Abstract
Background
Patients with heart failure and chronic kidney disease (CKD) may have an increased risk of death from causes competing with arrhythmic death, which could have implications for the efficacy of implantable cardioverter‐defibrillators (ICDs). We examined the long‐term effects of primary prophylactic ICD implantation, compared with usual care, according to baseline CKD status in an extended follow‐up study of DANISH (Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality).
Methods and Results
In the DANISH trial, 1116 patients with nonischemic heart failure with reduced ejection fraction were randomized to receive an ICD (N=556) or usual care (N=550). Outcomes were analyzed according to CKD status (estimated glomerular filtration rate ≥/<60 mL/min per 1.73 m2) at baseline. In total, 1113 patients had an available estimated glomerular filtration rate measurement at baseline (median estimated glomerular filtration rate 73 mL/min per 1.73 m2), and 316 (28%) had CKD. During a median follow‐up of 9.5 years, ICD implantation, compared with usual care, did not reduce the rate of all‐cause mortality (no CKD, HR, 0.82 [95% CI, 0.64–1.04]; CKD, HR, 1.02 [95% CI, 0.75–1.38]; P interaction=0.31) or cardiovascular death (no CKD, HR, 0.77 [95% CI, 0.58–1.03]; CKD, HR, 1.05 [95% CI, 0.73–1.51]; P interaction=0.20), irrespective of baseline CKD status. Similarly, baseline CKD status did not modify the beneficial effects of ICD implantation on sudden cardiovascular death (no CKD, HR, 0.57 [95% CI, 0.32–1.00]; CKD, HR, 0.65 [95% CI, 0.34–1.24]; P interaction=0.70).
Conclusions
ICD implantation, compared with usual care, did not reduce the overall mortality rate, but it did reduce the rate of sudden cardiovascular death, regardless of baseline kidney function in patients with nonischemic heart failure with reduced ejection fraction.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT00542945.
Keywords: chronic kidney disease, clinical trial, estimated glomerular filtration rate, heart failure, implantable cardioverter‐defibrillator
Subject Categories: Sudden Cardiac Death, Clinical Studies, Primary Prevention, Cardiomyopathy, Heart Failure
Nonstandard Abbreviations and Acronyms
- CRT
cardiac resynchronization therapy
- DANISH
Danish Study to Assess the Efficacy of Implantable Cardioverter Defibrillators in Patients With Nonischemic Systolic Heart Failure on Mortality
- HFrEF
heart failure with reduced ejection fraction
Clinical Perspective.
What Is New?
This extended follow‐up study of the DANISH (Danish Study to Assess the Efficacy of Implantable Cardioverter Defibrillators in Patients With Nonischemic Systolic Heart Failure on Mortality) trial, which included 1116 patients with nonischemic heart failure with reduced ejection fraction, confirmed the strong association between chronic kidney disease and mortality.
Implantable cardioverter‐defibrillator implantation, compared with usual care, did not reduce the overall mortality rate, regardless of baseline kidney function.
The beneficial effect of an implantable cardioverter‐defibrillator on sudden cardiac death was not modified by kidney function.
What Are the Clinical Implications?
Although our findings indicate that the effect of implantable cardioverter‐defibrillator implantation was not modified by kidney function, further study in a clinical trial designed and powered to answer this question, especially in those with severely impaired kidney function, is required.
Chronic kidney disease (CKD) is common in patients with heart failure (HF) with reduced ejection fraction (HFrEF). 1 , 2 The presence of CKD is associated with more severe HF and worse clinical outcomes, 3 , 4 and it influences the decision to initiate, uptitrate, and discontinue life‐saving guideline‐recommended medical therapies. Although kidney function does not appear to modify the effects of HFrEF therapies, 2 there is evidence to suggest that the effect of a primary prophylactic implantable cardioverter‐defibrillator (ICD) may be modified by CKD status. In a recent meta‐analysis of 3 primary prevention ICD trials, there was a significant interaction between estimated glomerular filtration rate (eGFR) and the effect of ICD implantation on all‐cause mortality, with a benefit in patients with an eGFR ≥60 mL/min per 1.73 m2 but not in those with an eGFR <60 mL/min per 1.73 m2. 5 However, the trials enrolled both patients with ischemic and nonischemic pathogenesis. More important, the trials included in this meta‐analysis were conducted decades ago, and the evidence‐based treatment armamentarium in HFrEF has evolved substantially since then. Therefore, it is important to examine the effects of ICD therapy in a more contemporary cohort of patients with HFrEF receiving guideline‐recommended therapies according to kidney function.
