Abstract
Background:
Cancer-related and traumatic stress symptoms, including Posttraumatic Stress Disorder (PTSD), can significantly impact cancer patients’ and survivors’ quality of life and psychological adjustment. Cognitive-behavioral therapy (CBT) is an effective intervention previously shown to ameliorate non-cancer-related PTSD. Due to some of the unique aspects of cancer-related traumatic stress, such as the internal and ongoing nature of the traumatic stressor, it is important to review the overall efficacy of CBT interventions in cancer populations.
Objective:
To review the findings of randomized clinical trials (RCTs) testing the efficacy of interventions with CBT components for cancer-related traumatic stress symptoms, such as intrusion and avoidance, in adults with cancer.
Methods:
Eligible RCTs were identified via search of OVID, PubMed, and Scopus. Bayesian random effects analysis of treatment effect sizes (ES) was conducted in a portion of the studies for which data were available.
Results:
Nineteen RCTs met search criteria. Six trials reported reductions in traumatic stress symptoms as a result of the intervention and thirteen studies reported null findings. Bayesian modeling based on thirteen studies showed no overall discernible effect of interventions with CBT components on intrusion and avoidance symptoms.
Conclusions:
The majority of studies were not designed to target traumatic stress symptoms in highly distressed cancer patients and did not include previously validated CBT components, such as cognitive restructuring and exposure.. Thus, there was insufficient evidence from which to draw definitive conclusions about the efficacy of CBT interventions for the treatment of cancer-related traumatic stress symptoms, including PTSD. However, interventions with CBT components may have potential for the reduction of PTSD symptoms in highly distressed patients. Future research should focus on testing trauma-focused interventions in demographically and clinically diverse samples.
Keywords: cancer, posttraumatic stress disorder, traumatic stress, cognitive behavioral therapy
Introduction
A large body of literature documents the efficacy of cognitive-behavioral therapy (CBT) for the treatment of posttraumatic stress disorder (PTSD) in non-cancer populations (Cahill & Foa, 2007; Foa, Keane, Friedman, & Cohen, 2009; U.S. Veterans Affairs/Department of Defense (VA/DoD), 2010). Existing guidelines recommend CBT as one of the treatments of choice in alleviating PTSD symptoms in survivors of a wide range of traumas, including sexual and physical assault, combat-related trauma, motor vehicle accidents, and natural disasters (VA/DoD, 2010; Foa et al., 2009; Forbes et al., 2010). Similar progress, however, has not been made with regards to recommendations for the treatment of cancer-related PTSD and traumatic stress symptoms, even though posttraumatic stress responses following cancer diagnosis have received increasing attention over the past three decades and may represent a significant source of distress for a subset of cancer patients and survivors (Deimling, Kahana, Bowman, & Scaefer, 2002; Gurevich, Devins, & Rodin, 2002; Kangas, Henry, & Bryant, 2002; Smith, Redd, Peyser, & Vogl, 1999; Mosher, Redd, Rini, Burkhalter & DuHamel, 2009). Advances in the management of distress during the cancer trajectory have largely focused on the development and evaluation of interventions targeting general distress symptoms and quality of life concerns. For example, several meta-analyses and review articles have addressed the efficacy of interventions with cognitive-behavioral components for some of the most common psychosocial issues in cancer patients and survivors, including depression and anxiety (Jacobsen & Jim, 2008; Osborn, Demoncada, & Feuerstein, 2006), fatigue (Kangas, Bovbjerg, & Montgomery, 2008) and pain (Tatrow & Montgomery, 2006). Yet a large gap exists in our knowledge and understanding of the clinical management of cancer-specific distress and PTSD symptoms in adults with cancer. The need for more research and empirically-based guidelines for the treatment of PTSD in cancer and other medically ill populations, such as HIV-infected adults, has been highlighted by experts in the field (Applebaum et al., under review; Kangas et al., 2002; Newell, Sanson-Fisher, & Savolainen, 2002).
The lack of treatment guidelines is, in part, a consequence of the relatively recent inclusion of life-threatening illness as a traumatogenic stressor in the 1994 edition of the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV; American Psychiatric Association, 1994) and, accordingly, the limited number of clinical trials designed to evaluate trauma-focused psychological interventions in cancer populations. Nevertheless, PTSD and traumatic stress symptoms have a considerable impact on cancer patients’ and survivors’ psychological and physical functioning and quality of life. Cancer-related traumatic stress symptoms can be associated with increased levels of depression and anxiety, desire for death, pain, disability, and treatment nonadherence (French-Rosas, Moye & Naik, 2011). Reported prevalence rates of PTSD and traumatic stress symptoms in the cancer literature vary due to the complex course and definition of PTSD in this population. Because the cancer trajectory may consist of a series of potentially traumatic events, including cancer detection and diagnosis, treatment, anticipation of test results, progression and recurrence, it is not always clear at which point a cancer survivor becomes truly post-trauma (Deimling et al., 2002; Gurevich et al., 2002; Kangas, Henry, & Bryant, 2005b; Smith et al., 1999). Additionally, posttraumatic stress responses exist on a continuum (Gurevich et al., 2002), with some patients meeting full DSM criteria for PTSD and others experiencing subsyndromal but clinically significant traumatic stress symptoms (Kangas et al., 2005b), although an appropriate threshold has not been established in cancer patients or survivors (Gurevich et al., 2002). The timing of symptom onset also varies, such that patients may meet criteria for PTSD soon after the cancer diagnosis or may develop PTSD at a later point in the disease trajectory (Kangas, Henry, & Bryant, 2005a). It is therefore not surprising that the prevalence of full-syndrome PTSD has been reported to range from 3% to 35% depending on the point in time at which the assessment was conducted, as well as on the measures and criteria used to assess PTSD symptoms (Gurevich et al., 2002). Similarly, subsyndromal posttraumatic stress symptoms of intrusion, avoidance and hyperarousal, have been found to range from 20% in patients with early-stage cancer to as high as 80% in patients with a recent recurrence (Gurevich et al., 2002). Finally, it is important to note that while specific symptoms of PTSD are common and may represent a normal temporary response to an acute traumatic stressor, such as cancer, it has been suggested that true PTSD may be characterized by the persistence of PTSD symptoms over time and, thus, a failure to adapt (Friedman, Resick, Bryant & Brewin, 2011).
The nature and intensity of the traumatic stress response can vary greatly from patient to patient. Typical posttraumatic stress symptoms are grouped into three main clusters: re-experiencing/intrusion, avoidance/numbing, and hyperarousal (Gurevich et al., 2002). Cancer patients may experience a wide range of symptoms during the continuum of care. For example, the response to the cancer diagnosis and treatment can include fear, horror, and helplessness (Fox, 1995). In addition, exposure to cues associated with diagnosis and treatment can produce intrusive thoughts, nightmares, and attempts to avoid such reminders (Smith et al., 1999). Many patients may continue to remain highly vigilant for recurrence, which, at times, may be an appropriate and reasonable response given the reality of possible recurrence in certain types of cancer (e.g., multiple myeloma; Deimling et al., 2002; Kornblith, Anderson, Cell, Tross, Zuckerman, et al., 1992). However, hypervigilance can also be a symptom of traumatic stress in the form of an exaggerated startle response and when accompanied by fear and preoccupation (Levine, Eckhardt & Targ, 2005). Even though posttraumatic stress reactions are now well-documented in cancer patients and survivors, little empirical data exists to guide the clinician in deciding when and how to intervene.
Empirically-Validated CBT Interventions for PTSD in Noncancer Populations
A number of CBT interventions have received empirical support for the treatment of non-cancer related PTSD and are included in current clinical practice guidelines. Although CBT interventions encompass a broad range of techniques, they can be classified into exposure-based and cognitive-based therapies (VA./DoD, 2010). Both exposure and cognitive-based treatments are considered trauma-focused in that they explicitly address and work through memories of the traumatic event as part of treatment. Exposure therapies can include imaginal exposure to the trauma memory or in vivo exposure to reminders of the trauma, or a combination of both, which has received the strongest empirical support (Foa et al., 2009). The main component of most cognitive-based therapies is cognitive restructuring, or the identification, challenging and modification of erroneous or dysfunctional cognitions with the goal of replacing them with more realistic and helpful thoughts and beliefs (Cahill & Foa, 2007). Oftentimes restructuring occurs around themes of safety and trust, relative danger, personal inadequacy, self-blame and worries about the future (VA/DoD, 2010). Several existing treatment packages consist of a combination of CBT techniques, most notably Cognitive Processing Therapy (CPT), which includes both cognitive restructuring and narrative exposure components and has been shown to be effective in female sexual assault survivors and combat veterans (VA/DoD, 2010). At least two clinical practice guidelines, the VA/DoD and International Society for Ttraumatic Stress Studies (ISTSS; Foa et al., 2009), recommend the above CBT interventions as first-line treatment for chronic PTSD based on “gold standard” evidence from randomized clinical trials (RCTs). While both exposure-based and cognitive-based therapies have produced significant improvement in PTSD symptomatology when compared with each other (Marks, Lovell, Noshirvani, Livanou & Thrasher, 1998; Cahill, Rothbaum, Resick & Follette, 2009) the evidence is particularly compelling for exposure-based therapies that combine imaginal and in vivo exposure (Foa et al., 2009), such as Prolonged Exposure (PE; Foa, Rothbaum, Riggs & Murdock, 1991). Various anxiety management and stress modulation techniques, including progressive muscle relaxation and breathing retraining, are commonly included as part of empirically-validated CBT interventions for PTSD. However, PTSD treatment guidelines clearly indicate that relaxation techniques are not recommended as stand-alone treatment given the empirical evidence for the superiority of cognitive and exposure-based therapies (Foa et al., 2009; VA/DoD, 2010; Forbes et al., 2010).
