Abstract
Regulation of the human papillomavirus type 16 (HPV-16) E6 promoter is a complex process in which transcriptional repression as well as activation plays an important role. Here, we identify a negative regulatory element that in the context of a continuous long control region fragment overcomes the activation of the HPV-16 enhancer. This silencing element, which we have termed a PSM (papillomavirus silencing motif), consists of two copies of the sequence 5′-TAYAATAAT-3′ that overlap the origin of replication. Each copy of this 9-bp sequence binds the same unknown cellular factor, which we refer to as PSM-BP (PSM binding protein). Both copies of the binding sequence are required for transcriptional repression, and we provide evidence that suggests that this particular organization results in the stabilization of a PSM-BP dimer. The silencing motif, while functioning in either orientation, showed a positional requirement between the enhancer and the promoter. Experiments with both a heterologous enhancer and a promoter also demonstrated a general ability of this element to function as a transcriptional silencer in non-HPV systems. Our findings provide an important addition to our understanding of HPV-16 gene regulation and an interesting model for the study of transcriptional repression.
Human papillomavirus type 16 (HPV-16) has a double-stranded circular DNA genome of 7,906 bp in length and is associated with the majority of cervical carcinomas (17, 51). In spite of this, the development of cancer in the host is a relatively rare event compared to the much greater prevalence of subclinical infections. This reflects the fact that HPV-16 has coevolved with humans and normally successfully replicates without causing disease. It has, therefore, been proposed that additional risk factors and genetic alterations are likely to be involved in the malignant progression of HPV-16 lesions (22).
It is thought that HPV-16 generally infects basal cells of the squamous epithelium, with vegetative replication and the production of new virions occurring in the terminally differentiated layers (8, 29). Since these terminally differentiated cells have exited the cell cycle, the virus needs to manipulate cellular events in order to induce the production of the replication machinery necessary for the viral life cycle. The two HPV proteins that have been implicated in reinitiating the cell cycle are E6 and E7. Both of these proteins target and abrogate the function of important negative regulators of the cell cycle (6, 16, 38, 49), thereby stimulating cell cycle progression. An increasing body of evidence suggests that under conditions of high levels of E6 and E7 expression, a cascade of events may be triggered, ultimately culminating in the development of cancer (21). Thus, it can be seen that the regulation of E6 and E7 expression is a fine balance between the need to stimulate cell cycle progression and the potential harm that can be incurred by the host.
Expression of the HPV-16 E6 and E7 open reading frames (ORFs) is regulated by the long control region (LCR) that makes up approximately 10% of the HPV-16 genome. These sequences contain a nuclear matrix attachment region (45), the epithelial cell-specific enhancer (10, 19), the origin of replication (7), and the promoter (denoted P97) that gives rise to the E6 and E7 transcripts (43). Regulation of P97 is a complex process involving many different transcription factors that exert either positive or negative effects (33). This complexity may reflect, at least in part, the need to express E6 and E7 only when and where it is needed, namely, in terminally differentiating cells, but not necessarily in mitotically active basal epithelial cells. Positive effectors of HPV-16 E6-E7 expression include AP-1 (4), NF-I (1, 2), Oct-1 (32), SP1 (18), TEF-1 (23), and the glucocorticoid receptor (19). Negative regulation of HPV-16 E6-E7 expression has been attributed to the effects of the viral E2 protein (28) and the transcription factor YY1 (27, 35). Studies of the mechanism of repression of P97 by E2 and the homologous promoters in other genital HPV types have shown that E2 represses E6-E7 transcription by binding to the promoter and displacing the transcription factors SP1 and TFIID (14, 46–48). Previous studies have also provided evidence for the role of the cellular transcription factor YY1 in the negative regulation of HPV-16 E6-E7 expression (27). More recently, we showed that multiple YY1 recognition sites were involved in the down-regulation of P97 and that the target of YY1-mediated repression was the transcriptional activator AP-1 (35).
The importance of negative regulators of E6 and E7 expression has become apparent from the characterization of HPV-16 genomes obtained from cervical carcinomas and related metastatic tumors. For example, approximately 60 to 70% of cervical tumor cells studied contain integrated HPV-16 genomes (11, 12, 26) that disrupt the E2 ORF or E2 expression (3). More recently, studies of primary tumors or metastases were found to contain nonintegrated HPV-16 episomes with mutated YY1 sites (15, 27). In both of these examples, an important negative regulator of the P97 promoter had been removed, and high cellular levels of E6 and E7 expression were detected.
In this study, we report the identification and characterization of a novel regulatory element within the LCR of HPV-16 that represses the E6-E7 promoter. We provide evidence that this element is capable of overcoming all other positive regulatory signals in the LCR and thus represents a bona fide silencing element and also show that the binding of an unknown cellular factor to the silencing motif correlates with transcriptional repression.
MATERIALS AND METHODS
Plasmid constructs.
All constructs used in functional assays were based on the chloramphenicol acetyltransferase (CAT) construct pBLCAT3 ΔH/N (32), a modified version of pBLCAT3 (39). The HPV-16 core promoter construct (previously described as p80 [35] or p16 [32]), which is referred to as P in this paper, contains HPV-16 promoter sequences from nucleotides 16 to 80 cloned into the BglII and XhoI sites of pBLCAT3 ΔH/N. Contiguous HPV-16 LCR fragments from positions 7451 to 15, 7526 to 15, or 7526 to 7855 were created by PCR and then cloned into the HindIII and BamHI sites of p80. p80:HPV-16 (positions 7526 to 7855), which is referred to as EP for enhancer-promoter construct, was used as the basis for a number of constructs in which intervening sequences were checked for their capacity to silence transcription. These were created by cloning double-stranded oligonucleotides containing the appropriate sequences and complementary BamHI and BglII ends into the BamHI site of EP. A restriction digest with BamHI and XhoI determined the orientation of the oligonucleotide insertion. The construct ESV40P was created by cloning the EcoRI fragment containing the simian virus 40 (SV40) enhancer from OVECS (50) into pBS (SK+). This fragment was then excised with HindIII and BamHI and cloned into p80. Either wild-type or mutated (m*) HPV-16 sequence (positions 7883 to 15) was then cloned into the BamHI site as described above to create ESV40(7883-15)P or ESV40(7883-15m*)P, respectively. The EPtk construct has been described previously (32). As with the other constructs, either wild-type or mutant sequence (positions 7883 to 15) was cloned into the BamHI site of this construct to create E(7883-15)Ptk or E(7883-15m*)Ptk, respectively. All of the constructs used in this study were sequenced according to the method described by Sanger et al. (37).
Cell culture and functional assays.
