Abstract
The murine retrovirus SL3-3 causes malignant transformation of thymocytes and thymic lymphoma in mice of the AKR and NFS strains when they are inoculated neonatally. The objective of the present study was to identify the primary target cells for the virus in the thymuses of these mice. Immunohistochemical studies of the thymus after neonatal inoculation of the SL3-3 virus showed that cells expressing the viral envelope glycoprotein (gp70+ cells) were first seen at 2 weeks of age. These virus-expressing cells were found in the cortex and at the corticomedullary junction in both mouse strains. The gp70+ cells had the morphology and immunophenotype of dendritic cells. They lacked macrophage-specific antigens. Cell separation studies showed that bright gp70+ cells were detected in a fraction enriched for dendritic cells. At 3 weeks of age, macrophages also expressed gp70. At that time, both gp70+ dendritic cells and macrophages were found at the corticomedullary junction and in foci in the thymic cortex. At no time during this 3-week period was the virus expressed in cortical and medullary epithelial cells or in thymic lymphoid cells. Infectious cell center assays indicated that cells expressing infectious virus were present in small numbers at 2 weeks after inoculation but increased at 5 weeks of age by several orders of magnitude, indicating virus spread to the thymic lymphoid cells. Thus, at 2 weeks after neonatal inoculation of SL3-3, thymic dendritic cells are the first cells to express the virus. At 3 weeks of age, macrophages also express the virus. In subsequent weeks, the virus spreads to the thymocytes. This pathway of virus expression in the thymus allows the inevitable provirus integration in a thymocyte that results in a clonal lymphoma.
Dendritic cells and macrophages are known to be primary targets for virus infection and serve as viral reservoirs for human immunodeficiency virus (HIV) (6, 7, 33), as well as for murine retroviruses (12, 18). This study was designed to determine if either or both of these cell types might be the first to express the murine oncogenic retrovirus SL3-3 (25). This virus, when inoculated into newborn mice, causes transformation of the lymphoid cells of the thymus, resulting in thymic lymphoma. The first transformed cells appear in the thymus at 5 to 6 weeks of age, and the mean time to clinical lymphoma development is 10 weeks (15, 19).
The AKR and NFS inbred strains, both highly susceptible to lymphomagenesis after neonatal inoculation with the SL3-3 virus (14), were evaluated for virus expression from week 1 to week 5 after virus inoculation at <24 h of age. AKR mice have an endogenous, ecotropic retrovirus which by genetic recombination becomes thymotropic and lymphomagenic in old age (10), and NFS mice do not have endogenous, ecotropic viruses. Yet, 100% of the animals of each strain inoculated with the SL3-3 virus neonatally develop clonal thymic lymphomas (14).
The essential role of the thymus in lymphomagenesis has been shown by studies in which thymectomy after virus inoculation prevents lymphoma development (13). Thymic stroma has been implicated in this process by studies showing that thymus grafts from high-incidence, but not low-incidence, strain mice given to thymectomized animals restore disease susceptibility. The lymphomas that develop in these grafts are of host bone marrow cell origin (16, 23). These findings suggest that stromal elements in the graft become infected with and express lymphomagenic retroviruses, which appear consistently in adult mice of high-incidence strains, and subsequently transfer the virus to thymocytes derived from host bone marrow progenitors which are maturing in the graft (32).
Thymic stromal cells are known to support thymocyte proliferation; they also induce thymocyte maturation, as well as positive and negative selection of maturing thymocytes (11, 35). The thymic stromal cells which carry out these functions consist of cortical epithelial cells, medullary epithelial cells, macrophages, and dendritic cells. In the present study, target cells for virus expression in the thymus during the first weeks after neonatal virus inoculation were identified by immunohistochemistry on frozen sections and in cell suspensions enriched for dendritic cells. The results show that thymic dendritic cells are the primary targets for the SL3-3 virus in neonatally inoculated AKR and NFS mice.
MATERIALS AND METHODS
Mice.
The AKR/J and NFS/N mice used in these studies were from inbred strains maintained in the laboratory. Pregnant females were observed daily to determine the time of birth of litters. The thymuses were removed at 1, 2, and 3 weeks after birth for the immunohistochemical studies and at 2 and 5 weeks after birth for the cell suspension studies. The experimental groups for immunohistochemical studies were as follows: SL3-3-injected and noninjected AKR mice, six animals at 1 week and three animals at 2 and 3 weeks; SL3-3-injected NFS mice, six animals each at 1, 2, and 3 weeks; noninjected NFS mice, three animals at 3 weeks.
Virus.
SL3-3 is a molecularly cloned ecotropic virus obtained from a cell line of an AKR spontaneous lymphoma (25). Virus stocks were harvested from supernatants of infected NIH 3T3 cells. Mice were infected with 103 PFU of virus per ml intraperitoneally at <24 h of age. This resulted in a 100% incidence of thymic lymphoma between 60 and 100 days of age (14).
Reagents and antibodies.
