Abstract
Background:
Urosepsis is a common disease in urology, which is characterized by high treatment costs and high mortality. In the treatment of sepsis, anti-infection therapy is the most important means. However, the effect of empirical anti-infection therapy is often not ideal. Therefore, it is necessary to continuously monitor the prevalence of bacterial isolates in the blood culture of patients with urinary sepsis and their sensitivity to antibacterial drugs. This is of great significance to improve the efficacy of empirical antibiotic therapy for urosepsis.
Objective:
To elucidate the landscape of prevailing bacterial profiles and their antimicrobial susceptibilities in urosepsis cases, and to furnish robust clinical evidence to underpin the timely initiation of empirical antibiotic treatment.
Methods:
Collect the basic information and blood culture results of patients with urosepsis hospitalized from 2017 to 2020. Retrospective analysis of bacterial species and antimicrobial susceptibility in urosepsis and changes over 4 years.
Results:
Gram-negative bacteria (178 isolates, 75.11%) constituted the main pathogens causing urosepsis, followed by Gram-positive bacteria (46 isolates, 19.41%) and fungus (13 isolates, 5.48%). The sensitivity of ertapenem, meropenem, amikacin, and imipenem to Gram-negative bacteria all exceeded 85%. The sensitivity rates of levofloxacin, gentamicin, and ciprofloxacin are decreasing every year (p < 0.05). Tigecycline, vancomycin, and linezolid exhibited excellent sensitivity against Gram-positive bacteria. Among fungi, fluconazole demonstrated universal sensitivity, while itraconazole-resistant isolates have been found, and amphotericin B is still effective.
Conclusion:
Analysis of blood culture results of patients more accurately reflected the etiology of urosepsis, mainly Escherichia coli, Enterococcus, and Klebsiella pneumoniae. If there are no definitive blood culture results, empiric treatment of urosepsis should not include fluoroquinolone antibiotics. Cefepime, cefoxitin, and ceftazidime are the most sensitive antibiotics to Gram-negative bacteria besides carbapenem antibiotics. In addition, the current situation regarding extended-spectrum β-lactamase-producing bacteria and carbapenem-resistant Enterobacteriaceae bacteria resistance is extremely concerning with limited therapeutic options available. Strengthening antibiotic management practices and exploring novel antibacterial agents can help mitigate this issue.
Keywords: antimicrobial susceptibility, blood culture, clinical evidence, pathogen distribution, urosepsis
Introduction
Sepsis is a significant global public health problem and an important reason for patients to be admitted to ICU. According to the literature review, there were 48.9 million cases of sepsis and 11 million sepsis-related deaths reported in 2017 alone, accounting for 19.7% of global mortality.1–3 Urosepsis accounts for about 25% of all cases of sepsis, 4 which is mainly caused by Escherichia coli (43%), Enterococcus (11%), and Klebsiella pneumoniae (10%). However, the distribution of bacterial flora and antibiotic sensitivities are different around the world.3,5 Rapid and appropriate treatment (including early intravenous injection of antibiotics that have been proven to be sensitive) is very important for managing life-threatening sepsis patients. 5 However, empirical antibacterial therapy sometimes fails to achieve a curative effect and leads to bacterial drug resistance. 6 The initial use of antibiotics often lacks the basis of drug sensitivity. Empirical antibiotic treatment for sepsis originating from genitourinary infections has been noted to have an inadequate coverage rate compared to other infections. 7 Therefore, it is very important to know more about the types of bacteria that cause urosepsis and their sensitivity to antibiotics. This approach is instrumental in bolstering the precision of initial therapeutic measures, thereby potentially curtailing the emergence of resistant isolates and optimizing patient outcomes.
In this study, the blood culture results of patients were retrospectively analyzed to understand the common bacteria and sensitive antibiotics that cause urosepsis and to provide valuable and reasonable drug selection for early empirical antibiotic treatment of urosepsis.
Methods
Diagnostic criteria for urosepsis
The International Consensus Definition of Sepsis and Septic Shock (Sepsis 3) characterizes sepsis as a life-threatening condition resulting from dysregulated host response to infection, leading to organ dysfunction. 8 Urosepsis is the term used to diagnose sepsis in patients with a genitourinary system infection. If the patient does not meet the diagnostic criteria of Sepsis-3, it indicates that the patient only has simple bacteremia or the specimen is contaminated. Such cases will be excluded from our research.
Data collection
The study included patients who were diagnosed with urosepsis based on positive blood cultures and were hospitalized in the Department of Urology at the Second Affiliated Hospital of Kunming Medical University between January 2017 and December 2020.
Inclusion criteria
(1) Patients were diagnosed with urosepsis. (2) The blood culture results of the patients were positive. (3) Antibiotic sensitivity tests of the patients yielded definitive results, including data on sensitivity, intermediation, and resistance. Only a few antibiotics exhibit intermediation in antibiotic susceptibility; therefore, instances where pharmaceutical susceptibility serves as a mediator between drug resistance and treatment are classified as insensitive.
Exclusion criteria
(1) Patients with infections affecting other organs, such as the lungs, abdomen, or brain, were excluded from the study. (2) Duplicate isolates originating from the same pathogen within a single patient were excluded. (3) Patients with incomplete healthcare records were excluded from the analysis.
Specimen testing method
According to the Chinese health regulatory requirements for bacterial culture, all blood samples were collected, sorted, and cultured. The Bact/Alert3D automated blood culture system (BioMérieux, France) and VITEK2-compact mass spectrometer were employed for automated blood culture, bacterial identification, and drug sensitivity studies, supporting both aerobic and anaerobic culture bottles.
