Abstract
Background
Muscle cramps can occur anywhere and for many reasons. Quinine has been used to treat cramps of all causes. However, controversy continues about its efficacy and safety. This review was first published in 2010 and searches were updated in 2014.
Objectives
To assess the efficacy and safety of quinine‐based agents in treating muscle cramps.
Search methods
On 27 October 2014 we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL, MEDLINE and EMBASE. We searched reference lists of articles up to 2014. We also searched for ongoing trials in November 2014.
Selection criteria
Randomised controlled trials of people of all ages with muscle cramps in any location and of any cause, treated with quinine or its derivatives.
Data collection and analysis
Three review authors independently selected trials for inclusion, assessed risk of bias and extracted data. We contacted study authors for additional information. For comparisons including more than one trial, we assessed the quality of the evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Main results
We identified 23 trials with a total of 1586 participants. Fifty‐eight per cent of these participants were from five unpublished studies. Quinine was compared to placebo (20 trials, n = 1140), vitamin E (four trials, n = 543), a quinine‐vitamin E combination (three trials, n = 510), a quinine‐theophylline combination (one trial, n = 77), and xylocaine injections into the gastrocnemius muscle (one trial, n = 24). The most commonly used quinine dosage was 300 mg/day (range 200 to 500 mg). We found no new trials for inclusion when searches were updated in 2014.
The risk of bias in the trials varied considerably. All 23 trials claimed to be randomised, but only a minority described randomisation and allocation concealment adequately.
Compared to placebo, quinine significantly reduced cramp number over two weeks by 28%, cramp intensity by 10%, and cramp days by 20%. Cramp duration was not significantly affected.
A significantly greater number of people suffered minor adverse events on quinine than placebo (risk difference (RD) 3%, 95% confidence interval (CI) 0% to 6%), mainly gastrointestinal symptoms. Overdoses of quinine have been reported elsewhere to cause potentially fatal adverse effects, but in the included trials there was no significant difference in major adverse events compared with placebo (RD 0%, 95% CI ‐1% to 2%). One participant suffered from thrombocytopenia (0.12% risk) on quinine.
A quinine‐vitamin E combination, vitamin E alone, and xylocaine injections into gastrocnemius were not significantly different to quinine across all outcomes, including adverse effects. Based on a single trial comparison, quinine alone was significantly less effective than a quinine‐theophylline combination but with no significant differences in adverse events.
Authors' conclusions
There is low quality evidence that quinine (200 mg to 500 mg daily) significantly reduces cramp number and cramp days and moderate quality evidence that quinine reduces cramp intensity. There is moderate quality evidence that with use up to 60 days, the incidence of serious adverse events is not significantly greater than for placebo in the identified trials, but because serious adverse events can be rarely fatal, in some countries prescription of quinine is severely restricted.
Evidence from single trials suggests that theophylline combined with quinine improves cramps more than quinine alone, and the effects of xylocaine injections into gastrocnemius are not significantly different to quinine across all outcomes. Low or moderate quality evidence shows no significant difference between quinine and vitamin E or quinine and quinine‐vitamin E mixture. Further research into these alternatives, as well other pharmacological and non‐pharmacological treatments, is thus warranted.
There is no evidence to judge optimal dosage or duration of quinine treatment. Further studies using different dosages and measurement of serum quinine levels will allow a therapeutic range to be defined for muscle cramp. Because serious adverse events are not common, large population studies are required to more accurately inform incidence. Longer lengths of follow‐up in future trials will help determine the duration of action following cessation of quinine as well as long‐term adverse events. The search for new therapies, pharmacological and nonpharmacological, should continue and further trials should compare vitamin E, quinine‐vitamin E combination, and quinine‐theophylline mixture with quinine.
Keywords: Humans; Drug Therapy, Combination; Lidocaine; Lidocaine/therapeutic use; Muscle Cramp; Muscle Cramp/drug therapy; Muscle Relaxants, Central; Muscle Relaxants, Central/adverse effects; Muscle Relaxants, Central/therapeutic use; Quinine; Quinine/adverse effects; Quinine/therapeutic use; Randomized Controlled Trials as Topic; Theophylline; Theophylline/therapeutic use; Vitamin E; Vitamin E/therapeutic use; Vitamins; Vitamins/therapeutic use
Plain language summary
Quinine for muscle cramps
Review question
We reviewed the evidence about the effect of quinine on muscle cramps.
Background
Muscle cramps can occur anywhere and in anyone; however, leg cramps are especially common in older people. Quinine is a medicine which has been used to treat cramps for many years. There is conflicting evidence for its ability to reduce cramps. Quinine can cause serious, even fatal adverse events, especially in overdosage.
Study characteristics
This review includes 23 trials, with 1586 participants. The trials compared quinine or quinine‐based medicines against inactive treatment (placebo) or other active treatments. We found no new studies when we searched the medical literature again and updated the review in 2014.
Key results and quality of the evidence
The risk of bias in the included trials varied considerably. All 23 trials claimed to be randomised, but many failed to clearly describe how participants were assigned to treatments. There is low quality evidence that quinine (200 mg to 500 mg daily) significantly reduces cramp number and cramp days and moderate quality evidence that quinine reduces cramp intensity. There is moderate quality evidence that there are more minor adverse events with quinine compared to placebo but no increase in major adverse events. However, there are reliable reports from other sources that an overdose of quinine can cause serious harm including death.
Low or moderate quality evidence shows there is no significant difference when comparing quinine to vitamin E or to a quinine‐vitamin E mixture. There is evidence from one trial that theophylline combined with quinine improves cramps more than quinine alone. In a single trial there was no significant difference when comparing quinine to xylocaine injections.
More research is needed to clarify the best dose and duration of treatment, as well as alternatives to quinine for cramps.
The evidence is current to October 2014.
Summary of findings
Summary of findings for the main comparison. Quinine versus placebo for muscle cramps.
| Quinine for muscle cramps | ||||||
| Patient or population: people with muscle cramps Settings: mainly outpatients Intervention: quinine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Quinine | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was 8.8 cramps | The mean number of cramps over 2 weeks in the intervention groups was 2.45 lower (1.36 to 3.54 lower) | 952 (13 studies) | ⊕⊕⊝⊝ low1 | The difference was statistically significant. | |
| Cramp intensity (on 3‐point scale; 1 = mild; 2 = moderate; 3 = severe) | The mean cramp intensity in the control groups was 1.2 units | The mean cramp intensity in the intervention groups was 0.12 lower (0.2 to 0.05 lower) | 666 (7 studies) | ⊕⊕⊕⊝ moderate1 | The difference was statistically significant. | |
| Participants suffering major adverse events | 14 per 1000 | 15 per 1000 (4 to 35) | See comment | 1103 (18 studies) | ⊕⊕⊕⊝ moderate2 | Risks were calculated from pooled risk differences. The difference was not statistically significant. |
| Participants suffering minor adverse events | 94 per 1000 | 127 per 1000 (94 to 154) | See comment | 969 (16 studies) | ⊕⊕⊕⊝ moderate3 | Risks were calculated from pooled risk differences. The difference was statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1There were significant shortcomings in study design in some trials, but the majority of those included in this meta‐analysis were of moderate to high quality, warranting a single downgrading for limitations in design and implementation. We further downgraded the evidence for this outcome to low quality because of the heterogeneity of the results.2Major adverse events were defined as those being severe enough to warrant participant withdrawal from the trial. As specific hypersensitivity reactions are so rare, larger studies are needed to clarify the incidence of such adverse events in particular. Some trials did not prespecify adverse events as an outcome but simply reported them retrospectively, thus compromising slightly on the quality of evidence. 3Minor adverse events were defined as being those that did not warrant participant withdrawal from the trial. Some trials did not prespecify adverse events as an outcome but simply reported them retrospectively, thus compromising slightly on the quality of evidence. Otherwise, a well‐reported outcome.
Summary of findings 2. Quinine versus vitamin E for muscle cramps.
| Quinine versus vitamin E for muscle cramps | ||||||
| Patient or population: people with muscle cramps Settings: outpatients Intervention: quinine versus vitamin E | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Vitamin E | Quinine | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was 7.22 | The mean number of cramps over 2 weeks in the intervention groups was 0.24 lower (1.29 lower to 0.81 higher) | 513 (3 studies) | ⊕⊕⊝⊝ low1,2 | The difference was not statistically significant. | |
| Cramp intensity (on 3‐point scale; 1 = mild; 2 = moderate; 3 = severe) | The mean cramp intensity in the control groups was 1.04 units | The mean cramp intensity in the intervention groups was 0.06 lower (0.17 lower to 0.04 higher) | 513 (3 studies) | ⊕⊕⊕⊝ moderate1 | The difference was not statistically significant. | |
| Participants suffering major adverse events | 3 per 1000 | 9 per 1000 (‐8 to 25) | See comment | 513 (3 studies) | ⊕⊕⊕⊝ moderate1 | Risks were calculated from pooled risk differences. The difference between the 2 groups was not statistically significant. |
| Participants suffering minor adverse events | 167 per 1000 | 189 per 1000 (127 to 257) | See comment | 483 (2 studies) | ⊕⊕⊕⊝ moderate3 | Risks were calculated from pooled risk differences. The difference between the 2 groups was not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Only 3 trials were available for this comparison, 2 of which were conducted by pharmaceutical investigators on behalf of manufacturers of quinine. A deficiency in the design of 1 of these trials meant that there was only a 2‐day washout between cross‐over treatments. 2The effect on cramp number was inconsistent among the 3 included trials. 3Only 2 studies were available for this comparison; 1 of them having a very short washout period (2 days) between treatments.
Summary of findings 3. Quinine versus a quinine‐vitamin E combination (Q‐Vel) for muscle cramps.
| Quinine versus a quinine‐vitamin E combination (Q‐Vel) for muscle cramps | ||||||
| Patient or population: people with muscle cramps Settings: outpatients Intervention: quinine versus a quinine‐vitamin E combination (Q‐Vel) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Quinine‐vitamin E combination (Q‐Vel) | Quinine | |||||
| Number of cramps over 2 weeks | The mean number of cramps over 2 weeks in the control groups was 8.37 | The mean number of cramps over 2 weeks in the intervention groups was 1.07 higher (1.08 lower to 3.23 higher) | 486 (2 studies) | ⊕⊕⊝⊝ low1,2 | The difference was not statistically significant. | |
| Cramp intensity (on 3‐point scale; 1 = mild; 2 = moderate; 3 = severe) | The mean cramp intensity in the control groups was 0.87 units | The mean cramp intensity in the intervention groups was 0.1 higher (0.06 lower to 0.26 higher) | 510 (3 studies) | ⊕⊕⊝⊝ low3,4 | The difference was not statistically significant. | |
| Participants suffering major adverse events | 8 per 1000 | 8 per 1000 (‐2 to 18) | See comment | 510 (3 studies) | ⊕⊕⊕⊝ moderate3 | Risks were calculated from pooled risk differences. |
| Participants suffering minor adverse events | 173 per 1000 | 202 per 1000 (133 to 273) | See comment | 510 (3 studies) | ⊕⊕⊕⊝ moderate3 | Risks were calculated from pooled risk differences. The difference was not statistically significant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The results for cramp number in these 2 trials were not consistent, each suggesting opposite effects. 2Only 2 studies were available for this comparison. Both were conducted by pharmaceutical investigators on behalf of manufacturers of quinine and the quinine‐vitamin E combination. 3All 3 trials were conducted by pharmaceutical companies who manufacture quinine and the quinine‐vitamin E combination. 4There was no consistency between the results for intensity in these 3 trials.
Background
Description of the condition
Muscle cramps are sudden episodes of painful involuntary muscle contractions that may be visible or palpable (Baldissera 1994). Episodes may last seconds or several minutes but are usually self limiting. The severity and duration of each attack varies from person to person, as does their frequency. They are a common distressing occurrence in elderly people. It has been estimated that between 33% and 50% of elderly people suffer from regular leg cramps (Abdulla 1999; Naylor 1994). Muscle cramps can occur at any time and in any body part, but frequently they occur at night in the legs. Most cases seem to be idiopathic (with no obvious underlying cause).
There are no explicit diagnostic criteria for muscle cramp or indeed a universally accepted definition. Despite its frequency and associated morbidity there has been relatively little research into its cause, treatment or prevention.
The aetiology of muscle cramp is unknown, and the many proposed mechanistic causes are speculative. Two principal mechanisms have been proposed. In one, the motor nerve terminals are abnormally excitable; in the other, groups of anterior horn cells may be unstable due to spinal disinhibition leading to explosive hyperactivity of motor neurons and consequent high‐frequency discharges of several motor units (Baldissera 1994; Jansen 1990; Layzer 1994). In symptomatic cramps, the pathophysiology may differ according to the underlying cause.
Suggested risk factors include motor neuron disease, peripheral neuropathy, radiculopathy, electrolyte disturbances, haemodialysis, uraemia, liver cirrhosis, hypothyroidism, as well as pregnancy and vigorous exercise (Miller TM 2005). Medications have also been implicated including diuretics, nifedipine, steroids, beta‐adrenoreceptor agonists, morphine and statins (Eaton 1989; Haskell 1997; McGee 1990).
Without a clear understanding of aetiology or pathophysiology, treatments have been empirical. It has been recommended that muscle cramps are treated by nonpharmacological interventions before any medications are commenced (Butler 2002). There is, however a significant paucity of good quality data on non‐drug therapies for cramp (Blyton 2012; Hallegraeff 2012). Drugs utilised to prevent muscle cramp include vitamin E (Ayres 1974), calcium channel blockers (Baltodano 1988; Peer 1983), naftidrofuryl oxalate (Young 1993), orphenadrine citrate (Latta 1989), magnesium sulphate (Dahle 1995; Garrison 2012; Young 2002), and quinine (Jones 1983). Vitamin E was considered effective in reducing cramps in three trials (Ayres 1969; Ayres 1974; Khajehdehi 2001), but not in another (Connolly 1992). Verapamil was tested in an uncontrolled trial of eight participants who were refractory to quinine treatment (Baltodano 1988). Of these eight, seven reported an improvement in their cramp symptoms over an eight‐week treatment period. Naftidrofuryl oxalate, a vasodilator, significantly reduced the frequency of cramps in a randomised control trial (RCT) involving 14 participants (Young 1993). Orphenadrine citrate, an anticholinergic with muscle relaxant properties, significantly reduced the frequency of cramps in a group of 59 participants in a double‐blind cross‐over trial (Latta 1989). Though magnesium salts have been shown to be effective in relieving the subjective distress caused by cramps in pregnancy (Dahle 1995; Young 2002), a RCT in nonpregnant sufferers reported no significant decrease in cramp frequency (Roffe 2002).
Description of the intervention
Quinine and its derivatives, quinine sulphate, hydroquinine and its optical isomer quinidine have been commonly prescribed to prevent cramps ever since a series of uncontrolled studies reported their beneficial effects in the 1940s (Gootnick 1943; Moss 1940; Nicholson 1945). Small groups of up to 30 people suffering from recurrent cramps were given quinine and the subjective outcome of no, partial or complete alleviation was reported on a case‐by‐case basis. In all three studies, the majority of participants reported improvement in their cramps, an effect that was reversed when the quinine was withdrawn.
Quinine (C₂₀H₂₄N₂O₂: molecular weight = 324), is a white crystalline alkaloid powder obtained from the bark of the cinchona tree which is native to the Andes region of South America (Krishna 1996). It comprises two benzene rings, and a covalently‐bonded nitrogenous carbon chain. Almost insoluble in water, it dissolves readily in alcohol and other organic solvents, and is used in the form of a salt, most commonly the sulphate. It is well known for its use in malaria and has been commercially synthesised from coal tar since 1944. Tonic water contains between 40 and 80 mg quinine/L. Quinine is inexpensive, bitter to taste, has excellent bioavailability and is predominantly excreted by the liver (Krishna 1996) but also by the kidneys to varying extents (Martindale 1996).
Quinine may have potentially serious adverse effects including fatal hypersensitivity reactions, particularly quinine‐induced thrombocytopenia (Barr 1990) which can occur idiosyncratically from the ingestion of even minimal amounts of quinine, such as are present in commercial tonic waters (Schneemann 2006). Other hypersensitivity reactions include angio‐oedema, disseminated intravascular coagulation, pancytopenia (Maguire 1993) and haemolytic uraemic syndrome (McDonald 1997). General and toxic reactions on the other hand are dose‐dependent and become common when plasma concentrations reach 10 mg/L (Schneemann 2006).
The dose of quinine used for the prevention of muscle cramps (200 to 300 mg daily) is significantly less than that used for the treatment of malaria (600 mg every eight hours); hence dose‐related adverse events are less common. However, gastrointestinal upset, abdominal pain, tinnitus and vertigo may occur, especially at higher doses, and quinine‐induced hypoglycaemia and renal insufficiency are also reported (Schneemann 2006). 'Cinchonism' is a symptom complex often linked to chronic use of quinine and consists of nausea, vomiting, vertigo, visual disturbances, tinnitus and hearing impairment (Bateman 1985). Quinine can interfere with the conduction pathways in the heart giving rise to arrhythmias, especially in overdosage (White 2007). Acute intoxication (ingestion of 4 to 12 g quinine) can cause convulsions followed by coma; death from respiratory arrest often results with doses exceeding 8 g. Permanent blindness has been reported in those with plasma concentrations over 10 mg/L (Prasad 2003).
Serious adverse effects including fatalities reported to the US Food and Drug Administration (FDA) led to the withdrawal of quinine from over‐the‐counter use and subsequently for all indications other than uncomplicated falciparum malaria (FDA 1982; FDA 1994; FDA 1995a; FDA 1995b; FDA 2006). A recent report from the American Academy of Neurology has recommended that quinine should not be used for the routine treatment of cramps, but only in cases of severe cramp where other treatments have failed and there is careful monitoring of side effects (Katzberg 2010). The FDA continue to be concerned about 'off label' use for nocturnal muscle cramp (www.fda.gov/ForHealthProfessionals/ArticlesofInterest/ucm317811.htm).
How the intervention might work
The precise mechanism of action of quinine is not known but it is believed to have a similar effect to curare on muscles and the neuromuscular junction. Quinine increases the refractory period of muscle, thereby reducing its response to repetitive stimulation (Goodman 2001). It also reduces the excitability of the motor end plate so that there is a diminished response to nerve stimulation and acetylcholine (Harvey 1939).
Why it is important to do this review
The first meta‐analysis of the efficacy of quinine for treating muscle cramps was published in 1995 and combined the results of six randomised, double‐blind, controlled trials investigating nocturnal leg cramps (Man‐Son‐Hing 1995). It concluded that treatment with quinine sulphate significantly reduced the number of cramps over a four‐week period by 8.83 (95% CI 4.2 to 13.5) cramps compared to placebo. However, the duration and intensity of individual cramps was not significantly affected by treatment. The same authors published a second meta‐analysis in 1998 that included three new unpublished trials which, when incorporated, decreased the magnitude of the reduction in the number of cramps to 3.6 (95% CI 2.2 to 5.1) fewer cramps than placebo (over four weeks) which remained significant (Man‐Son‐Hing 1998). The reduction in individual cramp intensity became significant, but the change in cramp duration remained nonsignificant. New data have been generated since the meta‐analyses of Man‐Song‐Hing (Man‐Son‐Hing 1995; Man‐Son‐Hing 1998). This systematic review includes these new studies. The review was first published in 2010 and this update in 2014.
Objectives
To assess the efficacy or safety of quinine‐based agents in treating muscle cramps.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐RCTs of quinine‐based agents for muscle cramps. Both cross‐over and parallel study designs were accepted and studies did not have to be double‐blinded. We included studies comparing quinine to placebo or to any other medication.
Types of participants
We included results from participants of all ages who suffered muscle cramps from any cause and in any setting. There is no universally accepted definition for muscle cramp and studies vary greatly in their diagnostic criteria. We defined a muscle cramp as a sudden, intense involuntary contraction of a muscle during rest or activity, accompanied by visible or palpable muscle hardening and pain. We included cramps in any body part, occurring at any time of day or night, and of any frequency.
