Abstract
Background
Measles outbreaks continue to occur in countries with high vaccination coverage. Passive immunisation is generally considered to prevent measles in someone who is not immune and has been exposed to infection. Estimates of effectiveness have varied and no minimum effective dose has been determined.
Objectives
To assess the effectiveness and safety of intramuscular injection or intravenous infusion of immunoglobulins (passive immunisation) for preventing measles when administered to exposed susceptible people before the onset of symptoms.
Search methods
We searched CENTRAL (2013, Issue 7), MEDLINE (1946 to July week 5, 2013), CINAHL (1981 to August 2013) and EMBASE (1974 to August 2013).
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs and prospective, controlled (cohort) studies if: participants were susceptible and exposed to measles, polyclonal immunoglobulins derived from human sera or plasma were administered intramuscularly or intravenously as the only intervention in at least one group and the number of subsequent measles cases was measured. We excluded studies of other sources of immunoglobulins.
Data collection and analysis
Two authors independently extracted data and critically appraised the included studies. We attempted to contact study authors for missing information. We described the results of studies not included in meta‐analyses.
Main results
We included one RCT, two quasi‐RCTs and 10 cohort studies (3925 participants). No studies were rated as low risk of bias for all criteria. Critical appraisal was constrained by a lack of information in most studies. The overall quality of the evidence was moderate.
Seven studies (1432 participants) assessed cases of measles after immunoglobulin versus no treatment. Heterogeneity was explained by subgrouping according to the blood product used as an approximation of dose of immunoglobulin. When given within seven days of exposure, immunoglobulins were effective at preventing measles: gamma globulin (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.08 to 0.36), convalescent serum (RR 0.21, 95% CI 0.15 to 0.29 to RR 0.49, 95% CI 0.44 to 0.54) and adult serum (RR 0.52, 95% CI 0.45 to 0.59). The differences in the effectiveness of different blood products were supported by studies not included in the meta‐analysis and by two studies (702 participants) that found gamma globulin more effective than serum (RR 0.56, 95% CI 0.46 to 0.69).
Based on three studies (893 participants) immunoglobulin was effective at preventing death due to measles compared to no treatment (RR 0.24, 95% CI 0.13 to 0.44).
Two studies included measles vaccine alone among the intervention groups. Meta‐analysis could not be undertaken. Both studies suggested the vaccine was more effective than gamma globulin.
No serious adverse events were observed in any of the included studies, although reporting of adverse events was poor overall. Non‐serious adverse events included transient fever, rash, muscle stiffness, local redness and induration.
Authors' conclusions
Passive immunisation within seven days of exposure is effective at preventing measles, with the risk for non‐immune people up to 83% less than if no treatment is given. Given an attack rate of 45 per 1000 (per the control group of the most recent included study), gamma globulin compared to no treatment has an absolute risk reduction (ARR) of 37 per 1000 and a number needed to treat to benefit (NNTB) of 27. Given an attack rate of 759 per 1000 (per the attack rate of the other included study assessing gamma globulin), the ARR of gamma globulin compared to no treatment is 629 and the NNTB is two.
It seems the dose of immunoglobulin administered impacts on effectiveness. A minimum effective dose of measles‐specific antibodies could not be identified.
Passive immunisation is effective at preventing deaths from measles, reducing the risk by 76% compared to no treatment. Whether the benefits of passive immunisation vary among subgroups of non‐immune exposed people could not be determined.
Due to a paucity of evidence comparing vaccine to passive immunisation, no firm conclusions can be drawn regarding relative effectiveness.
The included studies were not specifically designed to detect adverse events.
Future research should consider the effectiveness of passive immunisation for preventing measles in high‐risk populations such as pregnant women, immunocompromised people and infants. Further efforts should be made to determine the minimum effective dose of measles‐specific antibodies for post‐exposure prophylaxis and the relative effectiveness of vaccine compared to immunoglobulin.
Keywords: Humans; Cohort Studies; Immunization, Passive; Immunization, Passive/methods; Measles; Measles/prevention & control; Post‐Exposure Prophylaxis; Post‐Exposure Prophylaxis/methods; Randomized Controlled Trials as Topic; gamma‐Globulins; gamma‐Globulins/administration & dosage
Plain language summary
Antibodies for preventing measles after exposure
People who have had measles, or measles vaccine, have antibodies against the virus in their blood that protect them from developing measles should they come into contact with it. These antibodies can be extracted from blood donated by these individuals.
If people without antibodies come into contact with someone who is contagious with measles, they are likely to contract the disease. Measles is usually debilitating and can have serious consequences including death, so preventing it is desirable. One way of preventing measles in this group, when they do come into contact with a contagious person, is to inject them with antibodies that have been extracted from blood donations. This has been practised since the 1920s, but measures of its effectiveness have varied and the minimum amount of antibodies that we can give to prevent measles is unknown.
Based on seven studies (1432 people), of overall moderate quality, injecting antibodies into a muscle of people who came into contact with measles, but lacked their own antibodies, was effective at preventing them catching the disease compared to those who received no treatment. Using the modern day antibody preparation, people were 83% less likely to develop measles than those who were not treated. It was very effective at preventing them developing complications if they did contract measles and very effective at preventing death. The included studies generally did not intend to measure possible harms from the injections. Minor side effects were reported, such as muscle stiffness, redness around the injection site, fever and rash. Importantly, only two studies compared the measles vaccine with the antibody injection in this group of people, so no firm conclusions could be drawn about the relative effectiveness of these interventions.
The antibody injection is often recommended for pregnant women, infants and immunocompromised people (if they do not have their own antibodies to measles and come into contact with someone who is contagious with measles). The included studies did not include these groups of people, so it is unknown whether the effectiveness of antibody injections is different for them. We were also unable to identify the minimum dose of antibodies required as only one study measured the specific amount of measles antibodies in the injections and one other study estimated this figure; the results of these two studies were not consistent.
The evidence is current to August 2013.
Summary of findings
Summary of findings for the main comparison. Immunoglobulin compared to no treatment for preventing measles.
| Immunoglobulin compared to no treatment for preventing measles | ||||||
| Patient or population: susceptible people exposed to measles Settings: community and hospitals Intervention: immunoglobulin Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Immunoglobulin | |||||
| Measles cases ‐ convalescent serum | Study population | RR 0.21 (0.15 to 0.29) | 301 (3 studies) | ⊕⊕⊕⊝ moderate1,2,3,4,5,6 | ||
| 862 per 1000 | 181 per 1000 (129 to 250) | |||||
| Moderate | ||||||
| 1000 per 1000 | 210 per 1000 (150 to 290) | |||||
| Measles cases ‐ adult serum | Study population | RR 0.52 (0.45 to 0.59) | 586 (2 studies) | ⊕⊕⊕⊝ moderate3,5,7,8,9 | ||
| 860 per 1000 | 447 per 1000 (387 to 507) | |||||
| Moderate | ||||||
| 907 per 1000 | 472 per 1000 (408 to 535) | |||||
| Measles cases ‐ gamma globulin | Study population | RR 0.17 (0.08 to 0.36) | 545 (2 studies) | ⊕⊕⊕⊝ moderate4,5,10,11,12 | ||
| 110 per 1000 | 19 per 1000 (9 to 40) | |||||
| Moderate | ||||||
| 402 per 1000 | 68 per 1000 (32 to 145) | |||||
| Mortality due to measles | Study population | RR 0.24 (0.13 to 0.44) | 893 (3 studies) | ⊕⊕⊕⊕ high3,5,7 | ||
| 142 per 1000 | 34 per 1000 (18 to 62) | |||||
| Moderate | ||||||
| 40 per 1000 | 10 per 1000 (5 to 18) | |||||
| Complications due to measles | Study population | RR 0.18 (0.05 to 0.6) | 832 (3 studies) | ⊕⊕⊕⊝ moderate3,4,5,7 | ||
| 52 per 1000 | 9 per 1000 (3 to 31) | |||||
| Moderate | ||||||
| 71 per 1000 | 13 per 1000 (4 to 43) | |||||
| Adverse events | Study population | Not estimable | 0 (0) | See comment | Adverse events were poorly reported or not measured in all but one study comparing immunoglobulins and no treatment. No serious adverse events were reported.13 | |
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level for risk of bias. We pooled one quasi‐randomised trial and two cohort studies to give this estimate. Two further cohort studies also assessed convalescent serum versus no treatment for the prevention of measles. These latter studies had heterogenous results that may have resulted from differences in methodology and so were not included. We rated no studies at high risk of measurement bias, but lack of information about blinding and assessment of the outcome typically resulted in unclear risk. While any uncontrolled confounding would have decreased the effect size, measurement bias may have increased the effect size and so warrants a downgrade in quality here. 2Downgraded one level for inconsistency. The two cohort studies that assessed convalescent serum versus no treatment, which were left out of this pooled estimate, had heterogenous results, although still indicated a significant benefit of this intervention. 3Publication bias strongly suspected. The studies in this analysis were all published in the first half of the 20th century. Not as many journals existed and reporting standards were not as rigorous. It is very likely that many small studies were not published. 4Upgraded for very large effect size. The effect size was very large and reasonably precise. 5Upgraded as plausible confounding would reduce the demonstrated effect. 6Upgraded for dose‐response gradient. Convalescent serum was one subgroup of three in an analysis that examined the effect of an approximation of dose on the results. An apparent dose response could be seen across the three subgroups. 7Downgraded one level for risk of bias. We pooled one quasi‐randomised trial and two cohort studies to give this estimate. We rated no study at high risk of measurement bias, but lack of information about blinding and assessment of the outcome typically resulted in unclear risk. While any uncontrolled confounding would have decreased the effect size, measurement bias may have increased the effect size and so warrants a downgrade in quality here. 8Upgraded for large effect size. The effect size was large and precise. 9Upgraded for dose‐response gradient. Adult serum was one subgroup of three in an analysis that examined the effect of an approximation of dose on the results. An apparent dose response could be seen across the three subgroups. 10Downgraded two levels for risk of bias. Both of the studies contributing to this estimate were cohort studies. Any uncontrolled confounding would have decreased the effect size. We rated measurement bias as unclear for one study and this may have increased the effect size in this case. We rated measurement bias as low risk for the other study, but attrition bias was high risk for that study. Overall, a downgrading of two levels is warranted. 11Publication bias strongly suspected. Although one study in the analysis of this subgroup was published recently, the other was published in the first half of the 20th century. Not as many journals existed and reporting standards were not as rigorous at that time. It is very likely that many small studies were not published. 12Upgraded for dose‐response gradient. Gamma globulin was one subgroup of three in an analysis that examined the effect of an approximation of dose on the results. An apparent dose response could be seen across the three subgroups. 13One study recording 'vaccine reactions' reported 'fever and rash' at rates of 5% in the gamma globulin group, 4% in the vaccine group and 1% in the no treatment group. The differences between groups were not statistically significant. This study reported loss to follow‐up exceeding 20%.
Summary of findings 2. Gamma globulin compared to serum for preventing measles.
| Gamma globulin compared to serum for preventing measles | ||||||
| Patient or population: susceptible children exposed to measles Settings: community and hospitals Intervention: gamma globulin Comparison: serum | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Serum | Gamma globulin | |||||
| Measles cases | Study population | RR 0.56 (0.46 to 0.69) | 702 (2 studies) | ⊕⊕⊕⊕ high1,2,3,4,5 | ||
| 464 per 1000 | 260 per 1000 (214 to 320) | |||||
| Moderate | ||||||
| 554 per 1000 | 310 per 1000 (255 to 382) | |||||
| Mortality due to measles | Study population | Not estimable | 0 (0) | See comment | Mortality was not measured in the studies comparing gamma globulin and serum | |
| See comment | See comment | |||||
| Moderate | ||||||
| Complications due to measles | Study population | Not estimable | 0 (0) | See comment | Complications were not measured by one of the two studies comparing gamma globulin and serum. RCT results favoured gamma globulin (RR 0.41, 95% CI 0.08 to 2.07). | |
| See comment | See comment | |||||
| Moderate | ||||||
| Adverse events | Study population | Not estimable | 0 (0) | See comment | Adverse events were not specified as a measured outcome by the studies comparing gamma globulin and serum. Reporting was poor and the results are not amenable to meta‐analysis. | |
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Not downgraded for risk of bias. One of the studies contributing to this estimate is a randomised controlled trial, the other is a cohort study. Any uncontrolled confounding would have decreased the effect size. Measurement bias was low‐risk for the RCT and unclear for the cohort study. Overall, the downgrade of quality already applied for including cohort studies is all that is warranted. 2Publication bias strongly suspected. Both studies were published in the first half of the 20th century. Not as many journals existed and reporting standards were not as rigorous. It is likely that many small studies would not have been published. 3Upgraded for large effect size. Effect size is large and precise. 4Upgraded as plausible confounding would reduce the demonstrated effect. 5Upgraded for dose‐response gradient. Two doses of gamma globulin were used by the RCT. The higher dose was a smaller group and the confidence intervals overlap with that of the lower dose from this study, but the estimates of effect are consistent with a dose response.
Background
Before vaccination against measles was available, annual case numbers were estimated at 130 million (WHO 1999) and the disease caused between five and eight million deaths globally each year (Moss 2009). With the introduction of the vaccine, the worldwide number of cases began to decline (WHO 1999) and this trend continued with increasing vaccination coverage (WHO 2009a). However, the number of measles cases worldwide exceeded 300,000 in 2010 (WHO 2013), with the highest incidence occurring in the World Health Organization (WHO) African region at 238 cases per million population (WHO 2012). Measles is still an important cause of global mortality as identified by the joint WHO and United Nations International Children's Emergency Fund (UNICEF) Global Immunization Vision and Strategy 2005 to 2015 (WHO 2005). One of the strategy's goals is measles mortality reduction. In 2008, measles caused around 164,000 deaths (WHO 2009b).
Further to mortality reduction, most WHO regions have set measles elimination goals and reported on progress towards these (Castillo‐Solorzano 2011; Martin 2011; Sniadack 2011; WHO 2008). Many countries have noted continued reductions in incidence (WHO 2012) and even elimination of endemic transmission (Parker Fiebelkorn 2010). In 2010, the incidence in the WHO region of the Americas was just 0.3 cases per million population (WHO 2012). However, the WHO cautions that failure to maintain high vaccination coverage in all areas of a country results in resurgence of the disease (WHO 2009a). Certainly, there are many recent published reports of measles outbreaks among countries with high vaccination coverage (CDC 2011a; Delaporte 2011; DVD CDC 2011; Hoskins 2011; Parker Fiebelkorn 2010; Smithson 2010; Takimoto 2011; Vainio 2011) and the WHO confirms that the incidence of measles worldwide increased in 2010 because of large outbreaks in some regions (WHO 2012).
