Abstract
Background and Objectives:
Infectious agents are considered one of the possible etiological factors of systemic lupus erythematosus (SLE). It has been suggested that human herpesvirus type 6 (HHV-6) may trigger autoimmune disorders, but few studies have been conducted on the relationship between this virus and autoimmune diseases, especially SLE. The present study aimed to compare the frequency of HHV-6 infection between SLE patients and healthy individuals.
Materials and Methods:
Serum samples were collected from 60 healthy people and 60 SLE patients referred to the rheumatology clinic of Shahid-Beheshti Hospital, Kashan, Iran, from January 2020 to January 2021. The following data were collected from the medical records of patients: sex; age; duration of disease; SLE clinical manifestations; and disease activity. After the extraction of viral DNA from samples, a nested polymerase chain reaction (PCR) test was performed to detect HHV-6.
Results:
HHV-6 was detected in 12 SLE patients (20%) and in 8 healthy individuals (13.3%). A significant correlation was not obtained between SLE and the presence of HHV-6 (P = 0.09). There was no correlation between musculoskeletal involvements, skin lesions, renal manifestations, and hematological manifestations with the presence of HHV-6 (P>0.05). HHV-6 was detected more frequently in patients with active lupus than in patients with quiescent disease, but this difference was not significant (P=0.08).
Conclusion:
Although patients with SLE had a higher prevalence of HHV-6 compared with healthy people, there is no strong link between HHV-6 infection and SLE. Future research is necessary because this data does not support the hypothesis that human herpesvirus 6 plays a role in the pathogenesis of SLE.
Keywords: Autoimmune diseases, Systemic lupus erythematosus, Human herpesvirus 6
INTRODUCTION
Systemic lupus erythematosus (SLE), which is a complex autoimmune disease, is characterized by the production of pathogenic autoantibodies and a wide range of possible organ system injuries (1). Periodic flare-ups and the development of autoantibodies against nuclear antigens such as ribonucleoproteins (RNPs), ribosomal RNA, (Ro), and double-stranded DNA (dsDNA) are features of SLE (2). The global SLE prevalence in adults is estimated at around 61 per 100,000 people. In Iran, the prevalence of SLE is estimated to be 40 per 100,000 people (3). The exact etiology and pathogenesis of this disease is unknown. It seems that the etiology of lupus is multifactorial. Researchers believe that infectious agents including bacteria, fungi and viruses may play a key role in the etiology of SLE. According to previous research, some viruses have been reported to be associated with systemic lupus erythematosus such as Epstein–Barr virus (EBV), human T-lymphotropic virus type 1 (HTLV-1), parvovirus B19 and cytomegalovirus (CMV) (4). Similarities between foreign and self-peptides lead to a loss of tolerance to autoantigens and is a potential mechanism for the development of SLE (5).
Human herpesvirus 6 (HHV-6), which has a double stranded DNA, infects most adults and includes two distinct species HHV-6A and HHV-6B. Particularly in immunocompromised patients, such as those who have undergone hematopoietic stem cell transplantation (HSCT), solid organ transplant recipients, and AIDS patients, HHV-6A can be more deadly. HHV-6B can cause exanthema subitum and is a common virus that infects more than 90% of people globally. After initial infection, the virus can remain latent in the body lifelong, and it can be reactivated frequently (6).
There are several ways that viruses can trigger an autoimmune reaction. According to a theory known as “molecular mimicry”, viruses that contain structurally similar antigens to self-antigens stimulate B and T cells and cause a cross-reactive response against both self- and non-self-antigens. Molecular mimicry has been described for stromal keratitis caused by HSV, diabetes and myocarditis caused by Coxsackie virus and demyelinating disease caused by Theiler's murine encephalomyelitis virus. Another proposed mechanism for triggering autoimmunity by viruses is “bystander activation,” in which a non-specific and over-reactive antiviral immune response creates a localized pro-inflammatory environment and releases self-antigens from injured tissue. These self-antigens are then taken up and presented by antigen presentation cells (APC) to stimulate previously non-responsive, yet autoreactive T cells in the area, resulting in autoimmunity. Both molecular mimicry and bystander activation have been demonstrated in the experimental model of multiple sclerosis (MS) caused by EBV and myasthenia gravis (MG) caused by West Nile virus. In addition, some viruses such as EBV can immortalize autoreactive effector cells. While numerous hypotheses have been offered to elucidate the mechanisms underlying virus-induced autoimmunity, the specific role and mechanism of HHV-6 in autoimmunity is still unknown and this is a research gap (6).