The antimicrobial susceptibility analysis was conducted following the standards set by the American Institute of Clinical and Laboratory Standards (CLSI), and the interpretation of the results was based on CLSI standards.9–12
Statistical analysis
SPSS 24.0 software was utilized for statistical analysis. The chi-square test was employed to examine differences in categorical data between the two groups. The Rank-sum test and analysis of variance (ANOVA) were used for comparing count data. A significance level of p < 0.05 was considered indicative of a statistically significant difference.
About the Candida and Gram-positive coagulase-negative Staphylococci
The analysis of Candida and Gram-positive coagulase-negative Staphylococci was not conducted separately due to their insufficient quantity. Instead, they were respectively categorized and analyzed under the classifications of fungi and Gram-positive bacteria.
Results
Patient data
A total of 213 patients, consisting of 130 males and 83 females, with an average age of 57 years (ranging from 50 to 67 years) were included in the study. Among these patients, 120 cases were diagnosed with urolithiasis, 68 cases with hypertension, and 22 cases with diabetes. Before hospitalization, 52 patients underwent nephrostomy or cystostomy, and 57 patients were placed with ureteral stents. No significant differences were observed in age, body mass index (BMI), the incidence of hypertension and diabetes, pre-hospital catheter insertion (including nephrectomy and cystostomy), and indwelling rates of the ureteral stent (Table 1).
Table 1.
Patient characteristics.
| Year | 2017 | 2018 | 2019 | 2020 | p |
|---|---|---|---|---|---|
| Age, years | 56 (46–66) | 57 (50–67) | 59 (49–70) | 56 (53–64) | 0.900 |
| BMI | 23.30 (21.26–24.48) | 22.83 (20.57–25.39) | 24.11 (21.48–27.18) | 23.05 (21.11–25.35) | 0.270 |
| Sex | |||||
| Male | 24 | 42 | 30 | 34 | 0.697 |
| Female | 16 | 31 | 20 | 16 | |
| Hypertension | |||||
| Yes | 11 | 21 | 16 | 20 | 0.533 |
| No | 29 | 52 | 34 | 30 | |
| Diabetes | |||||
| Yes | 5 | 5 | 4 | 8 | 0.364 |
| No | 35 | 68 | 46 | 42 | |
| Catheterization | |||||
| Yes | 12 | 17 | 9 | 14 | 0.534 |
| No | 28 | 56 | 41 | 36 | |
| Ureteral stent | |||||
| Yes | 14 | 18 | 12 | 13 | 0.624 |
| No | 26 | 55 | 38 | 37 | |
| Diagnosis | |||||
| Urolithiasis | 26 | 41 | 28 | 25 | |
| BPH | 4 | 6 | 7 | 10 | |
| Ureterostenosis | 1 | 7 | 4 | 3 | |
| UTI | 2 | 4 | 4 | 0 | |
| Malignant tumor | 5 | 11 | 6 | 8 | |
| Else | 2 | 4 | 1 | 4 | |
BMI, body mass index; BPH, benign prostatic hyperplasia; UTI, urinary tract infection.
Pathogen species distribution
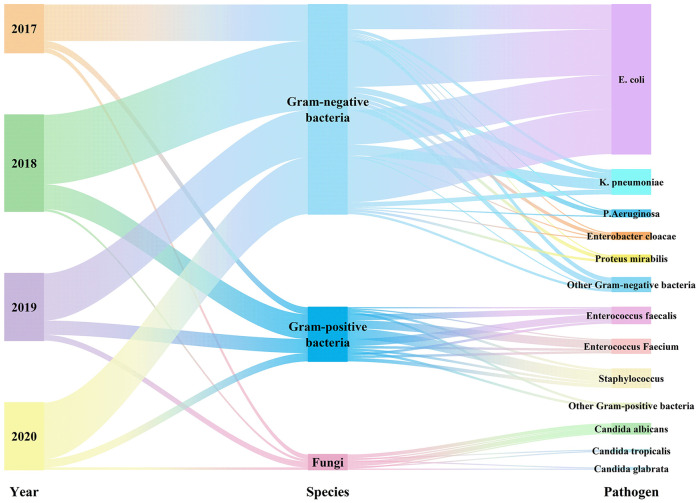
Between the years 2017 and 2020, a total of 237 non-duplicated isolates were obtained from positive blood culture samples. Among these isolates, 178 (75.11%) were identified as Gram-negative bacteria, while 46 (19.41%) were classified as Gram-positive bacterial isolates, and the remaining 13 (5.49%) were fungal isolates. Notably, E. coli accounted for the majority of the Gram-negative bacteria, constituting 53.59%, followed by K. pneumoniae (8.86%, 21/237), Pseudomonas aeruginosa (2.53%, 6/237), P. mirabilis (2.53%, 6/237), and so on. The main Gram-positive bacteria identified in this study were E. faecalis (5.91%, 14/237), E. calcium (5.06%, 12/237), Staphylococcus including Staphylococcus hominis (2.95%, 7/237), S. epidermis (1.68%, 4/237), and S. aureus (0.84%, 2/237), and so on. Candida albicans (3.80%, 9/237) was the most common fungus, followed by C. glabrata (0.84%, 2/237) and C. tropicalis (0.84%, 2/237) (Figure 1 and Supplemental Table 1).
Figure 1.
Distribution of pathogen.
