Abstract
Background
Human adenoviruses (HAdVs) can cause outbreaks of flu-like illness in university settings. Most infections in healthy young adults are mild; severe illnesses rarely occur. In Fall 2022, an adenovirus outbreak was identified in university students.
Methods
HAdV cases were defined as university students 17–26 years old who presented to the University Health Service or nearby emergency department with flu-like symptoms (eg, fever, cough, headache, myalgia, nausea) and had confirmed adenovirus infections by polymerase chain reaction (PCR). Demographic and clinical characteristics were abstracted from electronic medical records; clinical severity was categorized as mild, moderate, severe, or critical. We performed contact investigations among critical cases. A subset of specimens was sequenced to confirm the HAdV type.
Results
From 28 September 2022 to 30 January 2023, 90 PCR-confirmed cases were identified (51% female; mean age, 19.6 years). Most cases (88.9%) had mild illness. Seven cases required hospitalization, including 2 critical cases that required intensive care. Contact investigation identified 44 close contacts; 6 (14%) were confirmed HAdV cases and 8 (18%) reported symptoms but never sought care. All typed HAdV-positive specimens (n = 36) were type 4.
Conclusions
While most students with confirmed HAdV had mild illness, 7 otherwise healthy students had severe or critical illness. Between the relatively high number of hospitalizations and proportion of close contacts with symptoms who did not seek care, the true number of HAdV cases was likely higher. Our findings illustrate the need to consider a wide range of pathogens, even when other viruses are known to be circulating.
Keywords: adenovirus, outbreak, university, respiratory illness, students
A respiratory illness outbreak investigation at a university identified 90 laboratory-confirmed cases of human adenovirus (HAdV); 7 students were hospitalized. HAdV can cause moderate to critical illness. Many cases likely went undetected. HAdV should be considered in a respiratory outbreak.
Human adenoviruses (HAdVs) are medium-sized, double-stranded DNA viruses with 7 species (A–G) and >100 genome types; types 1–4, 7, and 14 account for most confirmed respiratory cases [1, 2]. HAdVs are primarily spread through respiratory droplets from the nose and throat, by directly inhaling droplets, or from contact with contaminated surfaces [1]. Infection can cause a wide range of signs and symptoms, including fever, cough, sore throat, conjunctivitis, pneumonia, bronchitis, and acute gastroenteritis (referred to in this article as flu-like symptoms) [1]. The incubation period varies from 2 to 14 days [1]. Infections generally result in mild illness, with symptoms lasting several days to weeks [1]. Treatment for HAdV illness remains limited to supportive care. Testing for HAdV is not common, especially among mild illness. Although rare, severe illness from HAdV among healthy individuals has been documented on college campuses [3–8]. While college students tend to be young and healthy, congregate settings and large social networks make them particularly susceptible to respiratory virus outbreaks, including HAdV.
In 2018 and 2019, 5 colleges in the United States found clusters of acute respiratory illnesses in students caused by HAdV-4 and HAdV-7 [7]. These 5 colleges identified a total of 168 cases including 11 hospitalizations and 2 deaths [7]. Another study among university students with influenza-like illness found 15% were positive for HAdV; types 3, 4, and 7 were predominant [3]. HAdV-4 has been implicated in multiple respiratory illness outbreaks in colleges and other congregate settings, such as long-term care facilities and military academies [4–6, 8, 9].
On 10 October 2022, the campus epidemiologist at a large university was notified of a student hospitalized for viral sepsis due to HAdV. The university's public health response team did a comprehensive review of laboratory testing results and identified 11 additional students who recently tested positive for HAdV. On 11 October 2022, the university notified local and state health departments of the cluster of cases. The university team initiated active monitoring to identify additional cases of HAdV among students who sought care at University Health Service (UHS) or nearby emergency departments (EDs) affiliated with the university. On October 25, the Centers for Disease Control and Prevention (CDC) was contacted to request adenovirus typing to determine if this cluster represented an outbreak, and for technical assistance in the response.
