Central Illustration
Key Words: coronary microvasculature, fibrosis, inflammation, microbiome, physiological cardiac hypertrophy
Highlights
-
•
This review discusses systemic and cardiac adaptations contributing to the benefits of exercise, including changes in cardiomyocyte function, growth and proliferation, coronary microvasculature and lymphatics, cardiac fibrosis, systemic and cardiac metabolism and inflammation, and effects related to the gut microbiome.
-
•
Insights from mechanistic and preclinical studies of exercise adaptation highlight the value of exercise as a platform for discovering potential therapeutic targets.
Summary
Among its many cardiovascular benefits, exercise training improves heart function and protects the heart against age-related decline, pathological stress, and injury. Here, we focus on cardiac benefits with an emphasis on more recent updates to our understanding. While the cardiomyocyte continues to play a central role as both a target and effector of exercise’s benefits, there is a growing recognition of the important roles of other, noncardiomyocyte lineages and pathways, including some that lie outside the heart itself. We review what is known about mediators of exercise’s benefits—both those intrinsic to the heart (at the level of cardiomyocytes, fibroblasts, or vascular cells) and those that are systemic (including metabolism, inflammation, the microbiome, and aging)—highlighting what is known about the molecular mechanisms responsible.
The importance of exercise in cardiovascular disease prevention and mitigation has long been recognized. While evidence of the cardiovascular benefits of exercise has come primarily from observational studies, it is further supported by some randomized trials,1,2 meta-analyses,3,4 and animal studies. As a result, physical activity guidelines codified by American College of Cardiology and the American Heart Association suggest moderate-intensity cardiorespiratory exercise training for at least 30 minutes per day, 5 days per week for healthy adults.5 In addition to reducing the risk of cardiovascular disease, exercise improves functional capacity and quality of life in populations with cardiovascular diseases such as heart failure and may improve clinical outcomes, although the latter has been difficult to establish unequivocally.1,6,7
Despite widespread acceptance of the cardiovascular benefits of exercise, many questions remain. In part, these reflect inherent limitations of studying lifestyle interventions. Bias and confounding limit observational studies and variability in adherence can undermine interventional trials. The optimal type, intensity, and duration of physical activity and how to optimize benefits for individuals remain unclear. In this context, animal studies can be particularly helpful. Not only can confounders be rigorously excluded, but also relevant tissues not available clinically can be accessed. Such studies can enhance understanding and identify new therapeutic targets. Although it is unlikely that any medicine will recapitulate all the benefits of exercise, it may be possible to manipulate specific downstream mediators to mimic some of the salutary effects. While those who can exercise should, exercise-mimetic therapeutics may be particularly helpful for patients who cannot exercise adequately. Another important practical goal is to identify biomarkers that correlate with exercise’s benefits to guide individualized exercise recommendations. If exercise can be considered medicine, we currently have no way to judge appropriate dosage or to know that we have moved the needle toward clinical benefit. Here, too, mechanistic understanding and animal models are important supplements to clinical studies.
In this review, we discuss cardiac benefits of exercise (Central Illustration). Exercise-induced changes in cardiomyocyte growth, proliferation, and function are central to many of the cardiac effects of exercise. However, there is a growing recognition of key roles for noncardiomyocyte lineages in the heart, including fibroblasts and vascular cells, which we discuss in the context of fibrosis, coronary microcirculation, and lymphatics. In addition, pathways and processes primarily outside the heart are increasingly appreciated as contributors to disease—and exercise benefits. We discuss effects on metabolism, intestinal microbiome, inflammation, and briefly, aging, which is likely an extrinsic and intrinsic contributor to cardiac effects. Where possible, we highlight molecular mechanisms and possible translational applications.
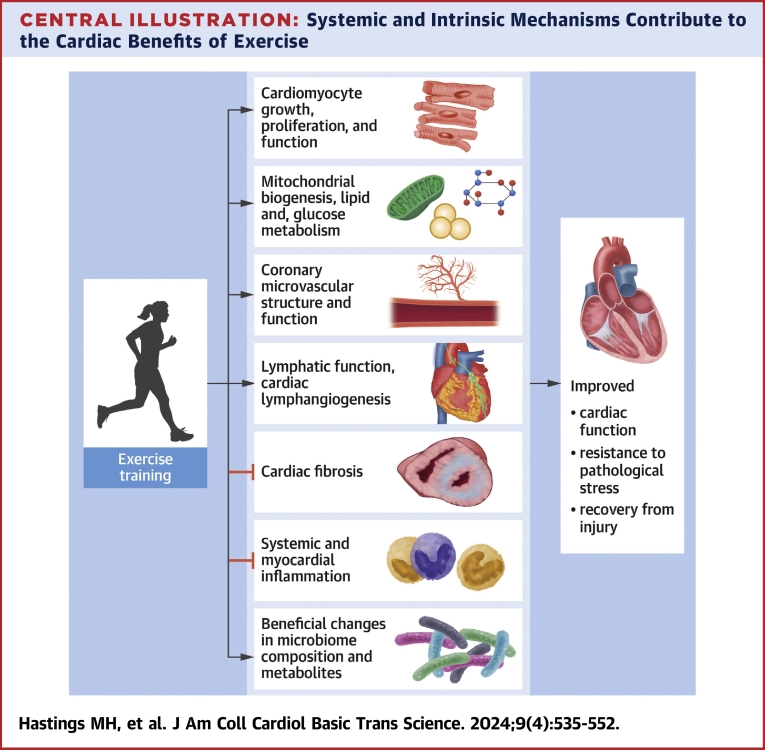
Central Illustration.
Systemic and Intrinsic Mechanisms Contribute to the Cardiac Benefits of Exercise
Mechanisms contributing to the benefits of exercise include effects on cardiomyocyte function, growth and proliferation, coronary microvasculature and cardiac lymphatics, cardiac fibrosis, systemic and cardiac metabolism and inflammation, and effects related to the gut microbiome. Created with BioRender.com.
Intrinsic cardiac effects
Understandably, cardiomyocytes have garnered much attention in the heart’s response to exercise. The cardiomyocyte is responsible for some of the most salient exercise phenotypes, including cardiac growth (hypertrophy) and alterations in function. The evolving understanding of these processes and their potential therapeutic relevance is discussed subsequently, followed by consideration of the role of fibrosis, the microvasculature, and lymphatics. These rely principally on other cell types, often influenced by crosstalk with cardiomyocytes and systemic factors. The potential therapeutic value of targeting some of these other cell types and processes is an area of active investigation.
Cardiomyocyte growth and proliferation in physiological cardiac hypertrophy
Cardiomyocytes represent only ∼30% to 40% of the heart's cells but account for ∼70% to 85% of its volume, and endurance training leads to cardiac enlargement (hypertrophy), due primarily to an increase in cardiomyocyte size.8 Despite appearing similar superficially to pathological cardiac growth that accompanies cardiovascular disease and often precedes heart failure,9 exercise-induced physiological hypertrophy10 differs in that it is reversible and, rather than dysfunction, is associated with protection from pathological stress.11 It also involves a distinct transcriptional profile10 without induction of pathological markers such as atrial natriuretic peptide or B-type natriuretic peptide.12 Exercise also increases cardiomyocyte length and width proportionately, while pathological hypertrophy disproportionately increases cardiomyocyte length.13 Notably, aerobic exercise training protects against11 and even reverses14 pathological hypertrophy in rodent models, supporting fundamental differences and dynamic tension between the two. Consistent with this, the underlying mechanisms also appear distinct, as detailed subsequently.
Physiological hypertrophy is associated with an increase in the adult mammalian heart’s limited capacity to form new cardiomyocytes. Swim training increases proliferation markers in cardiomyocytes.10 Because these markers do not unequivocally establish that new cardiomyocytes are formed, survive, and integrate into the myocardium, we used multi-isotope imaging mass spectrometry to demonstrate unambiguously that 8 weeks voluntary wheel running induced a 4.6-fold increase in cardiomyogenesis in adult mice.15 Furthermore, we found that exercise training restored declining rates of cardiomyogenesis in aging mice to levels comparable to sedentary young adult animals.15,16 Fewer new cardiomyocytes were induced than in young exercised animals, although this may be due to older animals running less.16 Others reported a lasting increase in the proportion of mononucleated cardiomyocytes, thought to be the cardiomyocytes that proliferate, in juvenile rats following treadmill training, although the effect was diminished in adolescence and not detected in the adult.17
Thus, cardiomyogenesis may contribute to the clinical benefits of exercise in part by counteracting cardiomyocyte loss thought to contribute to heart failure in multiple settings.18 However, it will be important to rigorously evaluate the functional implications of exercise-induced cardiomyogenesis given the low absolute number of cardiomyocytes formed.19,20 Treatment with antineoplastic agent 5-fluorouracil before swim training did not affect development of hypertrophy in mice but did reduce protection against subsequent ischemia reperfusion.21 This suggested an essential role for proliferation in exercise-induced cardioprotection, if not growth, although inhibition of proliferation in noncardiomyocytes may have contributed as 5-fluorouracil’s effects are not cell type specific. As discussed next, the protective effects of exercise can be mimicked with genetic or pharmacological interventions, and measures of cardiomyogenesis are increased by many of these. However, as with exercise, it is not clear whether this proliferation contributes to the protective effects.