Types of interventions
We included all the salts and derivatives of quinine such as quinine sulphate, quinine bisulphate, hydroquinine, hydroquinine hydrobromide and quinidine, the optical isomer of quinine. We excluded trials that did not contain a treatment arm solely composed of a quinine salt, as combinations with non‐quinine‐based drugs would mean the resultant effect could not be ascribed solely to the quinine component of the intervention. All doses and timing regimens of quinine administration were accepted.
Types of outcome measures
Primary outcomes
Absolute difference in number of cramps (occurring day or night) during a two‐week treatment period.
The number of cramps was selected as the primary outcome on the grounds that it is the most commonly used outcome in clinical trials. For trials that involved treatment periods greater than two weeks, the results were standardised to provide comparable data for combined analysis at two weeks. For example, studies with treatment periods of four weeks had the reported number of cramps divided by two.
Secondary outcomes
Difference in cramp intensity; there are a variety of 'pain scales' and so these were standardised to a three‐point scale (1 = mild, 2 = moderate, 3 = severe) to allow the results to be combined. This was done by scaling the scores proportionately (for example with a scale of 1 to 10, the score was divided by 3.33).
Difference in cramp duration (in minutes per cramp).
Absolute difference in number of 'cramp days' during a two‐week treatment period. This was the number of days in which the person suffered one or more cramps.
Participants with one or more minor adverse events. A 'minor' adverse event was defined as a reported side effect not severe enough to require withdrawal of treatment (e.g. diarrhoea or constipation).
Participants with one or more serious adverse events. A 'major' adverse event was defined as a side effect severe enough to require withdrawal of treatment (e.g. pancytopenia).
Search methods for identification of studies
Electronic searches
With the assistance of the Cochrane Neuromuscular Disease Group, we searched the Cochrane Neuromuscular Disease Group Specialized Register (27 October 2014), CENTRAL (2014, Issue 9 in The Cochrane Library), MEDLINE (January 1966 to October 2014) and EMBASE (January 1980 to October 2014). The detailed search strategies are in the appendices: Appendix 1 (MEDLINE), Appendix 2 (EMBASE), Appendix 3 (Cochrane Neuromuscular Disease Group Specialized Register) and Appendix 4 (CENTRAL).
On 3 November 2014 we also searched trial registries, ClinicalTrials.gov (www.clinicaltrials.gov/) and World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) for ongoing trials (Appendix 5).
Searching other resources
We contacted the American and British drug regulatory agencies and relevant pharmaceutical companies that manufacture quinine products to request any unpublished trials in their possession. We also contacted the authors of relevant trials and reviews to identify additional published or unpublished data. We checked the reference lists of these papers for further relevant material.
Data collection and analysis
Selection of studies
For the original review, three review authors independently checked the titles and abstracts of the articles identified by the search, obtaining the full text of all potentially relevant studies. The review authors selected the trials that satisfied the inclusion criteria for the review and graded their risk of bias and extracted data onto specially designed forms. There were no disagreements on which trials were to be excluded. For the update two authors (RB and SET) independently checked the titles and abstracts from the literature searches.
Data extraction and management
Four authors (SET, TAM, HV and TET) were involved in the data extraction, its checking and analysis. Three review authors independently extracted the data relating to the primary and secondary outcomes for all the included trials, and a fourth checked them.
One review author (SET) transferred study characteristics and outcome data into the Cochrane authoring and statistical software Review Manager (RevMan 2014) and a second author (TAM) carried out checks.
We transformed outcome data as described in Types of outcome measures to standardise reporting.
Assessment of risk of bias in included studies
Three review authors assessed the risk of bias of the trials independently using the recommended approach described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This addresses six specific domains: adequate sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and 'other sources of bias'. We gave each trial a classification of 'low risk', 'high risk' or 'unclear risk' (i.e. insufficient or no information). A fourth author resolved disagreements.
Measures of treatment effect
We combined the results of trials identified for inclusion in this review, where possible, using the Cochrane statistical package, RevMan. We combined continuous outcomes using the generic inverse variance (GIV) method which allows paired data from cross‐over trials (where each participant acts as their own control) to be combined with the results of two‐group parallel studies, producing a mean treatment effect with a 95% confidence interval (CI). For dichotomous outcomes (presence of major or minor adverse events), we calculated risk differences (RDs) with 95% CIs.
Unit of analysis issues
As noted above, we used GIV analysis to allow combination of parallel‐group and cross‐over studies. If multiple trial arms had been reported in a single trial, we would have included only the relevant arms.
Dealing with missing data
Where possible we derived standard errors (SE) from other data in order to perform GIV analyses. We contacted authors for missing data, but where we received no reply, we used the data within the studies to derive a standard error which could be used in the meta analysis.
Assessment of heterogeneity
We asssessed heterogeneity using the I2 statistic (Higgins 2003). Where we identified substantial unexplained heterogeneity, we reported it and explored possible causes by sensitivity analysis.
Assessment of reporting biases
Not done
Data synthesis
We undertook a fixed‐effect analysis initially and where the I² statistic exceeded 25%, undertook a sensitivity analysis. If heterogeneity remained unexplained, we used the random‐effects model of analysis. We regarded any outcome with a P value below 0.05 as significant. We also expressed all outcomes that reached statistical significance as relative percentage differences.
We discussed the adverse effects of quinine in the light of the results of this meta‐analysis. We used other sources of information for quinine's adverse event profile, including studies that were not randomised and texts such as Meyler's Adverse Events of Drugs (Schneemann 2006). We also discussed the costs and cost‐benefits of treating muscle cramp with quinine.
We created a 'Summary of findings' tables for comparisons where more than one trial was available. We presented the following outcomes:
Number of cramps over two weeks
Cramp intensity
Participants suffering major adverse events
Participants suffering minor adverse events
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence (studies that contribute data for the prespecified outcomes). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software. We justified all decisions to down‐ or up‐grade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
For the quinine versus placebo comparison and the outcome cramp number, we conducted a subgroup analysis by quinine dose. This was not a pre‐specified analysis.
Sensitivity analysis
We carried out a sensitivity analysis when I2 exceeded 25% by excluding:
trials that on examination had obvious potential sources of heterogeneity
trials at a high or unclear risk of bias for adequate sequence generation, allocation concealment, and blinding
We have described any deviations from the published protocol (El Tawil 2004) in Differences between protocol and review.
Results
Description of studies
The new, updated searches produced the following results: Cochrane Neuromuscular Disease (NMD) Group Specialized Register 32 papers, MEDLINE 186 papers, EMBASE 89 papers and CENTRAL 42 papers. There were no new studies satisfying our inclusion criteria. Searches of the trials registries ClinicalTrials.gov and ICTRP revealed no ongoing trials.
For the original review, search results from MEDLINE, EMBASE, CENTRAL and the Cochrane NMD Group Register revealed 259, 171, 43 and 22 papers, respectively. One hundred and twenty‐six titles were relevant to the topic and the abstracts of these were analysed. The authors reviewed the full texts of 28 studies. They eliminated five (see Excluded studies) leaving 23 studies that fulfilled our inclusion criteria. Quinine was compared to placebo (20 trials), to vitamin E (four trials), to a combination of quinine and vitamin E (also known as Q‐Vel) (three trials), to a combination of quinine and theophylline (one trial; Gorlich 1991) and to xylocaine injections (one trial; Prateepavanich 1999). Four trials compared quinine to more than one treatment (CIBA 1988; Connolly 1992; Gorlich 1991; Leo Winter 1986). See Table 4 for a tabulated summary of the trials and Included studies for further detail.
1. Study design of the 23 included trials.
| Study |
Number of participants Study design Patient focus |
Mean age (yrs) |
Female | Male | F: M ratio |
Quinine dose (mg) |
Treatment period (d) |
Washout period (d) | Treatment comparisons |
| BioDesign 1984a | n = 24 Study design = C Patient focus = I |
57 | 11 | 13 | 0.8 | 260 | 7 | 7 | Quinine‐vitamin E combination (Q‐Vel®)b ✓ |
| Bottner 1984a | n = 69 Study design = C Patient focus = I |
51 | 66 | 3 | 22.0 | 260 | 14 | 14 | Placebo ✓ |
| CIBA 1988a | n = 556 Study design = P Patient focus = I |
45 | 393 | 163 | 2.4 | 260 | 14 | n/a | Placebo ✓ Vitamin E ✓ Quinine‐vitamin E combination (Q‐Vel®) ✓ |
| Connolly 1992 | n = 30 Study design = C Patient focus = I |
59 | 0 | 30 | 0.0 | 500 | 28 | 28 | Placebo ✓ Vitamin E ✓c |
| Dunn 1993 | n = 28 Study design = C Patient focus = I |
67 | 17 | 11 | 1.5 | 300 | 30 | 3 | Placebo ✓ |
| Diener 2002 | n = 94 Study design = P Patient focus = I |
49 | 66 | 32 | 2.1 | 400 | 14 | n/a | Placebo ✓ |
| Fung 1989 | n = 9 Study design = C Patient focus = I |
63 | 7 | 1 | 7.0 | 200 | 28 | 7 | Placebo ✓ |
| Gorlich 1991 | n = 164 Study design = P Patient focus = I |
56 | 119 | 45 | 2.6 | 260 | 14 | n/a | Placebo ✓ Quinine‐theophylline combination (Limptar®)b ✓ |
| Hays 1986a | n = 62 Study design = C Patient focus = I |
47 | 49 | 13 | 3.8 | 325 | 14 | 14 | Placebo ✓ |
| Jansen 1994 | n = 20 Study design = P Patient focus = I |
55 | 14 | 6 | 2.3 | 300 | 14 | n/a | Placebo ✓ |
| Jansen 1997 | n = 106 Study design = P Patient focus = I |
51 | 68 | 44 | 1.5 | 300 | 14 | n/a | Placebo ✓ |
| Jones 1983 | n = 9 Study design = C Patient focus = I |
_ | _ | _ | _ | 300 | 14 | 14 | Placebo ✓ |
| Kaji 1976 | n = 9 Study design = C Patient focus = H |
_ | _ | _ | _ | 320d | 42d | 0 | Placebo ✓ |
| Lee 1991 | n = 31 Study design = P Patient focus = L |
62 | 5 | 26 | 0.2 | 400 | 28 | n/a | Placebo ✓ |
| Leo Winter 1986a | n = 205 Study design = C Patient focus = I |
44 | 173 | 32 | 5.4 | 260 | 5 | 2 | Placebo ✓ Vitamin E ✓ Quinine‐vitamin E combination (Q‐Vel®) ✓ |
| Lim 1986 | n = 25 Study design = P Patient focus = I |
_ | _ | _ | _ | 300 | ≤ 14 | n/a | Placebo ✓ |
| Maule 1990 | n = 16 Study design = C Patient focus = I |
76 | 10 | 6 | 1.7 | 300 | 21 | 0 | Placebo ✓ |
| Prateepavanich 1999 | n = 24 Study design = P Patient focus = Ie |
64 | 21 | 3 | 7.0 | 300 | 28 | n/a | Xylocaine injection ✓ |
| Roca 1992 | n = 30 Study design = P Patient focus = H |
48 | 10 | 19 | 0.5 | 325 | 60f | n/a | Vitamin E ✓ |
| Sidorov 1993 | n = 19 Study design = C Patient focus = I |
58 | 14 | 2 | 7.0 | 200 | 14 | 14 | Placebo ✓ |
| Smith 1985 | n = 21 Study design = C Patient focus = I |
73 | _ | _ | _ | 300 | 21 | 0 | Placebo ✓ |
| Warburton 1987 | n = 22 Study design = C Patient focus = I |
74 | 16 | 6 | 2.7 | 300 | 21 | 0 | Placebo ✓ |
| Woodfield 2005 | n = 13 Study design = N‐of‐1 Patient focus = I |
75 | 7 | 6 | 1.2 | 200 to 300 | 42 | 0 | Placebo ✓ |
Abbreviations: C: cross‐over, P: parallel, H: haemodialysis‐induced cramps, I: idiopathic, L: patients with liver cirrhosis; n/a: not available
aUnpublished. bQ‐Vel®: trade name for quinine‐vitamin E combination; Limptar®:trade name for quinine‐theophylline combination. cConnolly 1992 did not directly compare quinine versus vitamin E ‐ using the data provided we were able to draw comparison. dQuinine dose given at beginning of each dialysis session (3 times per wk) and not daily. eInclusion criteria included presence of myofascial trigger point in gastrocnemius. fA 60‐day trial but results only reported from first month of treatment.
Thirteen trials were cross‐over in design, nine were parallel studies, and one was a 'N‐of‐1' trial (see Table 4). The 23 studies involved a total of 1586 unique participants commencing the trials; 523 were from cross‐over trials and thus formed their own controls. The number of participants in each trial varied from 9 to 556, with only four trials containing more than 100 participants. Of the 23 trials included, five were unpublished studies acquired via the United States Food and Drug Administration (FDA). Indeed, the two largest trials included in this meta‐analysis were both large unpublished multicentre studies conducted by pharmaceutical companies in the United States: CIBA 1988 with 556 participants, and Leo Winter 1986 with 205 participants. The five unpublished studies contributed 58% of the total number of participants included in this meta‐analysis. The third largest trial was translated from German (Gorlich 1991). This was the only trial to compare quinine with a combination of quinine and theophylline, and was also conducted by a pharmaceutical company.
Twenty trials investigated idiopathic muscle cramps, most often in elderly participants suffering from nocturnal leg cramps. In one (Prateepavanich 1999), inclusion criteria included nocturnal calf cramps associated with a demonstrable myofascial trigger point on the medial head of the gastrocnemius muscle. Two studies (Kaji 1976; Roca 1992) recruited only participants who suffered haemodialysis‐related cramps, whilst one study (Lee 1991) recruited those with liver cirrhosis. The participants were all outpatients from general practice and medical clinics, except those in one trial (Lim 1986) who were inpatients on a general medical ward.
The typical format of the cross‐over trials consisted of an initial 'run‐in period' of around two weeks, allowing baseline characteristics to be assessed, inclusion criteria to be met, and any quinine from previous treatment to be washed out. Common inclusion criteria were: minimum cramp frequency of two per week, and the absence of conditions predisposing to cramps in those trials investigating idiopathic cramps. Common exclusion criteria included: electrolyte disturbances, renal or hepatic impairment, detectable quinine serum levels after the run‐in phase, and the use of concomitant medication interfering with quinine or cramp sensation. Time periods for trial washout and cross‐over varied. The washout interval ranged from 0 days (Kaji 1976; Maule 1990; Smith 1985; Warburton 1987) to 28 days (Connolly 1992) (see Table 4). Parallel group trials included a follow‐up assessment period.
The average age of the population under investigation in each study varied from 44 to 76 years (mean of these averages = 58 years). The youngest affected with cramp was 17 years and the oldest 87 years. Of the 19 trials that included gender demographics, 15 had a female preponderance, 11 of which contained more than twice as many women as men. The mean female to male ratio across the 19 trials describing sex distribution was 3.8. Of the four trials containing more men than women, one was conducted at a Veterans Affairs medical centre (Connolly 1992; 100% men), and another investigated people with liver cirrhosis resulting from chronic hepatitis and not alcohol intake (Lee 1991; 84% men).
Twenty trials used the sulphate salt of quinine as the active treatment, two studies (Jansen 1994; Jansen 1997) used hydroquinine hydrobromide, and one trial (Lee 1991) used the quinine isomer quinidine. Most of the trials used a daily quinine dose of 300 mg, closely followed by 200 mg. Two trials (Diener 2002: Lee 1991) used doses of 400 mg, and one trial (Connolly 1992) used a dose of 500 mg. The Woodfield 2005 trial maintained participants on the dosage of quinine which they had previously been prescribed (mainly 200 mg).
The duration of treatment with quinine ranged from five days (Leo Winter 1986) to 42 days (Kaji 1976; Woodfield 2005), with the majority (10 trials) treating for 14 days. One trial administered active medication for 60 days but only provided results for the first month of treatment (Roca 1992). The Leo Winter 1986 trial compared four treatments in cross‐over design over four weeks and so the treatment duration was limited to five days each with only two days washout in between treatments. A poorly designed trial treated patients on a general medical ward up to their discharge date or for up to two weeks, whichever was shorter (Lim 1986).
The timing of drug administration varied between trials; the majority of studies advised participants to take the therapy at or approaching bedtime, while the others divided the dose across the evening (BioDesign 1984; Leo Winter 1986) or in a morning/evening regimen (Lee 1991). Of the two haemodialysis trials, one (Kaji 1976) administered quinine or placebo at the beginning of each dialysis session only (three times per week), whilst the other (Roca 1992) administered the study drug daily. The parallel trial involving xylocaine injections (Prateepavanich 1999) gave one group daily 300 mg quinine at bedtime and the other group an injection of 1% xylocaine into the medial head of the gastrocnemius muscle at the start of the four‐week treatment period. Symptoms were reviewed on a fortnightly basis and further injections administered depending on the frequency of any ongoing symptoms.
Cramp number was the most common outcome measured in the trials. Some trials combined the cramp intensity with duration to give a 'cramp index' which was not an outcome in this meta‐analysis. All but two trials recorded adverse events as an outcome (Roca 1992; Smith 1985). In Kaji 1976, the frequency and severity of cramps was assessed only during dialysis sessions and not between them.
Risk of bias in included studies
The 'Risk of bias' assessment was performed as set out in the Methods.
The quality of the trials varied considerably. Newer trials were of better design, incorporating more appropriate statistical analysis, applying intention‐to‐treat analysis and taking into consideration baseline differences. The unpublished studies in general were much more detailed, each comprising several documents, and were conducted by professional pharmaceutical investigators.
All 23 trials claimed to be randomised, but only eight actually described the method of randomisation (Characteristics of included studies; Figure 1). Likewise, only eight trials stated how allocation was concealed.
1.
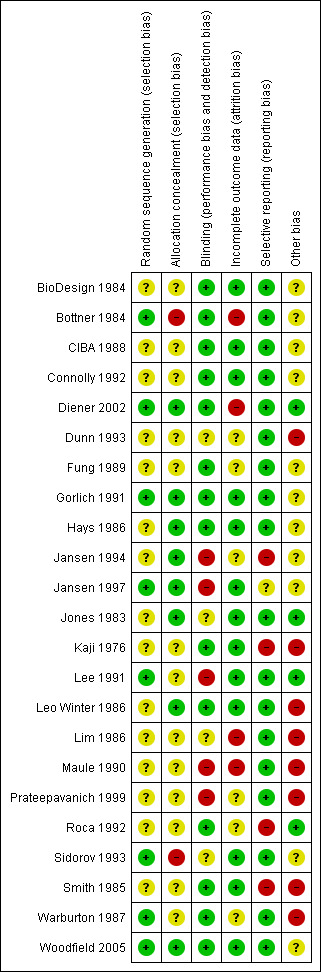
Methodological quality summary: review authors' judgements about each methodological quality item for each included study. Red = high risk of bias; yellow = unclear risk of bias; green = low risk of bias.
Fourteen studies were judged to be adequately double‐blinded (Figure 1). There was insufficient information to pass judgement in four trials, whilst five trials were not double‐blinded: quinine and placebo tablets looked different in one trial (Maule 1990), tasted different in two trials (Jansen 1994; Jansen 1997), physicians were not blinded in another (Lee 1991), and both participant and observer were aware of the treatments administered (xylocaine injections or oral quinine) in another (Prateepavanich 1999).
Thirteen studies were considered to have adequately addressed any incomplete outcome data. Two studies did not give complete details for participant withdrawal (Dunn 1993; Maule 1990) and two did not mention the drop‐outs at all (Bottner 1984; Diener 2002). In the Bottner 1984 trial, 15 drop‐outs were unaccounted for and one suffered quinine‐related adverse events and was not followed up. One study did not give the number of participants completing the trial (Lim 1986). Intention‐to‐treat analysis was not used in six studies (Fung 1989; Jansen 1994; Maule 1990; Prateepavanich 1999; Roca 1992; Warburton 1987).
The majority of studies were free of selective reporting (Figure 1), but five studies did not report on their adverse event outcomes (Roca 1992; Smith 1985) or other outcomes stipulated in their methods (Jansen 1994; Jansen 1997; Kaji 1976).