In the DANISH (Danish Study to Assess the Efficacy of ICDs in Patients With Nonischemic Systolic Heart Failure on Mortality) trial, a primary prophylactic ICD did not reduce the long‐term rate of death from any cause compared with usual care, but it did reduce sudden cardiovascular death (SCD). 6 Here, we provide a detailed report of the effects of primary prophylactic ICD implantation in an extended follow‐up study of the DANISH trial, adding 4 years of additional follow‐up, according to baseline eGFR level.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. DANISH was a randomized, controlled, unblinded multicenter trial, which evaluated the efficacy and safety of primary prevention ICD implantation compared with usual care in 1116 patients with nonischemic HFrEF. The design and main findings of the trial have been published and described in detail previously. 6 , 7 The ethics committee for the Capital Region of Denmark approved the protocol (H‐D‐2007‐0101), and all participants gave written informed consent.
Study Participants
Key inclusion criteria were nonischemic cause of HF (preferably determined by coronary angiography, but a normal computed tomographic angiogram or nuclear myocardial perfusion imaging was accepted), a left ventricular ejection fraction ≤35%, New York Heart Association functional class II or III (or class IV if cardiac resynchronization therapy [CRT] was planned), an NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) concentration >200 pg/mL, and optimal treatment with medical therapy for HF. Patients with preexisting pacemakers or CRT‐pacemakers were also eligible if they were willing to accept a potential upgrade. Patients fulfilling indications for a CRT device received a CRT‐defibrillator (if randomized to ICD arm) or CRT‐pacemaker (if randomized to control arm). Key exclusion criteria were a resting heart rate >100 beats per minute in patients with permanent atrial fibrillation and renal replacement therapy including dialysis. A full list of exclusion criteria is provided in the main paper. 6
eGFR Measurement
eGFR values were calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation. 8 , 9 Plasma creatinine was measured at baseline in all randomized patients with contemporary clinical routine assays at the local laboratories of the enrollment sites. The assessment of the effect of ICD implantation according to baseline eGFR was a prespecified subgroup analysis.
In the present study, patients were divided in 2 subgroups according to the presence of CKD (defined as an eGFR below 60 mL/min per 1.73 m2). eGFR was also analyzed as a continuous measure and a dichotomized measure at the median, as prespecified in the primary analysis of DANISH.
Follow‐Up and Outcomes
In the main trial, patients were followed until June 30, 2016. In the present study with extended follow‐up, patients were followed until May 18, 2020. No patients were lost to follow‐up. The primary outcome was death from any cause, and secondary outcomes were cardiovascular death, SCD, and noncardiovascular death. All outcomes were adjudicated by an event committee blinded to treatment allocation.
In addition, device complications among patients randomized to an ICD were also examined, although these data were available only for the original follow‐up duration.
Statistical Analysis
Baseline characteristics were summarized as frequencies with percentages or medians with interquartile ranges, and differences were tested using the χ 2 test for categorical variables and unpaired t test or the Wilcoxon test for normally distributed and nonnormally distributed continuous variables, respectively.
The association between eGFR and outcomes (regardless of treatment allocation) was examined with the Kaplan–Meier estimator (for death from any cause), Aalen–Johansen estimator (for cardiovascular death, SCD, and noncardiovascular death, taking the competing risk of other causes of death into account), and restricted cubic spline analyses based on Cox proportional hazards regression models, adjusted for treatment assignment, age, sex, systolic blood pressure, log of NT‐proBNP, left ventricular ejection fraction, prior HF hospitalization, New York Heart Association functional class, diabetes, atrial fibrillation, CRT implantation (preexisting or planned), and center. In these analyses, an eGFR of 60 mL/min per 1.73 m2 served as the reference.
The effect of ICD implantation versus usual care according to eGFR was examined with Cox proportional hazards regression models, stratified according to center and CRT implantation (preexisting or planned). In addition, models were adjusted for certain key variables that were significantly different between the ICD and control arm in each of the eGFR categories. The effect of ICD implantation was also examined according to continuous eGFR modeled as a fractional polynomial.
The effect of ICD implantation on death from any cause was also examined according to continuous age, modeled as a fractional polynomial, in patients with and without CKD, respectively.
Data were analyzed according to the intention‐to‐treat principle. All analyses were conducted using STATA version 17.0 and SAS version 9.4. A P value of 0.05 was considered statistically significant.
RESULTS
Of the 1116 patients randomized in DANISH, eGFR values at baseline were available in 1113 individuals. Median eGFR at baseline was 73 (25th–75th 58–92) mL/min per 1.73 m2. In total, 28.4% of the participants had CKD, of whom 61.4% had stage 3A CKD (ie, eGFR 45–59 mL/min per 1.73 m2), 31.6% stage 3B CKD (ie, 30–44 mL/min per 1.73 m2), and 7.0% stage 4 (ie, 15–29 mL/min per 1.73 m2). Median follow‐up was 9.5 years (interquartile range, 7.9–10.9 years).
Patient Characteristics
Patient characteristics according to CKD status (ie, eGFR above/below 60 mL/min per 1.73 m2) are presented in Table S1. Compared with patients without CKD, those with CKD were older and more likely to be men and have a higher body mass index, NT‐proBNP, longer QRS duration, and lower left ventricular ejection fraction. Patients with CKD were also more likely to have a history of hypertension, diabetes, and atrial fibrillation, worse New York Heart Association functional class, and longer duration of HF than those without CKD. With respect to background HF therapy, patients with CKD were less frequently treated with a renin‐angiotensin‐system inhibitor and a beta blocker, but they were more likely to be treated with amiodarone and a loop diuretic and more often had a preexisting or planned CRT implantation.