Pathways functionally important in the heart’s hypertrophic response to exercise also counteract pathological stress and injury
Many pathways functionally important in the cardiac exercise response protect the heart against pathological stress when exercise-related changes are mimicked experimentally. Most extensively studied is the insulin-like growth factor (IGF)-1/PI3K/Akt pathway. Plasma IGF-1 is higher in elite athletes than control subjects22 and cardiomyocyte-specific IGF-1 receptor knockout,23 dominant negative PI3K,24 or Akt1 knockout23,25 in mice disrupts exercise-induced cardiomyocyte growth, while activation of this pathway induces heart growth.26,27 Importantly, Akt activation reduces cardiomyocyte apoptosis,28 myocardial injury, and cardiac dysfunction after ischemic injury.29 Similarly, cardiac PI3K overexpression mitigates pathological remodeling and dysfunction after transverse aortic constriction (TAC).30
Similar results are seen with transcriptional pathways. The transcription factor CCAAT/enhancer-binding protein β (C/EBPβ) is down-regulated in hearts from exercised mice10 and heterozygous C/EBPβ knockout mice, which have cardiac C/EBPβ messenger RNA levels comparable to exercised mice, show signs of physiological cardiomyocyte hypertrophy and proliferation10 as well as improved heart function and survival after TAC.10 The transcriptional regulator CITED4 increases in exercised hearts, mediating some of the effects of the decrease in C/EBPβ,10 and its cardiomyocyte-specific deletion exacerbates TAC-induced dysfunction and pathological remodeling.31 These mice also have an altered, pathological response to exercise training, including modest cardiac dysfunction and dilation31 and impaired microstructural cardiac remodeling.32 Like C/EBPβ heterozygotes, cardiomyocyte-specific CITED4 overexpression recapitulates key features of physiological cardiac growth and reduced adverse remodeling and dysfunction after ischemia reperfusion.33
Noncoding RNAs regulate physiological and pathological cardiac growth. Cardiac microRNA miR-222 is increased by exercise and is necessary for exercise-induced hypertrophy34 and cardiomyogenesis.15 Cardiomyocyte-specific miR-222 overexpression reduces pathological remodeling and dysfunction after ischemic injury.34 We recently identified lncExACT1 as a long noncoding RNA up-regulated in pathological hypertrophy and down-regulated in physiological hypertrophy.12 Surprisingly, both overexpression and inhibition of lncExACT1 lead to cardiac hypertrophy but with pathological and physiological features, respectively, suggesting a critical role in toggling the heart between physiological and pathological growth. lncExACT1 inhibition also induced signs of cardiomyocyte proliferation and protected against TAC- or ischemia-reperfusion–induced cardiac dysfunction.12 Of note, the locked nucleic acid–antisense oligonucleotides used to inhibit miR-222 and lncExACT1 in these studies are not cardiomyocyte specific, and thus inhibition in other cell types may also contribute to effects observed.12,34 Recently, Gao et al35 identified another long noncoding RNA, cardiac physiological hypertrophy–associated regulator (CPhar), as up-regulated in exercised hearts but decreased in pathological hypertrophy. CPhar knockdown blocked swimming-induced cardiomyocyte hypertrophy and markers of cardiomyocyte proliferation, while overexpression protected against ischemia-reperfusion injury.35
These findings illustrate the value of animal exercise models for identifying therapeutic candidates, particularly as emerging technologies open new paths to translation. Of note, antisense inhibitors similar to those used to inhibit lncExACT1 are Food and Drug Administration approved for other indications.36 Viral vector–based gene delivery is improving, which may facilitate translation, particularly when systemic manipulation is problematic, such as for the IGF-1/PI3K/Akt pathway, given known roles in tumor development and metastasis.37
Cardiomyocyte function
Some exercise benefits reflect changes in cardiomyocyte function. Exercise training increases cardiac systolic and diastolic function in both health and disease, with important implications for quality of life.38 Although altered preload and afterload contribute to the improved cardiac function, changes in the heart’s chronotropic, inotropic, and lusitropic properties also contribute.38 Animal studies have confirmed that aerobic training can increase cardiomyocyte fractional shortening by up to 40% to 50%, contraction and relaxation rates by up to 20% to 40%, and maximal power output by up to 60%.39 These effects depend on exercise intensity and quickly regress with detraining.40,41
Mechanisms underlying improved cardiomyocyte function with exercise
Decades of animal studies have revealed exercise effects on nearly every component of the cardiomyocyte contractile machinery.41,42 Rodent studies have shown exercise training not only increases myofilament calcium (Ca2+) sensitivity, but also accelerates Ca2+ flux in the cardiomyocyte.42,43 The latter is likely due to more effective coupling of L-type Ca2+ channels and ryanodine receptors, increased SERCA2a or sodium-calcium exchanger expression, enhanced SERCA2a activity, and/or more efficient organization of T-tubules.39,41,44, 45, 46, 47
In a novel intersection between Ca2+ handling, epigenetic, and metabolic mechanisms, Lehmann et al48 recently showed that HDAC4-NT, a proteolytic fragment of HDAC4, is up-regulated in mouse hearts by exercise but down-regulated in failing hearts, and its myocardial overexpression mimics the protective effects of exercise. In contrast, cardiomyocyte HDAC4 deletion impairs exercise capacity. Mechanistic studies suggest that HDAC4-NT enhances cardiac function by reducing expression of nuclear orphan receptor NR4A1, a negative regulator of cardiomyocyte contraction, through a pathway that includes both the hexosamine biosynthetic pathway and the calcium sensor STIM1.48
Cardiomyocyte hypertrophy may itself contribute to enhanced mechanical function, in part through an increase in sarcomere functional units.49 Physiological hypertrophy is also associated with functionally favorable shifts in sarcomeric protein isoforms, such as an increased α- to β-myosin heavy chain ratio.50,51
Effects of exercise on cardiomyocyte function in disease and aging
Endogenous exercise-regulated pathways may provide therapeutic targets, not only for heart disease, but also for the decline in cardiac function that occurs in normal aging.52, 53, 54 Exercise can reverse left ventricular stiffness in sedentary aging53 and in middle-aged individuals with left ventricular hypertrophy at risk for heart failure.54 Furthermore, we demonstrated reversal by exercise of many—though not all—hallmark features of heart failure with preserved ejection fraction in an aging mouse model, including improved exercise capacity, diastolic function, and contractile reserves.55
Molecular mediators of cardiomyocyte functional adaptation to exercise in healthy animals also appear to contribute to exercise benefits in heart failure and aging. In ischemic and nonischemic heart failure models, exercise training improves systolic and diastolic function by modulating expression or activity of key regulators of cardiomyocyte contractility including SERCA2a, ryanodine receptors, phospholamban, and CaMKII.14,42,44,56 Many of these same mechanisms are implicated in exercise adaptation in aged cardiomyocytes, although there are distinctions including differing effects on cardiomyocyte β-adrenergic sensitivity,57 SERCA2a activity, Ca2+ cycling,58 and hypertrophic signaling.16,46,55,59 Notably, exercise modulates expression and/or activity of proteins in the activin/myostatin family, important regulators of skeletal muscle mass that are also implicated in age-related cardiac dysfunction60,61 through their effects on phospholamban and SERCA2a.60,61 A deeper understanding of this and other intersections between exercise-regulated pathways and those driving cardiac dysfunction in aging and disease may yield therapeutic insights.
Directly manipulating exercise-related targets, such as SERCA2a62 and activin/myostatin,61 improves cardiac function in models of heart failure and aging, suggesting that these may be valuable therapeutic targets. While clinical trials testing AAV1-mediated SERCA2a gene therapy for heart failure were not successful, this may relate to technical limitations that could be overcome as gene delivery technology advances (NCT01643330).63 Inhibitors of activin/myostatin signaling have also been evaluated in clinical trials for other indications, including a recent phase 3 trial in which a soluble ligand trap inhibitor of activin signaling (Sotatercept) increased exercise capacity relative to placebo in patients with pulmonary hypertension (NCT04576988).64 Further delineation of mechanisms by which exercise affects heart function will likely suggest new therapeutic strategies in heart failure and age-related heart disease.
Cardiac fibrosis
While pathological hypertrophy and age-related cardiac dysfunction are associated with interstitial fibrosis,55,65 physiological hypertrophy generally is not.66 Exercise-trained animals develop less fibrosis than sedentary control animals in response to cardiac injury or pathological stress including hypertension, anthracycline-induced cardiotoxicity, pressure overload, and ischemic injury, among others.67,68 Although the roles of fibroblasts are multifaceted and include important structural, mechanical, and repair functions, these observations suggest that, in addition to the favorable effects on cardiomyocytes discussed previously, exercise may reduce adverse cardiac remodeling in part by limiting fibrosis.
Mechanisms by which exercise suppresses fibrosis in pathological models
Mechanistic studies further support a role for fibroblasts and cardiomyocyte-fibroblast crosstalk in the benefits of exercise. Transcriptional profiling of noncardiomyocytes after exercise training, pressure overload, or myocardial infarction (MI) demonstrated activation of myofibroblast transformation gene programs in disease models but not in exercise.67 Some pathways were regulated inversely in exercise and pathological stress. For example, NRF2-dependent antioxidant genes, including metallothioneins Mt1 and Mt2, were up-regulated with exercise but suppressed in disease states by transforming growth factor (TGF)-β signaling.67 Interestingly, conditioned media from Mt1-overexpressing fibroblasts protected cardiomyocytes from oxidative injury and apoptosis, suggesting that, in addition to limiting fibrosis, exercise induced fibroblast-cardiomyocyte crosstalk to promote cardiomyocyte survival.67
Similarly, important crosstalk occurs downstream of exercise-induced transcriptional coactivator CITED4.33 As mentioned, cardiomyocyte-specific CITED4 knockdown resulted in a pathological response to exercise as well as injury.31 This included fibrosis and profibrotic gene expression (eg, collagens, CTGF, TGF-β2).31 CITED4 deletion also reduced cardiomyocyte miR30d expression and secretion via extracellular vesicles.31 Conditioned media from CITED4-deleted cardiomyocytes was sufficient to induce profibrotic gene programs in fibroblasts, an effect that was lost when cardiomyocyte miR30d expression was restored with miR-mimics.31
Mimicking exercised-related changes in noncoding RNA expression, including miR-222 and lncExACT1, also protects against fibrosis under pathological conditions.12,34 Interestingly, lncExACT1 acts through regulation of miR-222, calcineurin, and Hippo/Yap.12 Again highlighting the importance of cardiomyocyte-fibroblast crosstalk, manipulation of miR-222 in these studies was cardiomyocyte specific. Similarly, another exercise-induced microRNA, miR-29c, was inversely regulated with the fibrotic gene program, and its deletion in a murine pressure overload model also attenuated cardiac fibrosis.69
Across multiple preclinical models, suppression of cardiac fibrosis by exercise training has been linked to reduced inflammation and oxidative stress. For example, treadmill or swim training reduce fibrosis, oxidative stress, and inflammation in doxorubicin cardiotoxicity.68,70 Similarly, exercise reduces fibrosis when initiated 1 week after MI in rats, as well as in hypertensive rats, and is associated with reduced inflammatory and fibrotic gene expression (TGF-β, p-Smad2/3, CTGF, matrix metalloproteinase 9, and collagen I).71,72 Swim training mitigates isoproterenol-induced cardiac fibrosis in an adenosine monophosphate–activated protein kinase (AMPK)–dependent manner, consistent with an oxidative stress–related mechanism.73 Chronic exercise also reduces oxidative stress as well as profibrotic signaling (TGFβ, pSmad2/3, matrix metalloproteinase-2, CTGF) and fibrosis in rats with diet-induced type 2 diabetes and cardiac dysfunction.74 As oxidative stress and inflammation play important roles in the pathogenesis of fibrosis, these changes likely contribute to reduction of fibrosis by exercise.
Exercise likely influences fibrosis through multiple mechanisms in the aged heart. Treadmill exercise in aged rats reduced fibrosis and advanced glycation end-product accumulation that are associated with both aging and diabetes.75 In a recent study, 8 weeks of voluntary wheel running increased the number of fibroblasts without changing overall fibrosis in the hearts of aged mice.16 The relevance of fibroblast hyperplasia in this setting is unclear.
Possible benefits and risks of reducing fibrosis in disease and aging
Although fibrosis is a hallmark of adverse cardiac remodeling in the elderly,55 it remains challenging to define the contribution of fibrosis per se to these conditions. Excessive fibrosis can impair cardiac systolic and diastolic function as well as lead to arrhythmia, but fibroblasts likely also play important roles in homeostasis, generating the extracellular matrix that serves as the scaffold of the heart,76,77 contributing to the heart’s response to mechanical stress,78 replacing lost cardiomyocytes, and mediating scar formation after injury.79,80 Thus, benefits and risks of targeting fibrosis likely vary in different contexts, with concerns about potential rupture, especially after infarction. Interestingly, the Tallquist laboratory recently demonstrated that genetic ablation of substantial numbers of cardiac fibroblasts was remarkably well tolerated, and fibroblast-ablated mice even showed improved cardiac function under pathological conditions (angiotensin II/phenylephrine infusion).81 Thus, either fibroblasts are not as critical as generally thought or the system can compensate for considerable fibroblast loss. Further preclinical studies will be critical for evaluating risks and benefits of targeting fibrosis.
