Abstract
Exosomes (EXs) are emerging as novel players in the beneficial effects induced by exercise on vascular diseases. We have recently revealed that moderate exercise enhances the function of circulating endothelial progenitor cell-derived EXs (cEPC-EXs) on protecting endothelial cells against hypoxia injury. However, the relationship between the changes of cEPC-EXs and the effects of exercise on ischemic stroke (IS) is unknown. Here, we investigated whether exercise-regulated EPC-EXs contribute to the beneficial effects of exercise on IS. C57BL/6 mice received moderate treadmill exercise (10 m/min) for 4-wks and then were subjected to middle cerebral artery occlusion (MCAO) stroke. The neurologic deficit score (NDS), infarct volume, microvessel density, cell apoptosis, angiogenesis/neurogenesis, sensorimotor functions were determined on day 2 (acute stage) and/or day 28 (chronic stage) post-stroke. The miR-126 and EPC-EX levels were analyzed by RT-PCR or nanoparticle tracking analysis combined with microbeads and used for correlation analyses. The function of EPC-EXs from exercised mice was detected in a hypoxia neuron model. Cell apoptosis, axon growth ability and gene expressions (cas-3 and Akt) were measured. Our data showed that: i) On day 2, exercised mice had decreased NDS and infarct volume, reduced cell apoptosis rate and cleaved cas-3 level, and a higher microvessel density than those in control (no-exercise) mice. The levels of EPC-EXs in plasma and brain tissue were raised and positively correlated in exercised mice. Meanwhile, the miR-126 level in cEPC-EXs and in ischemic tissue were upregulated in exercised mice. The EPC-EXs and their carried miR-126 levels negatively correlated with the infarct volume and cell apoptosis, whereas positively correlated with microvessel density. In addition, cEPC-EXs from exercised mice elicited protective effects on neurons against hypoxia-induced apoptosis and compromised axon growth ability which were blocked by miR-126 and PI3k inhibitors in vitro. ii) On day 28, exercised mice had less infarct volume, higher microvessel density, angiogenesis/neurogenesis and better sensorimotor functions. The levels of BDNF, p-TrkB/TrkB and p-Akt/Akt were upregulated in the brain of exercised mice. These recovery indexes correlated with the levels of cEPC-EXs and their miR-126. In conclusion, our data suggest that moderate exercise intervention has protective effects on the brain against MCAO-induced ischemic injury in both acute and chronic stages which might via the release of miR-126 enriched EPC-EXs.
Keywords: Exercise, EPC-EXs, miR-126, Ischemic stroke
1. Introduction
Ischemic stroke (IS) is a leading cause of disability and mortality in the USA, associating with the increased economic burden (~$34 billion/year) (Rajsic et al., 2019). Thrombolytic and interventional therapies are used for acute IS, which are limited by a short therapeutic window (6 h). Exercise is a widely recognized non-pharmacological approach for vascular diseases and has been recommended by the American Heart Association as a part of stroke prevention and treatment strategy (Winstein et al., 2016). Clinical data has shown that physical inactivity is associated with increased stroke severity (Reinholdsson et al., 2018). The beneficial effects of exercise on stroke such as reduced stroke risk factors (Lee et al., 2003), promoted neurotrophic factor release, enhanced neural stem cell migration/differentiation (Zhao et al., 2017) and neural plasticity (Pan et al., 2017) have been observed. However, the cellular and mediator mechanisms of exercise on stroke are largely unknown.
Endothelial progenitor cells (EPCs), a vasculogenic subset of progenitors, are well known for their roles in contributing to endothelium repair and promoting angiogenesis after ischemic injury (Zhao et al., 2013). Infusion of EPCs alleviates ischemia-induced cerebral injury in a mouse IS model (Chen et al., 2013). Exercise has been shown to enhance the circulating numbers and functions of EPCs (Chang et al., 2015; Silva et al., 2012b) suggesting that EPCs participate in the protective effects of exercise on IS. Exosomes (EXs), a major type of extracellular vesicles, are newly recognized cell-cell communicators and could mediate tissue/organ cross-talk (Poe and Knowlton, 2018; Bellin et al., 2019)., Nowadays, increasing evidence indicates that stem cell-derived extracellular vesicles including EXs offer the benefits of their parent cells (Xin et al., 2014; Wang et al., 2015). Pluripotent stem cell-derived EXs have been shown to prevent cardiomyocyte apoptosis via delivering cardioprotective miRs (Wang et al., 2015). Our group has reported that EPC-derived microvesicles released under starvation protected endothelial cells against hypoxia/reoxygenation (H/R)-injury, mainly due to their carried miR-126 (Wang et al., 2013).
Of note, exercise has recently been shown to induce the elevation of total extracellular vesicle in circulation (Whitham et al., 2018). The plasma level of muscle-derived extracellular vesicles which carrying fatty acid transport proteins has been shown to be raised by exercise (Nielsen et al., 2019). Another study revealed that exercise enhanced the release of cardiomyocyte derived EXs from the heart of diabetic mice as compared to the hearts of sedentary control mice (Chaturvedi et al., 2015). Moreover, exercise can affect miR cargo packaging into EXs. A single bout of high-intensity interval cycling exercise has been shown to increase circulating exosomal miRs (miR-1-3p, −16-5p, −222-3p) (D’Souza et al., 2018). As we know, miR-126 could modulate angiogenesis and vascular integrity (Wang et al., 2008), whereas, its circulating level is decreased as observed from IS patients and animal studies (Long et al., 2013; Chen et al., 2017)., Interestingly, exercise like swimming training increases cardiac miR-126 expression in rats (Silva, et al., 2012a), and treadmill exercise upregulates miR-126 level in the vascular tissues (Wu et al., 2014). Our group has recently found that 4-wk moderate treadmill exercise intervention increases the miR-126 level in circulating EPC-EXs (cEPC-EXs) in C57 BL/6 J mice (Ma et al., 2018a). Meanwhile, we have revealed that the cEPC-EXs from exercised mice had better effects than those from sedentary mice on protecting endothelial cells against hypoxia injury in the co-culture system (Ma et al., 2018a). Nevertheless, whether these changes on cEPC-EXs play a role in modulating the beneficial effects of an exercise intervention on IS has not been clarified yet. In this study, we investigated the relationships of the level of cEPC-EXs and their carried miR-126 with exercise-induced beneficial effects on the brain against middle cerebral artery occlusion (MCAO)-ischemic injury.
2. Material and methods
A detailed experiment protocol section is available in the online Data Supplement.
2.1. Treadmill exercise
C57BL/6 J mice, 8–10-week old, were used in this study. Mice were randomly divided into control (no-exercise) and exercise groups (n = 11 per group). Treadmill exercise intensity (10 m/min for 4 wks, 5 days per wk) was based on our previous report (Ma et al., 2018a). All mice were maintained in a 22 °C room with a 12 h light/dark cycle and fed with standard chow and drinking water ad libitum. Their body weights were recorded weekly.
All experimental procedures were approved by the Wright State University Laboratory Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health.
Isolating and nanoparticle tracking analysis (NTA) of cEPC-EXs from peripheral blood.
