Abstract
We examined the effect of reduced water availability on the fatty acid composition of Pseudomonas putida strain mt-2 grown in a defined medium in which the water potential was lowered with the permeating solutes NaCl or polyethylene glycol (PEG) with a molecular weight of 200 (PEG 200) or the nonpermeating solute PEG 8000. Transmission electron microscopy showed that −1.0-MPa PEG 8000-treated cells had convoluted outer membranes, whereas −1.0-MPa NaCl-treated or control cells did not. At the range of water potential (−0.25 to −1.5 MPa) that we examined, reduced water availability imposed by PEG 8000, but not by NaCl or PEG 200, significantly altered the amounts of trans and cis isomers of monounsaturated fatty acids that were present in whole-cell fatty acid extracts. Cells grown in basal medium or under the −0.25-MPa water potential imposed by NaCl or PEG 200 had a higher trans:cis ratio than −0.25-MPa PEG 8000-treated cells. As the water potential was lowered further with PEG 8000 amendments, there was an increase in the amount of trans isomers, resulting in a higher trans:cis ratio. Similar results were observed in cells grown physically separated from PEG 8000, indicating that these changes were not due to PEG toxicity. When cells grown in −1.5-MPa PEG 8000 amendments were exposed to a rapid water potential increase of 1.5 MPa or to a thermodynamically equivalent concentration of the permeating solute, NaCl, there was a decrease in the amount of trans fatty acids with a corresponding increase in the cis isomer. The decrease in the trans/cis ratio following hypoosomotic shock did not occur in the presence of the lipid synthesis inhibitor cerulenin or the growth inhibitors chloramphenicol and rifampicin, which indicates a constitutively operating enzyme system. These results indicate that thermodynamically equivalent concentrations of permeating and nonpermeating solutes have unique effects on membrane fatty acid composition.
In soil, one of the more important environmental factors influencing the activity of microorganisms is the soil water potential (Ψ), which is the potential energy of water relative to the potential energy of pure water (17, 37). Generally, in saturated soils, the soil water potential is comprised almost exclusively of the solute potential, but as soils dry, the matric potential becomes the predominant factor contributing to the total soil water potential (17, 37). Consequently, the difference between these two stresses is that with a solute stress, bacteria are bathed in water (albeit water with diminished activity), whereas with a matric stress, bacteria become desiccated by the removal of water from its environment, and the availability of the water that is remaining is reduced through its interaction with the soil matrix. For dry nonsaline soils at −1.5 MPa of Ψ, which is the permanent wilting point for many agronomic plants, the water film thickness surrounding soil matrices has been estimated to be about 10 H2O molecules (3, 17).
Studies of bacterial water stress physiology have generally examined the genetic and physiological mechanisms of adaptation to osmotic stress caused by permeating solutes used to lower the water potential of the growth medium (11). In many nonsaline soils, however, lowering of the water potential is due primarily to a reduction in the water content and not to an increase in the concentration of permeating solutes. High-molecular-weight (i.e., a molecular weight [MW] of >3,000) polyethylene glycol (PEG) has been used extensively in plant (4, 24) and microbial (6, 31, 33) studies focused on responses to reduced water content. These PEGs are too large to penetrate cell walls and lower the water potential of a medium like a dry soil (46). PEG has also been used to demonstrate that osmoregulation of the proU operon in Salmonella enterica serovar Typhimurium (36) and synthesis of phosphocholine-substituted β-1,3;1,6 cyclic glucans in Bradyrhizobium japonicum (39) do not require solutes that can permeate into the periplasmic space of gram-negative bacteria.
The dehydration of membranes at a constant temperature causes a phase transition to a gel phase at a temperature at which they would be in a liquid crystalline phase if fully hydrated; that is, dehydration results in an increase in the transition temperature (9, 40). Further dehydration can cause a transition of the lamellar membranes to an inverted hexagonal II phase in which there are inverted phospholipids forming a micelle sandwiched between the bilayer (7, 10, 32). Sucrose and trehalose can depress the phase transition temperature during desiccation and may contribute to the ability of many microorganisms to survive desiccation by maintaining the fluidity of the membrane (9, 15, 40). Bacteria that are exposed to low matric water potentials may adjust membrane fatty acid composition or make other adaptations to offset the lipid-solidifying effects of dehydration in a fashion that is analogous to the homeoviscous adaptation of membrane fluidity to changes in temperature (30, 43) or hyperbaric pressure (12, 27). The phospholipid fatty acid (PLFA) profiles of subsurface bacteria have been previously shown to change during starvation and desiccation in a porous medium, although it is difficult to separate desiccation effects from starvation effects, since both stresses can occur simultaneously during air drying of a porous medium (28).
Our long-term goal is to elucidate the mechanisms that bacteria employ for responding to the forms of water deprivation they commonly encounter in soil. The objective of the work reported here was to assess whether the fatty acid composition of Pseudomonas putida mt-2 is differentially affected by permeating (NaCl or PEG 200 [i.e., a PEG with a MW of 200]) and nonpermeating PEG 8000 solutes, since they are frequently used to simulate solute and matric components of soil water potential, respectively. We used whole-cell fatty acid methyl ester (FAME) analysis to examine the effect of water deprivation on fatty acid composition, since this approach reflects the fatty acid composition of phospholipids (8, 48) and lipopolysaccharides (25). In these studies, we examined a range of water potentials that commonly occur in temperate and semiarid soils.
MATERIALS AND METHODS
Organisms, growth conditions, and culture media.
P. putida mt-2 was grown in 500-ml triple-baffle sidearm culture flasks (Bellco Biotechnology, Vineland, N.J.) at 27°C in an orbital shaker (150 rpm). Growth was monitored by measuring changes in culture density at 660 nm.
The basal medium consisted of 0.5 g of NH4Cl, 1.725 g of Na2HPO4 · 7H2O, 1.38 g of KH2PO4, and 100 ml of half-strength Hutner's mineral solution (44) per liter of deionized water. Glucose was provided at a concentration of 1.28 g per liter. The water potential of the basal medium was −0.15 MPa. To alter the water potential of the basal medium by −0.25, −0.5, −1.0, and −1.5 MPa, we added 3.2, 23.7, or 100 g; 6.4, 43.7, or 150 g; 12.8, 77.2, or 262 g; and 19.2, 105.3, or 330 g of NaCl, PEG 200, or PEG 8000, respectively (17, 45). All media were filter sterilized (with a 0.2-μm-pore-size filter) prior to use.
