Abstract
Background
Venoarterial extracorporeal membrane oxygenation (VA‐ECMO) is increasingly used for patients with cardiogenic shock. Although Impella or intra‐aortic balloon pump (IABP) is frequently used for left ventricular unloading (LVU) during VA‐ECMO treatment, there are limited data on comparative outcomes. We compared outcomes of Impella and IABP for LVU during VA‐ECMO.
Methods and Results
Using the Nationwide Readmissions Database between 2016 and 2020, we analyzed outcomes in 3 groups of patients with cardiogenic shock requiring VA‐ECMO based on LVU strategies: extracorporeal membrane oxygenation (ECMO) only, ECMO with IABP, and ECMO with Impella. Of 15 980 patients on VA‐ECMO, IABP and Impella were used in 19.4% and 16.4%, respectively. The proportion of patients receiving Impella significantly increased from 2016 to 2020 (6.5% versus 25.8%; P‐trend<0.001). In‐hospital mortality was higher with ECMO with Impella (54.8%) compared with ECMO only (50.4%) and ECMO with IABP (48.4%). After adjustment, ECMO with IABP versus ECMO only was associated with lower in‐hospital mortality (adjusted odds ratio [aOR], 0.83; P=0.02). ECMO with Impella versus ECMO only had similar in‐hospital mortality (aOR, 1.09; P=0.695) but was associated with more bleeding (aOR, 1.21; P=0.007) and more acute kidney injury requiring hemodialysis (aOR, 1.42; P<0.001). ECMO with Impella versus ECMO with IABP was associated with greater risk of acute kidney injury requiring hemodialysis (aOR, 1.49; P=0.002), higher in‐hospital mortality (aOR, 1.32; P=0.001), and higher 40‐day mortality (hazard ratio, 1.25; P<0.001).
Conclusions
In patients with cardiogenic shock on VA‐ECMO, LVU with Impella, particularly with 2.5/CP, was not associated with improved survival at 40 days but was associated with increased adverse events compared with IABP. More data are needed to assess Impella platform‐specific comparative outcomes of LVU.
Keywords: IABP, Impella, left ventricular unloading, VA‐ECMO
Subject Categories: Epidemiology, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Heart Failure, Quality and Outcomes, Clinical Studies
Nonstandard Abbreviations and Acronyms
- AKI
acute kidney injury
- CS
cardiogenic shock
- IABP
intra‐aortic balloon pump
- ICD‐10‐PCS
International Classification of Diseases, Tenth Revision, Procedure Coding System
- MCS
mechanical circulatory support
- NRD
Nationwide Readmissions Database
Clinical Perspective.
What Is New?
The proportion of cardiogenic shock patients on venoarterial extracorporeal membrane oxygenation managed with Impella for left ventricular unloading significantly increased from 7.3% in 2016 to 24.3% in 2020 while the temporal trend of use of intra‐aortic balloon pump had not significantly changed.
Left ventricular unloading using Impella, compared with intra‐aortic balloon pump, was independently associated with increased mortality and likelihood of acute kidney injury requiring renal replacement therapy.
What Are the Clinical Implications?
Further studies comparing outcomes of intra‐aortic balloon pump and Impella are needed to guide clinical practice of mechanical left ventricular unloading in patients on venoarterial extracorporeal membrane oxygenation for cardiogenic shock.
Cardiogenic shock (CS) is an acute clinical syndrome characterized by end‐organ hypoperfusion caused by low cardiac output and confers a high in‐hospital mortality rate (~50%). 1 , 2 In recent years, the incidence of CS has increased. 2 Despite lack of robust evidence from randomized clinical trials (RCT) demonstrating an improved survival, the use of temporary mechanical circulatory support (MCS) device in CS has substantially increased. 3 Venoarterial extracorporeal membrane oxygenation (VA‐ECMO) is increasingly used to manage CS. However, an increase in afterload during VA‐ECMO is associated with risks of worsening myocardial ischemia, delayed recovery of myocardium, left ventricular (LV) thrombus, and pulmonary edema. 4 To prevent these consequences, several LV unloading strategies are utilized in conjunction with VA‐ECMO. 5
Among various LV unloading strategies, mechanical unloading using either intra‐aortic balloon pump (IABP) or percutaneous transaortic ventricular assist device (eg, Impella) is frequently used based on their hemodynamic benefits. IABP can facilitate aortic valve opening and increase LV ejection by reducing afterload during systole. 6 Impella directly decompresses LV and decreases LV filling pressures using a microaxial flow pump. 7 While these percutaneous MCS devices can provide LV unloading, it is unclear whether the use of additional MCS for LV unloading leads to improved clinical outcomes. Indeed, observational studies have shown that ~50% of the survivors of CS treated with VA‐ECMO do not receive mechanical LV unloading. 8 There are limited data with mixed signals to guide the selection of MCS for LV unloading in those on VA‐ECMO for CS. The primary aim of our study was to compare the clinical outcomes of LV unloading using IABP versus Impella in patients treated with VA‐ECMO for CS in a large contemporary database.
Methods
Data Source and Study Population
The Nationwide Readmissions Database (NRD) was obtained from the Agency for Healthcare Research and Quality, which administers the Healthcare Cost and Utilization Project. The NRD is a national database that contains annual hospital discharge data with verified patient linkage numbers to track the patients across hospitals within a state during a given year. We identified study population, comorbidities, complications, causes of readmissions, and procedures based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) and Procedure Coding System (ICD‐10‐PCS) codes, respectively (Table S1). The data that support this study's findings are available from the corresponding author upon reasonable request.
From 2016 through 2020, all hospitalizations for CS coded either in primary or secondary diagnosis were identified based on ICD‐10‐CM code of R57.0. Among patients hospitalized for CS, those who required VA‐ECMO were selected based on ICD‐10‐PCS codes of 5A15223, 5A1522G, and 5A1522F. CS after cardiotomy was excluded, and patients who had both Impella and IABP were excluded. Also, to ensure that IABP or Impella were used for LV unloading in VA‐ECMO, patients who had IABP or Impella before VA‐ECMO were excluded because of the possibility of them being removed after escalation to VA‐ECMO. Finally, patients younger than 18 years of age, or those who were missing mortality or length of stay data, were excluded from the study. The study cohort consisted of 3 groups of patients on VA‐ECMO for CS, depending on the LV unloading strategy: (1) VA‐ECMO without LV unloading (ECMO only group), (2) IABP for LV unloading (IABP group), and (3) Impella for LV unloading (Impella group). Institutional review board approval and informed consent were not required for the current study because all data collection was derived from a deidentified administrative database.