Thy1.1 (clone 1A14), CD3, and CD4 (clone RL172.4) antibodies were used for depletion of thymocytes. The antibody to the viral envelope (referred to as gp70) was from hybridoma 24-8 supernatant provided by Miles Cloyd and recognizes the gp70-p15 complex (28). It binds to the envelope glycoprotein of Akv (endogenous, ecotropic virus of AKR mice) polytropic oncogenic murine leukemia viruses (MuLVs) of AKR mice, and the SL3-3 virus. Goat anti-mouse immunoglobulin G (IgG), anti-Iak–biotin, CD8-phycoerythrin (PE), B220-fluorescein isothiocyanate (FITC), and IgG (FITC, PE, and biotin) control antibodies were obtained from Pharmingen. ER-TR4 (cortical epithelium) and ER-TR5 (medullary epithelium) were used for identification of these thymic stromal cells (36). CD11c (clone N418) and MOMA-2 antibodies were used for the identification of dendritic cells. Bright staining with N418 and dim staining with MOMA-2 are characteristics of dendritic cells (1, 21). To detect macrophages, a cocktail of the antibodies F4/80, ER-HR3, MOMA-1, and ER-TR9 was used (22). The conjugates used were anti-rat immunoglobulin (Ig) and anti-hamster Ig (Dako, Copenhagen, Denmark, and Jackson Laboratory, Bar Harbor, Maine, respectively) coupled to horseradish peroxidase (HRP; Dako). Streptavidin-HRP (Dako) and streptavidin-alkaline phosphatase (Southern Biotechnology) were used as second-step reagents for gp70-biotin.
Immunoperoxidase staining of tissue sections.
Immunoperoxidase staining of cryostat tissue sections was performed as previously described (9). A hexazotized pararosaniline solution was used for tissue fixation (9). Monoclonal antibody (MAb) binding was detected by using either routine diaminobenzidine visualization of peroxidase or a modified protocol involving NiSO4-supplemented diaminobenzidine (9).
Immunohistochemical double labeling.
At the histological level, double labeling was performed by sequential incubation of sections with rat anti-mouse MOMA-2 or hamster anti-mouse N418, followed by peroxidase-conjugated anti-rat Ig or anti-hamster Ig, biotin-labeled anti-gp70, and finally streptavidin-alkaline phosphatase. MAbs were applied as undiluted hybridoma supernatants, whereas conjugates were optimally titrated. In the various steps, the sections were incubated for 20 to 30 min at room temperature in a moist chamber and washed in between at least twice in phosphate-buffered saline (PBS)–Tween (0.05%). Alkaline phosphatase activity was visualized first by 30 min of incubation in the dark with naphthol ASMX phosphate and Fast Blue BB base (final concentration of both, 0.025% in 200 mM Tris · HCl, pH 8.5) as the substrate and complexing agent, respectively. Levamisole (0.024%) was added to the reaction mixture to block endogenous alkaline phosphatase activity. After washing of the sections in tap water and PBS-Tween, 3-amino-9-ethylcarbazole (0.05% in 100 mM acetate buffer, pH 4.6, supplemented with 0.03% H2O2) was used in a 30-min incubation to detect peroxidase activity. The sections were then rinsed with PBS-Tween, imbedded in Kaiser’s gelatin, and coverslipped. Thus developed, alkaline phosphatase activity yielded a blue reaction product, whereas peroxidase activity appeared red.
Dendritic-cell enrichment.
A modification of the method described by Vremec et al. was used for dendritic-cell enrichment (37). Cells were prepared by mincing thymuses from 20 to 30 age-matched AKR mice into small fragments and pipetting the fragments vigorously in PBS-fetal calf serum (FCS). The suspended cells were removed and placed in a 50-ml conical tube. The remaining thymic fragments were digested with collagenase-dispase and DNase in RPMI 1640 medium (Irvine Scientific), and the digested cell suspension was combined with the previously dissociated cells to provide a whole-cell suspension (unseparated). To enrich for dendritic cells, these unseparated cells were purified by density gradient and MAb and magnetic bead depletion as described below.
FCS-EDTA (10 ml of FCS with 1 ml of 0.1 M EDTA) and EDTA-PBS-FCS (5% FCS–EDTA in PBS) were used throughout the density gradient procedure. Cells (2 × 109) were added to the gradient. The density gradient was prepared as follows. The cells were resuspended in a Nycodenz isotonic solution (Accurate Chemical & Scientific) at a density of 1.075 g/cm3. This dense solution with cells was overlaid with 3 ml of dense solution, followed by 3 ml of a Nycodenz isotonic solution at a density of 1.07 g/cm3 and 2 ml of FCS-EDTA. The gradient was centrifuged at 1,700 × g for 20 min. The band of low-density cells at the FCS-EDTA–low-density interface was removed and washed with FCS-EDTA.
Depletion of thymocytes bearing CD3 and CD4 from low-density cells was done by using magnetic immunobeads (34). Low-density cells were incubated with antibodies to CD3, CD4, and Thy1.1 and then with beads which were coated with goat anti-mouse IgG (Collaborative Research). Cells bound to the beads were removed with a magnet (Collaborative Research), leaving a population of enriched dendritic cells which could be tested by two-color flow cytometry for the presence of major histocompatibility complex (MHC) class II (IaK) and gp70.
Immunofluorescent staining and flow cytometry.
Cells were washed with PBS containing 0.1% sodium azide and 2% newborn calf serum (PBSA) before staining and incubated with optimal amounts of MAb for 30 min at 4°C. Combinations of FITC- and PE-conjugated MAbs were used for two-color analyses. Cells were incubated in a solution of 2-μg/ml 7-amino-actinomycin D in PBS–0.1% sodium azide–2% newborn calf serum to exclude dead cells (29).
For single-color, two-color, or three-color analyses, cells were acquired on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) as previously described; 5,000 to 10,000 events were collected for each sample (29, 30). For data analysis, isotype-matched control MAbs were used to determine appropriate cursor settings, and the gating region was determined by using a combination of forward-angle and 90° light scatter. Data were analyzed and displayed both by two-dimensional dot plots and single-dimensional histograms.
ICC assay.