In countries with low incidences of measles, elimination strategies typically include an urgent response to a single reported case, including confirmation of the diagnosis, contact tracing and post‐exposure prophylaxis (CDC 1998; CDNA 2009; NZ MoH 2011; UK DoH 2010). Post‐exposure prophylaxis may be a vaccination, which seems to be effective at preventing disease onset if administered within 72 hours of exposure (Barrabeig 2011), or may involve passive immunisation with immunoglobulin, particularly if outside this 72‐hour window (Heymann 2008).
Description of the condition
Measles is a highly communicable viral illness (Heymann 2008). The measles virus is an enveloped, single‐stranded RNA Morbillivirus of the family Paramyxoviridae (Heymann 2008; WHO 2009a). The virus is shed from the respiratory tract of infected persons and transmitted by aerosolised droplets or by direct contact with respiratory secretions (WHO 2009a). Someone with measles is contagious from one day before the symptoms start until four days after the rash appears. A susceptible person exposed to measles will usually develop symptoms after around 10 days, but this may range from 7 to 18 days after exposure (Heymann 2008).
Symptoms of measles include fever, conjunctivitis, runny nose, cough and a red blotchy rash (WHO 1999). The illness is often more severe in infants and adults than in children (Heymann 2008). Complications occur more frequently in cases in low‐income rather than high‐income countries (75% or more versus 10% to 15% of cases, respectively) (WHO 1999). Middle ear infection and pneumonia are fairly common complications, occurring in 5% to 15% and 5% to 10% of children with measles, respectively (WHO 2009a). Encephalitis is a serious, but rarer, complication of measles, occurring in about 1 out of every 1000 cases (WHO 2009a). A slowly progressing neurological disease, subacute sclerosing panencephalitis (SSPE), very rarely (1 out of 100,000 cases) occurs several years after the original measles infection, most often in children infected with measles under the age of two years (Heymann 2008). The mortality rate can be as high as 30% in some low‐income countries, although it is more typically estimated at 3% to 5% (WHO 1999). This compares with 0.1% in high‐income countries. The discrepancy has a significant association with the prevalence of vitamin A deficiency (WHO 1999).
Description of the intervention
The practice of passive immunisation against measles has been used since the 1920s (Haas 1926). Polyclonal immunoglobulins are administered parenterally to susceptible individuals, who have been in contact with an infectious case of measles, in an attempt to prevent the onset of disease or modify disease expression (Keller 2000).
A number of different immunoglobulin preparations have been used in the prophylaxis of measles. The serum or plasma of people recovering from measles or of adults who have previously suffered from the disease, whole blood from the same sources, the serous fluid obtained from placentas, ascites fluid and animal sera have all been trialled (Barenberg 1930; Karelitz 1937; Morales 1930; Thalhimer 1939; Zingher 1924). In the 1940s, methods were devised for concentrating the antibodies in human plasma and today the process of fractionation continues to be used to produce the blood product human immune globulin from pooled donated human plasma (Gonik 2011). Both intramuscular and intravenous preparations are in use. Product names vary from country to country; so too the concentration of disease‐specific immunoglobulins in the products will generally reflect circulating antibody levels in the donating populations (Sawyer 2000). However, in some countries, minimum neutralising antibody concentrations to measles may be regulated (Sawyer 2000).
Current recommendations for dose calculations vary by country, although they are all calculated according to body weight (CDC 2011b; CDNA 2009; ID HPA 2009; NZ MoH 2011). Regardless of the dose recommended, passive immunisation is not currently recommended if more than six days have elapsed since exposure to measles (CDC 2011b; CDNA 2009; ID HPA 2009; NZ MoH 2011).
How the intervention might work
Whether injected or infused, the administered immunoglobulins distribute throughout the recipient's body (Birdsall 2009). The mechanism by which the recipient is protected from disease involves interaction between the immunoglobulins, the invading measles virus particles and the cells and molecules of the recipient's immune system (Reading 2007). The exact mechanisms by which viral infectivity is mitigated by antibodies within the body are not comprehensively understood but vary according to the structure and functionality of the particular antibodies as they encounter the particular virus particles (Reading 2007). In general, measles‐specific antibodies bind to invading measles virus particles and this may prevent their entry into cells directly, or trigger other immune mechanisms that result in neutralisation or destruction of the virus (Birdsall 2009; Keller 2000; Reading 2007).
Why it is important to do this review
The effectiveness of post‐exposure prophylaxis against measles with immunoglobulins is generally accepted (ATAGI 2008; CDC 1998; NZ MoH 2011; Ramsay 2009). However, effectiveness rates vary considerably among identified reports (King 1991; Ordman 1944; Sheppeard 2009; Stokes 1944).
Further, national recommendations for the use of post‐exposure immunoglobulins for measles differ across a number of countries (Best 2011; CDC 1998; CDNA 2009; ID HPA 2009; NZ MoH 2011; Ramsay 2009) where disease incidences (WHO 2014), immunisation schedules (ATAGI 2008; Gustavo 2008; HPA 2011; NZ MoH 2011), measles‐containing vaccine coverage (WHO 2014) and relevant literature are similar. Differences in immunoglobulin dosage recommendations among these countries may reflect differences in the minimum levels of measles‐specific antibodies in intramuscular preparations (Best 2011; Ramsay 2009; Sawyer 2000).
We could not identify any systematic review evidence of the effectiveness of post‐exposure passive immunisation against measles, nor any systematic review evidence of the minimum effective dosage of immunoglobulin for post‐exposure prophylaxis against measles. Recent guidance from the United Kingdom on the required dosage of intramuscular immunoglobulin is based on a single study (Endo 2001; Ramsay 2009).
This review aimed to clarify the effectiveness rate, assess the evidence for a minimum effective dose and identify differences in benefit or harm across population groups. These outcomes would be valuable to guide public health practice in countries with low incidences of measles.
Objectives
To assess the effectiveness and safety of intramuscular injection or intravenous infusion of immunoglobulins for preventing measles when administered to exposed susceptible people before the onset of symptoms.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi‐RCTs and prospective non‐RCTs (cohort studies), irrespective of blinding, publication status, language or unit of randomisation. We included prospective non‐RCTs given that more recent studies, using current immunoglobulin preparations, were likely to be non‐randomised for ethical reasons. The intervention has been part of public health practice since the 1920s and, as such, any RCTs are likely to have been conducted at a time when the antibody levels of blood donors were due to infection with measles rather than vaccination. To inform practice appropriately, any evidence of the effectiveness of current immunoglobulin preparations should be included.
Types of participants
People of any age, sex or ethnic origin who were susceptible (no history of measles and not vaccinated against measles and/or measles immunoglobulin G (IgG) negative) and exposed to measles virus or exposed to someone diagnosed with measles and who were asymptomatic at the time of intervention or control administration. The primary study's definition of 'exposed' was accepted.
Types of interventions
Intervention: intramuscular injection of polyclonal immunoglobulins; intravenous infusion of polyclonal immunoglobulins. Only interventions using immunoglobulins derived from human sera or plasma were included.
Control: no intervention or placebo or live attenuated measles virus vaccine.
We also included studies assessing different brands or preparations of polyclonal immunoglobulins or different dosages of immunoglobulins. We only included studies where the intervention (and control) were administered to participants after exposure to measles and before the participants developed measles symptoms.
Types of outcome measures
Primary outcomes
Cases of measles. The diagnosis may be made by detection or isolation of measles virus in urine or respiratory secretions; by detection of measles virus antigen in urine or respiratory secretions; by serological detection of immunoglobulin M (IgM) to measles in the absence of vaccination eight days to eight weeks prior to testing; by IgG seroconversion or by a fourfold or greater rise in titre to measles virus in the absence of vaccination eight days to eight weeks prior to testing; or by symptoms consistent with measles (fever, a red blotchy rash, conjunctivitis, runny nose and cough) or modified measles (prolonged incubation period, milder fever, cough, runny nose, conjunctivitis and sparse discrete rash of short duration).
Mortality due to measles.
Secondary outcomes
Prevention of measles outbreak (higher than expected incidence) as identified by active surveillance.
Cessation of measles outbreak (return to expected incidence) as identified by active or passive surveillance (or both).
Complications due to measles such as otitis media, pneumonia or encephalitis.
Occurrence and type of adverse events. We proposed to analyse two types of adverse events: serious adverse events and non‐serious adverse events. A serious adverse event was defined as "any untoward medical occurrence that at any dose results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, or is a congenital anomaly/birth defect" (EMEA 1995). We classified all other events as non‐serious. We specifically sought to extract data on: blood‐borne virus infection; anaphylaxis; generalised hypersensitivity and injection site reactions. We also included any other adverse event reported as such by study authors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2012, Issue 7), which contains the Cochrane Acute Respiratory Infections (ARI) Group's Specialised Register, MEDLINE (via OVID) (1946 to July week 4, 2012), CINAHL (via EBSCO) (1981 to August 2012) and EMBASE (1974 to August 2012). We used the search strategy in Appendix 1 to search MEDLINE and CENTRAL. We adapted the strategy for EMBASE (Appendix 2) and CINAHL (Appendix 3). We combined the MEDLINE and EMBASE searches with the filter for study type in Appendix 4 as we considered the search results retrieved too large to be manageable. We updated the electronic searches on 14 August 2013 by searching CENTRAL (2013, Issue 7) from 2011 to 2013, MEDLINE from 1 June 2012 to July week 5 2013, CINAHL after June 2012 and EMBASE from 1 July 2012 to August 2013.
Searching other resources
We searched reference lists of identified relevant studies and reviews. We searched www.clinicaltrials.gov and WHO ICTRP (19 August 2013) using the search term 'measles'. To locate further published or unpublished studies, we attempted to contact companies manufacturing immunoglobulin products for countries with low measles incidences and attempted to contact the corresponding author of any included studies.
Data collection and analysis
Selection of studies
Two review authors (MY, GN) independently inspected the title and abstract (as available) of each reference identified by the electronic search and determined the potential relevance of each article. If identified by either review author as potentially relevant, we retrieved the full article. One author (MY) searched the reference lists of the relevant retrieved studies and retrieved the full articles of those that could not be excluded based on title (and abstract where available).
Both review authors independently inspected each full article using an eligibility checklist based on the inclusion criteria, to determine inclusion in the review. We resolved any disagreements through discussion. We excluded studies not meeting the eligibility criteria and stated the reasons for exclusion.
We did not identify any duplicate publications.
Data extraction and management
Two review authors (MY, AC) independently extracted data from the included studies using pre‐designed electronic data extraction forms. We resolved disagreements by discussion. We attempted to contact study authors for clarification or further information as necessary.
We attempted to extract the following data:
-
The study
First author, publication year/not published.
Location.
Date study undertaken.
Randomised/quasi‐randomised/non‐randomised.
-
Participants
Number in each group.
Age range in each group.
Proportion of adults, children, infants (aged < one year) in each group.
Gender distribution in each group.
Proportion of high‐risk individuals in each group: those with immunodeficiency; pregnancy or age under one year.
Range of time since exposure in each group.
Average time since exposure in each group.
Any measure of baseline comparability and result of this, if calculated.
-
Intervention
Intervention group: product used, concentration of measles antibody if known, volume given, route of administration.
Control group: placebo/vaccine/product/other, concentration of measles antibody if relevant and known, volume given, route of administration.
-
Outcomes
Primary and secondary (as above).
Length of follow‐up.
Loss to follow‐up.
Assessment of risk of bias in included studies
Two review authors (MY, AC) independently assessed the risk of bias of included studies. We resolved any disagreements by discussion. For randomised and quasi‐randomised studies, we assessed: randomisation sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting and other potential sources of bias. We reported the risk of bias using The Cochrane Collaboration's tool for assessing 'Risk of bias' (Higgins 2011). For non‐randomised studies, we allocated randomisation sequence generation and allocation concealment (selection bias) 'high risk'. We assessed: blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; management of confounders and other potential sources of bias.
We made the decision to include 'Summary of findings' tables in the review post‐protocol. We produced the tables using GRADEpro 2008 software. As per GRADE recommendations, where meta‐analyses included at least one cohort study, we initially considered the evidence of low quality and then upgraded and/or downgraded it according to GRADE criteria (Higgins 2011). Both primary outcomes and the secondary outcomes 'complications due to measles' and 'adverse events' were eligible for inclusion in the 'Summary of findings' tables.
Measures of treatment effect
Outcomes, as identified above, are dichotomous. We expressed these outcomes as risk ratios (RRs) and calculated 95% confidence intervals (CIs) for each.
Unit of analysis issues
No cluster‐randomised trials were identified for inclusion in the review.
For studies with multiple intervention groups, for example different doses or preparations of immunoglobulins compared to control, we split the shared group and included the relevant pair‐wise comparisons in the meta‐analysis (Higgins 2011).
Dealing with missing data
We attempted to contact the trial authors for any missing data. Where missing data exceeded 20% (one study ‐ Glyn‐Jones 1972), or where data were missing in different proportions in the treatment groups (one study ‐ Stillerman 1944), we excluded the study from meta‐analysis for the relevant outcomes. There were no studies (i.e. studies with smaller amounts of missing data) requiring sensitivity analysis (assuming worst‐case and best‐case scenarios).
Assessment of heterogeneity
We explored the presence of heterogeneity firstly by comparing studies' population groups and interventions. Where no clinically relevant heterogeneity was present, we proceeded to meta‐analysis. We considered the forest plots for each primary outcome and the secondary outcome "Complications due to measles" and proceeded to subgroup and sensitivity analyses where heterogeneity was clear visually. We re‐examined the heterogeneity of subgroup and sensitivity analyses separately. We considered an I2 statistic estimate of 60% or more, alongside a Chi2 test P value of 0.1 or less, to be important heterogeneity.
Our protocol indicated the secondary outcome 'serious adverse events' among those for meta‐analysis. However, this outcome was not reported in any included study.
Assessment of reporting biases
We examined each included study for indications that outcomes assessed had not been reported.
Our protocol indicated that, had multiple publications of the same study been retrieved, we would list the subsequent papers with the main paper and enter the data for meta‐analysis once only. However, we did not identify multiple publications of the same study.
Our protocol indicated that we would assess publication bias by examining funnel plots if sufficient studies (at least 10) were included. However, the maximum number of studies included in meta‐analyses was seven.
Data synthesis
We calculated the RR and 95% CI for each outcome measured in each study. We used a fixed‐effect model in meta‐analysis of each primary outcome and the secondary outcome 'complications due to measles' and examined the forest plots to assess heterogeneity. We explored possible reasons for apparent heterogeneity via subgroup and sensitivity analyses and reported the results of these using fixed‐effect models.
We reported the results of the secondary outcome 'adverse events' descriptively.
Subgroup analysis and investigation of heterogeneity
Our protocol listed the following subgroup analyses that we were unable to perform because of insufficient available information from the included studies:
proportion of high‐risk individuals;
dose of measles‐specific immunoglobulins.