According to findings of Francesco Broccolo et al. HHV-6 may be a pathogenic factor that predisposes people to developing autoimmune connective tissue illnesses (7). Several researchers have found HHV-6 to be associated with autoimmune diseases such as Hashimoto's Thyroiditis (HT), autoimmune hemolytic anemia/neutropenia, autoimmune acute hepatitis, and multiple sclerosis (MS) (8). Some studies have reported the prevalence of HHV-6 in patients with SLE more than in healthy people (9). However, few studies have been done in this field. It is not yet clear whether HHV6 is associated with SLE. More studies are needed to determine the relationship between this virus and autoimmune diseases such as SLE. Therefore, the present study aimed to estimate active HHV-6 infection among SLE patients to find the relationship between the virus and the disease. The result of the current study can make new ideas for the etiological research.
MATERIALS AND METHODS
Sample collection. To conduct this case-control study, serum samples were collected from 60 healthy people and 60 patients with SLE referred to the rheumatology clinic of Shahid-Beheshti Hospital, Kashan, Iran, from January 2020 to January 2022. Cases and controls were matched on age and sex.
Patients with at least four of the 11 American College of Rheumatology (ACR) criteria for the classification of SLE including positive ANA, 7 clinical (constitutional, hematologic, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, renal) and 3 immunological signs (antiphospholipid antibodies, complement proteins, SLE-specific antibodies) were selected by two rheumatologists. The patients who had other autoimmune diseases, malignancy, infectious diseases or hepatitis, and liver transplant recipients were excluded from the study. Each of the patients and participants in the study signed an informed consent form. This study was approved by the Ethics Committee of Kashan University of Medical Sciences (IR.KAUMS.MEDNT.REC.1400.015). The following data were collected from the medical records of patients: sex; age; duration of disease; SLE clinical manifestations; and disease activity. The activity of lupus disease was evaluated using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). SLEDAI score ≤ 6 was considered inactive lupus (10).
Extraction of HHV-6 DNA and detection by nested-PCR test. HHV-6 DNA was isolated from human serum samples by AmpliSens® RIBO-prep, Nucleic acid extraction kit (AmpliSens, Russia) according to the manufacturer's protocol. HHV-6 major capsid protein (MCP) gene was detected by a nested PCR test. The forward primer 5′-GCGTTTTCAGT-GTGTAGTTCGGCAG-3′ (11) and the reverse primer 5′-TGGCCGCATTCGTACAGATACGGAGG-3′ (11) were used in the first round of PCR which produced amplicon size equal 520 bp. The first set of primers amplifies the target DNA in the first round. The second round enhances the specificity and yield of the desired amplicons. The forward primer 5′-GCTAGAACGTATTTGC TGC AGAACG-3’ (11) and the reverse primer 5′-ATCCGAAACAACT-GTCTGACTGGCA-3′ (11) with an amplicon size of 258 bp were used for the second run of PCR. The reaction mix included 10 μL of template DNA or control, 0.5 μL of each primer (10 pM stock), 12.5 μL of the master mix (Sinaclon, Iran) and 1.5 μL of nuclease-free water. The second round of PCR was performed using 10 μL of the first round of PCR product and the reaction mix similar to the previous step. The amplification conditions were as follows: 95°C for 5 min, 35 cycles including, 95°C for 30s, 55°C for 30s, 72°C for 30s and one final extension step at 72°C for 5 min. The positive and negative controls in every run of PCR were used. PCR products were run on a 2% agarose gel (next to the 100–2000 bp DNA Ladder) and visualized using a UV transilluminator after staining with SYBR Safe DNA gel stain (Sinaclon, Iran).
Statistical analysis. Student’s t-test and chi-square statistical tests were performed in SPSS software version 22. A p value less than 0.05 was considered significant.
RESULTS
The SLE patients and control group had the mean age of 41.3 ± 13.6 and 39.5 ± 14.2, respectively (P=0.80). Each group under study included 50 women (84.5%) and 10 men (15.5%). HHV-6 was detected in 20 samples by the nested-PCR method and observation of a specific band on the electrophoresis gel (Fig. 1). HHV-6 was detected in 16 women and 4 men. No statistical relationship was found between gender and HHV-6 infection (P=0.12).
Fig. 1.