Study End Points
The primary end point of the study was all‐cause mortality, which was assessed for both in‐hospital mortality and early mortality with a follow‐up time up to 40 days. The secondary safety end points were in‐hospital vascular complications, bleeding complications, and acute kidney injury (AKI) requiring hemodialysis (Table S1). Vascular complications were a composite of arteriovenous fistula, rupture, dissection, other vascular complications following a procedure, and acute limb ischemia. Bleeding complications were a composite of cerebral, access site‐related, gastrointestinal, and pulmonary alveolar bleedings. Additionally, all‐cause 30‐day readmissions were examined as a secondary end point.
Statistical Analysis
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC). All analyses accounted for the complex survey design, including hospital‐level clustering of patients and sampling stratum, and discharge weight was used to obtain national estimates. For descriptive analyses, we compared baseline patient and hospital characteristics stratified by the LV unloading strategy. Categorical variables are shown as frequencies, and continuous variables are presented as mean or median. For comparison, the Rao‐Scott χ2 test was used for categorical variables and either the Mann–Whitney‐Wilcoxon nonparametric test or survey‐specific linear regression was used for continuous variables. We first examined the comparative outcomes between patients on ECMO only and those on either IABP or Impella for LV unloading. For the main comparison of our study, we compared the clinical outcomes between patients managed with IABP and Impella during VA‐ECMO treatment. To estimate the independent association between LV unloading strategies and study end points, we created multivariable logistic regression models by including covariates that had a univariate significance with each end point (P<0.1). Cox proportional hazards regression model was fitted to estimate the association between Impella versus IABP and early mortality. The proportional hazards assumption was met for all reported analyses based on the Kolmogorov‐type supremum test. 9
To further account for confounders, we used 3 propensity‐score methods. The individual propensities to receive IABP versus Impella were estimated using a multivariable logistic regression model that included all the measured covariates in this study. First, we conducted an analysis using the propensity score as an additional covariate in the multivariable regression models. Second, the 1:1 propensity score matching was performed between patients with IABP and Impella based on a caliper width of 0.2 of the standard deviation of the logit of the propensity score using a greedy algorithm. 10 Standardized differences for all covariates were computed before and after matching to examine the accomplishment of matching. After matching, the standardized difference for each covariate was <10%, indicating successful matching (Table S2). 11 Finally, we calculated inverse probability of treatment weights. We applied trimming to the inverse probability of treatment weights by excluding those lying outside of the 1st to 99th percentiles of the PS distribution. 12 Kaplan–Meier method was used to obtain early mortality risk in patients treated with Impella versus IABP among the propensity score matched patients.
For sensitivity analyses, we conducted a falsification end point analysis to examine the robustness of our findings. 13 , 14 Additionally, we calculated E values to assess the potential impact of an unmeasured confounding factor on the association between the LV unloading device and in‐hospital or early mortality. 15 Finally, given the possibility of the same‐day implantation and removal of Impella or IABP followed by escalation to VA‐ECMO, analyses were repeated in patients on ECMO only or whose LV unloading was started on days subsequent to ECMO cannulation day (n=6411). All 95% CIs and P values were corrected for multiple comparisons using Bonferroni method when appropriate. 16 All tests were 2‐sided, with P<0.05 indicating statistical significance.
Results
Study Population
During the study period from January 2016 to December 2020, a total of 15 980 patients were hospitalized with CS and underwent VA‐ECMO cannulation. CS caused by acute myocardial infarction accounted for 34.0% of the study cohort. With 35.8% of the study population being managed with mechanical LV unloading, IABP and Impella were used in 3098 (19.4%) and 2620 (16.4%) patients, respectively. Over time, the proportion of patients with CS on VA‐ECMO managed with Impella for LV unloading significantly increased from 7.3% (14 patients per 10 000 CS discharges) in 2016 to 24.3% (47 patients per 10 000 CS discharges) in 2020 (P for trend <0.001), while the temporal trend of use of IABP had not significantly changed (Figure 1). Baseline demographic and clinical characteristics of the weighted study population are listed in Table 1. Compared with those on ECMO only, patients managed with LV unloading (IABP group and Impella group) were more likely to be older, men, to present with acute coronary syndrome, and to have higher burden of comorbidities, including diabetes, coronary artery disease, and congestive heart failure. Percutaneous coronary intervention and pulmonary artery catheterization (PAC) were more frequently performed in patients managed with LV unloading versus ECMO only. Compared with patients on ECMO only, LV assist device was more frequently performed in patients managed with Impella. The cost of hospitalization was higher in patients who were managed with LV unloading compared with their counterparts. The comparison of baseline characteristics and treatments between patients managed with LV unloading using IABP versus Impella is listed in Tables S3 and S4. Among patients undergoing mechanical LV unloading, the Impella group versus IABP group was more likely to consist of men with a lower prevalence of valvular heart disease and more likely to receive PAC. Of the 2620 patients with Impella for LV unloading, about 1 in 6 patients received Impella (16.8%) with axillary or aortic grafts (Impella 5.0/LD/5.5). Depending on the platform, the Impella group fared differently from the IABP group: patients with Impella 2.5/CP had a lower burden of comorbidities (33.2% versus 39.9% with Elixhauser comorbidity scores ≥4; P=0.003) and more often presented with STEMI (ST‐segment–elevation myocardial infarction) and underwent percutaneous coronary intervention (Table S4). On the other hand, patients managed with Impella 5.0/LD/5.5 were younger and more likely to receive LV assist device or heart transplantation compared with those on IABP.
Figure 1. Temporal trends in utilization of intra‐aortic balloon pump and Impella for left ventricular unloading in adult patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation in the United States, 2016 to 2020.
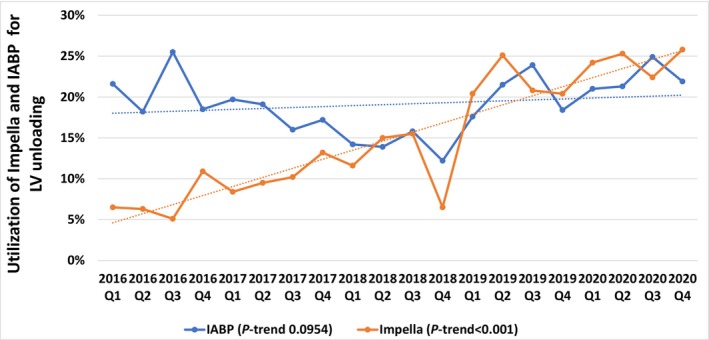
IABP indicates intra‐aortic balloon pump; and LV, left ventricular.
Table 1.