Further, the following subgroup analyses were not relevant to the review:
route of administration of immunoglobulins (all included studies administered immunoglobulins intramuscularly);
timing of administration of intervention in relation to exposure (included studies generally administered immunoglobulins within seven days of exposure where this was reported. Only Stillerman 1944 administered immunoglobulins within eight days, although Salomon 1923 and Wesselhoeft 1928 did not report the timing of the intervention in relation to exposure. Hence, rather than subgroup analysis, we undertook sensitivity analysis, by excluding each of these studies in turn and together. In addition, there were insufficient studies assessing the effect of the timing of the intervention (within seven days of exposure) on the prevention of measles to undertake a separate analysis);
differences in the primary study definition of exposed (with the exception of Cockburn 1950, Endo 2001 and Sheppeard 2009, all included studies had similar definitions of 'exposed'. Endo 2001 was not included in meta‐analyses. Cockburn 1950 was included with only one other study in a meta‐analysis. Thus we undertook sensitivity analysis, by excluding Sheppeard 2009, rather than subgroup analysis, to assess the impact of the difference in this study's exposure definition).
We undertook the following subgroup analyses:
study type (quasi‐RCTs and cohort type studies);
age of participants (although sufficient information was not available to divide the data as we had intended (infants/children/adults/combinations), we grouped studies according to age as follows: "included infants less than six months of age" and "did not include infants less than six months of age");
dose of immunoglobulins (studies generally reported administering a range of volumes of immunoglobulins and these were not uniform, hence studies were grouped by the type of intervention blood product (convalescent serum, adult serum and gamma globulin) as an approximation of dose).
Sensitivity analysis
Our protocol specified that we would undertake sensitivity analysis based on the risk of bias in included studies and studies with imputed missing data.
We examined the effect of the risk of bias of included studies on the results of meta‐analyses by excluding Sheppeard 2009 from the relevant outcome because of the high risk of attrition bias in this study. The risk of bias was otherwise similar across included studies.
We did not impute missing data for any study.
Post‐protocol sensitivity analyses
As indicated above, because most included studies identified the intervention dose of immunoglobulin by total volume and the ranges administered were not uniform between studies, we grouped the studies by the blood product used as an approximation of immunoglobulin dose. The rationale for this was: gamma globulin is manufactured as a concentrated preparation of immunoglobulins and is thus likely to have the highest concentration of measles‐specific antibodies per unit volume; the acute immune response following disease means that convalescent serum will contain the next highest concentration of measles‐specific antibodies per unit volume; and adult serum will contain the lowest concentration of measles‐specific antibodies per unit volume as disease would most likely have occurred in childhood for the donors of the serum at the time of the included studies. Given this approximation of dose, we undertook sensitivity analyses by excluding Stillerman 1944 as the outlier (largest volume range and highest volume) within the convalescent serum group and by excluding Salomon 1923 from the convalescent serum group as the volume of serum administered was not reported. Volume ranges within the subgroups were otherwise similar.
As indicated above, we also excluded Stillerman 1944, Salomon 1923 and Wesselhoeft 1928 alone and together to examine the impact of definite (Stillerman 1944) and possible (Salomon 1923; Wesselhoeft 1928) differences in the maximum time between exposure and intervention. We also excluded Sheppeard 2009 alone to assess the impact of this study's definition of exposure.
Results
Description of studies
Results of the search
Searches of MEDLINE, EMBASE, CENTRAL and CINAHL on 6 August 2012 identified 2369 unique records, of which we retrieved 55 full‐text articles resulting in five included studies. We updated the electronic searches on 14 August 2013 and identified 102 unique records, of which we retrieved two full‐text articles. No further studies met the inclusion criteria. Searching the reference lists of relevant retrieved full‐text articles identified a further 133 unique papers, of which we retrieved 89 full‐text articles resulting in eight included studies (Figure 1). Searching www.clinicaltrials.gov returned 158 records but no additional relevant studies. Searching WHO ICTRP returned 182 records but no additional relevant studies. We sent electronic written requests to 13 separate companies that manufacture immunoglobulin products (Appendix 5) and the Australian Technical Advisory Group on Immunisation (ATAGI). Four companies and the ATAGI responded. No additional studies were identified. The age of the included studies and absent up‐to‐date contact details for authors meant that we were only able to contact the authors of one study. No additional studies were identified as a result of this communication.
1.
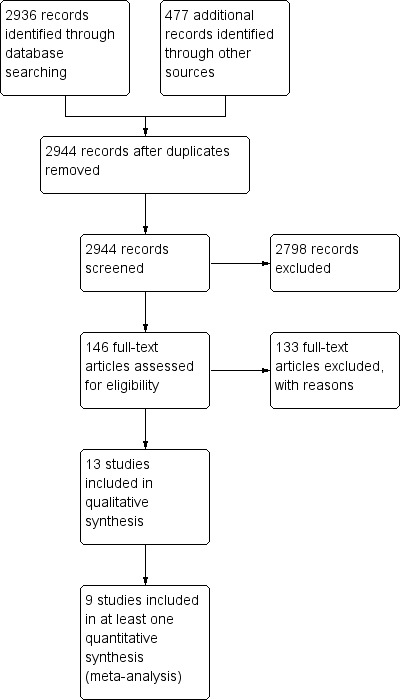
Flow diagram of retrieval, selection and exclusion of studies.
Included studies
A total of 13 studies were included in the review: one RCT, two quasi‐RCTs and 10 prospective, non‐randomised, controlled (cohort) studies (see Characteristics of included studies table). Included studies were published between 1920 and 2009. No unpublished studies were included.
Studies were undertaken in seven different countries: United States (Berkovich 1963; Ordman 1944; Stillerman 1944; Toomey 1926; Wesselhoeft 1928), Japan (Endo 2001), United Kingdom (Cockburn 1950; Hartley 1948), Australia (Sheppeard 2009), Germany (Degkwitz 1920; Salomon 1923), Zimbabwe (Glyn‐Jones 1972) and Puerto Rico (Morales 1930). A total of 3925 participants were recruited from hospitals, child care facilities and the community. Sample sizes ranged from 11 to 921.
Only one study included adults among the participants (Sheppeard 2009), although four studies (Berkovich 1963; Degkwitz 1920; Endo 2001; Wesselhoeft 1928) did not report the age of participants and five studies (Hartley 1948; Ordman 1944; Salomon 1923; Sheppeard 2009; Toomey 1926) did not report a clear age range. Two of these latter studies included participants less than six months of age (Hartley 1948; Salomon 1923). Ordman 1944 and Sheppeard 2009 specified that participants were aged six months and over. Toomey 1926 identified participants as 'children'. Participants of the remaining four included studies were aged no younger than six months, with maximum ages ranging from 35 months to 15 years (Cockburn 1950; Glyn‐Jones 1972; Morales 1930; Stillerman 1944).
The only study reporting gender distribution noted similar proportions of males and females in both the intervention and control groups (intervention 53% males; control 51% males) (Cockburn 1950).
The proportions of participants at high risk of measles complications were also poorly reported. Glyn‐Jones 1972 reported that between 40% and 50% of participants were aged less than 12 months, while this group was approximately one‐quarter of the participants of Hartley 1948, approximately 10% of the participants of Stillerman 1944 and around 5% of the participants of Cockburn 1950. No information was available on high‐risk groups in the other studies.
With the exception of Cockburn 1950, Endo 2001 and Sheppeard 2009, participants were exposed to measles either by living with someone diagnosed with measles or being in the same hospital ward as a person with measles. Cockburn 1950 defined 'intimate', 'close' and 'remote' contact. (Intimate ‐ played with and enrolled in the same section of the nursery as the primary case; close ‐ exposed for short periods at play or meals but enrolled in a different section of the nursery; remote ‐ contact usually confined to exposure in the entrance hall in the morning or evening or out of doors during the day). Endo 2001 defined close contact as: a household member with measles, exposure to a schoolmate or playmate with measles lasting at least one hour, or exposure to a person with measles in a medical facility. Sheppeard 2009 defined exposure as: anyone who was in the same room as the case, or the same room for up to two hours after the case, during the infectious period.
The interval between exposure and intervention or control was within seven days for 10 studies, within eight days for Stillerman 1944 and not reported for the other two studies (Salomon 1923; Wesselhoeft 1928). The intervention was convalescent serum given intramuscularly in six studies (Degkwitz 1920; Morales 1930; Salomon 1923; Stillerman 1944; Toomey 1926; Wesselhoeft 1928). Morales 1930 and Salomon 1923 also trialled adult serum intramuscularly. Doses ranged from 2.5 ml to 20 ml. The remaining seven studies trialled gamma globulin intramuscularly. With the exception of Glyn‐Jones 1972, whose participants received 2 ml every three weeks until discharge, studies trialling gamma globulin varied the single administered dose usually in response to participants' weight or age.
With the exception of Degkwitz 1920, all studies trialling convalescent serum included a 'no treatment' control group. Degkwitz examined 3 ml compared to 2.5 ml of convalescent serum both on day four after exposure in one trial and examined 6 ml to 7 ml of convalescent serum on day six after exposure compared to 7 ml to 8 ml of convalescent serum on day seven after exposure in a second trial.
Three studies trialling gamma globulin included 'no treatment' control groups (Glyn‐Jones 1972; Ordman 1944; Sheppeard 2009). Three studies administered measles vaccine to a control group (Berkovich 1963; Glyn‐Jones 1972; Sheppeard 2009), although Berkovich 1963 administered gamma globulin as well as vaccine to the same individuals. Hartley 1948 administered convalescent serum of doses between 2.5 ml and 5 ml or more to the control group. Cockburn 1950 administered adult serum or reconstituted dried plasma to the control group at a dose of 5 ml. Endo 2001 used four lots of gamma globulin, each with a different measles‐specific antibody titre (16 IU/ml, 33 IU/ml, 40 IU/ml and 45 IU/ml). The dose administered was 0.33 ml/kg for each participant.
All included studies assessed the number of measles cases in each group as the primary outcome. Five studies assessed complications due to measles in each study group (Cockburn 1950; Glyn‐Jones 1972; Morales 1930; Ordman 1944; Wesselhoeft 1928). One study ceased follow‐up of the 'no treatment' control group upon onset of rash and hence only assessed complications in the intervention group (Stillerman 1944). None of these studies described the criteria for determining that complications were due to measles. Four studies assessed mortality due to measles (Glyn‐Jones 1972; Morales 1930; Salomon 1923; Wesselhoeft 1928). None of these studies described the process for attributing participants' deaths to measles rather than another cause.
Adverse events were not considered in the majority of the included studies and only Glyn‐Jones 1972 specified adverse events as an outcome measure in the methods, but under the premise of reactions to measles vaccine rather than gamma globulin. However, Cockburn 1950 and Morales 1930 also reported on adverse events amongst their participants and Hartley 1948, Ordman 1944 and Toomey 1926 made mention of adverse events in their experience with passive immunisation more generally.
The effectiveness of passive immunisation for the prevention or cessation of measles outbreaks was not assessed by any included study.
Excluded studies
Out of the 146 full‐text papers retrieved, 108 were not prospective controlled studies. They included case reports, case series, reviews, retrospective designs and two studies where it was not clear that the comparison group originated from the same population as the intervention group. Another 21 of those excluded were studies where either the participants were not susceptible and exposed to measles or this was unclear. Three studies did not examine intramuscular or intravenous polyclonal immunoglobulins derived from human serum or plasma. One study did not assess the number of participants who developed measles.
The reasons for exclusion of individual studies where these were discussed by the authors, after comparison of their independent assessments, are given in the Characteristics of excluded studies table. For brevity, we have not listed studies where authors' independent assessments were in agreement.
Risk of bias in included studies
None of the included studies was determined to have a low risk of bias for all criteria (see Figure 2 and Characteristics of included studies table).
2.
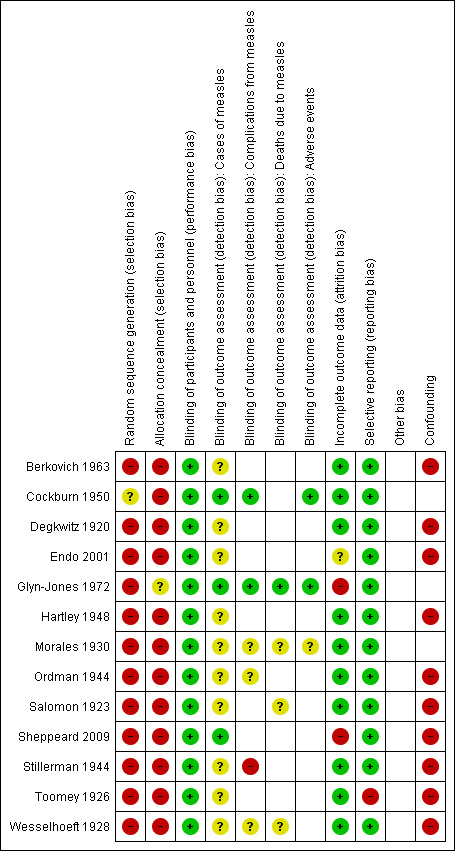
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The one included RCT described the random sequence generation in insufficient detail and we deemed it to have unclear risk of bias for this criterion (Cockburn 1950). All other studies were at high risk of bias for this criterion as they were either quasi‐randomised or non‐randomised studies.
Glyn‐Jones 1972 seemed to allocate participants to interventions using third parties with no knowledge of the participants. However, this was not explicitly stated and hence we deemed it to be unclear risk in terms of allocation concealment. The other studies, including the RCT, were at high risk of bias regarding the allocation of participants to interventions.
Blinding
The intervention, administration of polyclonal immunoglobulins, is very unlikely to be subject to variation due to performance and, as such, we deemed all studies at low risk of performance bias.
We assessed detection bias for the outcomes: cases of measles, complications due to measles, mortality due to measles and adverse events. With the exception of adverse events, each of these outcomes is objective provided appropriate pre‐study definitions are adopted. Unfortunately, sufficient information was rarely provided to determine whether pre‐study definitions had been set. Similarly, very limited information on blinding was provided in nearly all included studies.
Given this, we deemed the risk of detection bias to be unclear for the majority of included studies in relation to measles cases. We deemed Cockburn 1950, Glyn‐Jones 1972 and Sheppeard 2009 to have a low risk of detection bias with respect to cases of measles. Cockburn 1950 and Glyn‐Jones 1972 adequately described blinding procedures despite the lack of information on their case definition of measles and Sheppeard 2009 provided a very clear pre‐study case definition that was applied uniformly.
The outcome 'complications due to measles' was assessed by six studies. As for cases of measles, Cockburn 1950 and Glyn‐Jones 1972 were at low risk of detection bias. Stillerman 1944 assessed only the intervention group for complications due to measles as the study ceased follow‐up of controls upon the onset of rash. This study was clearly at high risk of detection bias for this outcome. The other three studies did not provide sufficient information and we deemed them at unclear risk (Morales 1930; Ordman 1944; Wesselhoeft 1928).
