Abstract
The white-rot fungus Pleurotus ostreatus was able to degrade the polycyclic aromatic hydrocarbons (PAHs) benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, and benzo[ghi]perylene in nonsterile soil both in the presence and in the absence of cadmium and mercury. During 15 weeks of incubation, recovery of individual compounds was 16 to 69% in soil without additional metal. While soil microflora contributed mostly to degradation of pyrene (82%) and benzo[a]anthracene (41%), the fungus enhanced the disappearance of less-soluble polycyclic aromatic compounds containing five or six aromatic rings. Although the heavy metals in the soil affected the activity of ligninolytic enzymes produced by the fungus (laccase and Mn-dependent peroxidase), no decrease in PAH degradation was found in soil containing Cd or Hg at 10 to 100 ppm. In the presence of cadmium at 500 ppm in soil, degradation of PAHs by soil microflora was not affected whereas the contribution of fungus was negligible, probably due to the absence of Mn-dependent peroxidase activity. In the presence of Hg at 50 to 100 ppm or Cd at 100 to 500 ppm, the extent of soil colonization by the fungus was limited.
Environmental pollution with polycyclic aromatic hydrocarbons (PAHs) has attracted much attention in recent decades because carcinogenic substances may be formed during biotransformation of PAHs in humans and microorganisms (23). PAHs are formed by incomplete burning of fossil fuels and can enter the soil via atmospheric deposition. Local contamination with PAHs is particularly due to industrial activities such as old gasification plants and wood-preserving plants where creosote and anthracene oil, partial distillates of oil with high concentrations of PAHs, are used (56, 63).
Study of the possible role of microorganisms in PAH degradation revealed that two main groups of microorganisms are involved in the oxidation and subsequent mineralization of these compounds: soil bacteria and white-rot fungi. The degradation of PAHs is limited by their low water solubility (56). Whereas soil bacteria were found to effectively degrade low-molecular-weight PAHs (19), white-rot fungi can also oxidize more condensed PAH molecules with up to six aromatic rings and limited water solubility (5, 8, 16, 64) and therefore decrease their toxicity (6, 7, 32).
The initial reactions of PAH degradation by white-rot fungi are usually ascribed to their extracellular ligninolytic enzymes, i.e., laccase, lignin peroxidase, and Mn-dependent peroxidase (MnP) (27, 59, 60). Under natural conditions, these enzymes attack the polyphenolic molecule of lignin—the principal component of wood. However, due to their low specificity, ligninolytic enzymes can also attack molecules structurally similar to lignin, including halogenated organic compounds and PAHs (41, 49). Purified enzymes are able to transform PAHs in vitro (11, 24, 55), and attempts have therefore been made to apply these fungi to the bioremediation of soils contaminated with compounds not sufficiently degradable by other soil microorganisms (2, 13, 34, 39).
Although many studies of PAH degradation in contaminated soils have been performed, little attention has been paid to the effect of environmental factors on that degradation. One of the serious problems for decontamination biotechnology is the existence of mixed pollution, i.e., the simultaneous presence of pollutants of different groups in soil. Near motorways or industrial facilities, soil contamination with PAHs is often accompanied by the presence of high levels of heavy metals (26, 31). Heavy metals like cadmium, copper, or mercury are known to be toxic for both white-rot fungi (3, 40) and soil microflora (17) and their negative effect on the activity of ligninolytic enzymes has been described under in vitro conditions (4). The presence of these substances in the environment can therefore negatively influence the effectiveness of bioremediation technologies.
The work presented here concentrated on the effect of cadmium and mercury on the biodegradative process performed by the white-rot fungus Pleurotus ostreatus in nonsterile soil containing PAHs. The ligninolytic system of P. ostreatus consists of laccase and MnP (62), and therefore the activity of these two enzymes was estimated. Cadmium and mercury were chosen because they are often found as soil contaminants (43) and are most toxic for white-rot fungi in liquid culture (3). Furthermore, both Cd and Hg are nonessential metals that differ in the proposed mechanism of toxicity. Whereas cadmium (similar to copper) acts as an inducer of oxidative stress, the toxicity of mercury stems mostly from its high affinity for thiol groups in proteins, which can lead to the inactivation of enzymes.
P. ostreatus was chosen due to its good applicability to PAH degradation. Recent studies have shown that this fungus is able to degrade a variety of PAHs in liquid culture (9, 10, 53, 54), as well as in a seminatural lignocellulosic substrate (64). The fungus shows highly competitive saprophytic ability against soil microbiota in soil-lignocellulose systems (35, 42) and is able to grow and produce ligninolytic enzymes in soil (48). Straw was used as the carrier for introduction of the fungus into soil. It was found to be best the substrate to promote the colonization of soil and the mineralization of 3,4-dichloroaniline and benzo[a]pyrene in soil by different white-rot fungi (45).
MATERIALS AND METHODS
Fungal strain and cultivation.
P. ostreatus DSMZ 11191 was used. For preparation of inocula, the fungus was grown on malt extract agar plates (15.0 g of agar per liter, 14.0 g of malt extract per liter) at 25°C for 7 days. Mycelium agar plugs 9 mm in diameter (cut along the edge of an actively growing colony) were used as inocula.
Soil samples.
The soil (acidic cambisol) was collected from the upper 10 cm (Ap layer) of an agricultural site at the Federal Research Center for Agriculture near Braunschweig, Germany. The soil is classified as silty loam with the following particle distribution: sand, 40.6%; silt, 52.8%; clay, 6.6%. The soil pH was 5.3, the organic carbon content was 0.8%, and the total nitrogen content was 0.08% (1). The soil sample was sieved (<2 mm), moistened to 45% of its maximum water-holding capacity, and left undisturbed at 22°C for 1 week before it was frozen (−25°C). Seven days before application, the required amount of soil was removed from the freezer and incubated for 2 days at 4°C and for another 5 days at 25°C. Before application, the soil was amended with an appropriate amount of Cd(NO3)2 or HgCl2. Aliquots of 0.75 g of dry soil for each flask were supplemented with 200 μl of a metal solution to give final Cd concentrations (after mixing with fresh soil) of 10, 100, and 500 ppm and Hg concentrations of 10, 50, and 100 ppm in dry soil. Aliquots for control flasks were supplemented with 200 μl of distilled water. After soaking, the soil aliquots were dried at 50°C overnight. Immediately before application, the dried aliquots were ground and mixed thoroughly with fresh soil to ensure a homogeneous metal concentration in the soil. For the PAH degradation experiment, a PAH solution containing pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, and benzo[ghi]perylene in toluene was prepared to give a final concentrations of each compound of 10 μg · g of dry soil−1. After addition of heavy metals, 0.75-g aliquots of dry soil were supplemented with 150 μl of the PAH solution. The toluene was allowed to evaporate overnight.
Reagents.
Pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, benzo[ghi]perylene, and ABTS [2,2′-azinobis(3-ethylbenzothiazolinesulfonic acid), diammonium salt] were purchased from Aldrich (Steinheim, Germany); dibenzo[a,h]anthracene, 3-methyl-2-benzothiazolinone hydrazone, and 3,3′-dimethylaminobenzoic acid were from Sigma (Deisenhofen, Germany). The purity of PAH compounds was 97% or higher. Acetonitrile, acetone, and n-hexane (Merck, Darmstadt, Germany) were of gradient grade. All other chemicals (Sigma) were of analytical grade.
Culture conditions.
Conical flasks (100 ml) containing 5 g of air-dried, milled wheat straw (particle size, <1 mm) were prepared. The straw was moistened with 15 ml of deionized water (water content, 75%) and covered with a nylon mesh. The flasks were stopped with cotton plugs, autoclaved (121°C for 40 min), and inoculated with two agar plugs with mycelium. The cultures were incubated at 25°C until the mycelia had colonized the substrate completely (14 days). Then, 12.25 g of moist, nonsterile, metal-supplemented soil (corresponding to 10.75 g of dry matter; water content, 14.0%) was spread on the surface of each straw culture to form a 4-mm-high layer. To improve contact between the soil and straw compartments, 1 ml of water was added dropwise to the surface of the soil. The flasks were incubated at 25°C in a dark, wet chamber. At each sampling time, four replicates of each metal concentration used and of the control were collected. In all replicates, activities of laccase and MnP were estimated.
Extraction of enzymes.
For extraction of enzymes from the culture, the soil layer was collected from the straw surface, which was facilitated by separating them with a nylon mesh. The straw layer was dried at 105°C until a constant weight was achieved for estimation of loss of organic matter. Each soil compartment, as a whole, was mixed with 10 ml of phosphate buffer (50 mM, pH 7.0) and incubated on ice for 1 h. The flasks were occasionally shaken by hand during this time. The suspensions were centrifuged at 15,000 × g for 15 min (15°C), and the resulting supernatants were centrifuged once more at 5,000 × g for 15 min (15°C). The clear supernatants were used immediately for estimation of enzyme activities (37).
Enzyme activity measurements.
Laccase activity was measured by monitoring the oxidation of ABTS (47) in citrate-phosphate (100 mM citrate, 200 mM phosphate) buffer (pH 5.0). The formation of green dye was followed spectrophotometrically. MnP activity was assayed as previously described (46) in succinate-lactate buffer (100 mM, pH 4.5). 3-Methyl-2-benzothiazolinone hydrazone and 3,3′-dimethylaminobenzoic acid were oxidatively coupled by the action of the enzyme, and formation of a purple indamine dye product was followed spectrophotometrically. The results were corrected by activities in test samples without manganese (the addition of manganese sulfate was replaced with an equimolar amount of EDTA). All measurements were done in quadruplicate in microtiter plates. The increase in absorption was measured using a microplate reader (Spectra; SLT GmbH, Grödig, Austria) at 1-min intervals for 6 min. The data were recorded by the Easy Fit program of the same manufacturer. One unit of enzyme activity was defined as the amount catalyzing the production of 1 μmol of colored product per ml per min. Statistical analysis was accomplished by one-way analysis of variance and t test.
Degradation of PAHs in soil.
The experimental design used to study PAH degradation by P. ostreatus in nonsterile soil was the same as in experiments with enzyme activity measurements except that the experiment was run for 15 weeks in order to obtain greater PAH degradation. Conical flasks were prepared and supplemented with soil as described for the experiment with enzyme activity measurements. For each treatment, four replicates were run. In addition to flasks inoculated with P. ostreatus, for each metal concentration, another four flasks were used containing only sterile straw and nonsterile soil for determination of PAH degradation by soil microflora. The flasks were incubated for 15 weeks at 25°C in the dark. At the end of the incubation, flasks were collected and the contents were dried at 60°C until a constant weight was achieved. The soil layer was then separated from the straw and used for PAH determination. The straw layer was dried at 105°C until a constant weight was achieved, and loss of organic matter was estimated for each replicate. Controls were collected immediately after inoculation, dried, and used for estimation of initial PAH contents. Statistical analysis of differences in the recovery of individual PAH molecules between inoculated versus control soil was accomplished by one-way analysis of variance.
Determination of PAHs.
Dried soil samples were homogenized with a mortar and pestle before extraction. Five grams of homogenized soil was extracted using a Soxhlet apparatus with acetone–n-hexane (1:4) for 6 h at 76°C. The extracts were evaporated and resuspended in 20 ml of acetonitrile. The high-pressure liquid chromatography system used for PAH determination consisted of an HP 1090L (Hewlett-Packard) liquid chromatograph and an HP 1046A (Hewlett-Packard) fluorescence detector. Separations were performed at 25°C isocratically with an analytical column (150 by 4.6 mm [inner diameter]; Hypersil PAH 5 μm). A mixture of acetonitrile and water (1,000:1) was used as the mobile phase at a flow rate of 0.5 ml · min−1. Fluorescence detection was performed using an excitation wavelength of 270 nm and an emission wavelength of 405 nm. Each sample was analyzed twice. PAH concentrations were determined using calibration with a mixture containing of all the PAHs involved in the experiment. Recovery of control samples was above 85% for all of the individual PAH compounds.
Colonization of soil.
Air-dried wheat straw was mixed with distilled water (1:3, wt/vol) and allowed to soak overnight at 4°C. Glass columns (length, 280 mm; inner diameter, 16 mm) were filled with 12 g of wet straw (90-mm layer, 50 mm from one end of the tube), sealed with cellulose stoppers, and autoclaved (121°C for 40 min). The straw was inoculated with an agar plug with mycelium. The cultures were incubated at 25°C until the mycelia had colonized the straw column completely (25 days). Then, 15.50 g of moist, nonsterile, metal-supplemented soil (corresponding to 13.54 g of dry matter; water content, 14.0%) was added to the top of each straw column, forming a 70-mm soil layer. The surface of the soil was slightly pressed to avoid dissipation and wetted with 0.5 ml of water. The tubes were incubated at 25°C in a dark, wet chamber. For each metal concentration, four replicates were run. On each sampling day, the increase in the mycelial colonization of the soil (mean of maximum and minimum values) was estimated visually.
RESULTS
Enzyme activity in soil.