Antimicrobial susceptibility results of pathogenic bacteria
Antibiotic susceptibility of Gram-negative bacteria to antibiotics
The sensitivity rates of ertapenem, meropenem, amikacin, and imipenem to Gram-negative bacteria were all above 85%, which were 92.70%, 91.57%, 89.89%, and 86.52% respectively. Cefepime demonstrated a sensitivity rate of 73.03%, while nitrofurantoin, cefoxitin, ceftazidime, piperacillin/tazobactam, cefoperazone/sulbactam, and amikacin had sensitivity rates of 67.42%, 65.73%, 62.92%, 58.99%, 51.69%, and 51.69%, respectively. All other antibiotics exhibited sensitivity rates below 50% (Table 2).
Table 2.
Antibiotic susceptibility rates in Gram-negative bacteria.
| Antibiotic strain, N (%) | Year | Total (N = 178) | χ2 | p | |||
|---|---|---|---|---|---|---|---|
| 2017 (N = 31) | 2018 (N = 59) | 2019 (N = 40) | 2020 (N = 48) | ||||
| Amoxicillin | 12 (38.71) | 20 (33.90) | 13 (32.5) | 26 (54.17) | 71 (39.89) | 5.893 | 0.117 |
| Amikacin | 28 (90.32) | 54 (91.53) | 36 (90.00) | 42 (87.50) | 160 (89.89) | 0.482 | 0.923 |
| Ampicillin | 0 (0.00) | 2 (3.39) | 1 (2.50) | 3 (6.25) | 6 (3.37) | 2.396 | 0.494 |
| Aztreonam | 13 (41.94) | 24 (40.68) | 25 (62.50) | 30 (62.50) | 92 (51.69) | 8.164 | *0.043 |
| Ceftazidime | 21 (67.74) | 31 (52.54) | 26 (65.00) | 34 (70.83) | 112 (62.92) | 4.395 | 0.222 |
| Ciprofloxacin | 13 (41.94) | 21 (35.59) | 7 (17.50) | 7 (14.58) | 48 (26.97) | 11.314 | *0.010 |
| Cefperazone/sulbactam | 16 (51.61) | 29 (49.15) | 20 (50.00) | 27 (56.25) | 92 (51.69) | 0.598 | 0.897 |
| Ceftriaxone | 8 (25.81) | 15 (25.42) | 14 (35.00) | 20 (41.67) | 57 (32.02) | 3.944 | 0.268 |
| Cefotaxime | 8 (25.81) | 14 (23.73) | 13 (32.50) | 13 (27.08) | 48 (26.97) | 0.957 | 0.812 |
| Cefuroxime | 9 (29.03) | 13 (22.03) | 5 (12.50) | 11 (22.92) | 38 (21.35) | 3.042 | 0.385 |
| Cefazolin | 2 (6.45) | 1 (1.69) | 0 (0.00) | 2 (4.17) | 5 (2.81) | 3.255 | 0.354 |
| ESBL detection | 16 (72.73) | 27 (58.70) | 22 (57.89) | 27 (64.29) | 92 (62.16) | 1.654 | 0.647 |
| Ertapenem | 29 (93.55) | 53 (89.83) | 37 (92.50) | 46 (95.83) | 165 (92.70) | 1.449 | 0.694 |
| Cefepime | 23 (74.19) | 35 (59.32) | 32 (80.00) | 40 (83.33) | 130 (73.03) | 9.255 | *0.026 |
| Cefoxitin | 21 (67.74) | 35 (59.32) | 22 (55.00) | 39 (81.25) | 117 (65.73) | 8.308 | *0.040 |
| Gentamycin | 19 (61.29) | 19 (32.20) | 19 (47.50) | 18 (37.50) | 75 (42.13) | 7.947 | *0.047 |
| Imipenem | 26 (83.87) | 49 (83.05) | 35 (87.50) | 44 (91.67) | 154 (86.52) | 1.918 | 0.590 |
| Levofloxacin | 13 (38.09) | 21 (28.21) | 5 (12.50) | 3 (4.25) | 46 (23.60) | 21.237 | *0.000 |
| Meropenem | 28 (90.32) | 52 (88.14) | 38 (95.00) | 45 (93.75) | 163 (91.57) | 1.870 | 0.600 |
| Nitrofurantoin | 22 (70.97) | 35 (59.32) | 28 (70.00) | 35 (72.92) | 120 (67.42) | 2.720 | 0.437 |
| Piperacillin | 5 (16.13) | 10 (16.95) | 5 (12.50) | 11 (22.92) | 31 (17.42) | 1.727 | 0.631 |
| Ampicillin/sulbactam | 8 (25.81) | 13 (22.03) | 8 (20.00) | 15 (31.25) | 35 (24.72) | 1.827 | 0.609 |
| Compound sulfamethoxazole | 16 (51.61) | 24 (40.68) | 15 (37.50) | 15 (31.25) | 70 (39.33) | 3.375 | 0.337 |
| Tetracycline | 11 (35.48) | 17 (28.81) | 10 (25.00) | 14 (29.17) | 52 (29.21) | 0.937 | 0.816 |
| Piperacillin/tazobactam | 14 (45.16) | 33 (55.93) | 26 (65.00) | 32 (66.67) | 105 (58.99) | 4.445 | 0.217 |
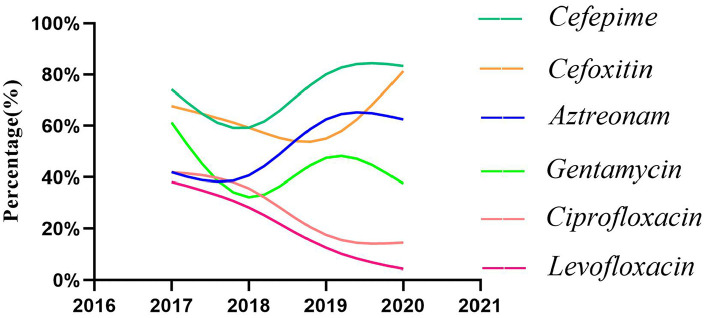
Notably, levofloxacin, gentamicin, and ciprofloxacin exhibited declining sensitivity rates over time (Figure 2). The sensitivity of all other antibiotics is lower than that of p > 0.05.