METHODS
Case Detection and Definition
Cases were identified through monitoring of university student visits to UHS and EDs starting on 28 September 2022. Confirmed cases were university students aged 17–26 years who presented to UHS or EDs with flu-like symptoms (Table 1) and who tested positive for HAdV by polymerase chain reaction (PCR) testing using a respiratory multipathogen panel; most students presented with respiratory symptoms. Probable cases were students who had close contact with a critically ill case during their period of infectivity and reported flu-like symptoms during contact investigation. Testing was not standardized and the decision to test was left to the clinical judgment of the treating clinician. No changes were made to clinical recommendations for testing during this time, although clinicians were aware of the outbreak. Case-based clinical and demographic data were collected until the outbreak was declared resolved, defined as no new confirmed HAdV student cases for 2 incubation periods (ie, 28 days).
Table 1.
Clinical Presentation of Students With Confirmed Human Adenovirus Infections
| Signs and Symptoms | No. (%) of Cases With Symptom (N = 90) |
|---|---|
| Fever | 72 (80.0) |
| Cough | 63 (70.0) |
| Sore throat | 56 (62.2) |
| Congestion | 45 (50.0) |
| Headache | 37 (41.1) |
| Myalgia/body aches | 25 (27.8) |
| Nausea and/or vomiting | 24 (26.7) |
| Chills | 22 (24.4) |
| Rhinorrhea/runny nose | 15 (16.7) |
| Neck pain/stiffness | 11 (12.2) |
| Ear pain/pressure | 10 (11.1) |
| Shortness of breath | 8 (8.9) |
| Malaise | 7 (7.8) |
| Diarrhea | 7 (7.8) |
| Sweats | 7 (7.8) |
| Conjunctivitis | 5 (5.6) |
| Othera | <5 (5)b |
aOther symptoms include tender/swollen nodes (n = 4 [4.4%]), rash (n = 3 [3.3%]), brain fog/confusion (n = 3 [3.3%]), lower back pain (n = 2 [2.2%]), dizziness (n = 2 [2.2%]), seizures (n = 2 [2.2%]), tonsillitis (n = 2 [2.2%]), fainting/syncope (n = 1 [1.1%]), shaky (n = 1 [1.1%]), fast heart rate (n = 1 [1.1%]), testicular pain (n = 1 [1.1%]), rhabdomyolysis (n = 1 [1.1%]).
bEach symptom in the “Other” category was noted for at least 1 student, with <5 total students experiencing each symptom.
Respiratory Pathogen Panel
The BioFire respiratory pathogen panel (RPP) [10] was ordered for individuals presenting with flu-like symptoms both by UHS (as a “send out” test to the university clinical laboratory) and EDs (available “in-house” at the same clinical laboratory) included in this investigation. UHS clinicians often performed a quad test (see description below) as the first diagnostic test, before ordering an RPP. The RPP assay tested for 19 pathogens: HAdV, seasonal coronaviruses (229E, HKU1, NL63, OC43), human metapneumovirus, human rhinovirus-enterovirus, influenza A and B, parainfluenza virus types 1–4, respiratory syncytial virus (RSV), Chlamydia pneumoniae, Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Co-detections were documented.
Quad Test Proxy Surveillance
For students presenting to UHS with flu-like symptoms or an exposure to coronavirus disease 2019 (COVID-19), point-of-care testing was done using the Xpert Xpress CoV-2/Flu/RSV plus test (“quad test”). The quad test is a multipathogen, rapid PCR test that includes SARS-CoV-2, influenza A, influenza B, and RSV [11]. For those who tested positive for any virus on the quad test, additional testing, including an RPP, was generally not ordered. During this outbreak, quad testing volumes at UHS were used to monitor trends of moderate flu-like illness in the student body (ie, students ill enough to seek healthcare). The percentage of quad tests negative for all 4 viruses suggested the possible presence of another pathogen and was utilized to indirectly monitor the possible burden of HAdV cases during the outbreak.
Demographic and Clinical Data Collection
Case-based clinical details (signs, symptoms, treatment, and laboratory results) and demographic characteristics were abstracted from electronic medical records and student enrollment records.
Clinical severity was categorized as mild, moderate, severe, or critical using the COVID-19 Clinical Severity Index, which ranks severity based on symptoms [12]. In brief, mild illness was defined as any flu-like illness except shortness of breath (dyspnea), or abnormal chest imaging. Moderate illness included individuals showing evidence of lower respiratory disease and an oxygen saturation (SpO2) ≥94%. Severe illness was defined as SpO2 <94%, ratio of arterial oxygen partial pressure to fractional inspired oxygen <300 mm Hg, respiratory rate >30 breaths/minute, or lung infiltrates >50%. Finally, critical illness was defined as respiratory failure, septic shock, and/or multiple organ failure. We modified the critical illness definition for use with HAdV cases to include intensive care unit (ICU) admission and intubation.