Of note, focal myocardial fibrosis has been reported in longtime athletes in some82,83 but not all studies,84,85 suggesting that exercise can promote fibrosis under some conditions, although causality is uncertain given confounders inherent in observational studies.86,87 These observations underscore the importance of reliable biomarkers to gauge exercise benefit.
Cardiac vasculature
Clinically, considerable attention is devoted to the epicardial vessels because of their role in acute coronary syndromes as well as myocardial ischemia and infarction. While exercise has important effects at this level, there is growing recognition of the importance of other vascular components, notably coronary microcirculation and cardiac lymphatics, which we discuss here.
Coronary microcirculation
The coronary microcirculation comprises an uninterrupted network of cardiac blood vessels with diameters decreasing in size from prearterioles (500-100 μm in diameter) to arterioles (<100 μm diameter) and capillaries. This microvasculature regulates myocardial perfusion to match blood supply with oxygen consumption.88 On short time scales, such as during acute exercise, rapid adjustments in blood flow are achieved primarily by changes in diameter of prearterioles and arterioles. However, the coronary microvasculature also undergoes long-term structural and functional adaptations to chronic exercise.89,90 Exercise training enhances both smooth muscle–dependent, pressure-induced myogenic constriction and endothelium-dependent/shear stress–induced dilation in coronary arterioles.90 Arteriole diameter and density as well as capillary surface area and permeability are increased.90, 91, 92, 93, 94 Exercise training decreases elastic modulus and increases wall thickness, wall stress, and distensibility and in rat coronary arterioles.95 In contrast to pathological hypertrophy, in which muscle growth can outpace angiogenesis, likely contributing to heart failure,96 exercise training induces capillary angiogenesis proportionate to cardiac growth.91,93 Exercise also may protect the coronary microcirculation indirectly by mitigating inflammation, platelet activation, autonomic dysfunction, and hemodynamic forces.90,97
Molecular mechanisms of exercise-induced coronary microcirculation adaptions
Exercise training promotes endothelium-dependent vascular relaxation as well as angiogenesis in part by increasing nitric oxide (NO) signaling. Endothelial nitric oxide synthase (eNOS) messenger RNA and protein expression are increased in arterioles after exercise training and contribute to enhanced endothelium-dependent dilation.98,99 Increased eNOS expression may be triggered by exercise-related flow and shear stress.100 Expression of Cu/Zn superoxide dismutase is also flow dependent,100 and superoxide dismutase activity is increased with exercise training in the mouse heart101 and rat ventricular myocardium,102 suggesting that it may also contribute to increased NO bioavailability, as well as to reducing oxidative stress. β3-adrenergic receptor–dependent modulation of eNOS phosphorylation also contributes to increased cardiac eNOS activity with exercise training.101
Ion channels also contribute to coronary microvascular adaptations during exercise training. Exercise training increases calcium currents through voltage-gated Ca2+ channels in smooth muscle from conduit arteries, small arteries, and large arterioles, likely contributing to enhanced myogenic constriction.103 In cultured endothelial cells, shear stress altered the distribution of transient receptor potential channel TRPV4,104 a Ca2+-permeable cation channel involved in endothelium-dependent dilation,105 consistent with possible modulation by exercise training.
Although still poorly understood, microvascular adaptation also involves a complex interplay of many vasodilators and vasoconstrictors, including neurohormones and endothelial and myocardial influences. Further investigation is needed to define fully how exercise regulates coronary microvascular structure and function.
Exercise-induced coronary microcirculation adaptations in aging and disease
Regular exercise benefits patients with diseases involving dysregulation of coronary microcirculation, including heart failure and coronary artery disease, among others.4,106 Supporting a role for microvascular changes in the clinical benefits of exercise, 12-week aerobic interval training increased coronary flow reserve in coronary artery disease patients.106 Aging is also associated with coronary microvascular dysfunction,107,108 and microvascular changes may also contribute to exercise benefits in aging. Moderate exercise improved leg microvascular function in older adults,109 and in rats, treadmill training reversed age-related aortic stiffness as well as impaired coronary blood flow responses, endothelium-dependent vasodilatation, and early to atrial filling velocity ratio.110
The clinical benefits of exercise have been linked to many of the molecular mediators described previously. The protective effects of exercise against myocardial ischemia-reperfusion injury were lost in mice deficient in eNOS or the β3-adrenergic receptor, although reduced exercise in these mice may be a confounder.101 Adrenergic modulation was also associated with exercise benefits in patients with microvascular angina and syndrome X.111,112 In porcine models, treadmill training reversed impaired NO-mediated dilation of arterioles distal to coronary artery occlusion, and this was dependent on enhanced H2O2 and NO production.113 Consistent with a possible role for TRPV4, exercise training reversed age-related decline in TRPV4-dependent, endothelium-derived hyperpolarizing factor–mediated dilation in rat aortic arteries.114 Molecular mechanisms underlying exercise benefits may also differ in the context of age or disease, for example, exercise training reduced vessel wall collagen-to-elastin ratio in coronary arterioles of old but not young rats.93,94 These observations suggest exercise-inspired therapeutics targeting the microvasculature could benefit cardiovascular diseases and cardiac aging.
Cardiac lymphatics
Recent findings suggest a role for cardiac lymphatics in the benefits of exercise. Lymphatic vessels play essential and dynamic roles in maintaining interstitial pressure, lipid transport, and clearance of antigens and immune cells, as well as organ-specific adaptation to the local microenvironment.115, 116, 117 Regular exercise improves impaired lymphatic function both in animal studies and in randomized controlled trials with human patients.118 Vascular endothelial growth factor C and D are the main drivers of lymphangiogenesis via the receptor vascular endothelial growth factor receptor 3 (VEGFR3), and all of these were elevated in mouse hearts after swim training.119 Lymphatic markers podoplanin and LYVE-1 were also increased in swim trained animals in a VEGFR3-dependent manner, as was the density of LYVE-1–positive vessels.119 Importantly, VEGFR3 inhibition attenuated exercise-induced cardiac and cardiomyocyte growth, suggesting a role of lymphangiogenesis in physiological cardiac hypertrophy.119 This role likely involves crosstalk between lymphatic endothelial cells and cardiomyocytes, as VEGFR3-dependent hypertrophy and proliferation was also induced in cultured neonatal rat cardiomyocytes treated with conditioned medium from lymphatic endothelial cells.119
The role of cardiac lymphatics in the therapeutic effects of exercise has not been directly examined; however, dysregulation of cardiac lymphatics in disease has been long recognized,120 and has been documented in hypertension,121 atherosclerosis and dyslipidemia,122,123 MI, and heart failure.124 Moreover, growing evidence supports cardiac lymphatic growth and remodeling as potential therapeutic targets.125,126 Vascular endothelial growth factor C gene delivery by adeno-associated virus or injection of protein reduced cardiac inflammation, infarct thinning, and cardiac dysfunction after MI.127,128 These benefits may in part reflect the role of cardiac lymphatics in transport of immune cells to and from the injury site after MI.129 Recently, analysis of the lymphatic endothelial cell secretome uncovered RELN as a lymphoangiocrine protein directing cardiomyocyte proliferation and survival during MI.130 Notably, RELN and IGF-1 were increased in mouse hearts by swim training.119 Further work is needed to define exercise-regulated lymphangiogenic pathways and crosstalk with cardiomyocytes and investigate their potential therapeutic relevance.
Systemic Effects with Important Cardiac Consequences
Metabolism
Exercise training, and physical activity more generally, induces systemic metabolic changes that reduce cardiovascular disease risk factors such as obesity and diabetes. In part, this may reflect changes in energy homeostasis although this effect is generally modest. Likely more important are improved insulin sensitivity and glucose uptake by skeletal muscle and other tissues, due in part to increased expression of the glucose transporter GLUT4131,132 and AMPK,133,134 a key kinase regulating glucose uptake. Another key systemic adaptation related to metabolic disease is skeletal muscle induction of transcriptional coactivator PGC-1α,135 important in mitochondrial biogenesis and oxidative metabolism.
In the myocardium, either increased substrate or more efficient energy utilization is necessary to support augmented cardiomyocyte size and function in response to exercise training, particularly as SERCA2a and other ion pumps involved in cardiomyocyte function account for the bulk of the heart’s adenosine triphosphate needs. Exercise training up-regulates metabolic modulatory enzymes in the heart including Akt1,136 NAD(+)-dependent deacetylases, SIRT-1137 and SIRT-3,138 eNOS,139 and the energy sensor, AMPK.139 Through targets including PGC-1α139,140 and transcription factors, FoxO1141 and FoxO3a,138 these interconnected signaling pathways activate transcriptional networks that increase measures of mitochondrial mass and function,139 improve cardiac fatty acid and glucose handling,139,142 and protect against oxidative stress.138,141
Exercise-mediated changes in metabolism in disease and aging
Exercise also directly counteracts metabolic changes seen in aging and diseased hearts. Pathological hypertrophy and cardiac aging are associated with impaired mitochondrial respiratory capacity, decreased mitochondrial biogenesis, a shift in substrate utilization from fatty acids to glucose, and excessive production of mitochondrial reactive oxygen species.143,144 These changes reduce metabolic reserve.145 In contrast, physiological cardiac remodeling is not associated with a shift from fatty acid metabolism to glycolysis and is associated with increased mitochondrial biogenesis and antioxidant mechanisms.146
Exercise training enhances cardiac metabolism in rodent heart failure models through more efficient fatty acid metabolism, restoration of autophagic flux, and increased mitochondrial biogenesis, essentially reprogramming the bioenergetic profile of the failing heart to improve function.147,148 The cardiac metabolic benefits of exercise also extend to aged subjects, but appear greater in young individuals,145 and this tracks with changes in the metabolic response to training. For example, exercise-induced muscle expression of PGC-1α is lower in older subjects.149
Pathways that modulate cardiac metabolic adaptation to exercise are also necessary and/or sufficient to protect the heart against pathological stress, injury, and aging. Cardiac-specific SIRT1 deletion exacerbated while overexpression protected against ischemia-reperfusion injury.141 SIRT3 overexpression in the heart blocked angiotensin II–induced pathological hypertrophy.138 Exercise training also ameliorated cardiac metabolic impairments in a diabetic cardiomyopathy model by increasing PGC-1α and Akt activation150 and reduced the age-related increase in mitochondrial reactive oxygen species production.144
These preclinical findings point to exercise-modulated metabolic regulators as clinically relevant targets. Notably, metformin, which activates AMPK, has been widely used for treatment of type 2 diabetes and may have protective effects on the cardiovascular system,151 and supplements such as resveratrol that have been reported to activate sirtuin, PGC-1α, and AMPK signaling are being investigated in clinical trials in the context of cardiovascular as well as metabolic disease152 (NCT03525379). New pharmacological approaches targeting these pathways warrant further investigation.