After 24 h of the last bout of exercise, blood samples were taken from the hearts of control and exercised mice using a 1 ml syringe with a small amount of anticoagulation solution. The isolation protocol of cEPC-EXs has been described in our previous report (Wang et al., 2016). The isolated cEPC-EXs were used for NTA by fluorescence Nanosight NS300 (Malvern Instruments, Malvern, UK) (Wang et al., 2016), for RNA extraction to determine their carried miR level or for co-culture experiments with neurons.
2.2. MCAO surgery and 5-bromo-2-deoxyuridine (BrdU) labeling
On the next day of the last bout of exercise, all the exercised and control mice were subjected to MCAO by inserting an intraluminal filament according to our previous reports (Chen et al., 2013; Chen et al., 2011). For those mice sacrificed on day 28, BrdU (IP, 65 μg/g per day) was intraperitoneally injected to label the newly generated cells for 7 continuous days before scarification (Chen et al., 2013).
2.3. Infarct volume and microvessel density analyses
The infarct volume was evaluated on day 2 post-MCAO by TTC staining (Chen et al., 2011) or day 28 post-MCAO surgery by cresyl violet (CV) staining (Mehta et al., 2015). For TTC staining, images of all stained slices were captured using a flatbed scanner. For CV staining, Images were acquired using a 2× objective under a bright-field microscope (Nikon, Eclipse E600). The area of infarction was measured by using Image J software.
For microvessel density analysis, brain sections were incubated with primary antibody rat anti-CD31 (1: 50, BD Pharmingen) overnight at 4 °C then followed by incubation with Cy3-conjugated anti-rat secondary antibody. CD31 positive cell counting was performed from photographs in 5 random microscopic fields. The average of 6 sections from rostral to caudal represented the data for one mouse.
2.4. Tunel assay of cell apoptosis and immunofluorescence analyses in the brain tissue
In situ apoptosis on day 2 post MCAO-surgery was measured by TUNEL assay kit (Roche, Switzerland) according to the manufacturer’s instruction. Tissue samples were examined under a fluorescence microscope (Nikon, Eclipse E600). Tunel+ cells were counted at five selected microscopic fields and averaged. The angiogenesis and neurogenesis were evaluated 28 days after MCAO surgery. The average of 6 sections from rostral to caudal represented the data for one mouse. In all experiments involving image analysis, the individual(s) taking the photographs and conducting the analysis was blinded to the grouping.
2.5. Neurological deficit and sensorimotor function evaluation
The neurological deficits score (NDS) was evaluated before (day −1) and after stroke (day 0), day 2 after MCAO surgery by using the 5-point scale method as previously described (Chen et al., 2013). The sensorimotor deficits were assessed by adhesive removal test and corner test as previously reported (Cai et al., 2017; Ma et al., 2018b). Mice were tested before (day −1) and after stroke (day 0), 14 or 28 days after MCAO surgery.
2.6. Isolation of EPC-EXs from the ischemic brain tissues
The protocol of isolating total EXs from the brain tissues has been described in a previous publication with slight modification (Polanco et al., 2016). The EPC-EXs were isolated from the total EXs on day 2 post-MCAO by using anti-CD34-conjugated and anti-VEGFR2-conjugated microbeads (Miltenyi Biotec).
2.7. Co-incubation of cEPC-EXs with hypoxia challenged N2a cells
To mimic in vivo ischemic condition, we produced an hypoxia/reoxygenation (H/R) injury model of N2a cells which was used for coculture experiments (Wang et al., 2013). The cEPC-EXs were sorted from the plasma of control mice and cEPC-EXsE were sorted from the plasma of exercised mice (Ma et al., 2018a). After 24 h co-culture, the BDNF level in the culture medium was determined by using the ELISA kit according to the manufacturer’s instructions.
2.8. Cell viability, apoptosis assay and neurite outgrowth of N2a cells
After 24 h of co-culture, the viability, apoptotic rate and neurite outgrowth of N2a cells were determined. The neurite lengths of N2a cells were measured using Image J software (Kamiya et al., 2006).
2.9. Quantitative RT-PCR analysis
The RNAs of cEPC-EXs, N2a cells, and the brain tissue were extracted using Trizol (Thermo Fisher Scientific). Reverse transcription (RT) reactions were performed using PrimeScriptTM RT reagent kit (TaKaRa, Japan) and PCR reactions were conducted using SYBR Premix EX TaqTM II kit (TaKaRa, Japan). The relative expression level of each gene was normalized to U6 and calculated using the 2−ΔΔCT method.
2.10. Western blot analysis
The proteins of EPC-EXs, N2a cells in different co-incubation groups and brain tissue were extracted with cell lysis buffer (Thermo Fisher Scientific). The protein lysates (20 μg) and EX protein lysate (2 μg) were electrophoresed, transferred onto PVDF membranes and incubated with primary antibody against anti- cleaved cas-3 (1: 50, Abcam), BDNF (1:1000), TrkB (1:1000), p-TrkB (1:1000), Akt (1: 1000), p-AKt (1: 1000), β-actin (1:4000; Sigma) at 4 °C overnight. All antibodies except cleaved caspase-3 and β-actin were purchased from Thermo Fisher Scientific. Then all membranes were washed and incubated with horseradish-peroxidase-conjugated anti-rabbit or anti-mouse IgG (1:40000; Jackson Immuno Research Lab) for 1 h at room temperature. Blots were developed with enhanced chemiluminescence developing solutions and images were quantified under ImageJ software.
2.11. Statistical analysis
All data are presented as mean ± SEM. The neurological deficit scores were expressed as median (range). The neurological deficit scores among different groups were compared by a Kruskal-Wallis test. When the Kruskal-Wallis test showed a significant difference, the Mann–Whitney U tests were applied as a post hoc test. Multiple comparisons of the data were analyzed by 2-way ANOVA followed by a Tukey post-hoc test (SPSS version 19.0; SPSS, Chicago, IL, USA). For all tests, a P < .05 was considered statistically significant.
3. Results
3.1. Exercised mice have reduced NDS and infarct volume, decreased cell apoptosis and a higher microvessel density on day 2 post-MCAO surgery
Exercised mice were subjected to MCAO-induced IS. Two days post-surgery, the neurological deficit was evaluated by using the 5-point NDS. Our data showed that exercised mice had a significantly lower score than that in control mice (Fig. 1A). According to the TTC staining (Fig. 1B), the infarct volume of exercised mice was 20 ± 2% which was smaller than that of the control mice (26 ± 3%) on day 2 after the MCAO surgery.
Fig. 1.
