Abstract
Background
Effective therapies for reducing cardiovascular disease (CVD) risk in people with elevated lipoprotein(a) are lacking, especially for primary prevention. Because of the potential association of lipoprotein(a) with thrombosis, we evaluated the relationship between aspirin use and CVD events in people with elevated lipoprotein(a).
Methods and Results
We used data from the MESA (Multi‐Ethnic Study of Atherosclerosis), a prospective cohort study of individuals free of baseline cardiovascular disease. Due to potential confounding by indication, we matched aspirin users to nonusers using a propensity score based on CVD risk factors. We then evaluated the association between aspirin use and coronary heart disease (CHD) events (CHD death, nonfatal myocardial infarction) stratified by baseline lipoprotein(a) level (threshold of 50 mg/dL) using Cox proportional hazards models with adjustment for CVD risk factors. After propensity matching, the study cohort included 2183 participants, including 1234 (57%) with baseline aspirin use and 423 (19%) with lipoprotein(a) >50 mg/dL. Participants with lipoprotein(a) >50 mg/dL had a higher burden of CVD risk factors, more frequent aspirin use (61.7% versus 55.3%, P=0.02), and higher rate of incident CHD events (13.7% versus 8.9%, P<0.01). Aspirin was associated with a significant reduction in CHD events among those with elevated lipoprotein(a) (hazard ratio, 0.54 [95% CI, 0.32–0.94]; P=0.03). Those with lipoprotein(a) >50 mg/dL and aspirin use had similar CHD risk as those with lipoprotein(a) ≤50 mg/dL regardless of aspirin use.
Conclusions
Aspirin use was associated with a significantly lower risk for CHD events in participants with lipoprotein(a) >50 mg/dL without baseline CVD. The results of this observational propensity‐matched study require confirmation in studies with randomization of aspirin use.
Keywords: aspirin, cardiovascular disease, Lipoprotein(a), primary prevention
Subject Categories: Lipids and Cholesterol, Biomarkers
Nonstandard Abbreviations and Acronyms
- ARRIVE
aspirin to reduce risk of initial vascular events
- ASCEND
effects of aspirin for primary prevention in persons with diabetes mellitus
- ASPREE
aspirin in reducing events in the elderly
- MESA
Multi‐Ethnic Study of Atherosclerosis
Clinical Perspective.
What Is New?
Elevated lipoprotein(a) is associated with increased risk for cardiovascular disease, but preventive therapies are lacking.
In a propensity‐matched cohort study, aspirin use was associated with a 46% reduced risk for coronary heart disease events in individuals with lipoprotein(a) >50 mg/dL.
What Are the Clinical Implications?
This study expands on prior studies as the first to use plasma lipoprotein(a) levels, which are more clinically applicable and available than genetic instruments, and was conducted in a more diverse population.
Aspirin may have a role for the primary prevention of cardiovascular events in individuals with elevated lipoprotein(a).
Lipoprotein(a) levels >50 mg/dL are present in ≈20% to 25% of the population. 1 In addition to its role in atherogenesis 2 and vascular inflammation, 3 , 4 lipoprotein(a) may play a role in thrombosis due to the antifibrinolytic effects of apolipoprotein(a), 5 and its interaction with platelets. 6 Despite the significantly increased risk associated with lipoprotein(a), therapies for primary prevention of cardiovascular disease (CVD) are lacking and increased risk attributable to lipoprotein(a) remains in patients treated with statins. 7 Given the high prevalence of elevated lipoprotein(a) and the associated CVD risk, there is a critical need for therapies that modify this risk, particularly for primary prevention.
Aspirin is a cornerstone of secondary prevention of CVD, but multiple large primary prevention aspirin trials have produced negative or marginally positive results. 8 , 9 , 10 However, a meta‐analysis of primary prevention trials of aspirin, including the 3 most recent large trials, showed a small benefit to aspirin use for CVD risk. 11 This has led to progressively narrower guideline recommendations for aspirin use in primary prevention, including the recent American College of Cardiology/American Heart Association and United States Preventive Services Task Force recommendations. 12 , 13 These results suggest that aspirin may be beneficial in primary prevention for specific subgroups of individuals at sufficiently high risk with a propensity for platelet‐mediated thrombosis. Given the atherothrombotic properties of lipoprotein(a), individuals with high levels may represent a subgroup of patients whose risk/benefit profile favors the use of aspirin for primary prevention. This was first suggested in the Women's Health Study, where carriers of an LPA single nucleotide polymorphism (SNP) associated with highly elevated lipoprotein(a) levels who were randomized to aspirin had significant benefit in the primary prevention of CVD. 14 A recent secondary analysis of the more contemporary ASPREE (aspirin in reducing events in the elderly) trial demonstrated a similar benefit to aspirin use in healthy elderly individuals with the same SNP as well as high risk based on a polygenic risk score. 15 However, lipoprotein(a) levels, used clinically to determine risk, were not measured, and it is unknown whether plasma lipoprotein(a) levels would yield similar results. To address this question, we evaluated the relationship between aspirin, elevated plasma lipoprotein(a), and incident coronary heart disease (CHD) events in the MESA (Multi‐Ethnic Study of Atherosclerosis).