Patient characteristics according to treatment assignment and CKD status are shown in Table 1. Overall, patient characteristics were similar between the ICD and usual care groups in both patients with and without CKD. When baseline characteristics were examined according to eGFR above or below the median level (73 mL/min per 1.73 m2), duration of HF was longer and atrial fibrillation was more common in the ICD arm than in the usual care arm in patients with CKD, whereas diabetes was more prevalent in the usual care arm than in the ICD arm in individuals without CKD (Table S2).
Table 1.
Baseline Characteristics of the Study Population According to Treatment Assignment in Patients With and Without Chronic Kidney Disease (eGFR Above/Below 60 mL/min per 1.73 m2)
| No CKD eGFR ≥60 mL/min per 1.73 m2 N=797 | CKD eGFR <60 mL/min/1.73 m2 N=316 | |||||
|---|---|---|---|---|---|---|
| Control group N=403 | ICD group N=394 | P value | Control group N=155 | ICD group N=161 | P value | |
| Age, y, median (interquartile range) | 61 (54–67) | 62 (54–70) | 0.40 | 67 (61–73) | 70 (63–75) | 0.10 |
| Male sex, N (%) | 274 (68.0) | 277 (70.3) | 0.48 | 128 (82.6) | 128 (79.5) | 0.49 |
| Physiologic measures, median (interquartile range) | ||||||
| Systolic blood pressure, mm Hg | 125 (112–139) | 123 (110–138) | 0.17 | 120 (109–135) | 123 (108–140) | 0.53 |
| Heart rate, bpm | 69 (61–78) | 69 (61–77) | 0.60 | 70 (60–78) | 68 (60–76) | 0.65 |
| Body mass index, kg/m2 | 26 (23–30) | 27 (24–30) | 0.19 | 28 (25–32) | 28 (24–31) | 0.35 |
| N‐terminal pro‐B‐type natriuretic peptide, pg/mL | 924 (501–1905) | 1095 (526–2135) | 0.17 | 1522 (808–3067) | 1795 (887–3191) | 0.73 |
| eGFR, mL/min per 1.73 cm2 | 83 (71–97) | 82 (72–97) | 0.88 | 50 (41–57) | 47 (39–54) | 0.05 |
| QRS duration, msec | 140 (108–164) | 145 (111–165) | 0.46 | 150 (119–166) | 150 (122–168) | 0.90 |
| Left ventricular ejection fraction, %, mean (SD) | 24.4 (6.3) | 24.2 (6.3) | 0.62 | 23.1 (5.5) | 23.9 (6.3) | 0.27 |
| Duration of HF, median (interquartile range), months | 15 (8–51) | 16 (8–58) | 0.65 | 20 (10–93) | 50 (12–96) | 0.14 |
| Main cause of HF, N (%) | 0.82 | 0.89 | ||||
| Idiopathic | 314 (77.9) | 309 (78.4) | 109 (70.3) | 114 (70.8) | ||
| Valvular | 12 (3.0) | 13 (3.3) | 9 (5.8) | 7 (4.4) | ||
| Hypertension | 33 (8.2) | 36 (9.1) | 22 (14.2) | 26 (16.2) | ||
| Other | 44 (10.9) | 36 (9.1) | 15 (9.7) | 14 (8.7) | ||
| New York Heart Association class, N (%) | 0.58 | 0.28 | ||||
| II | 239 (59.3) | 226 (57.4) | 59 (38.1) | 71 (44.1) | ||
| III/IV | 164 (40.7) | 168 (42.6) | 96 (61.9) | 90 (55.9) | ||
| Medical history, N (%) | ||||||
| Hospitalization for HF | 263 (65.8) | 246 (62.8) | 0.38 | 98 (64.1) | 113 (70.2) | 0.25 |
| Hypertension | 99 (24.6) | 114 (28.9) | 0.16 | 67 (43.5) | 67 (41.6) | 0.73 |
| Diabetes | 69 (17.1) | 53 (13.5) | 0.15 | 41 (26.5) | 46 (28.6) | 0.67 |
| Atrial fibrillation | 128 (31.8) | 145 (36.8) | 0.13 | 80 (51.6) | 92 (57.1) | 0.32 |
| Stroke | 45 (11.2) | 35 (8.9) | 0.28 | 19 (12.3) | 16 (10.0) | 0.52 |
| Treatment, N (%) | ||||||
| Angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker | 396 (98.3) | 388 (98.5) | 0.81 | 146 (94.2) | 144 (89.4) | 0.12 |
| Beta blocker | 378 (93.8) | 363 (92.1) | 0.36 | 137 (88.4) | 145 (90.1) | 0.63 |
| Mineralocorticoid receptor antagonist | 237 (58.8) | 237 (60.2) | 0.70 | 81 (52.3) | 88 (54.7) | 0.67 |
| Amiodarone | 15 (3.7) | 14 (3.6) | 0.90 | 17 (11.0) | 20 (12.4) | 0.69 |
| Loop diuretic | 278 (69.0) | 273 (69.3) | 0.93 | 138 (89.0) | 141 (87.6) | 0.69 |
| Thiazide | 31 (7.7) | 29 (7.4) | 0.86 | 14 (9.0) | 17 (10.6) | 0.65 |
| Metolazone | 4 (1.0) | 4 (1.0) | 0.97 | 2 (1.3) | 0 (0) | 0.15 |
| Erythropoietin | 0 (0) | 1 (0.3) | 0.31 | 3 (1.9) | 1 (0.6) | 0.30 |
| CRT‐P/CRT‐D | 222 (55.1) | 223 (56.6) | 0.67 | 100 (64.5) | 98 (60.9) | 0.50 |
CKD indicates chronic kidney disease; CRT‐D, cardiac resynchronization therapy defibrillator; CRT‐P, cardiac resynchronization therapy pacemaker; eGFR, estimated glomerular filtration rate; HF, heart failure; and ICD, implantable cardioverter‐defibrillator. According to the protocol, patients fulfilling criteria for CRT devices received a CRT‐D if randomized to the ICD arm or received a CRT‐P device if randomized to the control arm.