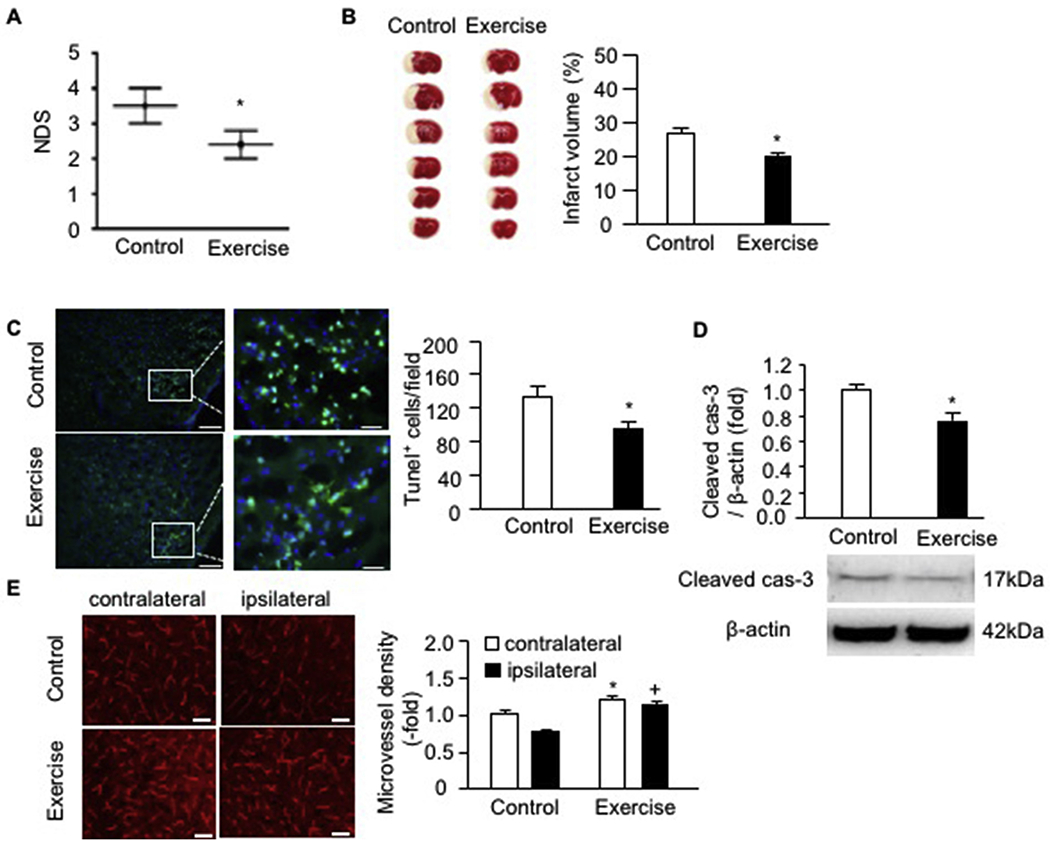
Exercised mice had reduced NDS and infarct volume, decreased cell apoptosis as well as a higher microvessel density on day 2 post-MCAO surgery. A, NDS; B, representative TTC images and summarized data showing the infarct volume of exercised and control mice; C, representative Tunel staining images and summarized data showing cell apoptosis in the peri-infarct area of exercised and control mice; Green: Tunel labeling; Blue: DAPI staining; scale bar in left panels: 100 um; scale bar in right panels (enlarged images of the box): 25 um; D, cleaved caspase-3 expression in ipsilateral brain of exercised and control mice; E, representative images and summarized data showing the microvessel density in the contralateral and ipsilateral brain; scale bar: 50 um. * p < .05, vs. control; + p < .05, vs. ipsilateral. Data are expressed as mean ± SEM. N = 11/group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To elucidate whether exercise has an effect on cell apoptosis, we conducted Tunel staining on brain tissue which was collected after 2 days of stroke surgery. As shown in Fig. 1C, there was a less amount of brain cells positively stained with Tunel in the peri-infarct area of exercised mice (92 ± 10 Tunel+ cells/field) than that in control mice (132 ± 15 Tunel+ cells/field). Meanwhile, we collected the proteins from the ipsilateral side and performed western blot analysis for the activated cas-3 expression. The data showed that the cleaved caspase-3 expression level was significantly down-regulated in the exercised mice (Fig. 1D), suggesting that exercise could protect the brain against MCAO-induced acute ischemic injury through preventing brain cells undergoing apoptosis.
In addition, we evaluated the microvessel density in the ipsilateral and contralateral brain of control and exercised mice by labeling the brain microvessels with CD31. As shown in Fig. 1E, the microvessel density was higher in the contralateral side of exercised mice than that in control mice. Similarly, in the ipsilateral brain, exercised mice had a higher density than that in control mice, suggesting exercise improved microvessel density in the ipsilateral brain which might contribute to the construction of bilateral blood flow and thereby alleviate acute injury.
3.2. Exercise intervention raises the levels of EPC-EXs and miR-126 in the plasma and ipsilateral brain tissue on day 2 post-MCAO surgery
After 2 days of stroke onset, we isolated and counted the number of cEPC-EXs using NTA. As shown in Fig. 2A, there was a higher number of cEPC-EXs in exercised mice than that in control mice. As revealed by qRT-PCR analysis (Fig. 2B), the miR-126 level in cEPC-EXs was upregulated by ~1.8 fold in exercised mice than that of control mice (p < .05). We also determined the number of tEPC-EXs and miR-126 levels in the ipsilateral brain tissue. Our data (Fig. 2C-D) showed that exercised mice had higher levels of EPC-EXs and miR-126 in the ipsilateral brain than those in control mice (p < .05). According to Pearson’s correlation analysis, the miR-126 level in the ipsilateral brain positively correlated with the numbers of EPC-EXs isolated from the ischemic brain of exercised mice (Fig. 2E), suggest that EPC-EXs are one of the sources of miR-126. Meanwhile, our data (Fig. 2F) showed that the level of cEPC-EXs positively correlated with the number of EPC-EXs in ischemic brain, indicating that the elevated cEPC-EXs level could be the potential mechanism of exercise on protecting the brain from acute ischemic injury.
Fig. 2.
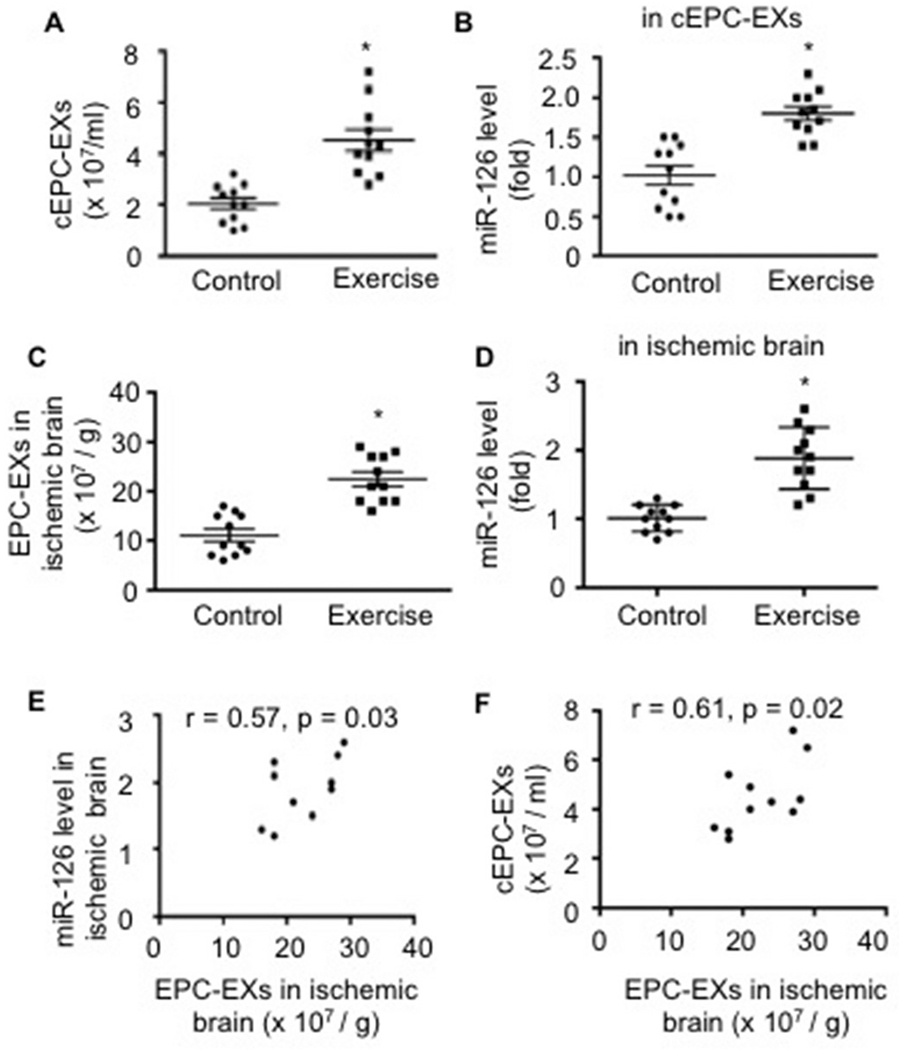
Analyses of the levels of EPC-EXs in the circulation and the brain, as well as the miR-126 expression on day 2 post-MCAO surgery. A-B, the level of cEPC-EXs and their carried miR-126 level in exercised and control mice; * p < .05, vs. control. C-D, EPC-EXs and miR-126 levels in the ischemic brain; * p < .05, vs. control; E-F, Pearson’s correlation analyses of the number of EPC-EXs with miR-126 in the ischemic brain, and the level of cEPC-EXs and EPC-EXs in the ischemic brain. Data are expressed as mean ± SEM. N = 11/group.
