Abstract
Background
Despite longstanding epidemiologic data on the association between increased serum triglycerides and cardiovascular events, the exact level at which risk begins to rise is unclear. The Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension has conceived a protocol aimed at searching for the prognostic cutoff value of triglycerides in predicting cardiovascular events in a large regional‐based Italian cohort.
Methods and Results
Among 14 189 subjects aged 18 to 95 years followed‐up for 11.2 (5.3–13.2) years, the prognostic cutoff value of triglycerides, able to discriminate combined cardiovascular events, was identified by means of receiver operating characteristic curve. The conventional (150 mg/dL) and the prognostic cutoff values of triglycerides were used as independent predictors in separate multivariable Cox regression models adjusted for age, sex, body mass index, total and high‐density lipoprotein cholesterol, serum uric acid, arterial hypertension, diabetes, chronic renal disease, smoking habit, and use of antihypertensive and lipid‐lowering drugs. During 139 375 person‐years of follow‐up, 1601 participants experienced cardiovascular events. Receiver operating characteristic curve showed that 89 mg/dL (95% CI, 75.8–103.3, sensitivity 76.6, specificity 34.1, P<0.0001) was the prognostic cutoff value for cardiovascular events. Both cutoff values of triglycerides, the conventional and the newly identified, were accepted as multivariate predictors in separate Cox analyses, the hazard ratios being 1.211 (95% CI, 1.063–1.378, P=0.004) and 1.150 (95% CI, 1.021–1.295, P=0.02), respectively.
Conclusions
Lower (89 mg/dL) than conventional (150 mg/dL) prognostic cutoff value of triglycerides for cardiovascular events does exist and is associated with increased cardiovascular risk in an Italian cohort.
Keywords: cardiovascular disease, cutoff value, hypertriglyceridemia, mortality, triglyceride
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Primary Prevention, Prognosis
Nonstandard Abbreviations and Acronyms
- URRAH
Uric Acid Right for Heart Health
Clinical Perspective.
What Is New?
Evidence indicates that elevated triglyceride levels are related to cardiovascular events and mortality. However, the exact level at which risk begins to increase is unclear.
In a large cohort of European subjects, a prognostic cutoff value of triglycerides lower (89 mg/dL) than the conventional one (150 mg/dL) was identified.
What Are the Clinical Implications?
Triglyceride measurement must be considered an important part of the routine evaluation to manage cardiovascular risk.
In primary prevention, subjects with triglycerides >89 mg/dL should be carefully observed to prevent possible cardiovascular events.
The global burden of dyslipidemia has increased over the past 30 years, with elevated plasma low‐density lipoprotein (LDL) cholesterol levels being the eighth most important risk factor for death in 2019. 1 In high‐income countries (mostly in Europe and North America), levels of LDL have been in steady decline in response to improvements in lifestyle and an increase in the use of lipid‐lowering drugs. 2 , 3 Based on risk stratification, LDL cholesterol lowering is addressed aggressively by statin and by nonstatin therapies (ezetimibe, inhibitors of proprotein convertase subtilisin/kexin type 9, bempedoic acid, evinacumab, and inclisiran) today. 4 , 5 Despite reduction in cardiovascular mortality over the past 2 decades, the number of deaths remains high and a residual risk persists. 6 This has compelled an interest in individuals with residually elevated triglyceride, who have higher concentrations of atherogenic cholesterol carried by circulating triglyceride‐rich lipoproteins. 7 , 8 , 9 , 10 It became more and more clear that if LDL is well controlled and non‐high‐density lipoprotein (HDL) cholesterol is not well controlled, the culprit is triglyceride‐rich lipoprotein, which is highly atherogenic. 9 , 10
The interest on the association between elevated triglycerides and cardiovascular events has fluctuated over the past many years, driven by changes in the evidence base that suggests that these either cause atherosclerotic cardiovascular disease or simply represent innocent bystanders. 8 Mendelian randomization studies have provided causal evidence for the role of triglyceride‐mediated pathways in coronary heart disease incidence. 11 In the large meta‐analysis from the Emerging Risk Factors Collaboration study, comprising 302 430 people without an initial vascular disease compiled from 68 long‐term prospective studies of Europe and North America, a total of 12 785 cases of coronary heart disease were recorded from a total of 2.79 million person‐years of follow‐up, showing a hazard ratio (HR) for nonfatal myocardial infarction and coronary heart disease death for triglycerides of 1.37 (95% CI, 1.31–1.42) after adjustment for nonlipid risk factors. 12 Raposeiras‐Roubin et al showed in a prospective cohort study including 3754 middle‐aged individuals with low to moderate cardiovascular risk that triglyceride levels ≥150 mg/dL were associated with subclinical atherosclerosis and vascular inflammation, even in participants with normal LDL‐C levels. 13
Despite epidemiologic data demonstrating the association between elevations in serum triglycerides and cardiovascular disease, 14 the exact level at which risk begins to increase is unclear. The first lipid guidelines for cardiovascular prevention defined elevated triglycerides as >250 mg/dL. 15 Since then, clinical trials evaluating the impact of triglyceride‐lowering therapies have used a cutoff around 150 mg/dL. 16 Therefore, in the recent European Society of Cardiology guidelines, the treatment target and goal on cardiovascular prevention indicates the conventional cutoff of 150 mg/dL. 4 However, specific prognostic cutoff value of triglycerides around which the rise of incident cardiovascular events associated with triglyceride changes appears has not been precisely evaluated in a European cohort. The Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension has conceived and designed an ad hoc protocol aimed at searching for prognostic cutoff values of triglycerides in predicting cardiovascular events in a large regional‐based Italian cohort of men and women.