For the infectious cell center (ICC) assay, 3,000 Mus dunni fibroblasts were used as target cells and plated in gelatin-coated wells of 24-well clusters in Dulbecco modified Eagle medium–10% calf serum–1% glutamine-penicillin-streptomycin. On the next day, cells obtained from the thymus were diluted in medium with 20 μg of Polybrene/ml (106 cells/ml) and 0.5 ml was added per well of target cells. After 24 h of incubation at 37°C, the supernatant including nonadherent cells was removed, the M. dunni target cells were washed, and the culture was continued. After 2 days of culture, when the M. dunni cells were confluent, the cells were washed with PBS and fixed for 10 min in 4% paraformaldehyde in 80% methanol before staining. The cells were stained overnight at 4°C with a 1:10 dilution of 24-8 hybridoma (anti-gp70) supernatant (28), followed by a 1:100 dilution of FITC-conjugated goat anti-mouse IgG, and fluorescent colonies were counted. This protocol allows counting of 100 infectious centers per well. Infectious centers were expressed as numbers of PFU per 106 thymus cells.
RESULTS
Immunohistochemistry of thymuses from mice inoculated neonatally with the SL3-3 virus.
Immunohistochemistry was used to determine which, if any, thymic stromal cells were targets of early expression of the SL3-3 virus. Virus-inoculated, as well as untreated control, AKR and NFS mice were evaluated. As previously mentioned, both strains, AKR with endogenous virus and NFS without it, are equally susceptible to the development of thymic lymphoma after neonatal inoculation with the SL3-3 virus. Spontaneous lymphoma occurs in AKR mice after 28 weeks, whereas lymphomas occur in both AKR and NFS mice between 8 and 14 weeks of age when induced by neonatal SL3-3 infection. Thus, the AKR predilection for spontaneous lymphoma does not interfere with the current experimental setup. Thymus specimens from mice inoculated with the SL3-3 virus and from uninoculated age-matched controls were removed at 1, 2, and 3 weeks of age, and frozen sections were prepared and stained with antibody to gp70. At 1 week after neonatal virus inoculation, no gp70+ cells could be detected in the thymus of either mouse strain. At 2 weeks after virus inoculation, small numbers of gp70+ cells were found in both strains of mice. These gp70+ cells were scattered in the cortex and concentrated at the corticomedullary junction and showed a dendritic morphology (Fig. 1). At 3 weeks after inoculation, increased numbers of gp70+ cells were found. The gp70+ cells at this time period were seen at the corticomedullary junction and were also present in foci in the cortex (Fig. 2). We could not detect gp70+ cells in the control uninfected AKR or NFS mice. Also, there were no strain differences between virus-inoculated AKR and NFS mice in the distribution of gp70+ cells (Fig. 1 and 2).
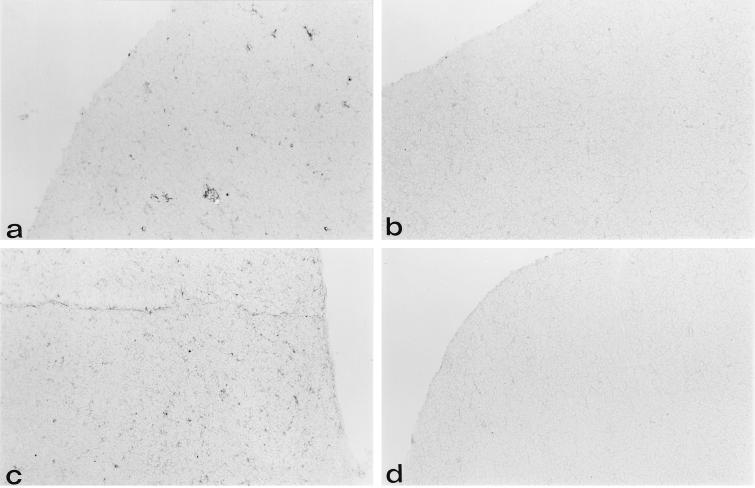
FIG. 1.
gp70+ cells are present at the corticomedullary junction and in the cortex of the thymus in AKR and NFS mice 2 weeks after neonatal inoculation with SL3-3. Immunohistochemical analysis was done on cryostat tissue sections of thymuses from 2-week-old AKR (a and b) and NFS (c and d) mice neonatally inoculated with the SL3-3 virus (a and c) as outlined in Materials and Methods or not inoculated (b and d). Anti-gp70–biotin and as a second step, streptavidin-HRP were used to identify SL3-3-expressing cells. Noninoculated control mice (b and d) did not show gp70+ cells, whereas gp70+ cells were present in inoculated mice at the corticomedullary junction and in clusters in the cortex. Original magnification, ×70.
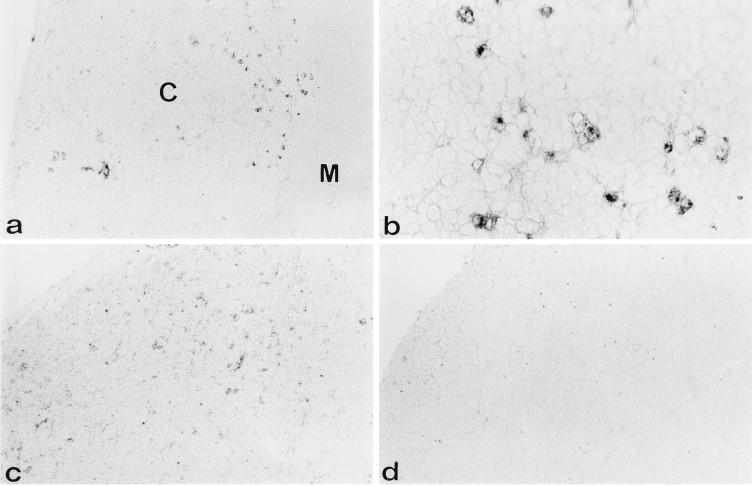
FIG. 2.