3.3. The levels of cEPC-EXs and their carried miR-126 correlate with NDS, infarct volume and the number of Tunel+ cells on day 2 post-MCAO surgery in exercised mice
As revealed by Pearson’s correlation analysis (Fig. 3A-C), the number of cEPC-EXs were found to be negatively correlated with the NDS (p = .01), the infarct volume (p = .01) and the number of Tunel+ cells in the peri-infarct area (p = .04) in exercised mice after 2 days of IS onset. Similarly, there were negative correlations between the relative miR-126 level in cEPC-EXs and the NDS (p = .02), the infarct volume (p = .03) and the number of Tunel+ cells (p = .04) (Fig. 3D-F).
Fig. 3.
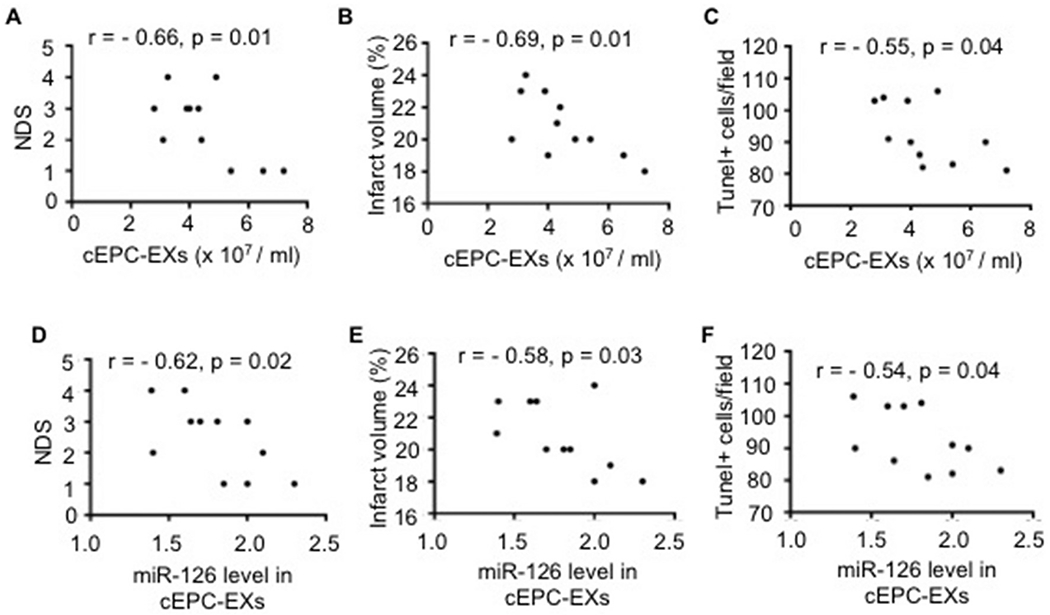
Correlation analyses of the levels of cEPC-EXs and their carried miR-126 with MCAO-induced brain injury on day 2 post-MCAO surgery.
A-F, Pearson’s correlation analysis of the level of cEPC-EXs and their carried miR-126 level with NDS, infarct volume and Tunel+ cells in exercised mice; Data is expressed as mean ± SEM. N = 11/group.
3.4. Exercised mice have a smaller infarct volume, increased microvessel density, angiogenesis and neurogenesis, and improved sensorimotor functions on day 28 post-MCAO surgery, accompanying with upregulated expressions of BDNF/TrkB/Akt signaling proteins
According to CV staining (Fig. 4A), the infarct volume was significantly decreased in exercised mice as compared to that of control mice on 28 days after IS onset (p < .05). Meanwhile, our data showed that the microvessel density (Fig. 4B), the numbers of BrdU + CD31 + cells (angiogenesis) and BrdU + NeuN + cells (neurogenesis) in the peri-infarct area (Fig. 4C) were increased in exercised mice compared to those in the control ones. The sensorimotor function recovery was assessed on day −1, day 0, days 14 and 28 after the MCAO surgery. According to the results of the corner test (Fig. 4D), exercised mice had a better sensorimotor functional recovery than that in control mice. This is indicated by the decreased number of left turns out of every 10 turn trials on day 28 post-surgery (P < .05). Similarly, as revealed by adhesive removal test analysis, exercised mice spent a shorter time to remove the adhesive tape than that of the control mice.
Fig. 4.
