Abstract
Tetrabromobisphenol A (TBBPA) is a flame retardant that is used as an additive during manufacturing of plastic polymers and electronic circuit boards. Little is known about the fate of this compound in the environment. In the current study we investigated biodegradation of TBBPA, as well as 2,4,6-tribromophenol (TBP), in slurry of anaerobic sediment from a wet ephemeral desert stream bed contaminated with chemical industry waste. Anaerobic incubation of the sediment with TBBPA and peptone-tryptone-glucose-yeast extract medium resulted in a 80% decrease in the TBBPA concentration and accumulation of a single metabolite. This metabolite was identified by gas chromatography-mass spectrometry (GC-MS) as nonbrominated bisphenol A (BPA). On the other hand, TBP was reductively dehalogenated to phenol, which was further metabolized under anaerobic conditions. BPA persisted in the anaerobic slurry but was degraded aerobically. A gram-negative bacterium (strain WH1) was isolated from the contaminated soil, and under aerobic conditions this organism could use BPA as a sole carbon and energy source. During degradation of BPA two metabolites were detected in the culture medium, and these metabolites were identified by GC-MS and high-performance liquid chromatography as 4-hydroxybenzoic acid and 4-hydroxyacetophenone. Both of those compounds were utilized by WH1 as carbon and energy sources. Our findings demonstrate that it may be possible to use a sequential anaerobic-aerobic process to completely degrade TBBPA in contaminated soils.
Tetrabromobisphenol A [4,4′-isopropylidenebis(2,6-dibromophenol)] (TBBPA) (Fig. 1) is a flame retardant that is used during production of many plastic polymers and electronic circuit boards. This compound is also incorporated into synthetic fabrics, and it is claimed that no carcinogenic or mutagenic effects of TBBPA are known (http://bromine.esi.be). However, TBBPA is toxic to aquatic life; the 50% lethal concentration for fish of is 0.4 or 0.54 mg/liter, and the 50% effective concentration for Daphnia magna is of 0.96 mg/liter (http://bromine.esi.be). At a contaminated site in Israel, the concentrations of TBBPA and 2,4,6-tribromophenol (TBP) in the upper 15 cm of the soil profile are as high as 450 and 110 mg/kg of soil, respectively (3). At pH 7.0, TBBPA is insoluble in water (<1 mg/liter) (3) and is nonvolatile; consequently, it is not mobile in the soil environment. Nevertheless, in calcareous soils in arid environments, the solubility and mobility of TBBPA increase tremendously as the soil solution pH increases. Thus, leaching can contaminate groundwater that is under soil contaminated with TBBPA (3). The contaminated site in Israel is located above a fractured chalk aquifer, and some of the fractures are exposed in an ephemeral stream bed (14). It is possible that TBBPA could be transported into these fractures during rain storms with the run-off water. Hence, it is worth studying the potential biodegradability of this compound in contaminated soils in order to reduce this hazard.
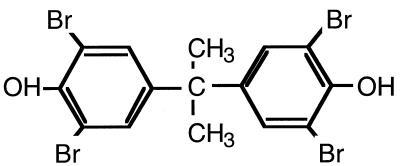
FIG. 1.
Molecular structure of TBBPA.
It is now a well-established fact that a whole range of microorganisms are capable of dehalogenating compounds like polychlorinated biphenyls polychlorinated phenols, and benzene, as well as many chlorinated solvents (4, 10, 18). Removal of the halogen atom from a molecule frequently makes the molecule more susceptible to complete mineralization (2, 10). For TBBPA, reports on the disappearance of TBBPA from wastewater have failed to distinguish between physical parameters (sedimentation) and biological parameters (biodegradation) as the cause of disappearance of the compound (15).
Reductive dehalogenation of multichlorinated compounds is a key step in the metabolism of these compounds in the environment (6, 18). For example, microbial degradation of highly chlorinated polychlorinated biphenyls occurs only via reductive dehalogenation (4). Likewise, microbial degradation of multichlorinated benzenes and phenols is more efficient if the halogen atoms are first removed anaerobically by reductive dehalogenation (18). Because aerobic degradation of the dehalogenation products is significantly faster than degradation under anaerobic conditions (2, 10), a multistage treatment process involving both anaerobic and aerobic stages may be the best solution for biodegradation of TBBPA.