Case Investigation
Thorough case investigation, including enhanced contact tracing, was undertaken for the 2 hospitalized students who were admitted to the ICU (cases A and B), because of their temporal proximity and critical clinical presentations. Identified close contacts were contacted to determine exposures and symptom status and to provide guidance and infection prevention methods.
Analysis Methods
Laboratory-confirmed HAdV cases were described using demographic characteristics (age, race, sex, type of student, on-campus vs off-campus housing), RPP co-detections, and clinical outcome. An epidemic curve was created using date of specimen collection; quad test results were aggregated and percentage negative was calculated. Both the epidemic curve and the quad test analyses included all test results in the corresponding timeframe of the outbreak. Secondary attack rates were calculated for each of the enhanced contact investigations as the number of confirmed or probable cases divided by the total number of close contacts. Data and visualizations were compiled and processed using R version 4.2.2 and RStudio version 2022.07.0.
Laboratory Typing
HAdV typing was conducted to determine if 1 type or multiple types were being transmitted among students. Early typing results (ie, identification of the same HAdV type) provided evidence that this was indeed an outbreak and justified dedication of additional campus and personnel resources to prevent or reduce transmission and to describe the outbreak. Residual specimens from a subset (n = 36) of the laboratory-confirmed HAdV cases were shipped frozen on dry ice to CDC (Atlanta, Georgia) for typing. The initial shipment included specimens from all identified cases at the time: 1 hospitalized case and 30 outpatient cases. A second shipment included 4 hospitalized cases and 1 outpatient case who was a close contact of a critical case to understand if these severe/critical cases were the same type as the earlier batch. HAdV molecular typing was performed based on amplification and sequencing of the hexon hypervariable regions 1–6 [13].
Ethical Considerations
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (see, eg, 45 Code of Federal Regulations [C.F.R.] part 46, 21 C.F.R. part 56; 42 United States Code [U.S.C.] §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq).
RESULTS
Demographics and Characteristics of Confirmed Cases
Ninety students had confirmed HAdV between 28 September 2022 and 30 January 2023. The mean age was 19.6 years (range, 17–24 years), 46 (51.1%) were female, and 88 (97.7%) were undergraduates (Table 2). Most cases lived off campus (n = 54 [60.0%]) and were White (n = 61 [67.8%]). Most cases were mild (n = 80 [88.9%]), with 7 (7.8%) moderate, 1 (1.1%) severe, and 2 (2.2%) critical.
Table 2.
Demographic and Clinical Characteristics of Confirmed Human Adenovirus Cases Identified as Part of University Outbreak, September 2022–January 2023
| Characteristic | Count (%) |
|---|---|
| All | 90 (100) |
| Gender | |
| Male | 44 (48.9) |
| Female | 46 (51.1) |
| Age, y, mean (range) | 19.6 (17–24) |
| Race | |
| White | 61 (67.8) |
| Asian | 14 (15.6) |
| Black | 4 (4.4) |
| Not specified | 6 (6.7) |
| Other (includes multiple races) | 5 (5.6) |
| Residence location | |
| On campus (residence hall) | 36 (40.0) |
| Off campus | 54 (60.0) |
| Student level | |
| Undergraduate | 88 (97.7) |
| Graduate/professional | 2 (2.2) |
| Symptom severitya | |
| Mild | 80 (88.9) |
| Moderate | 7 (7.8) |
| Severe | 1 (1.1) |
| Critical | 2 (2.2) |
| Hospitalization status | |
| Outpatient | 83 (92.2) |
| Inpatient, not ICU | 5 (5.6) |
| Inpatient, ICU | 2 (2.2) |
Abbreviation: ICU, intensive care unit.
aSymptom severity was determined based on a modified COVID-19 Clinical Severity Score. Mild illness was defined as signs or symptoms of human adenovirus except shortness of breath, dyspnea, or abnormal chest imaging. Moderate illness was defined as evidence of lower respiratory disease and oxygen saturation (SpO2) ≥94%. Severe illness was defined as SpO2 <94%, ratio of arterial oxygen partial pressure to fractional inspired oxygen <300 mg Hg, respiratory rate >30 breaths/minute, or lung infiltrates >50%. Critical illness was defined as respiratory failure, septic shock, multiple organ dysfunction, and/or ICU admission and intubation.