Other sources of bias included the lack of sufficient washout periods in cross‐over treatments (Dunn 1993; Kaji 1976; Leo Winter 1986; Maule 1990; Smith 1985; Warburton 1987), the fact that six studies were conducted by pharmaceutical companies (BioDesign 1984; Bottner 1984; CIBA 1988; Gorlich 1991; Hays 1986; Leo Winter 1986) (although this is not always considered a source of significant bias), gender bias (Bottner 1984; Connolly 1992; Fung 1989; Lee 1991; Jansen 1994; Sidorov 1993), and intra‐study variability in treatment dose (Prateepavanich 1999; Woodfield 2005) or duration (Lim 1986).
Not all data could be entered into the meta‐analysis. Two trials did not contain sufficient data for entry into the meta‐analysis (Maule 1990; Smith 1985). The Smith 1985 trial did not include any washout period between quinine and placebo and no data were given for the results. The Maule 1990 trial failed to give any detail on the cramp number outcome, whilst the information on adverse events could not be ascribed to a set number of participants.
The only data that could be entered from three of the trials were those of the adverse events experienced (Bottner 1984; Lim 1986; Kaji 1976). Kaji 1976 measured the number of cramps during dialysis sessions only and not over 24 hours, and this outcome was thus excluded from the meta‐analysis.The Lim 1986 trial presented very basic information on design and conduct. The trial did not have a set treatment duration and also failed to specify how many participants were randomised to each group. The results were not reported fully and carried no measure of spread. The GIV method of meta‐analysis is dependent upon the calculation of a standard error (SE) from standard deviations (SDs); where SDs were not available, the SE was approximated from a range, or CI, or from measures of significance (P values). None of these were available for the Bottner 1984 trial and so the results could not be entered into the meta‐analysis.
Effects of interventions
See: Table 1; Table 2; Table 3
Quinine versus placebo
Primary outcome measure: difference in number of cramps (occurring day or night) during a two‐week treatment period
Eighteen placebo‐controlled trials reported the absolute number of cramps occurring over their study period. To allow the results to be combined in a meta‐analysis, we standardised the data to the number of cramps occurring over two weeks.
Four trials could not be entered into the meta‐analysis for the reasons given above (Bottner 1984; Kaji 1976; Maule 1990; Smith 1985). The 14 trials (n = 982) entered into the meta‐analysis all investigated idiopathic muscle cramps except one (Lee 1991), which investigated cramps in people with liver cirrhosis. The results of the Lee 1991 trial were comparable with the rest. When the 14 trials were entered into the meta‐analysis, the combined mean change in the number of cramps was ‐1.81 (95% CI ‐2.20 to ‐1.42) (See Analysis 1.1).
1.1. Analysis.
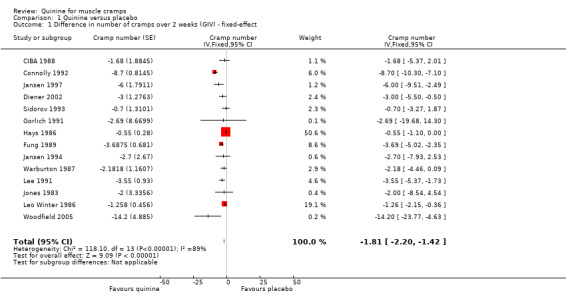
Comparison 1 Quinine versus placebo, Outcome 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect.
Significant heterogeneity was detected in the meta‐analysis (I² = 89%). Inspection of the included trials identified potential causes in two trials. The small trial of Woodfield 2005 contained large standard errors for the primary outcome and Connolly 1992 was the only trial to use 500 mg quinine and included only men. The exclusion of Woodfield 2005 did not reduce the heterogeneity but excluding Connolly 1992 reduced the I² index to 71%. We performed further sensitivity analyses excluding all trials at a high or unclear risk of bias for adequate sequence generation, allocation concealment, and blinding; the heterogeneity index changed to 50%, 67% and 91% respectively. Quinine remained significantly effective compared to placebo in all these sensitivity analyses. After discussion, we excluded Connolly 1992 only because of its unique participant selection and quinine dose, and used a random‐effects model. Quinine resulted in a significant decrease in cramp number (‐2.45 cramps, 95% CI ‐3.54 to ‐1.36, random‐effects), equivalent to a 28% (95% CI 15% to 40%) reduction over placebo (see Analysis 1.2, Figure 2). It is worth noting the persistent heterogeneity (I² = 71%) associated with this result.
1.2. Analysis.
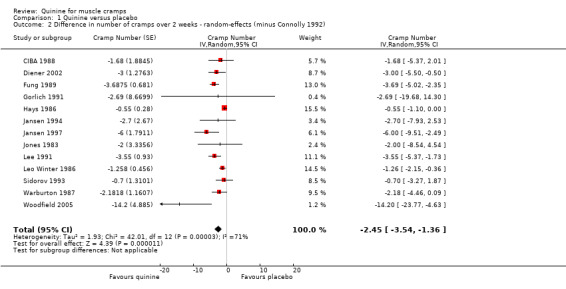
Comparison 1 Quinine versus placebo, Outcome 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992).
2.
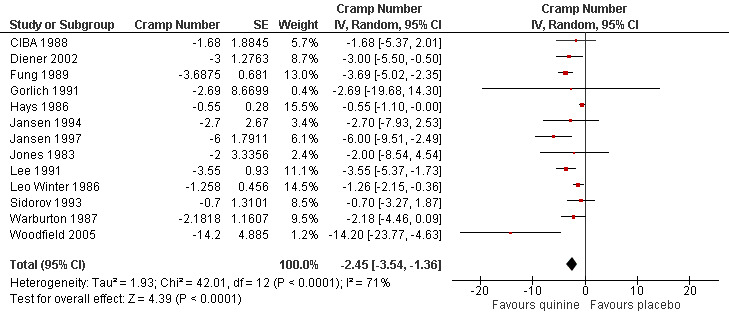
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992).
Subgroup analysis according to quinine dose
We analysed the data for the effect of quinine dose on cramp number over two weeks (see Analysis 1.3), grouping the studies according to the dose of quinine used. Although there seems to be an increasing effect with dose from 300 mg through 400 mg to 500 mg, the 200 mg and 260 mg doses go against this trend. It is notable that the reduction in cramp number reported by using the higher 500 mg dose (Connolly 1992) is more than double that achieved by any of the other doses. However, analysis of these data is severely undermined by the shortage of studies in each dose range; any conclusions drawn from these data would be highly speculative.
1.3. Analysis.
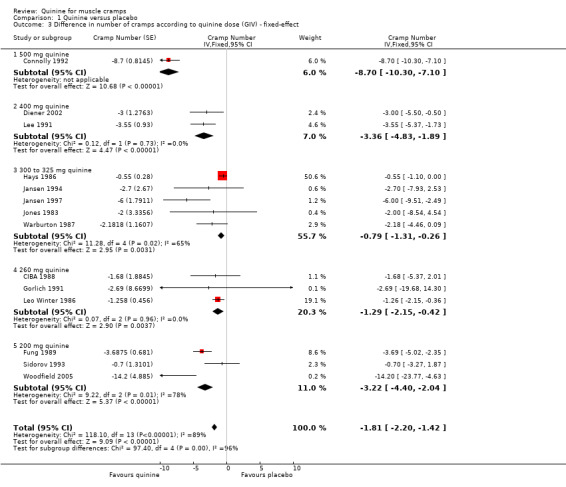
Comparison 1 Quinine versus placebo, Outcome 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect.
Change in cramp intensity during treatment period
Cramp intensity was reported in 13 of the 20 placebo‐controlled trials. A further two trials measured cramp intensity but combined it with duration to give an 'index' from which intensity itself could not be derived (Smith 1985; Warburton 1987).
From the 13 trials reporting intensity as an outcome, six trials could not be entered into the meta‐analysis. Three trials failed to report exact data (Diener 2002; Jansen 1994; Jansen 1997), standard errors could not be derived from two trials (Bottner 1984; Lim 1986), and one trial (Connolly 1992) measured only the most severe cramp experienced each night.
The remaining seven trials (n = 666) measured the intensity per cramp on different scales and so these were standardised to a three‐point scale (1 = mild pain, 2 = moderate pain, 3 = severe pain). Meta‐analysis of these trials (CIBA 1988; Fung 1989; Gorlich 1991; Hays 1986; Jones 1983; Leo Winter 1986; Sidorov 1993) demonstrated that quinine was significantly better than placebo in reducing cramp intensity (‐0.12 units, 95% CI ‐0.20 to ‐0.05), representing a 10% drop (95% CI 4% to 16%) compared to placebo (Analysis 1.4). There was no heterogeneity in this meta‐analysis (I² = 0).
1.4. Analysis.
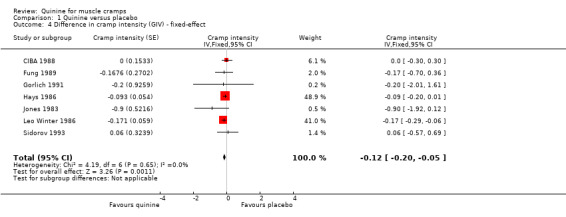
Comparison 1 Quinine versus placebo, Outcome 4 Difference in cramp intensity (GIV) ‐ fixed‐effect.
Change in cramp duration (in minutes)
This was an outcome in eight of the trials (CIBA 1988; Fung 1989; Gorlich 1991; Jansen 1994; Jansen 1997; Jones 1983; Kaji 1976; Sidorov 1993), but data from six trials (CIBA 1988; Gorlich 1991; Jansen 1994; Jansen 1997; Jones 1983; Kaji 1976) could not be entered into the meta‐analysis because the data could not be ascertained or converted into a suitable form or were unavailable from the trial authors. All except one (Gorlich 1991) of these excluded trials individually showed that there was no significant difference between quinine and placebo with regards to cramp duration. A further trial (Warburton 1987) combined cramp duration with mean severity to give a 'cramp index'; no significant difference in this index was found between quinine and placebo.
There remained two trials (n = 28; Fung 1989; Sidorov 1993) which could be combined into the meta‐analysis. Cramp durations were calculated from individual patient data provided in the Fung 1989 trial. We used a random‐effects model because of unexplained significant heterogeneity (I² = 31%), and there was no significant difference between quinine and placebo (‐1.35 minutes, 95% CI ‐4.00 to 1.30) (see Analysis 1.5).
1.5. Analysis.
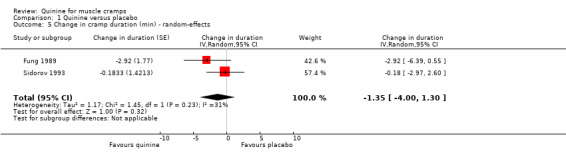
Comparison 1 Quinine versus placebo, Outcome 5 Change in cramp duration (min) ‐ random‐effects.
Change in number of cramp days
Ten trials measured this outcome and seven reported statistically significant reductions in the number of cramp days with quinine compared with placebo. However, one of these trials (Dunn 1993) could not be entered into the meta‐analysis as it was a cross‐over trial that was declared invalid by its authors because of a significant carry‐over effect. Lim 1986 could not be entered into the meta‐analysis as it failed to indicate the number of participants in each group.
Eight trials were combined (CIBA 1988; Connolly 1992; Diener 2002; Gorlich 1991; Hays 1986; Jansen 1997; Leo Winter 1986; Woodfield 2005). Results were standardised to two weeks. The three unpublished trials (CIBA 1988; Hays 1986; Leo Winter 1986) reported differences in the number of cramp days without details of standard deviation or confidence limits. These were approximately derived from the P values given. The trial of Connolly 1992 was excluded after sensitivity analysis because of the demonstrable heterogeneity that it contributed. The remaining seven trials (n = 842) showed that quinine significantly reduced cramp days compared to placebo (‐1.15 days, 95% CI ‐1.93 to ‐0.38, random‐effects) (see Analysis 1.6). This represents a 20% (95% CI 6% to 33%) reduction in cramp days when compared to the average number of affected days on placebo. It is worth noting that despite the exclusion of Connolly 1992, there remained significant heterogeneity between the trials (I² = 86%).
1.6. Analysis.
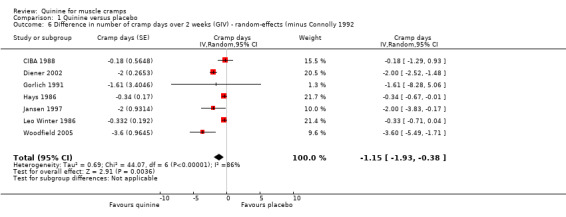
Comparison 1 Quinine versus placebo, Outcome 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992.
Participants with one or more adverse events
All but one of the included trials provided results for adverse events, investigated by participant diary or clinical assessment, or both. The trial that did not mention adverse events set out with the intention to do so but no mention of this was later given in the paper (Smith 1985). The data on adverse events in the Maule 1990 trial were unclear and could not be entered into the meta‐analysis. Only data for major adverse events from the Jansen 1997 trial could be entered into the meta‐analysis.
Minor adverse events
Sixteen of the 20 placebo‐controlled trials provided accurate data on minor adverse events. Quinine was free of all adverse events in six trials with a total of 106 participants (Dunn 1993; Jones 1983; Kaji 1976; Lim 1986; Warburton 1987; Woodfield 2005).
When the 16 trials were combined, 93 out of 725 participants on quinine suffered minor adverse events (12.8%), compared to 68 out of 722 on placebo (9.4%). The risk difference (RD) was small but significant at 3% (95% CI 0% to 6%) (Analysis 1.7, Figure 3). There was no heterogeneity in the results (I² = 0%).
1.7. Analysis.
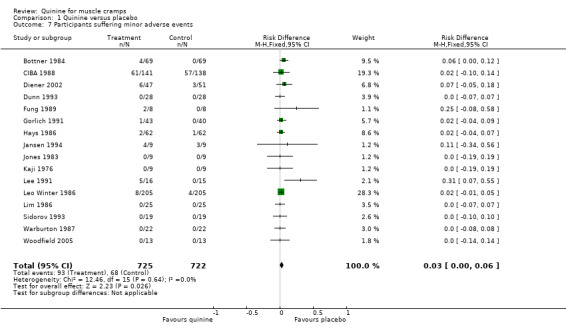
Comparison 1 Quinine versus placebo, Outcome 7 Participants suffering minor adverse events.
3.
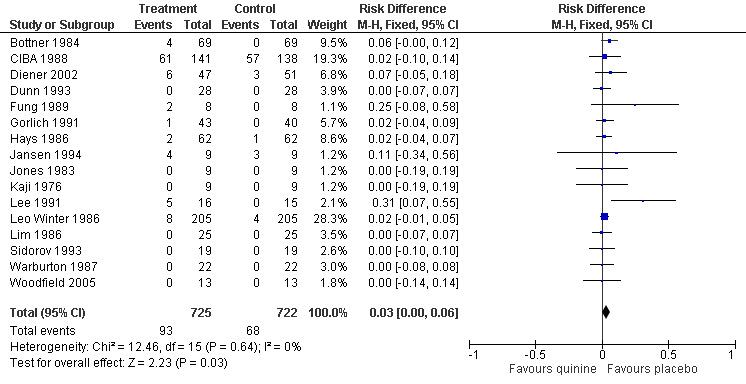
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.7 Participants suffering minor adverse events ‐ fixed‐effect.
In a separate analysis of specific minor adverse events, the Jansen 1997 and Maule 1990 trials could also be taken into account, giving a total of 790 participants in the quinine group and 791 in the placebo group. Comparing quinine with placebo, the following numbers of participants suffered the respective minor adverse events: gastrointestinal (39 quinine versus 16 placebo), headache (36 versus 33), tinnitus (10 versus 1), pruritis/scaly rash (9 versus 3), dizziness/drowsiness (8 versus 8), myalgia/paraesthesia (7 versus 10), visual disturbance (4 versus 2), and fever (3 versus 1). The only significant risk difference was found to be gastrointestinal‐related adverse events (RD 3%, 95% CI 1% to 5%) (Analysis 1.8, Figure 4). The apparent increase in incidence of tinnitus did not reach statistical significance.
1.8. Analysis.
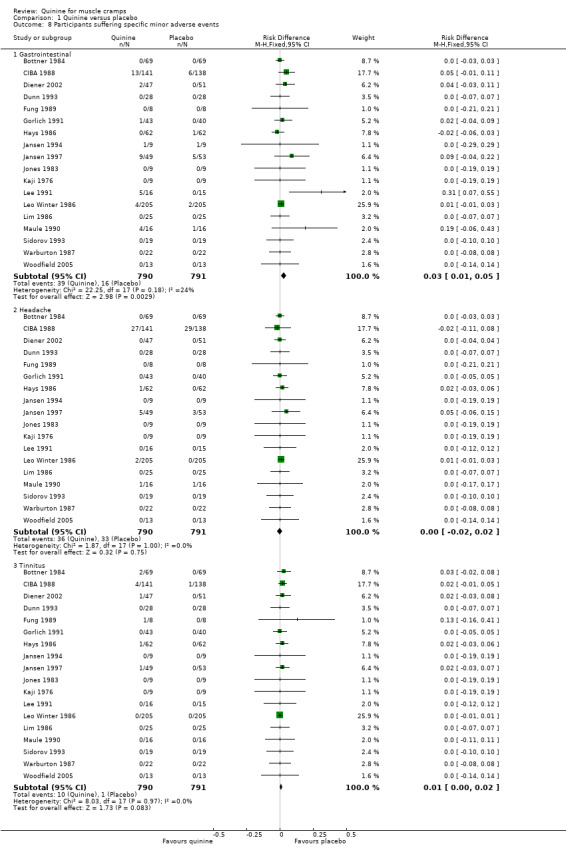
Comparison 1 Quinine versus placebo, Outcome 8 Participants suffering specific minor adverse events.
4.
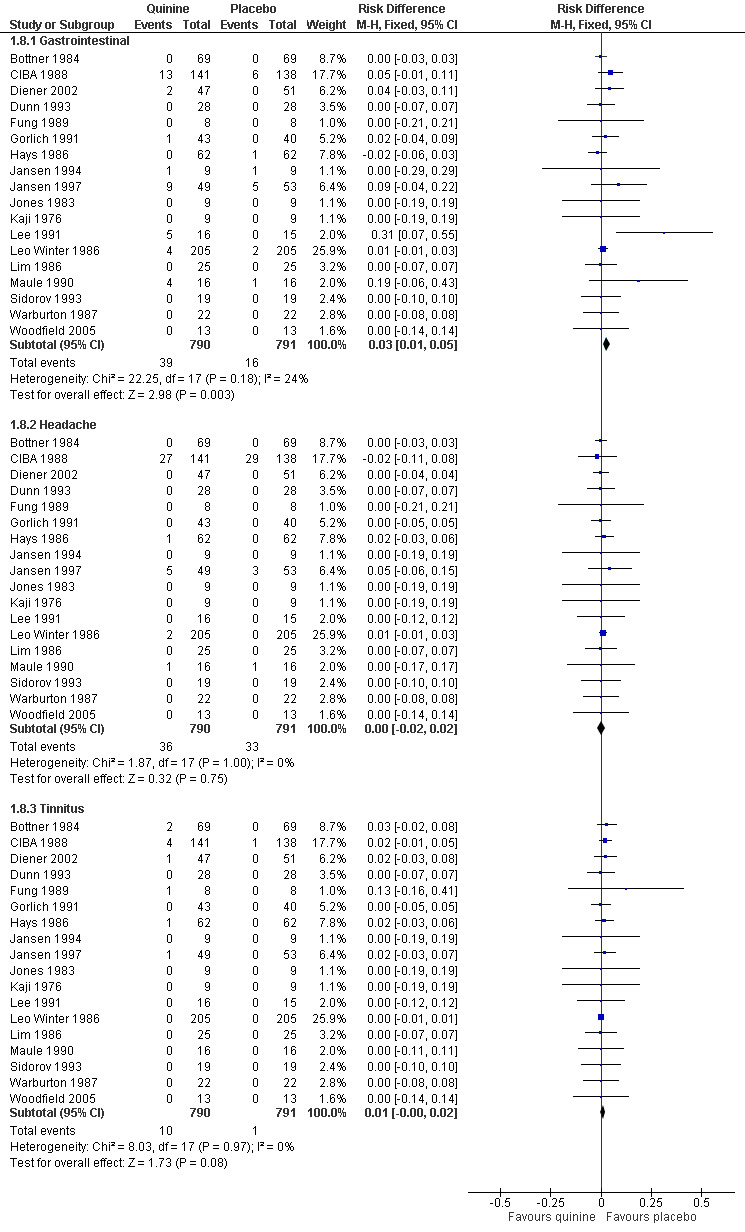
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.8 Participants suffering specific minor adverse events.