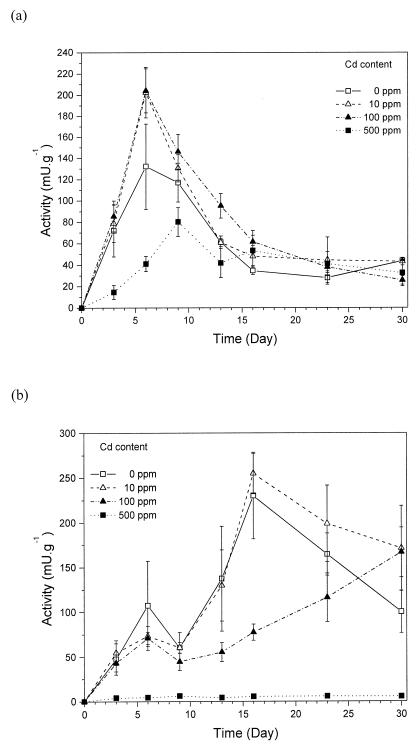
Enzyme activities were determined during the first 30 to 41 days, as the major changes in enzyme activity usually occur during the first weeks after contact of the mycelia with soil (37). In the presence of cadmium, peak laccase activity was detected on day 6 in soil containing the metal at 0 to 100 ppm (Fig. 1a). With Cd at 500 ppm, peak activity was detected later. After reaching a peak, laccase activity at all of the metal concentrations tested decreased, reaching stable values between days 16 and 30. The highest laccase activity on the peak day was detected in soil containing Cd at 10 and 100 ppm (200 mU · g−1), where the enzyme was substantially more active than in the control (130 mU · g−1). Soil containing Cd at 500 ppm showed lower laccase activity until day 13 (peak activity, 80 mU · g−1). In the course of the experiment, the laccase activity in all of the metal treatments and the control gradually reached the same value (between 30 and 40 mU · g−1).
FIG. 1.
Time course of enzyme activities during the growth of P. ostreatus in soil containing cadmium at 10, 100, and 500 ppm. Activities of laccase (a) and MnP (b) were measured. Averages and standard errors of four replicates are shown.
The MnP activity in soil containing Cd at up to 100 ppm (Fig. 1b) showed a first maximum on day 6. Control soil and soil containing Cd at 10 ppm showed a second maximum on day 16, and activity decreased after that time point. With Cd at 100 ppm, a second maximum of MnP activity was not detected and the enzyme activity increased until the end of the incubation (day 30). To the end of experiment, MnP activities in all treatments except Cd at 500 ppm reached roughly similar values (between 100 and 170 mU · g−1), although high variability among replicates was found. MnP activity in soil containing Cd at 500 ppm was negligible during the whole experiment (<5 mU · g−1).
The colonization of soil by fungal mycelia was completed within 9 days after soil addition with Cd at 0 to 100 ppm; however, the mycelial density in soil with Cd at 100 ppm was very low and the mycelium did not form continuous covers on the soil surface until day 30, which was the case with lower Cd concentrations. No soil colonization was apparent with Cd at 500 ppm until day 16. When the soil was harvested at the end of the experiment, the mycelium was visible in the soil layer although it did not reach the soil surface.
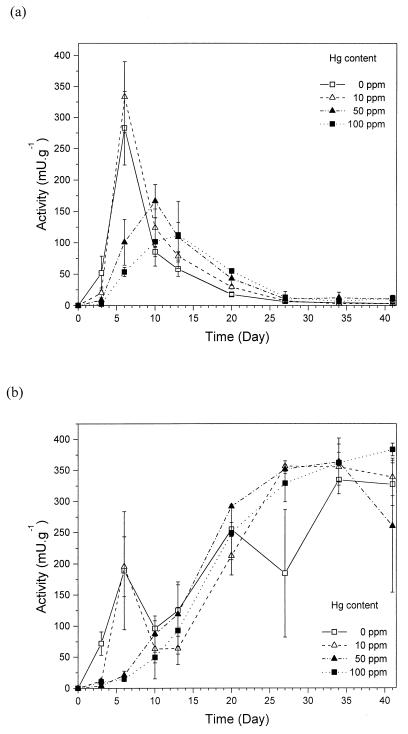
In soil containing mercury, laccase activity also reached a maximum within a few days after addition of soil to the fungal straw culture (Fig. 2a). However, at higher Hg concentrations, the laccase activity peak was delayed. The maximal values found in replicates with Hg at 50 and 100 ppm were also lower (170 and 110 mU · g−1, respectively) than in control soil and soil containing Hg at 10 ppm (280 and 330 mU · g−1). After reaching a maximum, laccase activity dropped and reached the same, relatively low value with all of the metal concentrations around day 27. Peak MnP activity was found in the control and Hg at 10 ppm on day 6 but not at higher mercury concentrations (Fig. 2b). Instead, there was a slow increase in MnP activity in soil containing Hg at 50 and 100 ppm in the first days of the experiment. MnP activity reached similar values in all of the metal treatments during the first 20 days of the experiment, and the activity increased with time. Already on day 10, mycelium was apparent at all mercury concentrations. However, until the end of experiments, the density of mycelia was lower at the two higher mercury concentrations.
FIG. 2.
Time course of enzyme activities during the growth of P. ostreatus in soil containing mercury at 10, 50, and 100 ppm. Activities of laccase (a) and MnP (b) were measured. Averages and standard errors of four replicates are shown.
Degradation of PAHs in soil.
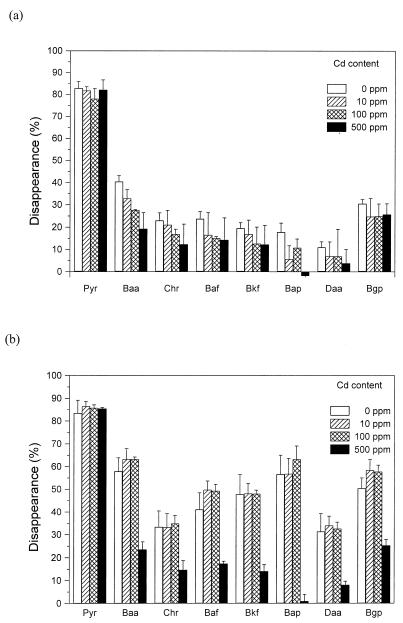
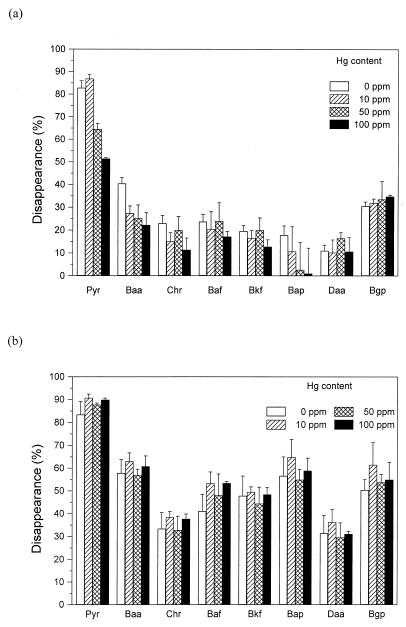
Degradation of PAHs by nonsterile soil with and without fungus is demonstrated in Fig. 3 and 4 for cadmium and mercury, respectively. In the absence of fungus, the two PAH compounds with the best water solubility, pyrene and benzo[a]anthracene, disappeared to the greatest extent. After 15 weeks, 83% pyrene and 41% benzo[a]anthracene disappeared in replicates without heavy metals. The presence of heavy metals in soil led to a decrease in disappearance, so that with cadmium at 100 ppm, 78% pyrene and 33% benzo[a]anthracene and in the same concentration of mercury, 51 and 22% of these compounds, respectively, was degraded. The disappearance of all other PAHs was below 20% and showed little or no response to metal addition.