Fifty-two (57.8%) cases were diagnosed through emergency services and were discharged, 31 (34.4%) were diagnosed through the outpatient UHS, 5 (5.6%) were diagnosed through the ED and were admitted to the university hospital but not to the ICU, and 2 (2.2%) were diagnosed through the ED and were admitted to the ICU. The most common clinical signs and symptoms included fever (n = 71 [79.8%]), cough (n = 62 [69.7%]), and sore throat (n = 56 [62.9%]) (Table 1).
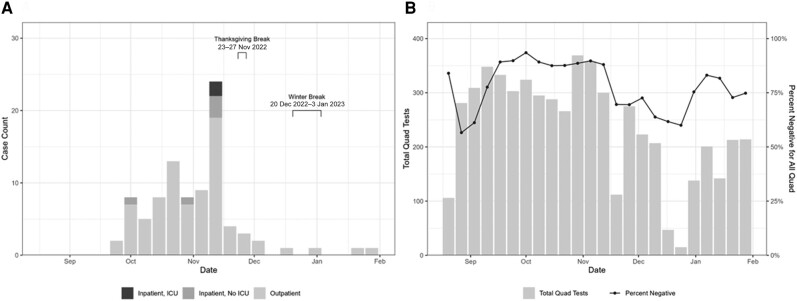
The majority of HAdV cases in this outbreak (n = 86 [95.6%]) occurred between 1 October 2022 and 10 December 2022, with the largest number of cases occurring during the week ending 19 November 2022 (n = 24 [30.0%]; Figure 1A). During the peak week, 5 students were hospitalized, triggering additional campus-wide messaging and facilities cleaning. The subsequent week was a national holiday (Thanksgiving), and the campus was largely vacated for several days. Case counts decreased dramatically following the peak, then decreased further during winter break, when few students were on campus for multiple weeks. The final laboratory-confirmed case was tested on 30 January 2023.
Figure 1.
A, Epidemic curve of laboratory-confirmed human adenovirus cases detected using BioFire Respiratory 2.1 Panel in the university campus outbreak—28 September 2022 to 30 January 2023. B, Number of weekly Xpert Xpress CoV-2/Flu/respiratory syncytial virus (RSV) plus tests (“quad tests”), which tested for severe acute respiratory syndrome coronavirus 2, influenza A, influenza B, and RSV [11], ordered and the percentage of negative results, as a proxy for potential human adenovirus infection, at University Health Service during the human adenovirus outbreak—28 September 2022 to 30 January 2023. Abbreviation: ICU, intensive care unit.
Respiratory Panel and Quad Laboratory Test Results
Among all RPP tests ordered for students presenting at UHS or the ED during the outbreak investigation (n = 194), 46.4% (n = 90) were positive for HAdV. Of the 90 HAdV-positive cases, 22 (24.4%) had additional pathogen(s) detected with RPP (21 positive for 2 total viruses and 1 positive for 3 total viruses) (Table 3). One student tested positive for influenza A, and none of the students in this outbreak tested positive for SARS-CoV-2 or RSV, all of which were circulating in the community during this time. None of the students with viral co-detections were admitted to the hospital.
Table 3.
Respiratory Virus Co-detections Identified Among Laboratory-Confirmed Adenovirus Cases Using the BioFire Respiratory Panel in the University Outbreak
| Virus | No. (%) (N = 90) |
|---|---|
| Total co-detections | 22 (24.4) |
| Enterovirus/rhinovirusa | 14 (15.6) |
| Parainfluenza virus 1 or 4 | 6 (6.6) |
| Influenza A | 2 (2.2) |
| Coronavirus HKU1 | 1 (1.1) |
The BioFire Respiratory 2.1 Panel tested for 19 viruses and bacteria, including human adenovirus, seasonal coronaviruses (229E, HKU1, NL63, OC43), human metapneumovirus, human rhinovirus-enterovirus, influenza A and B, parainfluenza virus types 1–4, respiratory syncytial virus, Chlamydia pneumoniae, Bordetella pertussis, Bordetella parapertussis, Mycoplasma pneumoniae, and severe acute respiratory syndrome coronavirus 2.
aEnterovirus and rhinovirus are not distinguishable on multipathogen panels.