Studies of reductive dehalogenation of monochlorinated phenols in both freshwater and marine sediments have shown that these compounds are metabolized under a variety of redox potential conditions, including nitrate-, iron-, and sulfate-reducing conditions, as well as under methanogenic conditions (7–9, 11, 13). It has been suggested that under sulfate-reducing conditions sulfate consumption is linked to mineralization of monochlorophenols and benzoates (7, 9). Recently, reductive dehalogenation of TBP by a Desulfovibrio strain (strain TBP1) linked to halorespiration was described (5). Therefore, we studied the effect of an anaerobic stage on cleanup of TBBPA-contaminated soil.
MATERIALS AND METHODS
Location of site.
In this study we focused on sediments obtained from the vicinity of an industrial complex in the northern Negev in Israel (Fig. 2). The site is located above Eocene chalk that is covered by thin Neogene and Pleistocene loess and unconsolidated sand (14). Ephemeral streams that drain runoff from the industrial zone dissect the site. Sediments from a Hovav stream bed were used throughout the study. The samples were stored covered with stream water at 4°C.
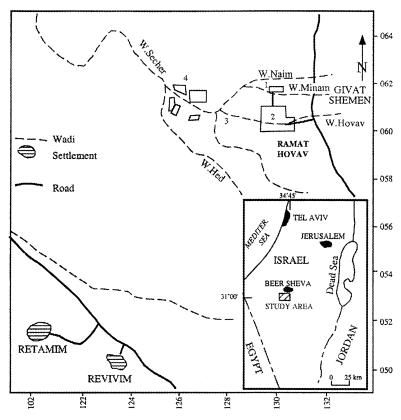
FIG. 2.
Study site location; 1, hazardous waste treatment plant; 2, industrial zone; 3, sediment sampling location; 4, wastewater storage ponds.
Experimental conditions.
We examined anaerobic metabolism of brominated phenols, phenol, and TBBPA by using 10 g (wet weight) of sediment from which the water was drained (7 g [dry weight] after drying at 105°C to a constant weight); the sediment was placed in a flask containing 90 ml of sterilized medium. The medium contained (per liter) 30 g of NaCl, 1.5 g of K2HPO4, 0.6 g of MgSO4, 0.5 g of peptone, 0.5 g of tryptone, 1 g of glucose, and 1 g of yeast extract. The pH was adjusted to 7.7 with 2 N NaOH. The resulting slurry was then supplemented with a substrate (100 mg/liter) obtained from a stock solution prepared in 0.2 N NaOH and incubated in an anaerobic chamber (Forma Scientific, Marietta, Ohio) containing 94% N2–6% H2 atmosphere at 30°C.
We studied aerobic degradation of bisphenol A [phenol 4,4′-(1-methylethyliden)bis] (BPA) by using a pure culture of a bacterium that was isolated from soil by using BPA as the sole carbon and energy source. Ten grams of contaminated soil from the site was used to inoculate mineral medium (16). The culture was incubated in the dark at 30°C on a rotary shaker at 200 rpm. After the substrate was depleted from the medium, 10 ml of the preparation was used to inoculate fresh culture medium. This enrichment procedure was repeated several times until repetitive streaking onto solidified medium (1.5% agar) resulted in isolation of a pure culture, which was characterized with a Biolog-GN identification kit (Biolog, Hayward, Calif.). Cell density during growth experiments was determined by determining the absorbance at 600 nm with a model 8452A diode array spectrophotometer (Hewlett-Packard, Palo Alto, Calif.); an optical density of 0.2 was equivalent to 7.0 × 108 cells/ml. Each experiment in which anaerobic sediment or an aerobic culture was used was performed in duplicate, and each treatment was repeated in triplicate. In all of the experiments autoclaved sediment (120°C for 30 min on 3 constitutive days) or uninoculated medium was used as the control.
Analysis.