Methods
Database and Study Protocol
The database called URRAH (Uric Acid Right for Heart Health) involves data on subjects aged 18 to 95 years collected on a regional community basis from all of Italy with a median follow‐up period of 11.2 years (interquartile range from 5.3–13.2 years) up to July 31, 2017. The study protocol has been previously extensively described, 17 , 18 , 19 and the Strengthening the Reporting of Observational Studies in Epidemiology cohort checklist was used to write the article. 20 The data that support the findings of this study are available from the corresponding author upon reasonable request. In brief, a nationwide Italian database was built by collecting data on subjects from representative cohorts having serum uric acid measurement and complete information about several variables, including outcomes. In the present analyses, 14 189 subjects were considered. For all subjects, a standardized set of items was recorded, including demographics; anthropometric measures; metabolic parameters; smoking habit; systolic and diastolic arterial blood pressure; renal function; history of cardiovascular, renal, and brain disease; concomitant treatments; and outcomes. Hypertension was defined by the presence of at least 2 blood pressure recordings ≥140 or ≥90 mm Hg or treatment with antihypertensive medications. Diabetes was defined if blood glucose was ≥126 mg/dL at fast or ≥200 mg/dL 2 hours after 75 g oral glucose load or if glycated hemoglobin was ≥48 mmol/mol. Kidney function was evaluated through estimation of the glomerular filtration rate, using a standardized serum creatinine assay and according to the Chronic Kidney Disease Epidemiology Collaboration equation. 21 Chronic kidney disease was defined for estimated glomerular filtration rate values <60 mL/min per 1.73 m2. Procedures for taking and preparing blood specimens and laboratory analysis were standardized. Blood specimens were taken by venipuncture after an overnight fast. Specimens were placed in edetic acid tubes in an ice bath. Plasma was then separated in a refrigerated centrifuge at 4 °C within 2 hours after collection; separated plasma was transferred into cryovials and frozen for later measurement of lipid concentration.
Ethics
The study data were collected routinely or ad hoc in previously authorized studies. Subjects underwent no extra tests or interventions, and there was no impact on subjects' care or outcome. The study was performed according to the Declaration of Helsinki for Human Research (41st World Medical Assembly, 1990). The processing of the patients' personal data collected in this study complies with the European Directive on the Privacy of Data. All data to be collected, stored and processed are anonymized, and all study‐related documents are retained in a secure location. No personal information is stored on local personal computers. Approval was sought from the Ethical Committee of the Coordinating Center at the Division of Internal Medicine of the University of Bologna (No. 77/2018/Oss/AOUBo). Informed consent was obtained from all subjects at recruitment.
Outcome
According to the study protocol, 18 incident events due to acute myocardial infarction, angina pectoris, heart failure, stroke, transient ischemic attack, and hypertensive complications were taken into consideration during the follow‐up (see Table S1 for International Classification of Diseases, Tenth Revision (ICD‐10) codes). Events were double‐checked with hospital and physicians' files.
Statistical Analysis
General Description
The SAS package version 9.4 (SAS Institute, Cary, NC) was used for statistical analysis. To calculate the sample size for the comparison of the area under receiver operating characteristic (ROC) curve (AUC) for 20‐year follow‐up based on the AUC of 0.576 as observed in other studies 22 with a null hypothesis of 0.5, we considered α=0.05, power (1‐β)=0.80 and a ratio of sample sizes in positive/negative groups of 1:8 (11% of cardiovascular disease [CVD] events). The Kolmogorov–Smirnov test was used to determine if all variables were normally distributed. Continuous variables were expressed as mean ± SD and compared among classes or categories by the analysis of covariance adjusted for proper confounders and followed by the Bonferroni's post hoc test. Categorical variables were compared by means of the Pearson χ2 test. The null hypothesis was rejected for values of P<0.05.
Preliminary Cox Analysis
Multivariable Cox proportional hazards regression models, having all combined cardiovascular events (fatal+nonfatal) as dependent variable and adjusted for plausible confounders (age, sex, body mass index, serum uric acid, serum HDL‐cholesterol, serum non‐HDL cholesterol, arterial hypertension, diabetes, chronic renal disease, smoking habit, alcohol consumption, and use of lipid‐lowering drugs), were used to search for an association between triglycerides as a continuous variable and cardiovascular events. We tested the interactions of triglycerides with sex, diabetes, arterial hypertension, and ethanol intake by incorporating corresponding interaction terms in the analysis. HRs with 95% CIs were produced. The null hypothesis was rejected for values of P<0.05.