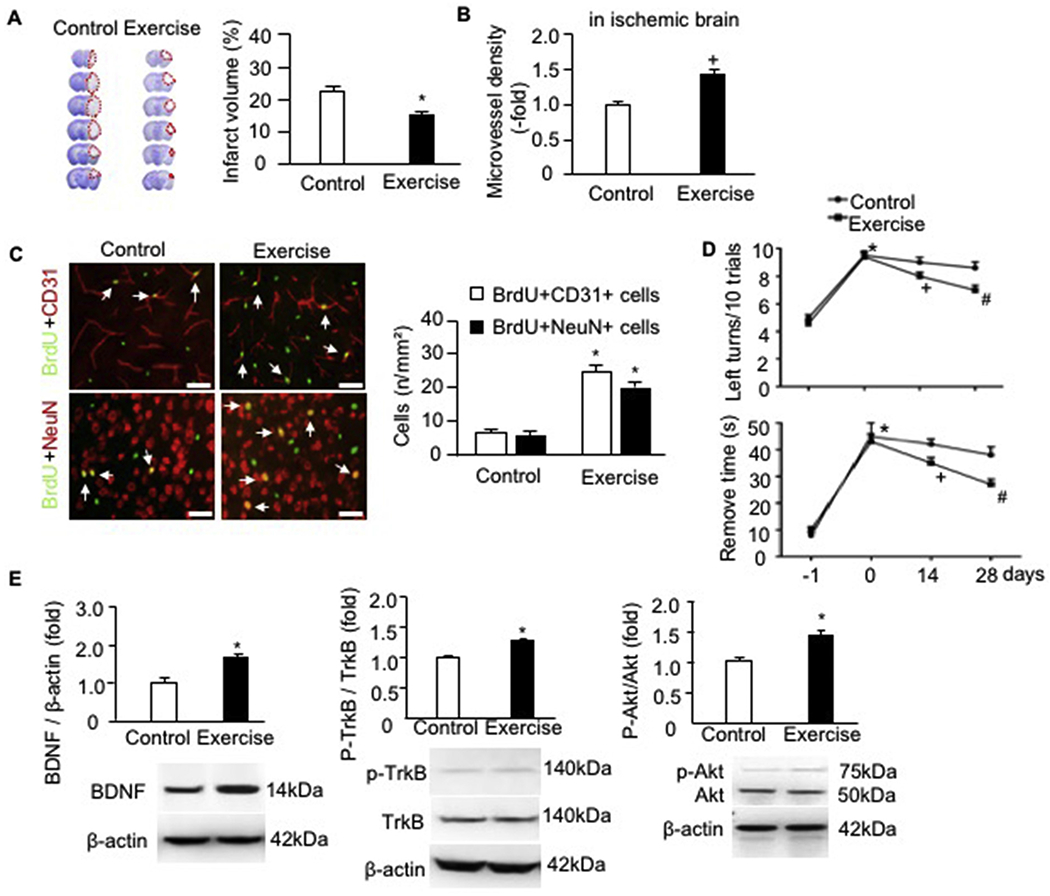
Exercised mice had alleviated infarct volume, increased microvessel density, angiogenesis and neurogenesis, improved sensorimotor functions as well as upregulated the protein expressions of BDNF/TrkB/Akt signaling pathway proteins in the ischemic brain of exercised mice day 28 post-MCAO surgery. A, representative CV images and summarized data showing the infarct volume of exercised and control mice; Red dash line indicate the infarct area in each brain slide; * p < .05, vs. control. B, microvessel density; C, representative immunofluorescence images and statistical data showing the angiogenesis and neurogenesis. Red: CD31 or NeuN; Green: BrdU; Double positive cells were labeled by arrows. Scale bar: 50 um. * p < .05, vs. control. D, corner test and adhesive remove test analyses. E, the protein levels of BDNF, p-TrkB/TrkB and p-Akt/Akt. * p < .05, vs. day −1, + p < .05, vs. day 0, # p < .05, vs. day 14. Data are expressed as mean ± SEM. N = 11/group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For mechanism study, we assessed the expressions of neurotrophic factor BDNF and its receptor TrkB. As shown in Fig. 4E, exercised mice had a higher level of BDNF in the ischemic brain than that in the control mice. The phosphorylation of TrkB is increased. Meanwhile, our data showed that the phosphorylation of the PI3k downstream molecular Akt was also raised, suggesting that the BDNF/TrkB/Akt pathway is activated in the ischemic brain of exercised mice.
According to Pearson’s correlation analysis (Fig. 5), the data showed that the number of cEPC-EXs and their relative miR-126 level were negatively correlated with the infarct volume, but positively correlated with the numbers of BrdU + CD31 + cells and BrdU + NeuN + cells in the peri-infarct area in exercised mice. What’s more, the number of cEPC-EXs and their relative miR-126 level in exercised mice had a significant correlation with the sensorimotor function performance. The higher levels of cEPC-EXs and their carried miR-126, the less chance of exercised mice made left turns every 10 trials, and the shorter time the exercised mice needed to remove the adhesive tape.
Fig. 5.
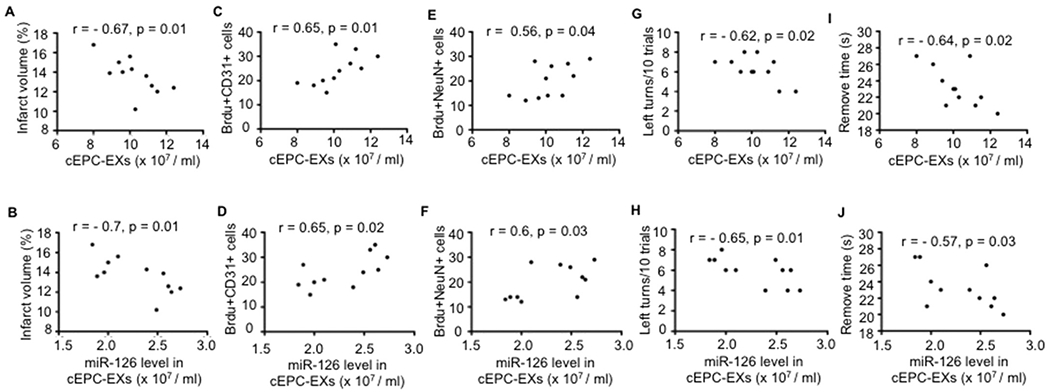
Correlation analyses of the levels of cEPC-EXs and their carried miR-126 with the infarct volume, angiogenesis, neurogenesis and sensorimotor function of exercised mice on day 28 post-MCAO surgery. A-J, Pearson’s correlation analysis of the level of cEPC-EXs and their carried miR-126 level with the infarct volume, the number of the number of BrdU + CD31 + cells and BrdU + NeuN + cells, and left turns/10 trials and adhesive remove time in exercised mice. Data is expressed as mean ± SEM. N = 11/group.
3.5. cEPC-EXs from exercised mice elevate miR-126 level and improve the viability of hypoxia-injured N2a cells
To explore the functions of exercise-regulated cEPC-EXs, we conducted in vitro experiments in a hypoxia neuron model. Our data (Fig. 6A) showed that both cEPC-EXs from control mice and from exercised mice (cEPC-EXE) raised the miR-126 level in H/R-injured N2a cells (~ 1.5 fold and ~ 2.3 fold, respectively) as compared to that in the veh (culture medium only). We found that cEPC-EXE had better effects than cEPC-EXs on protecting N2a cells against H/R injury as revealed by increased cell viability, decreased cell apoptosis and cleaved cas-3 level. Blocking miR-126 or the PI3K signal pathway by LY294002 could inhibit such effects elicited by cEPC-EXE (Fig. 6B-E).
Fig. 6.