The water and sediments obtained after extraction with water (1:1) were analyzed by using standard methods (1), and the results are shown in Table 1. A Dohramann model DC-190 total-organic-carbon (TOC) analyzer (Rosemount Analytical, Santa Clara, Calif.) was used to determine TOC contents. The standard used for TOC determinations was phthalic acid, and the standard deviation in the analysis was between 0.17 and 3.34%. In experiments performed with 2.9-ml portions of anaerobic sediments, 0.1 ml of 2 N NaOH was added to facilitate desorption and desolution of TBBPA (level of recovery, 101.2% ± 10.2%; n = 10). After centrifugation and filtration, the TBBPA content of each sample was determined by high-performance liquid chromatography (HPLC). Bromophenol concentrations were measured with a reverse-phase HPLC equipped with a diode array detector (model DAD-440; Kontron Instruments, Milan, Italy). The compounds were separated by using a Supelcosil LC-18 column (length, 25 cm; inside diameter, 4.6 mm; Supelco, Bellefonte, Pa.). The mobile phase used was a mixture of methanol supplemented with 1% acetic acid (phase A) and ammonium acetate buffer (18 mM) containing 1% acetic acid (phase B). Compounds were separated by using a gradient that started with 60% phase A; the phase A concentration was increased linearly to 100% over a 13-min period. The flow rate of the mobile phase was 1.5 ml/min. The different compounds were detected at the appropriate wavelengths (Table 2). Calibration was linear at concentrations between 0.1 and 100 mg/liter, and the R2 value was more than 0.98 for all of the compounds. The HPLC analysis was reproducible, and the coefficient of variation was between 0.71 and 2.19% for repeated injections of the same sample (n = 5). The identities of the different compounds in the culture medium were confirmed by comparing their retention times and UV spectra with the retention times and UV spectra of authentic standards (Table 2). The concentrations of the different chemicals were determined by the external standard method. Metabolites present in the anaerobic sediment and in aerobic culture media were extracted after acidification (pH 2.5) with ethyl acetate (1:1, vol/vol). The solvent was dried over Na2SO4 and then evaporated to dryness under an N2 stream. The residues were dissolved in 100 μl of ethyl acetate and then analyzed and identified by gas chromatography-mass spectrometry (GC-MS) (model Magnum; Finnigan Mat, San Jose, Calif.). Compounds were separated by using a DB-5 column (length, 30 m; inside diameter, 0.25 mm); the initial temperature used was 80°C, which was held for 4 min, and then the temperature was increased to 280°C at a rate of 10°C/min. The identities of metabolites were confirmed by comparing their retention times and mass spectrum fragmentation patterns to the retention times and mass spectrum fragmentation patterns of authentic compounds.
TABLE 1.
Chemistry of the water and sediment that were used in this study
| Prepn | pH | Conductivity (mS) | Dissolved organic carbon content (mg/liter or mg/kg)a | % of organic carbonb | Concn (mg/liter or mg/kg) ofa:
|
||
|---|---|---|---|---|---|---|---|
| Chloride | Bromide | Sulfate | |||||
| Water | 8.27 | 20 | 105.9 | 7,350 | 3,372 | 1,132 | |
| Sedimentc | 8.30 | 38 | 159.2d | 3.73 | 21,060 | 5,791 | 7,127 |
The concentration in water is expressed in milligrams per liter, and the concentration in sediment is expressed in milligrams per kilogram.
Value obtained after ashing.
Values were obtained by using 1:1 (wt/vol) water extracts, and the results are expressed on a wet weight basis unless indicated otherwise.
Value expressed on a dry weight basis.
TABLE 2.
HPLC retention times and λmax used for identification of the different compounds in this studya
| Compound | Retention time (min) | DAD λmax (nm) | Detection limit (μM) |
|---|---|---|---|
| HBZ | 2.61 | 254 | 0.73 |
| HAP | 2.97 | 275 | 0.72 |
| Phenol | 3.34 | 270 | 6.25 |
| 2-Bromophenol | 4.83 | 280 | 2.91 |
| 4-Bromophenol | 5.55 | 280 | 2.91 |
| BPA | 6.19 | 275 | 3.90 |
| 2,4-Dibromophenol | 6.50 | 280 | 1.99 |
| 2,6-Dibromophenol | 7.86 | 290 | 1.99 |
| TBP | 9.85 | 290 | 1.51 |
| TBBPA | 10.79 | 290 | 0.92 |
The analytical methods used are described in Materials and Methods.
Chemicals and reagents.
All chemicals were obtained from Aldrich (Milwaukee, Wis.) and were used as received. All of the organic solvents used were HPLC grade and were obtained from Merck (Darmstadt, Germany).
RESULTS
In this study we monitored the complete mineralization of TBBPA. At first, anaerobic microorganisms present in the sediments reductively dehalogenated TBBPA to BPA, and this product was further mineralized under aerobic conditions by a soil bacterium that closely resembled Sphingomonas sp.