FIG. 3.
Disappearance of PAHs (10 ppm) in nonsterile soil containing cadmium after 15 weeks of incubation. Panels: a, PAH disappearance in soil without fungus; b, PAH disappearance in soil with P. ostreatus. Abbreviations: Pyr, pyrene; Baa, benzo[a]anthracene; Chr, chrysene; Baf, benzo[a]fluoranthene; Bkf, benzo[k]fluoranthene; Bap, benzo[a]pyrene; Daa, dibenzo[a,h]anthracene; Bgp, benzo[ghi]perylene. The data are represented as percentages of the initial amount (PAHs extracted from a noncultivated control). Averages and standard errors of four replicates are shown.
FIG. 4.
Disappearance of PAHs (10 ppm) in nonsterile soil containing mercury after 15 weeks of incubation. Panels: a, PAH disappearance in soil without fungus; b, PAH disappearance in soil with P. ostreatus. Abbreviations: Pyr, pyrene; Baa, benzo[a]anthracene; Chr, chrysene; Baf, benzo[a]fluoranthene; Bkf, benzo[k]fluoranthene; Bap, benzo[a]pyrene; Daa, dibenzo[a,h]anthracene; Bgp, benzo[ghi]perylene. The data are represented as percentages of the initial amount (PAHs extracted from a noncultivated control). Averages and standard errors of four replicates are shown.
In soil inoculated with P. ostreatus, the disappearance of all individual PAHs was significantly greater than in treatments without fungus (Fig. 3b and 4b), except for Cd at 500 ppm. In the presence of fungus, more than 50% of the initial amount in controls disappeared in the case of four compounds: pyrene (83%), benzo[a]anthracene (58%), benzo[a]pyrene (57%), and benzo[ghi]perylene (50%). The contributions of P. ostreatus to PAH disappearance in nonsterile soil (i.e., the difference in PAH recovery between soil samples with and without fungus) are summarized in Tables 1 (for cadmium) and 2 (for mercury). It is apparent that the inoculation of soil with P. ostreatus led to selective enhancement of the disappearance of individual PAHs. In soil without heavy metals, the disappearance of PAH compounds with lower solubility and higher numbers of aromatic rings was enhanced. It is interesting that in the presence of both mercury and cadmium at 10 to 100 ppm, the fungal contribution to PAH disappearance was higher than in the absence of the heavy metals. However, it seems that only lower degradation of PAHs by soil microorganisms caused by the presence of metals was brought to essentially the same level as in the control (Fig. 3 and 4). A substantial negative effect on the degradation of all compounds was found only in the presence of cadmium at 500 ppm—the contribution of the fungus to PAH disappearance was not statistically significant. Mycelial colonization of soil was very low with cadmium at 500 ppm. The density of mycelia and the time course of soil colonization were also substantially altered with both metals at 100 ppm.
TABLE 1.
Contribution of P. ostreatus to PAH (10 ppm) disappearance in nonsterile soil containing cadmiuma
| Compound | Avg % disappearance ± SE at Cd concn (ppm) of:
|
|||
|---|---|---|---|---|
| 0 | 10 | 100 | 500 | |
| Pyrene | 0.5 ± 3.8 | 4.7 ± 1.4 | 7.7 ± 2.7 | 3.3 ± 2.8 |
| Benzo[a]anthracene | 17.4 ± 3.8 | 30.3 ± 3.2 | 35.6 ± 0.7 | 4.2 ± 4.6 |
| Chrysene | 10.4 ± 4.5 | 12.3 ± 4.8 | 18.0 ± 2.9 | 2.4 ± 5.7 |
| Benzo[a]fluoranthene | 17.3 ± 4.6 | 33.1 ± 6.1 | 34.2 ± 2.1 | 3.0 ± 5.9 |
| Benzo[k]fluoranthene | 28.2 ± 5.3 | 31.2 ± 4.3 | 35.3 ± 4.1 | 1.8 ± 5.3 |
| Benzo[a]pyrene | 38.7 ± 5.4 | 50.9 ± 4.9 | 52.2 ± 4.8 | 2.1 ± 9.2 |
| Dibenzo[a,h]anthracene | 20.3 ± 4.8 | 27.0 ± 4.3 | 25.6 ± 6.8 | 4.3 ± 3.9 |
| Benzo[ghi]perylene | 19.8 ± 2.9 | 33.6 ± 5.2 | 32.8 ± 3.6 | −0.4 ± 3.1 |
The data (represented as percentages of the initial amount) were calculated from PAH recoveries in treatments with and without fungus. The percentages are based on 100% recovery from the control at the beginning of the experiment.
Loss of straw dry weight during the biodegradation process is summarized in Table 3. In the absence of P. ostreatus, 20 to 25% of the straw was consumed during incubation. Only a slight decrease in straw utilization was found at higher metal concentrations. The straw consumption in the presence of fungus reached 40 to 55%. The highest rates of straw degradation were found at higher metal concentrations (100 to 500 ppm for Cd and 50 to 100 ppm for Hg), at which limited soil colonization occurred.
TABLE 3.
Loss of straw dry weight during PAH degradation in nonsterile soil supplemented with cadmium or mercury in the presence or absence of P. ostreatus
| Treatment | Avg % of original amt ± SEa
|
|
|---|---|---|
| Nonsterile soil | Nonsterile soil + P. ostreatus | |
| Control | 24.5 ± 3.4 | 38.6 ± 0.7 |
| Cd at: | ||
| 10 ppm | 22.5 ± 2.2 | 39.6 ± 1.0 |
| 100 ppm | 21.8 ± 1.8 | 53.3 ± 2.6 |
| 500 ppm | 22.1 ± 1.5 | 52.7 ± 3.9 |
| Hg at: | ||
| 10 ppm | 25.4 ± 3.0 | 42.6 ± 0.8 |
| 50 ppm | 22.9 ± 2.9 | 45.3 ± 0.3 |
| 100 ppm | 20.9 ± 1.4 | 47.1 ± 0.8 |
The experiment lasted 15 weeks, and the values shown are for four replicates.
Colonization of soil.
