirillum brasilense in soil. Can J Microbiol. 1987;33:459–461. [Google Scholar]
- 11.Jurkevitch E, Ramati B. Design and uses of a Bdellovibrio 16S rRNA-targeted oligonucleotide. FEMS Microbiol Lett. 2000;184:265–271. doi: 10.1111/j.1574-6968.2000.tb09025.x. [DOI] [PubMed] [Google Scholar]
- 12.Kannenberg E L, Reuhs B L, Forsberg L S, Carlson R W. Lipopolysaccharides and K-antigens: their structures, biosynthesis and functions. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 119–154. [Google Scholar]
- 13.Kelley J L, Turng B F, Williams H N, Baier M L. Effect of temperature, salinity, and substrate on the colonization of surfaces in situ by aquatic bdellovibrios. Appl Environ Microbiol. 1997;63:84–90. doi: 10.1128/aem.63.1.84-90.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J-S, Sakai M, Hosoda A, Matsuguchi T. Application of DGGE analysis to the study of bacterial community structure in plant roots and in nonrhizosphere soil. Soil Sci Plant Nutr. 1999;45:493–497. [Google Scholar]
- 15.Koval S F, Bayer M E. Bacterial capsules: no barrier against bdellovibrio. Microbiology. 1997;143:749–753. doi: 10.1099/00221287-143-3-749. [DOI] [PubMed] [Google Scholar]
- 16.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maloney P E, van Bruggen A H C, Hu S. Bacterial community structure in relation to the carbon environment in lettuce and tomato rhizospheres and in bulk soil. Microb Ecol. 1997;34:109–117. doi: 10.1007/s002489900040. [DOI] [PubMed] [Google Scholar]
- 18.Marchesi J R, Sato T, Weightman A J, Martin T A, Fry J C, Hiom S J, Wade W G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markelova N Y, Kerzhentsev A S. Isolation of a new strain of the genus Bdellovibrio from plant rhizosphere and its lytic spectrum. Microbiology (Engl Trans Mikrobiologiya) 1998;67:837–841. [Google Scholar]
- 20.Rice T D, Williams H N, Tung B F. Susceptibility of bacteria in estuarine environments to autochthonous bdellovibrios. Microb Ecol. 1998;35:256–264. doi: 10.1007/s002489900081. [DOI] [PubMed] [Google Scholar]
- 21.Rittenberg S C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972;109:432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruby E G. The genus Bdellovibrio. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer Verlag; 1991. pp. 3400–3415. [Google Scholar]
- 23.Seidler R J, Mandel M, Baptist J N. Molecular heterogeneity of the bdellovibrios: evidence of two new species. J Bacteriol. 1972;109:209–217. doi: 10.1128/jb.109.1.209-217.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seidler R J, Starr M. Isolation and characterization of host-independent bdellovibrios. J Bacteriol. 1969;100:769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolp H, Starr M P. Bdellovibrio bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- 26.Torrella F, Guerrero R, Seidler R J. Further taxonomic characterization of the genus Bdellovibrio. Can J Microbiol. 1978;24:1387–1394. doi: 10.1139/m78-222. [DOI] [PubMed] [Google Scholar]
- 27.Tsai Y L, Olson B H. Rapid method for separation of DNA from soil and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uematsu T. Ecology of Bdellovibrio parasitic to rice bacterial leaf blight pathogen, Xanthomonas oryzae. Rev Plant Prot Res. 1980;13:12–26. [Google Scholar]
- 29.Varon M, Shilo M. Advances in aquatic microbiology. Vol. 2. New York, N.Y: Academic Press; 1980. Ecology of aquatic bdellovibrios; pp. 1–48. [Google Scholar]
- 30.Varon M, Shilo M. Methods for separation of Bdellovibrio from mixed bacterial population by filtration through Millipore filters or by gradient differential centrifugation. Rev Int Oceanogr Med. 1970;18–19:145–152. [Google Scholar]
- 31.Williams H N, Scheoffield A J, Guether D, Kelley J, Shah D, Falker W A., Jr Recovery of bdellovibrios from submerged surfaces and other aquatic habitats. Microb Ecol. 1995;29:39–48. doi: 10.1007/BF00217421. [DOI] [PubMed] [Google Scholar]
- 32.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]