Anaerobic metabolism TBBPA.
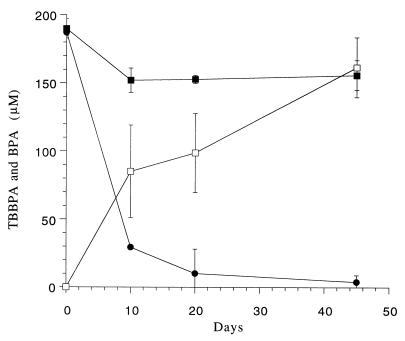
Our initial screening for anaerobic metabolism of various brominated phenols in sediment slurries revealed a distinct dehalogenation pattern for the different bromophenols (Table 3). 3-Bromophenol was not metabolized, while the sediment microorganisms degraded phenol. During dehalogenation of TBBPA a few intermediate metabolites were detected by HPLC. One of these had a retention time and UV spectrum similar to the retention time and UV spectrum of BPA. A GC-MS analysis of an ethyl acetate extract of the sediment slurry revealed that this metabolite had a molecular weight and fragmentation pattern identical to the molecular weight and fragmentation pattern of BPA (Fig. 3). Further GC-MS analysis of the extract revealed other metabolites with molecular weights of 449 and 372, which indicated that one and two bromine atoms, respectively, were lost from the original TBBPA molecule. The kinetics of TBBPA dehalogenation in the sediment slurries implied that most of the TBBPA was dehalogenated within 10 days (Fig. 4). However, a mass balance calculation showed that while 157.6 μM TBBPA was dehalogenated within 10 days, only 98.6 μM BPA were produced during that time. After 45 days the TBBPA was completely dehalogenated, and 161 μM BPA (88% of the initial TBBPA) was released into the medium. BPA persisted in the anaerobic slurry and was not degraded even after 3 months of incubation.
TABLE 3.
Anaerobic reductive debromination of different bromophenols in 10% (wt/vol) sediment slurries
| Compounda | Intermediate(s)b | Final productc |
|---|---|---|
| Phenol | NI | |
| 2-Bromophenol | Phenol | |
| 3-Bromophenol | No dehalogenation | |
| 4-Bromophenol | Phenol | |
| 2,4-Dibromophenol | 2BP, 4BP | Phenol |
| 2,6-Dibromophenol | 2BP | Phenol |
| TBP | 2BP, 4BP, 2,4DBP, 2,6DBP | Phenol |
| TBBPA | Tri- and dibrominated BPA | BPA |
The final concentration of each compound was 100 mg/liter.
The compounds were identified on the basis of HPLC retention times after 15 days of incubation. 2BP, 2-bromophenol; 4BP, 4-bromophenol; 2,4DBP, 2,4-dibromophenol; 2,6DBP, 2,6-dibromophenol.
The final debromination products were identified on the basis of HPLC retention times and GC-MS data. NI, not identified.
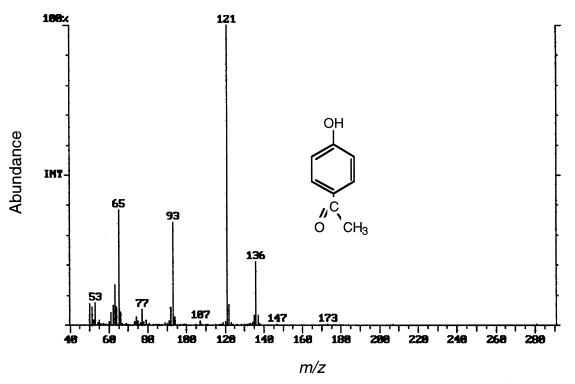
FIG. 3.
Mass spectrum of the metabolite that accumulated during anaerobic transformation of TBBPA in a sediment slurry. The spectrum of the metabolite is identical to that of BPA (inset).
FIG. 4.
Kinetics of TBBPA reductive dehalogenation in a 10% (wt/vol) sediment slurry incubated under anaerobic conditions. Symbols: ●, TBBPA; □, BPA; ■, TBBPA autoclaved control.
Anaerobic metabolism of TBP.