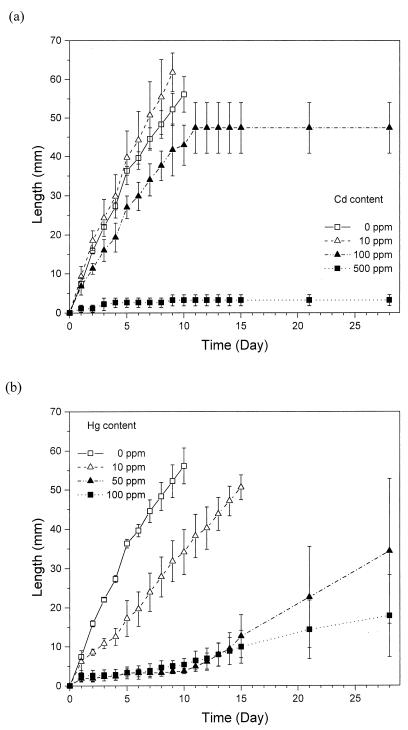
Because of the differences in soil colonization by mycelia at different mercury and cadmium concentrations, the influence of Cd and Hg on the penetration of a soil layer by P. ostreatus mycelium was studied in glass tubes. The results of this experiment are shown in Fig. 5. In the presence of cadmium, soil colonization was completely inhibited by 500 ppm—the fungus was only able to colonize the soil adjacent to the straw compartment (3 mm). At lower Cd concentrations, the fungus colonization rate did not differ significantly during the first days of growth. The growth rate in soil was 5.9 ± 0.5 mm · day−1 at control, 6.4 ± 1.3 mm · day−1 with Cd at 10 ppm, and 4.6 ± 0.9 mm · day−1 with Cd at 100 ppm. However, with Cd at 100 ppm, the fungal mycelium did not reach the end of the soil column and ceased to grow after 11 days, having colonized only 47 mm of the soil column (Fig. 5a). Later, a retreat (disappearance of mycelium) from soil colonized earlier was even apparent. Furthermore, the mycelial density was rather low at this metal concentration. Colonization of soil containing mercury was substantially slower at all metal concentrations (Fig. 5b). During the initial phase of soil colonization, the growth rates were 2.8 ± 1.2 mm · day−1 in Hg at 10 ppm (46% of the control growth rate) and only 0.3 and 0.2 mm · day−1 in Hg at 50 and 100 ppm (less than 6% of the control value). The fungus colonized the whole soil compartment during 16 days in Hg at 10 ppm. The growth of the fungus in Hg at 50 and 100 ppm increased significantly after a 10-day lag phase to 1.7 and 0.7 mm · day−1. However, this phase of faster growth was only temporary and the fungus did not colonize the soil column completely. In presence of mercury, the mycelial density also decreased with increased metal concentrations. At higher heavy-metal concentrations, the fungus is apparently able to colonize only a thin layer of soil, as was the case in experiments in which enzyme activities and PAH degradation were measured.
FIG. 5.
Colonization of soil containing cadmium and mercury by P. ostreatus. Panels: a, cadmium-containing soil; b, mercury-containing soil. The data points represent average mycelial progress. Averages and standard errors of four replicates are shown.
DISCUSSION
The time course of enzyme activities of Pleurotus sp. in sterile and nonsterile soil and straw was previously studied (37). Enzyme activities in sterile and nonsterile soil were similar, only laccase activity tended to be higher in nonsterile soil. Laccase activity reached a maximum during the first 2 weeks after addition of soil to straw colonized by fungus. Thereafter, laccase activity decreased and reached a constant minimal value maintained in the course of the experiment. MnP activity increased during the first 4 weeks and remained constant for several weeks. A similar time course of enzyme activities was also found in our experiment. The activities of both enzymes were higher in soil than in straw during soil colonization, and diffusive flux of enzymes from straw to soil can therefore be ruled out (37). Thus, the activity of enzymes extracted from the soil compartment reflects the activity of enzymes produced by mycelia growing in soil.
Thus far, only limited attention has been paid to the role of metal ions (including heavy-metal ions) on the activity of ligninolytic enzymes. In this regard, only manganese and copper, the metals with a known role in the catalytic action of MnP and laccase, have been studied. Manganese was found to increase MnP gene transcription and enzyme activity in several fungi, including Pleurotus spp. (15, 18, 52), whereas copper induces both the transcription and activity of laccase, a copper-containing enzyme (20, 25). The effect of a heavy metal on enzyme activity was studied only in the case of cadmium (4). It was found that the activities of ligninolytic enzymes from Phanerochaete chrysosporium and Stereum hirsutum cultivated in liquid media are decreased by cadmium. The most sensitive enzyme was MnP, where Cd at as little as 10 ppm caused a substantial decrease in activity and the activity of this enzyme was almost completely inhibited by Cd at 30 ppm. Activities of laccase from S. hirsutum and ligninase from P. chrysosporium were less sensitive to Cd. Cd also reduced the rate of decolorization of Poly R-478 dye in different fungi, including P. ostreatus (4).
The results presented here show that the situation in soil is different. Only during the first days of soil colonization was there a detectable influence of cadmium on laccase activity. Lower activity was found in Cd at 500 ppm, whereas in Cd at 10 and 100 ppm, enzyme activity was even higher than in the control. The activity of MnP was only slightly decreased in soil containing cadmium at 10 and 100 ppm during the onset of enzyme production. However, in the presence of Cd at 500 ppm, MnP activity was negligible throughout the whole experiment. In soil containing mercury, decreased peak activity of laccase and a temporal shift in activity maxima were observed in the presence of the metal at 50 and 100 ppm, probably due to the slower progress of soil colonization caused by mercury. The lower MnP activity measured during the first days of soil colonization in Hg at 50 and 100 ppm probably has the same cause. After colonization of soil by the fungus, laccase and MnP activities were the same in all of the mercury treatments. Lower toxicity of metals in soil can be caused by their limited bioavailability. The soil used in our experiment was found to have the highest metal bioavailability of all of the soils tested by Reber (51), due to the limited content of organic matter.
The degradation of PAHs by Pleurotus sp. has been studied in liquid culture (8, 9, 10), in a straw substrate (29, 36, 64), and in sterile soil (48). Degradation of more than 25% of the initial amount of PAHs containing four to six aromatic rings was found in straw with an initial PAH concentration of 10 ppm. The fungus was able to mineralize the labeled PAHs to 19 to 53% in 15 weeks. No correlation was observed between water solubility and recovery of PAHs (64). Recently, P. ostreatus was found to catalyze the humification of anthracene, benzo[a]pyrene, and fluoranthene in two PAH-contaminated soils, one from a former manufactured gas facility and another from an abandoned electric coking plant (12). The toxicity of PAHs to fungi is relatively low (48), and PAHs added to straw did not affect fungal growth and PAH degradation at concentrations of up to 250 ppm (64).
Although the degradation of PAHs by P. ostreatus has been studied in detail, there is little information about the degradation process in soil in the presence of indigenous microflora. In fact, under in situ conditions, soil microflora contributes to PAH degradation and influences the activity of the white-rot fungus inoculated into soil. Bacteria degrade bioavailable PAHs with up to four rings (19), and soil fungi are also capable of PAH degradation (38, 53, 58). The degradation of PAHs with three or four aromatic rings is probably mostly due to bacterial activity, and it was not found to be accelerated by the presence of a fungus (28). So far, only a few microorganisms have been shown to degrade higher PAHs (five or more rings). Since the low bioavailability of PAHs is a major rate-limiting factor in the degradation of these compounds by bacteria (57, 61), the increased bioavailability of the oxidized PAH metabolites produced by white-rot fungi can increase their mineralization by bacteria. Addition of indigenous microflora to benzo[a]pyrene oxidation products produced by Bjerkandera sp. led to a substantial increase in the mineralization rate (32). Therefore, the degradation process in soil proceeds as a cooperation between white-rot fungi (which mainly catalyze PAH oxidation) and bacteria (which mineralize PAHs with higher water solubility and oxidized metabolites of recalcitrant high-molecular-weight compounds).