gp70+ cells are present in unevenly distributed foci in the cortex 3 weeks after neonatal inoculation with SL3-3. Immunohistochemical analysis was done on cryostat tissue sections of thymuses from 3-week-old AKR (a and b) and NFS (c and d) mice neonatally inoculated with the SL3-3 virus as outlined in Materials and Methods. Anti-gp70–biotin and, as a second step, streptavidin-HRP were used to identify SL3-3-expressing cells. In this representative experiment, panels a and b show two magnifications (×70 and ×280, respectively) of a thymic section from an AKR mouse 3 weeks after infection. Panel b represents an area in the deep cortex (cf. panel a). Panels c and d show two different cortical areas of the thymus from an NFS mouse, demonstrating the heterogeneous distribution of foci of gp70+ cells. Original magnification of c and d, ×70. C, cortex; M, medulla.
To determine which cells in the thymus were expressing gp70, consecutive sections from 2- and 3-week-old, virus-inoculated mice were stained with antibodies to the various thymic stromal components. As can be seen in Fig. 3, gp70+ cells showed a staining pattern with some similarities to the staining with the N418 antibody, which identifies dendritic cells (1). The appearance of these patterns, however, suggested that at 3 weeks postinoculation, not all N418+ cells were gp70+ nor were all gp70+ cells N418+. The staining patterns obtained with the thymic epithelial cell antibody ER-TR4 (36), which identifies cortical epithelial cells (Fig. 3a), and with ER-TR5 (36), which identifies medullary epithelial cells (data not shown), were distinct from that obtained with gp70. Hence, virus replication apparently does not occur in thymic epithelial cells.
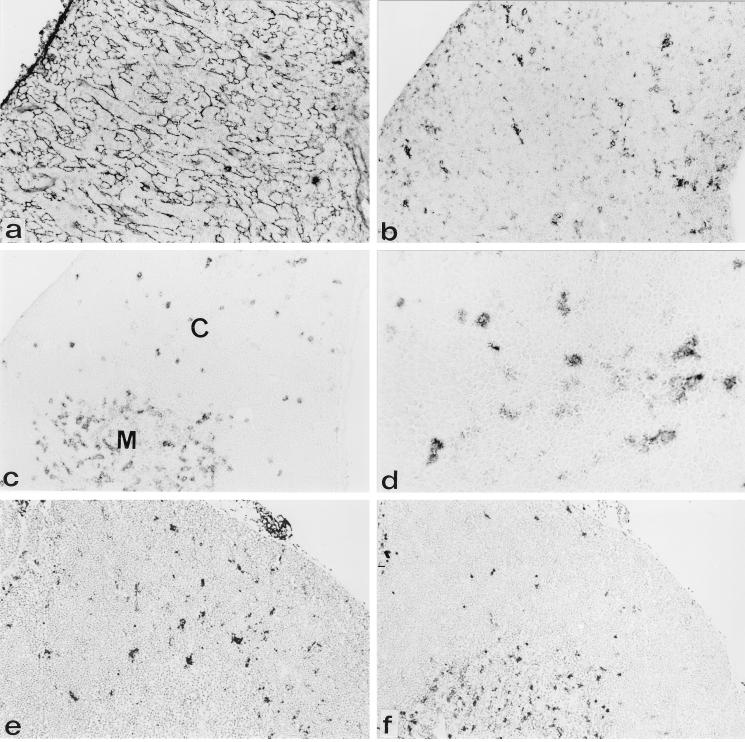
FIG. 3.
gp70+ cells have a dendritic morphology and a staining pattern similar to that of N418+ cells. Immunohistochemical analysis was done on consecutive cryostat tissue sections of thymuses from AKR (a to d) and NFS (e and f) mice 3 weeks after neonatal inoculation with the SL3-3 virus. Anti-gp70–biotin and streptavidin-alkaline phosphatase were used to identify SL3-3-expressing cells (b and e). Antibody ER-TR4 followed by anti-rat–HRP was used to identify cortical thymic epithelial cells (a). Antibody N418, followed by anti-hamster–HRP, was used to identify dendritic cells (c, d, and f), which are most abundantly present in the medulla and scattered in the cortex (d). Original magnification, ×70 (panel d, ×280). C, cortex; M, medulla.
To further identify the nature of the gp70+ cells, double-labeling experiments were performed by using the cocktail of antibodies identifying macrophages (F4/80, ER-HR3, MOMA-1, and ER-TR9) (22) or the antibody to MOMA-2 (21) in combination with gp70. Unfortunately, the double-labeling technique with the combination of N418 and gp70 antibodies could not be used for technical reasons. Two weeks after infection, a subset of the gp70+ cells expressed low levels of antigen recognized by the MOMA-2 antibody. This pattern of low-level expression of MOMA-2 has been described in (precursor) dendritic cells (21). The gp70 and dim MOMA-2 double-positive cells showed a dendritic morphology and were located at the corticomedullary junction of the thymus at 2 weeks after virus inoculation. Not all gp70+ cells expressed low levels of MOMA-2, suggesting that N418 and MOMA-2 may be expressed on dendritic cells at different stages of development or on cells of a different lineage. None of the gp70+ cells coexpressed the macrophage markers or high levels of MOMA-2 (MOMA-2 bright) at 2 weeks (Table 1). At 3 weeks after virus inoculation, gp 70+ macrophage marker+ and gp 70+ MOMA-2 bright cells, as well as gp70+ dendritic cells expressing low levels of MOMA-2, were demonstrated on the double-labeled thymus sections (Fig. 4 and Table 1). Thus, dendritic cells are the first to express the oncogenic retrovirus, and both dendritic cells and macrophages express the virus prior to thymocytes, the ultimate targets for transformation.