Outcomes According to Baseline eGFR
The cumulative incidence of outcomes according to CKD status (ie, eGFR above/below 60 mL/min per 1.73 m2) is shown in Figure S1. In restricted cubic spline analyses, after adjustment for prognostic variables, an eGFR <60 mL/min per 1.73 m2 was associated with a greater risk of all outcomes examined although the association was not statistically significant for noncardiovascular death (Figure 1). Among patients randomized to an ICD, there was no difference in the risk of device infection, and other ICD‐related complications, between patients with and without CKD (Table S3).
Figure 1. Absolute risk of outcomes according to chronic kidney disease.
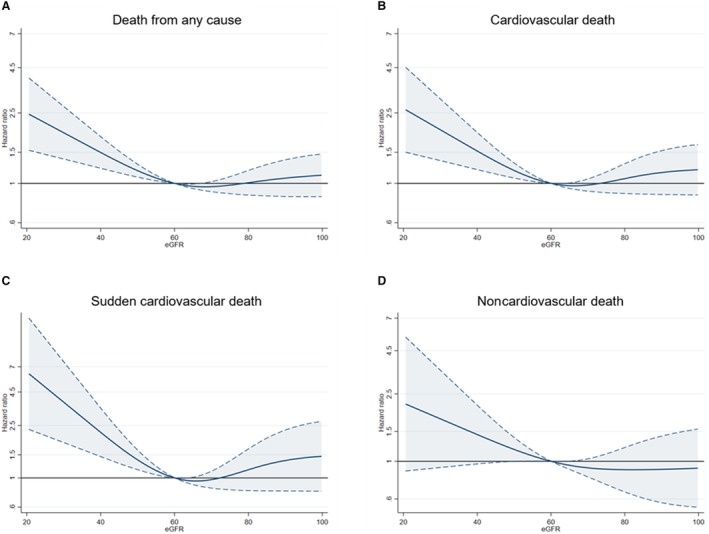
A, Death from any cause; B, Cardiovascular death; C, Sudden cardiovascular death; D, Noncardiovascular death. The figures show the association between eGFR (as a continuous variable) and outcomes. The reference is 60 mL/min per 1.73 m2. The Cox models were adjusted for treatment assignment, age, sex, systolic blood pressure, log of NT‐proBNP, LVEF, prior HF hospitalization, NYHA functional class, diabetes, atrial fibrillation, CRT implantation (preexisting or planned), and center. CRT indicates cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and NYHA, New York Heart Association.
Effect of ICD Implantation on Outcomes According to Baseline eGFR
The effects of ICD implantation on outcomes according to CKD status are shown in Figures 2 and 3 and Table S4. ICD implantation did not significantly reduce the rate of death from any cause or cardiovascular death in patients with and without CKD, and there was no significant interaction between CKD status and the effect of ICD implantation on either of these outcomes (P interaction=0.31 and 0.20, respectively). Similarly, there was no significant interaction between eGFR, examined as a continuous variable, and the effect of ICD implantation on death from any cause and cardiovascular death (Figure 4A and 4B).
Figure 2. Effect of ICD implantation compared with usual care according to CKD.