METHODS
Study Cohort
Data from the MESA were used for this study. Data are publicly available through MESA upon formal request. We performed a study using data from the MESA, a prospective cohort study of individuals free of known baseline CVD. We included individuals with both baseline lipoprotein(a) measurements and data on baseline aspirin use with follow‐up for adjudication of cardiovascular events. The design of the MESA has been described previously. 16 Initial recruitment was conducted between 2000 and 2002 at 6 centers across the United States. The institutional review boards at each center approved the study and participants provided written informed consent. In total, 6814 participants were recruited for the first examination.
Risk Factor Assessment and Outcomes
Characterization of the cohort at baseline, including demographics, medical comorbidities, and medication use was performed using standardized questionnaires. Cigarette smoking was defined as current, former, or never use, and quantified in pack years. Systolic blood pressure was recorded as the average of multiple seated blood pressure measurements. Fasting blood samples were obtained for laboratory measures including total cholesterol and high‐density lipoprotein cholesterol. Low‐density lipoprotein cholesterol was calculated using the Friedewald equation. Diabetes was defined using the 2003 American Diabetes Association criteria. Lipoprotein(a) was measured using a latex‐enhanced turbidimetric immunoassay (Denka Seiken) of mass concentration and reported in mg/dL. Aspirin use was defined as self‐report of use at least 3 days per week. Family history of atherosclerotic cardiovascular disease was defined as a history of myocardial infarction or stroke in a first‐degree relative.
Participants in MESA were followed prospectively for cardiovascular events, with adjudication of events available through 2018. For this study, the primary outcome was coronary heart disease (CHD) events (a composite of CHD death and nonfatal myocardial infarction). Major bleeding events were recorded based on International Classification of Diseases, Ninth Revision (ICD‐9) and ICD‐10 codes for bleeding during hospitalizations through 2018. Bleeding events were categorized as gastrointestinal, genitourinary, central nervous system, unspecified, or other bleeding. Postprocedural bleeding was excluded, and bleeding events occurring after a diagnosis of CVD were not included because prior nonaspirin users would be likely to be placed on aspirin after a CVD diagnosis.
Statistical Analysis
Due to anticipated confounding by indication for aspirin use without data regarding the initial indication for aspirin prescription by a physician, we used propensity score matching to evaluate the association between aspirin use and CHD events stratified by lipoprotein(a). A propensity score for aspirin use was created based on risk factors that may influence aspirin prescription (age, sex, race/ethnicity, hypertension, diabetes, cigarette smoking, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and total cholesterol). Aspirin users were then matched to nonaspirin users using 1:1 nearest neighbor matching.
After matching, baseline characteristics that were components of the propensity score were compared by baseline aspirin use to assess success of matching. Baseline demographics were compared by lipoprotein(a) level (above and below the guideline‐recommended threshold of 50 mg/dL 17 ). Continuous variables were compared with independent sample t tests or Mann–Whitney U tests, and categorical variables were compared with χ2 tests.
The association between baseline aspirin use and CHD was evaluated using Cox proportional hazards models, stratified by lipoprotein(a) level. We utilized multiple models with progressive multivariable adjustment: an unadjusted model containing a term for aspirin use at baseline; Model 1, which additionally adjusted for demographics (sex, race/ethnicity, and recruitment site), and Model 2, which additionally adjusted for CVD risk factors (diabetes, hypertension treatment, cigarette smoking, systolic blood pressure, high‐density lipoprotein cholesterol, total cholesterol, statin use, body mass index, family history of atherosclerotic cardiovascular disease, and C‐reactive protein).
Cumulative incidence curves were created for the association between aspirin use and CHD events using 4 categories stratified by lipoprotein(a) above or below 50 mg/dL, and aspirin use or nonuse. Potential effect modification by age (above and below 60 years), sex, or race/ethnicity was evaluated using multiplicative interaction tests. To confirm there was evidence of residual confounding, we also performed the same Cox proportional hazards models listed above, stratified by lipoprotein(a) level, using the whole cohort without propensity score matching.
Rates of major bleeding overall and by bleeding type were compared among aspirin users and nonaspirin users, stratified by lipoprotein(a) level. The association between aspirin use and time to first bleeding event, stratified by lipoprotein(a) level, was evaluated in Cox proportional hazards models adjusted for age, sex, race/ethnicity, liver disease, systolic blood pressure, treatment for hypertension, nonsteroidal anti‐inflammatory drug use, oral anticoagulant use, alcohol use, and estimated glomerular filtration rate.
Analyses were performed using R (v4.1, R Core Team 2021), and using packages survival, survminer, matchit, and ggplot2. A 2‐tailed P value of <0.05 was considered statistically significant. HB and ST had full access to the data and take responsibility for their integrity and the data analysis.