Primary human keratinocytes (PHKs) were grown in serum-free medium 154 (both cells and medium were obtained from Cascade Biologics, Inc.) according to the manufacturer’s recommendations. HeLa cells were cultured in minimal essential medium supplemented with 10% fetal calf serum. C33A cells were cultured in Dulbecco’s modified eagle’s medium containing 10% fetal calf serum. PHKs were plated out onto 10-cm culture dishes and transfected at 50 to 70% confluency with Lipofectin reagent (GIBCO-BRL). For each transfection, 30 μl of Lipofectin was mixed with 5 μg of DNA in 1 ml of medium 154 and left at room temperature for 15 min before being added to the 9 ml of medium covering the cells. After 18 to 24 h, the medium containing Lipofectin was replaced with 10 ml of normal fresh medium 154 and the cells were incubated for a further 24 h before harvesting. Transfection of HeLa or C33A cells was performed with either Lipofectamine (GIBCO-BRL) reagent under conditions similar to those described above or else by electroporation as described previously (35).
To determine CAT activity (20), assays were performed by essentially the same procedure as that used by Chan et al. (5), and CAT activities were determined as picomoles of chloramphenicol acetylated per minute per milligram of protein extract by quantification of radioactive spots on thin-layer chromatograms. Each value obtained represents between three and six independent transfections by using at least two different DNA preparations.
EMSA.
The double-stranded oligonucleotides used in the electrophoretic mobility shift assay (EMSA) described here were identical to those cloned into CAT expression vectors for functional studies. Approximately 50 ng of annealed oligonucleotide was labeled with [32P]dATP and dCTP nucleotides with Klenow polymerase. Approximately 250 pg of purified labeled probe was used with an activity of approximately 20,000 cpm in a standard reaction previously described (35). Samples were run on a 4% polyacrylamide gel containing 0.25× Tris-borate-EDTA acid (TBE) at 200 V for 2 h. The gels were then transferred onto blotting paper, dried for 1 h, and then exposed to autoradiographic film.
RESULTS
A region of HPV-16 encompassing the origin of replication contains sequences that repress transcription from the E6-E7 promoter.
Previously published data from our laboratory (19) and from others (36) have indicated the presence of an undefined negative regulatory element within the LCR of HPV-16. This is exemplified by the fact that subfragments of the LCR containing enhancer elements activate core HPV-16 promoter sequences (9, 10, 32, 35), while a contiguous LCR fragment containing the enhancer, origin of replication, and promoter shows very little activity (19).
In order to identify the sequences within the LCR responsible for this effect, we carried out functional assays using LCR deletion constructs driving the CAT reporter gene (see Materials and Methods). These initial experiments were carried out with HeLa cells that were originally obtained from a cervical carcinoma and contained integrated HPV sequences. Since we wished to rule out the possibility of any contribution by papillomavirus proteins or the transformed nature of the test cells, we also carried out our functional studies with C33A cells (transformed epithelial cells that do not contain integrated HPV sequences) and with PHKs.
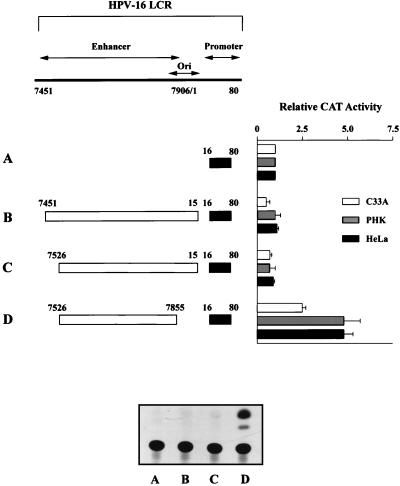
Figure 1 presents the results of these functional assays. The core HPV-16 promoter construct has been described previously (32) and consists of nucleotide sequences from positions 16 to 80 containing the natural TATA element and a proximal binding site for SP1 (18). The relative activity of the core promoter alone was set at one (see construct A), and all other activities were compared to it. The presence of an LCR fragment (genomic positions 7451 to 15) containing the enhancer and origin of replication (construct B) failed to show any activity above that obtained with the core promoter alone. The same result was obtained for all three cell types, thus providing evidence that neither HPV proteins nor the transformed state of the test cell is responsible for the lack of transcriptional activity. These data are in agreement with our previously published observation (19), and silencing of the LCR fragment was also observed when a construct containing the entire promoter sequence (positions 16 to 103) was used (34). Deletion of 5′ sequences from positions 7451 to 7526 (construct C) failed to activate the promoter. In contrast, the removal of 66 bp from 7856 to 15 which include the origin of replication (construct D) resulted in the activation of the HPV-16 promoter in all three cell types.
FIG. 1.
Deletion of sequences containing the origin of replication leads to an activation of the HPV-16 LCR. Indicated are the CAT activities of four HPV-16 constructs (A to D) in three different cell types, i.e., HeLa, C33A, and PHKs. Construct A represents the core promoter sequences cloned into the vector pBLCAT3 ΔH/N. The nucleotide numbers refer to the original viral DNA sequence (41), with corrections as previously described (31). The relative CAT activity of construct A has been set to 1, and the activities of constructs B to D are compared to this. LCR sequences from positions 7451 to 15 fail to activate the core promoter of HPV-16 (construct B), as do sequences from positions 7451 to 7525 (construct C). Deletion of sequences 7856 to 15 that include the origin of replication (construct D) leads to the activation of the core promoter, suggesting that these sequences may be responsible for the lack of activity demonstrated by the larger LCR fragment. Also shown is an autoradiograph depicting a representative result obtained with these four constructs, in this case from the transient transfection of C33A cells. The results depicted in the bar chart represent between three and six independent transfections for each construct with at least two different DNA preparations.
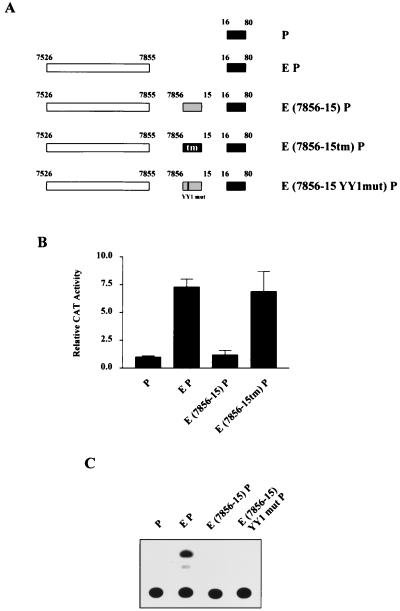
One possible explanation for this observation could be an alteration of the distance between upstream regulatory elements and the promoter. To rule out this possibility, we set up a cassette system in which the original sequence from positions 7856 to 15 could be replaced with a transversion mutant of an identical length. Consequently, for all nucleotides within this region, adenines were replaced with cytosines and guanines were replaced with thymidines, and vice versa. Figure 2A provides a schematic representation of the various constructs used to analyze any potential distance effect.
FIG. 2.