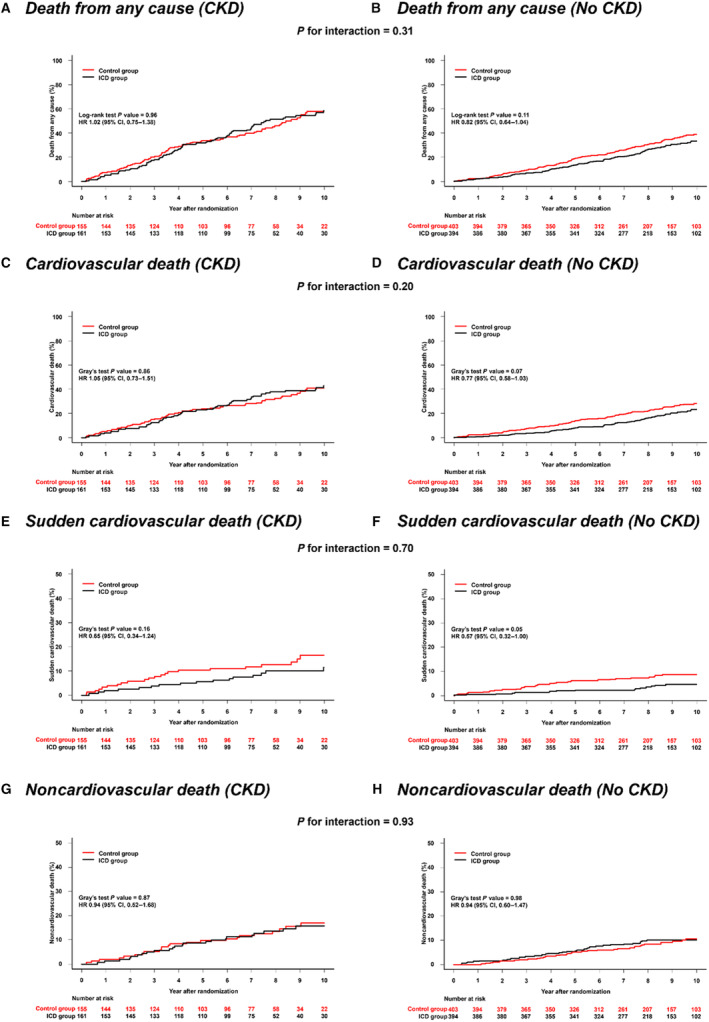
A, Death from any cause: eGFR <60 mL/min per 1.73 m2; B, Death from any cause: eGFR ≥60 mL/min per 1.73 m2; C, Cardiovascular death: eGFR <60 mL/min per 1.73 m2; D, Cardiovascular death: eGFR ≥60 mL/min per 1.73 m2; E, Sudden cardiovascular death: eGFR <60 mL/min per 1.73 m2; F, Sudden cardiovascular death: eGFR ≥60 mL/min per 1.73 m2; G, Noncardiovascular death: eGFR <60 mL/min per 1.73 m2; H, Noncardiovascular death: eGFR ≥60 mL/min per 1.73 m2. All hazard ratios are stratified according to center and cardiac resynchronization therapy implantation (preexisting or planned). CKD indicates chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; and ICD, implantable cardioverter defibrillator.
Figure 3. Effect of ICD implantation compared with usual care according to CKD.
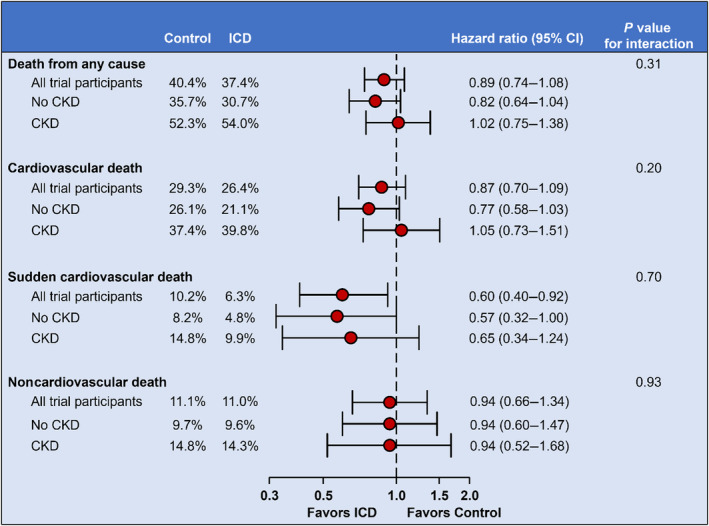
All hazard ratios are stratified according to center and cardiac resynchronization therapy implantation (preexisting or planned). CKD, chronic kidney disease; and ICD, implantable cardioverter defibrillator.
Figure 4. Effect of ICD implantation compared with usual care on outcomes according to continuous eGFR.