Univariate Prognostic Cutoff Values
The ROC curves method was used to search for prognostic cutoff of triglycerides for cardiovascular events in the whole database. The De Long et al method 23 was used. Ratio of cases in the positive group (prevalence), sensitivity, and specificity were calculated. ROC curve was generated in the whole database, and a prognostic cutoff value was identified as the curve point nearest to 100% of axis of the ordinates. 24 In practical terms, this was made by identifying the triglycerides value associated to the highest values of the sum sensitivity+specificity. Youden's index 25 defined for all points of ROC curves was used as a criterion for selecting the optimum cutoff. The cutoff point identified is the value corresponding with maximum of the Youden index J=max[Sei+SPi−1], where Sei and SPi are the sensitivity and specificity over all possible threshold values. The AUC was also shown for each ROC curves analysis. 26 The significance of AUC is tested using the Wald test statistic (Z statistic). In sensitivity analysis, we also validated the ROC curves in the survival models, 27 namely the integrated time‐dependent AUC and the designated ROC curves, and the AUC at 15 and 20 years of follow‐up.
Validation of the Conventional and Prognostic Cutoff Values and HR of Being Over Cutoff
The conventional (≥150 mg/dl) and the prognostic (identified by mean of the ROC curve) cutoff values of triglycerides were used as independent variables in separate multivariable Cox proportional hazards regression models adjusted for the confounders already identified, having combined cardiovascular events as time‐to‐event dependent variable in the whole database. A cutoff value identified via the ROC curves method was considered as valid if accepted in the model being the null hypothesis rejected, otherwise it was considered a false cutoff. The corresponding HRs with 95% CI were obtained. The conventional (≥150 mg/dL) and prognostic validated cutoff values were used in the whole database to stratify combined cardiovascular events in descriptive analysis and for generating outcome curves according to the Kaplan–Meier nonparametric estimator of limit product. Log‐rank tests were used to assess differences between curves.
Results
Descriptive Statistics
The general characteristics of the 14 189 subjects are shown in Table 1, also showing men and women separately. Very few participants (3.8%) were on lipid‐lowering therapy. Triglyceride was nonnormally distributed in the whole database with a median triglyceride level of 110 (25th–75th percentile 80–154) (Figure 1).
Table 1.
General Characteristics of the Study Participants Also Showing Sex Stratification
| Variables | Whole database (n=14 189) | Female sex (n=7912) | Male sex (n=6277) | P values between sexes |
|---|---|---|---|---|
| Triglycerides, mg/dL | 128.9 (77.5) | 125.0 (71.8) | 133.8 (84.0) | 0.008 |
| Age, y | 59.1 (15.3) | 59.3 (15.2) | 58.8 (14.8) | 0.003 |
| Body mass index, kg/m2 | 26.7 (4.3) | 26.7 (4.6) | 26.6 (3.9) | 0.06 |
| Waist circumference, cm | 88.9 (12.5) | 84.7 (12.4) | 93.9 (10.7) | <0.0001 |
| Systolic BP, mm Hg | 144.3 (24.6) | 144.6 (25.5) | 144.1 (23.4) | 0.24 |
| Diastolic BP, mm Hg | 84.6 (12.7) | 84.6 (13.1) | 84.7 (12.2) | 0.58 |
| Heart rate, bpm | 71.2 (11.4) | 72.3 (10.8) | 69.8 (11.9) | <0.0001 |
| Serum creatinine, mg/dL | 0.93 (0.23) | 0.89 (0.23) | 0.98 (0.21) | <0.0001 |
| Serum glucose, mg/dL | 99.2 (25.6) | 99.1 (27.1) | 99.3 (23.5) | 0.67 |
| Serum uric acid, mg/dL | 4.96 (1.41) | 4.69 (1.33) | 5.30 (1.42) | <0.0001 |
| Total cholesterol, mg/dL | 216.3 (38.7) | 218.1 (38.6) | 213.9 (38.7) | <0.0001 |
| HDL cholesterol, mg/dL | 53.4 (15.0) | 55.4 (15.4) | 50.7 (14.0) | <0.0001 |
| Non‐HDL cholesterol, mg/dL | 162.9 (39.2) | 162.7 (39.0) | 163.2 (39.4) | 0.37 |
| Smoking habit (yes, %) | 35.2 | 24.2 | 49.1 | <0.0001 |
| Ethanol intake (yes, %) | 62.4 | 58.3 | 67.6 | <0.0001 |
| Diabetes (yes, %) | 10.0 | 10.2 | 9.7 | 0.38 |
| Hypertension (yes, %) | 65.4 | 65.6 | 65.1 | 0.50 |
| Chronic kidney disease (yes, %) | 21.1 | 22.4 | 19.5 | <0.0001 |
| Antihypertensive use (yes, %) | 35.1 | 35.4 | 34.8 | 0.51 |
| Lipid‐lowering drugs (yes, %) | 3.8 | 3.7 | 4.0 | 0.33 |
Continuous variables are expressed as mean (SD). Categorical variables are in %. BP indicates arterial blood pressure; and HDL, high‐density‐lipoprotein.
Figure 1. Distribution of baseline serum triglycerides in the whole database.
