Heart failure has been increasing in prevalence, impacting 6.7 million Americans aged 20 years and above by 2020, and the mortality rates remain high (52.6% overall; 24.4% for those 60 years of age; and 54.4% for those 80 years of age).1 This underscores an urgent need for early detection, timely diagnosis, and consistent monitoring of heart failure after discharge. However, there are some “roadblocks” that lead to a gap between the standards of care recommended and what is delivered in practice. One major challenge is the lack of effective screening methods for early detection. Besides, the presence of nonspecific symptoms can complicate the diagnosis process, particularly when dealing with heart failure with preserved ejection fraction (HFpEF). Additionally, the follow-up care is often inconsistent, resulting in delayed medical responses after discharge. The current approach for monitoring the progression of heart conditions is limited to algorithms based on cardiac implantable electronic devices. There is a strong demand for noninvasive techniques for monitoring of both heart failure with reduced ejection fraction and HFpEF for ease of use.
In response to such demand, we have developed NIHA-HF (Neural Intelligent Heart Analyzer for Heart Failure), an artificial intelligence–driven solution that enables noninvasive and remote monitoring of heart failure conditions. The core of NIHA-HF is a deep learning–based algorithm to assist the diagnosis of heart failure using lead I electrocardiogram (ECG). The ECG is widely used to diagnose heart irregularities; however, relying solely on the ECG is not an effective method for diagnosing a patient with heart failure.2 The potential of using ECGs for heart failure detection based on machine learning has been verified by existing research.3 However, the reliance of the approaches on a multilead ECG limits their applicability in home monitoring environments.
We have already identified specific features in heartbeat waveforms from lead I ECG associated with heart failure progression, such as abnormalities in the P-wave, T-wave, and reduced amplitude and widening of the QRS complex.4 Motivated by this significant finding, we developed NIHA-HF and verified its effectiveness with preclinical studies. All studies were approved by the institutional ethical committee of the University of Tokyo. We trained the model using a dataset of 28,000 retrospective lead I ECGs extracted from standard 12-lead ECGs obtained from the University of Tokyo Hospital. This dataset contained approximately 11,000 subjects ≥20 years of age, including around 3,700 patients diagnosed with heart failure. Each ECG was labeled with a diagnosis of either non-heart failure or heart failure, along with a NYHA functional classification. The ECG signals underwent initial preprocessing and segmentation of heartbeats. Subsequently, feature extraction was performed using convolutional layers, leading to a forward pass through a convolutional neural network–based machine learning model. The final output not only provided an indication of the risk of heart failure, but also introduced the HF-Index, a novel metric derived from the output probabilities of the model, normalized between 0 and 100, serving as a scalable indicator of the heart failure condition.
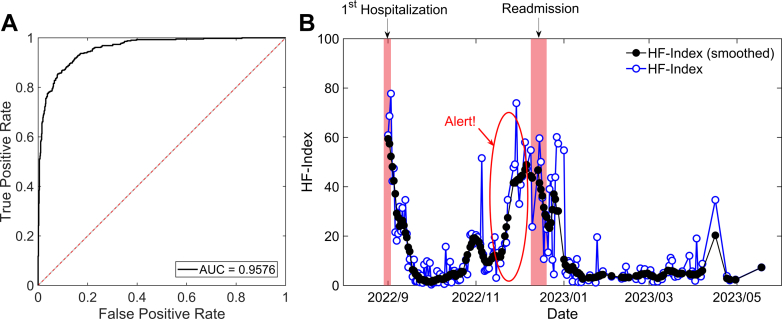
The performance was validated using 2 approaches: cross-sectional analysis of patient data at single time points, and longitudinal tracking of patient health over time. First, we assessed heart failure detection across an independent test dataset with 1,189 individuals (age 59.3 ± 14.5 years, female 44.2%). This data set comprised 776 control and 413 patients, with the latter group including heart failure with reduced ejection fraction (15%), heart failure with midrange ejection fraction (8%), and HFpEF (77%), featuring a diverse range of etiologies such as ischemic heart disease (44.1%), atrial fibrillation (17.9%), cardiomyopathy (10.4%), pulmonary hypertension (9.0%), and valvular disease (4.4%), among others. NIHA-HF showed a remarkable performance in detecting heart failure, achieving a high area under the curve score of 0.9576 (95% CI: 0.9458-0.9671) as shown in Figure 1A. Notably, the area under the curve score for the detection of HFpEF was 0.9525 (95% CI: 0.9388-0.9635).
Figure 1.
Validation Results for Heart Failure Detection and Monitoring
(A) Receiver-operating characteristic (ROC) curve for detecting heart failure. (B) A longitudinal monitoring of HF-Index variations after heart failure discharge. The black line with filled circles represents the raw HF-Index variations, whereas the blue line with open circles illustrates the smoothed HF-Index variations. Red shaded areas denote periods of hospitalization, with the red circle highlighting a significant rise in the HF-Index. AUC = area under the curve.
In the longitudinal studies, we monitored the variations of the HF-Index in heart failure patients after hospital discharge. The ECG was routinely obtained using a portable lead-I ECG device outside of the hospital. Figure 1B illustrates a particular case involving a 65-year-old female heart failure patient with reduced ejection fraction. Notably, we observe a significant increase in the HF-Index around 1 month before the readmission. Considering the high risk of recurrence in heart failure patients, this finding shows the effectiveness of NIHA-HF in catching worsening heart failure at an earlier stage.
Our preliminary findings provide evidence that NIHA-HF has the potential to enhance the process of heart failure management, aiding in both early detection and ongoing monitoring of heart failure. As NIHA-HF only requires lead I and can be provided as an on-demand software application, it can be integrated with any portable ECG devices, making it suitable for a wide range of health care settings.
We intend to broaden our preclinical studies, both retrospective and prospective, to the United States in 2024 to validate the performance and refine the machine learning model for the U.S. population. Furthermore, we plan to initiate a broader deployment of NIHA-HF under the 510(k) regulatory framework and investigate existing reimbursement pathways to facilitate our entry into the U.S. market.
Footnotes
Dr Chen is an employee of SIMPLEX QUANTUM Inc. Preclinical studies were conducted at the University of Tokyo Hospital in Japan by Dr Katsuhito Fujiu and Dr Eriko Hasumi.
This work was presented at the 2024 CRF THT Shark Tank, March 4-6, 2024, Boston, Massachusetts.
Editor’s Note: To view the authors’ full presentation at TCTMD Shark Tank, please visit https://www.jacc.org/journal/basic-translational/tht-2024-shark-tank.
The author attests they are in compliance with human studies committees and animal welfare regulations of the author’s institution and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93–621. doi: 10.1161/CIR.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 2.Taylor C., Hobbs R. Diagnosing heart failure–experience and ‘best pathways’. Eur Cardiol Rev. 2010;6(3):10. [Google Scholar]
- 3.Ulloa-Cerna A.E., Jing L., Pfeifer J.M., et al. rECHOmmend: an ECG-based machine learning approach for identifying patients at increased risk of undiagnosed structural heart disease detectable by echocardiography. Circulation. 2022;146(1):36–47. doi: 10.1161/CIRCULATIONAHA.121.057869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasumi E, Fujiu K, Chen Y, et al. Heart failure grading using single-lead electrocardiography. Preprint. Posted online October 13, 2020. medRxiv. https://doi.org/10.1101/2020.10.08.20209700