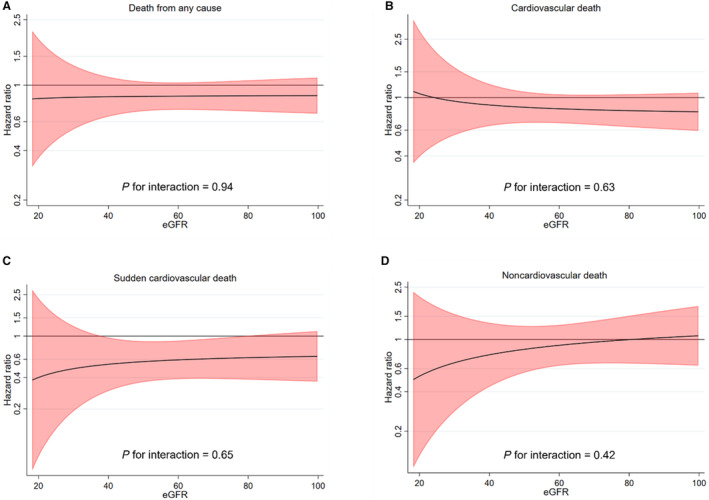
A, Death from any cause; B, Cardiovascular death; C, Sudden cardiovascular death; D, Noncardiovascular death. The figures show the effect of ICD implantation vs usual care on outcomes according to continuous eGFR modeled as a fractional polynomial. The model was stratified according to center and cardiac resynchronization therapy implantation (preexisting or planned). eGFR indicates estimated glomerular filtration rate; and ICD, implantable cardioverter defibrillator.
CKD status did not modify the beneficial effects of ICD implantation on the rate of SCD (P interaction=0.70). Similarly, there was no significant interaction between eGFR, examined as a continuous variable, and the effect of ICD implantation on SCD (P interaction=0.65) (Figure 4C).
ICD implantation neither reduced the rate of noncardiovascular death in patients with CKD nor in those without CKD, with no interaction between eGFR and the effect of treatment (Figure 4D).
We also examined the effect of ICD implantation according to baseline eGFR levels above or below median. In line with the main findings, there was no statistically significant interaction between eGFR and the effect of ICD implantation for any of the outcomes (Table S5).
Effect of ICD Implantation on Death From Any Cause According to Baseline eGFR and Age
In patients without CKD, age did not modify the effect of ICD implantation on death from any cause (P interaction=0.98; Figure S2A). In patients with CKD, there was a trend toward an interaction between age and the effect of ICD implantation on death from any cause, with a greater mortality reduction with ICD implantation in younger individuals (P interaction=0.11) (Figure S2B).
DISCUSSION
In this extended follow‐up study of the DANISH trial, we confirmed the strong association between CKD and mortality in patients with nonischemic HFrEF. ICD implantation, compared with usual care, did not reduce the overall mortality rate, regardless of baseline kidney function. The beneficial effect of an ICD on SCD was not modified by kidney function.
There were large differences in the clinical characteristics and outcomes between HFrEF patients with and without CKD, most of which confirmed prior findings. 5 , 10 Thus, patients with CKD were older and more often men, and they had a greater comorbidity burden, a longer duration of HF, and a higher mortality rate (irrespective of cause of death) than individuals without CKD. However, the proportion of patients with severe CKD was low, with only 2% of the DANISH population having stage 4 CKD (ie, 15–29 mL/min per 1.73 m2). The low proportion highlights the substantial underrepresentation of patients with severely impaired kidney function in HFrEF trials (including those of primary prevention ICD), and greater efforts should be made to increase the representation of these patients in clinical trials to establish the effect of therapies in individuals with more severe CKD.
Nevertheless, the effects of disease‐modifying, guideline‐recommended HFrEF therapies, including beta blockers, 11 , 12 , 13 , 14 renin‐angiotensin‐system inhibitors, 15 , 16 mineralocorticoid‐receptor antagonists, 17 , 18 angiotensin receptor‐neprilysin inhibitors, 10 , 19 and sodium‐glucose cotransporter 2 inhibitors, 20 , 21 , 22 appear to be consistent across the range of eGFRs included in landmark clinical trials. However, this may not be the case with primary prophylactic ICD implantation. In an individual participant‐level data meta‐analysis of MADIT‐I (Multicenter Automatic Defibrillator Implantation Trial), MADIT‐II, and SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial), there was a survival benefit with a primary prophylactic ICD, compared with usual care, in patients without CKD but not in those with CKD. 5 Similarly, the beneficial effect of an ICD on arrhythmic death was attenuated in patients with CKD. An ICD can prevent SCD caused by ventricular tachyarrhythmia, severe bradycardia, or complete heart block but cannot provide protection against other causes of death, and because patients with CKD have an increased risk of competing causes of death to arrhythmic death (ie, patients with a longer duration of HF or more severe HF more often die from pump failure or terminal HF, and those with more noncardiovascular comorbidities are more likely to die from noncardiovascular causes), this potential interaction between kidney function and the effect of an ICD is biologically plausible. However, our findings from a large and well‐treated cohort of patients with nonischemic HFrEF are in contrast with those from this meta‐analysis. Specifically, we found that the effects of ICD implantation were not modified by kidney function, and these results were supported by the statistically more powerful analyses of eGFR as a continuous variable, in which the treatment effect was entirely consistent across the range of baseline eGFR levels included in the DANISH trial.