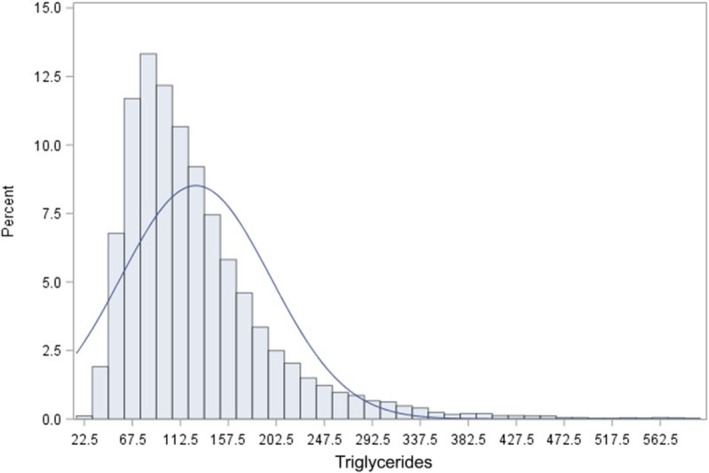
During 139 375 person‐years of follow‐up, 1601 participants experienced cardiovascular events (11.4 per 1000 age‐adjusted person‐years), 747 men (12.1 per 1000 age‐adjusted person‐years) and 854 women (11.0 per 1000 age‐adjusted person‐years).
Power analysis showed that the minimum sample size required (positive and negative groups together) was 1566 subjects. Therefore, the number of subjects in the database (n=14 189) represented a sample largely sufficient to avoid β error.
Multivariable Analysis
Preliminary Cox models having combined cerebrovascular events as dependent variable showed that, in the whole cohort, log transformed triglyceride as a continuous variable was a significant predictor of cardiovascular events (HR, 1.173 [95% CI, 1.035–1.330], P=0.01) with significant confounding factors age, body mass index, serum HDL cholesterol, serum uric acid, diabetes, arterial hypertension, alcohol consumption, and use of lipid‐lowering drugs (Table 2). Four interaction terms were tested (triglyceride × sex, triglyceride × diabetes, triglyceride × arterial hypertension, triglyceride × ethanol intake). Only triglycerides × diabetes was significant when included in the model (P=0.017; HR, 1.329 [95% CI, 1.051–1.680]).
Table 2.
Cox Model for Combined Cardiovascular Events Using Serum Triglycerides as a Continuous Independent Variable in the Whole Cohort (n=14 189)
| Independent variables | HR | 95% CI | P value |
|---|---|---|---|
| Serum triglycerides, mg/dL | 1.173 | 1.035–1.330 | 0.01 |
| Age, y | 1.061 | 1.056–1.067 | <0.0001 |
| Sex (1=men, 0=women) | 1.025 | 0.919–1.144 | 0.66 |
| Body mass index, kg/m2 | 0.970 | 0.957–0.982 | <0.0001 |
| HDL cholesterol, mg/dL | 0.992 | 0.989–0.996 | <0.0001 |
| Non‐HDL cholesterol, mg/dL | 0.999 | 0.998–1.001 | 0.44 |
| Serum uric acid, mg/dL | 2.047 | 1.681–2.492 | <0.0001 |
| Diabetes (1=yes, 0=no) | 1.908 | 1.689–2.156 | <0.0001 |
| Hypertension (1=yes, 0=no) | 1.469 | 1.281–1.684 | <0.0001 |
| Chronic kidney disease (1=yes, 0=no) | 0.910 | 0.808–1.025 | 0.12 |
| Smoking (1=yes, 0=no) | 1.051 | 0.936–1.180 | 0.40 |
| Ethanol (1=yes, 0=no) | 1.380 | 1.216–1.566 | <0.0001 |
| Lipid‐lowering drugs (1=yes, 0=no) | 0.621 | 0.477–0.809 | <0.001 |
HDL indicates high‐density‐lipoprotein; and HR, hazard ratios.
Search for Prognostic Cutoff Value of Triglycerides
ROC curve furnished plausible univariate cutoff value of triglyceride (>89 mg/dL [95% CI, 75.8–103.3], sensitivity 76.6, specificity 34.1) as prognostic cutoff value for cardiovascular events. When the conventional triglyceride value of 150 mg/dL is considered, the sensitivity and specificity parameters are 33.0 and 74.3, respectively (Figure 2 and Table 3). In sensitivity analysis, the integrated time‐dependent AUC was 0.569 (95% CI, 0.561–0.578; Z statistic 9.521, P<0.0001 for null hypothesis, AUC=0.50), and each time‐dependent AUC was 0.574 for 15 years of follow‐up and 0.601 for 20 years.
Figure 2. ROC curves of combined cardiovascular events.
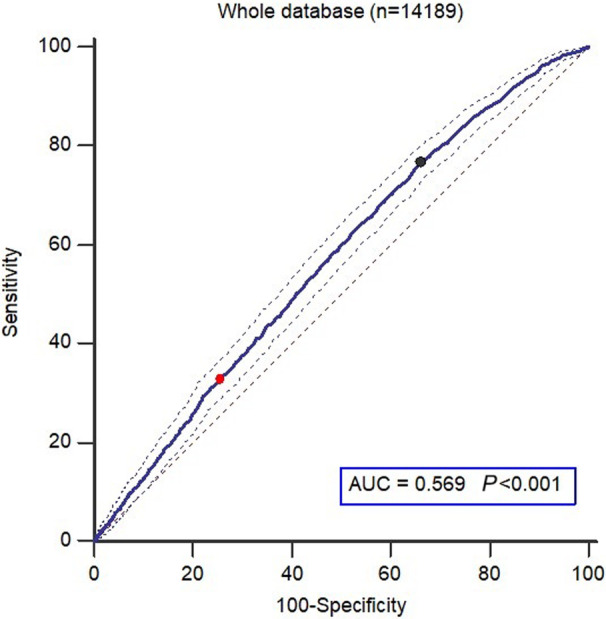
95% CIs are shown (thin lines). The black point corresponds to the criterion >89 mg/dL (sensitivity 76.6, specificity 34.1) and the red point corresponds to the criterion >150 mg/dL (sensitivity 33.0, specificity 74.3). AUC indicates area under the curve; and ROC, receiver‐operator‐characteristic; P, P value of the null hypothesis AUC=0.5.