A solid medium with altered water potentials was prepared in the following manner. The basal medium constituents, 1.0 g of MgSO4 · 7H2O, 8.0 g of Phytagel gellan gum (Sigma Chemical Co., St. Louis, Mo.), and 500 ml of deionized water, were boiled for 1 min prior to autoclaving. The MgSO4 · 7H2O was omitted when solid media containing high concentrations (−1.0 and −1.5 MPa) of PEG 8000 were made. To achieve the desired water potential, various concentrations of PEG or NaCl were dissolved in 500 ml of deionized water, and the solutions were each filter sterilized (with a 0.2-μm-pore-size filter) into a sterile bottle containing a magnetic stir bar and incubated at 75°C. While the PEG or NaCl solutions were gently stirred on a stir plate, 500 ml of warmed (75°C) gellan gum solution was added slowly. Once these solutions were thoroughly mixed, the plates were poured immediately. For all experiments using solid medium, bacteria were grown physically separated from the agar surface by placing a 1,000-MW exclusion membrane (Amicon, Inc., Beverley, Mass.) between the solid medium containing PEG 8000 or NaCl and a Magnagraph nylon membrane (MSI, Westboro, Mass.), which served as a surface for growth of the bacteria. Plates were incubated in sealable plastic containers at 27°C. Water potentials of all liquid and solid media were determined with a thermocouple psychrometer (Decagon Devices, Inc., Pullman, Wash.).
Preparation of hypo- and hyperosmotically shocked cells.
For preparation of hypoosmotically shocked cells, 50 ml of mid-exponential-phase cultures grown in −1.5-MPa PEG 8000-amended medium was harvested by centrifugation and resuspended in 1 ml of −1.5-MPa PEG 8000-amended medium. These cells were then added to 49 ml of either −1.5-MPa PEG 8000-amended, −1.5-MPa NaCl-amended, or unamended medium. For preparation of hyperosmotically shocked cells, 50 ml of exponential phase cultures grown in unamended basal medium was harvested by centrifugation and resuspended in 1 ml of unamended medium. These cells were then added to 49 ml of either −1.5-MPa PEG 8000-amended, −1.5-MPa NaCl-amended, or unamended medium. The cell suspensions were shaken at 150 rpm for 15 to 120 min. After the desired length of time, cells were harvested by centrifugation for FAME analysis.
Inhibition of lipid, protein, and mRNA synthesis.
To inhibit lipid, protein, or mRNA synthesis, 10 μg of cerulenin per ml, 100 μg of chloramphenicol per ml, or 100 μg of rifampicin per ml was added to mid-exponential-phase cultures, respectively. Fifty milliliters of exponential-phase cultures was harvested by centrifugation and resuspended in 1 ml of medium of the same water potential as the growth medium, and the cultures were incubated on an orbital shaker with the inhibitors for 30 min at 27°C. The cell suspensions were then added to either 49 ml of −1.5-MPa PEG 8000-amended, −1.5-MPa NaCl-amended, or unamended medium to create a hypo- or hyperosmotic shock condition. The cell suspensions were incubated on an orbital shaker for 5 to 120 min before the cells were harvested by centrifugation for FAME analysis. The concentration of the inhibitors was held constant throughout the duration of the experiment. Chloramphenicol and rifampicin completely inhibited growth, as determined by monitoring changes in culture density and protein content following addition of these inhibitors. Cerulenin pretreatments at 10 μg/liter have been previously shown to inhibit fatty acid synthesis in P. putida (13, 19).
Cellular protein content.
Cells were isolated by centrifugation (30,000 × g for 20 min at 5°C) within 0.75 to 1.5 h after the onset of stationary phase and then were solubilized by heating at 90°C for 10 min in 1 N NaOH. Protein content was determined by the method of Bradford with the Protein Assay kit (Bio-Rad, Hercules, Calif.), and bovine serum albumin was used as the standard.
FAME analysis.
To assess the effect of chronic exposure to solute or matric stress on fatty acid composition, mid-exponential-phase cultures were used to inoculate media with the same concentration of NaCl or PEG amendments. These cultures were grown to mid-exponential phase, harvested by centrifugation as described above, frozen in a dry ice-ethanol bath, and stored at −80°C until they were thawed for fatty acid analysis. Total cellular fatty acids were extracted from lipids by mixing the cell pellets with a 15% NaOH solution made in 1:1 methanol and water, and the fatty acids were methylated by incubating the cell mixture in a 6 N HCl-methanol solution (3.25:2.75) for 10 min at 80°C. FAMEs were extracted with a 1:1 mixture of hexane and methyl tert-butyl alcohol. Flame ionization detection gas chromatography was performed using the MIDI system (Newark, Del.) and in accordance with the manufacturer's recommended materials and protocols. Peaks were compared to known standards with the Sherlock-MIDI identification system and commercially available 16:1 ω7t and ω7c FAME standards (Sigma Chemical Co.), since the Sherlock-MIDI system lumps these fatty acids into one group (designated sum4). A subset of samples were subjected to PLFA analysis and gas chromatography-mass spectrometry analysis (Microbial Insights, Knoxville, Tenn.) to verify peak identity. Similar fatty acid profiles were generated by the MIDI-FAME whole-cell and PLFA procedures, except for the inclusion of hydroxy and 12:0 fatty acids in the MIDI procedure. As a control, we determined that PEG does not produce FAME peaks that could be mistaken for fatty acids.
Fatty acid nomenclature is as follows: the first number reflects the total number of carbon atoms, and the second number (separated by a colon from the first) is the number of double bonds. If double bonds are present, the location is designated by its distance from the aliphatic (ω) end and its orientation in cis (c) or trans (t) geometry. The suffixes OH, cyclo, and ISO denote hydroxy, cyclopropyl, and isobranched fatty acids, respectively. Results are reported as the mean percentage of the total amount of fatty acids or the ratio of particular types of fatty acids ± the standard error (SE) of three to five independent replications.
Transmission electron microscopy.
Early-exponential-phase cultures were harvested by centrifugation and resuspended in the same growth medium to one-tenth of the original volume. Cells were subjected to propane jet cryopreservation, fixed in 1% OsO4, embedded in Epon/Araldite 502 resin, and poststained with lead citrate and uranyl acetate. Transmission electron micrographs were taken with a JEOL electron microscope. Cells grown on a solid medium amended with either PEG or NaCl to attain a water potential of −1.0 MPa were used directly for electron microscopy.
Statistical analyses.
Statistical analyses were performed using Systat software (SPSS, Inc., Chicago, Ill.). For each type of solute, a one-factor analysis of variance was performed on each fatty acid to compare the effect of water potential on fatty acid composition. Fisher's least significant difference (LSD) (P = 0.01) was calculated by Systat software for comparison of treatment means. A paired t test was performed to compare the amount of fatty acid synthesized among the various types of solute amendments for each type of fatty acid. For cells exposed to an acute increase or decrease in water availability in the presence of various inhibitors (cerulenin, chloramphenicol, or rifampicin), a separate two-way analysis of variance was performed for each fatty acid. Factors noted included basal medium and NaCl- or PEG 8000-amended medium plus the inhibitors.