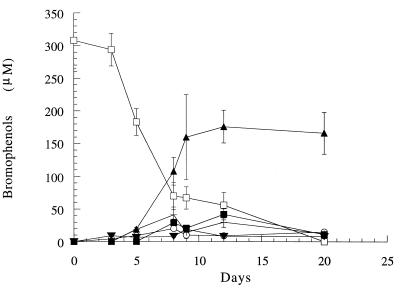
The anaerobic metabolism of TBP by sediment microorganisms is shown in Fig. 5. The main dehalogenation product formed in the slurry supplemented with TBP was phenol, and the maximum concentration of TBP was 171.6 μM, which corresponded to transformation of 57% of the TBP carbon. Transient dibromophenols and monobromophenols were also detected during anaerobic incubation of the sediment slurry (Fig. 5).
FIG. 5.
Kinetics of TBP dehalogenation and formation of bromophenol intermediates in a 10% (wt/vol) sediment slurry incubated under anaerobic conditions. Symbols: □, TBP; ▴, phenol; ▾, 2,6-dibromophenol; ○, 2,4-dibromophenol; ■, 2-bromophenol; +, 4-bromophenol.
Aerobic metabolism of BPA.
To evaluate whether aerobic microorganisms can degrade BPA, we prepared enrichment cultures containing BPA as the sole carbon and energy source. We isolated a gram-negative bacterium that closely resembled (41% similarity, based on the Biolog database) members of the genus Sphingomonas and designated this organism strain WH1. This strain utilized various aromatic compounds, such as benzoate, 4-hydroxybenzoate, and 4-hydroxyacetophenone, but not phenol, catechol, 4-bromophenol, or TBP. During BPA degradation few metabolites were detected in the culture medium. A GC-MS analysis of an ethyl acetate extract of the culture medium revealed that one of the metabolites had the same molecular weight and fragmentation pattern as 4-hydroxyacetophenone (Fig. 6). The identity of this metabolite was confirmed by comparing its GC-MS and HPLC data (retention time, mass spectrum, and UV spectrum) with data obtained for the authentic compound. Based on the metabolic pathway proposed for degradation of BPA (12, 17), we examined whether 4-hydroxybenzoic acid was present in the medium and found that the culture medium did contain a metabolite with a HPLC retention time, mass spectrum, and a UV spectrum identical to the HPLC retention time and UV spectrum of 4-hydroxybenzoic acid.
FIG. 6.
Mass spectrum of one of the metabolites detected during aerobic biodegradation of BPA by a pure culture of strain WH1. This spectrum was identical to the spectrum of authentic 4-hydroxyacetophenone (inset).
Utilization of BPA as a carbon and energy source.
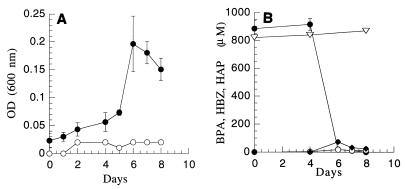
When strain WH1 was grown on BPA, the amount of biomass increased as the substrate was degraded (Fig. 7). The TOC concentration in the culture medium (biomass plus metabolites) after incubation for 7 days was 73 mg/liter (which accounted for 48% of the initial BPA carbon), while the concentration of dissolved TOC that remained was 38.5 mg/liter. The concentrations of 4-hydroxybenzoic acid (HBZ) and of 4-hydroxyacetophenone (HAP) that accumulated during incubation of strain WH1 with BPA were low; the highest concentration of HBZ was 73 μM, and the highest concentration of HAP was 17 μM (Fig. 7b).
FIG. 7.
(A) Growth of strain WH1 during metabolism of BPA as a sole carbon and energy source. Symbols: ●, active culture; ○, uninoculated control. (B) Degradation of BPA and formation of metabolites. Symbols: ●, BPA; ▿, BPA uninoculated control; ▾, HBZ; □, HAP. OD (600 nm), optical density at 600 nm.
DISCUSSION
In this paper we describe complete microbial mineralization of TBBPA. This compound is metabolized in two steps; the first step is reductive debromination of TBBPA under anaerobic conditions to BPA, and the second step is aerobic mineralization of BPA by a gram-negative aerobic bacterium (strain WH1).