Inflammation and immune cells
As mentioned previously, inflammation is important in the pathogenesis of many cardiovascular diseases,152 and the anti-inflammatory effects of exercise are important in its cardiovascular benefits (Figure 1). Controlled experiments in humans and animal models demonstrate an association between exercise benefits and a reduction in inflammatory markers systemically and in the heart, both during aging and in a range of pathological conditions including heart failure, MI, and atherosclerosis.68,71,72,153, 154, 155 Underscoring again the need for biomarkers of exercise benefit, strenuous acute exercise has been linked to increased inflammation, suggesting that the relationship between training and inflammation may change at high intensities.156
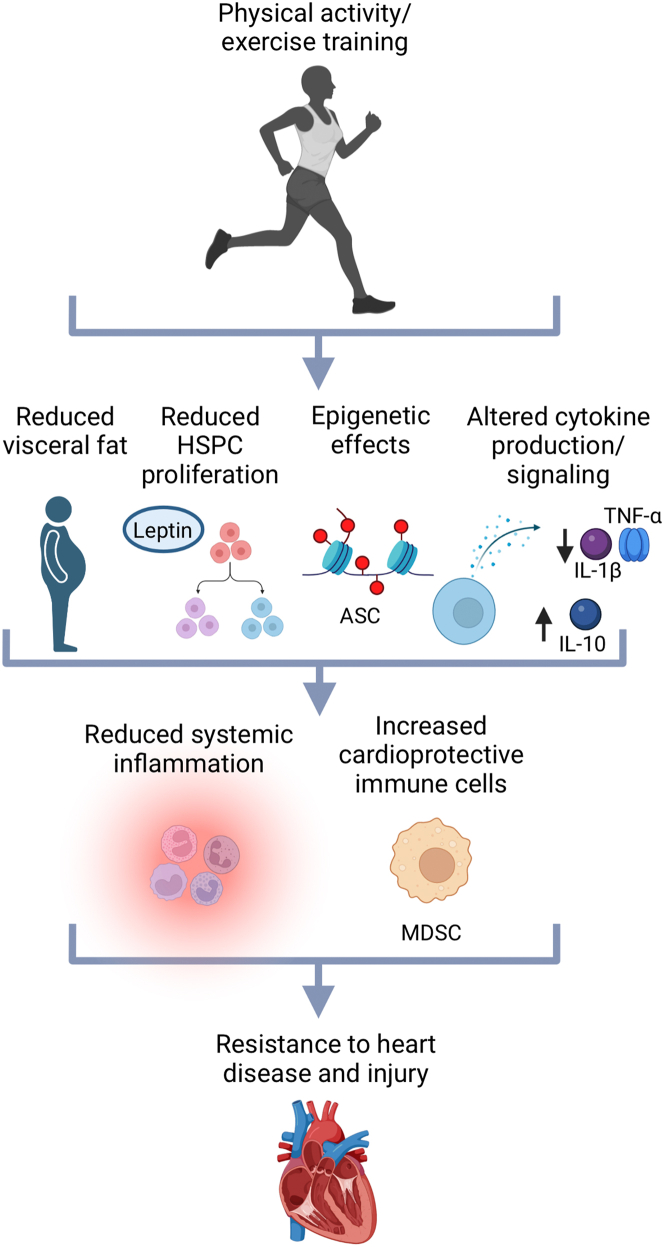
Figure 1.
Possible Inflammatory and Immune Mechanisms Underlying Exercise Effects on the Heart
Reduced systemic inflammation and expansion of cardioprotective immune cells, including myeloid derived suppressor cells (MDSCs), likely contribute to the cardiac benefits of exercise. Underlying mechanisms include reduced visceral fat, reduced hematopoietic stem and progenitor cell (HSPC) proliferation, epigenetic changes, and altered cytokine production/signaling. Created with BioRender.com. IL = interleukin; TNF = tumor necrosis factor.
The systemic anti-inflammatory benefits of exercise are likely due in part to reduced accumulation of visceral fat, which is associated with proinflammatory immune cell infiltration.157,158 However, exercise also appears to directly modulate the immune system.159 For example, in a mouse isoproterenol-induced heart failure model, exercise-induced cardioprotection was associated with increased serum and cardiac interleukin (IL)-10 and cardiac myeloid-derived suppressor cells, and protection was lost in myeloid-derived suppressor cell–depleted or IL-10 knockout mice.160
A recent study161 suggested that physical activity improved cardiac function in part through changes in the bone marrow microenvironment that reduced hematopoiesis. In mice, 6-week voluntary exercise triggered a 34% reduction in proliferation of hematopoietic stem and progenitor cells, which give rise to leukocytes, including lymphocytes, and macrophages. This in turn decreased circulating inflammatory leukocytes. Bone marrow mononuclear cells in exercise-trained mice were less able to differentiate into granulocytes, macrophages, and B cells. These effects were traced to decreased leptin signaling in bone marrow stromal cells, resulting from reduced leptin secretion from visceral adipose tissue. The reduction in circulating leukocytes and hematopoietic stem and progenitor cell proliferation was blocked by increasing leptin to sedentary levels, or mimicked by deleting the leptin receptor in bone marrow stromal cells. Decreased circulating leptin and leukocytes were also observed with exercise in atherosclerosis, both in humans and mice, and exercise benefits were mimicked in atherosclerotic mice lacking the bone marrow stromal cell leptin receptor. Mice lacking the leptin receptor also showed improved cardiac function and reduced cardiac and circulating leukocyte numbers after MI.
Other mechanisms of immune modulation have been indirectly implicated in the cardiovascular benefits of exercise. For example, one study reported that physical activity diminished cytokine production capacity of peripheral blood mononuclear cells in individuals at risk for cardiovascular disease.155 Other work pointed to epigenetic regulation of the gene encoding ASC, an adaptor protein that mediates proinflammatory signaling. Exercise increased methylation and decreased expression of ASC in peripheral blood from older individuals162 and heart failure patients.163 ASC methylation was associated with reduced plasma IL-1β and better performance on a 6-minute walk test in the heart failure patients. In contrast, aging162 and poor heart failure outcomes164 have been associated with decreased ASC methylation.
Systemic inflammation increases with aging and obesity and, due to increasing population age and obesity, represents a growing problem. Understanding the anti-inflammatory effects of exercise may provide new therapeutic targets for combatting these trends.
The microbiome
The intestinal microbiome is increasingly recognized as a possible contributor to exercise effects, including effects on inflammation and metabolism. In recent work from the Xiao laboratory, antibiotics abolished the protective effects of running in mice after MI, and fecal microbiota transplantation (FMT) from mice exercised post-MI attenuated postinfarction cardiac remodeling and improved heart function.165 Exercise was reported to increase microbial diversity, enrich beneficial bacterial genera, and reduce hypertension in spontaneously hypertensive rats.166 Indicating a causal role for the microbiome, FMT from exercised rats was also sufficient to decrease systolic blood pressure.166 The microbiome is also implicated in exercise benefits for prevention of diabetes, a cardiovascular risk factor, in humans. Prediabetics who derived glycemic benefits from exercise training could be discriminated from those who did not based on changes in their microbiome and associated metabolites,167 and FMT from responders but not nonresponders reproduced the glycemic benefits in obese mice.167 These observations suggest that microbiome changes may contribute to the cardiovascular benefits of exercise (Figure 2).
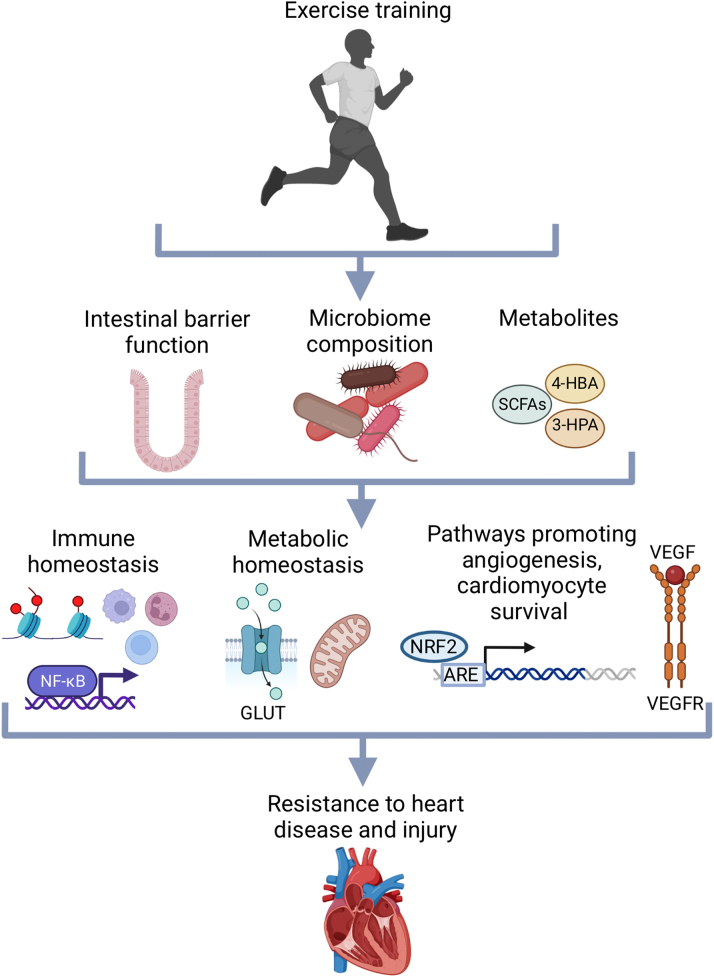
Figure 2.
Mechanisms by Which Gut Microbiota May Mediate the Effects of Exercise Training on the Heart
Exercise may reduce systemic inflammation, maintain metabolic homeostasis, and activate angiogenic and cardioprotective pathways in part through its effects on intestinal permeability, microbiota composition, and microbial metabolites. These processes could potentially be targeted through antibiotic treatment, fecal microbiota transplantation, or metabolite supplementation as therapeutic approaches for cardiovascular disease. Created with BioRender.com. NF-κB = nuclear factor-κB; SCFA = short-chain fatty acid; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
Potential mechanisms of microbiome-mediated exercise benefits
The effects of exercise likely reflect changes in microbiome diversity, composition, and metabolites. Increases and decreases in a range of fecal and serum metabolites are observed after acute exercise in amateur runners,168,169 with evidence of metabolite exchange between serum and fecal compartments.169 Regular exercise altered the gut microbiome in animals and humans, with effects on the most prevalent gut microbial phyla, Bacteroidetes and Firmicutes,170,171 although no consistent pattern has emerged at the genus level (Table 1). Some, but not all, reports suggest that physical activity also increases gut microbiome diversity.172
Table 1.