TABLE 1.
Immunohistochemistrya of thymuses from AKR and NFS mice inoculated with the SL3-3 virus at <24 h of age
| Mouse strain | Age (wk) | No. gp70+ | gp70+ MOMA2 dimb | gp70+ MOMA2 brightb | gp70+ macrophage marker+ |
|---|---|---|---|---|---|
| AKR | 2 | 9 | 7 | 0 | 0 |
| 3 | 13 | 5 | 2 | 3 | |
| NFS | 2 | 8 | 7 | 0 | 0 |
| 3 | 20 | 9 | 5 | 7 |
Dual-color immunohistochemical analysis was done on cryostat tissue sections of thymuses from AKR and NFS mice neonatally inoculated with the SL3-3 virus as outlined in Materials and Methods. To detect macrophages, a mixture of antibodies (F4/80, ER-HR3, MOMA-1, and ER-TR9) was used. To detect (precursor) dendritic cells, MOMA-2 was used. Anti-gp70–biotin and streptavidin-alkaline phosphatase were used to identify SL3-3-expressing cells. The cells were counted by using a 0.25-mm2 grid and a 40× objective. Results are expressed as the average number of positive cells per 10 mm2 of tissue area of thymus specimens from two to four animals per time point.
gp70+ cells expressing low levels of MOMA-2 had a dendritic cell morphology with extensive branching between the neighboring lymphocytes, while those expressing high levels of MOMA-2 had a macrophage morphology, i.e., a more rounded appearance with fewer cellular processes.
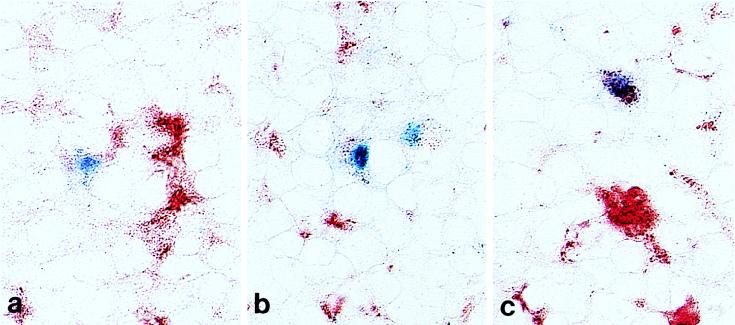
FIG. 4.
Dendritic cells express gp70 at 2 weeks after virus inoculation, while macrophages express gp70 at 3 weeks after virus inoculation. Immunohistochemical analysis using double labeling was done on cryostat tissue sections of thymuses from NFS mice 2 (a) and 3 (b and c) weeks after neonatal inoculation with the SL3-3 virus. Anti-gp70–biotin and streptavidin-alkaline phosphatase were used to identify SL3-3-expressing cells. The MOMA-2 antibody, followed by anti-rat–HRP, was used to identify dendritic (precursor) cells, which label dimly, whereas macrophages express MOMA-2 brightly. gp70 staining results in a blue precipitate, while MOMA-2 staining appears red. Panel a shows one gp70+ dim MOMA-2+ dendritic cell and two gp70− bright MOMA-2+ macrophages in the thymic medulla 2 weeks after virus inoculation. Panel b shows two gp70+ dim MOMA-2+ dendritic cells in the thymic medulla 3 weeks after virus inoculation. Panel c shows one gp70+ bright MOMA-2+ macrophage and two gp70− bright MOMA-2+ macrophages in the thymic cortex 3 weeks after virus inoculation.
Studies of thymocyte cell suspensions from thymuses of young AKR mice inoculated neonatally with the SL3-3 virus.
Our studies of thymic cell suspensions of 2- and 5-week-old mice looked at virus expression and the presence of infectious virus in unseparated cells and in a subpopulation enriched for dendritic cells. Thymic dendritic cells are a minor thymic population; there is one dendritic cell for every 2,000 thymocytes. They are low in density, nonadherent, and MHC class II+ (37). These characteristics were used to separate dendritic cells from the major population of thymocytes and from macrophages. The unseparated population was compared to a dendritic cell-enriched population obtained by density gradient for low-density, MHC class II+ cells, followed by depletion of the remaining thymocytes with MAbs to CD3, CD4, and Thy1.1 by using magnetic immunobeads. Immunophenotypic data from two experiments are presented in Table 2. There was a 7- to 10-fold enrichment of MHC class II+ cells by these procedures. In two experiments, the unseparated cells from mice at 2 weeks after inoculation contained <1% MHC class II+ cells expressing high levels of gp70 (bright gp70+). In contrast, the dendritic-cell-enriched populations in these experiments contained 1 to 3% MHC class II+ bright gp70+ cells. In experiment 2, the absolute numbers of cells which were MHC class II+ and gp70+ were calculated. The unseparated population had 3.6 × 105 MHC class II+ and gp70+ bright cells and the smaller, dendritic-cell-enriched, population had 2.7 × 105 MHC class II+ and gp70+ bright cells. Thus, nearly all of the cells with the latter phenotype in the starting population were present in the dendritic-cell-enriched fraction.
TABLE 2.