Table 3.
ROC Curve Parameters of the Cutoff Value for Combined Cardiovascular Events in a Regional Community‐Based Cohort of 14 189 Subjects
| Cutoff | >89 mg/dL | >150 mg/dL |
|---|---|---|
| Youden index (CI) | 0.1064 (0.0772–0.1233) | … |
| Sensitivity% (CI) | 76.6 (74.4–78.6) | 33.0 (30.7–35.4) |
| Specificity% (CI) | 34.1 (33.2–34.9) | 74,29 (73.5–75.0) |
Validation of Conventional and Prognostic Cutoff Values and HRs of Being Over Cutoff
The conventional (≥150 mg/dL) and the identified by the ROC curve (≥89 mg/dL) cutoff values of triglyceride were both accepted as predictors in separate multivariable Cox regression models, adjusted for the confounders already identified, demonstrating that being over the cutoff values led to higher risk with the HR 1.150 (95% CI, 1.021–1.295, P=0.02) and HR 1.211 (95% CI, 1.063–1.378, P=0.004), respectively (Table 4 and 5). Kaplan–Meier curves after stratification according to cutoff values of triglyceride are shown in Figure 3. The curves of subjects having triglyceride ≤cutoff and triglyceride >cutoff were clearly separate both for conventional (150 mg/dL) and prognostic (89 mg/dL) cutoff values.
Table 4.
Hazard Ratios of the Variable “Over Cutoff Value of Serum Triglycerides (≥150 mg/dL)” for Combined Cardiovascular Events in the Whole Database
| Independent variables | HR | 95% CI | P value |
|---|---|---|---|
| Specific cutoff of triglycerides (≥150 mg/dL) | 1.150 | 1.021–1.295 | 0.02 |
| Age, y | 1.062 | 1.057–1.068 | <0.0001 |
| Sex (1=men, 0=women) | 1.028 | 0.921–1.146 | 0.63 |
| Body mass index, kg/m2 | 0.968 | 0.956–0.981 | <0.0001 |
| HDL cholesterol, mg/dL | 0.992 | 0.989–0.996 | <0.0001 |
| Non‐HDL cholesterol, mg/dL | 0.999 | 0.998–1.001 | 0.72 |
| Serum uric acid, mg/dL | 2.005 | 1.650–2.436 | <0.0001 |
| Diabetes (1=yes, 0=no) | 1.935 | 1.727–2.183 | <0.0001 |
| Hypertension (1=yes, 0=no) | 1.317 | 1.141–1.523 | 0.0002 |
| Chronic kidney disease (1=yes, 0=no) | 0.838 | 0.740–0.949 | 0.005 |
| Smoking (1=yes, 0=no) | 1.047 | 0.936–1.175 | 0.44 |
| Ethanol (1=yes, 0=no) | 1.444 | 1.270–1.638 | <0.0001 |
| Statin use (1=yes, 0=no) | 0.598 | 0.458–0.779 | <0.0001 |
HDL indicates high‐density‐lipoprotein; and HR, hazard ratios.
Table 5.
Hazard Ratios of the Variable “Over Cutoff Value of Serum Triglycerides (≥89 mg/dL)” for Combined Cardiovascular Events in the Whole Database
| Independent variables | HR | 95% CI | P value |
|---|---|---|---|
| Specific cutoff of triglycerides (≥89 mg/dL) | 1.211 | 1.063–1378 | 0.004 |
| Age, y | 1.062 | 1.057–1.068 | <0.0001 |
| Sex (1=men, 0=women) | 1.028 | 0.921–1.146 | 0.62 |
| Body mass index, kg/m2 | 0.968 | 0.956–0.981 | <0.0001 |
| HDL cholesterol, mg/dL | 0.992 | 0.989–0.996 | <0.0001 |
| Non‐HDL cholesterol, mg/dL | 0.999 | 0.998–1.001 | 0.54 |
| Serum uric acid, mg/dL | 1.993 | 1.639–2.423 | <0.0001 |
| Diabetes (1=yes, 0=no) | 1.942 | 1.719–2.193 | <0.0001 |
| Hypertension (1=yes, 0=no) | 1.318 | 1.141–1.523 | 0.0002 |
| Chronic kidney disease (1=yes, 0=no) | 0.838 | 0.740–0.949 | 0.005 |
| Smoking (1=yes, 0=no) | 1.051 | 0.936–1.180 | 0.40 |
| Ethanol (1=yes, 0=no) | 1.442 | 1.270–1.638 | <0.0001 |
| Statin use (1=yes, 0=no) | 0.595 | 0.456–0.777 | <0.0001 |
HDL indicates high‐density‐lipoprotein; and HR, hazard ratios.