Psychosocial Care in Cancer
Provision of psychosocial care to cancer patients is complex and embedded in a multidisciplinary context where interventions often address a number of concerns that arise as a result of cancer. Thus, a typical psychosocial intervention for cancer patients may not consist of strictly CBT techniques, but rather may integrate CBT components with other therapeutic components not specific to CBT, such as support and medical education. Typical components of psychosocial interventions used in the cancer setting include psychoeducation about the impact of cancer on physical and emotional well-being, relaxation training, such as progressive muscle relaxation, mediation or guided imagery, problem-solving, cognitive restructuring, communication skills training and stress management training (Jacobsen & Jim, 2008). CBT has also been used in medically ill populations to change attitudes towards illness, improve adherence to medication (Safren et al., 2009), and to reduce the severity of pain and other bothersome physical symptoms (Antoni, Ironson, & Schneiderman, 2007). Thus, when implementing a treatment for cancer-related PTSD or clinically significant traumatic stress symptoms, the clinician must prioritize treatment goals by considering the many competing demands placed on the particular patient by the cancer experience.
Given the prevalence of cancer-related PTSD and traumatic stress symptoms and their substantial impact on quality of life, it is critical that psychosocial treatments are evaluated for their efficacy in this population and that consideration is given to their utility to address the full continuum and trajectory of posttraumatic symptoms. To our knowledge, no review to date has evaluated the efficacy of CBT interventions for cancer-related PTSD and traumatic stress symptoms. Therefore, the purpose of this study was to establish the state of the science of psychosocial interventions with CBT components in reducing traumatic stress symptoms, including PTSD, in adults with cancer at a variety of points in the cancer trajectory through a systematic literature search and a meta-analysis.
Methods
Search Criteria
This review of interventions with CBT components for traumatic stress symptoms following a cancer diagnosis is limited to articles published between 1994, when the criteria for PTSD were changed to include life-threatening illnesses, and 2010, when the search for this review was conducted. Articles were identified by entering the following search keywords in combination: cancer, (trauma or Posttraumatic Stress Disorder or PTSD) and (cognitive behavioral therapy or CBT) into several databases, including OVID, PubMed, Scopus, and EMBASE. Due to initial difficulty with identifying a sufficient number of relevant RCTs we refined our search further by manually checking the reference sections of articles reviewing interventions in cancer (Gurevich et al., 2002; Jacobsen & Jim, 2008; Kangas et al., 2002; Manne & Andrykowski, 2006; Newell et al., 2002; Osborn et al., 2006). We also used Scopus to search for intervention studies citing specific PTSD measures listed on the National Center for PTSD website (National Center for PTSD, 2009), including, but not limited to the Impact of Events Scale (IES; Horowitz, Wilner & Alvarez, 1979), the PTSD Checklist – Civilian (PCL-C; Weathers, Litz, Herman, Huska & Keane, 1993) and the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). This process consisted of separately entering the original references for each of the screens, interviews and self-report measures into Scopus, and then filtering the results by entering “cancer” in the search box, and manually selecting RCTs of interventions using CBT components. An intervention was considered as including a CBT component if it included at least one of the following: cognitive restructuring, imaginal or in vivo exposure, coping skills training, problem-solving or stress and anxiety management through relaxation training or mindfulness meditation. We did not exclude studies based on the format and modality of the intervention, and included interventions conducted in group, individual or couple settings, as well as interventions administered in person, over the phone or via the Internet. The combined database searches produced 4,551 articles whose abstracts were reviewed by at least two of the authors to remove impertinent subjects. We excluded articles that were focused on non-cancer populations or pediatric cancer. Further, RCTs using a PTSD scale as a moderator, mediator, or a measure of cognitive processing were also excluded. The final RCTs (n = 19) were selected based upon the agreement of at least two of the authors (MN, LM).
Effect Size Analyses
We obtained data for these analyses from each paper’s results section or, when relevant parameters were not published, we requested the data directly from the study’s primary author. Following these procedures we acquired necessary information for 13 out of the 19 RCTs included in this review. Effect sizes (ES) were derived using the delta procedures (Glass & Hopkins, 1984). We subtracted the mean of the post-treatment control group from the post-treatment experimental group, and then divided by the standard deviation of the control group at post-treatment. Dunlop, Cortina, Vaslow and Burke (1996) showed that this method yields unbiased ES estimates rather than potentially biased comparisons based on change scores and paired t-tests (Rosenthal, 1991). Thus, we chose this approach (rather than pre-post change scores, for example) so that greater confidence could be placed in the results. Effect sizes were estimated for each post-treatment primary endpoint. For example, we took data from the first row of Table 2 in Allen et al. (2002) and derived the ES estimate of the post-treatment IES-Intrusion scoreas follows: (10.8 – 12.6) / 8.4 = −0.078 (lower score indicating better status). We did not evaluate follow-up ES because studies varied in their follow-up schedule. For studies that included more than one intervention or control arm, we used the intervention condition most aligned with a CBT approach and the control group most similar to the rest of the studies, usually an inactive control condition.
Table 2.
Postintervention Study Descriptives, Effect Size (ES) Estimates, and 95% Confidence Intervals (CI)
| Experimental |
Control |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | N | Mean | (SD) | N | Mean | (SD) | ES | SE(ES) | Bayesian ES | Bayesian ES 95% CIs | |||
| Allen et al., 2002 | |||||||||||||
| Intrusion | 79 | 10.6 | 8.80 | 75 | 10.6 | 8.80 | 0 | 1.016 | −0.076 | −0.621 | .515 | ||
| avoidance | 79 | 10.8 | 7.40 | 75 | 12.6 | 8.40 | −.214 | 0.970 | −0.023 | −0.662 | 0.517 | ||
| Antoni et al., 2001 | |||||||||||||
| Intrusion | 47 | 14.0 | 4.70 | 53 | 14.2 | 5.30 | −0.042 | 0.733 | −0.085 | −0.617 | 0.480 | ||
| avoidance | 47 | 14.0 | 4.80 | 53 | 14.3 | 5.00 | −0.070 | 0.684 | −0.003 | −0.561 | 0.532 | ||
| Antoni et al., 2006 a | |||||||||||||
| Intrusion | 74 | 1.0 | 0.90 | 85 | 1.2 | 1.20 | −0.167 | 0.132 | −0.381 | −0.381 | 0.099 | ||
| avoidance | 74 | 1.0 | 1.00 | 85 | 1.0 | 0.90 | 0.065 | 0.097 | 0.048 | −0.136 | 0.232 | ||
| Arving et al., 2007 | |||||||||||||
| Intrusion | 51 | 9.0 | 8.00 | 42 | 13.0 | 10.00 | −0.400 | −0.094 | −0.094 | −0.708 | 0.491 | ||
| avoidance | 51 | 10.0 | 9.00 | 42 | 12.0 | 9.00 | 1.272 | 0.007 | −.0589 | −0.589 | 0.607 | ||
| Branstrom et al., 2010 | |||||||||||||
| Intrusion | 25 | 11.3 | 6.40 | 35 | 13.5 | 6.90 | −0.321 | 1.17 | −0.095 | −0.701 | 0.508 | ||
| avoidance | 25 | 7.4 | 7.00 | 35 | 11.6 | 7.30 | −0.565 | 1.235 | −0.025 | −0.733 | 0.573 | ||
| Chan et al., 2005 | |||||||||||||
| Intrusion | 60 | 10.3 | 7.70 | 53 | 10.2 | 8.30 | 0.012 | 1.144 | −0.083 | −0.616 | 0.505 | ||
| avoidance | 60 | 11.9 | 8.00 | 53 | 10.8 | 9.30 | 0.114 | 1.272 | 0.008 | −0.621 | 0.636 | ||
| Johansson et al., 2008 | |||||||||||||
| Intrusion | 109 | 8.0 | 7.00 | 103 | 9.0 | 7.00 | −0.143 | 0.690 | −0.091 | −0.625 | 0.453 | ||
| avoidance | 109 | 11.0 | 8.00 | 103 | 12.0 | 9.00 | −0.111 | 0.887 | −0.005 | −0.605 | 0.544 | ||
| Manne et al., 2005 | |||||||||||||
| Intrusion | 93 | 9.6 | 7.50 | 94 | 11.8 | 8.70 | −0.247 | 0.898 | −0.099 | −0.672 | 0.473 | ||
| avoidance | 93 | 9.7 | 7.80 | 94 | 9.1 | 7.60 | 0.072 | 0.783 | 0.009 | −0.567 | 0.559 | ||
| Manne et al., 2007 | |||||||||||||
| Intrusion | 98 | 9.6 | 7.80 | 100 | 10.7 | 8.90 | −0.124 | 0.891 | −0.094 | −0.673 | 0.463 | ||
| avoidance | 98 | 12.1 | 7.90 | 100 | 14.1 | 9.30 | −0.209 | 0.930 | −0.008 | −0.610 | 0.535 | ||
| Parker et al., 2009 | |||||||||||||
| Intrusion | 31 | 3.1 | 5.60 | 36 | 4.1 | 4.53 | −0.212 | 0.755 | −0.092 | −0.629 | 0.459 | ||
| avoidance | 31 | 3.7 | 5.70 | 36 | 5.1 | 5.30 | −0.264 | 0.883 | −0.012 | −0.615 | 0.553 | ||
| Scott et al., 2004 | |||||||||||||
| Intrusion | 31 | 7.4 | 6.60 | 31 | 8.9 | 8.80 | −0.170 | 1.580 | −0.087 | −0.684 | 0.467 | ||
| avoidance | 31 | 9.4 | 8.50 | 31 | 10.0 | 8.60 | −0.070 | 1.545 | −0.004 | −0.663 | 0.596 | ||
| Stanton et al., 2005 | |||||||||||||
| Intrusion | 143 | 4.3 | 4.30 | 136 | 3.3 | 3.60 | 0.267 | 0.309 | 0.006 | −0.368 | 0.512 | ||
| avoidance | 143 | 4.3 | 4.40 | 136 | 4.7 | 5.10 | −0.081 | 0.435 | −0.023 | −0.548 | 0.423 | ||
| Wengstrom et al., 1999 | |||||||||||||
| Intrusion | 67 | 3.1 | 3.80 | 67 | 3.3 | 4.80 | −0.042 | 0.586 | −0.083 | −0.553 | 0.426 | ||
| avoidance | 67 | 4.1 | 5.90 | 67 | 3.7 | 5.40 | 0.074 | 0.660 | 0.006 | −0.539 | 0.557 | ||
| Intrusion | −0.087 | −0.413 | 0.258 | ||||||||||
| Overall Bayesian ES Estimate | Avoidance | 0 | −0.375 | 0.317 | |||||||||
Note. Negative values denote an effect in favor of the intervention. ES estimates were calculated by dividing the difference in the postintervention means by the standard deviation of the control condition. The (ES) standard errors were calculated by dividing the standard deviation of the control condition by the square root of the control group sample size. SD = standard deviation.