Lack of LCR activity in constructs containing HPV-16 sequences 7856 to 15 is due neither to a distancing of regulatory elements from the promoter nor to the presence of a YY1 site. (A) Schematic representation of HPV-16 EP constructs used in the transient transfection of HeLa cells. The presence of wild-type or mutated sequences within the segment from positions 7856 to 15 is illustrated. The construct E(7856-15)P contains wild-type sequences, while E(7856-15tm)P represents the insertion of a transversion mutant segment and E(7856-15YY1mut)P represents a mutation of the putative YY1 site 5′-ACACATTTA-3′. (B) CAT assay results obtained after HeLa transfections indicate that while wild-type sequences from positions 7856 to 15 repress the HPV-16 EP construct, a fragment of identical size containing a transversion mutation of this sequence fails to silence transcription. (C) A representative example of a CAT assay experiment comparing the construct E(7856-15)P and the equivalent construct in which a YY1 site has been mutated. The YY1 mutation has no effect on the capacity of the segment from positions 7856 to 15 to repress transcription.
The core promoter sequences are denoted P, while enhancer sequences (from positions 7526 to 7855) are denoted E. Thus, the CAT construct containing both the enhancer and the core promoter will be referred to as EP. Sequences cloned between E and P are indicated in parentheses, with tm representing the transversion mutant sequences.
Functional data obtained from the transient transfection of HeLa cells with these constructs are shown in Fig. 2B. In the absence of sequence from positions 7856 to 15, the HPV-16 enhancer fragment activated the promoter by approximately sevenfold. However, the insertion of wild-type HPV sequences from 7856 to 15 between the enhancer and promoter resulted in an almost complete repression of this activity. This is consistent with the results presented in Fig. 1 with contiguous LCR fragments. Significantly, insertion of the transverse mutant fragment of the same size did not inhibit transcriptional activity. This indicates that the natural nucleotide sequences present within the segment from positions 7856 to 15 are responsible for the observed repression and not a distancing of regulatory elements from the promoter.
A role for the transcriptional repressor YY1 in the control of HPV gene expression has previously been documented (27, 35). Within the region from positions 7856 to 15 is the sequence 5′-ACACATTTA-3′ that has the potential to bind, albeit weakly, bacterially expressed YY1 protein (35). We wished to determine the involvement of this site, if any, in the repression observed in Fig. 1 and in Fig. 2B. Consequently, we introduced a mutation within this sequence that would abolish YY1 binding. We then tested the ability of a construct containing this YY1 mutation (Fig. 2A) to repress the HPV enhancer-promoter activity. Figure 2C demonstrates that the YY1 mutation had no effect on the capacity of this region to repress HPV transcription. This result is consistent with our previously published study in which this YY1 site, while binding recombinant YY1 weakly, failed to bind YY1 in EMSA experiments with HeLa nuclear extracts (35). Taken together, the results presented in Fig. 2 suggest that sequences within positions 7856 to 15, other than the YY1 binding site, are responsible for the observed repression of HPV-16 transcriptional activity.
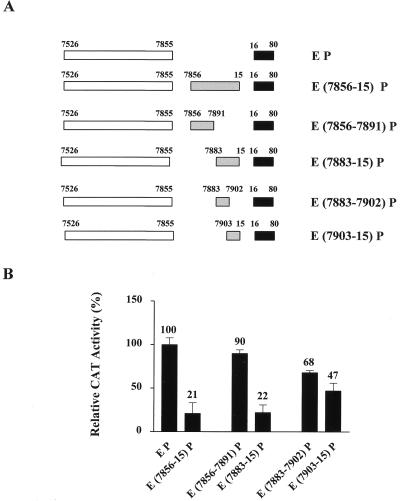
In order to identify the sequences responsible for repression, we made a number of deletion constructs (shown in Fig. 3A) that were subsequently tested in HeLa cells (Fig. 3B). In this experiment, the construct EP demonstrated a fivefold activation over that of the core promoter construct alone (the results for P are not shown in Fig. 3). The relative activity of the EP construct was then set to 100%, and the activities of other constructs were compared to this. It can be seen that the presence of the previously defined region (7856 to 15) resulted in a fivefold repression (down to the level of the core promoter alone). Sequences from positions 7856 to 7891 failed to show any significant repression of the HPV enhancer-promoter sequences. However, the segment from positions 7883 to 15 brought about the same level of repression as the sequences from 7856 to 15. This suggests that the repressive activity observed for the segment from positions 7856 to 15 lies within the 38 nucleotides from 7883 to 15. Interestingly, when this 38-nucleotide segment was further dissected into two, neither half demonstrated a full repressive capacity, although a certain modest level of repression was seen. This observation suggests that sequences present in the regions from 7883 to 7902 and from 7903 to 15 may interact synergistically to repress transcription. Therefore, we examined the sequences within the silencing region in the hope of gaining a further insight into the repression mechanism.
FIG. 3.
Deletion analysis of the HPV-16 region from positions 7856 to 15 suggests silencing results from the cooperative interaction between sequences in segments from positions 7883 to 7902 and from 7903 to 15. (A) Schematic representation of the different constructs transiently transfected into HeLa cells to identify the sequences responsible for silencing activity. (B) The CAT activity of the construct EP was five times that of the core promoter construct alone (not shown here) and was set to 100%. The activities of the remaining constructs are given as percentages of the EP construct. The segment from positions 7883 to 15 results in the same level of repression as the original sequence from positions 7856 to 15. Further dissection of this segment into two results in only a modest level of repression by each half.
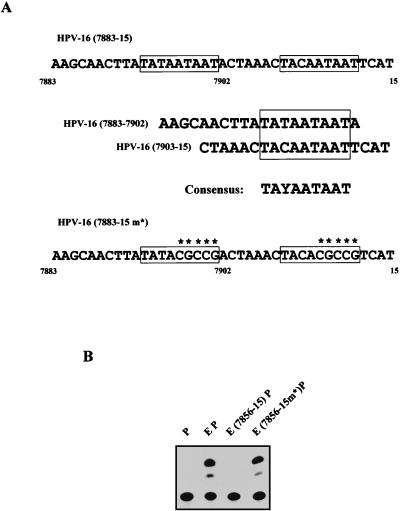
One striking feature of the sequence from positions 7883 to 15 is the presence of an almost perfect 9-bp direct repeat. The 9-bp sequence is present in both segments 7883 to 7902 and 7903 to 15 and is shown in Fig. 4A, in which it has been outlined. The consensus sequence for this repeat is 5′-TAYAATAAT-3′, where Y is either T or C. To determine if this sequence was responsible for the observed repressive activity, we introduced point mutations into the 3′ half of each repeat (Fig. 4A). The region from 7883 to 15 containing these point mutations was then cloned into the EP construct to generate the construct HPV-16 E(7883-15 m*)P. This construct was then tested in functional assays, the representative results of which are depicted in Fig. 4B. It can be seen that the point mutations within the 9-bp repeat completely abolish the repressive capacity of this region.