RESULTS
Influence of water stress on cell morphology.
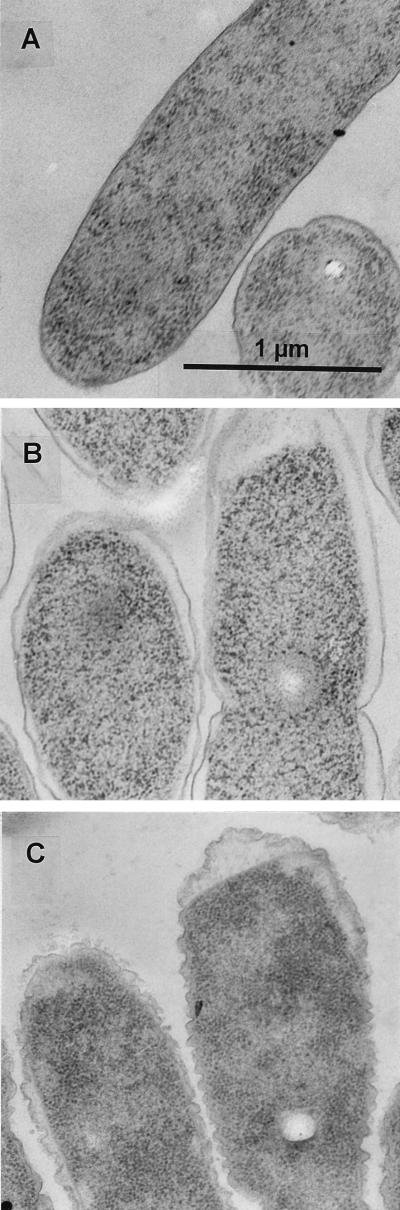
We assessed the effects of NaCl and PEG 8000 treatments on cell ultrastructure by transmission electron microscopy. The outer membranes of cells grown in the basal medium, −0.25-MPa PEG 8000-amended medium (data not shown), and −1.0-MPa NaCl-amended medium were smooth, whereas those grown in −1.0-MPa PEG 8000-amended medium appeared thicker and convoluted (Fig. 1). The apparently thicker outer membrane may be an artifact of viewing several planes of a convoluted membrane. We consistently observed that NaCl-treated cells exhibited a greater degree of plasmolysis than the PEG 8000-treated cells, which suggests that PEG 8000 did not penetrate the outer membrane (Fig. 1). The cytoplasmic membrane did not appear to be altered, even at higher magnifications (data not shown). Similar results were observed when cells were grown on a 1,000-MW exclusion ultrafiltration membrane overlaying a solid medium containing PEG 8000 or NaCl (data not shown); PEG 8000, but not NaCl, is too large to diffuse through the ultrafiltration membrane. These results suggest that the altered outer membranes observed in the PEG 8000 liquid cultures were not due to a direct physical interaction between PEG and the cell membrane.
FIG. 1.
Transmission electron micrographs of cell ultrastructure during water stress. (A) Basal medium; (B) −1.0-MPa NaCl-treated medium; (C) −1.0-MPa PEG 8000-treated medium. Magnification, ×36, 600. Bar, 1 μm.
Effects of permeating and nonpermeating solutes on cellular fatty acid composition.
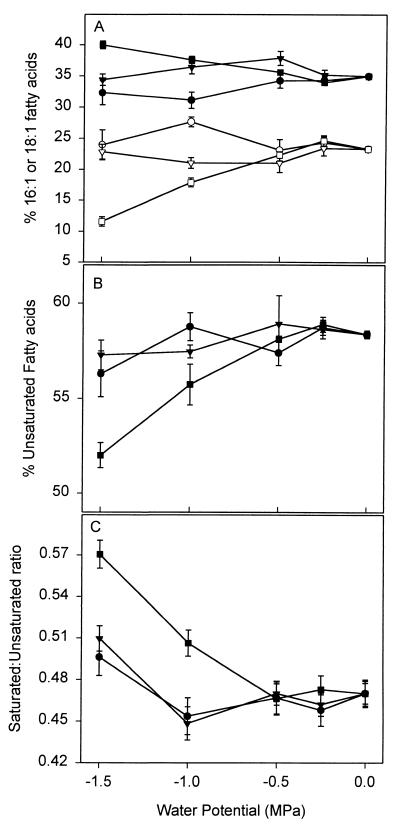
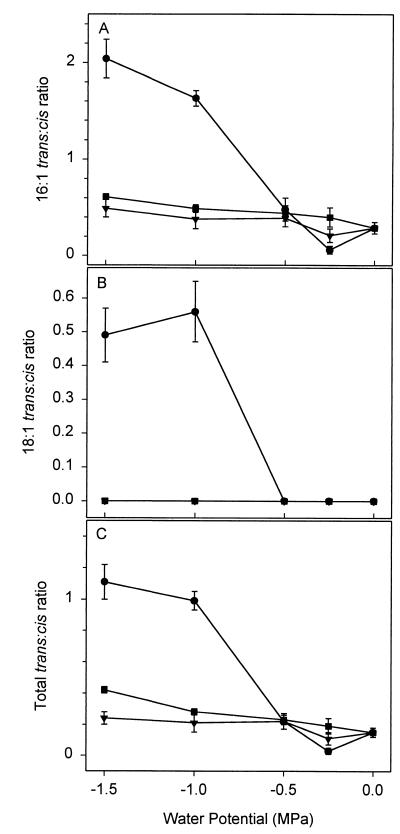
We performed FAME analysis on whole-cell fatty acid extracts of early- to mid-exponential-phase cultures grown in the presence of one of the permeating solutes (NaCl or PEG 200) or the nonpermeating solute PEG 8000. In general, the permeating and nonpermeating solutes at various water potentials primarily affected the relative amount of cis and trans isomers of monounsaturated fatty acids (Figs. 2–4) and, to a lesser extent, the ratio of saturated to unsaturated fatty acids (Fig. 4). The greatest differences between the effects of the nonpermeating solute, PEG 8000, and the permeating solutes were in the percentages of the trans and cis isomers of the 16:1 and 18:1 fatty acids (Fig. 2) and the trans:cis ratio of these fatty acids (Fig. 3). For example, for −0.25-MPa PEG 8000-treated cells, the trans:cis ratio was 0.03 ± 0.02 as compared to 0.15 ± 0.03 for the untreated cells and 0.11 ± 0.04 and 0.19 ± 0.05 for the −0.25-MPa NaCl- and PEG 200-treated cells, respectively (Fig. 3C). At a water potential of −0.25 MPa, the amount of the trans isomer 16:1 ω7t in PEG 8000-treated cells was significantly smaller (P = 0.05) than in the NaCl- or PEG 200-treated cells, based on a paired t test analysis. In contrast, at water potentials below −0.25 MPa, the PEG 8000-treated cells exhibited a reduction in the cis isomers and a corresponding increase in the trans isomers of the 16C and 18C monounsaturated fatty acids (Fig. 2). At water potentials of −1.0 MPa or lower in the PEG 8000 treatments, there was approximately a threefold increase in the amount of 16:1 ω7t compared to the no-stress control (0 MPa) and −0.25 MPa (Fig. 2). 18:1 ω7t was detected only at water potentials of −1.0 MPa or lower in PEG 8000-treated cells. Based on a paired t test at water potentials of −1.0 and −1.5 MPa, the PEG 8000-treated cells had a statistically significant increase in the amount of trans fatty acids and in the trans:cis ratio when compared to either PEG 200- or NaCl-treated cells (Fig. 2 and 3). The increase in the amounts of trans fatty acids at −1.0 and −1.5 MPa was not due to a reduced growth rate, since NaCl, sucrose, and PEG 200 treatments impose a reduction in growth rate similar to that of PEG 8000 treatments (21) without significantly altering the trans:cis ratio (Fig. 3 and data not shown).