Figure 3. Kaplan–Meier survival curves for combined cardiovascular events for the identified cutoff value of triglycerides 89 mg/dL (A) and the conventional cutoff value of triglycerides 150 mg/dL (B).
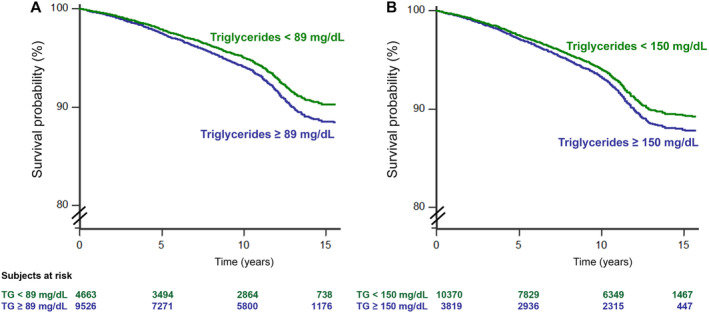
Trends of subjects having serum triglycerides > cutoff (blue line) and <cutoff (red line) are shown. Numbers of subjects at risk are shown in the 2 footnotes. Values of P indicate statistical difference vs reference.
Discussion
First, the results of this present analysis confirm that, in a large sample with a long follow‐up, fasting triglyceride is an independent risk factor for cardiovascular events. Adjusting for factors associated with both cardiovascular events and triglyceride such as diabetes, body mass index, alcohol use, and HDL and non‐HDL cholesterol attenuated but did not fully account for the association between triglycerids and cardiovascular events. This is consistent with a series of studies showing a positive association of incident cardiovascular events with triglyceride both fasting and nonfasting; in particular 3 large meta‐analyses provided adjusted odds ratio values ranging from 1.57 (95% CI, 1.10–2.24) to 1.80 (95% CI, 1.49–2.19) when individuals with triglycerides in the top tertile were compared with those with triglycerides in the bottom tertile. 28 , 29 , 30 , 31 Unlike our study, a number of studies have found that the association between triglycerides and cardiovascular risk is attenuated once adjusted for other lipid parameters, including HDL‐C and non‐HDL‐C. An analysis conducted by the Emerging Risk Factors Collaboration (n=302 430) demonstrated that the HR for coronary heart disease as a result of elevated triglyceride was 1.37 (95% CI, 1.31–1.42) when adjusted for non‐lipid factors and became nonsignificant (0.99 [95% CI, 0.94–1.05]) when adjusted for HDL cholesterol and non‐HDL cholesterol (0.99 [95% CI, 0.94–1.05]). 12 Elevated triglyceride levels are closely associated with higher levels of non‐HDL cholesterol and low levels of HDL cholesterol 32 and this may explain why this association is weakened after adjustment for these parameters.
No significant interaction was found according to sex. This finding suggests that the risk associated with increasing triglycerides was similar in men and women, excluding the need to identify different cutoffs of triglycerides by sex for a better risk prediction. Other observational data have demonstrated that triglycerides are more strongly associated with CVD risk in women than men. However, importantly, in both men and women, increasing triglycerides were associated with increased CVD risk even among those with triglycerides well below 150 mg/dL. 33
In the past 25 years, other authors have demonstrated a lower cut‐point for triglycerides (100 mg/dL) to be associated with increased risk of primary and secondary cardiovascular events. 34 , 35 , 36 In the present study, the prognostic cutoff value of triglycerides able to identify the subjects at risk of developing cardiovascular events was 89 mg/dL in the whole cohort: being over the cutoff, significantly led to HR >1 of developing a cardiovascular event. Therefore, our findings expand the role of triglycerides in predicting cardiovascular risk. Guidelines acknowledge that a fasting triglyceride level of <150 mg/dL is desirable. 4 , 37 Indeed, the American Heart Association/American College of Cardiology/multisociety cholesterol guideline recommended the use of elevated triglycerides as a “risk‐enhancing factor” in primary cardiovascular prevention and recommended optimizing diet and lifestyle as the first step. Emphasis on weight loss (5%–10% reduction in body weight) through healthy diet and physical activity (at least moderate 150 min/wk or vigorous 75 min/wk) can substantially lower triglycerides levels by 20% to 50%. A healthy dietary pattern includes lean protein, fish, fresh fruits and vegetables, legumes, avoidance of refined foods with high glycemic index and added sugars, and restriction of alcohol intake. 36 If elevated triglyceride or non‐HDL‐C levels remain following aggressive lifestyle intervention and statin therapy, guidelines recommend the use of triglycerides‐lowering agents. 4 , 37 , 38 The JELIS (Japan EPA Lipid Intervention Study) trial and the REDUCE‐IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial) trial used icosapent ethyl, a prescription grade purified eicosapentaenoic acid. 39 , 40 The JELIS trial looked at more than 18 000 individuals and showed that eicosapentaenoic acid reduced major coronary events by 19% compared with the control group. 39 The REDUCE‐IT trial included more than 8000 patients with CVD or diabetes with additional cardiovascular risk factors. 40 All participants were on statins with LDLs below 100 mg/dL and triglycerides between 135 and 499 mg/dL (with a mean triglyceride level of 216 mg/dL). The patients were randomly assigned to icosapent ethyl 4 g daily or mineral oil. The composite cardiovascular end point was reduced by 25% over ≈5 years, with a number needed to treat of 21.