Immunophenotype of unseparated and dendritic-cell-enriched cells from thymuses of 2-week-old AKR mice inoculated with the SL3-3 virus at <24 h of age
| Expt no. and cellsa | % of cells
|
||
|---|---|---|---|
| MHC class II+ | gp70+ | gp70+ MHC class II+ | |
| 1 | |||
| Unseparated | 2 | 3 (dim) | <1 |
| Dendritic cell enriched | 20 | 2 | 1 (bright) |
| 2 | |||
| Unseparated | 5 | 6 | <1b |
| Dendritic cell enriched | 37 | 4 | 3 (bright)b |
The cells were prepared from pooled thymuses of AKR mice inoculated with the SL3-3 virus at 2 weeks of age as outlined in Materials and Methods. Immunophenotype was determined by flow cytometry. Cell surface expression of MHC class II and gp70 was determined with a combination of anti-Iak (FITC) and anti-gp70–biotin (PE).
Absolute numbers of gp70+ MHC class II+ cells in experiment 2 were as follows: unseparated population, 3.6 × 105 bright gp70+ MHC class II+ cells; dendritic-cell-enriched population, 2.7 × 105 bright gp70+ MHC class II+ cells.
Indication of substantial virus spread involving thymocytes was found at 5 weeks after neonatal virus inoculation. The unseparated population contained 45% gp70+ cells, while the dendritic-cell-enriched population contained 7% gp70+ cells. This observation was confirmed by studies of infectious virus expression using ICC assays on the two cell populations from these animals at 5 weeks of age. The unseparated population, consisting mainly of thymocytes, contained 91,666 PFU/106 cells, while the dendritic-cell-enriched population contained only 1,244 PFU/106 cells. ICCs were present at low numbers among cells obtained from virus-treated 2-week-old animals (Table 3). ICCs were not found in control thymocyte pools from mice 2 and 5 weeks of age. These control data show that AKR endogenous viruses do not grow well in M. dunni cells and/or, as the immunohistochemical studies suggest, cells expressing these viruses are not present in the normal AKR thymus in the first 5 weeks of life.
TABLE 3.
ICC assays on cells from thymuses of 2- and 5-week-old AKR mice inoculated with the SL3-3 virus at <24 h of age
| Expt | Age (wk) | Cellsa | ICCs (no. of PFU/106 cells)b |
|---|---|---|---|
| 1 | 2 | Unseparated | 82 |
| 2 | 2 | Unseparated | 572 |
| 3 | 5 | Unseparated | 91,666 |
| Dendritic cell enriched | 1,244 | ||
| Control (noninoculated) | 5 | Unseparated | 0 |
| Dendritic cell enriched | 0 |
The cells were prepared from pooled thymuses of AKR mice neonatally inoculated with the SL3-3 virus as outlined in Materials and Methods.
ICC assays were done as outlined in Materials and Methods.
DISCUSSION
The objective of these experiments was to evaluate virus expression in the thymuses of AKR and NFS mice within the first weeks after inoculation of the lymphomagenic SL3-3 virus at <24 h of age. Our hypothesis was that thymic dendritic cells, which cluster and activate thymocytes and are known to be permissive for expression of retroviruses (6, 12, 18, 33), may be targets for early virus expression, providing a direct means for the critical infection leading to subsequent transformation of mature thymocytes.
The immunohistochemical studies reported here support this hypothesis. They show, in both strains, that the earliest virus envelope antigen expression is at the corticomedullary junction or in the cortex at 2 weeks. The cells involved are in a minor population with surface staining characteristics (N418+ and dim MOMA-2+) and the morphology of classical interdigitating cells (dendritic cells) (5). At 3 weeks after neonatal virus inoculation, gp70+ cells expressing low levels of MOMA-2 were again found at the corticomedullary junction. The gp70+ interdigitating cells were more abundant and also appeared in foci in the cortex of a minority of thymic lobules. gp70+ cells with a macrophage phenotype were seen for the first time at 3 weeks. Studies of cell suspensions from pooled thymuses from 2-week-old, virus-inoculated AKR mice are consistent with the immunohistochemical data and show that a minor population of bright gp70+ cells are concentrated in a nonadherent, low-density, MHC class II+ fraction, i.e., cells with the phenotype of dendritic cells (37, 38). The presence of infectious virus was confirmed by the finding of ICCs in the dendritic-cell-enriched fraction. The remarkable spread of virus to thymocytes, which comprise the majority of unseparated cells, was shown by the high number of ICCs (91,666) at 5 weeks. In summary, the immunohistochemical data show that the virus is expressed in dendritic cells at 2 and 3 weeks and in macrophages at 3 weeks. The cell suspension studies show that the virus is expressed in cells with the characteristics of dendritic cells at 2 weeks and that by 5 weeks, there are many thymocytes expressing the virus. Interestingly, it is at 5 weeks of age that we previously demonstrated the presence of the first transformed (lymphoma) cells in the thymuses of AKR mice neonatally inoculated with the SL3-3 virus (19).
Dendritic cells can present self peptides and foreign antigens to thymocytes and T cells (17, 38). During this activity, CD3-bearing thymocytes bind to and cluster around the minor population of dendritic cells. In this way, virus-expressing dendritic cells could initiate the infection of thymocytes, which results in their transformation and the development of clonal CD3+ lymphomas. At 3 weeks after virus inoculation, virus-expressing macrophages may also provide a source of virus for infection of thymocytes. Dendritic cells, and especially fused dendritic cells in syncytia, have been shown to be major stores of HIV (27). These infected human dendritic cells promote HIV replication in T cells which bind to them (27). In addition, thymic dendritic cells can be productively infected in vitro with macrophage-tropic HIV type 1 isolates (6).