Mean and standard deviation estimates provided by study author in z-scale.
We used a Bayesian random effects model to synthesize the ES estimates across the reviewed studies (Sutton, 2001). Specifically, we based our analytic strategy on Rubin’s original example (Rubin, 1981), which was further elaborated in a paper by Gelman and colleagues (Gelman, 2003). We used the WinBUGS-14 statistical software package to fit the Bayesian model by Gibbs Sampling. We based our WinBUGS syntax on Rubin’s example in the R2WinBUGS package. R2WinBUGS is an add-on software routine to the R statistical computing language to run WinBUGS within R (Sturtz, 2005). Rubin’s case study was named the ‘school’ example in R2WinBUGS. We fitted 3 chains of 10,000 iterations each, first 5,000 discarded, saving 334 iterations per chain after thinning. The number of 10,000 iterations was chosen so that all posterior parameter estimates must have an effective sample size (number of iterations adjusting for auto-correlations; Jackman, 2009) of at least 300. Our syntax is available upon request.
Results
Sample characteristics and results of the 19 studies can be found in Table 1.
Table 1.
Summary of Study Characteristics
| Study | Sample Characteristics | Intervention Description | Control Condition | PTSD Measure | Modality | Results |
|---|---|---|---|---|---|---|
| Allen et al., 2002 |
N = 164 Type: Breast Stage: I-IIIA Point in cancer Tx at baseline: Beginning chemotherapy Mean Age (SD): 42 (5.4) Ethnicity: Caucasian 83% Female: 100% |
Problem-solving skills training; Delivered by oncology nurses in two 2-hr in-person sessions and 4 phone sessions (of unknown duration) over a 12-week period. | Wait-list control | IES | Individual | No effect of intervention on IES-intrusion and avoidance scores. |
| Antoni et al., 2001 |
N = 100 Type: Breast Stage: 0-II Point in cancer Tx at baseline: Surgery within last 8 weeks Mean Age (SD): 50 (9.15) Ethnicity: Caucasian 74% Female: 100% |
Ten-week cognitive behavior stress management (CBSM) intervention with didactics; Delivered in weekly 2-hr sessions. | Day-long seminar (5–6 hrs) of condensed intervention administered 16–18 weeks after surgery. | IES | Group | No effect of intervention on IES-intrusion and avoidance scores. |
| Antoni et al., 2006 |
N = 199 Type: Breast Stage: 0-III Point in cancer Tx at baseline: Surgery within last 8 weeks Mean Age (SD): 50 (9) Ethnicity: Caucasian 69% Female: 100% |
Ten-week CBSM intervention with didactics; Delivered in weekly 2-hr sessions. | Day-long seminar (5–6 hr) of condensed intervention administered 16–18 weeks after surgery. | IES | Group | Significant reduction in IES-intrusion scores for participants in intervention arm; no effect on IES-avoidance. |
| Arving et al., 2007 |
N = 179 Type: Breast Stage: 0-III Point in cancer Tx at baseline: About to start adjuvant Tx Mean Age (range): 55 (23–87) Ethnicity: not reported Female: 100% |
I1) Individually tailored counseling derived from CBT consisting of problem solving, relaxation and distraction techniques, communication skills, and activity scheduling; administered by psychologists in 1–23 45–60 min sessions based on patient needs; (I2) same as I1 but delivered in 1–16 sessions by oncology nurses. | Standard care | IES | Individual | No effect of either intervention on IES-intrusion and avoidance scores. |
| Beatty et al., 2010 |
N = 40 Type: Breast Stage: I-II Point in cancer Tx at baseline: Completed Tx within 3 months Mean Age (SD): 53 (11.4) Ethnicity: Not reported Female: 100% |
Ten-chapter self-help work- book based on CBT and written emotional expression principles, utilizing psycho- education, worksheets and survivor stories/quotes. Included relaxation and a meditation tape; completed over 3 months. | Treatment as usual | PTSS | Individual | No effect of workbook on PTSS scores. |
| Branstrom et al., 2010 |
N = 71 Type: Mixed Stage: Not reported Point in cancer Tx at baseline: Not undergoing chemo or radiation Mean Age (SD): 52 (9.8) Ethnicity: Not reported Female: 99% |
Eight-session Mindfulness Based Stress Reduction (MBSR) program that included meditation, relaxation, and yoga exercises; delivered | Wait-list | IES-R | Group | Significant group 3 time interactions for IES-avoidance and IES- hyperarousal. No effect for IES-intrusion. |
| Chan et al., 2005 |
N = 155 Type: Gynecologic Stage: 0-IV Point in cancer Tx at baseline: Newly diagnosed Mean Age (SD): 45 (~10) Ethnicity: Chinese 100% Female: 100% |
Tailored CBT intervention that included psychoeducation and supportive care, stress management, brief crisis counseling, relaxation, pain, distress management, and management of specific symptom spectrum; delivered every 2 weeks during treatment and every 6 weeks (for up to 18 months) after treatment completion. | Assessment only | IES | Individual | No effect of intervention on IES-intrusion and avoidance |
| Duhamel et al., 2010 |
N = 89 Type: Mixed-HSCT survivors Stage: Not reported Point in cancer Tx at baseline: 12–36 months after HSCT Mean Age (SD): 51 (~11) Ethnicity: Caucasian 83% Female: 46% |
Ten-session telephone-based CBT (T-CBT) intervention; 60-min telephone sessions delivered in 10–16 weeks. Intervention included: self- monitoring and alteration of maladaptive beliefs, guided exposure to PTSD cues. | Assessment only | PCL-C, CAPS | Individual | Intervention group experienced a reduction in PCL-C intrusion and avoidance scores, but not in numbing or hyperarousal; participants in T-CBT were less likely to be diagnosed with PTSD based on the CAPS at 12-month follow-up. |
| Johansson et al., 2008 |
N = 481 Type: Prostate, GI or breast Stage: I-IV Point in cancer Tx at baseline: Newly diagnosed Mean Age (SD): 64 (13) Ethnicity: not reported Female: 58% |
(I1) Individual support (IS) derived from CBT: relaxation techniques, identification and challenging of negative thoughts, activity scheduling and daily planning; 1–24 in-person or telephone sessions as needed; (I2) Group rehabilitation (GR) that included CBT, light exercise and relaxation; delivered in 8 weekly sessions and one booster session; (I3) IS + GR delivered successively. | Standard care | IES | Individual and/or group | No effect of IS, GR, or IS 1 GR intervention conditions on IES-intrusion and avoidance scores. |
| Larson et al., 2000 |
N = 41 Type: Breast Stage: I-IV Point in cancer Tx at baseline: Newly diagnosed Mean Age (SD): 56 (13) Ethnicity: Caucasian 98% Female: 100% |
Two-session structured presurgical intervention consisting of psycho- education, problem-solving skills training, relaxation techniques, and psychosocial support; delivered in 90-min in-person individual or small group sessions. | Standard care | IES | Individual/ group | No effect of intervention on IES-intrusion and avoidance scores. |
| Levine et al., 2005 |
N = 181 Type: Breast Stage: I-IV Point in cancer Tx at baseline: 18 months since Dx Mean Age (SD): 48 (~9) Ethnicity: Caucasian 83% Female: 100% |
Twelve-week complementary and alternative medicine (CAM) program: included meditation and imagery, yoga and movement, support group and health lectures; delivered twice weekly in 2.5-hr sessions. | Twelve-week unstructured psychoeducatio- nal support group that included communication and coping skills training, problem- solving, anxiety management, body image, sexuality, grief, and anger discussions; delivered once weekly in 1.5-hr sessions. | PLC-C | Group | Control group participants showed greater decreases in total PCL scores, as well as in PCL reexperiencing and arousal symptoms, as compared to CAM participants. |
| Manne et al., 2005 |
N = 238 Type: Breast Stage: 0-IIIA Point in cancer Tx at baseline: Surgery in last 6 months Mean Age: 50 Ethnicity: Caucasian 90% Female: 100% |
Six-session couple-focused group intervention that addressed relaxation and stress management, coping skills, problem-solving, sexuality, and communication skills; delivered in weekly 90-min sessions. | Usual care | IES | Group | No significant effect of the intervention on IES scores; marginal significance for participants who were more physically impaired. |
| Manne et al., 2007 |
N = 353 Type: Gynecological Stage: 0-IIIA Point in cancer Tx at baseline: On active Tx or >3 months post-surgery Mean Age (SD): 50 (~11) Ethnicity: Caucasian 90% Female: 100% |
Seven-session coping and communication intervention (CCI) that included cognitive restructuring, coping skills training, and behavioral tasks as homework; delivered in 60-min weekly sessions plus 1 telephone booster. | (C1) Supportive Counseling (SC) consisting of six weekly 60-min sessions plus 1 booster; (C2) Usual care | IES | Group | No effect of CCI or SC on IES-intrusion and avoidance. |