Lowest PAH recoveries from nonsterile soil was found in the case of the four-ring molecules of pyrene and benzo[a]anthracene. Only a minor effect of metals on the disappearance of PAHs was found in soil without fungus. In addition to degradation, disappearance of PAHs was probably partly due to the formation of bound residues (30). The presence of fungus in soil led to increased disappearance of all PAH molecules. In addition to degradation (oxidation), the contribution of fungus might also be due to the limited toxicity of metals for soil microflora in the presence of mycelium. It has to be noted that Pleurotus spp. are strongly competitive fungal species which can readily colonize nonsterile soil (29, 37) and even decrease the population of soil bacteria (28). The disappearance of less-soluble compounds with five or six aromatic rings (benzo[a]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]anthracene, and benzo[ghi]perylene) was particularly enhanced in the presence of P. ostreatus. While the enhancement of PAH disappearance by the fungus led to almost similar recoveries of individual PAHs at all of the concentrations of mercury tested, in Cd at 500 ppm, the increase in PAH disappearance due to fungal inoculation was almost negligible (Table 1).
Although the role of individual ligninolytic enzymes in PAH degradation is still not completely understood and some investigators have suggested that the PAH-degrading and lignin-degrading systems of white-rot fungi can be distinct (8, 14, 22) it was shown that both laccase (21, 50) and MnP (13, 33, 44, 55) are capable of PAH degradation (oxidation) in the presence of appropriate mediators. No direct conclusion can be drawn about the role of ligninolytic enzymes in PAH disappearance; however, limited PAH degradation by P. ostreatus in Cd at 500 ppm can be due to low MnP activity, and thus, this enzyme is probably responsible for the bulk degradation of PAH compounds by the fungus.
The strong negative effect of heavy metals on the growth of wood-rotting fungi is already well documented in vitro (3, 40). In this work, we demonstrated that mycelial penetration into soil is also inhibited by cadmium and mercury. This is a serious problem for proposed in situ bioremediation, since incomplete colonization of soil also occurred at relatively low metal concentrations, at which PAH degradation and enzyme activities were still unaffected. The use of heavy-metal-resistant species of fungi at sites with combined pollution can be a solution to this problem. Interestingly, limited penetration into soil caused by heavy metals was accompanied by accelerated utilization of straw in the metal-free compartment.
It can be concluded that the effect of heavy metals on the activity of ligninolytic enzymes is less pronounced that in liquid culture. Because the effect of metals on the activity of soil microflora is also relatively small, the efficiency of a biodegradation process is limited mainly by interference from metals with the introduction and maintenance of white-rot fungi in soil. However, there is only limited information about the toxicity of soil metals to fungi and further research in this direction is necessary.
TABLE 2.
Contribution of P. ostreatus to the disappearance of PAHs at 10 ppm in nonsterile soil containing mercurya
| Compound | Avg % disappearance ± SE at Hg concn (ppm) of:
|
|||
|---|---|---|---|---|
| 0 | 10 | 50 | 100 | |
| Pyrene | 0.5 ± 3.8 | 3.8 ± 8.6 | 23.1 ± 11.9 | 38.4 ± 17.1 |
| Benzo[a]anthracene | 17.4 ± 3.8 | 35.5 ± 17.1 | 31.8 ± 16.8 | 38.5 ± 21.5 |
| Chrysene | 10.4 ± 4.5 | 23.2 ± 19.5 | 12.8 ± 18.4 | 26.4 ± 24.0 |
| Benzo[a]fluoranthene | 17.3 ± 4.6 | 32.7 ± 17.2 | 24.0 ± 16.3 | 36.1 ± 21.1 |
| Benzo[k]fluoranthene | 28.2 ± 5.3 | 33.1 ± 18.2 | 24.3 ± 17.2 | 35.6 ± 22.5 |
| Benzo[a]pyrene | 38.7 ± 5.4 | 54.2 ± 18.6 | 52.2 ± 18.7 | 57.8 ± 24.0 |
| Dibenzo[a,h]anthracene | 20.3 ± 4.8 | 26.1 ± 17.7 | 13.0 ± 16.7 | 20.4 ± 21.8 |
| Benzo[ghi]perylene | 19.8 ± 2.9 | 29.6 ± 14.7 | 20.1 ± 14.3 | 20.1 ± 18.2 |
The data (represented as percentages of the initial amount) were calculated from PAH recoveries in treatments with and without fungus. The percentages are based on 100% recovery from the control at the beginning of the experiment.
ACKNOWLEDGMENTS
This study was partly supported by the Deutsche Forschungsgemeinschaft (ZA 116/3-3), by the Bundesministerium für Bildung und Forschung, Germany (TSR-038-97), and by the Grant Agency of the Czech Republic (204/99/1528).