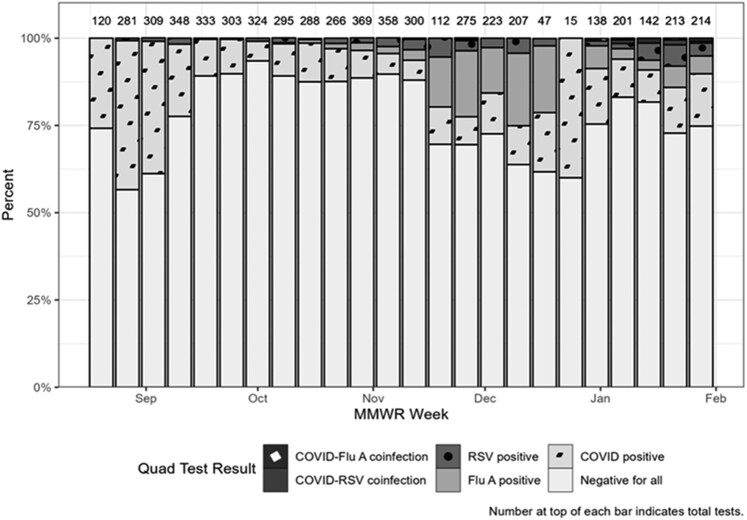
Quad testing volumes ranged from 15 to 369 tests per week and percentage negative ranged from 56.6% to 93.5% per week throughout this outbreak; the lowest number of weekly tests corresponded to Thanksgiving holiday and winter break (Figures 1B and 2). The highest number of weekly tests occurred between 30 October 2022 and 12 November 2022, which roughly corresponded to the peak of the outbreak (Figure 1A and 1B). Additionally, quad test negativity ranged from 88.6% to 89.7% during these weeks. Throughout the outbreak, SARS-CoV-2 and influenza A made up 90.3% of positive quad tests (Figure 2).
Figure 2.
Total Xpert Xpress severe acute respiratory syndrome coronavirus 2/Flu/respiratory syncytial virus (RSV) plus tests (“quad tests”) used and test results reported for all students tested at University Health Services between October 2022 and January 2023 by MMWR week [14]. Abreviation: MMWR, Morbidity and Mortality Weekly Report.
Case Investigation and Enhanced Contact Investigations
During the week ending 12 November 2022, 2 HAdV cases (cases A and B) presented to the ED with similar critical illness and required ICU admission. Cases A and B have already been described [15]. In brief, these individuals were both previously healthy and presented to the ED with neurologic symptoms (seizures and altered mental status) following 1 week of flu-like symptoms. Both were intubated for airway protection. Diagnostic infectious workup was positive only for HAdV for both cases. Both were extubated within 4 days and discharged within a week of hospital admission.
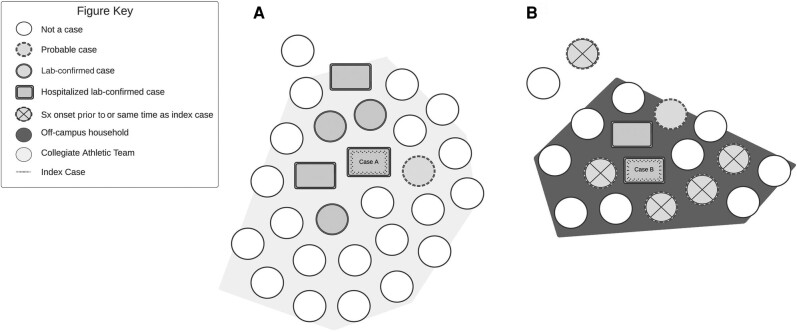
Twenty-seven close contacts were identified for case A, who was a member of a collegiate athletic team. Due to the amount of time case A spent with the team during the infectious period, all teammates were considered close contacts (n = 26). Five of the 26 teammates tested positive for HAdV and were classified as confirmed cases. One teammate was classified as a probable case as they exhibited symptoms but were not tested for HAdV. Of the 5 confirmed cases, 2 were hospitalized given concerns about initial symptoms but were promptly discharged following a negative workup. One nonteammate close contact was identified and remained asymptomatic and therefore was not tested for HAdV (Figure 3A). The secondary attack rate for case A was 22%.
Figure 3.