A study of adult AKR mice in their natural state showed that thymic macrophages in the cortex and medulla were the first cells expressing oncogenic envelope recombinant viruses (20). They did so approximately 12 weeks before the development of spontaneous lymphoma, i.e., at 5 to 6 months of age (20). This observation, compared to those of our study, may reflect fundamental differences between the adult thymus and the neonatal thymus. In any event, from both studies, it can be concluded that lymphomagenic virus expression in a nonlymphoid, nonepithelial cell of the thymus precedes expression in the lymphoid cell which is the target for neoplastic transformation.
It should be noted that the envelope antibody used in these studies identifies either ecotropic (Akv or SL3-3)- or polytropic-virus-expressing cells. Under natural conditions, young AKR mice have an endogenous ecotropic virus which, by recombination with endogenous sequences, is expressed as a polytropic lymphomagenic virus after 5 to 6 months of age (10). NFS mice have recombinant (polytropic) but no ecotropic sequences in their genome and do not express infectious MuLV. The lymphomagenic properties of SL3-3, which was isolated from an AKR spontaneous lymphoma cell line, are probably due to its ability to form recombinants with endogenous sequences from both of these strains, a well-known event associated with MuLV lymphomagenesis (8, 24). Support for this idea is found in molecular studies using Southern blot analysis of the cytoplasmic DNA from the thymuses of NFS mice neonatally inoculated with the SL3-3 virus (22a). Only ecotropic sequences were found at 2 weeks of age. Envelope recombinant, as well as ecotropic, sequences were present at 4 weeks of age. These findings suggest that virus expression associated with dendritic cells at 2 weeks of age was SL3-3 virus derived, while virus expression by cells of the thymus after this time was from both classes of virus.
The discovery that thymic dendritic cells, followed by macrophages, express virus prior to thymocytes in neonatally infected mice suggests the following series of events leading to transformation and lymphoma development in the thymus. First, virus entering the bloodstream during the first week after intraperitoneal inoculation infects the bone marrow compartment (4). This results in infection of some progenitors for dendritic cells which are developing from bone marrow stem cells and migrating to the thymus (2, 3, 38). This notion is compatible with the fact that the first evidence of thymic infection with virus was found in dendritic cells 2 weeks after neonatal inoculation. Evidence of virus transport to the thymus by bone marrow progenitors was found in our previous studies showing that donor-type thymic lymphomas result when radiation chimeras are produced by the inoculation of bone marrow cells from SL3-3 virus-infected mice (31). Thus, a dendritic cell arising from an infected progenitor will have the proviral integration resulting in virus expression, as shown by the immunohistochemical studies of the thymus at 2 weeks after neonatal inoculation. Although some bone marrow progenitors have been found to be common for dendritic cells and T cells or for dendritic cells and macrophages, others give rise to dendritic cells alone (3, 26, 38). Our data show that dendritic cells, which arise from the latter progenitors, are the first to express the virus after neonatal inoculation with SL3-3. This is followed by virus expression in macrophages at 3 weeks and by the extensive expression of the virus by thymocytes at 5 weeks. Thus, virus-expressing dendritic cells provide an effective way for spread of infection to the multiple thymocytes bound to them. Specific proviral integration in the thymocyte genome then results in transformation and the development of a clonal thymic lymphoma.
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Institutes of Health (HD 29341 and CA 12386) and the Department of Energy (contract DEFC 03-87-ER60615).
We thank Beth Jamieson for her critical review of the manuscript, Jane Voerman and Peter Paul Platenburg for their excellent technical assistance, and Justine Garakian in the laboratory of Harry Vinters for her help with the quantitative immunohistochemistry.
REFERENCES
- 1.Agger R, Crowley M T, Witmer-Pack M D. The surface of dendritic cells in the mouse as studied with monoclonal antibodies. Int Rev Immunol. 1990;6:89–101. doi: 10.3109/08830189009056621. [DOI] [PubMed] [Google Scholar]
- 2.Ardavin C. Thymic dendritic cells. Immunol Today. 1997;18:350–361. doi: 10.1016/s0167-5699(97)01090-6. [DOI] [PubMed] [Google Scholar]
- 3.Ardavin C, Wu L, Li C, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 4.Belli B, Fan H. The leukemogenic potential of an enhancer variant of Moloney murine leukemia virus varies with the route of inoculation. J Virol. 1994;68:6883–6889. doi: 10.1128/jvi.68.11.6883-6889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd R L, Tucek C L, Godfrey D I, Izon D J, Wilson T J, Davidson N J, Bean A G D, Ladyman H M, Ritter M A, Hugo P. The thymic microenvironment. Immunol Today. 1993;14:445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 6.Cameron P U, Lowe M G, Sotzik F, Coughlan A F, Crowe S M, Shortman K. The interaction of macrophage and non-macrophage tropic isolates of HIV-1 with thymic and tonsillar dendritic cells in vitro. J Exp Med. 1996;183:1851–1856. doi: 10.1084/jem.183.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron P U, Pope M, Granelli-Piperno A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukocyte Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 8.Cloyd M W, Hartley J W, Rowe W P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980;149:702–712. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong J P, Voerman J S, Leenen P J, van der Sluijs-Gelling A J, Ploemacher R E. Improved fixation of frozen lympho-haemopoietic tissue sections with hexazotized pararosanaline. Histochem J. 1991;23:392–401. doi: 10.1007/BF01042295. [DOI] [PubMed] [Google Scholar]