Summary of Studies Demonstrating Exercise Training Effects on the Composition of the Microbiome
| Model | Exercise Training | Effects of Exercise | Ref. # |
|---|---|---|---|
| Human: 32 previously sedentary women (n = 20) and men (n = 12) based on a lean or obese body mass index | 2 wk of baseline testing + 6 wk endurance-based exercise intervention + 6-wk washout period | Butyrate producers (Clostridiales spp., Lachnospira spp., Roseburia spp. f_Lachnospiraceae unclass, and Faecalibacterium spp.) increased. | 196 |
| Human: sedentary overweight women (n = 19) aged 36.8 ± 3.9 y | 6 wk of endurance training (40-60 min) | Dorea, Anaerofilum, and Akkermansia increased while unidentified Porphyromonadaceae, Odoribacter, unidentified Desulfovibrionaceae, and unidentified Enterobacteriaceae decreased. | 197 |
| Rat: male Sprague Dawley rats (5 wk old) | 6 d running wheels | Bifidobacterium and Lactobacillus increased; Bacteroides, Prevotella, Enteroccocus, and Clostridium decreased. | 198 |
| Mouse: male type 2 diabetic db/db mice (6 wk old) | 6 wk of low-intensity treadmill running (5 d/wk) | Bifidobacterium spp. and Methanobrevibacter spp. decreased; Lactobacillus spp. and Clostridium leptum increased. | 199 |
| Mouse: 8-wek-old male mice fed with HFD | 6 wk of treadmill running (1 h each day; 17-22 m/min) | Lactococcus was decreased 1 h after acute exercise, but change did not persist 1 wk after acute exercise. | 200 |
| Mouse: male C57BL/6 mice post-MI (8-10 wk old) | 8 wk of treadmill running (15 m/min) | Alistipes, Ruminococcus, Allobaculum, and Oscillospiraceae UCG-005 increased; Lachnospiraceae_UCG-001 decreased. | 165 |
HFD = high-fat diet; MI = myocardial infarction.
Although few studies have directly addressed mechanisms by which the microbiome mediates exercise effects on the heart, some microbiota-associated metabolites that are modulated by exercise training also impact cardiovascular disease phenotypes (Table 2). Fecal short-chain fatty acids (SCFAs) are increased in athletes,173 and SCFAs are associated with diverse, often protective roles in cardiovascular diseases, including atherosclerosis, hypertension, and heart failure.174,175 These effects may be related to SCFA modulation of inflammatory and immune phenotypes, including tumor necrosis factor and nuclear factor κB signaling, which SCFAs modulate through interaction with G protein–coupled receptors174 and by acting as histone deacetylase inhibitors, highlighting again the likely role of epigenetic mechanisms in the benefits of exercise training.176 Consistent with immune effects, SCFAs restore myeloid cell, macrophage, and neutrophil levels, as well as survival and favorable remodeling after MI in mice with depleted gut microbiota.177 Evidence supporting SCFAs as a link between exercise, microbiota, and cardiovascular risk comes from the observation that insulin resistance in obese mice was improved by FMT from prediabetic exercise responders but not nonresponders, and SCFA supplementation partially restored the beneficial effects in mice transplanted with “nonresponder” microbiota. In contrast, branched-chain amino acid supplementation decreased the beneficial effects in mice transplanted with “responder” microbiota.167
Table 2.
Cardiovascular Benefits and Mechanisms Associated With Exercise-Regulated Microbiome Metabolites
| Metabolite | Disease or Model | Mechanisms | Ref. # | |
|---|---|---|---|---|
| Short-chain fatty acids | Propionate | Hypertensive | Propionate affected immune homeostasis and beneficially modulated effector T cells. | 201 |
| Akt2 knockout–induced cardiac contractile and mitochondrial dysfunction | Propionate attenuated the decrease in G protein–coupled receptor GPR41 in this model. | 202 | ||
| Myocardial infarction | Propionate promoted macrophages reduction and inhibited JNK/P38/NFκB. | 203 | ||
| Butyrate | Diabetic cardiomyopathy mice (streptozotocin) | Butyrate inhibited HDAC4 and increased GLUT1 and GLUT4, as well as GLUT1 acetylation in the myocardium. | 204 | |
| Diabetic rats (HFD and low dose streptozotocin) | Sodium butyrate and exercise increased VEGF-A and VEGFR2. | 205 | ||
| Doxorubicin-induced cardiotoxicity | Butyrate derivative phenylalanine-butyramide inhibited oxidative and nitrosative stress and counteracted mitochondrial dysfunction. | 206 | ||
| 3-HPA | Myocardial infarction | 3-HPA and 4-HBA increased the expression of NRF2 in oxygen glucose deprivation/reoxygenation-induced neonatal rat cardiomyocytes. | 165 | |
| 4-HBA | ||||
HFD = high-fat diet; VEGF = vascular endothelial growth factor; VEGFR = vascular endothelial growth factor receptor.
Recently, metabolomic profiling of fecal samples identified 3-HPA and 4-HBA as candidate mediators of the protective effects of exercise in mice post-MI, and supplementation improved cardiac function and protected against cardiac dysfunction after MI.165 Mechanistically, 3-HPA and 4-HBA reduced cardiomyocyte apoptosis by activating NRF2,165 a new target of microbiota-derived metabolites.
Another microbiota-derived metabolite linked to both cardiovascular disease and exercise is trimethylamine. Trimethylamine is further metabolized to TMAO, which increased risk of atherosclerosis,178 cardiovascular events such as ischemic stroke,179 and heart failure.180 Interestingly exercise reversed the aggravation of cognitive dysfunction by TMAO in an Alzheimer’s mouse model.181
These data are consistent with the hypothesis that exercise contributes to cardiac benefits by increasing advantageous metabolites and/or reversing pathological metabolic changes. Supporting the latter possibility, cardiovascular diseases were associated with altered enterotypes,182 and FMT and microbiome-depletion experiments suggest causal roles for gut microbiota in cardiovascular diseases including hypertension and MI.177,182
Finally, effects on gut permeability may also contribute to the cardiovascular benefits of exercise. The intestinal mucosa serves as a selectively permeable barrier for nutrient absorption while preventing pathogen entry that could increase systemic inflammation, a driver of cardiovascular disease.183 While intense, acute exercise increases measures of intestinal permeability in humans,184 chronic exercise increased intestinal integrity and selective barrier function, possibly through changes in gut microbiota.185, 186, 187 In a recent study, lipopolysaccharide (LPS) and D-lactate, products of gut bacterial translocation, were increased in plasma after MI in patients and gut permeability was increased after MI in mice due to suppression of tight junction proteins and intestinal mucosal injury.188 The antibiotic polymyxin B inhibited gut microbial translocation and reduced cardiomyocyte injury.188 Interestingly, while voluntary wheel running alleviates symptoms and reduces inflammation in a mouse model of colitis, in an inflammatory disease involving increased gut permeability, forced treadmill running exacerbates it, possibly reflecting differences in intensity or stress in these exercise models.189
Metabolites and microbiota are readily manipulated, suggesting that interventions targeting the microbiome may be particularly amenable to translation. SCFAs in particular are already being investigated as a possible therapeutic for hypertension in clinical trials.190 While experiments in animal models will be essential for identifying therapeutic candidates, further work is also needed to characterize changes in the microbiome with exercise in patients with and without cardiovascular disease.
Exercise and aging
Advanced age is one of the strongest risk factors for cardiovascular disease in general and heart failure in particular,191 although precisely how aging contributes to the development of cardiovascular disease and whether it is possible to intervene in this process remain unclear. In part this reflects our still incomplete understanding of aging itself. While a detailed discussion of these issues is beyond the scope of this review, the interested reader is referred to a recent update cataloguing the phenotypic and molecular hallmarks of aging192 as well as recent reviews on targeting these pathways in heart disease and the role of exercise in this context.46,193 The same processes driving aging of the organism occur within the heart itself, so aging represents both a systemic and intrinsic contributor to heart disease. Interestingly, exercise counteracts many of these aging pathways. Consistent with this conceptual framework, exercise is one of the few interventions that appears effective in reducing age-related cardiac functional decline. On the other hand, in general, aged animals exercise at lower intensities than young animals, reducing the potential benefits of exercise. As noted previously, we found that forced treadmill exercise reversed many—but not all—of the phenotypes seen in age-related heart failure with preserved ejection fraction.55 Moreover, while voluntary wheel running induced cardiomyogenesis in both young adult and aged mice,15,16 the older mice ran less and only restored cardiomyogenesis to levels seen in sedentary younger mice. This illustrates one of the challenges of working with older animals because it is impossible to infer whether the lower rates of cardiomyogenesis reflect the lower activity level, a dampened response to exercise, or some combination.
With advanced age, hearts become hypertrophied with increases in cardiomyocyte size. However, the effect of exercise on age-related cardiac hypertrophy is controversial. Swim training for 8 weeks in 23-month-old mice was reported to increase capillary density without impacting cardiomyocyte size.194 In contrast, others have found that both short-term (10 weeks) and long-term (12 months) treadmill training increased heart weight and cardiomyocyte size in 24-month-old mice.195 Other reports demonstrated that 12 weeks of swim training reversed cardiac hypertrophy in 18-month-old mice.137 These inconsistencies may result from differences in age, exercise protocols, or animal strains used, but they also raise the possibility that exercise may be less effective in aged animals. While we have attempted to highlight what is known about the impact of exercise not only in young adults, but also in the context of advanced age, in many cases, our understanding remains incomplete.
Conclusions
A wealth of clinical and preclinical data have contributed to our appreciation of the cardiac benefits of exercise and physical activity. Yet, our insights into the responsible mechanisms and identification of reliable reporters of response remain limited. Here, we have reviewed our current understanding of the mediators of exercise benefits, including mechanisms intrinsic to the heart, involving cardiomyocytes and noncardiomyocyte, and those systemic processes that have important implications for cardiac biology. Efforts continue to better understand these contributions, notably including the Molecular Transducers of Physical Activity Consortium initiative, supported by the National Institutes of Health Common Fund. This large-scale, multidisciplinary consortium aims to comprehensively characterize the molecular changes induced by exercise across tissues in humans and preclinical models. In addition to identifying new mechanisms, the Molecular Transducers of Physical Activity Consortium's publicly available multiomics dataset will be a hypothesis-generating resource of unprecedented scope. Improved understanding and identification of molecular mediators as well as markers of benefit could lead to new therapeutic strategies, inspired by exercise, and ways to personalize general recommendations for physical activity. In the meantime, those who can should incorporate physical activity into their daily lives wherever possible as a route to preventing and mitigating disease as well as improving quality of life.