RESULTS
Baseline Characteristics
After excluding participants with missing aspirin (n=42), lipoprotein(a) (n=114), or follow‐up data (n=26), the study cohort contained 6632 participants. After propensity‐matching aspirin users and nonusers, there were 2183 participants in the matched cohort, 1760 (80.6%) with lipoprotein(a) ≤50 mg/dL, and 423 (19.4%) with lipoprotein(a) >50 mg/dL (Figure 1). Aspirin users and nonusers appeared well matched with standardized mean differences for components of the propensity score all <0.20 (Table 1). Those with lipoprotein(a) >50 mg/dL were more often female and of Black race with greater prevalence of hypertension, statin use, antihypertensive use, and aspirin use. Those with elevated lipoprotein(a) also had higher total cholesterol, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol. Incident CHD events were more frequent in those with elevated lipoprotein(a) (Table 2). The multiplicative interaction between aspirin use and lipoprotein(a) level for CHD events was borderline significant with a P value of 0.08. Continued regular use of aspirin was also evaluated in regular follow‐up examinations in those with available data; at median [interquartile range] follow‐up of 1.6 [1.4–1.8], 3.1 [3.0–3.3], and 4.8 [4.6–5.0] years; 946 (79.0%), 853 (78.8%), and 795 (74.4%) of participants were still using aspirin regularly, respectively. At the latest follow‐up (median 15.7 [15.3–16.2] years), data were available for 543 individuals, of whom 368 (67.8%) were still using aspirin regularly.
Figure 1. Study flow diagram.
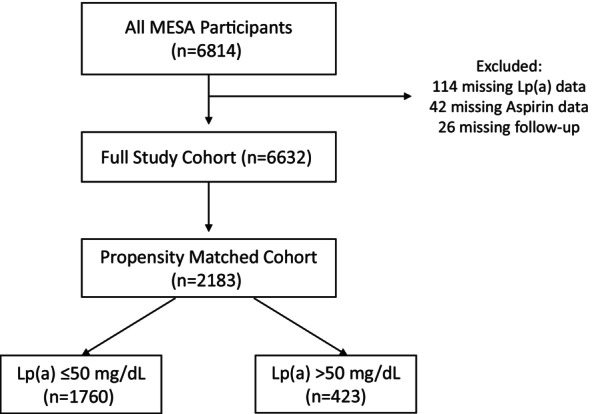
This figure illustrates the cohort selection process. There are 6814 participants in MESA, with 6632 after exclusions for missing data. After 1:1 propensity matching for aspirin users and nonaspirin users, there were 2183 participants (949 without baseline aspirin use and 1234 with baseline aspirin use), including 1760 with lipoprotein(a) ≤50 mg/dL and 423 with lipoprotein(a) >50 mg/dL. Lp(a) indicates lipoprotein(a); and MESA, Multi‐Ethnic Study of Atherosclerosis.
Table 1.
Baseline Characteristics and Propensity Score Components Stratified By Aspirin Use in Propensity‐Matched Cohort
| Nonaspirin (n=949) | Aspirin (n=1234) | P value | SMD | |
|---|---|---|---|---|
| Age, y | 65.34 (10.14) | 66.40 (9.11) | 0.01 | 0.11 |
| Female sex | 445 (46.9) | 541 (43.8) | 0.17 | 0.06 |
| Race or ethnicity | 0.89 | 0.03 | ||
| White | 489 (51.5) | 652 (52.8) | ||
| Asian | 89 (9.4) | 106 (8.6) | ||
| Black | 235 (24.8) | 298 (24.1) | ||
| Hispanic | 136 (14.3) | 178 (14.4) | ||
| Hypertension | 541 (57.0) | 722 (58.5) | 0.51 | 0.03 |
| Diabetes | 152 (16.0) | 212 (17.2) | 0.51 | 0.03 |
| Cigarette smoking | 0.49 | 0.05 | ||
| Never | 459 (48.4) | 565 (45.8) | ||
| Former | 403 (42.5) | 552 (44.7) | ||
| Current | 87 (9.2) | 117 (9.5) | ||
| Total cholesterol, mg/dL | 189.68 (34.08) | 188.04 (33.97) | 0.27 | 0.05 |
| LDL‐C, mg/dL | 113.58 (31.46) | 112.19 (30.41) | 0.30 | 0.05 |
| HDL‐C, mg/dL | 50.48 (14.77) | 50.55 (14.87) | 0.92 | 0.005 |
| Lipoprotein(a), mg/dL | 16.20 [7.00–37.50] | 15.90 [6.80–40.05] | 0.66 | 0.08 |
| Antihypertensive use | 433 (45.6) | 663 (53.7) | <0.001 | 0.16 |
| Statin use | 154 (16.3) | 325 (26.3) | <0.001 | 0.25 |
Results are presented as mean (SD), median [interquartile range], or n (%). HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and SMD, standardized mean difference.
Table 2.