FIG. 4.
A direct repeat of the sequence 5′-TAYAATAAT-3′ is responsible for the silencing activity of the HPV-16 region from positions 7883 to 15. (A) Present within each of the nonoverlapping segments 7856 to 7902 and 7903 to 15 is a 9-bp motif with the consensus 5′-TAYAATAAT-3′. Also shown is the sequence (from positions 7883 to 15) used in the construct E(7883-15m*)P that contains point mutations in each copy of this 9-bp motif (highlighted by the presence of an asterisk). (B) Functional studies with the E(7883-15m*)P construct indicate that the presence of the mutations within the 5′-TAYAATAAT-3′ motif abolishes the ability of the region from positions 7883 to 15 to silence transcription, as seen from this representative CAT assay result.
Taken together, these results suggest that two copies of a 9-bp sequence (5′-TAYAATAAT-3′) present at the origin of replication are responsible for the silencing of the LCR enhancer activity. We will refer to the region from positions 7893 to 11 that contains both copies of the 9-bp repeat as a papillomavirus silencing motif (PSM).
The binding of a cellular factor to the PSM correlates with repression of the HPV-16 P97 promoter.
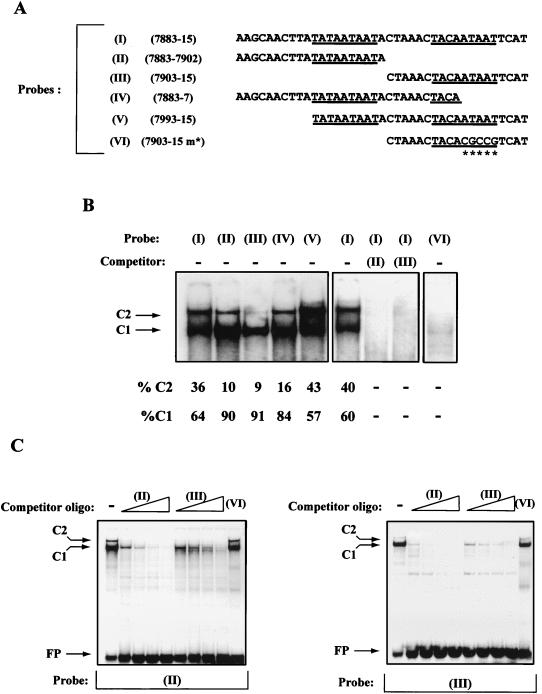
Our functional studies implied a role for the direct repeat of the sequence 5′-TAYAATAAT-3′ in the repression of HPV-16 transcription. This prompted us to investigate the binding of cellular factors to this sequence. Figure 5 shows the result of EMSA experiments using the silencing region of HPV-16 (positions 7883 to 15) shown to be responsible for repression. A magnified view of the shift obtained with an oligonucleotide probe containing this entire region (see probe I in Fig. 5B) shows that two complexes were obtained, which we have termed C1 and C2.
FIG. 5.
EMSA studies provide a correlation between the binding of a cellular factor to the PSM and transcriptional silencing of the HPV-16 P97 promoter. (A) Nucleotide sequences of the probes used in the EMSA studies. Probes are numbered I to VI and contain, in addition to the viral nucleotide sequence, BamHI (5′) and BglII (3′) complementary ends for labelling purposes. Underlined are the 5′-TAYAATAAT-3′ motifs. Double-stranded oligonucleotides containing these sequences were 32P labelled and examined in EMSA studies with HeLa nuclear extract (see Materials and Methods). (B) The result of the EMSA studies of probes I to VI. Probes I to V each give rise to two complexes indicated by the arrows and labelled C1 and C2. The percentages of these two complexes are given relative to the total C1 plus C2 shifts, as determined by densitometric analysis. Also shown is a competition between a 400-fold excess of unlabelled probe II or probe III and labelled probe I. The ability of both probes II and III to prevent the formation of complexes C1 and C2 with probe I suggests that the C1 and C2 complexes formed with probes II and III are the same as those formed with probe I. The oligonucleotide probe VI is based on the sequences present in probe III; however, probe VI contains the point mutations within the 5′-TAYAATAAT-3′ motif (Fig. 4A). Unlike probe III, probe VI fails to show any significant formation of complexes C1 and C2. (C) Competition studies between the nonoverlapping probes II and III. In each case, either probe is capable of successfully preventing the formation of complexes C1 and C2 with probes II and III. Labelled oligonucleotide probe was challenged with increasing amounts (50-, 100-, 200-, or 400-fold excess) of unlabelled double-stranded oligonucleotide. Probe II (that forms stronger C1 and C2 complexes than probe III) is a more efficient competitor than probe III. Probe VI, containing a mutant 5′-TAYAATAAT-3′ motif, fails to prevent the formation of complexes C1 and C2 with probes II and III, even at a 400-fold excess.
If the 5′-TAYAATAAT-3′ motif were responsible for these shifts, we would also expect nonoverlapping probes II and III, which contain the sequences 7883 to 7902 and 7903 to 15, to give rise to the same complexes, because a copy of this motif is present in each case (Fig. 5A). Figure 5B shows that this is indeed the case with both probes, giving rise to C1 and C2 complexes. It can be seen that both complexes (C1 and C2) had a higher affinity for probe II than for probe III, and this particular observation is reproducible. Also shown in Fig. 5B are the results obtained with probes IV and V that contain one or two complete 5′-TAYAATAAT-3′ motifs, respectively. Consistent with the results obtained from probes II and III, these probes also gave rise to the same two complexes. Interestingly, the relative levels of complexes C1 and C2 were not the same for all five probes. This difference was quantified by densitometric analysis and is given in Fig. 5B as the percentage of C1 and C2 relative to the total density of both C1 and C2 complexes. The significance of this observation will be discussed later.
To test if the C1 and C2 complexes obtained with the different probes were identical, we carried out a series of competition experiments, the first of which is shown in Fig. 5B. It can be seen that both the C1 and C2 complexes normally obtained with probe I were effectively prevented by the presence of an excess (400-fold) of either unlabelled probe II or probe III. This suggests that the C1 and C2 complexes obtained with probes II and III are, in fact, the same as those obtained with probe I. The finding that the probe I complex C2 was prevented by competitor probe III, even though the probe III C2 complex is weak, suggests two possibilities. Firstly, C2 complex formation on probe III, although weak, may be sufficient to compete with probe I when it is in excess. A second possibility is that the lower-mobility C2 complex may contain the protein that gives rise to C1, either as a dimer or in addition to another factor. If this were the case, then the removal of C1 by probe III would also result in the abolition of C2. While both possibilities may be correct, we currently favor a model in which complex C2 represents a dimer of the protein that gives rise to complex C1. The arguments for such a proposal are provided in the discussion. Further competition analysis confirmed the relationship between C1 and C2 in probes II and III. It can be seen from Fig. 5C that probe II prevented the formation of C1 and C2 on probe III, and vice versa. Consistent with the observation that probe II forms stronger C1 and C2 complexes than probe III is the fact that probe II was a much more effective competitor of C1 and C2 in these experiments.