Major adverse events
Quinine was stopped in a total of 12 participants in eight separate trials because of adverse events. Twelve participants (1.5%) withdrew from a total of 806 participants treated with quinine compared to 11 out of 807 on placebo (1.4%). There was no significant risk difference between the two groups (RD 0.0%; 95% CI ‐1% to 2%) (Analysis 1.9, Figure 5). There was no heterogeneity in the results (I² = 0%).
1.9. Analysis.
Comparison 1 Quinine versus placebo, Outcome 9 Participants suffering major adverse events.
5.
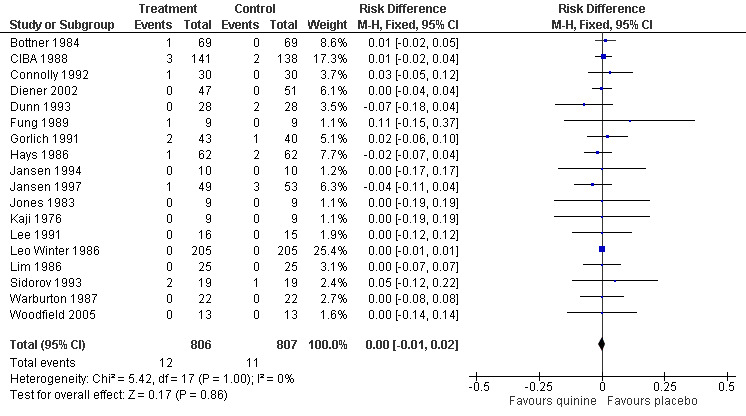
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.9 Participants suffering major adverse events.
The only truly serious adverse event occurred in the Sidorov 1993 cross‐over trial (n = 19), where a participant suffered leukopenia and thrombocytopenia with a severe rash, myalgia, and nausea, all of which resolved three days after stopping quinine. No further details are available regarding this participant.
The other adverse events encountered were mild or short‐lived, with authors usually reporting their resolution on cessation of treatment. Several participants suffered more than one major adverse event and by symptomatology these were: gastrointestinal (7 in quinine group versus 1 in placebo group), dizziness/drowsiness (3 versus 2), headache (2 versus 2), pruritis/scaly rash (2 versus 0), tinnitus (1 versus 0), myalgia/paraesthesia (1 versus 0), visual disturbance (0 versus 1), and fever (1 versus 0). Gastrointestinal symptoms were the commonest reason for participant withdrawal but this was not significantly different to placebo (Analysis 1.10, Figure 6).
1.10. Analysis.
Comparison 1 Quinine versus placebo, Outcome 10 Participants suffering specific major adverse events (gastrointestinal).
6.
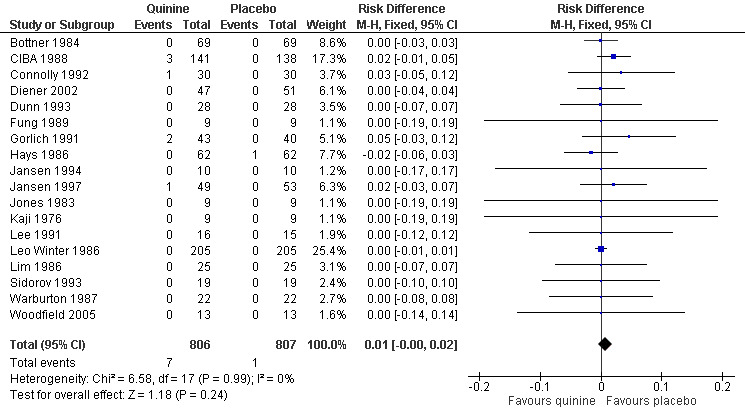
Forest plot of comparison: 1 Quinine versus placebo, outcome: 1.10 Participants suffering specific major adverse events (gastrointestinal).
Quinine versus vitamin E
Four trials (n = 543) compared quinine against vitamin E at doses of 1600 units (CIBA 1988; Leo Winter 1986) and 800 units (Connolly 1992; Roca 1992).
Primary outcome measure: difference in number of cramps (occurring day or night) during a two‐week treatment period
The inclusion of Connolly 1992 resulted in significant heterogeneity (I² = 94%, reduced to 29% on exclusion), so it was removed on the basis of its unique selection of participants and dose. With this persisting heterogeneity (I² = 29%), we used the random‐effects model and there was no significant difference between quinine and vitamin E in reducing cramps; ‐0.24 cramps (95% CI ‐1.29 to 0.81) (see Analysis 2.1, Figure 7).
2.1. Analysis.
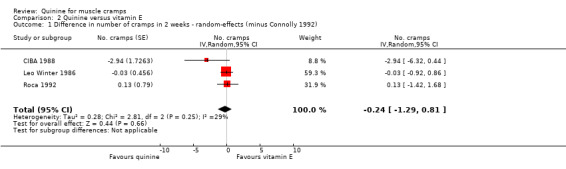
Comparison 2 Quinine versus vitamin E, Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992).
7.
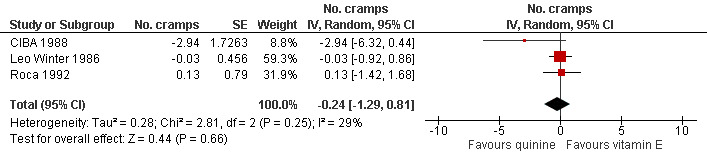
Forest plot of comparison: 2 Quinine versus vitamin E, outcome: 2.1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992).
Change in cramp intensity during treatment period
Four trials (CIBA 1988; Connolly 1992; Leo Winter 1986; Roca 1992) compared quinine to vitamin E, and in none was there a significant difference between the two treatments in reducing cramp intensity.
Following sensitivity analysis, Connolly 1992 was again removed, reducing I² to 0. The combined result from the remaining three trials (n = 513) showed that quinine was not significantly better than vitamin E in reducing cramp intensity (‐0.06 units, 95% CI ‐0.17 to 0.04, see Analysis 2.2).
2.2. Analysis.
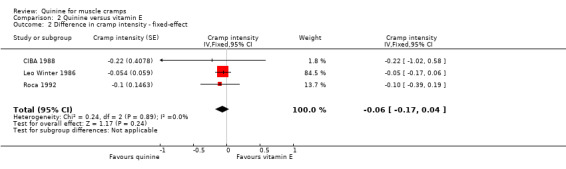
Comparison 2 Quinine versus vitamin E, Outcome 2 Difference in cramp intensity ‐ fixed‐effect.
Change in cramp duration (in minutes)
Only one trial (CIBA 1988, n = 256) measured cramp duration for this comparison. A descriptive scale was used (0 'none', 1 'short', 2 'moderate' and 3 'long') and the difference between quinine and vitamin E on this scale was nonsignificant at two weeks (‐0.06 units, 95% CI ‐0.13 to 0.01).
Change in number of cramp days
From the four trials comparing quinine with vitamin E, the number of cramp days was an outcome in three (CIBA 1988; Connolly 1992; Leo Winter 1986). The smaller study of Connolly 1992 did not provide statistical comparison of quinine with vitamin E, but did provide sufficient data for this analysis to be made without any statistical assumption; at two weeks, quinine was significantly better than vitamin E in reducing cramp days (‐2.85, 95% CI ‐3.32 to ‐2.38). However, the trial of Connolly 1992 was excluded from the final meta‐analysis (n = 483) due to high heterogeneity (I² dropping from 98% to 48% on exclusion). Accepting the persisting significant heterogeneity, there was no significant difference when quinine was compared to vitamin E (‐0.28 cramp days, 95% CI ‐0.98 to 0.43, random‐effects) (see Analysis 2.3).
2.3. Analysis.
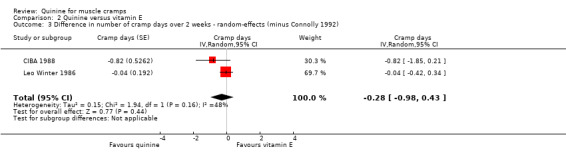
Comparison 2 Quinine versus vitamin E, Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992).
Participants with one or more adverse events
Three of the four vitamin E trials provided results for adverse events. The trial that did not mention adverse events set out with the intention to do so but no mention of this was later given in the paper (Roca 1992).
Minor adverse events
Two studies (CIBA 1988; Leo Winter 1986) were entered into the meta‐analysis as Connolly 1992 failed to give any detail on minor adverse events. Sixty‐nine participants (19.9%) from a total of 346 participants treated with quinine experienced minor adverse events compared to 57 out of 342 on vitamin E (16.7%). There was no significant risk difference between the two groups (RD 2%, 95% CI ‐4% to 9%, random‐effects) (Analysis 2.4). There was unexplained heterogeneity (I² = 36%).
2.4. Analysis.
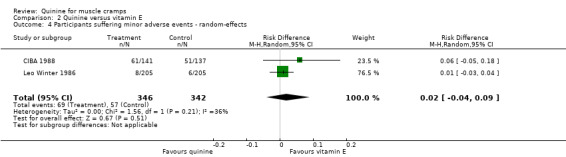
Comparison 2 Quinine versus vitamin E, Outcome 4 Participants suffering minor adverse events ‐ random‐effects.
Several participants suffered more than one minor adverse event and by symptomatology these were mainly: headache (29 in quinine group versus 24 in vitamin E group), gastrointestinal (17 versus 14), tinnitus (4 versus 0), and visual disturbance (1 versus 3).
Major adverse events
Three studies (CIBA 1988; Connolly 1992; Leo Winter 1986) could be entered into the meta‐analysis. Four participants (1.06%) withdrew from a total of 376 participants treated with quinine compared to one of 372 participants on vitamin E (0.27%). There was no significant risk difference between the two groups (RD 1%, 95% CI ‐1% to 2%) (Analysis 2.5). We used a random‐effects model, as the exclusion of Connolly 1992 did not reduce the significant heterogeneity found with the fixed‐effect model.
2.5. Analysis.
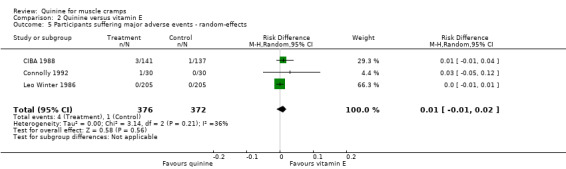
Comparison 2 Quinine versus vitamin E, Outcome 5 Participants suffering major adverse events ‐ random‐effects.
The four withdrawals from the quinine groups all complained of gastrointestinal symptoms, but also rash and paraesthesia (one participant), headache (two participants), and pruritus with bruising (one participant). This last participant would not permit follow‐up (Connolly 1992), whilst all symptoms resolved soon after cessation of quinine in the other three (CIBA 1988). The only participant to withdraw from the vitamin E group suffered from severe headache with nausea and vomiting, but was found to have a history of migraine.
Quinine versus a quinine‐vitamin E combination
Primary outcome measure: difference in number of cramps (occurring day or night) during a two‐week treatment period
Three unpublished studies (n = 510) compared quinine with a combination of quinine and vitamin E (Q‐Vel); two of these trials claimed superiority of the quinine‐vitamin E combination over quinine alone (BioDesign 1984; Leo Winter 1986), whilst one reported no significant difference (CIBA 1988). The BioDesign 1984 study did not report data that could be included in the meta‐analysis. We identified significant heterogeneity in the meta‐analysis of the remaining two studies (CIBA 1988; Leo Winter 1986). This could not be explained, so we used a random‐effects model (I² = 49%); there was a nonsignificant difference in cramp number when quinine alone was compared to the quinine‐vitamin E combination (1.07 cramps, 95% CI ‐1.08 to 3.23) (see Analysis 3.1, Figure 8).
3.1. Analysis.
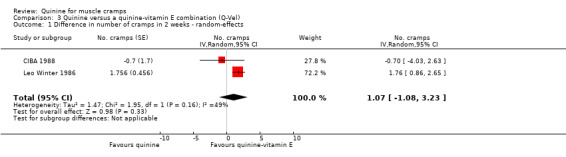
Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 1 Difference in number of cramps in 2 weeks ‐ random‐effects.
8.
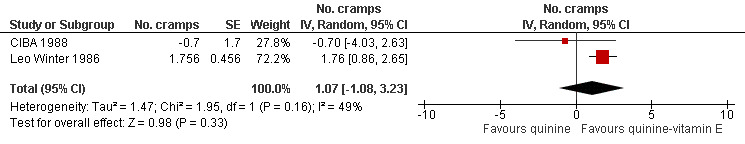
Forest plot of comparison: 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), outcome: 3.1 Difference in number of cramps in 2 weeks ‐ random‐effects.
Change in cramp intensity during treatment period
Three trials (n = 510), all unpublished, compared quinine to a quinine‐vitamin E combination (BioDesign 1984; CIBA 1988; Leo Winter 1986) and reported the change in cramp intensity.
We used a random‐effects model for the meta‐analysis because of unexplained heterogeneity. Accepting significant heterogeneity (I² = 74%) in the results, there was no significant difference between quinine and the quinine‐vitamin E combination with regards to cramp intensity (0.10, 95% CI ‐0.06 to 0.26) (see Analysis 3.2).
3.2. Analysis.
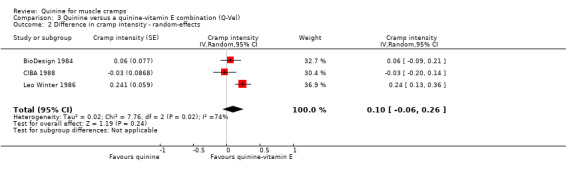
Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 2 Difference in cramp intensity ‐ random‐effects.
Change in cramp duration (in minutes)
Two trials (n = 299) measured cramp duration for this comparison, but could not be included in a meta‐analysis as grouped (BioDesign 1984) or descriptive (CIBA 1988) data only were available. There was no significant difference between quinine and quinine‐vitamin E combination in either study.
Change in number of cramp days
Two of the three trials involving a quinine‐vitamin E combination measured the number of cramp days as an outcome (CIBA 1988; Leo Winter 1986). The combined result of these two trials (n = 486) showed no significant difference between quinine and the quinine‐vitamin E combination (0.18 cramp days, 95% CI ‐1.13 to 1.49, random‐effects) (see Analysis 3.3). There was however significant unexplained heterogeneity (81%) in the results.
3.3. Analysis.
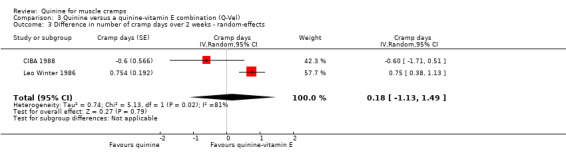
Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 3 Difference in number of cramp days over 2 weeks ‐ random‐effects.
Participants with one or more adverse events
Minor adverse events
Three trials (BioDesign 1984; CIBA 1988; Leo Winter 1986) were entered in the meta‐analysis. Seventy‐two participants out of the 370 (19.5%) taking quinine suffered from minor adverse events compared to 64 from 369 (17.3%) taking the quinine‐vitamin E combination. We used a random‐effects model as there was significant heterogeneity that could not be explained. The weighted RD was not significant at 3% (95% CI ‐4% to 10%) (Analysis 3.4). The adverse events mainly included: headache (29 participants on quinine versus 29 on quinine‐vitamin E), gastrointestinal (20 versus 17), tinnitus (4 versus 4), rashes (2 versus 0), pruritis (3 versus 1), visual disturbance (1 versus 1).
3.4. Analysis.
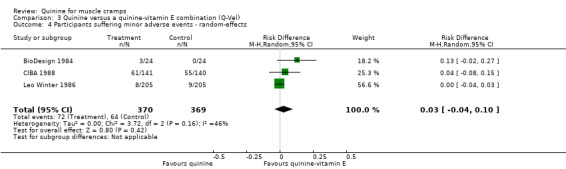
Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 4 Participants suffering minor adverse events ‐ random‐effects.
Major adverse events
Three trials (BioDesign 1984; CIBA 1988; Leo Winter 1986) were entered in the meta‐analysis. There were no major adverse events in the Leo Winter 1986 (n = 205) or BioDesign 1984 (n = 24) cross‐over trials. There were three participant withdrawals from each group in the CIBA 1988 trial. Thus, three participants (0.81%) withdrew from a total of 370 participants treated with quinine compared to three out of 369 on the quinine‐vitamin E combination (0.81%). The combined meta‐analysis thus showed no risk difference (RD 0%, 95% CI ‐1% to 1%) (Analysis 3.5).
3.5. Analysis.
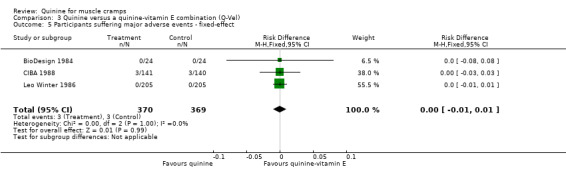
Comparison 3 Quinine versus a quinine‐vitamin E combination (Q‐Vel), Outcome 5 Participants suffering major adverse events ‐ fixed‐effect.
The adverse events suffered by those participants withdrawing from the quinine‐vitamin E group included: flu‐like symptoms, mild tinnitus, nausea, vomiting and diarrhoea, all of which resolved on cessation of medication. One participant was hospitalised with abdominal pain, headache, diffuse myalgias and fever, but their condition was never judged to be serious and improved with cessation of treatment (CIBA 1988). The three withdrawals from the quinine group all complained of gastrointestinal symptoms but also headache in one, and rash and paraesthesia in another (CIBA 1988). Again all resolved on cessation of treatment.
Quinine versus a quinine‐theophylline combination
Primary outcome measure: difference in number of cramps (occurring day or night) during a two‐week treatment period
A single parallel‐group study conducted by a pharmaceutical company (Gorlich 1991, n = 77 excluding placebo group) compared quinine to a quinine‐theophylline combination known as Limptar®. Standardised results from the second treatment week showed quinine was at a significant disadvantage compared to the quinine‐theophylline combination, giving 3.8 more cramps (95% CI 1.08 to 6.52) over two weeks, representing a 136% (95% CI 39% to 233%) difference.
Change in cramp intensity during treatment period
The Gorlich 1991 trial (n = 77 excluding placebo group) found that quinine alone was significantly less effective than the quinine‐theophylline combination in reducing cramp intensity (0.32 units, 95% CI 0.11 to 0.53) on a three‐point scale, representing a 168% (95% CI 58% to 279%) higher intensity.
Change in cramp duration (in minutes)
The Gorlich 1991 trial (n = 77) grouped cramp duration into three time intervals, meaning that individual cramp durations could not be ascertained. However, on their three‐point scale, quinine alone was significantly less effective than the quinine‐theophylline combination in reducing cramp duration (0.17 units, 95% CI 0.06 to 0.28), representing a 106% (95% CI 38% to 175%) longer cramp duration with quinine.
Change in number of cramp days
The Gorlich 1991 study (n = 77) reported a significantly higher incidence of cramp days over two weeks (2.2 cramp days, 95% CI 0.58 to 3.82) with the quinine group compared to the quinine‐theophylline combination, representing a 101% increase (95% CI 27% to 175%).
Participants with one or more adverse events
Minor adverse events
In the Gorlich 1991 trial, four participants suffered adverse events in the quinine‐theophylline combination group (n = 34). These included low blood pressure with dizziness (one participant), nausea and palpitations (two participants) and tinnitus (one participant). Three of these participants withdrew from the study but it is not clear from the study which participants these were. There was thus one minor adverse event in the quinine‐theophylline group (n = 34), which compares with one minor adverse event (nausea and vomiting) in the quinine group (n = 43) (Gorlich 1991).