FIG. 2.
Effects of permeating and nonpermeating solutes on trans-to-cis isomerization. (A) 16:1 fatty acids; (B) 18:1 fatty acids; (C) percent cis or trans isomers. Open symbols, cis isomer; closed symbol, trans isomer; ○ or ●, PEG 8000; □ or ■, PEG 200; ▵ or ▴, NaCl. Values are the means ± SE of three to five replications. LSDs for the percentage of 16:1 cis isomers (A) are 3.7, 3.8, and 3.2 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the percentage of 16:1 trans isomers (A) are 3.5, 3.9, and 3.9 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the percentage of 18:1 cis isomers (B) are 23.3, 1.9, and 2.2 for the PEG 8000, PEG 200, and NaCl treatments, respectively. The LSD for the percentage of 18:1 trans isomer for the PEG 8000 treatments is 1.4 (B). LSDs for the percentage of cis isomers (C) are 3.6, 4.2, and 4.2 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the percentage of trans isomers (C) are 3.5, 3.9, and 3.9 for PEG 8000, PEG 200, and NaCl treatments, respectively.
FIG. 4.
Effects of permeating and nonpermeating solutes on monounsaturated and saturated fatty acids. (A) Percentage of 16:1 and 18:1 fatty acids; (B) percentage of total monounsaturates; (C) saturated:unsaturated fatty acid ratio. Open symbols, 18:1 fatty acids; closed symbols, 16:1 fatty acids (A); ●, PEG 8000; ■, PEG 200, ▴, NaCl (B and C). Values are means ± SE of three to five replications. LSDs for the percentage of 16:1 fatty acids (A) are 2.6, 1.4, and 1.7 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the percentage of 18:1 fatty acids (A) are 2.7, 1.9, and 2.2 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the percentage of unsaturated fatty acids (B) are 1.3, 1.7, and 1.7 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSDs for the saturated:unsaturated fatty acids ratio (C) are 0.03, 0.03, and 0.02 for PEG 8000, PEG 200, and NaCl treatments, respectively.
FIG. 3.
Effects of permeating and nonpermeating solutes on trans:cis ratio. (A) 16:1 fatty acids; (B) 18:1 fatty acids; (C) total. ●, PEG 8000; ■, PEG 200; ▴, NaCl. Values are the means ± SE of three to five replications. LSDs for panel A are 0.22, 0.2, and 0.17 for PEG 8000, PEG 200, and NaCl treatments, respectively. LSD for panel B is 0.09 for the PEG 8000 treatments. LSDs for panel C are 0.11, 0.09, and 0.09 for PEG 8000, PEG 200, and NaCl treatments, respectively.
The total amounts of 16:1 or 18:1 fatty acids were influenced by the type of solute used to lower the medium's water potential. For example, as the water potential was lowered, the amount of 16:1 decreased in the PEG 8000 amendments, increased in the PEG 200 amendments, and did not change with the NaCl amendments (Fig. 4A); statistically significant differences were only observed at water potentials of −1.0 and −1.5 MPa. In contrast, the amount of 18:1 fatty acids was negatively correlated with the amount of 16:1 fatty acids (Fig. 4A). Although amounts of individual monounsaturated fatty acids were influenced by the severity of stress and type of solute used to impose stress, the total amount of unsaturated fatty acids and the ratio of saturated to unsaturated fatty acids generally remained constant at all water potentials for each solute (Fig. 4B). The exception was PEG 200-treated cells, which had smaller amounts of monounsaturated fatty acids (Fig. 4B) with a corresponding increase in saturated fatty acids (Fig. 4C). Although there was a reduction in the amount of monounsaturated fatty acids in PEG 200-treated cells as the water potential was lowered, there was no significant effect from lowering the water potential on the trans:cis ratio, compared to the PEG 8000-treated cells (Fig. 3).
PEG-cell interactions on fatty acid composition.
P. putida was physically separated from PEG 8000-amended solid medium by growth on a 1,000-MW exclusion membrane overlaying the solid medium. In general, fatty acid profiles of cells grown on a solid medium were similar to the profiles of cells grown in liquid batch culture, except for the presence of cyclopropyl fatty acids (Table 1). There were no statistically significant differences in the fatty acid composition of untreated and −1.0-MPa NaCl-treated cells (data not shown).
TABLE 1.
Effect of PEG-cell interactions on fatty acid compositiona
| Fatty acid | Mean ± SE (%) fatty acid content
|
|
|---|---|---|
| Basal medium | −1.0-MPa PEG-amended medium | |
| 16:1 ω7c | 5.45 ± 0.46 | 5.58 ± 2.47 |
| 16:1 ω7t | 6.64 ± 0.28 | 8.16 ± 1.81 |
| 17:0 cyclo | 18.70 ± 0.96 | 15.28 ± 4.46 |
| 18:1 ω7c | 15.18 ± 0.95 | 10.39 ± 0.02 |
| 18:1 ω7t | 0.00 ± 0.00 | 4.75 ± 0.23 |
| trans:cis ratio | 0.32 ± 0.01 | 0.81 ± 0.04 |
A 1,000-MW exclusion membrane was placed between solid medium containing PEG or NaCl (data not shown) and a nylon membrane, which served as a surface for bacterial growth.