| Marcus et al., 2010 |
N = 304 Type: Breast Stage: I-IIIA Point in cancer Tx at baseline: Completed definitive treatment Age: 50% were under 50 Ethnicity: Caucasian 91% Female: 100% |
Sixteen-session structured telephone counseling program consisting of psychoeducation, progressive muscle relaxation tapes, stress management skills training and cognition and emotion focused worksheets; Delivered in 45-min sessions spaced over 12 months. | Resource directory booklet | IES-intrusion | Individual | No effect of intervention on IES-intrusion scores |
| Owen et al., 2008 |
N = 62 Type: Breast Stage: 0-III Point in cancer Tx at baseline: Varied Mean Age (SD): 52 ( ~9) Ethnicity: Caucasian 98% Female: 100% |
Twelve-week self-guided internet-based coping skills training, discussion board, and education that included dictionary of medical terminology, database of breast cancer resources, and coping advice for management of common symptoms. | Wait-list | IES | Individual/ group | No effect of intervention on IES-intrusion and avoidance scores |
| Parker et al., 2009 |
N = 159 Type: Prostate Stage: I-IV Point in cancer Tx at baseline: up to 1 month prior to surgery Mean Age (SD): 60 (~6.5) Ethnicity: Caucasian 78% Female: 0% |
Four-session presurgical stress management intervention that included relaxation skills, imaginal exposure, and problem-focused coping strategies; delivered in two 60- to 90-min sessions plus 2 brief booster sessions. | (C1) Supportive Attention: unstructured supportive counseling delivered in equivalent time period; (C2) Standard care | IES | Individual | No effect of intervention on IES-intrusion and avoidance scores as compared to either control condition. |
| Scott et al., 2004 |
N = 84 Type: Breast or Gynecological Stage: I-II Point in cancer Tx at baseline: Beginning treatment Mean Age (SD): 51 (9.8) Ethnicity: Caucasian 98% Female: 100% |
(I1) Five-session couple-coping training (CanCOPE) at couples’ home: medical education, psychoeducation, extensive coping and communication skills training (i.e., problem-solving coping, effective communication), supportive counseling, sexual counseling, some existential discussion; delivered in five 2-hr in-person sessions and two 30-min phone sessions; (I2) Patient coping training (PC): same as I1 but conducted individually in four instead of five sessions. | Medical information (MI) education with no specific psychological intervention or coping skills training, consisting of a booklet and five 15-min telephone calls with a therapist. | IES | Group | Significant effect for CanCOPE on IES-avoidance- CanCOPE women had less avoidance than PC or MI women at the end of the intervention; no effect of either intervention on IES-intrusion. |
| Stanton et al., 2005 |
N = 279 Type: Breast Stage: I-II Point in cancer Tx at baseline: Completed treatment Mean Age (SD): 58 (~11) Ethnicity: Caucasian 87% Female: 100% |
(I1) One 80-min in-person session and one 30-min phone call of psychoeducational counseling (EDU) with a cancer educator, a 60-page manual, and a 23-min videotape; The intervention addressed common cancer related concerns in four life domains and helped participants develop an approach-oriented action plan; (I2) A video tape intervention (VID) consisting of the 23-min videotape in EDU. | Standard print control including 43-page booklet from NCI | IES-R | Individual | No effect of EDU or VID interventions on IES-R-intrusion, avoidance, and hyperarousal scores. |
| Wengstrom et al., 1999 |
N = 134 Type: Breast Stage: Not reported Point in cancer Tx at baseline: Beginning radiation therapy Mean Age (range): 60 (32 to 83) Ethnicity: Caucasian 100% Female: 100% |
Seven-session structured nursing intervention consisting of education and strategies for coping with emotional reactions, body image concerns, and variety of self-care activities related to radiation therapy and its side effects; delivered in five weekly 30-min sessions during radiation and two follow-up sessions (unspecified duration). | Standard care | IES | Individual | Significant time 3 group interaction for IES-intrusion scores; no effect on IES-avoidance. |
Note. PTSD = posttraumatic stress disorder; Tx = treatment; SD = standard deviation; IES = Impact of Events Scale; CBT = cognitive behavioral therapy; PTSS = Posttraumatic Stress Scale-Self Report; IES-R = Impact of Events Scale-Revised; PCL-C = PTSD Checklist-Civilian; CAPS = Clinician-Administered PTSD Scale; NCI = National Cancer Institute.
Methodology
Measurement.
Although we allowed for the inclusion of studies using a wide range of PTSD measures, PTSD assessment was highly uniform among the studies (Table 1) with all but three using the IES (Horowitz, Wilner & Alvarez, 1979) or the IES-R (Weiss & Marmar, 1996) as a measure of traumatic stress symptoms. The three studies that did not include a version of the IES used one or more of the following as outcome measures: the PCL-C (Weathers, Litz, Herman, Huska & Keane, 1993), the CAPS (Blake et al., 1995) and the Posttraumatic Stress Scale-Self Report (PTSS; Foa, Riggs, Dancu & Rothbaum, 1993).
Control conditions.
Most control groups did not consist of an active intervention and were described as either standard/usual care, treatment-as-usual, assessment-only or a wait-list control (Allen et al., 2002; Arving et al., 2007; Beatty et al., 2010; Branstrom et al, 2010; Chan et al., 2005; DuHamel et al, 2010; Larson et al., 2000; Manne et al., 2005; Owen et al., 2005; Wengstrom et al., 1999). Some control conditions were educational, in the form of a one-time condensed seminar (Antoni et al., 2001, 2006) or the distribution of educational materials (Marcus et al., 2010). One study (Levine et al., 2005) compared two active interventions and did not include a no-treatment control group. Four studies had three study arms and compared an active intervention against supportive counseling and standard care (Manne et al. 2007; Parker et al., 2009) or included two versions of an active intervention, which were compared with each other and with an educational control group (Scott et al., 2004; Stanton et al., 2005). Finally, one study had three intervention arms and a standard care control arm (Johansson et al., 2008).
Intervention Characteristics
Modality.
Four of the interventions were group interventions (Antoni et al., 2001, Antoni et al., 2006, Branstrom et al., 2010; Levine et al., 2005) and one was a couples’ group (Manne et al., 2005). The remaining interventions were conducted primarily in an individual format, although two were designed to be conducted either individually or in a group (Johansson et al., 2008; Larson et al., 2000). Finally, one study had a couples’ condition in addition to an individually delivered version of the intervention (Scott et al., 2004).
Duration, frequency and timing.
The interventions varied in their overall duration, frequency and the point in the cancer trajectory when they were initiated (Figure 1). Some interventions were very brief, consisting of only two sessions (Larson et al., 2000; Parker et al., 2009) while others included at least 10 sessions and lasted over the course of a year (Chan et al., 2005; Marcus et al., 2010). The timing of these interventions varied, such that some targeted newly diagnosed patients (Chan et al., 2005; Johansson et al., 2008), others focused on those who recently completed treatment and were at the point of “re-entry” (Beatty et al., 2010; Branstrom et al., 2010; Marcus et al., 2010; Stanton et al., 2005), and one targeted survivors (at least 12 months post-treatment; DuHamel et al., 2010). The remainder of the studies occurred at different points between cancer diagnosis and survivorship. Two were brief pre-surgical interventions confined to the peri-surgical period (Larson et al., 2000; Parker et al., 2009), while the majority targeted individuals receiving active treatment and typically started shortly post-surgery (Antoni et al., 2001; Antoni et al.,; 2006) or at the beginning or during adjuvant treatment (Allen et al., 2002; Arving et al., 2007; Manne et al., 2005; Manne et al., 2007; Scott et al., 2004; Wengstrom et al., 1999) and lasted through treatment completion or beyond. Finally, several studies did not restrict participation based on a specific point in the cancer trajectory and included a mixed sample of participants from newly diagnosed to long-term survivors (Levine et al., 2005; Owen et al., 2005).
Figure 1.