Major adverse events
There were three withdrawals from the quinine‐theophylline group (n = 34), and this compares with two drop‐outs from the quinine group (n = 43), with nausea and vomiting.
Quinine versus xylocaine injection
Prateepavanich 1999 (n = 24) was the only trial to compare quinine with 1% xylocaine injections into the medial head of the gastrocnemius muscle. Both treatments reduced cramp number significantly from baseline but there was no significant difference between the groups after four weeks treatment. However, xylocaine's beneficial effect lasted longer; four weeks after treatment cessation, quinine had a significant disadvantage compared to xylocaine (1.35 cramps, 95% CI 0.52 to 2.18).
Change in cramp intensity during treatment period
In the Prateepavanich 1999 trial (n = 24) both treatments reduced cramp intensity significantly from baseline, but there was no significant difference between the groups at four weeks. However, at the follow‐up appointment four weeks after treatment cessation, the quinine group was at a significant disadvantage compared to xylocaine (0.72 units on a three‐point scale, 95% CI 0.24 to 1.20).
Change in cramp duration (in minutes)
In the Prateepavanich 1999 trial (n = 24) both treatments reduced cramp duration significantly from baseline, but there was no significant difference between the groups at four weeks. However, at the follow‐up appointment four weeks after treatment cessation, quinine was at a significant disadvantage compared to xylocaine (0.5 minutes, 95% CI 0.09 to 0.91).
Change in number of cramp days
The number of cramp days was not an outcome in the Prateepavanich 1999 trial.
Participants with one or more adverse events
Minor adverse events
There were no minor adverse events in the Prateepavanich 1999 study.
Major adverse events
The Prateepavanich 1999 study reported the withdrawal of two participants from the quinine treatment group (n = 10) due to 'cinchonism'. Details of the specific adverse events experienced by each participant were not provided, and no follow‐up is given. No adverse events occurred in the xylocaine injection group (n = 12).
Discussion
Quinine versus placebo
There is low quality evidence of a statistically significant benefit of quinine in reducing cramp number over two weeks (the primary outcome) and moderate quality evidence for a reduction in cramp intensity (Table 1). Quinine also reduced the number of cramp days over two weeks. The only outcome which was not significantly different from placebo was that of cramp duration. The Summary of findings table does not present data for cramp duration and cramp days, but using GRADE criteria, we consider the quality of the evidence for these outcomes as moderate and low, respectively, owing to study design limitations and additionally for cramp days, heterogeneity.
Significant unexplained heterogeneity was notable in many of the meta‐analyses, reflecting the variable results between the included studies, and this may reduce the confidence in the conclusions. It is this heterogeneity which has led to the lack of a clear consensus on this subject. As little is known about the pathophysiology of cramps or the mechanism of action of quinine, it is difficult to explain why quinine should have no significant effect on cramp duration, yet seems to reduce cramp number and intensity. However, cramp duration is probably the most difficult to judge.
The first meta‐analysis by Man‐Son‐Hing included six small cross‐over trials (n = 107) (Man‐Son‐Hing 1995). Later, the same group performed a second meta‐analysis that included four published and three unpublished trials (n = 659) (Man‐Son‐Hing 1998); this demonstrated that quinine was effective in reducing cramp number (21% reduction; 95% CI 12% to 30%), and intensity (‐0.13 unit on a three‐point scale, P = 0.002). This is broadly in line with the results of our meta‐analysis.
The American Academy of Neurology reviewed a variety of treatments for muscle cramp and found that there was Level A evidence that quinine was effective (Katzberg 2010). As this was not a meta‐analysis, there are no figures for comparison. Only 12 studies containing quinine were included in the review, and none of these were unpublished trials. The report recommends the avoidance of quinine for leg cramps unless absolutely necessary, because of its potentially serious side‐effect profile. The review of adverse events also consisted of case reports and case series where high doses of quinine were being used for the treatment of malaria.
Quinine versus other treatments
This meta‐analysis showed there was no significant difference between quinine and vitamin E, or between quinine and a quinine‐vitamin E combination (Table 2 and Table 3). This would suggest that vitamin E alone may be as effective as quinine in reducing cramp number, intensity, and days, but that its effect when combined with quinine is not significantly additive.
We removed Connolly 1992 after sensitivity analyses for methodological reasons. However, inclusion of this study would make quinine seem significantly more effective than vitamin E at reducing cramp number and days. Vitamin E is generally regarded as safe, but a meta‐analysis (Miller 2005) suggested that high‐dose vitamin E (400 IU/d or more) may increase all‐cause mortality and should be avoided.
A single study (n = 77) performed by a pharmaceutical company suggested that a quinine‐theophylline combination was significantly more effective than quinine alone across all outcomes (Gorlich 1991). There were no additional adverse events. More trials are needed containing quinine‐theophylline to support or refute these findings.
Cramps with an identified myofascial trigger point may be more unusual. One trial (Prateepavanich 1999, n = 24) showed that quinine did not differ significantly from xylocaine injections into these trigger points after four weeks treatment, but that the xylocaine injections were significantly more effective at the eight‐week follow‐up (four weeks after treatment cessation). Further long‐term studies are required to replicate this finding and assess the feasibility of its application.
Effect of quinine dose and duration
Dose
Accumulation of quinine with daily dosing can be expected because its half‐life is between 9 and 11 hours in young people (Berlin 1975; Mutual 2006) and up to 19 hours in the elderly (Smith 1985; Warburton 1987). Despite these pharmacokinetic changes in the elderly, an alteration in the quinine dosage regimen in elderly people is not recommended by the manufacturers of one quinine product (Qualaquin) (Mutual 2006).
Two trials showed a significant positive correlation between serum quinine levels and reductions in cramp number (Lee 1991; Warburton 1987), whilst one trial showed no correlation (Woodfield 2005). In the Lee 1991 trial where 200 mg quinidine was given twice daily, the mean (SD) peak and trough serum levels at two weeks were 1.3 (0.1) mg/L and 0.7 (0.1) mg/L respectively. Two participants who did not show a significant response to quinidine were found to have low trough serum levels (0.2 mg/L and 0.3 mg/L); upon increasing the dose to 600 mg daily, trough levels improved to 0.6 and 0.7 mg/L and significant reductions in cramp number were then achieved. This indicates that quinine therapy may benefit from monitoring serum drug levels and adjusting dosage accordingly. Further trials are needed to elucidate the optimum therapeutic range for quinine in muscle cramps.
In the trials identified for this meta‐analysis, there was a trend of increasing effect with quinine doses of 300 mg to 500 mg. However, the results with lower doses (200 mg to 260 mg) go against this trend. This, however, is a very limited analysis as there was a shortage of studies in each dosage group and also several confounding variables. The Connolly 1992 trial was the only one to use a dose of 500 mg quinine; the adverse events experienced were comparable to those in the other trials.
Duration
The optimum treatment duration with quinine has equally not been investigated. The Connolly 1992 study suggested that quinine was rapidly effective, achieving a 50% reduction in cramp number in almost half the participants by three days. One trial (Leo Winter 1986) showed that a short five‐day course was sufficient to bring about significant change, whilst two trials recommended seven days (BioDesign 1984; CIBA 1988).
Most studies showed greater effect the longer quinine was continued; for example, cramp reductions were more significant in the second treatment week than the first in the Gorlich 1991 trial. However, the opposite was found in the CIBA 1988 trial where significant reductions after week one became nonsignificant in week two. The duration of treatment varied from 5 to 60 days in the included trials. Of the five trials with the greatest reductions in cramp number by quinine, four were achieved by the longest trials, with treatment durations of 42 days (Woodfield 2005) and 28 days (Connolly 1992; Fung 1989; Lee 1991).
Quinine was found to have significant lasting effects at two weeks after treatment cessation in three trials (Diener 2002; Jansen 1994; Jansen 1997), and at four weeks in another (Prateepavanich 1999). If continuous therapy is not needed, then knowing the duration of quinine's effect after cessation will allow the determination of suitable periods for 'drug holidays' and also for the required washout periods in new trials.
Adverse events due to quinine
The adverse events attributed to quinine can be divided into three types: those arising from hypersensitivity reactions which may occur immediately or after years of treatment (thrombocytopenia, disseminated intravascular coagulation, acute renal failure, haemolytic uraemic syndrome), those reversible adverse events that are dose‐dependent and can occur with normal use (gastrointestinal upset, tinnitus, vertigo, visual disturbance), and those that arise from toxic overdose (cardiac arrhythmias, blindness, seizures) (Bateman 1985; Knower 2003; Morton 2002; Prasad 2003; Schneemann 2006). All but one of the adverse events occurring from the trials included in this meta‐analysis fall into the second group; these dose‐related adverse events can occur with normal therapeutic use of quinine, but are more common when plasma quinine levels rise above 5 mg/L (Mandal 1995; Schneemann 2006).
Quinine has been implicated in accidental and intentional poisoning. The Scottish Poisons Information Bureau reported 96 such cases (out of a population of 5.2 million) over five years across a wide range of ages and overdosage; 38% of these patients were asymptomatic, 23% suffered visual toxicity, 19% auditory toxicity, 15% cardiotoxicity, 14% gastrointestinal symptoms, and 11% reduced Glasgow Coma Scale (Langford 2003).
Renal impairment and interactions with other medication (such as digoxin, anticoagulants and phenothiazines) must also be borne in mind (Pederson 1985), as well as the fact that memory loss in the elderly may make people vulnerable to overdosage of quinine.
This meta‐analysis showed that significantly more people suffered minor adverse events on quinine than placebo but no difference in major adverse events. Gastrointestinal upset in the form of nausea, vomiting and abdominal pain was the most common adverse event (5.7%, n = 806), followed by headache (4.7%), and tinnitus (1.4%). Pruritus, scaly rashes, dizziness, visual disturbances, and fever were rare. The only minor adverse events that were significantly higher with quinine were gastrointestinal events. This compares with Man‐Son‐Hing 1998 which showed that tinnitus was the only minor adverse event that was significantly more common with quinine.
Though 12 participants withdrew from quinine treatment due to side effects, these tended to be benign adverse events, and may or may not have been related to quinine; indeed, a total of 11 participants withdrew from placebo treatment, some with similar adverse events. There was however one serious adverse event which was likely to be attributed to quinine: one participant in the Sidorov 1993 trial suffered leukopenia and thrombocytopenia, with a severe rash, myalgia, and nausea. His symptoms resolved three days after stopping quinine.
In the placebo‐controlled trials, there were 1103 unique participants in the meta‐analysis of major adverse events and 969 unique participants in the minor adverse events meta‐analysis (the treatment groups contained many participants from cross‐over trials so the total numbers in both groups should not be summated). On the basis of these, quinine appears to be reasonably safe, but it is not possible to accurately calculate the true incidence of serious or life‐threatening side effects which are rare. A case‐control study in the USA estimated that quinine‐induced thrombocytopenia occurs in 26 of every 1 million users (Kaufman 1993).
Between 1969 and 1990, 110 serious adverse events with suspected links to quinine were reported to the Food and Drug Administration (FDA 1995b). However, the FDA judged that of the 110 reports, only 26 could reasonably be attributed to quinine; 21 of these cases involved quinine doses used in the treatment of leg cramps. There were no cases of overdose, but there were three deaths (FDA 1995b). There were six cases of severe skin reactions (two of which were erythema multiforme), 13 'haematological events' (two resulting in death), two cases of hepatitis, two cases of renal failure (one resulting in death, the other dialysis‐dependence), two cases of hypersensitivity syndrome ("chills, nausea, vomiting, diarrhoea"), and one case of anaphylaxis complicated by seizures and hypoxia following a single dose of quinine (FDA 1995b). Although this is invaluable information, the incidence of such adverse events still cannot be ascertained as the size of the treated population is unknown and not all serious side effects are reported to the FDA. It is however on the basis of these serious adverse effects that the FDA has banned the marketing of quinine for muscle cramps (FDA 2006) and that the American Academy of Neurology has recommended in their report (Katzberg 2010) that it only be used as a last resort in intractable cramps and with close monitoring.
Haemodialysis and liver cirrhosis
Muscle cramps are more common in people on haemodialysis (Chou 1985; Khajehdehi 2001) and in those with liver cirrhosis (Abrams 1996; Angeli 1996). Two trials (Kaji 1976; Roca 1992) involved participants with cramps related to dialysis, and quinine significantly reduced cramp number in both, along with cramp intensity in one (Roca 1992), and duration in the other (Kaji 1976). Serum levels of quinine were not monitored, but there were no reports of toxicity with doses of 325 mg daily (Roca 1992) and 320 mg thrice weekly (Kaji 1976). The manufacturers of a quinine product (Qualaquin) state that negligible amounts of quinine are removed from the blood during dialysis, and that the half‐life in people with severe renal impairment who are not on dialysis is increased to 26 hours, despite the vast majority of quinine being metabolised by the liver (Mutual 2006). The manufacturers thus recommend lower doses of quinine in people with severe renal impairment who require treatment for malaria (less than half the normal dose) (Mutual 2006).
One trial included 31 participants with liver cirrhosis and found the optical isomer of quinine, quinidine, to be significantly effective in reducing cramp number, with no sign of serum accumulation (Lee 1991). The manufacturers of Qualaquin state that although the elimination half‐life is increased in people with mild to moderate hepatic impairment, dosage adjustment is not needed as weight‐adjusted clearance remains the same (Mutual 2006).
Risk of bias and trial quality
Almost all of the trials included in this meta‐analysis had methodological limitations. As with all meta‐analyses, this leads to exclusion of some trials from some analyses, or the inclusion of data imputed from the published data. Each of the methodological flaws potentially impairs the quality of the data and the power of the meta‐analysis. Specific limitations included inadequate washout periods in cross‐over studies (Dunn 1993; Kaji 1976; Leo Winter 1986; Maule 1990; Smith 1985; Warburton 1987; Woodfield 2005), small number of participants (all but four trials contained less than 100 participants), inadequate explanations of methodology (for example randomisation and blinding), and poor statistical presentation (including lack of mean and standard error figures for outcomes).
A common problem was the lack of explanations for drop‐outs and the lack of intention‐to‐treat analyses. Many of the trials failed to elaborate on adverse events, which lie at the heart of the debate of quinine's benefit‐risk ratio. Only four trials (Lee 1991; Smith 1985; Warburton 1987; Woodfield 2005) measured serum quinine concentrations, which is not only helpful in determining the dose‐response relationship for muscle cramp, but allows the ceiling effect to be estimated (the level above which the risks of serious adverse events becomes significant).
It was impossible to exclude the confounding factor of physiological interventions. Some participants may have used various stretch exercises in addition to their allocated treatment and this may have influenced the outcomes to varying degrees. One study reported that seven out of the 10 participants used stretches to relieve cramp in the acute phase, although the outcomes in this trial did not include cramp duration or intensity (Woodfield 2005).
Other considerations
No study addressed the health economics of quinine; this is most likely because the trials were conducted when quinine was readily and cheaply available in the USA. Recently, however, the cost has risen sharply in the USA due to the FDA's ban on the marketing of quinine for muscle cramps, with the only approved brand being Qualaquin (Mutual Pharmaceutical) for falciparum malaria. Quinine in Europe, however, remains readily available and inexpensive; for instance in the UK each generic 300 mg tablet costs just under GBP 0.08 (USD 0.12). This compares with around USD 5.00 per 324 mg capsule of Qualaquin in the USA (Mutual Pharmaceutical). The difference between the USA and Europe with regards to quinine use in muscle cramps is striking. Whilst it can only be used off‐label in the USA for muscle cramps, in the UK quinine is fully licensed and regularly prescribed by general practitioners for people suffering from muscle cramp. It can be bought over the counter in Germany.
The British National Formulary (BNF) advises that it may take up to four weeks for the effect on cramp to become apparent and that if there is improvement, then quinine should be taken continuously with close monitoring for adverse events (BNF 2010). The BNF also advises interruption of treatment every three months for review of benefit. Some studies have suggested that there are people who benefit from quinine and some who do not (Connolly 1992; Woodfield 2005); this may be due to inadequate serum concentrations in some, or other factors which have yet to be identified. There is also no current evidence to predict who will suffer serious adverse events, and so it has been suggested that a trial of quinine be used on an individual basis to identify those who will safely benefit from therapy, and prevent others from unnecessary and possibly harmful long‐term medication (Woodfield 2005).
It has also been stated that before starting a trial of quinine, reversible causes of cramp such as hypothyroidism, electrolyte imbalance and the presence of cramp‐inducing medication should be considered (Guay 2008; Miller TM 2005). An audit of prescribing patterns in general practice showed that out of 70 people prescribed quinine for muscle cramp, over half were taking medications known to cause cramps (Mackie 1995).
There is a paucity of data concerning the use of quinine in pregnancy (Nosten 2006). Quinine crosses the placenta and is also excreted in breast milk, although breast feeding whilst on quinine is not contraindicated (AAP 2001). In very high doses, quinine can be teratogenic; in pregnant women who took overdoses of quinine, abortion was very rare but congenital anomalies occurred with central nervous system, limb, facial and cardiac defects (Nishimura 1976), optic nerve hypoplasia and deafness (McKinna 1966; Morgon 1971). However, at doses used in malaria, quinine appears to be safe in pregnancy (BNF 2010; McGready 2001; McGready 2002; Nosten 2002; Orton 2008) and does not increase the risk of abortion or preterm delivery (Phillips‐Howard 1996). There are, however, the expected side effects on the mother with the high doses used in malaria (Piola 2010). The World Health Organization recommends that quinine be used as first‐line treatment for uncomplicated falciparum malaria in the first trimester of pregnancy, and third‐line treatment in the second and third trimesters because of an increased risk of maternal hypoglycaemia in late pregnancy (WHO 2010). It is likely that the benefits of treatment outweigh the risks when treating pregnant women with malaria, but this may not be the case with muscle cramps. Magnesium salts may be a safer alternative for the treatment of cramps in pregnant women (Dahle 1995; Young 2002).
Authors' conclusions
Implications for practice.
There is low quality evidence that quinine (200 mg to 500 mg daily) significantly reduces cramp number and cramp days and moderate quality evidence that quinine reduces cramp intensity. Evidence from single trials suggests that theophylline combined with quinine improves cramps more than quinine alone and the effects of xylocaine injections into gastrocnemius are not significantly different to quinine across all outcomes. Low or moderate quality evidence shows no significant difference between quinine and vitamin E (four trials) or quinine and quinine‐vitamin E mixture (three trials). Major adverse events are rare with quinine (moderate quality evidence) but can be serious or fatal so that in some countries prescription of quinine is severely restricted. There is no evidence to judge optimal dosage or duration of quinine treatment.
Implications for research.
The optimum dose and duration of quinine treatment require elucidation. Measurement of serum quinine levels will allow a therapeutic range to be defined for muscle cramp. Longer lengths of follow‐up in trials will help determine the duration of its action following cessation as well as long‐term adverse events. Because serious adverse events are not common, large population studies are required to more accurately inform incidence. The search for new therapies, pharmacological and nonpharmacological, should continue and further trials should compare vitamin E, quinine‐vitamin E combination, and quinine‐theophylline mixture with quinine.
What's new
| Date | Event | Description |
|---|---|---|
| 10 May 2013 | New search has been performed | Minor update to background section. We revised the format of the review to current standards, including additional sections in the Methods. |
| 10 May 2013 | New citation required but conclusions have not changed | Searches updated to October 2014. No new trials identified. We reviewed and revised our assessments of the quality of the evidence for some outcomes. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 12, 2010
| Date | Event | Description |
|---|---|---|
| 5 April 2009 | Amended | Tables amended |
| 12 February 2009 | Amended | Converted to new review format. |
Acknowledgements
In loving memory of Dr Tariq El‐Tawil who sadly passed away in 2007 as this meta‐analysis was coming to an end. His valuable contribution as co‐author reflected the diligence and perfectionism that he strove for in all his work.