The outcome 'mortality due to measles' was assessed by four studies. Glyn‐Jones 1972 was again at low risk. The other three studies did not provide sufficient information and we deemed them at unclear risk (Morales 1930; Salomon 1923; Wesselhoeft 1928).
Glyn‐Jones 1972 was the only study to specify adverse events as an outcome measure in the methods, although Cockburn 1950 and Morales 1930 also reported on adverse events amongst their participants. As Cockburn 1950 and Glyn‐Jones 1972 were adequately blinded, these studies were at low risk of detection bias for this outcome. Morales 1930 did not provide sufficient information and was at unclear risk.
Incomplete outcome data
Most studies reported complete follow‐up for the primary outcome measures and were at low risk of attrition bias. Glyn‐Jones 1972 reported a loss to follow‐up of 20.6% overall, with rates of 19.4% to 22.4% across the three study groups. We considered this a high risk of bias and we excluded the study from meta‐analysis.
Endo 2001 did not specify whether parents who did not report illness in their child were actively followed up and the authors could not be contacted. We therefore deemed this study to be at unclear risk of attrition bias. The author of Sheppeard 2009 provided information that passive surveillance was the means of participant follow‐up. As such we deemed this study to be at high risk of attrition bias, although no loss to follow‐up was reported.
Selective reporting
Toomey 1926 presented some adverse event case series data but this outcome was not reported in relation to the cohort study participants. We therefore deemed this study to be at high risk of reporting bias. Each of the other included studies reported on all outcomes specified in the methods sections and we deemed them to be at low risk of reporting bias.
We did not identify multiple publications of the same study. As the maximum number of studies included in meta‐analysis was seven, we did not have sufficient studies to examine publication bias using funnel plots.
Other potential sources of bias
Ten of the included studies were non‐randomised 'cohort type' studies. Confounding was not well addressed in any of these studies and was typically not addressed at all. Confounding is therefore a likely source of bias in each of these studies and we deemed each to be at high risk for this criterion.
Effects of interventions
Three included studies could not be included in meta‐analyses because of heterogeneity among the comparison groups (Berkovich 1963; Degkwitz 1920; Endo 2001). One included study (Glyn‐Jones 1972) could not be included in meta‐analyses as per protocol because loss to follow‐up exceeded 20%.
Primary outcomes
1. Cases of measles
Seven included studies that examined the effect of immunoglobulin versus no treatment for the prevention of measles were included in a meta‐analysis of the primary outcome 'cases of measles' (Morales 1930; Ordman 1944; Salomon 1923; Sheppeard 2009; Stillerman 1944; Toomey 1926; Wesselhoeft 1928). Although all results favoured the intervention group, statistical heterogeneity was visually obvious upon examination of the initial forest plot (Analysis 1.1) and indeed the I² statistic was 87%. The sensitivity analyses conducted by excluding Salomon 1923, Sheppeard 2009, Stillerman 1944 and Wesselhoeft 1928 in turn did not alter these results.
1.1. Analysis.
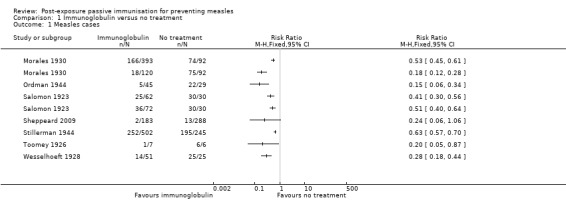
Comparison 1 Immunoglobulin versus no treatment, Outcome 1 Measles cases.
There was no significant difference in the results of the subgroup which included infants younger than six months of age compared to the subgroup that did not include infants younger than six months of age (test for subgroup differences: Chi² test = 0.36, df = 1 (P value = 0.55), I² statistic = 0%). No other subgroup analyses were able to examine possible differences in the benefit of the intervention.
Subgroup analyses examining study type and participant age did not explain the observed heterogeneity (Analysis 1.2). However, the subgroup analysis examining the blood product used, as an approximation of dose, revealed homogenous results for the adult serum group (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.45 to 0.59; heterogeneity: Chi² test = 0.02, df = 1 (P value = 0.88); I² statistic = 0%) and gamma globulin group (RR 0.17, 95% CI 0.08 to 0.36; heterogeneity: Chi² test = 0.34, df = 1 (P value = 0.56); I² statistic = 0%), although not the convalescent serum group (RR 0.49, 95% CI 0.44 to 0.54; heterogeneity: Chi² test = 49.53, df = 4 (P value < 0.001); I² statistic = 92%) (Analysis 1.2). Excluding Sheppeard 2009 from the gamma globulin group left only one study in this subgroup and only minimally altered the risk ratio from 0.17 to 0.15.
1.2. Analysis.
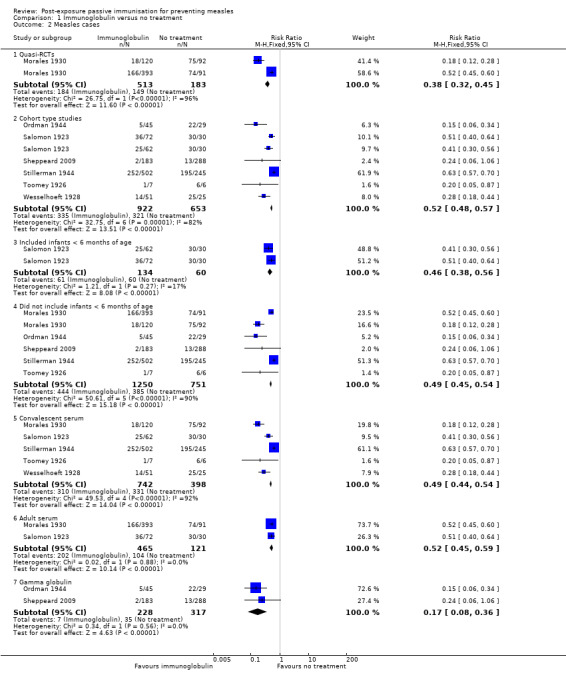
Comparison 1 Immunoglobulin versus no treatment, Outcome 2 Measles cases.
Sensitivity analyses that excluded Stillerman 1944, Salomon 1923 and Wesselhoeft 1928, in turn and together, demonstrated that the former two studies contributed most of the heterogeneity to the results for the convalescent serum subgroup (Analysis 1.3). The RR for this subgroup was 0.49 (95% CI 0.44 to 0.54) when the five eligible studies were included. Excluding Salomon 1923 did not alter the RR (0.49, 95% CI 0.45 to 0.55) and heterogeneity remained high (Chi² test = 46.69, df = 3 (P value < 0.001); I² statistic = 94%). Excluding Stillerman 1944 affected the RR considerably and also decreased the heterogeneity, although this was still significant (RR 0.26, 95% CI 0.21 to 0.33; heterogeneity: Chi² test = 11.40, df = 3 (P value = 0.010); I² statistic = 74%). Excluding Wesselhoeft marginally altered the RR 0.50 (95% CI 0.45 to 0.56) but again heterogeneity remained high (heterogeneity: Chi² test = 40.24, df = 3 (P value < 0.001); I² statistic = 93%). With both Salomon 1923 and Stillerman 1944 excluded, the RR for the convalescent serum group was 0.21 (95% CI 0.15 to 0.29) and heterogeneity was minimal (Chi² test = 2.11, df = 2 (P value = 0.35); I² statistic = 5%) (Analysis 1.3). Excluding Wesselhoeft as well altered the RR minimally (RR 0.19, 95% CI 0.12 to 0.28) and resulted in a further small reduction of heterogeneity (Chi² test = 0.01, df = 1 (P value = 0.90); I² statistic = 0%). Irrespective of these sensitivity analyses, differences in the subgroup estimates of effect were significant (P value < 0.001 to 0.02; I² statistic = 93.8% to 75.3%).
1.3. Analysis.
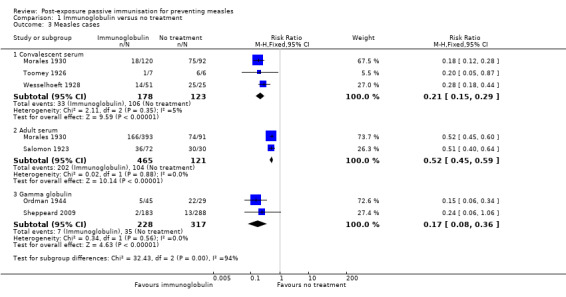
Comparison 1 Immunoglobulin versus no treatment, Outcome 3 Measles cases.
Two studies that examined the effect of gamma globulin compared to a comparison group administered serum (either convalescent or adult serum) for the prevention of measles were included in a meta‐analysis of the primary outcome 'cases of measles' (Cockburn 1950; Hartley 1948). Heterogeneity was not significant either visually or statistically (heterogeneity: Chi² test = 3.03, df = 2 (P value = 0.22); I² statistic = 34%). Similar to the comparison of immunoglobulin to no treatment, the result favoured gamma globulin (RR 0.56, 95% CI 0.46 to 0.69) (Analysis 2.1).
2.1. Analysis.
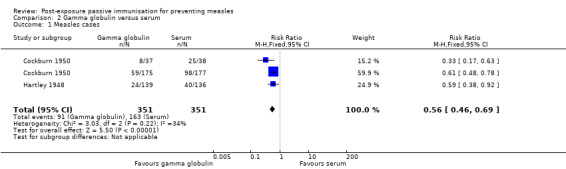
Comparison 2 Gamma globulin versus serum, Outcome 1 Measles cases.
The results of studies which could not be included in the meta‐analyses also supported the impact of the dose of immunoglobulins upon effectiveness. Endo 2001 reported that eight of 14 participants administered gamma globulin with a measles‐specific antibody concentration of 16 IU/ml developed measles as compared to one of six participants administered gamma globulin with a measles‐specific antibody concentration of 33 IU/ml and none of 13 participants administered gamma globulin with a measles‐specific antibody concentration of 40 IU/ml or more. This is a RR of 0.29 (95% CI 0.05 to 1.85) for the group given 33 IU/ml gamma globulin compared to the group given 16 IU/ml gamma globulin. Degkwitz 1920 reported that three of seven participants administered 2.5 ml of convalescent serum developed measles as compared to none of 12 participants administered 3 ml of convalescent serum. A RR could not be calculated for this comparison.
Degkwitz 1920 also examined the effect of the time since exposure on the effectiveness of immunoglobulins for preventing measles. None of eight participants administered 6 ml to 7 ml of convalescent serum at six days post‐exposure compared to one of three cases administered 7 ml to 8 ml of convalescent serum at seven days post‐exposure developed measles. Again, a RR could not be calculated for this comparison.
Berkovich 1963 compared measles vaccine and gamma globulin at 0.02 ml per pound of body weight to gamma globulin alone at 0.1 ml per pound of body weight and reported that nine of 14 participants given vaccine and gamma globulin and two of four participants given gamma globulin alone developed measles. This suggests less risk of developing measles in the gamma globulin only group but the RR was not statistically significant (RR 0.78, 95% CI 0.27 to 2.23).
Glyn‐Jones 1972 compared the effectiveness of gamma globulin, 2 ml every three weeks, with measles vaccine and no treatment. Twenty‐four of 68 participants who received gamma globulin, seven of 70 participants who received vaccine and 58 of 73 participants who received no measles prophylaxis developed measles. Thus, among those for whom data were available, the risk of measles was greater in the gamma globulin group than the vaccine group (RR 3.53, 95% CI 1.63 to 7.65) and less in the gamma globulin group than the no treatment group (RR 0.44, 95% CI 0.32 to 0.63).
In addition to comparing gamma globulin to no treatment, Sheppeard 2009 included a vaccine only group. None of the 82 participants who received vaccine within three days of exposure developed measles compared to two of the 183 participants who received gamma globulin within seven days and 13 of the 288 participants who received no treatment. A RR could not be calculated for comparison of vaccine to the other groups.
2. Mortality due to measles
Three studies were included in the meta‐analysis of the primary outcome 'mortality due to measles' (Morales 1930; Salomon 1923; Wesselhoeft 1928). The results were homogenous and favoured the intervention group (RR 0.24, 95% CI 0.13 to 0.44; heterogeneity: Chi² test = 0.65, df = 4 (P value = 0.96); I² statistic = 0%) (Analysis 1.4).
1.4. Analysis.
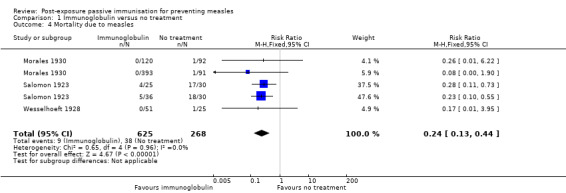
Comparison 1 Immunoglobulin versus no treatment, Outcome 4 Mortality due to measles.
Glyn‐Jones 1972, not included in the meta‐analysis due to loss to follow‐up in excess of 20%, reported that three of 68 participants in the gamma globulin group, 12 of 73 participants in the no treatment group and one of 70 participants in the vaccine group died as a result of measles. Thus gamma globulin reduced mortality compared to no treatment (RR 0.27, 95% CI 0.08 to 0.91) among those for whom results were available. Mortality seemed greater in the gamma globulin group compared to the vaccine group but the results were not statistically significant (RR 3.09, 95% CI 0.33 to 28.96).
Secondary outcomes
1. Prevention of measles outbreak
No included studies assessed the outcome 'prevention of measles outbreak'.
2. Cessation of measles outbreak
No included studies assessed the outcome 'cessation of measles outbreak'.
3. Complications due to measles
Three studies were included in the meta‐analysis of the secondary outcome 'complications due to measles' (Morales 1930; Ordman 1944; Wesselhoeft 1928). Stillerman 1944 was excluded from the analysis because of complete missing data in the control group. The results were homogenous and favoured the intervention group (RR 0.18, 95% CI 0.05 to 0.60; heterogeneity: Chi² test = 1.23, df = 3 (P value = 0.75); I² statistic = 0%) (Analysis 1.5).
1.5. Analysis.
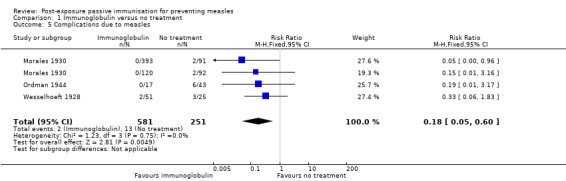
Comparison 1 Immunoglobulin versus no treatment, Outcome 5 Complications due to measles.
Two studies not included in the meta‐analysis because of heterogenous comparison groups also reported on 'complications from measles'. Cockburn 1950 reported that two of 212 participants given gamma globulin compared to five of 215 participants given adult serum developed complications from measles. This is a RR of 0.41 (95% CI 0.08 to 2.07). Endo 2001 reported no complications due to measles among any participants.