Baseline Individual‐ and Hospital‐Level Characteristics for Patients Hospitalized With Cardiogenic Shock Requiring VA‐ECMO, 2016 to 2020
| Characteristics (%) | All | ECMO only | IABP | P value* | Impella | P value† |
|---|---|---|---|---|---|---|
| No. of patients, weighted (%) | 15 980 | 10 262 (64.2) | 3098 (19.4) | 2620 (16.4) | ‐ | |
| Age, median (IQR) | 56 (45–65) | 55 (42–65) | 59 (50–68) | <0.001 | 58 (48–65) | <0.001 |
| Age group, y | <0.001 | <0.001 | ||||
| <50 | 5232 (32.8) | 3784 (36.9) | 734 (23.7) | 714 (27.3) | ||
| 50–64 | 6172 (38.6) | 3729 (36.3) | 1289 (41.6) | 1153 (44.0) | ||
| 65–79 | 4217 (26.4) | 2524 (24.6) | 988 (31.9) | 705 (26.9) | ||
| ≥80 | 359 (2.2) | 224 (2.2) | 87 (2.8) | 48 (1.8) | ||
| Women | 5441 (34.1) | 3775 (36.8) | 1006 (32.5) | 0.004 | 660 (25.2) | <0.001 |
| Causes of cardiogenic shock | ||||||
| STEMI | 3542 (22.2) | 1492 (14.5) | 927 (29.9) | <0.001 | 1123 (42.9) | <0.001 |
| NSTEMI | 1881 (11.8) | 1002 (9.8) | 507 (16.4) | <0.001 | 372 (14.2) | <0.001 |
| Nonischemic | 10 556 (66.0) | 7768 (75.7) | 1664 (53.7) | <0.001 | 1125 (42.9) | <0.001 |
| Previous cardiac arrest | 618 (3.9) | 404 (3.9) | 103 (3.3) | 0.344 | 111 (4.2) | 0.629 |
| Hypertension | 8696 (54.4) | 5379 (52.4) | 1863 (60.1) | <0.001 | 1453 (55.5) | 0.051 |
| Diabetes | 4625 (28.9) | 2730 (26.6) | 1028 (33.2) | <0.001 | 867 (33.1) | <0.001 |
| Known coronary artery disease | 6856 (42.9) | 3601 (35.1) | 1773 (57.2) | <0.001 | 1481 (56.5) | <0.001 |
| Prior PCI | 164 (1.0) | 92 (0.9) | 39 (1.3) | 0.211 | 33 (1.3) | 0.269 |
| Prior CABG | 867 (5.4) | 563 (5.5) | 201 (6.5) | 0.142 | 103 (3.9) | 0.018 |
| Iron deficiency anemia | 673 (4.2) | 438 (4.3) | 140 (4.5) | 0.690 | 95 (3.6) | 0.298 |
| Chronic kidney disease | 4558 (28.5) | 2762 (26.9) | 1059 (34.2) | <0.001 | 737 (28.1) | 0.376 |
| CHF | 7953 (49.8) | 4634 (45.2) | 1817 (58.7) | <0.001 | 1501 (57.3) | <0.001 |
| Atrial fibrillation/flutter | 5356 (33.5) | 3223 (31.4) | 1266 (40.9) | <0.001 | 867 (33.1) | 0.255 |
| Coagulopathy | 7071 (44.2) | 4522 (44.1) | 1420 (45.8) | 0.240 | 1129 (43.1) | 0.570 |
| COPD | 2635 (16.5) | 1734 (16.9) | 547 (17.6) | 0.510 | 354 (13.5) | 0.003 |
| Peripheral vascular disease | 3446 (21.6) | 2149 (20.9) | 725 (23.4) | 0.039 | 571 (21.8) | 0.515 |
| Valvular heart disease | 764 (4.8) | 546 (5.3) | 166 (5.4) | 0.964 | 52 (2.0) | <0.001 |
| Elixhauser comorbidity score≥4 | 5591 (35.0) | 3477 (33.9) | 1237 (39.9) | <0.001 | 877 (33.5) | 0.802 |
| Procedures/surgeries performed | ||||||
| Revascularization among ischemic cardiogenic shock | 3300 (60.8) | 1209 (48.5) | 1040 (72.5) | <0.001 | 1051 (70.3) | <0.001 |
| PCI | 2447 (45.1) | 836 (33.5) | 668 (46.6) | <0.001 | 943 (63.0) | <0.001 |
| CABG | 1080 (19.9) | 442 (17.7) | 495 (34.5) | <0.001 | 143 (9.6) | <0.001 |
| Pulmonary artery catheterization | 4600 (28.8) | 2251 (21.9) | 1104 (35.6) | <0.001 | 1245 (47.5) | <0.001 |
| LVAD | 1074 (6.7) | 622 (6.1) | 229 (7.4) | 0.101 | 222 (8.5) | 0.004 |
| Heart transplantation | 775 (4.8) | 404 (3.9) | 291 (9.4) | <0.001 | 79 (3.0) | 0.143 |
| Median household income | 0.035 | 0.011 | ||||
| 1st quartile | 4034 (25.2) | 2610 (25.4) | 804 (25.9) | 621 (23.7) | ||
| 2nd quartile | 4223 (26.4) | 2831 (27.6) | 761 (24.6) | 631 (24.1) | ||
| 3rd quartile | 4024 (25.2) | 2567 (25.0) | 755 (24.4) | 703 (26.8) | ||
| 4th quartile | 3699 (23.2) | 2254 (22.0) | 778 (25.1) | 666 (25.4) | ||
| Primary payer | 0.001 | 0.002 | ||||
| Medicare | 5677 (35.5) | 3583 (34.9) | 1259 (40.7) | 834 (31.8) | ||
| Medicaid | 2842 (17.8) | 1916 (18.7) | 503 (16.2) | 423 (16.1) | ||
| Private including | 6202 (38.8) | 3956 (38.6) | 1132 (36.6) | 1113 (42.5) | ||
| HMO | ||||||
| Self‐pay/no charge/other | 1260 (7.9) | 806 (7.8) | 203 (6.5) | 251 (9.6) | ||
| Hospital bed size | 0.043 | <0.001 | ||||
| Small | 355 (2.2) | 214 (2.1) | 83 (2.8) | 58 (2.2) | ||
| Medium | 1698 (15.6) | 939 (9.1) | 349 (11.3) | 409 (15.6) | ||
| Large | 13 927 (82.2) | 9109 (88.8) | 2665 (85.9) | 2153 (82.2) | ||
| Time to VA‐ECMO, median (IQR), d | 0.6 (0–4.2) | 0.8 (0–4.8) | 0.2 (0–3.4) | 0.047 | 0 (0–1.0) | <0.001 |
| Time to IABP, median (IQR), d | ‐ | ‐ | 0.8 (0–5.5) | ‐ | ‐ | ‐ |
| Time to Impella, median (IQR), d | ‐ | ‐ | ‐ | ‐ | 0.2 (0–2.7) | ‐ |
| Length of hospital stay, median (IQR), d | 16 (5–34) | 16 (5–34) | 18 (6–35) | 0.754 | 14 (4–30) | <00001 |
| Disposition‡ | 0.067 | 0.019 | ||||
| Home | 3738 (23.4) | 2487 (24.3) | 718 (23.2) | 533 (20.4) | ||
| Facility§ | 4097 (25.7) | 2579 (25.2) | 880 (28.4) | 638 (24.5) | ||
| Died | 8111 (50.9) | 5176 (50.5) | 1498 (48.4) | 1437 (55.1) | ||
| Cost of index hospitalization, median (IQR), $ | 153 768 (85885–265 632) | 145 651 (79792–254 479) | 169 158 (97903–280 557) | <0.001 | 171 386 (101507–288 212) | <0.001 |
CABG indicates coronary artery bypass graft surgery; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HMO, health maintenance organization; IABP, intra‐aortic balloon pump; IQR, interquartile range; LVAD, left ventricular assist device; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
P value for comparison between IABP and ECMO only.