Other than the silencing motif, the sequences present within the nonoverlapping segments 7883 to 7902 and 7903 to 15 have little similarity. The 5′-TAYAATAAT-3′ motif is, therefore, the most likely candidate sequence responsible for the formation of complexes C1 and C2. Further support for this idea comes from studies with probe VI, which consists of sequences from the segment 7903 to 15, in which the 5′-TAYAATAAT-3′ motif has been mutated (Fig. 5A). This probe fails to give rise to complexes C1 and C2 in EMSA experiments (Fig. 5B). Probe VI also fails to compete for the formation of complexes C1 and C2 with probes II and III, even at a 400-fold excess (Fig. 5C).
Together, our EMSA experiments provide a correlation between the formation of complexes C1 and C2 and the repression of the HPV-16 P97 promoter. In addition to the m* mutation in the segment from positions 7883 to 15, we have recently identified a double point mutation within the silencing motif (5′-TAYAATTGT-3′) that also abolishes complex C1 and C2 formation. Like 7883-15 m*, this mutation abolishes the silencing effect of this region in functional studies (34), providing further evidence for the correlation of C1 and C2 complex formation and transcriptional silencing.
Repression of the HPV-16 P97 promoter by the silencing region is orientation independent but position dependent.
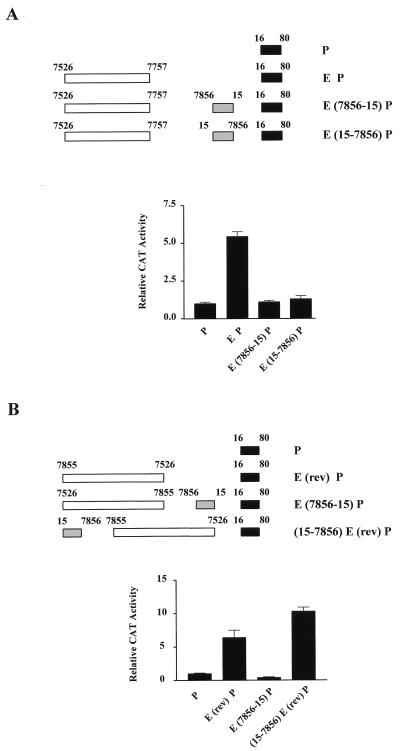
Having characterized the sequences responsible for the silencing of the HPV-16 LCR, we wished to investigate the properties of the silencing region as a whole in an attempt to approach an understanding of the mechanism involved. In particular, we were interested in whether the silencing region would function in either orientation and if the position of this region between the enhancer and promoter was important. The results of functional studies carried out after the transient transfection of HeLa cells are presented in Fig. 6 and provide answers to both of these questions.
FIG. 6.
The silencing region of HPV-16 is orientation independent but position dependent. (A) Illustrated are the HPV-16 CAT constructs tested in functional studies after transient transfection of HeLa cells. Construct E(15-7856)P contains the silencing region between the enhancer and promoter; however, the region is in reverse orientation. The lower part of the figure shows quantification of the CAT assays obtained with these constructs. Reversal of orientation does not affect the capacity of this region to silence transcription. (B) Constructs used to test the importance of position on the silencing region are indicated. A reversal of the orientation of enhancer does not affect its ability to activate the P97 promoter. Positioning of the silencer upstream of this enhancer fragment does, however, result in an inability of the silencer region to repress transcription.
First, it can be seen from Fig. 6A that when the silencing region (positions 7856 to 15) was placed between the enhancer fragment and the promoter in a reverse orientation (positions 15 to 7856), it was still capable of repressing transcriptional activity. Consequently, the orientation of the two silencing motifs does not appear to be a critical factor in the ability to repress transcription. Second, Fig. 6B addresses the question of the importance of position with respect to silencing activity. Previous studies have indicated that the HPV-16 enhancer will activate transcription in either orientation (5). This is confirmed here, where it can be seen that the construct E(rev)P containing sequences from positions 7526 to 7855 in reverse orientation activates transcription by approximately six- to sevenfold above the level of that of the promoter alone. This is comparable to results obtained with the same enhancer fragment in the normal orientation. As demonstrated previously, a lack of activity was observed for the construct E(7856-15)P, in which the silencing region was present between the enhancer and promoter. However, when the continuous enhancer and silencer regions were reversed in orientation, as indicated by the construct (15-7856)E(rev)P, such that the silencing region was now upstream of the enhancer, the repressive effect was abolished. This is not due to the fact that the silencing region was in reverse orientation, since we have demonstrated in Fig. 6A that this is not a critical factor in silencing. It would therefore appear that position is important, with the silencing region only functioning when it is positioned between the enhancer and the promoter.
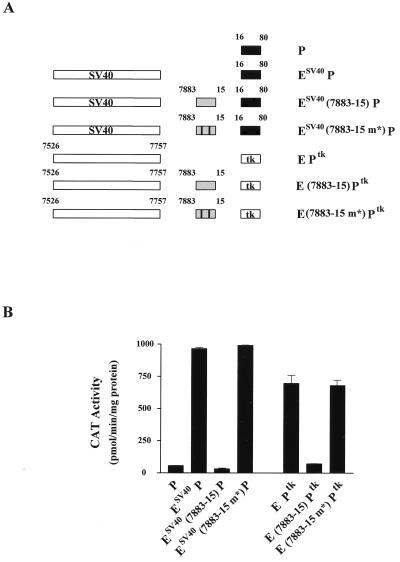
The silencing region represses the function of heterologous enhancers and promoters.
Thus far, we have studied the repressive effect of the silencing region only within the context of HPV-16. We were interested to know whether the silencing region would also affect heterologous enhancers and promoters. Therefore, we created constructs to look at the effect of this HPV-16 region on the SV40 enhancer and on the herpes simplex virus thymidine kinase (tk) promoter.
Figure 7A provides a schematic representation of the constructs used in transient transfection experiments of HeLa and C33A cells. The results of the subsequent CAT assays are shown in Fig. 7B. The activity of the SV40 enhancer driving the HPV-16 core promoter has been documented previously (47). In this experiment, we also observed a significant level of activation using the construct ESV40 P (Fig. 7B). However, the presence of the HPV-16 silencing region (positions 7883 to 15) between the SV40 enhancer and the HPV-16 promoter leads to a complete abolition of this activity, reducing it to that of the promoter alone. As a control, we also cloned into this SV40 enhancer construct the same region of HPV-16 containing point mutations within the two silencing motifs (7883-15 m*). Unlike the silencing region containing intact copies of the 5′-TAYAATAAT-3′ motif, the mutant version failed to repress the activity of the SV40 enhancer. This shows that repression of the SV40 enhancer activity is specific and requires intact silencing motifs.