Thank you to Dr AV Swan for statistical assistance, and to Prof R Hughes, K Jewitt, and R Brassington, for such valuable editorial support and guidance for the first version of this review. Editorial support from the Cochrane Neuromuscular Disease Group was funded by the TREAT NMD European Union Grant 036825 for the first version of this review.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Neuromuscular Disease Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The Cochrane Neuromuscular Disease Group is also supported by the MRC Centre for Neuromuscular Disease and Motor Neurone Disease Association.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to October Week 3 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (397325) 2 controlled clinical trial.pt. (90482) 3 randomized.ab. (292700) 4 placebo.ab. (154093) 5 drug therapy.fs. (1776484) 6 randomly.ab. (205493) 7 trial.ab. (304804) 8 groups.ab. (1306164) 9 or/1‐8 (3347080) 10 exp animals/ not humans.sh. (4079851) 11 9 not 10 (2851539) 12 cramp$.tw. or Muscle Cramp/ (6561) 13 spasm$.tw. or Spasm/ (24913) 14 Muscle Contraction/ or contraction$.tw. (170479) 15 or/12‐14 (198873) 16 quinine.tw. or Quinine/ (8989) 17 hydroquinine.mp. (24) 18 quinidine.tw. or quinidine/ (8267) 19 or/16‐18 (16456) 20 11 and 15 and 19 (191) 21 remove duplicates from 20 (186)
Appendix 2. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2014 Week 43> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (40422) 2 double‐blind procedure.sh. (115822) 3 single‐blind procedure.sh. (18928) 4 randomized controlled trial.sh. (351750) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1068811) 6 trial.ti. (163787) 7 or/1‐6 (1202625) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1295023) 9 animal/ or nonanimal/ or animal experiment/ (3263473) 10 9 not 8 (2732841) 11 7 not 10 (1104534) 12 limit 11 to embase (915914) 13 cramp$.mp. or Muscle Cramp/ (18311) 14 spasm$.mp. or Spasm/ (48366) 15 Muscle Contraction/ or contraction$.mp. (203664) 16 or/13‐15 (264699) 17 quinine.mp. or Quinine/ (16438) 18 hydroquinine.mp. (158) 19 quinidine.mp. or quinidine/ (18974) 20 or/17‐19 (33461) 21 12 and 16 and 20 (89) 22 remove duplicates from 21 (89)
Appendix 3. Cochrane Neuromuscular Disease Group Specialized Register (CRS) search strategy
#1 cramp* or spasm* or contraction* [REFERENCE] [STANDARD] #2 quinine or quinidine or hydroquinine [REFERENCE] [STANDARD] #3 #1 and #2 [REFERENCE] [STANDARD] #4 #1 and #2 [REFERENCE] [STANDARD] #5 (#1 and #2) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 4. CENTRAL SEARCH STRATEGY
#1 MeSH descriptor Muscle Cramp, this term only #2 cramp OR cramps #3 MeSH descriptor Spasm, this term only #4 spasm OR spasms #5 MeSH descriptor Muscle Contraction, this term only #6 contraction #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) #8 MeSH descriptor Quinine, this term only #9 MeSH descriptor Quinidine, this term only #10 quinine OR quinidine OR hydroquinine #11 (#8 OR #9 OR #10) #12 (#7 AND #11)
Appendix 5. ICTRP and ClinicalTrials.gov search strategy
quin* AND cramps OR hydroquinine AND cramps
Data and analyses
Comparison 1. Quinine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Difference in number of cramps over 2 weeks (GIV) ‐ fixed‐effect | 14 | Cramp number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| 2 Difference in number of cramps over 2 weeks ‐ random‐effects (minus Connolly 1992) | 13 | Cramp Number (Random, 95% CI) | ‐2.45 [‐3.54, ‐1.36] | |
| 3 Difference in number of cramps according to quinine dose (GIV) ‐ fixed‐effect | 14 | Cramp Number (Fixed, 95% CI) | ‐1.81 [‐2.20, ‐1.42] | |
| 3.1 500 mg quinine | 1 | Cramp Number (Fixed, 95% CI) | ‐8.7 [‐10.30, ‐7.10] | |
| 3.2 400 mg quinine | 2 | Cramp Number (Fixed, 95% CI) | ‐3.36 [‐4.83, ‐1.89] | |
| 3.3 300 to 325 mg quinine | 5 | Cramp Number (Fixed, 95% CI) | ‐0.79 [‐1.31, ‐0.26] | |
| 3.4 260 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐1.29 [‐2.15, ‐0.42] | |
| 3.5 200 mg quinine | 3 | Cramp Number (Fixed, 95% CI) | ‐3.22 [‐4.40, ‐2.04] | |
| 4 Difference in cramp intensity (GIV) ‐ fixed‐effect | 7 | Cramp intensity (Fixed, 95% CI) | ‐0.12 [‐0.20, ‐0.05] | |
| 5 Change in cramp duration (min) ‐ random‐effects | 2 | Change in duration (Random, 95% CI) | ‐1.35 [‐4.00, 1.30] | |
| 6 Difference in number of cramp days over 2 weeks (GIV) ‐ random‐effects (minus Connolly 1992 | 7 | Cramp days (Random, 95% CI) | ‐1.15 [‐1.93, ‐0.38] | |
| 7 Participants suffering minor adverse events | 16 | 1447 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 8 Participants suffering specific minor adverse events | 18 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Gastrointestinal | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 8.2 Headache | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.02, 0.02] |
| 8.3 Tinnitus | 18 | 1581 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 9 Participants suffering major adverse events | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 10 Participants suffering specific major adverse events (gastrointestinal) | 18 | 1613 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
Comparison 2. Quinine versus vitamin E.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects (minus Connolly 1992) | 3 | No. cramps (Random, 95% CI) | ‐0.24 [‐1.29, 0.81] | |
| 2 Difference in cramp intensity ‐ fixed‐effect | 3 | Cramp intensity (Fixed, 95% CI) | ‐0.06 [‐0.17, 0.04] | |
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects (minus Connolly 1992) | 2 | Cramp days (Random, 95% CI) | ‐0.28 [‐0.98, 0.43] | |
| 4 Participants suffering minor adverse events ‐ random‐effects | 2 | 688 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.09] |
| 5 Participants suffering major adverse events ‐ random‐effects | 3 | 748 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
Comparison 3. Quinine versus a quinine‐vitamin E combination (Q‐Vel).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Difference in number of cramps in 2 weeks ‐ random‐effects | 2 | No. cramps (Random, 95% CI) | 1.07 [‐1.08, 3.23] | |
| 2 Difference in cramp intensity ‐ random‐effects | 3 | Cramp intensity (Random, 95% CI) | 0.10 [‐0.06, 0.26] | |
| 3 Difference in number of cramp days over 2 weeks ‐ random‐effects | 2 | Cramp days (Random, 95% CI) | 0.18 [‐1.13, 1.49] | |
| 4 Participants suffering minor adverse events ‐ random‐effects | 3 | 739 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 5 Participants suffering major adverse events ‐ fixed‐effect | 3 | 739 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
BioDesign 1984.
| Methods | Double‐blind RCT of cross‐over design, based in Germany | |
| Participants | 24 participants (aged 51 to 64 years) who experienced at least 3 nocturnal leg muscle cramps per week | |
| Interventions | A quinine‐vitamin E combination (4 tablets taken daily, each containing 64.8 mg quinine sulphate and 400 IU vitamin E) or quinine sulphate 64.8 mg (4 tablets taken daily) alone taken for 1 week each. 7‐day placebo washout periods before, between and after treatments | |
| Outcomes | Cramp number, cramp duration, cramp intensity after treatment (graded 0 = better to 3 = much worse), adverse events | |
| Notes | Unpublished study conducted by BioDesign (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After randomization..." Comment: participants were allocated a number from 1 to14 and "randomized" to a specific group but no details of the randomization process are provided |
| Allocation concealment (selection bias) | Unclear risk | No details were given on how the allocation may have been concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The study was designed as a double blind..."; "...5 containers with 30 capsules labelled with the number of the treatment week were provided." Comment: probably done as quinine‐vitamin E combination and quinine capsules are similar by description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs from the trial |
| Selective reporting (reporting bias) | Low risk | All intended outcome measures were addressed in the results and analysis |
| Other bias | Unclear risk | Conducted by manufacturer of quinine tablets |
Bottner 1984.
| Methods | Double‐blind RCT of cross‐over design, based in Arizona, USA | |
| Participants | 69 participants (mean age 51 years, 3 men) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week baseline then 2 weeks of quinine sulphate (260 mg) or placebo, then 2‐week washout, then 2 weeks of cross‐over treatment, then 2‐week washout | |
| Outcomes | Cramp number, cramp intensity, cramp duration sleep disturbance, adverse events | |
| Notes | Out of the 69 participants, only 3 were men Unpublished (sponsored by Scholl Inc.) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were assigned a study number based upon sequential entry...The study numbers had been previously randomly assigned to either Group 1 or Group 2..." Comment: probably done |
| Allocation concealment (selection bias) | High risk | Quote: "The identity of the medication will be unknown to the patient and the Investigator but will be identifiable to the Clinical Monitor based on the randomization schedule."; "All patients which the Investigator judges eligible for admission into the study, must be approved by one of the Clinical Monitors either in person or by phone..." Comment: the clinical monitor overseeing the trial was responsible for vetting all candidates before entry into the study and also had access to the randomization schedule |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Placebo capsules will be of identical composition to the active capsule, except for the Quinine Sulfate content, and will be identical in appearance."; "The identity of the medications was unknown to the Investigator and the subjects" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 69 out of 84 participants completed the study but no mention is made of the drop‐outs or the underlying reasons |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in protocol addressed in analysis |
| Other bias | Unclear risk | The study was sponsored by Scholl who were marketing quinine as a treatment for cramps. Also 66 of the 69 participants were women although the significance of gender to outcome is not known |
CIBA 1988.
| Methods | Double‐blind RCT of parallel design, conducted in USA | |
| Participants | 556 participants (aged 18 to 84 years) who experienced at least 3 nights of nocturnal leg muscle cramps per week | |
| Interventions | 7‐day placebo washout period followed by 2 weeks of a quinine‐vitamin E combination (259.2 mg quinine sulphate and 1600 IU vitamin E daily) or quinine sulphate 259.2 mg alone or vitamin E (1600 IU) alone, or placebo | |
| Outcomes | Cramp number, cramp days, cramp intensity, cramp duration, sleep disturbance, adverse events | |
| Notes | Large multicentre trial. Approximately double number of women than men across all treatment groups. Unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...according to a predetermined randomized schedule" |
| Allocation concealment (selection bias) | Unclear risk | No details are given regarding how allocation may have been concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All capsules will be identical in appearance", "...weeks 2 and 3 will be double‐blind treatment periods" Comment: probably adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and those lost to follow up accounted for, and intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in detail |
| Other bias | Unclear risk | Conducted by manufacturer of quinine tablets |
Connolly 1992.
| Methods | Double‐blind RCT of cross‐over design, conducted at a Veterans Affairs Medical Center in USA | |
| Participants | 30 male participants (aged 38 to 73 years) who experienced at least 6 leg cramps per month | |
| Interventions | Quinine sulphate 500 mg daily (200 mg at supper, 300 mg at bedtime) or vitamin E 800 U daily or placebo for a 4‐week treatment period, followed by a 4‐week washout period before cross‐over to a second 4‐ week treatment period | |
| Outcomes | Cramp number, cramp nights, cramp intensity (graded 1 = no pain to 4 = severe), sleep disturbance (graded 1 = none to 4 = severe), adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Drug‐on periods were assigned in randomly permuted order..." Comment: details of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided on how allocation may have been concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "All medications were packaged in unit doses and dispensed by the same company." Comment: probably adequate blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 3 participants who failed to complete the study were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Other bias | Unclear risk | All subjects were men as all recruited from Veterans Affairs Medical Center |
Diener 2002.
| Methods | Double‐blind, parallel group RCT, set in Germany | |
| Participants | 94 participants (aged 18 to 70 years) who experienced at least 6 muscle cramps in 2 weeks | |
| Interventions | Quinine sulphate 400 mg daily or placebo for 2 weeks | |
| Outcomes | Cramp number, cramp days, cramp intensity (scale not stated), sleep disturbance (scale not stated), global efficacy rating by participant and doctor (scale not stated), adverse events | |
| Notes | Multicentre trial in Germany; participants taken from 17 general practices | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation used permuted blocks of four patients stratified by the centre...... when the sealed envelopes were collected and the blind review written, the code was revealed." Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quotes: "All investigators enrolled in the study and all participants were unaware of the treatment allocation, because tablets were identical..."; "A sealed envelope assigning either verum or placebo was available in each centre for each patient..." Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...because the quinine and placebo tablets were identical in appearance." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | According to the table of results ('Table 2' in the study), there were 6 drop‐outs; none of these are mentioned in the text |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Other bias | Low risk | Trial completed at designated time period. Well‐matched participant characteristics at baseline |
Dunn 1993.
| Methods | Double‐blind RCT of cross‐over design, carried out in 2 centres in the UK | |
| Participants | 28 participants (aged 51 to 82 years) selected from 2 general practices on the basis that they received regular repeat prescriptions for quinine | |
| Interventions | Quinine sulphate 300 mg daily or placebo for a 30‐day treatment period, followed by a 3‐day washout period before cross‐over to a second 30‐day treatment period | |
| Outcomes | Number of cramp nights, adverse events | |
| Notes | Results were invalidated by a significant carry‐over effect due to a short washout period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a randomised double blind cross‐over trial..." Comment: details of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No details regarding allocation concealment are provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details regarding methods of blinding are provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Of the 28 recruited, 25 completed the two parts of the trial and filled in diary cards successfully. Two of the three drop‐outs did so because of severe cramps during placebo period" Comment: 1 drop‐out not accounted for |
| Selective reporting (reporting bias) | Low risk | Both the intended outcome measures were addressed in the results and analysis |
| Other bias | High risk | Cross‐over trial with only 3 days allocated to washout period rendered a significant carry‐over effect of treatment |
Fung 1989.
| Methods | Double‐blind RCT of cross‐over design, set in Utah, USA | |
| Participants | 9 elderly outpatients with a history of night cramps of at least 1 year, with at least 2 cramps per week | |
| Interventions | Quinine sulphate 200 mg or placebo at bedtime for a 4‐week treatment period, followed by a 1‐week washout period before cross‐over to a second 4‐week treatment period | |
| Outcomes | Cramp number, cumulative duration of attacks (in minutes), cumulative score of cramp severity (graded 1 = mild to 3 = severe), adverse events | |
| Notes | The cumulative duration of cramps was calculated as was the score for intensity. Duration or intensity per cramp was not calculated. However, these were calculated from individual patient data. Recruitment in June to December 1987 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...and patients were randomly assigned.." Comment: no details of the randomisation process are provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided on how allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "in a double‐blind manner to begin receiving either quinine or a placebo." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Explanation given for the one drop‐out, but was not included in analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All intended outcome measures are addressed in the results |
| Other bias | Unclear risk | 7 of the 8 volunteers who completed the trial were women |
Gorlich 1991.
| Methods | Double‐blind, parallel group RCT, set in Germany | |
| Participants | 164 participants (mean age 56 years) suffering from at least 3 nights of leg cramps per week | |
| Interventions | Combination therapy of quinine sulphate plus theophylline ethylene diamine, or quinine alone, or placebo daily for 2 weeks. Before this treatment period, participants were put on placebo as a run‐in phase | |
| Outcomes | Cramp number, cramp nights, cramp intensity (graded 1 = mild to 3 = severe), cramp duration, adverse events | |
| Notes | Multicentre study in Germany conducted by Merrell Dow Pharma (now Sanofi‐Aventis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "für jedes Zentrum wurde eine Blockrandomisierung vorgenommen....."; [for each centre a blockwise randomization sequence was generated]......" Comment: probably adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Die Prüfärzte hatten für jeden Patienten einen verschlossenen Umschlag erhalten, in dem aussen die Randomnummer und innen das Prüfmedikament verzeichnet war" [Each prinicipal investigator was given a sealed envelope for each respective patient with the random number marked on the outside and the medication on the inside] Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: " 3‐fache blinde Studienanlage" [Triple blind study setting]:; die äusserlich indentischen und nicht voneinander zu unterscheidenen Tabletten..." [from the outside identical tablets indistinguishable with respect to form, taste, colour...] Comment: participants, principal investigators and statistician were all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Tab.5: Gründe für die fehlende Aufnahme in die inferenzstatistischen Zeitreihenanalysen.." [Tab.5: Reasons for exclusion from statistical analysis] Comment: reasons are given for all participants not included in the statistical analysis |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures are reported |
| Other bias | Unclear risk | Conducted by manufacturer of quinine and also the quinine‐theophylline combination |
Hays 1986.
| Methods | Double‐blind RCT of cross‐over design, set in Florida USA | |
| Participants | 62 participants (mean age 47 years) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week baseline then 2 weeks of quinine sulphate (325 mg) or placebo, then 2‐week washout, then 2 weeks of cross‐over treatment, then 2‐week washout | |
| Outcomes | Cramp number, cramp intensity, cramp days, adverse events | |
| Notes | Second trial by Scholl pharmaceuticals submitted to FDA, but with higher quinine dose of 325 mg. Unpublished. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...medications will be assigned according to a predetermined randomization schedule" Comment: no details of the "randomization schedule" are provided |
| Allocation concealment (selection bias) | Low risk | Quote: "Each subject's medications will be provided by the Sponsor and distributed by the Invesitgator" Comment: appears that allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Placebo capsules...will be identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Conducted by manufacturer of quinine tablets |
Jansen 1994.
| Methods | Double‐blind, parallel group RCT, set in the Netherlands. | |
| Participants | 20 adult volunteers (median age 55 years) from general population who suffered at least 3 muscle cramps per week | |
| Interventions | Hydroquinine hydrobromide 300 mg daily (200 mg at supper, 100 mg at bedtime) or placebo for 2 weeks. This was followed by a 2‐week intervention‐free period whereby persistence of drug effect was monitored | |
| Outcomes | Reduction in cramp number from baseline for each treatment group. Cramp severity (scale not stated), cramp duration, cramp location and adverse events were also outcomes | |
| Notes | Adult volunteers were recruited via a notice in a regional newspaper, with a "small financial reward". Randomisation led to quinine group being solely women whilst all men were randomised into placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty participants were randomly allocated to receive either active drug or placebo." Comment: no details of the randomisation process are provided |
| Allocation concealment (selection bias) | Low risk | Quote: "During the trial only the manufacturer knew the codes disclosing drug and placebo." Comment: probably adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "...three quinine users who complained of a bitter taste possibly were not blind to the type of medication..." Comment: inadequate blinding with high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One participant dropped out of the placebo group but it is unclear if an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | High risk | Quote: "Differences in severity, duration and location ... between placebo...and drug treatment were small." Comment: emphasis placed on cramp number, with no mention of the results for the 3 other outcomes |
| Other bias | Unclear risk | Volunteers were recruited via notice in a regional newspaper, for a "small financial reward". The quinine group was solely women, whilst all men were randomised into placebo group |
Jansen 1997.
| Methods | Double‐blind, parallel group RCT, set in the Netherlands | |
| Participants | 106 adult participants from general population who suffered at least 3 muscle cramps per week | |
| Interventions | Hydroquinine hydrobromide dihydrate 300 mg daily (200 mg at supper, 100 mg at bedtime) or placebo for 2 weeks | |
| Outcomes | Cramp number, cramp days, cramp intensity (graded 1 = mild to 10 = severe), cramp duration, cramp location, adverse events | |
| Notes | Adult volunteers were recruited via a notice in a regional newspaper, and posters in pharmacies and libraries | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent investigator used the random‐number generator of the SAS program to create the randomisation schedule." |
| Allocation concealment (selection bias) | Low risk | Quote: "All investigators involved in the study and all participants were unaware of the treatment allocation." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The only side‐effect definitely related to hydroquinine was a bitter taste or dry mouth (ten participants)..." Comment: inadequate blinding with high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who failed to complete the trial were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Data were collected with respect to cramp duration, severity and location, in addition to the primary outcome of frequency. However little actual data are presented to justify the "insignificant differences between drug and placebo" reported, and no mention of results for cramp location is made |
| Other bias | Unclear risk | Quote: "We recruited volunteers through notices in regional newspapers and posters in libraries and pharmacies." |
Jones 1983.