Dehalogenation of haloaromatic compounds appears to be a critical step in mineralization of these compounds in the environment (18). Reductive dehalogenation has been recognized as the only biotransformation process available for highly halogenated aryl halides of concern. Thus, the logical approach for biodegradation of TBBPA was an initial anaerobic step. The sediment which we collected had been exposed for years to different pollutants (3, 14), which resulted in increased organic material content, salinity, and sulfate concentration (Table 1); this creating conditions similar to a marine sediment environment. Reductive dehalogenation of halophenols has been observed previously in marine sediments in the presence of a variety of electron acceptors (5, 7–9, 13). Therefore, we expected that reductive debromination of brominated phenols would occur in these sediments under anaerobic conditions (Table 3 and Fig. 4 and 5). The major metabolite detected in sediment slurries after dehalogenation of TBBPA was BPA. A few other intermediates whose identities were not known were also detected. Based on the GC-MS data, we suggest that these metabolites were partially brominated BPA (tri-, di-, or monobrominated). If this is true, dehalogenation of TBBPA is probably a stepwise process in which bromine atoms are removed sequentially. Additional evidence which supports this hypothesis is the transient appearance of di- and monobrominated phenols during metabolism of TBP by sediment microorganisms. Tribromophenol is a copollutant in the sediment, and we assume that the same population of microorganisms that dehalogenates TBBPA mediates dehalogenation of this compound.
In a recently published report (5) on dehalogenation of TBP by a pure culture of Desulfovibrio strain TBP1, Boyle et al. proposed that this organism is capable of growth via halorespiration. The effect of sulfate on dehalogenation (sulfate may compete with TBP as an electron acceptor) was not determined in that study. In our sediments dehalogenation occurred in spite of high concentrations of sulfate (Table 1). This is consistent with other results describing dehalogenation of halophenols in sulfate-reducing enrichment cultures from marine and estuarine environments (7, 9). Furthermore, some studies have shown that mineralization of chlorophenols in sulfidegenic enrichment cultures is linked to sulfate reduction (7). Thus, it is possible that sulfate-reducing microorganisms mediate dehalogenation of the bromophenols in sediments.
The fact that sediment microorganisms are able to degrade phenol anaerobically (Table 3) suggests that tri- di-, and monobrominated phenols might be completely degraded under anaerobic conditions in sediment. However, the product of TBBPA dehalogenation (BPA) persists under anaerobic conditions and is degraded only aerobically. The metabolic pathway that results in BPA biodegradation has been described by Lobos et al. (12). Likewise, we identified two main metabolites in the strain WH1 culture medium, HBZ and HAP, that were further mineralized or incorporated into cell biomass, as indicated by the results of the TOC analysis (Fig. 7). Like strain MV1 (12), strain WH1 was not able to metabolize phenol or catechol, indicating that cleavage of the intermediate's aromatic ring occurs in the protocatechuic acid pathway and not the catechol pathway (12).
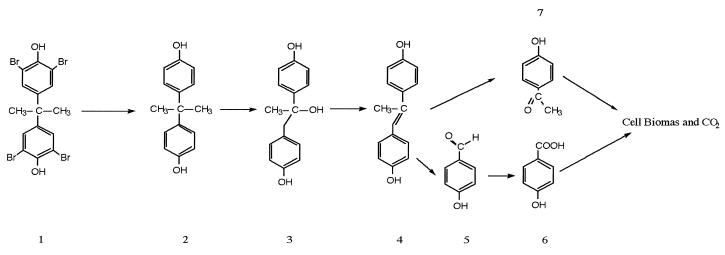
The results of this study clearly demonstrate that complete mineralization of TBBPA in the environment can occur in a sequence consisting of anaerobic and aerobic steps. This conclusion is supported by the results of a number of reports, which were summarized by Haggblom and Valo (10), on using a sequential anaerobic-aerobic process for bioremediation of chlorophenol wastes. The complete pathway for biodegradation of TBBPA, as determined in our study, is shown in Fig. 8. We propose that after bromine atoms are removed from TBBPA by reductive dehalogenation, BPA is degraded via the metabolic pathway proposed by Lobos et al. (12) and Spivak et al. (17). The first two metabolites of aerobic BPA degradation, 1,2-bis(4-hydroxyphenyl)-2-propanol and 4,4′-dihydroxy-α-methylstilbene, were not found in the strain WH1 culture. The fact that 4-hydroxybenzoic acid and 4-hydroxyacetophenone were formed led us to the conclusion that the route of metabolism of BPA by strain WH1 is the same as the route proposed for strain MV1.