P value for comparison between Impella and ECMO only.
Leaving against medial advice (AMA)/unknown not counted attributable to number of patients <10 accordingly to Healthcare Cost and Utilization Project guidelines.
Facility includes skilled nursing facility, intermediate care facility, and inpatient rehabilitation facility.
In‐Hospital Outcomes and Early Mortality at 40 Days
In‐hospital outcomes are presented in Figure 2 and Tables S5 through S7. In‐hospital mortality was 50.8% in the entire study population, while it was highest in the Impella group (54.8%), primarily driven by Impella 2.5/CP group (60.0%), compared with the ECMO only (50.4%) and IABP (48.4%) groups. Bleeding complications, particularly the access‐related bleeding and gastrointestinal bleeding, were more frequently observed in the Impella group (40.1%) than in the ECMO only (34.8%, P<0.001) and IABP (35.4%, P=0.045) groups. AKI requiring hemodialysis occurred more frequently in the Impella group (21.8%) compared with the ECMO only (16.5%, P<0.001) and IABP (16.0%, P=0.002) groups. After adjustment for confounding factors, LV unloading using IABP, compared with ECMO only, was associated with 17% lower odds of in‐hospital mortality (Table 2). The LV unloading with Impella, compared with ECMO only, had similar in‐hospital mortality, was associated with 21% higher odds of bleeding and 42% higher odds of AKI requiring hemodialysis. Furthermore, LV unloading with Impella was independently associated with 32% higher odds of in‐hospital mortality (odds ratio [OR], 1.32 [95% CI, 1.10–1.60]) and 49% higher odds of AKI requiring hemodialysis (OR, 1.49 [95% CI, 1.13–1.97]) compared with LV unloading with IABP. Of note, the association between Impella and in‐hospital outcomes, compared with other unloading strategies, differed depending on the platform of Impella: Impella 2.5/CP was associated with significantly increased in‐hospital mortality compared with ECMO only or IABP, whereas Impella 5.0/LD/5.5 was linked with reduced mortality (Table S8). There was no significant statistical interaction identified between the LV unloading device (Impella versus IABP) and causes of CS (acute myocardial infarction versus nonischemic) on in‐hospital mortality (P for interaction=0.811, Table S9). The association between Impella versus IABP use and in‐hospital mortality was consistently observed in the analyses based on the propensity score, including the propensity score matched analysis of 1149 unweighted pairs of patients (Table 3).
Figure 2. Comparison of early mortality and in‐hospital outcomes based on strategies of left ventricular unloading during venoarterial extracorporeal membrane oxygenation for cardiogenic shock.
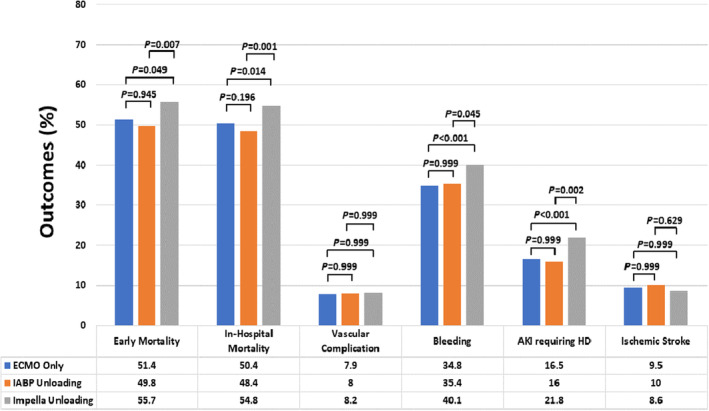
All P values are corrected for multiple comparisons using Bonferroni method. AKI indicates acute kidney injury; ECMO, extracorporeal membrane oxygenation; and HD, hemodialysis.
Table 2.
Association of LV Unloading Strategy With In‐Hospital Outcomes in Patients on VA‐ECMO for Cardiogenic Shock
| In‐hospital outcomes | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P Value* | Odds ratio (95% CI) | P value* | |
| In‐hospital mortality | ||||
| IABP vs ECMO only | 0.92 (0.79–1.07) | 0.587 | 0.83 (0.70–0.98) | 0.020 |
| Impella vs ECMO only | 1.19 (1.00–1.42) | 0.045 | 1.09 (0.91–1.31) | 0.695 |
| Impella vs IABP | 1.30 (1.07–1.57) | 0.004 | 1.32 (1.10–1.60) | 0.001 |
| Vascular complication | ||||
| IABP vs ECMO only | 1.01 (0.74–1.38) | 0.999 | 1.03 (0.74–1.43) | 0.999 |
| Impella vs ECMO only | 1.03 (0.79–1.35) | 0.999 | 1.06 (0.81–1.40) | 0.999 |
| Impella vs IABP | 1.02 (0.72–1.45) | 0.999 | 1.03 (0.73–1.47) | 0.999 |
| Bleeding† | ||||
| IABP vs ECMO only | 1.03 (0.87–1.21) | 0.999 | 0.99 (0.84–1.16) | 0.999 |
| Impella vs ECMO only | 1.25 (1.08–1.45) | <0.001 | 1.21 (1.04–1.41) | 0.007 |
| Impella vs IABP | 1.04 (1.00–1.49) | 0.047 | 1.23 (1.00–1.51) | 0.051 |
| AKI requiring hemodialysis | ||||
| IABP vs ECMO only | 0.96 (0.78–1.19) | 0.999 | 0.95 (0.76–1.18) | 0.999 |
| Impella vs ECMO only | 1.41 (1.13–1.76) | <0.001 | 1.42 (1.13–1.77) | <0.001 |
| Impella vs IABP | 1.47 (1.12–1.92) | 0.002 | 1.49 (1.13–1.97) | 0.002 |
| Ischemic stroke | ||||
| IABP vs ECMO only | 1.06 (0.83–1.35) | 0.999 | 0.99 (0.78–1.28) | 0.999 |
| Impella vs ECMO only | 0.90 (0.68–1.18) | 0.999 | 0.81 (0.61–1.08) | 0.240 |
| Impella vs IABP | 0.85 (0.61–1.17) | 0.634 | 0.82 (0.59–1.12) | 0.374 |
IABP indicates intra‐aortic balloon pump; and VA‐ECMO, venoarterial extracorporeal membrane oxygenation.