Characteristics of Participants Stratified by Lipoprotein(a) Level in Propensity‐Matched Cohort
| Lipoprotein(a)≤50 mg/dL (n=1760) | Lipoprotein(a)>50 mg/dL (n=423) | P value | |
|---|---|---|---|
| Age, y | 66.04 (9.53) | 65.50 (9.78) | 0.30 |
| Female sex | 765 (43.5) | 221 (52.2) | 0.001 |
| Race or ethnicity | <0.001 | ||
| White | 957 (54.4) | 184 (43.5) | |
| Asian | 176 (10.0) | 19 (4.5) | |
| Black | 352 (20.0) | 181 (42.8) | |
| Hispanic | 275 (15.6) | 39 (9.2) | |
| BMI, kg/m2 | 28.39 (5.19) | 28.44 (5.33) | 0.85 |
| Hypertension | 997 (56.6) | 266 (62.9) | 0.02 |
| Diabetes | 290 (16.5) | 74 (17.5) | 0.67 |
| Current smoker | 164 (9.3) | 40 (9.5) | 0.95 |
| Statin use | 354 (20.1) | 125 (29.6) | <0.001 |
| Antihypertensive use | 856 (48.6) | 240 (56.7) | 0.003 |
| SBP, mm Hg | 129.91 (21.54) | 130.98 (23.06) | 0.37 |
| Total cholesterol, mg/dL | 186.51 (32.70) | 198.08 (37.68) | <0.001 |
| LDL‐C, mg/dL | 110.48 (29.55) | 122.44 (34.27) | <0.001 |
| HDL‐C, mg/dL | 49.92 (14.68) | 53.04 (15.15) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 85.60 (20.38) | 84.98 (20.35) | 0.57 |
| CRP, mg/L | 3.67 (5.98) | 3.54 (5.20) | 0.70 |
| Oral anticoagulant use | 5 (0.3) | 1 (0.2) | >0.99 |
| NSAID use | 274 (15.6) | 55 (13.0) | 0.21 |
| History of blood clots | 55 (3.1) | 14 (3.3) | 0.96 |
| Liver disease | 70 (4.0) | 9 (2.1) | 0.09 |
| Family history ASCVD | 1080 (61.4) | 270 (63.8) | 0.38 |
| Aspirin use | 973 (55.3) | 261 (61.7) | 0.02 |
| CHD events | 156 (8.9) | 58 (13.7) | 0.004 |
| Follow‐up time, y | 16.5 [10.7–18.4] | 16.4 [9.1–17.2] | 0.04 |
Results are presented as mean (SD), median [interquartile range] or n (%). ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NSAID, nonsteroidal anti‐inflammatory drug; and SBP, systolic blood pressure.
Aspirin Use and CHD Outcomes by Lipoprotein(a) Level
In the propensity‐matched cohort, aspirin use was not significantly associated with a lower risk of CHD events in those with lipoprotein(a) ≤50 mg/dL (hazard ratio [HR], 0.80 [95% CI, 0.58–1.10). However, aspirin use was associated with significantly lower risk of CHD events among those with lipoprotein(a) >50 mg/dL (HR, 0.54 [95% CI, 0.31–0.93]; Table 3). Four participant categories were created based on lipoprotein(a) level (by threshold of 50 mg/dL) and aspirin use at baseline. Cumulative incidence curves for these categories and CHD events are shown in Figure 2. Those with lipoprotein(a) >50 mg/dL without aspirin use had the highest event rate, while those with lipoprotein(a) >50 mg/dL with aspirin use appeared similar to both groups with lipoprotein(a) ≤50 mg/dL (overall log‐rank test P=0.003 for comparing the 4 strata).
Table 3.
Association Between Aspirin Use and CHD Events, Stratified by Lipoprotein(a) Level, in Propensity‐Matched Cohort
| Lipoprotein(a) ≤50 mg/dL (n=1760) | Lipoprotein(a) >50 mg/dL (n=423) | |
|---|---|---|
| CHD events | 156 | 58 |
| HR | 95% CI | P value | HR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Aspirin use | ||||||
| Unadjusted | 0.89 | 0.65–1.22 | 0.48 | 0.65 | 0.39–1.09 | 0.10 |
| Model 1 | 0.80 | 0.59–1.10 | 0.18 | 0.62 | 0.37–1.06 | 0.08 |
| Model 2 | 0.80 | 0.58–1.10 | 0.17 | 0.54 | 0.31–0.93 | 0.03 |
Model 1 adjusts for sex, race/ethnicity and site. Model 2 adjusts for Model 1+diabetes, hypertension treatment, cigarette smoking, systolic blood pressure, high‐density lipoprotein, total cholesterol, statin use, body mass index, family history of atherosclerotic cardiovascular disease, and C‐reactive protein. CHD indicates coronary heart disease; and HR, hazard ratio.
Figure 2. Aspirin use and CHD events by lipoprotein(a) level in propensity‐matched cohort.
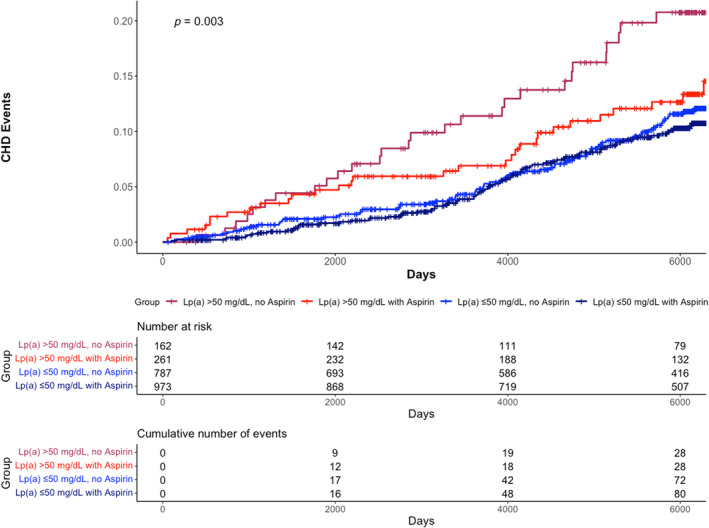
These cumulative incidence curves depict CHD risk for 4 categories based on lipoprotein(a) level and baseline aspirin use. Participants with lipoprotein(a) >50 mg/dL without aspirin use demonstrated the highest event rate, while participants with lipoprotein(a) >50 mg/dL with aspirin use demonstrated similar risk as those with lipoprotein(a) ≤50 mg/dL regardless of aspirin use. CHD indicates coronary heart disease; and Lp(a), lipoprotein(a).