Figure 2.
Antibiotic susceptibility rates in Gram-negative bacteria.
Antibiotic sensitivity rate of Gram-positive bacteria
In 2020, it was discovered that linezolid-resistant Gram-positive bacteria began to emerge, despite the previously observed high levels of sensitivity of tigecycline, vancomycin, and linezolid against Gram-positive bacteria. Other antibiotics, such as chloramphenicol (89.13%), teicoplanin (89.13%), nitrofurantoin (71.74%), quinoptin (63.04%), and gentamicin (56.52%), exhibited higher sensitivity. Conversely, the sensitivity of Gram-positive bacteria to erythromycin (10.87%), clindamycin (17.39%), and cotrimoxazole (21.74%) was notably low, with cotrimoxazole sensitivity decreasing annually (p < 0.05) (Supplemental Table 2).
Antibiotic sensitivity of fungi
During the 4 years, the number of fungi detected in blood culture was small, and the overall sensitivity rate of fungi to antibiotics was high. Only one C. tropicalis isolate was found to be resistant to itraconazole and moderately sensitive to voriconazole. In addition, one C. albicans to itraconazole was moderately sensitive (Supplemental Table 3).
Discussion
Urinary tract infections (UTIs) are a common infectious disease, which has great global influence, affecting about 130 million to 175 million people every year. 13 It has been observed that the incidence of urosepsis, a serious complication of urinary tract infection (UTI), has steadily increased, which may be due to the increasing application of endourological lithotripsy and the long-term use of prolonged utilization of catheters and ureteral stents.14,15 These invasive operations will damage the normal defense mechanism of the body and bring bacteria from external into the urinary system. The formation of a biofilm by the pathogen within and around urethral catheters is associated with an elevated risk of bacterial ascending infection, facilitating the entry of bacteria into the circulatory system and subsequently leading to sepsis.16,17 Once a UTI has progressed to urosepsis, rapid and effective treatment is essential. The timely administration of appropriate antibiotics is essential to improve the prognosis of sepsis. Studies have shown that the risk of death will increase by 7.6% for every hour of delay in the start of antibiotic treatment. 18
Blood cultures remain the preferred method for diagnosing urosepsis due to their consistent ability to identify the specific pathogen responsible for the infection and determine its antibiotic sensitivity. 19 In this study, a total of 237 blood culture-positive isolates were obtained from 213 patients with urosepsis. Among these patients, the culture results of 189 people showed that there was a single bacterium, while the culture results of 24 patients showed the existence of two kinds of bacteria. Gram-negative bacilli were the main pathogens causing urosepsis, accounting for 178 isolates (75.11%), followed by Gram-positive bacilli with 46 isolates (19.41%). Fungal pathogens were found to be less prevalent in causing urosepsis (13 isolates, 5.48%). In contrast to the ratio of Gram-negative bacteria to Gram-positive bacteria documented by the China Antibiotic Resistance Surveillance System and China Antibiotic Surveillance Network in 2018, which approximated 7:3, 20 the present study observed a ratio of approximately 8:2. This discrepancy can be attributed to the fact that the samples analyzed in this study were obtained from patients with sepsis resulting from UTIs, which are predominantly caused by Gram-negative bacteria. 21 Therefore, compared with the reported proportion, the detection rate of Gram-negative bacteria in this study is higher.
Consistent with prior studies, the principal pathogen implicated in urosepsis was E. coli. 22 Of the 178 isolates of Gram-negative bacilli uncovered, E. coli constituted a predominant 71.35% (127/178), single-handedly representing over half of all positive blood cultures, a finding that dovetails with the work by Rhee et al. 23 Furthermore, K. pneumoniae and P. aeruginosa were identified to account for 11.80% (21/178) and 3.37% (6/178) of the isolates, respectively, mirroring patterns observed in previous reports. 24 Analysis revealed the presence of 46 isolates of Gram-positive bacteria, encompassing 26 isolates of Enterococcus (56.52%), 14 isolates of E. faecalis (30.43%), 12 isolates of E. faecium (26.09%), and 20 isolates of other Gram-positive cocci, which included 5 isolates of S. hominis and 4 isolates of S. epidermidis. In contrast to the findings reported by Zhu, 25 no significant changes were observed in the incidence of Gram-positive bacteria. This disparity may be attributed to the relatively shorter duration of data collection. By continuing to collect the blood culture results in the following years, it may be better to explain the changing trend of Gram-positive bacteria in urosepsis. Among the 13 fungal isolates identified, C. albicans emerged as the most prevalent.