- 10.Elder J K. Recombinant retroviruses in the development of murine leukemia. In: Notkins A L, Oldstone M B A, editors. Concepts in viral pathogenesis. New York, N.Y: Springer Verlag; 1984. pp. 86–93. [Google Scholar]
- 11.Fairchild P J, Austyn J M. Thymic dendritic cells: phenotype and function. Int Rev Immunol. 1990;6:187–196. doi: 10.3109/08830189009056629. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich P I, Patterson S, Timofeev A V, Harvey J J, Knight S C. Mechanism for dendritic cell dysfunction in retroviral infection of mice. Clin Immunol Immunopathol. 1996;88:139–146. doi: 10.1006/clin.1996.0107. [DOI] [PubMed] [Google Scholar]
- 13.Hays E F. The role of thymus epithelial reticular cells in viral lymphomagenesis. Cancer Res. 1968;28:21–26. [PubMed] [Google Scholar]
- 14.Hays E F, Bristol G, McDougal S. Mechanisms of thymic lymphomagenesis by the retrovirus SL3-3. Cancer Res. 1990;50:5631s–5635s. [PubMed] [Google Scholar]
- 15.Hays E F, Bristol G C, McDougal S, Klotz J, Kronenberg M. Development of lymphoma in the thymus of AKR mice treated with the lymphomagenic virus SL3-3. Cancer Res. 1989;49:4225–4230. [PubMed] [Google Scholar]
- 16.Hays E F, Swanson S K, Hale L, Margaretten N. Thymic stroma in AKR mice. Its function and virus production. Leuk Res. 1984;8:637–645. doi: 10.1016/0145-2126(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Metlay J P, Crowley M T, Witmer-Pack M, Steinman R M. Dendritic cells as antigen presenting cells in vivo. Int Rev Immunol. 1990;6:197–206. doi: 10.3109/08830189009056630. [DOI] [PubMed] [Google Scholar]
- 18.Kast W M, Boog C J, Roep B O, Voordouw A C, Melief C J M. Failure or success in the restoration of virus-specific cytotoxic lymphocyte response defects of dendritic cells. Immunology. 1988;140:3186–3193. [PubMed] [Google Scholar]
- 19.Kato A, Hays E F. Development of virus-accelerated lymphoma in AKR mice. J Natl Cancer Inst. 1985;75:491–497. [PubMed] [Google Scholar]
- 20.Kim S Y, Evans L H, Malik F G, Rouse R V. Macrophages are the first cells to express polytropic retrovirus in AKR mouse leukemogenesis. J Virol. 1991;65:6238–6241. doi: 10.1128/jvi.65.11.6238-6241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraal G, Rep M, Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand J Immunol. 1987;26:653–661. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 22.Leenen P J, de Bruijn M F, Voerman J S, Campbell P A, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 22a.Marrero, P., and E. F. Hays. Unpublished data.
- 23.Miller J F A P. Studies on mouse leukemia. Fate of thymus homografts in immunologically tolerant mice. Br J Cancer. 1960;14:244–254. doi: 10.1038/bjc.1960.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowinski R C, Hays E F. Oncogenicity of AKR endogenous leukemia viruses. Virology. 1978;27:13–18. doi: 10.1128/jvi.27.1.13-18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pederson F S, Crowther D Y, Tenney A M, Niemold A M, Haseltine W A. Novel leukemogenic retroviruses isolated from a cell line derived from a spontaneous AKR tumor. Nature. 1981;262:167–170. doi: 10.1038/292167a0. [DOI] [PubMed] [Google Scholar]
- 26.Peters J H, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 27.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portis J L, McAtee F J, Cloyd M W. Monoclonal antibodies to xenotropic and MCF murine leukemia viruses derived during graft-versus-host reaction. Virology. 1982;118:181–190. doi: 10.1016/0042-6822(82)90331-2. [DOI] [PubMed] [Google Scholar]
- 29.Schmid I, Krall W J, Uittenbogaart C H, Braun J, Giorgi J V. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 30.Schmid I, Uittenbogaart C H, Giorgi J V. A gentle fixation and permeabilization method for combined cell surface and intracellular staining with improved precision in DNA quantification. Cytometry. 1991;12:279–285. doi: 10.1002/cyto.990120312. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi H, Kato A, Hays E F. Presence of prelymphoma cells in the bone marrow of the lymphomagenic virus-treated AKR mouse. Cancer Res. 1984;44:1008–1011. [PubMed] [Google Scholar]
- 32.Templis L D. Thymic epithelium controls thymocyte expression of preleukemic phenotype and leukemogenic retroviruses. J Immunol. 1987;138:3555–3565. [PubMed] [Google Scholar]
- 33.Tsunetsugu-Yokota Y, Akagawa K, Kimoto H, Suziki K, Iwasaki M, Yasuda S, Hausser G, Hultgren C, Meyerhans A, Takemori T. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J Virol. 1995;69:4544–4547. doi: 10.1128/jvi.69.7.4544-4547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueno Y, Hays E, Hultin L, Uittenbogaart C H. Human thymocytes do not respond to interleukin-2 after removal of mature “bright” CD5 positive cells. Cell Immunol. 1989;124:239–251. doi: 10.1016/0008-8749(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 35.van Ewijk W. T-cell differentiation is influenced by thymic microenvironments. Annu Rev Immunol. 1991;9:591–615. doi: 10.1146/annurev.iy.09.040191.003111. [DOI] [PubMed] [Google Scholar]
- 36.van Vliet E, Melis M, van Ewijk W. Monoclonal antibodies to stromal cell types of the thymus. Eur J Immunol. 1984;14:524–529. doi: 10.1002/eji.1830140608. [DOI] [PubMed] [Google Scholar]
- 37.Vremec D, Zorbas M, Scollay R, Saunders D J, Ardavin C F, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of CD8 expression on a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Vremec D, Ardavin C, Winkel K, Suss G, Georgiou H, Maraskivsky E, Cook W, Shortman K. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur J Immunol. 1995;25:418–425. doi: 10.1002/eji.1830250217. [DOI] [PubMed] [Google Scholar]