The main strength of the study described here is to have determined on a large Italian nationwide database with a long‐lasting follow‐up a clear prognostic cutoff of triglycerides, identified by the ROC curves methods and validated in multivariate models, able to identify subjects at higher cardiovascular risk. Further, if a test is used for the purpose of screening in an epidemiological context, then a cutoff value with a higher sensitivity and negative predictive value must be considered. 41 The limitations are represented by the fact that data are partially derived from a selected sample of patients referred by general practitioners to specialized centers, an underestimation of morbid events is quite likely as in other cohort studies, the design was fit to demonstrate an association but not a causality in the relationship between triglycerides and cardiovascular events, based on the univariate ROC curve, there is a large trade‐off between sensitivity and specificity, and the analysis was based on a single triglycerides measurement without taking into consideration the dilution bias. A recent paper based on 15 792 study participants from the Atherosclerosis Risk in Communities and Framingham Offspring studies, using fasting triglyceride measurements across multiple exams over time, showed that the average of several triglyceride readings provided incremental improvements for the prediction of CVD relative to a single triglyceride measurement. 33 However, regardless of the method of measurement, higher triglycerides were associated with increased CVD risk, even at levels previously considered “optimal” (<150 mg/dL). On the other hand, the collected database represents the largest number of Italian cases ever collected, and to our knowledge there is none more representative of the Italian situation. Furthermore, the present analysis was limited to Italian people and its results cannot be directly applied to other ethnicities. Validation of prediction requires testing in an independent data set as running analyses in the same data set will yield stronger associations than in an independent data set. Consequently, further studies are needed to confirm that the thresholds of triglycerides emerging from our analyses are valid also in general populations and in other ethnicities.
Conclusions
In conclusion, the Working Group on Uric Acid and Cardiovascular Risk of the Italian Society of Hypertension confirmed that, after adjusting for potential confounders, a lower than conventional prognostic cutoff of triglycerides able to separate subjects at risk of developing cardiovascular events can be identified in an Italian cohort.
Sources of Funding
This work has been conducted with an unrestricted grant from the Fondazione of the Italian Society of Hypertension (grant: MIOL).
Disclosures
Dr Borghi has received research grant support from Menarini Corporate and Novartis Pharma; has served as a consultant for Novartis Pharma, Alfasigma, Grunenthal, Menarini Corporate, and Laboratoires Servier; and received lecturing fees from Laboratoires Servier, Takeda, Astellas, Teijin, Novartis Pharma, Berlin Chemie, and Sanofi. The authors declare no competing interests. The remaining authors have no disclosures to report.
Supporting information
Table S1
Preprint posted on MedRxiv June 29, 2023. doi: https://doi.org/10.1101/2023.06.23.23291840.
This article was sent to Daniel Edmundowicz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030319
For Sources of Funding and Disclosures, see page 8.
References
- 1. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18:689–700. doi: 10.1038/s41569-021-00541-4 [DOI] [PubMed] [Google Scholar]
- 2. Barquera S, Pedroza‐Tobías A, Medina C, Hernández‐Barrera L, Bibbins‐Domingo K, Lozano R, Moran AE. Global overview of the epidemiology of atherosclerotic cardiovascular disease. Arch Med Res. 2015;46:328–338. doi: 10.1016/j.arcmed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 3. Hulmán A, Tabák AG, Nyári TA, Vistisen D, Kivimäki M, Brunner EJ, Witte DR. Effect of secular trends on age‐related trajectories of cardiovascular risk factors: the Whitehall II longitudinal study 1985‐2009. Int J Epidemiol. 2014;43:866–877. doi: 10.1093/ije/dyt279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones D, Morris P, Ballantyne CM, Birtcher KK, Covington AM, DePalma SM, Minissian MB, Orringer CE, Smith SC, Waring AA, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2022;80:1366–1418. doi: 10.1016/j.jacc.2022.07.006 [DOI] [PubMed] [Google Scholar]
- 6. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker‐Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. Heart disease and stroke statistics‐2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 7. Lawler PR, Kotrri G, Koh M, Goodman SG, Farkouh ME, Lee DS, Austin PC, Udell JA, Ko DT. Real‐world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86–94. doi: 10.1093/eurheartj/ehz767 [DOI] [PubMed] [Google Scholar]
- 8. Nordestgaard BG. Triglyceride‐rich lipoproteins and atherosclerotic cardiovascular disease. Circ Res. 2016;118:547–563. doi: 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 9. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6 [DOI] [PubMed] [Google Scholar]
- 10. Varbo A, Nordestgaard BG. Remnant cholesterol and triglyceride‐rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2016;36:2133–2135. doi: 10.1161/ATVBAHA.116.308305 [DOI] [PubMed] [Google Scholar]