Gram-negative bacteria exhibited the highest susceptibility to carbapenems. In addition to carbapenems, the sensitivity of cefepime, cefoxitin, and ceftazidime was higher, which were 73.03%, 65.73%, and 62.92% respectively. The sensitivities of cefazolin, ampicillin, piperacillin, cefuroxime, and levofloxacin were low, and the average sensitivity rates were 2.81%, 3.37%, 17.42%, 21.35%, and 23.60%, respectively. Notably, levofloxacin is recommended as a primary treatment for uncomplicated UTIs and pyelonephritis according to clinical guidelines. In 2017, the sensitivity rate to urosepsis was 38.09%, but by 2020 it was only 4.25%. The sensitivity rate of the same kind of antibiotic ciprofloxacin to Gram-negative bacteria has been decreasing year by year, with an average of 26.97%. The research conducted by Tumbarello et al. 26 shows that the use of fluoroquinolones as initial antimicrobial agents in sepsis caused by Enterobacteriaceae bacteria is associated with higher treatment failure rate and mortality. These findings indicate that fluoroquinolone antibiotics may not be appropriate for empirical treatment of urosepsis. In addition, the antibiotic sensitivity curves of aztreonam, cefepime, and cefoxitin showed an upward trend, but fluctuated greatly, which may be related to the insufficient sample size of the study.
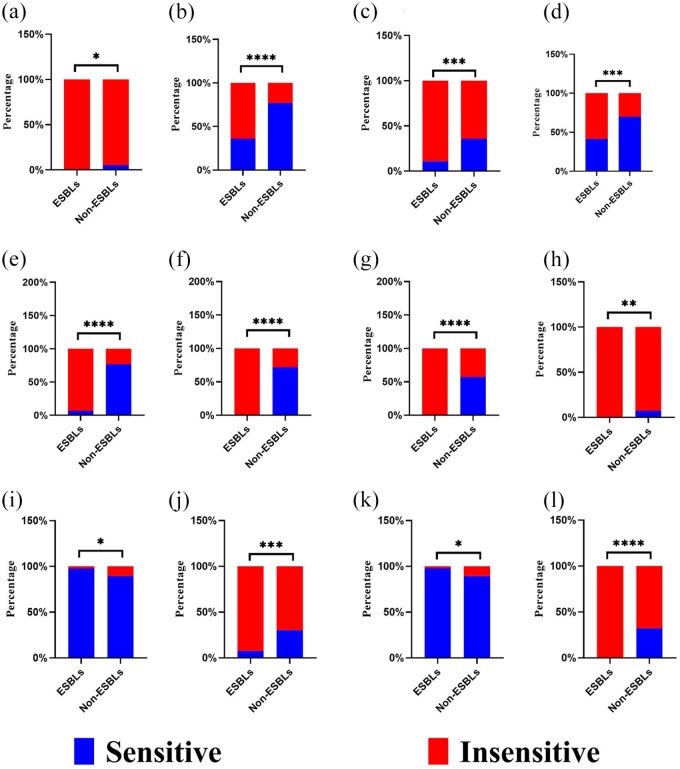
Bacterial resistance pertains to the ability of bacteria to withstand the effects of antimicrobial drugs. For instance, E. coli and K. pneumoniae can develop resistance to drugs by producing extended-spectrum β-lactamases (ESBLs). Among a total of 178 isolates of Gram-negative bacteria, 92 isolates were found to produce ESBL, resulting in an overall detection rate of 51.68% (92/178). Specifically, 81 isolates of E. coli accounted for 88.04% (81/92) of the ESBL-producing bacteria, while 11 isolates of K. pneumoniae constituted the remaining 11.96% (11/92). The detection rates of ESBL in E. coli and K. pneumoniae were 63.78% (81/127) and 52.38% (11/21), respectively. There was a significant difference in antimicrobial susceptibility between ESBL-producing bacteria and non-ESBL-producing bacteria (Figure 3 and Supplemental Table 4). The sensitivity of ESBL isolates to antibiotics such as ampicillin, cefotaxime, cefuroxime sodium, cefazolin, and piperacillin was found to be 0%. However, the sensitivity of non-ESBL isolates to cefotaxime and cefuroxime sodium remained above 50%, with a statistically significant difference (p < 0.05). This discrepancy in sensitivity can be attributed to the production of β-lactamases by the bacteria. Furthermore, the sensitivity of ampicillin, cefazolin, ampicillin/sulbactam, and tetracycline antibiotics to both ESBL and non-ESBL bacteria was all below 30%. These findings suggest that the aforementioned antibiotics may not be suitable for empirical treatment of urosepsis in the absence of accurate culture results.
Figure 3.
Drug susceptibility of ESBL and non-ESBL. (a) Ampicillin, (b) aztreonam, (c) ciprofloxacin, (d) cefoperazone/sulbactam, (e) ceftriaxone, (f) cefotaxime, (g) cefuroxime, (h) cefazolin, (i) ertapenem, (j) levofloxacin, (k) meropenem, and (l) piperacillin.
ESBL, extended-spectrum β-lactamase.
The selection of appropriate antibiotics for the treatment of urosepsis caused by ESBL and non-ESBL isolates is a significant concern among clinicians. The susceptibility of ESBL isolates to ertapenem, meropenem, imipenem, and amikacin exceeded 90%, suggesting that carbapenems remain the primary therapeutic option for urosepsis caused by ESBL isolates. Previous studies have reported that piperacillin sodium/tazobactam and amikacin exhibit high sensitivity toward ESBL isolates. 27 The susceptibility rates of piperacillin sodium/tazobactam to both ESBL and non-ESBL isolates were 58.7% and 60.71%, respectively, while the susceptibility rates of amikacin to ESBL and non-ESBL isolates were 94.57% and 91.07%, respectively. Consequently, piperacillin sodium/tazobactam and amikacin can be considered viable treatment options. In addition, the sensitivity rates of cefoxitin and cefepime were 71.74% and 67.39%, respectively, which made them suitable choices for the treatment of urosepsis caused by ESBL-producing bacteria. However, the use of cephalosporins in clinical environments is becoming more and more common, which may lead to a decline in the curative effect of such antibiotics. Clinicians can avoid using the third and fourth generations of cephalosporins as routine treatment drugs for UTIs, thus reducing the speed of bacterial resistance to the third and fourth generations of cephalosporins and reducing the emergence of ESBL-producing isolates.