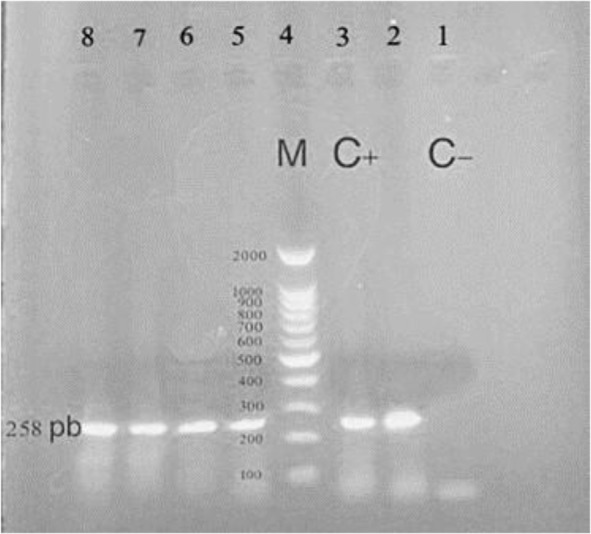
Gel electrophoresis showing the products of HHV-6 second round of nested-PCR amplification. In positive specimens a fragment of 258 base pairs (bp) was amplified. Lane 1: negative control, Lane 3: positive control, Lane 4: ladder 100–2000 bp, Lane 2, 5,6,7,8: positive samples
Twelve patients with SLE were HHV-6-positive (20%) and 48 were HHV-6-negative (80%, Table 1). In the control group, 8 individuals (13.3%) were positive for HHV-6, while 52 individuals (86.7%) were HHV-6-negative. Although the frequency of HHV-6 infection among lupus patients was higher than among healthy individuals, this difference was not statistically significant (P = 0.09).
Table 1.
Frequency of HHV-6 among patients with SLE according to the clinical signs, lupus activity and disease duration.
| Characteristic | HHV-6 positive | HHV-6 negative | P value | |
|---|---|---|---|---|
| Group | SLE | 12 (20%) | 48 (80%) | 0.09 |
| Control | 8 (13.3%) | 52 (86.7%) | ||
| Clinical sign | Musculoskeletal involvement | 5 | 43 | 0.09 |
| Skin lesion | 3 | 27 | 0.14 | |
| Renal manifestation | 2 | 18 | 0.17 | |
| Hematological manifestation | 4 | 20 | 0.19 | |
| Lupus activity | 7 | 5 | 0.08 | |
| Disease duration (mean year ± SD) | 6/3 ± 3/5 | 6/1 ± 3/4 | 0.12 |
The frequency of HHV-6 among SLE patients according to clinical signs showed that there is no correlation between musculoskeletal involvements, skin lesions, renal manifestations, or hematological manifestations with the presence of HHV-6 (Table 1, P>0.05). HHV-6 was detected more frequently in patients with active SLE than in patients with quiescent disease, but this difference was not significant (P=0.08). The mean of disease duration in the HHV-6 positive and negative patients was 6/3 ± 3/5 and 6/1 ± 3/4 years, respectively.
DISCUSSION
Autoimmune connective tissue diseases (ACTDs) are a wide spectrum of diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), dermatomyositis (DM), discoid lupus erythematosus (DLE), systemic sclerosis (SSc), and other disorders with chronic inflammation, which can impact numerous organs and systems (12). Although the cause of ACTD is unknown, clinical, epidemiological, and laboratory evidence suggest that various viral infections may be implicated (13–16). The results of studies in this field are contradictory, and no conclusive evidence has been discovered to support this theory. In the present study, the frequency of HHV-6 infection was compared between patients with SLE and healthy individuals. HHV-6 was detected in 12 of 60 patients (20%) and 8 of 60 controls (13.3%). The difference in the frequency of the HHV-6 infection between the two groups was not statistically significant (P=0.09).
In recent years, HHV-6 has been recognized as one of the infectious agents associated with autoimmune diseases. This virus frequently is detected in patients with Multiple Sclerosis, Hashimoto’s Thyroiditis and ACTD (6, 8). The few studies conducted have not been able to show a direct link between HHV-6 and lupus diseases. Hoffmann and colleagues detected active HHV-6 infection in a 37-year-old woman with SLE for the first time (17). Krueger et al. isolated HHV-6 from patients with collagen vascular diseases. They detected HHV-6 in 55% of the SLE patients and 6.5% of the RA patients by serology methods (18). These two studies only showed that HHV-6 can be present in SLE patients, but could not prove the role of HHV-6 in triggering of disease.