Effect of hyperosmotic shock on fatty acid composition.
Exponentially growing cells of P. putida were exposed to a sudden decrease in water availability by the resuspension of pelleted cells grown in a basal medium in either −1.5-MPa NaCl- or PEG 8000-amended or unamended medium. There was no change in culturability of the cell population or in protein content before and after the hyperosmotic shock. Within 15 min after this sudden decrease in water availability, there was approximately a 100 and 200% increase in the amount of 16:1 ω7t in the NaCl- and PEG 8000-treated cells, respectively, with a corresponding decrease in 16:1ω7c in both treatments (Table 2). Unlike cells growing in the presence of NaCl (Fig. 2 and 3), the −1.5-MPa (NaCl-treated) osmotically shocked cells exhibited a statistically significant increase (P = 0.05) in the amount of 16:1 ω7t. There was no significant change in the total amount of 16:1 and 18:1 monounsaturated, unsaturated, or hydroxy fatty acids (data not shown). Also, unlike the PEG 8000-treated cells, the untreated and NaCl-treated (shocked) cells did not produce detectable amounts of 18:1 ω7t (Table 2). Neither fatty acyl chain length nor saturation of fatty acids was affected by the sudden decrease in water availability (data not shown).
TABLE 2.
Effect of hyperosmotic shock on cis-to-trans isomerizationa
| Treatment | Fatty acid content (%)b
|
||||||
|---|---|---|---|---|---|---|---|
| 16:1ω7c | 16:1ω7t | 18:1ω7c | 18:1ω7t | Total cis | Total trans | Total trans:cis ratio | |
| None | 29.1 ± 3.6 A | 5.7 ± 1.9 A | 22.3 ± 0.2 A | 0 ± 0 A | 51.3 ± 3.8 A | 5.7 ± 1.9 A | 0.10 ± 0.04 A |
| NaCl | 23.4 ± 1.1 B | 10.7 ± 1.5 B | 22.6 ± 0.4 A | 0 ± 0 A | 46.0 ± 1.5 B | 10.7 ± 1.5 B | 0.24 ± 0.03 B |
| PEG 8000 | 16.0 ± 0.8 C | 17.3 ± 0.8 C | 20.0 ± 1.1 B | 2.6 ± 1.6 B | 36.0 ± 2.7 C | 19.9 ± 2.4 C | 0.55 ± 0.09 C |
Mid-exponential-phase cells were grown in a basal medium and suddenly exposed to a −1.5-MPa reduction in solution water potential by the addition of the permeating solute NaCl or the nonpermeating solute PEG 8000. Fatty acids were extracted from cells within 15 min after the addition of NaCl or PEG 8000. Values are the means ± SE of three replications.
Values followed by the same letter in a column are not statistically different, based on Fisher's LSD (P ≤ 0.05).
Effect of hypoosmotic shock on fatty acid composition.
Cells were exposed to a sudden increase in water availability by resuspending pelleted cells grown in PEG 8000-amended medium in the basal medium. There was no change in culturability or protein content before or after hypoosmotic shock. Within 15 min, there was a 53% decrease in the amount of trans fatty acids with a corresponding increase (46%) in the amount of cis fatty acids (Table 3). Most of the changes in the amount of trans fatty acids were due to a disappearance of 18:1 ω7t with a corresponding increase in 18:1 ω7c. Preincubation of cells in the presence of either an inhibitor of fatty acid synthase (cerulenin), protein synthesis (chloramphenicol), or transcription (rifampicin) did not prevent the trans-to-cis isomerization from occurring during conditions of hypoosmotic shock (Table 4). Preliminary studies indicated that pretreatment with these inhibitors reduced the incorporation of sodium [1-14C]acetate into lipids or a combined protein and nucleic acid pool to 0.8 to 2.3% of the amount of label incorporated into untreated cells (L. J. Halverson, unpublished data). Also, the concentrations of chloramphenicol and rifampicin were sufficient to inhibit the growth of P. putida mt-2 completely. This suggests that there is a constitutively operating trans-to-cis enzyme system that is able to change the orientation of the hydrogen atoms at the double bond following hypoosmotic shock. Furthermore, a sudden change in the form of water deprivation (from nonpermeating to permeating solute) also resulted in an increase in the amount of the cis isomer with a corresponding decrease in the trans isomer (Table 3). However, this change was not as dramatic as that observed when cells were exposed to a sudden increase in water availability. Again, cerulenin, chloramphenicol, and rifampicin pretreatments did not prevent the increase in the amount of cis isomers (L. J. Halverson, unpublished data). There was no affect of hypoosmotic shock or change from permeating to nonpermeating solute on fatty acyl chain length or saturation of fatty acids (data not shown).
TABLE 3.
Effect of hypoosmotic shock or exposure to the permeating solute NaCl on fatty acid compositiona
| Treatment | Fatty acid content (%)b
|
||||||
|---|---|---|---|---|---|---|---|
| 16:1ω7c | 16:1ω7t | 18:1ω7c | 18:1ω7t | Total cis | Total trans | Total trans:cis ratio | |
| Control | 12.0 ± 0.9 A | 18.5 ± 1.1 A | 16.9 ± 2.7 A | 4.0 ± 1.3 A | 28.9 ± 3.6 A | 22.5 ± 2.9 A | 0.78 ± 0.11 A |
| Basal | 19.4 ± 1.5 B | 10.5 ± 1.7 B | 23.2 ± 0.3 B | 0 ± 0 B | 42.6 ± 1.5 B | 10.5 ± 1.7 B | 0.25 ± 0.05 B |
| NaCl | 14.3 ± 0.9 A | 16.9 ± 1.4 A | 21.3 ± 0.4 B | 0 ± 0 B | 35.6 ± 1.2 C | 16.9 ± 1.4 C | 0.47 ± 0.05 C |
Mid-exponential-phase cultures grown in −1.5-MPa PEG 8000-amended medium were either exposed to a sudden increase in solution water potential (basal treatment), no increase in water potential but a change from a nonpermeating to a permeating solute (NaCl treatment), or no change in the form or magnitude of water deprivation (control). Fatty acids were extracted from cells within 15 min after the treatments were initiated. Values are the mean percentage ± SE of three replications.
Values followed by the same letter in a column are not statistically different, based on Fisher's LSD (P ≤ 0.05).
TABLE 4.