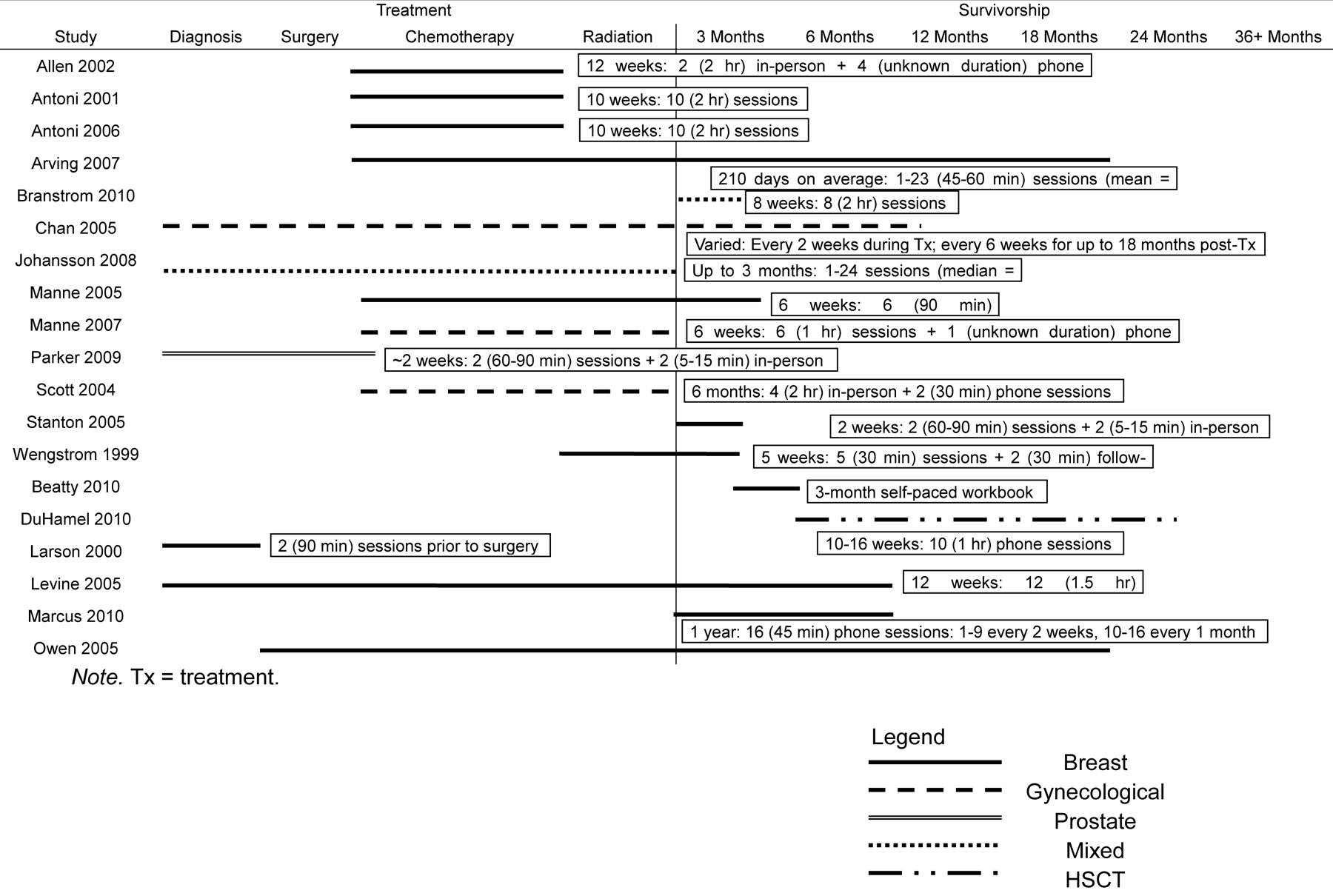
Study start point and duration throughout cancer trajectory grouped by inclusion in meta-analysis
Mode of administration.
Mode of administration was fairly homogenous with the majority of the interventions delivered in person. However, some interventions included brief phone calls as an adjunct to in-person meetings (Allen et al., 2002; Scott et al., 2004; Stanton et al., 2005; Wengstrom et al., 1999), and some were conducted entirely over the phone (DuHamel et al., 2010; Marcus et al., 2010) or internet (Owen et al., 2005). Additionally, one intervention involved the dissemination of a workbook, which patients completed at home with minimal therapist contact (Beatty et al., 2010). In contrast to these individual interventions, the group interventions had a much more uniform format; the majority included six to ten 60-, 90- or 120-minute sessions administered on a weekly basis in person.
Treatment components.
It was difficult to determine the exact content or “dose” of every intervention from the descriptions of the interventions supplied in each manuscript (or in additional references provided in the manuscripts) as descriptions varied in their level of detail and completeness. Therefore, the following represents the main CBT components included in the 19 studies reviewed. The most common component was anxiety management through various types of relaxation training, including progressive muscle relaxation, diaphragmatic breathing, guided imagery and meditation. Only six RCTs did not include some form of relaxation practice (Allen et al., 2002; Marcus et al., 2010; Owen et al., 2005, Scott et al., 2004; Stanton et al., 2005; Wengstrom et al., 1999). One intervention (Branstrom et al., 2010) tested a Mindfulness Based Stress Reduction (MBSR) program. Another was described as a strictly problem-solving intervention (Allen et al., 2002). The remaining studies combined a broad range of CBT components, such as psychoeducation, self-monitoring, coping skills training, stress management, problem-solving, assertiveness training, communication skills training, activity scheduling and homework assignments. It is important to note that only one study included imaginal and/or in vivo exposure to a past-oriented event (systematic desensitization was used in DuHamel et al., 2010), and that few explicitly indentified cognitive restructuring as a component of their intervention (Antoni 2001, 2006; DuHamel et al., 2010, Manne 2007; Marcus et al., 2010; Scott et al., 2004). Thus, the majority of studies were not explicitly described as including cognitive and exposure-based techniques with the strongest empirical support in the general PTSD literature. Common intervention components not specific to CBT were psychological support, mobilization and use of social support resources, medical information regarding cancer and its treatment, nutritional counseling, stories or vignettes from other cancer survivors, discussion of existential and spiritual concerns and encouragement of emotional expression.
Study findings
Of the 19 interventions reviewed, 68% (n = 13) did not report an effect on cancer-related traumatic stress symptoms. However, four studies did find that participants in the intervention arm experienced some reductions in intrusion, avoidance or hyperarousal symptoms, as assessed by the IES or the IES-R when compared to control participants (Antoni et al., 2006; Wengstrom et al., 1999; Scott et al., 2004; Branstrom et al., 2010). Additionally, one study found reductions in PCL-C intrusion and avoidance, but not in numbing or hyperarousal (DuHamel et al., 2010). This study also found that participants in the intervention group were less likely to be diagnosed with PTSD based on the CAPS at the 12 month follow-up. Another study observed that a CBT-based support group resulted in greater reductions in PCL-C re-experiencing and arousal, but not in avoidance subscale scores as compared with a complementary and alternative medicine intervention (Levine et al., 2005).
Effect Size Estimates
The results of the quantitative analysis further supported these findings. Six studies were not included in the meta-analytic component of this review because data could not be obtained from the manuscript or by request from the authors (Larson et al., 2000; Marcus et al., 2010; Owen et al., 2005) or because the IES was not used as an outcome measure (Beatty et al., 2010; DuHamel et al., 2010; Levine et al., 2005). The results of the ES calculations are displayed in Table 2. The ES analyses confirmed that the effects of the interventions were small and ranged from −0.138 to 0.006 for intrusion and from −0.025 to 0.048 for avoidance, where a negative number indicates an effect in favor of the intervention. Further, the overall Bayesian ES estimate indicated that the interventions with CBT components included in the meta-analytic portion of this review did not have a significant effect on either intrusion or avoidance scores [µintrusion= −0.087 (SD = 0.169), 95% CI = −0.413 to 0.258; µavoidance= 0.000 (SD = 0.170), 95% CI = −0.375 to 0.317]. Figure 2 illustrates the individual and overall ES results with 95% CIs.
Figure 2.
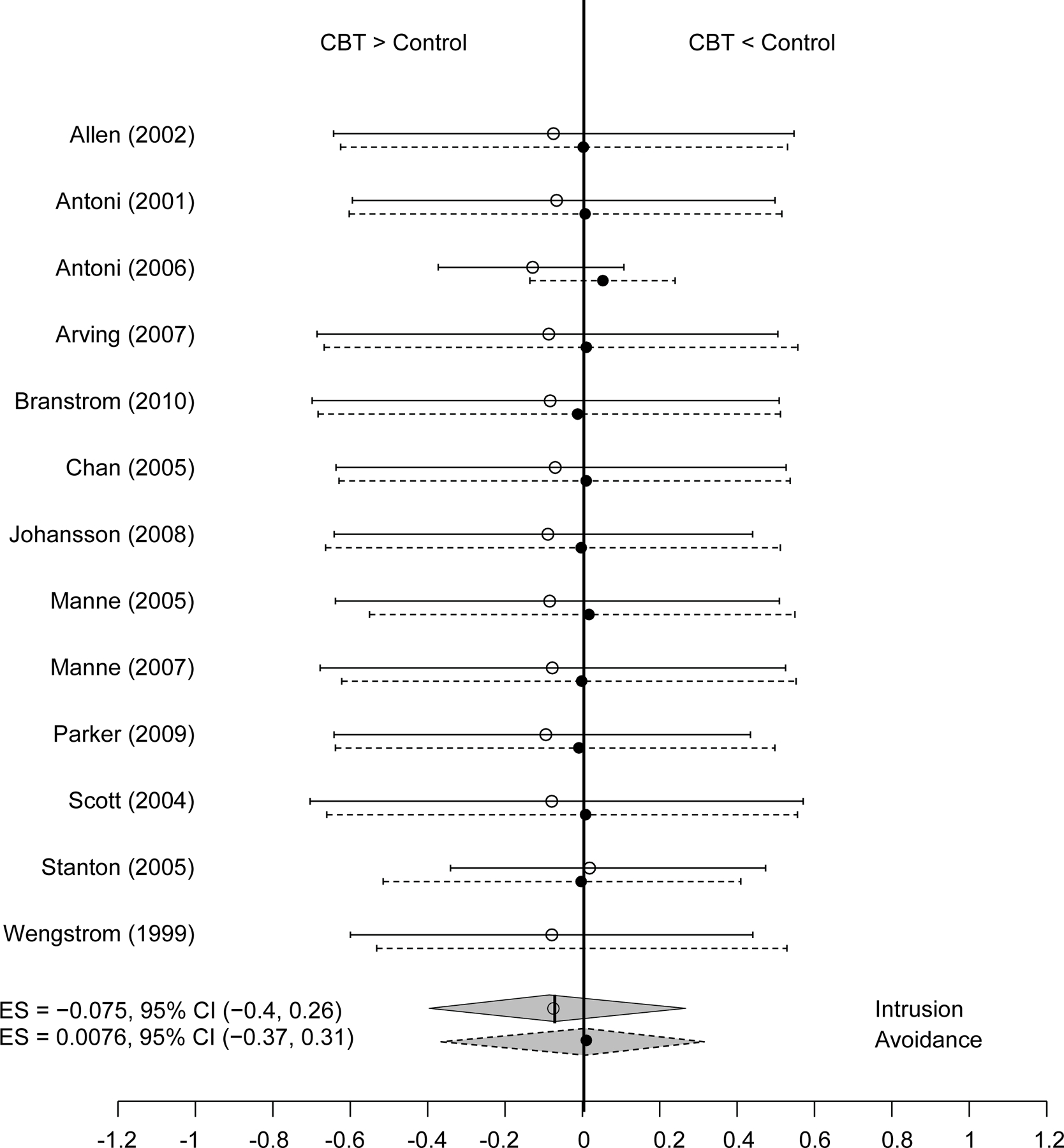
Bayesian Posterior Effect Size Estimate.