Reis et al. determined the frequency of active herpes virus infections in SLE patients. HHV-DNA was found in 15 of 71 SLE patients (21.1%), while HHV-6 was not found in any of the patients (19). Therefore, the role of the HHV-6 in the etiology of the disease was not proven. Although the virus was found in lupus patients in our investigation, no link was found between the incidence of viral infection and the disease.
HHV-6 can become dormant in bone marrow, lymphocytes, monocytes, and the central nervous system with the potential to become active again later. Based on previous results, HHV-6 can be reactive in patients with active ACTD, especially SLE. In the study of Broccolo et al. the frequency of HHV-6 DNA in patients with SSc, DLE, SLE and DM were 70%, 40%, 45%, and 25%, respectively. They reported that HHV-6 viremia in the serum of patients with lupus is higher than that of healthy individuals. HHV-6 serum viremia was seen in 22 of 38 individuals with active ACTD and only 4 of 20 patients with inactive ACTD (9). In another study, active HHV-6 infection was found in a significant percentage of patients with autoimmune connective tissue diseases (27.1%), especially in systemic lupus erythematosus (36%) (7). These findings support the concept that HHV-6 may act as a pathogen to the development of autoimmune connective tissue disorders. The findings of our study were not consistent with the results of the mentioned studies because the prevalence of the virus among SLE patients was not statistically higher than that of healthy individuals. These inconsistent findings could be due to differences in sample size, geographic area, and virus detection techniques in different studies. In addition, it is possible that the selected lupus patients were in different stages of the disease, and therefore the virus had better conditions for reactivation in some patients. However, HHV-6 reactivation is probably a secondary phenomenon in SLE patients mostlikely through triggering some cytokines. Indeed, several studies have shown that cytokine production, specifically tumor necrosis factor (TNF) and IL-6 are involved in CMV reactivation, which has similar biological characteristics to HHV-6 (20). Thus, proinflammatory cytokines that are markedly enhanced in active SLE patients may play a key role in HHV-6 reactivation (9).
Molecular mimicry, endothelial cell damage, super-antigen stimulation, and microchimerism are four pathogenic hypotheses that can interpret the possible role of the virus in causing ACTD (8). Several studies have proposed linking viral agents to ACTD, but evidence for a direct association between HHV-6 and SLE remains inadequate (5, 21). The results of the present study also did not show a relationship between HHV-6 and SLE.
Viruses can cause auto reactivity through molecular mimicry, but in the majority of viral infections, it seems to happen seldom (22). It is described that HHV-6 may trigger Multiple Sclerosis by molecular mimicry mechanism. The researchers discovered that residues 96–102 of myelin basic protein, a potential autoantigen in multiple sclerosis, and residues 4–10 of HHV-6 pU24 are similar (23). It is possible that this virus can trigger SLE through a similar mechanism, which should be discovered in the future.
Viruses more frequently cause autoimmunity by cell death, mostly by enhanced apoptosis, which releases self-antigens (8). It has been proposed that one of the main pathogenetic mechanisms for ACTD is enhanced apoptosis (24). It is believed that HHV-6 causes Multiple Sclerosis by destroying neurons. Therefore, it can be suggested that this virus may play a role in triggering SLE with the same mechanism mentioned above.
It has been reported in previous studies that HHV-6 can upregulate human leukocyte antigen (HLA) class II expression on thyrocytes and induce an immune response to virus antigens in patients with Hashimoto's thyroiditis. It is reported a significant increase in CD4+ T cells recognizing HHV-6 antigens in Hashimoto's thyroiditis patients, especially in the subgroup of polyfunctional T cells that secrete both IFN-γ and IL-2 (11). IFN may function as a general trigger for the onset of autoimmunity. HHV-6 may cause lupus disease by using the mentioned mechanism
Our study had limitations. Although the sample size was sufficient, it would have been better if the study had a larger sample size. Also, we did not have the viral load and serological status of the patients.
CONCLUSION
The findings of the current study demonstrated that although patients with SLE had a higher prevalence of human herpesvirus 6 compared with healthy people, there is no strong link between HHV-6 infection and SLE. Future research is necessary because this data does not support the hypothesis that human herpesvirus 6 plays a role in the pathogenesis of SLE.
ACKNOWLEDGEMENTS
This study was funded by Kashan University of Medical Sciences with grant number 99223.