P values are corrected based on Bonferroni correction method for multiple comparison.
Bleeding: Composite of central nervous system bleeding, access‐related bleeding including hematoma and retroperitoneal bleeding, gastrointestinal bleeding, and pulmonary alveolar bleeding.
Table 3.
Association Between Left Ventricular Unloading Device and In‐Hospital Mortality Based on Propensity Score Analyses
| Left ventricular unloading device | Model 1* | Model 2† | Model 3‡ | |||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Impella vs IABP | 1.40 (1.19–1.64) | <0.001 | 1.38 (1.17–1.63) | <0.001 | 1.25 (1.15–1.37) | <0.001 |
IABP indicates intra‐aortic balloon pump.
Model 1: Propensity score included as a covariate in the multivariable logistic regression model.
Model 2: Analysis based on the propensity score matching.
Model 3: Inverse probability of treatment weights analysis based on the propensity score after trimming by excluding those lying outside of the 1st to 99th percentiles of the propensity score distribution. Adjusted odds ratio (95% CI) without trimming: 1.27 (1.17–1.39), P<0.001.
Among patients who survived the index hospitalization with CS, 18.8% (17.8% in ECMO only, 20.7% in IABP, and 20.7% in Impella groups) were readmitted to a hospital within 30 days of discharge for a variety of reasons. Specifically, infection/sepsis was the most common cause of 30‐day readmissions, followed by heart failure and respiratory failure. During the 30‐day readmission, cardiac catheterization/percutaneous coronary intervention were performed in 11.2% of the patients, and 2.7% underwent heart transplantation (Figures S1 and S2). Median time to 30‐day readmission was 10 (interquartile range [IQR], 4–20) days.
Over a median follow‐up of 17 (interquartile range [IQR], 5–42) days (18 [IQR, 6–43] days in IABP group and 14 [IQR, 4–39] in Impella group), early mortality in the study population was 51.8%. Early mortality was highest in the Impella group (55.7%) compared with ECMO only (51.4%) and IABP (49.8%) groups (Figure 2). After adjustment for confounders with regression and propensity score analyses, Impella use was associated with at least a 25% higher hazard of early mortality (hazard ratio [HR], 1.25 [95% CI, 1.17–1.32]) compared with IABP use (Table S10; Figure 3).
Figure 3. Kaplan–Meier curves for early mortality among propensity matched patients with cardiogenic shock on venoarterial extracorporeal membrane oxygenation receiving Impella versus intra‐aortic balloon pump for left ventricular unloading.
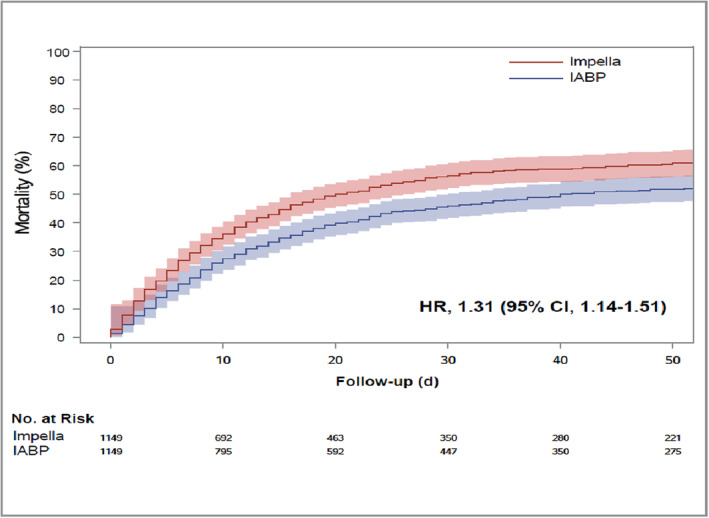
Median follow‐up time = 17 (interquartile range, 5–42) days. HR indicates hazard ratio; and IABP, intra‐aortic balloon pump.
In falsification end point analysis, we found no association of Impella versus IABP use with community acquired pneumonia and a composite end point of acute diarrhea, cellulitis, or intestinal obstruction, suggesting that the significant association between Impella versus IABP and mortality end point was unlikely because of unmeasured confounding factors (Table S11). Furthermore, based on the point estimates from propensity score‐matched cohort, E values for in‐hospital mortality and early mortality were 1.63 with 1.38 for lower bound of CI and 1.7 with 1.42 for lower bound of CI, respectively. This suggests that an unmeasured confounder would have to be associated with 1.63‐fold increased odds of in‐hospital mortality or 1.7‐fold increased hazard of early mortality to explain our findings. These point estimates are much greater than any measured confounding factors in our study. Therefore, it is unlikely that an unmeasured confounding factor would overcome the observed association of Impella versus IABP with in‐hospital and early mortality. The sensitivity analyses among those on ECMO only or who had LV unloading started on following days of ECMO cannulation day showed results consistent with the main analyses (Table S12).
Discussion
In this observational study of a large, real‐world, nationally representative database in the United States, several important findings were identified. First, the use of LV unloading device steadily increased in patients treated with VA‐ECMO for CS. Particularly, from 2016 through 2020, there was more than a 3‐fold increase in the number of patients managed with Impella for LV unloading during VA‐ECMO treatment. Second, the highest in‐hospital mortality was observed with Impella + ECMO compared with IABP + ECMO or ECMO only cohort. Third, LV unloading with IABP, compared with ECMO only, was associated with reduced in‐hospital mortality. Fourth, LV unloading with Impella, compared with ECMO only, was associated with increased likelihood of bleeding and AKI requiring hemodialysis. Finally, LV unloading with Impella versus IABP was associated with 49% higher odds of AKI requiring hemodialysis, 32% higher odds of in‐hospital mortality, and 25% higher hazard of early mortality. To our knowledge, this is the largest study of the comparative outcomes of LV unloading between Impella and IABP among patients on VA‐ECMO for CS.