Discussion
In 2002, Kangas, Henry and Bryant highlighted the “marked need for controlled outcome studies that (a) index the relative efficacy of CBT in reducing cancer-related PTSD symptoms; (b) examine the specific components of CBT that mediate recovery; and (c) determine when is the most appropriate time in the course of an individual’s cancer experience to implement CBT components in treating cancer-related PTSD” (p. 519). The current study reviewed the empirical literature in light of the objectives above. Based on the findings of this review, it appears that researchers are in the early stages of establishing the relative efficacy of CBT interventions for the treatment of cancer-related traumatic stress symptoms, as few RCTs were specifically designed to evaluate trauma-focused interventions with distressed cancer patients and survivors. The present review revealed mixed findings, with six studies (Antoni et al., 2006; Branstrom et al, 2010; DuHamel et al, 2010; Levine et al., 2005; Scott et al., 2004; Wengstrom et al., 1999) demonstrating some reduction in traumatic stress symptoms as a result of the intervention tested and 13 studies failing to demonstrate significant changes in cancer-related traumatic stress following psychosocial treatment (Allen et al., 2002; Antoni et al., 2001; Arving et al., 2007; Beatty et al., 2010; Chan et al., 2005; Johannson Larson et al., 2000; Manne et al., 2005, 2007; Marcus et al., 2010; Owen et al., 2005; Parker et al., 2009; Stanton et al., 2005). Furthermore, no intervention successfully reduced traumatic stress symptoms across all symptom clusters, and only three interventions reported an effect on more than one cluster of symptoms (Branstrom et al., 2010; DuHamel study et al., 2010; Levine et al., 2005). This high rate of non-significant findings should not be interpreted as evidence for the ineffectiveness of interventions with CBT components for the treatment of traumatic stress and PTSD in cancer patients and survivors. Rather, the mixed results may be due to a number of study design limitations in the RCTs, such as low distress levels at study entry and the non-trauma focus of the interventions.
In this review, consideration was given to a range of study characteristics that may have influenced the results of each RCT in a potentially systematic way. Specifically, we considered the average and baseline levels of distress of the participants, whether the participants were screened for traumatic stress symptoms prior to enrollment, the specific intervention components, whether the intervention was trauma-focused or targeted general distress, the duration and intensity of the intervention, and the point of the cancer trajectory when the intervention was delivered. Examination of these study dimensions allowed us to identify limitations in the available literature that restrict conclusions about the efficacy of interventions with CBT components for cancer-related traumatic stress symptoms, and to generate suggestions for future research directions.
Interpretation of Main Findings
Baseline distress level.
After considering the above characteristics across all nineteen RCTs, it appears that there were differences in the baseline distress levels of participants between the trials reporting an effect of the intervention on traumatic symptoms and those with null findings. Five of the six studies that found an effect of the intervention on traumatic symptoms had either pre-screened their participants for distress (DuHamel et al., 2010), or included participants with relatively high levels of distress (Antoni et al., 2006; Branstrom et al., 2010; Scott et al., 2004) or the PCL-C (Levine et al., 2005). In contrast, many of the trials which failed to find a significant effect of their intervention on traumatic stress symptoms concluded that their sample included participants who were well-adjusted and could not further benefit from a psychosocial intervention, much like individuals without pain who have no need for a pain-management intervention. This finding is consistent with extant literature, including a meta-analysis of diverse psychosocial intervention studies for cancer patients, that demonstrates the moderating role of baseline distress on psychosocial treatment efficacy for a number of distress outcomes, including depression and anxiety (Schneider, Moyer, Knapp-Oliver, Sohl, S. Canella, D., & Targhetta, V., 2010; Moyer, Sohl, Knapp-Oliver, & Schneider, 2009). Thus, it is likely that some of the RCTs in this review may have failed to report an effect of the intervention due to the over-inclusion of individuals without clinically significant symptoms of cancer-specific distress. On the basis of this finding we conclude that pre-screening participants for distress is an essential methodological aspect that is largely missing in current clinical trials in the area of cancer-related traumatic stress, negatively impacting the status of research on the efficacy of CBT interventions.
Intervention components and focus.
Next, we considered the content and focus of the interventions, in order to explore whether certain components consistently produced significant improvements in cancer-related traumatic stress symptoms. Three out of six of the effective interventions included elements of cognitive restructuring (Antoni et al., 2006; DuHamel et al., 2010; Scott et al., 2004), a CBT component with established treatment efficacy in non-cancer PTSD populations. In contrast, only four of the 13 studies with non-significant findings included some form of cognitive restructuring. However, of these, two were administered to a low distress sample as reported by each study author (Antoni et al., 2001; Marcus et al., 2010), one was part of a self-administered workbook intervention with no therapist feedback (Beatty et al., 2010), and one did not specify how extensive or prominent the cognitive restructuring component was (Manne 2007). Thus, on the basis of this review, there is preliminary evidence that cognitive restructuring may offer some benefit to individuals with high levels of cancer-specific distress when administered systematically by a therapist. Further examinations of the efficacy of cognitive restructuring in cancer patients and survivors with clinically significant traumatic stress symptoms at various points of the cancer trajectory are needed to strengthen this conclusion.
With regards to the focus of the intervention, all but one of the studies that showed an effect on traumatic stress symptoms explicitly considered the experience of cancer from a trauma perspective, targeted traumatic stress symptoms, or used a measure of cancer-specific distress as a primary outcome (Antoni et al., 2006; DuHamel et al., 2010; Levine et al., 2005; Scott et al., 2004; Wengstrom et al., 1999). The only study that did not explicitly target traumatic stress reactions but found significant reductions in avoidance and hyperarousal symptoms nonetheless, was a Mindfulness-Based Stress Reduction (MBSR) program, which taught participants to develop “awareness towards mental states and processes” and to cultivate a “non-evaluative openness and acceptance towards moment-to-moment experiences” (p. 151, Branstrom et al., 2010) - skills that are likely directly relevant to managing attempts at suppressing or avoiding distressing thoughts. In contrast, the majority of the studies that did not report significant findings on traumatic stress symptoms had a very broad focus (i.e., addressing multiple aspects of adjustment to cancer) and/or targeted a primary outcome other than traumatic stress symptoms, such as depression, anxiety or quality of life. Thus, this review points to the need for additional, targeted studies that evaluate trauma-focused interventions and relevant outcomes.
Intervention timing.
Another important consideration when evaluating the efficacy of psychosocial interventions for cancer-related traumatic stress symptoms is the point at which the intervention is administered. It is possible that targeting individuals too early (i.e., before traumatic symptomatology has emerged as a persistent and disruptive problem) may result in administering potentially expensive and time consuming interventions to those who are not likely to benefit from them, resulting in poor allocation of resources. Alternatively, intervening too late may also be problematic and lead to unnecessary suffering on the part of the distressed individual who is in need of an effective and appropriate intervention. It is also possible that specific CBT components may be particularly efficacious at certain points in the cancer trajectory, while others may be contraindicated due to their potential to exacerbate distress. Therefore, having empirical data on what treatment components are appropriate at various points in the cancer trajectory is an important aspect of efficacy research that will guide future treatment guidelines in the area of cancer-related traumatic stress and PTSD.
The results of the current review offer some guidance in terms of the timing of interventions with CBT components for cancer-related traumatic stress symptoms. The RCTs reviewed can be roughly grouped into those that target patients from the time of diagnosis through active adjuvant treatment, and those that focus on patients after treatment completion into the re-entry and long-term survivorship period. Many of the interventions were administered at a point in the cancer trajectory prior to treatment completion (n = 13), while a smaller subset focused on treating individuals in the survivorship period (n = 6). This alone represents a gap in the literature and highlights the need for additional interventions that target survivors who have completed treatment, as they are more likely to be considered “post-trauma,” as well as to exhibit lingering and persisting traumatic stress symptoms that require intervention (Kangas, Henry & Bryant, 2002). Additionally, the majority of interventions targeting newly diagnosed patients or those undergoing treatment (surgery, chemotherapy or radiation) had no significant findings (n = 10), which may be a reflection of several factors. First, the overwhelming majority of these studies were not designed to ameliorate traumatic stress symptoms. Second, because intrusion and avoidance symptoms are often highest near diagnosis, during treatment and shortly after treatment completion (Levine et al., 2005), the majority of individuals who present with cancer-specific distress may experience a natural resolution of symptoms within three months of diagnosis or upon completion of treatment without the need of intervention (Kangas, Henry & Bryant, 2002). This may explain why studies at earlier points in the cancer trajectory consistently fail to show a significant difference between participants in the intervention and control conditions at follow-up. Only three interventions targeting patients prior to survivorship showed significant results, of which, one (Scott et al., 2004) had a marginal effect on IES-avoidance symptoms. Another nursing intervention (Wengstrom et al., 1999), which was administered to a low distress sample of patients undergoing radiation therapy, had a “protective” effect on intrusion symptoms at only one assessment point, such that levels of intrusive thoughts in participants in the intervention were maintained low throughout the study, while participants in the control condition experienced an increase in intrusive thoughts at week five of radiation treatment. Since this intervention was focused specifically on preparing patients for the potential physical and emotional side effects of radiation therapy, it is plausible that the intervention contained an element of imaginal exposure in the form of “explicit instructions on how simulation and treatment felt, [and] what sensations the patient might experience” (p. 765, Wengstrom et al., 1999) that could have served as a protective factor for participants in the intervention group. Finally, the only intervention administered during adjuvant treatment with an effect on intrusions that was maintained at 9 months post-intervention, was the cognitive-behavior stress management group therapy trial by Antoni and colleagues (2006). As previously mentioned, the authors attributed the success of their intervention to the level of intrusion symptoms in their sample, which was significantly higher than that of participants in their previous trial, as well than that of similar studies. Together, these findings suggest that offering interventions with CBT components during the active phase of treatment may have limited benefits for the average cancer patient in terms of reducing cancer-specific distress. If interventions are tested in this early period of the cancer trajectory, researchers may need to focus on identifying and targeting individuals at high-risk for developing PTSD symptomatology, whose symptoms are likely to persist or worsen upon adjuvant treatment completion.