Testing for interaction between aspirin use and key demographics was performed. The interaction tests between aspirin use and age (P=0.83), sex (P=0.61), and race/ethnicity (P=0.33) were not significant. Additionally, analyses by race/ethnicity would likely be underpowered due to small samples sizes. Bleeding events were not adjudicated in MESA.
Aspirin Use and CHD Outcomes by Lipoprotein(a) Level in Unmatched Cohort
In the whole cohort without propensity matching, there were 5314 (80.1%) participants with lipoprotein(a) ≤50 mg/dL and 1318 (19.9%) with lipoprotein(a) >50 mg/dL. There was a nonsignificant trend towards greater risk of CHD events with aspirin use in unadjusted models with lipoprotein(a) ≤50 mg/dL (HR, 1.26 [95% CI, 0.99–1.61]) and lipoprotein(a) >50 mg/dL (HR, 1.38 [95% CI, 0.91–2.09]). With multivariable adjustment, the association between aspirin use and CHD events became inverse, though nonsignificant, in both lipoprotein(a) strata: (HR, 0.80 (95% CI, 0.62–1.04]) for lipoprotein(a) ≤50 mg/dL and (HR, 0.88 [95% CI, 0.56–1.38]) for lipoprotein(a) >50 mg/dL, suggestive of possible confounding by indication (Table S1).
Bleeding Events
In the overall cohort, aspirin users were observed to have a higher rate of major bleeding events (n=227, 17.5%) than nonaspirin users (n=655, 12.5%, P<0.001). Among aspirin users, there was no difference in the rate of major bleeding (P=0.224) or the type of bleeding (P=0.070) by lipoprotein(a) level. Similarly, there were no significant differences observed by lipoprotein(a) level among nonaspirin users (Table S2).
Aspirin use was associated with major bleeding in those without elevated lipoprotein(a) (HR, 1.51 [95% CI, 1.27–1.80]) and those with lipoprotein(a) >50 mg/dL (HR, 1.49 [95% CI, 1.09–2.04]) in an unadjusted analysis. After multivariable adjustment, however, aspirin use was positively but nonsignificantly associated with major bleeding in those without elevated lipoprotein(a) (HR, 1.08 [95% CI, 0.90–1.29]) and those with elevated lipoprotein(a) (HR, 1.21 [95% CI, 0.86–1.69]). Similar results were observed for gastrointestinal bleeding, but aspirin use was not significantly associated with bleeding events even in the unadjusted analysis (Table 4).
Table 4.
Association Between Aspirin Use and Bleeding Events, Stratified by Lipoprotein(a) Level
| Lipoprotein(a) ≤50 mg/dL (n=5100) | Lipoprotein(a) >50 mg/dL (n=1261) | |
|---|---|---|
| Major bleeding events | 667 | 197 |
| HR | 95% CI | P value | HR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Aspirin use | ||||||
| Unadjusted | 1.51 | 1.27–1.80 | <0.001 | 1.49 | 1.09–2.04 | 0.013 |
| Adjusted* | 1.08 | 0.90–1.29 | 0.439 | 1.21 | 0.86–1.69 | 0.265 |
| Gastrointestinal bleeding events | 266 | 91 | ||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Aspirin use | ||||||
| Unadjusted | 1.69 | 1.29–2.20 | <0.001 | 1.25 | 0.77–2.01 | 0.368 |
| Adjusted* | 1.18 | 0.89–1.56 | 0.252 | 1.14 | 0.69–1.91 | 0.604 |
Adjusted for age, sex, race/ethnicity, liver disease, hypertension treatment, nonsteroidal anti‐inflammatory drug use, oral anticoagulant use, alcohol use, systolic blood pressure, and estimated glomerular filtration rate. HR indicates hazard ratio.
DISCUSSION
In a propensity‐matched cohort of individuals without baseline cardiovascular disease, aspirin use was associated with a significant reduction in risk for cardiovascular events among those with lipoprotein(a) >50 mg/dL. These findings using plasma lipoprotein(a) are complementary to recent LPA genetic data suggesting a similar association and add to the evidence base that patients with elevated lipoprotein(a) may uniquely benefit from aspirin therapy in the primary prevention setting.