LV Unloading Using IABP Versus VA‐ECMO Only
A meta‐analysis of 7 observational studies found a reduced mortality risk associated with LV unloading during VA‐ECMO treatment for CS. The study cohort in this meta‐analysis consisted mostly of patients receiving IABP (n=1555; 92%) for LV unloading, and the reduced mortality was predominantly observed with the use of IABP (risk ratio [RR], 0.81 [95% CI, 0.73–0.89]). 17 Another meta‐analysis of 37 observational studies by Al‐Fares et al reported a significant reduction in in‐hospital mortality in patients receiving IABP for LV unloading during VA‐ECMO for CS (RR, 0.86 [95% CI, 0.77–0.95]). 18 Consistent with these meta‐analyses, our study also demonstrated a reduction in in‐hospital mortality in patients managed with IABP for LV unloading (OR, 0.83 [95% CI, 0.70–0.98]) compared with those on VA‐ECMO only. Application of IABP in VA‐ECMO has been shown to further improve regional microcirculation and provides enhanced hemodynamic support by increasing pulsatility as well as reducing LV end‐diastolic dimension and pulmonary capillary wedge pressure by an average of ~4 mm Hg. 19 , 20
LV Unloading Using Impella Versus ECMO Only
Previous studies have reported a substantial increase in the overall utilization of Impella devices. From 2007 through 2012, a 30‐fold increase in the utilization of Impella was noted mostly in patients with CS or acute myocardial infarction. 21 In addition, an analysis of the United Network for Organ Sharing database between 2015 and 2019 found that the proportion of patients receiving Impella while awaiting heart transplantation significantly increased from 1% to 4%. 22 The most significant increase occurred after the United Network for Organ Sharing policy change in the donor heart allocation system in 2018, giving higher priority to those on MCS. Adding to the existing body of literature, our study found a steady increase in the use of Impella for LV unloading among patients with CS on VA‐ECMO from 2016 to 2020, with the utilization of Impella exceeding that of IABP by 2020. Although improved surrogate outcomes have been reported, including hemodynamic parameters, there is a lack of data from RCTs that support a survival benefit of Impella in patients with CS. Therefore, the use of Impella for LV unloading during VA‐ECMO needs to be judicious and individualized until informed by further trial data.
Conflicting results of LV unloading with Impella compared with ECMO only have been reported in several observational studies. An international, multicenter study, reported by Schrage et al of 686 patients with CS on VA‐ECMO, demonstrated an association of LV unloading using Impella (n=255) with lower 30‐day mortality (HR, 0.79 [95% CI, 0.63–0.98]). 23 Complications occurred more frequently in patients with LV unloading using Impella versus without LV unloading, including severe bleeding and renal replacement therapy. Another meta‐analysis of 8 observational studies found a signal toward improved early mortality with versus without Impella in patients with CS on VA‐ECMO (RR, 0.90 [95% CI, 0.78–1.03]). 24 However, there was also an increased risk of complications associated with Impella, including renal replacement therapy (RR, 1.54; 95% CI, 1.19–1.99) and bleeding (RR, 1.45 [95% CI, 1.20–1.75]). On the other hand, a meta‐analysis by Al‐Fares et al showed no difference in in‐hospital mortality with LV unloading using Impella (RR, 1.13 [95% CI, 0.85–1.50]). 18
In our study of real‐world data, LV unloading with Impella was also associated with similar in‐hospital mortality (OR, 1.09 [95% CI, 0.91–1.31]) but was associated with higher risk of bleeding likely due to the additional large‐bore access for Impella placement and AKI requiring renal replacement therapy compared with VA‐ECMO only. Our cohort study strongly parallels the demographic and clinical characteristics of patients in the study reported by Schrage et al, which was based on international, multicenter registry data derived from large, experienced centers in the use of MCS. The lack of survival benefit with LV unloading using Impella in our study, compared with the international study, is likely multifactorial. International, large, experienced centers are more likely to have standardized protocols for managing patients with CS requiring MCS. The standardized shock protocols, incorporating hemodynamic monitoring, have been shown to improve outcomes in the prospective single‐arm National Cardiogenic Shock Registry. 25 However, marked variation across the US hospitals has been observed in the use of Impella, and in associated outcomes of death, bleeding, and AKI. 26 Importantly, previous studies reported a considerable variation in anticoagulation strategies and how monitoring in Impella patients was conducted across the hospitals. 26 , 27 Accordingly, significant interhospital variation in bleeding complications was noted among patients receiving Impella. Notably, the use of PAC in the Impella cohort in our study was only 47.5%, indicating lack of standardized protocols for hemodynamic monitoring in patients with CS requiring MCS. The use of PAC in caring for patients with critical illness has been an area of much debate and controversy. In the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) trial, the use of PAC did not improve survival but increased adverse events. 28 In parallel with that trial, the use of PAC significantly decreased from 1993 to 2013 in the United States. 29 , 30 Although the impact of PAC on outcomes among patients with CS on MCS has not been established by RCTs, there is growing evidence of clinical benefit of hemodynamic monitoring with PAC, linked with lower mortality based on recent retrospective studies. 31 , 32 , 33 Accordingly, a Society for Cardiovascular Angiography and Interventions/Heart Failure Society of America clinical expert consensus document endorsed continuous hemodynamic monitoring with PAC for management of patients receiving MCS. 34 Our findings highlight the adverse outcomes associated with LV unloading using Impella in the real world and the need to implement standardized protocols for mechanical LV unloading.
LV Unloading Using Impella Versus IABP
Substantial gaps remain in the literature regarding comparative outcomes of LV unloading using Impella versus IABP during VA‐ECMO therapy. The baseline characteristics of our contemporary US study population strongly resemble those of the cohort with CS on VA‐ECMO of a large prospective RCT, conducted in the intensive care units at 20 French cardiac shock care centers between 2016 and 2019. 35 Furthermore, the early mortality and bleeding risks observed in our study are consistent with the report of that RCT, supporting the generalizability of our findings. A recent study of 3399 patients managed with mechanical LV unloading (n=580 [17.1%] with Impella) on VA‐ECMO for CS showed a signal toward lower in‐hospital mortality with IABP versus Impella (OR, 0.80 [95% CI, 0.64–1.01]; P=0.06). 36 While that study also reported increased risks of bleeding and renal injury with Impella versus IABP for LV unloading, it was limited by a relatively small sample size to assess the association with mortality. Using a large, nationally representative database, we report higher in‐hospital and 40‐day mortality rates associated with Impella versus IABP for LV unloading. The underlying mechanism of increased mortality associated with Impella on VA‐ECMO requires further investigation. However, there are clearly increased risks of bleeding and acute kidney injury requiring hemodialysis associated with Impella compared with IABP. Indeed, bleeding events have been shown to be associated with an increase in in‐hospital mortality in patients receiving ECMO. 37 Our study showed an association of Impella with 49% increased odds of AKI requiring hemodialysis compared with IABP for LV unloading. The NRD database does not provide data on specific platforms of Impella or the degree of hemolysis. Nonetheless, Impella has been shown in previous studies to be associated with an increased risk of hemolysis, which is the second most common cause of pigment nephropathy that can lead to AKI. 38 Therefore, we speculate that the increased incidence of the AKI observed in the Impella group in our study might be due to increased pigment nephropathy. 39 Notably, AKI is associated with poor outcomes in patients with CS. 40 , 41 In some centers, Impella devices are routinely placed for preemptive LV unloading at the time of VA‐ECMO implantation. 42 However, our study suggests that the selection of an LV unloading device should be based on a careful assessment of the net clinical benefit for individual patients, weighing the hemodynamic benefits against risks of adverse events.