REFERENCES
- 1.Anderson T-H, Domsch K H. Effects of soil structure on microbial biomass. In: Hartger K H, Stewart B A, editors. Soil structure—its development and function. Boca Raton, Fla: Lewis Publishers; 1995. pp. 325–345. [Google Scholar]
- 2.Andersson B E, Henrysson T. Accumulation and degradation of dead-end metabolites during treatment of soil contaminated with polycyclic aromatic hydrocarbons with five strains of white-rot fungi. Appl Microbiol Biotechnol. 1996;46:647–652. [Google Scholar]
- 3.Baldrian P, Gabriel J. Effect of heavy metals on the growth of selected wood-rotting basidiomycetes. Folia Microbiol. 1997;42:521–523. [Google Scholar]
- 4.Baldrian P, Gabriel J, Nerud F. Effect of cadmium on the ligninolytic activity of Stereum hirsutum and Phanerochaete chrysosporium. Folia Microbiol. 1996;41:363–367. [Google Scholar]
- 5.Barr D P, Aust S D. Mechanisms white-rot fungi used to degrade pollutants. Environ Sci Technol. 1994;28:78–87. doi: 10.1021/es00051a724. [DOI] [PubMed] [Google Scholar]
- 6.Baud-Grasset F, Baud-Grasset S, Saffernan S I. Evaluation of the bioremediation of a contaminated soil with phytotoxicity tests. Chemosphere. 1993;26:1365–1374. [Google Scholar]
- 7.Baud-Grasset S, Baud-Grasset F, Bifulco J M, Meier J R, Ma T-H. Reduction of genotoxicity of a creosote-contaminated soil after fungal treatment determined by the Tradescantia-micronucleus test. Mutat Res. 1993;303:77–82. doi: 10.1016/0165-7992(93)90098-g. [DOI] [PubMed] [Google Scholar]
- 8.Bezalel L, Hadar Y, Cerniglia C E. Mineralization of polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:292–295. doi: 10.1128/aem.62.1.292-295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezalel L, Hadar Y, Fu P P, Freeman J P, Cerniglia C E. Metabolism of phenanthrene by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:2547–2553. doi: 10.1128/aem.62.7.2547-2553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezalel L, Hadar Y, Fu P P, Freeman J P, Cerniglia C E. Initial oxidation products in the metabolism of pyrene, anthracene, fluorene, and dibenzothiophene by the white rot fungus Pleurotus ostreatus. Appl Environ Microbiol. 1996;62:2554–2559. doi: 10.1128/aem.62.7.2554-2559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogan B W, Lamar R T. Polycyclic aromatic hydrocarbon-degrading capabilities of Phanerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol. 1996;62:1597–1603. doi: 10.1128/aem.62.5.1597-1603.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogan B W, Lamar R T, Burgos W D, Tien M. Extent of humification of anthracene, fluoranthene, and benzo[a]pyrene by Pleurotus ostreatus during growth in PAH-contaminated soils. Lett Appl Microbiol. 1999;28:250–254. [Google Scholar]
- 13.Bogan B W, Schoenike B, Lamar R T, Cullen D. Manganese peroxidase mRNA and enzyme activity levels during bioremediation of polycyclic aromatic hydrocarbon-contaminated soil with Phanerochaete chrysosporium. Appl Environ Microbiol. 1996;62:2381–2386. doi: 10.1128/aem.62.7.2381-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle D, Wiesner C, Richardson A. Factors affecting the degradation of polyaromatic hydrocarbons in soil by white-rot fungi. Soil Biol Biochem. 1998;30:873–882. [Google Scholar]
- 15.Brown J A, Alic M, Gold M H. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J Bacteriol. 1991;173:4101–4106. doi: 10.1128/jb.173.13.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bumpus J A, Tien M, Wright D, Aust S D. Oxidation of persistent environmental pollutants by a white-rot fungus. Science. 1985;228:1434–1436. doi: 10.1126/science.3925550. [DOI] [PubMed] [Google Scholar]
- 17.Burkhardt C, Insam H, Hutchinson T C, Reber H H. Impact of heavy metals on the degradative capabilities of soil bacterial communities. Biol Fertil Soils. 1993;16:154–156. [Google Scholar]
- 18.Buswell J A, Cai Y, Chang S. Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Lentinula (Lentinus) edodes. FEMS Microbiol Lett. 1995;128:81–88. [Google Scholar]
- 19.Cerniglia C E, Heitkamp M A. Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment. In: Varanasi U, editor. Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 41–68. [Google Scholar]
- 20.Collins P J, Dobson A D W. Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol. 1997;63:3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins P J, Kotterman M J J, Field J A, Dobson A D W. Oxidation of anthracene and benzo[a]pyrene by laccases from Trametes versiocolor. Appl Environ Microbiol. 1996;62:4563–4567. doi: 10.1128/aem.62.12.4563-4567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhawale S W, Dhawale S S, Dean-Ross D. Degradation of phenanthrene by Phanerochaete chrysosporium occurs under ligninolytic as well as nonligninolytic conditions. Appl Environ Microbiol. 1992;58:3000–3006. doi: 10.1128/aem.58.9.3000-3006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dipple A, Cheng S C, Bigger C A H. Polycyclic aromatic hydrocarbon carcinogens. In: Pariza M W, Aeschbacher H U, Felton J S, Sato S, editors. Mutagens and carcinogens in the diet. New York, N.Y: Wiley-Liss; 1990. pp. 109–127. [Google Scholar]
- 24.Field J A, Vledder R H, van Zeist J G, Rulkens W H. The tolerance of lignin peroxidase and manganese-dependent peroxidase to miscible solvents and the in vitro oxidation of anthracene in solvent:water mixtures. Enzyme Microb Technol. 1996;18:300–308. [Google Scholar]
- 25.Froehner S C, Eriksson K-E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974;120:450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giersig K, Schinner F. Abbau von polyzyklischen aromatischen Kohlenwasserstoffen in metallbelasteten Boden. In: Margesin R, Schneider M, Schinner F, editors. Praxis der mikrobiellen Bodensanierung. Berlin, Germany: Springer; 1995. pp. 107–117. [Google Scholar]
- 27.Glenn J K, Morgan M A, Mayfield M B, Kuwahara M, Gold M H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983;114:1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- 28.Gramss G, Voigt K D, Kirsche B. Degradation of polycyclic aromatic hydrocarbons with three to seven aromatic rings by higher fungi in sterile and unsterile soils. Biodegradation. 1999;10:51–62. doi: 10.1023/a:1008368923383. [DOI] [PubMed] [Google Scholar]
- 29.in der Wiesche C, Martens R, Zadrazil F. Two-step degradation of pyrene by white-rot fungi and soil microorganisms. Appl Microbiol Biotechnol. 1996;46:653–659. doi: 10.1007/s002530050876. [DOI] [PubMed] [Google Scholar]
- 30.Kästner M, Streibich S, Beyrer M, Richnow H H, Fritsche W. Formation of bound residues during microbial degradation of [14C]anthracene in soil. Appl Environ Microbiol. 1999;65:1834–1842. doi: 10.1128/aem.65.5.1834-1842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeleman M, van der Laak W J, Ietswaart H. Dispersion of PAH and heavy metals along motorways in The Netherlands—an overview. Sci Total Environ. 1999;235:347–349. doi: 10.1016/s0048-9697(99)00253-3. [DOI] [PubMed] [Google Scholar]