Effect of physiological inhibitors on cellular fatty acid composition, following hypoosmotic shocka
| Treatment | Fatty acid content
|
|||||
|---|---|---|---|---|---|---|
| 16:1 ω7t
|
18:1 ω7t
|
Total trans/cis ratio
|
||||
| Controlb | Shockc | Control | Shock | Control | Shock | |
| No inhibitor | 18.5 ± 1.1 | 10.5 ± 1.7 | 4.0 ± 1.3 | 0.0 ± 0 | 0.78 ± 0.11 | 0.27 ± 0.05 |
| Rifampicin | 19.0 ± 1.2 | 12.8 ± 1.9 | 3.7 ± 1.9 | 0.0 ± 0 | 0.85 ± 0.18 | 0.33 ± 0.06 |
| Chloramphenicol | 18.3 ± 1.1 | 11.3 ± 1.5 | 4.1 ± 2.1 | 0.0 ± 0 | 0.83 ± 0.19 | 0.28 ± 0.04 |
| Cerulenin | 18.3 ± 1.0 | 11.7 ± 1.4 | 3.4 ± 1.7 | 0.0 ± 0 | 0.82 ± 0.17 | 0.29 ± 0.04 |
Values are the means ± SE of three replications. Cultures were exposed to the inhibitors for 30 min prior to the hypoosmotic shock. Fatty acids were extracted 15 min after the osmotic shock.
No change in water potential.
Hypoosmotic shock (1.5-MPa water potential increase).
DISCUSSION
Our findings show that there is a differential effect of permeating and nonpermeating solutes on the fatty acid composition of P. putida mt-2, which suggests that there is a differential effect of matric and solute components of soil water potential on cellular fatty acid composition. First, a cis-to-trans isomerization of monounsaturated fatty acids occurred in response to growth in the presence of the nonpermeating solute PEG 8000 but did not occur in response to growth in the permeating solutes (Fig. 2 to 4). These results support and expand an earlier observation that a desiccated (air-dried) Pseudomonas aureofaciens strain in sand cultures exhibits a twofold increase in the amount of trans fatty acids at approximately a 5% water content (28). It has been previously shown that the cis-to-trans isomerase is constitutively expressed in P. putida P8 (14, 19), and more recently, a genetic approach has been used to demonstrate constitutive expression of the cti (cis-to-trans isomerase) gene in a solvent-resistant P. putida strain (26). The cis-to-trans isomerase is localized in the periplasm (26, 38) and apparently requires the involvement of other factors for its activity (38), possibly of a cytochrome c-type protein (22). Second, there was a greater increase in the cis-to-trans isomerization of monounsaturated fatty acids when cells were shocked with nonpermeating solutes than with permeating solutes (Table 2). Lastly, the trans/cis ratio decreases when cells were hypoosmotically shocked and when the form of stress is switched from a nonpermeating to a permeating solute (Table 3). The trans-to-cis isomerization appears to be due to a constitutively operating enzyme system, although it cannot completely reverse the cis-to-trans isomerization that occurs when cells are exposed to a sudden decrease in water availability. A trans-to-cis isomerization has been previously suggested to occur in response to cold shock, although a corresponding increase in the amount of cis isomer was not observed (34). Further work is necessary to firmly establish that the trans-to-cis isomerization is the result of a constitutively operating enzyme system.
There is strong evidence indicating that PEG is excluded from membrane surfaces (1, 2), and our results clearly show that alterations in membrane ultrastructure and fatty acid composition are not due to PEG-cell interactions, since these responses also occurred when cells were physically separated from PEG 8000. Ultrastructural changes may be the result of osmotic pressure being exerted on the outer membrane rather than the cytoplasmic membrane, since nonpermeating sugars can also impose similar effects on cell ultrastructure (51).
Presumably, these changes reduce the damaging effects of PEG 8000-mediated dehydration on membrane integrity that have been observed in other systems (5, 9, 23). The poorly dehydrating properties of NaCl (49) may explain the differential effects of NaCl and PEG 8000 on the fatty acid composition of P. putida (Fig. 2 to 4 and Table 3). An increase from a millimolar to molar NaCl concentration has a very small effect on the gel-to-liquid crystalline phase transition temperature (42), and only with toxic concentrations of NaCl is there a significant increase in the trans/cis ratio of P. putida strain S12 (18). It is unlikely that the cis-to-trans isomerization we observed in PEG 8000-treated cells was due to the trace amounts of impurities in the PEG (<0.0005 to 0.05%), since it has been previously shown that millimolar concentrations of those elements are required for the cis-to-trans isomerization (18).
Since trans monounsaturated fatty acids have a packing arrangement similar to that of saturated fatty acids, isomerization of cis fatty acids into trans fatty acids reduces the spacing between phospholipids. This could explain the increase in trans unsaturated fatty acids at matric water potentials of ≤−1.0 MPa (Fig. 2 to 4); however, this does not explain the decrease in trans fatty acids at −0.25 MPa (Fig. 2 to 4). Rand and Parsegian (41) reported that one-third to one-half of the water of a hydrated phosphatidylcholine bilayer could be removed with a relative humidity equivalent to a −0.1-MPa water potential. The thickness of the water film surrounding soil microorganisms at a −0.1-MPa matric water potential has been estimated to be 1.5 μm thick (17). It is conceivable that at a −0.25-MPa matric water potential, membranes are sufficiently dehydrated that the gel-to-liquid crystalline phase transition temperature is raised. Thus, P. putida may adapt by increasing the proportion of cis monounsaturated fatty acids and lowering the phase transition temperature, therefore maintaining the membrane in a liquid crystalline phase to counteract the membrane-solidifying effects of dehydration. Further reductions in matric water potential could lower the liquid crystalline-to-hexagonal phase transition temperature. By increasing the amount of trans unsaturated fatty acids, P. putida may raise this transition temperature (7, 29, 50) and prevent the formation of hexagonal II phases, which are favored by cis monounsaturated fatty acids, and thus again maintain the membrane in a liquid crystalline phase.
The synthesis of trans monounsaturated fatty acids has been reported to increase in several species of gram-negative bacteria in response to various physiologically stressful conditions, such as pH, temperature, starvation, desiccation, and heavy metal and pollutant toxicity (14, 18–20, 28, 34, 35, 50; H. C. Pinkart, D. C. White, J. Wolfram, and R. Rodgers, Abstr. 94th Gen. Meet. Am. Soc. Microbiol. 1994, abstr. K-85, p. 290, 1994). Desiccation of P. aureofaciens in air-dried sand cultures resulted in an increase in the ratio of saturated to unsaturated fatty acids, an increase in the ratio of trans to cis monounsaturated fatty acids, and an increase in the ratio of cyclopropyl fatty acids to their monoenoic precursors (28). We did not observe an increase in the ratio of saturated to unsaturated fatty acids or in the ratio of cyclopropyl fatty acids to their monoenoic precursors when P. putida was exposed to simulated matric water stress conditions. This is probably due to differences in the growth phase (exponential versus stationary phase) in these studies. Furthermore, the extent of cis-to-trans isomerization in response to nonpermeating solutes and desiccation in porous medium is comparable to the extent of the cis-to-trans isomerization observed with cells exposed to organic pollutants or elevated temperature.