| Methods | Double‐blind RCT of cross‐over design, UK | |
| Participants | 9 elderly participants seeking treatment from GP for at least 2 cramp nights per week | |
| Interventions | Quinine sulphate 300 mg or placebo daily for a 2‐week treatment period, followed by a 2‐week washout period before cross‐over to a second 2‐week treatment period. A 2‐week run‐in period (of placebo) preceded the first phase of treatment | |
| Outcomes | Improvement in sleep induction (graded 1 = difficult to 10 = easy), sleep quality (graded 1 = poor to 10 = good), cramp severity (graded 1 = mild to 10 =severe), cramp timing (before or after 2 am), cramp duration and adverse events | |
| Notes | Table of results for cramp duration contradicts commentary in Results section | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was double‐blind and crossed over within patients, and randomised..." Comment: details of randomisation not provided |
| Allocation concealment (selection bias) | Low risk | Quote: "...the study was... randomised and balanced by an independent observer." Comment: probably adequate concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The study was double‐blind.... the two weeks between treatments were single‐blind with patients taking placebo." Comment: no explicit mention of how blinding was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 9 participants completed the trial |
| Selective reporting (reporting bias) | Low risk | All outcome measures commented upon in analysis, including adverse events |
| Other bias | Low risk | Adequate washout periods. Trial ended at designated time period |
Kaji 1976.
| Methods | Double‐blind RCT of cross‐over design, set in New York, USA | |
| Participants | 9 participants with chronic renal failure on maintenance haemodialysis 3 times per week, and with frequent muscle cramps | |
| Interventions | Participants given quinine sulphate 320 mg or placebo at the beginning of each dialysis treatment, for a period of 12 weeks | |
| Outcomes | Cramp frequency, cramp intensity (graded mild = cramp lasting < 5 minutes and disappeared spontaneously, moderate = cramp lasting between 5 and 10 minutes and ceased after reduction of dialysis pump‐rate and severe = cramp lasting > 15 minutes and unrelieved despite reduction in pump rate), cramp duration, adverse events | |
| Notes | Study conducted in New York. Frequency of muscle cramps expressed as number of dialyses affected by cramps, rather than number or cramps during a fixed period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...a randomised double blind cross‐over trial..." Comment: details of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No details regarding methods of concealment are provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Quinine sulphate and placebo were placed in identical gelatin capsules and delivered from the hospital pharmacy...The pharmacy kept a record of the content of the capsule...but this information was withheld from the dialysis staff..." Comment: adequately blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial |
| Selective reporting (reporting bias) | High risk | The distribution and timing of cramps, and the blood pressure and dialysis pump rate during an episode were said to be outcomes but these are not mentioned in the Results/Discussion sections |
| Other bias | High risk | Only cramps during dialysis sessions were assessed; effect of treatment on cramps outside of dialysis sessions was not measured. Also, there was no washout period between cross‐over treatments |
Lee 1991.
| Methods | Single‐blind, parallel group RCT, set in Taiwan | |
| Participants | 31 cirrhotic participants with an average of over 3 muscle cramps per week | |
| Interventions | 4‐week run‐in period, followed by a 4‐week treatment period of either quinidine sulphate 200 mg twice‐daily or placebo twice‐daily | |
| Outcomes | Cramp number, adverse events | |
| Notes | Study conducted on an outpatient basis in Taiwan. 31 participants (mean age 62 years) completed the study. 84% participants were men | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...were allocated, using a table of random numbers." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was conveyed to the investigators, though as this was a single‐blinded study, concealment may not have been attempted at all |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Patients were not aware of which drug was being prescribed, but physicians were." Comment: single‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 out of 43 participants completed the study and withdrawals are accounted for (excluded due to low cramp frequency or poor record keeping) and were excluded before randomisation |
| Selective reporting (reporting bias) | Low risk | All intended outcome measures are addressed in the results |
| Other bias | Low risk | Except for the lack of double‐blinding counted above, nil else significant |
Leo Winter 1986.
| Methods | Double‐blind RCT of cross‐over design, set in New York and California, USA | |
| Participants | 205 participants (mean age 44 years) who experienced at least 2 leg cramps per week | |
| Interventions | 1‐week washout then 4 blocks of 5‐day treatment periods separated by 2‐day washouts. Treatments consisted of 129.6 mg quinine sulphate twice daily, or a quinine‐vitamin E combination (129.6 mg quinine sulphate plus 800 Units vitamin E) twice daily, or 800 units vitamin E twice daily | |
| Outcomes | Cramp number, cramp intensity, cramp days, sleep disturbance, adverse events. | |
| Notes | The second largest trial. 2‐centre trial (New York & California). NB short treatment periods. Unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...patients were assigned at random to 24 treatment sequences according to a randomisation schedule" Comment: no details of how the "randomisation schedule" was generated |
| Allocation concealment (selection bias) | Low risk | Quote: "...according to a randomisation schedule prepared by an independent person who did not participate in the study" Comment: appears adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "...under the double‐blind condition for identically appearing study medications" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All withdrawals and those lost to follow‐up were fully accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in the results |
| Other bias | High risk | High risk of bias caused by very short washout periods between treatments. Also, trial conducted by manufacturer of quinine tablets |
Lim 1986.
| Methods | Double‐blind RCT of parallel design | |
| Participants | 25 participants on a general medical ward, experiencing at least 2 leg cramps per week | |
| Interventions | Nightly quinine sulphate (300 mg) or placebo for 2 weeks (or less if discharged earlier) | |
| Outcomes | Cramp days, cramp intensity, adverse events | |
| Notes | Poorly designed study with no mention of number of participants in each group. Scanty data also | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each volunteer was randomly allocated to receive either 300 mg quinine or a placebo..." Comment: no description of randomisation protocol |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation may have been concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details regarding methods of blinding are provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Impossible to assess as no mention of number of participants in each group nor of how many actually completed the trial |
| Selective reporting (reporting bias) | Low risk | Both the intended outcome measures were addressed in the results and analysis |
| Other bias | High risk | Participants were recruited from a general medical ward as inpatients. Some were discharged before the 2‐week follow‐up |
Maule 1990.
| Methods | RCT of cross‐over design | |
| Participants | 16 participants from general practice (mean age 76 years) who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week washout then 4 blocks of 3‐week treatment periods consisting of quinine bisulphate (300 mg) or placebo or cork or wood in woollen bags | |
| Outcomes | Cramp number, adverse events | |
| Notes | Quinine compared against placebo and folklore. Only data provided is that for adverse events, but how many participants suffered these is not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were allocated to receive the three treatments and placebo in random order." Comment: details of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "the two tablets (quinine & placebo) should have been physically identical, but owing to lack of funds this criterion was not met." Comment: treatments were clearly distinguishable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 6 withdrawals from the trial; it is not clear from which treatment group they withdrew from, and the precise causes of the withdrawals are not given |
| Selective reporting (reporting bias) | Low risk | Quote: "During analysis of the data only average cramp number was considered because the duration section of the form was inadequately filled in by the majority of patients." Commment: suggests authors would have, as planned, analysed such data if they were available |
| Other bias | High risk | Treatments were sequential with no dedicated washout period between each phase, raising the possibility of significant carry‐over/withdrawal effects |
Prateepavanich 1999.
| Methods | Single‐blind, parallel group RCT, set in Thailand | |
| Participants | 24 adult outpatients (mean age 64 years) with nocturnal calf cramps associated with myofascial pain syndrome and gastrocnemius trigger points with at least 4 cramps per month | |
| Interventions | Quinine sulphate 300 mg orally daily at bedtime or 1 to 2 ml 1% xylocaine injection at the gastrocnemius trigger point at the start of the trial. Treatment period for 4 weeks, followed by follow‐up 4 weeks later. All subjects assigned to perform calf stretches daily | |
| Outcomes | Cramp number, cramp intensity (graded 0 = no pain to 10 = severe), cramp duration (minutes), adverse events | |
| Notes | Participants recruited from several outpatient clinics in Thailand. 2 participants withdrew from the study due to cinchonism during the treatment period. 20 of the 22 participants who completed the study were women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomly divided into two groups..." Comment: no description of randomisation protocol |
| Allocation concealment (selection bias) | Unclear risk | No details regarding how allocation may have been concealed is given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "...a single‐blinded comparative clinical study." Comment: no control group was used for the injection treatment. The reviewing physician was blinded to the treatment received by the participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for the 2 participant withdrawals are given but these were not counted in an intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All intended outcome measures were addressed |
| Other bias | High risk | The number of treatments received by participants in the injection group varied depending on individual cramp frequencies during the follow‐up period; there was therefore no uniform dose/regimen for the injections. Significant confounder in the fact that all participants were to perform calf stretches daily |
Roca 1992.
| Methods | Double‐blind, parallel group RCT, set in Ohio, USA | |
| Participants | 30 participants on dialysis, with a history of leg cramps | |
| Interventions | 2‐month placebo run‐in period, then active phase of either daily quinine 325 mg at bedtime with a vitamin E placebo, or vitamin E 400 IU at bedtime with a quinine placebo, for 2 months | |
| Outcomes | Cramp number, cramp intensity (graded 1 = no pain to 6 = excruciating), adverse events | |
| Notes | 29 participants (aged 21 to 73 years) from a community‐based academic hospital in Ohio, USA, completed the study. Study compares quinine to vitamin E as well as vitamin E and quinine to placebo. Although researchers state adverse effects of interventions will be investigated, no mention is made of these in the Results or Discussion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then randomized into two groups..." Comments: no description of randomisation protocol |
| Allocation concealment (selection bias) | Unclear risk | No details regarding how allocation may have been concealed is given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "quinine 325 mg at bedtime with a vitamin E placebo or 2) vitamin E 400 IU at bedtime with a quinine placebo." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 of 40 participants did not complete the trial, all of whom were accounted for. However, 1 participant died after randomisation but no details were given about which treatment was received or whether the death was related to the medication given |
| Selective reporting (reporting bias) | High risk | Adverse events were an outcome but no results given. Also results are given only for first month of treatment, despite treatment duration being 60 days |
| Other bias | Low risk | |
Sidorov 1993.
| Methods | Double‐blind RCT of cross‐over design, set in USA | |
| Participants | 19 adult participants from general medicine clinic who experienced at least 2 leg cramps per week | |
| Interventions | 2‐week run‐in period, followed by either quinine bisulphate 200 mg at night or placebo daily for 3 weeks before cross‐over to a second 3‐week treatment period | |
| Outcomes | Cramp number, cramp intensity (graded 1 = mild to 10 = severe), cramp duration (seconds), adverse events | |
| Notes | Single centre. Conducted in USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished using a simple random numbers table..." Comment: probably adequate |
| Allocation concealment (selection bias) | High risk | No evidence that allocations were concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Patients were blinded to all study periods. However, study personnel were aware that periods one and three used placebo." Comment: details of how investigators and participants were blinded not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 6 participants who left the study were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the methods were addressed in the analysis |
| Other bias | Unclear risk | The study group who successfully completed the study consisted of 14 women and only 2 men |
Smith 1985.
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 21 elderly participants who experienced at least 2 cramps per week | |
| Interventions | 2‐week run‐in period, followed by either quinine bisulphate 300 mg at night or placebo daily for 3 weeks before cross‐over to a second 3‐week treatment period | |
| Outcomes | Cramp number, cramp index (incorporating cramp duration and intensity) | |
| Notes | Only 18 participants (mean age 73 years) completed the study ‐ full reasons for withdrawal given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " Patients were randomly allocated..." Comment: no description of randomisation protocol |
| Allocation concealment (selection bias) | Unclear risk | No details given regarding allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Treatments were quinine bisulphate in a dose of one tablet (300 mg) at night and an identical sugar‐coated placebo..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 18 participants completed the study, but reasons for withdrawal were given |
| Selective reporting (reporting bias) | High risk | Adverse events was an outcome but no results given |
| Other bias | High risk | There was no washout period between the treatment phases, thus leaving open the possibility of a carry‐over effect |
Warburton 1987.
| Methods | Double‐blind RCT of cross‐over design | |
| Participants | 22 elderly outpatients (mean age 74 years), seeking treatment for leg cramps | |
| Interventions | Quinine bisulphate 300 mg or placebo daily for a 3‐week treatment period, followed by immediate cross‐over onto another 3‐week treatment period (i.e. no washout period inbetween). A 2‐week run‐in period before the trial involved quinine abstention | |
| Outcomes | Cramp number, "cramp index" (the product of intensity score 1 = mild to 3 = severe and duration < 1 min = 1, 1 to 10 min = 2, 11 to 20 min = 3, 21 to 60 min = 4, or > 60 min = 5), adverse events | |
| Notes | Cramp duration and intensity could not be separated from the "cramp index". Individual patient data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the remainder were allocated, using a table of random numbers..." Comment: probably adequate |
| Allocation concealment (selection bias) | Unclear risk | No details given regarding allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Treatments were quinine, 300 mg, at night, or an identical, sugar coated placebo tablet..." Comment: satisfactory blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant dropped out during the placebo stage for an unspecified reason and was not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All outcome measures mentioned in the protocol were addressed in the analysis |
| Other bias | High risk | Quote: "followed by two, sequential, 3‐week treatment periods." Comment: no washout period between each treatment phase raises the possibility of significant carry‐over effect |
Woodfield 2005.
| Methods | Double‐blind, randomised controlled 'N‐of‐1' trial, set in New Zealand | |
| Participants | 13 elderly participants (median age 75 years), suffering at least 2 cramps per week | |
| Interventions | 2‐week washout period followed by 3 x 4‐week treatment blocks in which participants are randomised to either placebo or quinine sulphate (200 to 300 mg) for 2 weeks and then the other treatment for 2 weeks | |
| Outcomes | Cramp number, cramp days | |
| Notes | General practices in New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of eight possible treatment sequences..."; "...copy of the randomisations code..." Comment: probably done as description suggests centrally‐organised randomisation codes |
| Allocation concealment (selection bias) | Low risk | Quote: "A sealed copy of the randomisation code..."; " A master copy of the randomisation codes was also held by the research supervisor..." Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Both the patients and the researcher interacting with them and conducting the analyses were blinded..." Comment: adequate double‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full explanation provided for the 3 drop‐outs |
| Selective reporting (reporting bias) | Low risk | All outcomes measured that were described in the initial protocol were addressed in the analysis |
| Other bias | Unclear risk | Participants continued with their most recent dose of quinine, thus this varied between participants |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baltodano 1988 | This was an observational study where participants already on quinine were started on verapamil instead, and seen to improve |
| Coppin 2005 | The focus of this RCT was on the cessation of quinine and effect of exercise |
| Morl 1980 | The active treatment in this RCT comprised quinine with aminophylline. The effect of quinine alone could therefore not be ascertained |
| Sandoval 1980 | This Spanish paper was translated into English. There was no evidence of randomisation and all cramps were treated with hypertonic saline, meaning that quinine was not given alone. Also the outcome measured was the number of dialysis sessions affected by cramp rather than the cramp number itself |
| Wessely 1984 | The active treatment in this RCT of haemodialysis patients comprised quinine with aminophylline. The effect of quinine alone could therefore not be ascertained |
RCT: randomised controlled trial
Differences between protocol and review
Since publication of the protocol, new 'Risk of bias' methodology and 'Summary of findings' tables have been introduced. Within the methods section, three criteria for including trials in the meta‐analysis were dropped, as these were deemed to be unnecessarily restrictive: minimum cramp number of two experienced in two weeks, participants taking analgesics, and minimum duration of quinine treatment of two weeks.
We did not prespecify subgroup analyses but performed a subgroup analysis by dose.
We activated standard headings in the Methods section and described methods for dealing with studies with multiple treatment arms, added a statement about contacting trial authors for missing data, and described the subgroup analysis and sensitivity analyses.
In the 2014 update we included searches for ongoing trials. RB joined the authors for this update.
Contributions of authors
Sherif El‐Tawil prepared the first draft of the review. Four authors (SET, TAM, HV, TET) were involved in the data extraction, its checking and analysis. Two authors (ML and MW) checked and edited the final manuscript. RB checked search results, redrafted the plain language summary and carried out minor revisions for this update.
Sources of support
Internal sources
No funding was received from any internal or external organisations or companies, Other.
External sources
No funding was received from any internal or external organisations or companies, Other.
Declarations of interest
Ruth Brassington is Managing Editor of the Cochrane Neuromuscular Disease Group and Michael Lunn is Joint Co‐ordinating Editor. He was not involved in acceptance of the review for publication.
There are no potential conflicts of interest.
Tariq El‐Tawil: Author deceased; declaration of interest published in the protocol: “No potential conflicts of interest".
Deceased
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
BioDesign 1984 {unpublished data only}
- BioDesign (for Bio Products Inc.). Clinical evaluation of Q‐Vel® in patients with nocturnal leg muscle cramps. Federal Register, Docket No. 77N‐0094. 1984.
Bottner 1984 {unpublished data only}
- Bottner M (for Scholl Inc.). Clinical trial of the efficacy of quinine sulphate in the treatment of nocturnal leg muscle cramps. Federal Register, Docket No. 77N‐0094. 1984.
CIBA 1988 {unpublished data only}
- CIBA Consumer Pharmaceuticals. A short‐term randomized, double‐blind parallel study of Q‐Vel® versus quinine sulfate versus vitamin E versus placebo in the prevention and treatment of nocturnal leg cramps. Federal Register, Docket No. 77N‐0094. 1988.
Connolly 1992 {published data only}
- Connolly PS, Shirley EA, Wasson JH, Nierenberg DW. Treatment of nocturnal leg cramps. A crossover trial of quinine vs vitamin E. Archives of Internal Medicine 1992;152(9):1877‐80. [PUBMED: 1520054] [PubMed] [Google Scholar]
Diener 2002 {published data only}
- Diener HC, Dethlefsen U, Dethlefsen‐Gruber S, Verbeek P. Effectiveness of quinine in treating muscle cramps: a double‐blind, placebo‐controlled, parallel‐group, multicentre trial. International Journal of Clinical Practice 2002;56(4):243‐6. [PUBMED: 12074203] [PubMed] [Google Scholar]
Dunn 1993 {published data only}
- Dunn NR. Effectiveness of quinine for night cramps. British Journal of General Practice 1993;43(368):127‐8. [PUBMED: 8323792] [PMC free article] [PubMed] [Google Scholar]
Fung 1989 {published data only}
- Fung MC, Holbrook JH. Placebo‐controlled trial of quinine therapy for nocturnal leg cramps. Western Journal of Medicine 1989;151(1):42‐4. [PUBMED: 2669346] [PMC free article] [PubMed] [Google Scholar]
Gorlich 1991 {published data only}
- Gorlich HD, Gablenz E, Steinberg HW. Treatment of nocturnal leg cramps. A multicenter, double blind, placebo controlled comparison between the combination of quinine and theophylline ethylene diamine with quinine. Arzneimittel‐Forschung 1991;41(2):167‐75. [PUBMED: 2043179] [PubMed] [Google Scholar]
Hays 1986 {unpublished data only}
- Hays RM, Goodman JJ (for Scholl Inc.). Clinical trial of the efficacy of quinine sulfate in the treatment of nocturnal leg muscle cramps. Federal Register, Docket No. 77N‐0094. 1986.