- 32.Kotterman M J J, Vis E H, Field J A. Successive mineralization and detoxification of benzo[a]pyrene by the white rot fungus Bjerkandera sp. strain BOS55 and indigenous microflora. Appl Environ Microbiol. 1998;64:2853–2858. doi: 10.1128/aem.64.8.2853-2858.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kotterman M J J, Wasseveld R A, Field J A. Hydrogen peroxide production as a limiting factor in xenobiotic compound oxidation by nitrogen-sufficient cultures of Bjerkandera sp. strain BOS55 overproducing peroxidases. Appl Environ Microbiol. 1996;62:880–885. doi: 10.1128/aem.62.3.880-885.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamar R T, Davis M W, Dietrich D M, Glaser J A. Treatment of pentachlorophenol- and creosote-contaminated soil using the lignin-degrading fungus Phanerochaete sordida: a field demonstration. Soil Biol Biochem. 1994;26:1603–1611. [Google Scholar]
- 35.Lang E, Eller G, Zadrazil F. Lignocellulose decomposition and production of ligninolytic enzymes during interaction of white rot fungi with soil microorganisms. Microb Ecol. 1997;34:1–10. doi: 10.1007/s002489900029. [DOI] [PubMed] [Google Scholar]
- 36.Lang E, Nerud F, Novotná E, Zadražil F, Martens R. Production of ligninolytic exoenzymes and C-14-pyrene mineralization by Pleurotus sp. in lignocellulose substrate. Folia Microbiol. 1996;41:489–493. [Google Scholar]
- 37.Lang E, Nerud F, Zadrazil F. Production of ligninolytic enzymes by Pleurotus sp. and Dichomitus squalens in soil and lignocellulose substrate as influenced by soil microorganisms. FEMS Microbiol Lett. 1998;167:239–244. [Google Scholar]
- 38.Lange B, Kremer S, Sterner O, Anke H. Metabolism of pyrene by basidiomycetous fungi of the genera Crinipellis, Marasmius and Marasmiellus. Can J Microbiol. 1996;42:1179–1183. [Google Scholar]
- 39.Loske D, Hüttermann A, Majerczyk A, Zadrazil F, Lorsen H, Waldinger P. Use of white rot fungi for the clean-up of contaminated sites. In: Coughlan M P, Collaco P, editors. Advances in biological treatment of lignocellulosic materials. London, United Kingdom: Elsevier; 1990. pp. 311–321. [Google Scholar]
- 40.Mandal T K, Baldrian P, Gabriel J, Nerud F, Zadražil F. Effect of mercury on the growth of wood-rotting basidiomycetes Pleurotus ostreatus, Pycnoporus cinnabarinus and Serpula lacrymans. Chemosphere. 1998;36:435–440. [Google Scholar]
- 41.Martens R, Wetzstein H G, Zadrazil F, Capelari M, Hoffmann P, Schmeer N. Degradation of the fluoroquinolone enrofloxacin by wood-rotting fungi. Appl Environ Microbiol. 1996;62:4206–4209. doi: 10.1128/aem.62.11.4206-4209.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martens R, Zadrazil F. Screening of white-rot fungi for their ability to mineralize polycyclic aromatic hydrocarbons in soil. Folia Microbiol. 1998;43:97–103. doi: 10.1007/BF02815552. [DOI] [PubMed] [Google Scholar]
- 43.Meschede T, Vogelsberger R. Remediation of a mercury contaminated site in Egypt. In: van den Brink W J, Bosman R, Arendt F, editors. Contaminated soil '95. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 237–238. [Google Scholar]
- 44.Moen M A, Hammel K E. Lipid peroxidation by the manganese peroxidase of Phanerochaete chrysosporium is the basis for phenanthrene oxidation by the intact fungus. Appl Environ Microbiol. 1994;60:1956–1961. doi: 10.1128/aem.60.6.1956-1961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan P, Lee S A, Lewis S T, Sheppard A N, Watkinson R J. Growth and biodegradation by white-rot fungi inoculated into soil. Soil Biol Biochem. 1993;25:279–287. [Google Scholar]
- 46.Ngo T T, Lenhoff H M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem. 1980;105:389–397. doi: 10.1016/0003-2697(80)90475-3. [DOI] [PubMed] [Google Scholar]
- 47.Niku-Paavola M L, Raaska L, Itävaara M. Detection of white-rot fungi by a non-toxic stain. Mycol Res. 1990;94:27–31. [Google Scholar]
- 48.Novotný Č, Erbanová P, Šašek V, Kubátová A, Cajthaml T, Lang E, Krahl J, Zadražil F. Extracellular oxidative enzyme production and PAH removal in soil by exploratory mycelium of white rot fungi. Biodegradation. 1999;10:159–168. doi: 10.1023/a:1008324111558. [DOI] [PubMed] [Google Scholar]
- 49.Paszczynski A, Crawford R L. Potential for bioremediation of xenobiotic compounds by the white-rot fungus Phanerochaete chrysosporium. Biotechnol Prog. 1995;11:368–379. [Google Scholar]
- 50.Pickard M A, Roman R, Tinoco R, Vazquez-Duhalt R. Polycyclic aromatic hydrocarbon metabolism by white rot fungi and oxidation by Coriolopsis gallica UAMH 8260 laccase. Appl Environ Microbiol. 1999;65:3805–3809. doi: 10.1128/aem.65.9.3805-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reber H H. Threshold levels of cadmium for soil respiration and growth of spring wheat (Triticum aestivum L.) and difficulties with their determination. Biol Fertil Soils. 1989;7:152–157. [Google Scholar]
- 52.Ruiz-Duenas F J, Guillén F, Camarero S, Pérez-Boada M, Martínez M J, Martínez A T. Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl Environ Microbiol. 1999;65:4458–4463. doi: 10.1128/aem.65.10.4458-4463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sack U, Günther T. Metabolism of PAH by fungi and correlation with extracellular enzymatic activities. J Basic Microbiol. 1993;33:269–277. doi: 10.1002/jobm.3620330411. [DOI] [PubMed] [Google Scholar]
- 54.Sack U, Heinze T M, Deck J, Cerniglia C E, Martens R, Zadrazil F, Fritsche W. Comparison of phenanthrene and pyrene degradation by different wood-decaying fungi. Appl Environ Microbiol. 1997;63:3913–3925. doi: 10.1128/aem.63.10.3919-3925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sack U, Hofrichter M, Fritsche W. Degradation of polycyclic aromatic hydrocarbons by manganese peroxidase of Nematoloma frowardii. FEMS Microbiol Lett. 1997;152:227–234. doi: 10.1111/j.1574-6968.1997.tb10432.x. [DOI] [PubMed] [Google Scholar]
- 56.Sims R C, Overcash M R. Fate of polynuclear aromatic compounds (PNAs) in soil-plant systems. Residue Rev. 1983;88:1–68. [Google Scholar]
- 57.Stucki G, Alexander M. Role of dissolution rate and solubility in biodegradation of aromatic compounds. Appl Environ Microbiol. 1987;53:292–297. doi: 10.1128/aem.53.2.292-297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutherland J B, Rafii F, Khan A A, Cerniglia C E. Mechanism of polycyclic aromatic hydrocarbon degradation. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss; 1995. pp. 269–306. [Google Scholar]
- 59.Thurston C F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- 60.Tien M, Kirk T K. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci USA. 1984;81:2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Volkering F, Breure A M, Sterkenburg A, van Andel J G. Microbial degradation of polycyclic aromatic hydrocarbons: effect of substrate bioavailability on bacterial growth kinetics. Appl Microbiol Biotechnol. 1992;36:548–552. [Google Scholar]
- 62.Waldner R, Leisola M S A, Fiechter A. Comparison of ligninolytic activities of selected white-rot fungi. Appl Microbiol Biotechnol. 1988;29:400–407. [Google Scholar]
- 63.Wilson S C, Jones K C. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ Pollut. 1993;81:681–696. doi: 10.1016/0269-7491(93)90206-4. [DOI] [PubMed] [Google Scholar]
- 64.Wolter M, Zadrazil F, Martens R, Bahadir M. Degradation of eight highly condensed polycyclic aromatic hydrocarbons by Pleurotus sp. Florida in solid wheat straw substrate. Appl Microbiol Biotechnol. 1997;48:398–404. [Google Scholar]