Glyn‐Jones 1972, not included in the meta‐analysis due to loss to follow‐up in excess of 20%, reported that four of 68 participants in the gamma globulin group, 11 of 73 participants in the no treatment group and two of 70 participants in the vaccine group developed complications due to measles. Thus, the gamma globulin group seemed to be at less risk of complications from measles than the no treatment group and more at risk of complications than the vaccine group, but the risk ratios were not statistically significant (RR for gamma globulin versus no treatment 0.39 (95% CI 0.13 to 1.17); RR for gamma globulin versus vaccine 2.06 (95% CI 0.39 to 10.87)).
4. Occurrence and type of adverse events
Of the included studies that mentioned or recorded adverse events, no 'serious adverse events' were reported. Glyn‐Jones 1972 recorded adverse events rates of 5% in the gamma globulin group, 4% in the vaccine group and 1% in the no treatment group. These 'probable vaccine reactions' were described as rash and fever. The differences between groups were not statistically significant. Glyn‐Jones 1972 also noted no statistically significant differences in mortality rates due to presenting illness, or in exacerbations of presenting illness, between these groups of children who were hospital inpatients. Morales 1930 noted that two participants in the intervention group given convalescent serum had a slight fever and urticarial rash. The control group for this study was 'no treatment' and data on adverse events were not collected or reported. Cockburn 1950 noted a few cases (intervention group unspecified) of transient limb stiffness lasting one or two hours among participants.
Referring to their experience within and beyond the included study, Ordman 1944 noted no severe adverse reactions to gamma globulin, with less than 5% of recipients experiencing mild reactions of slight muscle stiffness, local redness and induration. One recipient of 'several hundred' experienced fever two days after gamma globulin administration. Hartley 1948, also referring to observations within and beyond the included study, noted no local or general adverse events among gamma globulin recipients. Toomey 1926, reporting on recipients of convalescent serum over a two‐year period prior to the included study, noted no local reactions, although reported that mild fever within 24 hours of administration and lasting not more than 24 hours was common.
Discussion
Summary of main results
A total of 13 studies were included in the review: one randomised controlled trial (RCT), two quasi‐RCTs and 10 prospective, non‐randomised, controlled (cohort) studies. No unpublished studies were included.
Seven studies were included in meta‐analysis of immunoglobulin versus no treatment for measles cases. Heterogeneity was explained by subgrouping studies according to the blood product used as an approximation of the dose of immunoglobulin and then excluding two studies among the convalescent serum group thought to have different dosing and intervention timing to the other studies. Gamma globulin was most effective at preventing measles (risk ratio (RR) 0.17, 95% confidence interval (CI) 0.08 to 0.36), followed by convalescent serum (RR 0.21, 95% CI 0.15 to 0.29 to RR 0.49, 95% CI 0.44 to 0.54) and then adult serum (RR 0.52, 95% CI 0.45 to 0.59).
One study was particularly influential on the convalescent serum group estimate of effect (Stillerman 1944). This study had a very large sample size and diverged from the other studies in this group on some points of methodology, namely the volume range of convalescent serum administered was the largest (5 ml to 20 ml) and the intervention was administered up to eight days post‐exposure to measles rather than up to seven days. The estimate of effect of this study was smaller than the other studies in this group. Factors contributing to this may have included: the delay between exposure and intervention for some participants; the fact that although the maximum volume of serum administered was much larger than the other studies, the volume range was not applied uniformly according to age or weight and was not applied consistently across the duration of the study; and the serum was collected from convalescents up to four months after illness (average two months), which is longer than for other studies where this was reported (Toomey 1926: eighth day after the rash began to disappear; Morales 1930: fifth to tenth day of convalescence).
The results of the blood product subgroup analyses were supported by a meta‐analysis of gamma globulin versus serum (either convalescent or adult serum) including two studies. Gamma globulin was more effective than serum at preventing measles (RR 0.56, 95% CI 0.46 to 0.69).
The apparent dose‐effect was further supported by studies not included in the meta‐analyses. However, only two studies provided sufficient information to calculate the dose of measles‐specific antibodies administered to participants and as the attack rates in their intervention groups were not congruous, no minimum effective dose could be concluded.
Three studies were included in meta‐analysis of immunoglobulin versus no treatment for mortality due to measles. Immunoglobulin was effective at preventing death due to measles (RR 0.24, 95% CI 0.13 to 0.44).
Three studies were included in meta‐analysis of immunoglobulin versus no treatment for complications due to measles. Immunoglobulin was effective at preventing complications due to measles (RR 0.18, 95% CI 0.05 to 0.60).
Only two studies included vaccine only comparison groups. Their results suggested greater effectiveness of vaccine given within three days of exposure compared to gamma globulin given within seven days of exposure, but meta‐analysis could not be undertaken.
No serious adverse events were observed in any of the included studies. Non‐serious adverse events reported included: transient fever, rash, muscle stiffness, local redness and induration.
Overall completeness and applicability of evidence
The ethnic diversity of the populations of the included studies supports the generalisability of the results. However, 'high‐risk individuals' were not well represented and, in particular, pregnant women and immunocompromised people were not identified among study participants. Further, only one included study identified adults among their participants. While it is highly likely that passive immunisation would also be effective for these groups, no conclusions can be drawn about possible differences in the magnitude of effect.
Our investigation of the influence of age on the effectiveness of immunoglobulins compared to no treatment was limited to subgrouping studies that included infants younger than six months of age among participants and those that did not. No difference in the magnitude of effect was observed between these subgroups.
Two included studies were conducted this century and therefore examined gamma globulin that was likely to contain concentrations of measles‐specific antibodies similar to those used in current practice. These were the only two studies that provided sufficient information to allow calculation of the dose of measles‐specific antibodies administered to participants. One of these studies administered gamma globulin of different measles‐specific antibody concentrations to different groups and did not include a no treatment group (Endo 2001). The other obtained an estimate of the measles‐specific antibody concentration from the manufacturer and included a no treatment group (Sheppeard 2009). Despite overlapping estimates of the administered doses of measles‐specific antibody, no conclusions about the minimum effective dose could be drawn as the attack rates in these intervention groups across the two studies were not consistent with a unified dose‐response relationship. There are a number of possible reasons for this. Firstly, as mentioned, Sheppeard 2009 did not measure measles‐specific antibody levels in the blood product used for passive immunisation but reported an estimate from the manufacturer. Secondly, the intervention group sizes were very small in Endo 2001. Thirdly, study methodology was different across these studies and Sheppeard 2009, in particular, was known to be at high risk of attrition bias and may have underestimated the number of measles cases in the group administered gamma globulin if this led to modified measles which was not identified as such.
Only two studies examined the effectiveness of active vaccination alone compared to passive immunisation (Glyn‐Jones 1972; Sheppeard 2009). We were unable to combine these studies in meta‐analysis as per our protocol because the loss to follow‐up in Glyn‐Jones 1972 exceeded 20%. Both studies suggested vaccination was more effective at preventing measles cases than passive immunisation when administered within three days of exposure. However, study quality, low event rates in Sheppeard 2009 and the questionable external validity of Glyn‐Jones 1972 limit the conclusions that can be drawn.
No studies specifically examined measles outbreak prevention or cessation and this is perhaps not unexpected given that we did not include interrupted time series study designs in the review. In retrospect, the question of the impact of passive immunisation (and vaccination) on measles outbreaks is distinct from the individual focus of the questions we asked and may be better posed in a separate review.
Quality of the evidence
We rated no included studies at a low risk of bias for all criteria. Critical appraisal was constrained by a lack of information in most studies, yet study authors could not be contacted to supplement the information reported, mostly because of the age of the studies.
Despite these limitations, we have rated the overall quality of the evidence as moderate (see Table 1; Table 2). This is for the following reasons:
Although only one study randomised participants and none of the non‐randomised studies adequately controlled for confounders, all prespecified confounders, if present and not controlled for would be expected to cause an underestimation of effect. The prespecified confounders were: dose according to weight, time between exposure and intervention, 'high risk' of poor outcome (immunosuppression, pregnancy, infancy), other comorbidity and age. For comparison with no treatment, dose according to weight and time between exposure and intervention are not applicable. Non‐random allocation to groups would likely distribute those at 'high risk', including those with comorbidity or of particularly susceptible age, into the treatment group because of the tendency to present for preventive treatment and because of the clinician's desire for a good outcome. If we consider that this group is most likely to become ill with measles, random allocation would have increased the estimate of the effect of treatment. The non‐randomised study included in the comparison of gamma globulin and serum controlled for time between exposure and intervention by restriction, and demonstrated even distribution according to age group between treatment groups. As gamma globulin was thought to be the better product as outlined in the study's introduction, those at 'high risk', including participants with comorbidity, would have a tendency to be allocated to the gamma globulin group, meaning that random allocation would result in an increased estimate of effect. Similarly, as gamma globulin was thought to be 'more potent', the study shows that the proportion of older children who were given the smallest volume of gamma globulin was larger than the proportion of older children given the smallest volume of serum. Confounding because of failing to dose per unit of weight is thus likely (more of the gamma globulin group would have received a smaller dose per unit weight), but would result in an underestimate of the effect of gamma globulin.
The other important point of potential bias for these studies was measurement bias in relation to the outcome. There was only one study that we assessed as at high risk of bias under this criterion and this was for the outcome 'measles complications'. All other studies were at low or unclear risk of bias. Lack of information usually resulted in the unclear rating. For most studies, measles was diagnosed by a physician but blinding to treatment group was unknown. The age of the studies meant that the diagnosis was not usually confirmed by laboratory testing. If the assessors were not blind, there may be a bias operating that would overestimate the effect of passive immunisation. However, the effect size was very large and therefore likely still to be significant even if this bias was realised.
The gamma globulin estimates of effect are particularly pertinent to current practice. Meta‐analytic comparison of gamma globulin compared to another immunoglobulin preparation for the outcome 'measles cases' consisted of two studies, one at low risk of measurement bias and the other at unclear risk. In this comparison, the study at unclear risk had a smaller estimate of effect than the one at low risk. Meta‐analytic comparison of gamma globulin compared to no treatment consisted of two studies, again one at low risk of measurement bias in relation to the outcome 'measles cases' and one at unclear risk. In this comparison, the study at unclear risk did have a slightly larger effect size but the results of both studies were still homogenous.
Acknowledging that dose was approximated, an apparent dose effect was observed, increasing confidence in the results.
Potential biases in the review process
We used a filter for study design to reduce the results of the electronic searches to a manageable number. However, the use of the filter may have excluded relevant studies.
We were unable to contact the study authors of many of the retrieved studies, therefore we necessarily relied on reported information. We therefore may have excluded relevant studies because of the lack of information reported about participant exposure and/or susceptibility and/or the populations from which participants (mainly controls) were selected.
We were not aware, prior to retrieving studies, that immunoglobulins had been sourced from other than human sera or plasma in the early days of passive immunisation and this resulted in a narrowing of the intervention inclusion criteria during the review process.
It may be argued that the inclusion of non‐randomised studies introduces a bias into the review. However, as outlined above, non‐even distribution of confounders between study groups is likely to have underestimated rather than overestimated the effect size in this case.
In the absence of reported doses of measles‐specific immunoglobulins administered to intervention groups, we used blood product as an approximation of dose, acknowledging the inherent imprecision.
Agreements and disagreements with other studies or reviews
No previous systematic reviews have examined passive immunisation for the prevention of measles. Ramsay 2009 presented an account of some studies that have contributed to the field, noting the varying estimates of effectiveness. Some of the studies cited by Ramsay 2009 were included in our review (Endo 2001; Ordman 1944), while others did not meet our inclusion criteria (Black 1960; King 1991; Stokes 1944). Ramsay 2009 also suggested that the dose of measles‐specific antibody is important to the estimates of effect.
Authors' conclusions
Implications for practice.
Compared to no treatment, passive immunisation is of benefit for preventing measles up to seven days after exposure. Considering the results for gamma globulin (the current immunoglobulin preparation used in practice) and the attack rate of measles in the control group of the most recent included study (45 per 1000) (Sheppeard 2009), the absolute risk reduction for passive immunisation is 37 and the number needed to treat to benefit (NNTB) is 27 compared to no treatment. Adopting the attack rate of the control group of the other study comparing gamma globulin to no treatment (759 per 1000) (Ordman 1944), the absolute risk reduction would be 629 and the NNTB would be two.
The data for a dose‐response effect in our review has come from subgroup estimates of the different blood products used in the included studies for preventing measles. There is insufficient evidence to conclude a minimum effective dose of measles‐specific antibodies.
There is insufficient evidence to make firm conclusions regarding the relative effectiveness of passive immunisation compared to vaccination at this time.
Implications for research.
With the evidence available (of moderate quality), it is clear that passive immunisation has a large protective effect against measles for those who are exposed and not immune. However, the available evidence does not include pregnant women nor people who are immunocompromised and does not adequately distinguish infants from older participants. This 'high‐risk' group are particularly mentioned in existing public health recommendations about passive immunisation from countries with low incidences of measles. Future research should consider the effectiveness of passive immunisation for preventing measles in this defined 'high‐risk' population and include careful recording of any potential adverse events.
As a dose effect is clearly observed, further efforts should be made to determine the minimum effective dose of measles‐specific antibodies. If sufficient information exists, this may be possible to do via retrospective cohort studies. In the absence of routinely collected data that include the measles‐specific antibody level of any immunoglobulin administered, ethical considerations would likely limit this avenue of study to in vitro experiments.
In this era where measles vaccination is recommended for post‐exposure prophylaxis for those not at 'high risk', future studies should also consider the comparative effectiveness of measles vaccine if possible.
Acknowledgements
We would like to thank Liz Dooley and Clare Dooley for their support and assistance. We also wish to thank the following people for commenting on the draft protocol: Theresa Wrangham, Sushil Kabra, Segun Bello, Viviana Rodriguez and Taixiang Wu. Finally we thank the following people for commenting on the draft review: Theresa Wrangham, Sushil Kabra, Segun Bello, Robert Ware and Taixiang Wu.