Apart from ESBL, another group of bacteria worthy of attention is carbapenem-resistant Enterobacteriaceae (CRE), which are resistant to all carbapenem antibiotics. The emergence of CRE isolates was initially documented in the 1990s. Since then, carbapenemase-producing Enterobacteriaceae isolates have disseminated globally. Notably, the prevalence of the CRE population has experienced a substantial surge in recent years, including in China.28,29 A total of 16 isolates of CRE were identified, accounting for (16/178) of the Gram-negative bacteria. Among these, there were eight isolates of K. pneumoniae (8/16) and three isolates of P. aeruginosa (3/16) (Table 3). In addition, one strain of Acinetobacter baumannii, E. arabii, K. oxytoca, Providencia, and E. cloacae was also detected. According to previous studies, carbapenem-resistant bacteria are more common in K. pneumoniae. 30 This observation aligns with the findings of our study. K. pneumoniae displayed significantly lower sensitivity to carbapenems compared to E. coli, and it consistently exhibited resistance to carbapenems each year. Notably, amikacin demonstrated effective treatment for carbapenem-resistant K. pneumoniae infection. 27 Our results showed that amikacin was the most sensitive to CRE, and 9 of the 16 CRE isolates were sensitive to amikacin. However, amikacin is nephrotoxic, and renal function should be closely monitored when it is used in patients with severe sepsis or septic shock. 31 The scarcity of CRE treatment schemes reflects the urgency of developing new antibiotics to treat it.
Table 3.
Antibiotic susceptibility in carbapenem-resistant Enterobacteriaceae.
| Antibiotic strain, N | Year | Total (N = 16) | χ2 | p | |||
|---|---|---|---|---|---|---|---|
| 2017 (N = 4) | 2018 (N = 7) | 2019 (N = 2) | 2020 (N = 3) | ||||
| Amoxicillin | 0 | 0 | 0 | 0 | 0 | – | – |
| Amikacin | 1 | 6 | 1 | 1 | 9 | 4.729 | 0.193 |
| Ampicillin | 0 | 0 | 0 | 1 | 1 | 4.622 | 0.202 |
| Aztreonam | 1 | 0 | 0 | 1 | 2 | 3.048 | 0.384 |
| Ceftazidime | 0 | 0 | 0 | 1 | 1 | 4.622 | 0.202 |
| Ciprofloxacin | 0 | 1 | 0 | 1 | 1 | 1.371 | 0.712 |
| Cefperazone/sulbactam | 0 | 1 | 0 | 1 | 1 | 1.371 | 0.712 |
| Ceftriaxone | 0 | 0 | 1 | 0 | 1 | 7.467 | 0.058 |
| Cefotaxime | 0 | 0 | 0 | 0 | 0 | – | – |
| Cefuroxime | 0 | 0 | 0 | 0 | 0 | – | – |
| Cefazolin | 0 | 0 | 0 | 0 | 0 | – | – |
| Ertapenem | 0 | 0 | 0 | 0 | 0 | – | – |
| Cefepime | 0 | 1 | 1 | 1 | 3 | 2.716 | 0.438 |
| Cefoxitin | 0 | 0 | 0 | 0 | 0 | – | – |
| Gentamycin | 0 | 5 | 0 | 0 | 5 | 9.351 | 0.025 |
| Imipenem | 0 | 0 | 0 | 0 | 0 | – | – |
| Levofloxacin | 0 | 1 | 0 | 0 | 1 | 1.371 | 0.712 |
| Meropenem | 0 | 0 | 0 | 0 | 0 | – | – |
| Nitrofurantoin | 1 | 0 | 1 | 0 | 2 | 4.571 | 0.206 |
| Piperacillin | 0 | 0 | 0 | 1 | 1 | 4.622 | 0.202 |
| Ampicillin/sulbactam | 0 | 0 | 0 | 0 | 0 | – | – |
| Compound sulfamethoxazole | 1 | 1 | 0 | 0 | 2 | 1.306 | 0.728 |
| Tetracycline | 1 | 0 | 0 | 1 | 2 | 3.048 | 0.384 |
| Piperacillin/tazobactam | 0 | 2 | 0 | 1 | 3 | 2.247 | 0.523 |
E. faecium and E. faecalis are the predominant Gram-positive bacteria responsible for urosepsis. In this study, 46 isolates of Gram-positive bacteria were identified, accounting for 19.4% (46/237) of the total bacterial isolates. Of all the Gram-positive bacteria, E. faecalis accounted for 30.34% (14/46) and E. faecium accounted for 26.08% (12/46), which is consistent with Elena et al.’s findings. 32 It is worth noting that all Gram-positive bacteria isolated from 2017 to 2019 exhibited sensitivity to tigecycline, vancomycin, and linezolid, and no resistant isolates were detected. However, in 2020, the first linezolid-resistant isolate emerged. Linezolid is typically employed for severe infections caused by multidrug-resistant Gram-positive bacteria, including vancomycin-resistant Enterococci. 33 In the case of linezolid-resistant Gram-positive cocci, our results suggest that tigecycline can be considered as a last resort treatment. The sensitivity rates for other antibiotics against Gram-positive bacteria, such as teicoplanin, nitrofurantoin, quinupristin/dalfopristin, and gentamicin were 89.13%, 71.74%, 63.04%, and 56.52%, respectively. In our study, the sensitivity rate of nitrofurantoin to Gram-positive bacteria was 71.74%, and it also had a definite curative effect on vancomycin-resistant Enterococci. 34 However, it is basically excreted from the kidney and has a high concentration in urine, so it is more suitable for the treatment of UTI. But nitrofurantoin exhibits heightened sensitivity in both Gram-negative and Gram-positive bacteria, making it a potential choice for community UTIs. Although chloramphenicol demonstrated higher sensitivity in this study, its use is uncommon for urosepsis due to its toxic side effects, particularly on the hematological system. Moreover, the frequency of chloramphenicol use in China is low, and the rate of bacterial resistance to chloramphenicol is slow, which is one of the reasons for the high sensitivity of chloramphenicol. Other antibiotics, such as erythromycin (10.87%), clindamycin (17.39%), co-trimoxazole (21.74%), and ampicillin (36.96%) displayed lower sensitivity, with co-trimoxazole sensitivity decreasing year by year (p < 0.05). None of these antibiotics are suitable for empirical treatment of urosepsis caused by Gram-positive cocci. Compared to Gram-negative bacilli, Gram-positive bacteria have fewer available antimicrobial options.