Results from enhanced contract tracing investigations from 2 hospitalized university student adenovirus cases including sports teammates (A) and household members (B). Abbreviation: Sx, symptom.
Case B had 17 close contacts, including 15 housemates and 2 nonhousemates. Six housemates reported flu-like symptoms but never sought clinical assessment (probable cases); 4 were symptomatic before case B and 2 developed symptoms after case B. Only 1 close contact tested positive for HAdV (confirmed case); this individual was hospitalized for HAdV-associated rhabdomyolysis and discharged in stable condition. Of the 2 nonhousehold close contacts, 1 developed symptoms (probable case) and 1 remained asymptomatic (Figure 3B). The secondary attack rate for case B was 47.1%.
Laboratory Typing
All 36 samples submitted for sequencing were identified as HAdV-4 (Supplementary Figure 1) and exhibited 100.0% sequence homology in hypervariable regions 1–6.
DISCUSSION
We identified 90 cases of flu-like illness caused by HAdV through multipathogen testing; however, we suspect many cases remained undetected in this outbreak. HAdV surveillance is limited as it is not a notifiable disease. Because infections present similarly to other flu-like illnesses (eg, influenza and SARS-CoV-2) and there is no specific treatment, clinicians do not routinely order HAdV testing. It was fortunate this outbreak was identified via the university's use of multipathogen testing; however, this testing may not be readily available in other settings as multipathogen testing is expensive and does not change clinical management in most cases. Additionally, individuals may not seek care for mild illnesses. This was highlighted in the contact investigation where most students, despite exposure to a confirmed case and symptoms, did not seek care. Thus, the HAdV cases we confirmed in this outbreak likely represent only a fraction of the cases circulating during the investigation.
To estimate the possible extent of disease burden on campus, we reviewed UHS quad test results. While the quad test was useful for diagnosing 4 common respiratory viruses, a negative result in a symptomatic student may have indicated the presence of an alternate pathogen, which during this outbreak may have been HAdV. Percentage negative was useful as a proxy surveillance tool to complement existing clinical testing and to understand the potential outbreak magnitude. During this outbreak, quad test percentage negative tracked HAdV trends reasonably well, during the peak of the outbreak (ie, negative quad tests increased), and signaled a change in circulating virus as the outbreak was coming to an end (ie, negative quad tests decreased). Future research could consider creative use of other human test results (eg, negative SARS-CoV-2 or quad tests) and environmental monitoring (eg, surface or wastewater testing) to augment surveillance activities and determine absence or presence of viruses of interest circulating in a certain population [16].
Although we did not type every case in this outbreak, among the 36 typed cases, the hypervariable region was identical and no other HAdV types were detected, providing strong evidence that this outbreak was caused by only HAdV-4. While HAdV-4 infections usually cause mild disease, this outbreak was unusual because of the number of moderate to critical cases detected within a short timeframe. The hospitalized cases did not possess known comorbidities that increased their risk for moderate to critical disease. All typed cases, regardless of severity, were infected with HAdV-4. Although seizures have been described among pediatric cases infected with HAdV-E4, this clinical presentation is rare. Furthermore, there have not been strong associations between a specific virus type and seizures, and it is unclear what predisposes individuals infected with HAdV to seizures [17–20].
This investigation underscored the importance of outbreak preparedness and response to a viral pathogen in college settings. By detecting an increase in HAdV cases, the public health response team responded with a campus-wide notification advising students to seek clinical assessment at UHS if symptomatic. Information specific to HAdV was highlighted, but campus-wide messages also provided broader information on infection prevention measures (eg, masking and handwashing) applicable to other circulating viruses. Environmental Protection Agency List G cleaning agents, effective in neutralizing HAdV on surfaces, were used by janitorial staff in high-traffic areas such as athletics facilities and residence hall bathrooms [21], and bleach (an effective and inexpensive disinfectant) was recommended for off-campus students to disinfect their own spaces. There was concern that mitigation messages to students would be ignored due to the mental toll of following mitigations during the pandemic, or “COVID fatigue” [22]. Therefore, messaging was distributed to students sparingly.