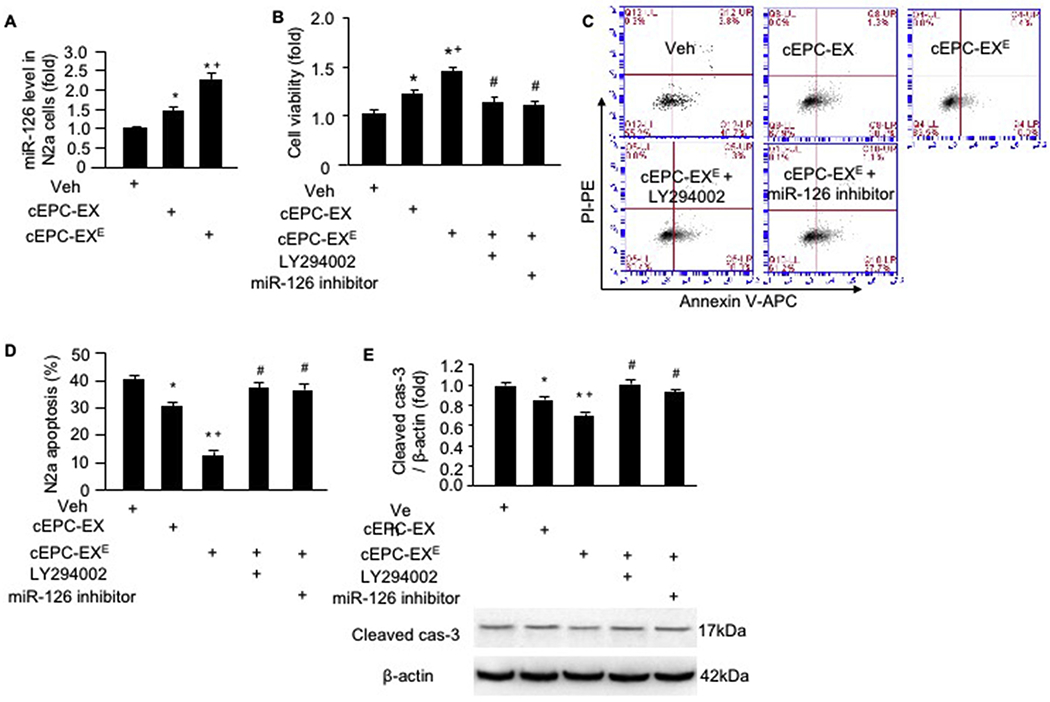
cEPC-EX of exercised mice elevated the miR-126 level and improved the survival ability of H/R-injured N2a cells. A, miR-126 level in different co-culture groups; B, cell viability as revealed by MTT assay; C-D, representative flow cytometry plot and summarized data showing the apoptotic rate of N2a cells; E, cleaved cas-3 level in N2a cells. * p < .05, vs. veh, + p < .05, vs. cEPC-EX, # p < .05, vs. cEPC-EXE. Data are expressed as mean ± SEM. N = 5/group.
3.6. cEPC-EXs from exercised mice restore the neurite length and promote BDNF secretion as well as activate the PI3k/Akt signal pathway of N2a cells, which are significantly reduced by PI3k or miR-126 inhibitor
As shown in Fig. 7A, N2a cells treated by cEPC-EX had longer neurite length than that of the cells in the vehicle group (~ 9.5 um and ~ 18 um, respectively). What’s more, the neurite length of N2a cells was even longer in those treated by cEPC-EXE (~29 um). Whereas, miR- 126 inhibitor or LY294002 (an inhibitor of the PI3K signal pathway) significantly attenuated such effect.
Fig. 7.
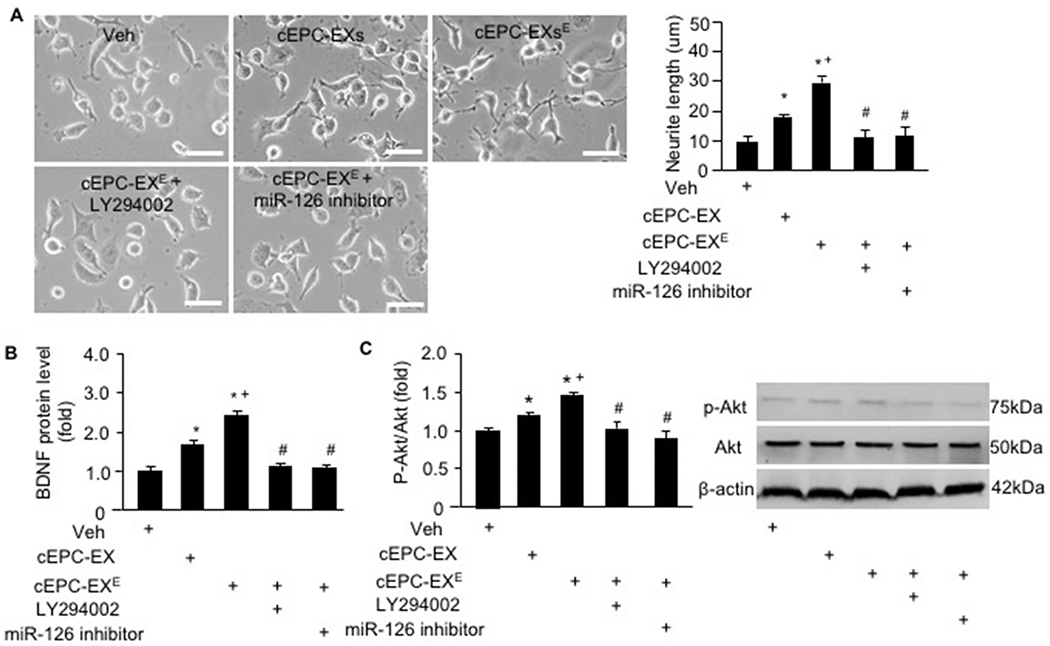
cEPC-EX of exercised mice restored the neurite length, promoted BDNF secretion and activated the PI3k/Akt signal pathway of N2a cells. A, representative phase-contrast images and summarized data showing the neurite length of N2a cells in different co-culture groups; B, BDNF level in the culture medium of N2a cells in different co-culture groups; C, representative western blot bands and summarized data showing p-AKt/Akt expression of N2a cells in different co-culture groups. * p < .05, vs. veh, + p < .05, vs. cEPC-EX, # p < .05, vs. cEPC-EXE. Data are expressed as mean ± SEM. N = 5/group.
Since neurotrophic factors such as BDNF is important for neuron cell growth, we determined its level in the culture medium of N2a cells. Our data (Fig. 7B) showed that cEPC-EXs promoted the secretion of BDNF (by ~1.6-fold), and cEPC-EXE had a profound effect as compared to cEPC-EXs. The phosphorylation of PI3K downstream molecular AKt had found to be changed in N2a cells after the treatment with EXs. The data (Fig. 7C) showed that cEPC-EXE had a better effect than cEPC-EXs on raising the phosphorylation of Akt, which was inhibited by miR-126 inhibitor and LY294002.
4. Discussion
The major finding of the present study is that moderate exercise intervention elicits beneficial effects on MCAO-induced ischemia injury, as evidenced by alleviated acute brain cell injury/apoptosis and improved neurological functional recovery which are ascribed to the release of miR-126 enriched cEPC-EXs.