FIG. 7.
The silencer region is able to repress both heterologous enhancers and promoters. (A) Schematic representation of the EP segments inserted into CAT vectors. The HPV-16 core promoter was driven by either one repeat of the 72-bp SV40 enhancer from OVECS (see Materials and Methods) or by the HPV-16 enhancer. These constructs contained either no silencing region, one in which the PSM was intact, or, alternatively, a silencing region in which the 5′-TAYAATAAT-3′ motifs were mutated. Similar sets of constructs in which the tk promoter replaced the core promoter of HPV-16 were created. (B) Results of CAT assays after transfection of these constructs into C33A cells. Activation of the HPV-16 core promoter by the SV40 enhancer is abrogated by the presence of a wild-type version of the silencing element. However, a similarly constructed plasmid containing a mutated PSM shows no evidence of transcriptional silencing. Similar results are seen for constructs containing the tk promoter.
Likewise, we tested the ability of the HPV-16 silencing region to interfere with the activation of the tk promoter. The results presented in Fig. 7B clearly show that the region of HPV-16 (positions 7883 to 15) containing intact silencing motifs prevents the activation of the tk promoter by the HPV-16 enhancer fragment. This is in contrast to the construct that contains point mutations within the silencing motifs. Together, these results demonstrate that the silencing region is capable of disrupting transcriptional activation involving either heterologous enhancers or promoters.
DISCUSSION
The transcriptional control of the HPV-16 E6-E7 promoter involves a complex interaction of both positive and negative regulators. Activators provide the capacity to express viral proteins in an appropriate host cell type. The roles of negative regulators of P97 activity are less clear. One suggestion is that such repressors may provide a means of timing E6 and E7 expression to when it is most appropriate, namely, in postmitotic epithelial cells of the suprabasal layers. There are in fact a number of examples in which viruses involved in persistent or latent infections utilize cellular repressors to keep viral gene expression at low levels. Regulated expression of viral genes is achieved when a specific trigger or phase in the viral life cycle leads to the removal of these negative signals. DNA viruses that make use of the nuclear factor YY1 in this way include the adeno-associated virus (AAV) (42), Epstein-Barr virus (30), and human cytomegalovirus (25). Negative control exerted by YY1 in HPV transcription has also been reported (15, 27, 35). However, if and by what means this repression is alleviated in HPV-16 have yet to be determined. One of the problems encountered in HPV transcription research is the difficulty in examining gene regulation in differentiating epithelia, and this remains an important challenge for the future, although progress in this area has already begun (8, 13, 24, 29).
In this study, we have defined a previously undiscovered negative regulatory element within the LCR of HPV-16 that may also play an important role in regulating the expression of the viral genes E6 and E7. The ability of this element to overcome all other positive activators, in the context of a continuous LCR fragment, suggests that it can be thought of as a silencer of transcription. Such a claim is supported by the ability of this regulatory region to completely abolish the high level of transcriptional activity normally provided by another viral regulatory region, the SV40 enhancer.
We have defined the silencing element, or PSM, as consisting of two directly repeated 9-bp motifs (5′-TAYAATAAT-3′) separated by 7 bp. The results presented here indicate that both copies of the 9-bp motif are necessary for full functional repression, since neither one alone is able to silence transcription. In addition to identifying the DNA sequence responsible for transcriptional repression, we have also presented evidence for the specific interaction of an unknown cellular factor with the PSM that correlates with transcriptional repression.
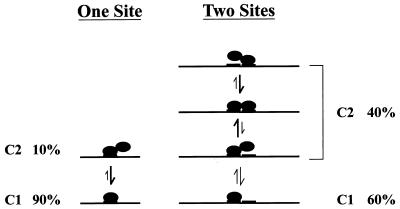
We propose that the complexes C1 and C2 seen in Fig. 5 represent the respective binding of a monomer and dimer of this unknown factor, which we will refer to as PSM-BP (PSM binding protein). We have come to this conclusion for the following reasons. First, we have never observed a C2 complex in the absence of C1, even when using probes containing a number of different mutations in the silencer element (34). Second, there are no other significant lower-molecular-weight complexes binding to probes such as II and III (Fig. 6C) that could form the higher-molecular-weight complex C2. Together, these observations suggest that C2 contains the factor that gives rise to C1. Third, we believe that C2 consists of a dimer of C1 because of the relationship that exists between the number of 5′-TAYAATAAT-3′ motifs present on the oligonucleotide probe and the ratio of complex C2 to complex C1.
In Fig. 5, a number of probes (I to V) were described and tested in EMSA experiments that contained one or more of the 5′-TAYAATAAT-3′ motifs. For the probes II and III that contain only one motif, a low ratio of C2 to C1 (1:9) was observed. This ratio was slightly higher in probe IV, which contains a partial second motif. However, it can be seen that for probes containing two complete motifs (as is the case for both probes I and V), a much higher ratio of C2 to C1 (1:1.5) exists, and thus far this observation has been consistent. This finding is not a consequence of probe size, since probe V is of a length comparable to that of probe IV and probe IV does not demonstrate this high ratio of C2 to C1.
In Fig. 8, we present a model that attempts to explain these observations by defining the complex C2 in terms of a dimeric form of the protein that gave rise to C1. In this model, the cellular factor represented by complex C1 (PSM-BP) can bind a single motif either as a monomer or as a dimer. When a single motif is present, the dimer is not very stable, as suggested by the low dimer-to-monomer (C2-C1) ratio. When bound to DNA containing two motifs, however, the dimer state is stabilized, since each partner can bind to DNA, resulting in the observed higher proportion of dimer-to-monomer (C2-C1) complexes. Such a model is not only consistent with our current EMSA data but also provides a potential explanation for our functional results. These results (Fig. 3) indicate a requirement for two copies of the 5′-TAYAATAAT-3′ motif in order to achieve transcriptional silencing. One interpretation of these data would be that only the dimer form of PSM-BP is capable of effective repression.
FIG. 8.
A model for the proposed interactions between the PSM and PSM-BP. Line, segment of the HPV-16 LCR; boldface lines, the 9-bp 5′-TAYAATAAT-3′ motif; filled circles, monomer of the unidentified factor PSM-BP. In the presence of a single 5′-TAYAATAAT-3′ motif, we observed only a low C2-to-C1 ratio, which could be explained by the fact that the dimeric form of PSM-BP is inherently unstable. With two complete 5′-TAYAATAAT-3′ motifs, the dimeric form of PSM-BP is stabilized, since both molecules of PSM-BP can bind independently to the DNA.