There is not a state or national definition for what constitutes an adenovirus outbreak nor how to determine start or end of an outbreak. Therefore, our definition for the end of the outbreak was 28 days, or 2 incubation periods for respiratory infections from adenovirus, following the last confirmed HAdV case. Using this definition, the outbreak was determined to have ended on 27 February 2023, with the last case occurring on 30 January 2023 (Figure 1A). Multiple factors may have contributed to the end of the outbreak including public health messaging and environmental cleaning, but the breaks, when students vacated campus for periods of time, likely had a significant impact on interrupting transmission of disease.
This investigation had several limitations. First, initial cases were identified through regular monitoring of student health records. Detection relied heavily on students’ decision to seek medical care and healthcare providers’ discretion to order multipathogen testing. Even after active case finding for laboratory-confirmed HAdV was implemented, most cases were likely not tested and likely went undetected. Second, because this investigation was descriptive in its analysis (instead of a case-control design), we were not able to identify risk factors for severe/critical disease (ie, hospitalizations) or areas of high transmission. Third, the investigation was focused on students. It is unknown how staff, faculty, and community members were affected by this outbreak, although a previous study found that during a large outbreak of SARS-CoV-2, college students did not significantly contribute to the community disease burden [23]. Fourth, the quad test was used as a screening tool for students presenting to UHS with flu-like symptoms. For students who tested positive for SARS-CoV-2, influenza A/B, or RSV, an RPP was not ordered, thus limiting information regarding HAdV co-detections with these viruses, 3 of which were known to have been circulating in the community at the time of the outbreak. Finally, whole genome sequencing was not conducted, so we cannot definitively say that all HAdV-4 identified were clonal as our typing methods only include partial sequencing of the hypervariable hexon region.
Our study describes HAdV-4 as a cause of flu-like illness in otherwise healthy, young adults. Due to limitations of HAdV surveillance—testing not routinely conducted and the infection not considered a notifiable condition—there were likely many cases that were undetected in our investigation. HAdV should be considered in the differential among flu-like illness outbreaks in congregate settings, even when other viruses are known to be circulating. Efforts to disrupt transmission of adenovirus are important because although most illness are relatively mild, large outbreaks may result in severe/critical illnesses and/or outcomes.
Supplementary Material
Contributor Information
JoLynn P Montgomery, University Health Service, University of Michigan, Ann Arbor, Michigan, USA.
Juan Luis Marquez, Washtenaw County Health Department, Ypsilanti, Michigan, USA.
Jennifer Nord, Environment Health and Safety, University of Michigan, Ann Arbor, Michigan, USA.
Aleksandra R Stamper, University Health Service, University of Michigan, Ann Arbor, Michigan, USA.
Elizabeth A Edwards, University Health Service, University of Michigan, Ann Arbor, Michigan, USA.
Nicholas Valentini, Department of Emergency Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Christopher J Frank, University Health Service, University of Michigan, Ann Arbor, Michigan, USA.
Laraine L Washer, Department of Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA.
Robert D Ernst, University Health Service, University of Michigan, Ann Arbor, Michigan, USA.
Ji In Park, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Deanna Price, Washtenaw County Health Department, Ypsilanti, Michigan, USA.
Jim Collins, Michigan Department of Health and Human Services, Communicable Disease Division, Lansing, Michigan, USA.
Sarah E Smith-Jeffcoat, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Fang Hu, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Christine L Knox, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Rebia Khan, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Xiaoyan Lu, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Hannah L Kirking, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Christopher H Hsu, Centers for Disease Control and Prevention, Coronavirus and Other Respiratory Viruses Division, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. P. M., J. L. M., J. N., A. R. S., E. A. E., L. L. W., R. D. E., J. C., D. P., S. E. S.-J., H. L. K., and C. H. H. provided investigation technical support, analysis, and manuscript writing and editing. N. V., C. J. F., and R. K. supported manuscript writing and editing. J. I. P., F. H., C. L. K., and X. L. provided specimen processing, typing, and phylogenetic analysis.
Patient consent. This is a report of an outbreak investigation led by the local health department, for which consent is generally not obtained. This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy. Patient consent was obtained from patients classified as critical, for whom in-depth investigations were conducted.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Financial support. No specific funding was received for this project. All work was supported by each author's affiliated institution as part of the institution’s public health response infrastructure.