FIG. 8.
Proposed metabolic pathway for complete biodegradation of TBBPA that involves a sequence of anaerobic and aerobic steps. 1, TBBPA; 2, BPA; 3, 1,2-bis(4-hydroxyphenyl)-2-propanol; 4, 4,4′-dihydroxy-α-methylstilbene; 5, 4-hydroxybenzaldhyde; 6, HBZ; 7, HAP.
ACKNOWLEDGMENT
This study was funded in part by a grant from the Israel Ministry of Environmental Quality.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C.: American Public Health Association, American Water Works Association, Water Environment Federation; 1992. [Google Scholar]
- 2.Armeneate P M, Kafkewitz D, Laewandowski G L, Jou C-J. Anaerobic-aerobic treatment of halogenated phenolic compounds. Water Res. 1999;33:681–692. [Google Scholar]
- 3.Arnon S. Transport of organic and inorganic contaminants in desert soil—evaluation of contaminants flushing from a contaminated soil near Ramat-Hovav industrial park. M.Sc. thesis. Sede Boker Campus, Israel: Ben-Gurion University of the Negev; 1999. . (In Hebrew.) [Google Scholar]
- 4.Bedard D L, Quensen J F. Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 127–216. [Google Scholar]
- 5.Boyle A W, Phelps C D, Young L Y. Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4,6-tribromphenol. Appl Environ Microbiol. 1999;65:1133–1140. doi: 10.1128/aem.65.3.1133-1140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolfing J, Beurskens J E. The microbial logic and environmental significance of reductive dehalogenation. Adv Microb Ecol. 1994;14:143–205. [Google Scholar]
- 7.Haggblom M M, Young L Y. Chlorophenol degradation coupled to sulfate reduction. Appl Environ Microbiol. 1990;56:3255–3260. doi: 10.1128/aem.56.11.3255-3260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haggblom M M, Rivera M D, Young L Y. Influence of alternative electron acceptors on the anaerobic biodegradability of chlorinated phenols and benzoic acid. Appl Environ Microbiol. 1993;59:1162–1167. doi: 10.1128/aem.59.4.1162-1167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggblom M M, Young L Y. Anaerobic degradation of halogenated phenols by sulfate-reducing consortia. Appl Environ Microbiol. 1995;61:1546–1550. doi: 10.1128/aem.61.4.1546-1550.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haggblom M M, Valo R J. Bioremediation of chlorophenol wastes. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 389–434. [Google Scholar]
- 11.Kazumi J, Haggblom M M, Young L Y. Diversity of anaerobic microbial processes in chlorobenzoate degradation: nitrate, iron, sulfate, and carbonate as electron acceptors. Appl Microbiol Biotechnol. 1995;43:929–936. doi: 10.1007/BF02431930. [DOI] [PubMed] [Google Scholar]
- 12.Lobos J K, Leib T K, Su T-M. Biodegradation of bisphenol A and other bisphenols by a gram-negative aerobic bacterium. Appl Environ Microbiol. 1992;58:1823–1831. doi: 10.1128/aem.58.6.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monserrate E, Haggblom M M. Dehalogenation and biodegradation of brominated phenols and benzoic acids under iron-reducing, sulfidogenic, and methanogenic conditions. Appl Environ Microbiol. 1997;63:3911–3915. doi: 10.1128/aem.63.10.3911-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nativ R, Adar E M, Becker A. Designing a monitoring network for contaminated groundwater in fractured chalk. Ground Water. 1999;37:38–47. [Google Scholar]
- 15.Sellstrom U, Jansson B. Analysis of tetrabromobisphenol A in product and environmental samples. Chemosphere. 1995;31:3085–3092. [Google Scholar]
- 16.Shochat E, Hermoni I, Cohen Z, Abliovich A, Belkin S. Bromoalkane-degrading Pseudomonas strains. Appl Environ Microbiol. 1993;59:1403–1409. doi: 10.1128/aem.59.5.1403-1409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spivack J, Leib T K, Lobos J H. Noval pathway for bacterial metabolism of bisphenol A. J Biol Chem. 1994;269:7323–7329. [PubMed] [Google Scholar]
- 18.Suflita J M, Townsend G T. The microbial ecology and physiology of aryl dehalogenation and implications for bioremediation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 243–268. [Google Scholar]