- 11. Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–555. doi: 10.1093/eurheartj/eht571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emerging Risk Factors Collaboration , Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raposeiras‐Roubin S, Rosselló X, Oliva B, Fernández‐Friera L, Mendiguren JM, Andrés V, Bueno H, Sanz J, Martínez de Vega V, Abu‐Assi E, et al. Triglycerides and residual atherosclerotic risk. J Am Coll Cardiol. 2021;77:3031–3041. doi: 10.1016/j.jacc.2021.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–557. doi: 10.1210/er.2018-00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assmann G, Schulte H, von Eckardstein A. Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle‐aged men. Am J Cardiol. 1996;77:1179–1184. doi: 10.1016/S0002-9149(96)00159-2 [DOI] [PubMed] [Google Scholar]
- 16. Bezafibrate Infarction Prevention (BIP) Study . Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. doi: 10.1161/01.CIR.102.1.21 [DOI] [PubMed] [Google Scholar]
- 17. Desideri G, Virdis A, Casiglia E, Borghi C. Exploration into uric and cardiovascular disease: Uric Acid Right for heArt Health (URRAH) project. A study protocol for a retrospective observational study. High Blood Press Cardiovasc Prev. 2018;25:197–202. doi: 10.1007/s40292-018-0250-7 [DOI] [PubMed] [Google Scholar]
- 18. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, Rivasi G, Salvetti M, Barbagallo CM, Bombelli M, et al. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. 2020;75:302–308. doi: 10.1161/HYPERTENSIONAHA.119.13643 [DOI] [PubMed] [Google Scholar]
- 19. Casiglia E, Tikhonoff V, Virdis A, Masi S, Barbagallo CM, Bombelli M, Bruno B, Cicero AFG, Cirillo M, Cirillo P, et al. Serum uric acid and fatal myocardial infarction: detection of prognostic cut‐off values: the URRAH (Uric Acid Right for Heart Health) study. J Hypertens. 2020;38:412–419. doi: 10.1097/HJH.0000000000002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet. 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imano H, Li J, Tanaka M, Yamagishi K, Muraki I, Umesawa M, Kiyama M, Kitamura A, Sato S, Iso H. Optimal cut‐off points of nonfasting and fasting triglycerides for prediction of ischemic heart disease in Japanese general population: the Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb. 2023;30:110–130. doi: 10.5551/jat.63358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 24. Kamarudin AN, Cox T, Kolamunnage‐Dona R. Time‐dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17:53. doi: 10.1186/s12874-017-0332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: [DOI] [PubMed] [Google Scholar]
- 26. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut‐point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi: 10.1097/01.ede.0000147512.81966.ba [DOI] [PubMed] [Google Scholar]
- 27. Uno H, Cai T, Tian L, Wei LW. Evaluating prediction rules for t‐year survivors with censored regression models. J Am Stat Assoc. 2007;478:527–537. doi: 10.1198/016214507000000149 [DOI] [Google Scholar]
- 28. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high‐density lipoprotein cholesterol level: a meta‐analysis of population‐based prospective studies. J Cardiovasc Risk. 1996;3:213–219. [PubMed] [Google Scholar]
- 29. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;11:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793 [DOI] [PubMed] [Google Scholar]
- 30. Patel A, Barzi F, Jamrozik K, Lam TH, Ueshima H, Whitlock G, Woodward M; Asia Pacific Cohort Studies Collaboration . Serum triglycerides as a risk factor for cardiovascular diseases in the Asia‐Pacific region. Circulation. 2004;110:2678–2686. doi: 10.1161/01.CIR.0000145615.33955.83 [DOI] [PubMed] [Google Scholar]
- 31. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg‐Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;29:299–308. doi: 10.1001/jama.298.3.299 [DOI] [PubMed] [Google Scholar]
- 32. Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Borén J, Catapano AL, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, et al. Triglyceride‐rich lipoproteins and high‐density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aberra T, Peterson ED, Pagidipati NJ, Mulder H, Wojdyla DM, Philip S, Granowitz C, Navar AM. The association between triglycerides and incident cardiovascular disease: what is "optimal"? J Clin Lipidol. 2020;14:438–447. doi: 10.1016/j.jacl.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller M, Seidler A, Moalemi A, Pearson TA. Normal triglyceride levels and coronary artery disease events: the Baltimore coronary observational long‐term study. J Am Coll Cardiol. 1998;31:1252–1257. doi: 10.1016/S0735-1097(98)00083-7 [DOI] [PubMed] [Google Scholar]
- 35. Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–2275. doi: 10.1016/j.jacc.2015.03.544 [DOI] [PubMed] [Google Scholar]
- 36. Klempfner R, Erez A, Sagit BZ, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated triglyceride level is independently associated with increased all‐cause mortality in patients with established coronary heart disease: twenty‐two‐year follow‐up of the Bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104 [DOI] [PubMed] [Google Scholar]
- 37. Virani S, Morris P, Agarwala A, Ballantyne CM, Birtcher KK, Kris‐Etherton PM, Ladden‐Stirling AB, Miller M, Orringer CE, Stone NJ. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia. J Am Coll Cardiol. 2021;78:960–993. doi: 10.1016/j.jacc.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 38. Koneru SC. Fellow's voice: hypertriglyceridemia: understanding the current guideline. Am J Prev Cardiol. 2022;9:100322. doi: 10.1016/j.ajpc.2022.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 41. Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94:557–592. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