Appendices
Appendix 1. CENTRAL and MEDLINE (OVID) search strategy
1 exp Measles/ (12564) 2 exp Measles virus/ (5522) 3 measles.tw. (17121) 4 (rubeola or rubeolla).tw. (280) 5 or/1‐4 (20953) 6 exp Immunoglobulins/ (703484) 7 (immunoglobulin* or immuno‐globulin* or immun* globulin*).tw,nm. (282301) 8 (gammaglobulin* or gamma‐globulin* or gamma globulin*).tw,nm. (24894) 9 exp Immunization, Passive/ (27269) 10 (passiv* adj2 (immunotherap* or immuni*)).tw. (4143) 11 (passiv* transfer* adj2 antibod*).tw. (294) 12 passive antibody transfer.tw. (35) 13 Post‐Exposure Prophylaxis/ (255) 14 ((post exposur* or post‐exposur* or postexposur*) adj2 (prophyla* or prevent*)).tw. (1708) 15 or/6‐14 (755709) 16 5 and 15 (4922)
Appendix 2. Embase.com search strategy
#16 #5 AND #15 1938 #15 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 298227 #14 ((postexposur* OR 'post exposure' OR 'post‐exposure') NEAR/2 (prophyla* OR prevent*)):ab,ti 1589 #13 'post exposure prophylaxis'/exp 279 #12 (passiv* NEAR/2 (immunother* OR immuni*)):ab,ti 3659 #11 'passive antibody transfer':ab,ti 35 #10 (antibod* NEAR/2 'passive transfer'):ab,ti OR (antibod* NEAR/2 'passively transferred'):ab,ti OR (antibod* NEAR/2 'passively transfer'):ab,ti 251 #9 'passive immunization'/de 5281 #8 gammaglobulin*:ab,ti OR 'gamma‐globulin':ab,ti OR 'gamma‐globulins':ab,ti OR (gamma NEXT/1 globulin*):ab,ti 5413 #7 immunoglobulin*:ab,ti OR 'immuno‐globulin':ab,ti OR 'immuno‐globulins':ab,ti OR (immun* NEXT/1 globulin*):ab,ti 110314 #6 'immunoglobulin'/exp 252149 #5 #1 OR #2 OR #3 OR #4 16468 #4 rubeola:ab,ti OR rubeolla:ab,ti 240 #3 measles:ab,ti 12401 #2 'measles virus'/de 5656 #1 'measles'/de 9241
Appendix 3. CINAHL (EBSCO) search strategy
S14 S4 and S13 108 S13 S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 8108 S12 TI (((post exposur* or post‐exposur* or postexposur*) N2 (prophyla* or prevent*))) OR AB (((post exposur* or post‐exposur* or postexposur*) N2 (prophyla* or prevent*))) 413 S11 (MH "Postexposure Follow‐Up") 952 S10 TI passive antibody transfer OR AB passive antibody transfer 6 S9 TI passiv* transfer* N2 antibod* OR AB passiv* transfer* N2 antibod* 11 S8 TI (passiv* N2 (immunotherap* or immuni*)) OR AB (passiv* N2 (immunotherap* or immuni*)) 82 S7 TI (gammaglobulin* or gamma‐globulin* or gamma globulin*) OR AB (gammaglobulin* or gamma‐globulin* or gamma globulin*) 97 S6 TI (immunoglobulin* or immuno‐globulin* or immun* globulin*) OR AB (immunoglobulin* or immuno‐globulin* or immun* globulin*) 3229 S5 (MH "Immunoglobulins") 5000 S4 S1 or S2 or S3 1902 S3 TI ( rubeola or rubeolla ) OR AB ( rubeola or rubeolla ) 17 S2 TI Measles OR AB Measles 1512 S1 (MH "Measles") 1276
Appendix 4. EMBASE and MEDLINE filter for study type
We combined the following filter for non‐randomised prospective intervention studies (not before and after and not time series studies) with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Higgins 2011).
1. exp Cohort Studies/ 2. Epidemiologic Studies/ 3. Intervention Studies/ 4. Evaluation Studies/ 5. Program Evaluation/ 6. Random Allocation/ 7. Clinical Trial/ 8. Single‐Blind Method/ 9. Double‐Blind Method/ 10. Control Groups/ 11. Pilot Projects/ 12. controlled clinical trial.pt. 13. clinical trial.pt. 14. comparative study.pt. 15. multicenter study.pt. 16. evaluation studies.pt. 17. Comparative Study/ 18. Multicenter Study/ 19. Follow‐Up Studies/ 20. Prospective Studies/ 21. (cohort adj (study or studies)).tw. 22. cohort analy*.tw. 23. cohort*.tw. 24. (("follow up" or follow‐up) adj (study or studies or assessment)).tw. 25. (observational adj (study or studies)).tw. 26. longitudinal.tw. 27. prospective.tw. 28. ((single or double* or triple* or treb*) and (blind* or mask*)).tw. 29. trial*.tw. 30. placebo.tw. 31. groups.tw. 32. ("pre test" or pretest or pre‐intervention or preintervention or "pre intervention" or "post test" or posttest or post‐intervention or postintervention or "post intervention").tw. 33. (pre adj5 post).tw. 34. ((evaluat* or intervention or interventional or treatment) and (control or controlled or study or studies or program* or comparison or comparative or "usual care")).tw. 35. ((intervention or interventional or process or program) adj8 (evaluat* or effect* or outcome*)).tw. 36. (program or programme or secondary analyse*).tw. 37. (quasi‐experiment* or Quasiexperiment* or "quasi random*" or quasirandom* or "quasi control*" or quasi control* or ((quasi* or experimental) adj3 (method* or study or studies or trial or design*))).tw. 38. random*.tw. 39. (study adj3 aim*).ab. 40. "our study".ab. 41. multivariate.ab. 42. compared.ab. 43. intervention*.ti. 44. pilot.ti. 45. (multicentre or multicenter or multi‐centre or multi‐center).ti. 46. controlled.ti. 47. (rat or rats or cow or cows or chicken* or horse or horses or mice or mouse or bovine or animal*).ti. 48. exp animals/ not humans.sh. 49. (or/1‐46) not (47 or 48)
Appendix 5. Companies manufacturing immunoglobulin products contacted for unpublished studies
Bayer Healthcare Pharmaceuticals
BDI Pharma (a business unit of Baxter Healthcare corporation)
Bio Products Laboratory*
CSL Behring
Grifols*
Haffkine Bio‐Pharmaceutical Corporation Ltd
Kedrion Biopharma
LFB Biotechnologies
Link Medical Products Pty Ltd
Mirren*
Octapharma*
Sanofi Aventis*
Taj Pharmaceuticals Limited
Companies contacted using details available publicly on their websites in October 2012.
*Companies that responded indicated with an asterisk.
Data and analyses
Comparison 1. Immunoglobulin versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Measles cases | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Measles cases | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Quasi‐RCTs | 1 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.32, 0.45] |
| 2.2 Cohort type studies | 6 | 1575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.48, 0.57] |
| 2.3 Included infants < 6 months of age | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.38, 0.56] |
| 2.4 Did not include infants < 6 months of age | 5 | 2001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.45, 0.54] |
| 2.5 Convalescent serum | 5 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.44, 0.54] |
| 2.6 Adult serum | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.45, 0.59] |
| 2.7 Gamma globulin | 2 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.08, 0.36] |
| 3 Measles cases | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Convalescent serum | 3 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.15, 0.29] |
| 3.2 Adult serum | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.45, 0.59] |
| 3.3 Gamma globulin | 2 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.08, 0.36] |
| 4 Mortality due to measles | 3 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.13, 0.44] |
| 5 Complications due to measles | 3 | 832 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.60] |
Comparison 2. Gamma globulin versus serum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Measles cases | 2 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.46, 0.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berkovich 1963.
| Methods | Non‐RCT undertaken in December 1961 | |
| Participants | Tuberculous patients housed together in a children's ward of a New York hospital, USA and exposed to a symptomatic case of measles on the ward. Age and gender not reported | |
| Interventions | 1. Commercially produced gamma globulin of 512 neutralising measles titre intramuscularly at 0.1 ml/pound of body weight 2. Ender's live measles virus vaccine and same lot of commercially produced gamma globulin at 0.02 ml/pound body weight intramuscularly at separate sites |
|
| Outcomes | Cases of measles | |
| Total length of follow up | 20 days | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Those with parental consent received live virus vaccine while those without consent for the vaccine received gamma globulin only |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of both interventions is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not clear who assessed the participants for signs of measles and no standard definition of measles was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in the results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | No measurement of or control for potential confounders |
Cockburn 1950.
| Methods | RCT undertaken in early 1949 | |
| Participants | Children aged between 6 and 60 months of age who attended or resided at child care institutions in England and Scotland. Around 5% were under the age of 1 year. Just over half the participants were male | |
| Interventions | 1. 225 mg to 450 mg of freeze‐dried gamma globulin dissolved in 3 ml to 6 ml of sterile, distilled water immediately before intramuscular injection 2. 5 ml adult serum containing 0.5% phenol or reconstituted dried plasma intramuscularly |
|
| Outcomes | Cases of measles Complications due to measles Adverse events |
|
| Total length of follow up | 21 days | |
| Notes | Adverse events not specified as an outcome in the methods but reported for both groups collectively: "Apart from transient limb stiffness lasting one or two hours in a few cases, no local reactions were observed in the globulin or adult‐serum groups. One child in the globulin group developed, three days after inoculation, an urticarial rash which disappeared in twenty‐four hours" (pg 735) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A person who was not giving the injections provided for each study locality a list of pairs of letters, "G" for gamma globulin and "A" for adult serum. The order in which the letters appeared in the pair was determined by "random sampling numbers". No further information was reported on how numbers were generated |
| Allocation concealment (selection bias) | High risk | Person giving injections made a list of all eligible consenting contacts, dividing them into groups according to predefined "intimacy of exposure" levels. Within each subgroup participants were listed in order of increasing age. From the top of the list, those in each pair were then allocated based on the letter list. Once used, the pair of letters was crossed off the list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the interventions means they are not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Low risk | The details of the intervention were recorded and then filed away, with subsequent clinical observations recorded on a separate sheet (pg 733). While the doctors who gave the injections were the assessors of the outcomes, the authors tested recall of which child had which intervention in a preliminary study and "it was practically impossible for the observer to remember after inoculating the children whether a particular child had been given gamma globulin or adult serum" |
| Blinding of outcome assessment (detection bias) Complications from measles | Low risk | As with cases of measles outcome |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | As with cases of measles outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up apparent. Each child was observed for 21 days at the child care institutions. If the child was absent, they were visited at their home. If no cases of measles occurred in the contacts within 21 days, the trial at that institution was closed |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
Degkwitz 1920.
| Methods | Non‐RCT. Year study undertaken not known | |
| Participants | Children aged 8 months to 13.5 years, exposed to measles in a hospital in Germany | |
| Interventions | 1. Convalescent serum administered on day 4 after exposure, 3 ml versus 2.5 ml as control 2. Convalescent serum 6 ml to 7 ml administered on day 6 after exposure versus day 7 after exposure as control |
|
| Outcomes | Measles cases | |
| Total length of follow up | Not reported | |
| Notes | Article in German ‐ assessment based on translation form information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Not reported how participants were allocated to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not clear who assessed the participants for signs of measles and no standard definition of measles was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in the results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | No measurement of or control for potential confounders |
Endo 2001.
| Methods | Non‐RCT undertaken in 1999 to 2000 | |
| Participants | Susceptible infants and toddlers in Japan, of average age 1.5 years, who had close contact with someone with measles and whose parents consented to participate in the study. 24 boys and 9 girls were enrolled | |
| Interventions | Intramuscular immunoglobulin 0.33 ml/kg within 5 days of exposure. 4 different lots of commercially obtained immunoglobulins were used. The concentrations of measles‐specific antibody in each were: 16 IU/ml, 33 IU/ml, 40 IU/ml, 45 IU/ml | |
| Outcomes | Cases of measles | |
| Total length of follow up | 14 days | |
| Notes | Adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. It is not reported how participants were allocated to the different lots of immunoglobulin |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Parents were asked to report fever or rash over the 2 weeks subsequent to intervention. Upon report, a physician examined the child to confirm the diagnosis. It is not reported whether the physician was aware of which lot of IG was administered |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is not clear whether those parents who did not report illness of their child were actively followed up although the results suggest follow‐up was complete |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | Comparison is made between the 9 children with clinical measles and the 24 children without clinical measles over the follow‐up period in terms of age, body weight, interval between exposure and intervention, dose of IG in ml/kg and measles‐specific antibody titre, suggesting no difference in these characteristics between the 2 groups apart from the mean measles‐specific antibody titre administered. There is no control for confounding according to lot of IG administered |
Glyn‐Jones 1972.
| Methods | Quasi‐RCT undertaken in 1968 to 1969 | |
| Participants | Susceptible children aged 6 to 35 months admitted to the paediatric unit at Mpilo Hospital in Zimbabwe ('Rhodesia' at the time of the study) who were alive the day after admission | |
| Interventions | 1. Human immune globulin 2 ml intramuscularly on the day after admission, repeated at 3‐weekly intervals until discharge 2. No treatment 3. Measles vaccine, 1 dose intramuscularly on the day after admission |
|
| Outcomes | Cases of measles Deaths due to measles Complications from measles Adverse events ‐ measles vaccine reactions |
|
| Total length of follow up | At least 2 weeks after discharge from hospital | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assigned sequentially according to admission order |
| Allocation concealment (selection bias) | Unclear risk | Treatment group assigned by author's colleague and given to senior ward nurses who administered the treatment (pg 4). Unclear if the colleague was involved in the care of the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the interventions means they are not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Low risk | All assessment of participants carried out by author who was not aware of group allocation |
| Blinding of outcome assessment (detection bias) Complications from measles | Low risk | All assessment of participants carried out by author who was not aware of group allocation |
| Blinding of outcome assessment (detection bias) Deaths due to measles | Low risk | All assessment of participants carried out by author who was not aware of group allocation |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk | All assessment of participants carried out by author who was not aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants accounted for in results. Loss to follow‐up very similar across groups but exceeded 20% |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
Hartley 1948.
| Methods | Non‐RCT undertaken in 1943 | |
| Participants | Susceptible children aged 0 to 10+ years exposed to 'an undoubted case' of measles in their home or child care institution or hospital ward in England or Scotland | |
| Interventions | 1. Gamma globulin manufactured in the USA by Cohn cold ethanol fractionation and administered intramuscularly in doses of 1.2 ml or less, 1.5 ml to 2 ml and 2.5 ml or more 2. Reconstituted dried human convalescent serum (single batch) intramuscularly in doses of 2.5 ml, 3.3 ml to 3.5 ml and 5 ml or more |
|
| Outcomes | Cases of measles | |
| Total length of follow up | 3 weeks post‐intervention | |
| Notes | Adverse reactions not specified as an outcome in the methods; very poorly reported and not specific for this cohort: "no untoward results were noted" (pg 43); "no local or general reactions followed the injection of gamma‐globulin" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Not reported how contacts were allocated to intervention group |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the interventions means they are not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not reported who assessed the participants for signs of measles and measles is not defined for the purposes of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in the results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified in the methods that were not reported |
| Confounding | High risk | Did not dose according to weight and did not account for 'high risk' of illness or other comorbidity. Restricted based on time between exposure and intervention and stratified by dose of gamma globulin/serum, age and place of exposure |
Morales 1930.