Effect of Impella Platform on Outcomes of LV Unloading
In our exploratory analysis that accounted for the different platforms of Impella, LV unloading using Impella 2.5/CP was associated with a significantly increased in‐hospital mortality compared with ECMO only or IABP. On the other hand, Impella 5.0/LD/5.5 was associated with a decreased in‐hospital mortality despite the increased bleeding risk. While the findings of improved survival and increased provision of LV assist device/heart transplantation in patients managed with Impella 5.0/LD/55 are consistent with previous observations, these findings are subject to the selection and survival biases and need a cautious interpretation in the context of the observational study design. 43 We speculate that the patients with an established plan to undergo LV assist device or heart transplantation or those who survived until the conversion to Impella 5.0/LD/5.5 were more likely to be assigned to this group, leading to an increased survival.
Limitations
There are several limitations in our study, primarily because of the observational nature of the study design. First, despite the use of rigorous statistical methods based on multivariable regression, propensity score, and sensitivity analyses, there are still residual unmeasured confounding factors. For example, we were unable to account for the race, ethnicity, timing of the CS, and the Society for Cardiovascular Angiography and Interventions shock classification despite these being important predictors of mortality in CS due to lack of such data in the NRD. 44 Second, the follow‐up duration is limited to a given year because the NRD does not track patients across the years. Third, there are no ICD‐10‐PCS codes to identify the removal of MCS, precluding the inclusion of patients who received IABP or Impella before ECMO. Therefore, we were unable to assess the impact of LV unloading initiated before VA‐ECMO on outcomes. Schrage et al demonstrated lower 30‐day mortality in a subgroup that received Impella before or shortly after VA‐ECMO as opposed to nonsignificant association with mortality when Impella was implanted >2 hours after VA‐ECMO cannulation. 23 Further studies are needed to assess the impact of implantation timing of LV unloading devices on the association of LV unloading and outcomes during VA‐ECMO. Fourth, death occurring out of hospital after index hospitalization discharge was not captured in NRD. Also, patients admitted to 1 state and readmitted to another state would not be tracked by the NRD. Therefore, the early mortality rate in our study might be underestimated. Although early mortality observed in our study is consistent with that reported in a large multicenter French randomized clinical trial, supporting the robustness of our findings, it is still possible that the early mortality might be higher in the real world. 35 Finally, selection and misclassification biases cannot be excluded given the observational design. Although the Medicare Severity Diagnosis Related Group codes were used to assess the outcomes separately for Impella 2.5/CP and 5.0/LD/5.5 groups, we were unable to identify specific Impella platforms because of lack of ICD‐10‐PCS codes during the study period, which raises a possibility of misclassification bias and selection bias without randomization.
Conclusions
In this large, real‐world cohort study, LV unloading with Impella compared with IABP was not associated with improved survival at 40 days in patients receiving VA‐ECMO for CS. Increased risks of adverse events were observed with Impella compared with IABP placed for LV unloading. Our findings call for further high‐quality studies to guide clinical practice of LV unloading in patients on VA‐ECMO for CS.
Sources of Funding
This work was supported by grants from the Michael Wolk Heart Foundation, the New York Cardiac Center, Inc, and the New York Weill Cornell Medical Center Alumni Council. The Michael Wolk Heart Foundation, the New York Cardiac Center, Inc, and the New York Weill Cornell Medical Center Alumni Council had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Disclosures
Dr Cheung has received consulting fees from Abbott, Biotronik, and Boston Scientific, research grant support from Boston Scientific, and fellowship grant support from Abbott, Biosense, Biotronik, Boston Scientific, and Medtronic. The remaining authors have no disclosures to report.
Supporting information
Data S1
This manuscript was sent to Sakima A. Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032607
For Sources of Funding and Disclosures, see page 11.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/cir.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2. Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST‐elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/jaha.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short‐term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol. 2014;64:1407–1415. doi: 10.1016/j.jacc.2014.07.958 [DOI] [PubMed] [Google Scholar]
- 4. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66:2663–2674. doi: 10.1016/j.jacc.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 5. Geller BJ, Sinha SS, Kapur NK, Bakitas M, Balsam LB, Chikwe J, Klein DG, Kochar A, Masri SC, Sims DB, et al. Escalating and de‐escalating temporary mechanical circulatory support in cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2022;146:e50–e68. doi: 10.1161/cir.0000000000001076 [DOI] [PubMed] [Google Scholar]
- 6. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, et al. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; affirmation of value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65:e7–e26. doi: 10.1016/j.jacc.2015.03.036 [DOI] [PubMed] [Google Scholar]
- 7. Raess DH, Weber DM. Impella 2.5. J Cardiovasc Transl Res. 2009;2:168–172. doi: 10.1007/s12265-009-9099-4 [DOI] [PubMed] [Google Scholar]
- 8. Aso S, Matsui H, Fushimi K, Yasunaga H. The effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: an analysis using a nationwide inpatient database. Crit Care Med. 2016;44:1974–1979. doi: 10.1097/ccm.0000000000001828 [DOI] [PubMed] [Google Scholar]
- 9. Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale‐based residuals. Biometrika. 1993;80:557–572. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 10. Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52:249–264. doi: 10.2307/2533160 [DOI] [PubMed] [Google Scholar]
- 11. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conover MM, Rothman KJ. Propensity score trimming mitigates bias due to covariate measurement error in inverse probability of treatment weighted analyses: a plasmode simulation. Stat Med. 2021;40:2101–2112. doi: 10.1002/sim.8887 [DOI] [PubMed] [Google Scholar]
- 13. Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. doi: 10.1001/jama.2012.96867 [DOI] [PubMed] [Google Scholar]
- 14. Wimmer NJ, Resnic FS, Mauri L, Matheny ME, Yeh RW. Comparison of transradial versus transfemoral percutaneous coronary intervention in routine practice: evidence for the importance of "falsification hypotheses" in observational studies of comparative effectiveness. J Am Coll Cardiol. 2013;62:2147–2148. doi: 10.1016/j.jacc.2013.07.036 [DOI] [PubMed] [Google Scholar]