Urosepsis caused by fungal pathogens is a relatively uncommon occurrence, but it still accounts for a certain proportion. Our research shows that C. albicans is still the most common pathogen of hospital-acquired fungal infections. We identified 13 fungal isolates (5.49%, 13/237), encompassing 9 C. albican isolates, with C. tropicalis and C. glabrata accounting for 2 isolates each. Most of these isolated fungi showcased substantial susceptibility toward antifungal agents. Since 2019, we have observed itraconazole-resistant C. glabrata isolates, with a C. albican isolate demonstrating intermediate sensitivity to itraconazole. Such trends warn us of the potential rise of fungal resistance, warranting continuous surveillance. The results also show that fluconazole is a commonly used and effective drug for fungal urosepsis.
Our present study is not without limitations. First, it is a single-center study. Second, it is a retrospective study, so it has limitations. In the future, conducting multi-center and prospective studies may be the way to address these limitations. Considering that our investigation is an observational retrospective study, a power analysis for sample size calculation was not executed, a common characteristic of this type of research design.
Conclusion
Analysis of blood culture results of patients more accurately reflected the etiology of urosepsis, mainly E. coli, Enterococcus, and K. pneumoniae. Empiric treatment of urosepsis should not include fluoroquinolone antibiotics without definitive blood culture results. Cefepime, cefoxitin, and ceftazidime are the most sensitive antibiotics to Gram-negative bacteria besides carbapenem antibiotics. In addition, the current situation regarding ESBL-producing bacteria and CRE bacteria resistance is extremely concerning with limited therapeutic options available. Strengthening antibiotic management practices and exploring novel antibacterial agents can help mitigate this issue.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-2-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-3-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-4-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Acknowledgments
None.
Footnotes
ORCID iD: Jiong-ming Li  https://orcid.org/0000-0001-8680-3872
https://orcid.org/0000-0001-8680-3872
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yu-yun Wu, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Pei Li, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Zi-ye Huang, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Jian-he Liu, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Bo-wei Yang, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Wen-bo Zhou, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Fei Duan, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, P. R. China.
Guang Wang, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, No. 374 Dian-Mian Avenue, Kunming, Yunnan, 650101, P. R. China.
Jiong-ming Li, The Department of Urology, The Second Affiliated Hospital of Kunming Medical University, No. 374 Dian-Mian Avenue, Kunming, Yunnan, 650101, P. R. China.
Declarations
Ethics approval and consent to participate: This study was approved by the Ethics Committee of the Second Affiliated Hospital of Kunming Medical University (approval number: 审-PJ-科-2023-153). Because this is a retrospective study, the Ethics Committee waived the requirement for patient informed consent.
Consent for publication: Not applicable.
Author contributions: Yu-yun Wu: Data curation; Writing – original draft.
Pei Li: Writing – review & editing.
Zi-ye Huang: Writing – review & editing.
Jian-he Liu: Writing – review & editing.
Bo-wei Yang: Methodology; Resources; Writing – review & editing.
Wen-bo Zhou: Methodology; Resources.
Fei Duan: Methodology; Resources.
Guang Wang: Writing – review & editing.
Jiong-ming Li: Funding acquisition; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Yunnan Provincial Science and Technology Department/Yunnan Medical Science Specialist Training Project (grant no. H-2017045), and the Yunnan Provincial Science and Technology Department Zhangqun Ye Expert’s Workstation (grant no. 202105AF150063).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Please contact the corresponding author if required.