References
- 1. Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev 2014; 27:441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binder AM, Biggs HM, Haynes AK, et al. Human adenovirus surveillance—United States, 2003–2016. MMWR Morb Mortal Wkly Rep 2017; 66:1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biggs HM, Lu X, Dettinger L, Sakthivel S, Watson JT, Boktor SW. Adenovirus-associated influenza-like illness among college students, Pennsylvania, USA. Emerg Infect Dis 2018; 24:2117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chu VT, Simon E, Lu X, et al. Outbreak of acute respiratory illness associated with human adenovirus type 4 at the United States Coast Guard Academy, 2019. J Infect Dis 2022; 225:55–64. [DOI] [PubMed] [Google Scholar]
- 5. Hang J, Vento TJ, Norby EA, et al. Adenovirus type 4 respiratory infections with a concurrent outbreak of coxsackievirus A21 among United States Army Basic Trainees, a retrospective viral etiology study using next-generation sequencing. J Med Virol 2017; 89:1387–94. [DOI] [PubMed] [Google Scholar]
- 6. Kolavic-Gray SA, Binn LN, Sanchez JL, et al. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin Infect Dis 2002; 35:808–18. [DOI] [PubMed] [Google Scholar]
- 7. Kujawski SA, Lu X, Schneider E, et al. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin Infect Dis 2021; 72:1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McNeill KM, Benton FR, Monteith SC, Tuchscherer MA, Gaydos JC. Epidemic spread of adenovirus type 4–associated acute respiratory disease between U.S. Army installations. Emerg Infect Dis J 2000; 6:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kajon AE, Lamson DM, Bair CR, et al. Adenovirus type 4 respiratory infections among civilian adults, northeastern United States, 2011–2015. Emerg Infect Dis 2018; 24:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biomérieux . The BioFire Respiratory 2.1 (RP2.1) Panel. Available at: https://www.biofiredx.com/products/the-filmarray-panels/filmarrayrp/. Last accessed 11 April 2024.
- 11. Cepheid . Xpert Xpress CoV-2/Flu/RSV plus. Available at: https://www.cepheid.com/en_US/tests/Critical-Infectious-Diseases/Xpert-Xpress-CoV-2-Flu-RSV-plus. Last accessed 11 April 2024.
- 12. National Institutes of Health, COVID-19 Treatment Guidelines Panel . Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. Last accessed 11 April 2024. [PubMed]
- 13. Lu X, Erdman DD. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch Virol 2006; 151:1587–602. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. MMWR weeks. Available at: https://ndc.services.cdc.gov/wp-content/uploads/MMWR_Week_overview.pdf. Last accessed 11 April 2024.
- 15. Valentini N, Breeden M, Felley LE, et al. A cluster of neuroinvasive adenovirus infections on a college campus: case series. Clin Pract Cases Emerg Med 2023; 7:64–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levy JI, Andersen KG, Knight R, Karthikeyan S. Wastewater surveillance for public health. Science 2023; 379:26–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz KL, Richardson SE, MacGregor D, Mahant S, Raghuram K, Bitnun A. Adenovirus-associated central nervous system disease in children. J Pediatr 2019; 205:130–7. [DOI] [PubMed] [Google Scholar]
- 18. Probst V, Datyner EK, Haddadin Z, et al. Human adenovirus species in children with acute respiratory illnesses. J Clin Virol 2021; 134:104716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang YC, Huang SL, Chen SP, et al. Adenovirus infection associated with central nervous system dysfunction in children. J Clin Virol 2013; 57:300–4. [DOI] [PubMed] [Google Scholar]
- 20. Zhang XF, Tan CB, Yao ZX, Jiang L, Hong SQ. Adenovirus infection-associated central nervous system disease in children. Pediatr Infect Dis J 2021; 40:205–8. [DOI] [PubMed] [Google Scholar]
- 21. Environmental Protection Agency . List G: antimicrobial products registered with EPA for claims against norovirus (feline calicivirus). Available at: https://www.epa.gov/pesticide-registration/list-g-antimicrobial-products-registered-epa-claims-against-norovirus-feline. Last accessed 11 April 2024.
- 22. Ball H, Wozniak TR. Why do some Americans resist COVID-19 prevention behavior? An analysis of issue importance, message fatigue, and reactance regarding COVID-19 messaging. Health Commun 2022; 37:1812–9. [DOI] [PubMed] [Google Scholar]
- 23. Valesano AL, Fitzsimmons WJ, Blair CN, et al. SARS-CoV-2 genomic surveillance reveals little spread from a large university campus to the surrounding community. Open Forum Infect Dis 2021; 8:ofab518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.