The reasons for the discrepancy in results between the meta‐analysis and the DANISH trial are not clear, although there are several possible explanations. First, the evidence‐based treatment armamentarium in HFrEF has evolved and expanded substantially since the landmark ICD trials included in the meta‐analysis were conducted, 23 , 24 , 25 , 26 and due to the cumulative benefit of these evidence‐based, disease‐modifying therapies, the incidence of SCD has declined in patients with HFrEF during the past decades. 27 Indeed, the use of renin‐angiotensin‐system inhibitors, beta blockers, and mineralocorticoid‐receptor antagonists was markedly higher in the DANISH trial than in any previous ICD trial, and more than 50% of the DANISH participants received CRT. 6 , 28 , 29 , 30 Therefore, the lack of interaction between an ICD and kidney function (and the lack of efficacy of ICD implantation in patients without CKD) in the DANISH trial may be due to the low rate of SCD. Second, the majority of the patients in the meta‐analysis had HF of ischemic origin, 28 , 29 whereas individuals enrolled in the DANISH trial were required to have HF of nonischemic origin, and patients with ischemic HF may be more susceptible to ventricular arrhythmias, originating from myocardial scar tissue, than those with nonischemic HF. 31 , 32 Finally, given the limitations of subgroup analyses, a significant interaction (or the lack hereof) could simply have resulted by chance, and the findings should therefore be considered as hypothesis generating. Thus, it remains uncertain whether kidney function, especially in the lower eGFR range or in patients on dialysis, modifies the effect of an ICD. Therefore, there is a need for a randomized clinical trial specifically designed and powered to address this question. Although the DANISH trial and the ICD trials included in the meta‐analysis described here excluded patients undergoing dialysis, the ICD2 (Implantable Cardioverter‐Defibrillator in Dialysis Patients) trial examined the effects of prophylactic ICD implantation, compared with usual care, in dialysis‐treated patients with a left ventricular ejection fraction ≥35%. In this trial, ICD implantation did not reduce the rate of SCD or all‐cause mortality, but <200 patients were included, and because <5% had HF, these findings cannot be extrapolated to patients with HFrEF. 33
Limitations
This study has some limitations that need to be acknowledged. First, although the assessment of the effect of ICD implantation on death from any cause according to baseline eGFR, dichotomized at the median, was prespecified, the examination of secondary outcomes was done post hoc, as was the assessment of the effect of ICD implantation according CKD status. Because the trial was not powered to examine the effect of ICD in a subgroup of patients, the results should be interpreted with caution and considered as hypothesis generating at best. Second, as in any clinical trial, the prespecified inclusion and exclusion criteria in DANISH precluded the enrollment of very high‐risk patients, and the study population was predominantly White. Third, the number and proportion of patients with stage 4 CKD were low (2% of the DANISH population; 7% of the DANISH population with CKD) and patients on dialysis were excluded. Consequently, these findings cannot be generalized to individuals with severely impaired kidney function. Fourth, the requirement of optimal medical therapy might have resulted in a lower proportion of patients with CKD in the DANISH trial, because these patients have a lower tolerance to HF therapies. Fifth, although it would have been interesting to examine longitudinal changes in eGFR, we did not have data on eGFR during follow‐up. Finally, in the analysis comparing outcomes between patients with and without CKD, the risk of residual confounding cannot be excluded despite comprehensive adjustment. Therefore, causal inference cannot be drawn from these analyses.
CONCLUSIONS
In an extended follow‐up study of the DANISH trial, ICD implantation, in comparison with usual care, did not reduce the overall mortality rate, but it did reduce the rate of SCD, regardless of baseline kidney function in patients with nonischemic HFrEF.
Sources of Funding
S.N. Doi was supported by the Japanese College of Cardiology Overseas Research Fellowship and Scandinavia‐Japan Sasakawa Foundation.
Disclosures
Dr Butt reports advisory board honoraria from Bayer, consultant honoraria from Novartis and AstraZeneca, and travel grants from AstraZeneca outside the submitted work. Dr Nielsen reports grant from the Novo Nordisk Foundation (NNF16OC0018658 and NNF17OC0029148) outside the submitted work. Dr Svendsen reports honoraria from Medtronic (speaker fee and membership of advisory committee) and research grant from Medtronic outside the submitted work. Dr Pehrson reports honoraria (speaker fees) from AstraZeneca, Bristol Myers Squibb, and Abbott, outside the submitted work. Dr Hassager reports grant from the Novo Nordisk Foundation, the Lundbeck Foundation, and the Danish Heart Foundation outside the submitted work. Dr Bruun reports grants from the Novo Nordisk Foundation, Health Insurance Denmark, the Augustinus Foundation, and the Kaj Hansen Foundation outside the submitted work. Dr Gustafsson reports research grant from Pfizer and the Novo Nordisk Foundation, advisory board honoraria from Bayer, Pfizer, Abbott, Ionis, and Alnylam and speakers fee from AstraZeneca, Novartis, and Orion Pharma outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figures S1–S2
This work was presented in part at the European Society of Cardiology Congress, August 25–28, 2023, in Amsterdam, Netherlands.