References
- 1. Bauer M, Gerlach H, Vogelmann T, et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care 2020; 24: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020; 395: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang Y, Li J, Zhang Y, et al. Clinical situations of bacteriology and prognosis in patients with urosepsis. Biomed Res Int 2019; 2019: 3080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wagenlehner FM, Pilatz A, Weidner W. Urosepsis-from the view of the urologist. Int J Antimicrob Agents 2011; 38: 51–57. [DOI] [PubMed] [Google Scholar]
- 5. Tandoğdu Z, Bartoletti R, Cai T, et al. Antimicrobial resistance in urosepsis: outcomes from the multinational, multicenter global prevalence of infections in urology (GPIU) study 2003-2013. World J Urol 2016; 34: 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grabe MJ, Resman F. Antimicrobial stewardship: what we all just need to know. Eur Urol Focus 2019; 5: 46–49. [DOI] [PubMed] [Google Scholar]
- 7. Flaherty SK, Weber RL, Chase M, et al. Septic shock and adequacy of early empiric antibiotics in the emergency department. J Emerg Med 2014; 47: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute. CLSI document M100S. Performance standards for antimicrobial susceptibility testing, twenty-sixth informational supplement[S]. Wayne, PA: CLSI, 2016. [Google Scholar]
- 10. Clinical and Laboratory Standards Institute. CLSI document M100S. Performance standards for antimicrobial susceptibility testing, twenty-seventh informational supplement[S]. Wayne, PA: CLSI, 2017. [Google Scholar]
- 11. Clinical and Laboratory Standards Institute. CLSI document M100S. Performance standards for antimicrobial susceptibility testing, twenty-eight informational supplement[S]. Wayne, PA: CLSI, 2018. [Google Scholar]
- 12. Clinical and Laboratory Standards Institute. CLSI document M100S. Performance standards for antimicrobial susceptibility testing, twenty-nine informational supplement[S]. Wayne, PA: CLSI, 2019. [Google Scholar]
- 13. Asadi Karam MR, Habibi M, Bouzari S. Urinary tract infection: pathogenicity, antibiotic resistance and development of effective vaccines against uropathogenic Escherichia coli. Mol Immunol 2019; 108: 56–67. [DOI] [PubMed] [Google Scholar]
- 14. Wu H, Wang Z, Zhu S, et al. Uroseptic shock can be reversed by early intervention based on leukocyte count 2 h post-operation: animal model and multicenter clinical cohort study. Inflammation 2018; 41: 1835–1841. [DOI] [PubMed] [Google Scholar]
- 15. Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 16. Scotland KB, Lo J, Grgic T, et al. Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling 2019; 35: 117–127. [DOI] [PubMed] [Google Scholar]
- 17. Yuan F, Huang Z, Yang T, et al. Pathogenesis of proteus mirabilis in catheter-associated urinary tract infections. Urol Int 2021; 105: 354–361. [DOI] [PubMed] [Google Scholar]
- 18. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 19. Bromiker R, Elron E, Klinger G. Do neonatal infections require a positive blood culture? Am J Perinatol 2020; 37(Suppl. 2): S18–S21. [DOI] [PubMed] [Google Scholar]
- 20. Hu F, Zhu D, Wang F, et al. Current status and trends of antibacterial resistance in China. Clin Infect Dis 2018; 67(Suppl_2): S128–S134. [DOI] [PubMed] [Google Scholar]
- 21. Yang H, Smith RD, Sumner KP, et al. A matrix-assisted laser desorption ionization-time of flight mass spectrometry direct-from-urine-specimen diagnostic for Gram-negative pathogens. Microbiol Spectr 2022; 10: e0373022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonkat G, Cai T, Veeratterapillay R, et al. Management of urosepsis in 2018. Eur Urol Focus 2019; 5: 5–9. [DOI] [PubMed] [Google Scholar]
- 23. Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open 2020; 3: e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shields RK, Zhou Y, Kanakamedala H, et al. Burden of illness in US hospitals due to carbapenem-resistant gram-negative urinary tract infections in patients with or without bacteraemia. BMC Infect Dis 2021; 21: 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Q, Yue Y, Zhu L, et al. Epidemiology and microbiology of Gram-positive bloodstream infections in a tertiary-care hospital in Beijing, China: a 6-year retrospective study. Antimicrob Resist Infect Control 2018; 7: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tumbarello M, Sanguinetti M, Montuori E, et al. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51: 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodrigues D, Baldissera GS, Mathos D, et al. Amikacin for the treatment of carbapenem-resistant Klebsiella pneumoniae infections: clinical efficacy and toxicity. Braz J Microbiol 2021; 52: 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lutgring JD. Carbapenem-resistant enterobacteriaceae: an emerging bacterial threat. Semin Diagn Pathol 2019; 36: 182–186. [DOI] [PubMed] [Google Scholar]
- 29. Wang M, Earley M, Chen L, et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): a prospective, multicentre, cohort study. Lancet Infect Dis 2022; 22: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect 2019; 25: 943–950. [DOI] [PubMed] [Google Scholar]
- 31. Zohar I, Schwartz O, Yossepowitch O, et al. Aminoglycoside versus carbapenem or piperacillin/tazobactam treatment for bloodstream infections of urinary source caused by gram-negative ESBL-producing enterobacteriaceae. J Antimicrob Chemother 2020; 75: 458–465. [DOI] [PubMed] [Google Scholar]
- 32. Rosselli Del Turco E, Bartoletti M, Dahl A, et al. How do I manage a patient with enterococcal bacteraemia? Clin Microbiol Infect 2021; 27: 364–371. [DOI] [PubMed] [Google Scholar]
- 33. Liu BG, Yuan XL, He DD, et al. Research progress on the oxazolidinone drug linezolid resistance. Eur Rev Med Pharmacol Sci 2020; 24: 9274–9281. [DOI] [PubMed] [Google Scholar]
- 34. Mercuro NJ, Davis SL, Zervos MJ, et al. Combatting resistant enterococcal infections: a pharmacotherapy review. Expert Opin Pharmacother 2018; 19: 979–992. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-2-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-3-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease
Supplemental material, sj-docx-4-tai-10.1177_20499361241248058 for Four-year variation in pathogen distribution and antimicrobial susceptibility of urosepsis: a single-center retrospective analysis by Yu-yun Wu, Pei Li, Zi-ye Huang, Jian-he Liu, Bo-wei Yang, Wen-bo Zhou, Fei Duan, Guang Wang and Jiong-ming Li in Therapeutic Advances in Infectious Disease