- 15. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/m16-2607 [DOI] [PubMed] [Google Scholar]
- 16. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, Visintini S, Simard T, Di Santo P, Mathew R, et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J Am Coll Cardiol. 2019;73:654–662. doi: 10.1016/j.jacc.2018.10.085 [DOI] [PubMed] [Google Scholar]
- 18. Al‐Fares AA, Randhawa VK, Englesakis M, McDonald MA, Nagpal AD, Estep JD, Soltesz EG, Fan E. Optimal strategy and timing of left ventricular venting during veno‐arterial extracorporeal life support for adults in cardiogenic shock: a systematic review and meta‐analysis. Circ Heart Fail. 2019;12:e006486. doi: 10.1161/circheartfailure.119.006486 [DOI] [PubMed] [Google Scholar]
- 19. Jung C, Lauten A, Roediger C, Fritzenwanger M, Schumm J, Figulla HR, Ferrari M. In vivo evaluation of tissue microflow under combined therapy with extracorporeal life support and intra‐aortic balloon counterpulsation. Anaesth Intensive Care. 2009;37:833–835. doi: 10.1177/0310057x0903700517 [DOI] [PubMed] [Google Scholar]
- 20. Petroni T, Harrois A, Amour J, Lebreton G, Brechot N, Tanaka S, Luyt CE, Trouillet JL, Chastre J, Leprince P, et al. Intra‐aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 2014;42:2075–2082. doi: 10.1097/ccm.0000000000000410 [DOI] [PubMed] [Google Scholar]
- 21. Khera R, Cram P, Lu X, Vyas A, Gerke A, Rosenthal GE, Horwitz PA, Girotra S. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175:941–950. doi: 10.1001/jamainternmed.2014.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pahwa S, Dunbar‐Matos C, Slaughter MS, Trivedi JR. Use of Impella in patients listed for heart transplantation. ASAIO J. 2022;68:786–790. doi: 10.1097/mat.0000000000001679 [DOI] [PubMed] [Google Scholar]
- 23. Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/circulationaha.120.048792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cappannoli L, Galli M, Zito A, Restivo A. Venoarterial extracorporeal membrane oxygenation (VA‐ECMO) with vs without left ventricular unloading by Impella: a systematic review and meta‐analysis. Eur Heart J Qual Care Clin Outcomes. 2023;9:358–366. doi: 10.1093/ehjqcco/qcac076 [DOI] [PubMed] [Google Scholar]
- 25. Basir MB, Kapur NK, Patel K, Salam MA, Schreiber T, Kaki A, Hanson I, Almany S, Timmis S, Dixon S, et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2019;93:1173–1183. doi: 10.1002/ccd.28307 [DOI] [PubMed] [Google Scholar]
- 26. Amin AP, Spertus JA, Curtis JP, Desai N, Masoudi FA, Bach RG, McNeely C, Al‐Badarin F, House JA, Kulkarni H, et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–284. doi: 10.1161/circulationaha.119.044007 [DOI] [PubMed] [Google Scholar]
- 27. Reed BN, DiDomenico RJ, Allender JE, Coons JC. Survey of anticoagulation practices with the Impella percutaneous ventricular assist device at high‐volume centers. J Interv Cardiol. 2019;2019:3791307. doi: 10.1155/2019/3791307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625 [DOI] [PubMed] [Google Scholar]
- 29. Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993‐2004. JAMA. 2007;298:423–429. doi: 10.1001/jama.298.4.423 [DOI] [PubMed] [Google Scholar]
- 30. Seifi A, Elliott RJ, Elsehety MA. Usage of Swan‐Ganz catheterization during the past 2 decades in United States. J Crit Care. 2016;35:213–214. doi: 10.1016/j.jcrc.2016.05.024 [DOI] [PubMed] [Google Scholar]
- 31. Garan AR, Kanwar M, Thayer KL, Whitehead E, Zweck E, Hernandez‐Montfort J, Mahr C, Haywood JL, Harwani NM, Wencker D, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in‐hospital mortality. JACC Heart Fail. 2020;8:903–913. doi: 10.1016/j.jchf.2020.08.012 [DOI] [PubMed] [Google Scholar]
- 32. Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team‐based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 33. O'Neill WW, Grines C, Schreiber T, Moses J, Maini B, Dixon SR, Ohman EM. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J. 2018;202:33–38. doi: 10.1016/j.ahj.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 34. Sorajja P, Borlaug BA, Dimas VV, Fang JC, Forfia PR, Givertz MM, Kapur NK, Kern MJ, Naidu SS. SCAI/HFSA clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. Catheter Cardiovasc Interv. 2017;89:E233–E247. doi: 10.1002/ccd.26888 [DOI] [PubMed] [Google Scholar]
- 35. Levy B, Girerd N, Amour J, Besnier E, Nesseler N, Helms J, Delmas C, Sonneville R, Guidon C, Rozec B, et al. Effect of moderate hypothermia vs normothermia on 30‐day mortality in patients with cardiogenic shock receiving venoarterial extracorporeal membrane oxygenation: a randomized clinical trial. JAMA. 2022;327:442–453. doi: 10.1001/jama.2021.24776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grandin EW, Nunez JI, Willar B, Kennedy K, Rycus P, Tonna JE, Kapur NK, Shaefi S, Garan AR. Mechanical left ventricular unloading in patients undergoing venoarterial extracorporeal membrane oxygenation. J Am Coll Cardiol. 2022;79:1239–1250. doi: 10.1016/j.jacc.2022.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aubron C, DePuydt J, Belon F, Bailey M, Schmidt M, Sheldrake J, Murphy D, Scheinkestel C, Cooper DJ, Capellier G, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care. 2016;6:97. doi: 10.1186/s13613-016-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakthirajan R, Dhanapriya J, Varghese A, Saravanakumar K, Dineshkumar T, Balasubramaniyan T, Gopalakrishnan N, Abraham KA. Clinical profile and outcome of pigment‐induced nephropathy. Clin Kidney J. 2018;11:348–352. doi: 10.1093/ckj/sfx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Annamalai SK, Jorde LE, Davila CD, Kapur NK. Effect of acute mechanical circulatory support on kidney function. In: Rangaswami J, Lerma E, McCullough P, eds Kidney Disease in the Cardiac Catheterization Laboratory: A Practical Approach. Cham: Springer; 2020:259–273. doi: 10.1007/978-3-030-45414-2_15 [DOI] [Google Scholar]
- 40. Fuernau G, Poenisch C, Eitel I, Denks D, de Waha S, Pöss J, Heine GH, Desch S, Schuler G, Adams V, et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP‐SHOCK II‐trial. Int J Cardiol. 2015;191:159–166. doi: 10.1016/j.ijcard.2015.04.242 [DOI] [PubMed] [Google Scholar]
- 41. Koreny M, Karth GD, Geppert A, Neunteufl T, Priglinger U, Heinz G, Siostrzonek P. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med. 2002;112:115–119. doi: 10.1016/s0002-9343(01)01070-1 [DOI] [PubMed] [Google Scholar]
- 42. Rob D, Bělohlávek J. The mechanical support of cardiogenic shock. Curr Opin Crit Care. 2021;27:440–446. doi: 10.1097/mcc.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 43. U.S. Food and Drug Administration . Impella Ventricular Support System 5.5 ‐ Instructions for Use. 2020. Accessed December 20, 2023. https://www.fda.gov/media/140766/download.
- 44. Ya'qoub L, Lemor A, Dabbagh M, O'Neill W, Khandelwal A, Martinez SC, Ibrahim NE, Grines C, Voeltz M, Basir MB. Racial, ethnic, and sex disparities in patients with STEMI and cardiogenic shock. J Am Coll Cardiol Interv. 2021;14:653–660. doi: 10.1016/j.jcin.2021.01.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