This article was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031977
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, Maggioni AP, Voors AA. Co‐morbidities in patients with heart failure: an analysis of the European heart failure pilot survey. Eur J Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30 [DOI] [PubMed] [Google Scholar]
- 2. Beldhuis IE, Lam CSP, Testani JM, Voors AA, Van Spall HGC, Ter Maaten JM, Damman K. Evidence‐based medical therapy in patients with heart failure with reduced ejection fraction and chronic kidney disease. Circulation. 2022;145:693–712. doi: 10.1161/circulationaha.121.052792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 4. Patel RB, Fonarow GC, Greene SJ, Zhang S, Alhanti B, DeVore AD, Butler J, Heidenreich PA, Huang JC, Kittleson MM, et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2021;78:330–343. doi: 10.1016/j.jacc.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pun PH, Al‐Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, et al. Implantable cardioverter‐defibrillators for primary prevention of sudden cardiac death in CKD: a meta‐analysis of patient‐level data from 3 randomized trials. Am J Kidney Dis. 2014;64:32–39. doi: 10.1053/j.ajkd.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029 [DOI] [PubMed] [Google Scholar]
- 7. Thune JJ, Pehrson S, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al. Rationale, design, and baseline characteristics of the DANish randomized, controlled, multicenter study to assess the efficacy of implantable cardioverter defibrillators in patients with non‐ischemic systolic heart failure on mortality (DANISH). Am Heart J. 2016;179:136–141. doi: 10.1016/j.ahj.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens LA, Schmid CH, Zhang YL, Coresh J, Manzi J, Landis R, Bakoush O, Contreras G, Genuth S, Klintmalm GB, et al. Development and validation of GFR‐estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant. 2010;25:449–457. doi: 10.1093/ndt/gfp510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 11. Kotecha D, Gill SK, Flather MD, Holmes J, Packer M, Rosano G, Böhm M, McMurray JJV, Wikstrand J, Anker SD, et al. Impact of renal impairment on beta‐blocker efficacy in patients with heart failure. J Am Coll Cardiol. 2019;74:2893–2904. doi: 10.1016/j.jacc.2019.09.059 [DOI] [PubMed] [Google Scholar]
- 12. Ghali JK, Wikstrand J, Van Veldhuisen DJ, Fagerberg B, Goldstein S, Hjalmarson A, Johansson P, Kjekshus J, Ohlsson L, Samuelsson O, et al. The influence of renal function on clinical outcome and response to beta‐blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in chronic HF (MERIT‐HF). J Card Fail. 2009;15:310–318. doi: 10.1016/j.cardfail.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 13. Castagno D, Jhund PS, McMurray JJ, Lewsey JD, Erdmann E, Zannad F, Remme WJ, Lopez‐Sendon JL, Lechat P, Follath F, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II) trial. Eur J Heart Fail. 2010;12:607–616. doi: 10.1093/eurjhf/hfq038 [DOI] [PubMed] [Google Scholar]
- 14. Wali RK, Iyengar M, Beck GJ, Chartyan DM, Chonchol M, Lukas MA, Cooper C, Himmelfarb J, Weir MR, Berl T, et al. Efficacy and safety of carvedilol in treatment of heart failure with chronic kidney disease: a meta‐analysis of randomized trials. Circ Heart Fail. 2011;4:18–26. doi: 10.1161/circheartfailure.109.932558 [DOI] [PubMed] [Google Scholar]
- 15. Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/circulationaha.105.580506 [DOI] [PubMed] [Google Scholar]
- 16. Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB, Rich MW, Pitt B, White M, Bakris GC, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD treatment trial. Int J Cardiol. 2013;167:151–156. doi: 10.1016/j.ijcard.2011.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone evaluation study investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/nejm199909023411001 [DOI] [PubMed] [Google Scholar]
- 18. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207 [DOI] [PubMed] [Google Scholar]
- 19. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 21. Jhund PS, Solomon SD, Docherty KF, Heerspink HJL, Anand IS, Böhm M, Chopra V, de Boer RA, Desai AS, Ge J, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA‐HF. Circulation. 2021;143:298–309. doi: 10.1161/circulationaha.120.050391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 24. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 25. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 26. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/cir.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 27. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Køber L, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. doi: 10.1056/NEJMoa1609758 [DOI] [PubMed] [Google Scholar]
- 28. Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/nejm199612263352601 [DOI] [PubMed] [Google Scholar]
- 29. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 30. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 31. Ebinger MW, Krishnan S, Schuger CD. Mechanisms of ventricular arrhythmias in heart failure. Curr Heart Fail Rep. 2005;2:111–117. doi: 10.1007/s11897-005-0018-y [DOI] [PubMed] [Google Scholar]
- 32. Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA‐HF. Eur Heart J. 2021;42:3727–3738. doi: 10.1093/eurheartj/ehab560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Krol‐van Straaten MJ, et al. Prophylactic use of implantable cardioverter‐defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation. 2019;139:2628–2638. doi: 10.1161/circulationaha.119.039818 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2