Funding Support and Author Disclosures
This work was supported by the National Institutes of Health (R01AG061034 and R35HL155318 [to Dr Rosenzweig], R21AG077040 [to Dr Li], K08HL140200 [to Dr Rhee], T32HL007208 [to Dr Xia], and K76AG064328 [to Dr Roh]), the American Heart Association (20CDA35310184 [to Dr Li]), the German Research Foundation (grant number LE3257/1-1 [to Dr Lerchenmüller]), the Olympia Morata Fellowship and project support by the University of Heidelberg Medical Faculty (to Dr Lerchenmüller), the Else-Kröner-Fresenius-Stiftung (2019-A07 [to Dr Lerchenmüller]), the National Natural Science Foundation of China (82020108002 [to Dr Xiao] and 82200321 [to Dr Zhou]), and the Shanghai Sailing Program (21YF1413200 [to Dr Zhou]). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.O'Connor C.M., Whellan D.J., Lee K.L., et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannuzzi P., Temporelli P.L., Marchioli R., et al. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med. 2008;168:2194–2204. doi: 10.1001/archinte.168.20.2194. [DOI] [PubMed] [Google Scholar]
- 3.Lawler P.R., Filion K.B., Eisenberg M.J. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2011;162:571–584.e2. doi: 10.1016/j.ahj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Piepoli M.F., Davos C., Francis D.P., Coats A.J., ExTraMatch Collaborative Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman D.W., Brubaker P., Morgan T., et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelmann F., Gelbrich G., Dungen H.D., et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Pinto A.R., Ilinykh A., Ivey M.J., et al. Revisiting cardiac cellular composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao C.W., Gona P.N., Salton C.J., et al. Left ventricular structure and risk of cardiovascular events: a Framingham Heart Study Cardiac Magnetic Resonance Study. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bostrom P., Mann N., Wu J., et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H., Zhu Y., Zheng C., et al. Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779. Circulation. 2021;143:2277–2292. doi: 10.1161/CIRCULATIONAHA.120.047000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Trager L.E., Liu X., et al. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation. 2022;145:1218–1233. doi: 10.1161/CIRCULATIONAHA.121.056850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura M., Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 14.Wisloff U., Loennechen J.P., Currie S., Smith G.L., Ellingsen O. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA-2 in rat after myocardial infarction. Cardiovasc Res. 2002;54:162–174. doi: 10.1016/s0008-6363(01)00565-x. [DOI] [PubMed] [Google Scholar]
- 15.Vujic A., Lerchenmuller C., Wu T.D., et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerchenmuller C., Vujic A., Mittag S., et al. Restoration of cardiomyogenesis in aged mouse hearts by voluntary exercise. Circulation. 2022;146:412–426. doi: 10.1161/CIRCULATIONAHA.121.057276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asif Y., Wlodek M.E., Black M.J., Russell A.P., Soeding P.F., Wadley G.D. Sustained cardiac programming by short-term juvenile exercise training in male rats. J Physiol. 2018;596:163–180. doi: 10.1113/JP275339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wencker D., Chandra M., Nguyen K., et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann O., Bhardwaj R.D., Bernard S., et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazar E., Sadek H.A., Bergmann O. Cardiomyocyte renewal in the human heart: insights from the fall-out. Eur Heart J. 2017;38:2333–2342. doi: 10.1093/eurheartj/ehx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bei Y., Fu S., Chen X., et al. Cardiac cell proliferation is not necessary for exercise-induced cardiac growth but required for its protection against ischaemia/reperfusion injury. J Cell Mol Med. 2017;21:1648–1655. doi: 10.1111/jcmm.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koziris L.P., Hickson R.C., Chatterton R.T., Jr., et al. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J Appl Physiol (1985) 1999;86:1436–1442. doi: 10.1152/jappl.1999.86.4.1436. [DOI] [PubMed] [Google Scholar]
- 23.Kim J., Wende A.R., Sena S., et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullen J.R., Shioi T., Zhang L., et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeBosch B., Treskov I., Lupu T.S., et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 26.Shioi T., McMullen J.R., Kang P.M., et al. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui T., Li L., Wu J.C., et al. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 28.Matsui T., Li L., delMonte F., et al. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 29.Matsui T., Tao J., del Monte F., et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 30.Weeks K.L., Gao X., Du X.J., et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–534. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 31.Lerchenmuller C., Rabolli C.P., Yeri A., et al. CITED4 protects against adverse remodeling in response to physiological and pathological stress. Circ Res. 2020;127:631–646. doi: 10.1161/CIRCRESAHA.119.315881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eder R.A., van den Boomen M., Yurista S.R., et al. Exercise-induced CITED4 expression is necessary for regional remodeling of cardiac microstructural tissue helicity. Commun Biol. 2022;5:656. doi: 10.1038/s42003-022-03635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezzerides V.J., Platt C., Lerchenmuller C., et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X., Xiao J., Zhu H., et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21 doi: 10.1016/j.cmet.2015.02.014. 584-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao R., Wang L., Bei Y., et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation. 2021;144:303–317. doi: 10.1161/CIRCULATIONAHA.120.050446. [DOI] [PubMed] [Google Scholar]
- 36.Gales L. Tegsedi (Inotersen): an antisense oligonucleotide approved for the treatment of adult patients with hereditary transthyretin amyloidosis. Pharmaceuticals (Basel) 2019;12:78. doi: 10.3390/ph12020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nwabo Kamdje A.H., Seke Etet P.F., Kipanyula M.J., et al. Insulin-like growth factor-1 signaling in the tumor microenvironment: carcinogenesis, cancer drug resistance, and therapeutic potential. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.927390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavie C.J., Arena R., Swift D.L., et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisloff U., Ellingsen O., Kemi O.J. High-intensity interval training to maximize cardiac benefits of exercise training? Exerc Sport Sci Rev. 2009;37:139–146. doi: 10.1097/JES.0b013e3181aa65fc. [DOI] [PubMed] [Google Scholar]
- 40.Kemi O.J., Haram P.M., Loennechen J.P., et al. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67:161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Kemi O.J., Ellingsen O., Smith G.L., Wisloff U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Front Biosci. 2008;13:356–368. doi: 10.2741/2685. [DOI] [PubMed] [Google Scholar]
- 42.Wisloff U., Loennechen J.P., Falck G., et al. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res. 2001;50:495–508. doi: 10.1016/s0008-6363(01)00210-3. [DOI] [PubMed] [Google Scholar]
- 43.Diffee G.M., Seversen E.A., Titus M.M. Exercise training increases the Ca(2+) sensitivity of tension in rat cardiac myocytes. J Appl Physiol (1985) 2001;91:309–315. doi: 10.1152/jappl.2001.91.1.309. [DOI] [PubMed] [Google Scholar]
- 44.Kemi O.J., Ellingsen O., Ceci M., et al. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007;43:354–361. doi: 10.1016/j.yjmcc.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stolen T.O., Hoydal M.A., Kemi O.J., et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 46.Roh J., Rhee J., Chaudhari V., Rosenzweig A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ Res. 2016;118:279–295. doi: 10.1161/CIRCRESAHA.115.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemi O.J., Hoydal M.A., Macquaide N., et al. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J Cell Physiol. 2011;226:2235–2243. doi: 10.1002/jcp.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehmann L.H., Jebessa Z.H., Kreusser M.M., et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med. 2018;24:62–72. doi: 10.1038/nm.4452. [DOI] [PubMed] [Google Scholar]
- 49.Seo D.Y., Kwak H.B., Kim A.H., et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch. 2020;472:155–168. doi: 10.1007/s00424-019-02266-3. [DOI] [PubMed] [Google Scholar]
- 50.Emter C.A., McCune S.A., Sparagna G.C., Radin M.J., Moore R.L. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol. 2005;289:H2030–H2038. doi: 10.1152/ajpheart.00526.2005. [DOI] [PubMed] [Google Scholar]
- 51.Lowes B.D., Gilbert E.M., Abraham W.T., et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 52.Pandey A., Kraus W.E., Brubaker P.H., Kitzman D.W. Healthy aging and cardiovascular function: invasive hemodynamics during rest and exercise in 104 healthy volunteers. J Am Coll Cardiol HF. 2020;8:111–121. doi: 10.1016/j.jchf.2019.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howden E.J., Sarma S., Lawley J.S., et al. Reversing the cardiac effects of sedentary aging in middle age-a randomized controlled trial: implications for heart failure prevention. Circulation. 2018;137:1549–1560. doi: 10.1161/CIRCULATIONAHA.117.030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hieda M., Sarma S., Hearon C.M., Jr., et al. One-year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage B heart failure with preserved ejection fraction. Circulation. 2021;144:934–946. doi: 10.1161/CIRCULATIONAHA.121.054117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roh J.D., Houstis N., Yu A., et al. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell. 2020;19 doi: 10.1111/acel.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu L., Mei D.F., Gu A.G., et al. Exercise training normalizes altered calcium-handling proteins during development of heart failure. J Appl Physiol (1985) 2002;92:1524–1530. doi: 10.1152/japplphysiol.00405.2001. [DOI] [PubMed] [Google Scholar]
- 57.Scarpace P.J., Shu Y., Tumer N. Influence of exercise training on myocardial beta-adrenergic signal transduction: differential regulation with age. J Appl Physiol (1985) 1994;77:737–741. doi: 10.1152/jappl.1994.77.2.737. [DOI] [PubMed] [Google Scholar]
- 58.Tate C.A., Taffet G.E., Hudson E.K., Blaylock S.L., McBride R.P., Michael L.H. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol. 1990;258:H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 59.Kwak H.B., Song W., Lawler J.M. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–793. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- 60.Morissette M.R., Stricker J.C., Rosenberg M.A., et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roh J.D., Hobson R., Chaudhari V., et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmidt U., del Monte F., Miyamoto M.I., et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 63.Greenberg B., Butler J., Felker G.M., et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 64.Hoeper M.M., Badesch D.B., Ghofrani H.A., et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388:1478–1490. doi: 10.1056/NEJMoa2213558. [DOI] [PubMed] [Google Scholar]
- 65.Porter K.E., Turner N.A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan M.L., Cheslow Y., Vikstrom K., et al. Cardiac adaptations to chronic exercise in mice. Am J Physiol. 1994;267:H1167–H1173. doi: 10.1152/ajpheart.1994.267.3.H1167. [DOI] [PubMed] [Google Scholar]
- 67.Lighthouse J.K., Burke R.M., Velasquez L.S., et al. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4 doi: 10.1172/jci.insight.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang H.L., Hsieh P.L., Hung C.H., et al. Early moderate intensity aerobic exercise intervention prevents doxorubicin-caused cardiac dysfunction through inhibition of cardiac fibrosis and inflammation. Cancers (Basel) 2020;12:1102. doi: 10.3390/cancers12051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sassi Y., Avramopoulos P., Ramanujam D., et al. Cardiac myocyte miR-29 promotes pathological remodeling of the heart by activating Wnt signaling. Nat Commun. 2017;8:1614. doi: 10.1038/s41467-017-01737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ascensao A., Magalhaes J., Soares J., et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100:451–460. doi: 10.1016/j.ijcard.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Xu X., Wan W., Powers A.S., et al. Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. J Mol Cell Cardiol. 2008;44:114–122. doi: 10.1016/j.yjmcc.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin Y.Y., Hong Y., Zhou M.C., et al. Exercise training attenuates cardiac inflammation and fibrosis in hypertensive ovariectomized rats. J Appl Physiol (1985) 2020;128:1033–1043. doi: 10.1152/japplphysiol.00844.2019. [DOI] [PubMed] [Google Scholar]