Confirmation of this model, as well as further insights into the mechanism of the transcriptional silencing described here, will require the identification of PSM-BP. Until that time, we are left with a number of interesting questions about the mechanism by which the silencer element represses transcription and also about the role of the silencer element in HPV biology.
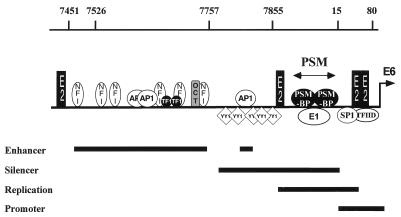
An initial inspection of other HPV replication origins has not indicated the presence of two copies of the 5′-TAYAATAAT-3′ motif in these viruses. However, we do not know exactly what nucleotide substitutions within this motif will still permit PSM-BP binding. Consequently, at this time it is too early to say if this regulatory mechanism is specific for HPV-16 or is present and functional in other HPVs. A search for the 5′-TAYAATAAT-3′ motif elsewhere within the HPV-16 genome also failed to identify further sites. Thus, it would appear that the PSM is limited to the position that overlaps the replication origin between the enhancer and the promoter (see Fig. 9). The fact that the PSM overlaps with the HPV E1 binding site (7, 40) suggests that the binding to this region by E1 and the cellular factor PSM-BP may be mutually exclusive events. We have not yet addressed this question experimentally; however, should PSM-BP prevent E1 binding and vice versa, this would raise the interesting prospect that HPV-16 DNA replication and transcriptional silencing by the PSM-BP are antagonistic events.
FIG. 9.
Schematic representation of the HPV-16 LCR illustrating some of the transcription factors that influence the activity of the P97 promoter, namely, NFI, AP-1, TEF-1 (TF1), Oct-1 (OCT), YY1, SP1, and the general transcription factor TFIID, as well as the HPV proteins E1 and E2. Overlapping with the HPV-16 E1 binding site is the PSM containing two binding sites for the cellular factor PSM-BP which may result in the mutually exclusive binding of these two factors. Black bars at the bottom, regions responsible for the regulation of HPV replication, mRNA initiation (the promoter), promoter activation (the enhancer), and promoter repression (the silencer region). The genomic positions of constructs examined in this study are indicated at the top.
Figure 9 illustrates that the region of the LCR between the enhancer and promoter of HPV-16 is a negative regulatory region that includes both the YY1 sites previously identified and the PSM described here. Experiments testing positional dependence (Fig. 6B) have suggested that transcriptional repression occurs when the silencing motif is placed between the enhancer and promoter, but not when it is moved upstream of the enhancer. One interpretation of this is that the PSM prevents the cross-talk between enhancer-bound activators and potential targets in the basal transcription machinery. The ability of the PSM to inhibit transcription involving heterologous enhancers and promoters (Fig. 7) suggests that the mechanism may be a general one. One possibility, therefore, might be that PSM-BP directly targets general transcription factors (GTFs) or other components of the basal transcription machinery. Another possibility is that PSM-BP affects the chromatin structure of the HPV-16 LCR.
Support for the latter idea comes from two observations. First, we have found no difference between the transcriptional activity of HPV-16 EP constructs with and without the silencer region (positions 7883 to 15) in in vitro transcription studies (34). These studies were carried out with nuclear extracts that for the most part have been depleted of the histone proteins necessary for the packaging of DNA into nucleosomes. Second, our recent studies have provided in vivo evidence for the specific positioning of nucleosomes within the LCR of HPV-16, including one site just downstream of the PSM in the vicinity of the P97 promoter (34). These findings suggest that there could be a link between the activity of the silencer element and the nucleosome organization of the HPV-16 LCR. One possibility is that the PSM-BP, once bound to the silencer motif, could stabilize a nucleosome organization that repressed P97 promoter activity. Alternatively, recent investigations have demonstrated that a number of different transcriptional repressors recruit deacetylase complexes that can modify nucleosomes, thereby decreasing the accessibility of transcription factors to a promoter (see reference 44 for a review). It is also possible that the PSM-BP dimer could recruit a deacetylase complex and in a similar manner repress HPV-16 transcription. Future studies should provide evidence as to whether any of these models are correct.
In summary, the discovery of a novel transcriptional regulatory element that is capable of silencing the expression of the HPV-16 E6 and E7 genes is an important addition to our understanding of both HPV-16 regulation and transcriptional control in general. While much effort has been spent in understanding transcriptional activation, relatively few examples of transcriptional repression have been investigated at the molecular level. The ability of the HPV-16 silencer element to inhibit heterologous enhancers and promoters suggests that this may represent another example of how the study of viruses provides important insights into the complexities of mammalian transcriptional regulation.
ACKNOWLEDGMENTS
We thank Bernd Gloss and Edward Manser for helpful discussions and Chiew-Hoon Tan for technical assistance.