| Methods | Quasi‐RCT undertaken in 1928 to 1929 | |
| Participants | Susceptible children aged 6 months to 15 years old in Porto Rico exposed to a household case of measles | |
| Interventions | 1. "Injection" of 4 ml to 6 ml pooled convalescent serum obtained from the 5th to 10th day of convalescence 2. "Injection" of 10 ml to 40 ml pooled serum from adult donors with a history of measles between 1 and 10 years ago 3. No treatment |
|
| Outcomes | Cases of measles Complications due to measles Deaths due to measles Adverse events |
|
| Total length of follow up | 8 weeks following exposure | |
| Notes | Adverse events very poorly reported: "Among more than 500 who received injections of serum, only 2 children showed reactions; they had slight fever, accompanied by an urticarial rash" (pg 1218) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Every third child was regarded as a control and received no treatment, while each of the remainder received an injection of either convalescent or immune serum" (pg 1216) |
| Allocation concealment (selection bias) | High risk | Not reported who allocated participants to groups or whether the person allocating had any knowledge of participant characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the interventions means they are not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Inspectors visited the participants at home at regular intervals and reported illness to one author who then visited the participant immediately to determine the cause of illness. Not reported whether the author was blinded to intervention group |
| Blinding of outcome assessment (detection bias) Complications from measles | Unclear risk | No definition of complications from measles is reported for the purposes of study. Not reported who assessed participants for complications or whether they were blind to intervention group |
| Blinding of outcome assessment (detection bias) Deaths due to measles | Unclear risk | Unclear whether the author who confirmed measles diagnosis was blind to intervention status. Unclear whether the deaths reported are only those felt to be connected to measles or all deaths |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Inspectors visited the participants at home at regular intervals and reported illness to one author who then visited the participant immediately to determine the cause of illness. Not reported whether the author was blinded to intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
Ordman 1944.
| Methods | Non‐RCT undertaken in 1942 to 1943 | |
| Participants | Susceptible children 6 months of age and older living in Boston, USA exposed to a household member with measles | |
| Interventions | 1. Single batch of gamma globulin prepared by Cohn cold ethanol fractionation 2 ml to 5 ml IM in the gluteal region 2. No treatment |
|
| Outcomes | Cases of measles Complications due to measles |
|
| Total length of follow up | 3 weeks after intervention | |
| Notes | Adverse reactions not specified as an outcome in the methods, poorly reported and not specific for this cohort. "No severe reactions have been observed in the several hundred individuals inoculated with it. In less than 5 per cent of these, mild reactions occurred. With a single exception, the reactions consisted of a slight feeling of stiffness in the muscle injected or a little local erythema and induration. In one case, the individual had a rise in temperature to 102°F 2 days after inoculation but no other systemic or local manifestation. Whether or not this febrile reaction was due to the globulin cannot be stated" (pg 547) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | "When there were 2 or more susceptible contacts in a family, they were divided into 2 groups composed of persons as nearly alike as possible with respect to age and degree of exposure. Children over 15 years of age were placed in the control group" (pg 542) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Family was visited by one of the authors at each follow‐up. Not reported whether assessors were different to those who had given the interventions or whether they were blinded to intervention group |
| Blinding of outcome assessment (detection bias) Complications from measles | Unclear risk | As for cases of measles outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified in the methods that were not reported |
| Confounding | High risk | Adjusted dose to age as in methodological protocol but no other measurement or control for confounding |
Salomon 1923.
| Methods | Non‐RCT. Year study undertaken not reported | |
| Participants | Children aged older than 3 months in a hospital in Germany | |
| Interventions | 1. Convalescent serum (dose not reported) 2. Adult serum 10 ml to 15 ml 3. No treatment |
|
| Outcomes | Measles cases Death due to measles |
|
| Total length of follow up | Not reported | |
| Notes | Article in German. Assessment based on translation form information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Interventions administered sequentially in blocks of time according to availability |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not clear who assessed the participants for signs of measles and no standard definition of measles was reported |
| Blinding of outcome assessment (detection bias) Deaths due to measles | Unclear risk | Not clear who assessed the participants regarding death due to measles or whether they were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | No measurement of or control for potential confounders |
Sheppeard 2009.
| Methods | Non‐RCT undertaken in 2006 | |
| Participants | Susceptible contacts (aged 6 months to 40+ years) of confirmed cases of measles notified to New South Wales public health units | |
| Interventions | 1. MMR if within 3 days of exposure 2. Normal human immunoglobulin (gamma globulin) within 7 days of exposure, 0.2 ml/kg up to 15 ml 3. No treatment |
|
| Outcomes | Cases of measles | |
| Total length of follow up | Passive surveillance until 2 incubation periods after the last notified case | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Allocation determined by length of time from exposure at point of contact with participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the interventions means they are not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Low risk | Case of measles defined for the purposes of the study, criteria objective and likely assessed by other than the authors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up by passive surveillance |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | No control of potential confounders. Measured setting of exposure only |
Stillerman 1944.
| Methods | Non‐RCT undertaken in 1938‐1941 | |
| Participants | Healthy, susceptible children living in New York city, USA, aged 6 months to 15 years who were exposed to a family member in their household who had been diagnosed with measles | |
| Interventions | 1. Pooled convalescent serum (collected from adolescents and adults up to 4 months after the onset of measles) 5 ml to 20 ml administered IM into the upper outer aspect of the buttock or the thigh 2. No treatment |
|
| Outcomes | Cases of measles Complications from measles |
|
| Total length of follow up | Up to 23 days after exposure | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Not reported how participants were allocated to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not reported who assessed participants for measles or whether they were blind to group allocation |
| Blinding of outcome assessment (detection bias) Complications from measles | High risk | Complications only reported for intervention group. Control only followed up until rash onset if they became ill |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results for cases of measles |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | Restricted against 'high‐risk' contacts in terms of comorbidity. Measured most prespecified confounders but only undertook univariate analyses. Did not dose according to weight |
Toomey 1926.
| Methods | Non‐RCT undertaken in 1925 | |
| Participants | Cohort of susceptible 'children' exposed on the same hospital ward in the 2 days prior. Age and gender not reported | |
| Interventions | 1. Convalescent serum (obtained 8 days after the rash began to disappear) 2.5 ml to 5 ml administered intramuscularly 2. No treatment |
|
| Outcomes | Cases of measles | |
| Total length of follow up | 60 days | |
| Notes | Some children had a second exposure Adverse reactions not specified as an outcome in the methods, poorly reported and not specific for this cohort. "There was no local reaction to the injection. In most instances there was a rise in temperature of from 1 to 1.5C, beginning within twenty‐four hours after the injection and lasting rarely longer than twenty‐four hours. In six susceptible subjects, diarrhea was noted" (pg 401) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Not reported how participants allocated to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not reported who assessed participants for signs of measles or whether they were blind to group allocation; no standard definition of measles was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up of all patients while on the ward and at 60 days |
| Selective reporting (reporting bias) | High risk | Report contains case series data for which adverse events were reported but this outcome not reported in relation to this cohort |
| Confounding | High risk | No measurement of or control for potential confounders |
Wesselhoeft 1928.
| Methods | Non‐RCT undertaken in 1928 | |
| Participants | Susceptible children exposed to measles in the diptheria and scarlet fever wings of a hospital in Boston, USA. Age and gender not reported | |
| Interventions | 1. Convalescent serum (collected from older children and adults) 5 ml administered intramuscularly 2. No treatment |
|
| Outcomes | Cases of measles Complications due to measles Deaths due to measles |
|
| Total length of follow up | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised |
| Allocation concealment (selection bias) | High risk | Not randomised. Not reported how participants were allocated to groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nature of the intervention means it is not subject to variation due to performance |
| Blinding of outcome assessment (detection bias) Cases of measles | Unclear risk | Not reported who assessed participants for signs of measles or whether they were blind to group allocation and no standard definition of measles was reported |
| Blinding of outcome assessment (detection bias) Complications from measles | Unclear risk | Not reported who assessed participants for complications due to measles or whether they were blind to group allocation |
| Blinding of outcome assessment (detection bias) Deaths due to measles | Unclear risk | Not reported who assessed participants regarding death due to measles or whether they were blind to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | No outcomes specified that were not reported |
| Confounding | High risk | No measurement of or control for potential confounders |
RCT = randomised controlled trial IG = immunoglobulin IM = intramuscularly MMR = measles, mumps and rubella vaccine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barenberg 1930 | Unclear whether all participants were exposed |
| Benson 1927 | Intervention and control groups not recruited over similar and overlapping time periods |
| Blackfan 1923 | Unclear whether all participants were susceptible |
| Christensen 1953 | Unclear whether all controls were susceptible and placental and plasma globulin were combined as one intervention (results for each not separable) |
| Gunn 1928 | No definable control group for intervention of relevance |
| Haas 1926 | Unclear whether all controls were susceptible |
| Karelitz 1938 | Unclear whether all participants susceptible |
| King 1991 | Retrospective cohort study |
| Kingsbury 1927 | Unclear whether all participants exposed and unclear whether all susceptible |
| Laning 1935 | Immunoglobulin used was derived from placentas |
| LeBlanc 2012 | Communication from author that study was retrospective |
| Lempriere 1939 | Unclear whether all participants were exposed |
| McGuinness 1943 | Unclear whether control group were comparable and unclear whether all were susceptible |
| Monnet 1954 | Intervention and control groups not recruited over similar and overlapping time periods |
| Rivera 1991 | Unable to contact study author for further details. Review authors agreed retrospective study from published details |
| Weaver 1924 | Unclear whether controls came from the same exposed population as the intervention group |
| Zingher 1924 | Unclear whether all were susceptible |
We retrieved 144 full‐text articles for assessment and excluded 131 of these from the review. Studies included in the above table are those that were discussed by the authors, after comparison of their independent assessments, because either one or both authors listed the study as 'unsure' for inclusion or because there was disagreement in the results of independent assessment.
Differences between protocol and review
We had not identified in the protocol that we would search www.clinicaltrials.gov or WHO ICTRP.
We recognised during the search and retrieval of studies that a number of different sources of immunoglobulin had been studied in the early practice of passive immunisation. GN and MY collectively decided to include only studies where the immunoglobulins used were sourced from human sera or plasma and exclude studies of immunoglobulins from other sources. This decision was made as these interventions were felt to be analogous to the current practice of using a blood product manufactured from human plasma, whereas the other sources are not comparable to current practice.
We recognised during the application of the inclusion criteria, particularly to older studies where no further information was going to be obtainable from authors, that we needed to increase the specificity of the criteria for inclusion so that they could be applied consistently. GN and MY discussed the criteria and collectively determined that to be considered a prospective non‐RCT (cohort) study, the intervention and control groups of relevance needed to be recruited over the same (or similar and overlapping) timeframe and from the same exposed population. Further, to be included, the study must specify that the intervention and control populations of relevance had been exposed to measles and were susceptible to measles. If any of these points could not be determined from the information available, either in the publication or from the authors, the study was excluded.
We found that in at least one study with multiple intervention groups the risk ratio for each intervention group compared to control was clearly heterogenous. We therefore chose, for studies with multiple intervention groups, to split the control group and add each pair‐wise comparison to the relevant meta‐analyses rather than calculate a weighted average of the relevant pair‐wise comparisons as we had outlined in the protocol.
We had not listed the secondary outcome 'complications from measles' among the outcomes for meta‐analysis in the protocol but found this was warranted given the available evidence.
We undertook a number of sensitivity analyses that were not listed in the protocol in response to our inability to undertake subgroup analyses that were specified in the protocol.
We made the decision to include 'Summary of findings' tables in the review along with the eligible outcomes for the tables post‐protocol.
Contributions of authors
Dr Megan Young (MY) and Prof Graeme Nimmo (GN) obtained copies of the studies and selected studies for inclusion in the review. MY and Prof Allan Cripps (AC) extracted the data and assessed the risk of bias in the studies. MY and Dr Mark Jones (MJ) entered and analysed the data and interpreted the analysis. All authors completed the final review.
Sources of support
Internal sources
-
Griffith University, Australia.
In‐kind employee time
-
The University of Queensland, Australia.
In‐kind employee time
-
Queensland Health, Australia.
In‐kind employee time
External sources
No sources of support supplied
Declarations of interest
Dr Megan Young is a public health physician in Queensland, Australia who is involved in the public health management of measles and a PhD student whose thesis topic is the effectiveness and efficiency of passive immunisation with NHIG (normal human immunoglobulin) for the public health management of communicable disease. She is collaborating with staff of CSL Biotherapies, Australia on a study related to the review topic. She receives no financial benefits from CSL or any other pharmaceutical company.
Prof Allan Cripps and Prof Graeme Nimmo are PhD supervisors for Dr Megan Young.
Dr Mark Jones has no known conflicts of interest.