REFERENCES
- 1.Caielli S, Wan Z, Pascual V. Systemic lupus erythematosus pathogenesis: interferon and beyond. Annu Rev Immunol 2023; 41: 533–560. [DOI] [PubMed] [Google Scholar]
- 2.Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, et al. An overview of systemic lupus erythematosus (SLE) pathogenesis, classification, and management. Cureus 2022; 14(10): e30330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Shujairi A, Elbadawi F, Al-Saleh J, Hamouda M, Vasylyev A, Khamashta M. Literature review of lupus nephritis from the Arabian Gulf region. Lupus 2023; 32: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundaresan B, Shirafkan F, Ripperger K, Rattay K. The role of viral Infections in the onset of autoimmune diseases. Viruses 2023; 15: 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus 2014; 23: 596–605. [DOI] [PubMed] [Google Scholar]
- 6.Darvish Molla Z, Kalbasi S, Kalantari S, Bidari Zerehpoosh F, Shayestehpour M, Yazdani S. Evaluation of the association between human herpes virus 6 (HHV-6) and Hashimoto's thyroiditis. Iran J Microbiol 2022; 14: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broccolo F, Drago F, Cassina G, Fava A, Fusetti L, Matteoli B, et al. Selective reactivation of human herpesvirus 6 in patients with autoimmune connective tissue diseases. J Med Virol 2013; 85: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 8.Broccolo F, Fusetti L, Ceccherini-Nelli L. Possible role of human herpesvirus 6 as a trigger of autoimmune disease. ScientificWorldJournal 2013; 2013: 867389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broccolo F, Drago F, Paolino S, Cassina G, Gatto F, Fusetti L, et al. Reactivation of human herpesvirus 6 (HHV-6) infection in patients with connective tissue diseases. J Clin Virol 2009; 46: 43–46. [DOI] [PubMed] [Google Scholar]
- 10.Petri M. Review of classification criteria for systemic lupus erythematosus. Rheum Dis Clin North Am 2005; 31: 245–254. [DOI] [PubMed] [Google Scholar]
- 11.Darvish Molla Z, Kalbasi S, Kalantari S, Bidari Zerehpoosh F, Shayestehpour M, Yazdani S. Evaluation of the association between human herpes virus 6 (HHV-6) and Hashimoto's thyroiditis. Iran J Microbiol 2022; 14: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose J. Autoimmune connective tissue diseases: systemic lupus erythematosus and rheumatoid arthritis. Emerg Med Clin North Am 2022; 40: 179–191. [DOI] [PubMed] [Google Scholar]
- 13.Zamani B, Moeini Taba S-M, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep 2021; 15: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shayestehpour M, Zamani B. Systemic lupus erythematosus and varicella-like rash following COVID-19 in a previously healthy patient. J Med Virol 2021; 93: 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shayestehpour M, Zamani B. The first case of systemic lupus erythematosus (SLE) triggered by COVID-19 infection. Eur Rev Med Pharmacol Sci 2020; 24: 11474. [DOI] [PubMed] [Google Scholar]
- 16.Houen G, Trier NH. Epstein-Barr virus and systemic autoimmune diseases. Front Immunol 2021; 11: 587380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quaglia M, Merlotti G, De Andrea M, Borgogna C, Cantaluppi V. Viral Infections and systemic Lupus Erythematosus: new players in an old story. Viruses 2021; 13: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Li H, Wu C, Zhang Y. Epstein–Barr virus and human herpesvirus 6 infection in patients with systemic lupus erythematosus. Virol J 2023; 20: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis AD, Mudinutti C, de Freitas Peigo M, Leon LL, Costallat LTL, Rossi CL, et al. Active human herpesvirus infections in adults with systemic lupus erythematosus and correlation with the SLEDAI score. Adv Rheumatol 2020; 60: 42. [DOI] [PubMed] [Google Scholar]
- 20.Chun H-Y, Chung J-W, Kim H-A, Yun J-M, Jeon J-Y, Ye Y-M, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007; 27: 461–456. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, You X, Zeng X. Research progress of BK virus and systemic lupus erythematosus. Lupus 2022; 31: 522–531. [DOI] [PubMed] [Google Scholar]
- 22.Illescas-Montes R, Corona-Castro CC, Melguizo-Rodríguez L, Ruiz C, Costela-Ruiz VJ. Infectious processes and systemic lupus erythematosus. Immunology 2019; 158: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Bolle L, Naesens L, De Clercq E. Update on human Herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 2005; 18: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay IR, Leskovsek NV, Rose NR. Cell damage and autoimmunity: a critical appraisal. J Autoimmun 2008; 30: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