In an earlier report (21), we showed that growth rates of P. putida mt-2 were slightly higher in PEG 8000-amended medium (−0.25 MPa) than in an unamended medium or media amended with various permeating solutes (−0.25 MPa). This higher growth rate correlates with a reduction in the total amount of trans monounsaturated fatty acids and with a corresponding increase in the amount of cis isomer. Membrane fluidity can strongly influence nutrient transport rates (52, 53), membrane enzyme activities (16), and electron transfer rates (47). It is possible that with −0.25-MPa PEG 8000 treatments, there are similar changes in membrane protein activities due to increased membrane fluidity; as a consequence, P. putida grows faster.
To the best of our knowledge, this is the first report of the differential effect of thermodynamically equivalent concentrations of permeating and nonpermeating solutes on cellular fatty acid composition. Our results also indicate that the amount of trans isomers of monounsaturated fatty acids can decrease, following an increase in water availability or in response to a change from a nonpermeating solute to a permeating solute. We cannot state conclusively that these observations represent homeoviscous adaptation of membrane fluidity until physical measurements of membrane viscosity are made. However, studies of other organisms have shown a strong correlation between temperature- and pressure-induced changes in lipid composition and maintenance of membrane fluidity (27, 43). Additional studies will allow us to determine whether membrane fluidity is altered by matric water stress, whether other physiological strategies are employed to counter the membrane destabilizing effects of cellular dehydration, and whether the same enzyme is responsible for the cis-to-trans and trans-to-cis isomerization activities.
ACKNOWLEDGMENTS
We thank the UC Berkeley Electron Microscopy Lab for their assistance with the electron microscopy. We also thank Trish Holden, Martijn van de Mortel, Steve Lindow, and Gwyn Beattie for critically reading an earlier draft of the paper and Joe Miller for technical assistance.
This research was supported in part by National Institute of Environmental Health Science Superfund Program grant 3P42 ES04705-07 (M.K.F.) and an ISU University Research Grant (L.J.H.). This work was also supported by the Hatch Act and the State of Iowa.
Footnotes
Journal paper no. J-18636 of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, project no. IOW03439.
REFERENCES
- 1.Arnold K. Cation-induced vesicle fusion modulated by polymers and proteins. In: Lipowsky R, Sackmann E, editors. Handbook of biological physics. 1B. Structure and dynamics of membranes. Amsterdam, The Netherlands: Elsevier Science; 1995. [Google Scholar]
- 2.Arnold K, Zschoernig O, Barthel D, Herold W. Exclusion of poly-(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990;1022:303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- 3.Baver L D, Gardner W H, Gardner W R. Soil physics. New York, N.Y: John Wiley and Sons; 1972. [Google Scholar]
- 4.Blackman S A, Wettlaufer S H, Obendorf R L, Leopold A C. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol. 1991;96:868–874. doi: 10.1104/pp.96.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boss W F, Mott R L. Effects of divalent cations and polyethylene glycol on the membrane fluidity of protoplasts. Plant Physiol. 1980;66:835–837. doi: 10.1104/pp.66.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse M D, Bottomly P J. Growth and nodulation responses of Rhizobium meliloti to water stress induced by permeating and nonpermeating solutes. Appl Environ Microbiol. 1989;55:2431–2436. doi: 10.1128/aem.55.10.2431-2436.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffrey M. The influence of water on the phase properties of membrane lipids: relevance to anhydrobiosis. In: Leopold A C, editor. Membranes, metabolism, and dry organisms. Ithaca, N.Y: Comstock Publishing Associates, University Press; 1986. pp. 242–258. [Google Scholar]
- 8.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids. In: Neidhart F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 474–497. [Google Scholar]
- 9.Crowe J H, Crowe L M, Carpenter J F, Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe L M, Crowe J H. Hydration-dependent phase transitions and permeability properties of biological membranes. In: Leopold A C, editor. Membranes, metabolism, and dry organisms. Ithaca, N.Y: Comstock Publishing Associates; 1986. pp. 211–230. [Google Scholar]
- 11.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong E F, Yayanos A A. Adaptation of the membrane lipids of a deep-sea bacterium to changes in hydrostatic pressure. Science. 1985;228:1101–1103. doi: 10.1126/science.3992247. [DOI] [PubMed] [Google Scholar]
- 13.Diefenbach R, Heipieper H-J, Keweloh H. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl Microbiol Biotechnol. 1992;38:382–387. [Google Scholar]
- 14.Diefenbach R, Keweloh H. Synthesis of trans unsaturated fatty acids in Pseudomonas putida P8 by direct isomerization of the double bonds of lipids. Arch Microbiol. 1994;162:120–125. doi: 10.1007/s002030050112. [DOI] [PubMed] [Google Scholar]
- 15.Eleutherio E C A, de Araujo P S, Panek A D. Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochim Biophys Acta. 1993;1156:263–266. doi: 10.1016/0304-4165(93)90040-f. [DOI] [PubMed] [Google Scholar]
- 16.Gordon L M, Sauerheber R D, Esgate J A, Dipple I, Marchmont R J, Houslay M. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J Biol Chem. 1980;255:4519–4527. [PubMed] [Google Scholar]
- 17.Harris R F. Effect of water potential on microbial growth and activity. In: Parr J F, Gardner W R, Elliot L F, editors. Water potential relations in soil microbiology. Madison, Wis: Soil Science Society of America; 1981. pp. 23–96. [Google Scholar]
- 18.Heipieper H-J, Meulenbeld G, van Oirschot Q, de Bont J A M. Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl Environ Microbiol. 1996;62:2773–2777. doi: 10.1128/aem.62.8.2773-2777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heipieper H-J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heipieper H-J, Weber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 21.Holden P A, Halverson L J, Firestone M K. Water stress effects on toluene biodegradation by Pseudomonas putida. Biodegradation. 1997;8:143–151. doi: 10.1023/a:1008237819089. [DOI] [PubMed] [Google Scholar]
- 22.Holtwick R, Keweloh H, Meinhardt F. cis/trans isomerase of unsaturated fatty acids of Pseudomonas putida P8: evidence for a heme protein of the cytochrome c type. Appl Environ Microbiol. 1999;65:2644–2649. doi: 10.1128/aem.65.6.2644-2649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui S-W, Boni L T. Membrane fusion induced by polyethylene glycol. In: Wilschut J, Hoekstra D, editors. Membrane fusion. New York, N.Y: Dekker; 1991. pp. 231–253. [Google Scholar]