New
References
References to studies included in this review
Berkovich 1963 {published data only}
- Berkovich S, Starr S. Use of live‐measles‐virus vaccine to abort an expected outbreak of measles within a closed population. New England Journal of Medicine 1963;269(2):75‐7. [DOI] [PubMed] [Google Scholar]
Cockburn 1950 {published data only}
- Cockburn WC, Harrington JA. Gamma globulin in the prevention and attenuation of measles: controlled trials in day and residential nurseries. Lancet 1950;2(6641):732‐6. [PubMed] [Google Scholar]
Degkwitz 1920 {published data only}
- Degkwitz R. Over measles convalescent serum [Uber Masern‐Rekonvaleszentenserum]. Zeitschrift fur Kinderheilkunde 1920;27:171‐94. [Google Scholar]
Endo 2001 {published data only (unpublished sought but not used)}
- Endo A, Izumi H, Miyashita M, Taniguchi K, Okubo O, Harada K. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. Journal of Pediatrics 2001;138(6):926‐8. [DOI] [PubMed] [Google Scholar]
Glyn‐Jones 1972 {published data only}
- Glyn‐Jones R. Measles vaccine gamma globulin in the prevention of cross infection with measles in an acute paediatric ward. Central African Journal of Medicine 1972;18(1):4‐9. [PubMed] [Google Scholar]
Hartley 1948 {published data only}
- Hartley P, Anderson T, Begg ND, Gunn W, Cruickshank R, Martin WJ, et al. Gamma‐globulin in the prevention and attenuation of measles: report of a subcommittee to the blood transfusion research committee of the medical research council. Lancet 1948;2:41‐4. [Google Scholar]
Morales 1930 {published data only}
- Morales EG, Mandry OC. Relative prophylactic value of convalescent and immune adult measles serums. American Journal of Diseases of Children 1930;39:1214‐20. [Google Scholar]
Ordman 1944 {published data only}
- Ordman CW, Jennings CG, Janeway CA. Chemical, clinical, and immunological studies on the products of human plasma fractionation. XII. The use of gamma globulin (human immune serum globulin) in the prevention and attenuation of measles. Journal of Clinical Investigation 1944;23(4):541‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salomon 1923 {published data only}
- Salomon G. The prophylactic injection of normal serum as a measles protection [Die prophylaktische Injektion von Normalserum als Masernschutz]. Deutsche Medizinische Wochenschrift 1923;49(35):1151‐2. [Google Scholar]
Sheppeard 2009 {published and unpublished data}
- Sheppeard V, Forssman B, Ferson MJ, Moreira C, Campbell‐Lloyd S, Dwyer DE, et al. The effectiveness of prophylaxis for measles contacts in NSW. NSW Public Health Bulletin 2009;20(5‐6):81‐5. [DOI] [PubMed] [Google Scholar]
Stillerman 1944 {published data only}
- Stillerman M, Marks HH, Thalhimer W. Prophylaxis of measles with convalescent serum: principal factors influencing the results. American Journal of Diseases of Children 1944;67(1):1‐14. [Google Scholar]
Toomey 1926 {published data only}
- Toomey JA. Prophylaxis of measles. American Journal of Diseases of Children 1926;32:401‐6. [Google Scholar]
Wesselhoeft 1928 {published data only}
- Wesselhoeft C, Gordon FF. Report of convalescent measles serum administration in cases exposed to measles during the course of scarlet fever and diptheria. New England Journal of Medicine 1928;198(14):752‐4. [Google Scholar]
References to studies excluded from this review
Barenberg 1930 {published data only}
- Barenberg LH, Lewis JM, Messer WH. Measles prophylaxis: comparative results with the use of adult blood, convalescent serum and immune goat serum (Tunnicliff). Journal of the American Medical Association 1930;95:4‐8. [Google Scholar]
Benson 1927 {published data only}
- Benson WT, Lawrie JDH. The seroprophylaxis of measles. Edinburgh Medical Journal 1927;34:216‐22. [Google Scholar]
Blackfan 1923 {published data only}
- Blackfan KD, Peterson MF, Conroy FC. Use of convalescent serum as a prophylaxis in measles and chickenpox. Ohio State Medical Journal 1923;19:97‐100. [Google Scholar]
Christensen 1953 {published data only}
- Christensen PE, Schmidt H. An epidemic of measles in southern Greenland 1951; measles in virgin soil. IV the significance of specific prophylaxis. Acta Medica Scandinavica 1953;145(2):126‐42. [DOI] [PubMed] [Google Scholar]
Gunn 1928 {published data only}
- Gunn W. A critical investigation of the value of immune sera in the prophylaxis of measles. Lancet 1928;212(5484):690‐4. [Google Scholar]
Haas 1926 {published data only}
- Haas SV, Blum J. The prophylactic value of blood of convalescents in measles. Journal of the American Medical Association 1926;87:558‐61. [Google Scholar]
Karelitz 1938 {published data only}
- Karelitz S, Karelitz RF. The significance of the conditions of exposure in the study of measles prophylaxis: An added criterion in the evaluation of measles prophylactic agents. Journal of Pediatrics 1938;13:195‐207. [Google Scholar]
King 1991 {published data only}
- King GE, Markowitz LE, Patriarca PA, Dales LG. Clinical efficacy of measles vaccine during the 1990 measles epidemic. Pediatric Infectious Disease Journal 1991;10(12):883‐8. [DOI] [PubMed] [Google Scholar]
Kingsbury 1927 {published data only}
- Kingsbury AN. Serum prophylaxis in measles. An evaluation of the resulting immunity. Lancet 1927;209(5392):7‐9. [Google Scholar]
Laning 1935 {published data only}
- Laning GM, Horan TN. Immune globulin used as preventive and modifier of measles. Journal of the Michigan State Medical Society 1935;34:772‐4. [Google Scholar]
LeBlanc 2012 {published and unpublished data}
- LeBlanc J, Stinchfield P. Measles outbreak management at a Minnesota Children's Hospital in 2011. American Journal of Infection Control 2012;40:e67. [Google Scholar]
Lempriere 1939 {published data only}
- Lempriere LR. Adult serum in a school epidemic of measles. British Medical Journal 1939;1:1136‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuinness 1943 {published data only}
- McGuinness AC, Stokes J, Armstrong JG. Vacuum dried human serums in the prevention and treatment of certain of the common communicable diseases ‐ an 8‐year study. American Journal of Medical Sciences 1943;205:826‐34. [Google Scholar]
Monnet 1954 {published data only}
- Monnet P, Levy M, Gilly R. Trial of prevention of measles by adult human plasma or placental gamma‐globulins: addressing epidemics in the hospital environment [Essai de prevention de la rougeole par le plasma humain d'adultes ou les gamma‐globulines d'origine placentaire: A propos d'epidemies en milieu hospitalier]. Pediatrie 1954;9(8):854‐7. [PubMed] [Google Scholar]
Rivera 1991 {published data only (unpublished sought but not used)}
- Rivera ME, Mason WH, Ross LA, Wright HT. Nosocomial measles infection in a pediatric hospital during a community‐wide epidemic. Journal of Pediatrics 1991;119:183‐6. [DOI] [PubMed] [Google Scholar]
Weaver 1924 {published data only}
- Weaver GH, Crooks TT. The use of convalescent serum in the prophylaxis of measles. Journal of the American Medical Association 1924;82:204‐6. [Google Scholar]
Zingher 1924 {published data only}
- Zingher A. Convalescent whole blood, plasma and serum in prophylaxis of measles. Journal of the American Medical Association 1924;82:1180‐7. [Google Scholar]
Additional references
ATAGI 2008
- Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook. Canberra: Australian Government, 2008. [Google Scholar]
Barrabeig 2011
- Barrabeig I, Rovira A, Rius C, Munoz P, Soldevila N, Batalla J, et al. Effectiveness of measles vaccination for control of exposed children. Pediatric Infectious Disease Journal 2011;30:78‐80. [DOI] [PubMed] [Google Scholar]
Best 2011
- Best, Voss, Roberts, Freeman. Starship Children's Health Clinical Guideline: Measles ‐ Infection Control Definitions & Guidelines. www.adhb.govt.nz/starshipclinicalguidelines/_Documents/Measles.pdf (accessed 30 April 2012).
Birdsall 2009
- Birdsall HH. Antibodies. In: Mandell GL, Bennett JE, Dolin R editor(s). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th Edition. Philadelphia: Churchill Livingstone, 2009. [Google Scholar]
Black 1960
- Black FL, Yannet H. Inapparent measles after gamma globulin administration. JAMA 1960;173(11):1183‐8. [DOI] [PubMed] [Google Scholar]
Castillo‐Solorzano 2011
- Castillo‐Solorzano C, Reef S, Morice A, Andrus J, Ruiz M, Tambini G, et al. Guidelines for the documentation and verification of measles, rubella, and congenital rubella syndrome elimination in the region of the Americas. Journal of Infectious Diseases 2011;204(Suppl 2):683‐9. [DOI] [PubMed] [Google Scholar]
CDC 1998
- Centers for Disease Control and Prevention. Measles, mumps, and rubella ‐ vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 1998;47(RR‐8):1‐57. [PubMed] [Google Scholar]
CDC 2011a
- Centers for Disease Control and Prevention. Increased transmission and outbreaks of measles ‐ European Region, 2011. Morbidity and Mortality Weekly Report 2011;60:1605‐10. [PubMed] [Google Scholar]
CDC 2011b
- Centers for Disease Control and Prevention. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2011;60(RR‐2):1‐61. [PubMed] [Google Scholar]
CDNA 2009
- Communicable Diseases Network Australia. Measles: National Guidelines for Public Health Units. Canberra: Commonwealth Department of Health and Ageing, 2009. [Google Scholar]
Delaporte 2011
- Delaporte E, Richard J, Wyler Lazarevic C, Lacour O, Girard M, Ginet C, et al. Ongoing measles outbreak, Geneva, Switzerland, January to March 2011. Euro Surveillance: European Communicable Disease Bulletin 2011;16:10. [DOI] [PubMed] [Google Scholar]
DVD CDC 2011
- Division of Viral Diseases, National Centre for Immunization and Respiratory Diseases, CDC. Measles ‐ United States, January ‐ May 20, 2011. Morbidity and Mortality Weekly Report 2011;60(20):666‐8. [PubMed] [Google Scholar]
EMEA 1995
- European Medicines Agency. ICH Topic E 2 A ‐ Clinical safety data management: definitions and standards for expedited reporting. www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf (accessed 10 June 2010).
Gonik 2011
- Gonik B. Passive immunization: the forgotten arm of immunologically based strategies for disease containment. American Journal of Obstetrics and Gynecology 2011;205(5):444. [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schunemann H. GRADEpro. Version 3.2 for Windows. Brozek J, Oxman A, Schunemann H, 2008.
Gustavo 2008
- Gustavo H, Dayan M, Rota J, Bellini W, Redd S. Chapter 7: Measles (updated April 2009). Manual for the Surveillance of Vaccine‐Preventable Diseases. Atlanta: Centers for Disease Control and Prevention, 2008. [Google Scholar]
Heymann 2008
- Heymann DL. Control of Communicable Diseases Manual. Washington DC: American Public Health Association, 2008. [Google Scholar]
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org 2011.
Hoskins 2011
- Hoskins R, Vohra R, Vlack S, Young MK, Humphrey K, Selvey C, et al. Multiple cases of measles after exposure during air travel ‐ Australia and New Zealand, January 2011. Morbidity and Mortality Weekly Report 2011;60:851. [PubMed] [Google Scholar]
HPA 2011
- Health Protection Agency. Vaccination schedule: routine childhood immunisation schedule. www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/VaccineCoverageAndCOVER/VaccinationSchedule/ (accessed 2 January 2012).
ID HPA 2009
- Immunisation Department. Measles. Immunoglobulin Handbook. UK: Health Protection Agency, 2009. [Google Scholar]
Karelitz 1937
- Karelitz S, Greenwald CK, Klein AJ. Placental fluid in measles prophylaxis. Journal of Pediatrics 1937;10:170‐4. [Google Scholar]
Keller 2000
- Keller M, Stiehm E. Passive immunity in prevention and treatment of infectious diseases. Clinical Microbiology Reviews 2000;13(4):602‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2011
- Martin R, Wassilak S, Emiroglu N, Uzicanin A, Deshesvoi S, Jankovic D, et al. What will it take to achieve measles elimination in the World Health Organization European Region: progress from 2003‐2009 and essential accelerated actions. Journal of Infectious Diseases 2011;204(Suppl 1):325‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moss 2009
- Moss W, Scott S. The Immunological Basis for Immunization Series Module 7: measles. Geneva: World Health Organization, 2009. [Google Scholar]
NZ MoH 2011
- New Zealand Ministry of Health. Immunisation Handbook 2011. Wellington: Ministry of Health, 2011. [Google Scholar]
Parker Fiebelkorn 2010
- Parker Fiebelkorn A, Redd S, Gallagher K, Rota P, Rota J, Bellini W, et al. Measles in the United States during the postelimination era. Journal of Infectious Diseases 2010;202(10):1520‐8. [DOI] [PubMed] [Google Scholar]
Ramsay 2009
- Ramsay M, Manikkavasagan G, Brown K, Craig L. Post exposure prophylaxis for measles: revised guidance May 2009. Health Protection Agency, UK 2009.
Reading 2007
- Reading SA, Dimmock NJ. Neutralization of animal virus infectivity by antibody. Archives of Virology 2007;152:1047‐59. [DOI] [PubMed] [Google Scholar]
Sawyer 2000
- Sawyer L. Antibodies for the prevention and treatment of viral diseases. Antiviral Research 2000;47(2):57‐77. [DOI] [PubMed] [Google Scholar]
Smithson 2010
- Smithson R, Irvine N, Hutton C, Doherty L, Watt A. Spotlight on measles 2010: ongoing measles outbreak in Northern Ireland following an imported case, September‐October 2010. Euro Surveillance: European Communicable Disease Bulletin 2010;15:43. [DOI] [PubMed] [Google Scholar]
Sniadack 2011
- Sniadack D, Mendoza‐Aldana J, Jee Y, Bayutas B, Lorenzo‐Mariano K. Progress and challenges for measles elimination by 2012 in the Western Pacific Region. Journal of Infectious Diseases 2011;204(Suppl 1):439‐46. [DOI] [PubMed] [Google Scholar]
Stokes 1944
- Stokes J, Maris E, Gellis S. Chemical, clinical, and immunological studies on the products of human plasma fractionation. XI. The use of concentrated normal human serum gamma globulin (human immune serum globulin) in the prophylaxis and treatment of measles. Journal of Clinical Investigation 1944;23(4):531‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takimoto 2011
- Takimoto N, Takahashi Y, Ishiyama A, Kishimoto K, Iwama R, Nakano M. Control of a measles outbreak by prohibiting non‐vaccinated susceptible students from attending school in Akita Prefecture, Japan. Japanese Journal of Infectious Diseases 2011;64(4):309‐11. [PubMed] [Google Scholar]
Thalhimer 1939
- Thalhimer W, Stillerman M. Prevention and modification of measles with concentrated pooled ascites fluid and with its globulin fraction. Proceedings of the Society for Experimental Biology and Medicine 1939;42:683‐7. [Google Scholar]
UK DoH 2010
- UK Department of Health. Chapter 21: Measles ‐ updated December 2010. Immunisation Against Infectious Disease ‐ "The Green Book". UK: Department of Health, UK, 2006. [Google Scholar]
Vainio 2011
- Vainio K, Ronning K, Steen T, Arnesen T, Anestad G, Dudman S. Ongoing outbreak of measles in Oslo, Norway, January ‐ February 2011. Euro Surveillance: European Communicable Disease Bulletin 2011;16:8. [PubMed] [Google Scholar]
WHO 1999
- World Health Organization, Communicable Disease Surveillance and Response. WHO Guidelines for Epidemic Preparedness and Response to Measles Outbreaks. www.who.int/csr/resources/publications/measles/whocdscsrisr991.pdf (accessed 10 June 2012).
WHO 2005
- World Health Organization, UNICEF. GIVS: Global Immunization Vision and Strategy 2006‐2015. Switzerland: WHO and UNICEF, 2005. [Google Scholar]
WHO 2008
- World Health Organization. Progress towards reducing measles mortality and eliminating measles, WHO Eastern Mediterranean Region, 1997‐2007. Weekly Epidemiological Record 2008;83(11):97‐104. [PubMed] [Google Scholar]
WHO 2009a
- World Health Organization. Measles vaccines: WHO position paper. Weekly Epidemiological Record 2009;84(35):349‐60. [Google Scholar]
WHO 2009b
- World Health Organization. Global reductions in measles mortality 2000‐2008 and the risk of measles resurgence. Weekly Epidemiological Record 2009;84(49):509‐16. [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Progress in global measles control, 2000‐2010. Weekly Epidemiological Record 2012;87(5):45‐52. [Google Scholar]
WHO 2013
- World Health Organization. Global health observatory data repository: World Health Statistics ‐ selected infectious diseases, measles number of reported cases, data by WHO region. http://apps.who.int/gho/data/view.main.1520_62?lang=en (accessed 21 March 2014) 2013 (accessed 21 March 2014).
WHO 2014
- World Health Organization. WHO Vaccine Preventable Diseases Monitoring System 2013 Global Summary. http://apps.who.int/immunization_monitoring/globalsummary (accessed 21 March 2014) 2014.