- 73.Ma X., Fu Y., Xiao H., et al. Cardiac fibrosis alleviated by exercise training is AMPK-dependent. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S.Q., Li D., Yuan Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-beta1/Smad pathway. J Physiol Sci. 2019;69:861–873. doi: 10.1007/s12576-019-00696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright K.J., Thomas M.M., Betik A.C., Belke D., Hepple R.T. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp Gerontol. 2014;50:9–18. doi: 10.1016/j.exger.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Travers J.G., Kamal F.A., Robbins J., Yutzey K.E., Blaxall B.C. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowers S.L., Banerjee I., Baudino T.A. The extracellular matrix: at the center of it all. J Mol Cell Cardiol. 2010;48:474–482. doi: 10.1016/j.yjmcc.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abramochkin D.V., Lozinsky I.T., Kamkin A. Influence of mechanical stress on fibroblast-myocyte interactions in mammalian heart. J Mol Cell Cardiol. 2014;70:27–36. doi: 10.1016/j.yjmcc.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz-Villalba A., Romero J.P., Hernandez S.C., et al. Single-cell RNA sequencing analysis reveals a crucial role for CTHRC1 (collagen triple helix repeat containing 1) cardiac fibroblasts after myocardial infarction. Circulation. 2020;142:1831–1847. doi: 10.1161/CIRCULATIONAHA.119.044557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holmes J.W., Borg T.K., Covell J.W. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–253. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 81.Kuwabara J.T., Hara A., Bhutada S., et al. Consequences of PDGFRalpha(+) fibroblast reduction in adult murine hearts. Elife. 2022;11 doi: 10.7554/eLife.69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.La Gerche A., Burns A.T., Mooney D.J., et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 83.Wilson M., O'Hanlon R., Prasad S., et al. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985) 2011;110:1622–1626. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdullah S.M., Barkley K.W., Bhella P.S., et al. Lifelong physical activity regardless of dose is not associated with myocardial fibrosis. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bohm P., Schneider G., Linneweber L., et al. Right and left ventricular function and mass in male elite master athletes: a controlled contrast-enhanced cardiovascular magnetic resonance study. Circulation. 2016;133:1927–1935. doi: 10.1161/CIRCULATIONAHA.115.020975. [DOI] [PubMed] [Google Scholar]
- 86.Shave R., Oxborough D. Endurance exercise and myocardial fibrosis: let us keep the risk in perspective. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005730. [DOI] [PubMed] [Google Scholar]
- 87.Malek L.A., Bucciarelli-Ducci C. Myocardial fibrosis in athletes: additional considerations. Clin Cardiol. 2020;43:1208. doi: 10.1002/clc.23466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Buono M.G., Montone R.A., Camilli M., et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heinonen I., Kalliokoski K.K., Hannukainen J.C., Duncker D.J., Nuutila P., Knuuti J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology (Bethesda) 2014;29:421–436. doi: 10.1152/physiol.00067.2013. [DOI] [PubMed] [Google Scholar]
- 90.Koller A., Laughlin M.H., Cenko E., et al. Functional and structural adaptations of the coronary macro- and microvasculature to regular aerobic exercise by activation of physiological, cellular, and molecular mechanisms: ESC Working Group on Coronary Pathophysiology and Microcirculation position paper. Cardiovasc Res. 2022;118:357–371. doi: 10.1093/cvr/cvab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncker D.J., Bache R.J. Regulation of coronary blood flow during exercise. Physiol Rev. 2008;88:1009–1086. doi: 10.1152/physrev.00045.2006. [DOI] [PubMed] [Google Scholar]
- 92.Jones C.J., Kuo L., Davis M.J., Chilian W.M. Myogenic and flow-dependent control mechanisms in the coronary microcirculation. Basic Res Cardiol. 1993;88:2–10. doi: 10.1007/BF00788525. [DOI] [PubMed] [Google Scholar]
- 93.White F.C., Bloor C.M., McKirnan M.D., Carroll S.M. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol (1985) 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 94.Hanna M.A., Taylor C.R., Chen B., et al. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol (1985) 2014;117:616–623. doi: 10.1152/japplphysiol.01296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szekeres M., Nádasy G.L., Dörnyei G., Szénási A., Koller A. Remodeling of wall mechanics and the myogenic mechanism of rat intramural coronary arterioles in response to a short-term daily exercise program: role of endothelial factors. J Vasc Res. 2018;55:87–97. doi: 10.1159/000486571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isner J.M., Losordo D.W. Therapeutic angiogenesis for heart failure. Nat Med. 1999;5:491–492. doi: 10.1038/8374. [DOI] [PubMed] [Google Scholar]
- 97.Padro T., Manfrini O., Bugiardini R., et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on 'coronary microvascular dysfunction in cardiovascular disease'. Cardiovasc Res. 2020;116:741–755. doi: 10.1093/cvr/cvaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bove A.A., Dewey J.D. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation. 1985;71:620–625. doi: 10.1161/01.cir.71.3.620. [DOI] [PubMed] [Google Scholar]
- 99.Laughlin M.H., Pollock J.S., Amann J.F., Hollis M.L., Woodman C.R., Price E.M. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol (1985) 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- 100.Woodman C.R., Muller J.M., Rush J.W., Laughlin M.H., Price E.M. Flow regulation of ecNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol. 1999;276:H1058–H1063. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- 101.Calvert J.W., Condit M.E., Aragón J.P., et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β(3)-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Powers S.K., Criswell D., Lawler J., et al. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993;265:H2094–H2208. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- 103.Bowles D.K., Hu Q., Laughlin M.H., Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol. 1998;275:H2159–H2169. doi: 10.1152/ajpheart.1998.275.6.H2159. [DOI] [PubMed] [Google Scholar]
- 104.Baratchi S., Knoerzer M., Khoshmanesh K., Mitchell A., McIntyre P. Shear stress regulates TRPV4 channel clustering and translocation from adherens junctions to the basal membrane. Sci Rep. 2017;7 doi: 10.1038/s41598-017-16276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sonkusare S.K., Bonev A.D., Ledoux J., et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olsen R.H., Pedersen L.R., Jürs A., Snoer M., Haugaard S.B., Prescott E. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int J Cardiol. 2015;185:229–235. doi: 10.1016/j.ijcard.2015.03.118. [DOI] [PubMed] [Google Scholar]
- 107.Fisher J.P., Young C.N., Fadel P.J. Autonomic adjustments to exercise in humans. Compr Physiol. 2015;5:475–512. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 108.Masi S., Rizzoni D., Taddei S., et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. 2021;42:2590–2604. doi: 10.1093/eurheartj/ehaa857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hurley D.M., Williams E.R., Cross J.M., et al. Aerobic exercise improves microvascular function in older adults. Med Sci Sports Exerc. 2019;51:773–781. doi: 10.1249/MSS.0000000000001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hotta K., Chen B., Behnke B.J., et al. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol. 2017;595:3703–3719. doi: 10.1113/JP274172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur Heart J. 2013;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eriksson B.E., Tyni-Lennè R., Svedenhag J., et al. Physical training in syndrome X: physical training counteracts deconditioning and pain in syndrome X. J Am Coll Cardiol. 2000;36:1619–1625. doi: 10.1016/s0735-1097(00)00931-1. [DOI] [PubMed] [Google Scholar]
- 113.Thengchaisri N., Shipley R., Ren Y., Parker J., Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol. 2007;27:791–798. doi: 10.1161/01.ATV.0000258416.47953.9a. [DOI] [PubMed] [Google Scholar]
- 114.Huang J., Zhang H., Tan X., Hu M., Shen B. Exercise restores impaired endothelium-derived hyperpolarizing factor-mediated vasodilation in aged rat aortic arteries via the TRPV4-K(Ca)2.3 signaling complex. Clin Interv Aging. 2019;14:1579–1587. doi: 10.2147/CIA.S220283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huggenberger R., Siddiqui S.S., Brander D., et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117:4667–4678. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 117.Petrova T.V., Koh G.Y. Biological functions of lymphatic vessels. Science. 2020;369 doi: 10.1126/science.aax4063. [DOI] [PubMed] [Google Scholar]
- 118.Baumann F., Reike A., Reimer V., et al. Effects of physical exercise on breast cancer-related secondary lymphedema: a systematic review. Breast Cancer Res Treat. 2018;170:1–13. doi: 10.1007/s10549-018-4725-y. [DOI] [PubMed] [Google Scholar]
- 119.Bei Y., Huang Z., Feng X., et al. Lymphangiogenesis contributes to exercise-induced physiological cardiac growth. J Sport Health Sci. 2022;11:466–478. doi: 10.1016/j.jshs.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bradham R.R., Parker E.F., Barrington B.A., Jr., Webb C.M., Stallworth J.M. The cardiac lymphatics. Ann Surg. 1970;171:899–902. doi: 10.1097/00000658-197006010-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Machnik A., Neuhofer W., Jantsch J., et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 122.Brakenhielm E., Alitalo K. Cardiac lymphatics in health and disease. Nat Rev Cardiol. 2019;16:56–68. doi: 10.1038/s41569-018-0087-8. [DOI] [PubMed] [Google Scholar]
- 123.Lim H.Y., Thiam C.H., Yeo K.P., et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 124.Henri O., Pouehe C., Houssari M., et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. 2016;133:1484–1497. doi: 10.1161/CIRCULATIONAHA.115.020143. discussion 1497. [DOI] [PubMed] [Google Scholar]
- 125.Aspelund A., Robciuc M.R., Karaman S., Makinen T., Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res. 2016;118:515–530. doi: 10.1161/CIRCRESAHA.115.306544. [DOI] [PubMed] [Google Scholar]
- 126.Liu X., Cui K., Wu H., et al. Promoting lymphangiogenesis and lymphatic growth and remodeling to treat cardiovascular and metabolic diseases. Arterioscler Thromb Vasc Biol. 2023;43:e1–e10. doi: 10.1161/ATVBAHA.122.318406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Houssari M., Dumesnil A., Tardif V., et al. Lymphatic and immune cell cross-talk regulates cardiac recovery after experimental myocardial infarction. Arterioscler Thromb Vasc Biol. 2020;40:1722–1737. doi: 10.1161/ATVBAHA.120.314370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klotz L., Norman S., Vieira J.M., et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nahrendorf M., Swirski F.K., Aikawa E., et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu X., De la Cruz E., Gu X., et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature. 2020;588:705–711. doi: 10.1038/s41586-020-2998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Christ-Roberts C.Y., Pratipanawatr T., Pratipanawatr W., et al. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects. Metabolism. 2004;53:1233–1242. doi: 10.1016/j.metabol.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 132.Goodyear L.J., Kahn B.B. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 133.Spaulding H.R., Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–227. doi: 10.1146/annurev-physiol-060721-095517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sriwijitkamol A., Coletta D.K., Wajcberg E., et al. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yuan D., Xiao D., Gao Q., Zeng L. PGC-1α activation: a therapeutic target for type 2 diabetes? Eat Weight Disord. 2019;24:385–395. doi: 10.1007/s40519-018-0622-y. [DOI] [PubMed] [Google Scholar]