In this study, we found that moderate treadmill exercise regimen improved the injury indexes in the acute stage (2 days post-IS) of our mouse stroke model, such as decreased NDS, infarct size and cell apoptosis. Our findings are supported by two previous studies in rat stroke models. Mayhan and colleagues showed that vigorous exercise training (25 m/min, 1 h, 10% grade of incline) carried out 5 days/wk. for several weeks could reduce acute brain injury in a MCAO followed by reperfusion model (Arrick et al., 2014). Another study revealed that an exercise regimen (> 3 times/wk., 25 m/min, 30 mins) for 3 weeks prior to stroke could mitigate neuronal apoptosis that accompanies stroke in the acute stage, but the 1 time a week of exercise did not (Terashi et al., 2019). The possible mechanisms in the two studies are either related to the increased contribution of nitric oxide (Arrick et al., 2014) or the downregulation of caspase-3 signal pathway (Terashi et al., 2019). There are lacking information regarding to the effects of exercise on mice IS models. Here, we have demonstrated that 4-wk of moderate exercise regimen (10 m/min, 5 bouts per week) induces favorable effects in alleviating acute ischemic injury in mice subjected to MCAO. Moreover, in order to clarify whether the alleviated acute injury is related to the brain microvessel system, we analyzed the microvessel density in the brain. Our data revealed that this moderate exercise regimen not only increased the microvessel density in the contralateral brain but also in the ipsilateral brain, suggesting the cerebral vessel could response to the moderate exercise regimen stimulus. The potential mechanism might be related to the raised number of circulating EPCs and their released trophic factors such as vascular endothelial growth factor and BDNF which could elicit favorable effects on angiogenesis (Volaklis et al., 2013; Ma et al., 2018a). Importantly, the increased density of microvessels contributes to the establishment of collateral blood flow and increases the availability of salvage tissue which alleviates acute ischemic injury.
We have recently demonstrated that moderate exercise promoted the release of cEPC-EXs (Ma et al., 2018a). EPC-derived extracellular vesicles have been shown to promote angiogenesis (Chen et al., 2013), vascular repair (Li et al., 2016) and recovery of hindlimb ischemia (Ranghino et al., 2012), suggesting the potential of EPC-EXs for ischemia tissue repair. In the present study, we clarified the relationship between cEPC-EXs and the effects of exercise on IS. Upon the Pearson’s correlation analysis, we have revealed that the levels of cEPC-EXs correlated with the effects of exercise in acute stage of IS (NDS, infarct size and apoptotic cells), supporting our hypothesis that exercise-regulated cEPC-EXs participate in cerebral tissue repair. Of note, we can’t rule out other potential mechanisms of the beneficial effects elicited by exercise intervention on IS such as the enhanced migration/differentiation ability of neural stem cell (Zhao et al., 2017) or promoted neural plasticity (Pan et al., 2017).
To further understand the mechanism of the effects of exercise on the acute stage of stroke, we focused on the miRs carried by EPC-EXs. The miR-126 has been shown to decrease endothelial apoptosis (Fish et al., 2008) and promote neuron survival (Kong et al., 2017). Notably, exercise can increase miR-126 level in circulation, the heart and vascular tissues (Baggish et al., 1985; Silva et al., 2012a; Wu et al., 2014). In exercised mice, we found that miR-126 level was significantly raised in the ipsilateral tissue after 2 days of IS onset. Moreover, its level was positively correlated with the EPC-EX level in the brain tissue. Although we did not assess whether the exercise regimen (60 mins at 10 m/min for 4 wks) could directly affect the miR-126 level in brain microvessels, our data suggest that the EPC-EXs might be one of the sources of releasing miR-126 into the ipsilateral brain tissue. These results are in line with our observations showing that the microvessel density was increased and cell apoptosis was decreased in the acute stage of exercised mice subjected to MCAO. Previous reports showing that EPC-extracellular vesicles play an important role in protecting endothelial cells against hypoxia through their carried miR-126 (Chen et al., 2013; Li et al., 2016) also support our finding. In addition, we further illustrated the relationship between the levels of EPC-EXs in the brain and plasma. Our data showed that the EPC-EX level in ischemic brain positively correlated with the level of cEPC-EXs, suggesting that cEPC-EXs is a source of EPC-EXs in the local brain. All of these results support our hypothesis that cEPC-EXs could serve as a novel mechanism which is responsible for exercise intervention-induced beneficial effects in the acute phase of IS.
To explore how EPC-EXs rescue neurons against ischemic injury, we conducted co-culture experiments on a neural hypoxia model. Our data revealed that cEPC-EXs of exercised mice significantly elevated miR-126 level in N2a cells and promoted the secretion of BDNF which participates in the processes of angiogenesis and neurogenesis in vivo (Apte et al., 2019; Bath et al., 2012). The cEPC-EXs of exercised mice exhibited the anti-apoptotic effect on hypoxia-injured N2a cells which is in consistent with our in vivo finding showing less apoptotic cells in the ischemic brain of exercised mice on acute stage. All of these effects were blocked by PI3k inhibitor and miR-126 inhibitor, indicating that the beneficial effects of cEPC-EXs on neurons is mediated by miR-126/PI3k signal pathway.
Here, we also revealed that 4-wk of moderate exercise treadmill regimen can improve the recovery indexes in the chronic stage (28 days post-MCAO) of IS stroke including infarct size, and sensorimotor functions. The neurological functional improvements of exercised mice are in line with our findings showing the repair of histological damage as evidenced by the increased brain microvessel density, improved neurogenesis and angiogenesis in the peri-infarct area. Our findings are supported by other reports (Pianta et al., 2019; Rezaei et al., 2018)., One study shows that a short bout of 30-min or 60-min forced running wheel exercise before a stroke can provide an improvement on functional outcomes by enhancing angiogenesis, with a trend towards a prolonged duration of exercise (Pianta et al., 2019). Otsuka and his colleagues suggest that 3-wk of daily exercise prior to MCAO is insufficient to induce neurological improvements in IS (Rezaei et al., 2018), which indicates that a longer timeframe preconditioning exercise regimen might provide the enhancements in neurological function. Indeed, we found 4-wk moderate exercise intervention elicited long-term favorable effects on day 28 after stroke onset. Of note, we firstly revealed that cEPC-EXs and their carried miR-126 negatively correlated with the infarct volume, whereas positively correlated to angiogenesis, neurogenesis and neurological functional recovery in the chronic stage in exercised mice subjected to MCAO-induced IS. These findings support the concept that EXs are novel mediators of the system adaption to exercise intervention (Safdar and Tarnopolsky, 2018). However, optimizing and translating exercise durations from animal models to humans requires additional research.
Besides, we found that the moderate exercise regimen promoted the secretion of neurotrophic factor BDNF. Sleiman et al. have previously reported that BDNF production in the brain was raised in mice that did running wheel exercise for 30 days (Sleiman et al., 2016), which is consistent with our study. Another study indicates that the phosphorylation of BDNF cognate TrkB receptor is largely involved in the positive effect of aerobic exercise on brain functioning (Pedard et al., 2019). In the present study, we have revealed that the TrkB phosphorylation was significantly increased in exercised mice, which might contribute to the neurological recovery in the chronic phase since the BDNF/TrkB signal pathway is involved in neurogenesis (Numakawa et al., 2018). Activation of TrkB is also coupled to the stimulation of PI3k pathway. We determined the PI3k downstream protein Akt expression and found its expression was upregulated, suggesting the PI3k/Akt pathway is involved in the beneficial effects of exercise in IS.
In conclusion, moderate treadmill exercise prior to IS elicited beneficial effects including reducing brain cell apoptosis in the acute stage and improving sensorimotor function by enhancing angiogenesis and neurogenesis in the chronic stage. These effects are significantly correlated with the cEPC-EXs and their carried miR-126.
Supplementary Material
Acknowledgment
This work was supported by the American Heart Association (18POST33990433, USA), National Natural Science Foundation of China (81700280, 81970261, China).
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.expneurol.2020.113325.