Aspirin use is well established for the secondary prevention of atherosclerotic cardiovascular disease events, with an estimated 25% relative risk reduction in yearly risk of serious vascular events in a meta‐analysis of secondary prevention studies. 18 In the modern era, the use of aspirin for primary prevention in the general population, however, is less well established. The reasons for a less clear benefit of aspirin use in primary prevention include lower population risk and lower event rates, which make it more difficult to balance the associated increased risk of bleeding, as well as the evolution of other primary preventive therapies such as lipid‐lowering therapy and more aggressive management of diabetes and hypertension. In a 2009 meta‐analysis of primary prevention trials involving aspirin use, aspirin resulted in a 12% relative risk reduction in yearly serious vascular events, but only a 0.07% absolute risk reduction (as compared with a 19% relative risk reduction, and 1.5% absolute risk reduction for secondary prevention). 18 In 2016, the United States Preventive Services Task Force gave a relatively broad recommendation for aspirin in adults aged 50 to 59 (grade B) or 60 to 69 (grade C) with at least 10% 10‐year atherosclerotic cardiovascular disease risk. 19
There were subsequently 3 large randomized controlled trials of aspirin use for primary prevention in different populations, published in 2018. The ASCEND (effects of aspirin for primary prevention in persons with diabetes mellitus) trial demonstrated a benefit in a composite outcome of serious vascular events with a rate ratio of 0.88 (95% CI, 0.79–0.97) in diabetic persons assigned aspirin 100 mg daily. However, this was offset by increased major bleeding (relative risk, 1.29 [95% CI, 1.09–1.52). 9 The ASPREE trial, which randomized healthy elderly participants to 100 mg daily aspirin or placebo, failed to show a benefit in a composite outcome of cardiovascular disease (HR, 0.95 [95% CI, 0.83–1.08), while also demonstrating an increased risk of major hemorrhage (HR, 1.38 [95% CI, 1.18–1.62]). 10 Finally, the ARRIVE (aspirin to reduce risk of initial vascular events) trial of participants at moderate vascular risk failed to show a benefit in its primary end point with aspirin 100 mg daily (HR, 0.96 [95% CI, 0.81–1.13]) but again demonstrated increasing gastrointestinal bleeding (HR. 2.11 [95% CI, 1.36–3.28]). 8 While the latter 2 trials failed to show a benefit to aspirin, ASCEND demonstrated that there is likely a benefit in a group that is of sufficiently higher risk, analogous to benefit in secondary prevention patients who are also high risk. A 2019 meta‐analysis, including the above studies, again demonstrated a benefit to aspirin use in 13 randomized primary prevention trials (HR, 0.89 [95% CI, 0.84–0.94]) with associated increased bleeding risk (HR, 1.43 [95% CI, 1.30–1.56]). 11 While there is some evidence of ischemic benefit in these studies, this tends to be counterbalanced by major bleeding episodes, leading to lack of a net clinical benefit.
The findings from the 2018 trials led to relatively narrow guideline recommendations for aspirin in primary prevention, including the American College of Cardiology/American Heart Association primary prevention guidelines, which recommend consideration of low‐dose aspirin use for select adults aged 40 to 70 at higher atherosclerotic cardiovascular disease risk but not bleeding risk (class IIb recommendation), 12 and the recent United States Preventive Services Task Force recommendations, which provide significantly more narrow recommendations than in 2016. 13 However, there may be a group that is at high enough cardiovascular risk to derive a benefit from aspirin that outweighs bleeding risk. Given lipoprotein(a)'s interaction with platelets and thrombosis, there is biological plausibility that aspirin may benefit individuals with high lipoprotein(a). In a secondary prevention study, prolonged dual antiplatelet therapy (>1 year) after percutaneous coronary intervention reduced subsequent ischemic cardiovascular events in people with elevated lipoprotein(a), 20 suggesting a particular benefit to antiplatelet therapy in this population. In a secondary analysis of the Women's Health Study (WHS), which randomized healthy women to aspirin 100 mg every other day or placebo, participants were genotyped for rs3798220, a SNP of the LPA gene. Carriers of the rs3798220‐C variant assigned to the placebo group had very elevated lipoprotein(a) levels and significantly elevated CVD risk (HR, 2.24 [95% CI, 1.36–3.68]) compared with noncarriers. Carriers who were assigned aspirin had a significant reduction in events (HR, 0.44 [95% CI, 0.20–0.94]) and a similar risk profile as noncarriers assigned aspirin or placebo. Bleeding risk was not described. 14 The findings of this study, however, were limited to White women who were carriers of the rs3798220‐C variant (which was only present in 3.7% of women in the study) 14 and require genetic testing for clinical application. A recent secondary analysis of the ASPREE trial demonstrated similar results, supporting the findings of the current study. Using the same SNP as the WHS study, as well as a polygenic risk score including many lipoprotein(a) SNPs, neither the presence of the lipoprotein(a) SNP nor the highest quintile of lipoprotein(a) genetic risk score were associated with risk for major adverse cardiovascular events in those assigned aspirin, while significant risk was present in those assigned placebo. 15