- 136.Matsui T., Nagoshi T., Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- 137.Lai C.H., Ho T.J., Kuo W.W., et al. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordr) 2014;36:9706. doi: 10.1007/s11357-014-9706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sundaresan N.R., Gupta M., Kim G., Rajamohan S.B., Isbatan A., Gupta M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vettor R., Valerio A., Ragni M., et al. Exercise training boosts eNOS-dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab. 2014;306:E519–E528. doi: 10.1152/ajpendo.00617.2013. [DOI] [PubMed] [Google Scholar]
- 140.Lehman J.J., Barger P.M., Kovacs A., Saffitz J.E., Medeiros D.M., Kelly D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hsu C.P., Zhai P., Yamamoto T., et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arany Z., He H., Lin J., et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 143.Escobales N., Nuñez R.E., Jang S., et al. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J Mol Cell Cardiol. 2014;77:136–146. doi: 10.1016/j.yjmcc.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Judge S., Jang Y.M., Smith A., et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 145.Moreira J.B.N., Wohlwend M., Wisløff U. Exercise and cardiac health: physiological and molecular insights. Nat Metab. 2020;2:829–839. doi: 10.1038/s42255-020-0262-1. [DOI] [PubMed] [Google Scholar]
- 146.Lehman J.J., Kelly D.P. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin Exp Pharmacol Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 147.Tao L., Bei Y., Lin S., et al. Exercise training protects against acute myocardial infarction via improving myocardial energy metabolism and mitochondrial biogenesis. Cell Physiol Biochem. 2015;37:162–175. doi: 10.1159/000430342. [DOI] [PubMed] [Google Scholar]
- 148.Kraljevic J., Marinovic J., Pravdic D., et al. Aerobic interval training attenuates remodelling and mitochondrial dysfunction in the post-infarction failing rat heart. Cardiovasc Res. 2013;99:55–64. doi: 10.1093/cvr/cvt080. [DOI] [PubMed] [Google Scholar]
- 149.Lanza I.R., Short D.K., Short K.R., et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang H., Bei Y., Lu Y., et al. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell Physiol Biochem. 2015;35:2159–2168. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 151.Schernthaner G., Brand K., Bailey C.J. Metformin and the heart: Update on mechanisms of cardiovascular protection with special reference to comorbid type 2 diabetes and heart failure. Metabolism. 2022;130 doi: 10.1016/j.metabol.2022.155160. [DOI] [PubMed] [Google Scholar]
- 152.Coggins M., Rosenzweig A. The fire within: cardiac inflammatory signaling in health and disease. Circ Res. 2012;110:116–125. doi: 10.1161/CIRCRESAHA.111.243196. [DOI] [PubMed] [Google Scholar]
- 153.Jakic B., Carlsson M., Buszko M., et al. The effects of endurance exercise and diet on atherosclerosis in young and aged ApoE–/–and wild-type mice. Gerontology. 2019;65:45–56. doi: 10.1159/000492571. [DOI] [PubMed] [Google Scholar]
- 154.Liao P.H., Hsieh D.J., Kuo C.H., et al. Moderate exercise training attenuates aging-induced cardiac inflammation, hypertrophy and fibrosis injuries of rat hearts. Oncotarget. 2015;6:35383–35394. doi: 10.18632/oncotarget.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Noz M.P., Hartman Y.A., Hopman M.T., et al. Sixteen-week physical activity intervention in subjects with increased cardiometabolic risk shifts innate immune function towards a less proinflammatory state. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morici G., Zangla D., Santoro A., et al. Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1496–R1503. doi: 10.1152/ajpregu.00338.2005. [DOI] [PubMed] [Google Scholar]
- 157.Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. 2022;20:494. doi: 10.1186/s12916-022-02672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinto A.J., Bergouignan A., Dempsey P.C., et al. The physiology of sedentary behavior. Physiol Rev. 2023;103:2561–2622. doi: 10.1152/physrev.00022.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duggal N.A., Niemiro G., Harridge S.D., Simpson R.J., Lord J.M. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 160.Feng L., Li G., An J., et al. Exercise training protects against heart failure via expansion of myeloid-derived suppressor cells through regulating IL-10/STAT3/S100A9 pathway. Circ Heart Fail. 2022;15 doi: 10.1161/CIRCHEARTFAILURE.121.008550. [DOI] [PubMed] [Google Scholar]
- 161.Frodermann V., Rohde D., Courties G., et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med. 2019;25:1761–1771. doi: 10.1038/s41591-019-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nakajima K., Takeoka M., Mori M., et al. Exercise effects on methylation of ASC gene. Int J Sports Med. 2010;31:671–675. doi: 10.1055/s-0029-1246140. [DOI] [PubMed] [Google Scholar]
- 163.Butts B., Butler J., Dunbar S.B., Corwin E., Gary R.A. Effects of exercise on ASC methylation and IL-1 cytokines in heart failure. Med Sci Sports Exerc. 2018;50:1757–1766. doi: 10.1249/MSS.0000000000001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Butts B., Gary R.A., Dunbar S.B., Butler J. Methylation of apoptosis-associated speck-like protein with a caspase recruitment domain and outcomes in heart failure. J Card Fail. 2016;22:340–346. doi: 10.1016/j.cardfail.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou Q., Deng J., Pan X., et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome. 2022;10:82. doi: 10.1186/s40168-022-01271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xia W.J., Xu M.L., Yu X.J., et al. Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat. Gut Microbes. 2021;13:1–24. doi: 10.1080/19490976.2020.1854642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Y., Wang Y., Ni Y., et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31:77–91.e5. doi: 10.1016/j.cmet.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Zhao X., Zhang Z., Hu B., Huang W., Yuan C., Zou L. Response of gut microbiota to metabolite changes induced by endurance exercise. Front Microbiol. 2018;9:765. doi: 10.3389/fmicb.2018.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tabone M., Bressa C., Garcia-Merino J.A., et al. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep. 2021;11:3558. doi: 10.1038/s41598-021-82947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen J., Guo Y., Gui Y., Xu D. Physical exercise, gut, gut microbiota, and atherosclerotic cardiovascular diseases. Lipids Health Dis. 2018;17:17. doi: 10.1186/s12944-017-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mailing L.J., Allen J.M., Buford T.W., Fields C.J., Woods J.A. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47:75–85. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 172.Aya V., Florez A., Perez L., Ramirez J.D. Association between physical activity and changes in intestinal microbiota composition: a systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Barton W., Penney N.C., Cronin O., et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 174.Chen X.F., Chen X., Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci (Lond) 2020;134:657–676. doi: 10.1042/CS20200128. [DOI] [PubMed] [Google Scholar]
- 175.Zhou W., Cheng Y., Zhu P., Nasser M.I., Zhang X., Zhao M. Implication of gut microbiota in cardiovascular diseases. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/5394096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tang T.W.H., Chen H.C., Chen C.Y., et al. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation. 2019;139:647–659. doi: 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 178.Koeth R.A., Wang Z., Levison B.S., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nam H.S. Gut microbiota and ischemic stroke: the role of trimethylamine N-oxide. J Stroke. 2019;21:151–159. doi: 10.5853/jos.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Y., Wang Y., Ke B., Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–125. doi: 10.1016/j.trsl.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 181.Zhang Y., Wang G., Li R., et al. Trimethylamine N-oxide aggravated cognitive impairment from APP/PS1 mice and protective roles of voluntary exercise. Neurochem Int. 2023;162 doi: 10.1016/j.neuint.2022.105459. [DOI] [PubMed] [Google Scholar]
- 182.Li J., Zhao F., Wang Y., et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Di Tommaso N., Gasbarrini A., Ponziani F.R. Intestinal barrier in human health and disease. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph182312836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pires W., Veneroso C.E., Wanner S.P., et al. Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Med. 2017;47:1389–1403. doi: 10.1007/s40279-016-0654-2. [DOI] [PubMed] [Google Scholar]
- 185.Keirns B.H., Koemel N.A., Sciarrillo C.M., Anderson K.L., Emerson S.R. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol. 2020;319:G512–G518. doi: 10.1152/ajpgi.00232.2020. [DOI] [PubMed] [Google Scholar]
- 186.Feng V., Bawa K.K., Marzolini S., et al. Impact of 12-week exercise program on biomarkers of gut barrier integrity in patients with coronary artery disease. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Karhu E., Forsgard R.A., Alanko L., et al. Exercise and gastrointestinal symptoms: running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur J Appl Physiol. 2017;117:2519–2526. doi: 10.1007/s00421-017-3739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou X., Li J., Guo J., et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6:66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cook M.D., Martin S.A., Williams C., et al. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav Immun. 2013;33:46–56. doi: 10.1016/j.bbi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rhys-Jones D., Climie R.E., Gill P.A., et al. Microbial Interventions to Control and Reduce Blood Pressure in Australia (MICRoBIA): rationale and design of a double-blinded randomised cross-over placebo controlled trial. Trials. 2021;22:496. doi: 10.1186/s13063-021-05468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bui A.L., Horwich T.B., Fonarow G.C. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Li H., Hastings M.H., Rhee J., Trager L.E., Roh J.D., Rosenzweig A. Targeting age-related pathways in heart failure. Circ Res. 2020;126:533–551. doi: 10.1161/CIRCRESAHA.119.315889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iemitsu M., Maeda S., Jesmin S., Otsuki T., Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol. 2006;291:H1290–H1298. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 195.Walton R.D., Jones S.A., Rostron K.A., et al. Interactions of short-term and chronic treadmill training with aging of the left ventricle of the heart. J Gerontol A Biol Sci Med Sci. 2016;71:1005–1013. doi: 10.1093/gerona/glv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Allen J.M., Mailing L.J., Niemiro G.M., et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 197.Munukka E., Ahtiainen J.P., Puigbo P., et al. Six-week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over-weight women. Front Microbiol. 2018;9:2323. doi: 10.3389/fmicb.2018.02323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Queipo-Ortuno M.I., Seoane L.M., Murri M., et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lambert J.E., Myslicki J.P., Bomhof M.R., Belke D.D., Shearer J., Reimer R.A. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab. 2015;40:749–752. doi: 10.1139/apnm-2014-0452. [DOI] [PubMed] [Google Scholar]
- 200.Denou E., Marcinko K., Surette M.G., Steinberg G.R., Schertzer J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310:E982–E993. doi: 10.1152/ajpendo.00537.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bartolomaeus H., Balogh A., Yakoub M., et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li L., Hua Y., Ren J. Short-chain fatty acid propionate alleviates Akt2 knockout-induced myocardial contractile dysfunction. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/851717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhou M.M., Li D.W., Xu L., et al. Propionate alleviated post-infarction cardiac dysfunction by macrophage polarization in a rat model. Int Immunopharmacol. 2023;115 doi: 10.1016/j.intimp.2022.109618. [DOI] [PubMed] [Google Scholar]
- 204.Chen Y., Du J., Zhao Y.T., et al. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc Diabetol. 2015;14:99. doi: 10.1186/s12933-015-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dariushnejad H., Pirzeh L., Roshanravan N., Ghorbanzadeh V. Sodium butyrate and voluntary exercise through activating VEGF-A downstream signaling pathway improve heart angiogenesis in type 2 diabetes. Microvasc Res. 2023;147 doi: 10.1016/j.mvr.2023.104475. [DOI] [PubMed] [Google Scholar]
- 206.Russo M., Guida F., Paparo L., et al. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur J Heart Fail. 2019;21:519–528. doi: 10.1002/ejhf.1439. [DOI] [PubMed] [Google Scholar]