Jansen 1994 {published data only}
- Jansen PH, Veenhuizen KC, Verbeek AL, Straatman H. Efficacy of hydroquinine in preventing frequent ordinary muscle cramp outlasts actual administration. Journal of the Neurological Sciences 1994;122(2):157‐61. [PUBMED: 8021700] [DOI] [PubMed] [Google Scholar]
Jansen 1997 {published data only}
- Jansen PH, Veenhuizen KC, Wesseling AI, Boo T, Verbeek AL. Randomised controlled trial of hydroquinine in muscle cramps. Lancet 1997;349(9051):528‐32. [PUBMED: 9048790] [DOI] [PubMed] [Google Scholar]
Jones 1983 {published data only}
- Jones K, Castleden CM. A double‐blind comparison of quinine sulphate and placebo in muscle cramps. Age and Ageing 1983;12(2):155‐8. [PUBMED: 6346830] [DOI] [PubMed] [Google Scholar]
Kaji 1976 {published data only}
- Kaji DM, Ackad A, Nottage WG, Stein RM. Prevention of muscle cramps in haemodialysis patients by quinine sulphate. Lancet 1976;2(7976):66‐7. [PUBMED: 59150] [DOI] [PubMed] [Google Scholar]
Lee 1991 {published data only}
- Lee FY, Lee SD, Tsai YT, Lai KH, Chao Y, Lin HC, et al. A randomized controlled trial of quinidine in the treatment of cirrhotic patients with muscle cramps. Journal of Hepatology 1991;12(2):236‐40. [PUBMED: 2051002] [DOI] [PubMed] [Google Scholar]
Leo Winter 1986 {unpublished data only}
- Leo Winter Associates Inc (for Bio Products Inc.). Double blind randomized crossover study of Q‐Vel® versus quinine sulfate versus vitamin E versus placebo in the treatment of nocturnal leg muscle cramps. Federal Register, Docket No. 77N‐0094. 1986.
Lim 1986 {published data only}
- Lim SH. Randomised double‐blind trial of quinine sulphate for nocturnal leg cramp. British Journal of Clinical Practice 1986;40(11):462. [PUBMED: 3307858] [PubMed] [Google Scholar]
Maule 1990 {published data only}
- Maule B. Nocturnal cramp: quinine versus folklore. Practitioner 1990;234(1487):420‐1. [PUBMED: 2367299] [PubMed] [Google Scholar]
Prateepavanich 1999 {published data only}
- Prateepavanich P, Kupniratsaikul V, Charoensak T. The relationship between myofascial trigger points of gastrocnemius muscle and nocturnal calf cramps. Journal of the Medical Association of Thailand 1999;82(5):451‐9. [PUBMED: 10443094] [PubMed] [Google Scholar]
Roca 1992 {published data only}
- Roca AO, Jarjoura D, Blend D, Cugino A, Rutecki GW, Nuchikat PS, et al. Dialysis leg cramps. Efficacy of quinine versus vitamin E. ASAIO Journal 1992;38(3):M481‐5. [PUBMED: 1457907] [PubMed] [Google Scholar]
Sidorov 1993 {published data only}
- Sidorov J. Quinine sulfate for leg cramps: does it work?. Journal of the American Geriatrics Society 1993;41(5):498‐500. [PUBMED: 8486881] [DOI] [PubMed] [Google Scholar]
Smith 1985 {published data only}
- Smith C, Jee R, O'Neill C, Dobbs SM. Double‐blind, placebo controlled, cross‐over study of maintenance treatment with quinine bisulphate for night cramps. British Journal of Clinical Pharmacology 1986;21(1):108P. [Google Scholar]
Warburton 1987 {published data only}
- Warburton A, Royston JP, O'Neill CJ, Nicholson PW, Jee RD, Denham MJ, et al. A quinine a day keeps the leg cramps away?. British Journal of Clinical Pharmacology 1987;23(4):459‐65. [PUBMED: 3555580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodfield 2005 {published data only}
- Woodfield R, Goodyear‐Smith F, Arroll B. N‐of‐1 trials of quinine efficacy in skeletal muscle cramps of the leg. British Journal of General Practice 2005;55(512):181‐5. [PUBMED: 15808032] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baltodano 1988 {published data only}
- Baltodano N, Gallo BV, Weidler DJ. Verapamil vs quinine in recumbent nocturnal leg cramps in the elderly. Archives of Internal Medicine 1988;148(9):1969‐70. [PubMed] [Google Scholar]
Coppin 2005 {published data only}
- Coppin, RJ, Wicke DM, Little PS. Managing nocturnal leg cramps ‐ calf‐stretching exercises and cessation of quinine treatment. British Journal of General Practice 2005;55(512):186‐91. [PMC free article] [PubMed] [Google Scholar]
Morl 1980 {published data only}
- Morl H, Dieterich HA. Nocturnal leg cramps‐ their causes and treatment. Medizinische Klinik 1980;75(7):264‐67. [PubMed] [Google Scholar]
Sandoval 1980 {published data only}
- Sandoval Pandero J, Perez Garcia A, Martin Abad L, Piqueras A, Garces L, Chacon JC, et al. Action of quinine sulphate on the incidence of muscle cramps during hemodialysis. Medicina Clinica (Barcelona) 1980;75(6):247‐9. [PubMed] [Google Scholar]
Wessely 1984 {published data only}
- Wessely S, Dieterich HA. Quinine sulfate plus aminophylline in treatment of muscle cramps in dialysis patients. Therapiewoche 1984;34(29):4356‐9. [Google Scholar]
Additional references
AAP 2001
- Committee on Drugs, American Academy of Pediatrics. The transfer of drugs and other chemicals into human milk. Pediatrics 2001;108(3):776‐89. [DOI] [PubMed] [Google Scholar]
Abdulla 1999
- Abdulla AJ, Jones PW, Pearce VR. Leg cramps in the elderly: prevalence, drug and disease associations. International Journal of Clinical Practice 1999;53(7):494‐6. [PubMed] [Google Scholar]
Abrams 1996
- Abrams GA, Concato J, Fallon MB. Muscle cramps in patients with cirrhosis. American Journal of Gastroenterology 1996;91(7):1363‐6. [PubMed] [Google Scholar]
Angeli 1996
- Angeli P, Albino G, Carraro P, Dalla Pria M, Merkel C, Caregaro L, et al. Cirrhosis and muscle cramps: evidence of a causal relationship. Hepatology 1996;23(2):264‐73. [DOI] [PubMed] [Google Scholar]
Ayres 1969
- Ayres S Jr, Mihan R. Leg cramps (systremma) and "restless legs" syndrome. Response to vitamin E (tocopherol). California Medicine 1969;111(2):87‐91. [PMC free article] [PubMed] [Google Scholar]
Ayres 1974
- Ayres S Jr, Mihan R. Nocturnal leg cramps (systremma): a progress report on response to vitamin E. Southern Medical Journal 1974;67(11):1308‐12. [DOI] [PubMed] [Google Scholar]
Baldissera 1994
- Baldissera F, Cavallari P, Dworzak F. Motor neuron 'bistability'. A pathogenetic mechanism for cramps and myokymia. Brain 1994;117(Pt 5):929‐39. [DOI] [PubMed] [Google Scholar]
Barr 1990
- Barr E, Douglas JF, Hill CM. Recurrent acute hypersensitivity to quinine. BMJ 1990;301(6747):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 1985
- Bateman DN, Blain PG, Woodhouse KW, Rawlins MD, Dyson H, Heyworth R, et al. Pharmacokinetics and clinical toxicity of quinine overdosage: lack of efficacy of techniques intended to enhance elimination. Quarterly Journal of Medicine 1985;54(214):125‐31. [PubMed] [Google Scholar]
Berlin 1975
- Berlin CM, Stackman JM, Vesell ES. Quinine‐induced alterations in drug disposition. Clinical Pharmacology and Therapeutics 1975;18(6):670‐9. [DOI] [PubMed] [Google Scholar]
Blyton 2012
- Blyton F, Chuter V, Walter KE, Burns J. Non‐drug therapies for lower limb muscle cramps. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD008496.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2010
- Joint Formulary Committee. British National Formulary. 9th Edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2010. [Google Scholar]
Butler 2002
- Butler JV, Mulkerrin EC, O'Keeffe ST. Nocturnal leg cramps in older people. Postgraduate Medical Journal 2002;78(924):596‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 1985
- Chou CT, Wasserstein A, Schumacher HR Jr, Fernandez P. Musculoskeletal manifestations in hemodialysis patients. Journal of Rheumatology 1985;12(6):1149‐53. [PubMed] [Google Scholar]
Dahle 1995
- Dahle LO, Berg G, Hammar M, Hurtig M, Larsson L. The effect of oral magnesium substitution on pregnancy‐induced leg cramps. American Journal of Obstetrics and Gynecology 1995;173(1):175‐80. [DOI] [PubMed] [Google Scholar]
Eaton 1989
- Eaton JM. Is this really a muscle cramp?. Postgraduate Medical Journal 1989;86(3):227‐32. [DOI] [PubMed] [Google Scholar]
FDA 1982
- Food, Drug Administration. Advance notice of proposed rulemaking to reopen the rulemaking for OTC internal analgesic, antipyretic, and antirheumatic drug products. Federal Register Oct 1, 1982; Vol. Docket No: 47 FR 43562:Sec 330.10(a).
FDA 1994
- Food, Drug Administration. Drug Products for the Treatment and/or Prevention of Nocturnal Leg Muscle Cramps for Over‐the‐Counter Human Use; Final Rule. Federal Register, Docket No. 77N‐0094 Aug 22, 1994.
FDA 1995a
- Food, Drug Administration. FDA orders stop to marketing of quinine for night leg cramps. FDA Homepage www.fda.gov (accessed 20 July 2008).
FDA 1995b
- Food, Drug Administration. Drug products containing quinine for the treatment and/or prevention of malaria for over‐the‐counter human use; proposed rule. Federal Register 19 April 1995;60:19649‐55. [Google Scholar]
FDA 2006
- Food, Drug Administration. Quinine: important warning. www.fda.gov/bbs/topics/news/2006/new01521 12 December 2006 (accessed 20 July 2008).
Garrison 2012
- Garrison SR, Allan GM, Sekhon RK, Musini VM, Khan KM. Magnesium for skeletal muscle cramps. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009402.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 2001
- Goodman L, Gilman A. The Pharmacological Basis of Therapeutics. 10th Edition. New York: McGraw‐Hill, 2001. [Google Scholar]
Gootnick 1943
- Gootnick A. Night cramps and quinine. Archives of Internal Medicine 1943;71(4):555‐62. [Google Scholar]
Guay 2008
- Guay DR. Are there alternatives to the use of quinine to treat nocturnal leg cramps?. Consultant Pharmacist 2008;23(2):141‐56. [DOI] [PubMed] [Google Scholar]
Hallegraeff 2012
- Hallegraeff JM, Schans CP, Ruiter R, Greef MH. Stretching before sleep reduces the frequency and severity of nocturnal leg cramps in older adults: a randomised trial. Journal of Physiotherapy 2012;58(1):17‐22. [DOI] [PubMed] [Google Scholar]
Harvey 1939
- Harvey A. The mechanism of action of quinine in myotonia and myasthenia. JAMA 1939;112:1562‐3. [Google Scholar]
Haskell 1997
- Haskell SG, Fiebach NH. Clinical epidemiology of nocturnal leg cramps in male veterans. American Journal of the Medical Sciences 1997;313(4):210‐14. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jansen 1990
- Jansen PH, Joosten EM, Vingerhoets HM. Muscle cramp: main theories as to aetiology. European Archives of Psychiatry & Neurological Sciences 1990;239(5):337‐42. [DOI] [PubMed] [Google Scholar]
Katzberg 2010
- Katzberg HD, Khan AH, So YT. Assessment: symptomatic treatment for muscle cramps (an evidence‐based review). Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;74(8):691–6. [DOI] [PubMed] [Google Scholar]
Kaufman 1993
- Kaufman DW, Kelly JP, Johannes CB, Sandler A, Harmon D, Stolley PD, et al. Acute thrombocytopenic purpura in relation to the use of drugs. Blood 1993;82(9):2714‐8. [PubMed] [Google Scholar]
Khajehdehi 2001
- Khajehdehi P, Mojerlou M, Behzadi S, Rais‐Jalali GA. A randomized, double‐blind, placebo‐controlled trial of supplementary vitamins E, C and their combination for treatment of haemodialysis cramps. Nephrology Dialysis Transplantation 2001;16(7):1448‐51. [DOI] [PubMed] [Google Scholar]
Knower 2003
- Knower MT, Bowton DL, Owen J, Dunagan DP. Quinine‐induced disseminated intravascular coagulation: case report and review of the literature. Intensive Care Medicine 2003;29(6):1007‐11. [DOI] [PubMed] [Google Scholar]
Krishna 1996
- Krishna S, White NJ. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clinical implications. Clinical Pharmacokinetics 1996;30(4):263‐99. [DOI] [PubMed] [Google Scholar]
Langford 2003
- Langford NJ, Good AM, Laing WJ, Bateman DN. Quinine intoxications reported to the Scottish Poisons Information Bureau 1997‐2002: a continuing problem. British Journal of Clinical Pharmacology 2003;56(5):576‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latta 1989
- Latta D, Turner E. An alternative to quinine in nocturnal leg cramps. Current Therapeutic Research, Clinical and Experimental 1989;45:833‐7. [Google Scholar]
Layzer 1994
- Layzer RB. The origin of muscle fasciculations and cramps. Muscle and Nerve 1994;17(11):1243‐9. [DOI] [PubMed] [Google Scholar]
Mackie 1995
- Mackie MA, Davidson J. Prescribing of quinine and cramp inducing drugs in general practice. BMJ 1995;311(7019):1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 1993
- Maguire RB, Stroncek DF, Campbell AC. Recurrent pancytopenia, coagulopathy, and renal failure associated with multiple quinine‐dependent antibodies. Annals of Internal Medicine 1993;119(3):215‐17. [DOI] [PubMed] [Google Scholar]
Man‐Son‐Hing 1995
- Man‐Son‐Hing M, Wells G. Meta‐analysis of efficacy of quinine for treatment of nocturnal leg cramps in elderly people. BMJ 1995;310(6971):13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Man‐Son‐Hing 1998
- Man‐Son‐Hing M, Wells G, Lau A. Quinine for nocturnal leg cramps: a meta‐analysis including unpublished data. Journal of General Internal Medicine 1998;13(9):600‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandal 1995
- Mandal AK, Abernathy T, Nelluri SN, Stitzel V. Is quinine effective and safe in leg cramps?. Journal of Clinical Pharmacology 1995;35(6):588‐93. [DOI] [PubMed] [Google Scholar]
Martindale 1996
- Martindale W, Westcott W. In: Martindale W, Westcott W editor(s). The Extra Pharmacopoeia. 1st Edition. London: Royal Pharmaceutical Society, 1996:474‐77. [Google Scholar]
McDonald 1997
- McDonald SP, Shanahan EM, Thomas AC, Roxby DJ, Beroukas D, Barbara JA. Quinine‐induced hemolytic uremic syndrome. Clinical Nephrology 1997;47(6):397‐400. [PubMed] [Google Scholar]
McGee 1990
- McGee SR. Muscle cramps. Archives of Internal Medicine 1990;150(3):511‐18. [PubMed] [Google Scholar]
McGready 2001
- McGready R, Cho T, Samuel, Villegas L, Brockman A, van Vugt M, et al. Randomized comparison of quinine‐clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Transactions of The Royal Society of Tropical Medicine and Hygiene 2001;95(6):651‐6. [DOI] [PubMed] [Google Scholar]
McGready 2002
- McGready R, Thwai KL, Cho T, Samuel, Looareesuwan S, White NJ, et al. The effects of quinine and chloroquine antimalarial treatments in the first trimester of pregnancy. Transactions of The Royal Society of Tropical Medicine and Hygiene 2002;96(2):180‐4. [DOI] [PubMed] [Google Scholar]
McKinna 1966
- McKinna AJ. Quinine induced hypoplasia of the optic nerve. Canadian Journal of Ophthalmology 1966;1(4):261. [PubMed] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142(1):37‐46. [DOI] [PubMed] [Google Scholar]
Miller TM 2005
- Miller TM, Layzer RB. Muscle cramps. Muscle and Nerve 2005;32(4):431‐42. [DOI] [PubMed] [Google Scholar]
Morgon 1971
- Morgon A, Charachon D, Brinquier N. Disorders of the auditory apparatus caused by embryopathy or foetopathy: prophylaxis and treatment. Acta Otolaryngology (Suppl) 1971;291:1‐27. [PubMed] [Google Scholar]
Morton 2002
- Morton AP. Quinine‐induced disseminated intravascular coagulation and haemolytic‐uraemic syndrome. Medical Journal of Australia 2002;176(7):351. [DOI] [PubMed] [Google Scholar]
Moss 1940
- Moss HK, Herrmann LG. The use of quinine for relief of night cramps in the extremities. Journal of the American Medical Association 1940;115:1358‐9. [Google Scholar]
Mutual 2006
- Mutual Pharmaceutical Company. Quinine sulfate [package insert]. Philadelphia, PA: 2006. thomsonweb.esourcegroup.com/files/PI_0.pdf (accessed 2 February 2009).
Naylor 1994
- Naylor RJ, Young JB. A general population survey of leg cramps. Age and Ageing 1994;23(5):418‐20. [DOI] [PubMed] [Google Scholar]
Nicholson 1945
- Nicholson JH, Falk A. Night cramps in young men. New England Journal of Medicine 1945;233:556‐9. [Google Scholar]
Nishimura 1976
- Nishimura H, Tanimura T. Clinical Aspects of The Teratogenicity of Drugs. Amsterdam: Excerpta Medica, 1976. [Google Scholar]
Nosten 2002
- Nosten F, McGready R, D'Alessandro U, Bonell A, Verhoeff F, Menendez C, et al. The effects of quinine and chloroquine antimalarial treatments in the first trimester of pregnancy. Transactions of the Royal Society of Tropical Medicine & Hygiene 2002;96:180‐4. [DOI] [PubMed] [Google Scholar]
Nosten 2006
- Nosten F, McGready R, d'Alessandro U, Bonell A, Verhoeff F, Menendez C, et al. Antimalarial drugs in pregnancy: a review. Current Drug Safety 2006;1:1‐15. [DOI] [PubMed] [Google Scholar]
Orton 2008
- Orton LC, Omari AAA. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004912.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pederson 1985
- Pedersen KE, Lysgaard Madsen J, Klitgaard NA, Kjaer K, Hvidt S. Effect of quinine on plasma digoxin concentration and renal digoxin clearance. Acta Medica Scandinavica 1985;218(2):229‐32. [DOI] [PubMed] [Google Scholar]
Peer 1983
- Peer G, Blum M, Aviram A. Relief of hemodialysis‐induced muscular cramps by nifedipine. Dialysis and Transplantation 1983;12:180‐1. [Google Scholar]
Phillips‐Howard 1996
- Phillips‐Howard PA, Wood D. The safety of antimalarial drugs in pregnancy. Drug Safety 1996;14(3):131‐45. [DOI] [PubMed] [Google Scholar]
Piola 2010
- Piola P, Nabasumba C, Turyakira E, Dhorda M, Lindegardh N, Nyehangane D, et al. Efficacy and safety of artemether‐lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open‐label, randomised, non‐inferiority trial. Lancet Infectious Diseases 2010;10(11):762‐9. [DOI] [PubMed] [Google Scholar]
Prasad 2003
- Prasad RS, Kodali VR, Khuraijam GS, Cho M, Travers JP. Acute confusion and blindness from quinine toxicity. European Journal of Emergency Medicine 2003;10(4):353‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roffe 2002
- Roffe C, Sills S, Crome P, Jones P. Randomised, cross‐over, placebo controlled trial of magnesium citrate in the treatment of chronic persistent leg cramps. Medical Science Monitor 2002;8(5):326‐30. [PubMed] [Google Scholar]
Schneemann 2006
- Schneemann M. Quinine. In: Aronson JK editor(s). Meyler's Side Effects of Drugs. 15th Edition. New York: Elsevier Science, 2006:3002‐7. [Google Scholar]
White 2007
- White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infectious Diseases 2007;7(8):549‐58. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Guidelines for the treatment of malaria. 2nd Edition. Geneva: WHO Press, 2010. [Google Scholar]
Young 1993
- Young JB, Connolly MJ. Naftidrofuryl treatment for rest cramp. Postgraduate Medical Journal 1993;69:624‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2002
- Young GL, Jewell D. Interventions for leg cramps in pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD000121] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
El Tawil 2004
- El‐Tawil S, Musa TA, El‐Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD005044] [DOI] [PubMed] [Google Scholar]
El Tawil 2010
- El‐Tawil S, Al Musa T, Valli H, Lunn MP, El‐Tawil T, Weber M. Quinine for muscle cramps. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD005044.pub2] [DOI] [PubMed] [Google Scholar]