In contrast, half of the trials conducted with survivors or with individuals at least 18 months post cancer diagnosis (n = 3), showed some significant results (Branstrom et al., 2010, DuHamel et al., 2010; Levine et al., 2005), suggesting that intervening later in the cancer trajectory and during the survivorship period may be a more appropriate time to target posttraumatic stress symptoms. Potential problems with the remainder of the studies that did target survivors but did not produce significant findings are the short duration of the intervention (i.e. two sessions, Stanton et al., 2005), the lack of therapist involvement (i.e., self-guided workbook intervention, Beatty et al., 2010), and the low distress levels of the participants (Marcus et al., 2010).
Effect size analyses.
The quantitative analyses we conducted on a portion of the studies in this review using the IES confirmed that the majority of trials did not produce significant findings, and that, for the few studies that did have significant results, the effect sizes were generally low. On the basis of our conservative ES estimates using data from each study’s primary analyses only (i.e. not considering post-hoc adjustments, such as sample stratification or removal of data from cases within one year of death), we cannot conclude that at this time there is evidence for the overall efficacy of interventions with CBT components for the treatment of cancer-related intrusion and avoidance symptoms. Our analyses, however, did not include studies using measures other than the IES, such as the PCL-C and the CAPS. Thus, the quantitative portion of this study may in fact underestimate the significance of existing cancer-related traumatic stress efficacy research.
Limitations
This review has several limitations. First, it is possible that relevant RCTs were not retrieved through the search strategies employed. Due to the relatively limited state of the intervention literature on cancer-related traumatic stress and PTSD and the initial difficulty in locating RCTs on the topic, a broad search strategy was chosen and retrieved articles were manually filtered. Additionally, no formal evaluation of the methodological quality of the RCTs was performed through the use of standard assessment measures. While reviewing only high quality trials is preferable, this approach was not employed due to the small number of existing RCTs. More generally, systematic reviews have received some criticism from authors who note that the practice of relying on RCTs as the definitive empirical evidence for the utility of a particular intervention downplays the value of other types of study designs, such as case studies, which can offer insight into important treatment considerations, including matching the treatment to the person (Hunt, 2012).
Future Directions and Conclusions
The results of this review highlight the need for more targeted studies that evaluate the efficacy of interventions in cancer patients and survivors at high risk for developing PTSD symptoms or those already displaying significant posttraumatic stress reactions. First, our finding that baseline distress levels likely influenced the efficacy of the reviewed interventions underscores the importance of screening participants for distress prior to enrollment. Enrolling distressed cancer patients as indicated by measures of PTSD or cancer-specific distress would parallel methods employed by intervention trials with non-cancer PTSD populations and increase the statistical power of future clinical trials. Additionally, the inclusion of clinician-administered interviews (e.g., the CAPS or the SCID) and self-report measures other than the IES would strengthen study design by improving diagnostic accuracy and increasing the relevance of study findings to the entire continuum of posttraumatic stress reactions, from elevated distress to true cases of PTSD. Second, our review revealed a paucity of studies that targeted PTSD symptoms in cancer patients and survivors by the systematic application of CBT components previously validated in non-cancer trauma populations (i.e., cognitive restructuring and imaginal/in-vivo exposure). It is important that more studies evaluate and compare the efficacy of trauma-focused cognitive restructuring and exposure techniques at different points along the cancer trajectory, as the relative efficacy of each of these components is currently unknown in cancer populations. Although a combination of imaginal and in vivo exposure has been validated as frontline treatment for PTSD symptoms in non-cancer populations, Kangas, Henry and Bryant (2002) caution that introducing exposure-based strategies during active medical treatment may burden the cancer patient by compounding traumatic stress symptoms. Thus, it is crucial that future studies examine the utility and safety of exposure techniques. Finally, it will be important for future studies to enroll participants with diverse demographic and medical characteristics as a large majority of studies in this review were conducted primarily with highly-educated, Caucasian breast cancer survivors.
This review illustrates the potential of interventions with CBT components to reduce symptoms of cancer-related traumatic stress and PTSD. However, the results also indicated that efficacy research of PTSD interventions following cancer diagnosis and treatment is in its early stages of development and identified important gaps in the literature that must be addressed if treatment guidelines for cancer-related traumatic stress and PTSD are to be established. While the majority of cancer patients and survivors do not develop PTSD, research on trauma-focused CBT interventions is critical for those who do exhibit persistent and debilitating posttraumatic stress symptoms and may offer hope to patients and their families, who may be unaware that cancer can be associated with traumatic stress and that effective treatments for these symptoms are available.
References
*References marked with an asterisk indicate study was included in the review and/or meta-analysis
- *Allen SM, Shah AC, Nezu AM, Nezu CM, Ciambrone D, Hogan J, et al. (2002). A problem-solving approach to stress reduction among younger women with breast carcinoma. Cancer, 94(12), 3089–3100. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th Ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Emery CF, Glaser R, Crespin T, & Carson W (2007). Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brian, Behavior and Immunity, 21:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Slferi SM, et al. (2001). Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology, 20(1), 20–32. [DOI] [PubMed] [Google Scholar]
- *Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, et al. (2006). Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry, 163(10), 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum A, Bedoya A, Wilkinson Hendrikson, E., J, Safren S, & O’Cleirigh C (under review). Are tailored PTSD interventions needed in HIV? The state of the science of interventions for targeting PTSD in HIV-infected adults. Manuscript submitted to Psychological Trauma: Theory, Research, Practice, and Policy. [Google Scholar]
- *Arving C, Sjoden PO, Bergh J, Hellbom M, Johansson B, Glimelius B, et al. (2007). Individual psychosocial support for breast cancer patients. Cancer Nursing, 30(3), E10–19. [DOI] [PubMed] [Google Scholar]
- *Beatty L, Oxlad M, Koczwara B, & Wade TD (2010). A randomized pilot of a self-help workbook intervention for breast cancer survivors. Support Care Cancer, 18, 1597–1603. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a clinician-administered PTSD scale. Journal of Traumatic Stress, 8, 75–90. [DOI] [PubMed] [Google Scholar]
- *Branstrom R, Kvillemo P, Brandberg Y & Moskowitz JT (2010). Self-report Mindfulness as a Mediator of Psychological Well-being in a Stress Reduction Intervention for Cancer Patients—A Randomized Study. Annals of Behavioral Medicine, 39, 151–161. [DOI] [PubMed] [Google Scholar]
- Cahill SP, & Foa EB “PTSD: Treatment efficacy and future directions.” Psychiatric Times 1 Mar. 2007: 32. Health Reference Center Academic. Web. 26 Sep. 2011., [Google Scholar]
- Cahill SP, Rothbaum BO, Resick P, & Follette V (2009). Cognitive behavior therapy for adults. In Foa EB, Keane TM, Friedman MJ & Cohen JA (Eds.), Effective treatments for PTSD (2nd ed, pp 139–222). New York: Guilford. [Google Scholar]
- Chan YM, Lee PWH, Fong DYT, Fung ASM, Wu LYF, Choi AYY, et al. (2005). Effect of individual psychological intervention in Chinese women with gynecologic malignancy: A randomized trial. Journal of Clinical Oncology, 23(22), 4913–4924. [DOI] [PubMed] [Google Scholar]
- Deimling GT, Kahana B, Bowman KF & Schaefer ML (2002). Cancer survivorship and psychological distress in later life. Psycho-oncology, 11, 479–494. [DOI] [PubMed] [Google Scholar]
- *DuHamel KN et al. (2010). Randomized Clinical Trial of Telephone-Administered Cognitive-Behavioral Therapy to Reduce Post-Traumatic Stress Disorder and Distress Symptoms After Hematopoietic Stem-Cell Transplantation. Journal of Clinical Oncology, 28 (23), 3754–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop WP, Cortina JM, Vaslow JB, & Burke MJ (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1, 170–177. [Google Scholar]
- Foa. EB, Rothman BO, Riggs DS, & Murdock TB (1991). Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. Journal of Consulting and Clinical Psychology, 59(5), 715–723. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, & Rothbaum BO (1993). Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. Journal of Traumatic Stress, 6: 459–473. [Google Scholar]
- Foa EB, Keane TM, Friedman MJ, & Cohen JA (Eds.). (2009). Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies (2nd ed.). New York: Guilford Press. [Google Scholar]