References
- Apte RS, Chen DS, Ferrara N, 2019. VEGF in Signaling and disease: beyond discovery and development. Cell. 176 (6), 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick DM, Yang S, Li C, Cananzi S, Mayhan WG, 2014. Vigorous exercise training improves reactivity of cerebral arterioles and reduces brain injury following transient focal ischemia. Microcirculation. 21 (6), 516–523. [DOI] [PubMed] [Google Scholar]
- Baggish AL, Park J, Min PK, et al. , 2014. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J. Appl. Physiol 116 (5), 522–531 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Akins MR, Lee FS, 2012. BDNF control of adult SVZ neurogenesis. Dev. Psychobiol 54 (6), 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilin G, Gardin C, Ferroni L, et al. , 2019. Exosome in cardiovascular diseases: a complex world full of hope. Cells 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Zhang W, Weng Z, et al. , 2017. Promoting neurovascular recovery in aged mice after ischemic stroke - prophylactic effect of omega-3 polyunsaturated fatty acids. Aging Dis. 8 (5), 531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Paterno J, Duscher D, et al. , 2015. Exercise induces stromal cell-derived factor-1 alpha-mediated release of endothelial progenitor cells with increased vasculogenic function. Plast. Reconstr. Surg 135 (2), 340e–350e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC, 2015. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J. Cell. Mol. Med 19 (9), 2153–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen S, Chen Y, et al. , 2011. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am. J. Physiol. Endocrinol. Metab 301 (1), E62–E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao X, Chen S, et al. , 2013. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 61 (3), 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui C, Yang X, et al. , 2017. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl. Stroke Res 8 (4), 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DA, ND J, Fernandes T, Soci UP, et al. , 2012a. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med. Sci. Sports Exerc 44 (8), 1453–1462. [DOI] [PubMed] [Google Scholar]
- D’Souza RF, Woodhead JST, Zeng N, et al. , 2018. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol. Endocrinol. Metab 315 (4), E723–E733. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, et al. , 2008. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 15 (2), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, et al. , 2006. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum. Mol. Genet 15 (22), 3313–3323. [DOI] [PubMed] [Google Scholar]
- Kong F, Zhou J, Zhou W, et al. , 2017. Protective role of microRNA-126 in intracerebral hemorrhage. Mol. Med. Rep 15 (3), 1419–1425. [DOI] [PubMed] [Google Scholar]
- Lee CD, Folsom AR, Blair SN, 2003. Physical activity and stroke risk: a meta-analysis. Stroke. 34 (10), 2475–2481. [DOI] [PubMed] [Google Scholar]
- Li X, Chen C, Wei L, et al. , 2016. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 18 (2), 253–262. [DOI] [PubMed] [Google Scholar]
- Long G,, Wang F, Li H, et al. , 2013. Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol. 13, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wang J, Liu H, et al. , 2018a. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med. Sci. Sports Exerc 50 (10), 2024–2032. [DOI] [PubMed] [Google Scholar]
- Ma S, Wang J, Wang Y, et al. , 2018b. Diabetes mellitus impairs white matter repair and long-term functional deficits after cerebral ischemia. Stroke. 49 (10), 2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SL, Kim T, Vemuganti R, 2015. Long noncoding RNA FosDT promotes ischemic brain injury by interacting with REST-associated chromatin-modifying proteins. J. Neurosci 35 (50), 16443–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MH, Sabaratnam R, Pedersen AJT, Hojlund K, Handberg A, 2019. Acute exercise increases plasma levels of muscle-derived microvesicles carrying fatty acid transport proteins. J. Clin. Endocrinol. Metab 104 (10), 4804–4814. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Odaka H, Adachi N, 2018. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci 19 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Jiang T, Zhang L, et al. , 2017. Physical exercise promotes novel object recognition memory in spontaneously hypertensive rats after ischemic stroke by promoting neural plasticity in the entorhinal cortex. Front. Behav. Neurosci 11, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedard M, Cefis M, Ennequin G, et al. , 2019. Brain-derived neurotrophic factor pathway after downhill and uphill training in rats. Med. Sci. Sports Exerc 51 (1), 27–34. [DOI] [PubMed] [Google Scholar]
- Pianta S, Lee JY, Tuazon JP, et al. , 2019. A short bout of exercise prior to stroke improves functional outcomes by enhancing angiogenesis. NeuroMolecular Med. 21 (4), 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe AJ, Knowlton AA, 2018. Exosomes and cardiovascular cell-cell communication. Essays Biochem. 62 (2), 193–204. [DOI] [PubMed] [Google Scholar]
- Polanco JC, Scicluna BJ, Hill AF, Gotz J, 2016. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem 291 (24), 12445–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsic S, Gothe H, Borba HH, et al. , 2019. Economic burden of stroke: a systematic review on post-stroke care. Eur. J. Health Econ 20 (1), 107–134. [DOI] [PubMed] [Google Scholar]
- Ranghino A, Cantaluppi V, Grange C, et al. , 2012. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int. J. Immunopathol. Pharmacol 25 (1), 75–85. [DOI] [PubMed] [Google Scholar]
- Reinholdsson M, Palstam A, Sunnerhagen KS, 2018. Prestroke physical activity could influence acute stroke severity (part of PAPSIGOT). Neurology. 91 (16), e1461–e1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei R, Nasoohi S, Haghparast A, et al. , 2018. High intensity exercise preconditioning provides differential protection against brain injury following experimental stroke. Life Sci. 207, 30–35. [DOI] [PubMed] [Google Scholar]
- Safdar A, Tarnopolsky MA, 2018. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb. Perspect Med 8 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JF, Rocha NG, Nobrega AC, 2012b. Mobilization of endothelial progenitor cells with exercise in healthy individuals: a systematic review. Arq. Bras. Cardiol 98 (2), 182–191. [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, et al. , 2016. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body beta-hydroxybutyrate. Elife. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashi T, Otsuka S, Takada S, et al. , 2019. Neuroprotective effects of different frequency preconditioning exercise on neuronal apoptosis after focal brain ischemia in rats. Neurol. Res 41 (6), 510–518. [DOI] [PubMed] [Google Scholar]
- Volaklis KA, Tokmakidis SP, Halle M, 2013. Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin. Res. Cardiol 102 (4), 249–257. [DOI] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, et al. , 2008. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 15 (2), 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen S, Ma X, et al. , 2013. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxidative Med. Cell. Longev 2013, 572729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Li Y, et al. , 2015. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol 192, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Guo R, Yang Y, et al. , 2016. The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells Int. 2016, 2639728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, Parker BL, Friedrichsen M, et al. , 2018. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 27 (1), 237–251 (e234). [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Stein J, Arena R, et al. , 2016. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 47 (6), e98–e169. [DOI] [PubMed] [Google Scholar]
- Wu XD, Zeng K, Liu WL, et al. , 2014. Effect of aerobic exercise on miRNA-TLR4 signaling in atherosclerosis. Int. J. Sports Med 35 (4), 344–350. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Chopp M, 2014. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front. Cell. Neurosci 8, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YH, Yuan B, Chen J, et al. , 2013. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci. Ther 19 (2), 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Pang Q, Liu M, et al. , 2017. Treadmill exercise promotes neurogenesis in ischemic rat brains via Caveolin-1/VEGF signaling pathways. Neurochem. Res 42 (2), 389–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