Importantly, there is currently no approved medical therapy for treating lipoprotein(a)‐mediated risk, but there is evidence that targeting lipoprotein(a) may result in a reduction in CVD risk. Statins increase lipoprotein(a) 10% to 25%, and CVD risk remains in statin‐treated patients with elevated lipoprotein(a). 7 , 21 , 22 Proprotein convertase subtilisin/kexin type 9 inhibitors achieve modest lowering of lipoprotein(a), and secondary analyses of clinical trials suggest that this results in a reduction in events, 23 , 24 but this has not been assessed in primary prevention, and proprotein convertase subtilisin/kexin type 9 inhibitors are not approved for the indication of elevated lipoprotein(a). Antisense oligonucleotides and short interfering RNA produce potent lipoprotein(a) lowering and are in clinical development, including 2 ongoing phase 3 secondary prevention clinical trials, Lp(a) HORIZON (NCT04023552) and OCEAN(a) is Olpasiran Trials of Cardiovascular Events and Lipoprotein(a) Reduction ‐ Outcomes Trial (NCT05581303). However, there are no ongoing trials for the use of these drugs in primary prevention. Our study suggests a benefit to aspirin therapy for primary prevention of CHD in people with elevated lipoprotein(a). 14
Our study has multiple novel findings. To our knowledge, this is the first study to address the use of aspirin for the primary prevention of cardiovascular disease in individuals with elevated lipoprotein(a) levels. Both the prior WHS and ASPREE analyses mentioned above focused on the use of SNPs of the LPA gene. However, the use of lipoprotein(a) levels is much more clinically relevant and broadly applicable because lipoprotein(a) levels summate all known and unknown genetic, environmental, and dietary influences on plasma levels. For example, the polygenic risk score used in the ASPREE study estimated ≈60% of the variation in lipoprotein(a) levels in a cohort of European ancestry, and requires measurements of 43 SNPs. However, the association between the genetic risk score with CVD outcomes is significantly reduced when adjusting for lipoprotein(a) levels. 25 Furthermore, circulating lipoprotein(a) is the likely culprit for atherothrombotic risk, rather than the underlying genetics influencing lipoprotein(a) in plasma. Plasma lipoprotein(a) levels are also more clinically applicable because testing is more readily available, and at lower cost. Additionally, the WHS analysis focused on a single SNP present in 3.7% of White women in the study. The ASPREE study focused only on individuals of European ancestry, and the primary SNP evaluated was again only present in 3.2% of individuals included. Our findings, in contrast, are applicable to a diverse, multi‐ethnic cohort, and >19% of individuals in the propensity‐matched cohort had elevated lipoprotein(a) levels >50 mg/dL. Finally, the WHS study did not describe bleeding events, 14 while the ASPREE analysis observed a small increase in clinically significant bleeding in SNP carriers with aspirin use. 15 Our study extends these results by observing an overall increased rate of bleeding with aspirin use, but finding no significant difference between those with elevated versus nonelevated lipoprotein(a).
Our study has important limitations due to its observational nature, particularly confounding by indication. The initial indication for aspirin therapy was not collected as part of the MESA and could not be directly controlled for. This is supported by the analysis in the whole cohort without propensity matching; the positive association between aspirin use and CHD in unadjusted analyses, and the lower hazard ratios for aspirin with progressive multivariable adjustment may represent confounding by indication. We attempted to mitigate this by using propensity score matching, but the results must be interpreted in this context as hypothesis generating. Additionally, aspirin use was recorded by self‐report, which may be subject to recall bias. Dosage of aspirin and adherence to aspirin therapy could not be fully assessed as part of this study. However, over follow‐up of nearly 5 years, >74% of individuals continued to report regular aspirin use. Over extended follow‐up of >15 years, >67% of individuals continued to report regular aspirin use. Despite these limitations, the study results also align with the prior WHS and ASPREE studies. In addition, subsequent bleeding events were collected based on hospitalization billing codes, which may be inaccurate. Finally, stratified analyses were limited by sample size and number of events, though tests for interaction between aspirin use and age, sex, and race/ethnicity were not significant.
Further study is needed to confirm these findings and to inform clinical practice and guidelines. In particular, randomization of aspirin use will be an important aspect of future studies to address the issue of confounding by indication. Studies with larger sample sizes could also better address stratified analyses. Finally, studies involving well‐adjudicated bleeding events are needed to assess the net risk/benefit of aspirin therapy in this population.
CONCLUSIONS
In a propensity‐matched analysis, aspirin use was associated with a lower risk for cardiovascular events among participants with elevated lipoprotein(a) and free of baseline cardiovascular disease. These results suggest that aspirin may have potential as a therapy for primary prevention in a large population with currently limited therapeutic options. However, further studies, particularly with randomization of aspirin, are needed.
Sources of Funding
HB was supported by the National Institutes of Health, Grants 5T32HL079891, 1KL2TR001444, and 1K08HL166962. ST is supported by NIH R01 HL159156. PT is supported by NIH SC1 GM139730. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute, and by grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420 from the National Center for Advancing Translational Sciences (NCATS).
Disclosures
HB received consulting fees from Kaneka Medical and Novartis Pharmaceuticals. ST is a co‐inventor and receives royalties from patents owned by UCSD and is a co‐founder and has an equity interest in Oxitope, Inc and Kleanthi Diagnostics, LLC and has a dual appointment at UCSD and Ionis Pharmaceuticals. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict‐of‐interest policies. The remaining authors have no disclosures to report.
Supporting information
Tables S1‐S2
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org.