REFERENCES
- 1.Apt D, Chong T, Liu Y, Bernard H U. Nuclear factor I and epithelial cell-specific transcription of human papillomavirus type 16. J Virol. 1993;67:4455–4463. doi: 10.1128/jvi.67.8.4455-4463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apt D, Liu Y, Bernard H U. Cloning and functional analysis of spliced isoforms of human nuclear factor I-X: interference with transcriptional activation by NFI/CTF in a cell-type specific manner. Nucleic Acids Res. 1994;22:3825–3833. doi: 10.1093/nar/22.19.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker C C, Phelps W C, Lindgren V, Braun M J, Gonda M A, Howley P M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987;61:962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan W K, Chong T, Bernard H U, Klock G. Transcription of the transforming genes of the oncogenic human papillomavirus-16 is stimulated by tumor promoters through AP1 binding sites. Nucleic Acids Res. 1990;18:763–769. doi: 10.1093/nar/18.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan W K, Klock G, Bernard H U. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J Virol. 1989;63:3261–3269. doi: 10.1128/jvi.63.8.3261-3269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chellappan S, Kraus V B, Kroger B, Munger K, Howley P M, Phelps W C, Nevins J R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow L T, Broker T R. Papillomavirus DNA replication. Intervirology. 1994;37:150–158. doi: 10.1159/000150373. [DOI] [PubMed] [Google Scholar]
- 8.Chow L T, Broker T R. In vitro experimental systems for HPV: epithelial raft cultures for investigations of viral reproduction and pathogenesis and for genetic analyses of viral proteins and regulatory sequences. Clin Dermatol. 1997;15:217–227. doi: 10.1016/s0738-081x(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 9.Cripe T P, Alderborn A, Anderson R D, Parkkinen S, Bergman P, Haugen T H, Pettersson U, Turek L P. Transcriptional activation of the human papillomavirus-16 P97 promoter by an 88-nucleotide enhancer containing distinct cell-dependent and AP-1-responsive modules. New Biol. 1990;2:450–463. [PubMed] [Google Scholar]
- 10.Cripe T P, Haugen T H, Turk J P, Tabatabai F, Schmid P G, Durst M, Gissmann L, Roman A, Turek L P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987;6:3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen A P, Reid R, Campion M, Lorincz A T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65:606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel B, Rangarajan A, Mukherjee G, Vallikad E, Krishna S. The link between integration and expression of human papillomavirus type 16 genomes and cellular changes in the evolution of cervical intraepithelial neoplastic lesions. J Gen Virol. 1997;78:1095–1101. doi: 10.1099/0022-1317-78-5-1095. [DOI] [PubMed] [Google Scholar]
- 13.Dollard S C, Wilson J L, Demeter L M, Bonnez W, Reichman R C, Broker T R, Chow L T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. Genes Dev. 1992;6:1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- 14.Dong G, Broker T R, Chow L T. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J Virol. 1994;68:1115–1127. doi: 10.1128/jvi.68.2.1115-1127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X P, Stubenrauch F, Beyer-Finkler E, Pfister H. Prevalence of deletions of YY1-binding sites in episomal HPV 16 DNA from cervical cancers. Int J Cancer. 1994;58:803–808. doi: 10.1002/ijc.2910580609. [DOI] [PubMed] [Google Scholar]
- 16.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 17.Galloway D A, McDougall J K. Human papillomaviruses and carcinomas. Adv Virus Res. 1989;37:125–171. doi: 10.1016/s0065-3527(08)60834-9. [DOI] [PubMed] [Google Scholar]
- 18.Gloss B, Bernard H U. The E6/E7 promoter of human papillomavirus type 16 is activated in the absence of E2 proteins by a sequence-aberrant Sp1 distal element. J Virol. 1990;64:5577–5584. doi: 10.1128/jvi.64.11.5577-5584.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloss B, Bernard H U, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howley P M. Role of the human papillomaviruses in human cancer. Cancer Res. 1991;51:5019s–5022s. [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer (IARC) Human papillomaviruses. Vol. 64. Lyon, France: IARC; 1995. The evaluation of carcinogenic risks to humans; pp. 233–250. [Google Scholar]
- 23.Ishiji T, Lace M J, Parkkinen S, Anderson R D, Haugen T H, Cripe T P, Xiao J H, Davidson I, Chambon P, Turek L P. Transcriptional enhancer factor (TEF)-1 and its cell-specific co-activator activate human papillomavirus-16 E6 and E7 oncogene transcription in keratinocytes and cervical carcinoma cells. EMBO J. 1992;11:2271–2281. doi: 10.1002/j.1460-2075.1992.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyo S, Klumpp D J, Inoue M, Kanaya T, Laimins L A. Expression of AP1 during cellular differentiation determines human papillomavirus E6/E7 expression in stratified epithelial cells. J Gen Virol. 1997;78:401–411. doi: 10.1099/0022-1317-78-2-401. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Baillie J, Sissons J G, Sinclair J H. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 1994;22:2453–2459. doi: 10.1093/nar/22.13.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsukura T, Koi S, Sugase M. Both episomal and integrated forms of human papillomavirus type 16 are involved in invasive cervical cancers. Virology. 1989;172:63–72. doi: 10.1016/0042-6822(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 27.May M, Dong X P, Beyer-Finkler E, Stubenrauch F, Fuchs P G, Pfister H. The E6/E7 promoter of extrachromosomal HPV16 DNA in cervical cancers escapes from cellular repression by mutation of target sequences for YY1. EMBO J. 1994;13:1460–1466. doi: 10.1002/j.1460-2075.1994.tb06400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride A A, Romanczuk H, Howley P M. The papillomavirus E2 regulatory proteins. J Biol Chem. 1991;266:18411–18414. [PubMed] [Google Scholar]
- 29.Meyers C, Frattini M G, Hudson J B, Laimins L A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 30.Montalvo E A, Shi Y, Shenk T E, Levine A J. Negative regulation of the BZLF1 promoter of Epstein-Barr virus. J Virol. 1991;65:3647–3655. doi: 10.1128/jvi.65.7.3647-3655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers G, Bernard H U, Delius H, Icenogle J, Baker C C, Halpern A, Wheeler C. Human papillomaviruses. Los Alamos, N.Mex: Los Alamos National Laboratory; 1995. [Google Scholar]
- 32.O’Connor M, Bernard H U. Oct-1 activates the epithelial-specific enhancer of human papillomavirus type 16 via a synergistic interaction with NFI at a conserved composite regulatory element. Virology. 1995;207:77–88. doi: 10.1006/viro.1995.1053. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor M, Chan S Y, Bernard H U. Transcription factor binding sites in the long control region of genital HPVs. In: Myers G, Bernard H U, Delius H, Icenogle J, Baker C C, Halpern A, Wheeler C, editors. Human papillomaviruses. Los Alamos, N.Mex: Los Alamos National Laboratory; 1995. pp. 21–40. [Google Scholar]
- 34.O’Connor, M. J., W. Stünkel, and H. U. Bernard. 1998. Unpublished observations.
- 35.O’Connor M J, Tan S H, Tan C H, Bernard H U. YY1 represses human papillomavirus type 16 transcription by quenching AP-1 activity. J Virol. 1996;70:6529–6539. doi: 10.1128/jvi.70.10.6529-6539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romanczuk H, Villa L L, Schlegel R, Howley P M. The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J Virol. 1991;65:2739–2744. doi: 10.1128/jvi.65.5.2739-2744.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 39.Schule R, Muller M, Otsuka-Murakami H, Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988;332:87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- 40.Sedman T, Sedman J, Stenlund A. Binding of the E1 and E2 proteins to the origin of replication of bovine papillomavirus. J Virol. 1997;71:2887–2896. doi: 10.1128/jvi.71.4.2887-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seedorf K, Krammer G, Durst M, Suhai S, Rowekamp W G. Human papillomavirus type 16 DNA sequence. Virology. 1985;145:181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y, Seto E, Chang L S, Shenk T. Transcriptional repression by YY1, a human GLI-Kruppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 43.Smotkin D, Wettstein F O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci USA. 1986;83:4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 45.Tan S H, Bartsch D, Schwarz E, Bernard H U. Nuclear matrix attachment regions of human papillomavirus type 16 point toward conservation of these genomic elements in all genital papillomaviruses. J Virol. 1998;72:3610–3622. doi: 10.1128/jvi.72.5.3610-3622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan S H, Gloss B, Bernard H U. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res. 1992;20:251–256. doi: 10.1093/nar/20.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan S H, Leong L E, Walker P A, Bernard H U. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J Virol. 1994;68:6411–6420. doi: 10.1128/jvi.68.10.6411-6420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thierry F, Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987;6:3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 50.Westin G, Gerster T, Muller M M, Schaffner G, Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987;15:6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.zur Hausen H, de Villiers E M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]