- 24.Iraki N M, Bressan R A, Hasegawa P M, Carpita N C. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 1989;91:39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jantzen E, Bryn K. Whole-cell and lipopolysaccharide fatty acids and sugars of gram-negative bacteria. In: Goodfellow M, Minnikin D E, editors. Chemical methods in bacterial systematics. London, England: Academic Press; 1985. pp. 145–171. [Google Scholar]
- 26.Junker F, Ramos J L. Involvement of the cis/trans isomerase Cti in solvent resistance of Pseudomonas putida DOT-T1E. J Bacteriol. 1999;181:5693–5700. doi: 10.1128/jb.181.18.5693-5700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneshiro S M, Clark D S. Pressure effects on the composition and thermal behavior of lipids from the deep-sea thermophile Methanococcus jannaschii. J Bacteriol. 1995;177:3668–3672. doi: 10.1128/jb.177.13.3668-3672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieft T L, Ringleberg D B, White D C. Changes in ester-linked phospholipid fatty acid profiles of subsurface bacteria during starvation and desiccation in a porous medium. Appl Environ Microbiol. 1994;60:3292–3299. doi: 10.1128/aem.60.9.3292-3299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdonald P M, Sykesma B D, McElhaney R N. Fluorin-19 nuclear magnetic resonance studies of lipid fatty acyl chain order and dynamics in Acholeplasma laidlawii B membranes. A direct comparison of the effects of cis and trans cyclopropane ring and double-bond substituents on oriental order. Biochemistry. 1985;24:4651–4659. doi: 10.1021/bi00338a026. [DOI] [PubMed] [Google Scholar]
- 30.Marr A G, Ingraham J L. Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol. 1962;84:1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAneney K J, Harris R F, Gardner W R. Bacterial water relations using polyethylene glycol 4000. Soil Sci Soc Am J. 1982;46:542–547. [Google Scholar]
- 32.Monteoliva-Sanchez M, Ramos-Cormenzana A, Russell N J. The effect of salinity and compatible solutes on the biosynthesis of cyclopropane fatty acids in Pseudomonas halosaccharolytica. J Gen Microbiol. 1993;139:1877–1884. [Google Scholar]
- 33.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okuyama H, Okajima N, Sasaki S, Higashi S, Murata N. The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochem Biophys Acta. 1991;1084:13–20. doi: 10.1016/0005-2760(91)90049-n. [DOI] [PubMed] [Google Scholar]
- 35.Okuyama H, Sasaki S, Higashi S, Murata N. A trans-unsaturated fatty acid in a psychrophilic bacterium, Vibrio sp. strain ABE-1. J Bacteriol. 1990;172:3515–3518. doi: 10.1128/jb.172.6.3515-3518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overdier D G, Fletcher S, Csonka L N. Osmotic control of transcription of the proU operon of Salmonella typhimurium. In: Somero G N, Osmond C B, Bolis L, editors. Water and life. Berlin, Germany: Springer-Verlag; 1992. pp. 61–69. [Google Scholar]
- 37.Papendick R I, Campbell G S. Theory and measurement of water potential. In: Parr J F, Gardner W R, Elliot L F, editors. Water potential relations in soil microbiology. Madison, Wis: Soil Science Society of America; 1981. pp. 1–22. [Google Scholar]
- 38.Pedrota V, Witholt B. Isolation and characterization of the cis-trans-unsaturated fatty acid isomerase of Pseudomonas oleovorans Gpo12. J Bacteriol. 1999;181:3256–3261. doi: 10.1128/jb.181.10.3256-3261.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfeffer P E, Bécard G, Rolin D B, Uknalis J, Cooke P, Tu S-I. In vivo nuclear magnetic resonance study of the osmoregulation of phosphocholine-substituted β-1,3;1,6 cyclic glucan and its associated carbon metabolism in Bradyrhizobium japonicum USDA 110. Appl Environ Microbiol. 1994;60:2137–2146. doi: 10.1128/aem.60.6.2137-2146.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potts M. Desiccation tolerance of prokaryotes. Microbiol Rev. 1994;58:755–805. doi: 10.1128/mr.58.4.755-805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rand R P, Parsegian V A. Hydration forces between phospholipid bilayers. Biochem Biophys Acta. 1989;988:351–376. [Google Scholar]
- 42.Russel N J. Adaptive modifications of halotolerant and halophilic microorganisms. J Bioenerg Biomembr. 1989;21:93–113. doi: 10.1007/BF00762214. [DOI] [PubMed] [Google Scholar]
- 43.Sinensky M. Homeoviscous adaptation: a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:522–528. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smibert R M, Krieg N R. Phenotypic characterization. In: Gerhardt P, et al., editors. Methods for general and molecular bacteriology. Washington, D.C.: ASM Press; 1994. pp. 607–654. [Google Scholar]
- 45.Soroker E J. Ph.D. dissertation. Berkeley: University of California; 1990. [Google Scholar]
- 46.Steuter A A, Mozafar A, Goodin J R. Water potential of aqueous polyethylene glycol. Plant Physiol. 1981;67:64–67. doi: 10.1104/pp.67.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strittmatter P, Rogers M J. Apparent dependence of interactions between cytochrome b5 and cytochrome b5 reductase upon translational diffusion in dimyristoyl lecithin liposomes. Proc Natl Acad Sci USA. 1975;72:2658–2661. doi: 10.1073/pnas.72.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki K, Goodfellow M, O'Donnell A G. Cell envelopes and classification. In: Goodfellow M, O'Donnell A G, editors. Handbook of new bacterial systematics. London, England: Academic Press; 1993. pp. 195–250. [Google Scholar]
- 49.Tatulian S A. Ionization and ion binding. In: Gregor C, editor. Phospholipid handbook. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 511–552. [Google Scholar]
- 50.Weber F J, Isken S, de Bont J A M. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994;140:2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- 51.Yoneyama H, Akatsuka A, Nakae T. The outer membrane of Pseudomonas aeruginosa is a barrier against the penetration of disaccharides. Biochem Biophys Res Commun. 1986;134:106–112. doi: 10.1016/0006-291x(86)90533-4. [DOI] [PubMed] [Google Scholar]
- 52.Yull I, Wilbrandt W, Shinitzky M. Glucose transport through cell membranes of modified lipid fluidity. Biochemistry. 1981;20:4250–4256. doi: 10.1021/bi00518a003. [DOI] [PubMed] [Google Scholar]
- 53.Zheng T, Driessen A J M, Konings W N. Effect of cholesterol on the branched-chain amino acid transport system of Streptococcus cremoris. J Bacteriol. 1988;170:3194–3198. doi: 10.1128/jb.170.7.3194-3198.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]