This manuscript was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033562
For Sources of Funding and Disclosures, see page 9.
References
- 1. Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36:2239–2245. doi: 10.1161/atvbaha.116.308011 [DOI] [PubMed] [Google Scholar]
- 2. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. doi: 10.1016/j.jacc.2016.11.042 [DOI] [PubMed] [Google Scholar]
- 3. Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis E, Witztum JL, Koschinsky ML, et al. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and Lipoprotein (a). J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Valk FM, Bekkering S, Kroon J, Yeang C, Van den Bossche J, van Buul JD, Ravandi A, Nederveen AJ, Verberne HJ, Scipione C, et al. Oxidized phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134:611–624. doi: 10.1161/circulationaha.116.020838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boffa MB, Koschinsky ML. Lipoprotein (a): truly a direct prothrombotic factor in cardiovascular disease? J Lipid Res. 2016;57:745–757. doi: 10.1194/jlr.R060582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martínez C, Rivera J, Loyau S, Corral J, González‐Conejero R, Lozano ML, Vicente V, Anglés‐Cano E. Binding of recombinant apolipoprotein(a) to human platelets and effect on platelet aggregation. Thromb Haemost. 2001;85:686–693. doi: 10.1055/s-0037-1615654 [DOI] [PubMed] [Google Scholar]
- 7. Willeit P, Ridker PM, Nestel PJ, Simes J, Tonkin AM, Pedersen TR, Schwartz GG, Olsson AG, Colhoun HM, Kronenberg F, et al. Baseline and on‐statin treatment lipoprotein(a) levels for prediction of cardiovascular events: individual patient‐data meta‐analysis of statin outcome trials. Lancet. 2018;392:1311–1320. doi: 10.1016/s0140-6736(18)31652-0 [DOI] [PubMed] [Google Scholar]
- 8. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;392:1036–1046. doi: 10.1016/s0140-6736(18)31924-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Group ASC , Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. doi: 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 10. McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379:1509–1518. doi: 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng SL, Roddick AJ. Association of Aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta‐analysis. Jama. 2019;321:277–287. doi: 10.1001/jama.2018.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guirguis‐Blake JM, Evans CV, Perdue LA, Bean SI, Senger CA. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US preventive services task Force. Jama. 2022;327:1585–1597. doi: 10.1001/jama.2022.3337 [DOI] [PubMed] [Google Scholar]
- 14. Chasman DI, Shiffman D, Zee RY, Louie JZ, Luke MM, Rowland CM, Catanese JJ, Buring JE, Devlin JJ, Ridker PM. Polymorphism in the apolipoprotein(a) gene, plasma Lipoprotein(a), cardiovascular disease, and low‐dose aspirin therapy. Atherosclerosis. 2009;203:371–376. doi: 10.1016/j.atherosclerosis.2008.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lacaze P, Bakshi A, Riaz M, Polekhina G, Owen A, Bhatia HS, Natarajan P, Wolfe R, Beilin L, Nicholls SJ, et al. Aspirin for primary prevention of cardiovascular events in relation to Lipoprotein(a) genotypes. Je Am Coll Cardiol. 2022;80:1287–1298. doi: 10.1016/j.jacc.2022.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, et al. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 17. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella‐Tommasino J, Forman DE, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association task Force on clinical practice guidelines. Circulation 2019;139:e1082‐e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Antithrombotic Trialists' (ATT) Collaboration , Colin Baigent LB, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/s0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bibbins‐Domingo K; Force USPST . Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. preventive services task Force recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577 [DOI] [PubMed] [Google Scholar]
- 20. Cui K, Wang H‐Y, Yin D, Zhu C, Song W, Wang H, Jia L, Zhang D, Song C, Feng L, et al. Benefit and risk of prolonged dual antiplatelet therapy after percutaneous coronary intervention with drug‐eluting stents in patients with elevated Lipoprotein(a) concentrations. Front Cardiovasc Med. 2021;8:807925. doi: 10.3389/fcvm.2021.807925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pirillo A, Catapano AL. Statins increase Lp(a) plasma level: is this clinically relevant? Eur Heart J. 2020;41:2285–2287. doi: 10.1093/eurheartj/ehz505 [DOI] [PubMed] [Google Scholar]
- 22. Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41:2275–2284. doi: 10.1093/eurheartj/ehz310 [DOI] [PubMed] [Google Scholar]
- 23. Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, et al. Effect of alirocumab on Lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–144. doi: 10.1016/j.jacc.2019.10.057 [DOI] [PubMed] [Google Scholar]
- 24. Szarek M, Bittner VA, Aylward P, Baccara‐Dinet M, Bhatt DL, Diaz R, Fras Z, Goodman SG, Halvorsen S, Harrington RA, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low‐density lipoprotein cholesterol lowering: ODYSSEY OUTCOMES trial. Eur Heart J. 2020;41:4245–4255. doi: 10.1093/eurheartj/ehaa649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trinder M, Uddin MM, Finneran P, Aragam KG, Natarajan P. Clinical utility of lipoprotein(a) and LPA genetic risk score in risk prediction of incident atherosclerotic cardiovascular disease. JAMA Cardiol. 2021;6:287–295. doi: 10.1001/jamacardio.2020.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S2