- Forbes D, Creamer M, Bisson JI, Cohen JA, Crow BE, Foa EB, … Ursano J (2010). A Guide to the guidelines for the treatment of PTSD and related conditions. Journal of Traumatic Stress, 23(5), 537–552. [DOI] [PubMed] [Google Scholar]
- Fox BH (1995). The role of psychological factors in cancer incidence and prognosis. Oncology, 9, 245–255. [PubMed] [Google Scholar]
- French-Rosas LN, Moye J, & Naik AD (2011). Improving the recognition and treatment of cancer-related posttraumatic stress disorder. Journal of Psychiatric Practice, 17(4), 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, & Brewin CR (2011). Considering PTSD for DSM-5. Depression and Anxiety, 28, 750–769. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, & Rubin DB Bayesian data analysis. New York: Chapman & Hall. [Google Scholar]
- Glass GV, & Hopkins KD (1984). Statistical methods in education and psychology (2nd ed.). Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Gurevich M, Devins GM, & Rodin GM (2002). Stress response syndromes and cancer: Conceptual and assessment issues. Psychosomatics, 43(4), 259 – 279. [DOI] [PubMed] [Google Scholar]
- Guthrie E (2006). Psychological treatments in liaison psychiatry: the evidence base. Clinical Medicine, 6, 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MJ, Wilner NR, & Alvarez W (1979). Impact of Events Scale: a measure of subjective stress. Psychosomatic Medicine, 41, 209–218. [DOI] [PubMed] [Google Scholar]
- Hunt N (2012). Methodological limitations of the RCT in determining the efficacy of psychological therapy for trauma. Journal of Traumatic Stress, 1: 1–3. [Google Scholar]
- Jackman S (2009). Bayesian analysis for the social sciences. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- Jacobsen PB, & Jim HS (2008). Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA: A Cancer Journal for Clinicians, 58, 214–230. [DOI] [PubMed] [Google Scholar]
- *Johansson B, Brandberg Y, Hellbom M, Persson C, Petersson LM, Berglund G, et al. (2008). Health-related quality of life and distress in cancer patients: Results from a large randomized study. British Journal of Cancer, 99, 1975–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas M, Bovbjerg DH, & Montgomery GH (2008). Cancer-related fatigue: A systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychological Bulletin. 134(5): 700–741. [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry JL, & Bryant RA (2002). Psychosocial perspectives on post-traumatic theories of post-traumatic stress. Clinical Psychology Review, 15, 515–544. [Google Scholar]
- Kangas M, Henry JL, & Bryant RA (2005a). The course of psychological disorders in the 1st year after cancer diagnosis. Journal of Consulting and Clinical Psychology, 73(4), 763–8. [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry JL, & Bryant RA (2005b). The relationship between acute stress disorder and posttraumatic stress disorder following cancer. Journal of Consulting and Clinical Psychology, 73(2), 360–364. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Anderson J, Cell DF, Tross S, Zuckerman E, Cherin E, et al. (1992). Comparison of psychosocial adaptation and sexual function of survivors of advanced Hodgkin disease treated by MOPP, ABVD, or MOPP alternating with ABVD. Cancer, 70, 2508–2516. [DOI] [PubMed] [Google Scholar]
- *Larson MR, Duberstein PR, Talbot NL, Galdwell C, & Moynihan JA (2000). A presurgical psychosocial intervention for breast cancer patients: Psychological distress and the immune response. Journal of Psychosomatic Research, 48, 187–194. [DOI] [PubMed] [Google Scholar]
- *Levine EG, Eckhardt J, & Targ E (2005). Change in post-traumatic symptoms following psychosocial treatment for breast cancer. Psycho-Oncology, 14, 618–635. [DOI] [PubMed] [Google Scholar]
- *Manne SL, Winkel G, Grana G, Ross S, Ostroff JS, Fox K, et al. (2005). Couple-focused group intervention for women with early stage breast cancer. Journal of Consulting and Clinical Psychology, 4, 634–646. [DOI] [PubMed] [Google Scholar]
- *Manne SL, Rubin S, Edelson M, Rosenblum N, Bergman C, Hernandez E, et al. (2007). Coping and communication-enhancing intervention versus supportive counseling for women diagnosed with gynecological cancers. Journal of Consulting and Clinical Psychology, 4, 615–628. [DOI] [PubMed] [Google Scholar]
- Manne SL, & Andrykowski MA (2006). Are psychological interventions effective and accepted by cancer patients? II. Using empirically supported therapy guidelines to decide. Annals of Behavioral Medicine, 32(2), 98–103. [DOI] [PubMed] [Google Scholar]
- *Marcus AC, Garrett KM, Cella D, Wenzel L, Brady MJ, Fairclough D, et al. (2009). Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors? Psycho-Oncology, 19, 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks I, Lovell K, Noshirvani H, Livanou M, & Thrasher S (1998). Treatment of posttraumatic stress disorder by exposure and/or cognitive restructuring: A controlled study. Archives of General Psychiatry, 55, 317 – 325. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Redd WH, Rini CM, Burkhalter JE, & DuHamel KN (2009). Physical, psychological and social sequelae following hematopoietic stem cell transplantation: A review of the literature. Psychooncology, 18(2), 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer A, Sohl SJ, Knapp-Oliver SK, & Schneider S (2009). Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients. General and Supportive Care, 35(5), 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for PTSD, (n.d.). List of PTSD screening instruments. Retrieved November 9, 2009, from http://www.ptsd.va.gov/professional/pages/assessments/list-screening-instruments.asp
- Newell SA, Sanson-Fisher RW, & Savolainen NJ (2002). Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. Journal of the National Cacner Institute, 94(8), 558–579. [DOI] [PubMed] [Google Scholar]
- Osborn RL, Demoncada AC, & Feuerstein M (2006). Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta analyses. The International Journal of Psychiatry in Medicine, 36(1), 13–34. [DOI] [PubMed] [Google Scholar]
- *Owen JE, Klapow JC, Roth DL, Shuster JL, Bellis J, Meredith R, et al. (2005). Randomized pilot of a self-guided internet coping group for women with early-stage breast cancer. Annals of Behavioral Medicine, 30(1), 54–64. [DOI] [PubMed] [Google Scholar]
- *Parker PA, Pettaway CA, Babaian RJ, Pisters LL, Miles B, Fortier A, et al. (2009). The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. Journal of Clinical Oncology, 27(19), 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R (1991). Meta-analytic procedures for social research. Newbury Park, CA: Sage. [Google Scholar]
- Rothbaum BO, Foa EB, Riggs DS, Murdock T, & Walsh W (1992). A prospective examination of post-traumatic stress disorder in rape victims. Journal of Traumatic Stress, 5, 455–475. [Google Scholar]
- Rubin DB (1981). Estimation in parallel randomized experiments. Journal of Educational Statistics, 6(4), 377–401. [Google Scholar]
- Safren SA, O’Cleirigh C, Tan JY, Raminani SR, Reilly LC, Otto MW & Mayer KH (2009). A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology, 28(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Moyer A, Knapp-Oliver S, Sohl S Canella D, & Targhetta V (2010). Pre-intervention distress moderates the efficacy of psychosocial treatment for cancer patients: a meta-analysis. Journal of Behavioral Medicine, 33(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Scott JL, Halford WK, & Ward BG (2004). United we stand? The effects of a couple-coping intervention on adjustment to early stage breast or gynecological cancer. Journal of Consulting and Clinical Psychology, 72(6), 1122–1135. [DOI] [PubMed] [Google Scholar]
- Smith MY, Redd WH, Peyser C, & Vogl D (1999). Post-traumatic stress disorder in cancer: A review. Psycho-Oncology, 8, 521–537. [DOI] [PubMed] [Google Scholar]
- *Stanton AL, Ganz PA, Kwan L, Meyerowitz BE, Bower JE, Krupnick JL, et al. (2005). Outcomes from the moving beyond cancer psychoeducational, randomized, controlled trial with breast cancer patients. Journal of Clinical Oncology, 23(25), 6009–6018. [DOI] [PubMed] [Google Scholar]
- Sturtz S, Ligges U, & Gelman A (2005). R2WinBUGS: A package for running WinBUGS from R. Journal of Statistical Software, 12(3), 1–16. [Google Scholar]
- Sutton AJ, & Abrams KR (2001). Bayesian methods in meta-analysis and evidence synthesis. Statistical Methods in Medical Research, 10(4), 277–303. [DOI] [PubMed] [Google Scholar]
- Tatrow K & Montgomery GH (2006). Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. Journal of Behavioral Medicine, 29, 17–27. [DOI] [PubMed] [Google Scholar]
- Targ EF, & Levine EG (2002). The efficacy of a mind-body-spirit group for women with breast cancer: a randomized controlled trial. General Hospital Psychiatry, 24, 238–248. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs, U.S. Department of Defense. (2010). VA/DoD clinical practice guideline for the management of post-traumatic stress. Version 2. Retrieved November 15, 2011, from http://www.healthquality.va.gov/ptsd/PTSD-FULL-2010a.pdf.
- Weathers F, Litz B, Herman D, Huska J, & Keane T (October 1993). The PTSD Checklist (PCL): Reliability, Validity, and Diagnostic Utility. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies, San Antonio, TX. [Google Scholar]
- Weiss DS, & Marmar CR (1996). The Impact of Event Scale - Revised. In Wilson J & Keane TM (Eds.), Assessing psychological trauma and PTSD (pp. 399–411). New York: Guilford. [Google Scholar]
- *Wengstrom Y, Haggmark C, Strander H, & Forsberg C (1999). Effects of a nursing intervention on subjective distress, side effects and quality of life of breast cancer patients receiving curative radiation therapy. Acta Oncologica, 38(6), 763–770. [DOI] [PubMed] [Google Scholar]


